Abstract
The consolidation of motor sequence learning is known to depend on sleep. Work in our laboratory and others have shown that the striatum is associated with this off-line consolidation process. In this study, we aimed to quantify the sleep-dependent dynamic changes occurring at the network level using a measure of functional integration. We directly compared changes in connectivity before and after sleep or the simple passage of daytime. As predicted, the results revealed greater integration within the cortico-striatal network after sleep, but not an equivalent daytime period. Importantly, a similar pattern of results was also observed using a data-driven approach; the increase in integration being specific to a cortico-striatal network, but not to other known functional networks. These findings reveal, for the first time, a new signature of motor sequence consolidation: a greater between-regions interaction within the cortico-striatal system.
Keywords: Functional connectivity, Consolidation, Sleep, Motor learning, fMRI, Networks
Introduction
Contemporary theories of motor skill learning advocate that following encoding of a new motor ability, the memory undergoes “off-line” transformations allowing the initially labile trace to become somewhat fixed into the physical structure of the brain through a cascade of events occurring at both cellular and systems levels: a phase called “memory consolidation” (Dudai, 2004). A large number of studies have now convincingly demonstrated that sleep, during nighttime or daytime, plays a critical role in the off-line consolidation of some, but not all types of motor skills (see Diekelmann et al., 2009; Born and Wilhelm, 2012 for reviews). Indeed, sleep-dependent consolidation has particularly been observed following the acquisition of a new sequence of movements, as opposed to tasks requiring subjects to adapt to visuomotor changes in the environment (Albouy et al., 2013c; Doyon et al., 2009b). This mnemonic process has also been reported in conditions where motor sequences were acquired explicitly (Fischer et al., 2002; Walker et al., 2002) rather than when they are learned implicitly (Robertson et al., 2004), and more so for the allocentric (spatial) compared to the egocentric (motor) representation of a newly learned sequence of movements (Albouy et al., 2013a; Cohen et al., 2005; Witt et al., 2010).
Up to now, functional neuroimaging studies in the field have mainly attempted to identify the specific brain regions mediating motor sequence learning (MSL) and consolidation. While the results have corroborated the contribution of both cortico-striatal and cortico-cerebellar systems in the acquisition of such skilled behaviours (Doyon and Benali, 2005; Doyon et al., 2009a; Floyer-Lea and Matthews, 2005), the off-line consolidation phase has been associated with increased activity in the striatum, and the putamen in particular (Debas et al., 2010), the hippocampus (Albouy et al., 2008; Walker et al., 2005), the cerebellum (Steele and Penhune, 2010; Walker et al., 2005) as well as other cortical regions including the primary motor (Steele and Penhune, 2010) and the medial prefrontal cortices (Walker et al., 2005). Interestingly, however, recent neuroimaging work has also begun to characterize the dynamic learning-dependent functional changes between cerebral regions through connectivity analyses, which are based upon correlations between time courses of brain areas (Friston et al., 1993; Marrelec et al., 2008). Most of these studies have used hypothesis-driven, predefined motor networks or specific seed regions in order to identify the connectivity changes within or between networks during learning. Hence, MSL has been associated with greater connectivity between motor-related regions in the early learning phase of a new sequence of movements, followed by stabilization within the 2nd and 4th weeks of the acquisition process (Ma et al., 2010). Similarly, Sun et al. (2007) have also found greater connectivity between the sensorimotor, premotor and the supplementary motor areas (SMA) within and between hemispheres during early learning of a bimanual motor sequence task. Yet, although very informative, such an a priori approach is limited by the fact that the pattern of changes in connectivity during learning can vary a great deal depending on the seed motor areas chosen within a given network. For example, Coynel et al. (2010) have reported decreases of integration within the associative, but not within the sensorimotor cortico-striatal network across 28 days of acquisition of an explicit sequence, hence demonstrating that the choice of motor regions within a motor network does have a critical effect on pattern of connectivity changes observed with MSL. To overcome this limitation, some authors have employed data-driven approaches. For example, using a graph-theoretical network analysis strategy in groups of young healthy subjects who learned to execute a bimanual learning task, Heitger et al. (2012) have reported that improved motor performance over a 5-day period was associated with increased functional network connectivity metrics. Using a multi-variate model-free method to analyse changes in functional networks related to practice of a motor sequence, another study has revealed an increase in connectivity within a network comprising the premotor and posterior parietal cortices within a first training session (Tamas Kincses et al., 2008). Yet very little is known with respect to the change in functional connectivity related to the consolidation process of a newly learned sequential motor skill (see Dayan and Cohen, 2011).
Task-related changes in connectivity between motor brain regions before and after sleep have previously been measured in order to gain insight into their interaction in association with motor memory consolidation. For instance, a dynamic interplay between the hippocampus and the striatum during MSL training has been shown to predict overnight gains in performance (Albouy et al., 2008, 2013b). While activity in the dorsal premotor cortex, posterior parietal cortex and pre-SMA was significantly correlated during REM sleep following sequence learning, no correlation was observed between these structures in a group that had not learned the motor sequence (Laureys et al., 2001). Despite such advances, however, it remains unclear whether such increase in connectivity (possibly reflecting the consolidation process following sequence learning) was strictly dependent on sleep, as no daytime control condition was used in both of these investigations. Furthermore, considering that the latter researchers analysed connectivity changes through the use of specific seed regions based on a priori hypothesis, it is thus unknown whether the sleep-related changes in brain connectivity described above are specific to the motor regions mediating the learning process in the first place, or whether they can be observed in other brain networks. Finally, no study, to our knowledge, has described connectivity changes following sleep at the systemic level using a data-driven approach.
The aim of the present study was thus to compare directly the changes in functional connectivity related to the consolidation process of a motor memory trace in two groups of young adults who participated in a test-retest paradigm, where motor sequence learning was measured before and after a 12-hour delay filled with either night sleep or the simple passage of daytime. Participants belonging to the Day/awake group were scanned in the morning and evening using functional magnetic resonance imaging (fMRI) while they executed a 5-element version of the finger sequence learning task (Karni et al., 1995), whereas those in the Night/sleep group were first tested on the same task in the evening, and then retested the following morning. Two different approaches in connectivity analysis were applied: First, similar to studies summarized above, we used a hypothesis-driven method including motor brain regions known to contribute to MSL and/or motor sequence consolidation. Based on Doyon and colleagues’ model, which predicts that the cortico-striatal system contributes to the consolidation process of a new sequence of movements (Doyon and Benali, 2005; Doyon et al., 2009a; Doyon and Ungerleider, 2002), we hypothesized that subjects in the night group would show increased correlations (i.e., greater integration) between the learning-dependent motor regions of that system when compared to subjects in the day group. Second, we used a data-driven approach that allowed functional network reorganization to be quantified without a priori assumptions. This method permitted us to measure the change of integration not only within the cortico-striatal system before and after a night of sleep, but also within other large- scale, functionally distinct networks extracted through an independent component analysis (ICA). It was predicted that the changes in integration after sleep, associated with off-line consolidation, would be spatially specific as it would only be observed within the cortico-striatal system, and not within the other extracted networks.
