Abstract
Background:
A high risk subgroup of older depressed patients has slowed processing and gait speeds. This study examined whether carbidopa/levodopa (L-DOPA) monotherapy increased dopamine availability, increased processing/gait speed, and relieved depressive symptoms.
Methods:
Depressed adult outpatients >59 years old underwent baseline [11C]raclopride positron emission tomography (PET) followed by open L-DOPA for three weeks (one week each of 150mg, 300mg, and 450mg). Generalized estimating equations tested the pre- and post-L-DOPA differences in processing and gait speed measures, depressive symptoms, and reported side effects. The decrease in binding potential (ΔBPND) between the pre-and post-treatment scans indexed enhanced synaptic dopamine availability induced by L-DOPA treatment.
Results:
36 subjects participated who were 75.3 ± 7.5 years old and 44.4% male. Significant, dose dependent increases in processing and gait speed were observed with L-DOPA (450mg dose: processing speed factor score effect size [ES] = 0.41, p = 0.001; dual task gait speed ES = 0.43, p = 0.002). [11C]raclopride ΔBPND was significantly different from 0 in sensorimotor (t = −4.85, df = 24, p < 0.001) and associative striatum (t = −2.52, df 24, p = 0.019) but not in limbic striatum (t = 0.265, df = 24, p = 0.793). Depressive symptoms decreased significantly on the Hamilton Rating Scale for Depression (ES = −0.37, p = 0.002). Drop-out rate was 8.3%, and nausea was the most frequently-reported side effect.
Conclusions:
By enhancing availability of dopamine, L-DOPA improved processing and gait speed in depressed older adults and significantly decreased [11C]raclopride binding in selected striatal subregions.
Keywords: late life depression, processing speed, gait speed, levodopa, raclopride, positron emission tomography
Introduction
Depression occurring in older adults (i.e., Major Depressive Disorder, Persistent Depressive Disorder, and clinically significant subthreshold depressive symptoms—collectively referred to here as Late Life Depression [LLD]), is prevalent (1–3), disabling (4–5), and associated with high rates of completed suicide (6). LLD is one of the foremost reversible risk factors for cognitive decline and the development of dementia (7–8), is frequently chronic and recurrent (9), and is often difficult to treat (10). Among the LLD patients at highest risk of these adverse outcomes are those who manifest significant psychomotor slowing, often indexed by decreased processing speed and/or decreased gait speed. Decreased processing speed has been referred to as “the core cognitive deficit” in LLD (11) because it mediates performance on tests of nearly all cognitive domains (12), predicts poorer acute response to antidepressants (13), and increases risk for dementia (14) and dependence in activities of daily living (15). Slowed gait speed leads to incident depression in older adults (16) and has been associated with a greater risk of falls (17), disability (18), and hospital admission (19). In depressed older adults who are otherwise healthy, the development of slowed gait speed increases mortality risk over ten-year follow up by approximately 20% (16).
Even when treatment with antidepressant medication successfully reduces depressive symptoms, it often does not improve slowed processing speed (20) or restore cognitive functioning to normal levels of performance (21–22). No studies have investigated the effects of antidepressant treatment on depressed older adults with gait slowing. In order to develop urgently needed novel therapeutics, a reasonable approach is to target brain systems underlying the development and persistence of psychomotor slowing. Post-mortem experiments and in vivo neuroimaging studies have implicated age-related dopaminergic decline in the development of slowing, since aging is associated with reduced dopamine levels, decreased D1/D2 receptor density, and loss of dopamine transporters (DAT) (23–25). Mesolimbic dopaminergic tone modulates processing speed in both humans and animal models (26), and decreased striatal dopamine transmission has been associated with decreased gait speed (27) and impaired balance (28). These age-associated declines in dopaminergic functioning are topographically distinct from the denervation pattern typical of Parkinson’s disease (PD), being observed diffusely across the striatum rather than predominantly posterior putaminal in location (29).
Multiple case series from the 1960s onward report improved depressive symptoms (particularly psychomotor retardation) with levodopa (hereafter referred to as L-DOPA) monotherapy or augmentation (30–31). L-DOPA is the immediate precursor of dopamine, is converted to dopamine in presynaptic dopaminergic nerve terminals, and enhances dopaminergic transmission in multiple brain regions. L-DOPA has been reported to relieve depressive symptoms in new onset PD, improving symptoms in 90.3% of patients (N=31) and resulting in a mean Hamilton Rating Scale for Depression (HRSD) decrease of 11.7 points in one study (32). As opposed to other dopaminergic interventions (i.e., dopamine receptor agonists and stimulants), a large literature shows beneficial effects of L-DOPA on cognitive performance and gait in patients with PD (33–34), whereas the few available studies in elderly patients show minimal effects of dopamine agonists or stimulants. L-DOPA, especially at lower doses, is a safe and well-tolerated medication that is difficult to differentiate from placebo in terms of side effects (35), while adverse cardiac effects and impulsive behavior are important potential risks for stimulants and dopamine agonists, respectively.
The goal of the present combined clinical trial and positron emission tomography (PET) study was to examine whether monotherapy with L-DOPA increases dopamine release in the striatum, increases processing and gait speed, and reduces depressive symptoms in a sample of older adults with LLD and psychomotor slowing. We hypothesized that enrolled patients would demonstrate dose-dependent increases of at least moderate effect size in both processing speed and gait speed over the three-week duration open study. Further, we anticipated that [11C]raclopride binding potential (BPND) in the sensorimotor striatum measured before and after subacute L-DOPA administration would be significantly reduced due to enhanced dopamine availability effectively competing with [11C]raclopride for binding to the D2 receptor. While the three-week duration study is briefer than most antidepressant trials, we were interested to determine whether significant change would occur in both rater-administered and self-reported depressive symptoms.
Methods and Materials
Subjects
This study was conducted in the Adult and Late Life Depression Research Clinic at the New York State Psychiatric Institute (NYSPI) and approved by the NYSPI Institutional Review Board. All participants met eligibility criteria and signed informed consent for the study. Eligible subjects were adult outpatients aged ≥ 60 years who were diagnosed with Diagnostic and Statistical Manual (DSM) 5 MDD, Dysthymia, or Depression Not Otherwise Specified (NOS), had Center for Epidemiologic Studies-Depression Rating scale (CES-D) score ≥ 10, and decreased gait speed (average walking speed over 15’ course < 1m/s). Subjects were excluded for substance abuse or dependence (excluding Tobacco Use Disorder) within the past 12 months, history of psychosis, mania, or bipolar disorder, diagnosis of probable Alzheimer’s Disease, Vascular Dementia, or PD, Mini Mental Status Examination (MMSE) ≤ 24, HRSD suicide item > 2 or Clinical Global Impressions (CGI)-Severity score of 7 at baseline, current or within the past 4 weeks treatment with psychotropic or other medications known to affect dopamine, history of intolerance to L-DOPA, physical or intellectual disability adversely affecting ability to complete assessments, acute or severe medical illness, or mobility limiting osteoarthritis, spine disease, or history of joint or spine surgery. Subjects undergoing neuroimaging procedures did not have contraindication to magnetic resonance imaging (MRI), inability to tolerate the scanning procedures, or history of significant radioactivity exposure.
