Abstract
Background:
We previously demonstrated the normal mouse epididymal lumen contains a nonpathological amyloid matrix that surrounds spermatozoa and which we propose plays important roles in sperm maturation and protection.
Objective:
The objective herein is to present a review of this work including studies showing the amyloid structures of four members of the CRES (cystatin-related epididymal spermatogenic) subgroup are integral and essential components of the amyloid matrix.
Methods:
We used conformation-dependent reagents that recognize the cross-β-sheet structure characteristic of amyloid including thioflavin S (ThS), thioflavin T (ThT), anti-amyloid antibodies, and X-ray diffraction, as well as negative stain electron microscopy (TEM) to visualize amyloid structures in the epididymal lumen. Antibodies that specifically detect each CRES subgroup family member were also used in indirect immunofluorescence analysis.
Results and Discussion:
The epididymal lumen contains an amyloid matrix that surrounds maturing spermatozoa and which represents a functional amyloid. Alterations in the structure of the amyloid matrix by the loss of the CRES subgroup members or the overexpression of cystatin C results in epididymal pathologies including infertility. Preliminary data suggests the epididymal amyloid matrix is structurally and functionally similar to bacterial biofilms.
Conclusion:
Together, these results suggest the amyloid matrix serves important roles in epididymal function including sperm maturation and protection.
Keywords: epididymis, amyloid, sperm maturation, antimicrobial, mouse, bacterial biofilm
Introduction
Following spermatogenesis, spermatozoa exit the testis and enter a long convoluted tubule known as the epididymis. As spermatozoa transit the epididymis they undergo maturation and acquire the functions of progressive motility and the ability to fertilize an oocyte. Since spermatozoa are synthetically inactive, maturation requires the interactions of spermatozoa with proteins secreted into the lumen by the surrounding epididymal epithelial cells. The epididymal epithelium secretes proteins in a highly regionalized manner creating unique luminal microenvironments along the tubule which are necessary for maturation (Cornwall 2009). In addition to forming an environment that will mature spermatozoa, the epididymis must also ensure that spermatozoa are protected from pathogens that can ascend the male reproductive tract. However, the mechanism by which these diverse processes occur is not known.
As luminal fluid enters the efferent ducts and initial segment region of the epididymis greater than 90% of the water is removed. This produces a luminal environment that is macromolecularly crowded and allows a close association between luminal components and spermatozoa. Indeed, examination of the epididymal lumen by transmission electron microscopy (TEM) shows it to be highly particulate in nature with a dense matrix-like material surrounding the spermatozoa (SP) (Fig 1A). Extracellular vesicles (EVs) of various sizes, including epididymal exosomes (epididymosomes) are also found within this matrix and their delivery of cargo to spermatozoa is an essential part of the maturation process (Fig 1A, arrows) (Caballero et al., 2013; Sullivan 2015; Whelly et al., 2016). Various other aggregate structures including electron dense bodies which contain heat shock protein 1 (HSPD1, HSP60) and tumor rejection antigen (TRA1), a member of the heat shock protein 90 family, are also in the epididymal lumen; however their functions have not been determined (Asquith et al., 2005). Thus particulate/insoluble structures are an integral part of the epididymal lumen and likely important for epididymal function.
Figure 1. An amyloid matrix is present in the mouse epididymal lumen.
A) Transmission electron micrograph of the initial segment region of the mouse epididymis showing particulate material within the lumen. SP, spermatozoon. Arrows, extracellular vesicles. Micrograph kindly provided by A. Parent and L. Hermo, McGill University, Montreal, Quebec Canada. Reprinted from (Cornwall et al., 2011). Bar, 0.5 μm. B) Indirect immunofluorescence analysis of the initial segment epididymal lumen using the anti-oligomeric amyloid antibody (A11) (green fluorescence). DAPI, blue fluorescence, indicates staining of epithelial cell nuclei. Bar, 20 μm. C) Thioflavin S staining of amyloid matrix isolated from the mouse initial segment; and D) cauda epididymis. Bar, 5 μm. E) X-ray diffraction of epididymal amyloid matrix isolated from the initial segment region of the epididymis. Reprinted from (Whelly et al., 2012).