Methods
The present study uses a subset of the behavioural and fMRI data that we previously published where the results of a standard univariate approach were reported (see Debas et al., 2010, for more details).
Subjects
24 young healthy subjects aged between 19 and 30 years old (13 women) participated in the present study. Participants had no experience playing a musical instrument, nor had received previous training for speed typing. Participants reported having a regular cycle of bedtime starting around 11:00 p.m. (±1 h) and waking-up around 7:00 (±1 h), which was confirmed through a sleep diary for a full week prior to the study. Subjects with depressive symptoms (score above 4) as measured by the short version of the Beck Depression Scale were excluded. All subjects participated in an adaptation night at our laboratory, during which polysomnographic (PSG) measures of their sleep were recorded. Subjects with an apnea-hypoapnea index >5 or a periodic limb movements index >5 were excluded. None of the subjects worked night shifts or were engaged in trans-meridian trips 3 months prior to the study. During the entire period of the experiment, subjects were asked to abstain from alcohol. They were nonsmokers and remained caffeine free prior to scanning sessions. They were also right handed, had no history of neurological or psychiatric disorders as well as no history of sleep disturbances or medication intake. They gave their written informed consent to participate in the study. The project was approved by the “Regroupement Neuroimagerie/Québec” Ethics Review Committee at the “Centre de recherche de l’Institut universitaire de gériatrie de Montréal, Université de Montréal”.
Experimental design
To study the effect of sleep on the integration of cerebral networks, we used a between (Night/sleep-Day/awake) and within-subject (test and retest sessions) design (see Debas et al., 2010 for more details). Briefly, the Night/sleep group (n = 13, 7 female) was first trained in the evening (9:00 p.m. approximately) on the motor sequence learning task, in which subjects were asked to practice an explicitly known 5-item sequence (4–1-3–2-4) of finger movements of the left, non-dominant hand (Karni et al., 1995) until they reached asymptotic performance. This training session took place in a mock scanner to simulate the MRI environment. Participants then moved to the MRI room and were scanned (i.e., test session) in a blocked paradigm while executing the newly learned motor sequence using a MRI-compatible response box. Following this first scanning session, which lasted a total of about 20 min depending on each participant’s speed to complete the sequence, subjects were required to sleep in the laboratory and polysomnographic (PSG) data were recorded (see Barakat et al., 2010 for a report of the EEG results). They were then rescanned the following morning 12 h later (9:00 a.m. approximately) in a retest session. The training, test and retest sessions consisted of 8 blocks during which subjects had to practice 20 sequences each (i.e., 100 finger movements). All experimental blocks started off with a 2.5 s instruction period where the word “Sequence” appeared in the middle of the screen seen through mirrors inserted in the head coil, followed by a green square indicating that subjects could start producing the known sequence as fast and accurately as possible. After having completed 20 sequences, the colour of the square on the screen changed to red, indicating the beginning of a 15 s rest period. The Day/awake group (n = 11, 6 female) followed a similar procedure, except that the first training and scanning sessions took place in the morning (around 9:00 a.m.), whereas the retest session was completed 12 h later in the evening (around 9:00 p.m.). Time per sequence and the number of correct sequences were recorded for each block in the two groups of subjects.
Behavioural analyses
As it is commonly found when subjects are requested to practice a short sequence of movements that is already known explicitly, participants made very few errors during task execution in the scanner. Thus we used speed as our main behavioural metric to measure offline gains in performance (Doyon et al., 2009b; Fischer et al., 2002; Walker et al., 2002). Time to execute a sequence was averaged for each of the 8 blocks of the test and delayed retest sessions. Data from both sessions were normalised based upon the results of the last 5 blocks of the test session, a period during which subjects had reached asymptotic performance. Thus, the mean reaction time of each block was subtracted from the averaged reaction time of these 5 blocks, and then divided by their standard deviation. We then used the first two blocks of the delayed retest session to directly compare the behavioural performance between the two groups with an independent sample t-test.
MRI acquisition and analyses
Brain imaging data were acquired with a 3T scanner (Magnetom Trio Siemens AG, Germany) equipped with an 8-channel head coil. A high resolution anatomical T1-weighted scan was obtained for each subject (voxel size = 1 × 1 × 1 mm3, TR = 23 ms, TE = 2.98 ms, FA = 9°; matrix 256 × 256; 176 slices). Functional T2*-weighted images were also acquired using a standard gradient echo-planar sequence sensitive to the BOLD signal (voxel size = 3.75 × 3.75 × 5 mm3; TR = 2.5 s; TE = 30 ms; FA = 90°; FOV = 240 × 240 mm2, matrix size = 64 × 64; 28 slices). Pre-processing steps using Statistical Parametric Mapping (SPM5) (http://www.fil.ion.ucl.ac.uk/spm/software) included realignment and smoothing using a Gaussian Kernel of 6 mm full-width at half-maximum (FWHM). Then, as part of the connectivity toolbox Netbrainwork (http://sites.google.com/site/netbrainwork/), the data was registered into the 152-MNI stereotaxic space using nonlinear spatial transformations as implemented in SPM5.
Connectivity analyses
Hypothesis-driven functional network identification
The hypothesis-driven cortico-striatal network was built based on regions known to contribute to the learning and consolidation of a new motor sequence. To do so, we used the coordinates corresponding to the local peaks of activity during task execution that were found in our standard main effect analyses (Debas et al., 2010) as well as in other relevant studies. Spheres of 7 and 4 voxels in the cortical and sub-cortical areas, respectively, were then formed around these peak coordinates to create the ROIs implicated in the hypothesis-driven network. This network was thus composed of two ROIs in the precentral area, as the latter has been shown to play a critical role in the consolidation of motor sequences (Robertson et al., 2005; Steele and Penhune, 2010). Similarly, the putamen, globus pallidus, SMA and the superior parietal cortex were also included for their contribution to motor sequence learning and/or consolidation (Debas et al., 2010; Doyon et al., 2002; Fischer et al., 2005; Grafton and Ivry, 1995). Finally, because we aimed to study the levels of information exchange within the cortico-striatal network via levels of integration, the thalamus, which constitutes a primordial relay station (Alexander and Crutcher, 1990) was also added to the network.
Data-driven functional network identification
For this approach, we used the NetBrainWork toolbox (http://sites.google.com/site/netbrainwork/). This software implements the NEDICA method for spatial independent component analysis (sICA) at the group level (Perlbarg et al., 2008). The default number of spatial components (i.e., 40) was calculated for each subject using the infomax ICA algorithm. After registration into the MNI standardized space, a hierarchical clustering on the independent components from all participants yielded a tree diagram. Selection of the components at the group level was then based on the maximization of two indices: the degree of representativity (i.e., the proportion of subjects represented in the group component) and the degree of unicity (i.e., the proportion of subjects contributing to only one component in the group component). Once the group components were found, a t-score map was computed from the spatial components belonging to this group-component.
