Abstract
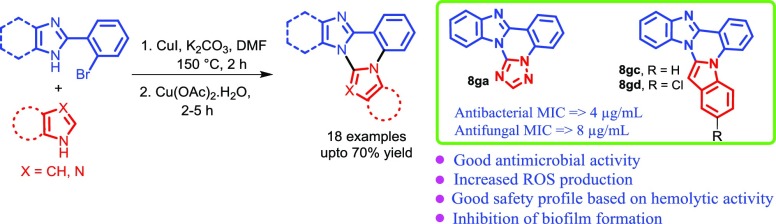
A new class of fused quinazolines has been designed and synthesized via copper-catalyzed Ullmann type C–N coupling followed by intramolecular cross-dehydrogenative coupling reaction in moderate to good yields. The synthesized compounds were tested for in vitro antibacterial activity against three Gram negative (Escherichia coli, Pseudomonas putida, and Salmonella typhi) and two Gram positive (Bacillus subtilis, and Staphylococcus aureus) bacteria. Among all tested compounds, 8ga, 8gc, and 8gd exhibited promising minimum inhibitory concentration (MIC) values (4–8 μg/mL) for all bacterial strains tested as compared to the positive control ciprofloxacin. The synthesized compounds were also evaluated for their in vitro antifungal activity against Aspergillus niger and Candida albicans and compounds 8ga, 8gc, and 8gd having potential antibacterial activity also showed pronounced antifungal activity (MIC values 8–16 μg/mL) against both strains. The bactericidal assay by propidium iodide and live–dead bacterial cell screening using a mixture of acridine orange/ethidium bromide (AO/Et·Br) showed considerable changes in the bacterial cell membrane, which might be the cause or consequence of cell death. Moreover, the hemolytic activity for most potent compounds (8ga, 8gc, and 8gd) showed their safety profile toward human blood cells.
1. Introduction
Bacterial and fungal infections have become a global challenge for human health because of the lack of sufficient and effective antimicrobial drugs, especially in immune compromised patients. The increase in prevalence and emergence of multidrug resistance among bacteria including methicillin-resistant Staphylococcus aureus (MRSA) has endangered the efficacy of antibiotics in the advent of modern medicine.1 Antimicrobial resistant bacteria result in increased mortality rate and increased healthcare cost. According to a study by World Health Organization (WHO), microbial infection can become one of the most serious causes for human death in near future.2 Considering these facts, there is an urgent need to develop new effective antibacterial agents that circumvent the emergence of resistance and thus, there have been tremendous efforts by researchers to find new antibiotics to combat microbial resistance.3
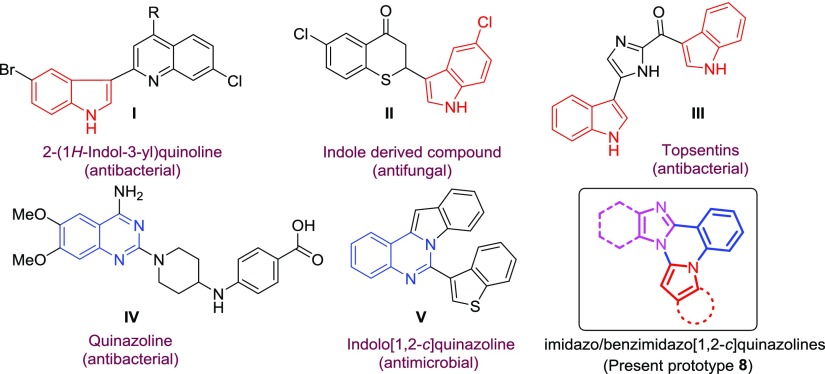
Nitrogen-containing heterocyclic compounds are the most abundant and integral scaffolds because of their distinct biological and industrial activities.4 In the past, a number of indole and quinazoline-based compounds have been shown to be promising antimicrobial agents (Figure 1).5 Hoemann et al. found that 2-(1H-indol-3-yl)quinolines (I) are effective against methicillin-resistant S. aureus with minimum inhibitory concentration (MIC) values <1.0 μg/mL.6 Yu and group synthesized a series of 2-(indole-3-yl)-thiochroman-4-ones (II) and found that these indole derivatives show good in vitro antifungal activity with MIC in the range of 4–8 μg/mL.7 The bis(indole)alkaloid, topsentin (III) has received growing attention for their interesting antibacterial properties.8 Kung et al. found that quinazoline-derived compounds (IV) have exhibited good antibacterial activity against Escherichia coli, S. aureus, and K. pneumonia with MIC values ranging from 0.2–12 μM.9 A few quinazoline-fused molecules such as indolo-, imidazo-, and benzimidazo-quinazolines have also been found to exhibit potential antifungal and antibacterial activities (Figure 1).10 For example, indolo[1,2-c]quinazoline (V) has been found to possess good antimicrobial activity with MIC values ranging from 2.5–20 μg/mL.10a On the basis of the high degree of bioactivity shown by molecules based on indole, quinazoline, and imidazole moieties and also in continuation of our ongoing research interest for the synthesis of novel-fused heterocyclic molecules under mild reaction conditions,11 we became interested toward designing a novel imidazo/benzimidazo[1,2-c]quinazoline structural framework (8) that incorporates all these three moieties into a single molecular framework and evaluate their potential additive effects on the antimicrobial activities.
Figure 1.
Antimicrobial agents with indole and quinazoline motifs and designed imidazo/benzimidazo[1,2-c]quinazolines.
2. Results and Discussion
2.1. Chemistry
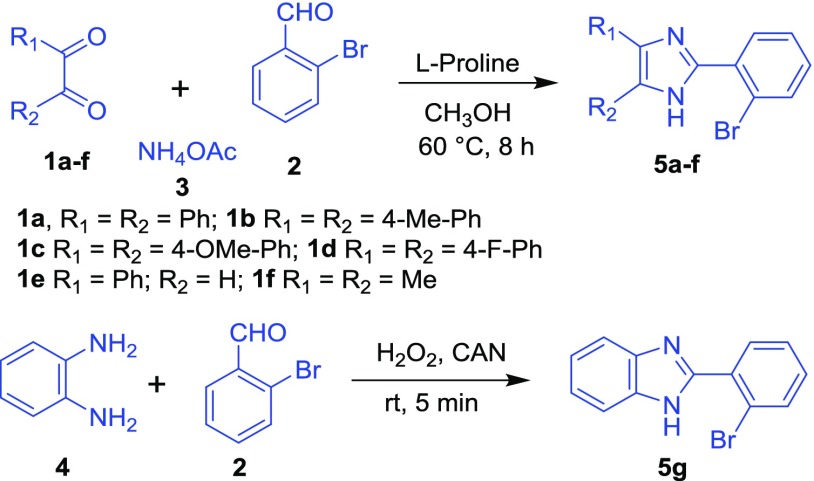
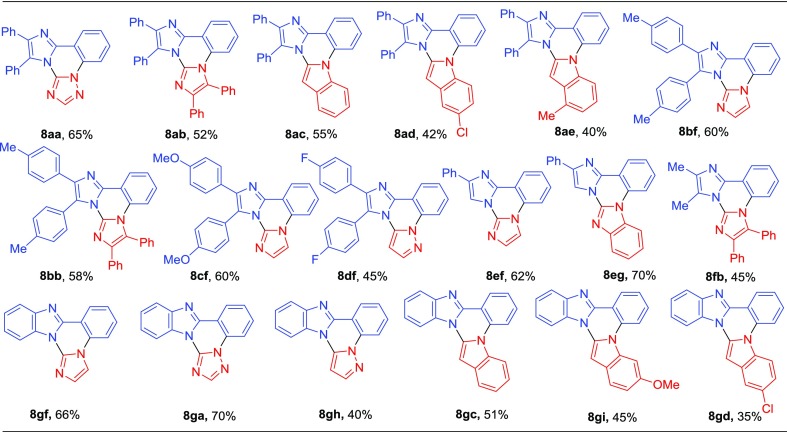
Initially, benzil and related dicarbonyl compounds (1a–f), 2-bromoaryl aldehyde (2), and ammonium acetate (3) were reacted in the presence of l-proline/MeOH to provide a series of 2-(2-bromoaryl)-1H-imidazole (5a–f) via a multicomponent strategy.12 Similarly, the reaction of 2-bromoaryl aldehyde (2) with 1,2-diaminobenzene (4) in the presence of H2O2/CAN gave 2-(2-bromophenyl)-1H-benzo[d]imidazole (5g) (Scheme 1).13
Scheme 1. Synthesis of 2-(2-Bromoaryl)-1H-imidazole and 2-(2-Bromophenyl)-1H-benzo[d]imidazole.
