Abstract
The all-thiophene-based double helix DH-1 was designed and prepared originally from the selective deprotonation of cyclooctatetrathiophene (tetra[3,4]thienylene, COTh) and following the Negishi coupling reaction with 3,3′-bithiophene. The X-ray crystallographic studies revealed that DH-1 has a double-helical scaffold. The arylations including tetraphenylation and tetrathienylation were efficiently employed to replace the four α-protons of the central COTh of DH-1 with phenyl and thiophenyl groups via cross-coupling reactions. The chiral resolution of rac-DH-1 was fulfilled via chiral high-performance liquid chromatography, and the chiroptical properties were characterized by circular dichroism spectra and optical rotation. Ultraviolet–visible absorption and fluorescence behaviors of DH-1 and its arylation products were also characterized to describe the extended conjugated scaffold.
Introduction
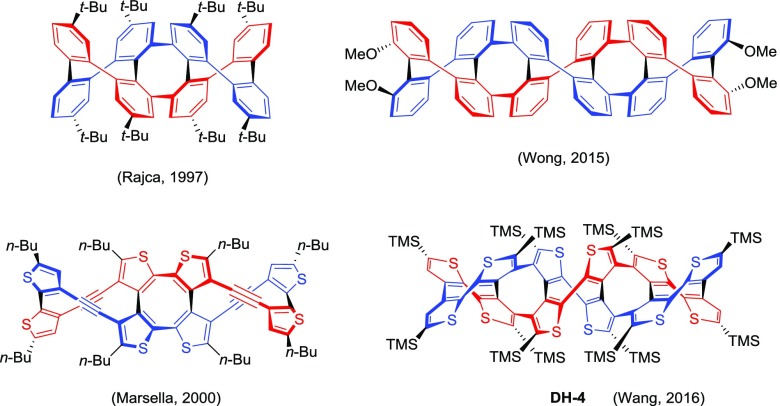
Organic double helices are one of the significant species because of their characteristic molecular configurations and extensive applications. Structurally, double helices are constituted of two flexible helical molecular chains which are linked by multiple modes, such as the van der Waals forces,1 hydrogen bonds,2 π–π intermolecular interactions,3 coordinate bonds,4 and covalent bonds.5 Among these, covalent bond-linked double helices could exhibit higher stability and could be more practical for applications such as chiral materials and catalysis. As their pioneering work, Rajca6 and Marsella7 reported covalent bond-linked double helices on the basis of two tetraphenyl and tetrathienyl chains (Figure 1) in 1997 and 2000, respectively. As the continuation of the work, Wong8 and Wang9 extended the helical chains along with the horizontal (one-dimensional) trend recently and obtained two longest double helices, which contain two hexaphenyl and hexathienyl chains (Figure 1), respectively. However, the two-dimensional cross-derivatization of the double helices have been rarely reported. One of the main reasons is that the reported double helices including tetra- and hexa-aryl double helices do not have applicable active Ar–H for further vertical derivatization.
Figure 1.
Molecular structures of covalent bond-linked double helices based on phenyl and thienyl chains.
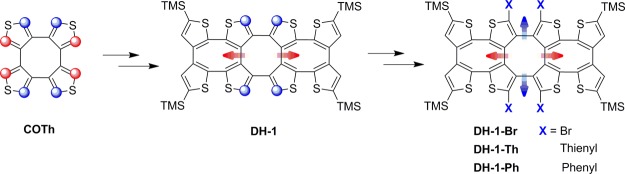
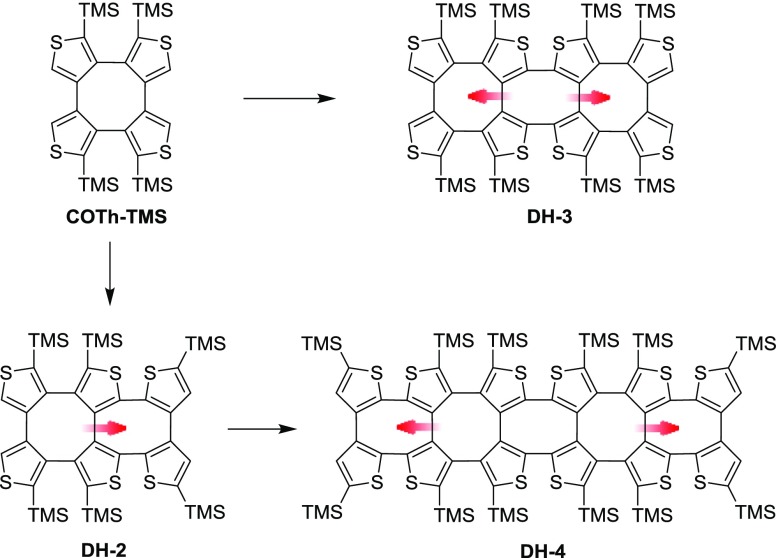
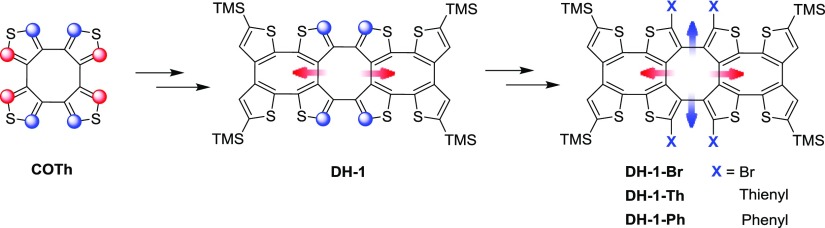
In our previous work, the high selectivity for ipsilateral deprotonation of COTh was studied, and a stepwise lithiation procedure was proved.9,10 For utilizing this selective deprotonation, three one-dimensional double-helical shaped oligothiophenes were synthesized (Figure 2).9 Meanwhile, a systematic synthetic strategy of one-dimensional oligothiophenes based on the selective deprotonation of COTh was empolyed. As the extension to these amazing works, herein, we have designed and synthesized a novel one-dimensional thiophene-based double helix (DH-1) by the Negishi coupling reaction between bithiophene and COTh. DH-1 has four active α-H on the central COTh unit for further two-dimensional derivatization. Besides the efficient chiral resolution of DH-1, the tetrabromination and tetra-arylation have effectively occurred at the central COTh unit of DH-1 (Figure 3). Furthermore, the crystal structure of DH-1 and the spectroscopic properties of DH-1 and its aryl derivatives are also described.
