Significance
Immunoglobulin isotype switching plays an important role in adaptive immune responses. It requires transcription of switch regions from inducible promoters controlled by a long-range superenhancer (3′RR). Most stimuli induce more than one promoter on both chromosomes. Thus, it is unknown whether isotype (I) promoters compete for the 3′RR on the same chromosome. By performing allele-specific RT-qPCR assays, we show that the 3′RR activates promoters by either co-activation or competition and that the nature of the inducing signal plays a pivotal role in determining the activation mode. The two activation paths may have evolved to cope with different kinetics of primary immune responses.
Keywords: B lymphocyte, immunoglobulin locus, enhancer, promoter, switch transcription
Abstract
B cell isotype switching plays an important role in modulating adaptive immune responses. It occurs in response to specific signals that often induce different isotype (I) promoters driving transcription of switch regions, located upstream of the Ig heavy chain (IgH) constant genes. The transcribed switch regions can recombine, leading to a change of the constant gene and, consequently, of antibody isotype. Switch transcription is controlled by the superenhancer 3′ regulatory region (3′RR) that establishes long-range chromatin cis-interactions with I promoters. Most stimuli induce more than one I promoter, and switch transcription can occur on both chromosomes. Therefore, it is presently unknown whether induced I promoters compete for the 3′RR on the same chromosome. Here we performed single-chromosome RT-qPCR assays to examine switch transcription monoallelically in the endogenous context. We show that there are two modes of 3′RR-mediated activation of I promoters: coactivation and competition. The nature of the inducing signal plays a pivotal role in determining the mode of activation. Furthermore, we provide evidence that, in its endogenous setting, the 3′RR has a bidirectional activity. We propose that the coactivation and competition modes mediated by the 3′RR may have evolved to cope with the different kinetics of primary immune responses.
The IgH locus is one of the most complex loci in mammals, and its expression is regulated in a cell-type and developmental-stage manner (1, 2). Two types of recombination take place at the IgH locus. In developing B cells, variable, (diversity), and joining segments [V(D)J] assembly generates the variable region genes encoding antigen-binding sites. Upon antigen challenge, mature B cells undergo class switch recombination (CSR) in which a selected constant (C) gene is brought next to a rearranged VDJ gene, enabling isotype switch from the initially expressed IgM to one of the downstream isotypes (IgG, IgE, or IgA), diversifying antibody effector functions (3, 4).
CSR takes place between highly repetitive sequences [called switch (S) sequences] that must be transcribed for CSR to occur. Switch transcription initiates from upstream I promoters (Fig. 1A) and is induced by various stimuli, including antigens, mitogens, and cytokines. These stimuli direct which isotype (I) promoter is activated, and therefore the S sequence that will undergo recombination. Switch transcription produces long noncoding RNAs that render S sequences accessible to the Activation-Induced cytidine Deaminase enzyme, which initiates DNA breaks at the Sµ donor region and the transcribed downstream acceptor S regions (Sγ, Sε, Sα) (3, 4). CSR often occurs on both alleles, and Sµ donor regions generally recombine with the same acceptor switch regions on the two chromosomes (e.g., refs. 5 and 6).
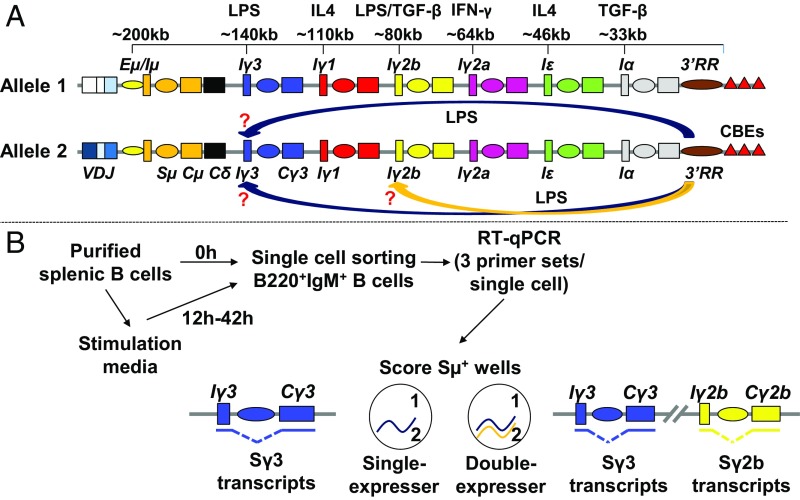
Fig. 1.
