Abstract
Accurate genome duplication during cell division is essential for life. This process is accomplished by the close collaboration between replication factors and many additional proteins that provide assistant roles. Replication factors establish the replication machineries capable of copying billions of nucleotides, while regulatory proteins help to achieve accuracy and efficiency of replication. Among regulatory proteins, protein modification enzymes can bestow fast and reversible changes to many targets, leading to coordinated effects on replication. Recent studies have begun to elucidate how one type of protein modification, sumoylation, can modify replication proteins and regulate genome duplication through multiple mechanisms. This chapter summarizes these new findings, and how they can integrate with the known regulatory circuitries of replication. As this area of research is still at its infancy, many outstanding questions remain to be explored, and we discuss these issues in light of the new advances.
Keywords: DNA replication initiation, Replication progression, Posttranslational modifications, Sumoylation, Ubiquitination, Phosphorylation
1. Overview of Eukaryotic DNA Replication
1.1. Replication Initiation
DNA replication occurs in three stages, namely, initiation, progression, and termination. Each of these stages entails multi-step DNA transactions carried out by dozens of proteins. Most of the replication steps and proteins are highly conserved from simple model organisms, such as yeasts, to humans. Replication initiation begins with the licensing of genomic sites called origins. Origin licensing takes place in late M to G1 phase when origins become bound by the MCM complex, a ring-shaped complex composed of MCM2–7 subunits (Fig. 1). This process is achieved by interaction between MCM and MCM-loading factors. In budding yeast, wherein origin licensing is best understood, the MCM-loading factors include the origin recognition complex (ORC), Cdc6, and the MCM binding partner Cdt1 (Fig. 1) [reviewed in (Diffley et al. 1994; Kelly and Brown 2000; Bell and Dutta 2002; Sclafani and Holzen 2007; Remus and Diffley 2009; Li and Araki 2013; Bell and Labib 2016)]. ORCs demarcate origins by interacting with specific DNA sequences and chromatin components [reviewed in (Hoggard and Fox 2016; Gutiérrez and MacAlpine 2016)]. Cdc6 recruits the MCM-Cdt1 complex to ORC through interactions with both factors (Santocanale and Diffley 1996; Donovan et al. 1997; Speck et al. 2005; Randell et al. 2006; Sun et al. 2013). Subsequently, ATP hydrolysis by ORC, Cdc6, and MCM enables a pair of MCM rings to enclose DNA at origins (Fig. 1) (Bowers et al. 2004; Randell et al. 2006; Remus et al. 2009; Fernández-Cid et al. 2013; Frigola et al. 2013; Coster et al. 2014). This multistep process produces an MCM double hexamer with its central channel enclosing DNA [reviewed in (Diffley et al. 1994; Kelly and Brown 2000; Bell and Dutta 2002; Sclafani and Holzen 2007; Remus and Diffley 2009; Wei and Zhao 2016b; Li and Araki 2013; Bell and Labib 2016)].
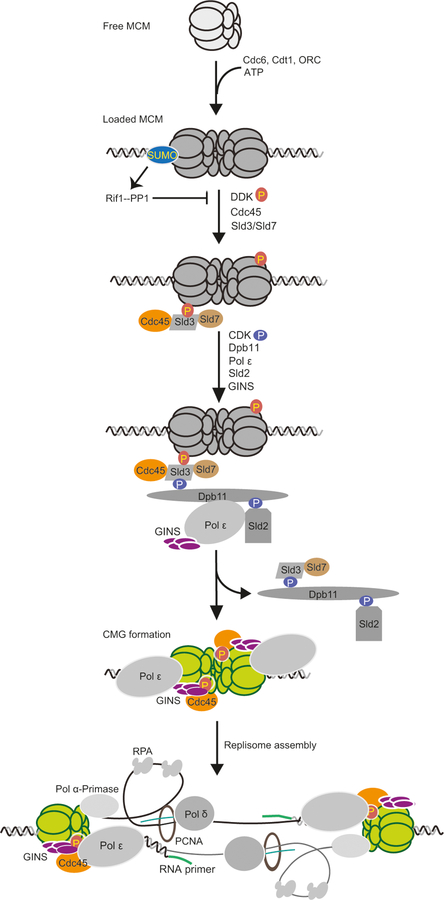
Fig. 1.
Summary of main steps of DNA replication initiation in budding yeast. The origin licensing step during late M to G1 phases entails free MCM being loaded onto replication origins as a double hexamer. This process requires several MCM-loading factors, including Cdc6, Cdt1, and the ORC complex. During the origin firing step in S phase, DDK and CDK kinases activate the loaded MCM. DDK-mediated phosphorylation of loaded MCM recruits Sld3 and its binding partners Cdc45 and Sld7. CDK-mediated phosphorylation of Sld2 and Sld3 promotes the recruitment of Pol ε, Dpb11, and the GINS complex. Cdc45, MCM, and GINS form the active replicative helicase CMG. Subsequent recruitment of additional protein factors results in the formation of the replisome. SUMO can counteract DDK-mediated MCM phosphorylation. A fraction of loaded Mcm2–7 subunits are sumoylated to prevent premature replication initiation. This is partly achieved as SUMO aids the recruitment of the Rif1-PP1 phosphase complex that can antagonize DDK action in G1. As S phase starts, rising DDK levels are associated with increased MCM phosphorylation and decreased MCM sumoylation levels. Such a switch of MCM modification states is also seen in other organisms. Note that recently findings suggest that MCM also switches from enclosing dsDNA to enclosing mostly leading strand ssDNA template when DNA synthesis begins and that the N-terminal tier of the MCM ring faces the moving replication fork
Once loaded, the MCM double hexamer must be kept inactive until the onset of S phase when origin firing takes place. One critical event during origin firing is the conversion of MCM into the replicative helicase, composed of MCM and its cofactors Cdc45 and the four-subunit GINS complex. This conversion requires two protein kinases, DNA polymerase ε, and several scaffolding proteins, including Sld2, Sld3–Sld7, and Dpb11 in budding yeast and their homologs in other organisms (Gambus et al. 2006; Moyer et al. 2006; Pacek et al. 2006; Ilves et al. 2010; Muramatsu et al. 2010; Kang et al. 2012). The first kinase, the Dbf4-dependent kinase (DDK), phosphorylates MCM subunits (Fig. 1). Mcm4 phosphorylation is particularly important as it is recognized by Sld3 in partnership with Sld7 and Cdc45 (Fig. 1) (Sheu and Stillman 2006; Sheu and Stillman 2010; Deegan et al. 2016). Subsequent to this step, the second kinase, cyclin-dependent kinase (CDK), phosphorylates Sld2 and Sld3, enabling their interaction with Dpb11 in cooperation with Pol ε and GINS (Fig. 1) (Tanaka et al. 2007; Zegerman and Diffley 2007; Muramatsu et al. 2010). As a result of this cascade of protein interactions, Cdc45 and GINS are delivered to MCM, resulting in the formation of the replicative helicase CMG (Cdc45-MCM-GINS) (Fig. 1) (Gambus et al. 2006; Moyer et al. 2006; Pacek et al. 2006; Ilves et al. 2010; Kang et al. 2012).
Subsequent to CMG formation, DNA polymerase α, the chromatin remodeling complex FACT, and several scaffolding proteins are recruited to CMG and Pol ε to form the replisome (Fig. 1) (Gambus et al. 2006, 2009; Morohashi et al. 2009). In the meantime, the origin firing scaffolds, such as Sld2, Sld3, and Dpb11, leave the CMG and are recycled to additional origins that fire later in S phase (Fig. 1) (Mantiero et al. 2011). The temporal order of the origin firing program is determined by multiple factors, such as the affinity between ORCs and origin sequences and local chromatin environment [reviewed in (Masai et al. 2010; Fragkos et al. 2015)]. At both early- and late-fired origins, the formation of a pair of replisomes establishes divergent replication forks that travel in opposite directions.
1.2. Replication Progression
As DNA synthesis begins, the DNA primase-Pol α complex generates primer sequences (Fig. 1). These primers can be extended by Pol ε for continuous leading strand synthesis and by Pol δ to produce many Okazaki fragments during discontinuous lagging strand synthesis (Fig. 1) [reviewed in (Kelly and Brown 2000; Bell and Dutta 2002; Sclafani and Holzen 2007; Bell and Labib 2016)]. The maturation of Okazaki fragments requires several additional conserved enzymes. In budding yeast, these include the flap endonuclease Rad27, the DNA helicase-nuclease Dna2, the exonuclease Exo1, the DNA helicase Pif1, and the ligase Cdc9 [reviewed in (Waga and Stillman 1998; Bell and Labib 2016)]. Collaboration among these factors enables ligation of Okazaki fragments.