Figure 2.
Reported double helices, the horizontal derivatives from COTh–TMS, as building blocks.
Figure 3.
Molecular structures of the target double helix (DH-1) and its two-dimensional derivatives.
Results and Discussion
Syntheses of DH-1 and Its Arylation
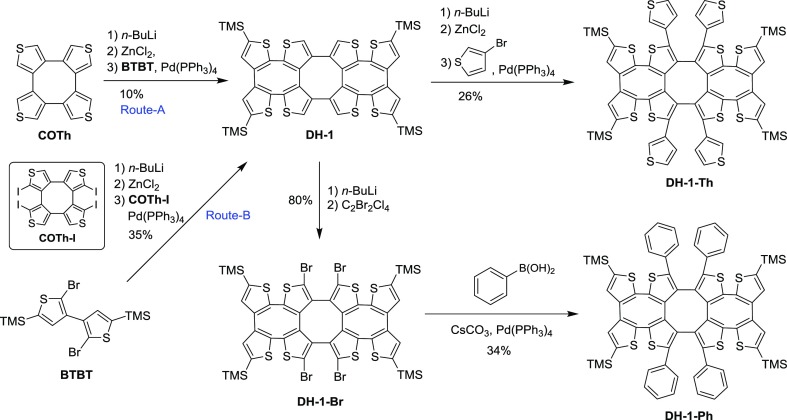
There are two synthetic routes to DH-1. According to the ipsilateral selectivity of deprotonation of COTh in the presence of n-BuLi in tetrahydrofuran (THF),9,10 the obtained tetralithiated intermediate was converted to an aryl zinc intermediate in Route-A (Scheme 1) for a further Negishi reaction with 2,2′-dibromo-5,5′-bistrimethylsilanyl[3,3′]bithiophenyl (BTBT) at 120 °C. Such a palladium-catalyzed cross-coupling generated DH-1 in a low yield of 10%. The low yield might be from the steric hindrance of two ZnCl groups and the weak activity of the C–Br bond of BTBT in the Negishi reaction.
Scheme 1. Synthetic Routes to DH-1 and Its Aryl Derivatives.
To avoid the adverse factors and increase the reaction yield, the Negishi reagent was changed from the COTh zinc intermediate to the BTBT zinc intermediate and then reacted with COTh–I(10) (Route-B, Scheme 1). Under the same palladium-catalyzed reaction conditions as shown in Route-A, the yield of DH-1 was enhanced from 10% to 35%. The improvement of the synthetic yield may be attributed to the higher reactivity of the C–I bond from the aryl iodide.
Arylation of DH-1 is very important to the double helix, so that the functionalized derivatives may be employed in some fields, such as opt-electric materials, supermolecular chemistry, and so on. The big challenge is in the organic synthesis itself because of the activity of the four α-H on the central COTh unit of DH-1 and the steric hindrance for bond formation. In our case, DH-1 was first treated with n-BuLi in THF at −78 °C. After the deprotonation at 60 °C for 2 h and the following quenching with 1,2-dibromotetrachloroethane, DH-1–Br was obtained in 80% yield (Scheme 1). The respective results indicate that α-H on the central COTh unit of DH-1 have enough activity for deprotonation, which is helpful for further vertical derivatization.
The tetrathienylation of DH-1 took place between the organozinc intermediate prepared from tetradeprotonated DH-1 and 3-bromothiophene in the presence of Pd(PPh3)4. Such a coupling reaction generated DH-1–Th in a yield of 26%. Besides the Negishi coupling, Suzuki reaction was also employed for tetraphenylation. The reaction of DH-1–Br and phenylboronic acid in the presence of CsCO3 and Pd(PPh3)4 was performed, and the target product DH-1–Ph was yielded in 34% (Scheme 1).