Analysis of switch transcription by single-chromosome RT-qPCR assay. (A) Scheme of a rearranged mouse IgH locus. Except for Cδ, the constant (C) genes are structurally similar and are composed of an I promoter driving transcription across the I exon, intron S sequence, and terminating downstream of the C exons. The eight C genes are represented with different colors. The inducible I promoters and switch (S) sequences, the Eµ enhancer (at the 5′ end), the 3′RR superenhancer, and the 3′ elements that bind the CCCTC-binding factor [CTCF] (CBEs) are also shown. The average distance between elements and the stimulations activating each promoter are indicated. Activation of Iγ2b and Iγ3 promoters upon LPS stimulation is shown. Single cells transcribe Sγ2b and Sγ3, and different scenarios are possible. For example, the 3′RR could activate one promoter per allele (e.g., Iγ3 on allele 1 and Iγ2b on allele 2), or one promoter on one allele and both promoters on the other allele (e.g., Iγ3 on allele 1 and both promoters on allele 2), or both promoters on the two alleles, etc. (B) Overview of the single-cell RT-qPCR assay. Purified splenic B cells were either immediately single-sorted into 96-well plates or instead cultured, for up to 42 h, under appropriate stimulation and then single-sorted. Each single cell was then subjected to RT-qPCR with three primer sets. Because the two alleles derive from mice with distinct genotypes, the percentages of single cells transcribing one (single-expressers) or two transcripts (double-expressers) from the same allele could be determined. For clarity, only transcripts originating from allele 2 are displayed.
Complex loci in higher eukaryotes often contain multiple gene promoters that are activated by distant enhancers. Despite extensive efforts, it remains unclear whether a shared enhancer coactivates two promoters or if target promoters compete for enhancer activity (e.g., refs. 7 and 8). The major element controlling switch transcription is a superenhancer called 3′ regulatory region (3′RR) located downstream of the IgH locus. The 3′RR is composed of four enhancers that synergistically activate the I promoters (9), and deletion of 3′RR severely impairs switch transcription and CSR (10).
In cultured B cells, most stimuli activate more than one I promoter and previous mutational studies on B cell populations concluded that I promoters compete for 3′RR activity (e.g., refs. 11–15). Notably, insertional studies suggested that, for example, while lipopolysaccharide (LPS) stimulation activates Iγ3 and Iγ2b promoters, IL-4 stimulation activates Iγ1 and Iε, which out-compete Iγ3 or Iγ2b promoters for 3′RR and extinguish Sγ3 and Sγ2b transcription (13). Whereas a recent single-cell study using tagged I promoters proposed that allelic I promoters are stochastically activated (16), activation of I promoters within each allele has never been addressed. It therefore remains uncertain whether I promoters that are located on the same chromosome and that respond to the same stimulus compete for 3′RR activity. Here, we have designed a single-chromosome RT-qPCR assay to examine, on a monoallelic basis, I promoter activation by the 3′RR in the endogenous context. We show that there are two modes of 3′RR long-range activity, depending on the inducing signal.
Results
Single-Chromosome RT-qPCR Analysis of Allele-Specific Transcription.
We used stimulation conditions that robustly induce two I promoters (Fig. 1 A and B). To detect allele-specific switch transcription, we purified IgM-expressing splenic B cells from different mouse lines and then immediately single-sorted them or placed them in culture in stimulation media (Fig. 1B). We were able to efficiently perform RT-qPCR with three distinct primer pairs per single cell (SI Appendix, Fig. S1 and Datasets S1–S3). Sµ transcription is constitutive (17), and therefore all cells, regardless of stimulation, transcribe it. We have chosen to examine only single cells where we could detect Sµ transcripts. Therefore, hereafter, “single-expresser cells” refers to single cells cotranscribing Sµ and another S transcript and “double-expressers” to single cells transcribing Sµ and two other S transcripts (Fig. 1B). Finally, although not terminologically rigorous, the terms “transcription” and “expression” of S regions will be used interchangeably.
The 3′RR Activates Two I Promoters on the Same Allele.
To determine whether I promoters compete for 3′RR activity or whether the 3′RR coactivates promoters on the same allele, we used a mouse line carrying one WT allele from 129Sv1 (WT) mice, and one mutant allele (ZI) in which the chicken β-globin core insulator (18) had been inserted upstream of the 3′RR (Fig. 2A). Because, in this genetic setting, the insulator completely inhibits activation of the Iγ3 and Iγ2b promoters by the 3′RR after LPS stimulation, any detected Sγ3 and/or Sγ2b transcripts originate only from the WT allele (Fig. 2A). Before or 12, 18, 36, or 42 h after LPS stimulation, splenic B cells were single-sorted and subjected to RT-qPCR, and the number of single- versus double-expressers was scored (Fig. 2 B and C and SI Appendix, Figs. S2 and S3).
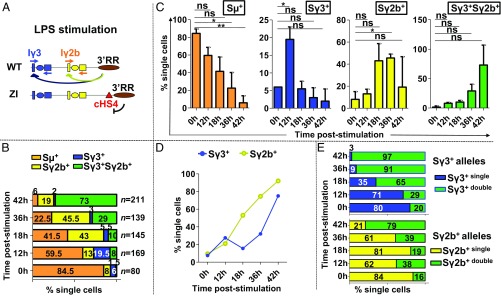
Fig. 2.
