Abstract
Alum adjuvanted formalin-inactivated respiratory syncytial virus (RSV) vaccination resulted in enhanced respiratory disease in young children upon natural infection. Here, we investigated the adjuvant effects of monophosphoryl lipid A (MPL) and oligodeoxynucleotide CpG (CpG) on vaccine-enhanced respiratory disease after fusion (F) protein prime vaccination and RSV challenge in infant and adult mouse models. Combination CpG+MPL adjuvant in RSV F protein single dose priming of infant and adult age mice was found to promote the induction of IgG2a isotype antibodies and neutralizing activity, and lung viral clearance after challenge. CpG+MPL adjuvanted F protein (Fp) priming of infant and adult age mice was effective in avoiding lung histopathology, in reducing interleukin-4+ CD4 T cells and cellular infiltration of monocytes and neutrophils after RSV challenge. This study suggests that combination CpG and MPL adjuvant in RSV subunit vaccination might contribute to priming protective immune responses and preventing inflammatory RSV disease after infection.
Keywords: RSV, enhanced disease, safety, RSV F protein, MPL, CpG, adjuvant
Introduction
Respiratory syncytial virus (RSV) is a negative sense strand RNA virus belonging to the Pneumoviridae family. RSV is the leading cause of approximately 3.5 million hospital admissions in the extreme age populations, particularly in infants and children below 5 years old, resulting in 66,000 to 199,000 deaths, in low- and middle-income countries (Nair et al., 2010). Also, approximately 14,000 to 60,000 hospitalizations happen in elderly populations and 10,000 deaths are estimated due to RSV disease in the US annually (Branche and Falsey, 2015; Falsey et al., 2005). Despite the extensive effort for over 50 years, no effective vaccine against RSV is licensed yet. Enhanced RSV disease was observed in infants and young children after vaccination with a formalin- inactivated alum-adjuvanted whole virus (FI-RSV) vaccine in the 1960s upon natural infection (Kim et al., 1969). FI-RSV vaccine-enhanced pulmonary histopathology has been reported in various animal models including mice (Connors et al., 1992), cotton rats (Prince et al., 1986), cattle (Gershwin et al., 1998), and African green monkeys (Kakuk et al., 1993). Other platforms of RSV vaccines have been also known to cause enhanced disease after RSV challenge. Mice that were vaccinated with recombinant vaccinia virus expressing RSV attachment G or fusion (F) proteins (rVV-F, to a lesser degree) developed lung disease after RSV challenge (Openshaw et al., 1992).
Palivizumab, a monoclonal antibody (mAb) against F proteins has been licensed as a prophylactic drug to prevent severe RSV disease in high-risk infants (Simoes et al., 2007). RSV F protein vaccines are under clinical investigation, targeting to older populations and high-risk children or maternal immunization. Alum adjuvanted purified F protein vaccines based on F in the post-fusion conformation (post-F) were tested in early clinical phase I and II trials of different age groups including healthy adults, children over 12 months of age, older persons, and pregnant women (Munoz et al., 2003). A phase II trial of alum-adjuvanted post F protein vaccines in seropositive children showed a modest increase in neutralizing titers but no reduction in the incidence of RSV infections (Esposito and Pietro, 2016). A subunit vaccine using F protein displayed in rosettes has been advanced to Phase III trials in pregnant women (Esposito and Pietro, 2016; Neuzil, 2016). Vaccine candidates utilizing F stabilized in its pre-fusion conformation (pre-F) are under Phase I and II clinical studies (Esposito and Pietro, 2016; Neuzil, 2016).
Although RSV F is being developed as an important subunit vaccine candidate, protein-based immunogens in antigen-naïve hosts can promote T helper type 2 (Th2) biased immune response. Subunit F protein-based vaccines have been reported to cause enhanced lung histopathology in antigen-naïve animal models (Murphy et al., 1990; Palomo et al., 2016; Schneider-Ohrum et al., 2017). Therefore, adjuvants that modulate immune responses to avoid enhanced RSV disease after vaccination and RSV challenge would be highly significant for advancing safe RSV vaccination in antigen-naïve infants.
Synthetic oligodeoxynucleotides containing unmethylated cytosine-phosphate-guanosine (CpG), a Toll-like receptor (TLR)-9 agonist, are known to activate Th1 immune responses to RSV F or killed RSV vaccination with high dose (10 – 100 μg) of CpG (Garlapati et al., 2012; Hancock et al., 2001; Oumouna et al., 2005) but no details on safety aspects on lung inflammation and RSV disease after RSV challenge were reported. A high dose of CpG (20 – 100 μg) included in the intranasal vaccination of cotton rats with RSV F protein was reported to have safety concern on promoting pulmonary pathology after RSV challenge (Prince et al., 2003). Monophosphoryl lipid A (MPL) is a TLR4 agonist and included in human vaccines (Rappuoli et al., 2011). RSV F protein vaccination adjuvanted with ranges of high dose MPL (10 – 50 μg/rat) protected cotton rats against RSV and lung pathology (Blanco et al., 2014). A high dose MPL was shown to attenuate FI-RSV-induced histopathology and proinflammatory cytokines (Boukhvalova et al., 2006; Prince et al., 2001). A post-fusion F protein vaccine formulated with an MPL analog of TLR4 agonist in stable emulsion was tested under phase I and planning for phase II in older adults (Esposito and Pietro, 2016). TLR4 agonist MPL in stable emulsion was reported to result in the different outcomes of protection and safety concerns after RSV vaccination and challenge, depending on the animal models (Lambert et al., 2015; Schneider-Ohrum et al., 2017).
In previous studies on adjuvanted influenza vaccination, we found that combination adjuvant at low doses of MPL (0.5 – 1 μg) +CpG (2 – 4 μg) was effective in promoting homologous and heterologous protective efficacy of influenza vaccines (Ko et al., 2018; Ko et al., 2017). Based on previous studies, we wanted to determine whether a low dose of combined MPL+CpG adjuvant would be effective in enhancing protective efficacy of RSV F protein-based subunit vaccine while preventing pulmonary histopathology after challenge in a mouse model. Herein, we investigated the adjuvant effects of CpG, MPL, and combined CpG and MPL on promoting RSV vaccine efficacy and inflammatory histopathology after priming with F protein and RSV challenge in infant (2 weeks old) and adult mouse models in comparison with alum adjuvant. Combined CpG+MPL adjuvanted F protein priming of infant and adult age mice was found to be highly effective in avoiding lung histopathology as well as in enhancing the efficacy of RSV F protein vaccination.
Material and Methods
Cells, Virus and Vaccine formulation with adjuvants.
The RSV A2 was provided from Dr. Martin Moore (Emory University, GA) and propagated in HEp 2 Cells (American Type Culture Collection). The virus stocks were tittered as the plaque-forming units (PFU) on the Hep2 cells by immunostaining with an horse radish peroxidase (HRP)-labeled F-monoclonal antibody as described (Lee et al., 2017). The HEp 2 Cells were grown in DMEM supplemented with 5% fetal bovine serum and penicillin/streptomycin antibiotics. The RSV A2 F glycoprotein was obtained from Biodefense and Emerging Infections Research Resources (NR-28908, BEI Resources, Manassas, VA). In brief, this recombinant form of soluble F glycoprotein with C-terminal histidine tag was expressed by transfection of 293F cells with a plasmid encoding a codon-optimized F gene engineered to produce a stable post fusion conformation of the F trimer and purified from the cell culture supernatant using nickel chromatography. Antigenic analysis indicates that the F protein used in this study is in a form of post-fusion conformation (Supplementary Fig. S1). Adjuvant Alum (Sigma Aldrich, St. Louis, MO), MPL (Sigma Aldrich, St. Louis, MO), or CpG (oligodeoxynucleotide 1826, 5’-TCC ATG ACG TTC CTG ACG TT-3’, DNA Technologies) was mixed with 0.1 μg F protein vaccine to vaccinate the mice.
Animals, Immunization and RSV challenge.
BALB/c mice (n=5, Charles River Laboratories, Inc.) were intramuscularly (i.m.) primed (a single dose) in the thigh muscle of the leg with 0.1 μg of F protein only or in the presence of adjuvant (50 μg Alum, 1μg MPL, 4 μg CpG and, 1μg MPL+ 4 μg CpG), or phosphate buffered saline (PBS, naïve control). The infant mice were 2 weeks old and adult mice were 6 to 8weeks old at the time of priming vaccination. For immunogenicity study, blood samples were collected from individual mice at 3 weeks after vaccination. Mice were intranasally (i.n.) infected with 3.5 × 105 PFU/ml of RSV A2 under isoflurane anesthesia to determine the efficacy of protection and histopathology at 4 weeks after vaccination. Individual lung, bronchiolar alveolar lavage fluids (BALF), mediastinal lymph nodes (MLN), and spleens were collected at 5 days after challenge. All animal experimental procedures were approved and performed by following the guidelines of Georgia State University Institutional Animal Care and Use Committee.