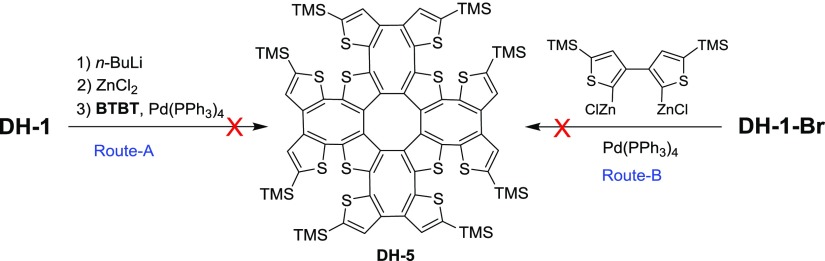
With the good successes of bromination and arylation of DH-1, a more synthetic challenging work for making a cross-double helix, DH-5, is of high interest to us because of its cross-dimensional double-helical configuration. Referred to the reaction conditions of thienylation and phenylation of DH-1, two approaches (Route-A and Route-B, Scheme 2) from DH-1 and DH-1–Br under the conditions of Negishi reaction were empolyed to synthesize DH-5. Unfortunately, DH-5 has not been produced after many attempts by changing the reaction conditions. One of the possible reasons is the torsional strain of the double-helical skeleton, which may be too huge to lead the harsh building of the new vertical COThs.
Scheme 2. Unsuccessful Synthetic Routes to DH-5.
X-ray Crystal Structure Analyses of DH-1
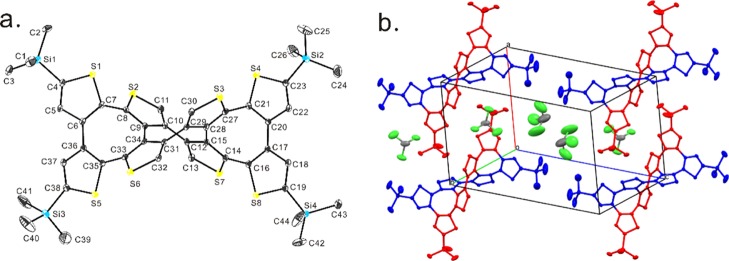
The single crystal of DH-1 was obtained by slow evaporation from the chloroform/methanol (1:3, v/v) solution. Its crystal belongs to the triclinic space group P1̅. The crystal structure of DH-1 is shown in Figure 4a, from which the double-helical molecular configuration can be visualized. Similar to the reported DH-3,9DH-1 has three COTh moieties, which are sequentially annelated together in the molecule, and the two quadruple thiophene chains are intertwined with each other via the C–C single bonds. Different from the case of DH-3, which has one tetra[2,3]thienylene and two tetra[3,4]thienylene,9 however, DH-1 shows an alternation of two tetra[2,3]thienylene and one tetra[3,4]thienylene. Additionally, the molecular length of DH-1 is 12.325 Å (C5···C22), which is longer than that of DH-3 (11.921 Å). Simultaneously, the average value of the dihedral angles of the two terminal thiophene rings of each strand in the double-helical oligomer is decreased from 196° of DH-3 to 168° of DH-1. These correlative changes are due to the twist degree of the COTh core of each double helix, which is influenced by the steric hindrance of the introduced TMS groups.
Figure 4.
X-ray crystal structures (a) and crystal packing (b) of DH-1. Carbon, silicon, sulfur, and chlorine atoms are depicted with the thermal ellipsoids set at the 30% probability level. All hydrogen atoms are omitted for clarity.
Figure 4b shows the packing of DH-1. Two pairs of enantiomers are stacked in a unit cell. Multiple interactions between the molecules of the CHCl3 solvent and the molecules of DH-1 lend the molecular arrangements, such as Cl3···C18 (3.421 Å), Cl4···H24C (2.848 Å), H46A···S1 (2.892 Å), and H46A···H2B (2.358 Å). It is clear that the CHCl3 solvent shows an important factor for the formation of the DH-1 crystal.
Resolution of Double Helix rac-DH-1 and the Chiroptical Properties
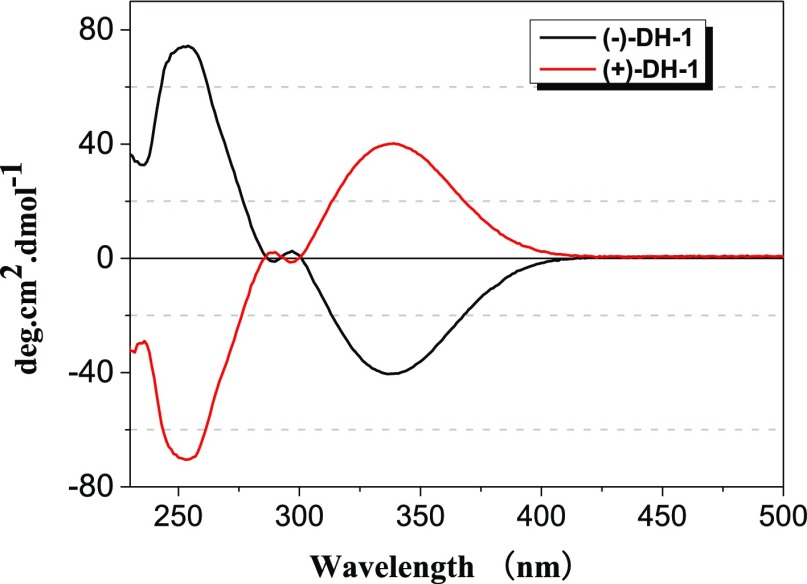
The resolution of rac-DH-1 was carried out by chiral high-performance liquid chromatography, with n-hexane/isopropanol (99.5:0.5, v/v) as the eluent. The two enantiomers were obtained on a semipreparative-scale chiral column (Figure S17, Supporting Information). From the 5 mg scale of rac-DH-1, 2.1 mg (ee = 84%) of (+)-DH-1 and 1.2 mg (ee = 89%) of (−)-DH-1 were obtained. The optical rotations of (+)-DH-1, [α]D23 = +200° (c = 0.0013 g/mL in dichloromethane) and (−)-DH-1, [α]D = −210° (c = 0.0013 g/mL in dichloromethane) were observed, respectively. The circular dichroism (CD) spectra of (+)-DH-1 and (−)-DH-1 were taken in dichloromethane. Two enantiomers, (+)-DH-1 and (−)-DH-1, show nearly identical symmetric image spectra (Figure 5).