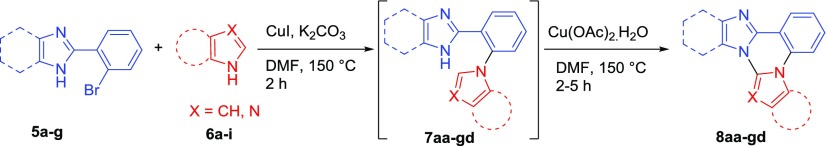
The designed prototype that is fused imidazo/benzimidazo quinazolines (8) were synthesized by using our previous approach with a slight modification in the reaction conditions.13 Interestingly, in the present approach, we have successfully established copper-catalyzed cross-dehydrogenative coupling (CDC) instead of a palladium-catalyzed CDC strategy reported earlier. Copper-catalyzed Ullmann type C–N coupling between 2-(2-bromophenyl)-1H-imidazole/benzimidazole (5) with azoles (6) in the presence of CuI (20 mol %), K2CO3 in dimethylformamide (DMF) at 150 °C, followed by CDC in the presence of Cu(OAc)2·H2O resulted in the desired fused imidazo/benzimidazo quinazolines (8). As shown in Table 1, aryl substitution at C4 and C5- position on 2-(2-bromophenyl)-1H-imidazole was treated with different azoles such as 1,2,4-triazole, imidazole and indoles to afford corresponding imidazo[1,2-c]quinazolines (8aa−ae) in 40−65% yields. The 2-(2-bromophenyl)-1H-imidazole having different substituents such as -Me, -OMe and -F at the p-position of aryl ring at C4 and C5- position of imidazole smoothly reacted with imidazoles and pyrazole to give respective imidazo[1,2-c]quinazolines (8bf−df) in 45−60% yields. Similarly, the reaction of C4-arylated 2-(2-bromophenyl)-1H-imidazole with imidazole and benzimidazole produced corresponding imidazo[1,2-c]quinazolines (8ef−eg) in 62−70% yields. Furthemore, methyl substituted imidazole at C4 and C5- position well tolerated under standard reaction conditions and afforded the target product 8fb in 45% yield. Similarly, 2-(2-bromophenyl)benzimidazole (5g) also reacted with different azoles (6) to afforded corresponding benzimidazo[1,2-c]quinazolines (8gf, 8ga, 8gh, 8gc, 8gi, and 8gd) in 35–70% yields. The molecular structure of the synthesized imidazo/benzimidazo[1,2-c]quinazoline derivatives (8) was confirmed by 1H and 13C NMR and ESI-HRMS analysis.
Table 1. Synthesis of Fused Imidazo/Benzimidazo[1,2-c]quinazolines (8)a,b.
Reaction conditions: 5 (1.0 mmol), 6 (1.2 mmol), CuI (20 mol %), K2CO3 (2.0 mmol), and DMF (2.0 mL) under air at 150 °C for 2 h followed by the addition of Cu(OAc)2·H2O (0.5 mmol), 150 °C, 2–5 h.
Isolated yield.
2.2. Biology
2.2.1. Antimicrobial Activity
Antibacterial evaluation of imidazo/benzimidazo[1,2-c]quinazoline derivatives (8) was carried out against a panel of three Gram negative strains, viz., E. coli, Pseudomonas putida (P. putida), and Salmonella typhi (S. typhi) and two Gram positive strains, viz., Bacillus subtilis (B. subtilis) and S. aureus using ciprofloxacin as a standard drug. Antifungal efficacy was evaluated against pathogenic strains, viz., Candida albicans (C. albicans) and Aspergillus niger (A. niger) using amphotericin B as a positive control. The results of antimicrobial activity are presented in Table 2. Initially, to understand the antibacterial effect of the synthesized compounds, zone of inhibition (ZOI) was measured through the disc diffusion method. Presence of these compounds in the culture medium increased the ZOI diameter by 1 to 7 mm. Compounds 8ga, 8gc, and 8gd, with an increase of 6–7 mm in the inhibition zone diameter were found to be the most promising candidate for antibacterial activity. Next, for quantitative measurement of the antibacterial activity of 8aa–gd, MIC was evaluated. It was found that the compounds (8aa–gd) effectively inhibited the growth of pathogenic strains with MIC in the range of 4–64 μg/mL. Among all tested compounds, 8ga, 8gc, and 8gd were found to be most effective compounds with MIC in the range of 4–8 μg/mL.
Table 2. Antimicrobial Activity of Fused Imidazo/Benzimidazo[1,2-c]quinazolines (8)a.
| MIC (μg/mL) |
|||||||
|---|---|---|---|---|---|---|---|
| Bacteria |
|
||||||
| Gram negative |
Gram positive |
fungi |
|||||
| compounds | E. coli | P. putida | S. typhi | B. subtilis | S. aureus | C. albicans | A. niger |
| 8aa | >32 | >32 | 32 | 64 | 64 | >32 | >32 |
| 8ab | 64 | >32 | 64 | 32 | 32 | b | b |
| 8ac | 64 | >64 | 64 | >64 | 64 | 128 | >64 |
| 8ad | 32 | 32 | >32 | 32 | 32 | b | b |
| 8ae | >64 | 64 | 64 | >32 | >32 | 64 | 64 |
| 8bf | >32 | 32 | >32 | 32 | 32 | 32 | 32 |
| 8bb | >16 | 32 | 32 | >16 | >16 | >32 | >32 |
| 8cf | 32 | >32 | 32 | 32 | >32 | >128 | >128 |
| 8df | >16 | 16 | 16 | >16 | >16 | 64 | >64 |
| 8ef | 32 | >32 | >32 | >32 | >32 | 32 | 32 |
| 8eg | 32 | 32 | 32 | 32 | >32 | 64 | 64 |
| 8fb | 64 | 64 | >64 | >32 | >32 | 128 | 128 |
| 8gf | >8 | 8 | >8 | 8 | >8 | 16 | 16 |
| 8ga | 4 | 4 | >4 | 8 | 8 | 8 | >8 |
| 8gh | 16 | 16 | 16 | 16 | 16 | 32 | 32 |
| 8gc | 4 | 4 | 8 | 4 | >4 | 8 | 8 |
| 8gi | 16 | >16 | >16 | 16 | >16 | >16 | >16 |
| 8gd | 4 | >4 | 4 | >4 | >4 | 16 | 16 |
| ciprofloxacin | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | ||
| amphotericin B | 30 | 30 | |||||
MIC: minimum inhibitory concentration in μg/mL.
No activity.
With respect to antifungal activity, compounds 8bf, 8ef, 8gf, 8ga, 8gh, 8gc, 8gi, and 8gd exhibited good to excellent activity against A. niger and C. albicans. Compounds 8bf, 8ef, and 8gh were found equally potent (MIC in the range of 32 μg/mL) in comparison to the reference drug amphotericin B, whereas compounds 8gf, 8ga, 8gc, 8gi, and 8gd even showed better activity than the positive control with MIC in the range of 8–16 μg/mL.
It was observed that benzimidazole derivatives substituted with indoles (14gc–gd) and azoles [1,2,4-triazole (8ga), imidazole (8gf), and pyrazole (8gh)] exhibited better antibacterial activity in comparison to the imidazole substrate containing similar motifs (8aa–fb). More specifically, benzimidazole fused with indole-bearing electron-withdrawing group (−Cl) exhibited comparatively better activity than the electron neutral or electron-donating (−OMe) group. Similarly, the benzimidazole substrate fused with indole and azoles (1,2,4-triazole, imidazole, and pyrazole) exhibited better antifungal activity in comparison to the imidazole substrate containing a similar scaffold.
2.2.2. Bactericidal Assay by Propidium Iodide
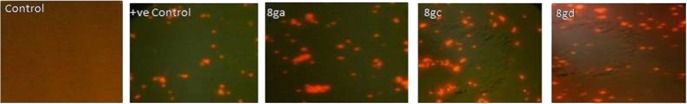
The propidium iodide (PI) dye penetrates only the damaged or compromised membrane and hence can be used to detect dead cells.14 The dye PI intercalates with double-stranded DNA, and subsequent fluorescence detection allows assessment of the number of nonviable/dead cells.15 The most potent compounds 8ga, 8gc, and 8gd were further tested for their effect against the total cell population of P. putida using the PI dye. The microscopic images of the bacterial culture treated with the compounds showed significant cell death at 2× MIC, which is similar to the number of cells for the positive control treated with the tert-butyl hydroperoxide (TBHP) bacterial culture (Figure 2). The result revealed that the compounds 8ga, 8gc, and 8gd have potent bactericidal activity.
Figure 2.
Bactericidal assay against P. putida for compounds 8ga, 8gc, and 8gd.