Monoallelic coactivation of Iγ3 and Iγ2b promoters upon LPS stimulation. (A) Scheme representing the two alleles of ZI/WT mice. On the ZI allele, a constitutive insulator (cHS4) has been inserted upstream of 3′RR, thus blocking activation of Iγ3 and Iγ2b promoters. Switch transcription can occur only on the WT allele. (B) Percentages of single- and double-expressers upon single-chromosome RT-qPCR. Purified B cells from n = 2–3 ZI/WT mice were grown in the absence (0 h) or presence of LPS for 12–42 h. The percentages of Sµ+ (orange), Sγ3+ (blue), Sγ2b+ (yellow), and Sγ2b+Sγ3+ (green) change with time (n indicates the number of single cells analyzed per time point). (C) Evolution of the total number of single- versus double-expressers with time after stimulation (same data as for B). (D) Evolution of the total number of Sγ3+ or Sγ2b+ alleles (single- plus double-expressers) with time after stimulation (same data as for B). (E) Graphic representation of the percentage of single Sγ3 (blue) or single Sγ2b (yellow) expressers versus double-expressers (green) contributing to the total number of Sγ3+ or Sγ2b+ alleles with time post stimulation (same data as for B).
Before stimulation (0 h), as expected, the vast majority (84.5%) of single cells transcribed Sµ exclusively, whereas 6–8% transcribed either Sγ3 (Sγ3+) or Sγ2b (Sγ2b+), and only a minority (1.5%) cotranscribed Sγ3 and Sγ2b (Sγ3+Sγ2b+) (Fig. 2B). At 12 h post LPS, the percentage of Sµ+ single-expressers started to diminish, reaching a minimum of 6% at 42 h (P = 0.0012 between 0 and 42 h) (Fig. 2 B and C). This coincided with an increasing number of alleles transcribing Sγ3 and/or Sγ2b. While the percentage of Sγ2b+ single-expressers initially increased (from 8% at 0 h to 45.5% at 36 h, P = 0.0215) (Fig. 2 B and C), only 19% of alleles transcribed Sγ2b alone at 42 h post LPS (Fig. 2B). The number of Sγ3+ single-expressers peaked at 12 h (19.5%) (P = 0.0364 between 0 and 12 h) and then started to decrease until a minimum of 2% at 42 h post stimulation (Fig. 2 B and C and SI Appendix, Figs. S2 and S3). Remarkably, with increasing time following LPS stimulation, the percentage of Sγ3+Sγ2b+ double-expressers increased from 8% at 12 h to 73% at 42 h (Fig. 2 B and C and SI Appendix, Figs. S2 and S3). Because of this, the total number of Sγ3+ and Sγ2b+ alleles (single- plus double-expressers) continuously increased with time (Fig. 2D). In fact, when we determined the contribution of single- versus double-expressers to the total percentage of Sγ3+ and Sγ2b+ alleles (Fig. 2E), we noted that almost all (91%) of the Sγ3+ alleles were Sγ3+Sγ2b+ coexpressing alleles at 36 h post stimulation already, whereas only at 42 h did the majority (79%) of Sγ2b+ alleles also transcribe Sγ3.
To exclude that the above data reflect different stabilities of Sγ3 and Sγ2b transcripts, we determined their half-lives and found that they were comparable (SI Appendix, Fig. S4; see also SI Appendix, Supplementary Results and Fig. S5).
Thus, following LPS stimulation, 3′RR-mediated coactivation of Iγ3 and Iγ2b promoters leads to a majority of B cells cotranscribing Sγ3 and Sγ2b on the same allele.
Activation of Iγ3 Promoter Does Not Preclude Activation of Iγ2b Promoter on the Same Allele.
The above data indicate that the Sγ3+Sγ2b+ double-expressers likely derive from Sγ3+ alleles initially and subsequently from Sγ2b+ alleles. This suggests that initial Iγ3 activation does not prevent subsequent Iγ2b activation. To test this, we used a mouse genetic setting that magnifies initial monoallelic activation of the Iγ3 promoter. We have recently shown that deletion of the inducible insulator 5′hs1RI in Cα leads to premature activation of the Iγ3 and Iγ2b promoters (15). In resting (nonactivated) 5′hs1RIΔ/Δ splenic B cells, Sγ3 and Sγ2b transcript levels are unusually high compared with WT counterparts, with Sγ3 transcripts displaying the biggest difference (15). We reasoned that that might reflect an initial higher number of Sγ3-expressing alleles.