Amyloids are proteins that self-assemble into highly ordered cross-β-sheet rich structures. Although amyloids historically have been thought to be pathological entities associated with neurodegenerative disease and prionopathies (Cline et al., 2018), increasing evidence reveals that most proteins can adopt the amyloid fold given the correct environmental conditions and that many amyloids carry out biological roles in the absence of pathology. These structures, known as functional amyloids, perform many different roles including in host defense/invasion and cell adhesion (Pham et al., 2014; Hewetson et al., 2017). In bacteria, a family of proteins known as curli forms amyloid that plays an important structural role in the formation of an extracellular matrix that contributes to biofilm formation (Chapman et al., 2002; Wang and Chapman, 2008). Despite that functional amyloids were only first described in mammals in 2006, their number has grown considerably and now includes amyloids that function as a biological scaffolds, signaling complexes, and storage depots (Fowler et al., 2006; Maj et al., 2009; Li et al., 2012).
Amyloids exhibit SDS and/or protease resistance and often require harsh denaturants such as formic acid, guanidine, or DMSO for solubilization (Loksztejn and Dzwolak 2009; Whelly et al. 2014, Whelly et al., 2016). The extreme stability of amyloid can be problematic in pathological states but functionally beneficial for its biological roles as scaffolds and structures that provide protection and mediate cell-cell interactions including the chorion surrounding oocytes in fish and its equivalent, the zona pellucida in mammals (Iconomidou 2000; Podradsky 2001; Louros et al. 2013; Egge et al., 2015). Proteins that form pathological and functional amyloids follow similar aggregation pathways and progress from oligomers to protofibrils and fibrils. Other amyloid forms including matrix, films, and ribbons have also been observed which may represent higher ordered structures (Hatters et al., 2000). While both functional and pathological amyloids have cross β-sheet structure and often are indistinguishable by TEM, it is not clear if pathological and functional amyloids are distinct at the atomic level. Structural biological approaches including solid state NMR and cryo-EM are currently being carried out to answer this question.
We previously established that the mouse epididymal lumen contains an amyloid-rich extracellular matrix that surrounds the maturing spermatozoa (Whelly et al., 2012). In this review, we will discuss our work on this functional epididymal amyloid matrix including its formation and putative roles in sperm protection and maturation. We will also discuss the possibility it is an evolutionarily conserved structure, functionally similar to bacterial biofilms.
Epididymal amyloid matrix
We used several biochemical and biophysical approaches including thioflavin S (ThS), anti-amyloid antibodies (Kayed et al., 2010), and X-ray diffraction, all of which detect cross-β-sheet structures characteristic of amyloid, to establish the epididymal luminal particulate material is rich in amyloid (Fig 1B-E). Because this amyloid matrix is nonpathological and a normal component of the mouse epididymal lumen, it likely represents a functional amyloid (Whelly et al., 2012). Similar to maturational changes in spermatozoa, the epididymal amyloid matrix also seems to change along the epididymal tubule as anti-amyloid A11 antibody and ThS staining decrease from the proximal (initial segment/caput) to the distal (cauda) epididymal regions (Fig 1C,D, and Muthusubramanian and Cornwall, unpublished observations). We have not yet determined if this change indicates an amyloid matrix that has become more highly ordered and is less able to bind these reagents or is an amyloid matrix that is starting to disassemble. Nonetheless these maturational changes in amyloid matrix structure may be functionally relevant as they correlate with changes in epididymal function since the proximal epididymis participates in sperm maturation while the cauda serves primarily to store the mature spermatozoa.
Cystatin amyloids
Multiple members of the family 2 cystatins of cysteine protease inhibitors contribute to the formation of the epididymal amyloid matrix. The CRES (cystatin-related epididymal spermatogenic) protein is the defining member of a reproductive subgroup within the family 2 cystatins (Cornwall et al., 1992; Töhönen et al., 1998; Turk and Bode, 1991; Cornwall and Hsia, 2003). Subsequent to our initial description of CRES, work from our lab as well as others has established that the CRES subgroup consists of 8 family members all exhibiting overlapping yet distinctive patterns of expression in the reproductive tract (Cornwall, et al., 1992; Cornwall and Hsia, 2003; Frygelius et al., 2010). Within the epididymis, CRES, CRES2, CRES3, and cystatin E2 are synthesized and secreted by the initial segment region suggesting interrelated functions (Cornwall & Hann, 1995; Hsia and Cornwall, 2003; Li et al., 2005; Li et al., 2003; Li et al., 2002). In support of this, using antibodies that specifically recognize each subgroup member, we established that CRES, CRES2, CRES3, and cystatin E2 colocalize with each other and with the prototypical cystatin, cystatin C, in amyloid matrix isolated from the mouse epididymal initial segment lumen, and within the luminal amyloid matrix in situ, as represented by the colocalization of CRES and CRES3 in immunofluorescence studies (Whelly et al., 2016; Fig 2). Additional biochemical approaches including pulldown of epididymal amyloid using the protein aggregation disease (PAD) ligand followed by immunoblot with anti-CRES antibodies further indicated the four epididymal CRES subgroup members were indeed present as amyloids in the epididymal lumen (Whelly et al., 2016).