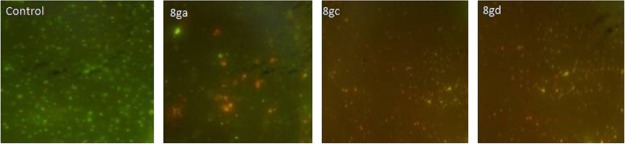
2.2.3. Live–Dead Bacteria Cell Screening
Acridine orange (AO) is a vital dye and will stain both live and dead cells, whereas ethidium bromide (Et·Br) will stain only cells that have lost membrane integrity.16 The cell death assay caused by most efficient compounds 8ga, 8gc, and 8gd was evaluated by the AO/Et·Br dual staining assay (Figure 3). In this assay, P. putida cells were stained with a mixture of AO and Et·Br. The dye AO can enter inside living cells and binds with DNA of living cells to emit green fluorescence, whereas Et·Br enters only through the modified cell membrane of dead cells and emits red fluorescence. The untreated bacterial cells displayed green fluorescence, whereas the cells treated with compounds 8ga, 8gc, and 8gd exhibited red fluorescence along with minor green fluorescence (Figure 3). This bacterial cell viability assay demonstrated that compounds 8ga, 8gc, and 8gd cause cell damage by making loss of cell membrane integrity and thus illustrates the bactericidal potential of these compounds.
Figure 3.
Live–dead bacterial cell screening by compounds 8ga, 8gc, and 8gd using AO/Et·Br dual staining.
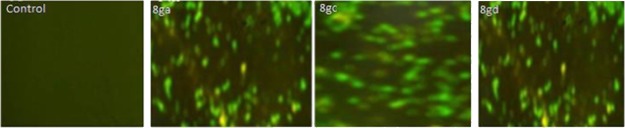
2.2.4. Evaluation of Reactive Oxygen Species Production
A moderate reactive oxygen species (ROS) generation plays a crucial role in cell proliferation and differentiation, whereas the excess production of ROS induces oxidative damage to cellular lipids, proteins, and DNA in bacteria which leads to cell death. Therefore, the effect of compounds 8ga, 8gc, and 8gd on cellular ROS level of P. putida bacterial cells was measured using the 2′,7′-dichlorofluorescein diacetate dye (DCFH-DA). A significant increase in the ROS level was observed by the chemically synthesized compounds 8ga, 8gc, and 8gd against P. putida cells (Figure 4). Compared to the untreated control cells, the compound-treated bacterial cell showed enhanced generation of intracellular ROS. This intracellular-accumulated ROS may also be responsible for bactericidal activity.
Figure 4.
Evaluation of ROS production against P. putida using the fluorescent dye DCFH-DA as a probe.
2.2.5. Hemolytic Activity
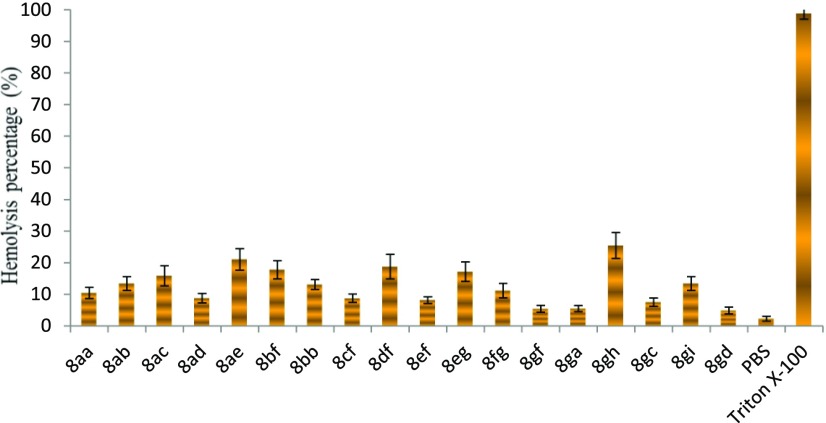
To ascertain the toxicity profile of synthesized compounds, hemolytic activity was evaluated. The hemolytic activity can occur by several mechanisms, from increased permeability of cell membranes to complete cell lysis. The evaluation of hemolytic activity is to check the damage caused by synthesized compounds to the membranes of RBCs (erythrocytes), which leads to release of hemoglobin. It is an additional tool to verify the importance of synthesized compounds against red blood cells (RBCs) and may also give an idea to promote such compounds as a drug level (Figure 5). The toxicity profile of chemically synthesized compounds on human RBCs was determined at a fixed concentration of 100 μM. The observed results showed that most compounds caused 3–28% hemolysis. Interestingly, the efficient antibacterial compounds 8ga, 8gc, and 8gd showed less than 10% hemolysis at a high concentration of 100 μM, suggesting that these compounds are negligibly toxic to human blood cells (Figure 5). The results of hemolytic activity further support the significance of the present study.
Figure 5.
Hemolytic activity on human RBCs using 8aa–8gd at 100 μM. Data were analyzed by mean ± SD (standard deviation) of triplicate (n = 3) samples.
After the preliminary hemolytic activity of tested compounds at 100 μM concentration, the concentration-dependent activity of one of the most potent compounds 8ga was performed at different concentrations (20–100 μM). It was found that the hemolysis activity of 8ga increased with increasing concentration and was less than 10% hemolysis for the concentration range of 20–100 μM. The observed results of compound 8ga at different concentrations showed its negligible toxicity to human blood cells (Figure 6).
Figure 6.
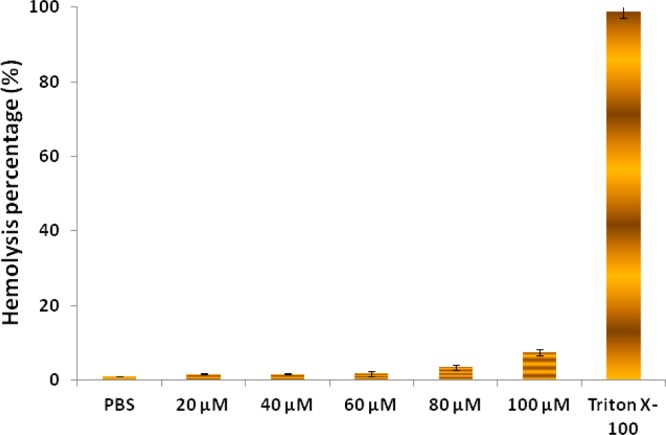
Hemolytic activity of compound 8ga on human RBCs at different concentrations 20–100 μM. Data were analyzed by mean ± SD (standard deviation) of triplicate (n = 3) samples.
2.2.6. Biofilm Inhibition
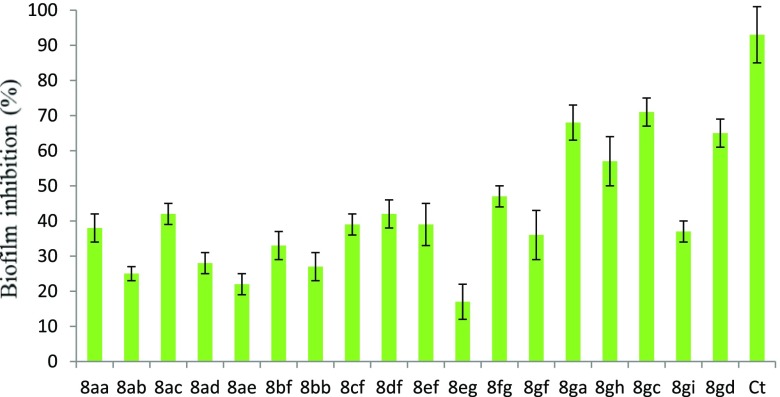
Bacterial biofilm is an extracellular polymeric substance and composed of extracellular polysaccharides and proteins, causing chronic infections in humans via hospital and community environments. Biofilm formation provides resistance to microbes against phagocytosis and other components of the body’s defense system. Thus, compounds having antibiofilm activity could prevent the contamination of biomedical implants. In our study, we observed that most of the compounds showed antibiofilm activity in the range of 20–70%, as compared to positive control ciprofloxacin (Figure 7). The highest inhibitory activity was shown by compounds 8gc (70%), followed by 8ga (67%) and 8gd (63%) (Figure 7). Compounds can modulate the biofilm formation through the nonmicrobicidal or biocidal mechanism. Moreover, these molecules weaken the biofilm either by degradation of the extracellular matrix or targeting of extracellular and intracellular signaling molecules.
Figure 7.
Biofilm inhibition assay against strain S. aureus by 8aa–8gd at 50 μg/mL. All experiments were carried out in triplicates, and the values are indicated as mean ± SD.