During replication progression, a major challenge is coping with many types of template blockages. These can include (i) the topological stress generated by DNA unwinding, (ii) tightly bound nonhistone proteins, (iii) difficult-to-replicate genomic loci, (iv) collision with transcription machinery, and (v) DNA lesions generated from both intrinsic and extrinsic sources. Topological stress is largely relieved by replisome-associated topoisomerases. Several scaffold proteins within the replisome play pivotal roles in dealing with other replication impediments. Depending on the types of blocks, different proteins and strategies are used, and in many cases, additional DNA metabolism proteins are recruited to overcome template blocks. For example, tightly bound nonhistone proteins can be removed by DNA helicases, such as Rrm3 in budding yeast, allowing the resumption of DNA synthesis (Ivessa et al. 2000; Calzada et al. 2005; Azvolinsky et al. 2006). In the case of template damage, such as UV-induced thymidine dimers, translesion polymerases can mediate synthesis bypass of these sites [reviewed in (Waters et al. 2009)].
Besides template blockage, other issues that must be managed during replication progression include the removal of template nucleosomes ahead of replication forks and reestablishment or recycling of nucleosomes with correct positioning behind replication forks. In addition, replication progression is coupled with the establishment of sister chromatid cohesion and inheritable epigenetic markers. These topics have been recently summarized, and we refer the readers to several reviews for details [reviewed in (Jeppsson et al. 2014; Almouzni and Cedar 2016; Bell and Labib 2016)].
1.3. Replication Termination
As two opposing replication forks from adjacent origins converge, replication terminates. In general, replication termination sites are determined by the meeting point of two forks, but in some instances, termination occurs at replication pausing sites where one fork has more retention time (Labib and Hodgson 2007; Fachinetti et al. 2010). Three major events are required for replication termination, including the completion of local DNA synthesis, decatenation of the two daughter strands by topoisomerases, and disassembly of replisome. Compared with replication initiation and progression, replication termination is less well understood. More recently, new findings have implicated MCM ubiquitination in the disassembly of replisomes during termination (Maric et al. 2014; Priego Moreno et al. 2014; Dewar et al. 2015).
2. The SUMO Modification Cycle
Protein modifications underpin many regulatory mechanisms during the three stages of replication. Phosphorylation and ubiquitination have been found to be critical in all stages of replication [reviewed in (Wei and Zhao 2016b; Kelly and Brown 2000; Diffley 2004; Vodermaier 2004; Blow and Dutta 2005; Arias and Walter 2007; Sclafani and Holzen 2007; Moreno and Gambus 2015; Sivakumar and Gorbsky 2015; Garcia-Rodriguez et al. 2016)]. More recently, sumoylation has also been found to influence replication and is important for genome integrity. In this chapter, we summarize the findings that begin to unravel the mechanisms of SUMO-based replication regulation after a brief introduction of protein sumoylation.
2.1. Principles of the Sumoylation Process
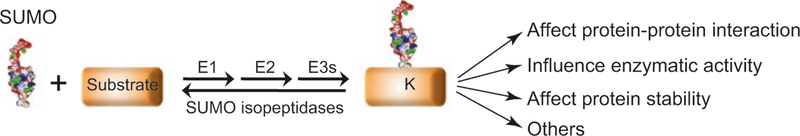
SUMO (small ubiquitin-like modifier) is a highly conserved member of the ubiquitin family of protein modifiers. With approximately 100 amino acids, SUMO assumes an ubiquitin fold but with a distinct surface charge distribution. The SUMO-specific E1 (or activating enzyme), E2 (or conjugating enzyme), and E3 (or ligase) enzymes conjugate SUMO to the ε-amino group lysine residue of a substrate (Johnson 2004) (Fig. 2). Most organisms contain one SUMO E1 and E2 but multiple SUMO E3s. SUMO E2 can directly bind to the so-called sumoylation consensus or reverse consensus sequences, [ΨKX(D/E)] or [(D/E)XKΨ] (Ψ, a hydrophobic residue; X, any residue). With the help of E3s, SUMO is then transferred from E2 to the lysine within such sequences (Gareau and Lima 2010; Lamoliatte et al. 2014). However, proteomic studies found that many of these sites are not sumoylated, suggesting that additional factors must also influence the sumoylation process (Gareau and Lima 2010; Lamoliatte et al. 2014). For example, it has been noted that sumoylation sites are often in loop regions (Gareau and Lima 2010), likely because of the ability of such regions to adopt local conformational changes that favor productive contact between the E2~SUMO thioester bond and the acceptor lysine. This principle could also explain findings that while sumoylation is conserved among many protein homologs, the sites of modification often vary (Golebiowski et al. 2009; Dou et al. 2010; Elrouby and Coupland 2010; Cremona et al. 2012; Psakhye and Jentsch 2012; Hendriks et al. 2014; Ma et al. 2014; Tammsalu et al. 2014).
Fig. 2.
The SUMO conjugation cycle and SUMO’s effects on substrate proteins. SUMO E1, E2, and E3 enzymes can conjugate a SUMO molecule to a lysine residue of a substrate. Sumoylation can also occur on multiple lysines of a substrate or in the form of SUMO chain (not shown). Sumoylation can have several biological effects on the substrates, and three frequently observed molecular consequences are indicated. Sumoylation can be reversed by SUMO isopeptidases
SUMO E3s play important roles partly by bridging E2 and substrates. For instance, budding yeast has the homologous Siz1 and Siz2 SUMO E3s and the Mms21 E3, all of which possess an SP-RING domain that can associate with Ubc9 (Johnson and Gupta 2001; Takahashi et al. 2001; Zhao and Blobel 2005; Gareau and Lima 2010). In addition, each E3 can associate with specific substrates. For example, Siz2 interacts with the ssDNA-binding protein RPA, promoting RPA sumoylation and Siz2 localization to DNA breaks (Chung and Zhao 2015). Mms21 is a part of the Smc5/Smc6 complex that localizes to several genomic loci to influence sumoylation events in these places (Murray and Carr 2008; De Piccoli et al. 2009; Hang et al. 2015; Bonner et al. 2016; Bermudez-Lopez et al. 2016). These interactions and the ability of E3s to enable productive alignment between E2 and substrates for SUMO transfer make SUMO E3s indispensible for sumoylation in vivo [reviewed in (Gareau and Lima 2010)]. Based on studies from several organisms, SUMO E3s often exhibit substrate redundancy, likely reflecting their similar SUMO transfer mechanisms (Hang et al. 2014; Sarangi et al. 2014).
Mammalian cells have larger numbers of SUMO E3s than yeast. At least ten E3s have been described in human cells thus far (Gareau and Lima 2010; Cappadocia et al. 2015; Eisenhardt et al. 2015). These can be divided into several groups, including (1) PIAS proteins that are homologs of the yeast Siz1 and Siz2, (2) the Mms21 homolog NSMCE2, (3) the ZNF451 type of SUMO E3s that utilize tandem SUMO-interacting motifs (SIMs) to enable sumoylation (Cappadocia et al. 2015; Eisenhardt et al. 2015), and (4) more specialized SUMO E3s that target specific processes, such as the nuclear pore protein Ran binding protein 2 (RanBP2), the polycomb group protein Pc2, and the promyelocytic leukemia (PML) protein. The increased numbers of SUMO E3s in human cells are associated with the presence of multiple SUMO isoforms (Gareau and Lima 2010; Liang et al. 2016). SUMO2 and SUMO3 have 97% sequence identity and can form SUMO chains, while the more divergent SUMO1 is less frequently found in SUMO chains [reviewed in (Gareau and Lima 2010)]. The acquirement of different SUMO isoforms and the many types of SUMO chains that they can form, in conjunction with the multiple types of SUMO E3s in human cells, can meet the needs of more complex genomes and increased demands for regulation.
2.2. Principles of the Desumoylation Process
Sumoylation can be reversed by multiple SUMO-specific cysteine proteases, known as desumoylation enzymes (Mukhopadhyay and Dasso 2007; Hickey et al. 2012) (Fig. 2). The substrate selectivity of desumoylation enzymes is partly achieved by their distinct localizations. Using budding yeast as an example, one of its desumoylation enzymes, Ulp1, primarily associates with nuclear pore complexes, whereas the other enzyme, Ulp2, can be seen concentrated in the nucleolus (Li and Hochstrasser 1999, 2000; Panse et al. 2003; Kroetz et al. 2009; Srikumar et al. 2013). Consistent with these localization patterns, Ulp1 and Ulp2 have different substrates (Makhnevych et al. 2009; de Albuquerque et al. 2016; Wei and Zhao 2016a). In addition, Ulp1 enables SUMO maturation by removing the tail of the precursor SUMO molecule (Li and Hochstrasser 1999), while Ulp2 has a major role in removing SUMO chains (Li and Hochstrasser 2000). Both Ulp1 and Ulp2 are required for cell fitness and resistance to a broad range of genotoxins (Li and Hochstrasser 1999, 2000; Schwartz et al. 2007). As Ulp2 mutant defects are suppressed to a large degree by mutating the lysine residues on SUMO, which prevents SUMO chain formation, accumulation of SUMO chains is deleterious (Bylebyl et al. 2003). Human cells contain at least six desumoylation enzymes, called sentrin-/SUMO-specific proteases (SENPs) (Hannoun et al. 2010; Hickey et al. 2012). SENP1, 2, 3, and 5 are more related to Ulp1, whereas SENP6 and 7 are similar to Ulp2 [reviewed in (Mukhopadhyay and Dasso 2007)]. As is the case for Ulp1 and Ulp2 in yeast, SENPs have distinct activities and cellular localization patterns, and their mutants cause a wide range of defects [reviewed in (Mukhopadhyay and Dasso 2007; Hickey et al. 2012)].