Networks were extracted for each individual using the time courses of functional volumes derived from the test session while participants were performing the MSL task (i.e., active state). A first analysis using fMRI BOLD data collected during the test session from both Night/sleep and Day/awake groups gave rise to the group components. Two indices (representativity and unicity) as well as guidelines (Kelly et al., 2010) were used to visually screen the group components in order to exclude maps comprising regions distributed in and around ventricles, sinus or blood vessels. The remaining seven group components are reported in the Results section and are referred to as the functional networks. Before quantifying network integration, structured noise (related to, e.g., respiration, heartbeat, and movements) was removed with CORSICA (Perlbarg et al., 2007), which uses the ability of spatial ICA decomposition to separate processes related to structured noise from other components. Automatic selection of noise-related components was based on their spatial distribution. CORSICA identifies maps with a spatial distribution of Z values maximal around ventricles or edges of the brain as likely movements or physiological noise and subtracts these components of signal from the original data. This method allows improvement of signal to noise ratio evaluated within the cardiac and respiratory frequencies, while preserving fluctuations related to neural activity.
Region extraction
The data-driven large-scale networks were built using an automatic approach. ROIs were formed automatically around the peak signal of each of the seven large-scale networks, using a region-growing algorithm. At each step of this algorithm, the voxel included in the ROI corresponded to the local highest t-score. Although it is possible to choose the size of the ROI, only one ROI size can be selected for a given large-scale network. Thus ROIs comprising 7 voxels, with a minimal distance between 2 ROIs of 30 mm, were established for networks that were composed primarily of cortical areas; whereas ROIs extending over 4 voxels with a minimal distance of 25 mm between 2 ROIs were chosen for the network with sub-cortical areas primarily.
Integration analysis
Large-scale integration is the term used to represent the idea that neural mechanisms select and coordinate distributed brain activity to produce a flow of adapted and unified cognitive movements (Varela et al., 2001). Quantification of the level of network integration implemented in NetBrainWork was previously defined by Tononi et al. (1994) and has successfully been applied to fMRI results in Marrelec et al. (2008). Integration refers to a multivariate measure based on the correlations between the temporal courses of the ROI of a given network, as the amplitude of the signal of the ROI is normalised (Marrelec et al., 2008). This metric allows one to quantify the global level of statistical dependence within the network. For a multivariate normal distribution (see Marrelec et al., 2008), Integration (I) is equal to: I = −½ ln|R| where R is the correlation matrix and |.| is the matrix determinant.
This expression summarizes the within system organisation using all correlation coefficients that can be computed into a single number, which can then be appropriately statistically compared between sessions and between groups. The underlying assumption is that the larger the correlation coefficient, the greater the integration and information exchange within the system. As the absolute measure of integration is dependent upon the number of ROIs selected within a given network, no statistical comparison was done between networks. The latter measure of integration was solely assessed between groups and across session. Independent t-test between the Day/awake group and the Night/sleep groups’ test session tested whether there was any time of day effects. Then, relative changes of integration between the test and retest sessions were calculated for the two groups. Independent sample t-tests were first used to assess the difference in connectivity changes associated with sleep vs. the simple passage of daytime for the hypothesis-driven cortico-striatal network.
Because the present study constitutes the first attempt to investigate dynamic changes in network integration after either sleep or the simple passage of time, we then assessed the spatial specificity between networks. This allowed us to verify whether the pattern of results described above within the cortico-striatal network was specific to the interaction between sleep/passage of time and the cortico-striatal network; or whether it was simply the result of a non-specific effect of sleep on brain regions at large (i.e., if all functional systems of the brain were generally more synchronous after sleep). Thus a measure of integration was computed for each subject and each session for the 7 identified data-driven networks. Seven independent t-tests were then performed to test the effect of sleep vs. daytime. We then used Pearson’s correlation analyses to assess the possible relationship between the subject’s behavioural improvements in performance from test to retest with changes in the levels of integration within networks identified through the hypothesis- and data-driven approaches.
Finally, we investigated whether the response to the task was a driving factor in the observed changes in integration of brain networks. To do so, we modelled the fluctuations associated with the block design during the experimental task using SPM’s canonical hemodynamic response, and regressed them out from the functional images used above, i.e. for each participant’s test session. The analysis of functional networks and integration described above were replicated using the residuals of the regression, i.e. after the direct influence of the task on fMRI time series was modelled and removed.
Results
Behaviour
There was no group difference in speed to produce sequences during the first testing session (t(22) = −.24, p = .23), suggesting that their performance was not influenced by the time of day, nor by a change in circadian rhythms. Second, to test for the amount of offline gains in performance following sleep as opposed to daytime, a t-test was carried out to directly compare groups’ performance on the first two blocks of practice only after normalisation (see Behavioural analyses section above for more details). This allowed us to restrain our search on blocks of trials that reflect the off-line consolidation phase and not the slow learning or re-learning processes that usually occur during the second testing session. As expected, the results revealed a significant difference between the two groups (t(1,22) = 2.33; p = .03) at retest, with the Night/sleep group performing faster than the Day/awake group.