The analysis of antibacterial activity confirmed their efficacy against tested bacteria because of damage on the bacterial membranes and the effects of oxidative stress as evident by the live–dead bacterial assay and ROS production, respectively. Generation of ROS leads to a lethal effect on bacterial cells and can increase the bactericidal process. The acute change in response to compound treatment may cause the release and degradation of membrane proteins; this leads to the loss of membrane integrity and bacterial cell morphology, formation of fissures, and perforations in the cell membrane. The formation of fissures and perforation leads to the leakage of cytoplasmic contents from the cell; this increases the death consequences.
The overall study concludes that benzimidazole derivatives bearing the indole, 1,2,4-triazole, imidazole, and pyrazole group induced greater oxidative stress and morphological changes like membrane damage. Moreover, pathological outcomes such as DNA damage, alterations to specific proteins, and enzymes responsible for different physiological processes at the molecular level need further investigation.
3. Conclusions
A new series of imidazo/benzimidazo[1,2-c]quinazolines has been synthesized via copper-catalyzed Ullmann type C–N coupling followed by the intramolecular CDC reaction. All of the synthesized compounds were evaluated for their antimicrobial activity. Among all, compounds 8gf, 8ga, 8gc, and 8gd exhibited most promising antibacterial (MIC 4–8 μg/mL) and antifungal (MIC 4–16 μg/mL) activities. The structure activity relationship of synthesized compounds revealed that benzimidazo[1,2-c]quinazolines showed better antimicrobial activity than imidazo[1,2-c]quinazoline derivatives. Among benzimidazo[1,2-c]quinazolines, 1,2,4-triazole (8ga) and indole (8gc and 8gd)-fused benzimidazo[1,2-c]quinazolines exhibited better antimicrobial activity as compared to other azoles. The live–dead bacteria cell screening and increased ROS production upon treatment with most active compounds 8ga, 8gc, and 8gd, illustrates their efficient membrane penetration ability which might be the main cause of bacterial cell death. Furthermore, synthesized compounds were also evaluated for hemolytic activity which showed a negligible toxicity profile toward human blood cells. The overall study concludes that compounds 8ga, 8gc, and 8gd may serve as potential antimicrobial candidates for lead optimization.
4. Experimental Section
All reagents and solvents were obtained from commercial suppliers and used without further purification unless otherwise mentioned. Progress of the reactions was monitored by using thin layer chromatography (TLC) on 0.2 mm silica gel F254 plates. The chemical structures of the final products were determined by the NMR (1H and 13C) and HRMS analysis. 1H NMR and 13C NMR spectra were recorded on a 400 and 100 MHz spectrometer. Chemical shifts are reported in parts per million (ppm) using tetramethylsilane as an internal standard or the deuterated solvent peak (CDCl3 and DMSO-d6). HRMS data were recorded on a mass spectrometer with an electrospray ionization and TOF mass analyzer. Melting points were determined in open capillary tubes on an automated melting point apparatus and are uncorrected. All tested compounds were ≥95% pure by high-performance liquid chromatography (HPLC) with detection at 270 nm. Starting substrates 2-(2-bromophenyl)-4,5-diaryl-1H-imidazole and 2-(2-bromophenyl)-1H-benzo[d]imidazole were prepared by the following reported procedure.12,13
4.1. General Procedure for the Synthesis of Imidazo/Benzimidazo[1,2-c]quinazolines
A clean oven-dried 10 mL round-bottom flask was charged with 5 (1.0 mmol), 6 (1.2 mmol), K2CO3 (2.0 mmol), CuI (0.20 mmol), and DMF (2 mL). The resulting solution was stirred at 150 °C for 2 h. On completion of the first step monitored by TLC, Cu(OAc)2·H2O (0.5 mmol) was added in the same flask without isolating the intermediate, and the reaction mass was further stirred at 150 °C for 2–5 h. The reaction progress was monitored by TLC. After completion, the reaction mass was allowed to cool at ambient temperature, diluted with water (10 mL), and extracted with EtOAc (2 × 10 mL). The combined organic layer was dried over anhydrous Na2SO4 and concentrated under reduced pressure. The crude material was purified by column chromatography on silica gel (100–200 mesh) using ethyl acetate/hexane (15%, v/v) as an eluant.
4.1.1. 5,6-Diphenylimidazo[1,2-c][1,2,4]triazolo[1,5-a]quinazoline (8aa)
Off-white solid; yield 65%; mp 260–261 °C (lit. = 260–261 °C);131H NMR (400 MHz, CDCl3): δ 8.75 (d, J = 8.0 Hz, 1H), 8.31 (d, J = 8.3 Hz, 1H), 8.05 (s, 1H), 7.78 (t, J = 7.6 Hz, 1H), 7.69–7.56 (m, 8H), 7.33–7.29 (m, 3H); 13C NMR (100 MHz, CDCl3): δ 151.2, 143.8, 142.0, 140.4, 133.2, 131.8, 130.8, 130.7, 129.5, 129.0, 128.5, 128.3, 127.9, 127.7, 127.0, 124.7, 115.5, 114.6; HRMS (ESI): calcd for C23H16N5, 362.1400; found, 362.1400 [M + H]+.
4.1.2. 2,3,6,7-Tetraphenyldiimidazo[1,2-a:1′,2′-c]quinazoline (8ab)
Off-white solid; yield 52%; mp 255–257 °C; 1H NMR (400 MHz, CDCl3): δ 8.73 (d, J = 7.9 Hz, 1H), 7.77–7.71 (m, 4H), 7.65–7.57 (m, 8H), 7.43 (t, J = 7.6 Hz, 1H), 7.35–7.28 (m, 3H), 7.23–7.12 (m, 6H), 6.93 (d, J = 8.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 141.2, 139.8, 136.7, 136.7, 133.8, 133.6, 132.2, 132.1, 132.0, 131.8, 130.5, 130.0, 129.3, 128.8, 128.3, 128.0, 128.0, 127.8, 127.3, 126.9, 126.6, 125.4, 125.2, 125.0, 122.6, 116.0, 115.9; HRMS (ESI): calcd for C36H25N4, 513.2074; found, 513.2073 [M + H]+.
4.1.3. 1,2-Diphenylimidazo[1,2-c]indolo[1,2-a]quinazoline (8ac)
Off-white solid; yield 55%; mp 240–243 °C; 1H NMR (400 MHz, CDCl3): δ 8.71 (dd, J = 7.9, 1.6 Hz, 1H), 8.47 (d, J = 8.5 Hz, 1H), 8.29 (d, J = 8.4 Hz, 1H), 7.70–7.66 (m, 4H), 7.63–7.61 (m, 4H), 7.51–7.45 (m, 2H), 7.39–7.34 (m, 1H), 7.32–7.30 (m, 2H), 7.26–7.23 (m, 2H), 5.48 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 140.9, 139.1, 134.2, 133.8, 131.9, 131.7, 130.9, 130.7, 130.1, 129.8, 129.1, 128.8, 128.3, 127.3, 127.2, 125.3, 125.0, 124.0, 122.4, 122.3, 121.0, 115.2, 113.4, 87.9; HRMS (ESI): calcd for C29H20N3, 410.1652; found, 410.1672 [M + H]+.
4.1.4. 11-Chloro-1,2-diphenylimidazo[1,2-c]indolo[1,2-a]quinazoline (8ad)
Brown solid; yield 42%; mp 259–261 °C; 1H NMR (400 MHz, CDCl3): δ 8.73 (d, J = 7.8 Hz, 1H), 8.37 (d, J = 8.5 Hz, 1H), 8.18 (d, J = 9.0 Hz, 1H), 7.70–7.60 (m, 8H), 7.51–7.47 (m, 2H), 7.32–7.28 (m, 4H), 5.41 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 141.0, 139.0, 133.8, 133.6, 132.7, 131.8, 130.5, 130.2, 130.1, 129.9, 129.2, 128.3, 128.0, 127.3, 125.4, 125.0, 124.4, 122.3, 120.3, 115.3, 115.0, 114.3, 87.3; HRMS (ESI): calcd for C29H19ClN3, 444.1262; found, 444.1237 [M + H]+.
4.1.5. 12-Methyl-1,2-diphenylimidazo[1,2-c]indolo[1,2-a]quinazoline (8ae)
White solid; yield 40%; mp 272–274 °C; 1H NMR (400 MHz, CDCl3): δ 8.57 (d, J = 7.8 Hz, 1H), 8.35 (d, J = 8.5 Hz, 1H), 8.24 (d, J = 8.4 Hz, 1H), 7.54–7.30 (m, 15H), 1.47 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 134.3, 132.4, 131.0, 130.9, 130.7, 130.2, 128.9, 128.4, 128.2, 127.2, 126.3, 125.2, 123.6, 123.0, 122.2, 119.0, 115.3, 114.6, 113.8, 99.9, 9.3; HRMS (ESI): calcd for C30H22N3, 424.1808; found, 424.1808 [M + H]+.
