Abstract
Objectives
Type 2 diabetes mellitus (T2DM) and osteoarthritis (OA) are common diseases that frequently co-exist, along with overweight/obesity. While the mechanical impact of excess body weight on joints may explain lower limb OA, we sought to explore whether T2DM is linked to OA outside of excess weight and whether T2DM may play a role in OA pathophysiology. The consequence of T2DM on OA outcomes is a question of research interest.
Methods
We conducted a critical review of the literature to explore the association between T2DM and OA, whether any association is site-specific for OA, and whether the presence of T2DM impacts on OA outcomes. We also reviewed the literature to assess the safety of anti-OA treatments in patients with T2DM.
Results
T2DM has a pathogenic effect on OA through 2 major pathways involving oxidative stress and low-grade chronic inflammation resulting from chronic hyperglycemia and insulin resistance. T2DM is a risk factor for OA progression and has a negative impact on arthroplasty outcomes. Evidence is mounting for safety concerns with some of the most frequently prescribed anti-OA medications, including paracetamol, non-steroidal anti-inflammatory drugs, and corticosteroid injections, while other anti-OA medications may be safely prescribed in OA patients with T2DM, such as glucosamine and intra-articular hyaluronic acid.
Conclusions
Future research is needed to better understand whether diabetes control and prevention can modulate OA occurrence and progression. The selection of therapy to treat OA symptoms in patients with T2DM may require careful consideration of the evidence based to avoid untoward safety issues.
Keywords: type 2 diabetes mellitus, osteoarthritis, obesity, pathophysiology, safety
1.0. Introduction
Type 2 diabetes mellitus (T2DM) and osteoarthritis (OA) are common diseases that are predicted to increase in prevalence [1, 2]. OA and T2DM frequently co-exist simply by chance due to their high prevalence and shared risk factors. For example, the association of OA with obesity is well-supported [3], and obesity occurs in the majority of people with T2DM [4, 5]. Aging is a well-known risk factor for both T2DM and OA. The estimated prevalence in the US of T2DM is 4.6 million among individuals aged 18–44, and rises to 14.3 million people aged 45–64 and 12.0 million people aged ≥65 years [6]. Similarly, radiographically-defined knee OA increases dramatically with age, affecting 14% of adults aged over 25 years and 37% of those over the age of 60 years [7].
T2DM is a highly prevalent complex disease with a genetic background and the intervention of environmental risk factors, especially poor lifestyle habits that lead to overweight and obesity. The prevalence of the disease markedly increases with age, with >10% of the population aged ≥65 years having T2DM. The disease combines several defects, among which include a defect in insulin secretion by pancreatic beta-cells, and cellular insulin resistance mainly present in skeletal muscles and the liver but also in other tissues [8, 9]. Prolonged hyperglycemia, both in fasting and postprandial states, leads to advanced glycated end products (AGEs), oxidative stress and low-grade inflammation, and results in damage to the vessels, mainly in the heart, kidneys, eyes, nerves, but also other tissues [10].
Nearly half (47.3%) of patients with T2DM have some form of arthritis [11]. OA is a heterogeneous disorder affecting joints of the hand, hip and knee. Beside the various localizations, different phenotypes of OA have been proposed that include age-related, metabolic syndrome (MetS)-related (closely linked to abdominal adiposity), genetic-related, and post-traumatic OA [12, 13]. In MetS-associated OA, the mechanical impact of overweight/obesity on joints may easily explain lower limb OA [14]. Other components of MetS, including dysglycemia (that may be considered as equivalent to a prediabetic state), high blood pressure and atherogenic dyslipidemia may together or independently participate in OA pathophysiology [15–17]. Of note, more than three-quarters of patients with T2DM have MetS according to the unifying definition [18]. So far, the severity of symptomatic knee OA is found to be significantly associated with hypertension, dyslipidemia, and the number of MetS factors present; although no association between the severity of radiographic knee OA and MetS factors was found in the same study [19].
In this critical literature review, we seek to explore whether T2DM is linked to OA outside of weight overload and whether T2DM may play a role in OA pathophysiology. The consequence of T2DM on OA outcomes is also a question of research interest. There are multiple pharmacologic treatment options available which may provide adequate management of the symptoms of OA. However, evidence is mounting for safety concerns with some of the most frequently prescribed anti-OA medications, including paracetamol and non-steroidal anti-inflammatory drugs (NSAIDs) [20–23]. In addition, we have reviewed the available evidence to explore whether the co-presence of T2DM poses any additional safety issues for the treatment of OA.
2.0. Methods
Articles included in this narrative review were identified through literature searches of PubMed using the following MeSH items or free words: “osteoarthritis”, “type 2 diabetes mellitus”, “incidence”, “progression”, “epidemiology”, “pathophysiology”, “antidiabetic agent”, “paracetamol”, “NSAIDs”, “SYSADOA”, “corticosteroid”, “hyaluronic acid”, “bariatric surgery”. The search strategy was limited to studies conducted in humans, publications in English language, and full-length articles published from inception until January 15, 2018. A similar search was made in Scopus, EMBASE and Google Scholar in order to find relevant studies in this field. Finally, the reference lists of relevant articles (particularly review articles) were scrutinized for potentially interesting articles.
3.0. Incidence and progression of OA in people with T2DM
An association between the occurrence of T2DM and OA has been demonstrated, although a causal link is not well established. In a meta-analysis of 49 studies involving more than 1 million participants (N = 1,192,518), Louati et al. found that OA and T2DM were significantly associated [14]. The odds ratio (OR) of T2DM in the OA population vs. non-OA population was 1.41 (95% CI 1.21, 1.65), and the prevalence of T2DM among patients with OA was 14%. The overall presence of OA in the T2DM population vs. non-T2DM population (OR) was 1.46 (95% CI 1.08, 1.96). In this population (mean age 61 years), the prevalence of OA among patients with T2DM was 30% (38% hand OA, 12% hip OA, and 17% knee OA) [14]. In another meta-analysis including only studies controlling for weight or body mass index (BMI), the presence of OA in patients with T2DM was also high, with an OR of 1.25 vs. non-T2DM population (95% CI 1.05, 1.46) [24].
Data for an association between T2DM and site of OA gives variable outcomes; an association between T2DM and hand OA is found in some studies and a meta-analysis [14, 3]. The association is robust, especially for younger patients: those aged 55-62 years with T2DM had a two-times higher rate of hand OA than non-diabetics, which was associated with pain in erosive hand OA [25, 26]. However, a recent case-control study using a UK population-based database of people aged 30-90 years found no significant association between T2DM and hand OA, although the number of OA and/or diabetic patients may have been underestimated due to diagnostic uncertainties [27]. The association between diabetes and erosive hand OA warrants further investigation.
T2DM may be a risk factor for OA progression; T2DM is found to be a risk factor for disease progression in men with established knee OA based on annual assessment of joint space narrowing (JSN), while no relationships were found between MetS or other metabolic factors and radiographic progression [28]. T2DM is an independent risk predictor for arthroplasty. After controlling for age, BMI, and other potential confounders, the presence of T2DM doubles the risk of severe OA necessitating arthroplasty (HR = 2.1; 95% CI 1.1,3.8; p=0.023). T2DM was associated with more severe clinical symptoms of OA and structural joint changes [29].
Conversely, in a population-based case-control study (N = 94,609), no association was found between DM (almost 20 times more T2DM than type 1 diabetes in this cohort) and total joint replacement (TJR) of either the hip or knee among OA patients with or without DM [24]. T2DM may have a negative impact on arthroplasty outcomes including an increased risk of post-surgical death, decreased functional outcomes for arthroplasty, increased rate of infection, and increased need for revision arthroplasty [10]. Some arthroplasty outcomes may pertain to metabolic effects on joint tissues such as defective bone healing, while others reflect general risks of T2DM associated with major surgical procedures, while all can add considerable cost to clinical care [10].
3.1. Could anti-diabetic medications be important?
Whether anti-diabetic medications impact on OA outcomes has been investigated in an analysis of longitudinal data from the Osteoarthritis Initiative study, finding that medication-treated diabetes has no effect on knee OA incidence (OR = 0.53; 95% CI 0.23, 1.5), but reduces knee OA progression, measured as JSN or knee replacement therapy (OR = 0.66; 95% CI 0.44–0.98) [30].
Metformin is the first recommended antidiabetic drug for the management of T2DM. In a UK cohort study set within the Consultations in Primary Care Archive, of 3,217 patients with T2DM, there was no association between prescribed metformin treatment at baseline and OA outcome during follow up (adjusted HR = 1.02; 95% CI: 0.91, 1.15) [31]. However, in a case-control study performed in Taiwan, patients who have OA and T2DM receiving combination NSAIDs and metformin therapy had lower joint replacement surgery rates than those without metformin (adjusted HR = 0.742; 95% CI 0.601, 0.915; p = 0.005) [32]. It has been suggested that this effect may be attributable to a reduction in pro-inflammatory factors associated with combined therapy with metformin. Indeed, metformin and even more thiazolidinediones are antidiabetic agents that have been shown to exert some anti-inflammatory activity [33]. However, a large population based case-control study was performed in UK using the Clinical Practice Research Datalink; it did not find any evidence for a disease modifying osteoarthritic effect of thiazolidinediones [34], despite promising results from animal in vivo studies [34]. Finally, there are no clinical data available yet with new antidiabetic agents such as incretin-based therapies - dipeptidyl peptidase-4 (DPP-4) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists or sodium-glucose cotransporter type 2 (SGLT2) inhibitors.
4.0. Pathophysiology of OA and association with diabetes
OA is a complex disease affecting all joint tissues of articular cartilage, subchondral bone, and synovium. OA is associated with a local and systemic low-grade inflammation state [35]. Articular cartilage comprises an extracellular matrix containing chondrocytes, responsible for synthesis of the extracellular matrix. One role of cartilage is to absorb the mechanical stresses between two mobile bone surfaces; in OA the stress is followed by an increase of pro-inflammatory mediator production by chondrocytes, including cytokines (interleukin-1β [IL-1β]), tumor necrosis factor-α (TNF-α), radical oxygen species, AGEs, and prostaglandins. This local inflammation induces an increase in production of proteolytic enzymes (matrix metalloproteinases [MMPs] and aggrecanases) that will digest the cartilage matrix.
T2DM has a pathogenic effect on OA through 2 major pathways: 1) Chronic hyperglycemia, which induces oxidative stress, overproduction of pro-inflammatory cytokines and AGEs in joint tissues; and 2) Insulin resistance, which could play a role locally but also through the systemic low-grade inflammatory state [35]. Leptin, a major adipokine secreted mostly by adipose tissue, is able to promote chondrocyte apoptosis and also increase cytokine and MMP production by chondrocytes [36]. An insulin-resistant state and obesity are also associated with elevated free fatty acids (FFAs), which may modulate OA progression [37].
4.1. Role of insulin/insulin resistance
The role of insulin on OA remains controversial, especially because high insulin levels are associated with insulin resistance in T2DM, so that it is not easy to distinguish between the effects of insulin per se and the effects related to insulin resistance [38, 39]. Human chondrocytes express functional insulin receptors that respond to physiologic insulin concentrations. The insulin receptors seem to be more abundant in normal than in OA chondrocytes, and some responses are impaired while others appear fully activated [40]. It has been suggested that excess insulin as seen in T2DM patients may damage cartilage.