Figure 2. Colocalization of CRES and CRES3 in amyloid matrix in the initial segment epididymal lumen.
Indirect immunofluorescence analysis was performed using an affinity-purified rabbit anti-mouse CRES antibody followed by an Alexa Fluor 594 conjugated secondary antibody (red fluorescence) and an Alexa Fluor 488 conjugated CRES3 antibody (green fluorescence) on paraformaldehyde-fixed paraffin embedded mouse epididymal tissue sections. Images were captured with a Nikon Ti-E confocal microscope. Bar, 20 μm.
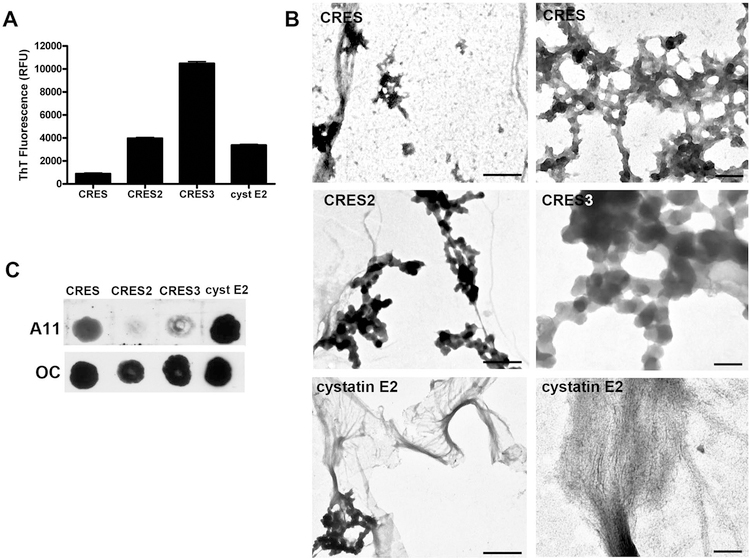
All four epididymal CRES subgroup members are also highly amyloidogenic in vitro and exhibit distinct kinetics of amyloidogenesis and form unique amyloid structures including matrices, films, and polygons (Fig 3B) (Whelly et al., 2016). Similarly, cystatin C is an established amyloid in vitro and in vivo (Wahlbom et al., 2007). ThT fluorescence, negative stain TEM, and dot blot analysis using anti-amyloid antibodies (anti-A11 antibody recognizes immature amyloids while anti-OC antibody recognizes mature amyloids (Kayed et al., 2010)) showed that, of the four proteins, CRES is the least amyloidogenic while CRES3 is the most amyloidogenic. Immediately following dilution out of 6M guanidine CRES3 rapidly transitioned into stable amyloid polygons, highly thioflavin T reactive structures with little or no oligomeric forms present, while CRES was distributed between both immature oligomeric and mature fibrillar amyloid forms (Fig 3A-C). CRES2 and cystatin E2 also immediately transitioned to amyloid after dilution and formed matrices, films and fibrils (Fig 3B). The unique aggregation properties that we observed for each CRES subgroup member in vitro is similar to that in vivo. In addition to its presence in the amyloid matrix, CRES is also in the epididymal lumen as soluble monomers, dimers, and SDS-sensitive oligomers while CRES3 is only present in the amyloid matrix-containing particulate fraction (von Horsten et al., 2007; Whelly et al., 2016). Although we do not yet know the biological significance of the various amyloid structures that CRES members can adopt, they may allow the epididymal amyloid matrix to have some plasticity which may be important for function. Indeed, several peptides/proteins including amyloid- β and α-synuclein will adopt very different amyloid conformations depending on environmental conditions or even under the same growth conditions (Colletier et al., 2011; Gath et al., 2014). This suggests that amyloids in general may have the ability to be “shape shifters” for reasons that remain unclear but which possibly could be evolutionarily advantageous.
Figure 3. CRES subgroup members form amyloid in vitro.