4.1.6. 2,3-Di-p-tolyldiimidazo[1,2-a:1′,2′-c]quinazoline (8bf)
Brown solid; yield 60%; mp 224–226 °C (lit. = 224 °C);131H NMR (300 MHz, CDCl3): δ 8.70 (d, J = 7.8 Hz, 1H), 7.70–7.60 (m, 3H), 7.55–7.47 (m, 5H), 7.3 (d, J = 7.8 Hz, 2H), 7.18 (s, 1H), 7.10 (d, J = 7.9 Hz, 2H), 2.49 (s, 3H), 2.35 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 141.4, 139.5, 138.7, 137.3, 137.0, 131.6, 130.9, 130.3, 129.9, 129.1, 129.0, 128.9, 127.7, 127.0, 126.0, 125.2, 124.6, 115.2, 114.6, 109.9, 21.7, 21.3; HRMS (ESI): calcd for C26H21N4, 389.1761; found, 389.1771 [M + H]+.
4.1.7. 6,7-Diphenyl-2,3-di-p-tolyldiimidazo[1,2-a:1′,2′-c]quinazolines (8bb)
White solid; yield 58%; mp 251–253 °C; 1H NMR (400 MHz, CDCl3): δ 8.73 (d, J = 7.9 Hz, 1H), 7.70–7.57 (m, 9H), 7.42–7.37 (m, 4H), 7.25–7.15 (m, 7H), 6.94 (d, J = 8.7 Hz, 1H), 2.57 (s, 3H), 2.38 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 141.1, 139.6, 138.5, 137.0, 136.7, 136.7, 133.7, 132.2, 132.1, 131.9, 131.8, 131.1, 129.9, 129.9, 129.1, 129.0, 128.7, 128.0, 127.7, 126.8, 126.7, 125.4, 125.2, 124.8, 122.52, 116.0, 115.9, 21.6, 21.3; HRMS (ESI): calcd for C38H29N4, 541.2387; found, 541.2382 [M + H]+.
4.1.8. 2,3-Bis(4-methoxyphenyl)diimidazo[1,2-a:1′,2′-c]quinazoline (8cf)
Brown solid; yield 60%; mp 223 °C (lit. = 223 °C);131H NMR (300 MHz, CDCl3): δ 8.69 (d, J = 7.8 Hz, 1H), 7.70–7.49 (m, 8H), 7.18–7.16 (m, 1H), 7.03 (d, J = 7.8 Hz, 2H), 6.84 (d, J = 8.0 Hz, 2H), 3.91 (s, 3H), 3.81 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.9, 158.9, 141.0, 139.3, 137.2, 133.1, 130.3, 129.9, 129.0, 128.8, 126.3, 126.0, 125.2, 123.8, 122.0, 115.0, 114.6, 113.8, 113.7, 109.9, 55.2, 55.2; HRMS (ESI): calcd for C26H21N4O2, 421.1659; found, 421.1676 [M + H]+.
4.1.9. 2,3-Bis(4-fluorophenyl)imidazo[1,2-c]pyrazolo[1,5-a]quinazoline (8df)
Off-white solid; yield 45%; mp 257–260 °C; 1H NMR (400 MHz, CDCl3 + formic acid): δ 8.60 (d, J = 8.0 Hz, 1H), 8.36 (d, J = 8.3 Hz, 1H), 7.78–7.74 (m, 2H), 7.62–7.51 (m, 5H), 7.30 (t, J = 8.5 Hz, 2H), 7.03 (t, J = 8.6 Hz, 2H), 5.41 (d, J = 2.3 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 163.9 (d, J = 252.0 Hz), 162.8 (d, J = 249.0 Hz), 141.5, 139.2, 138.7, 133.7 (d, J = 8.4 Hz), 133.4, 132.6, 132.1, 130.0 (d, J = 8.3 Hz), 127.0, 126.9, 124.7, 123.9 (d, J = 3.6 Hz), 123.4, 116.8 (d, J = 21.8 Hz), 115.7 (d, J = 21.7 Hz), 115.6, 112.3, 92.5; HRMS (ESI): calcd for C24H15F2N4, 397.1259; found, 397.1262 [M + H]+.
4.1.10. 2-Phenyldiimidazo[1,2-a:1′,2′-c]quinazoline (8ef)
Off-white solid; yield 62%; mp 190 °C (lit. = 188–190 °C);131H NMR (400 MHz, CDCl3): δ 8.55 (d, J = 7.6 Hz, 1H), 8.24 (s, 1H), 7.99 (d, J = 7.3 Hz, 2H), 7.66 (d, J = 1.32 Hz, 1H), 7.63–7.53 (m, 2H), 7.50–7.45 (m, 3H), 7.36 (t, J = 7.3 Hz, 1H), 7.31 (d, J = 1.6 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 144.6, 140.2, 136.6, 133.1, 130.3, 130.0, 128.8, 128.7, 127.9, 126.0, 125.8, 125.1, 114.8, 114.7, 110.7, 108.0; HRMS (ESI): calcd for C18H13N4, 285.1135; found, 285.1112 [M + H]+.
4.1.11. 2-Phenylbenzo[4,5]imidazo[1,2-a]imidazo[1,2-c]quinazoline (8eg)
Brown solid; yield 70%; mp 208–210 °C; 1H NMR (400 MHz, CDCl3): δ 8.70 (dd, J = 7.9, 1.6 Hz, 1H), 8.40 (s, 1H), 8.37 (d, J = 8.4 Hz, 1H), 8.19 (dd, J = 7.6, 1.7 Hz, 1H), 8.04 (dd, J = 8.4, 1.5 Hz, 2H), 7.92–7.90 (m, 1H), 7.77–7.73 (m, 1H), 7.59–7.55 (m, 1H), 7.52–7.47 (m, 4H), 7.41–7.37 (m, 1H); 13C NMR (100 MHz, CDCl3): δ 145.4, 142.0, 141.2, 140.8, 132.8, 132.7, 130.9, 130.6, 128.9, 128.2, 125.9, 125.5, 125.4, 124.6, 123.3, 120.0, 115.0, 114.9, 113.1, 108.8; HRMS (ESI): calcd for C22H15N4, 335.1291; found, 335.1287 [M + H]+.
4.1.12. 2,3-Dimethyl-6,7-diphenyldiimidazo[1,2-a:1′,2′-c]quinazoline (8fb)
White solid; yield 45%; mp 271–273 °C; 1H NMR (400 MHz, CDCl3):δ 8.49 (dd, J = 8.0, 1.6 Hz, 1H), 7.65–7.56 (m, 7H), 7.35 (t, J = 7.5 Hz, 1H), 7.26–7.22 (m, 3H), 7.15–7.10 (m, 1H), 6.93 (d, J = 8.6 Hz, 1H), 3.00 (s, 3H), 2.46 (s, 3H); 13C NMR (100 MHz, CDCl3):δ 138.3, 137.8, 137.5, 137.2, 133.8, 132.0, 131.8, 131.6, 129.8, 128.4, 128.2, 127.1, 127.0, 125.4, 124.3, 122.5, 121.4, 116.0, 116.0, 12.9, 11.2; HRMS (ESI): calcd for C26H21N4, 389.1761; found, 389.1777 [M + H]+.
4.1.13. Benzo[4,5]imidazo[1,2-c]imidazo[1,2-a]quinazoline (8gf)
Brown solid; yield 66%; mp 192–195 °C (lit. = 192–195 °C);131H NMR (400 MHz, CDCl3): δ 8.72–8.69 (m, 1H), 8.64–8.61 (m, 1H), 8.00–7.98 (m, 1H), 7.72–7.71 (m, 3H), 7.58–7.52 (m, 3H), 7.42 (d, J = 1.7 Hz, 1H); 13C NMR (100 MHz, CDCl3) δ 143.9, 143.4, 137.7, 132.0, 131.8, 129.8, 129.0, 126.5, 126.2, 125.0, 124.2, 119.6, 114.9, 114.6, 114.3, 110.1; HRMS (ESI): calcd for C16H11N4, 259.0978; found, 259.0972 [M + H]+.
4.1.14. Benzo[4,5]imidazo[1,2-c][1,2,4]triazolo[1,5-a]quinazoline (8ga)
Off-white solid; yield 70%; mp 170–172 °C (lit. = 170–172 °C);131H NMR (300 MHz, CDCl3): δ 8.60 (d, J = 7.9 Hz, 1H), 8.39–8.36 (m, 1H), 8.20–8.17 (m, 2H), 7.92–7.89 (m, 1H), 7.75 (t, J = 7.5 Hz, 1H), 7.57 (t, J = 7.7 Hz, 1H), 7.50–7.48 (m, 2H); 13C NMR (75 MHz, CDCl3): δ 151.5, 144.1, 143.9, 143.5, 132.3, 132.1, 129.2, 127.1, 125.8, 125.6, 124.6, 120.0, 115.6, 114.0, 113.6; HRMS (ESI): calcd for C15H10N5, 260.0931; found, 260.0941 [M + H]+.