In immortalized human chondrocytes and cultures of primary human chondrocytes, it is found that that insulin downregulates autophagy by reducing LC3 II expression and increasing Akt and rpS6 phosphorylation. Autophagy is an essential homeostasis mechanism in articular cartilage, that is defective in T2DM and OA. Loss of proteoglycans and increased MMP-13 and IL-1β expression was observed after insulin treatment. Furthermore, chondrocytes from diabetic patients with OA showed decreased LC3 and increased p-rpS6 expression compared to healthy subjects and non-diabetic OA patients [41].
Insulin resistance and T2DM are often a consequence of visceral obesity, which is an important source of pro-inflammatory cytokines, causing low-grade chronic metabolic inflammation that can lead to structural damage in the joints [42]. Insulin resistance might impair joint tissue because of a local insulin resistance of diabetic synovial membrane [43], but also by the systemic low-grade inflammation state [35]. The synovium is shown to develop insulin resistance in obese OA patients with T2DM [44]. Insulin is a critical negative regulator of synovial inflammation and catabolism, and the development of insulin resistance in obese individuals would diminish the ability of insulin to suppress the production of inflammatory and catabolic mediators that promote OA [43].
The systemic role of MetS associated with insulin resistance in OA pathophysiology is now better understood, but new avenues of research are being pursued to better decipher the insulin resistance/MetS-associated OA phenotype [45].
4.2. Diabetes and cartilage/chondrocytes
Cartilage is a non-vascularized and non-innervated tissue and receives nutrients from its connection with subchondral bone and synovial fluid through joint cavity. Chondrocytes are glycolytic cells that express glucose transporters (GLUT) (especially GLUT-1, GLUT-3 and GLUT-9) and are able to sense glucose concentration in the media and adapt GLUT expression under normal conditions [46]. The capacity of chondrocytes to adapt themselves to local glucose level is lost during OA, which is responsible for high glucose uptake and potential glucose toxicity [47]. Local high glucose concentration leads to a reduction in chondrogenic differentiation of mesenchymal, muscle, and adipose-derived stem cells, which may further decrease the potential regeneration of cartilage that is already decreased in OA [35].
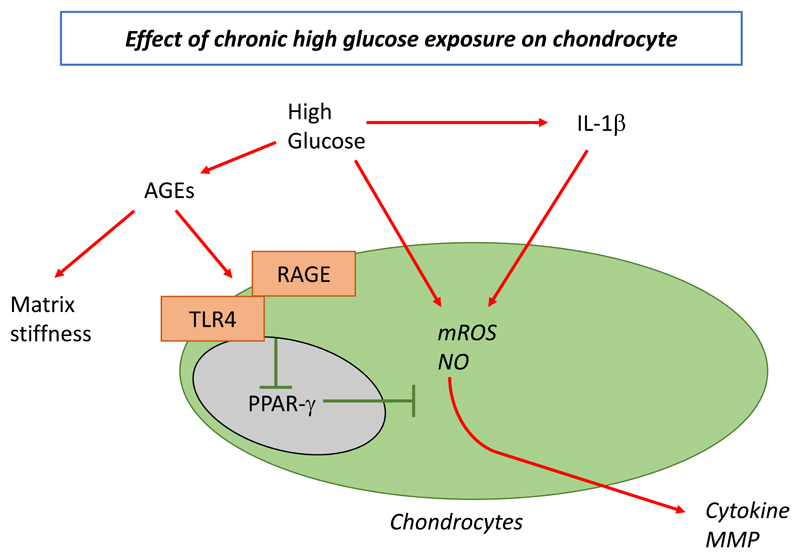
Chronic high glucose environment also has a noxious effect on chondrocyte metabolism. High glucose concentration has a pro-inflammatory and pro-degradative effect on human OA chondrocytes. AGEs are known to accumulate in cells and tissues under high glucose concentration and accumulate with age in OA cartilage modifying its mechanical properties, including stiffness and resistance. An association between the levels of AGEs and degradation of cartilage has been observed [48], although this finding is not universal [49]. AGEs can also induce a pro-inflammatory and pro-catabolic phenotype of chondrocytes via RAGE (receptor of AGE) and toll-like receptors (Figure 1). Activation of these receptors decreases peroxisome proliferator-activated receptor gamma (PPAR-γ), which induces oxidative stress (mitochondrial reactive oxygen species and nitric oxide) and cytokine release by chondrocytes [50]. High glucose rate increases MMP expression and oxidative stress in chondrocytes and also elevates the effect of IL-1β on cytokine release.
Figure 1.
Pathologic association of type 2 diabetes mellitus and osteoarthritis: involvement of hyperglycemia in local chondrocyte activation
AGEs, advanced glycation end-products; IL-1β, interleukin-1 beta; MMP matrix metalloproteinase; mROS, mitochondrial reactive oxygen species; NO, nitric oxide; PPAR-γ, peroxisome proliferator-activated receptor gamma; RAGE, receptor of AGE; TLR4, toll-like receptor 4.
Reprinted from Diabetes Research and Clinical Practice, 122, Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: What are the links? 198-206, Copyright (2016), with permission from Elsevier.
4.3. Diabetes and synovium
The effect of hyperglycemia on the synovium is less well understood. High glucose concentration increases pro-angiogenic factor expression in synovial fibroblasts via oxidative stress [51]. Synovial angiogenesis is known to induce pro-inflammatory cells locally. Diabetes induces more synovial inflammation in in vivo models [52], which is in line with clinical observations of more synovitis in knee OA in diabetic than in non-diabetic patients [29]. The synovium from OA patients with T2DM contained markedly more macrophages and showed elevated TNFα levels as compared to the synovium from OA patients without diabetes. Moreover, insulin-dependent phosphorylation of insulin receptors and serine/threonine kinase Akt (a key player in the intracellular cascade of insulin action) was blunted in cultures of OA fibroblast-like synoviocytes from patients with T2DM, supporting the presence of insulin resistance in the synovium of T2DM patients with OA [44].
4.4. Diabetes and subchondral bone
Diabetes is known to decrease bone remodeling; high fasting glucose concentration is associated with bone marrow lesions in the knee joint, which are known to predict OA structural damage [53]. The loss of subchondral bone in diabetic advanced knee OA is identified by a lower bone mineral density and a higher porosity, independent of weight [54]. AGE accumulate in subchondral bone of diabetic patients more than in non-diabetic patients, which may impact the mechanical resistance of subchondral bone and display pro-inflammatory effects [55].
4.5. Diabetes and microvascular changes
It is widely known that diabetes can lead to microvascular changes that increase the risk of some osteoarticular conditions, such as inflammatory peri-arthritis of the shoulder [56], Dupuytren’s contracture [57], shoulder hand syndrome of upper extremities and Charcot joints [58], often involving the lower extremities (e.g. ankle, disappearing bones of the foot) [58].
The same mechanism (i.e. the presence of microvascular changes) seems to contribute to the association between diabetes and OA. Sub-chondral bone has multiple arterial inlets and venous outlets. In the case of long bones (such as hip), four arterial inputs, the nutrient artery, periosteal arteries, metaphyseal arteries and epiphyseal arteries are present [59]. In particular, subchondral areas of long bones are highly vascularized, suggesting high nutrient requirements [60]. Since diabetes is able to lead to relevant microvascular changes it is likely that diabetes can increase the risk of OA also through this pathway.
5.0. Role of physical exercise in the prevention and management of T2DM
Lifestyle measures, such as physical activity and body weight control, are increasingly recognized as important approaches for the prevention and treatment of non-communicable diseases [61]. However, while physical exercise is recognized as an important component of the management of OA [62], the presence of lower limb OA (hip or knee) can restrict the possibility to perform physical exercise. Both optimal nutrition and physical activity can form part of a healthy lifestyle for people with T2DM and may play a role in the prevention of T2DM. The American Diabetes Association (ADA) recognizes lifestyle management as a fundamental aspect of diabetes care, which includes diabetes self-management, education and support, medical nutrition, and physical activity [63]. The ADA recommends that most adults with T2DM should engage in 150 min or more of moderate-to vigorous intensity aerobic activity per week, along with resistance exercise of 2-3 sessions/week, and flexibility training and balance training 2-3 times/week for older adults with T2DM.
However, concerns over a lack of physical activity in the general population has led to a call to action for making physical activity assessment and prescription a medical standard of care in daily practice in the US [64]. While there is no strong evidence that diet alone or physical activity alone can influence the risk of T2DM, the combination of diet plus physical activity reduces or delays the incidence of T2DM in people with impaired glucose tolerance (‘pre-diabetic’ people) [65, 66].
Intervention programs that include physical activity may halve the risk of developing T2DM among people at high-risk [67–69], and the reduction in incidence can persist for 10 years following the initial intervention [70]. There are several biologically-plausible mechanisms by which physical activity may be linked to a lower risk of T2DM, including weight loss, improved insulin sensitivity, improved endothelial function, and improved autonomic nervous system function [71]. Exercise has a positive effect on systemic glucose homeostasis and, in T2DM, exercise does not decrease insulin secretion [72]. Physical activity and exercise are known to have a beneficial effect on a variety of factors relevant to diabetes and cardiovascular disease, including blood pressure, lipid profiles, and body composition [73]. A significant reduction in both cardiovascular and all-cause mortality has been shown in long-term follow-up of a lifestyle intervention among people with impaired glucose intolerance [74].
6.0. Safety of anti-osteoarthritis medicines in diabetic patients
6.1. Paracetamol
Paracetamol is widely used as first-line rescue analgesia in OA, even though the benefit of paracetamol on pain, physical function and stiffness is minimal [62, 75, 76]. Moreover, increasing evidence of gastrointestinal, cardiovascular, hepatic and renal adverse events with paracetamol raise questions over its routine, chronic use, especially at the upper end of standard analgesic doses [20].
Reports of non-overdose paracetamol-associated acute liver failure leading to transplantation, and staggered paracetamol overdoses leading to paracetamol-induced hepatotoxicity are a cause of concern with widespread, unrestricted paracetamol use [77, 78]. People taking paracetamol are nearly 4-times more likely to have abnormal liver function tests [79]. Non-alcoholic fatty liver disease (NAFLD) and the more severe form steatohepatitis (NASH) occur frequently in around 50-70% of patients with T2DM [80, 81]. They affect both those with elevated aminotransferases and those with normal levels [82]. Obesity and T2DM are also often associated with NAFLD and both obesity and NAFLD are able to increase the risk and severity of hepatotoxicity of different drugs, including paracetamol [83, 84]. Although there is limited evidence as yet [85], because paracetamol hepatotoxicity and liver alterations in NAFLD/NASH-associated liver alterations share some common mechanisms, potentiation of harm cannot be excluded [86]. Furthermore, data suggest that paracetamol toxicity is increased in diabetic animal models [87]; conversely, metformin may provide some protection against paracetamol hepatotoxicity [88, 89]. Nevertheless, in the absence of further clinical data, caution should be recommended when considering the use of high-dose paracetamol for the management of OA-related pain in patients with T2DM and advanced NAFLD/NASH.
While it is currently viewed that paracetamol can be administered to patients with liver impairment, as the prevalence of lifestyle-related liver diseases such as NAFLD is likely to increase, studies to further the understanding of changes to paracetamol metabolism, efficacy and toxicity will be invaluable [90]. A small study reported that people with T2DM eliminate paracetamol with more difficulty than healthy controls [91]. Indeed, further studies are warranted to answer these research questions: Is the risk of liver toxicity with paracetamol increased in the diabetic population? And, can paracetamol be prescribed in patients with T2DM and NAFLD/NASH?