A) Recombinant proteins in 6 M guanidine-Cl were diluted to 10 µM in buffer, ThT added to 20 µM and fluorescence immediately determined using a Synergy HT plate reader with excitation at 450 nm and emission at 485 nm. Values represent the mean relative fluorescence units (RFU) + SEM of four replicates. B) Negative stain EM of recombinant proteins following dilution into buffer. Scale bar, CRES left, CRES2, 366 nm; cystatin E2 left, 616 nm; CRES right, CRES3, cystatin E2 right, 100 nm. C) Dot blot analysis of immature (A11 positive) and mature (OC positive) forms of amyloid in CRES subgroup proteins. Five micrograms of each recombinant CRES subgroup protein were diluted into buffer and immediately spotted onto nitrocellulose membrane in a dot blot apparatus. The membranes were incubated with the conformation-dependent anti-oligomer A11 and anti-fibrillar OC antibodies. (Modified and reprinted from Whelly et al., 2016).
It is possible that interactions between CRES subgroup members may coordinate and control assembly of the epididymal amyloid matrix. Functional amyloids are thought to form under controlled conditions and having specific family members that interact could be a mechanism to determine when and where amyloid formation occurs. In preliminary studies we observed that recombinant CRES and CRES3 can heterooligomerize and form unique amyloid structures distinct from those formed by the individual proteins (Do, Hewetson and Cornwall, unpublished observations). Further, CRES3 amyloid facilitates amyloid formation in CRES monomers suggesting that it could mediate amyloid matrix formation possibly by serving as a nucleator to initiate assembly.
Alterations in the epididymal amyloid matrix are associated with epididymal pathologies
Using several different mouse models, we demonstrated that disruption of the amyloid matrix can result in epididymal pathology including infertility. Transgenic mice that overexpress a mutant, highly amyloidogenic form of human cystatin C (L68Q mutation), are unable to produce offspring due to profoundly impaired sperm motility and viability (Whelly et al., 2014). Examination of the epididymal amyloid matrix revealed a structure that had increased levels of cystatin C amyloid and that was more resistant to DMSO denaturation. Large agglutinated clumps of spermatozoa were often observed in the epididymal lumen. Incubation of epididymal luminal fluid containing the amyloid matrix from the L68Q mice recapitulated the motility defect in wildtype spermatozoa while luminal fluid depleted of the amyloid matrix did not (Whelly et al., 2014). These observations suggested the altered amyloid matrix had become cytotoxic to spermatozoa resulting in the poor viability and motility.
Knockout mice lacking the CRES gene (CRES KO) also have an epididymal phenotype. Mice developed an age-dependent lysosomal storage disease-like phenotype in the epididymal epithelium and an increased appearance of abnormal aggregates in the epididymal lumen (Parent et al., 2011). Intriguingly, the loss of the CRES gene also resulted in the profound down-regulation of the other CRES subgroup members CRES2, CRES3, and cystatin E2 in the epididymis supporting our idea that the four CRES proteins may carry out coordinated functions in the epididymal lumen (Whelly et al., 2016). Cystatin C in the epididymis was unaffected by the loss of CRES. Preliminary studies examining the structure of the amyloid matrix isolated from the KO mice revealed a matrix that was sparse and structurally distinct from that in wildtype mice (Fig. 4). This included an increased appearance of ball-like structures associated with fibrils. While we have yet to determine what these are, they may represent immature, oligomeric amyloid forms suggesting the loss of the CRES subgroup results in an altered, perhaps immature amyloid matrix. Although cystatin C is still present in the amyloid matrix from CRES KO mice, it is possible that other amyloidogenic proteins may also contribute to amyloid matrix formation and function; studies are ongoing to determine this. Together our studies suggest that cystatin C and CRES subgroup members are integral components of the epididymal amyloid matrix and that alterations in their levels can have a significant impact on amyloid matrix structure and likely function. In addition to a lysosomal storage disease phenotype in the epididymis, CRES KO mice also exhibit reduced fertility in vitro since spermatozoa are unable to undergo a progesterone-induced acrosome reaction (Chau and Cornwall, 2011). However, this phenotype is detected in both young and older mice and we believe is a result of alterations in CRES-containing amyloid structures in the mouse sperm acrosome rather than the epididymal luminal amyloid matrix (Guyonnet et al. 2012).
Figure 4. Epididymal amyloid matrix structure is altered in CRES KO mice.