4.1.15. Benzo[4,5]imidazo[1,2-c]pyrazolo[1,5-a]quinazoline (8gh)
Off-white solid; yield 40%; mp 146–148 °C; 1H NMR (400 MHz, CDCl3): δ 8.65 (dd, J = 7.8, 1.4 Hz, 1H), 8.38 (dd, J = 8.4, 1.1 Hz, 1H), 8.01–7.99 (m, 2H), 7.87–7.85 (m, 1H), 7.80–7.75 (m, 1H), 7.58–7.50 (m, 3H), 6.77 (d, J = 2.1 Hz, 1H); 13C NMR (100 MHz, CDCl3): δ 143.8, 143.8, 141.9, 134.4, 133.9, 132.3, 129.9, 126.2, 125.8, 124.8, 123.9, 120.3, 115.5, 113.6, 111.3, 90.4; HRMS (ESI): calcd for C16H11N4, 259.0978; found, 259.1002 [M + H]+.
4.1.16. Benzo[4,5]imidazo[1,2-c]indolo[1,2-a]quinazolines (8gc)
Off-white solid; yield 51%; mp 178–180 °C; 1H NMR (400 MHz, CDCl3): δ 8.65 (d, J = 7.8 Hz, 1H), 8.34 (d, J = 8.5 Hz, 1H), 8.15 (d, J = 7.7 Hz, 1H), 7.98–7.93 (m, 2H), 7.72–7.66 (m, 2H), 7.50–7.48 (m, 2H), 7.41 (t, J = 7.6 Hz, 1H), 7.34–7.31 (m, 2H), 6.86 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 144.2, 144.0, 135.6, 131.9, 131.8, 130.9, 130.7, 129.4, 126.6, 124.4, 124.0, 123.7, 122.6, 122.4, 120.8, 120.0, 115.3, 114.3, 113.3, 112.1, 86.4; HRMS (ESI): calcd for C21H14N3, 308.1182; found, 308.1165 [M + H]+.
4.1.17. 3-Methoxybenzo[4,5]imidazo[1,2-c]indolo[1,2-a]quinazoline (8gi)
White solid; yield 45%; mp 198–200 °C; 1H NMR (400 MHz, CDCl3): δ 8.72 (dd, J = 7.9, 1.6 Hz, 1H), 8.38 (d, J = 8.5 Hz, 1H), 8.14 (d, J = 9.1 Hz, 1H), 8.04–8.00 (m, 2H), 7.76–7.72 (m, 1H), 7.54–7.52 (m, 2H), 7.46 (t, J = 7.5 Hz, 1H), 7.24 (d, J = 2.6 Hz, 1H), 7.01 (dd, J = 9.1, 2.6 Hz, 1H), 6.92 (s, 1H), 3.95 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 155.7, 144.2, 144.2, 135.5, 132.4, 132.0, 130.7, 130.6, 126.7, 125.9, 124.5, 123.9, 123.6, 120.0, 114.9, 114.2, 112.1, 111.3, 103.0, 86.4, 55.7; HRMS (ESI): calcd for C22H16N3O, 338.1288; found, 338.1285 [M + H]+.
4.1.18. 2-Chlorobenzo[4,5]imidazo[1,2-c]indolo[1,2-a]quinazoline (8gd)
Off-white solid; yield 35%; mp 245–247 °C; 1H NMR (400 MHz, CDCl3): δ 8.64 (d, J = 7.8 Hz, 1H), 8.23 (d, J = 8.5 Hz, 1H), 8.04 (d, J = 9.0 Hz, 1H), 7.96 (d, J = 7.2 Hz, 1H), 7.88 (d, J = 7.4s Hz, 1H), 7.68 (t, J = 7.2 Hz, 1H), 7.63 (d, J = 2.2 Hz, 1H), 7.53–7.47 (m, 2H), 7.43 (t, J = 7.6 Hz, 1H), 7.26–7.25 (m, 1H), 6.76 (s, 1H); 13C NMR (100 MHz, CDCl3): δ 144.1, 143.8, 135.0, 132.7, 132.0, 130.6, 130.6, 129.2, 128.2, 126.7, 124.6, 124.5, 123.9, 122.4, 120.1, 115.0, 114.3, 114.2, 112.04, 85.7; HRMS (ESI): calcd for C21H13ClN3, 342.0793; found, 342.0799 [M + H]+.
4.2. Antimicrobial Activity
4.2.1. Antibacterial Activity
The compounds 8 were tested for antibacterial activity against three Gram negative bacteria including E. coli (MTCC 1652), P. putida (MTCC 102), and S. typhi (98) and two Gram positive bacteria including B. subtilis (MTCC 121) and S. aureus (MTCC 96) as per the standard method.17 The tested strains were collected from the Microbial Type Culture Collection and Gene Bank (MTCC, India). The autoclaved Luria–Bertani agar medium was poured into sterile glass Petri dishes (90 mm) under aseptic conditions. After solidification of the medium, 100 μL culture of each pathogenic culture (107 cfu/mL) was spread using a sterile glass spreader and left for 15 min for complete adsorption. After adsorption, a well size of 6 mm diameter was made by the sterile metallic borer, and the solution of the working compound of different concentrations was poured into the wells. After incubation at 37 °C for 24 h, the diameter of ZOI was measured in comparison with standard antibiotic “ciprofloxacin”. The solvent, DMSO was used as the negative control, whereas antibiotic “ciprofloxacin” as the positive control. For the MIC assay, test compounds were prepared in concentrations of 2, 4, 8, 16, 32, 64, 128, and 256 μg/mL in DMSO and serial diluted test samples of each compound (200 μL) were added in 96-well microtrays. The test microorganism was added to microtrays well to obtain a final volume of 400 μL and incubated at 37 °C for 24 h. The MIC value is defined as the lowest concentration of the compound that inhibits the visible growth of bacteria (OD600 less than 0.06). Each assay was performed in duplicate sets.
4.2.2. Antifungal Activity
The compounds 8 were tested for antifungal activity by the agar well diffusion method against the fungal strains C. albicans (MTCC 3958) and A. niger (MTCC 9933). For the experimental work, a loopful of each strain was grown in the potato dextrose broth (PDB, HiMedia, India) medium at 28 °C for 4–5 days. Following optimal growth of each fungal strain, 100 μL of culture was uniformly spread on the potato dextrose agar medium plate. Following adsorption, wells of 6 mm were prepared by the sterile metallic borer and a solution of the working compound of different concentrations was poured into the wells. Plates were incubated at 28 °C for 4–5 days under dark conditions. The mean diameter of the inhibition zone was measured to determine antifungal activity. For the MIC assay, sterile test tubes containing 5 mL of sterilized Czapeks Dox broth medium was inoculated with 100 μL of freshly grown culture of each test strain and appropriate amount of the compound was added to achieve the desired concentrations. The tubes were incubated at 28 °C for five days under dark conditions and carefully observed for the presence of turbidity. Amphotericin B was used as the positive control. The experiment was performed in duplicate sets.
4.3. Bactericidal Assay by PI
The single colony of bacterium P. putida was inoculated into the sterile nutrient broth medium and kept in an incubator shaker (150 rpm) till the optical density (OD) of the culture reached up to 0.8. The culture was treated with selected compounds at 2× MIC for 4 h. After treatment, the culture was centrifuged at 5000g for 10 min, and the cell pellet was stained with 1.0 mg/mL of PI (Sigma-Aldrich, USA). The stained colony was streaked on the clean glass slide and covered with a glass slip. The population of PI-positive bacterial cells was compared with untreated cells by epifluorescence microscopy. During the experiment, the bacterial culture treated with TBHP was taken as the positive control.
4.4. Live–Dead Bacteria Screening through Fluorescence Microscopy
To discriminate live and dead bacterial cells, freshly grown culture of P. putida (107 cfu mL–1) was treated with compounds 8ga, 8gc, and 8gd for 4 h. Following treatment, 5 μL each of AO (15 μg mL–1) and Et·Br (50 μg mL–1) was added in a 500 μL of the P. putida culture. The working solution of acridine orange and ethidium bromide were prepared in the phosphate buffer saline (PBS) buffer. The suspension was centrifuged at 5000g for 10 min, and the supernatant was discarded. The cell pellet was washed with the 1× PBS buffer (pH 7.2) three times to remove any traces of unbound dyes. The washed cell pellet was streaked on the glass slide with a cover slip on top of it and viewed under an epifluorescence microscope (Olympus-CKX41, Olympus, Japan) at intensity between 450 and 490 nm using a 100× objective lens and 10× eyepiece lens.