2.3. Biochemical Effects of Sumoylation
Protein sumoylation affects a myriad of biological processes, such as transcription, nuclear transport, DNA metabolism, and protein quality control [reviewed in (Sarangi and Zhao 2015)]. The conjugation and removal of SUMO from proteins can alter protein-protein interactions, partly because SUMO modules can interact with SIMs on other proteins or the substrate itself. As such, SUMO-SIM interactions can promote the assembly of protein complexes and the formation of a membrane-free nuclear compartment [reviewed in (Shen et al. 2006)]. On the other hand, SUMO sometimes disrupts existing protein-protein interactions or protein aggregation, possibly due to steric hindrance posed by the SUMO moiety. Additionally, sumoylation can alter a protein’s interaction with DNA or chromatin, its enzymatic activities, or its protein levels. Each of these effects has been observed in DNA metabolism processes, particularly in DNA repair. For example, sumoylation enhances inter-subunit association among the DNA helicase-topoisomerase Sgs1-Top3-Rmi1 complex and promotes its function in resolving Holliday junctions (Bermudez-Lopez et al. 2016; Bonner et al. 2016). On the other hand, sumoylation of the DNA nuclease cofactor Sae2 helps convert the protein from insoluble aggregates to a soluble form, which is required for DNA-end resection (Sarangi et al. 2015). In the case of the recombination mediator protein Rad52, sumoylation leads to association with the segregase Cdc48/p97 that removes proteins from DNA using its ATPase activity (Bergink et al. 2013). These examples illustrate some of the mechanisms by which sumoylation regulates genome maintenance. Recent studies have begun to reveal the roles of sumoylation during DNA replication, and the following sections summarize findings in this area.
3. SUMO-Based Regulation of Replication Initiation
Proteomic studies in multiple organisms have shown that protein factors that help replisomes cope with template obstacles are enriched among SUMO substrates (Golebiowski et al. 2009; Elrouby and Coupland 2010; Cremona et al. 2012; Hendriks et al. 2014; Ma et al. 2014; Tammsalu et al. 2014). Genetic and biochemical studies of individual SUMO substrates and SUMO enzymes have revealed some mechanisms of SUMO-based regulation of these proteins and how they affect DNA replication.
3.1. MCM Sumoylation Inhibits Replication Initiation in Budding Yeast
In budding yeast, all six subunits of MCM are sumoylated (Cremona et al. 2012; de Albuquerque et al. 2016; Wei and Zhao 2016a). MCM sumoylation has a distinct spatial and temporal pattern relative to the cycle of DNA replication (Wei and Zhao 2016a). Spatially, MCM is only sumoylated when loaded onto origins (Wei and Zhao 2016a). Temporally, Mcm2–6 sumoylation levels peak during G1 phase prior to DDK-mediated Mcm4 phosphorylation, then decline as cells enter S phase, and again increase during G2/M phase, coincident with the next MCM-loading cycle (Wei and Zhao 2016a). The opposing patterns of Mcm2–6 sumoylation and MCM phosphorylation during the cell cycle indicate a negative role of MCM sumoylation during replication initiation. Indeed, increased MCM sumoylation causes a reduction in the levels of Mcm4 phosphorylation, CMG, and origin firing (Wei and Zhao 2016a). These defects are partly because hyper-sumoylated MCM has increased association with the PP1 phosphatase, which reverses Mcm4 phosphorylation (Wei and Zhao 2016a; Davé et al. 2014; Hiraga et al. 2014; Mattarocci et al. 2014). MCM sumoylation levels subside at the start of S phase, partly through the action of Ulp2 (Wei and Zhao 2016a; de Albuquerque et al. 2016). These findings suggest that MCM sumoylation serves as a safeguard to prevent premature helicase function before S phase and that initiation of DNA synthesis requires removing this modification (Wei and Zhao 2016a). Future work is needed to examine whether the observed effects are due to a particular MCM subunit or contributions from multiple subunits and whether sumoylation alters other MCM features in addition to PP1 regulation.
3.2. MCM Sumoylation in Higher Eukaryotes
MCM sumoylation has also been detected in higher eukaryotes (Golebiowski et al. 2009; Elrouby and Coupland 2010; Hendriks et al. 2014; Ma et al. 2014; Schimmel et al. 2014; Tammsalu et al. 2014). For example, human MCM2, 3, 4, and 7 proteins are sumoylated. Importantly, Mcm4 sumoylation levels exhibit a similar pattern to that of yeast Mcm2–6 during the cell cycle, peaking in G1, declining in S phase, and increasing again during the subsequent G1 phase (Schimmel et al. 2014). It is reasonable to envision that human MCM sumoylation also provides a regulatory mechanism to restrain origin firing. A negative role for sumoylation in replication initiation can also be inferred from findings in Xenopus, wherein increased origin firing occurs after reducing sumoylation, either by expression of a dominant-negative SUMO E2 or addition of SUMO-specific proteases (Bonne-Andrea et al. 2013). Given Xenopus MCM subunits are sumoylated (Ma et al. 2014), it is worthy of consideration whether this modification underlies the negative effect of sumoylation in replication initiation in this system. Considering that PP1- and DDK-mediated MCM regulation is conserved across species (Wotton and Shore 1997; Lee et al. 2003; Cho et al. 2006; Masai et al. 2006; Montagnoli et al. 2006; Tsuji et al. 2006; Cornacchia et al. 2012; Hayano et al. 2012; Yamazaki et al. 2012), the targeting of this pathway by MCM sumoylation to prevent premature initiation, as seen in yeast, may be conserved. Direct tests of these ideas will clarify the roles of MCM sumoylation in higher eukaryotes.
3.3. ORC2 Sumoylation Prevents Re-replication at Centromeric Regions
ORC, composed of ORC1–6 subunits, binds to replication origins and is critical for MCM loading during origin licensing (Diffley et al. 1994; Kelly and Brown 2000; Bell and Dutta 2002; Sclafani and Holzen 2007; Remus and Diffley 2009; Li and Araki 2013). In addition, ORC2 can dissociate from replication origins and localize to centromeric regions during G2/M phase (Craig et al. 2003; Prasanth et al. 2004; Lee et al. 2012), coincident with the appearance of SUMO2-modified ORC2 in human cells (Huang et al. 2016). Elimination of ORC2 sumoylation by mutating its two sumoylation sites leads to re-replication, polyploidy, and genome damage (Huang et al. 2016). Mechanistically, ORC2 sumoylation promotes the recruitment of KDM5A (Huang et al. 2016), a histone H3 lysine 4 (H3K4) demethylase (Defeo-Jones et al. 1991; Christensen et al. 2007; Klose et al. 2007). Loss of ORC2 sumoylation results in elevated levels of tri-methylated H3K4 (H3K4me3) in centromeric chromatin, reduced transcription of α-satellites at centromeres, and decondensation of pericentric heterochromatin, which correlates with re-replication of the pericentric region (Huang et al. 2016). It remains to be determined how the change in chromatin environment caused by the perturbation of the ORC2-KMD5A axis leads to re-replication, despite the presence of multiple mechanisms that prevent re-replication. In budding yeast, multiple ORC subunits are sumoylated (Cremona et al. 2012), but the functions of this modification have yet to be determined.
3.4. Other Potential SUMO Substrates Affecting Replication Initiation
Several other proteins involved in replication initiation are SUMO substrates, such as the ssDNA-binding protein RPA and CDK (Dou et al. 2010; Cremona et al. 2012; Bonne-Andrea et al. 2013). Sumoylation of both human and yeast RPA has been reported to promote homologous recombination during DNA repair, though whether it also has a role in replication initiation has not been tested (Dou et al. 2010; Psakhye and Jentsch 2012). Cyclin E has been shown to be a SUMO2/SUMO3 substrate in Xenopus (Bonne-Andrea et al. 2013). Its sumoylation is detectable during replication and is independent of its kinase activity (Bonne-Andrea et al. 2013). A direct effect of cyclin E sumoylation in replication initiation remains to be determined. Because multiple proteins involved in origin licensing and firing are SUMO substrates, we anticipate the presence of multiple mechanisms through which SUMO regulates timing and efficiency of origin firing and prevents harmful rereplication events.