Functional connectivity
Network identification
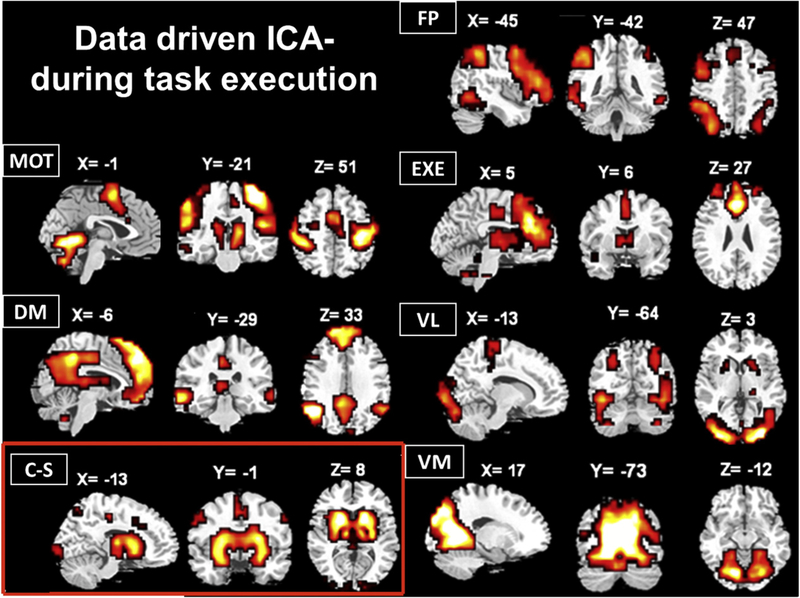
The hypothesis-driven network consisted of 7 selected ROIs (see Table 1), whereas the data-driven approach through the ICA analysis allowed the identification of seven, spatially independent, functional networks (Fig. 1). Each data-driven network was present in more than 72% of the participants, except for the “Executive network”, which was detected in only 54% of them. Despite this, a measure of integration within the latter is nevertheless reported here, as it is a functional network often identified in the literature (Beckmann et al., 2005; Damoiseaux et al., 2006). Six of the networks (i.e., default-mode, motor, executive, fronto-parietal, visual lateral and visual medial) were spatially very similar, and thus reminiscent to those previously reported in studies investigating resting-state networks (Beckmann et al., 2005), further supporting the relative stability of these functional networks regardless of the participant’s state being either at rest or in action (Bianciardi et al., 2009; Buckner et al., 2009; Calhoun et al., 2008; Wang et al., 2012). A 7th large-scale network labelled “cortico-striatal” was also identified (see Table 1 for more details). Such a network has rarely been identified with the use of ICA (Robinson et al., 2009), but its detection may be related to the fact that subjects were required to execute a motor sequence learning task, which potentiated the activity within this network and increased its independence in relation to the other functional connectivity networks. This cortico-striatal network included multiple cortical areas known to be associated with it (see Doyon and Benali, 2005; Doyon and Ungerleider, 2002), notably the pre- and post-central gyri as well as the superior and inferior parietal cortices. Admittedly, however, this network comprised additional brain regions that are usually thought to be unrelated to such a cortico-subcortical system. For example, we found a certain number of visual cortical areas, which might be due to the fact that subjects were required to start executing the sequence once a green square was presented on the screen. Thus, as visual information was used to trigger motor sequence performance, this could explain why the ICA combined all these brain regions into a single network. Yet, because the strongest signals within this network came from basal ganglia structures, notably the putamen, caudate nucleus and globus pallidus, and because it was the only network that showed such subcortical activity, the latter was categorized as the cortico-striatal network.
Table 1.
Regions of interest (ROI) being part of the cortico-striatal (C-S) networks. Coordinates are presented in the Talaraich space. SMA = supplementary motor area; CB = cerebellum; Ant = anterior; Mid = middle; Inf = inferior; Sup = superior.
| ROIs | X | Y | Z |
|---|---|---|---|
| A. Hypothesis-driven C-S network | |||
| Precentral | 39 | −22 | 51 |
| Precentral | 31 | −12 | 63 |
| Putamen | 26 | 7 | 5 |
| SMA | −1 | −4 | 53 |
| Parietal Sup | 22 | −54 | 63 |
| Globus pallidus | 30 | −13 | −1 |
| Thalamus | 16 | −18 | 6 |
| B. Data-driven C-S network | |||
| CB Crus II | 35 | −80 | −30 |
| CB Crus II | 10 | −79 | −28 |
| Vermis | 2 | −60 | −33 |
| Putamen | −19 | 6 | 7 |
| Globus pallidus | 16 | 6 | 1 |
| Globus pallidus | 30 | −13 | −1 |
| Amygdala | −26 | −2 | −13 |
| Amygdala | 24 | −1 | −17 |
| Thalamus | −10 | −15 | 7 |
| Hippocampus | −29 | −18 | −4 |
| Caudate nucleus | 19 | 1 | 18 |
| Post-central | −50 | −10 | 36 |
| Precentral | −57 | 4 | 18 |
| Precentral | 60 | 10 | 21 |
| Cingulum Ant | −4 | 18 | 27 |
| Cingulum Mid | −9 | −25 | 43 |
| Cingulum Mid | −3 | −2 | 42 |
| Parietal Inf | −47 | −33 | 49 |
| Parietal Sup | −10 | −67 | 54 |
| Cuneus | −1 | −76 | 37 |
| Frontal Mid | −34 | 49 | 13 |
| Insula | −27 | −22 | 11 |
| Insula | −39 | 11 | 2 |
| Fusiform | 28 | −73 | −9 |
| Lingual | −22 | −85 | −8 |
| Calcarine | −8 | −97 | −2 |
| Occipital Inf | −42 | −86 | 1 |
| Occipital Inf | −48 | −63 | −9 |
| Calcarine | 18 | −97 | 5 |
Fig. 1.
Large scale networks isolated through ICA during the first testing session of both sleep and wake groups. As in Beckmann et al. (2005), spatial maps correspond to the best sagittal, coronal and axial views to display the most representative brain regions composing each network. The coordinates refer to mm distances from the anterior commissure. MOT = motor; DM = default-mode; C-S = cortico-striatal; FP = fronto-parietal; EXE = executive; VL = visual lateral; VM = visual medial.
Functional integration
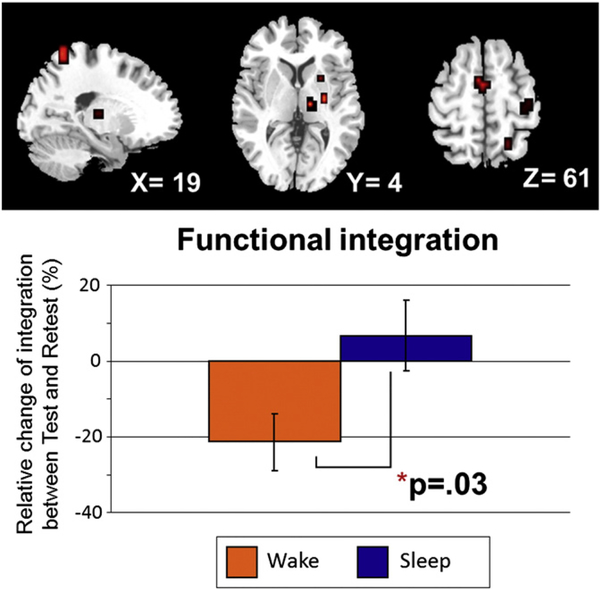
Changes in network integration relative to sleep or daytime were first measured within the hypothesis-driven, cortico-striatal network. The level of integration between the two groups (Day/awake and Night/sleep) in the first test session, i.e., morning vs. evening test groups, did not significantly differ (t(22), p = .87), hence suggesting that there was no significant effect of time of day on this measure of connectivity at baseline. By contrast, an independent t-test comparing groups showed that connectivity within the hypothesis-driven network changed significantly depending on whether participants had slept or not. Results revealed that there was a decrease of 21% in integration level after the passage of time, as opposed to an increase of 7% following a night of sleep (t(22), p = .03) Fig. 2). The latter findings are consistent with our predictions and thus suggest a preserved level of synchrony in signals between regions of the hypothesis-driven cortico-striatal network following sleep-dependent motor memory consolidation.
Fig. 2.
Hypothesis-driven network analysis. Relative change in integration within the cortico-striatal system between the test and retest sessions. The results show a differential effect of sleep on the level of interaction amongst regions of this network. The group who remained awake during daytime shows a 21% decrease in the level of network integration, as opposed to the sleep group who demonstrates a 7% increase in integration from test to retest.