ELISA of IgG antibodies and cytokines
Blood samples collected at 3 weeks after prime were used to determine RSV F specific IgG antibodies (IgG, IgG1 and IgG2a) by enzyme-linked immunosorbent assay (ELISA). Post-fusion F protein (NR- 28908, BEI resources) or pre-fusion conformation stabilized protein with DsCav mutations (McLellan et al., 2013) was coated on 96-well plates as an ELISA antigen. The pre-fusion F protein with DS-Cav1 mutations was kindly provided by Vaccine Research Center (NIAID, NIH). Pre-fusion F conformation-stabilizing mutations (DS-Cav1) contain additional disulfide bonds (DC) residues [S155C, S290C] and amino acid changes with cavity filling residues [S190F, V207L], and the trimer-stabilizing foldon domain at the c-terminus of pre-fusion DS-Cav1 F protein, which stabilizes the pre-fusion conformation, (McLellan et al., 2013). Secondary antibodies conjugated with horseradish peroxidase (Southern Biotechnology) were used to detect IgG isotype antibodies. The substrate TMB (3, 3′, 5, 5′-tetramethylbenzidine, Sigma Aldrich) was treated and stopped with 1M H3PO4. Optical densities (O.D) were read by spectrophotometer reader at 450nm. The lung homogenates and bronchoalveolar lavage fluid (BALF) were used to quantify cytokine concentrations by using cytokine ELISA kits of interleukin (IL)-4, IL-5, interferon (IFN)-γ, IL-13 (eBioscience, SanDiego, CA).
Lung viral titers and Neutralizing-antibody assays.
Serum samples were collected 3 weeks after prime immunization and heat-inactivated at 56°C for 45 min. In 48-well plates. test sera (starting dilution 1:4) were serially diluted by 2-fold increments in cell culture media for a final volume of 50 μL. Each serum serial dilution was mixed with 50 μL RSV A2 (200 PFU per well), incubated for 2 h, and added to the monolayer cultures of HEp-2 cells (2.5 × 104 cells in each well). Cells plus virus and cells only on the wells without sera served as controls. After 3 days of incubation at 37°C with 5% CO2, the cell culture medium was removed, and the monolayer was fixed with chilled 4% neutral paraformaldehyde. Plaque formation units indicating RSV replication were visualized and quantitated by immunostaining with an HRP-labeled F-monoclonal antibody. The reciprocal dilutions in log2, inhibiting 50% plaque forming units of virus (IC50) was determined for each serum sample (Supplementary Fig S2). The lower limit of background titer in this assay is 2 log2. Individual lung samples were collected day 5 post challenge and RSV lung viral titers determined. RSV plaque-forming units (PFU) were determined using an immuno-plaque assay as previously described (Lee et al., 2017). RSV control and lung homogenates mixed with RSV were incubated on HEp-2 cells for 1 h 37°C, 5% CO2. After 4% formalin fixation, the plaques were detected with anti RSV F monoclonal antibody (mAb) (Millipore).
Flow cytometry
BALF were collected by flushing the lungs with 1 ml of PBS supplemented with protease inhibitors using a winged shielded catheter (1.3630 mm, BD Utah) inserted, through an incision, in the trachea of euthanized mice. Lung cells were harvested day 5 post challenge and isolated by homogenization as previously described (Lee et al., 2017). For intracellular cytokine staining analysis of T cell responses, lung and BALF cells were stimulated with F51–66 CD4 T cell epitope of RSV F (5μg/ml), and then the cells were fixed and permeabilized according to the manufacturer’s instructions (BD Biosciences) as described in our previous studies (Ko et al., 2015; Lee et al., 2017). Intracellular cytokine and surface makers for T cells were stained with antibodies for IFN-γ-APC/Cy7, IL-4-FITC, CD4-APC, CD8-PE (eBioscience/BD Biosciences). Infiltrating innate immune cells were detected with antibodies for CD11b-APC, F4/80-FITC, Ly6c-A700 or Siglec F-PE (BD Biosciences). Cellular phenotypes were collected with the Becton-Dickinson LSR-II/Fortessa flow cytometer (BD, San Diego, CA). Flow cytometry data acquired were analyzed by using Flowjo software (Tree Star Inc.).
Lung histology and inflammation scoring
Left lung tissues were harvested on day 5 post challenge and fixed with 10% neutral buffered formalin. They were embedded in paraffin in the dorsoventral position. Subsequently, sections of tissue blocks were obtained and stained with hematoxylin and eosin (H & E), periodic acid–Schiff (PAS), and hematoxylin- congo red (H&CR). Histopathology of lung tissue slides were analyzed using a semiquantitative scale (0 to 3) (0 = absent and 3 = maximum/severe) by light microscope as previously described (Hwang et al., 2014; Hwang et al., 2016; Zhang et al., 2017). Assigned score 0 was the surrounding space which is free or has few infiltrating cells, score 1 contains focal aggregates of infiltrating cells or the structure is cuffed by one definite layer of infiltrating cells, score 2 is cuffed by two defined layers of infiltrating cells, and score 3 when structure is cuffed by three or more definite layers of infiltrating cells with or without focal aggregates. PAS was used to identify mucus-producing goblet cell metaplasia, which was indicated by magenta staining as described (Miller et al., 2003; Stokes et al., 2013). Randomly 10 to 12 airways were selected to quantify for PAS positive stain with mucin expression area as automatically calculated in the selected airways by using the Adobe Photoshop CS5.1 software (Hwang et al., 2014; Hwang et al., 2016; Lee et al., 2017). H&E stained slides were evaluated for inflammatory infiltration and histopathology around peribronchial and/or peribronchiolar, perivascular, and interstitial regions. The presence of pulmonary eosinophilia was quantified by H&CR stain with 400× magnified microscope and flow cytometry by using antibodies for eosinophil phenotypic markers (CD11b+, CD11c+, SiglecF+) as previously described (Hwang et al., 2014; Hwang et al., 2016; Jia et al., 2017).
Statistical Analysis.
Statistical differences were performed using GraphPad prim version 5 (Graph Pad software Inc, San Diego, CA). Data were analyzed for significance using one-way ANOVA with Tukey’s test for multiple comparisons or two-way ANOVA with Bonferroni posttests. The difference was considered statistically significant when the P value was less than 0.05.
Result
Combination CpG+MPL adjuvant in RSV F protein prime vaccination mediates the effective induction of IgG2a antibody and neutralizing activity in infant and adult mice.
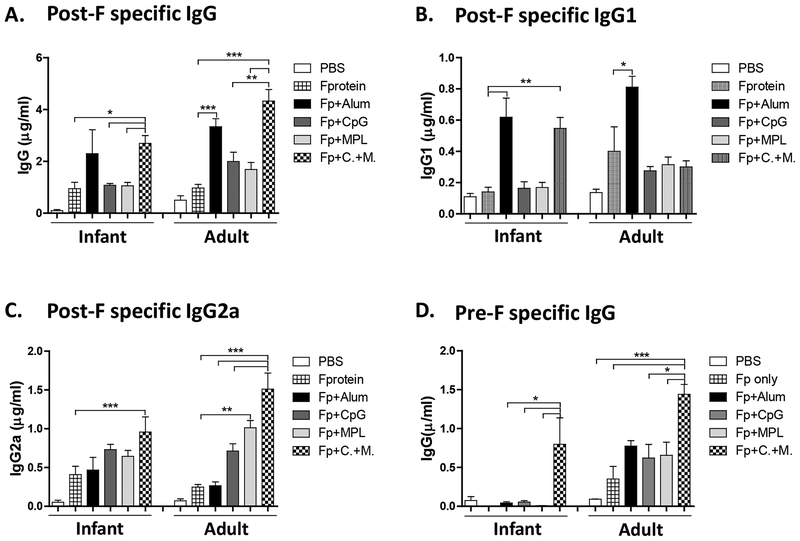
Purified RSV A2 F glycoproteins used in this study were found to be highly reactive to 131–2a monoclonal antibody (mAb) specific for post-fusion F conformation and Palivizumab recognizing site II epitope, but poorly reactive to the pre-fusion specific 5C4 mAb (Supplementary Fig. S1), suggesting that F proteins are mostly in a post-fusion conformation. We reasoned that F proteins would serve as a model antigen to test the adjuvant effects on the efficacy and lung inflammatory disease after subunit F vaccination and RSV challenge. RSV F protein vaccine doses of 0.1 and 0.3 μg were tested and found to have similar immunogenicity between the 0.1 and 0.3 μg groups after prime immunization of mice (Lee et al., 2017). Also, a low dose range (0.03 – 0.3 μg) post-fusion F protein vaccination was shown to be immunogenic in the presence of adjuvants in cotton rats (Schneider-Ohrum et al., 2017). Based on these previous studies, we rationed that RSV F protein vaccine dose of 0.1 μg would be an optimal dose to test the adjuvant effects. Infant (2weeks old) and adult (5~6 weeks old) mice were intramuscularly prime immunized with RSV F protein (Fp, 0.1μg) or in the presence of alum (50 μg), CpG (4μg), MPL (1μg) or combination CpG (4μg) + MPL (1μg) adjuvant (Fig. 1). Substantial levels of RSV F specific antibodies were induced after vaccination with F proteins in the presence of adjuvants from the mice that were primed at 2-week age although higher levels of RSV F specific antibodies were observed in the mice primed at adult age (Fig. 1A). Combination CpG+MPL adjuvant in F protein vaccination was more effective in increasing post-F specific IgG antibodies than the MPL or CpG alone adjuvanted F protein vaccine group, and similar to those in the Alum adjuvant group (Fig. 1A). The induction of IgG2a and IgG1 immunoglobulin isotypes as markers for Th1 and Th2 lymphocytes respectively has been previously reported in mice, implicating different T helper cell types induced by vaccination (Feltquate et al., 1997; Maloy et al., 1995; Mountford et al., 1994). The ratios of IgG2a/IgG1 indicate a profile of Th1 and Th2 immune responses. IgG1 isotype antibodies were highly induced in the alum adjuvanted group whereas higher levels of IgG2a were induced in the CpG, MPL, and CpG+MPL groups (Fig. 1B, 1C, Supplementary Table S1). Adult mice induced higher levels of IgG2a isotype antibodies than those in infant age mice in response to CpG, MPL, and CpG+MPL adjuvanted F protein vaccination (Fig. 1C). In particular, the CpG+MPL adjuvanted F protein group induced the highest levels of IgG2a isotype antibodies in primed infant and adult mice (Fig. 1C, Supplementary Table S1).
