Abstract
To evaluate the role of atmospheric heterogeneous reactions on the ice nucleation ability of airborne dust particles, we investigated the systematic study of ice nucleation microphysics with a suite of atmospherically relevant metals (10), halides (4), and oxyhalides (2). Within a minute, a kaolin–iron oxide composite (KaFe) showed efficient reactions with aqueous mercury salts. Among the different mercury salts tested, only HgCl2 reacting with KaFe generated HgKaFe, a highly efficient ice nucleating particle (HEIN). When added to water, HgKaFe caused water to freeze at much warmer temperatures, within a narrow range of −6.6 to −4.7 °C. Using a suite of optical spectroscopy, mass spectrometry, and microscopy techniques, we performed various experiments to decipher the physical and chemical properties of surface and bulk. KaFe was identified as a mixture of different iron oxides, namely, goethite, hematite, magnetite, and ε-Fe2O3, with kaolin. In HgKaFe, HgCl2 was reduced to Hg2Cl2 and iron was predominantly in maghemite form. Reduction of Fe2+ by NaBH4, followed by aerial oxidation, helped KaFe to be an exact precursor for the synthesis of HEIN HgKaFe. Kaolin served as a template for synthesizing iron oxide, opposing unwanted aggregation. No other metal or metal halide was found to have more efficient nucleating particles than HgCl2 with KaFe composite. The chelation of Hg(II) hindered the formation of HEIN. This study is useful for investigating the role of morphology and how inorganic chemical reactions on the surface of dust change morphology and thus ice nucleation activity. The understanding of the fundamentals of what makes a particle to be a good ice nucleating particle is valuable to further understand and predict the amount and types of atmospheric ice nucleating particles.
1. Introduction
Iron-rich kaolin is found in many places on Earth.1 Kaolin seldom possesses aluminum oxides or hydroxides. Weathering and degradation of feldspars and other aluminosilicates result in the formation of kaolin, transported by the wind in the atmosphere. This process includes the elimination of various cations and the product with contaminants.1 Fe is a common contaminant of kaolin and often leaches from kaolin to precipitate as oxide or oxyhydroxide.2 The laminated limestones of the Late Triassic Mohila Formation (Makhtesh Ramon, Israel) are interbedded with dolomite, gypsum, and some illite, kaolinite (mostly Fe-rich), and mixed-layer clay.3,4 Various mining and fossil fuel power generation activities have also shown the concurrent emission of dusts containing iron and various clay minerals like kaolin, as well as trace metals, such as mercury.5
Nanoparticles are ubiquitous in the atmosphere and are known to be of the utmost importance by both health and climate organizations. Adams et al.6 vividly explained the role nanoparticles play in climate change. Similarly, Gwinn and Vallyathan demonstrated the pros and cons of nanoparticles on health.7 As a matter of fact, nanoparticle-induced ice nucleation is an important field of study, in relation to climate and health.8
The ice nucleation phenomenon is something that we experience almost regularly in a variety of scenarios, including climate change and intracellular freezing.9−12 Pure supercooled water can be cooled to ca. −40 °C without freezing.9 However, the ice nucleation phenomena in nature occurs very often due to the nucleating substances, ranging from nanoparticles and biological compounds to crystalline surfaces. The remarkable diversity in nucleating compounds makes the mechanism of ice nucleation by efficient ice nucleating particles very complicated.13 Since the last few decades, a great deal of experimental and theoretical research has been undertaken to reveal the unequivocal driving force for efficient ice nucleation. However, the understanding of heterogeneous ice nucleation is quite complex, and its physicochemical intricacy has yet to be deciphered.
During the last five decades, several organic and inorganic materials have been suggested to contribute to ice nucleation.14−18 To date, the best ice nucleating particles have been selected bacteria, including Gram-negative Pseudomonas syringae, which freeze at about −2 °C.14 Yet, due to the propensity of dust particles globally, the overall impact of dust particles on ice nucleation has been considered to be very important.
Mineral dusts are ubiquitous in the atmosphere and contain various metals and minerals, including a suite of metals, metal oxides, and clay minerals, such as kaolin. Many studies related to the efficiency of mineral dust and acid-coated mineral dust have been conducted for heterogeneous ice nucleation at cirrus temperatures. Because of the abundance in observed aerosols around the world, various types of iron oxides have been employed as a surrogate for environmental mineral dust particles.15 Furthermore, some studies have provided identification of efficiency of ice nucleation by generating active sites of clay particles, like feldspar, kaolin, and montmorillonite, with or without metal particles.16−18
It has been increasingly clear that atmospheric aerosols are mostly not homogenous in composition or phase. Many studies indicate that several ice nucleating particles in the atmosphere and at atmospheric interfaces (air/snow/ice/water/soil) can be heterogeneous in composition with multiphase configurations.19 Yet, the impact of co-pollutants and the physical and chemical processes leading to the change of nucleation processes are still unknown.
Along with the possible uptake of aerosols, mercury species may partake in nucleation, leading to a phase alteration, from a less condensed to a more condensed phase.20 Iron particles in the nanoregime have been proven to be excellent platforms to uptake many kinds of metal ions.21,22 Liu et al.23 demonstrated density functional theory (DFT) as well as conducted experimental studies on the mechanism of elemental mercury capture, in the presence of HCl on α-Fe2O3(001). Further experimental and theoretical works are yet to be done to understand the effect of a toxic metal, like mercury, on clay particles containing other metals.
In this study, we investigated the ice nucleation ability of the combination of a suite of metal and metal oxides as well as inorganic halogenated compounds of relevance to the atmosphere and to atmospheric surfaces. Among several freezing modes, we followed immersion freezing mode using drop-freezing experiments to understand the ice nucleation efficiency.24,25 We provide the evidence for a kaolin–iron oxide composite (KaFe) that absorbs HgCl2 in water. Through a complementary suite of spectroscopic and microscopic techniques, we provide physical and chemical insights of our observations and a potential reaction mechanism.
2. Results and Discussion
2.1. Characterization of KaFe and HgKaFe
We performed complementary synthesis, characterization, and microphysics studies. The following section explores all systematic steps that led to the identification of effective ice nucleating particles. This can expand the range of known ice nucleating materials, particularly with more diverse emissions of emerging nanoparticle contaminants in the atmosphere coming to light. The mean freezing temperature (MFT) is a measure of the ice nucleation ability of nucleating particles. Brooks et al. compared ice nucleation activity of soot and polycyclic aromatic hydrocarbons using the mean freezing temperature (MFT).26 For the purpose of clarity in describing the relative ice nucleation temperature patterns of various ice nucleating particles studied in this work, particles with a mean freezing temperature <7 °C were classified as highly efficient ice nucleating particles (HEINs), those with a mean freezing temperature in between −14 and −7 °C were termed as average ice nucleating particles, and the particles with a mean freezing temperature in between −21 and −14 °C were termed as poor ice nucleating particles.
KaFe was a gray solid, synthesized from off-white kaolin and greenish yellow FeCl2·4H2O, after treatment with sodium borohydride. Our experiment showed that the freezing range of KaFe was −20.2 to −8.8 °C (poor ice nucleating particles with MFT of −16.7 °C) when HgKaFe (KaFe@HgCl2) was a HEIN. No other metal chloride, except HgCl2, demonstrated high ice nucleation ability with KaFe, as illustrated in Figure 1. To comprehend its ice nucleation efficiency, knowledge on its physicochemical state is pivotal. Therefore, we characterized KaFe and HgKaFe via microscopic and spectroscopic techniques to understand the drastic differences of ice nucleation properties.
Figure 1.
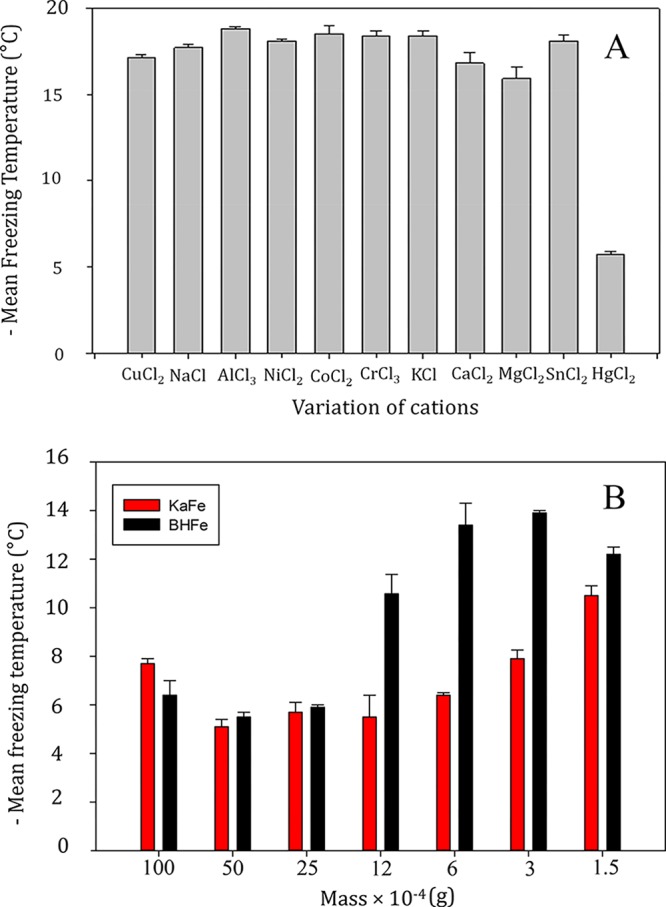
(A) Bar diagram representing the mean freezing temperature of 3.69 × 10–3 M different metal chloride solution with 0.0025 g of KaFe in 10 mL of Milli-Q water. (B) Bar diagram representing the mean freezing temperature of 3.69 × 10–3 M HgCl2 with different amounts of BHFe and KaFe in 10 mL of Milli-Q water.