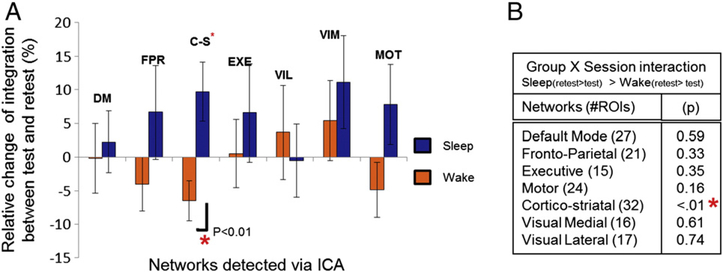
Similarly, we also calculated the relative change in integration between the test and retest sessions in both groups based upon the data-driven large-scale connectivity network detected through ICA. This was done for each of our 7 large-scale networks, which comprised between 15 and 32 ROIs (default-mode, fronto-parietal, motor, cortico- striatal, executive, visual lateral, visual medial). Independent t-tests comparing the relative change in integration for each network following either sleep vs. the simple passage of daytime, revealed a difference that was only significant for the large-scale cortico-striatal network. Again, there was no significant difference in integration within this network between our two groups in the first test session (t(22), p = .11), hence suggesting that there was no time of day effect at baseline. Yet we found a decrease in integration of 7% after the passage of daytime as opposed to an increase of 10% following sleep (t(22) = −3.1, p = .006), supporting the idea that sleep reorganizes flow of activity within this network and plays a role in consolidation. Only the motor network also showed a tendency for a greater integration after sleep as opposed to the passage of time (t(22) = −1.7, p = .10), yet the difference was not significant.
Qualitatively, we found that night sleep generally resulted in a greater level of integration as compared to daytime (see Fig. 3A). Although we were limited by the fact that we cannot directly compare the extent of change in integration between networks, it is important to note that only the cortico-striatal system revealed a strong tendency for an increase in integration following sleep (t(12) = 2.04, p = .063). These results thus suggest that sleep profited specifically to the data-driven, large-scale cortico-striatal network, as opposed to other functional connectivity networks present during task execution. Unfortunately, however, there was no correlation between the changes in integration within the networks and the observed overnight improvement in performance for the sleep group (r = .14, p = .66 and r = .40, p = .23 for hypothesis-driven and data-driven network, respectively).
Fig. 3.
Data-driven network analysis. A. Relative change in integration from test to retest for all data-driven networks. When compared to daytime, sleep had a significant beneficial effect on within network integration for the cortico-striatal network only. B. Results (p values) of the group (sleep vs. wake) and session (test vs. retest) interaction in integration for each of the identified network.
Finally, when we tested for the changes in integration after sleep or wake, and after regressing out the effect of the block design, the results of the ICA analysis at the group level now revealed the existence of six networks (visual medial, visual lateral, default-mode, fronto-parietal, motor and executive) instead of seven. These were visually similar to the evoked networks reported above, except that the large-scale cortico-striatal network was no longer isolated. These results suggest the presence of two superimposed types of fluctuations; those that are evoked in which networks include task-related brain regions and others that are intrinsic, comprising little regions of interest for the task at hand. This approach, however, did not reveal any differential effect of sleep vs. the passage of daytime on any of the networks, hence suggesting that the observed changes in network integration were to some large degree driven by the canonical response to the task.
Discussion
As predicted, connectivity changes measured during an active state (i.e., practice of a motor sequence) using both hypothesis and data-driven methods revealed that off-line motor sequence consolidation is associated with a greater level of integration within the cortico-striatal system. Importantly, the two approaches confirm that the increased integration within this task-related network is sleep-dependent, as no such change in integration was observed after a similar period of daytime. Furthermore, our findings show that this within-network dynamic found following motor sequence consolidation is specific to the cortico-striatal system as none of the other networks identified through the data-driven analysis demonstrated significant sleep-related changes in integration. Altogether, our findings are suggestive of a new mechanism associated with the off-line sleep-dependent motor memory consolidation process, that is, a greater synchrony of activity between regions forming the cortico-striatal network.
The present increase in interactions between regions of the cortico-striatal network, as shown with the hypothesis-driven network analysis is in line with previous studies that have demonstrated greater strength in regional brain connectivity during the initial learning phase of a MSL task (Coynel et al., 2010; Ma et al., 2010; Sun et al., 2007). For example, our findings are in accord with those from Ma et al. (2010) who have reported that the connectivity between the basal ganglia and M1 is strengthened across 2 and 4 weeks of learning a sequence of movements. They also corroborate the results from Sun et al. (2007) who have shown that activity in the parietal cortex becomes significantly more correlated with other motor-related brain regions during early vs. late MSL. And finally, they are consistent with conclusions from Peigneux et al. (2003) who have demonstrated that probabilistic sequence learning increases connectivity between the striatum and cuneus. Such a hypothesis-driven approach does not exclude the possibility that other structures like the cerebellum (Steele and Penhune, 2010) and the hippocampus (Albouy et al., 2008,2013a) may also contribute to the consolidation process of sequential types of skills. Yet our findings suggest that the brain regions constituting the cortico-striatal network, namely the primary motor cortex, SMA, superior parietal cortex, putamen, globus pallidus and thalamus, are not only involved in the acquisition of a new motor sequence; but also that the increased coherence in activity between these brain structures following sleep can be a good marker (and thus a possible mechanism) of the sleep-dependent off-line consolidation process of MSL.
As a complementary approach, we also used spatial independent component analysis (sICA) at the group level during task execution in order to test the specificity of the sleep-dependent increase in cortico- striatal integration. Remarkably, this method allowed the isolation of a large-scale cortico-striatal system (see Table 1), in which more than half of the automatically detected seeds have previously been directly associated with motor learning and/or motor memory consolidation (Albouy et al., 2008; Dayan and Cohen, 2011; Doyon et al., 2002, 2003; Fischer et al., 2005; Hikosaka et al., 1999; Walker et al., 2005). This data-driven approach did not only recruit multiple motor cortical regions, but also a large number of sub-cortical structures like the putamen, globus pallidus and the hippocampus. Interestingly, similar to the hypothesis-driven analysis, this network demonstrated a significant increase in integration after sleep, while the simple passage of daytime provoked a decrease of the within-system integration. Such pattern of findings is in accord with our previous work demonstrating that the putamen is related to motor sequence consolidation (Debas et al., 2010). It is also consistent with an increasing number of studies, which are suggesting that the hippocampus is implicated in this consolidation process, as it appears to be responsible for consolidating contextual information and high-order associations in motor sequence learning (Albouy et al., 2008, 2013a; Gheysen et al., 2010; Rose et al., 2011; Schendan et al., 2003). More importantly, however, only this large- scale cortico-striatal network revealed a sleep-dependent increase in integration, while daytime provoked a decrease in integration. Indeed, no other functional network presented a significant change in integration associated with sleep or daytime, hence further confirming the specificity of the association between sleep-dependent consolidation and the cortico-striatal system. Altogether, our results support the hypothesis elaborated in Doyon’s model (e.g., Doyon and Benali, 2005), endorsing a role for the cortico-striatal system in the consolidation of motor sequences.