Study Design and Assessments
At baseline, patients were screened for significant medical problems with a history and physical examination, blood tests, electrocardiogram, and urine toxicology. Vital signs were recorded at baseline and weekly thereafter. Following screening, participants who were eligible for neuroimaging procedures had pre-treatment PET and MRI scans prior to a Week 0 visit. Participants who were ineligible for PET/MRI scanning (e.g., on the basis of having MRI incompatible metal in their body) returned 1 week after screening for Week 0. Open L-DOPA treatment was initiated as described below and continued for the three-week duration study. For participants undergoing neuroimaging, post-treatment PET scans were scheduled to coincide with Day 21 of the study insofar as was possible.
Processing and gait speed were assessed at screening, Week 0, and weekly upon initiation of L-DOPA treatment (i.e., Weeks 1–3). Assessments were performed at approximately 1pm to control for time of day effects and the duration since the last morning L-DOPA dose (anticipated to be 4 hours). Therefore, Week 3 measures were performed before the midday L-DOPA dose in order to maintain consistency across study weeks in the timing of assessments relative to last L-DOPA dose. Multiple pre-L-DOPA assessments of processing and gait speed were conducted in order to control for practice effects, which generally occur between the first and second assessment and decline thereafter. Processing speed was assessed using the Digit Symbol test from the Wechsler Adult Intelligence Scale-III (WAIS-III) (36) and the Pattern and Letter Comparison tests (37). A latent factor constructed from these three measures was chosen as the primary outcome measure for processing speed.
Gait speed was measured in m/s both as a single task in which study participants walked at their usual or normal speed on a 15 foot walking course and as a dual task in which participants walked at their usual speed while naming as many animals as possible. Two trials (each, for single and dual tasks) were completed, and the final gait speed measurement was recorded as the average of these two trials. Measuring gait speed while subjects perform one or more concurrent tasks (dual task) more accurately reflects the reality of physical functioning and may be a better predictor of fall risk compared to single task measurements (38). Thus, dual task gait speed was chosen as the primary outcome measure for gait speed.
Other study measurements performed weekly included the 24-item HRSD, the Inventory of Depressive Symptomatology 30-item self-report (IDS-SR), the CGI-Severity and CGI-Improvement scales, a rating scale for treatment-emergent side effects, and weekly pill counts.
L-DOPA Administration
Taking into account L-DOPA’s pharmacokinetics in the plasma as well as its pharmacodynamics in the central nervous system, we devised a dosing schedule allowing us to assess the effects and tolerability of three different L-DOPA doses (150mg, 300mg, and 450mg). Subjects began taking 37.5mg carbidopa/150mg levodopa once daily (9am) at the Week 0 appointment. After one week at this dosage, subjects were instructed to take 37.5mg carbidopa/150mg levodopa at 9am and 5pm (twice daily). For the third week of treatment, subjects took 37.5mg carbidopa/150mg levodopa three times daily (9am, 12pm, 5pm). Participants were instructed to maintain the same timing of doses throughout the study. Based on published work using L-DOPA for PD, we expected effects on cognition and gait to be apparent at each dosing level within the first 24 hours of administration or dosage increase and then remain relatively stable (39–40). We allowed one week between dosage increases to ensure tolerability as per standard neurologic clinical practice and to permit L-DOPA effects on mood to be measured.
Positron Emission Tomography scanning
Dynamically acquired [11C]raclopride scans were performed on a Biograph mCT hybrid PET/CT scanner (Siemens, Knoxville, TN). Initially, a brief (< 10s) CT scan of the head was acquired for attenuation correction. Following intravenous injection of [11C]raclopride, emission data were collected in list mode for 60 min. List mode data were binned into a sequence of frames of increasing duration (from 20s to 10 min) and reconstructed by filtered backprojection, with correction for attenuation, random coincidences, scatter and dead time, using manufacturer-provided software. High resolution anatomical T1-weighted MRI scans were acquired for each subject and PET data were coregistered to the MRIs using maximization of mutual information (SPM12, Wellcome Centre for Human Neuroimaging). Regions of interest (ROIs) were applied to the MRIs and transferred to the PET emission data and included the sensorimotor striatum (post-commissural putamen, SMST), associative striatum (whole caudate and pre-commissural putamen, AST) and the limbic striatum (nucleus accumbens and the most ventral aspects of the pre-commissural caudate and putamen, LST) as previously described (41). Additionally, an ROI was drawn on cerebellum as a reference tissue. ROI time activity curves were derived as the average activity in each ROI in each frame. The primary outcome measure was BPND, the binding potential with respect to the non-displaceable compartment (42), derived by the simplified reference tissue model (SRTM) (43). The effect of L-DOPA treatment was measured as the percent difference in BPND (ΔBPND) between the pre-and post-treatment scans, ΔBPND = 100%*[BPND (post-treatment) - BPND(pre-treatment]/BPND(pre-treatment).
Statistical Analyses
Differences in baseline demographic and clinical characteristics between subjects receiving at least one dose of L-DOPA (i.e., the analyzed sample) and those lost to follow-up prior to taking a pill were compared using t-tests or chi-squared tests. A latent factor analysis was conducted to create a processing speed factor from digit symbol, pattern comparison, and letter comparison test scores. Each outcome variable was collected at five time points (i.e., screening, Week 0, and Weeks 1–3) and modeled with a general linear model including a 5-category fixed effect predictor for time. Estimation was performed using generalized estimating equations (GEE) to account for the correlated observations within person using an independent working correlation matrix. Dose effects were estimated and tested from this model by forming contrasts of means at each time point compared to Week 0. Effect sizes were calculated by scaling the mean differences by the standard deviation of the measure at Week 0. 95% confidence intervals for the mean differences and the effect sizes and associated p-values are presented to assess statistical significance. All analyses were performed in SAS 9.4.