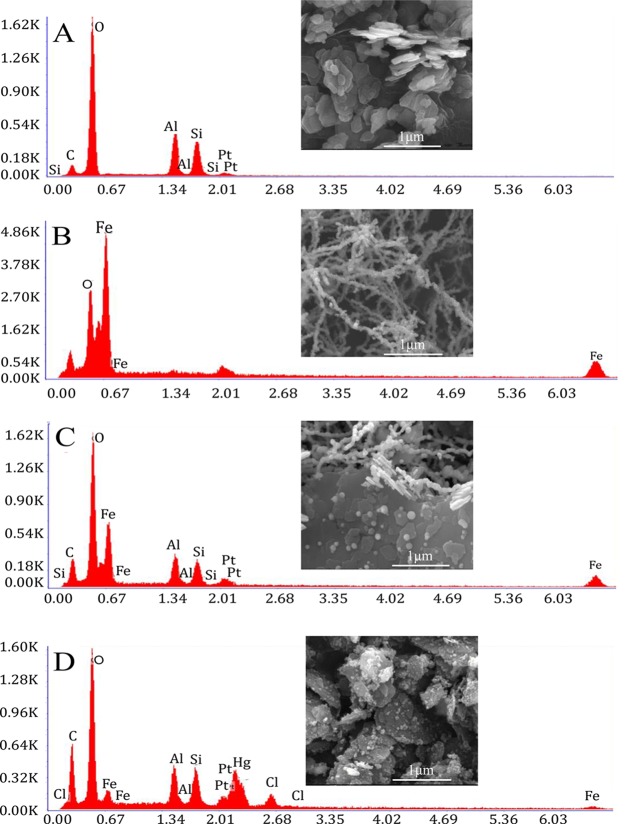
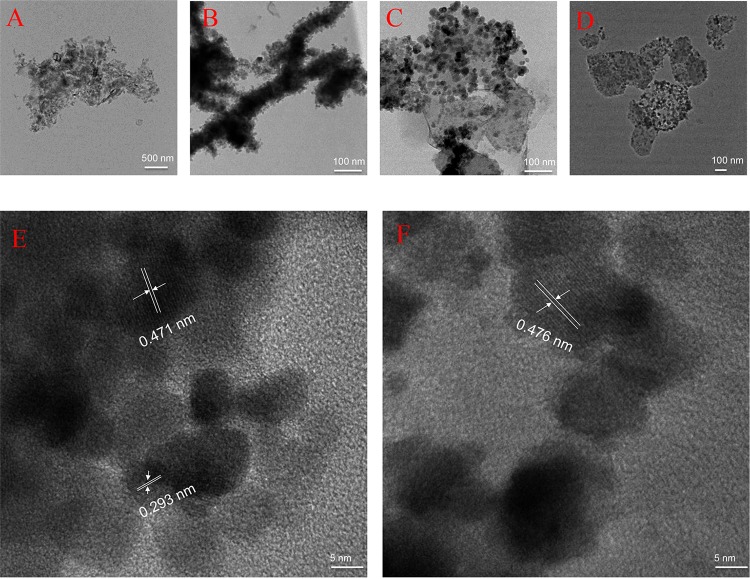
To understand the surface morphology, scanning electron microscopy (SEM) analysis was performed. Pure kaolin was found to be as a stacked nanodish under a scanning electron microscope, whereas in KaFe, particles were attached to the dish as spheres and long wires (made of a small unit sphere of ca. 60–80 nm). However, in BHFe (HEIN), obtained by NaBH4 treatment with FeCl2·4H2O, particles were arranged mostly as aggregated nanowire. Nanospheres and nanowires in KaFe were converted to nanodots on the surface of kaolin in HgKaFe. Energy-dispersive X-ray spectrometry analysis indicated that HgKaFe had more oxygen than KaFe (Figures 2 and S1, Supporting Information). X-ray diffraction (XRD) analyses, discussed below, indicated that KaFe possesses iron oxide. Thereby, iron was further oxidized in HgKaFe. Also, HgCl2 was reduced to Hg2Cl2 in the presence of KaFe. Transmission electron microscopy (TEM) analysis supported BHFe to be more aggregated in comparison to KaFe. From high-resolution transmission electron microscopy (HRTEM) analysis, it became evident that KaFe contained magnetite (Fe3O4) as well as goethite (FeOOH). A lattice fringe of 0.29 nm corresponds to the (220) lattice plane of Fe3O4, whereas a lattice fringe of 0.47 nm corresponds to FeOOH.27−29 Furthermore, a lattice fringe of 0.47 nm may likely be due to the (002) plane of ε-Fe2O3 (reported lattice fringe of 0.48 nm).30 On the other hand, a lattice fringe of 0.47 nm was found at different regions of HgKaFe for the (111) plane of maghemite (γ-Fe2O3), as depicted in Figure 3.27
Figure 2.
Selected SEM images and EDAX images of (A) kaolin, (B) BHFe, (C) KaFe, and (D) HgKaFe.
Figure 3.
TEM images of (A) kaolin, (B) BHFe, (C) KaFe, and (D) HgKaFe. HRTEM images of (E) KaFe and (F) HgKaFe.
XRD analysis indicated that KaFe contained ε-Fe2O3 (20.3%), hematite (16.1%), goethite (10.2%), and magnetite (4.6%). However, HgKaFe contained maghemite-C, syn (11.9%). Therefore, HgKaFe predominantly possessed one type of iron oxide, i.e., maghemite. Figures 4 and S2–S4, in the Supporting Information, represent the XRD spectra and structural fitting pattern details of KaFe and HgKaFe. In HgKaFe, HgCl2 was reduced to Hg2Cl2. As such, during the in situ process, a maghemite–Hg2Cl2 composite was produced with HEIN characteristics.
Figure 4.
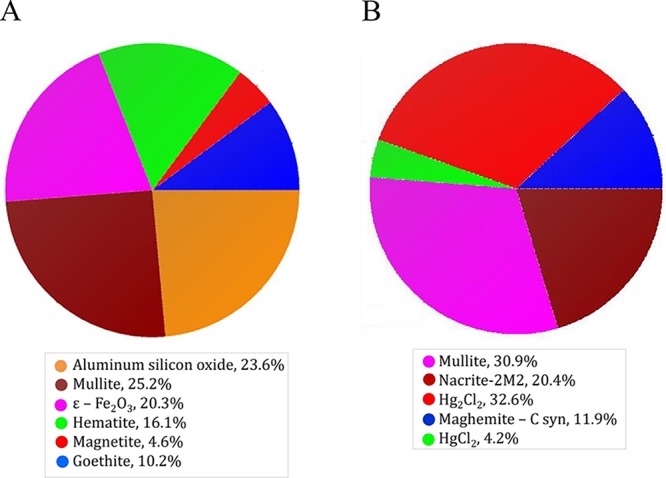
Pie diagram representing the composition of (A) KaFe and (B) HgKaFe, obtained from XRD analysis.