Figure 1. F protein prime vaccination of infant and adult age mice with Alum, CpG, MPL or CpG+MPL adjuvants elicits different IgG isotype antibodies.
Infant (2 weeks old) or adult (5~6weeks old) BALB/c mice (n=5) were intramuscularly primed with F protein or F protein with Alum, CpG, MPL or CpG+MPL. Immune sera were collected 3 weeks after prime. The levels of IgG isotypes were determined using a post-fusion F (A, B, C) or pre-fusion Ds-Cav1 F protein (D). PBS: unimmunized mice, F protein: 0.1 μg of F protein, Fp+Alum: F protein 0.1 μg with alum 50 μg, Fp+CpG: F protein 0.1 μg with CpG 4 μg, Fp+MPL 1 μg: F protein 0.1 μg with MPL, Fp+C.+M.: F protein 0.1 μg with CpG 4 μg and MPL 1 μg combination adjuvants. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; *** p<0.001, ** p<0.01, * p<0.05 comparing F to F + adjuvant in mice.
Next, we determined whether adjuvants would promote the induction of IgG antibodies recognizing pre-fusion conformation-stabilized F protein with Ds-Cav1 mutations (McLellan et al., 2013). It is notable that CpG+MPL adjuvanted F protein vaccination induced IgG antibodies specific for Ds-Cav1 pre-F antigen at higher levels than alum, CpG, or MPL adjuvanted F protein vaccine groups particularly after priming of mice at an infant age (Fig. 1D).
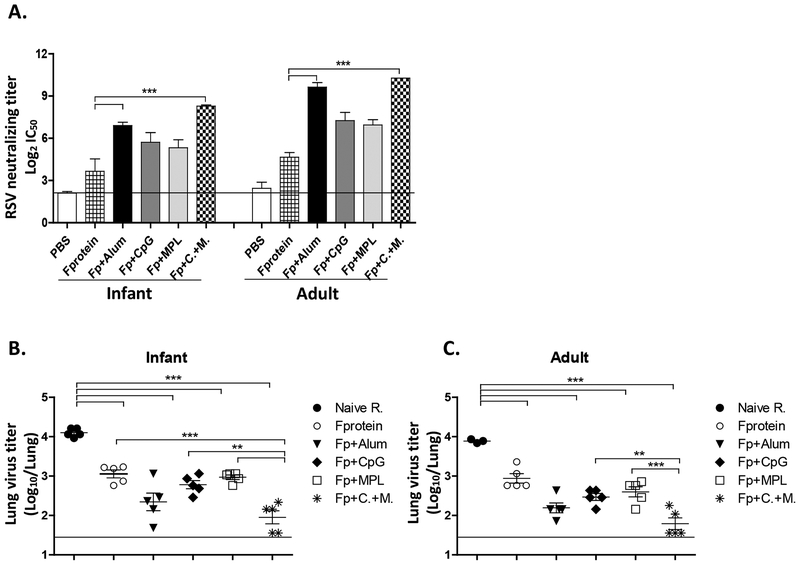
Adult mice showed approximately 2-fold higher levels of RSV neutralizing antibody titers than those in infant mice after prime immunization with adjuvanted F proteins (Fig. 2A, Supplementary Fig. S2). The CpG+MPL adjuvanted F protein group showed highest RSV neutralizing antibody titers among the adjuvanted F protein vaccination groups in infant mice. RSV neutralizing antibody titers were similarly high in both alum and CpG+MPL adjuvanted F protein immunized adult mice (Fig. 2A). These data indicate that combination CpG+MPL adjuvant in RSV F protein single vaccination effectively induces IgG2a antibodies and neutralizing activity in infant and adult mice.
Figure 2. RSV neutralizing titers in primed mice and lung viral clearance after challenge.
(A) Neutralization titers at 3 weeks after prime vaccination of 2 weeks (2wks) old and adult mice. A line indicates a background titer. (B-C) Lung RSV titers after challenge. naïve R. (unimmunized mice) and immunized mice were challenged with 3.5×105 PFU RSV A2. BALB/c mice (n=5) were immunized with F protein or F protein with alum, CpG, MPL or CpG+MPL. Individual lungs were collected at 5 days after RSV challenge and RSV titers were determined by an immunoplaque assay. The linear line represents the lower limit of detection (LLOD). PBS: unimmunized mice, Naïve R: unimmunized+RSV, F protein: 0.1 μg of F protein+RSV, Fp+Alum: F protein 0.1 μg with alum 50 μg+RSV, Fp+CpG: F protein 0.1 μg with CpG 4 μg+RSV, Fp+MPL 1 μg: F protein 0.1 μg with MPL+RSV, Fp+C.+M.: F protein 0.1 μg with CpG 4 μg and MPL 1 μg combination adjuvants+RSV. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; *** p<0.0001.
Combination CpG+MPL adjuvant improves lung viral clearance and protection of F protein vaccine.
Protective efficacy of adjuvanted F proteins at 3 weeks after single vaccination was assessed by determining lung virus titers after RSV challenge. Both young age (5 weeks old) and adult (9 to 11 weeks old) naïve mice showed high lung viral titers at day 5 after RSV infection (104 PFU/g lung tissues, Fig. 02B, C). In the F protein primed groups at an infant age, the CpG+MPL group was most effective in clearing lung viral loads by 100 folds, whereas the alum, CpG, and MPL groups showed 10 – 20 folds lower RSV titers compared to the equivalent ae naïve mice (Fig. 2B). In the adult age F protein primed groups, the CpG+MPL group was most effective in clearing lung viral loads by 100 folds, followed by the alum, CpG, and MPL groups (Fig. 2C). Adjuvants F protein primed mice at adult age were more effective in clearing RSV lung viral titers than adjuvanted F protein primed mice at an infant age (Fig. 2B, C), which is consistent with IgG antibody levels specific for post-F protein (Fig. 1). Overall, combination CpG+MPL adjuvant was more effective than other adjuvants in improving the protective efficacy after a single dose F protein vaccination in 2 weeks old or adult mice.
CpG+MPL adjuvant suppresses lung histopathology after F protein vaccination and RSV challenge.
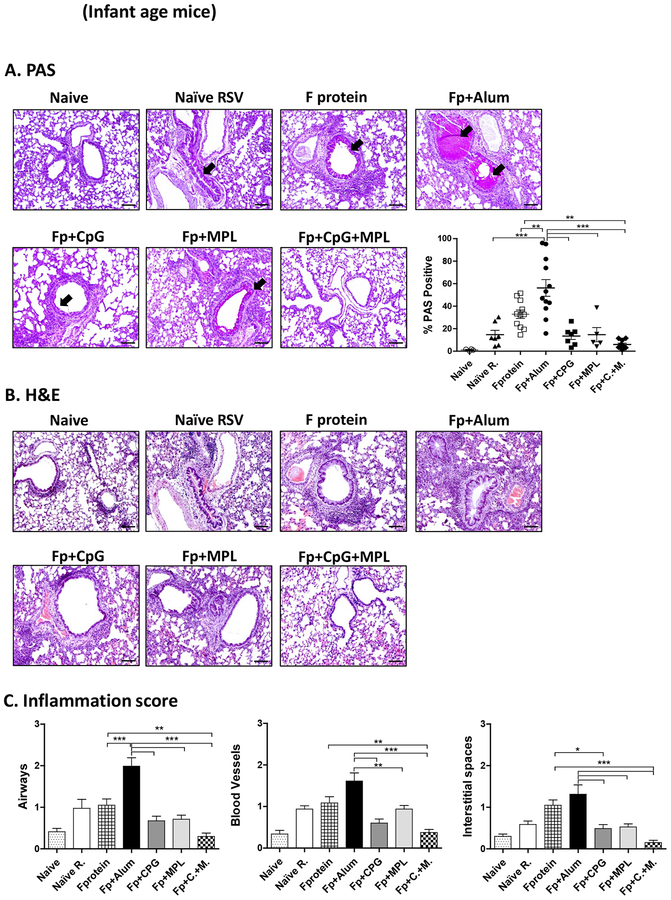
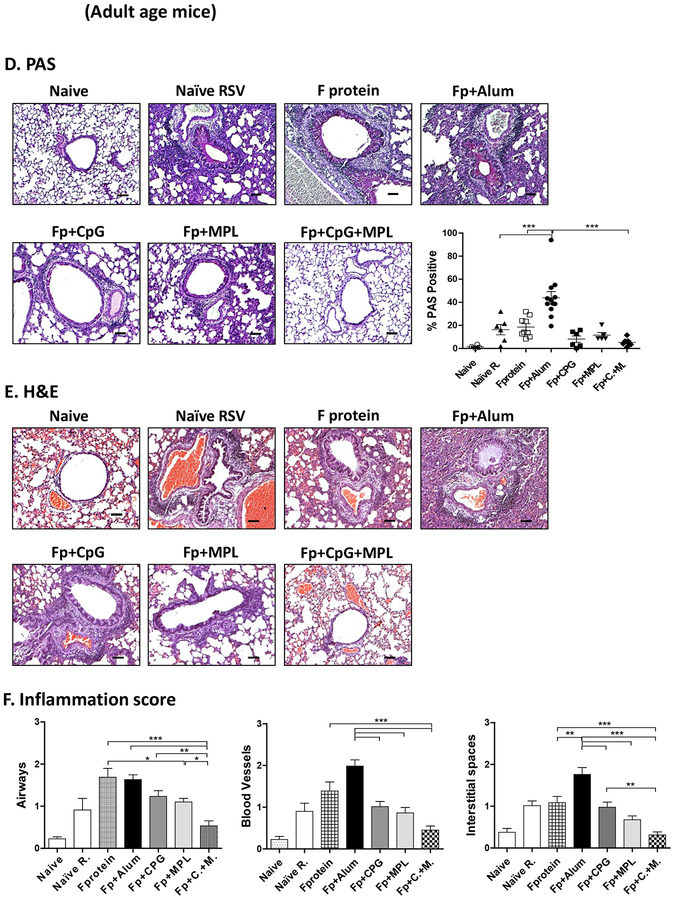
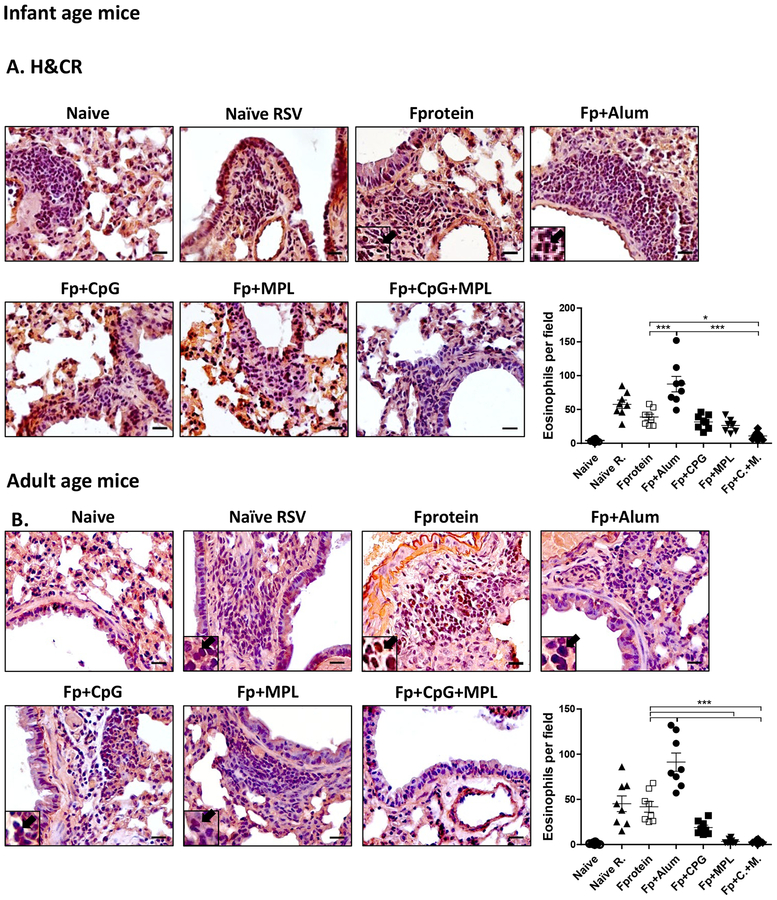