When testing for integration changes after regressing out the canonical response to the task, no significant difference between the sleep and wake groups was observed in the cortico-striatal network. This suggests some redundancy between the SPM analysis performed in Debas et al. (2010) and the present findings, to some degree. However, we relied here on a model-free multivariate analysis, as opposed to a mass univariate parametric analysis in Debas et al. (2010) with two major implications: (1) increased sensitivity to distributed network effects; and, (2) sensitivity to task-evoked and intrinsic fluctuations that depart from the canonical response. The increased sensitivity difference (1) is important by itself, since we found only one motor region in Debas et al. (2010), the putamen, showing greater activity following sleep vs. daytime. By contrast, in the present work, we found an influence of consolidation on a widespread distributed network involving the cortico-striatal system. Furthermore, the fact that no significant change was observed after regressing out the effect of the canonical response to the task does not necessarily imply that (2) it does not play a role in this finding, i.e. some sources of fluctuations orthogonal to the canonical response to the task may still contribute to the observed change in network integration when no regression is performed. In fact, the regression experiment only shows that these additional sources are not large enough to drive significant changes in integration when isolated from the main effect of the task. Whether the differences between the analysis reported here and in Debas et al. (2010) originate from the multivariate or model-free characteristics of the method, which cannot be fully disambiguated here, these differences lead nevertheless to important new conclusions regarding the network-level interactions involved in the memory trace of a newly acquired motor skill after sleep, which go beyond those reported in Debas et al. (2010).
Systemic sleep-dependent consolidation
The present study is the first, to our knowledge, to look at changes in connectivity related to motor memory consolidation at the systemic level following sleep. Qualitatively, we found that almost all functional networks identified through the data-driven approach revealed higher connectivity following sleep, compared to after the simple passage of daytime (Fig. 3). This observation supports the idea that sleep provokes a diffuse increase in integration within networks at the whole brain level (Boly et al., 2012). In addition to this global increased interaction, however, the specificity of this effect on the cortico-striatal system also suggests that consolidation during sleep provides an active protection, by facilitating the cohesion of the functional system mediating the type of learning that previously occurred during daytime.
The physiological mechanism responsible for this increase in integration within the cortico-striatal system after sleep remains unknown. Furthermore, the present study does not admittedly allow to address this issue directly as group network analyses were only measured before and after, but not during sleep. Yet because such a change in integration was only found after a night of sleep, but not following a similar amount of awake time, three hypothetical physiological processes could account for the sleep-dependent consolidation manifestation reported here. First, it is possible that such an increase of integration within the cortico-striatal system was due to the reactivation process known to occur during sleep. Indeed, brain regions initially involved during training have shown to be reactivated together during the post-learning sleep period (see Rauchs et al., 2005 for a review). For example, in several electrophysiological studies in rodents, recordings of place cells in the hippocampus have revealed that the firing pattern of these cells is repeated in the same sequence during sleep (e.g. Wilson and McNaughton, 1994). Importantly, hippocampal replay has also been shown to cause reactivation and increased firing rates in other task-relevant brain structures such as the striatum (Lansink et al., 2009), hence suggesting that depending on the task, the striatum may also be part of the network reactivated after learning. Additional support for the role of the striatum in the “reactivation hypothesis” has come from human neuroimaging studies using positron by emission tomography (PET), which revealed that regional activity recorded during training on a probabilistic serial reaction time task was re-expressed in the post-training night (Maquet et al., 2000; Peigneux et al., 2003), notably within the cuneus and striatum (Peigneux et al., 2003).
Second, although conjectural because we did not measure functional connectivity during the post-learning rest period, nor during the night, it is also possible that maintenance of the motor memory trace during rest after learning, in addition to a sleep-dependent reactivation of the cortico-striatal system, also favoured optimal interaction between the regions forming that specific network. Consequently, the accumulation of those post-learning processes could thus allow the newly acquired motor skill to be consolidated, hence explaining the gains in performance after sleep. Such interpretation is consistent with previous work, which has shown that motor learning influences resting-state network activity (e.g., Albert et al., 2009, Vahdat et al., 2011), and that the functional dynamics observed during a resting state period can also impact subsequent motor learning (Fox et al., 2007).
A third possible mechanism, which could explain our pattern of results is based on the work by Boly et al (2012), who have demonstrated that during non-rapid eye movement (NREM) sleep, each functional network detected via ICA is characterized by greater within - as compared to between - systems connectivity. Interestingly, the increased functional clustering of brain activity, suggesting an independent operation of networks during NREM sleep, might actually support the specificity of changes in integration that we found in the cortico-striatal system. The latter hypothesis is also supported by the fact that different sleep characteristics of NREM sleep in particular (see Diekelmann and Born, 2010), notably spindles (Fogel and Smith, 2011; Fogel et al., 2012), have been linked to memory consolidation, and have been shown to correlate with activity in the putamen in association with motor sequence consolidation (Barakat et al., 2013).
Conclusion
The results of the present study suggest a new mechanism by which motor sequence consolidation is processed in the brain, i.e., through an increased level of integration within the task-related cortico-striatal network. Importantly, this effect was observed with both hypothesis- and data-driven approaches. Furthermore, our findings indicate that such change in integration is specific to the cortico-striatal system, and not to other independent functional networks. Altogether, the present findings extend those from Debas et al. (2010), and thus suggest that greater coherence between the striatum and motor-related brain regions is critical for a motor memory trace to become durable over time.
Acknowledgment
Support for this research was provided by a Canadian Institutes of Health Research (86463) grant to JD,JC, AHT, AK, HB and LGU, and by a fellowship from the Fonds de recherche du Québec santé to KD (folder number # 20882). The authors are grateful to Vo An Nguyen, Estelle Breton and Laurence Girouard for their help in data acquisition.