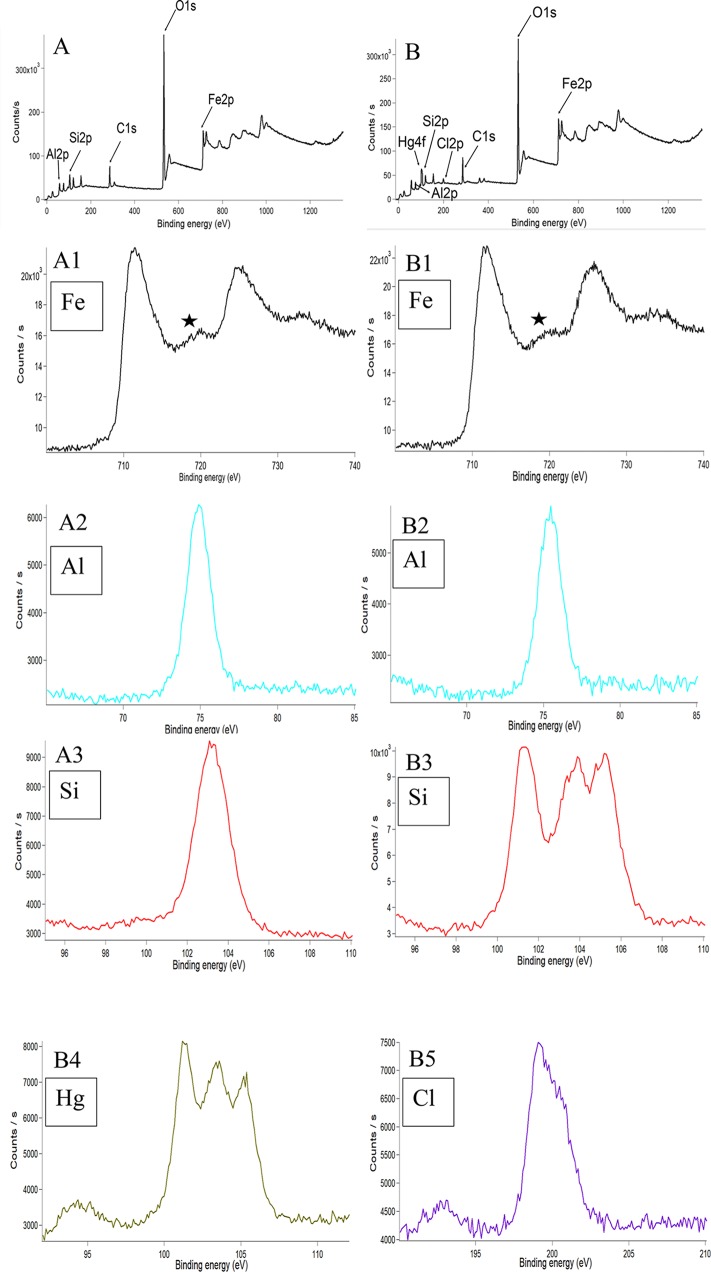
From X-ray photoelectron spectroscopy (XPS) analysis, we found that both KaFe (711.48 eV) and HgKaFe (711.38 eV) had similar oxidation states to Fe(III) 2p3/2 oxide. Satellite peaks found in both KaFe and HgKaFe indicated that neither compound contained only magnetite.31 The HgKaFe peak for the binding energy of Hg was 101.08–101.48 eV. This peak might be due to the presence of some unreacted HgCl2, along with Hg2Cl2 (calomel).32 A shift in binding energy of Hg(I) 4f7/2 toward a higher-energy region was the result of the anchoring of Hg2Cl2 to electron-deficient iron oxide maghemite. The main components of kaolin are Al and Si. A decrease in the binding energies of Al 2p and Si 2p in HgKaFe (75.20 eV for Al and 103.06 eV for Si) in comparison to KaFe (75.37 eV for Al and 103.93 eV for Si) indicated that Al and Si were electron-rich by further oxidizing magnetite (Fe3O4) to maghemite in HgKaFe. More metal–oxide interactions (∼530.3 eV) in HgKaFe than in KaFe implied that a higher concentration of iron(III) oxide was formed mainly in HgKaFe compared to KaFe.33 It is to be mentioned that Cl– 2p in HgCl2 did not alter its electronic state in HgKaFe (binding energy, 199.53 eV)34 (Figures 5 and S5, Supporting Information).
Figure 5.
(A, B) Wide-range XPS images of KaFe and HgKaFe. (A1–A3) XPS images of elements Fe, Al, and Si, respectively, in KaFe. (B1–B5) XPS images of elements Fe, Al, Si, Hg, and Cl, respectively, in HgKaFe. The symbol ★ in A1 and B1 represents a satellite peak.
It is important to note that no detectable Hg(0) was emitted from HgKaFe, as determined by mass spectral analysis (shown in Figure 6) under our experimental conditions. Instead of using NaBH4, the use of NH4OH during the preparation of KaFe resulted in HgKaFe, which is a poor ice nucleating particle (MFT, −16.3 °C). As such, the reduction of iron chloride with NaBH4, followed by aerial oxidation of KaFe, produces HgKaFe (HEIN) with aqueous HgCl2, as illustrated in Figure S6 in the Supporting Information.
Figure 6.
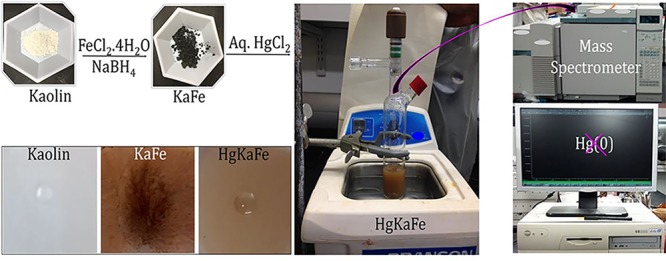
Schematic representation of no Hg(0) vapor evolution from HgKaFe and digital images of 10 μL water droplet on thin films (of kaolin, KaFe, and HgKaFe).
Thin films of kaolin, KaFe, and HgKaFe were made from their aqueous suspensions via spin coating. Then, 10 μL of Milli-Q water was dropped onto each of the films. Subsequently, using a goniometer, we measured the contact angles of the drops. Kaolin and HgKaFe were hydrophilic compounds with measured contact angles of 33 and 32°, respectively. On the contrary, KaFe was very much hydrophilic and its contact angle was 12°, as shown in Figure 6. However, HgKaFe was shown to be the HEIN. A low contact angle generally refers to a higher affinity toward water. Lupi and Molinero35 discovered that the increment of hydrophilicity can exhibit adverse effects, due to the incorporation of OH groups, reducing water–surface interaction strength. The experiment performed by Whale et al.36 also supports the aforementioned proposition. Notwithstanding these data, contact angle measurement using thin films should not be the sole conclusive measure to evaluate ice nucleating potentials of particles.
2.2. HEIN HgKaFe
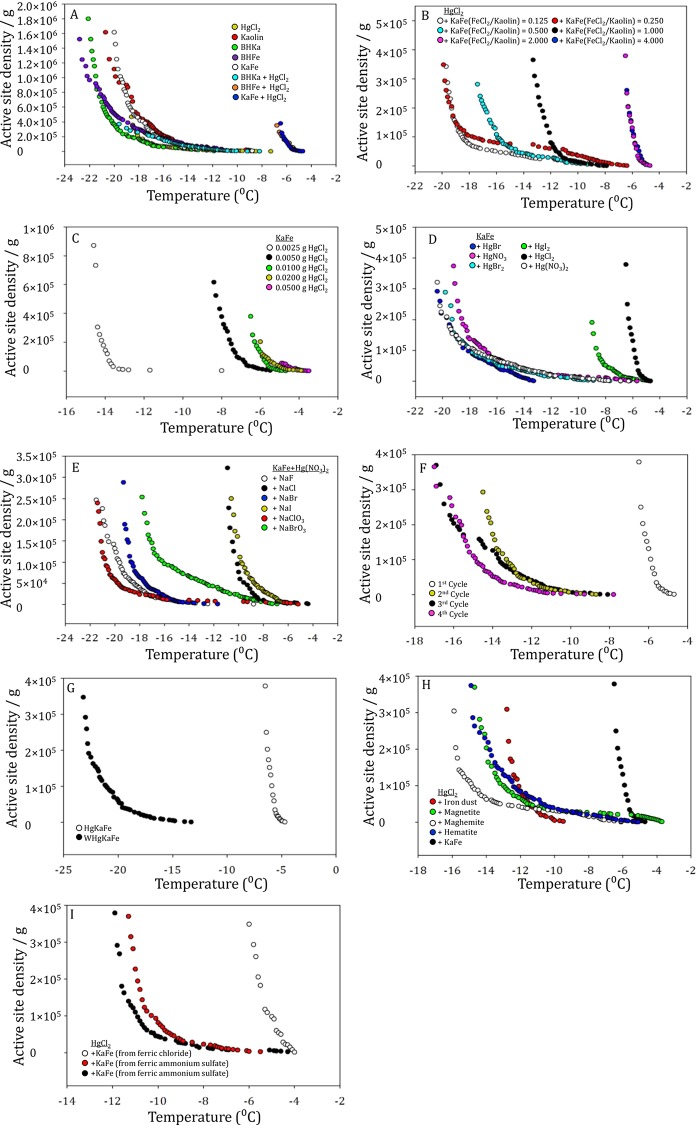
KaFe in water was classified as a poor ice nucleating material, as mentioned above. Similarly, kaolin showed ice nucleation ability within the range of −20 to −12 °C (MFT, −16.2 °C). We subsequently sonicated 0.0025 g of KaFe inside an aqueous solution of 10 mL of 3.69 × 10–3 M HgCl2 for 10 min. Further ice nucleation experiments were carried out, and it was discovered that water froze at much warmer temperatures (−6.6 to −4.7 °C; MFT, −5.6 °C). We note that pure HgCl2 (3.69 × 10–3 M) was also found to be a poor ice nucleating material. An aqueous solution of 3.69 × 10–3 M HgCl2 froze within a range of −17 to −6 °C (MFT, −15.3 °C). HgKaFe, as HEIN, was obtained when 0.0025 g of KaFe was sonicated with 10 mL of a 3.69 × 10–3 M aqueous HgCl2 solution for 10 min. At this point, water froze very quickly within a narrow temperature range of −6.6 to −4.7 °C. NaBH4-treated kaolin (BHKa) was a poor ice nucleating material in water (−21 to −11 °C; MFT, −18.7 °C) or an average ice nucleating material with aqueous HgCl2 (−20 to −8 °C; MFT, −13.5 °C). Interestingly, it was discovered that NaBH4-treated FeCl2·4H2O (BHFe), without kaolin, was a poor ice nucleating material (−22 to −12 °C; MFT, −17.4 °C). On the contrary, BHFe was a HEIN in aqueous HgCl2 solution (−7.5 to −4.8 °C; MFT, −5.8 °C). Thus, the Fe–Hg composite was indeed a HEIN, whether kaolin was present in the system or not, as depicted in Figure 7A.