After RSV challenge week 3 post vaccination, alum adjuvanted F protein vaccination of mice at infant ages resulted in high PAS positive mucus production in the airway epithelium and inflammation scores in the lung histology when examined day 5 post RSV challenge (Fig. 3A). Also, age matching infected naïve mice displayed significant histopathology of inflammation in the airways (scores 1.5), blood vessels (1.3) and interstitial spaces (1.0) compared to those from uninfected mice (Fig. 3B, C). We found lower levels of inflammation in the lung histology from CpG or MPL adjuvanted F protein immunized mice after RSV challenge compared to those in F protein alone or alum adjuvanted F protein vaccine mice (Fig. 3B, C). Similar to infant age primed mice, the Alum adjuvanted F protein primed adult group displayed the highest level of PAS+ mucus production, followed by F protein and naïve mice (Fig. 3D). Infant age mice (Fig. 3A, B, C) with CpG adjuvanted F protein prime were found to be more effective in suppressing inflammation in the lung histology (airways, blood vessels, interstitial spaces) than adult age mice with CpG adjuvanted F protein prime (Fig. 3D, E, F). Interestingly, MPL appeared to be more effective in suppressing lung inflammatory for adult mice (Fig. 3D, E, F) with F protein vaccination than CpG although there is no statistical difference between the 2 groups. Most notably, in both infant and adult (Fig. 3) age mice with CpG+MPL adjuvanted F protein prime vaccination, lung inflammatory histopathology and PAS positive mucus production in the airway epithelium were not observed after RSV challenge. We carried out H&CR staining of lung histology as a measure of eosinophilic infiltration (Fig. 4) and flow cytometry of SiglecF+ phenotypic eosinophils in lung cells (Supplementary Fig. S3) at 5 days after RSV challenge. Both CpG+MPL adjuvanted RSV F primed infant (Fig. 4A, B) and adult (Fig. 4E, F) age mouse groups did not show eosinophil infiltrations after RSV challenge, which is similar to naïve mice without RSV infection. In contrast, alum adjuvanted F protein vaccination exhibited high levels of eosinophilic H&CR positive mucus staining cell spots, followed by naïve mice with RSV infection (Fig. 4A, 4B). The MPL adjuvanted F protein primed adult mice showed a low to moderate level of eosinophilic staining and infiltration, compared to those in the CpG adjuvanted F protein primed mice.
Figure 3. Lung histopathology and mucus production in primed 2-week old and adult mice after RSV challenge.
Lung tissue histopathology of the mice primed at 2 weeks (2wks) old (A-C) or at 5–6 weeks old adult age (D-F) at 5 days after RSV challenge. (A, D) PAS-stained lung tissues from individual mice (n=5) were dissected to assess peribronchiolar, alveolar pneumonia, and mucus production. Photographs of PAS. Scale bars indicate 100μm. The percentage of PAS stained lung section area that were PAS positive for each airway was determined; individual airways are shown per group. (B, C, E, F) Hematoxyline and Eosin (H&E) stained lung tissue were dissected to assess peribronchiolar, alveolar pneumonia. Photographs of H&E. Scale bars indicate 100 μm. (C, F) The inflammation scores of histopathology were blindly scored using a 1–3 scoring system where 1= minimal pathology and 3 = maximum pathology for airway, interstitial spaces, blood vessels. Naïve R: Unvaccinated naïve mice with RSV infection. The group labels are the same as in the Figure 2 legend except for RSV challenge. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; *** p<0.0001, ** p<0.005, * p<0.05.
Figure 4. Eosinophilic infiltration into the lungs of adjuvanted F protein primed infant and adult mice after RSV challenge.
The eosinophil infiltration in hematoxyline and congo red (H&CR) stained sections of individual lung tissue of the mice (n=5) primed at 2 weeks old (A) or at 5–6 weeks old adult age (E to C since flow data to Supplementary Fig S3?) at 5 days after RSV challenge. Scale bars indicate 400 μm. (B, F to D?) The infiltrated eosinophils per field were quantified in the airways. (A, B) Arrows: eosinophil granulocytes. Individual airways shown per group. The group labels are the same as in the Figure 2 legend. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA in GraphPad Prism; *** p<0.0001, ** p<0.005, * p<0.05.
To quantitate eosinophil infiltration, BALF and lung cells from immunized mice were collected day 5 post challenge and Siglec F+ eosinophils were determined by flow cytometry staining (Supplementary Fig. S3). Consistent with the results from H&CR staining of lung histology, MPL or CpG +MPL adjuvanted RSV F immunized group showed the lowest level or no eosinophils (Supplementary Fig. S3). The F protein only or CpG adjuvanted F protein primed adult mice showed a moderate level of eosinophils (Supplementary Fig. S3). The mice primed with F protein at 2-week old showed lower infiltrated eosinophils in the BALF than the mice primed at an adult age, whereas lung eosinophils were approximately 3-fold higher in 2-week old primed mice with Alum adjuvanted F protein than those in adult age primed mice, after RSV challenge, as determined by flow cytometry (Supplementary Fig. S3). Taken together, these results suggest that CpG+MPL adjuvanted F protein vaccination suppresses inflammatory mucus production, lung histopathology, and eosinophil infiltration after RSV challenge in infant and adult age mouse models.
CpG+MPL adjuvanted F protein priming of infant and adult mice effectively prevents the induction of IL-4+ CD4 T cells after RSV challenge.