4. SUMO-Based Regulation of Replication Progression
Genetic studies using SUMO E2 and E3 mutants have shown that reducing sumoylation retards replication progression, particularly under replicative stress (Cremona et al. 2012; Schimmel et al. 2014; Hang et al. 2015). For example, mutating SUMO E3s in budding yeast impairs replication when cells are treated with the DNA-alkylating agent methyl methanesulfonate (MMS) (Cremona et al. 2012). In human cell lines, reducing UBC9 function leads to a prolonged S phase (Schimmel et al. 2014). The effects of sumoylation in replication progression may be broad, as many proteins central for this process are subject to sumoylation. Aside from MCM, several other replisome components are sumoylated, including Pol ε, Pol δ, the processivity factor PCNA, topoisomerases, DNA primase, and the nucleosome remodeling factor FACT (Golebiowski et al. 2009; Elrouby and Coupland 2010; Cremona et al. 2012; Hendriks et al. 2014; Ma et al. 2014; Tammsalu et al. 2014). In addition, several proteins that collaborate with replisome for DNA synthesis are sumoylated (Golebiowski et al. 2009; Elrouby and Coupland 2010; Cremona et al. 2012; Hendriks et al. 2014; Ma et al. 2014; Tammsalu et al. 2014). Some examples include subunits of the SMC complexes (cohesin, condensin, and Smc5/Smc6), the SMC-like Mre11 complex, and the clamp loader RFC complex (Golebiowski et al. 2009; Elrouby and Coupland 2010; Cremona et al. 2012; Hendriks et al. 2014; Ma et al. 2014; Tammsalu et al. 2014). Among these proteins, the sumoylation of PCNA has been well examined and shown to recruit the anti-recombinase Srs2 to impaired replication forks in order to prevent toxic recombination events (Papouli et al. 2005; Armstrong et al. 2012). The molecular function of this modification, in conjunction with other types of PCNA modifications, has been extensively reviewed elsewhere [reviewed in (Mailand et al. 2013; Ulrich and Takahashi 2013)]. Below we focus on recent findings regarding additional effects SUMO has on replication progression, mostly derived from studying the combined effects of loss of sumoylation of many substrates, but with a few mechanistic studies as well.
4.1. SUMO-Based Regulation of Replisome Components
Following up earlier observations that Mms21 SUMO ligase mutations impair replication, our group showed that under MMS conditions, Mms21 and the associated Smc5/Smc6 complex promote sumoylation of Mcm6 and Pol2, the catalytic subunit of Pol ε (Hang et al. 2015). As physical interactions are detected between the Smc5/Smc6 complex and these substrates, the effects seen are likely to be direct (Hang et al. 2015). In addition, as Smc5/Smc6 deficiency impairs replication at regions far from fired origins, the Smc5/Smc6 complex may use sumoylation to facilitate later stages of replication. Future tests can help us understand whether MCM sumoylation has a distinct role during replication stress and whether Pol2 sumoylation influences DNA polymerization.
Several replisome members have been found to be sumoylated in human cells (Golebiowski et al. 2009; Hendriks et al. 2014; Schimmel et al. 2014; Tammsalu et al. 2014). Interestingly, under ATR inhibition conditions, sumoylation of replisome components can lead to fork collapse (Ragland et al. 2013). This largely depends on the SUMO-targeted ubiquitin ligase RNF4 and endonuclease scaffold protein SLX4 (Ragland et al. 2013). It is thought that under such conditions, RNF4 can target sumoylated replisomes for degradation, rendering replication forks accessible for SLX4-mediated cleavage (Ragland et al. 2013). In another study, sumoylation of two Fanconi anemia (FA) proteins, namely, FANCD2 and FANCI, was shown to trigger RNF4-mediated ubiquitination of these proteins and subsequent removal from DNA damage sites (Gibbs-Seymour et al. 2015). Lack of this regulation reduces the ability of cells to cope with replication stress, likely due to blockage of FANCD2 and FANCI from recycling among different damage sites (Gibbs-Seymour et al. 2015). Both of these studies highlight the complex interplay between sumoylation and ubiquitination for replication fork management.
The above notion is further extended by another study utilizing iPOND (isolation of proteins on nascent DNA) coupled with mass spectrometry-based protein analyses in human cells. It has been reported that SUMO is enriched on nascent chromatin, while ubiquitin molecules are enriched on mature chromatin (Lopez-Contreras et al. 2013). A follow-up study showed that the SUMO deubiquitinase, USP7, contributes to the establishment of this SUMO-high and ubiquitin-low nascent chromatin environment (Lecona et al. 2016). USP7 can remove ubiquitin molecules that are conjugated to SUMO2 in vitro and in vivo and is associated with nascent chromatin and MCM4 (Lecona et al. 2016). The authors suggest that through this mechanism, USP7 reduces ubiquitin levels and allows enrichment of SUMO2 on replisome components. This role likely contributes to USP7’s function in maintaining normal rates of fork speed and origin firing (Lecona et al. 2016). These studies suggest that enrichment of SUMO and reduction of ubiquitin at or near replisomes can be advantageous for replication progression. Further investigation is needed to provide mechanistic insights into the relevant sumoylation events critical for DNA synthesis and the full spectrum of the effects of USP7 and RNF4 in keeping a balance between sumoylation and ubiquitination of replisome components under different conditions.
4.2. SUMO-Based Regulation of Lagging Strand Synthesis
Lagging strand Okazaki fragment processing involves sequential reactions of gap filling by polymerase δ, flap cleavage by flap endonuclease 1 (FEN1), and nick ligation by DNA ligase 1 (LIG1) (Waga and Stillman 1998). In human cells, modification of FEN1 by SUMO3 at lysine 168 occurs in S phase and peaks in G2/M phase (Guo et al. 2012). The sumoylation of FEN1 is dependent on its phosphorylation, which occurs during G1 phase, peaking in late S phase. Sumoylation of FEN1 appears to occur after its phosphorylation, as the non-phosphorylatable FEN1 mutant (S187A) is not sumoylated (Guo et al. 2012). Furthermore, sumoylation of FEN1 triggers its ubiquitination and subsequent proteasome-mediated degradation, presumably by recruiting the SIM-containing ubiquitin E3 ligase PRP19 (Guo et al. 2012). The timely degradation of FEN1 via this cascade of modifications is critical for maintaining genome stability, as its deregulation leads to cell cycle delay and polyploidy (Guo et al. 2012). Other than FEN1, polymerase δ is also reported to be SUMO substrate in organisms from yeast to humans (Cremona et al. 2012; Hendriks et al. 2014; Tammsalu et al. 2014). It remains to be determined if polymerase δ sumoylation has a role in DNA lagging strand synthesis.
4.3. SUMO-Based Regulation of Sister Chromatid Cohesion
As replication progresses, the two sister chromatids stay close together partly through the function of cohesin [reviewed in (Blow and Tanaka 2005; Sherwood et al. 2010)]. The ring-shaped cohesin complex is loaded onto chromatin before S phase and encloses sister chromatids to keep them connected during S phase [reviewed in (Blow and Tanaka 2005; Sherwood et al. 2010)]. The resulting sister chromatid cohesion is important for supplying faithful templates for DNA repair and for ensuring each daughter cell receives one set of chromosomes during mitosis [reviewed in (Blow and Tanaka 2005; Sherwood et al. 2010)]. Four subunits of cohesin, including the rod-shaped Smc1 and Smc3 proteins and the associated Scc1 and Scc3 proteins, are sumoylated in yeast and human cells, partly in an Mms21-dependent manner (Denison et al. 2005; Potts et al. 2006; Almedawar et al. 2012; Golebiowski et al. 2009; Hendriks et al. 2014; Tammsalu et al. 2014). In budding yeast, sumoylation of Smc1 and Scc1 occurs after cohesin loading, and Scc1 sumoylation is independent of another important modification for cohesion establishment, namely, Smc3 acetylation (Almedawar et al. 2012). Decreasing cohesin sumoylation impairs cohesion, without affecting the integrity of the cohesin complex (Almedawar et al. 2012). Based on these observations, it was proposed that cohesin sumoylation is required for the establishment of cohesion.
Another study further highlights the importance of SUMO-based regulation of cohesion. When a cohesion establishment factor, Pds5, is mutated in budding yeast, Scc1 becomes hyper-sumoylated and is degraded (D’Ambrosio and Lavoie 2014). This correlates with precocious separation of sister chromatids (D’Ambrosio and Lavoie 2014). Such a defect can be suppressed through removal of the SUMO E3 ligase Siz2 or the SUMO-targeted ubiquitin ligase Slx5/Slx8, supporting the hypothesis that toxic sumoylation events underlie pds5–1 defects (D’Ambrosio and Lavoie 2014). The authors suggest that Pds5 plays a role in preventing hyper-sumoylation of cohesin and maintaining cohesin levels until mitosis. The full picture of how SUMO regulates cohesion is likely more complex. For example, Pds5 itself is sumoylated by Siz2 from S to G2/M phase, though the biochemical effect of this modification is not known (Stead et al. 2003). The timing of sumoylation and ubiquitination of cohesin is critical, and it will be of interest to determine how their temporal order is established, perhaps through the regulation of sumoylation and desumoylation enzymes or through other cohesion regulators.
4.4. SUMO-Based Regulation of Topoisomerase Function
Topoisomerases are essential for releasing topological stress and promoting replication fork progression (Champoux 2001; Wang 2002; Vos et al. 2011). They also contribute to the removal of transcription-generated DNA-RNA hybrids, known as R-loops, which pose a barrier for replication (Gan et al. 2011; Aguilera and Garcia-Muse 2012). A recent study showed that sumoylation of human TOP1 provides a means to reduce R-loop-mediated replication fork stalling via two distinct mechanisms (Li et al. 2015). Upon sumoylation by PIAS1, TOP1 showed improved interactions with the active form of RNA polymerase II (RNAPIIo), leading to recruitment of splicing factors to avoid R-loop formation (Li et al. 2015). In addition, sumoylation of TOP1 reduces its enzymatic activity, potentially leading to reduced TOP1-induced DNA nicking at transcriptionally active regions (Li et al. 2015). Both effects of TOP1 sumoylation could contribute to lessening barriers for replication forks. It remains to be tested if these effects of TOP1 sumoylation are conserved in other organisms.