PET data were analyzed in the mixed model framework with ROIs as repeated measures and ΔBPND as dependent variable (SPSS 24, IBM). A critical value of α = 0.05 was chosen as a significance level. To understand the relationship between changes in [11C]raclopride binding and changes in processing/gait speed change, Spearman correlation coefficients were estimated between ΔBPND and the Week 0–3 change in processing and gait speed tests.
Results
Subject Disposition and Characteristics
The final CONSORT diagram for the study is shown in Figure S1. 47 subjects signed consent to participate in the study, of whom 11 were lost to follow up prior to taking study medication and were excluded from the analyses. As shown in Table 1, participants receiving L-DOPA had a mean age of 75.3 ± 7.5 years, were 44.4% male, and suffered from c depressive symptoms at a level corresponding to syndromal MDD (i.e., mean baseline CES-D 22.5 ± 10.0, where CES-D scores ≥16 are associated with the presence of MDD). Twenty-one subjects (58.3%) were diagnosed with MDD, N=13 (36.1%) with Depression NOS, and N=2 (5.6%) with Dysthymia. Most individuals suffered from chronic depression (self-reported mean duration current episode was over 10 years), and the average number of failed antidepressant medication trials in the current depressive episode was 1.4 ± 2.2. Mean baseline processing speed as measured by the Digit Symbol test was 31.9 ± 9.7, which is >1 SD below norms for individuals having similar age, sex, and education characteristics (44). Mean dual task gait speed was 0.7 ± 0.2, which is >1 SD below the age-adjusted norm for age 74 (closest published norm to the mean age of the current sample) (45). No significant differences were observed between subjects receiving L-DOPA (i.e., the analyzed sample) and those lost to follow up during the screening process.
Table 1.
Baseline characteristics of analyzed study subjects (N=36) and those lost to follow up before receiving L-DOPA.
| Characteristic | Received L-DOPA (N=36) | Did not receive L-DOPA (N=11) | |
|---|---|---|---|
| Mean ± SD | Mean ± SD | p | |
| Age (years) | 75.3 ± 7.5 | 72.1 ± 6.6 | 0.21 |
| Gender (N male) | 16 (44.4%) | 2 (18.1%) | 0.12 |
| Ethnicity (N Hispanic) | 7 (19.4%) | 2 (18.1%) | 0.93 |
| Other | 3 (8.3%) | ||
| Depression NOS | 13 (36.1%) | 2 (18.2%) | |
| Duration of current depressive episode (weeks) | 528.9 ± 854.6 | 702.4 ± 1040.4 | 0.60 |
| Number of prior antidepressant medications | 1.4 ± 2.2 | 1.2 ± 1.3 | 0.77 |
| Hamilton Rating Scale for Depression (24 item) | 15.5 ± 6.0 | 19.4 ± 4.2 | 0.056 |
| Inventory of Depressive | 26.4 ± 12.6 | 30.5 ± 12.4 | 0.47 |
| Symptomatology—Self Report | |||
| Clinical Global Impressions—Severity | 3.3 ± 0.7 | 3.7 ± 0.8 | 0.11 |
| Center for Epidemiologic Studies—Depression | 22.5 ± 10.0 | 24.9 ± 8.7 | 0.47 |
Effects on Psychomotor Speed and Depressive Symptoms
Significant practice effects were observed from screening to Week 0 (see Table S1) on Digit Symbol (effect size [ES] = 0.55, 95% confidence interval [CI] 0.35–0.75, p < 0.001), the composite processing factor (ES=0.28, 95% CI= 0.11–0.45, p = 0.001), single task gait speed (ES = 0.46, 95% CI = 0.18–0.73 p = 0.001), and dual task gait speed (ES = 0.22, 95% CI = 0.03–0.40, p = 0.022). Consistent with the pattern of diminishing practice effects that is frequently observed with repeated testing, a smaller amount of improvement occurred from Week 0 to Week 1 on these measures despite the initiation of L-DOPA.
As hypothesized, L-DOPA treatment was associated with dose-dependent increases in both processing speed and gait speed on multiple outcome measures (see Table 2). For the 450mg L-DOPA dose, statistically significant improvements of moderate effect size or greater relative to Week 0 pre-treatment were observed on the primary outcome measures for both processing speed (processing speed factor ES = 0.41, 95% CI = 0.24–0.58, p < 0.001) and gait speed (dual task ES = 0.43, 95% CI = 0.16–0.70 p = 0.002). Plots depicting individual participants’ change on the Digit Symbol and dual task gait speed measures as well as means and 95% confidence intervals at each time point are shown in Figures S2 and S3, respectively. A significant decrease in depressive symptoms was observed from Week 0 pre-treatment to Week 3 (450mg) on the Hamilton Rating Scale for Depression (HRSD, ES = −0.37, 95% CI = −0.61 - −0.14, p = 0.002) and Inventory of Depressive Symptomatology—Self Report (IDS-SR, ES = −0.54, 95% CI = −0.74 - −0.34, p < 0.001). Remission rate defined by final HRSD < 10 was 48.5%.
Table 2.
Change in slowing and depressive symptoms before and after L-DOPA treatment. ‘Screening’ refers to assessments performed at screening visit, ‘Week 0’ refers to pre-medication baseline, and Week 1–3 refer to assessments performed after 150mg, 300mg, and 450mg L-DOPA, respectively. Dose effects are estimated by tests of change from pre-treatment (Week 0). Effect size (ES) is calculated by scaling the mean change by the standard deviation at Week 0. HRSD=Hamilton Rating Scale for Depressive Symptoms, IDS-SR=Inventory for Depressive Symptomatology—Self Report. Subjects contributing data to this table were N=36 (Screen, Week 0, and Week 1), N=35 at Week 2, and N=33 at Week 3.