To analyze monoallelic transcription, we created hemizygous mice in which one chromosome carried the 5′hs1RI deletion whereas the other chromosome was derived from ZI mice (Fig. 3A). Purified resting or LPS-activated splenic B cells were single-cell–sorted and assayed for monoallelic transcription of Sγ3 and Sγ2b, as described above. Interestingly, and in agreement with the high levels of Sγ3 transcripts detected in resting B cell populations (15), already at 0 h, over 40% of alleles expressed Sγ3, while only 6.5% transcribed Sγ2b and 4% coexpressed Sγ3 and Sγ2b (Fig. 3 B–D). Upon LPS stimulation, while the number of Sγ2b expressers tended to increase, the number of Sγ3 expressers kept decreasing to a minimum of 5% at 42 h (P = 0.005 between 0 and 42 h) (Fig. 3 B–D). Importantly, there was a dramatic increase in the number of Sγ3+Sγ2b+ alleles with time (P = 0.0006 between 0 and 42 h) (Fig. 3 B–D), and at 42 h 67% of alleles were double-expressers (Fig. 3B). Accordingly, the majority of Sγ3+ and Sγ2b+ at this time corresponded to Sγ3+Sγ2b+ alleles (Fig. 3D). The percentage of single- and double-expressers remained virtually unchanged between 36 and 42 h (P = 0.8956) (Fig. 3 B and C and SI Appendix, Figs. S3 and S6), indicating that the population had reached equilibrium at 36 h. Moreover, since the number of Sγ3+ alleles kept decreasing (Fig. 3E), while the percentage of both Sγ2b+ and Sγ3+Sγ2b+ increased (Fig. 3 B and C), the Iγ2b promoter was likely activated on alleles that had already activated the Iγ3 promoter.
Fig. 3.
Premature activation of Iγ3 does not preclude activation of Iγ2b. (A) Scheme representing the two alleles of ZI/5′hs1RIΔ mice. While in the ZI allele the 3′RR is unable to activate the Iγ3 and Iγ2b promoters after LPS stimulation, in the 5′hs1RIΔ allele, the two promoters are already active before induction due to deletion of the 5′hs1RI insulator. (B) Percentages of single- and double-expressers upon single-chromosome RT-qPCR. Purified B cells from n = 2–3 ZI/5′hs1RIΔ mice were grown in the absence (0 h) or presence of LPS for 12–42 h. The percentages of Sµ+ (orange), Sγ3+ (blue), Sγ2b+ (yellow), and Sγ2b+Sγ3+ (green) change with time (n indicates the number of single cells analyzed per time point). (C) Evolution of the total number of single- versus double-expressers with time after stimulation (same data as for B). (D) Graphic representation of the percentage of single Sγ3 (blue) or single Sγ2b (yellow) expressers versus double-expressers (green) contributing to the total number of Sγ2b+ or Sγ3+ alleles with time post stimulation (same data as for B). (E) Comparison of the percentage of single Sγ3 expressers with time post stimulation in ZI/WT and ZI/5′hs1RIΔ mice (same data as for B and Fig. 2B).
Thus, initial activation of the Iγ3 promoter did not preclude monoallelic activation of the Iγ2b promoter.
I Promoters Induced by Different Stimuli Compete for 3′RR Activity.
The data above suggest that, after LPS stimulation, Sγ3+Sγ2b+ coexpressing alleles are in the majority. However, stimuli other than LPS trigger activation of more than one promoter pair (Fig. 1A). We wondered whether 3′RR-mediated coactivation of I promoters is a general feature or if instead I promoters activated by other stimulations compete for the 3′RR. In the absence of mutations that fully repress transcription from promoter pairs, we took advantage of the existence of polymorphisms between the γ1, γ2b, ε, and α constant genes of 129Sv1 and C57BL/6J mouse strains and designed polymorphic primers that distinguish transcripts originating from one or the other allele (SI Appendix, Table S1). We obtained “heterozygous” mice (here called 129/Bl6) resulting from the crossing between 129Sv1 (129) and C57BL/6J (Bl6) WT mice (Fig. 4A) and validated the strain specificity of the polymorphic primers at the population and single-cell levels (SI Appendix, Fig. S7).
Fig. 4.
Promoters that respond to stimuli other than LPS compete for the 3′RR activity. (A) Scheme representing the two WT alleles from 129Sv1 and C57BL6J strains. Iγ1 and Iε promoters are induced by IL4. The Sγ1 and Sε transcripts originating from the two alleles can be distinguished by using polymorphic primers. (B) Percentages of single- and double-expressers upon single-chromosome RT-qPCR. Purified B cells from n = 2 129Sv1/C57BL6J mice were grown in the absence (0 h) or presence of IL4 for 42 h. The percentages of Sµ+ (orange), Sγ1 (red), Sε (green), or both Sγ1 and Sε (purple) are indicated (n indicates the number of single cells analyzed). (C) Graphic representation of the percentage of Sγ1 single-expressers (red) or Sε single-expressers (green) versus double-expressers (purple) contributing to the total number of Sγ1+ and Sε+ cells in each allele (same data as in B). (D) Iγ2b and Iα promoters are induced by TGF-β. (E) Percentages of single- and double-expressers upon single-chromosome RT-qPCR. Purified B cells from n = 3 129Sv1/C57BL6J mice were grown in the absence (0 h) or presence of TGF-β for 42 h. The percentages of Sµ+ (orange), Sγ2b+ (yellow), Sα+ (gray), or Sγ2b+Sα+ (green) are indicated (n indicates the number of single cells analyzed). (F) Graphic representation of the percentage of Sγ2b single-expressers (yellow) or Sα single-expressers (gray) versus double-expressers (green) contributing to the total number of Sγ2b+ and Sα+ cells in each allele (same data as in E). (G) Comparison of the percentage of Sγ2b double-expressers after LPS (Sγ3+Sγ2b+) or TGF-β (Sγ2b+Sα+) stimulation (same data as for B and Fig. 2B).