4.5. Evaluation of ROS Production
To evaluate the intracellular reactive oxygen species formation after compound treatment, fluorescent dye DCFH-DA was used as probes. The DCFH is a nonfluorescent dye, but is oxidized to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) by intracellular H2O2 or nitric oxide. At the mid log phase of bacterial growth, compounds were added to the bacterial suspension (P. putida) for 2 h and then treated with 2 μM of DCFH-DA at 37 °C for 30 min. The emitted fluorescence of DCF was measured at 530 nm after excitation at 485 nm by fluorescence microscopy and qualitatively screened for ROS production.
4.6. Hemolytic Activity
The compounds 8 were tested for hemolytic activity on human RBCs following the standard protocol with minor modifications.18 The healthy human blood samples of males between 25 and 28 age groups were collected from the hospital of BITS Pilani campus following Institutional Ethics Committee guidelines and washed with the sterile PBS solution at least three times. After washing, blood was centrifuged at 2500 rpm for 6 min at room temperature and RBC pellet was suspended in the PBS buffer. The RBC suspension of 0.1 mL was mixed with 0.5 mL buffer solution containing 100 μg/mL of each compound in DMSO (1%, v/v) in the PBS buffer. DMSO (1%, v/v) in the PBS buffer and Triton X-100 (1%) were used as negative and positive controls, respectively. The samples were incubated at room temperature for 3 h. Following incubation, samples were centrifuged at 8000 rpm and absorbance of the supernatant was recorded at 540 nm. The percentage hemolysis was calculated by using the following equation:
[% Hemolysis = (A540 test sample – A540 negative control/A540 positive control – absorbance 540 negative control) × 100]. Each sample was analyzed in triplicate sets.
4.7. Biofilm Inhibition Assay
The pathogenic bacterial strain S. aureus was cultured in the tryptic soy broth medium and OD of the bacterial suspension was adjusted to 1 × 106 cfu mL–1. The synthesized compounds having concentrations of 50 μg/mL were mixed to the grown bacterial culture and properly mixed. The aliquots of 100 μL were distributed into the 96-well polystyrene microtiter plates (Tarson, India) and kept under static conditions at 37 °C for 24 h. The medium was discarded with micropipettes, and the plate was washed with PBS buffer (1×, pH 7.2) to wash away the nonadherent bacterial culture. The well of microtiter plates were stained with 100 μL of 0.1% crystal violet solution for 30 min at room temperature. After incubation, the staining solution was discarded, and wells were washed with autoclaved Milli-Q water and kept for air drying at room temperature. The stained biofilm were solubilized with 100 μL of 95% ethanol and absorbance was taken at 540 nm. Blank wells were taken as the background control. All experiments were carried out in triplicates, and the values are indicated as mean ± SD.
Acknowledgments
The authors sincerely acknowledge financial support [EMR/2016/002242 and PDF/2017/001910] by Science and Engineering Research Board (SERB), New Delhi, to carry out this work, DST-FIST [SR/FST/CSI-270/2015] for HRMS facility and Central Analytical Lab, BITS Pilani, for NMR. N.K.N., S.D., and H.K.S. are grateful to the Council of Scientific and Industrial Research (CSIR), New Delhi, India, for senior research fellowship. The authors also acknowledge Shahid Khan, Department of Biological Science, BITS Pilani, Pilani campus Rajasthan, India for concentration-dependent hemolytic activity.
Glossary
Abbreviations
- MRSA
methicillin-resistant Staphylococcus aureus
- MIC
minimum inhibitory concentration
- ZOI
zone of inhibition
- PI
propidium iodide
- AO
acridine orange
- Et·Br
ethidium bromide
- ROS
reactive oxygen species
- DCFH-DA
2′,7′-dichlorofluorescein diacetate
- RBC
red blood cell
- PBS
phosphate buffer saline
- CDC
cross-dehydrogenative coupling
- CAN
ceric ammonium nitrate
- TBHP
tert-butyl hydroperoxide
- DMSO
dimethyl sulphoxide
- PDB
potato dextrose broth
- OD
optical density
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01592.
Copies of 1H and 13C NMR, ESI-HRMS, and HPLC purity spectra of compounds 8aa–8gd (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Lowy F. D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. 10.1056/nejm199808203390806. [DOI] [PubMed] [Google Scholar]
- Shallcross L. J.; Davies S. C. The world health assembly resolution on antimicrobial resistance. J. Antimicrob. Chemother. 2014, 69, 2883–2885. 10.1093/jac/dku346. [DOI] [PubMed] [Google Scholar]
- a Wencewicz T. A.; Miller M. J. Biscatecholate–monohydroxamate mixed ligand siderophore–carbacephalosporin conjugates are selective sideromycin antibiotics that target acinetobacter baumannii. J. Med. Chem. 2013, 56, 4044–4052. 10.1021/jm400265k. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Carosso S.; Liu R.; Miller P. A.; Hecker S. J.; Glinka T.; Miller M. J. Methodology for monobactam diversification: Syntheses and studies of 4-thiomethyl substituted β-lactams with activity against gram-negative bacteria, including carbapenemase producing acinetobacter baumannii. J. Med. Chem. 2017, 9, 8933–8944. 10.1021/acs.jmedchem.7b01164. [DOI] [PubMed] [Google Scholar]; c Ghosh M.; Miller P. A.; Möllmann U.; Claypool W. D.; Schroeder V. A.; Wolter W. R.; Suckow M.; Yu H.; Li S.; Huang W.; Zajicek J.; Miller M. J. Targeted antibiotic delivery: Selective siderophore conjugation with daptomycin confers potent activity against multidrug resistant acinetobacter baumannii both in vitro and in vivo. J. Med. Chem. 2017, 60, 4577–4583. 10.1021/acs.jmedchem.7b00102. [DOI] [PubMed] [Google Scholar]
- a Zhang H.-Z.; He S.-C.; Peng Y.-J.; Zhang H.-J.; Gopala L.; Tangadanchu V. K. R.; Gan L.-L.; Zhou C.-H. Design, synthesis and antimicrobial evaluation of novel benzimidazole-incorporated sulfonamide analogues. Eur. J. Med. Chem. 2017, 136, 165–183. 10.1016/j.ejmech.2017.04.077. [DOI] [PubMed] [Google Scholar]; b Zhang W.; Liu J.; Macho J. M.; Jiang X.; Xie D.; Jiang F.; Liu W.; Fu L. Design, synthesis and antimicrobial evaluation of novel benzoxazole derivatives. Eur. J. Med. Chem. 2017, 126, 7–14. 10.1016/j.ejmech.2016.10.010. [DOI] [PubMed] [Google Scholar]; c Ullah A.; Iftikhar F.; Arfan M.; Kazmi S. T. B.; Anjum M. N.; Haq I.-u.; Ayaz M.; Farooq S.; Rashid U. Amino acid conjugated antimicrobial drugs: Synthesis, lipophilicity-activity relationship, antibacterial and urease inhibition activity. Eur. J. Med. Chem. 2018, 145, 140–153. 10.1016/j.ejmech.2017.12.089. [DOI] [PubMed] [Google Scholar]; d Sekhar M. M.; Nagarjuna U.; Padmavathi V.; Padmaja A.; Reddy N. V.; Vijaya T. Synthesis and antimicrobial activity of pyrimidinyl 1, 3, 4-oxadiazoles, 1, 3, 4-thiadiazoles and 1, 2, 4-triazoles. Eur. J. Med. Chem. 2018, 145, 1–10. 10.1016/j.ejmech.2017.12.067. [DOI] [PubMed] [Google Scholar]; e El-Gohary N. S.; Shaaban M. I. Synthesis and biological evaluation of a new series of benzimidazole derivatives as antimicrobial, antiquorum-sensing and antitumor agents. Eur. J. Med. Chem. 2017, 131, 255–262. 10.1016/j.ejmech.2017.03.018. [DOI] [PubMed] [Google Scholar]; f Anand D.; Yadav P. K.; Patel O. P. S.; Parmar N.; Maurya R. K.; Vishwakarma P.; Raju K. S. R.; Taneja I.; Wahajuddin M.; Kar S. Antileishmanial activity of pyrazolopyridine derivatives and their potential as an adjunct therapy with miltefosine. J. Med. Chem. 2017, 60, 1041–1059. 10.1021/acs.jmedchem.6b01447. [DOI] [PubMed] [Google Scholar]