Figure 7.
Comparison of active-site density per gram against temperature (in 10 mL of Milli-Q water) of (A) 3.69 × 10–3 M pure HgCl2, 0.0025 g of kaolin, 0.0025 g of BHKa, 0.0025 g of BHFe, 0.0025 g of KaFe, 0.0025 g of BHKa with 3.69 × 10–3 M HgCl2, 0.0025 g of BHFe with 3.69 × 10–3 M HgCl2, 0.0025 g of KaFe with 3.69 × 10–3 M HgCl2; (B) 3.69 × 10–3 M HgCl2 with 0.0025 g of KaFe [prepared with a different ratio of kaolin to FeCl2·4H2O]; (C) HgCl2 of different concentrations (including 3.69 × 10–3 M, i.e., 0.01 g) with 0.0025 g of KaFe; (D) 3.69 × 10–3 M different Hg salts with 0.0025 g of KaFe; (E) 3.69 × 10–3 M Hg(NO3)2 with 0.0025 g of KaFe in the presence of different sodium salts of 1.7 × 10–2 M; (F) HgKaFe at different cycles; (G) HgKaFe (first cycle) and WHgKaFe; (H) iron dust and different iron oxide (0.0025 g) with HgCl2 (3.69 × 10–3 M); and (I) HgKaFe (KaFe prepared from different iron precursors).
Kaolin in KaFe was used as a scaffold for the synthesis of Fe(0) particles, formed initially after the addition of NaBH4. Then, rapid aerial oxidation took place, forming kaolin-passivated iron oxide. It was previously reported that modified zero-valent iron nanoparticles enhance the rapidity and efficacy of remediation.37,38 During the reduction of an aqueous iron salt, support materials were introduced to prevent the usual aggregation of iron nanoparticles, as have been previously reported by Ponder et al.21 and Zhang et al.37 Therefore, the dispersion of kaolin-embedded iron oxide nanoparticles (KaFe) exhibited a higher specific surface area. Consequently, a higher reactivity of iron toward the aqueous HgCl2 was observed, and 0.0025 g of solid KaFe (containing less iron than 0.0025 g of solid BHFe) showed improved ice nucleation ability with aqueous HgCl2, compared to 0.0025 g of BHFe with aqueous HgCl2 of a similar concentration.
2.3. Impact of the Change of Concentration and Mixing Ratios of Composites on Ice Nucleation
In this study, both KaFe and BHFe were HEIN, with HgCl2. Ice nucleation efficiency was investigated at a wide range of concentrations of KaFe and BHFe with HgCl2. It was discovered that the efficiency of BHFe with HgCl2 as an ice nucleating particle decreased rapidly with decreasing concentration, unlike KaFe. KaFe was a much more efficient nucleating particle (−6.4 to −4.2 °C; MFT, −5.4 °C when 0.0012 g of KaFe was employed) with HgCl2 than BHFe (−11.9 to −7.4 °C; MFT, −10.5 °C when 0.0012 g of BHFe was employed), when the amount was <0.0025 g. This experiment provided evidence for the importance of kaolin as a template and the importance of making KaFe more efficient by protecting the rapid aggregation of iron particles.39,40 Thereby, under our experimental conditions, kaolin enhanced the surface-to-volume ratio (and active sites at the surface) of iron particles (Figures 1B and S7, Supporting Information).
During the synthesis of KaFe, we varied the concentration ratio of kaolin to FeCl2·4H2O, keeping the amount of kaolin and NaBH4 unchanged. KaFe, prepared from higher [FeCl2·4H2O], was found to be a more efficient ice nucleating particle with HgCl2 [KaFe from FeCl2·4H2O/kaolin = 0.125 with a freezing range of −19.7 to −8.1 °C (MFT, −15.3 °C) with HgCl2, whereas KaFe from FeCl2·4H2O/kaolin = 4.000 with a freezing range of −6.5 to −4.9 °C (MFT, −4.4 °C) with HgCl2]. This indicated that the iron particle was the key player for ice nucleation and kaolin was its template, as depicted in Figure 7B. Besides, change of surface area, morphology, or size of the particles might be altered in KaFe (of altered iron-to-kaolin ratio), resulting in altered ice nucleation ability.
2.4. Importance of HgCl2
Under our experimental conditions, no other studied metal chloride except mercury(II) chloride, namely, copper(II) chloride, sodium(I) chloride, aluminum(III) chloride, nickel(II) chloride, cobalt(II) chloride, chromium(II) chloride, potassium(I) chloride, calcium(II) chloride, magnesium(II) chloride, or tin(II) chloride, could produce HEIN with KaFe. In other words, the Fe–Hg composite was very selective to produce efficient ice nucleating particles, as shown in Figures 1 and S8, in the Supporting Information.
When we increased [HgCl2] gradually with a fixed amount of KaFe (0.0025 g), it was observed that KaFe increased its efficiency as an ice nucleating particle. When 0.0025 g of HgCl2 is used in 10 mL of an aqueous KaFe suspension, the freezing range is −14.9 to −8 °C (MFT, −14 °C). On the other hand, when 0.05 g of HgCl2 was employed in 10 mL of an aqueous KaFe suspension, the freezing range was −5 to −3.5 °C (MFT, −4.2 °C). This indicated that Fe–HgCl2 composites were responsible for HEIN. In other words, iron oxide and HgCl2 were both pivotal to produce HEIN (Figure 7C).
The importance of the chloride ion to form HEIN with KaFe was also evaluated. The experiments above were repeated with other Hg salts, like Hg(NO3)2, HgBr2, HgI2, Hg2(NO3)2, and HgBr. However, they exhibited relatively poor ice nucleation ability with KaFe, as shown in Figure 7D. It is to be noted that insoluble HgI2 itself was an average ice nucleating particle (−13.5 to −5.1 °C; MFT, −10.6 °C), due to the remarkable size difference between Hg2+ and I–, as shown in Figure S9 in the Supporting Information. HgI2 with KaFe was also found to be significantly efficient, but not more efficient than HgCl2. The freezing range of KaFe–HgI2 (−9.3 to −4.7 °C; MFT, −7.4 °C) was much wider than that of KaFe–HgCl2. However, the improvement of the ice nucleation ability of HgI2, after the association with KaFe, was not as impressive as with HgCl2. The small improvement in ice nucleation ability of HgI2 is attributed to the size difference between the cation and anion in the compound, which is related to the ease of ionization. Thus, both Hg and Cl were needed to generate HEIN with KaFe.
After the addition of Hg(NO3)2 to the aqueous suspension of KaFe, the mixture was sonicated. Subsequently, different sodium salts (NaF, NaCl, NaBr, NaI, NaClO3, and NaIO3) were added individually and sonicated further for 10 min. We found that only NaCl created HEIN, supporting the idea of the pivotal role that Cl– has in the formation of HEIN. Interestingly, NaClO3 did not produce a HEIN, indicating that the electronic environment of Cl– was crucial, as shown in Figure 7E. Therefore, free Cl– might also be a key player, along with Fe and Hg, in the formation of HEIN.
We note that under our experimental conditions, [Hg(NO3)2 + NaI] with KaFe was found to be an average ice nucleating material (−10.7 to −6.1 °C; MFT, −8.3 °C). After further experiments, we observed that [Hg(NO3)2 + NaI] without KaFe produced a similar nucleation range of −12.5 to −5.5 °C (MFT, −8.9 °C) (Figure S10, Supporting Information). However, pure HgI2, due to its hydrophobicity, showed freezing points from −13.5 to −5.1 °C (MFT, −10.5 °C). The size discrepancy between Na+ and I– is much higher than that between Hg2+ and I–, facilitating ease of ionization and surface buckling for NaI.
2.5. Effect of Washing of the Precipitate of HgKaFe
HgKaFe (HEIN) was formed when 0.01 g of HgCl2 was added to 10 mL of Milli-Q water, which contained 0.0025 g of KaFe. The precipitate was then centrifuged and later washed several times with Milli-Q water. Ice nucleation experiments were then performed in the presence of 10 mL of Milli-Q water, containing 0.01 g of HgCl2. The efficiency of HgKaFe as an ice nucleating particle was reduced remarkably after the first cycle. We observed freezing points within the range of −6.6 to −4.7 °C (MFT, −5.7 °C) for the first cycle, −14.6 to −8.7 °C (MFT, −12.4 °C) for the second cycle, −16.9 to −7.7 °C (MFT, −12.5 °C) for the third cycle, and −20.5 to −13.3 °C (MFT, −13.8 °C) for the fourth cycle. As such, we could confirm that there was a permanent change in KaFe, after the treatment of HgCl2. Our results indicated that there was an inactivation of the active sites of KaFe. Even after several washes, the original KaFe, which produced HEIN with HgCl2, could not be obtained, as illustrated in Figure 7F.