| Visit | Dose | Mean±SD | Mean change±SE | Mean change 95% CI | ES±SE | ES 95% CL | P-value | |
|---|---|---|---|---|---|---|---|---|
| Processing speed Measures | ||||||||
| Digit symbol | Screening | 31.86±9.69 | . | |||||
| Week 0 | Pre-treatment | 37.19±8.73 | . | |||||
| Week 1 | 150mg | 38.89±9.43 | 1.69±0.77 | 0.19,3.20 | 0.19±0.09 | 0.02,0.37 | 0.0271 | |
| Week 2 | 300mg | 41.09±8.54 | 3.89±0.88 | 2.17,5.62 | 0.45±0.10 | 0.25,0.64 | <.0001 | |
| Week 3 | 450mg | 41.42±9.56 | 4.23±0.94 | 2.38,6.08 | 0.48±0.11 | 0.27,0.70 | <.0001 | |
| Pattern comparison | Screening | 24.72±5.42 | . | |||||
| Week 0 | Pre-treatment | 25.39±5.57 | . | |||||
| Week 1 | 150mg | 26.89±4.87 | 1.5±0.63 | 0.27,2.73 | 0.27±0.11 | 0.05,0.49 | 0.0171 | |
| Week 2 | 300mg | 27.29±5.7 | 1.91±0.69 | 0.55,3.27 | 0.34±0.12 | 0.10,0.59 | 0.006 | |
| Week 3 | 450mg | 27.61±4.94 | 2.22±0.56 | 1.12,3.32 | 0.40±0.10 | 0.20,0.60 | <.0001 | |
| Letter comparison | Screening | 14.36±4.73 | . | |||||
| Week 0 | Pre-treatment | 15±4.53 | . | |||||
| Week 1 | 150mg | 15.33±4.64 | 0.33±0.52 | −0.69,1.36 | 0.07±0.12 | −0.15,0.3 | 0.5248 | |
| Week 2 | 300mg | 15.65±4.66 | 0.65±0.5 | −0.33,1.62 | 0.14±0.11 | −0.07,0.36 | 0.194 | |
| Week 3 | 450mg | 15.69±4.54 | 0.69±0.54 | −0.37,1.74 | 0.15±0.12 | −0.08,0.38 | 0.2016 | |
| Processing factor (composite of DS, PC, and LC) | Screening | −2.87±7.05 | . | |||||
| Week 0 | Pre-treatment | −0.91±6.79 | . | |||||
| Week 1 | 150mg | 0.63±6.33 | 1.53±0.58 | 0.40,2.67 | 0.23±0.09 | 0.06,0.39 | 0.008 | |
| Week 2 | 300mg | 1.54±6.47 | 2.45±0.61 | 1.26,3.64 | 0.36±0.09 | 0.19,0.54 | <.0001 | |
| Week 3 | 450mg | 1.85±6.72 | 2.76±0.59 | 1.60,3.91 | 0.41±0.09 | 0.24,0.58 | <.0001 | |
| Gait speed measures | ||||||||
| Single task gait speed | Screening | 0.80±0.18 | . | |||||
| Week 0 | Pre-treatment | 0.88±0.21 | . | |||||
| Week 1 | 150mg | 0.89±0.19 | 0.01±0.02 | −0.02,0.05 | 0.07±0.08 | −0.09,0.22 | 0.4066 | |
| Week 2 | 300mg | 0.91±0.21 | 0.04±0.03 | −0.01,0.09 | 0.18±0.12 | −0.06,0.42 | 0.1465 | |
| Week 3 | 450mg | 0.92±0.21 | 0.04±0.02 | 0.00,0.08 | 0.19±0.09 | 0.01,0.38 | 0.0369 | |
| Dual task gait speed | Screening | 0.69±0.23 | . | |||||
| Week 0 | Pre-treatment | 0.74±0.20 | . | |||||
| Week 1 | 150mg | 0.74±0.23 | 0.00±0.02 | −0.05,0.05 | 0±0.12 | −0.23,0.23 | 0.9999 | |
| Week 2 | 300mg | 0.80±0.24 | 0.06±0.03 | 0.01,0.12 | 0.32±0.15 | 0.03,0.62 | 0.0314 | |
| Week 3 | 450mg | 0.82±0.24 | 0.09±0.03 | 0.03,0.14 | 0.43±0.14 | 0.16,0.70 | 0.0018 | |
| Depression measures | ||||||||
| Hamilton Rating Scale for Depression (24 item) | Screening | 15.53±6.04 | . | |||||
| Week 0 | Pre-treatment | 13.42±6.63 | . | |||||
| Week 1 | 150mg | 11.97±6.96 | −1.44±0.94 | −3.29,0.40 | −0.22±0.14 | −0.5,0.06 | 0.125 | |
| Week 2 | 300mg | 11.06±6.17 | −2.36±1.03 | −4.37,−0.35 | −0.36±0.16 | −0.66,−0.05 | 0.0216 | |
| Week 3 | 450mg | 10.94±6.87 | −2.48±0.80 | −4.05,−0.90 | −0.37±0.12 | −0.61,−0.14 | 0.002 | |
| Inventory of Depressive Symptomatology—Self Report | Screening | 26.42±12.57 | . | |||||
| Week 0 | Pre-treatment | 22.47±13.61 | . | |||||
| Week 1 | 150mg | 19.17±11.44 | −3.31±1.9 | −7.03,0.42 | −0.24±0.14 | −0.52,0.03 | 0.0819 | |
| Week 2 | 300mg | 17.57±11.71 | −4.9±1.86 | −8.55,−1.25 | −0.36±0.14 | −0.63,−0.09 | 0.0084 | |
| Week 3 | 450mg | 15.09±10.64 | −7.38±1.38 | −10.09,−4.67 | −0.54±0.1 | −0.74,−0.34 | <.0001 | |
Effects on [11C]Raclopride Binding
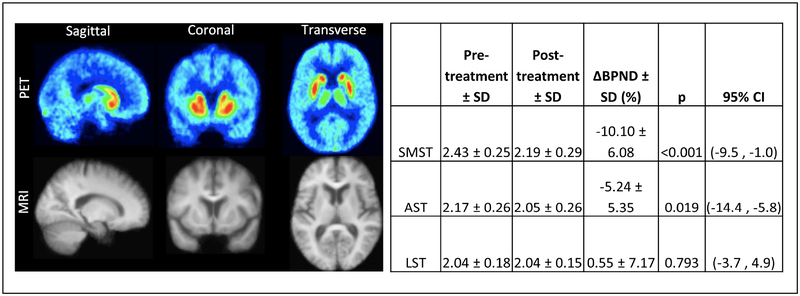
Ten subjects underwent neuroimaging procedures. One subject had to be excluded immediately after undergoing PET scanning when it became known that the subject had covertly continued taking a prohibited psychotropic medication (duloxetine) during the pre-treatment PET scan and early phases of the clinical trial. There was no significant difference in injected activity between the pre- and post-treatment scans (pre-treatment 11.2 ± 2.3 mCi, post treatment 10.0 ± 1.5 mCi, p = 0.30). There was a small but significant difference in injected cold raclopride mass between conditions (pre-treatment 2.3 ± 0.9 μg, post-treatment 1.5 ± 0.6 μg, p = 0.04) but all mass was within the tracer dose range. Figure 1 shows BPND and ΔBPND across the three striatal subregions. ΔBPND was uncorrelated across ROIs (|r| <0.14, p > 0.6 in all cases) and significantly different from 0 in SMST (t = −4.85, df = 24, p < 0.001) and AST (t = −2.52, df 24, p = 0.019) but not in LST (t = 0.265, df = 24, p = 0.793).