- a Hong W.; Li J.; Chang Z.; Tan X.; Yang H.; Ouyang Y.; Yang Y.; Kaur S.; Paterson I. C.; Ngeow Y. F.; Wang H. Synthesis and biological evaluation of indole core-based derivatives with potent antibacterial activity against resistant bacterial pathogens. J. Antibiot. 2017, 70, 832. 10.1038/ja.2017.55. [DOI] [PubMed] [Google Scholar]; b Lepri S.; Buonerba F.; Goracci L.; Velilla I.; Ruzziconi R.; Schindler B. D.; Seo S. M.; Kaatz G. W.; Cruciani G. Indole based weapons to fight antibiotic resistance: A structure–activity relationship study. J. Med. Chem. 2016, 59, 867–891. 10.1021/acs.jmedchem.5b01219. [DOI] [PubMed] [Google Scholar]; c Jafari E.; Khajouei M. R.; Hassanzadeh F.; Hakimelahi G. H.; Khodarahmi G. A. Quinazolinone and quinazoline derivatives: Recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci. 2016, 11, 1–14. [PMC free article] [PubMed] [Google Scholar]
- Hoemann M. Z.; Kumaravel G.; Xie R. L.; Rossi R. F.; Meyer S.; Sidhu A.; Cuny G. D.; Hauske J. R. Potent in vitro methicillin-resistant staphylococcus aureus activity of 2-(1H-indol-3-yl) quinoline derivatives. Bioorg. Med. Chem. Lett. 2000, 10, 2675–2678. 10.1016/s0960-894x(00)00542-4. [DOI] [PubMed] [Google Scholar]
- Song Y.-L.; Wu F.; Zhang C.-C.; Liang G.-C.; Zhou G.; Yu J.-J. Ionic liquid catalyzed synthesis of 2-(indole-3-yl)-thiochroman-4-ones and their novel antifungal activities. Bioorg. Med. Chem. Lett. 2015, 25, 259–261. 10.1016/j.bmcl.2014.11.056. [DOI] [PubMed] [Google Scholar]
- Bartik K.; Braekman J.-C.; Daloze D.; Stoller C.; Huysecom J.; Vandevyver G.; Ottinger R. Topsentins, new toxic bis-indole alkaloids from the marine sponge topsentia genitrix. Can. J. Chem. 1987, 65, 2118–2121. 10.1139/v87-352. [DOI] [Google Scholar]
- Kung P.-P.; Casper M. D.; Cook K. L.; Wilson-Lingardo L.; Risen L. M.; Vickers T. A.; Ranken R.; Blyn L. B.; Wyatt J. R.; Cook P. D. Structure–activity relationships of novel 2-substituted quinazoline antibacterial agents. J. Med. Chem. 1999, 42, 4705–4713. 10.1021/jm9903500. [DOI] [PubMed] [Google Scholar]
- a Rohini R.; Reddy P. M.; Shanker K.; Hu A.; Ravinder V. Antimicrobial study of newly synthesized 6-substituted indolo[1, 2-c]quinazolines. Eur. J. Med. Chem. 2010, 45, 1200–1205. 10.1016/j.ejmech.2009.11.038. [DOI] [PubMed] [Google Scholar]; b Rohini R.; Shanker K.; Reddy P. M.; Ho Y.-P.; Ravinder V. Mono and bis-6-arylbenzimidazo[1, 2-c]quinazolines: A new class of antimicrobial agents. Eur. J. Med. Chem. 2009, 44, 3330–3339. 10.1016/j.ejmech.2009.03.022. [DOI] [PubMed] [Google Scholar]; c Khan I.; Zaib S.; Batool S.; Abbas N.; Ashraf Z.; Iqbal J.; Saeed A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016, 24, 2361–2381. 10.1016/j.bmc.2016.03.031. [DOI] [PubMed] [Google Scholar]; d Khan I.; Ibrar A.; Ahmed W.; Saeed A. Synthetic approaches, functionalization and therapeutic potential of quinazoline and quinazolinone skeletons: The advances continue. Eur. J. Med. Chem. 2015, 90, 124–169. 10.1016/j.ejmech.2014.10.084. [DOI] [PubMed] [Google Scholar]; e Khan I.; Ibrar A.; Abbas N.; Saeed A. Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: Synthetic approaches and multifarious applications. Eur. J. Med. Chem. 2014, 76, 193–244. 10.1016/j.ejmech.2014.10.084. [DOI] [PubMed] [Google Scholar]; f Fröhlich T.; Reiter C.; Ibrahim M. M.; Beutel J.; Hutterer C.; Zeitträger I.; Bahsi H.; Leidenberger M.; Friedrich O.; Kappes B. Synthesis of novel hybrids of quinazoline and artemisinin with high activities against plasmodium falciparum, human cytomegalovirus, and leukemia Cells. ACS Omega 2017, 2, 2422–2431. 10.1021/acsomega.7b00310. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Jadhavar P. S.; Dhameliya T. M.; Vaja M. D.; Kumar D.; Sridevi J. P.; Yogeeswari P.; Sriram D.; Chakraborti A. K. Synthesis, biological evaluation and structure–activity relationship of 2-styrylquinazolones as anti-tubercular agents. Bioorg. Med. Chem. Lett. 2016, 26, 2663–2669. 10.1016/j.bmcl.2016.04.012. [DOI] [PubMed] [Google Scholar]
- a Nandwana N. K.; Dhiman S.; Shelke G. M.; Kumar A. Copper-catalyzed tandem Ullmann type C-N coupling and dehydrative cyclization: Synthesis of imidazo[1,2-c]quinazolines. Org. Biomol. Chem. 2016, 14, 1736–1741. 10.1039/c5ob02469b. [DOI] [PubMed] [Google Scholar]; b Nandwana N. K.; Dhiman S.; Saini H. K.; Kumar I.; Kumar A. Synthesis of quinazolinones, imidazo[1, 2-c]quinazolines and imidazo[4, 5-c]quinolines through tandem reductive amination of aryl halides and oxidative amination of C(sp3)–H bonds. Eur. J. Org. Chem. 2017, 514–522. 10.1002/ejoc.201601329. [DOI] [Google Scholar]; c Kumar A.; Nandwana N. K.; Saini H. K.; Shinde V. N. Copper-catalysed one-pot tandem reaction for the synthesis of imidazo[1,2-c][1,2,3]triazolo[1,5-a]quinazolines. Eur. J. Org. Chem. 2017, 6445–6449. 10.1002/ejoc.201701221. [DOI] [Google Scholar]
- Samai S.; Nandi G. C.; Singh P.; Singh M. L-Proline: an efficient catalyst for the one-pot synthesis of 2, 4, 5-trisubstituted and 1, 2, 4, 5-tetrasubstituted imidazoles. Tetrahedron 2009, 65, 10155–10161. 10.1016/j.tet.2009.10.019. [DOI] [Google Scholar]
- Nandwana N. K.; Pericherla K.; Kaswan P.; Kumar A. Synthesis of novel azole-fused quinazolines via one-pot, sequential Ullmann-type coupling and intramolecular dehydrogenative C–N bonding. Org. Biomol. Chem. 2015, 13, 2947–2950. 10.1039/c4ob02375g. [DOI] [PubMed] [Google Scholar]
- Tyagi P.; Singh M.; Kumari H.; Kumari A.; Mukhopadhyay K. Bactericidal activity of curcumin I is associated with damaging of bacterial membrane. PLoS One 2015, 10, e0121313 10.1371/journal.pone.0121313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel P.; Schmidt-Emrich S.; Maniura-Weber K.; Ren Q. Critical aspects of using bacterial cell viability assays with the fluorophores SYTO9 and propidium iodide. BMC Microbiol. 2015, 15, 36. 10.1186/s12866-015-0376-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Kasibhatla S.; Amarante-Mendes G. P.; Finucane D.; Brunner T.; Bossy-Wetzel E.; Green D. R. Acridine orange/ethidium bromide (AO/EB) staining to detect apoptosis. Cold Spring Harb. Protoc. 2006, 3, prot4493. 10.1101/pdb.prot4493. [DOI] [PubMed] [Google Scholar]; b Ribble D.; Goldstein N. B.; Norris D. A.; Shellman Y. G. A simple technique for quantifying apoptosis in 96-well plates. BMC Biotechnol. 2005, 5, 12. 10.1186/1472-6750-5-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson E. H.Prevalence of Penicillin-Resistant Streptococcus Pneumoniae-Connecticut, 1992–1993; Library Publications and Presentations, 1994; Vol. 43. [Google Scholar]
- Sathishkumar G.; Jha P. K.; Vignesh V.; Rajkuberan C.; Jeyaraj M.; Selvakumar M.; Jha R.; Sivaramakrishnan S. Cannonball fruit (Couroupita guianensis, Aubl.) extract mediated synthesis of gold nanoparticles and evaluation of its antioxidant activity. J. Mol. Liq. 2016, 215, 229–236. 10.1016/j.molliq.2015.12.043. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.