We examined the ice nucleation efficiency of the precipitate obtained in the HgKaFe solution. The precipitate was washed several times with Milli-Q water and ice nucleation experiments were performed in Milli-Q water. We found that the precipitate (WHgKaFe) was frozen within the range of −23.5 to −13.3 °C (MFT, −19.5 °C). This indicated that Hg was not strongly adsorbed in the precipitate, as illustrated in Figure 7G. From infrared spectra, we confirmed the existence of KaFe and WHgKaFe using their fingerprinting spectral signatures, as depicted in Figure S11 in the Supporting Information. There was no evidence for the presence of HgCl2 in WHgKaFe.41
2.6. Effect of Different Iron Compounds with HgCl2 on Ice Nucleation
To examine potential atmospheric reactions of dust and mercury chlorides, commercially available iron dust particles were used, as well as magnetite (Fe3O4), maghemite (γ-Fe2O3), and hematite (α-Fe2O3), to evaluate their ice nucleation efficiency in the presence of HgCl2. We observed the freezing ranges of −12.9 to −6.1 °C (MFT, −11 °C), −14.9 to −3.9 °C (MFT, −10.3 °C), −16 to −5.2 °C (MFT, −12.2 °C), and −15.2 to −5.1 °C (MFT, −10.5 °C) for iron dust particles, magnetite, maghemite, and hematite, respectively. However, the reduction of FeCl2·4H2O with NaBH4 (in KaFe), followed by aerial oxidation, generated active sites for HgCl2 to sit on, producing HEIN, as illustrated in Figure 7H. From the XRD spectra, it was evident that in KaFe, Fe is in both +2 and +3 oxidation states. However, in HgKaFe, Fe was always observed to be at the +3 oxidation state. Fe in KaFe was further oxidized (OKaFe) and thus completely oxidized to its +3 oxidation state. When using a strong oxidizing agent, hydrogen peroxide (H2O2), OKaFe showed a decrease in its ice nucleation ability (−20.1 to −9.2 °C; MFT, −16.5 °C), in the presence of HgCl2 (Figure S12, Supporting Information).
During the synthesis of KaFe, we altered the iron precursors, to explore their impacts on ice nucleation processes. Instead of FeCl2·4H2O, we employed FeCl3·6H2O, ferrous ammonium sulfate, and ferric ammonium sulfate. Our nucleation experiments indicated that FeCl3.6H2O also produced KaFe, which was capable of originating HEIN with HgCl2 (−6 to −4 °C; MFT, −4.8 °C). Ferrous ammonium sulfate and ferric ammonium sulfate also produced KaFe, KaFe from ferrous ammonium sulfate with HgCl2, and KaFe from ferric ammonium sulfate with HgCl2, nucleated within the ranges of −12.4 to −4.3 °C (MFT, −9.7 °C) and −11.7 to −5.5 °C (MFT, −9.2 °C), respectively, as shown in Figure 7I. The decreased nucleation efficiency of KaFe from ferric/ferrous ammonium sulfate with HgCl2 is attributed to ammonium sulfate-induced aggregation of iron (rendering lower surface area).
2.7. Absorption of HgCl2 in KaFe
To further provide physical insights into the absorption of HgCl2 in KaFe, several UV–vis spectroscopic experiments were performed. First, we added different amounts of KaFe (0.0025–0.25 g) into 10 mL of 3.69 × 10–3 M HgCl2 and sonicated the solutions for 10 min. These solutions were then centrifuged and diluted 13 times with Milli-Q water to record UV–vis spectra. With increasing KaFe, the absorption of [HgCl2] was increased and an increasing amount of Hg(II) was removed from the solution, as seen in Figure 8A. Again, for a fixed amount of KaFe (0.01 g), we repeated a few experiments with varying concentrations of HgCl2, as illustrated in Figure 8B. We discovered that the extent of absorption remains almost unchanged with the variation of [HgCl2]. Figure 8C shows almost parallel lines of absorbance for only HgCl2 and HgCl2+KaFe, at different concentrations of HgCl2. Hg(NO3)2 and HgBr2 were also tested in the context of absorption with KaFe. It was discovered that all Hg(II) salts were absorbed significantly in KaFe, as illustrated in Figure S13. However, these Hg(II) salts, except for HgCl2, could not form HEIN.
Figure 8.
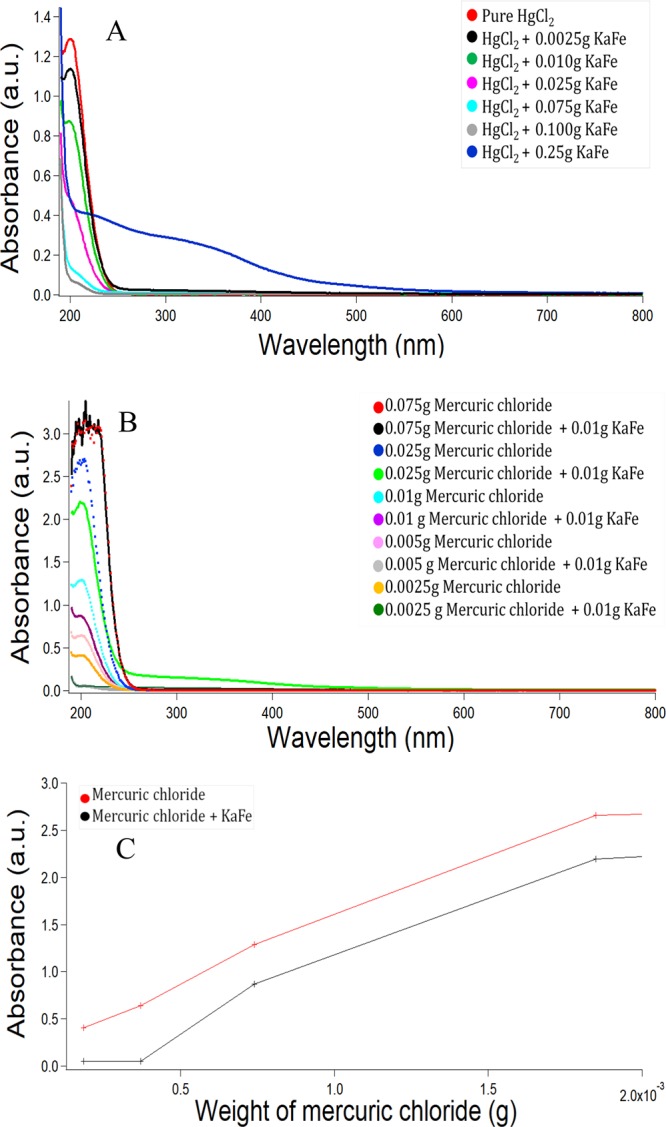
(A) UV–vis spectra of HgCl2 in the presence of different amounts of KaFe (spectra have been recorded after 13 times dilution), [HgCl2] = 2.84 × 10–4 M. (B) UV–vis spectra of different amounts of HgCl2 in the presence and absence of fixed amount of KaFe (spectra have been recorded after 13 times dilution). (C) Comparative study of absorbance variation with different amounts of HgCl2 (with and without KaFe) as mentioned in (B).
2.8. Impact of Other Metal Halides on the Absorption in KaFe
We attempted to evaluate the impacts of different metal chlorides [copper(II) chloride, sodium(I) chloride, aluminum(III) chloride, nickel(II) chloride, cobalt(II) chloride, chromium(II) chloride, potassium(I) chloride, calcium(II) chloride, magnesium(II) chloride, tin(II) chloride] in the context of absorption in KaFe. We observed from UV–vis spectral analysis that SnCl2, CuCl2, CrCl3, and AlCl3 were also absorbed significantly in KaFe, like HgCl2. However, no metal chloride (except HgCl2) could produce HEIN, as shown in Figure S14.
2.9. Effect of Chelation
Hg(II) forms a strong chelate with disodium ethylene diamine tetra acetic acid (Na2-EDTA).42,43 Having this idea in mind, we prepared a Hg(II)–EDTA complex by mixing Na2–EDTA and HgCl2 in a 1:1 molar ratio in Milli-Q water. Therefore, the water solution containing [Hg(II)-EDTA]Cl2 and KaFe resulted in an average ice nucleating material (−14.9 to −8.7 °C; MFT, −12.3 °C) compared to HgKaFe. Experimental results indicated the necessity of having free Hg(II) in a solution to produce HEIN. From the UV–vis spectroscopic data, it was found that the absorption spectrum of [Hg(II)–EDTA]Cl2 was almost unaltered after sonication with KaFe, implying that [Hg(II)–EDTA]Cl2 would likely not be absorbed by KaFe (Figure 9).
Figure 9.
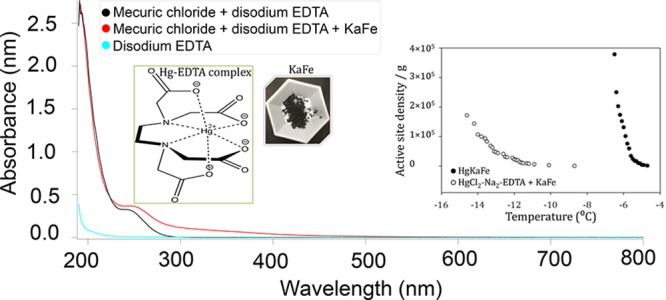
UV–vis spectra showing the absorption of Hg–EDTA complex on KaFe; [Hg(II)] = 1.42 × 10–4 M, KaFe = 0.01 g. The inset shows the comparison of the active-site density per gram and temperature to show the effect of Na2–EDTA; [Hg(II)] = 3.69 × 10–3 M, KaFe = 0.0025 g, Milli-Q water = 10 mL.