6.2. Non-steroidal anti-inflammatory drugs (NSAIDs)
6.2.1. NSAIDs and renal risk
NSAIDs are widely used and have been linked to acute kidney injury (AKI), chronic kidney disease, and cardiovascular disease [21]. T2DM causes renal failure, and T2DM and hypertension have become the most common causes of end-stage renal disease responsible for more than 50% of cases [92]. NSAID use is associated with an increased risk of hospitalization in high-risk populations; for example, for every 10,000 people treated for 30 days with NSAIDs, there were 20 extra hospitalizations in the diabetes population (incidence rate ratio [IRR] 1.31; 95% CI 1.08, 1.60), compared with those not treated with NSAIDs [93, 94].
NSAID users have a 3-fold greater risk for developing a first-ever diagnosis of AKI compared with non-NSAID users in the general population [95]. Hypertension (83%), arthritis (71%), heart failure (44%), CKD (36%) and diabetes (35%) are prevalent among NSAID users [21]. Although antihypertensive drugs have cardiovascular benefits, vigilance may be warranted when they are used concurrently with NSAIDs. Moreover, AKI caused by drug-drug interactions between NSAIDs and angiotensin-converting enzyme inhibitors (ACEIs), angiotensin receptor blockers (ARBs), or diuretics is a frequently reported adverse drug reaction, of which around one-quarter of cases is serious AKI [96]. The use of selected cardiovascular drugs is associated with a 5-fold increase in risk for AKI. Diuretics present the greatest risk, and the risk increases with concomitant use of NSAIDs and diuretics (relative risk [RR] = 11.6; 95% CI 4.2, 32.2) and NSAIDs and calcium channel blockers (RR = 7.8; 95% CI 3.0, 20.5) [95]. AKI risk is highest among users of loop diuretic/aldosterone antagonist combinations, in those over 75 years of age, and in those with renal impairment [97, 98].
NSAID use among survivors of an AKI event is common, with around 68% of survivors found taking NSAIDs both before and after the AKI event. A history of arthritis (OR: 3.00; 95% CI: 1.92, 4.68) and paracetamol use (OR: 2.43; 95% CI: 1.50, 3.93) is significantly associated with NSAID use, while prevalent CKD (OR: 0.63; 95% CI: 0.41, 0.98) and diabetes (OR: 0.44; 95% CI: 0.29, 0.69) are not [21].
Antidiabetic agents are frequently associated with diuretics (~25-35%) and blockers of the renin-angiotensin system (ACEI/ARB) (~50–80%) [99]. SGLT2 inhibitors (dapagliflozin, canagliflozin, empagliflozin and ipragliflozin) represent a novel approach for the management of T2DM promoting glucosuria, osmotic diuresis and natriuresis [100]. SGLT2 inhibitors are generally well-tolerated, even if some severe adverse events have also been reported [101]. Caution is particularly recommended in the elderly population, even more treated with (loop-)diuretics, because of a higher risk of orthostatic hypotension and dehydration, which may lead to renal impairment. Indeed, despite encouraging renal safety outcomes reported in clinical cardiovascular outcome trials, scattered reports suggested that there might be a risk for AKI [102]. As recently discussed, a major decline in kidney function occasionally occurs, often associated with an acute illness or with specific co-administered medications, including NSAIDs [102]. Thus, the safety of SGLT2 inhibitors in combination with NSAIDs certainly deserves further specific investigation. So far, the avoidance of NSAIDs in patients on SGLT2 inhibitors is recommended [22].
6.2.2. NSAIDs and cardiovascular risk
The burden of cardiovascular disease among patients with T2DM is substantial; around one third to a half of all adults with T2DM has coronary heart disease, which is the leading cause of early death in patients with T2DM. T2DM is an independent risk factor for all manifestations of coronary heart disease, particularly heart failure, angina pectoris, re-infarction disability and sudden cardiac death for which rates among patients with T2DM are at least twice that observed in non-diabetic patients [103].
All non-selective NSAIDs and COX-2 inhibitors alike have the potential for gastrointestinal and cardiovascular (CV) toxicity [23, 104, 105]. Drug choice within the available NSAIDs class has therefore been dictated by safety profile, according to different risk factors and patient’s concomitant diseases and medical conditions [62]. While it was previously thought that selectivity of the NSAID for the COX-2 enzyme governed the CV toxicity profile, recent results suggest that CV risk may be drug specific; rofecoxib is the only NSAID associated with an increased risk of CV events compared to all other NSAIDs (OR = 1.61; 95% CI 1.31, 1.98) [106], although etoricoxib may have a greater risk than celecoxib, while celecoxib poses a similar risk to naproxen and a reduced risk of major CV event compared with ibuprofen in OA patients (HR = 0.84; 95% CI 0.72, 0.99) [107, 108]. In patients with T2DM, hypertension, and OA, at equally effective doses for OA management, treatment with rofecoxib but not celecoxib or naproxen induced a significant increase in 24-hour systolic blood pressure [109].
In a recent study using Danish population-based health registries, the cardiovascular risk of diclofenac initiation was compared with initiation of other traditional NSAIDs, paracetamol, and no initiation. The event rate of major adverse cardiovascular events within 30 days of initiation among diclofenac users increased by 50% compared with non-initiators (IRR = 1.5; 95% CI 1.4, 1.7), 20% compared with paracetamol or ibuprofen initiators (both IRR = 1.2; 95% CI 1.1, 1.3), and 30% compared with naproxen initiators (IRR 1.3; 95% CI 1.1, 1.5). Although the relative risk of major adverse cardiovascular events was highest in individuals with low or moderate baseline risk (i.e., diabetes mellitus), the absolute risk was highest in individuals with high baseline risk (i.e., previous myocardial infarction or heart failure) [110]. In a study of diabetes patients treated in primary care in Saudi Arabia (N=443; March 2016), inappropriate prescribing of NSAIDs was found in 66% of patients at high cardiovascular risk, contravening current clinical guidelines and recommendations of regulatory agencies [111].
6.3. Topical NSAIDs
For OA of the hands and knees, topical NSAIDs provide efficacy, with far less systemic distribution than oral NSAIDs [112, 113]. Observational studies and post-hoc analysis of pooled data from placebo-controlled trials suggested that long-term topical diclofenac sodium 1% treatment is safe in the subpopulation of patients with an elevated risk of NSAID-related adverse events, such as the elderly and those with the comorbidities of T2DM, hypertension, and cardiovascular disease [114, 115].
6.4. SYSADOAs
Slow Acting Symptomatic Drugs in Osteoarthritis (SYSADOAs) include diverse medications possibly able to interfere with disease pathogenetic processes, inducing a moderate symptomatic effect with slow onset of action and, in some cases, a joint structure-modifying effect in long-term trials [116–121]. They include mainly glucosamine (prescription crystalline glucosamine sulfate [pCGS] and over-the-counter glucosamine sulfate or hydrochloride), chondroitin sulfate, diacerein and avocado soybean unsaponifiables, with different levels of evidence for efficacy [122–125]. Clinical trials and systematic reviews outline a very good safety profile of the SYSADOAs glucosamine and chondroitin sulfate [126–129], and some adverse events with diacerein and avocado soybean unsaponifiables [130, 131]. No concern with respect to T2DM or any interaction with glucose metabolism has been noted in clinical trials or meta-analyses for chondroitin sulfate, diacerein or avocado soybean unsaponifiables. However, questions have been raised over time on a possible interference of glucosamine with glucose metabolism, based on precise mechanistic hypotheses.
Insulin resistance, a major contributing factor to the pathogenesis of T2DM, is characterized by reduced rates of insulin-mediated glucose uptake e.g. into skeletal muscle or adipocytes. Hyperglycemia induces or worsens insulin resistance, and in vitro studies show that glucose-induced insulin resistance involves impaired recruitment of intracellular glucose transporters to the cell surface. Glucosamine is a product of glucose metabolism and, in vitro, was shown to substitute for glucose and be more potent in promoting glucose transport desensitization [132]. Actually, in vitro studies showed that high glucosamine concentrations may increase the activity of the hexosamine pathway, a metabolic process functioning as a nutrient sensor and modulating insulin sensitivity and glucose uptake [133]. Effects of glucosamine on insulin resistance via the hexosamine pathway can be seen in vitro (in rodents), mainly at dramatically high concentrations [134]. Excessive emphasis was given to this pathway for postulated glucosamine toxicity, since it was wrongly believed that this was also the mechanism of action for the therapeutic effect in OA (stimulation of proteoglycans synthesis).
However, parenteral administration of glucosamine to humans at similarly high concentrations in repeated studies failed to show similar results, indicating species differences in sensitivity, or possible mechanistic differences i.e. less operational relevance of the hexosamine pathway in the regulation of insulin sensitivity in humans [135, 136]. Clinical trials at oral recommended doses for OA treatment showed no interference with glucose metabolism in normoglycemic subjects and in most subjects with hyperglycemia, insulin sensitivity impairment, pre-diabetes or diabetes [137, 138]. Additional analyses of the GUIDE study found that pCGS (1500 mg/day) for 6 months does not modify plasma glucose levels in the overall population, or in hyperglycemic patients [139, 140]. Systematic reviews on the effects of glucosamine on glucose metabolism in humans find that administration of glucosamine at usual oral doses in humans or OA patients in clinical trials is well-tolerated by normal, diabetic, or pre-diabetic patients [141, 142]. However, a minority of clinical studies have indicated non-significant trends for interference in some of the latter subjects. A non-significant increase in glycated hemoglobin levels was found in overweight women who received pCGS treatment for 2.5 years in the PROOF trial, and with up to 6.5 years of follow-up [143, 144]. Thus, in accordance with the summary of Product Characteristics for pCGS, it seems reasonable to advise caution at the start of treatment with glucosamine in diabetic patients, with no specific recommendations for other patients [145].
6.5. IA corticosteroids
Intra-articular (IA) corticosteroid injections may be used for the local symptomatic control of joint arthritis due to their anti-inflammatory properties. However, it has been shown that locally-injected corticosteroids are absorbed into the systemic circulation [146]. Parentally-administered steroids are known to affect glucose metabolism and can cause abnormal blood glucose levels in patients with diabetes, which may be a concern when administering IA corticosteroid injections.
Choudhry et al. recently performed a systematic review to identify studies that examined the effect of IA corticosteroid injections on blood glucose levels in patients with diabetes [147]. They identified 532 citations, however, after inclusion criteria were applied only 7 studies with a total of 72 patients were included in the meta-analysis. All studies showed a rise in blood glucose levels following IA corticosteroid injections, which was described as substantial in 4 studies, reaching a peak as high as 500 mg/dL. The timing of the peak glucose levels may vary from several hours post injection to 10-15 days. Consequently, patients with diabetes should monitor their blood glucose levels following IA corticosteroid injections for 24-48 hours post-injection for a potential risk of hyperglycemia, and antihyperglycemic therapies should be adjusted accordingly. Insulin-treated patients with T2DM commonly require transient up-titration of insulin doses after IA corticosteroid injections. Patients with diabetes who are taking insulin should be encouraged to check their blood glucose levels at a minimum of twice daily after receiving an intra-articular corticosteroid injection in order to determine whether they need short-acting insulin.
6.6. Hyaluronic acid
Intra-articular hyaluronic acid (IAHA) is a local treatment modality targeted to avoid the systemic side effects usually observed after IA corticosteroid injection (of particular difficulty in patients with T2DM), or after oral administration of analgesics and NSAIDs [148]. It has been reported that the results obtained with IAHA are not significantly different from continuous oral NSAID treatment used in the short and medium term, i.e. providing moderate symptomatic relief of knee OA pain, function and stiffness [149]. IAHA was superior to IA corticosteroids from 8 to 26 weeks post-treatment regarding knee pain [150]. IAHA is a safe alternative to oral NSAIDs and opioids for OA; a systematic review and meta-analysis of the safety of 18 HA products involving over 13,000 patients found a very low incidence of adverse events, most commonly transient local reactions that subsided rapidly [151].