References
- Albert N, Robertson E, Miall R, 2009. The resting human brain and motor learning. Curr. Biol 19, 1023–1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Balteau E, Vandewalle G, Desseilles M, Dang-Vu T, Darsaud A, Ruby P, Luppi PH, Degueldre C, Peigneux P, Luxen A, Maquet P, 2008. Both the hippocampus and striatum are involved in consolidation of motor sequence memory. Neuron 58, 261–272. [DOI] [PubMed] [Google Scholar]
- Albouy G, Fogel S, Pottiez H, Nguyen VA, Ray L, Lungu O, Carrier J, Robertson E, Doyon J, 2013a. Daytime sleep enhances consolidation of the spatial but not motoric representation of motor sequence memory. PLoS One 8, e52805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Sterpenich V, Vandewalle G, Darsaud A, Gais S, Rauchs G, Desseilles M, Boly M, Dang-Vu T, Balteau E, Degueldre C, Phillips C, Luxen A, Maquet P, 2013b. Interaction between hippocampal and striatal systems predicts subsequent consolidation of motor sequence memory. PLoS One 8, e59490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albouy G, Vandewalle G, Sterpenich V, Rauchs G, Desseilles M, Balteau E, et al. , 2013c. Sleep stabilizes visuomotor adaptation memory: a functional magnetic resonance imaging study. J. Sleep Res 22, 144–154. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, 1990. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 13, 266–271. [DOI] [PubMed] [Google Scholar]
- Barakat M, Doyon J, Debas K, Vandewalle G, Morin A, Poirier G, Martin N, Lafortune M, Karni A, Ungerleider LG, Benali H, Carrier J, 2010. Fast and slow spindle involvement in the consolidation of a new motor sequence. Behav. Brain Res 217 (1), 117–121. [DOI] [PubMed] [Google Scholar]
- Barakat M, Carrier J, Debas K, Lungu O, Fogel S, Vandewalle G, Hoge RD, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J, 2013. Sleep spindles predict neural and behavioral changes in motor sequence consolidation. Hum. Brain Mapp 34 (11), 2918–2928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann CF, DeLuca M, Devlin JT, Smith SM, 2005. Investigations into resting-state connectivity using independent component analysis. Philos. Trans. R. Soc. Lond. B Biol. Sci 360, 1001–1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianciardi M, Fukunaga M, van Gelderen P, Horovitz SG, de Zwart JA, Duyn JH, 2009. Modulation of spontaneous fMRI activity in human visual cortex by behavioral state. Neurolmage 45, 160–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boly M, Perlbarg V, Marrelec G, Schabus M, Laureys S, Doyon J, Pelegrini-Issac M, Maquet P, Benali H, 2012. Hierarchical clustering of brain activity during human nonrapid eye movement sleep. Proc. Natl. Acad. Sci. U. S. A 109, 5856–5861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Born J, Wilhelm l., 2012. System consolidation of memory during sleep. Psychol. Res 76, 192–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckner RL, Sepulcre J, Talukdar T, Krienen FM, Liu H, Hedden T, Andrews-Hanna JR, Sperling RA, Johnson KA, 2009. Cortical hubs revealed by intrinsic functional connectivity: mapping, assessment of stability, and relation to Alzheimer’s disease. J. Neurosci. Off. J. Soc. Neurosci 29, 1860–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun VD, Kiehl KA, Pearlson GD, 2008. Modulation of temporally coherent brain networks estimated using ICA at rest and during cognitive tasks. Hum. Brain Mapp 29, 828–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen DA, Pascual-Leone A, Press DZ, Robertson EM, 2005. Off-line learning of motor skill memory: a double dissociation of goal and movement. Proc. Natl. Acad. Sci. U. S. A 102, 18237–18241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coynel D, Marrelec G, Perlbarg V, Pelegrini-Issac M, Van de Moortele PF, Ugurbil K, Doyon J, Benali H, Lehericy S, 2010. Dynamics of motor-related functional integration during motor sequence learning. Neuroimage 49, 759–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damoiseaux JS, Rombouts SA, Barkhof F, Scheltens P, Stam CJ, Smith SM, Beckmann CF, 2006. Consistent resting-state networks across healthy subjects. Proc. Natl. Acad. Sci. U. S. A 103, 13848–13853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayan E, Cohen LG, 2011. Neuroplasticity subserving motor skill learning. Neuron 72, 443–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debas K, Carrier J, Orban P, Barakat M, Lungu O, Vandewalle G, Hadj Tahar A, Bellec P, Karni A, Ungerleider LG, Benali H, Doyon J, 2010. Brain plasticity related to the consolidation of motor sequence learning and motor adaptation. Proc. Natl. Acad. Sci. U. S. A 107, 17839–17844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diekelmann S, Born J, 2010. The memory function of sleep. Nat. Rev. Neurosci 11, 114–126. [DOI] [PubMed] [Google Scholar]
- Diekelmann S, Wilhelm I, Born J, 2009. The whats and whens of sleep-dependent memory consolidation. Sleep Med. Rev 13, 309–321. [DOI] [PubMed] [Google Scholar]
- Doyon J, Ungerleider LG, 2002. Functional anatomy of motor skill learning, In: Squire LR, Schacter DL (Eds.), Neuropsychology of memory, third ed. The Guilford Press, New York, pp. 225–238. [Google Scholar]
- Doyon J, Benali H, 2005. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol 15, 161–167. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG, 2002. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc. Natl. Acad. Sci. U. S. A 99, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG, Doyon J, Penhune V, Ungerleider LG, 2003. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 41, 252–262. [DOI] [PubMed] [Google Scholar]
- Doyon J, Bellec P, Amsel R, Penhune V, Monchi O, Carrier J, Lehericy S, Benali H, 2009a. Contributions of the basal ganglia and functionally related brain structures to motor learning. Behav. Brain Res 199, 61–75. [DOI] [PubMed] [Google Scholar]
- Doyon J, Korman M, Morin A, Dostie V, Tahar AH, Benali H, Karni A, Ungerleider LG, Carrier J, 2009b. Contribution of night and day sleep vs. simple passage of time to the consolidation of motor sequence and visuomotor adaptation learning. Exp. Brain Res. (Experimented Hirnforschung) 195, 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudai Y, 2004. The neurobiology of consolidations, or, how stable is the engram? Annu. Rev. Psychol 55, 51–86. [DOI] [PubMed] [Google Scholar]
- Fischer S, Hallschmid M, Elsner AL, Born J, 2002. Sleep forms memory for finger skills. Proc. Natl. Acad. Sci. U. S. A 99, 11987–11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer S, Nitschke MF, Melchert UH, Erdmann C, Born J, 2005. Motor memory consolidation in sleep shapes more effective neuronal representations. J. Neurosci 25 (49), 11248–11255 (December). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floyer-Lea A, Matthews PM, 2005. Distinguishable brain activation networks for short- and long-term motor skill learning. J. Neurophysiol 94 (1), 512–518 (July). [DOI] [PubMed] [Google Scholar]
- Fogel SM, Smith CT, 2011. The function of the sleep spindle: a physiological index of intelligence and a mechanism for sleep-dependent memory consolidation. Neurosci. Biobehav. Rev 35, 1154–1165. [DOI] [PubMed] [Google Scholar]