2.10. Potential Reaction Mechanism
HgKaFe is a HEIN and is more efficient than any mineral dust or metal particle, which has been previously studied. The efficiency of HgKaFe, as an ice nucleating particle, is very close to the most efficient, but most rare ice nucleating particles in nature, biological particles. It was found that maghemite and Hg2Cl2 were the main constituents of HgKaFe, as mentioned below. However, it is still being explored if the mixture of the final products together or alone (in the presence of the other constituents, i.e., synergism) or any secondary product is the potential ice nucleating particle.
The two highly conversed “criteria” for efficient ice nucleating particles are the crystallographic match with respect to bulk ice and the strength of the water–surface interactions. As water–surface interaction is poorer for HgKaFe than for KaFe (the contact angle is less for KaFe), the first criterion, in this case, may be the dominant criterion. The arrangement of different heights of atoms in the contact layer is called buckling, which is a significant factor for efficient nucleation. It has been observed that if the first water overlayer is not icelike, then efficient ice nucleation is possible for pronounced buckling of the contact layer. When the atomic arrangement in the contact region is similar, small lattice mismatch (δ) is the pivotal factor for being an efficient nucleating particle. Despite serious challenges from recent experiments and simulations for validation and the generalization of the concept, small lattice mismatch is still a primary cause of efficient ice nucleation.44−50 When the arrangements of atoms in the contact area are alike, δ between the corresponding surface unit cells can be explained in a more rationalized manner as
| 1 |
where ai and as represent the lattice parameters of a certain face of ice and the surface unit cells of the substrate, respectively.
All of these factors individually or together generate HgKaFe to be HEIN. The extraordinarily narrow freezing range of −6.6 to −4.7 °C is an indication of one kind of dominant ice nucleating particle in HgKaFe. Further experiments and theoretical studies are to be conducted, to design an unequivocal mechanism behind the formation of the HEIN.
From the ice nucleation data, it is evident that HgKaFe is an efficient ice nucleating agent. The goal of the paper is to understand the physicochemical properties of HgKaFe in the context of ice nucleation. We know that iron/iron oxides in different forms (namely, magnetite,51,52 maghemite,52 hematite,52,53 Fe dust,52 etc.) are commonly available in the atmosphere. Silver iodide (AgI) is a well-known cloud seeding material.54 We measured ice nucleation efficiency of iron oxides (Table 1, Figure S15, Supporting Information) and AgI (Table 1, Figure S15, Supporting Information) in comparison to HgKaFe. The efficiency of the HgKaFe nucleation rate, with the same size and mass of droplet, is much better (>7 °C warmer) than iron oxides and closer to AgI.
Table 1. MFT of Different Samples of 0.0025 g in 10 mL of Milli-Q Watera.
| sample | Fe dust | magnetite | hematite | maghemite | AgI | HgMtFe |
|---|---|---|---|---|---|---|
| MTF (°C) | –12.9 | –19.3 | –14.2 | –13.6 | –3.8 | –5.8 |
HgKaFe was diluted 5 times to obtain total mass of 0.0025 g in 10 mL of milli-Q water.
In this paper, we are unveiling the formation of highly efficient ice nucleating particles, where environmentally abundant iron oxide has a key role in the presence of toxic mercury chloride. Moreover, kaolin and iron oxide are very common ingredients in dust particles. Mercury in different forms is being emitted every day, creating a great threat to the environment.55 The importance of the contact angle, surface sites, composition, and morphology on ice nucleation efficiency and untangling these effects from each other were the main theme of the work. These insights can be applied to atmospheric particles.
3. Conclusions and Future Directions
Maghemite and Hg2Cl2 were key players in the preparation of highly efficient, completely inorganic ice nucleating particles. This new heterogeneous highly efficient ice nucleus was not associated with any biomolecule, which has been previously shown to be an efficient ice nucleating particle. No other studied metal ion was found to be able to make such an efficient ice nucleating particle, except mercury. Chloride also showed to play a pivotal role in ice nucleation. Kaolin–iron oxide composites absorbed HgCl2 in an aqueous medium, and as a corollary, highly efficient ice nucleating particles were generated. Interactions of mineral dust particles (containing mostly iron oxide) with water vapor may alter heterogeneous reactivity toward toxic metals. Immersion freezing (ice nucleation by particles immersed in supercooled water) is a crucial route for creating ice in mixed-phase clouds. Immersion freezing experiments with particles in microliter-sized water droplets are often used for identifying very small numbers of nucleating particles. Additionally, atmospheric heterogeneous and multiphase reactions may influence the chemical compositions when mineral dust particles reach the troposphere. Therefore, interactions of mineral dust particles with water vapor are changed.
A complete and in-depth knowledge of the interactions between dust and dust containing toxic metals with water vapor will help us to understand the role mineral dust aerosols have in many facets of atmospheric chemistry. These important facets include the increasing threat of climate change and airborne nanoparticles near emission sources, including urban areas. These areas are major sites of important pollutants in climate change and environmental health. To conclude, a better understanding of nanoparticles in the atmosphere and ice nucleation may lead to an improvement in climate change and public health knowledge.
4. Experimental Section
In this section, we describe the synthesis methods, as well as various analytical techniques for chemical and physical analyses. Also, the materials and supplies required are discussed.
4.1. Synthesis
4.1.1. Synthesis of KaFe
FeCl2·4H2O (2.67 g) was fully dissolved in a 4:1 ethanol/water mixture (24 mL of ethanol and 6 mL of water). Consequently, 1.5 g of kaolin was added to the solution, creating a muddy beige mixture. This suspension was then placed in an ultrasonic shaker for 2 h to disperse the kaolin particles. In the meantime, a 1 M NaBH4 solution was prepared by dissolving 3.05 g of the powder in 100 mL of ice-cold Milli-Q water. When the ultrasonic shaking was completed, the freshly prepared sodium borohydride solution was added dropwise into the suspension with vigorous shaking, showing an instant color change from beige to black. When the entire NaBH4 solution was transferred, the reaction mixture was shaken for 10 min and left to settle for 1 day. After 24 h, the supernate was decanted, while keeping the solid inside the container with a strong magnet, then the solid was washed eight times with 200 mL of Milli-Q water and twice with 200 mL of ethanol. The synthesized iron–kaolin composite was then placed in a vacuum oven at 50 °C to dry completely. Finally, when the drying process was complete, the solid was crushed and mixed thoroughly using a mortar and pestle to obtain KaFe.
4.1.2. Synthesis of BHFe
BHFe was synthesized using the same procedure as the KaFe synthesis; however, kaolin was not used.
4.1.3. Synthesis of HgKaFe
KaFe (0.0025 g) was sonicated with 10 mL of a 3.69 × 10–3 M aqueous HgCl2 solution for 10 min to form HgKaFe.
4.2. Analytical Techniques for Physical and Chemical Characterization
4.2.1. Drop-Freezing Assays
Drop-freezing experiments are among the most common techniques to judge the ice nucleation efficiency of a heterogeneous ice nucleating particle in immersion mode.19,24 A better method for gauging immersion freezing in large droplets over a wide temperature range has been shown by Tobo.25 For each sample, droplets were placed on a cooling plate (in-house-made copper plate) and the plate (starting at ∼0 °C) was cooled at a rate of 1 °C/min. The temperature at which each droplet froze was recorded. The samples were loaded onto the plate as 10 μL droplets. Before adding the droplets, a thin layer of petroleum jelly was spread on the copper plate to minimize the reactions between the copper plate and the drops and to maintain hydrophobicity.
We followed the Tobo protocol25 to plot ice nucleation data. It was assumed that the ice nucleation process by immersed particles in a supercooled drop was both a temperature-dependent and time-independent process. So, the cumulative number of ice nucleation active sites per unit volume of water (K) at a certain temperature (T) can be presented by the following equation (eq 2)
| 2 |
When the mass of particles per unit volume of water is known, the ice nucleation active-site density per unit mass (nm) can also be expressed by eq 3
| 3 |
where Cm represents the mass concentration of the particles in the initial suspension and d represents the dilution ratio of the suspension relative to Cm. We have performed background corrections by subtracting the ice nucleation data of pure Milli-Q water.
4.2.2. UV–Vis Spectroscopy
All UV–vis absorption spectra were recorded on a Varian Cary 50 Bio UV spectrophotometer. The solutions were put into a 1 cm, well-stoppered quartz cuvette. The spectra were recorded against a reference solvent.
4.2.3. Contact Angle
Thin films of kaolin, KaFe, and HgKaFe were prepared on a glass slide using a spin coater. The contact angle was measured by placing 10 μL of Milli-Q water on the thin films using a Rame-Hart goniometer (model number 100-00115).
4.2.4. Scanning Electron Microscopy
Particle morphology was examined using an FEI Helios NanoLab 660 DualBeam (focused ion beam) extreme high-resolution scanning electron microscope. The microscope contains Leica Microsystems EM VCT100 Cryo Transfer System, MultiChem Gas Injection System, and EDAX Octane Ultra 100 mm2 SDD and TEAM 3D EDS Analysis System. Samples of kaolin, KaFe, BHFe, and HgKaFe were vacuum-dried for 24 h before measurements were taken. The compounds were then coated with Pt and put on carbon tape before SEM studies were conducted.