Figure 5.
CD spectra of (−)-DH-1 (black line, ee = 89%, 1.4 × 10–5 M in dichloromethane) and (+)-DH-1 (red line, ee = 84%, 1.4 × 10–5 M in dichloromethane) at room temperature.
Electronic Absorption Spectra of DH-1 and the Arylation Products
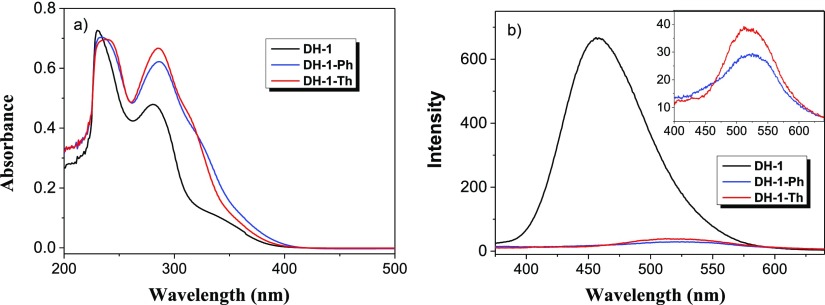
The ultraviolet–visible (UV–vis) absorption spectra of DH-1, DH-1–Th, and DH-1–Ph in dichloromethane are shown in Figure 6a. DH-1 has two major absorption peaks at 231 and 282 nm and a weak absorption band peaked at 344 nm. In the absorption spectra of DH-1–Th, the introduction of thienyl leads the major absorption peaks bathochromic-shifted to 239 and 286 nm compared with the case of DH-1. In the case of DH-1–Ph, its absorption behavior is very similar to that of DH-1–Th. Compared with the case of DH-1, DH-1–Ph and DH-1–Th show high intensity and broad absorption band in the long-wavelength region. Such spectroscopic behaviors are attributed to the extended conjugations of DH-1–Ph and DH-1–Th. Meanwhile, the phenyl groups and thiophenyl groups show conjugation effects similar to that of DH-1 because of their twist configurations in the molecules of DH-1–Ph and DH-1–Th.
Figure 6.
(a) UV–vis spectra of DH-1, DH-1–Ph, and DH-1–Th in dichloromethane ([C] = 1 × 10–5 M). (b) Fluorescence spectra of DH-1, DH-1–Ph, and DH-1–Th in dichloromethane ([C] = 1 × 10–5 M), λex = 330 nm, slit: 5/5.
The fluorescence behaviors of DH-1, DH-1–Ph, and DH-1–Th in dichloromethane are also characterized. As shown in Figure 6b, DH-1 shows a much strong emission peaked at 457 nm. Compared with the case of DH-1, DH-1–Ph and DH-1–Th exhibit a much weak emission, and their emission peaks are bathochromic-shifted to 525 and 516 nm, respectively. The obvious bathochromic shifts of emission are attributed to the increased conjugation extension of DH-1–Ph and DH-1–Th. The single bond rotation between the aryl groups and the central COTh unit might be the main reason for the increase in the nonradiative deactivation processes and decrease in the intensity of emission.
Theoretical Calculations for Electronic Absorption Spectra of DH-1 and the Arylation Products
To obtain the effects of molecular structure and electronic structure on the spectroscopic properties of DH-1 and its derivatives, their electronic structures and excited-state calculations were performed by density functional theory (DFT) and time-dependent DFT calculations using Gaussian 09 program package.11 The geometries were fully optimized at the B3LYP/6-31G* level of theory.12 Subsequently, frequency analysis was also performed at the same level of theory to ensure that the structures correspond to a true minimum. On the basis of the optimized geometries, the excited-state calculations were performed by using the MPW1B95 functional13 and the polarizable continuum model14 at the 6-31G* level. As depicted in Figure S18 (Supporting Information) and Figure 6a, the shapes and positions of absorption peaks (listed in Table S2, Supporting Information) from the predicted UV–vis spectra were consistent with the experimental curves. The excitation energies, oscillator strengths, and transition contributions of the most important states are listed in Table S2. In the predicted UV–vis spectrum of DH-1, the two major absorption peaks located at 231 and 282 nm were in agreement with the experimental results. Further, the largest contributions to the major absorption peaks were attributed to S0 → S37 and S0 → S7, respectively. The lowest energy absorption band of DH-1 was associated to the S0 → S2 transition, which was dominated by the highest occupied molecular orbital (HOMO) – 1 → lowest occupied molecular orbital (LUMO) + 1 and HOMO → LUMO transitions. Compared with DH-1, there were slight bathochromic shifts for the values of the two major absorption peaks in the spectra of DH-1–Ph and DH-1–Th. Further, the high-intensity absorption bands in the long-wavelength region in DH-1–Ph and DH-1–Th were attributed to the large transition dipole moments between S0 and S2, as listed in Table S2.