The epididymal amyloid matrix was isolated from the initial segment region from age-matched CRES wildtype (WT) and CRES knockout (KO) mice and incubated with the protein aggregation disease (PAD) reagent (Microsens Biotechnologies, London, UK) to pulldown amyloid structures (Whelly et al., 2012). Proteins were eluted from the PAD beads by incubation in Laemmli buffer at 65°C for 15 min, spotted on to formvar/carbon coated 200 mesh nickel grids (Ted Pella, Redding, CA, USA) and stained with 2% uranyl acetate. Images were captured with a Hitachi H-8100 transmission electron microscope. Bar, top panels, 500 nm; Bar, bottom panels, 100 nm.
Finally, a recent review describing how the loss of androgens can affect the production of Aβ peptide and its role in the pathogenesis of Alzheimer’s disease raises the possibility that androgens might also impact epididymal amyloid matrix structure and function, particularly in aged animals (Lei and Renyuan, 2018).
Modeling epididymal amyloid matrix structure and function based on bacterial biofilms
We hypothesize the epididymal amyloid matrix may, in a broad sense, be structurally and functionally similar to a bacterial biofilm. In E.coli coordinated interactions between amyloidogenic curli family members coordinate assembly of the extracellular amyloid matrix that contributes to biofilm formation (Chapman et al., 2002). Functionally the biofilm unifies the resident cells into a community to protect them from host responses as well as to nurture the cells including providing nutrients to bacteria deep within the biofilm, allowing their survival. Although their roles are still poorly understood, extracellular vesicles (EVs) are part of bacterial biofilms and thought to be a means to deliver nutrients to the cells. EVs have also been shown to transport extracellular DNA, which is an essential component of some biofilms and which contributes to its stability and serves as a gene pool for horizontal gene transfer (Whitechurch et al.,2002; Liao et al., 2014).
Similar to bacterial biofilm, the epididymal amyloid matrix seems to require multiple amyloidogenic cystatin family members for appropriate formation; further our preliminary studies suggest that coordinated interactions between the amyloidogenic CRES subgroup members may be important for matrix assembly. We also have determined that EVs are within the epididymal amyloid matrix and that CRES subgroup members, including their amyloid forms, are associated with EVs in addition to their presence in the amyloid matrix (Whelly et al., 2016, and Cornwall, unpublished observations). Our preliminary studies also suggest that extracellular DNA is a component of the epididymal amyloid matrix (Myers and Cornwall, unpublished observations). Together these studies reveal striking structural similarities between the epididymal amyloid matrix and biofilm.
Analogous to biofilm function, we hypothesize the epididymal amyloid matrix may integrate spermatozoa into a community of cells to facilitate maturation and protection during epididymal transit. As a structure that is intimately associated with spermatozoa roles in sperm maturation are possible including participating in protein delivery to sperm, possibly via EVs, as part of the maturation process. It is also possible the amyloid matrix plays an important role in host defense and protects spermatozoa and the epididymal epithelium from ascending pathogens. CRES, CRES2, and cystatin C have established antimicrobial or antiviral activities in vitro and may represent a new class of antimicrobial proteins (AMPs) (Kasprzykowski et al., 2000; Hamil et al., 2002; Wang et al., 2012). This is supported by the fact that cystatins and the well-established cathelicidin family of AMPs are both cysteine protease inhibitors and based on structure belong to the same superfamily (Zhu 2008). In addition to cystatins and cathelicidin hCAP-18, the epididymal lumen is particularly rich in other AMPs including over 40 β-defensins and HE2/SPAG11 (Patil et al., 2005; Hall et al., 2007; Malm et al., 2000; Pujianto et al., 2013). It is possible these AMPs may also be a part of the amyloid matrix.
Finally, although the majority of our work on the epididymal amyloid matrix has been carried out in the mouse, CRES and CRES2 are also expressed in the human epididymis (Wassler et al., 2002; Hamil et al., 2002). Although their amyloidogenic properties have not yet been determined, a cystatin-rich amyloid matrix may also be present in the human epididymal lumen.
Summary
Taken together, we hypothesize the epididymal amyloid matrix represents an evolutionarily conserved structure that is related to bacterial biofilms and whose function is to protect and nurture spermatozoa. Studies are ongoing to further examine the biofilm properties of the epididymal amyloid matrix including determining if other antimicrobial proteins contribute to its structure, possibly as amyloids. Defining the mechanisms of how the CRES subgroup amyloids mediate amyloid matrix formation will also be important for understanding the regulation of functional amyloids and could provide insight of what may go wrong in neurodegenerative disease and prionopathies caused by pathological amyloids.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (HD056182 to G.A.C). The authors declare there are no conflicts of interest.