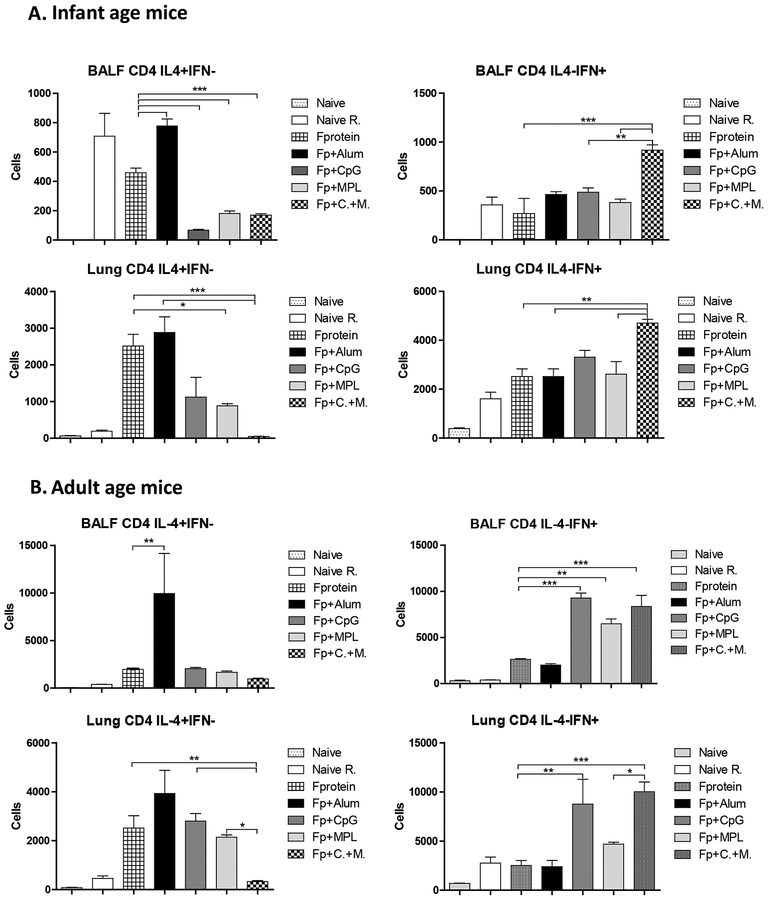
To determine whether adjuvants such as alum, CpG and MPL would modulate IFN-ɤ or IL-4 producing T cell responses, BALF and lung cells at 5 days after challenge week 4 post prime vaccination were analyzed by intracellular cytokine staining flow cytometry after stimulation with a known CD4 T cell epitope F51–66 (Fig. 5). Both groups of infant and adult age mice primed with alum adjuvanted F protein showed the highest levels of CD4 T cells secreting IL-4 cytokines in BALF and lung samples (Fig. 5A, B). CpG, MPL and CpG+MPL adjuvanted F protein primed mice induced relatively low levels of BALF IL-4+ CD4 T cells but CpG or MPL adjuvanted F protein groups displayed 3- to 4-fold higher levels of lung IL-4+ CD4 T cells respectively than those in CpG+MPL adjuvanted F protein group (Fig. 5A, B). The low cellularity of IL-4+ CD4 T cells was observed in BALF and lung samples from infant age-primed and adult age-primed mice with combination CpG+MPL adjuvanted F proteins (Fig. 5A, B). The CD4 T cell numbers secreting cytokines were higher in primed adult mice than 2-week old infant age primed mice as presented in different Y axis scales (Fig. 5). The mice that were primed with CpG+MPL adjuvanted F protein at 2 weeks old responded to induce BALF and lung IFN-ɤ+ CD4 T cells at 1.5- to 2-fold higher levels than Naïve (PBS), F protein, alum, CpG, or MPL adjuvanted F protein groups respectively after RSV infection (Fig. 5A). The groups of CpG, MPL and CpG+MPL but not alum adjuvanted F protein primed mice at an adult age induced IFN-ɤ+ CD4 T cells after challenge (Fig. 5B). These results suggest that CpG+MPL adjuvanted F protein prime vaccination effectively prevents the induction of IL-4+ CD4 T cells, while promoting the induction of IFN-ɤ+ CD4 T cells after RSV challenge.
Figure 5. IL-4 and IFN-γ secreting CD4+ T cells in BALF and lung samples of infant and adult age primed mice after RSV challenge.
BALF and lung cells from infant (A) and adult (B) age primed mice (n=5) were collected at 5 days after challenge. CD4+ T cell epitope peptide F51−66 was used to stimulate BALF and lung cells in vitro, which were stained with phenotypic marker antibodies and cytokine antibodies, and then subsequently analyzed by Flowcytometry. The group labels are the same as in the Figure 2 legend. The results are presented as means with the SEM, and statistical significance was determined using one-way ANOVA with Tukey’s multiple comparison test performed in GraphPad Prism. ***, p < 0.0001, **p<0.005, * p<0.05.
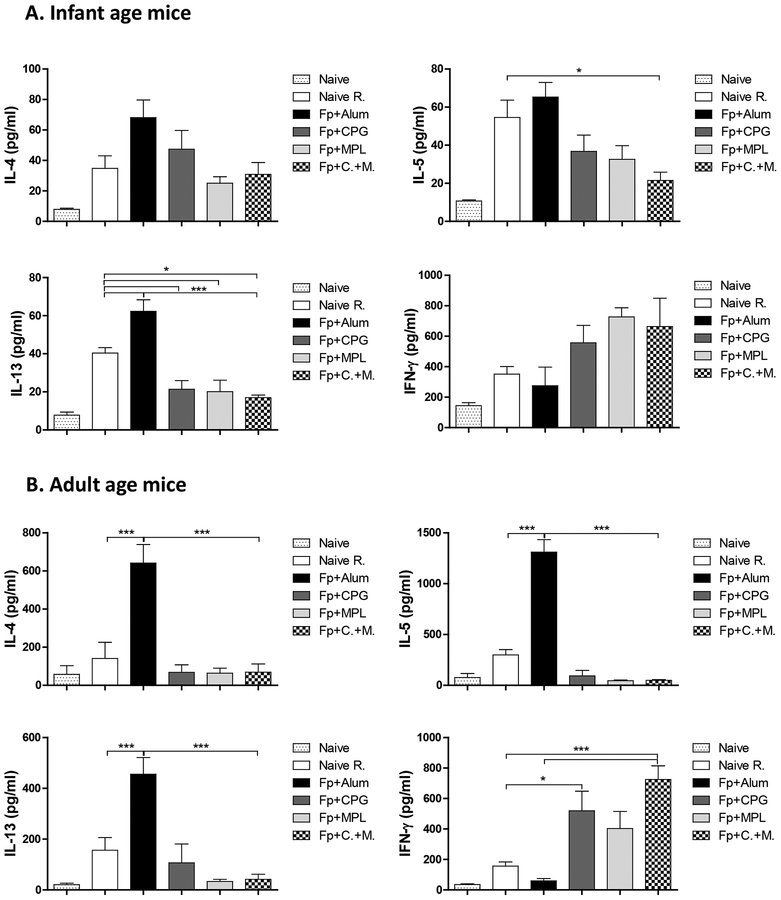
The alum adjuvanted F protein group of mice primed at adult age responded with higher levels of Th2 type cytokines (IL-4, IL-5, IL-13) by adjuvanted F protein compared to those in the infant age after RSV challenge as presented in different Y axis scales (Fig. 6A, B). The CpG, MPL, and CpG+MPL adjuvanted F protein immunization groups showed relatively low levels of Th2 type cytokines but high levels of Th1 cytokine IFN-γ+ in the BALF samples although significant differences in IFN-γ+ levels among the groups were observed in the adult but not infant age primed mice (Fig. 6A, B).
Figure 6. Cytokines in the airway BALF from the primed mice at infant and adult age after RSV challenge.
Airway BALF samples were collected from the primed mice (n=5) at infant age (A) and adult age (B) at 5 days after RSV challenge. IL-4, IL-5, IL-13 and IFN-γ cytokines in BALF were determined by corresponding cytokine ELISA kits. The group labels are the same as in the Figure 2 legend. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA and Tukey’s multiple-comparison test in GraphPad Prism; *** p<0.0001, **p<0.001, *p<0.05. The Fp +/− adjuvanted vaccination group labels are the same as in the legends of Figures 1 and 2.
Monocytes and Neutrophils are not infiltrated into the lungs from CpG+MPL adjuvanted F protein primed infant and adult mice after RSV challenge
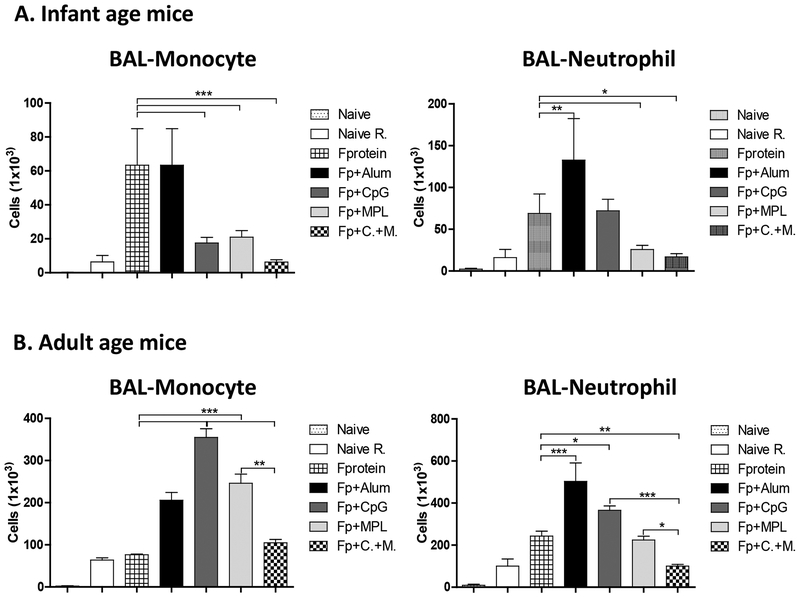
We analyzed the cellular infiltrates in the airway BALF day 5 post challenge by flow cytometry using phenotypic markers of monocytes (CD11b+Ly6chiF4/80+) and neutrophils (CD11b+Ly6c+F4/80−) (Fig. 7). The alum adjuvanted F protein priming of infant age mice induced highest levels of monocytes and neutrophils in BALF, whereas CpG+MPL adjuvanted F protein priming of infant age mice avoided the cellular infiltrates of monocytes and neutrophils after RSV challenge (Fig. 7A). MPL adjuvanted F protein priming was more effective than CpG adjuvanted F protein priming in preventing the cellular infiltrates of particularly airway monocytes (Fig. 7). In F protein priming of adult age mice, combination CpG+MPL adjuvant was most effective in reducing the infiltrates into the airways and lungs compared to alum, CpG, or MPL (Fig. 7B). Overall, priming vaccination of adult mice induced higher cellular infiltrates than infant age primed mice approximately by 4 folds as shown in different Y axis scales (Fig. 7). These results suggest that CpG+MPL adjuvanted F protein priming is effective in avoiding inflammatory cellular infiltrates of monocytes and neutrophils into the lungs in infant and adult age mice after challenge.