Conclusions
In summary, we have explored the efficient synthesis of the all-thiophene-based double helix, DH-1, and studied its crystal structure, chiral resolution, and chiroptical property. Such covalent bond-linked double helices could be more practical for applications such as chiral materials and catalysis after functionalization. Furthermore, bromination and arylation efficiently occur at the four active α-positions on the central COTh unit of DH-1, which means DH-1 is a potential candidate for making the amazing two-dimensional cross-double helix, DH-5. It has been proved in our current work that making DH-5 is a huge challenge, but the attempts have not been given up in our lab.
Experimental Section
General Procedures and Materials
THF was freshly distilled from sodium/benzophenone prior to use. The compound BTBT was prepared according to our previous research work.15 The concentration of n-BuLi (in hexane) was determined by titration with N-pivaloyl-o-toluidine.16 Silica gel (300–400 mesh) was employed for column chromatography. Analytical thin-layer chromatography was performed on glass plates of silica gel GF-254 with UV detection. The standard techniques for synthesis under an inert atmosphere, using gasbag and Schlenk glassware equipped with an 8 mm polytetrafluoroethylene vacuum stopcock, were employed. All starting materials and reagents were commercially available. The two enantiomers (+)-DH-1 and (−)-DH-1 were obtained on a semipreparative-scale chiral column (RC-SCDP5).
The proton nuclear magnetic resonance (1H NMR) and carbon (13C) NMR spectra were recorded on a 400 MHz NMR instrument using CDCl3 as the solvent. The IR spectra were obtained using a Fourier transform infrared instrument. High-resolution mass spectrometry (HRMS) analysis was carried out on a mass spectrometer equipped with an FTMS and LTQ FT Ultra. The melting point was determined on a Melt-Temp apparatus and was uncorrected. A slow evaporation of the solutions of DH-1 in CHCl3–CH3OH (1:3, v/v) was employed for growing single crystals. The X-ray crystallographic analysis was performed using the crystal of DH-1 with the size 0.33 × 0.21 × 0.17 mm3. The intensity data were collected with the ω scan mode (296 K) on a diffractometer with a CCD detector using Mo Kα radiation (λ = 0.71073 Å). The data were corrected for Lorentz and polarization effects, and the absorption corrections were performed using the SADABS program.17 The crystal structures were solved using the SHELXTL program and refined using full-matrix least-squares.18 Further details are deposited in the CIFs (see the Supporting Information).
Synthesis of DH-1
Method A
To a solution of COTh (104 mg, 0.32 mmol) in dry THF (8 mL), n-BuLi (0.58 mL, 2.33 M in hexane, 1.35 mmol, 4.5 equiv) was added dropwise at −78 °C. The reaction temperature was allowed to warm to room temperature and then was heated to 60 °C. After 2 h, ZnCl2 (205 mg, 1.5 mmol, 5.0 equiv) was added at −78 °C, and then the reaction mixture was warmed to room temperature. The prepared organozinc was added to the mixture of BTBT (282 mg, 0.60 mmol, 2.0 equiv), Pd(PPh3)4 (35 mg, 0.03 mmol, 0.1 equiv), and THF (5 mL) in a glovebox. The reaction mixture was heated to 120 °C for 48 h and was quenched with methanol at 0 °C. The mixture was extracted with CH2Cl2 (3 × 10 mL) and then washed with saturated NaCl (10 mL) and water (2 × 10 mL). After the mixture dried over MgSO4, the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/CHCl3 (4/1) as a white solid to yield DH-1 (28 mg, 10%). mp: >300 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.21 (s, 4 H), 7.09 (s, 4 H), 0.34 (s, 36 H). 13C NMR (100 MHz, CDCl3): δ 142.31, 138.53, 138.47, 137.59, 136.28, 135.19, 133.53, 126.98, 0.02. MS (EI, 70 eV) m/z: 922.16 [M+]. HRMS (MALDI MS EI+) m/z: calcd for [C44H44Si4S8+], 940.0286; found, 940.0280. IR (KBr): 2954 cm–1 (C–H).
Method B
To a solution of BTBT (105 mg, 0.22 mmol) in dry THF (10 mL), n-BuLi (0.19 mL, 2.4 M in hexane, 0.46 mmol, 2.05 equiv) was added dropwise at −78 °C. After the temperature was maintained at −78 °C for 2 h, ZnCl2 (64 mg, 0.47 mmol, 2.1 equiv) was added at −78 °C, and the reaction temperature was warmed to room temperature. The prepared organozinc was added to the mixture of COTh–I (281.6 mg, 0.60 mmol, 2.0 equiv), Pd(PPh3)4 (25 mg, 0.02 mmol, 0.1 equiv), and THF (5 mL) in a glovebox. The reaction mixture was heated to 120 °C for 48 h and was quenched with methanol at 0 °C. The mixture was extracted with CH2Cl2 (3 × 10 mL) and then washed with saturated NaCl (10 mL) and water (2 × 10 mL). After the mixture dried over MgSO4, the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/CH2Cl2 (5/1) as a white solid to yield DH-1 (28.3 mg, 35%).