To determine the extent of coactivation of Iγ1 and Iε promoters on the same allele, purified splenic B cells from 129/Bl6 mice were stimulated with IL4 for 42 h, single-sorted, and assayed for allele-specific transcription of Sγ1 and Sε. Each single-cell plate was tested for one allele; that is, in “129 plates,” we detected 129-specific Sγ1 (Sγ1129) and Sε (Sε129) transcripts, while in “Bl6 plates,” we checked transcription of Bl6-specific Sγ1 (Sγ1Bl6) and Sε (SεBl6) (Fig. 4A). We found that, while half the cells transcribed Sγ1 alone (43% from the 129 allele and 55% from the Bl6 allele), a minority expressed Sε from either the 129 or the Bl6 allele (5 versus 4%, respectively) (Fig. 4B and SI Appendix, Fig. S8). The percentage of Sγ1+Sε+ cells was slightly higher for the 129 allele (11%) versus the Bl6 allele (6%) (P = 0.3292) (Fig. 4B and SI Appendix, Fig. S8). Remarkably, while most Sγ1+ cells did not transcribe Sε, the majority of cells transcribing Sε also transcribed Sγ1 (68% for the 129 allele and 65% for the Bl6 allele) (Fig. 4C). Once more, these numbers do not reflect significantly different half-lives for Sγ1 and Sε transcripts (SI Appendix, Fig. S9).
The Iγ2b promoter is unique in that it can be induced by both LPS and TGF-β stimulations (Fig. 1A). While LPS induces activation of Iγ2b and Iγ3, TGF-β leads to activation of Iγ2b and Iα. This offered us the opportunity to explore the behavior of the same promoter when induced by different stimuli and when coactivated with different promoters. We have shown that coactivation of Iγ3 and Iγ2b, after LPS treatment, is the norm (Fig. 2B), so next we tested coactivation of Iγ2b and Iα by treating 129/Bl6 splenic B cells for 42 h with TGF-β (Fig. 4D). We obtained similar results for the two alleles (Fig. 4E and SI Appendix, Fig. S8). About one-third of single cells transcribed Sγ2b from either the 129 allele (32%) or the Bl6 allele (27%), while a relatively smaller percentage expressed Sα (21% from the 129 allele and 15.5% from the Bl6 allele) (Fig. 4E). Notably, only ∼6% of single alleles cotranscribed Sγ2b and Sα from either the 129 or the Bl6 allele (Fig. 4 E and F). This contrasts with the high percentages of alleles coexpressing Sγ2b and Sγ3 (P = 0.0272 between Sγ3+Sγ2b+ and Sγ2b+Sα+) (Fig. 4G), suggesting that the Iγ2b promoter behaves differently under LPS and TGF-β stimulation. Once again, the half-lives of Sγ2b and Sα transcripts are comparable (SI Appendix, Figs. S4 and S12).
These results show that, while coactivation of the Iγ1 and Iε promoters or of the Iγ2b and Iα promoters can occur on the same allele, the majority of alleles display single promoter activation, suggesting promoter competition.
The 3′RR Can Bidirectionally Activate I Promoters on the Same Allele.
To explore whether the 3′RR displays a bidirectional activity, we used a mouse line in which the Iα promoter has been duplicated downstream of the 3′RR (2Iα mice) (15, 19). We derived a heterozygous mouse line, here called 2Iα/Bl6 mice, carrying a WT allele and a mutant 2Iα allele, of C57BL/6J and 129Sv1 origin, respectively (SI Appendix, Fig. S10A). In this genetic setting, endogenous Sα transcripts are transcribed from both alleles (SI Appendix, Fig. S10A) but can be distinguished by using polymorphic primers (SI Appendix, Fig. S7). In addition, the 2Iα allele transcribes human β-globin transcripts that can be detected with specific primers (15, 19) (SI Appendix, Fig. S10A).
We show that, at the single-cell level, transcription of the ectopic cassette does not interfere with activation of the Iα promoter in cis (129 allele) or trans (Bl6 allele). Furthermore, since transcription of β-globin and Sα are driven by identical Iα promoters, each located on one side of the 3′RR, these results suggest that the 3′RR displays bidirectional activity (SI Appendix, Supplementary Results, Supplementary Discussion, and Figs. S10–S12).