IAHA injections have demonstrated clinical benefit in knee OA and seem to offer a good benefit-risk balance among the pharmacologic options [152, 153]. Based on the available evidence and guidelines, the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) recommends using IAHA in patients with knee OA remaining symptomatic despite continuous or intermittent treatment with conventional pharmacologic treatment modalities, i.e., paracetamol, SYSADOAs and NSAIDs, as well as in patients with co-morbidities precluding the use of NSAIDs or IA corticosteroids, such as diabetic patients [148, 154, 155]. No interaction of IAHA use in OA with glucose metabolism or association with hyperglycemia has been reported in the current literature to date, even in patients who received repeated HA cycles over several years among whom diabetes was a common comorbidity (16% of patients) [156]. HA appears to be safe in diabetic patients and a meta-analysis reported that HA is beneficial in treating diabetic foot by increasing the rate of wound healing [157] suggesting a potential (albeit peculiar) role of HA in treating OA in diabetic people.
7.0. Bariatric surgery
Obesity is a well-known risk factor for both T2DM [5] and OA [158, 159]. Obesity accelerates the development of OA of the knee and hip by exerting deleterious effects on joints through both biomechanical and also systemic inflammatory changes [159]. Bariatric/metabolic surgery has proven to be the most successful procedure to improve glucose control and sustained diabetes remission has been reported in many patients. The success is explained not only by the remarkable and sustained weight reduction, but also by several endocrine and metabolic improvements [160]. Besides the correction of hyperglycemia, bariatric surgery also improves the different components of MetS, hypertension, atherogenic dyslipidemia and insulin resistance, leading to better CV outcomes [161]. In addition, several studies reported a reduction in low-grade inflammation and various inflammatory markers [162–164].
The inflammatory pathway reduction following weight loss obtained through bariatric surgery is connected both with the improvement of some rheumatic diseases and a reduction in medication use (steroids and NSAIDs) [165]. Bariatric surgery with subsequent marked weight loss is likely to improve knee pain, joint function and stiffness in (morbidly) obese and obese adult patients with T2DM. However, with the current available evidence, there is further need for high-quality studies [159, 166]. Among a cohort of participants with severe obesity undergoing bariatric surgery, a large percentage experienced improvement, compared with baseline, in pain, physical function, and walk time over 3 years, but the percentage with improvement in pain and joint function decreased between year 1 and year 3 [167].
According to a systematic review and meta-analysis, for most peri-operative outcomes, bariatric surgery prior to total hip or total knee arthroplasty does not significantly reduce the complication rates or improve the clinical outcome [168]. However, decreased operative time and length of hospital stay were observed among patients who underwent total hip or knee arthroplasty after versus before their bariatric surgery [169]. Optimal timing of orthopedic and bariatric surgery has yet to be established. There are also financial and ethical implications of use of bariatric surgery for risk reduction before total joint arthroplasty [170]. Fundamental clinical questions remain regarding the optimal management of obesity with T2DM and lower extremity OA, which should be the focus of future collaborations across disciplines providing care to patients with both conditions [159].
8.0. Discussion
While T2DM and OA are known to frequently coincide (along with obesity/overweight, and frequently in the context of MetS), whether any causal relationship between the two disorders exists is a question of research interest. Analysis of studies involving more than 1 million participants shows a clear association of T2DM and OA, and an elevated risk of OA is demonstrated in patients with T2DM even when controlled for body weight.
Studies finding an association between T2DM and hand OA raise the question of whether DM impacts on the pathophysiology of OA beyond that which may easily be explained, e.g. the mechanical impact of overweight/obesity (which often accompanies T2DM) on lower limb OA. An association between pain in erosive hand OA and diabetes (in type 2 but also in type 1, may be linked to low grade inflammation related to MetS) requires further investigation.
Evidence supports a role for T2DM on OA pathogenesis through two major pathways: oxidative stress resulting from chronic hyperglycemia and leading to overproduction of pro-inflammatory cytokines and AGEs in joint tissue; and insulin resistance which may impact both locally and through systemic low-grade chronic inflammation. Dysglycemia, including T2DM, is only one factor of MetS, and whether other components of MetS including high blood pressure and atherogenic dyslipidemia together or independently impact on OA pathophysiology remains to be explored.
The impact of T2DM on OA outcomes is also of research interest. The presence of T2DM is associated with OA progression, severe clinical symptoms, and joint structural changes, while treatment of T2DM may reduce the progression of knee OA. T2DM is shown to have a negative impact on arthroplasty, including decreased functional outcomes and greater need for revision arthroplasty.
Future research is needed to understand if diabetes control and prevention can modulate OA occurrence and progression in humans. Interventions involving physical activity and body weight control are known to have positive outcomes in the prevention and treatment of T2DM. Concerns over a lack of physical activity in the US population has led to a call to action for making physical activity prescription a medical standard of care [64]. Recommendations for management of OA also include a core set of treatment modalities including weight management and exercise programs [62].
In this review, we have sought to explore whether the co-presence of T2DM poses any additional safety issues for the pharmacologic treatment of OA. Due to the fragility of patients with diabetes, the recommendation to prioritize drugs for which the benefits are not outweighed by harms is of critical importance. Evidence is mounting for safety concerns with some of the most frequently prescribed anti-OA medications, including paracetamol and NSAIDs. Paracetamol is associated with some degree of liver toxicity. Liver disease (NAFLD) occurs frequently in people with T2DM, and whether paracetamol can be prescribed safely in OA patients with co-morbid T2DM and NAFLD remains to be determined. NSAIDs are linked to renal impairment, and drug-drug interactions between NSAIDs and other drug classes, e.g. anti-hypertensive agents, resulting in renal injury have been reported. The antidiabetic agent class of SGLT2 inhibitors has potential to impact on renal function, especially when conditions leading to dehydration are present; thus, the co-administration of NSAIDs and SGLT2 inhibitors is not recommended.
NSAIDs increase the risk of cardiovascular disease in the general population, and although there is little direct evidence for elevated cardiovascular risk with NSAIDs in people with diabetes, as T2DM is a prominent risk factor for cardiovascular disease we may speculate on a higher risk in patients with T2DM. The same may be true for the renal risk of NSAIDs.
A concern raised over potential interference of the SYSADOA glucosamine on glucose metabolism is not borne out by the majority of the clinical evidence. There is limited evidence for a non-significant increase in glycated hemoglobin levels in overweight women prescribed glucosamine for OA prevention. While in systematic reviews, glucosamine administered to OA patients at usual oral doses is well-tolerated by both diabetic and pre-diabetic patients.
IA corticosteroid injections are associated with a rise in blood glucose levels and hence an elevated risk of hyperglycemia in OA patients with T2DM; thus, monitoring of blood glucose levels following IA corticosteroid injections is recommended for 24-48 hours post-injection with corticosteroids and antihyperglycemic therapy may be transiently intensified if necessary. Alternatively, IAHA may be used in knee OA patients remaining symptomatic following treatment with paracetamol, SYSADOAs and NSAIDs, as well as in patients with co-morbidities precluding the use of NSAIDs or IA corticosteroids, such as diabetic patients. HA appears to be safe in diabetic patients and may have a potential role promoting wound healing in diabetic foot ulceration.
In conclusion, the selection of therapy to treat OA symptoms in patients with co-morbid T2DM may require careful consideration of the evidence base to avoid untoward safety issues. Further research is needed to better identify any safety concerns for treatment of OA patients with T2DM, and to optimize long-term patient management.
Acknowledgements
Authors’ statement
The views expressed in this article represent the outcomes of a Working Group jointly organized by the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis and Musculoskeletal Diseases (ESCEO) and the World Health Organization Collaborating Center for Public Health Aspects of Musculoskeletal Health and Aging, Liège, Belgium, and held in Zurich, Switzerland, on January 17th, 2018.
The expert group comprised a global representation of clinicians, endocrinologists, surgeons, researchers, clinical pharmacologists, epidemiologists, geriatricians, and members of regulatory agencies. They were invited for their expertise and knowledge regarding osteoarthritis and diabetes mellitus. Agreement on the principles outlined in this article were based on an exchange of peer-reviewed publications prior to the meeting and a one-day interactive meeting. All authors meet the ICMJE criteria for authorship for this manuscript, take responsibility for the integrity of the work as a whole, and have given final approval to the version to be published.
Role of the funding source
The meeting was funded by the ESCEO, a Belgian not-for-profit organization.
The authors thank the Chair for Biomarkers of Chronic Diseases and the International Scientific Partnership Program (ISPP#0111) at King Saud University, Riyadh, Saudi Arabia for their support.
Role of medical writer/editor
Editorial assistance in the preparation of this manuscript was provided by Lisa Buttle, PhD, of Medscript Ltd., which was funded by the ESCEO asbl, Belgium.
Abbreviations
- MetS
metabolic syndrome
- NAFLD
Non-alcoholic fatty liver disease
- NSAID
non-steroidal anti-inflammatory drug
- T2DM
type 2 diabetes mellitus
- OA
osteoarthritis
Footnotes
Declaration of interests
O. Bruyere reports grants from Biophytis, IBSA, MEDA, Servier, SMB, and Theramex, outside of the submitted work.
C. Cooper reports personal fees from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB, outside of the submitted work.
R. Rizzoli reports personal fees for lecture or advisory boards from Radius Health, Labatec, Nestlé and Danone, outside of the submitted work.
A. J. Scheen declares no conflict of interest with the content of this paper. He has received lecturer/scientific advisor/clinical investigator fees from AstraZeneca, Boehringer Ingelheim, Eli Lilly, Janssen, Merck Sharp & Dohme, Novartis, NovoNordisk, Sanofi and Servier.
J. Branco declares no conflict of interest with the content of this paper. He has received scientific advisor and/or speaker and/or clinical investigator fees from AstraZeneca, BIAL, Biogen, Eli Lilly, Novartis and Pfizer.
L.C. Rovati is a former employee of Rottapharm, the company that developed and commercialized prescription crystalline glucosamine sulfate, now commercialized by Mylan. He is currently Chief Scientific Officer of Rottapharm Biotech, which has no commercial interests in glucosamine or any other drugs for OA discussed here.
E. Dennison reports personal fees for lectures or advisory boards from UCB and Pfizer, outside of the submitted work.
J.F. Kaux reports a grant from Eli Lilly, outside of the submitted work.
E. Maheu reports personal fees from Expanscience, FIDIA, IBSA - GENEVRIER, LCA (France), TRB Chemedica, Rottapharm - Meda, outside of the submitted work.
N. Al-Daghri, J-Y. Reginster, G. Herrero-Beaumont, D. Uebelhart, N. Veronese, M. Hochberg, R. Roth, R. Chapurlat, and M. Vlaskovska report nothing to disclose.