5. Potential SUMO Substrates Affecting Replication Termination
Although a full understanding of replication termination is still elusive, several new discoveries have shed light into this process. In budding yeast, C. elegans, and Xenopus, ubiquitination of Mcm7 has been shown to be a key event for disassembly of the replisome. In budding yeast, the Dia2 ubiquitin ligase that is part of the replisome can ubiquitinate Mcm7 when replication forks converge. This modification is then recognized by the segregase Cdc48/p97, leading to removal of MCM from chromatin and replisome disassembly (Maric et al. 2014; Priego Moreno et al. 2014). In C. elegans and Xenopus, replisome-associated E3 ligase CUL-2LRR−1 and the segregase remove CMG during termination (Sonneville et al. 2017; Dewar et al. 2017). Interestingly, in budding yeast, Mcm7 sumoylation appears to be regulated distinctly from that of Mcm2–6, with its levels only declining when the bulk of DNA replication has been completed (Wei and Zhao 2016a). It will be interesting to investigate whether Mcm7 sumoylation could trigger its ubiqutination or contribute to replication termination.
During replication termination, decatenation of sister chromatids requires Top2. Top2 sumoylation has been found in human, mouse, Xenopus, and yeast [reviewed in (Lee and Bachant 2009)]. Sumoylation of Top2 in vertebrates promotes the recruitment of Top2 or the chromosomal passenger complex to kinetochores during mitosis to facilitate chromosome segregation [reviewed in (Lee and Bachant 2009)]. Whether Top2 sumoylation plays a role in decatenation during replication termination is not known. With more molecular details of replication termination becoming available in the future, the examination of SUMO substrates involved in replication termination will reveal more details of this process.
6. Concluding Remarks
Each stage of DNA replication is intricately regulated to ensure precise genome duplication. Posttranslational modifications provide a dynamic regulatory means at multiple stages of the replication process. Phosphorylation- and ubiquitination-based modes of regulation are essential for replication, and the role of sumoylation in replication is emerging from several recent studies. During the replication initiation stage, sumoylation of MCM (yeast) and ORC2 (human) can influence origin firing. In addition, sumoylation promotes replication progression through multiple mechanisms, such as lagging strand synthesis, reducing R-loops, replication fork metabolism, and sister chromatid cohesion. However, only a small number of SUMO substrates have been studied thus far as summarized in Table 1, and our understanding of how sumoylation regulates replication is still at an early stage. With more advanced methods to map sumoylation sites and tools to alter the sumoylation status of substrates, detailed molecular mechanisms of how sumoylation regulates each substrate will be elucidated. Future work will also help to establish a clear picture of how sumoylation is coordinated with other types of protein modifications during DNA replication. In addition, examination of how sumoylation and desumoylation enzymes are themselves regulated can also reveal how SUMO modification cycles facilitate DNA replication. As SUMO enzyme deficiencies, such as SUMO E1 and E2 depletion or SUMO E3 mutations, have been implicated in cancer and inherited human syndromes (Eifler and Vertegaal 2015; He et al. 2015; Yu et al. 2015), understanding their roles in genome duplication will provide new avenues for disease detection and treatment strategies.
Table 1.
Summary of sumoylated substrates involved in DNA replication
| Replication | Substrate (function) | Molecular effect(s) of sumoylation |
|---|---|---|
| Initiation | scMCM (replicative helicase) | Inhibit replication initiation via phosphatase recruitment |
| hsORC2 (replication origin recognition) | Prevent centromeric region re-replication by recruitment of the histone demethylase KDM5A | |
| Progression | hsFANCD2 and hsFANCI (Fanconi anemia proteins) | Promote replication stress survival by triggering self-ubiquitination and removal from DNA damage sites |
| hsFEN1 (flap endonuclease) | Promote genome stability, prerequisite for its ubiquitination and degradation | |
| scCohesin subunits (chromatid cohesion) | Promote sister chromatid cohesion | |
| hsTOP1 (topoisomerase) | Prevent R-loop formation by promoting interaction with splicing factors; reduce DNA nicking |
Note that only the major function is indicated for each substrate
sc Saccharomyces cerevisiae, hs Homo sapiens
References
- Aguilera A, Garcia-Muse T (2012) R loops: from transcription byproducts to threats to genome stability. Mol Cell 46:115–124 [DOI] [PubMed] [Google Scholar]
- Almedawar S, Colomina N, Bermudez-Lopez M, Pocino-Merino I, Torres-Rosell J (2012) A SUMO-dependent step during establishment of sister chromatid cohesion. Curr Biol 22:1576–1581 [DOI] [PubMed] [Google Scholar]
- Almouzni G, Cedar H (2016) Maintenance of epigenetic information. Cold Spring Harb Perspect Biol 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias EE, Walter JC (2007) Strength in numbers: preventing rereplication via multiple mechanisms in eukaryotic cells. Genes Dev 21:497–518 [DOI] [PubMed] [Google Scholar]
- Armstrong AA, Mohideen F, Lima CD (2012) Recognition of SUMO-modified PCNA requires tandem receptor motifs in Srs2. Nature 483:59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azvolinsky A, Dunaway S, Torres JZ, Bessler JB, Zakian VA (2006) The S. cerevisiae Rrm3p DNA helicase moves with the replication fork and affects replication of all yeast chromosomes. Genes Dev 20:3104–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71:333–374 [DOI] [PubMed] [Google Scholar]
- Bell SP, Labib K (2016) Chromosome duplication in Saccharomyces cerevisiae. Genetics 203:1027–1067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergink S, Ammon T, Kern M, Schermelleh L, Leonhardt H, Jentsch S (2013) Role of Cdc48/p97 as a SUMO-targeted segregase curbing Rad51-Rad52 interaction. Nat Cell Biol 15:526–532 [DOI] [PubMed] [Google Scholar]
- Bermudez-Lopez M, Villoria MT, Esteras M, Jarmuz A, Torres-Rosell J, Clemente-Blanco A, Aragon L (2016) Sgs1’s roles in DNA end resection, HJ dissolution, and crossover suppression require a two-step SUMO regulation dependent on Smc5/6. Genes Dev 30:1339–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Dutta A (2005) Preventing re-replication of chromosomal DNA. Nat Rev Mol Cell Biol 6:476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blow JJ, Tanaka TU (2005) The chromosome cycle: coordinating replication and segregation. Second in the cycles review series. EMBO Rep 6:1028–1034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonne-Andrea C, Kahli M, Mechali F, Lemaitre J-M, Bossis G, Coux O (2013) SUMO2/3 modification of cyclin E contributes to the control of replication origin firing. Nat Commun 4:1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner JN, Choi K, Xue X, Torres NP, Szakal B, Wei L, Wan B, Arter M, Matos J, Sung P et al. (2016) Smc5/6 mediated sumoylation of the Sgs1-Top3-Rmi1 complex promotes removal of recombination intermediates. Cell Rep 16:368–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowers JL, Randell JCW, Chen S, Bell SP (2004) ATP hydrolysis by ORC catalyzes reiterative Mcm2–7 assembly at a defined origin of replication. Mol Cell 16:967–978 [DOI] [PubMed] [Google Scholar]
- Bylebyl GR, Belichenko I, Johnson ES (2003) The SUMO isopeptidase Ulp2 prevents accumulation of SUMO chains in yeast. J Biol Chem 278:44113–44120 [DOI] [PubMed] [Google Scholar]
- Calzada A, Hodgson B, Kanemaki M, Bueno A, Labib K (2005) Molecular anatomy and regulation of a stable replisome at a paused eukaryotic DNA replication fork. Genes Dev 19:1905–1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappadocia L, Pichler A, Lima CD (2015) Structural basis for catalytic activation by the human ZNF451 SUMO E3 ligase. Nat Struct Mol Biol 22:968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux JJ (2001) DNA topoisomerases: structure, function, and mechanism. Annu Rev Biochem 70:369–413 [DOI] [PubMed] [Google Scholar]
- Cho W-H, Lee Y-J, Kong S-I, Hurwitz J, Lee J-K (2006) CDC7 kinase phosphorylates serine residues adjacent to acidic amino acids in the minichromosome maintenance 2 protein. Proc Natl Acad Sci U S A 103:11521–11526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen J, Agger K, Cloos PA, Pasini D, Rose S, Sennels L, Rappsilber J, Hansen KH, Salcini AE, Helin K (2007) RBP2 belongs to a family of demethylases, specific for tri-and dimethylated lysine 4 on histone 3. Cell 128:1063–1076 [DOI] [PubMed] [Google Scholar]