Figure 1.
Baseline (Week 0) and post-L-DOPA (Week 3) mean [11C]raclopride binding (N=10). To the left, mean sagittal, coronal, and transverse images for [11C]raclopride PET and structural MRI are shown. Canonical striatal brain regions demonstrating [11C]raclopride are evident. To the right, mean changes from pre- to post-L-DOPA in [11C]raclopride BPND are provided. Significantly decreased [11C]raclopride was observed in sensorimotor striatum (SMST) and associative striatum (AST), but not limbic striatum (LST).
Exploratory analyses examined relationships between improvements in processing/gait speed and [11C]raclopride ΔBPND in corresponding brain regions (i.e., AST and processing speed, SMST and gait speed). These revealed that improvement on the processing speed factor was significantly correlated with reduced BPND in the AST (r = −0.75, df 7, p = 0.02), while increased single task gait speed was correlated at a trend level with decreased BPND in the SMST (r = −0.63, df 7, p = 0.07). No significant correlations were observed between [11C]raclopride ΔBPND and depressive symptoms measured by the HRSD or IDS-SR.
L-DOPA Adverse Effects and Attrition
The overall drop-out rate was 8.3%, with 5.6% subjects dropping out due to adverse effects of L-DOPA. Thirty of the total N=36 analyzed subjects reached the final L-DOPA dose of 450mg. As shown in Table 3, nausea was the most frequently reported treatment-emergent side effect. The frequency of subjects reporting nausea decreased over the course of the trial, from 19.4% of subjects reported for 150mg L-DOPA, to 17.1% for 300mg L-DOPA, and 9.1% for 450mg L-DOPA. The only other side effects reported by more than one subject were insomnia (150mg/300mg/450mg L-DOPA: 8.3%/5.7%/3.0%) and headache (150mg/300mg/450mg L-DOPA: 2.8%/2.9%/6.1%). The emergence of dyskinesias during L-DOPA treatment was evaluated using items 32 and 33 of the Unified Parkinson’s Disease Rating Scale (UPDRS), and no significant change from baseline was observed on either these items. Mean scores were 0.0 at Week 3 for both items.
Table 3.
Treatment emergent adverse events associated with L-DOPA. Items 32–33 on the Unified Parkinson’s Disease Rating Scale assess the presence or absence of motor side effects. Below, the total number of study subjects (N) and proportion of the sample (%) experiencing specific side effects are listed. All side effects experienced by at least one study subject at any time point are listed.
| Variable | Week 1 | Week 2 | Week 3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Unified Parkinson’s Disease Rating Scale | Mean | SD | p vs. W0 | Mean | ES vs. W0 | p vs. W0 | Mean | ES vs. W0 |
p vs. W0 |
| Item 32 | 0.18 | 0.58 | 0.17 | 0.18 | 0.18 | 0.17 | 0.0 | 0.0 | 0.32 |
| Item 33 | 0.09 | 0.38 | 0.09 | 0.09 | 0.09 | 0.09 | 0.0 | 0.0 | 0.32 |
| Treatment Emergent Side Effect Scale | n | % | n | % | n | % | |||
| Nausea | 7 | 19.4 | 6 | 17.1 | 3 | 9.1 | |||
| Insomnia | 3 | 8.3 | 2 | 5.7 | 1 | 3.0 | |||
| Headache | 1 | 2.8 | 1 | 2.9 | 2 | 6.1 | |||
| Drowsiness | 0 | 0.0 | 1 | 2.9 | 1 | 3.0 | |||
| Dystonia | 0 | 0.0 | 1 | 2.9 | 0 | 0 | |||
| Akathisia | 1 | 2.8 | 0 | 0 | 0 | 0 | |||
| Dry mouth | 0 | 0.0 | 0 | 0 | 1 | 3.0 | |||
| Dizziness | 0 | 0.0 | 0 | 0 | 1 | 3.0 | |||
Discussion
The primary finding of this open study of L-DOPA for psychomotor slowing and depression in older adults was a dose-dependent increase in both processing and gait speed, which coincided with significantly decreased [11C]raclopride binding in selected striatal subregions. While L-DOPA effects on processing and gait speed were modest (ES = 0.41 and 0.43, respectively), these effects are significant given the functional disability and increased risk for adverse outcomes associated with slowing and its lack of responsivity to standard antidepressant treatments. Though depressive symptom change was not the focus of this study, we found a significant decrease from baseline on both rater-administered (HRSD) and self-report measures of depression across this relatively brief, three-week duration study. L-DOPA was remarkably well-tolerated in this patient sample. The most common side effect, nausea, decreased in frequency as the study progressed, and no persistent motor outcomes such as dyskinesias were observed.
While the number of individuals undergoing PET scanning in this study was relatively small, significantly decreased [11C]raclopride binding following subacute L-DOPA treatment was observed, and these regionally specific BPND reductions were associated with characteristic behavioral outputs. For example, reduced AST binding was specifically correlated with increased processing speed, which is consistent with prior literature linking maladaptive changes in AST (i.e., caudate) structure and function to slowed processing speed in neuropsychiatric disorders such as PD and normal aging (46–47). Similarly, the trend-level correlation between reduced SMST binding following L-DOPA and increased single task gait speed is consistent with a large PD literature demonstrating links between putaminal dopamine release, gait speed, and responses to L-DOPA treatment (48–49). Larger samples of depressed, non-PD older adults are needed to further examine the behavioral consequences of L-DOPA-induced changes in specific striatal subregions and how change in specific subregions interact with one another.
More generally, the conceptual framework undergirding this line of research—that novel therapeutic development for later life neuropsychiatric disorders should be guided by an understanding of the causative age-related physiologic processes—may merit broader application. Historically, treatments for disorders such as LLD have been based on pathophysiologic models of depression in younger adults and have been efficacy-tested in clinical trials mostly enrolling younger patients. As development and validation of the Vascular Depression Hypothesis in the 1990s made clear (50) distinct processes (e.g., cerebrovascular aging and development of deep white matter hyperintensities [WMH]) may cause and perpetuate depressive disorders in later life as compared to earlier in the life course. More recently, other processes, such as dopaminergic decline, age-related hearing loss (51), and development of the syndrome of frailty (52), have been identified as pathophysiologic routes to developing or perpetuating LLD. By developing precision interventions for these age-related disease mechanisms and testing them in etiologically homogenous patient samples, better outcomes for older adults may be achievable.