4.2.5. Transmission Electron Microscopy
Samples of kaolin (0.0025 g in 10 mL of Milli-Q water), KaFe (0.0025 g in 10 mL of Milli-Q water), BHFe (0.0025 g in 10 mL of Milli-Q water), and HgKaFe (5 μL) were deposited on glow-discharged carbon film-coated copper electron microscopy grids. The droplets were kept on the grids for 1 min, followed by blotting off the excess liquid with the edge of a piece of filter paper. The samples were imaged using an FEI Tecnai 12 Biotwin TEM microscope (FEI Electron Optics) equipped with a tungsten filament at 120 kV, containing an AMT XR80C CCD Camera System.
4.2.6. X-ray Diffraction
X-ray diffraction (XRD) was performed with a Bruker D8 Discovery X-ray diffractometer (VANTEC Detector Cu-Source λ = 1.5418 Å). XRD patterns were recorded for 3° ≤ 2θ ≤ 80° with increments of 0.005°. Samples of kaolin, KaFe, BHFe, and HgKaFe were vacuum-dried at room temperature before measurements were taken.
4.2.7. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy (XPS) measurement was carried out with a Thermo Scientific K-Alpha X-ray photoelectron spectrometer. The samples were loaded onto carbon tape to be placed on a grid for the analysis. Samples of KaFe and HgKaFe were vacuum-dried at room temperature before measurement.
4.3. Materials and Supplies
All of the reagents were of analytical reagent grade, and Milli-Q water was used throughout the experiment. Kaolin, all metal salts, iron dust, all iron oxides, disodium ethylene diamine tetra acetic acid (Na2-EDTA), and hydrogen peroxide were purchased from Sigma-Aldrich. Sodium borohydride was obtained from Alfa Aesar. All glassware was cleaned with freshly prepared aqua regia, subsequently rinsed with copious amounts of distilled water, and dried well before use. All of the reagents were used without further purification.
Acknowledgments
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC)—NSERC CREATE Mine of Knowledge, Fonds de recherche du Québec—Nature et Technologies (FRQNT), and Environment Canada. The authors are thankful to Rangel-Alvarez for helping with thin-film preparation. They also thank Mr. Kurien and Ms. Morris for their valuable comments to improve the manuscript.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01830.
Elemental analysis; XRD; XPS; ice nucleation data; IR; UV–vis spectra (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Grim R. E.Clay Mineraology, 2nd ed.; McGraw Hill: New York, 1968; p 596. [Google Scholar]
- Rozenson I.; Spiro B.; Zak I. Transformation of ironbearing kaolinite to iron-free kaolinite, goethite, and hematite. Clays Clay Miner. 1982, 30, 207–214. 10.1346/CCMN.1982.0300307. [DOI] [Google Scholar]
- Goldbery R. Sedimentology of the Lower Jurassic flint clay bearing Mishhor Formation, Makhtesh Ramon, Israel. Sedimentology 1979, 26, 229–251. 10.1111/j.1365-3091.1979.tb00352.x. [DOI] [Google Scholar]
- Slatkine A.; Heller L. In A Petrological Study of the Flint Clay from Makhtesh Ramon, International Geological Congress 21st Session. Norden Part, 1961; Vol. 24, pp 88–107.
- Air Pollution from Electricity-Generating Large Combustion Plants; European Environment Agency (EEA): Copenhagen, 2008, ISBN 978-92-9167-355-1. [Google Scholar]
- Adams P. J.; Donahue N. M.; Pandis S. N. Atmospheric nanoparticles and climate change. AIChE J. 2013, 59, 4006–4019. 10.1002/aic.14242. [DOI] [Google Scholar]
- Gwinn M. R.; Vallyathan V. Nanoparticles: health effects—pros and cons. Environ. Health Perspect. 2006, 114, 1818–1825. 10.1289/ehp.8871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders R. W.; Mohler O.; Schnaiter M.; Benz S.; Wagner R.; Saathoff H.; Connolly P. J.; Burgess R.; Gallagher M.; Wills R.; Murray B. J.; Plane J. M. C. Plane An aerosol chamber investigation of the heterogeneous ice nucleating potential of refractory nanoparticles. Atmos. Chem. Phys. Discuss. 2009, 9, 23271–23318. 10.5194/acpd-9-23271-2009. [DOI] [Google Scholar]
- Harrison R. G. Cloud formation and the possible significance of charge for atmospheric condensation and ice nuclei. Space Sci. Rev. 2000, 94, 381–396. 10.1023/A:1026708415235. [DOI] [Google Scholar]
- Bartels-Rausch T. Chemistry: Ten things we need to know about ice and snow. Nature 2013, 494, 27–29. 10.1038/494027a. [DOI] [PubMed] [Google Scholar]
- Mazur P. Cryobiology: The freezing of biological systems. Science 1970, 168, 939–949. 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- McNeill V. F.; Grannas A. M.; Abbatt J. P. D.; Ammann M.; Ariya P.; Bartels-Rausch T.; Domine F.; Donaldson D. J.; Guzman M. I.; Heger D.; Kahan T. F.; Klan P.; Masclin S.; Toubin C.; Voisin D. Organics in environmental ices: sources, chemistry, and impacts. Atmos. Chem. Phys. 2012, 12, 9653–9678. 10.5194/acp-12-9653-2012. [DOI] [Google Scholar]
- Vali G.; DeMott P. J.; Möhler O.; Whale T. F. Technical note: a proposal for ice nucleation terminology. Atmos. Chem. Phys. 2015, 15, 10263–10270. 10.5194/acp-15-10263-2015. [DOI] [Google Scholar]
- Mortazavi R.; Hayes C. T.; Ariya P. A. Ice nucleation activity of bacteria isolated from snow compared with organic and inorganic substrates. Environ. Chem. 2008, 5, 373–381. 10.1071/EN08055. [DOI] [Google Scholar]
- Archuleta C. M.; DeMott P. J.; Kreidenweis S. M. Ice nucleation by surrogates for atmospheric mineral dust and mineral dust/sulfate particles at cirrus temperatures. Atmos. Chem. Phys. Discuss. 2005, 5, 3391–3436. 10.5194/acpd-5-3391-2005. [DOI] [Google Scholar]
- Zolles T.; Burkart J.; Hausler T.; Pummer B.; Hitzenberger R.; Grothe H. Identification of Ice Nucleation Active Sites on Feldspar Dust Particles. J. Phys. Chem. A 2015, 119, 2692–2700. 10.1021/jp509839x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welti A.; Kanji Z. A.; Lüönd F.; Stetzer O.; Lohmann U. Exploring the Mechanisms of Ice Nucleation on Kaolinite: From Deposition Nucleation to Condensation Freezing. J. Atmos. Sci. 2014, 71, 16–36. 10.1175/JAS-D-12-0252.1. [DOI] [Google Scholar]
- Salam A.; Lesins G.; Lohmann U. Laboratory study of heterogeneous ice nucleation in deposition mode of montmorillonite mineral dust particles aged with ammonia, sulfur dioxide, and ozone at polluted atmospheric concentrations. Air Qual., Atmos. Health 2008, 1, 135–142. 10.1007/s11869-008-0019-6. [DOI] [Google Scholar]
- Rangel-Alvarado R. B.; Nazarenko Y.; Ariya P. A. Snow-borne nanosized particles: Abundance, distribution, composition, and significance in ice nucleation processes. J. Geophys. Res.: Atmos. 2015, 120, 11760–11774. 10.1002/2015JD023773. [DOI] [Google Scholar]
- Ariya P. A.; Amyot M.; Dastoor A.; Deeds D.; Feinberg A.; Kos G.; Poulain A.; Ryjkov A.; Semeniuk K.; Subir M.; Toyota K. Mercury physicochemical and biogeochemical transformation in the atmosphere and at atmospheric interfaces: a review and future directions. Chem. Rev. 2015, 115, 3760–3802. 10.1021/cr500667e. [DOI] [PubMed] [Google Scholar]
- Ponder S. M.; Darab J. G.; Mallouk T. E. Remediation of Cr(VI) and Pb(II) aqueous solutions using supported, nanoscale zero-valent iron. Environ. Sci. Technol. 2000, 34, 2564–2569. 10.1021/es9911420. [DOI] [Google Scholar]
- Kanel S. R.; Manning B.; Charlet L.; Choi H. Removal of arsenic(III) from groundwater by nanoscale zero-valent iron. Environ. Sci. Technol. 2005, 39, 1291–1298. 10.1021/es048991u. [DOI] [PubMed] [Google Scholar]
- Liu T.; Xue L.; Guo X.; Huang Y.; Zheng C. DFT and experimental study on the mechanism of elemental mercury capture in the presence of HCl on α-Fe2O3(001). Environ. Sci. Technol. 2016, 50, 4863–4868. 10.1021/acs.est.5b06340. [DOI] [PubMed] [Google Scholar]