Figure 7. Monocytes and Neutrophils in the airways (BALF) of the infant and adult age primed mice after RSV challenge.
Airway BALF samples were collected from the primed mice (n=5) at infant age (A) and adult age (B) at 5 days after RSV challenge, stained with antibodies against monocyte and neutrophil cell type phenotypic markers, and analyzed by flow cytometry. The group labels are the same as in the Figure 2 legend. Results are presented as mean ± SEM. Statistical significances were performed by one-way ANOVA and Tukey’s multiple-comparison test in GraphPad Prism; *** p<0.0001, **p<0.001, *p<0.05. The Fp +/− adjuvanted vaccination group labels are the same as in the legends of Figures 1 and 2.
Discussion
It is a challenge to develop safe non-live RSV subunit protein vaccines for RSV-naïve infants due to the potential safety concerns of enhanced inflammatory disease after RSV infection. RSV rosetted post-F protein subunit nanoparticle vaccines were shown to be safe and immunogenic in adults in clinical studies (Glenn et al., 2015; Glenn et al., 2013). Another version of RSV post-F delivered as a soluble trimer and formulated with TLR4 agonist glucopyranosyl lipid A (GLA) integrated into stable emulsion (GLA-SE) was found to be immunogenic in adults but did not protect against RSV mediated illness (Falloon et al., 2017). F protein vaccines have been reported to cause vaccine-enhanced lung histopathology in animal models after challenge (Murphy et al., 1990; Palomo et al., 2016; Schneider-Ohrum et al., 2017). Also, in previous studies, vaccine-associated inflammatory histopathology was not attenuated by addition of various adjuvants to RSV vaccination, including alum, squalene oil-in-water emulsion (Lambert et al., 2015), GLA-SE (Schneider-Ohrum et al., 2017), polysaccharides-based delta-inulin (Wong et al., 2016), natural killer (NK) T cell agonist α-GalCer (Johnson et al., 2002). RSV F protein vaccination of mice in the presence of TLR3 agonist poly I:C adjuvants did not suppress lung histopathology after RSV challenge despite adjuvant effects on enhancing the immunogenicity of F protein vaccine and significant reduction in lung viral titers (Supplementary Fig. S4). A previous study reported a gradient of IgG1/IgG2a and IL-4 with various adjuvants with RSV FG protein vaccine with heightened inflammatory response to RSV challenge (Neuzil et al., 1997). In this study, we demonstrated the adjuvant effects of combined MPL+CpG on immunogenicity, the efficacy of lung viral titers, and particularly on suppressing pulmonary histopathology after F protein single dose priming of infant and adult age mice and RSV challenge.
A previous study reported that CpG TLR9 agonist but not TLR7/8 agonists played adjuvant roles during vaccination in diminishing formalin-inactivated RSV (FI-RSV) vaccine-enhanced lung pathology and cytokines after RSV challenge (Johnson et al., 2009). Therapeutic administration during primary RSV infection caused severe RSV disease, suggesting anti-viral innate immune responses might also be associated with RSV immunopathology (Johnson et al., 2009). Co-delivery of RSV F protein trimer vaccine and TLR7/8 agonists in polymer nanoparticles was shown to elicit high titers of RSV F specific binding and neutralizing antibodies but histopathology was not investigated (Francica et al., 2016). In comparison with other TLR agonists, we compared the adjuvant effects of TLR3 agonist poly I:C and combination MPL+CpG on reducing RSV F protein vaccine-enhanced lung histopathology after RSV challenge. TLR3 agonist poly I:C in RSV F protein prime vaccination of adult age mice did not diminish RSV F protein vaccine-enhanced lung histopathology (Supplementary Fig. S4). Different outcomes between TLR3 agonist poly I:C and combination MPL+CpG adjuvant were also observed with FI-RSV vaccination and RSV challenge of mice (data not shown). It is significant to find that combination CpG+MPL adjuvanted F protein priming of infant and adult age mice was effective in avoiding lung histopathology, IL-4+ CD4 T cell responses, and cellular infiltration of monocytes and neutrophils after RSV challenge, as well as enhancing RSV neutralizing antibodies and lung viral clearance. A recent study demonstrated that DS-Cav1 pre-fusion F protein vaccine adjuvanted with a Sigma adjuvant system, an oil-in-water adjuvant plus Carbopol, Alum, or Poly (I:C) significantly enhanced the levels of RSV neutralizing titers in mice after prime boost vaccination but vaccine-enhanced disease after RSV challenge was not assessed (Sastry et al., 2017). It is expected that use of combination CpG+MPL adjuvant in the RSV subunit vaccines such as pre-fusion F proteins would be effective in priming protective immune responses and preventing inflammatory lung pathology after RSV infection, but the efficacy of the pre-fusion F (DS-Cav1 F) with this combination MPL+CpG adjuvant remains to determined.
Combination of alum (100 μg) + CpG (20–100 μg) adjuvants in F protein vaccination was reported to increase neutralization titers, Th1 and Th2 immune responses, and lung viral clearance (Hancock et al., 2001). Triple components of adjuvants CpG (10 μg) and an innate defense peptide in polyphosphazene microparticles in the RSV F protein vaccination of mice resulted in controlling virus replication and no evidence of vaccine-induced pathology after challenge (Garg et al., 2014; Garlapati et al., 2012). The outcomes of CpG adjuvant appear to be different in other studies. CpG inclusion in a polysaccharide adjuvant derived from delta inulin promoted the induction of predominately IgG2a antibodies to RSV whole virus vaccination of mice but exacerbated lung pathology after RSV challenge (Wong et al., 2016). Cotton rats that were immunized with CpG (20 – 100 μg) adjuvanted F protein increased the induction of RSV neutralizing titers but developed enhanced pulmonary pathology consisting of alveolitis and interstitial pneumonitis after RSV challenge (Prince et al., 2003). CpG (4 μg) adjuvant effects on suppressing lung pathology after RSV challenge were found to be prominent in F protein primed mice at an infant age. Consistent with these studies (Prince et al., 2003; Wong et al., 2016), CpG adjuvanted F protein priming of mice at an adult age resulted in substantial inflammation scores in lung histology, and infiltration of eosinophils, monocytes and neutrophils after RSV challenge despite of inducing IgG2a Th1 type immune responses.
TLR4 agonist MPL included in the alum adjuvanted FI-RSV vaccination was demonstrated to reduce the polymorphonuclear leukocyte infiltration within the alveoli and moderately attenuate peribronchiolitis in cotton rats post-challenge (Prince et al., 2001). Inclusion of MPL in the FI-RSV vaccination was found to mitigate the lung pathology with a reduction in the levels of Th1-and Th2-type cytokines and chemokines (Boukhvalova et al., 2006). Post-fusion or pre-fusion F protein low dose vaccination of cotton rats even in the presence of a TLR4 agonist Th1-biasing (GLA- stabilized emulsion) adjuvant was reported to prime inflammatory vaccine-enhanced alveolitis although lung viral titers were below the detection limit after RSV challenge (Schneider-Ohrum et al., 2017). In this study, MPL and CpG adjuvant effects on RSV F protein vaccination were similarly effective in suppressing histopathology and eosinophil infiltration at moderately low levels in infant age primed mice. In adult age priming of mice with F protein, MPL was more prominent in suppressing histopathology compared to CpG after RSV challenge although there was no statistically difference between the two adjuvants. Consistent with previous studies (Lambert et al., 2015; Prince et al., 2003; Schneider-Ohrum et al., 2017; Wong et al., 2016), separate MPL or CpG adjuvant alone in F protein priming of mice at infant and adult age exhibited a certain degree of histopathology and monocyte infiltration upon RSV challenge, despite the induction of Th1 type immune responses and significant reduction in lung viral titers.
MPL triggers MyD88-dependent (TIRAP/MyD88) and TRAM/TRIF (Toll-interleukin 1 receptor domain–containing adapter)-dependent pathways, leading to activation of nuclear factor (NF)-κB and IFN-response factor 3 (IRF3) and inducing type 1 IFN innate immune responses (Kagan et al., 2008; Kawai and Akira, 2010). Meanwhile intracellular TLR9 ligand CpG interaction recruits MyD88-dependent pathway leading to the activation of NF-κB and IRF7, eventually inducing inflammatory cytokines and type 1 IFNs (Kawai and Akira, 2010). Synergistic effects of combination TLR4 and 9 agonists on IL-1β production but not on IL-6 were demonstrated with stimulation of peripheral blood mononuclear cells isolated from healthy adults (Timmermans et al., 2013). Inclusion of CpG+MPL adjuvnated RSV F protein priming of infant and adult age mice was most prominent for avoiding lung histopathology, IL-4+ CD4 T cell responses, and cellular infiltration of monocytes and neutrophils after RSV challenge, in addition to increasing protective efficacy of lung viral clearance after challenge. The levels of IFN-γ+ CD4 T cells were similarly observed in F protein primed infant age mice that displayed different degrees of histopathology, suggesting that an inverse correlation between IFN-γ+ CD4 T cells and histopathology is not always predicted. In contrast, priming adult mice with alum adjuvanted F protein showed a typical pattern of high IL-4+ CD4 T cells and low IFN-γ+ CD4 T cells, which is correlating with inflammatory histopathology after RSV challenge.