- Chung I, Zhao X (2015) DNA break-induced sumoylation is enabled by collaboration between a SUMO ligase and the ssDNA-binding complex RPA. Genes Dev 29:1593–1598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornacchia D, Dileep V, Quivy JP, Foti R, Tili F, Santarella-Mellwig R, Antony C, Almouzni G, Gilbert DM, Buonomo SBC (2012) Mouse Rif1 is a key regulator of the replication-timing programme in mammalian cells. EMBO J 31:3678–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coster G, Frigola J, Beuron F, Morris EP, Diffley JF (2014) Origin licensing requires ATP binding and hydrolysis by the MCM replicative helicase. Mol Cell 55:666–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig JM, Earle E, Canham P, Wong LH, Anderson M, Choo KH (2003) Analysis of mammalian proteins involved in chromatin modification reveals new metaphase centromeric proteins and distinct chromosomal distribution patterns. Hum Mol Genet 12:3109–3121 [DOI] [PubMed] [Google Scholar]
- Cremona CA, Sarangi P, Yang Y, Hang LE, Rahman S, Zhao X (2012) Extensive DNA damage-induced sumoylation contributes to replication and repair and acts in addition to the mec1 checkpoint. Mol Cell 45:422–432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Ambrosio LM, Lavoie BD (2014) Pds5 prevents the PolySUMO-dependent separation of sister chromatids. Curr Biol 24:361–371 [DOI] [PubMed] [Google Scholar]
- Davé A, Cooley C, Garg M, Bianchi A (2014) Protein phosphatase 1 recruitment by Rif1 regulates DNA replication origin firing by counteracting DDK activity. Cell Rep 7:53–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Albuquerque CP, Liang J, Gaut NJ, Zhou H (2016) Molecular circuitry of the SUMO (Small Ubiquitin-like Modifier) pathway in controlling Sumoylation homeostasis and suppressing genome rearrangements. J Biol Chem 291:8825–8835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Piccoli G, Torres-Rosell J, Aragón L (2009) The unnamed complex: what do we know about Smc5-Smc6? Chromosom Res 17:251–263 [DOI] [PubMed] [Google Scholar]
- Deegan TD, Yeeles JT, Diffley JF (2016) Phosphopeptide binding by Sld3 links Dbf4-dependent kinase to MCM replicative helicase activation. EMBO J 35:961–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defeo-Jones D, Huang PS, Jones RE, Haskell KM, Vuocolo GA, Hanobik MG, Huber HE, Oliff A (1991) Cloning of cDNAs for cellular proteins that bind to the retinoblastoma gene product. Nature 352:251–254 [DOI] [PubMed] [Google Scholar]
- Denison C, Rudner AD, Gerber SA, Bakalarski CE, Moazed D, Gygi SP (2005) A proteomic strategy for gaining insights into protein sumoylation in yeast. Mol Cell Proteomics 4:246–254 [DOI] [PubMed] [Google Scholar]
- Dewar JM, Budzowska M, Walter JC (2015) The mechanism of DNA replication termination in vertebrates. Nature 525:345–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewar JM, Low E, Mann M, Räschle M, Walter JC (2017) CRL2Lrr1 promotes unloading of the vertebrate replisome from chromatin during replication termination. Genes Dev 31(3):275–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diffley JFX (2004) Regulation of early events in chromosome replication. Curr Biol 14:R778–R786 [DOI] [PubMed] [Google Scholar]
- Diffley JF, Cocker JH, Dowell SJ, Rowley A (1994) Two steps in the assembly of complexes at yeast replication origins in vivo. Cell 78:303–316 [DOI] [PubMed] [Google Scholar]
- Donovan S, Harwood J, Drury LS, Diffley JF (1997) Cdc6p-dependent loading of Mcm proteins onto pre-replicative chromatin in budding yeast. Proc Natl Acad Sci U S A 94:5611–5616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dou H, Huang C, Singh M, Carpenter PB, Yeh ET (2010) Regulation of DNA repair through deSUMOylation and SUMOylation of replication protein A complex. Mol Cell 39:333–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eifler K, Vertegaal AC (2015) SUMOylation-mediated regulation of cell cycle progression and cancer. Trends Biochem Sci 40:779–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenhardt N, Chaugule VK, Koidl S, Droescher M, Dogan E, Rettich J, Sutinen P, Imanishi SY, Hofmann K, Palvimo JJ et al. (2015) A new vertebrate SUMO enzyme family reveals insights into SUMO-chain assembly. Nat Struct Mol Biol 22:959–967 [DOI] [PubMed] [Google Scholar]
- Elrouby N, Coupland G (2010) Proteome-wide screens for small ubiquitin-like modifier (SUMO) substrates identify Arabidopsis proteins implicated in diverse biological processes. Proc Natl Acad Sci U S A 107:17415–17420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fachinetti D, Bermejo R, Cocito A, Minardi S, Katou Y, Kanoh Y, Shirahige K, Azvolinsky A, Zakian VA, Foiani M (2010) Replication termination at eukaryotic chromosomes is mediated by Top2 and occurs at genomic loci containing pausing elements. Mol Cell 39:595–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Cid A, Riera A, Tognetti S, Herrera MC, Samel S, Evrin C, Winkler C, Gardenal E, Uhle S, Speck C (2013) An ORC/Cdc6/MCM2–7 complex is formed in a multistep reaction to serve as a platform for MCM double-hexamer assembly. Mol Cell 50:577–588 [DOI] [PubMed] [Google Scholar]
- Fragkos M, Ganier O, Coulombe P, Mechali M (2015) DNA replication origin activation in space and time. Nat Rev Mol Cell Biol 16:360–374 [DOI] [PubMed] [Google Scholar]
- Frigola J, Remus D, Mehanna A, Diffley JFX (2013) ATPase-dependent quality control of DNA replication origin licensing. Nature 495:339–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K (2006) GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol 8:358–366 [DOI] [PubMed] [Google Scholar]
- Gambus A, van Deursen F, Polychronopoulos D, Foltman M, Jones RC, Edmondson RD, Calzada A, Labib K (2009) A key role for Ctf4 in coupling the MCM2–7 helicase to DNA polymerase alpha within the eukaryotic replisome. EMBO J 28:2992–3004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan W, Guan Z, Liu J, Gui T, Shen K, Manley JL, Li X (2011) R-loop-mediated genomic instability is caused by impairment of replication fork progression. Genes Dev 25:2041–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Rodriguez N, Wong RP, Ulrich HD (2016) Functions of ubiquitin and SUMO in DNA replication and replication stress. Front Genet 7:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau JR, Lima CD (2010) The SUMO pathway: emerging mechanisms that shape specificity, conjugation and recognition. Nat Rev Mol Cell Biol 11:861–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbs-Seymour I, Oka Y, Rajendra E, Weinert BT, Passmore LA, Patel KJ, Olsen JV, Choudhary C, Bekker-Jensen S, Mailand N (2015) Ubiquitin-SUMO circuitry controls activated fanconi anemia ID complex dosage in response to DNA damage. Mol Cell 57:150–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golebiowski F, Matic I, Tatham MH, Cole C, Yin Y, Nakamura A, Cox J, Barton GJ, Mann M, Hay RT (2009) System-wide changes to SUMO modifications in response to heat shock. Sci Signals 2:ra24. [DOI] [PubMed] [Google Scholar]
- Guo Z, Kanjanapangka J, Liu N, Liu S, Liu C, Wu Z, Wang Y, Loh T, Kowolik C, Jamsen J et al. (2012) Sequential posttranslational modifications program FEN1 degradation during cell-cycle progression. Mol Cell 47:444–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutiérrez MP, MacAlpine DM (2016) The initiation of DNA replication in eukaryotes. In: Kaplan DL (ed) Chapter 5: chromatin determinants of origin selection and activation Springer International Publishing, ISBN 978–3-319–24696-3 [Google Scholar]
- Hang LE, Lopez CR, Liu X, Williams JM, Chung I, Wei L, Bertuch AA, Zhao X (2014) Regulation of Ku-DNA association by Yku70 C-terminal tail and SUMO modification. J Biol Chem 289:10308–10317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hang LE, Peng J, Tan W, Szakal B, Menolfi D, Sheng Z, Lobachev K, Branzei D, Feng W, Zhao X (2015) Rtt107 is a multi-functional scaffold supporting replication progression with partner SUMO and ubiquitin ligases. Mol Cell 60:268–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannoun Z, Greenhough S, Jaffray E, Hay RT, Hay DC (2010) Post-translational modification by SUMO. Toxicology 278:288–293 [DOI] [PubMed] [Google Scholar]