References
- 1.Guariguata L, Whiting DR, Hambleton I, Beagley J, Linnenkamp U, Shaw JE. Global estimates of diabetes prevalence for 2013 and projections for 2035. Diabetes Research and Clinical Practice. 2014;103(2):137–49. doi: 10.1016/j.diabres.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2163–96. doi: 10.1016/s0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Visser AW, de Mutsert R, le Cessie S, den Heijer M, Rosendaal FR, Kloppenburg M, et al. The relative contribution of mechanical stress and systemic processes in different types of osteoarthritis: the NEO study. Ann Rheum Dis. 2015;74(10):1842–7. doi: 10.1136/annrheumdis-2013-205012. [DOI] [PubMed] [Google Scholar]
- 4.Teodoro JS, Varela AT, Rolo AP, Palmeira CM. High-fat and obesogenic diets: current and future strategies to fight obesity and diabetes. Genes Nutr. 2014;9(4):406. doi: 10.1007/s12263-014-0406-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scheen AJ, Van Gaal LF. Combating the dual burden: therapeutic targeting of common pathways in obesity and type 2 diabetes. Lancet Diabetes Endocrinol. 2014;2(11):911–22. doi: 10.1016/S2213-8587(14)70004-X. [DOI] [PubMed] [Google Scholar]
- 6.CDC. Centers for Disease Control and Prevention: National Diabetes Statistics Report. Atlanta: GA Centers for Disease Control and Prevention, U.S. Dept of Health and Human Services; 2017. [Google Scholar]
- 7.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defronzo RA. Banting Lecture. From the triumvirate to the ominous octet: a new paradigm for the treatment of type 2 diabetes mellitus. Diabetes. 2009;58(4):773–95. doi: 10.2337/db09-9028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stumvoll M, Goldstein BJ, van Haeften TW. Type 2 diabetes: pathogenesis and treatment. Lancet. 2008;371(9631):2153–6. doi: 10.1016/S0140-6736(08)60932-0. [DOI] [PubMed] [Google Scholar]
- 10.King KB, Rosenthal AK. The adverse effects of diabetes on osteoarthritis: update on clinical evidence and molecular mechanisms. Osteoarthritis Cartilage. 2015;23(6):841–50. doi: 10.1016/j.joca.2015.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.CDC. Prevalence of doctor-diagnosed arthritis and arthritis-attributable activity limitation--United States, 2010-2012. MMWR Morb Mortal Wkly Rep. 2013;62(44):869–73. [PMC free article] [PubMed] [Google Scholar]
- 12.Bijlsma JW, Berenbaum F, Lafeber FP. Osteoarthritis: an update with relevance for clinical practice. Lancet. 2011;377(9783):2115–26. doi: 10.1016/S0140-6736(11)60243-2. [DOI] [PubMed] [Google Scholar]
- 13.Yusuf E, Nelissen RG, Ioan-Facsinay A, Stojanovic-Susulic V, DeGroot J, van Osch G, et al. Association between weight or body mass index and hand osteoarthritis: a systematic review. Ann Rheum Dis. 2010;69(4):761–5. doi: 10.1136/ard.2008.106930. [DOI] [PubMed] [Google Scholar]
- 14.Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1(1):e000077. doi: 10.1136/rmdopen-2015-000077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alberti KG, Zimmet P, Shaw J, Group IDFETFC The metabolic syndrome--a new worldwide definition. Lancet. 2005;366(9491):1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 16.Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121(6):9–20. doi: 10.3810/pgm.2009.11.2073. [DOI] [PubMed] [Google Scholar]
- 17.Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nat Rev Rheumatol. 2012;8(12):729–37. doi: 10.1038/nrrheum.2012.135. [DOI] [PubMed] [Google Scholar]
- 18.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–5. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 19.Yasuda E, Nakamura R, Matsugi R, Goto S, Ikenaga Y, Kuroda K, et al. Association between the severity of symptomatic knee osteoarthritis and cumulative metabolic factors. Aging Clinical and Experimental Research. 2018;30(5):481–8. doi: 10.1007/s40520-017-0808-6. [DOI] [PubMed] [Google Scholar]
- 20.Roberts E, Delgado Nunes V, Buckner S, Latchem S, Constanti M, Miller P, et al. Paracetamol: not as safe as we thought? A systematic literature review of observational studies. Ann Rheum Dis. 2016;75(3):552–9. doi: 10.1136/annrheumdis-2014-206914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lipworth L, Abdel-Kader K, Morse J, Stewart TG, Kabagambe EK, Parr SK, et al. High prevalence of non-steroidal anti-inflammatory drug use among acute kidney injury survivors in the southern community cohort study. BMC Nephrol. 2016;17(1):189. doi: 10.1186/s12882-016-0411-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heyman SN, Khamaisi M, Rosen S, Rosenberger C, Abassi Z. Potential hypoxic renal injury in patients with diabetes on SGLT2 inhibitors: caution regarding concomitant use of nsaids and iodinated contrast media. Diabetes Care. 2017;40(4):e40–e1. doi: 10.2337/dc16-2200. [DOI] [PubMed] [Google Scholar]
- 23.Bhala N, Emberson J, Merhi A, Abramson S, Arber N, Baron JA, et al. Vascular and upper gastrointestinal effects of non-steroidal anti-inflammatory drugs: meta-analyses of individual participant data from randomised trials. Lancet. 2013;382(9894):769–79. doi: 10.1016/s0140-6736(13)60900-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nielen JT, Emans PJ, Dagnelie PC, Boonen A, Lalmohamed A, de Boer A, et al. Severity of diabetes mellitus and total hip or knee replacement: a population-based case-control study. Medicine (Baltimore) 2016;95(20):e3739. doi: 10.1097/MD.0000000000003739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dahaghin S, Bierma-Zeinstra SM, Koes BW, Hazes JM, Pols HA. Do metabolic factors add to the effect of overweight on hand osteoarthritis? The Rotterdam Study. Ann Rheum Dis. 2007;66(7):916–20. doi: 10.1136/ard.2005.045724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Magnusson K, Hagen KB, Osteras N, Nordsletten L, Natvig B, Haugen IK. Diabetes is associated with increased hand pain in erosive hand osteoarthritis: data from a population-based study. Arthritis Care Res (Hoboken) 2015;67(2):187–95. doi: 10.1002/acr.22460. [DOI] [PubMed] [Google Scholar]
- 27.Frey N, Hugle T, Jick SS, Meier CR, Spoendlin J. Type II diabetes mellitus and incident osteoarthritis of the hand: a population-based case-control analysis. Osteoarthritis Cartilage. 2016;24(9):1535–40. doi: 10.1016/j.joca.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Eymard F, Parsons C, Edwards MH, Petit-Dop F, Reginster JY, Bruyere O, et al. Diabetes is a risk factor for knee osteoarthritis progression. Osteoarthritis Cartilage. 2015;23(6):851–9. doi: 10.1016/j.joca.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 29.Schett G, Kleyer A, Perricone C, Sahinbegovic E, Iagnocco A, Zwerina J, et al. Diabetes is an independent predictor for severe osteoarthritis: results from a longitudinal cohort study. Diabetes Care. 2013;36(2):403–9. doi: 10.2337/dc12-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shirinsky IV, Shirinsky VS. Effects of medication-treated diabetes on incidence and progression of knee osteoarthritis: a longitudinal analysis of the Osteoarthritis Initiative data. Rheumatol Int. 2017;37(6):983–91. doi: 10.1007/s00296-017-3676-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnett LA, Jordan KP, Edwards JJ, van der Windt DA. Does metformin protect against osteoarthritis? An electronic health record cohort study. Prim Health Care Res Dev. 2017;18(6):623–8. doi: 10.1017/S1463423617000287. [DOI] [PubMed] [Google Scholar]
- 32.Lu CH, Chung CH, Lee CH, Hsieh CH, Hung YJ, Lin FH, et al. Combination COX-2 inhibitor and metformin attenuate rate of joint replacement in osteoarthritis with diabetes: A nationwide, retrospective, matched-cohort study in Taiwan. PLoS One. 2018;13(1):e0191242. doi: 10.1371/journal.pone.0191242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Scheen AJ, Esser N, Paquot N. Antidiabetic agents: Potential anti-inflammatory activity beyond glucose control. Diabetes Metab. 2015;41(3):183–94. doi: 10.1016/j.diabet.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 34.Nielen JT, de Vries F, Dagnelie PC, van den Bemt BJ, Emans PJ, Lalmohamed A, et al. Use of thiazolidinediones and the risk of elective hip or knee replacement: a population based case-control study. Br J Clin Pharmacol. 2016;81(2):370–8. doi: 10.1111/bcp.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract. 2016;122:198–206. doi: 10.1016/j.diabres.2016.10.021. [DOI] [PubMed] [Google Scholar]
- 36.Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthritis Cartilage. 2015;23(11):1955–65. doi: 10.1016/j.joca.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 37.Wang Y, Wluka AE, Hodge AM, English DR, Giles GG, O'Sullivan R, et al. Effect of fatty acids on bone marrow lesions and knee cartilage in healthy, middle-aged subjects without clinical knee osteoarthritis. Osteoarthritis Cartilage. 2008;16(5):579–83. doi: 10.1016/j.joca.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 38.Askari A, Ehrampoush E, Homayounfar R, Bahramali E, Farjam M. Serum insulin in pathogenesis and treatment of osteoarthritis. Med Hypotheses. 2017;99:45–6. doi: 10.1016/j.mehy.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 39.Al-Jarallah K, Shehab D, Abdella N, Al Mohamedy H, Abraham M. Knee osteoarthritis in type 2 diabetes mellitus: does insulin therapy retard osteophyte formation? Med Princ Pract. 2016;25(1):12–7. doi: 10.1159/000441418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosa SC, Rufino AT, Judas F, Tenreiro C, Lopes MC, Mendes AF. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthritis Cartilage. 2011;19(6):719–27. doi: 10.1016/j.joca.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 41.Ribeiro M, Lopez de Figueroa P, Blanco FJ, Mendes AF, Carames B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthritis Cartilage. 2016;24(4):731–9. doi: 10.1016/j.joca.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 42.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–45. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 43.Griffin TM, Huffman KM. Editorial: Insulin resistance: releasing the brakes on synovial inflammation and osteoarthritis? Arthritis Rheumatol. 2016;68(6):1330–3. doi: 10.1002/art.39586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hamada D, Maynard R, Schott E, Drinkwater CJ, Ketz JP, Kates SL, et al. Suppressive effects of insulin on tumor necrosis factor-dependent early osteoarthritic changes associated with obesity and type 2 diabetes mellitus. Arthritis Rheumatol. 2016;68(6):1392–402. doi: 10.1002/art.39561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Courties A, Sellam J, Berenbaum F. Metabolic syndrome-associated osteoarthritis. Curr Opin Rheumatol. 2017;29(2):214–22. doi: 10.1097/BOR.0000000000000373. [DOI] [PubMed] [Google Scholar]
- 46.Mobasheri A, Neama G, Bell S, Richardson S, Carter SD. Human articular chondrocytes express three facilitative glucose transporter isoforms: GLUT1, GLUT3 and GLUT9. Cell Biol Int. 2002;26(3):297–300. doi: 10.1006/cbir.2001.0850. [DOI] [PubMed] [Google Scholar]