MPL injection was shown to transiently induce inflammatory cytokines, leading to increases in numbers of activated antigen-carrying dendritic and monocytes in the draining lymph nodes within a day and retaining those antigen presenting cells in the draining lymph nodes for over 3 days (Didierlaurent et al., 2009). Injection of CpG or an oil-in-water emulsion MF59 adjuvant was demonstrated to promote the expression of a cluster of adjuvant core response genes including cytokines, chemokines, and cellular recruitment at the injection sites (Mosca et al., 2008). Peritoneal injection of combination MPL+CpG resulted in acute induction of chemokines (MCP-1, RANTES, KC, IP-10) and recruiting dendritic cells and neutrophils but depleting macrophages in the site 24 h post-injection within a day (Ko et al., 2018), which is a similar pattern with MF59 (Calabro et al., 2011; Ko et al., 2016). It was also demonstrated that MF59 intramuscular injection increased the number of antigen-carrying cells (B cells, dendritic cells, neutrophils) in the draining lymph nodes within 24 h post treatment (Calabro et al., 2011). Therefore, it is possible that MPL+CpG adjuvants promote the recruitment of neutrophils and dendritic cells, antigen update, and trafficking antigen presenting cells to the draining lymph nodes. Effective long-term protection is important. It was reported in a previous study that intranasal immunization of mice with wild type RSV F soluble protein vaccine in triple adjuvant formulations containing CpG (10 μg) and an immunostimulatory peptide in a synthetic water-soluble polymer carrier induced immunity for 1 year via prime boost regimen (Garg et al., 2014). Further studies on the longevity of protection and the mechanisms of how combination MPL+CpG adjuvant work on priming Th1 responses and preventing enhanced RSV disease after challenge in mice will provide important insight into developing effective adjuvanted RSV subunit protein vaccines in future.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health/National Institute of Allergy and Infectious Diseases grants AI093772, AI105170, and AI134132 to SMK. The following reagent was obtained through BEI Resources, NIAID, NIH: Purified recombinant F glycoprotein (RSV A2) with C-Terminal His tag produced in 293F cells (BEI NR-28908). The authors acknowledge the provision of RSV F purified proteins in post-fusion and pre-fusion conformation, and 5C4 m6onoclonal antibody from Vaccine Research Center (NIAID, NIH, Bethesda, MD 20892, USA) used for ELISA coating antigens.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Blanco JC, Boukhvalova MS, Pletneva LM, Shirey KA, Vogel SN, 2014. A recombinant anchorless respiratory syncytial virus (RSV) fusion (F) protein/monophosphoryl lipid A (MPL) vaccine protects against RSV-induced replication and lung pathology. Vaccine 32, 1495–1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boukhvalova MS, Prince GA, Soroush L, Harrigan DC, Vogel SN, Blanco JC, 2006. The TLR4 agonist, monophosphoryl lipid A, attenuates the cytokine storm associated with respiratory syncytial virus vaccine-enhanced disease. Vaccine 24, 5027–5035. [DOI] [PubMed] [Google Scholar]
- Branche AR, Falsey AR, 2015. Respiratory syncytial virus infection in older adults: an under-recognized problem. Drugs & aging 32, 261–269. [DOI] [PubMed] [Google Scholar]
- Calabro S, Tortoli M, Baudner BC, Pacitto A, Cortese M, O’Hagan DT, De Gregorio E, Seubert A, Wack A, 2011. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine 29, 1812–1823. [DOI] [PubMed] [Google Scholar]
- Connors M, Kulkarni AB, Firestone CY, Holmes KL, Morse HC 3rd, Sotnikov AV, Murphy BR, 1992. Pulmonary histopathology induced by respiratory syncytial virus (RSV) challenge of formalin-inactivated RSV-immunized BALB/c mice is abrogated by depletion of CD4+ T cells. Journal of virology 66, 7444–7451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didierlaurent AM, Morel S, Lockman L, Giannini SL, Bisteau M, Carlsen H, Kielland A, Vosters O, Vanderheyde N, Schiavetti F, Larocque D, Van Mechelen M, Garcon N, 2009. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. Journal of immunology 183, 6186–6197. [DOI] [PubMed] [Google Scholar]
- Esposito S, Pietro GD, 2016. Respiratory syncytial virus vaccines: an update on those in the immediate pipeline. Future microbiology 11, 1479–1490. [DOI] [PubMed] [Google Scholar]
- Falloon J, Yu J, Esser MT, Villafana T, Yu L, Dubovsky F, Takas T, Levin MJ, Falsey AR, 2017. An Adjuvanted, Postfusion F Protein-Based Vaccine Did Not Prevent Respiratory Syncytial Virus Illness in Older Adults. The Journal of infectious diseases 216, 1362–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE, 2005. Respiratory syncytial virus infection in elderly and high-risk adults. N Engl J Med 352, 1749–1759. [DOI] [PubMed] [Google Scholar]
- Feltquate DM, Heaney S, Webster RG, Robinson HL, 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J Immunol 158, 2278–2284. [PubMed] [Google Scholar]
- Francica JR, Lynn GM, Laga R, Joyce MG, Ruckwardt TJ, Morabito KM, Chen M, Chaudhuri R, Zhang B, Sastry M, Druz A, Ko K, Choe M, Pechar M, Georgiev IS, Kueltzo LA, Seymour LW, Mascola JR, Kwong PD, Graham BS, Seder RA, 2016. Thermoresponsive Polymer Nanoparticles Co-deliver RSV F Trimers with a TLR-7/8 Adjuvant. Bioconjug Chem 27, 2372–2385. [DOI] [PubMed] [Google Scholar]
- Garg R, Latimer L, Gerdts V, Potter A, van Drunen Littel-van den Hurk S, 2014. Vaccination with the RSV fusion protein formulated with a combination adjuvant induces long-lasting protective immunity. The Journal of general virology 95, 1043–1054. [DOI] [PubMed] [Google Scholar]
- Garlapati S, Garg R, Brownlie R, Latimer L, Simko E, Hancock RE, Babiuk LA, Gerdts V, Potter A, van Drunen Littel-van den Hurk S, 2012. Enhanced immune responses and protection by vaccination with respiratory syncytial virus fusion protein formulated with CpG oligodeoxynucleotide and innate defense regulator peptide in polyphosphazene microparticles. Vaccine 30, 5206–5214. [DOI] [PubMed] [Google Scholar]
- Gershwin LJ, Schelegle ES, Gunther RA, Anderson ML, Woolums AR, Larochelle DR, Boyle GA, Friebertshauser KE, Singer RS, 1998. A bovine model of vaccine enhanced respiratory syncytial virus pathophysiology. Vaccine 16, 1225–1236. [DOI] [PubMed] [Google Scholar]
- Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, Flyer D, Jani D, Hickman SP, Piedra PA, 2015. A Randomized, Blinded, Controlled, Dose-Ranging Study of a Respiratory Syncytial Virus Recombinant Fusion (F) Nanoparticle Vaccine in Healthy Women of Childbearing Age. The Journal of infectious diseases. [DOI] [PubMed] [Google Scholar]
- Glenn GM, Smith G, Fries L, Raghunandan R, Lu H, Zhou B, Thomas DN, Hickman SP, Kpamegan E, Boddapati S, Piedra PA, 2013. Safety and immunogenicity of a Sf9 insect cell-derived respiratory syncytial virus fusion protein nanoparticle vaccine. Vaccine 31, 524–532. [DOI] [PubMed] [Google Scholar]
- Hancock GE, Heers KM, Smith JD, Scheuer CA, Ibraghimov AR, Pryharski KS, 2001. CpG containing oligodeoxynucleotides are potent adjuvants for parenteral vaccination with the fusion (F) protein of respiratory syncytial virus (RSV). Vaccine 19, 4874–4882. [DOI] [PubMed] [Google Scholar]
- Hwang HS, Kwon YM, Lee JS, Yoo SE, Lee YN, Ko EJ, Kim MC, Cho MK, Lee YT, Jung YJ, Lee JY, Li JD, Kang SM, 2014. Co-immunization with virus-like particle and DNA vaccines induces protection against respiratory syncytial virus infection and bronchiolitis. Antiviral research 110C, 115–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang HS, Lee YT, Kim KH, Park S, Kwon YM, Lee Y, Ko EJ, Jung YJ, Lee JS, Kim YJ, Lee YN, Kim MC, Cho M, Kang SM, 2016. Combined virus-like particle and fusion protein-encoding DNA vaccination of cotton rats induces protection against respiratory syncytial virus without causing vaccine-enhanced disease. Virology 494, 215–224. [DOI] [PubMed] [Google Scholar]
- Jia R, Lu L, Liang X, Sun Z, Tan L, Xu M, Su L, Xu J, 2017. Poly(U) and CpG ameliorate the unbalanced T cell immunity and pneumonia of mice with RSV vaccine-enhanced disease. Biosci Trends 11, 450–459. [DOI] [PubMed] [Google Scholar]
- Johnson TR, Hong S, Van Kaer L, Koezuka Y, Graham BS, 2002. NK T cells contribute to expansion of CD8(+) T cells and amplification of antiviral immune responses to respiratory syncytial virus. Journal of virology 76, 4294–4303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TR, Rao S, Seder RA, Chen M, Graham BS, 2009. TLR9 agonist, but not TLR7/8, functions as an adjuvant to diminish FI-RSV vaccine-enhanced disease, while either agonist used as therapy during primary RSV infection increases disease severity. Vaccine 27, 3045–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagan JC, Su T, Horng T, Chow A, Akira S, Medzhitov R, 2008. TRAM couples endocytosis of Toll-like receptor 4 to the induction of interferon-beta. Nat Immunol 9, 361–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakuk TJ, Soike K, Brideau RJ, Zaya RM, Cole SL, Zhang JY, Roberts ED, Wells PA, Wathen MW, 1993. A human respiratory syncytial virus (RSV) primate model of enhanced pulmonary pathology induced with a formalin-inactivated RSV vaccine but not a recombinant FG subunit vaccine. The Journal of infectious diseases 167, 553–561. [DOI] [PubMed] [Google Scholar]