References
- 1.Asquith KL, Harman AJ, McLaughlin EA, Nixon B & Aitken RJ. (2005) Localization and significance of molecular chaperones, heat shock protein 1, and tumor rejection antigen gp96 in the male reproductive tract and during capacitation and acrosome reaction. Biol Reprod 72, 328–337. [DOI] [PubMed] [Google Scholar]
- 2.Caballero JN, Frenette G, Belleannée C & Sullivan R. (2013) CD9-positive microvesicles mediate the transfer of molecules to bovine spermatozoa during epididymal maturation. PLoS One 8:e65364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chapman MR, Robinson LS, Pinkner JS, Roth R, Heuser J, Hammar M, Normark S & Hultgren SJ. (2002) Role of Escherichia coli curli operons in directing amyloid fiber formation. Science 295, 851–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chau KM & Cornwall GA. (2011) Reduced fertility in vitro in mice lacking the cystatin CRES (cystatin-related epididymal spermatogenic): rescue by exposure of spermatozoa to dibutyryl cAMP and isobutylmethylxanthine. Biol Reprod 84,140–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cline EN, Bicca MA, Viola KL, & Klein WL. (2018) The Amyloid-β Oligomer Hypothesis: Beginning of the Third Decade. J Alzheimers Dis 64, S567–S610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colletier JP Laganowsky A, Landau M, Zhao M, Soriaga AB, Goldschmidt L, Flot D, Cascio D, Sawaya MR & Eisenberg D. (2011) Molecular basis for amyloid-beta polymorphism. Proc Natl Acad Sci USA 108,16938–16943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cornwall GA, Orgebin-Crist MC & Hann SR. (1992) The CRES gene: a unique testis-regulated gene related to the cystatin family is highly restricted in its expression to the proximal region of the mouse epididymis. Mol Endocrinol 6,1653–1664. [DOI] [PubMed] [Google Scholar]
- 8.Cornwall GA & Hann SR. (1995) Transient appearance of CRES protein during spermatogenesis and caput epididymal sperm maturation. Mol Reprod Dev 41, 37–46. [DOI] [PubMed] [Google Scholar]
- 9.Cornwall GA & Hsia N. (2003) A new subgroup of the family 2 cystatins. Mol Cell Endocrinol 200, 1–8. [DOI] [PubMed] [Google Scholar]
- 10.Cornwall GA. (2009) New insights into epididymal biology and function. Hum Reprod Update 15, 213–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cornwall GA, Von Horsten HH & Whelly S. (2011) Cystatin-related epididymal spermatogenic aggregates in the epididymis. J Androl 32, 679–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Egge N, Muthusubramanian A & Cornwall GA. (2015) Amyloid properties of the mouse egg zona pellucida. PLoS One 10:e0129907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fowler DM, Koulov AV, Alory-Jost C, Marks MS, Balch WE & Kelly JW. (2006) Functional amyloid formation within mammalian tissue. PLoS Biol 4:e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Frygelius J, Arvestad L, Wedell A & Töhönen V. (2010) Evolution and human tissue expression of the Cres/Testatin subgroup genes, a reproductive tissue specific subgroup of the type 2 cystatins. Evol Dev 12, 329–342. [DOI] [PubMed] [Google Scholar]
- 15.Gath J, Bousset L, Habenstein B, Melki R, Meier BH & Böckmann A.(2014) Yet another polymorph of α-synuclein: solid-state sequential assignments. Biomol NMR Assign 8, 395–404. [DOI] [PubMed] [Google Scholar]
- 16.Guyonnet B, Zabet-Moghaddam M, SanFrancisco S, & Cornwall GA. (2012). Isolation and proteomic characterization of the mouse sperm acrosomal matrix. Mol Cell Proteomics 11, 758–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hall SH, Yenugu S, Radhakrishnan Y, Avellar MC, Petrusz P & French FS. (2007) Characterization and functions of beta defensins in the epididymis. Asian J Androl 9, 453–462. [DOI] [PubMed] [Google Scholar]
- 18.Hamil KG, Liu Q, Sivashanmugam P, Yenugu S, Soundararajan R, Grossman G, Richardson RT, Zhang YL, O’Rand MG, Petrusz P, French FS & Hall SH. (2002) Cystatin 11: a new member of the cystatin type 2 family. Endocrinology 143, 2787–2796. [DOI] [PubMed] [Google Scholar]