- Vali G. Quantitative Evaluation of Experimental Results and the Heterogeneous Freezing Nucleation of Supercooled Liquids. J. Atmos. Sci. 1971, 28, 402–409. . [DOI] [Google Scholar]
- Tobo Y. An improved approach for measuring immersion freezing in large droplets over a wide temperature range. Sci. Rep. 2016, 6, 32930 10.1038/srep32930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks S. D.; Suter K.; Olivarez L. Effects of Chemical Aging on the Ice Nucleation Activity of Soot and Polycyclic Aromatic Hydrocarbon Aerosols. J. Phys. Chem. A 2014, 118, 10036–10047. 10.1021/jp508809y. [DOI] [PubMed] [Google Scholar]
- Salazar-Alvarez G.; Sort J.; Uheida A.; Muhammed M.; Surinach S.; Baro M. D.; Nogues J. Reversible post-synthesis tuning of the superparamagnetic blocking temperature of γ-Fe2O3 nanoparticles by adsorption and desorption of Co(II) ions. J. Mater. Chem. 2007, 17, 322–328. 10.1039/B613026G. [DOI] [Google Scholar]
- Han C.; Ma J.; Wu H.; Wei Y.; Hu K. A low-cost and high-yield production of magnetite nanorods with high saturation magnetization. J. Chil. Chem. Soc. 2015, 60, 2799–2802. 10.4067/S0717-97072015000100005. [DOI] [Google Scholar]
- Ivanova A. S.; Slavinskaya E. M.; Stonkus O. A.; Gulyaev R. V.; Glazneva T. S.; Noskova A. S.; Boronin A. I. Highly active and durable Pd/Fe2O3 catalysts for wet CO oxidation under ambient conditions. Catal. Sci. Technol. 2016, 6, 3918–3928. 10.1039/C5CY01588J. [DOI] [Google Scholar]
- David B.; Pizúrová N.; Synek P.; Kudrle V.; Jašekb O.; Schneeweissa O. epsilon-Fe2O3 nanoparticles synthesized in atmospheric-pressure microwave torch. Mater. Lett. 2014, 116, 370–373. 10.1016/j.matlet.2013.11.057. [DOI] [Google Scholar]
- Moussy J.-B. From epitaxial growth of ferrite thin films to spinpolarized tunnelling. J. Phys. D: Appl. Phys. 2013, 46, 143001 10.1088/0022-3727/46/14/143001. [DOI] [Google Scholar]
- Liu J.; Du X. Fast removal of aqueous Hg(II) with quaternary ammonium-functionalized magnetic mesoporous silica and silica regeneration. J. Mater. Chem. 2011, 21, 6981–6987. 10.1039/c1jm10111k. [DOI] [Google Scholar]
- Sharma R. K.; Rastogi A. C.; Desu S. B. Manganese oxide embedded polypyrrole nanocomposites for electrochemical supercapacitor. Electrochim. Acta 2008, 53, 7690–7695. 10.1016/j.electacta.2008.04.028. [DOI] [Google Scholar]
- Chopra T. P.; Longo R. C.; Cho K.; Halls M. D.; Thissen P.; Chabal Y. J. Ethylenediamine grafting on oxide-free H-, 1/3 ML F-, and Cl-terminated Si(111) surfaces. Chem. Mater. 2015, 27, 6268–6281. 10.1021/acs.chemmater.5b03156. [DOI] [Google Scholar]
- Lupi L.; Molinero V. Does hydrophilicity of carbon particles improve their ice nucleation ability?. J. Phys. Chem. A 2014, 118, 7330–7337. 10.1021/jp4118375. [DOI] [PubMed] [Google Scholar]
- Whale T. F.; Rosillo-Lopez M.; Murray B. J.; Salzmann C. G. Ice nucleation properties of oxidized carbon nanomaterials. J. Phys. Chem. Lett. 2015, 6, 3012–3016. 10.1021/acs.jpclett.5b01096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. X. J. Nanoscale iron particles for environmental remediation: an overview. J. Nanopart. Res. 2003, 5, 323–332. 10.1023/A:1025520116015. [DOI] [Google Scholar]
- Tratnyek P. G.; Johnson R. L. Nanotechnologies for environmental cleanup. Nano Today 2006, 1, 44–48. 10.1016/S1748-0132(06)70048-2. [DOI] [Google Scholar]
- Üzüm Ç; Shahwan T.; Eroğlu A. E.; Hallam K. R.; Scott T. B.; Lieberwirth I. Synthesis and characterization of kaolinite-supported zero-valent iron nanoparticles and their application for the removal of aqueous Cu2+ and Co2+ ions. Appl. Clay Sci. 2009, 43, 172–181. 10.1016/j.clay.2008.07.030. [DOI] [Google Scholar]
- Zhang H.; Jin Z. H.; Han L.; Qin C. H. Synthesis of nanoscale zero-valent iron supported on exfoliated graphite for removal of nitrate. Trans. Nonferrous Met. Soc. China 2006, 16, S345–S349. 10.1016/S1003-6326(06)60207-0. [DOI] [Google Scholar]
- Zhou X.; Zeng K.; Wanga Q.; Yang X.; Wang K. In vitro studies on dissolved substance of cinnabar: Chemical species and biological properties. J. Ethnopharmacol. 2010, 131, 196–202. 10.1016/j.jep.2010.06.018. [DOI] [PubMed] [Google Scholar]
- Ganguly M.; Mondal C.; Pal J.; Pal A.; Negishi Y.; Pal T. Fluorescent Au(I)@Ag2/Ag3 giant cluster for selective sensing of mercury(II) ion. Dalton Trans. 2014, 43, 11557–11565. 10.1039/C4DT01158A. [DOI] [PubMed] [Google Scholar]
- Ganguly M.; Mondal C.; Jana J.; Pal A.; Pal T. Selective dopamine chemosensing using silver-enhanced fluorescence. Langmuir 2014, 30, 4120–4128. 10.1021/la404982t. [DOI] [PubMed] [Google Scholar]
- Fitzner M.; Sosso G. C.; Cox S. J.; Michaelides A. The many faces of heterogeneous ice nucleation: interplay between surface morphology and hydrophobicity. J. Am. Chem. Soc. 2015, 137, 13658–13669. 10.1021/jacs.5b08748. [DOI] [PubMed] [Google Scholar]
- Murray B. J.; O’Sullivan D.; Atkinson J. D.; Webb M. E. Ice nucleation by particles immersed in supercooled cloud droplets. Chem. Soc. Rev. 2012, 41, 6519–6554. 10.1039/c2cs35200a. [DOI] [PubMed] [Google Scholar]
- Zettlemoyer A. C.; Tcheurekdjian N.; Chessick J. Surface properties of silver iodide. Nature 1961, 192, 653. 10.1038/192653a0. [DOI] [Google Scholar]
- Fraux G.; Doye J. P. K. Heterogeneous ice nucleation on silver-iodide-like surfaces. J. Chem. Phys. 2014, 141, 216101 10.1063/1.4902382. [DOI] [PubMed] [Google Scholar]
- Cabriolu R.; Li T. Ice nucleation on carbon surface supports the classical theory for heterogeneous nucleation. Phys. Rev. E 2015, 91, 052402 10.1103/PhysRevE.91.052402. [DOI] [PubMed] [Google Scholar]
- Cox S. J.; Kathmann S. M.; Purton J. A.; Gillan M. J.; Michaelides A. Non-hexagonal ice at hexagonal surfaces: the role of lattice mismatch. Phys. Chem. Chem. Phys. 2012, 14, 7944–7949. 10.1039/c2cp23438f. [DOI] [PubMed] [Google Scholar]
- Cox S. J.; Raza Z.; Kathmann S. M.; Slater B.; Michaelides A. The microscopic features of heterogeneous ice nucleation may affect the macroscopic morphology of atmospheric ice crystals. Faraday Discuss. 2013, 167, 389–403. 10.1039/c3fd00059a. [DOI] [PubMed] [Google Scholar]
- Maher B. A.; Ahmed I. A. M.; Karloukovski V.; MacLaren D. A.; Foulds P. G.; Allsop D.; Mann D. M. A.; Torres-Jardon R.; Calderon-Garciduenas L. Magnetite pollution nanoparticles in the human brain. Proc. Natl. Acad. Sci. U. S. A. 2016, 113, 10797–10801. 10.1073/pnas.1605941113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno T.; Martins V.; Querol X.; Jones T.; BeruBe K.; Minguillon M. C.; Amato F.; Capdevila M.; de Miguel E; Centelles S.; Gibbons W. A new look at inhalable metalliferous airborne particles on rail subway platforms. Sci. Total Environ. 2015, 505, 367–375. 10.1016/j.scitotenv.2014.10.013. [DOI] [PubMed] [Google Scholar]
- Flament P.; Mattielli N.; Aimoz L.; Choel M.; Deboudt K.; de Jong J.; Rimetz-Planchon J.; Weis D. Iron isotopic fractionation in industrial emissions and urban aerosols. Chemosphere 2008, 73, 1793–1798. 10.1016/j.chemosphere.2008.08.042. [DOI] [PubMed] [Google Scholar]
- Marcolli C.; Nagare B.; Welti A.; Lohman U. Iron isotopic fractionation in industrial emissions and urban aerosols. Atmos. Chem. Phys. 2016, 16, 8915–8937. 10.5194/acp-16-8915-2016. [DOI] [Google Scholar]
- Streets D. G.; Devane M. K.; Lu Z.; Bond T. C.; Sunderland E. M.; Jacob D. J. All-time releases of mercury to the atmosphere from human activities. Environ. Sci. Technol. 2011, 45, 10485–10491. 10.1021/es202765m. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.