Discussion
Deciphering the mechanisms underlying long-range activation of I promoters by the 3′RR is challenging, and using cell populations or even single cells is not conclusive because switch transcription can occur on both alleles (16, 20). Here we used single-chromosome RT-qPCR assays to investigate cis-activation of I promoters by the 3′RR superenhancer in response to different inducers.
Upon LPS stimulation, the 3′RR coactivated mainly Iγ3 and Iγ2b promoters, and the Iγ2b promoter was often activated on alleles that had previously activated Iγ3. The lack of significant competition between the two promoters suggests that 3′RR activity, RNA polymerase II, and transcription factors are not limiting, that both promoters are in close proximity to the 3′RR, and that initial activation of one promoter does not exclude activation of the other. The latter point was made clear when we examined 5′hs1RIΔ alleles, which are initially almost exclusively Sγ3+ and progressively become Sγ3+Sγ2b+ double-expressers. The large number of 5′hs1RIΔ alleles transcribing Sγ3 before LPS stimulation suggests that the Iγ3 promoter already lies in close proximity to the 3′RR at this time. Indeed, chromosome conformation capture studies detected a relatively high frequency of cross-linking between the 3′RR and Iγ3 in resting B cells (21–23). These findings are consistent with a model in which the Iγ3 promoter is first activated due to its proximity to the 3′RR (15), while Iγ2b is recruited to the 3′RR-Iγ3 hub after LPS stimulation only. This indicates that 3′RR, Iγ3, and Iγ2b lie in an LPS-induced productive spatial proximity where factors such as RNA polymerase II and transcriptional/architectural factors may contribute to bridge (8) the 3′RR with Iγ3 and Iγ2b.
Transcription occurs in bursts (8, 24) and, suggesting that coactivation and bursting are not mutually exclusive processes, it has been reported that promoters sharing a distant enhancer are activated in coordinated transcriptional bursts (25). Our assay cannot determine if the Iγ3 and Iγ2b promoters are activated simultaneously in double-expressing alleles. However, nonsynchronized activation would require fast shift of the 3′RR from one promoter to the other, and given the high percentage of cotranscribing alleles and the comparable half-lives of transcripts, this is unlikely.
Following IL4 or TGF-β stimulation, the majority of alleles displayed promoter competition. Nonetheless, the patterns were different. While, similarly to previous findings (16), Sγ1+ single-expressers prevailed over Sε+ single-expressers, there were similar percentages of single Sγ2b+ and Sα+ alleles. This might be due to the intensity of the signal, the promoters’ strength and their affinity for transcription factors, the kinetics of transcription factor assembly, preferential interactions between individual 3′RR enhancers and I promoters (22, 26), or, as is likely the case for Iγ1, association with a proximal (27) and a distal enhancer (28, 29).
CSR to IgE can occur directly (Sµ/Sε) or sequentially (Sµ/Sγ1 and then Sε) (e.g., refs. 30–33). While direct Sµ/Sε switching could occur on Sε+ alleles, sequential switching could occur on Sγ1+Sε+ double-expressers. This does not exclude the possibility that Iε promoter can be activated in an IgG1-expressing B cell intermediate (having deleted Iγ1 subsequent to Sµ/Sγ1 switching), which may be relevant to IgG1-driven generation of IgE-producing cells in secondary responses (34).
Previous analyses of the well-studied β-globin locus led to conflicting results regarding promoter competition and the effect of proximity between promoter and β-globin superenhancer for promoter activation (e.g., refs. 35–37). Nevertheless, the most recent evidence shows that, in their endogenous context, two distant but developmentally synchronized promoters can be coactivated by the β-globin superenhancer and that the previously activated proximal promoters do not interfere (37). The β-globin and IgH superenhancers differ not only in terms of tissue specificity, but also in the fact that, while the former is developmentally regulated, the latter is induced, at least in activated mature B cells. Significantly, prevalence of Iγ1 over Iε and the relative balance between activation of Iγ2b and Iα occur despite the 3′RR-proximal location of the Iε and Iα promoters. Moreover, coactivation of Iγ3 and Iγ2b takes place despite initial activation of the more distant Iγ3 promoter. Additionally, Iγ1, located between Iγ3 and Iγ2b promoters, does not interfere with Iγ3 and Iγ2b coactivation not because it is activated at a distinct developmental stage, but due to the nature of the inducer. Clearly, the physical arrangement of I promoters along the locus does not play a significant role in the mode of 3′RR-mediated activation. The type of activation may instead be the most important factor regarding how the promoter is activated. The Iγ2b promoter was almost always coactivated with Iγ3, after LPS stimulation, and almost never coactivated with Iα, upon TGF-β stimulation.