- Kawai T, Akira S, 2010. The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nat Immunol 11, 373–384. [DOI] [PubMed] [Google Scholar]
- Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH, 1969. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol 89, 422–434. [DOI] [PubMed] [Google Scholar]
- Ko EJ, Kwon YM, Lee JS, Hwang HS, Yoo SE, Lee YN, Lee YT, Kim MC, Cho MK, Lee YR, Quan FS, Song JM, Lee S, Moore ML, Kang SM, 2015. Virus-like nanoparticle and DNA vaccination confers protection against respiratory syncytial virus by modulating innate and adaptive immune cells. Nanomedicine : nanotechnology, biology, and medicine 11, 99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee Y, Lee YT, Kim YJ, Kim KH, Kang SM, 2018. MPL and CpG combination adjuvants promote homologous and heterosubtypic cross protection of inactivated split influenza virus vaccine. Antiviral Res 156, 107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee YT, Kim KH, Jung YJ, Lee Y, Denning TL, Kang SM, 2016. Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4+ T Cells. Journal of virology 90, 6976–6988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko EJ, Lee YT, Lee Y, Kim KH, Kang SM, 2017. Distinct Effects of Monophosphoryl Lipid A, Oligodeoxynucleotide CpG, and Combination Adjuvants on Modulating Innate and Adaptive Immune Responses to Influenza Vaccination. Immune Netw 17, 326–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert SL, Aslam S, Stillman E, MacPhail M, Nelson C, Ro B, Sweetwood R, Lei YM, Woo JC, Tang RS, 2015. A novel respiratory syncytial virus (RSV) F subunit vaccine adjuvanted with GLA-SE elicits robust protective TH1-type humoral and cellular immunity in rodent models. PloS one 10, e0119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Lee YT, Ko EJ, Kim KH, Hwang HS, Park S, Kwon YM, Kang SM, 2017. Soluble F proteins exacerbate pulmonary histopathology after vaccination upon respiratory syncytial virus challenge but not when presented on virus-like particles. Hum Vaccin Immunother 13, 2594–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloy KJ, Donachie AM, Mowat AM, 1995. Induction of Th1 and Th2 CD4+ T cell responses by oral or parenteral immunization with ISCOMS. European journal of immunology 25, 2835–2841. [DOI] [PubMed] [Google Scholar]
- McLellan JS, Chen M, Joyce MG, Sastry M, Stewart-Jones GB, Yang Y, Zhang B, Chen L, Srivatsan S, Zheng A, Zhou T, Graepel KW, Kumar A, Moin S, Boyington JC, Chuang GY, Soto C, Baxa U, Bakker AQ, Spits H, Beaumont T, Zheng Z, Xia N, Ko SY, Todd JP, Rao S, Graham BS, Kwong PD, 2013. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 342, 592–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller AL, Strieter RM, Gruber AD, Ho SB, Lukacs NW, 2003. CXCR2 regulates respiratory syncytial virus-induced airway hyperreactivity and mucus overproduction. J Immunol 170, 3348–3356. [DOI] [PubMed] [Google Scholar]
- Mosca F, Tritto E, Muzzi A, Monaci E, Bagnoli F, Iavarone C, O’Hagan D, Rappuoli R, De Gregorio E, 2008. Molecular and cellular signatures of human vaccine adjuvants. Proc Natl Acad Sci U S A 105, 10501–10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountford AP, Fisher A, Wilson RA, 1994. The profile of IgG1 and IgG2a antibody responses in mice exposed to Schistosoma mansoni. Parasite Immunol 16, 521–527. [DOI] [PubMed] [Google Scholar]
- Munoz FM, Piedra PA, Glezen WP, 2003. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 21, 3465–3467. [DOI] [PubMed] [Google Scholar]
- Murphy BR, Sotnikov AV, Lawrence LA, Banks SM, Prince GA, 1990. Enhanced pulmonary histopathology is observed in cotton rats immunized with formalin-inactivated respiratory syncytial virus (RSV) or purified F glycoprotein and challenged with RSV 3–6 months after immunization. Vaccine 8, 497–502. [DOI] [PubMed] [Google Scholar]
- Nair H, Nokes DJ, Gessner BD, Dherani M, Madhi SA, Singleton RJ, O’Brien KL, Roca A, Wright PF, Bruce N, Chandran A, Theodoratou E, Sutanto A, Sedyaningsih ER, Ngama M, Munywoki PK, Kartasasmita C, Simoes EA, Rudan I, Weber MW, Campbell H, 2010. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: a systematic review and meta-analysis. Lancet 375, 1545–1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil KM, 2016. Progress toward a Respiratory Syncytial Virus Vaccine. Clinical and vaccine immunology : CVI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuzil KM, Johnson JE, Tang YW, Prieels JP, Slaoui M, Gar N, Graham BS, 1997. Adjuvants influence the quantitative and qualitative immune response in BALB/c mice immunized with respiratory syncytial virus FG subunit vaccine. Vaccine 15, 525–532. [DOI] [PubMed] [Google Scholar]
- Openshaw PJ, Clarke SL, Record FM, 1992. Pulmonary eosinophilic response to respiratory syncytial virus infection in mice sensitized to the major surface glycoprotein G. International immunology 4, 493–500. [DOI] [PubMed] [Google Scholar]
- Oumouna M, Mapletoft JW, Karvonen BC, Babiuk LA, van Drunen Littel-van den Hurk S, 2005. Formulation with CpG oligodeoxynucleotides prevents induction of pulmonary immunopathology following priming with formalin-inactivated or commercial killed bovine respiratory syncytial virus vaccine. Journal of virology 79, 2024–2032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palomo C, Mas V, Thom M, Vazquez M, Cano O, Terron MC, Luque D, Taylor G, Melero JA, 2016. Influence of Respiratory Syncytial Virus F Glycoprotein Conformation on Induction of Protective Immune Responses. Journal of virology 90, 5485–5498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Denamur F, Deschamps M, Garcon N, Prieels JP, Slaoui M, Thiriart C, Porter DD, 2001. Monophosphoryl lipid A adjuvant reverses a principal histologic parameter of formalin-inactivated respiratory syncytial virus vaccine-induced disease. Vaccine 19, 2048–2054. [DOI] [PubMed] [Google Scholar]
- Prince GA, Jenson AB, Hemming VG, Murphy BR, Walsh EE, Horswood RL, Chanock RM, 1986. Enhancement of respiratory syncytial virus pulmonary pathology in cotton rats by prior intramuscular inoculation of formalin-inactiva ted virus. Journal of virology 57, 721–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince GA, Mond JJ, Porter DD, Yim KC, Lan SJ, Klinman DM, 2003. Immunoprotective activity and safety of a respiratory syncytial virus vaccine: mucosal delivery of fusion glycoprotein with a CpG oligodeoxynucleotide adjuvant. Journal of virology 77, 13156–13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappuoli R, Mandl CW, Black S, De Gregorio E, 2011. Vaccines for the twenty-first century society. Nature reviews. Immunology 11, 865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry M, Zhang B, Chen M, Joyce MG, Kong WP, Chuang GY, Ko K, Kumar A, Silacci C, Thom M, Salazar AM, Corti D, Lanzavecchia A, Taylor G, Mascola JR, Graham BS, Kwong PD, 2017. Adjuvants and the vaccine response to the DS-Cav1-stabilized fusion glycoprotein of respiratory syncytial virus. PLoS One 12, e0186854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Ohrum K, Cayatte C, Bennett AS, Rajani GM, McTamney P, Nacel K, Hostetler L, Cheng L, Ren K, O’Day T, Prince GA, McCarthy MP, 2017. Immunization with Low Doses of Recombinant Postfusion or Prefusion Respiratory Syncytial Virus F Primes for Vaccine-Enhanced Disease in the Cotton Rat Model Independently of the Presence of a Th1-Biasing (GLA-SE) or Th2-Biasing (Alum) Adjuvant. Journal of virology 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoes EA, Groothuis JR, Carbonell-Estrany X, Rieger CH, Mitchell I, Fredrick LM, Kimpen JL, Palivizumab Long-Term Respiratory Outcomes Study, G., 2007. Palivizumab prophylaxis, respiratory syncytial virus, and subsequent recurrent wheezing. J Pediatr 151, 34–42, 42 e31. [DOI] [PubMed] [Google Scholar]
- Stokes KL, Currier MG, Sakamoto K, Lee S, Collins PL, Plemper RK, Moore ML, 2013. The respiratory syncytial virus fusion protein and neutrophils mediate the airway mucin response to pathogenic respiratory syncytial virus infection. Journal of virology 87, 10070–10082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmermans K, Plantinga TS, Kox M, Vaneker M, Scheffer GJ, Adema GJ, Joosten LA, Netea MG, 2013. Blueprints of signaling interactions between pattern recognition receptors: implications for the design of vaccine adjuvants. Clin Vaccine Immunol 20, 427–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TM, Petrovsky N, Bissel SJ, Wiley CA, Ross TM, 2016. Delta inulin-derived adjuvants that elicit Th1 phenotype following vaccination reduces respiratory syncytial virus lung titers without a reduction in lung immunopathology. Human vaccines & immunotherapeutics, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Li H, Hai Y, Yin W, Li W, Zheng B, Du X, Li N, Zhang Z, Deng Y, Zeng R, Wei L, 2017. CpG in Combination with an Inhibitor of Notch Signaling Suppresses Formalin-Inactivated Respiratory Syncytial Virus-Enhanced Airway Hyperresponsiveness and Inflammation by Inhibiting Th17 Memory Responses and Promoting Tissue-Resident Memory Cells in Lungs. Journal of virology 91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.