- 47.Rosa SC, Goncalves J, Judas F, Mobasheri A, Lopes C, Mendes AF. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther. 2009;11(3):R80. doi: 10.1186/ar2713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Eaton CB, Sayeed M, Ameernaz S, Roberts MB, Maynard JD, Driban JB, et al. Sex differences in the association of skin advanced glycation endproducts with knee osteoarthritis progression. Arthritis Res Ther. 2017;19(1):36. doi: 10.1186/s13075-017-1226-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vos PA, Welsing PM, deGroot J, Huisman AM, Oostveen JC, Reijman M, et al. Skin pentosidine in very early hip/knee osteoarthritis (CHECK) is not a strong independent predictor of radiographic progression over 5 years follow-up. Osteoarthritis Cartilage. 2013;21(6):823–30. doi: 10.1016/j.joca.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 50.Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One. 2013;8(6):e66611. doi: 10.1371/journal.pone.0066611. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Tsai CH, Chiang YC, Chen HT, Huang PH, Hsu HC, Tang CH. High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim Biophys Acta. 2013;1830(3):2649–58. doi: 10.1016/j.bbagen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 52.Ribeiro M, Lopez de Figueroa P, Nogueira-Recalde U, Centeno A, Mendes AF, Blanco FJ, et al. Diabetes-accelerated experimental osteoarthritis is prevented by autophagy activation. Osteoarthritis Cartilage. 2016;24(12):2116–25. doi: 10.1016/j.joca.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 53.Davies-Tuck ML, Wang Y, Wluka AE, Berry PA, Giles GG, English DR, et al. Increased fasting serum glucose concentration is associated with adverse knee structural changes in adults with no knee symptoms and diabetes. Maturitas. 2012;72(4):373–8. doi: 10.1016/j.maturitas.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 54.Wen CY, Chen Y, Tang HL, Yan CH, Lu WW, Chiu KY. Bone loss at subchondral plate in knee osteoarthritis patients with hypertension and type 2 diabetes mellitus. Osteoarthritis Cartilage. 2013;21(11):1716–23. doi: 10.1016/j.joca.2013.06.027. [DOI] [PubMed] [Google Scholar]
- 55.Franke S, Ruster C, Pester J, Hofmann G, Oelzner P, Wolf G. Advanced glycation end products affect growth and function of osteoblasts. Clin Exp Rheumatol. 2011;29(4):650–60. [PubMed] [Google Scholar]
- 56.Charnley J. Periarthritis of the shoulder. Postgraduate Med J. 1959;35(405):384. doi: 10.1136/pgmj.35.405.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kischer CW, Speer DP. Microvascular changes in Dupuytren's contracture. J Hand Surgery. 1984;9(1):58–62. doi: 10.1016/s0363-5023(84)80185-9. [DOI] [PubMed] [Google Scholar]
- 58.Schramm JC, Dinh T, Veves A. Microvascular changes in the diabetic foot. Int J Low Extrem Wounds. 2006;5(3):149–59. doi: 10.1177/1534734606292281. [DOI] [PubMed] [Google Scholar]
- 59.Johnson EO, Soultanis K, Soucacos PN. Vascular anatomy and microcirculation of skeletal zones vulnerable to osteonecrosis: vascularization of the femoral head. Orthopedic Clinics. 2004;35(3):285–91. doi: 10.1016/j.ocl.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 60.Findlay DM. Vascular pathology and osteoarthritis. Rheumatology. 2007;46(12):1763–8. doi: 10.1093/rheumatology/kem191. [DOI] [PubMed] [Google Scholar]
- 61.Kushner RF, Sorensen KW. Lifestyle medicine: the future of chronic disease management. Curr Opin Endocrinol Diabetes Obes. 2013;20(5):389–95. doi: 10.1097/01.med.0000433056.76699.5d. [DOI] [PubMed] [Google Scholar]
- 62.Bruyere O, Cooper C, Pelletier JP, Branco J, Brandi ML, Guillemin F, et al. An algorithm recommendation for the management of knee osteoarthritis in Europe and internationally: a report from a task force of the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Semin Arthritis Rheum. 2014;44(3):253–63. doi: 10.1016/j.semarthrit.2014.05.014. [DOI] [PubMed] [Google Scholar]
- 63.ADA. American Diabetes Association. 4. Lifestyle management: Standards of Medical Care in Diabetes 2018. Diabetes Care. 2018;41(Suppl. 1) doi: 10.2337/dc18-S004. [DOI] [PubMed] [Google Scholar]
- 64.Sallis RE, Matuszak JM, Baggish AL, Franklin BA, Chodzko-Zajko W, Fletcher BJ, et al. Call to action on making physical activity assessment and prescription a medical standard of care. Curr Sports Med Rep. 2016;15(3):207–14. doi: 10.1249/JSR.0000000000000249. [DOI] [PubMed] [Google Scholar]
- 65.Hemmingsen B, Gimenez-Perez G, Mauricio D, Roque IFM, Metzendorf MI, Richter B. Diet, physical activity or both for prevention or delay of type 2 diabetes mellitus and its associated complications in people at increased risk of developing type 2 diabetes mellitus. Cochrane Database Syst Rev. 2017;12:CD003054. doi: 10.1002/14651858.CD003054.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kriska A. Can a physically active lifestyle prevent type 2 diabetes? Exerc Sport Sci Rev. 2003;31(3):132–7. doi: 10.1097/00003677-200307000-00006. [DOI] [PubMed] [Google Scholar]
- 67.Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, et al. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20(4):537–44. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- 68.Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344(18):1343–50. doi: 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- 69.Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346(6):393–403. doi: 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Diabetes Prevention Program Research G. Knowler WC, Fowler SE, Hamman RF, Christophi CA, Hoffman HJ, et al. 10-year follow-up of diabetes incidence and weight loss in the Diabetes Prevention Program Outcomes Study. Lancet. 2009;374(9702):1677–86. doi: 10.1016/S0140-6736(09)61457-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Carnethon MR, Craft LL. Autonomic regulation of the association between exercise and diabetes. Exerc Sport Sci Rev. 2008;36(1):12–8. doi: 10.1097/jes.0b013e31815e3dc5. [DOI] [PubMed] [Google Scholar]
- 72.Dela F, Prats C, Helge JW. Exercise interventions to prevent and manage type 2 diabetes: physiological mechanisms. Med Sport Sci. 2014;60:36–47. doi: 10.1159/000357334. [DOI] [PubMed] [Google Scholar]
- 73.Lumb A. Diabetes and exercise. Clin Med (Lond) 2014;14(6):673–6. doi: 10.7861/clinmedicine.14-6-673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li G, Zhang P, Wang J, An Y, Gong Q, Gregg EW, et al. Cardiovascular mortality, all-cause mortality, and diabetes incidence after lifestyle intervention for people with impaired glucose tolerance in the Da Qing Diabetes Prevention Study: a 23-year follow-up study. Lancet Diabetes Endocrinol. 2014;2(6):474–80. doi: 10.1016/S2213-8587(14)70057-9. [DOI] [PubMed] [Google Scholar]
- 75.Towheed TE, Maxwell L, Judd MG, Catton M, Hochberg MC, Wells G. Acetaminophen for osteoarthritis. Cochrane Database Syst Rev. 2006;(1):CD004257. doi: 10.1002/14651858.CD004257.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang W, Nuki G, Moskowitz RW, Abramson S, Altman RD, Arden NK, et al. OARSI recommendations for the management of hip and knee osteoarthritis: part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthritis Cartilage. 2010;18(4):476–99. doi: 10.1016/j.joca.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 77.Gulmez SE, Larrey D, Pageaux GP, Bernuau J, Bissoli F, Horsmans Y, et al. Liver transplant associated with paracetamol overdose: results from the seven-country SALT study. Br J Clin Pharmacol. 2015;80(3):599–606. doi: 10.1111/bcp.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Craig DG, Bates CM, Davidson JS, Martin KG, Hayes PC, Simpson KJ. Staggered overdose pattern and delay to hospital presentation are associated with adverse outcomes following paracetamol-induced hepatotoxicity. Br J Clin Pharmacol. 2012;73(2):285–94. doi: 10.1111/j.1365-2125.2011.04067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Machado GC, Maher CG, Ferreira PH, Pinheiro MB, Lin CW, Day RO, et al. Efficacy and safety of paracetamol for spinal pain and osteoarthritis: systematic review and meta-analysis of randomised placebo controlled trials. BMJ. 2015;350:h1225. doi: 10.1136/bmj.h1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williams KH, Shackel NA, Gorrell MD, McLennan SV, Twigg SM. Diabetes and nonalcoholic Fatty liver disease: a pathogenic duo. Endocr Rev. 2013;34(1):84–129. doi: 10.1210/er.2012-1009. [DOI] [PubMed] [Google Scholar]
- 81.Dai W, Ye L, Liu A, Wen SW, Deng J, Wu X, et al. Prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus: A meta-analysis. Medicine (Baltimore) 2017;96(39):e8179. doi: 10.1097/md.0000000000008179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Portillo-Sanchez P, Bril F, Maximos M, Lomonaco R, Biernacki D, Orsak B, et al. High prevalence of nonalcoholic fatty liver disease in patients with type 2 diabetes mellitus and normal plasma aminotransferase levels. J Clin Endocrinol Metab. 2015;100(6):2231–8. doi: 10.1210/jc.2015-1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Massart J, Begriche K, Moreau C, Fromenty B. Role of nonalcoholic fatty liver disease as risk factor for drug-induced hepatotoxicity. J Clin Transl Res. 2017;3(Suppl 1):212–32. doi: 10.18053/jctres.03.2017S1.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Michaut A, Moreau C, Robin MA, Fromenty B. Acetaminophen-induced liver injury in obesity and nonalcoholic fatty liver disease. Liver Int. 2014;34(7):e171–9. doi: 10.1111/liv.12514. [DOI] [PubMed] [Google Scholar]
- 85.Caparrotta TM, Antoine DJ, Dear JW. Are some people at increased risk of paracetamol-induced liver injury? A critical review of the literature. Eur J Clin Pharmacol. 2018;74(2):147–60. doi: 10.1007/s00228-017-2356-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ajith TA. Role of mitochondria and mitochondria-targeted agents in non-alcoholic fatty liver disease. Clin Exp Pharmacol Physiol. 2018;45(5):413–21. doi: 10.1111/1440-1681.12886. [DOI] [PubMed] [Google Scholar]
- 87.Kon K, Ikejima K, Okumura K, Arai K, Aoyama T, Watanabe S. Diabetic KK-A(y) mice are highly susceptible to oxidative hepatocellular damage induced by acetaminophen. Am J Physiol Gastrointest Liver Physiol. 2010;299(2):G329–37. doi: 10.1152/ajpgi.00361.2009. [DOI] [PubMed] [Google Scholar]