We have previously proposed that the 3′RR, Iγ3, and Iγ2b proximal positioning evolved, at least in part, to enable rapid generation of IgG3 and IgG2b by marginal-zone B cells, which mainly switch to IgG3 and, to a lesser extent, IgG2b, in response to T cell-independent antigens (15). Fast coactivation of Iγ3 and Iγ2b promoters may have also evolved as a safety mechanism to cope with the presence of a nonexpressed allele in the same cell. In contrast, it takes days for germinal centers to form and CSR and selection mechanisms to proceed in T-dependent responses. Therefore, the fact that Iγ3 and Iγ2b are mainly coactivated while other promoters mostly compete for 3′RR activity may relate to the kinetics of primary immune responses against T-independent and T-dependent antigens.
Our results showing that the IgH superenhancer can activate transcription from two promoters in the same allele, regardless of the positon of the promoter, may also be relevant for the onset of cancer. The 3′RR can long-range–activate the translocated c-myc oncogene (38). The translocated c-myc can accompany CSR to downstream S regions during tumor progression (39), and the coactivating capacity of the 3′RR may facilitate this process by activating both the translocated c-myc and the I promoter of the target S sequence.
In conclusion we show that the IgH superenhancer cis-activates I promoters through two modes, coactivation and promoter competition. The nature of the inducer is critical in determining the prevalence of one mode over the other.
Materials and Methods
Mice and Ethical Guidelines.
Two to three mice of each genotype were used per experiment, and each experiment was repeated at least two times. We used mice of both genders at 5–7 wk. All experiments on mice were carried out according to the CNRS ethical guidelines and were approved by the Regional Ethical Committee (accreditation no. E31555005).
Additional materials and methods are provided in SI Appendix.
Supplementary Material
Acknowledgments
We thank the Institut de Pharmacologie et de Biologie Structurale (IPBS) animal facility, the Imaging Core Facility TRI-IPBS, in particular Emmanuel Nasser, and the Purpan CPTP cytometry platform for their excellent work. TRI-IPBS has the financial support of ITMO Cancer Aviesan (Alliance Nationale Pour les Sciences de la Vie et de la Santé, National Alliance for Life Science and Health) within the framework of Cancer Plan. This work was supported by the Institut National du Cancer (INCA_9363, PLBIO15-134), the Agence Nationale de la Recherche (ANR-16-CE12-0017), and the Fondation ARC pour la Recherche sur le Cancer (PJA 20141201647).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1902250116/-/DCSupplemental.
References
- 1.Jung D., Giallourakis C., Mostoslavsky R., Alt F. W., Mechanism and control of V(D)J recombination at the immunoglobulin heavy chain locus. Annu. Rev. Immunol. 24, 541–570 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Schatz D. G., Ji Y., Recombination centres and the orchestration of V(D)J recombination. Nat. Rev. Immunol. 11, 251–263 (2011). [DOI] [PubMed] [Google Scholar]
- 3.Stavnezer J., Guikema J. E., Schrader C. E., Mechanism and regulation of class switch recombination. Annu. Rev. Immunol. 26, 261–292 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boboila C., Alt F. W., Schwer B., Classical and alternative end-joining pathways for repair of lymphocyte-specific and general DNA double-strand breaks. Adv. Immunol. 116, 1–49 (2012). [DOI] [PubMed] [Google Scholar]
- 5.Hummel M., Berry J. K., Dunnick W., Switch region content of hybridomas: The two spleen cell Igh loci tend to rearrange to the same isotype. J. Immunol. 138, 3539–3548 (1987). [PubMed] [Google Scholar]
- 6.Winter E., Krawinkel U., Radbruch A., Directed Ig class switch recombination in activated murine B cells. EMBO J. 6, 1663–1671 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bulger M., Groudine M., Functional and mechanistic diversity of distal transcription enhancers. Cell 144, 327–339 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furlong E. E. M., Levine M., Developmental enhancers and chromosome topology. Science 361, 1341–1345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khamlichi A. A., Pinaud E., Decourt C., Chauveau C., Cogné M., The 3′ IgH regulatory region: A complex structure in a search for a function. Adv. Immunol. 75, 317–345 (2000). [DOI] [PubMed] [Google Scholar]
- 10.Vincent-Fabert C., et al. , Genomic deletion of the whole IgH 3′ regulatory region (hs3a, hs1,2, hs3b, and hs4) dramatically affects class switch recombination and Ig secretion to all isotypes. Blood 116, 1895–1898 (2010). [DOI] [PubMed] [Google Scholar]
- 11.Cogné M., et al. , A class switch control region at the 3′ end of the immunoglobulin heavy chain locus. Cell 77, 737–747 (1994). [DOI] [PubMed] [Google Scholar]