Future studies should examine more closely the relationship between slowing and depression in older adults, which is facilitated by the recent addition of a Sensorimotor Domain to the Research Domain Criteria (RDoC) matrix. One possibility is that slowed processing and gait speed are not causally related to depression but are simply markers of a low dopamine state, which leads to the development of depressive disorders via other processes such as impaired reinforcement learning (53–54) or reduced hedonic capacity (55–56). Alternatively, slowing may reside on a causal sequence leading to depression in older adults, possibly through increased fatigability leading to alterations in effort-based decision making (57–58). It is intuitive to hypothesize that psychomotor slowing increases the effort cost of voluntary behavior, such that slowed older adults are less willing to work for rewards of a given value relative to older adults without slowing. This state may lead to reduced behavioral activation and contribute to social isolation and loneliness, all of which increase risk for depression (59).
A significant limitation to the above-presented results is the open administration of L-DOPA utilized in this study. Although some evidence suggests depressed older adults experience diminished expectancy-based placebo effects relative to younger adults (60), a portion of the improvement observed on processing speed, gait speed, and depressive symptoms may be attributable to participants’ expectation of improvement, therapeutic interactions with the research staff, and spontaneous improvement. Given that striatal dopamine release has been shown to mediate placebo responses of various types (61), a portion of the change in [11C]raclopride binding potential observed with L-DOPA treatment also may have been caused by non-specific factors. Another limitation to be considered when interpreting results from this study is its relatively small sample size, both for the clinical and molecular effects of L-DOPA. Larger sample sizes will permit confirmation of the findings reported above and provide the requisite power for mediation analyses testing whether a greater degree of slowing improvement predicts greater reductions in depressive symptoms.
Given that this study was designed to identify L-DOPA effects on molecular and functional targets, its ability to detect change in depressive symptoms was limited. In particular, the brief treatment duration and mild depression severity of study participants pose limitations to detecting L-DOPA treatment effects as well as finding significant correlations between change in [11C]raclopride binding and change in depression outcomes. In addition, there was variability in L-DOPA effects on psychomotor speed among participants in this study, which may relate to the nonspecific nature of slowed gait speed as a marker for age-associated dopaminergic decline. This limitation is mitigated by the inherently scaleable and non-invasive nature of gait speed as a marker of dopamine availability in older adults, but future studies may consider how individual differences in dopamine metabolism contribute to slowing and may moderate response to L-DOPA (e.g., catechol-O-methyl-transferase [COMT] genotype).
To address these limitations and focus on depression-related outcomes, a double-blind, placebo-controlled study with increased sample size and longer duration is now underway in our laboratories. However, the above data already suggest the exciting possibility that age-associated dopaminergic decline and its adverse functional consequences can be remediated in this high risk and therapeutically challenging subgroup of LLD patients. Restoring psychomotor speed to age-appropriate norms may improve cognitive and physical functioning, relieve symptoms of depression, and reduce long term risk for adverse health outcomes associated with slowing. Existing treatments (e.g., antidepressant medication) largely do not address the loss of dopaminergic tone hypothesized to cause psychomotor slowing in older adults, which may explain why they are often unsuccessful in achieving response or remission.
Supplementary Material
Acknowledgements
This study was supported by NIMH R61 MH110029 (Principal Investigator Rutherford).
Disclosures
Dr. Abi-Dargham reports being a stockholder in Systems 1 Bio and Storm Biosciences, receiving honoraria for lectures from Otsuka, and being a member of scientific advisory boards with Roche and Sunovion. Dr. Slifstein reports being a stockholder in Systems 1 Bio and Storm Biosciences. All other authors report no biomedical financial interests or potential conflicts of interet.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study is registered on Clinicaltrials.gov ().
Contributor Information
Bret R Rutherford, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Mark Slifstein, Stony Brook University College of Medicine.
Chen Chen, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Anissa Abi-Dargham, Stony Brook University College of Medicine.
Patrick J. Brown, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Melanie W. Wall, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
Nora Vanegas-Arroyave, Columbia University College of Physicians and Surgeons.
Yaakov Stern, Columbia University College of Physicians and Surgeons.
Veronika Bailey, New York State Psychiatric Institute.
Emily Valente, New York State Psychiatric Institute.
Steven P. Roose, Columbia University College of Physicians and Surgeons, New York State Psychiatric Institute.
References
- 1.Kessler RC, Birnbaum H, Bromet E, Hwang I, Sampson N, Shahly V. Age differences in major depression: results from the National Comorbidity Survey Replication (NCS-R). Psychol Med 2010; 40:225–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blazer DG, Landerman LR, Hays JC, Simonsick EM, Saunders WB. Symptoms of depression among community-dwelling elderly African-American and white older adults. Psychol Med 1998; 28:1311–1320. [DOI] [PubMed] [Google Scholar]