- Fogel S, Martin N, Lafortune M, Barakat M, Debas K, Laventure S, Latreille V, Gagnon JF, Doyon J, Carrier J, 2012. NREM sleep oscillations and brain plasticity in aging. Front. Neurol 3, 176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MD, Snyder AZ, Vincent JL, Raichle ME, 2007. Intrinsic fluctuations within cortical systems account for intertrial variability in human behavior. Neuron 56, 171–184. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Liddle PF, Frackowiak RS, 1993. Functional connectivity: the principal-component analysis of large (PET) data sets. J. Cereb. Blood Flow Metab 13, 5–14. [DOI] [PubMed] [Google Scholar]
- Gheysen F, Van Opstal F, Roggeman C, Van Waelvelde H, Fias W, 2010. Hippocampal contribution to early and later stages of implicit motor sequence learning. Exp. Brain Res. (Experimentelle Hirnforschung Experimentation cerebrale) 202, 795–807. [DOI] [PubMed] [Google Scholar]
- Grafton ST, Hazeltine E, Ivry R, 1995. Functional mapping of sequence learning in normal humans. J. Cogn. Neurosci 7, 497–510. [DOI] [PubMed] [Google Scholar]
- Heitger MH, Ronsse R, Dhollander T, Dupont P, Caeyenberghs K, Swinnen SP, 2012. Motor learning-induced changes in functional brain connectivity as revealed by means of graph-theoretical network analysis. NeuroImage 61, 633–650. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Nakahara H, Rand MK, Sakai K, Lu X, Nakamura K, Miyachi S, Doya K, 1999. Parallel neural networks for learning sequential procedures. Trends Neurosci. 22, 464–471. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG, 1995. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377, 155–158. [DOI] [PubMed] [Google Scholar]
- Kelly RE Jr., Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ, 2010. Visual inspection of independent components: defining a procedure for artifact removal from fMRI data. J. Neurosci. Methods 189, 233–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM, 2009. Hippocampus leads ventral striatum in replay of place-reward information. PLoS. Biol 7, e1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laureys S, Peigneux P, Phillips C, Fuchs S, Degueldre C, Aerts J, Del Fiore G, Petiau C, Luxen A, van der Linden M, Cleeremans A, Smith C, Maquet P, 2001. Experience- dependent changes in cerebral functional connectivity during human rapid eye movement sleep. Neuroscience 105, 521–525. [DOI] [PubMed] [Google Scholar]
- Ma L, Wang B, Narayana S, Hazeltine E, Chen X, Robin DA, Fox PT, Xiong J, 2010. Changes in regional activity are accompanied with changes in inter-regional connectivity during 4 weeks motor learning. Brain Res. 1318, 64–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, Luxen A, Franck G, Van Der Linden M, Smith C, Cleeremans A, 2000. Experience-dependent changes in cerebral activation during human REM sleep. Nat. Neurosci 3, 831–836. [DOI] [PubMed] [Google Scholar]
- Marrelec G, Bellec P, Krainik A, Duffau H, Pelegrini-Issac M, Lehericy S, Benali H, Doyon J, 2008. Regions, systems, and the brain: hierarchical measures of functional integration in fMRI. Med. Image Anal 12, 484–496. [DOI] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, Luxen A, Cleeremans A, Maquet P, 2003. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage 20, 125–134. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Bellec P, Anton JL, Pelegrini-Issac M, Doyon J, Benali H, 2007. CORSICA: correction of structured noise in fMRI by automatic identification of ICA components. Magn. Reson. Imaging 25, 35–46. [DOI] [PubMed] [Google Scholar]
- Perlbarg V, Marrelec G, Doyon J, Pelegrini-Issac M, Lehericy S, Benali H, 2008. NEDICA: detection of group functional networks in fMRI using spatial independent component analysis. 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, 2008 ISBI, pp. 1247–1250. [Google Scholar]
- Rauchs G, Desgranges B, Foret J, Eustache F, 2005. The relationships between memory systems and sleep stages. J. Sleep Res 14,123–140. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Pascual-Leone A, Press DZ, 2004. Awareness modifies the skill-learning benefits of sleep. Curr. Biol 14, 208–212. [DOI] [PubMed] [Google Scholar]
- Robertson EM, Press DZ, Pascual-Leone A, 2005. Off-line learning and the primary motor cortex. J. Neurosci. Off. J. Soc. Neurosci 25, 6372–6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson S, Basso G, Soldati N, Sailer U, Jovicich J, Bruzzone L, Kryspin-Exner I, Bauer H, Moser E, 2009. A resting state network in the motor control circuit of the basal ganglia. BMC Neurosci. 10, 137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose M, Haider H, Salari N, Buchel C, 2011. Functional dissociation of hippocampal mechanism during implicit learning based on the domain of associations. J. Neurosci. Off. J. Soc. Neurosci 31, 13739–13745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendan HE, Searl MM, Melrose RJ, Stern CE, 2003. An fMRI study of the role of the medial temporal lobe in implicit and explicit sequence learning. Neuron 37, 1013–1025. [DOI] [PubMed] [Google Scholar]
- Steele CJ, Penhune VB, 2010. Specific increases within global decreases: a functional magnetic resonance imaging investigation of five days of motor sequence learning. J. Neurosci 30, 8332–8341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun FT, Miller LM, Rao AA., D’Esposito M, 2007. Functional connectivity of cortical networks involved in bimanual motor sequence learning. Cereb. Cortex 17, 1227–1234. [DOI] [PubMed] [Google Scholar]
- Tamas Kincses Z, Johansen-Berg H, Tomassini V, Bosnell R, Matthews PM, Beckmann CF, 2008. Model-free characterization of brain functional networks for motor sequence learning using fMRI. Neuroimage 39, 1950–1958. [DOI] [PubMed] [Google Scholar]
- Tononi G, Sporns O, Edelman GM, 1994. A measure for brain complexity: relating functional segregation and integration in the nervous system. Proc. Natl. Acad. Sci. U. S. A 91, 5033–5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahdat S, Darainy M, Milner TE, Ostry DJ, 2011. Functionally specific changes in resting-state sensorimotor networks after motor learning. J. Neurosci 31, 16907–16915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela F, Lachaux JP, Rodriguez E, Martinerie J, 2001The brainweb: phase synchronization and large-scale integration. Nat. Rev. Neurosci 2, 229–239. [DOI] [PubMed] [Google Scholar]
- Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R, 2002. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron 35, 205–211. [DOI] [PubMed] [Google Scholar]
- Walker MP, Stickgold R, Alsop D, Gaab N, Schlaug G, 2005. Sleep-dependent motor memory plasticity in the human brain. Neuroscience 133, 911–917. [DOI] [PubMed] [Google Scholar]
- Wang Z, Liu J, Zhong N, Qin Y, Zhou H, Li K, 2012. Changes in the brain intrinsic organization in both on-task state and post-task resting state. NeuroImage 62, 394–407. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL, 1994. Reactivation of hippocampal ensemble memories during sleep. Science (New York, N.Y.) 265, 676–679. [DOI] [PubMed] [Google Scholar]
- Witt K, Margraf N, Bieber C, Born J, Deuschl G, 2010. Sleep consolidates the effectorindependent representation of a motor skill. Neuroscience 171, 227–234. [DOI] [PubMed] [Google Scholar]