Synthesis of DH-1–Br
To a solution of DH-1 (35 mg, 0.04 mmol) in dry THF (15 mL), n-BuLi (0.06 mL, 2.4 M in hexane, 0.15 mmol, 4.1 equiv) was added dropwise at −78 °C. The reaction temperature was allowed to warm to room temperature and then was heated to 60 °C. After 2 h, C2Br2Cl4 (50 mg, 0.15 mmol, 4.1 equiv) was added at −78 °C, and then the reaction mixture was slowly warmed to room temperature overnight. The mixture was quenched with methanol at 0 °C, extracted with CH2Cl2 (3 × 10 mL), and then washed with saturated NaCl (10 mL) and water (2 × 10 mL). After the mixture dried over MgSO4, the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/CHCl3 (4/1) as a white solid to yield DH-1–Br (37 mg, 80%). mp: >300 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.07 (s, 4 H), 0.34 (s, 36 H). 13C NMR (100 MHz, CDCl3): δ 143.34, 136.39, 1350.82, 135.62, 135.02, 133.95, 115.88, −0.01. HRMS (ESI) m/z: calcd for [C44H41Br4Si4S8+], 1252.6779; found, 1252.6848. IR (KBr): 2953 cm–1 (C–H).
Synthesis of DH-1–Th
To a solution of DH-1 (20 mg, 0.21 mmol) in dry THF (10 mL), n-BuLi (0.04 mL, 2.5 M in hexane, 0.1 mmol, 4.5 equiv) was added dropwise at −78 °C. The reaction temperature was allowed to warm to room temperature and then was heated to 60 °C. After 2 h, ZnCl2 (14.5 mg, 0.11 mmol, 5.0 equiv) was added at −78 °C, and then the reaction mixture was warmed to room temperature. The prepared organozinc was added to the mixture of 3-bromothiophene (17 mg, 0.11 mmol, 5.0 equiv), Pd(PPh3)4 (2.5 mg, 0.02 mmol, 0.1 equiv), and THF (5 mL) in a glovebox. The reaction mixture was heated to 120 °C for 48 h and was quenched with methanol at 0 °C. The mixture was extracted with CH2Cl2 (3 × 10 mL) and then washed with saturated NaCl (10 mL) and water (2 × 10 mL). After the mixture dried over MgSO4, the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/CH2Cl2 (5/1) as a white solid to yield DH-1–Th (7 mg, 26%). mp: >300 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.20 (s, 4H), 6.84 (dd, J = 5.0, 3.0 Hz, 4H), 6.50 (dd, J = 2.9, 1.1 Hz, 4H), 6.39 (dd, J = 5.0, 1.1 Hz, 4H), 0.37 (s, 36H). 13C NMR (100 MHz, CDCl3): δ 144.49, 142.53, 138.84, 138.68, 137.41, 136.28, 133.60, 133.43, 131.53, 128.61, 127.79, 127.11, 0.05. HRMS (ESI) m/z: calcd for [C60H53Si4S12+], 1268.9867; found, 1268.9887. IR (KBr): 2955 cm–1 (C–H).
Synthesis of DH-1–Ph
The mixture of DH-1–Br (24 mg, 0.02 mmol), phenylboronic acid (12.3 mg, 0.1 mmol, 5 equiv), Pd(PPh3)4 (9 mg, 0.008 mmol, 0.4 equiv), and Cs2CO3 (65 mg, 0.2 mmol, 10 equiv) in THF (10 mL) and degassed H2O (0.2 mL) was heated to 100 °C. The mixture was extracted with CH2Cl2 (3 × 10 mL) and then washed with saturated NaCl (10 mL) and water (2 × 10 mL). After the mixture dried over MgSO4, the solvent was removed under vacuum. The residue was purified by column chromatography on silica gel with petrol ether (60–90 °C)/CH2Cl2 (5/1) as a white solid to yield DH-1–Ph (9 mg, 34%). mp >300 °C. 1H NMR (CDCl3, 400 MHz): δ (ppm) 7.24 (s, 1H), 7.00 (t, J = 7.4 Hz, 1H), 6.79 (t, J = 7.7 Hz, 2H), 6.56 (d, J = 7.5 Hz, 2H), 0.39 (s, 8H). 13C NMR (100 MHz, CDCl3): δ 142.74, 139.05, 138.79, 138.01, 137.14, 136.29, 133.95, 132.88, 130.85, 127.04, 124.75, 122.07, 0.02. HRMS (ESI) m/z: calcd for [C68H61Si4S8+], 1245.1611; found, 1245.1615. IR (KBr): 2955 cm–1 (C–H).
Acknowledgments
This research was supported by NSFC (21672054, 21672053, and 21703055) as well as Innovation Scientists and Technicians Troop Construction Projects of Henan Province (C20150011).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02492.