- 3.Sutin AR, Terracciano A, Milaneschi Y, An Y, Ferrucci L, Zonderman AB. The Trajectory of Depressive Symptoms across the Adult Life Span. JAMA Psychiatry 2013; 70:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Penninx B, Leveille S, Ferrucci L, van Eijk J, Guralnik J. Exploring the effect of depression on physical disability: Longitudinal evidence from the Established Populations for Epidemiologic Studies of the Elderly. Am J Publ Health 1999; 89:1346–1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Callahan CM, Wolinsky FD, Stump TE, Nienaber NA, Hui SL, Tierney WM. Mortality, symptoms, and functional impairment in late-life depression. J Gen Intern Med 1998; 13:746–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conwell Y, Lyness J, Duberstein P, Cox C, Seidlitz L, DiGiorgio A. Completed suicide among older patients in primary care practices: a controlled study. J Am Geriatr Soc 2000; 48:23–29. [DOI] [PubMed] [Google Scholar]
- 7.Kaup AR, Byers AL, Falvey C, Simonsick EM, Satterfield S, Ayonayon HN, et al. Trajectories of Depressive Symptoms in Older Adults and Risk of Dementia. JAMA Psychiatry 2016; 73:525–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mirza SS, Wolters FJ, Swanson SA, Koudstaal PJ, Hofman A, Tiemeier H, Ikram MA. 10-year trajectories of depressive symptoms and risk of dementia: a population-based study. Lancet Psychiatry 2016; 3:628–635. [DOI] [PubMed] [Google Scholar]
- 9.Alexopoulos GS, Meyers BS, Young RC, Kakuma T, Feder M, Einhorn A, Rosedahl E. Recovery in geriatric depression. Arch Gen Psychiatry 1996; 53:305–312. [DOI] [PubMed] [Google Scholar]
- 10.Sneed JR, Rutherford BR, Rindskopf D, Roose SP. Design makes a difference: antidepressant response rates in placebo-controlled versus comparator trials in late life depression. Am J Geri Psychiatry 2008; 16:65–73. [DOI] [PubMed] [Google Scholar]
- 11.Sheline YI, Barch DM, Garcia K, Gersing K, Pieper C, Welsh-Bohmer K, et al. Cognitive Function in Late Life Depression: Relationships to Depression Severity, Cerebrovascular Risk Factors and Processing Speed. Biol Psychiatry 2006; 60:58–65. [DOI] [PubMed] [Google Scholar]
- 12.Salthouse TA. The Processing-Speed Theory of Adult Age Differences in Cognition. Psychol Rev 1996; 103:403–428. [DOI] [PubMed] [Google Scholar]
- 13.Pimontel MA, Culang-Reinlieb ME, Morimoto SS, Sneed JR. Executive dysfunction and treatment response in late life depression. Int J Geriatr Psychiatry 2012; 27:893–899. [DOI] [PubMed] [Google Scholar]
- 14.Rapp MA, Reischies FM. Attention and executive control predict Alzheimer disease in late life: results from the Berlin Aging Study (BASE). Am J Geriatr Psychiatry 2005; 13:134–141. [DOI] [PubMed] [Google Scholar]
- 15.Iwasa H, Gondo Y, Yoshida Y, Kwon J, Inagaki H, Kawaai C, et al. Cognitive performance as a predictor of functional decline among the non-disabled elderly dwelling in a Japanese community: A 4-year population-based prospective cohort study. Arch Gerontol Geriatr 2008; 47:139–149. [DOI] [PubMed] [Google Scholar]
- 16.Brown PJ, Roose SP, Zhang J, Wall M, Rutherford BR, Ayonayon HN, et al. Inflammation, Depression, and Slow Gait: A High Mortality Phenotype in Later Life. J Gerontol Med Sci 2016; 71:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Verghese J, Holtzer R, Lipton RB, Wang C. Quantitative Gait Markers and Incident Fall Risk in Older Adults. J Gerontol Med Sci 2009; 64:896–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci 2000; 55:M221–M231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.White DK, Neogi T, Nevitt MC, Peloquin CE, Zhu Y, Boudreau RM, et al. Trajectories of Gait Speed Predict Mortality in Well-Functioning Older Adults: The Health, Aging, and Body Composition Study. J Gerontol A Biol Sci Med Sci 2013; 68:456–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, Etkin A. Effect of antidepressant treatment on cognitive impairments associated with depression: a randomized longitudinal study. Lancet Psychiatry 2016; 3:425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butters MA, Becker JT, Nebes RD, Zmuda MD, Mulsant BH, Pollock BG, Reynolds CF 3rd. Changes in Cognitive Functioning Following Treatments of Late-Life Depression. Am J Psychiatry 2000; 157:1949–1954. [DOI] [PubMed] [Google Scholar]
- 22.Nebes RD, Pollock BG, Houck PR, Butters MA, Mulsant B, Zmuda MD, Reynolds CF 3rd. Persistence of cognitive impairment in geriatric patients following antidepressant treatment: a randomized double-blind clinical trial with nortriptyliine and paroxetine. J Psychiatr Res 2003; 37:99–108. [DOI] [PubMed] [Google Scholar]
- 23.Backman L, Nyberg L, Lindenberger U, Li SC, Farde L. The correlative triad among aging, dopamine, and cognition: Current status and future prospects. Neurosci Biobehav Rev 2006; 30:791–807. [DOI] [PubMed] [Google Scholar]
- 24.Volkow ND, Gur RC, Wang GJ, Fowler JS, Moberg PJ, Ding YS, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998; 155, 344–349. [DOI] [PubMed] [Google Scholar]
- 25.Kaasinen V, Vilkman H, Hietala J, Nagren K, Helenius H, Olsson H, et al. Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain. Neurobiol Aging 2000; 21:683–688. [DOI] [PubMed] [Google Scholar]
- 26.Eckart C, Bunzeck N. Dopamine modulates processing speed in the human mesolimbic system. Neuroimage 2013; 66:293–300. [DOI] [PubMed] [Google Scholar]
- 27.Cham R, Studenski SA, Perara S, Bohnen NI. Striatal dopaminergic denervation and gait in healthy adults. Exp Brain Res 2008; 185:391–398. [DOI] [PubMed] [Google Scholar]
- 28.Cham R, Perera S, Studenski SA, Bohnen NI. Striatal dopamine denervation and sensory integration for balance in middle-aged and older adults. Gait Posture 2007; 26:516–525. [DOI] [PubMed] [Google Scholar]
- 29.Bohnen NI, Albin RL, Koeppe RA, Wernette KA, Kilbourn MR, Minoshima S, Frey KA. Positron emission tomography of monoaminergic vesicular binding in aging and Parkinson disease. J Cereb Blood Flow Metab 2006; 26:1198–1212. [DOI] [PubMed] [Google Scholar]
- 30.Klerman GL, Schildkraut JJ, Hasenbush LL, Greenblatt VL, Friend DG. Clinical experience with dihydroxyphenylalanine (DOPA) in depression. J Psychiatr Res 1963; 1:289–297. [DOI] [PubMed] [Google Scholar]
- 31.Sanghvi I, Urquiaga X, Gershon S. Exploration of the Anti-Depressant Potential of L-DOPA. Psychopharmacologia 1971; 20:118–127. [DOI] [PubMed] [Google Scholar]
- 32.Kashihara K, Imamura T, Ohno M. Effect of levodopa on depression in de novo patients with Parkinson’s disease. Movement Disord 2013, 28(Suppl 1):407.23401107 [Google Scholar]