- 19.Hatters DM, MacPhee CE, Lawrence LJ, Sawyer WH, Howlett GJ. (2000). Human apolipoprotein C-II forms twisted amyloid ribbons and closed loops. Biochemistry 39, 8276–8283. [DOI] [PubMed] [Google Scholar]
- 20.Hewetson A, Do HQ, Myers C, Muthusubramanian A, Sutton RB, Wylie BJ & Cornwall GA. (2017) Functional amyloids in reproduction. Biomolecules 29, 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsia N & Cornwall GA. (2003) Cres2 and Cres3: new members of the cystatin-related epididymal spermatogenic subgroup of family 2 cystatins. Endocrinology 144, 909–915. [DOI] [PubMed] [Google Scholar]
- 22.Iconomidou VA, Vriend G & Hamodrakas SJ. (2000) Amyloids protect the silkmoth oocyte and embryo. FEBS Lett 479, 141–145. [DOI] [PubMed] [Google Scholar]
- 23.Kasprzykowski F, Schalen C, Kasprzykowska R, Jastrzebska B & Grubb A. (2000). Synthesis and antibacterial properties of peptidyl derivatives and cyclopeptides structurally based upon the inhibitory centre of human cystatin C. Dissociation of antiproteolytic and antibacterial effects. APMIS 108,473–481. [DOI] [PubMed] [Google Scholar]
- 24.Kayed R, Canto I, Breydo L, Rasool S, Lukacsovich T, Wu J, et al. (2010) Conformation dependent monoclonal antibodies distinguish different replicating strains or conformers of prefibrillar Aβ oligomers. Mol Neurodegener 5, 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, McQuade T, Siemer AB, Napetschnig J, Moriwaki K, Hsiao YS, Damko E, Moquin D, Walz T, McDermott A, Chan FK & Wu H. (2012) The RIP1/RIP3 necrosome forms a functional amyloid signaling complex required for programmed necrosis. Cell 150, 339–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li Y, Friel PJ, Robinson MO, McLean DJ & Griswold MD. (2002) Identification and characterization of testis- and epididymis-specific genes: cystatin SC and cystatin TE-1. Biol Reprod 67,1872–1880. [DOI] [PubMed] [Google Scholar]
- 27.Li Y, Friel PJ, McLean DJ & Griswold MD. (2003) Cystatin E1 and E2, new members of male reproductive tract subgroup within cystatin type 2 family. Biol Reprod 69, 489–500. [DOI] [PubMed] [Google Scholar]
- 28.Li Y, Putnam-Lawson CA, Knapp-Hoch H, Friel PJ, Mitchell D, Hively R & Griswold MD. (2005) Immunolocalization and regulation of cystatin 12 in mouse testis and epididymis. Biol Reprod 73, 872–880. [DOI] [PubMed] [Google Scholar]
- 29.Liao S, Klein MI, Heim KP, Fan Y, Bitoun JP, Ahn SJ, Burne RA, Koo H, Brady LJ & Wen ZT. (2014) Streptococcus mutans extracellular DNA is upregulated during growth in biofilms, actively released via membrane vesicles, and influenced by components of the protein secretion machinery. J Bacteriol 196, 2355–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loksztejn A & Dzwolak W. (2009) Noncooperative dimethyl sulfoxide-induced dissection of insulin fibrils. Toward soluble building blocks of amyloid. Biochemistry 48, 4846–4851. [DOI] [PubMed] [Google Scholar]
- 31.Louros NN, Iconomidou VA, Giannelou P & Hamodrakas SJ. (2013) Structural analysis of peptide-analogues of human zona pellucida ZP1 protein with amyloidogenic properties: insights into mammalian zona pellucida formation. PLoS One 8, e73258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maji SK, Perrin MH, Sawaya MR, Jessberger S, Vadodaria K, Rissman RA, Singru PS, Nilsson KP, Simon R, Schubert D, et al. (2009) Functional amyloids as natural storage of peptide hormones in pituitary secretory granules. Science 325, 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Malm J, Sørensen O, Persson T, Frohm-Nilsson M, Johansson B, Bjartell A, Lilja H, Ståhle-Bäckdahl M, Borregaard N & Egesten A. (2000) The human cationic antimicrobial protein (hCAP-18) is expressed in the epithelium of human epididymis, is present in seminal plasma at high concentrations, and is attached to spermatozoa. Infect Immun 68, 4297–4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parent AD, Cornwall GA, Liu LY, Smith CE & Hermo L. (2011) Alterations in the testis and epididymis associated with loss of function of the cystatin-related epididymal spermatogenic (CRES) protein. J Androl 32, 444–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patil AA, Cai Y, Sang Y, Blecha F & Zhang G. (2005) Cross-species analysis of the mammalian beta-defensin gene family: presence of syntenic gene clusters and preferential expression in the male reproductive tract. Physiol Genomics 23, 5–17. [DOI] [PubMed] [Google Scholar]