Experimental procedures and characterization data for all compounds (CIF)
Experimental procedures and characterization data for all compounds NMR, HRMS, and IR spectra of DH-1, DH-1–Br, DH-1–Ph, DH-1–Th; CIF of rac-DH-1; and calculated absorption spectra of DH-1, DH-1–Ph, and DH-1–Th in dichloromethane solution using TDDFT/PCM approaches (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Schomaker E.; Challa G. Complexation of stereoregular poly (methyl methacrylates). 14. The basic structure of the stereocomplex of isotactic and syndiotactic poly (methyl methacrylate). Macromolecules 1989, 22, 3337–3341. 10.1021/ma00198a025. [DOI] [Google Scholar]; b Kumaki J.; Kawauchi T.; Yashima E. Two-dimensional folded chain crystals of a synthetic polymer in a Langmuir-Blodgett film. J. Am. Chem. Soc. 2005, 127, 5788–5789. 10.1021/ja050457e. [DOI] [PubMed] [Google Scholar]; c Kumaki J.; Kawauchi T.; Okoshi K.; Kusanagi H.; Yashima E. Supramolecular helical structure of the stereocomplex composed of complementary isotactic and syndiotactic poly (methyl methacrylates) as revealed by atomic force microscopy. Angew. Chem., Int. Ed. 2007, 46, 5348–5351. 10.1002/anie.200700455. [DOI] [PubMed] [Google Scholar]
- a Tanaka Y.; Katagiri H.; Furusho Y.; Yashima E. A modular strategy to artificial double helices. Angew. Chem., Int. Ed. 2005, 44, 3867–3870. 10.1002/anie.200501028. [DOI] [PubMed] [Google Scholar]; b Ito H.; Ikeda M.; Hasegawa T.; Furusho Y.; Yashima E. Synthesis of complementary double-stranded helical oligomers through chiral and achiral amidinium-carboxylate salt bridges and chiral amplification in their double-helix formation. J. Am. Chem. Soc. 2011, 133, 3419–3432. 10.1021/ja108514t. [DOI] [PubMed] [Google Scholar]; c Hasegawa T.; Furusho Y.; Katagiri H.; Yashima E. Enantioselective synthesis of complementary double-helical molecules that catalyze asymmetric reactions. Angew. Chem., Int. Ed. 2007, 46, 5885–5888. 10.1002/anie.200701735. [DOI] [PubMed] [Google Scholar]; d Miwa K.; Furusho Y.; Yashima E. Ion-triggered spring-like motion of a double helicate accompanied by anisotropic twisting. Nat. Chem. 2010, 2, 444–449. 10.1038/nchem.649. [DOI] [PubMed] [Google Scholar]; e Li J.; Wisner J. A.; Jennings M. C. A self-associating ADADA hydrogen-bonded double helix. Org. Lett. 2007, 9, 3267–3269. 10.1021/ol071171c. [DOI] [PubMed] [Google Scholar]
- a Berl V.; Huc I.; Khoury R. G.; Krische M. J.; Lehn J.-M. Interconversion of single and double helices formed from synthetic molecular strands. Nature 2000, 407, 720–723. 10.1038/35037545. [DOI] [PubMed] [Google Scholar]; b Berl V.; Huc I.; Khoury R. G.; Lehn J.-M. Helical molecular programming: supramolecular double helices by dimerization of helical oligopyridine-dicarboxamide strands. Chem.—Eur. J. 2001, 7, 2810–2820. . [DOI] [PubMed] [Google Scholar]
- a Lehn J. M.; Rigault A.; Siegel J.; Harrowfield J.; Chevrier B.; Moras D. Spontaneous assembly of double-stranded helicates from oligobipyridine ligands and copper (I) cations: structure of an inorganic double helix. Proc. Natl. Acad. Sci. U.S.A. 1987, 84, 2565–2569. 10.1073/pnas.84.9.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lehn J.-M.; Rigault A. Helicates: tetra- and pentanuclear double helix complexes of CuI and poly (bipyridine) strands. Angew. Chem., Int. Ed. Engl. 1988, 27, 1095–1097. 10.1002/anie.198810951. [DOI] [Google Scholar]; c Hasenknopf B.; Lehn J.-M.; Kneisel B. O.; Baum G.; Fenske D. Self-assembly of a circular double helicate. Angew. Chem., Int. Ed. Engl. 1996, 35, 1838–1840. 10.1002/anie.199618381. [DOI] [Google Scholar]; d Hasenknopf B.; Lehn J. M.; Baum G.; Fenske D. Self-assembly of a heteroduplex helicate from two different ligand strands and Cu(II) cations. Proc. Natl. Acad. Sci. U.S.A. 1996, 93, 1397–1400. 10.1073/pnas.93.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Hasenknopf B.; Lehn J.-M.; Kneisel B. O.; Baum G.; Fenske D. Self-assembly of a circular double helicate. Angew. Chem., Int. Ed. Engl. 1996, 35, 1838–1840. 10.1002/anie.199618381. [DOI] [Google Scholar]