- 88.Kim YH, Hwang JH, Kim KS, Noh JR, Choi DH, Kim DK, et al. Metformin ameliorates acetaminophen hepatotoxicity via Gadd45beta-dependent regulation of JNK signaling in mice. J Hepatol. 2015;63(1):75–82. doi: 10.1016/j.jhep.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 89.Du K, Ramachandran A, Weemhoff JL, Chavan H, Xie Y, Krishnamurthy P, et al. Editor's Highlight: metformin protects against acetaminophen hepatotoxicity by attenuation of mitochondrial oxidant stress and dysfunction. Toxicol Sci. 2016;154(2):214–26. doi: 10.1093/toxsci/kfw158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hayward KL, Powell EE, Irvine KM, Martin JH. Can paracetamol (acetaminophen) be administered to patients with liver impairment? Br J Clin Pharmacol. 2016;81(2):210–22. doi: 10.1111/bcp.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kamali F, Thomas SH, Ferner RE. Paracetamol elimination in patients with non-insulin dependent diabetes mellitus. Br J Clin Pharmacol. 1993;35(1):58–61. doi: 10.1111/j.1365-2125.1993.tb05672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nasri H, Rafieian-Kopaei M. Diabetes mellitus and renal failure: Prevention and management. Journal of Research in Medical Sciences : The Official Journal of Isfahan University of Medical Sciences. 2015;20(11):1112–20. doi: 10.4103/1735-1995.172845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pratt N, Roughead EE, Ryan P, Gilbert AL. Differential impact of NSAIDs on rate of adverse events that require hospitalization in high-risk and general veteran populations: a retrospective cohort study. Drugs Aging. 2010;27(1):63–71. doi: 10.2165/11531250-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 94.Roughead EE, Ramsay E, Pratt N, Gilbert AL. NSAID use in individuals at risk of renal adverse events: an observational study to investigate trends in Australian veterans. Drug Saf. 2008;31(11):997–1003. doi: 10.2165/00002018-200831110-00004. [DOI] [PubMed] [Google Scholar]
- 95.Huerta C, Castellsague J, Varas-Lorenzo C, Garcia Rodriguez LA. Nonsteroidal anti-inflammatory drugs and risk of ARF in the general population. Am J Kidney Dis. 2005;45(3):531–9. doi: 10.1053/j.ajkd.2004.12.005. [DOI] [PubMed] [Google Scholar]
- 96.Fournier JP, Sommet A, Durrieu G, Poutrain JC, Lapeyre-Mestre M, Montastruc JL, et al. Drug interactions between antihypertensive drugs and non-steroidal anti-inflammatory agents: a descriptive study using the French Pharmacovigilance database. Fundam Clin Pharmacol. 2014;28(2):230–5. doi: 10.1111/fcp.12014. [DOI] [PubMed] [Google Scholar]
- 97.Dreischulte T, Morales DR, Bell S, Guthrie B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015;88(2):396–403. doi: 10.1038/ki.2015.101. [DOI] [PubMed] [Google Scholar]
- 98.Lapi F, Azoulay L, Yin H, Nessim SJ, Suissa S. Concurrent use of diuretics, angiotensin converting enzyme inhibitors, and angiotensin receptor blockers with non-steroidal anti-inflammatory drugs and risk of acute kidney injury: nested case-control study. BMJ. 2013;346:e8525. doi: 10.1136/bmj.e8525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wong HK, Ong KL, Cheung CL, Cheung BM. Utilization of glucose, blood pressure, and lipid lowering medications among people with type II diabetes in the United States, 1999-2010. Ann Epidemiol. 2014;24(7):516–21 e1. doi: 10.1016/j.annepidem.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 100.Scheen AJ. Pharmacodynamics, efficacy and safety of sodium-glucose co-transporter type 2 (SGLT2) inhibitors for the treatment of type 2 diabetes mellitus. Drugs. 2015;75(1):33–59. doi: 10.1007/s40265-014-0337-y. [DOI] [PubMed] [Google Scholar]
- 101.Scheen AJ. SGLT2 Inhibitors: Benefit/Risk Balance. Curr Diab Rep. 2016;16(10):92. doi: 10.1007/s11892-016-0789-4. [DOI] [PubMed] [Google Scholar]
- 102.Szalat A, Perlman A, Muszkat M, Khamaisi M, Abassi Z, Heyman SN. Can SGLT2 inhibitors cause acute renal failure? plausible role for altered glomerular hemodynamics and medullary hypoxia. Drug Saf. 2018;41(3):239–52. doi: 10.1007/s40264-017-0602-6. [DOI] [PubMed] [Google Scholar]
- 103.Thomas MC. Type 2 Diabetes and Heart Failure: Challenges and Solutions. Current Cardiology Reviews. 2016;12(3):249–55. doi: 10.2174/1573403X12666160606120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Amer M, Bead VR, Bathon J, Blumenthal RS, Edwards DN. Use of nonsteroidal anti-inflammatory drugs in patients with cardiovascular disease: a cautionary tale. Cardiol Rev. 2010;18(4):204–12. doi: 10.1097/CRD.0b013e3181ce1521. [DOI] [PubMed] [Google Scholar]
- 105.Maillard M, Burnier M. Comparative cardiovascular safety of traditional nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2006;5(1):83–94. doi: 10.1517/14740338.5.1.83. [DOI] [PubMed] [Google Scholar]
- 106.Gunter BR, Butler KA, Wallace RL, Smith SM, Harirforoosh S. Non-steroidal anti-inflammatory drug-induced cardiovascular adverse events: a meta-analysis. J Clin Pharm Ther. 2017;42(1):27–38. doi: 10.1111/jcpt.12484. [DOI] [PubMed] [Google Scholar]
- 107.Nissen SE, Yeomans ND, Solomon DH, Luscher TF, Libby P, Husni ME, et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–29. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 108.Solomon DH, Husni ME, Wolski KE, Wisniewski LM, Borer JS, Graham DY, et al. Differences in safety of nonsteroidal antiinflammatory drugs in patients with osteoarthritis and patients with rheumatoid arthritis: a randomized clinical trial. Arthritis Rheumatol. 2018;70(4):537–46. doi: 10.1002/art.40400. [DOI] [PubMed] [Google Scholar]
- 109.Sowers JR, White WB, Pitt B, Whelton A, Simon LS, Winer N, et al. The Effects of cyclooxygenase-2 inhibitors and nonsteroidal anti-inflammatory therapy on 24-hour blood pressure in patients with hypertension, osteoarthritis, and type 2 diabetes mellitus. Arch Intern Med. 2005;165(2):161–8. doi: 10.1001/archinte.165.2.161. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt M, Sorensen HT, Pedersen L. Diclofenac use and cardiovascular risks: series of nationwide cohort studies. BMJ. 2018;362:k3426. doi: 10.1136/bmj.k3426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mazhar F, Haider N, Sultana J, Akram S, Ahmed Y. Prospective study of NSAIDs prescribing in Saudi Arabia: Cardiovascular and gastrointestinal risk in patients with diabetes mellitus. Int J Clin Pharmacol Ther. 2018;56(2):64–71. doi: 10.5414/cp203071. [DOI] [PubMed] [Google Scholar]
- 112.Barthel HR, Axford-Gatley RA. Topical nonsteroidal anti-inflammatory drugs for osteoarthritis. Postgrad Med. 2010;122(6):98–106. doi: 10.3810/pgm.2010.11.2227. [DOI] [PubMed] [Google Scholar]
- 113.Rannou F, Pelletier JP, Martel-Pelletier J. Efficacy and safety of topical NSAIDs in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S18–21. doi: 10.1016/j.semarthrit.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 114.Peniston JH, Gold MS, Wieman MS, Alwine LK. Long-term tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Clin Interv Aging. 2012;7:517–23. doi: 10.2147/CIA.S35416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baraf HS, Gold MS, Petruschke RA, Wieman MS. Tolerability of topical diclofenac sodium 1% gel for osteoarthritis in seniors and patients with comorbidities. Am J Geriatr Pharmacother. 2012;10(1):47–60. doi: 10.1016/j.amjopharm.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 116.Reginster JY, Deroisy R, Rovati LC, Lee RL, Lejeune E, Bruyere O, et al. Long-term effects of glucosamine sulphate on osteoarthritis progression: a randomised, placebo-controlled clinical trial. Lancet. 2001;357(9252):251–6. doi: 10.1016/S0140-6736(00)03610-2. [DOI] [PubMed] [Google Scholar]
- 117.Pavelka K, Gatterova J, Olejarova M, Machacek S, Giacovelli G, Rovati LC. Glucosamine sulfate use and delay of progression of knee osteoarthritis: a 3-year, randomized, placebo-controlled, double-blind study. Arch Intern Med. 2002;162(18):2113–23. doi: 10.1001/archinte.162.18.2113. [DOI] [PubMed] [Google Scholar]
- 118.Zegels B, Crozes P, Uebelhart D, Bruyere O, Reginster JY. Equivalence of a single dose (1200 mg) compared to a three-time a day dose (400 mg) of chondroitin 4&6 sulfate in patients with knee osteoarthritis. Results of a randomized double blind placebo controlled study. Osteoarthritis Cartilage. 2013;21(1):22–7. doi: 10.1016/j.joca.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 119.Kahan A, Uebelhart D, De Vathaire F, Delmas PD, Reginster JY. Long-term effects of chondroitins 4 and 6 sulfate on knee osteoarthritis: the study on osteoarthritis progression prevention, a two-year, randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2009;60(2):524–33. doi: 10.1002/art.24255. [DOI] [PubMed] [Google Scholar]
- 120.Altman RD, Abramson S, Bruyere O, Clegg D, Herrero-Beaumont G, Maheu E, et al. Commentary: osteoarthritis of the knee and glucosamine. Osteoarthritis Cartilage. 2006;14(10):963–6. doi: 10.1016/j.joca.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 121.Maheu E, Cadet C, Marty M, Moyse D, Kerloch I, Coste P, et al. Randomised, controlled trial of avocado-soybean unsaponifiable (Piascledine) effect on structure modification in hip osteoarthritis: the ERADIAS study. Ann Rheum Dis. 2014;73(2):376–84. doi: 10.1136/annrheumdis-2012-202485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Reginster JY. The efficacy of glucosamine sulfate in osteoarthritis: financial and nonfinancial conflict of interest. Arthritis Rheum. 2007;56(7):2105–10. doi: 10.1002/art.22852. [DOI] [PubMed] [Google Scholar]
- 123.Reginster JY, Dudler J, Blicharski T, Pavelka K. Pharmaceutical-grade Chondroitin sulfate is as effective as celecoxib and superior to placebo in symptomatic knee osteoarthritis: the ChONdroitin versus CElecoxib versus Placebo Trial (CONCEPT) Ann Rheum Dis. 2017;76(9):1537–43. doi: 10.1136/annrheumdis-2016-210860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pavelka K, Bruyere O, Cooper C, Kanis JA, Leeb BF, Maheu E, et al. Diacerein: Benefits, risks and place in the management of osteoarthritis. An opinion-based report from the ESCEO. Drugs Aging. 2016;33(2):75–85. doi: 10.1007/s40266-016-0347-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Christiansen BA, Bhatti S, Goudarzi R, Emami S. Management of osteoarthritis with avocado/soybean unsaponifiables. Cartilage. 2015;6(1):30–44. doi: 10.1177/1947603514554992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Towheed TE, Maxwell L, Anastassiades TP, Shea B, Houpt J, Robinson V, et al. Glucosamine therapy for treating osteoarthritis. Cochrane Database Syst Rev. 2009;(2):CD002946. doi: 10.1002/14651858.CD002946.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wandel S, Juni P, Tendal B, Nuesch E, Villiger PM, Welton NJ, et al. Effects of glucosamine, chondroitin, or placebo in patients with osteoarthritis of hip or knee: network meta-analysis. BMJ. 2010;341:c4675. doi: 10.1136/bmj.c4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hathcock JN, Shao A. Risk assessment for glucosamine and chondroitin sulfate. Regul Toxicol Pharmacol. 2007;47(1):78–83. doi: 10.1016/j.yrtph.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 129.Singh JA, Noorbaloochi S, MacDonald R, Maxwell LJ. Chondroitin for osteoarthritis. Cochrane Database Syst Rev. 2015;1:CD005614. doi: 10.1002/14651858.CD005614.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Pelletier JP, Martel-Pelletier J. Diacerein-containing products: same risk of diarrhoea? Aging Clin Exp Res. 2018;30(4):411–2. doi: 10.1007/s40520-018-0911-3. [DOI] [PubMed] [Google Scholar]
- 131.Olivier P, Montastruc JL, Reseau francais des centres regionaux de p [Post-marketing safety profile of avocado-soybean unsaponifiables] Presse Med. 2010;39(10):e211–6. doi: 10.1016/j.lpm.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 132.Garvey WT, Olefsky JM, Matthaei S, Marshall S. Glucose and insulin co-regulate the glucose transport system in primary cultured adipocytes. A new mechanism of insulin resistance. J Biol Chem. 1987;262(1):189–97. [PubMed] [Google Scholar]