- Hayano M, Kanoh Y, Matsumoto S, Renard-Guillet C, Shirahige K, Masai H (2012) Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes Dev 26:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X, Riceberg J, Pulukuri SM, Grossman S, Shinde V, Shah P, Brownell JE, Dick L, Newcomb J, Bence N (2015) Characterization of the loss of SUMO pathway function on cancer cells and tumor proliferation. PLoS One 10:e0123882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendriks IA, D’Souza RC, Yang B, Verlaan-de Vries M, Mann M, Vertegaal AC (2014) Uncovering global SUMOylation signaling networks in a site-specific manner. Nat Struct Mol Biol 21:927–936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey CM, Wilson NR, Hochstrasser M (2012) Function and regulation of SUMO proteases. Nat Rev Mol Cell Biol 13:755–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S-I, Alvino GM, Chang F, Lian H-Y, Sridhar A, Kubota T, BREWER BJ, Weinreich M, Raghuraman MK, Donaldson AD (2014) Rif1 controls DNA replication by directing protein phosphatase 1 to reverse Cdc7-mediated phosphorylation of the MCM complex. Genes Dev 28:372–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoggard T, Fox C (2016) The initiation of DNA replication in eukaryotes. In: Kaplan DL (ed) Chapter 9: the origin recognition complex in the initiation of DNA replication Springer International Publishing, ISBN 978–3-319–24696-3 [Google Scholar]
- Huang C, Cheng J, Bawa-Khalfe T, Yao X, Chin YE, Yeh ET (2016) SUMOylated ORC2 recruits a histone demethylase to regulate centromeric histone modification and genomic stability. Cell Rep 15:147–157 [DOI] [PubMed] [Google Scholar]
- Ilves I, Petojevic T, Pesavento JJ, Botchan MR (2010) Activation of the MCM2–7 helicase by association with Cdc45 and GINS proteins. Mol Cell 37:247–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivessa AS, Zhou JQ, Zakian VA (2000) The Saccharomyces Pif1p DNA helicase and the highly related Rrm3p have opposite effects on replication fork progression in ribosomal DNA. Cell 100:479–489 [DOI] [PubMed] [Google Scholar]
- Jeppsson K, Kanno T, Shirahige K, Sjogren C (2014) The maintenance of chromosome structure: positioning and functioning of SMC complexes. Nat Rev Mol Cell Biol 15:601–614 [DOI] [PubMed] [Google Scholar]
- Johnson ES (2004) Protein modification by SUMO. Annu Rev Biochem 73:355–382 [DOI] [PubMed] [Google Scholar]
- Johnson ES, Gupta AA (2001) An E3-like factor that promotes SUMO conjugation to the yeast septins. Cell 106:735–744 [DOI] [PubMed] [Google Scholar]
- Kang YH, Galal WC, Farina A, Tappin I, Hurwitz J (2012) Properties of the human Cdc45/Mcm2–7/GINS helicase complex and its action with DNA polymerase epsilon in rolling circle DNA synthesis. Proc Natl Acad Sci U S A 109:6042–6047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly TJ, Brown GW (2000) Regulation of chromosome replication. Annu Rev Biochem 69:829–880 [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yan Q, Tothova Z, Yamane K, Erdjument-Bromage H, Tempst P, Gilliland DG, Zhang Y, Kaelin WG Jr (2007) The retinoblastoma binding protein RBP2 is an H3K4 demethylase. Cell 128:889–900 [DOI] [PubMed] [Google Scholar]
- Kroetz MB, Su D, Hochstrasser M (2009) Essential role of nuclear localization for yeast Ulp2 SUMO protease function. Mol Biol Cell 20:2196–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labib K, Hodgson B (2007) Replication fork barriers: pausing for a break or stalling for time? EMBO Rep 8:346–353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamoliatte F, Caron D, Durette C, Mahrouche L, Maroui MA, Caron-Lizotte O, Bonneil E, Chelbi-Alix MK, Thibault P (2014) Large-scale analysis of lysine SUMOylation by SUMO remnant immunoaffinity profiling. Nat Commun 5:5409. [DOI] [PubMed] [Google Scholar]
- Lecona E, Rodriguez-Acebes S, Specks J, Lopez-Contreras AJ, Ruppen I, Murga M, Munoz J, Mendez J, Fernandez-Capetillo O (2016) USP7 is a SUMO deubiquitinase essential for DNA replication. Nat Struct Mol Biol 23:270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M-T, Bachant J (2009) SUMO modification of DNA topoisomerase II: trying to get a CENse of it all. DNA Repair 8:557–568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J-K, Seo Y-S, Hurwitz J (2003) The Cdc23 (Mcm10) protein is required for the phosphorylation of minichromosome maintenance complex by the Dfp1-Hsk1 kinase. Proc Natl Acad Sci U S A 100:2334–2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Bang SW, Yoon SW, Lee SH, Yoon JB, Hwang DS (2012) Phosphorylation of ORC2 protein dissociates origin recognition complex from chromatin and replication origins. J Biol Chem 287:11891–11898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Araki H (2013) Loading and activation of DNA replicative helicases: the key step of initiation of DNA replication. Genes Cells 18:266–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li SJ, Hochstrasser M (1999) A new protease required for cell-cycle progression in yeast. Nature 398:246–251 [DOI] [PubMed] [Google Scholar]
- Li SJ, Hochstrasser M (2000) The yeast ULP2 (SMT4) gene encodes a novel protease specific for the ubiquitin-like Smt3 protein. Mol Cell Biol 20:2367–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Pokharel S, Wang JT, Xu X, Liu Y (2015) RECQ5-dependent SUMOylation of DNA topoisomerase I prevents transcription-associated genome instability. Nat Commun 6:6720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YC, Lee CC, Yao YL, Lai CC, Schmitz ML, Yang WM (2016) SUMO5, a novel poly-SUMO isoform, regulates PML nuclear bodies. Sci Rep 6:26509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Contreras AJ, Ruppen I, Nieto-Soler M, Murga M, Rodriguez-Acebes S, Remeseiro S, Rodrigo-Perez S, Rojas AM, Méndez J, Muñoz J et al. (2013) A proteomic characterization of factors enriched at nascent DNA molecules. Cell Rep 3:1105–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Aslanian A, Sun H, Jin M, Shi Y, Yates JR 3rd, Hunter T (2014) Identification of small ubiquitin-like modifier substrates with diverse functions using the Xenopus egg extract system. Mol Cell Proteomics 13:1659–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailand N, Gibbs-Seymour I, Bekker-Jensen S (2013) Regulation of PCNA-protein interactions for genome stability. Nat Rev Mol Cell Biol 14:269–282 [DOI] [PubMed] [Google Scholar]
- Makhnevych T, Sydorskyy Y, Xin X, Srikumar T, Vizeacoumar FJ, Jeram SM, Li Z, Bahr S, Andrews BJ, Boone C et al. (2009) Global map of SUMO function revealed by protein-protein interaction and genetic networks. Mol Cell 33:124–135 [DOI] [PubMed] [Google Scholar]
- Mantiero D, Mackenzie A, Donaldson A, Zegerman P (2011) Limiting replication initiation factors execute the temporal programme of origin firing in budding yeast. EMBO J 30:4805–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maric M, Maculins T, De Piccoli G, Labib K (2014) Cdc48 and a ubiquitin ligase drive disassembly of the CMG helicase at the end of DNA replication. Science 346:1253596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masai H, Taniyama C, Ogino K, Matsui E, Kakusho N, Matsumoto S, Kim J-M, Ishii A, Tanaka T, Kobayashi T et al. (2006) Phosphorylation of MCM4 by Cdc7 kinase facilitates its interaction with Cdc45 on the chromatin. J Biol Chem 281:39249–39261 [DOI] [PubMed] [Google Scholar]
- Masai H, Matsumoto S, You Z, Yoshizawa-Sugata N, Oda M (2010) Eukaryotic chromosome DNA replication: where, when, and how? Annu Rev Biochem 79:89–130 [DOI] [PubMed] [Google Scholar]
- Mattarocci S, Shyian M, Lemmens L, Damay P, Altintas DM, Shi T, Bartholomew CR, Thomä NH, Hardy CFJ, Shore D (2014) Rif1 controls DNA replication timing in yeast through the PP1 phosphatase Glc7. Cell Rep 7:62–69 [DOI] [PubMed] [Google Scholar]
- Montagnoli A, Valsasina B, Brotherton D, Troiani S, Rainoldi S, Tenca P, Molinari A, Santocanale C (2006) Identification of Mcm2 phosphorylation sites by S-phase-regulating kinases. J Biol Chem 281:10281–10290 [DOI] [PubMed] [Google Scholar]
- Moreno SP, Gambus A (2015) Regulation of unperturbed DNA replication by ubiquitylation. Gene 6:451–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morohashi H, Maculins T, Labib K (2009) The amino-terminal TPR domain of Dia2 tethers SCF(Dia2) to the replisome progression complex. Curr Biol 19:1943–1949 [DOI] [PubMed] [Google Scholar]
- Moyer SE, Lewis PW, Botchan MR (2006) Isolation of the Cdc45/Mcm2–7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci U S A 103:10236–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukhopadhyay D, Dasso M (2007) Modification in reverse: the SUMO proteases. Trends Biochem Sci 32:286–295 [DOI] [PubMed] [Google Scholar]
- Muramatsu S, Hirai K, Tak Y-S, Kamimura Y, Araki H (2010) CDK-dependent complex formation between replication proteins Dpb11, Sld2, Pol epsilon, and GINS in budding yeast. Genes Dev 24:602–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JM, Carr AM (2008) Smc5/6: a link between DNA repair and unidirectional replication? Nat Rev Mol Cell Biol 9:177–182 [DOI] [PubMed] [Google Scholar]
- Pacek M, Tutter AV, Kubota Y, Takisawa H, Walter JC (2006) Localization of MCM2–7, Cdc45, and GINS to the site of DNA unwinding during eukaryotic DNA replication. Mol Cell 21:581–587 [DOI] [PubMed] [Google Scholar]