- a Mascal M.; Wood I. G.; Begley M. J.; Batsanov A. S.; Walsgrove T.; Slawin A. M. Z.; Williams D. J.; Drake A. F.; Siligardi G. Indole-fused tetraazacyclooctadecanes: double-helical macrocycles. J. Chem. Soc., Perkin Trans. 1 1996, 2427–2433. 10.1039/p19960002427. [DOI] [Google Scholar]; b An D. L.; Nakano T.; Orita A.; Otera J. Enantiopure double-helical alkynyl cyclophanes. Angew. Chem., Int. Ed. 2002, 41, 171–173. . [DOI] [PubMed] [Google Scholar]; c Nakatani Y.; Furusho Y.; Yashima E. Synthesis of helically twisted [1+1]macrocycles assisted by amidinium-carboxylate salt bridges and control of their chiroptical properties. Org. Biomol. Chem. 2013, 11, 1614–1623. 10.1039/c2ob27054d. [DOI] [PubMed] [Google Scholar]
- Rajca A.; Safronov A.; Rajca S.; Shoemaker R. Double helical octaphenylene. Angew. Chem., Int. Ed. Engl. 1997, 36, 488–491. 10.1002/anie.199704881. [DOI] [Google Scholar]
- Marsella M. J.; Kim I. T.; Tham F. Toward conjugated double helical ladder polymers: cyclooctatetrathiophene as a highly versatile double helical scaffold. J. Am. Chem. Soc. 2000, 122, 974–975. 10.1021/ja9939340. [DOI] [Google Scholar]
- Chen J.-X.; Han J.-W.; Wong H. N. C. Synthesis and Chiroptical Properties of Double-Helical (M)- and (P)-o-Oligophenylenes. Org. Lett. 2015, 17, 4296–4299. 10.1021/acs.orglett.5b02102. [DOI] [PubMed] [Google Scholar]
- Zhang S.; Liu X.; Li C.; Li L.; Song J.; Shi J.; Morton M.; Rajca S.; Rajca A.; Wang H. Thiophene-Based Double Helices: Syntheses, X-ray Structures, and Chiroptical Properties. J. Am. Chem. Soc. 2016, 138, 10002–10010. 10.1021/jacs.6b05709. [DOI] [PubMed] [Google Scholar]
- Li L.; Li B.; Li C.; Ma Z.; Xu L.; Wang H. Selective deprotonation of tetra[3,4]thienylene in the presence of n-BuLi. Org. Chem. Front. 2017, 4, 1019–1023. 10.1039/c6qo00754f. [DOI] [Google Scholar]
- Frisch M. J.; Trucks G. W.; Schlegel H. B.; Scuseria G. E.; Robb M. A.; Cheeseman J. R.; Scalmani G.; Barone V.; Mennucci B.; Petersson G. A.; Nakatsuji H.; Caricato M.; Li X.; Hratchian H. P.; Izmaylov A. F.; Bloino J.; Zheng G.; Sonnenberg J. L.; Hada M.; Ehara M.; Toyota K.; Fukuda R.; Hasegawa J.; Ishida M.; Nakajima T.; Honda Y.; Kitao O.; Nakai H.; Vreven T.; Montgomery J. A. Jr.; Peralta J. E.; Ogliaro F.; Bearpark M.; Heyd J. J.; Brothers E.; Kudin K. N.; Staroverov V. N.; Kobayashi R.; Normand J.; Raghavachari K.; Rendell A.; Burant J. C.; Iyengar S. S.; Tomasi J.; Cossi M.; Rega N.; Millam J. M.; Klene M.; Knox J. E.; Cross J. B.; Bakken V.; Adamo C.; Jaramillo J.; Gomperts R.; Stratmann R. E.; Yazyev O.; Austin A. J.; Cammi R.; Pomelli C.; Ochterski J. W.; Martin R. L.; Morokuma K.; Zakrzewski V. G.; Voth G. A.; Salvador P.; Dannenberg J. J.; Dapprich S.; Daniels A. D.; Farkas O.; Foresman J. B.; Ortiz J. V.; Cioslowski J.; Fox D. J.. Gaussian 09, D.01; Gaussian, Inc.: Wallingford, CT, 2013.
- Stephens P. J.; Devlin F. J.; Chabalowski C. F.; Frisch M. J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Phys. Chem. 1994, 98, 11623–11627. 10.1021/j100096a001. [DOI] [Google Scholar]
- Zhao Y.; Truhlar D. G. Hybrid Meta Density Functional Theory Methods for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions: The MPW1B95 and MPWB1K Models and Comparative Assessments for Hydrogen Bonding and van der Waals Interactions. J. Phys. Chem. A 2004, 108, 6908–6918. 10.1021/jp048147q. [DOI] [Google Scholar]
- Scalmani G.; Frisch M. J. Continuous surface charge polarizable continuum models of solvation. I. General formalism. J. Chem. Phys. 2010, 132, 114110–114124. 10.1063/1.3359469. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Wang Z.; Zhao D.; Wang Z.; Cheng Y.; Wang H. Efficient Synthesis of Trimethylsilyl-Substituted Dithieno[2,3-b:3′,2′-d]thiophene, Tetra[2,3-thienylene] and Hexa[2,3-thienylene] from Substituted [3,3′]Bithiophenyl. Synlett 2007, 2390–2394. 10.1055/s-2007-985582. [DOI] [Google Scholar]
- Suffert J. Simple direct titration of organolithium reagents using N-pivaloyl-o-toluidine and/or N-pivaloyl-o-benzylaniline. J. Org. Chem. 1989, 54, 509–510. 10.1021/jo00263a052. [DOI] [Google Scholar]
- Sheldrick G. M.SADABS; University of Gottingen: Germany, 1996.
- Sheldrick G. M.SHELXTL, version 5.1; Bruker Analytical X-ray Systems, Inc.: Madison, WI, 1997.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.