- 33.Hayes AE, Davidson MC, Keele SW. Towards a functional analysis of the basal ganglia. J Cogn Neurosci 1998; 10:178–198. [DOI] [PubMed] [Google Scholar]
- 34.Costa A, Peppe A, Dell’Agnello G, Carlesimo G, Murri L, Bonuccelli U, Caltarigone C. Dopaminergic modulation of visual-spatial working memory in Parkinson’s disease. Dem Geriatr Cogn Disord 2003; 15:55–66. [DOI] [PubMed] [Google Scholar]
- 35.The Parkinson Study Group. Levodopa and the Progression of Parkinson’s Disease. New Engl J Med 2004; 351:2498–2508. [DOI] [PubMed] [Google Scholar]
- 36.Wechsler D Wechsler Memory Scale – Third Edition. San Antonio, TX: Psychological Corporation, 1997. [Google Scholar]
- 37.Salthouse TA, Babcock RL. Decomposing adult age differences in working memory. Devel Psychol 1991; 27:763–776. [Google Scholar]
- 38.Muir W, Speechley M, Wells J, Borrie M, Gopaul Ktero-Odasso M. Gait assessment in mild cognitive impairment and Alzheimer’s disease: The effect of dual-task challenges across the cognitive spectrum. Gait & Posture 2012; 35:96–100. [DOI] [PubMed] [Google Scholar]
- 39.Nutt JG, Holford NH. The response to levodopa in Parkinson’s disease: imposing pharmacological law and order. Ann Neurol 1996; 39:561–573. [DOI] [PubMed] [Google Scholar]
- 40.Malloy SA, Rowan EN, O’Brien JT, McKeith IG, Wesnes K, Burn DJ. Effect of levodopa on cognitive function in Parkinson’s disease with and without dementia and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry 2006; 77:1323–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, et al. Cocaine dependence and D-2 receptor availability in functional subdivisions of the striatum: Relationship with cocaine-seeking behavior. Neuropsychopharmacology 2004; 29:1190–1202. [DOI] [PubMed] [Google Scholar]
- 42.Innis RB, Cunningham VJ, Delforge J, Fujita M, Gjedde A, Gunn RN, et al. Consensus nomenclature for in vivo imaging of reversibly binding radioligands. J Cereb Blood Flow Metab 2007; 27:1533–1539. [DOI] [PubMed] [Google Scholar]
- 43.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage 1996; 4:153–158. [DOI] [PubMed] [Google Scholar]
- 44.Gaertner B, Wagner M, Luck T, Buttery AK, Fuchs J, Busch MA. Normative Data for the Digit Symbol Substitution Test in a population-based Sample aged 65 to 79 Years: Results from the German Health Interview and Examination Survey for Adults (DEGS1). Clin Neuropsychol 2018; doi: 10.1080/13854046.2018. [DOI] [PubMed] [Google Scholar]
- 45.Beauchet O, Allali G, Sekhon H, Verghese J, Guilain S, Steinmetz JP, et al. Guidelines for Assessment of Gait and Reference Values for Spatiotemporal Gait Parameters in Older Adults: The Biomathics and Canadian Gait Consortiums Initiative. Front Hum Neurosci 2017; 11: 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price CC, Tanner J, Nguyen PT, Schwab NA, Mitchell S, Slonena E, et al. Gray and White Matter Contributions to Cognitive Frontostriatal Deficits in Non-Demented Parkinson’s Disease. PLoS ONE 11(1): e0147332 Doi: 10.1371/journal.pone.01473332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Backman L, Lindenberger U, Li SC, Nyberg L. Linking cognitive aging to alterations in dopamine neurotransmitter functioning: Recent data and future avenues. Neurosci Biobehav Rev 2010; 34: 670–677. [DOI] [PubMed] [Google Scholar]
- 48.Pavese N, Evans AH, Tai YF, Hotton G, Brooks DJ, Lees AJ, Piccini P. Clinical correlates of levodopa-induced dopamine release in Parkinson’s disease: A PET Study. Neurology 2006; 67:1612–1617. [DOI] [PubMed] [Google Scholar]
- 49.Kumar A, Mann S, Sossi V, Ruth TJ, Stoessl AJ, Schulzer M, Lee CS. [11C}DTBZ-PET correlates of levodopa responses in asymmetric Parkinson’s disease. Brain 2003; 126:2648–2655. [DOI] [PubMed] [Google Scholar]
- 50.Sneed JR, Roose SP, Sackeim HA. Vascular Depression: A Distinct Diagnostic Subtype? Biol Psychiatry 2006; 60:1295–1298. [DOI] [PubMed] [Google Scholar]
- 51.Rutherford BR, Brewster K, Golub JS, Kim AH, Roose SP. Sensation and Psychiatry: Linking Age-Related Hearing Loss to Late-Life Depression and Cognitive Decline. Am J Psychiatry 2018; 175:215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown PJ, Rutherford B, Yaffe K, Tandler J, Ray JL, Pott E, et al. The Depressed Frail Phenotype: The Clinical Manifestation of Increased Biological Aging. Am J Geriatr Psychiatry 2016; 24:1084–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vrieze E, Pizzagalli DA, Demyttenaere K, Hompes T, Sienaert P, de Boer P, et al. Reduced reward learning predicts outcome in major depressive disorder. Biol Psychiatry 2013; 73: 639–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen C, Takahashi T, Nakagawa S, Inoue T, Kusumi I. Reinforcement learning in depression: a review of computational research. Neurosci Biobehav Rev 2015; 55: 247–67. [DOI] [PubMed] [Google Scholar]
- 55.Russo SJ, Nestler EJ. The brain reward circuitry in mood disorders. Nat Rev Neurosci 2013; 14: 609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang WN, Chang SH, Guo LY, Zhang KL, Wang J. The neural correlates of reward-related processing in major depressive disorder: a meta-analysis of functional magnetic resonance imaging studies. J Affect Disord 2013; 151: 531–9. [DOI] [PubMed] [Google Scholar]
- 57.Moreh E, Jacobs JM, Stessman. Fatigue, Function, and Mortality in older Adults. J Gerontol A Med Sci Biol Sci 2010; 65A: 887–895. [DOI] [PubMed] [Google Scholar]
- 58.Treadway MT, Bossaller N, Shelton RC, Zald DH. Effort-Based Decsion Making in Major Depressive Disorder: A Translational Model of Motivational Anhedonia. J Abnorm Psychol 2012; 12:553–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alpass FM, Neville S. Loneliness, health and depression in older males. Aging Mental Health 2010; 7:212–216. [DOI] [PubMed] [Google Scholar]
- 60.Rutherford BR, Wall MM, Brown PJ, Choo TH, Wager TD, Peterson BS, et al. Patient Expectancy as a Mediator of Placebo Effects in Antidepressant Clinical Trials. Am J Psychiatry 2017; 174:135–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Scott DJ, Stohler CS, Egnatuk CM, Wang H, Koeppe RA, Zubieta JK. Individual Differences in Reward Responding Explain Placebo-Induced Expectations and Effects. Neuron 2007; 55:325–336. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.