- 133.Marshall S, Bacote V, Traxinger RR. Discovery of a metabolic pathway mediating glucose-induced desensitization of the glucose transport system. Role of hexosamine biosynthesis in the induction of insulin resistance. J Biol Chem. 1991;266(8):4706–12. [PubMed] [Google Scholar]
- 134.Baron AD, Zhu JS, Zhu JH, Weldon H, Maianu L, Garvey WT. Glucosamine induces insulin resistance in vivo by affecting GLUT 4 translocation in skeletal muscle. Implications for glucose toxicity. J Clin Invest. 1995;96(6):2792–801. doi: 10.1172/JCI118349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pouwels MJ, Jacobs JR, Span PN, Lutterman JA, Smits P, Tack CJ. Short-term glucosamine infusion does not affect insulin sensitivity in humans. J Clin Endocrinol Metab. 2001;86(5):2099–103. doi: 10.1210/jcem.86.5.7470. [DOI] [PubMed] [Google Scholar]
- 136.Monauni T, Zenti MG, Cretti A, Daniels MC, Targher G, Caruso B, et al. Effects of glucosamine infusion on insulin secretion and insulin action in humans. Diabetes. 2000;49(6):926–35. doi: 10.2337/diabetes.49.6.926. [DOI] [PubMed] [Google Scholar]
- 137.Muniyappa R, Karne RJ, Hall G, Crandon SK, Bronstein JA, Ver MR, et al. Oral glucosamine for 6 weeks at standard doses does not cause or worsen insulin resistance or endothelial dysfunction in lean or obese subjects. Diabetes. 2006;55(11):3142–50. doi: 10.2337/db06-0714. [DOI] [PubMed] [Google Scholar]
- 138.Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, Hooper MM, et al. Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med. 2006;354(8):795–808. doi: 10.1056/NEJMoa052771. [DOI] [PubMed] [Google Scholar]
- 139.Palma Dos Reis R, Giacovelli G, Girolami F, Andre R, Bonazzi A, Rovati LC. Crystalline glucosamine sulfate in the treatment of osteoarthritis: evidence of long-term cardiovascular safety from clinical trials. Open Rheumatol J. 2011;5:69–77. doi: 10.2174/1874312901105010069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Herrero-Beaumont G, Ivorra JA, Del Carmen Trabado M, Blanco FJ, Benito P, Martin-Mola E, et al. Glucosamine sulfate in the treatment of knee osteoarthritis symptoms: a randomized, double-blind, placebo-controlled study using acetaminophen as a side comparator. Arthritis Rheum. 2007;56(2):555–67. doi: 10.1002/art.22371. [DOI] [PubMed] [Google Scholar]
- 141.Dostrovsky NR, Towheed TE, Hudson RW, Anastassiades TP. The effect of glucosamine on glucose metabolism in humans: a systematic review of the literature. Osteoarthritis Cartilage. 2011;19(4):375–80. doi: 10.1016/j.joca.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 142.Simon RR, Marks V, Leeds AR, Anderson JW. A comprehensive review of oral glucosamine use and effects on glucose metabolism in normal and diabetic individuals. Diabetes Metab Res Rev. 2011;27(1):14–27. doi: 10.1002/dmrr.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gommans YMM, Runhaar J, Jacobs ML, Bierma-Zeinstra SMA. The effect of prolonged glucosamine usage on HbA1c Levels and new-onset diabetes mellitus in overweight and obese middle-aged women. Am J Med. 2017;130(6):731–7 e6. doi: 10.1016/j.amjmed.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 144.Runhaar J, Deroisy R, van Middelkoop M, Barretta F, Barbetta B, Oei EH, et al. The role of diet & exercise and of glucosamine sulfate in the prevention of knee osteoarthritis: further results from the Prevention of Knee Osteoarthritis in Overweight Females (PROOF) study. Semin Arthritis Rheum. 2016;45(Suppl 4S):S42–S8. doi: 10.1016/j.semarthrit.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 145.Mylan. DONA (crystalline glucosamine sulfate1500 mg powder): Summary of Product Characteristics. 2018 Apr; 2018. [Google Scholar]
- 146.Habib GS. Systemic effects of intra-articular corticosteroids. Clin Rheumatol. 2009;28(7):749–56. doi: 10.1007/s10067-009-1135-x. [DOI] [PubMed] [Google Scholar]
- 147.Choudhry MN, Malik RA, Charalambous CP. Blood glucose levels following intra-articular steroid injections in patients with diabetes: a systematic review. JBJS Rev. 2016;4(3) doi: 10.2106/JBJS.RVW.O.00029. [DOI] [PubMed] [Google Scholar]
- 148.Cooper C, Bardin T, Brandi M-L, Cacoub P, Caminis J, Civitelli R, et al. Balancing benefits and risks of glucocorticoids in rheumatic diseases and other inflammatory joint disorders: new insights from emerging data. An expert consensus paper from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) Aging Clinical and Experimental Research. 2016;28(1):1–16. doi: 10.1007/s40520-015-0522-1. [DOI] [PubMed] [Google Scholar]
- 149.Bannuru RR, Vaysbrot EE, Sullivan MC, McAlindon TE. Relative efficacy of hyaluronic acid in comparison with NSAIDs for knee osteoarthritis: a systematic review and meta-analysis. Semin Arthritis Rheum. 2014;43(5):593–9. doi: 10.1016/j.semarthrit.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 150.Bannuru RR, Natov NS, Obadan IE, Price LL, Schmid CH, McAlindon TE. Therapeutic trajectory of hyaluronic acid versus corticosteroids in the treatment of knee osteoarthritis: a systematic review and meta-analysis. Arthritis Rheum. 2009;61(12):1704–11. doi: 10.1002/art.24925. [DOI] [PubMed] [Google Scholar]
- 151.Bannuru RR, Osani M, Vaysbrot EE, McAlindon TE. Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis Cartilage. 2016;24(12):2022–41. doi: 10.1016/j.joca.2016.07.010. [DOI] [PubMed] [Google Scholar]
- 152.Bannuru RR, Schmid CH, Kent DM, Vaysbrot EE, Wong JB, McAlindon TE. Comparative effectiveness of pharmacologic interventions for knee osteoarthritis: a systematic review and network meta-analysis. Ann Intern Med. 2015;162(1):46–54. doi: 10.7326/M14-1231. [DOI] [PubMed] [Google Scholar]
- 153.Maheu E, Bannuru RR, Herrero-Beaumont G, Allali F, Bard H, Migliore A. Why we should definitely include intra-articular hyaluronic acid as a therapeutic option in the management of knee osteoarthritis: Results of an extensive critical literature review. Semin Arthritis Rheum. 2018 doi: 10.1016/j.semarthrit.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 154.Cooper C, Rannou F, Richette P, Bruyere O, Al-Daghri N, Altman RD, et al. Use of intraarticular hyaluronic acid in the management of knee osteoarthritis in clinical practice. Arthritis Care Res (Hoboken) 2017;69(9):1287–96. doi: 10.1002/acr.23204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Maheu E, Rannou F, Reginster JY. Efficacy and safety of hyaluronic acid in the management of osteoarthritis: Evidence from real-life setting trials and surveys. Semin Arthritis Rheum. 2016;45(4 Suppl):S28–33. doi: 10.1016/j.semarthrit.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 156.Altman R, Lim S, Steen RG, Dasa V. Hyaluronic acid injections are associated with delay of total knee replacement surgery in patients with knee osteoarthritis: Evidence from a large U.S. health claims database. PLoS One. 2015;10(12):e0145776. doi: 10.1371/journal.pone.0145776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Chen CP, Hung W, Lin SH. Effectiveness of hyaluronic acid for treating diabetic foot: a systematic review and meta-analysis. Dermatol Ther. 2014;27(6):331–6. doi: 10.1111/dth.12153. [DOI] [PubMed] [Google Scholar]
- 158.Wluka AE, Lombard CB, Cicuttini FM. Tackling obesity in knee osteoarthritis. Nat Rev Rheumatol. 2013;9(4):225–35. doi: 10.1038/nrrheum.2012.224. [DOI] [PubMed] [Google Scholar]
- 159.Springer BD, Carter JT, McLawhorn AS, Scharf K, Roslin M, Kallies KJ, et al. Obesity and the role of bariatric surgery in the surgical management of osteoarthritis of the hip and knee: a review of the literature. Surg Obes Relat Dis. 2017;13(1):111–8. doi: 10.1016/j.soard.2016.09.011. [DOI] [PubMed] [Google Scholar]
- 160.Rubino F, Nathan DM, Eckel RH, Schauer PR, Alberti KG, Zimmet PZ, et al. Metabolic surgery in the treatment algorithm for type 2 diabetes: a joint statement by international diabetes organizations. Diabetes Care. 2016;39(6):861–77. doi: 10.2337/dc16-0236. [DOI] [PubMed] [Google Scholar]
- 161.Pareek M, Schauer PR, Kaplan LM, Leiter LA, Rubino F, Bhatt DL. Metabolic surgery: weight loss, diabetes, and beyond. J Am Coll Cardiol. 2018;71(6):670–87. doi: 10.1016/j.jacc.2017.12.014. [DOI] [PubMed] [Google Scholar]
- 162.Lindegaard KK, Jorgensen NB, Just R, Heegaard PM, Madsbad S. Effects of Roux-en-Y gastric bypass on fasting and postprandial inflammation-related parameters in obese subjects with normal glucose tolerance and in obese subjects with type 2 diabetes. Diabetol Metab Syndr. 2015;7:12. doi: 10.1186/s13098-015-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hafida S, Mirshahi T, Nikolajczyk BS. The impact of bariatric surgery on inflammation: quenching the fire of obesity? Curr Opin Endocrinol Diabetes Obes. 2016;23(5):373–8. doi: 10.1097/MED.0000000000000277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yadav R, Hama S, Liu Y, Siahmansur T, Schofield J, Syed AA, et al. Effect of Roux-en-Y bariatric surgery on lipoproteins, insulin resistance, and systemic and vascular inflammation in obesity and diabetes. Front Immunol. 2017;8:1512. doi: 10.3389/fimmu.2017.01512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gallo G, Candilio G, De Luca E, Iannicelli A, Sciaudone G, Pellino G, et al. Bariatric surgery and rheumatic diseases: a literature review. Rev Recent Clin Trials. 2018;13(3):176–83. doi: 10.2174/1574887113666180314095445. [DOI] [PubMed] [Google Scholar]
- 166.Groen VA, van de Graaf VA, Scholtes VA, Sprague S, van Wagensveld BA, Poolman RW. Effects of bariatric surgery for knee complaints in (morbidly) obese adult patients: a systematic review. Obes Rev. 2015;16(2):161–70. doi: 10.1111/obr.12236. [DOI] [PubMed] [Google Scholar]
- 167.King WC, Chen JY, Belle SH, Courcoulas AP, Dakin GF, Elder KA, et al. Change in pain and physical function following bariatric surgery for severe obesity. JAMA. 2016;315(13):1362–71. doi: 10.1001/jama.2016.3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Smith TO, Aboelmagd T, Hing CB, MacGregor A. Does bariatric surgery prior to total hip or knee arthroplasty reduce post-operative complications and improve clinical outcomes for obese patients? Systematic review and meta-analysis. Bone Joint J. 2016;98-B(9):1160–6. doi: 10.1302/0301-620X.98B9.38024. [DOI] [PubMed] [Google Scholar]
- 169.Nearing EE, 2nd, Santos TM, Topolski MS, Borgert AJ, Kallies KJ, Kothari SN. Benefits of bariatric surgery before elective total joint arthroplasty: is there a role for weight loss optimization? Surg Obes Relat Dis. 2017;13(3):457–62. doi: 10.1016/j.soard.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 170.Hooper JM, Deshmukh AJ, Schwarzkopf R. The role of bariatric surgery in the obese total joint arthroplasty patient. Orthop Clin North Am. 2018;49(3):297–306. doi: 10.1016/j.ocl.2018.02.003. [DOI] [PubMed] [Google Scholar]