- Panse VG, Kuster B, Gerstberger T, Hurt E (2003) Unconventional tethering of Ulp1 to the transport channel of the nuclear pore complex by karyopherins. Nat Cell Biol 5:21–27 [DOI] [PubMed] [Google Scholar]
- Papouli E, Chen S, Davies AA, Huttner D, Krejci L, Sung P, Ulrich HD (2005) Crosstalk between SUMO and ubiquitin on PCNA is mediated by recruitment of the helicase Srs2p. Mol Cell 19:123–133 [DOI] [PubMed] [Google Scholar]
- Potts PR, Porteus MH, Yu H (2006) Human SMC5/6 complex promotes sister chromatid homologous recombination by recruiting the SMC1/3 cohesin complex to double-strand breaks. EMBO J 25:3377–3388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth SG, Prasanth KV, Siddiqui K, Spector DL, Stillman B (2004) Human Orc2 localizes to centrosomes, centromeres and heterochromatin during chromosome inheritance. EMBO J 23:2651–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priego Moreno S, Bailey R, Campion N, Herron S, Gambus A (2014) Polyubiquitylation drives replisome disassembly at the termination of DNA replication. Science 346:477–481 [DOI] [PubMed] [Google Scholar]
- Psakhye I, Jentsch S (2012) Protein group modification and synergy in the SUMO pathway as exemplified in DNA repair. Cell 151:807–820 [DOI] [PubMed] [Google Scholar]
- Ragland RL, Patel S, Rivard RS, Smith K, Peters AA, Bielinsky AK, Brown EJ (2013) RNF4 and PLK1 are required for replication fork collapse in ATR-deficient cells. Genes Dev 27:2259–2273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell JCW, Bowers JL, Rodríguez HK, Bell SP (2006) Sequential ATP hydrolysis by Cdc6 and ORC directs loading of the Mcm2–7 helicase. Mol Cell 21:29–39 [DOI] [PubMed] [Google Scholar]
- Remus D, Diffley JFX (2009) Eukaryotic DNA replication control: lock and load, then fire. Curr Opin Cell Biol 21:771–777 [DOI] [PubMed] [Google Scholar]
- Remus D, Beuron F, Tolun G, Griffith JD, Morris EP, Diffley JFX (2009) Concerted loading of Mcm2–7 double hexamers around DNA during DNA replication origin licensing. Cell 139:719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santocanale C, Diffley JF (1996) ORC- and Cdc6-dependent complexes at active and inactive chromosomal replication origins in Saccharomyces cerevisiae. EMBO J 15:6671–6679 [PMC free article] [PubMed] [Google Scholar]
- Sarangi P, Zhao X (2015) SUMO-mediated regulation of DNA damage repair and responses. Trends Biochem Sci 40:233–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi P, Bartosova Z, Altmannova V, Holland C, Chavdarova M, Lee SE, Krejci L, Zhao X (2014) Sumoylation of the Rad1 nuclease promotes DNA repair and regulates its DNA association. Nucleic Acids Res 42:6393–6404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarangi P, Steinacher R, Altmannova V, Fu Q, Paull TT, Krejci L, Whitby MC, Zhao X (2015) Sumoylation influences DNA break repair partly by increasing the solubility of a conserved end resection protein. PLoS Genet 11:e1004899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel J, Eifler K, Sigurethsson JO, Cuijpers SA, Hendriks IA, Verlaan-de Vries M, Kelstrup CD, Francavilla C, Medema RH, Olsen JV et al. (2014) Uncovering SUMOylation dynamics during cell-cycle progression reveals FoxM1 as a key mitotic SUMO target protein. Mol Cell 53:1053–1066 [DOI] [PubMed] [Google Scholar]
- Schwartz DC, Felberbaum R, Hochstrasser M (2007) The Ulp2 SUMO protease is required for cell division following termination of the DNA damage checkpoint. Mol Cell Biol 27:6948–6961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sclafani RA, Holzen TM (2007) Cell cycle regulation of DNA replication. Annu Rev Genet 41:237–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen TH, Lin HK, Scaglioni PP, Yung TM, Pandolfi PP (2006) The mechanisms of PML-nuclear body formation. Mol Cell 24:331–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood R, Takahashi TS, Jallepalli PV (2010) Sister acts: coordinating DNA replication and cohesion establishment. Genes Dev 24:2723–2731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y-J, Stillman B (2006) Cdc7-Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol Cell 24:101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu Y-J, Stillman B (2010) The Dbf4-Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 463:113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivakumar S, Gorbsky GJ (2015) Spatiotemporal regulation of the anaphase-promoting complex in mitosis. Nat Rev Mol Cell Biol 16:82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonneville R, Moreno SP, Knebel A, Johnson C, Hastie CJ, Gartner A, Gambus A, Labib K (2017) CUL-2LRR-1 and UBXN-3 drive replisome disassembly during DNA replication termination and mitosis. Nat Cell Biol 10.1038/ncb3500 [DOI] [PMC free article] [PubMed]
- Speck C, Chen Z, Li H, Stillman B (2005) ATPase-dependent cooperative binding of ORC and Cdc6 to origin DNA. Nat Struct Mol Biol 12:965–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srikumar T, Lewicki M, Baught B (2013) A global S. cerevisiae small ubiquitin-related modifier (SUMO) system interactome. Mol Syst Biol 9:668–676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stead K, Aguilar C, Hartman T, Drexel M, Meluh P, Guacci V (2003) Pds5p regulates the maintenance of sister chromatid cohesion and is sumoylated to promote the dissolution of cohesion. J Cell Biol 163:729–741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J, Evrin C, Samel SA, Fernandez-Cid A, Riera A, Kawakami H, Stillman B, Speck C, Li H (2013) Cryo-EM structure of a helicase loading intermediate containing ORC-Cdc6-Cdt1-MCM2–7 bound to DNA. Nat Struct Mol Biol 20:944–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Toh-e A, Kikuchi Y (2001) A novel factor required for the SUMO1/Smt3 conjugation of yeast septins. Gene 275:223–231 [DOI] [PubMed] [Google Scholar]
- Tammsalu T, Matic I, Jaffray EG, Ibrahim AFM, Tatham MH, Hay RT (2014) Proteome-wide identification of SUMO2 modification sites. Sci Signal 7:rs2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H (2007) CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 445:328–332 [DOI] [PubMed] [Google Scholar]
- Tsuji T, Ficarro SB, Jiang W (2006) Essential role of phosphorylation of MCM2 by Cdc7/Dbf4 in the initiation of DNA replication in mammalian cells. Mol Biol Cell 17:4459–4472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulrich HD, Takahashi T (2013) Readers of PCNA modifications. Chromosoma 122:259–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vodermaier HC (2004) APC/C and SCF: controlling each other and the cell cycle. Curr Biol 14:R787–R796 [DOI] [PubMed] [Google Scholar]
- Vos SM, Tretter EM, Schmidt BH, Berger JM (2011) All tangled up: how cells direct, manage and exploit topoisomerase function. Nat Rev Mol Cell Biol 12:827–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waga S, Stillman B (1998) The DNA replication fork in eukaryotic cells. Annu Rev Biochem 67:721–751 [DOI] [PubMed] [Google Scholar]
- Wang JC (2002) Cellular roles of DNA topoisomerases: a molecular perspective. Nat Rev Mol Cell Biol 3:430–440 [DOI] [PubMed] [Google Scholar]
- Waters LS, Minesinger BK, Wiltrout ME, D’Souza S, Woodruff RV, Walker GC (2009) Eukaryotic translesion polymerases and their roles and regulation in DNA damage tolerance. Microbiol Mol Biol Rev 73:134–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Zhao X (2016a) A new MCM modification cycle regulates DNA replication initiation. Nat Struct Mol Biol 23:209–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Zhao X (2016b) The initiation of DNA replication in eukaryotes. In: Kaplan DL (ed) Chapter 18: role of posttranslational modifications in replication initiation Springer International Publishing, ISBN 978–3-319–24696-3 [Google Scholar]
- Wotton D, Shore D (1997) A novel Rap1p-interacting factor, Rif2p, cooperates with Rif1p to regulate telomere length in Saccharomyces cerevisiae. Genes Dev 11:748–760 [DOI] [PubMed] [Google Scholar]
- Yamazaki S, Ishii A, Kanoh Y, Oda M, Nishito Y, Masai H (2012) Rif1 regulates the replication timing domains on the human genome. EMBO J 31:3667–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B, Swatkoski S, Holly A, Lee LC, Giroux V, Lee CS, Hsu D, Smith JL, Yuen G, Yue J et al. (2015) Oncogenesis driven by the Ras/Raf pathway requires the SUMO E2 ligase Ubc9. Proc Natl Acad Sci U S A 112:E1724–E1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegerman P, Diffley JFX (2007) Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445:281–285 [DOI] [PubMed] [Google Scholar]
- Zhao X, Blobel G (2005) A SUMO ligase is part of a nuclear multiprotein complex that affects DNA repair and chromosomal organization. Proc Natl Acad Sci U S A 102:4777–4782 [DOI] [PMC free article] [PubMed] [Google Scholar]