- 12.Manis J. P., et al. , Class switching in B cells lacking 3′ immunoglobulin heavy chain enhancers. J. Exp. Med. 188, 1421–1431 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seidl K. J., et al. , Position-dependent inhibition of class-switch recombination by PGK-neor cassettes inserted into the immunoglobulin heavy chain constant region locus. Proc. Natl. Acad. Sci. U.S.A. 96, 3000–3005 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oruc Z., Boumédiène A., Le Bert M., Khamlichi A. A., Replacement of Igamma3 germ-line promoter by Igamma1 inhibits class-switch recombination to IgG3. Proc. Natl. Acad. Sci. U.S.A. 104, 20484–20489 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braikia F. Z., et al. , Inducible CTCF insulator delays the IgH 3′ regulatory region-mediated activation of germline promoters and alters class switching. Proc. Natl. Acad. Sci. U.S.A. 114, 6092–6097 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu Y. L., Stubbington M. J., Daly M., Teichmann S. A., Rada C., Intrinsic transcriptional heterogeneity in B cells controls early class switching to IgE. J. Exp. Med. 214, 183–196 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li S. C., et al. , Expression of I mu-C gamma hybrid germline transcripts subsequent to immunoglobulin heavy chain class switching. Int. Immunol. 6, 491–497 (1994). [DOI] [PubMed] [Google Scholar]
- 18.Chung J. H., Bell A. C., Felsenfeld G., Characterization of the chicken beta-globin insulator. Proc. Natl. Acad. Sci. U.S.A. 94, 575–580 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos J. M., et al. , Duplication of a germline promoter downstream of the IgH 3′ regulatory region impairs class switch recombination. Sci. Rep. 8, 9164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delpy L., Le Bert M., Cogné M., Khamlichi A. A., Germ-line transcription occurs on both the functional and the non-functional alleles of immunoglobulin constant heavy chain genes. Eur. J. Immunol. 33, 2108–2113 (2003). [DOI] [PubMed] [Google Scholar]
- 21.Wuerffel R., et al. , S-S synapsis during class switch recombination is promoted by distantly located transcriptional elements and activation-induced deaminase. Immunity 27, 711–722 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sellars M., Reina-San-Martin B., Kastner P., Chan S., Ikaros controls isotype selection during immunoglobulin class switch recombination. J. Exp. Med. 206, 1073–1087 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thomas-Claudepierre A. S., et al. , Mediator facilitates transcriptional activation and dynamic long-range contacts at the IgH locus during class switch recombination. J. Exp. Med. 213, 303–312 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hnisz D., Shrinivas K., Young R. A., Chakraborty A. K., Sharp P. A., A phase separation model for transcriptional control. Cell 169, 13–23 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fukaya T., Lim B., Levine M., Enhancer control of transcriptional bursting. Cell 166, 358–368 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laurencikiene J., Deveikaite V., Severinson E., HS1,2 enhancer regulation of germline epsilon and gamma2b promoters in murine B lymphocytes: Evidence for specific promoter-enhancer interactions. J. Immunol. 167, 3257–3265 (2001). [DOI] [PubMed] [Google Scholar]
- 27.Xu M. Z., Stavnezer J., Regulation of transcription of immunoglobulin germ-line gamma 1 RNA: Analysis of the promoter/enhancer. EMBO J. 11, 145–155 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Medvedovic J., et al. , Flexible long-range loops in the VH gene region of the Igh locus facilitate the generation of a diverse antibody repertoire. Immunity 39, 229–244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Predeus A. V., et al. , Targeted chromatin profiling reveals novel enhancers in Ig H and Ig L chain loci. J. Immunol. 192, 1064–1070 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yoshida K., et al. , Immunoglobulin switch circular DNA in the mouse infected with Nippostrongylus brasiliensis: Evidence for successive class switching from mu to epsilon via gamma 1. Proc. Natl. Acad. Sci. U.S.A. 87, 7829–7833 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mandler R., Finkelman F. D., Levine A. D., Snapper C. M., IL-4 induction of IgE class switching by lipopolysaccharide-activated murine B cells occurs predominantly through sequential switching. J. Immunol. 150, 407–418 (1993). [PubMed] [Google Scholar]
- 32.Zhang T., et al. , Downstream class switching leads to IgE antibody production by B lymphocytes lacking IgM switch regions. Proc. Natl. Acad. Sci. U.S.A. 107, 3040–3045 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wesemann D. R., et al. , Immature B cells preferentially switch to IgE with increased direct Sμ to Sε recombination. J. Exp. Med. 208, 2733–2746 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He J. S., et al. , IgG1 memory B cells keep the memory of IgE responses. Nat. Commun. 8, 641 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu X., et al. , Promoters of the murine embryonic beta-like globin genes Ey and betah1 do not compete for interaction with the beta-globin locus control region. Proc. Natl. Acad. Sci. U.S.A. 100, 1111–1115 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deng W., et al. , Reactivation of developmentally silenced globin genes by forced chromatin looping. Cell 158, 849–860 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Allahyar A., et al. , Enhancer hubs and loop collisions identified from single-allele topologies. Nat. Genet. 50, 1151–1160 (2018). [DOI] [PubMed] [Google Scholar]
- 38.Gostissa M., et al. , Long-range oncogenic activation of Igh-c-myc translocations by the Igh 3′ regulatory region. Nature 462, 803–807 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Janz S., Myc translocations in B cell and plasma cell neoplasms. DNA Repair (Amst.) 5, 1213–1224 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.