- 36.Pham CLL, Kwan AH & Sunde M. (2014) Functional amyloid: widespread in Nature, diverse in purpose. Essays Biochem 56, 207–219. [DOI] [PubMed] [Google Scholar]
- 37.Podrabsky JE, Carpenter JF & Hand SC. (2001) Survival of water stress in annual fish embryos: dehydration avoidance and egg envelope amyloid fibers. Am J Physiol Regul Integr Comp Physiol 280, R123–131. [DOI] [PubMed] [Google Scholar]
- 38.Pujianto DA, Loanda E, Sari P, Midoen YH & Soeharso P. (2013) Sperm-associated antigen 11A is expressed exclusively in the principal cells of the mouse caput epididymis in an androgen-dependent manner. Reprod Biol Endocrinol 11, 59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sullivan R (2015) Epididymosomes: a heterogeneous population of microvesicles with multiple functions in sperm maturation and storage. Asian J Androl 17, 726–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Töhönen V, Osterlund C & Nordqvist K. (1998) Testatin: a cystatin-related gene expressed during early testis development. Proc Natl Acad Sci USA 95, 14208–14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turk V & Bode W. (1991) The cystatins: protein inhibitors of cysteine proteinases. FEBS Lett 285, 213–219. [DOI] [PubMed] [Google Scholar]
- 42.von Horsten HH, Johnson SS, SanFrancisco SK, Hastert MC, Whelly SM & Cornwall GA. (2007) Oligomerization and transglutaminase cross-linking of the cystatin CRES in the mouse epididymal lumen: potential mechanism of extracellular quality control. J Biol Chem 282, 32912–32923. [DOI] [PubMed] [Google Scholar]
- 43.Wahlbom M, Wang X, Lindström V, Carlemalm E, Jaskolski M & Grubb A. (2007) Fibrillogenic oligomers of human cystatin C are formed by propagated domain swapping. J Biol Chem 282, 18318–18326. [DOI] [PubMed] [Google Scholar]
- 44.Wang L, Yuan Q, Chen S, Cai H, Lu M, Liu Y & Xu C. (2012) Antimicrobial activity and molecular mechanism of the CRES protein. PLoS One 7:e48368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang X & Chapman MR. (2008) Curli provide the template for understanding controlled amyloid propagation. Prion 2, 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wassler M, Syntin P, Sutton-Walsh HG, Hsia N, Hardy DM & Cornwall GA. (2002) Identification and characterization of cystatin-related epididymal spermatogenic protein in human spermatozoa: localization in the equatorial segment. Biol Reprod 67, 795–803. [DOI] [PubMed] [Google Scholar]
- 47.Whelly S, Johnson S, Powell J, Borchardt C, Hastert MC & Cornwall GA. (2012) Nonpathological extracellular amyloid is present during normal epididymal sperm maturation. PLoS One 7: e36394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whelly S, Serobian G, Borchardt C, Powell J, Johnson S, Hakansson K, Lindstrom V, Abrahamson M, Grubb A & Cornwall GA. (2014) Fertility defects in mice expressing the L68Q variant of human cystatin C: a role for amyloid in male infertility. J Biol Chem 289, 7718–7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whelly S, Muthusubramanian A, Powell J, Johnson S, Hastert MC, Cornwall GA.(2016) Cystatin-related epididymal spermatogenic subgroup members are part of an amyloid matrix and associated with extracellular vesicles in the mouse epididymal lumen. Mol Hum Reprod 22, 729–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitechurch CB, Tolker-Nielsen T, Ragas PC & Mattick JS. (2002) Extracellular DNA required for bacterial biofilm formation. Science 295; 1487. [DOI] [PubMed] [Google Scholar]
- 51.Zhu S (2008) Did cathelicidins, a family of multifunctional host-defense peptides, arise from a cysteine protease inhibitor? Trends in Microbiology 16, 353–360. [DOI] [PubMed] [Google Scholar]