Abstract
Despite the significant progress, C–H arylation with aryldiazonium salts is a major challenge because of the faster rate of oxidative addition compared to the C–H insertion, leading to a deleterious homocoupling product. Recently, this limitation has been overcome by merging a photoredox catalyst with transition-metal catalysts which proceeds through a distinct single electron-transfer mechanism. However, we have observed that the photoredox catalyst is not necessary for the C–H arylation of aniline rather chemical reactivity can be controlled by tuning the electronic nature of the substrate. We report, herein, a palladium-catalyzed C–H arylation of aniline carbamates with aryldiazonium salts under external oxidant, acid, base free conditions at room temperature. Mechanistic studies suggest that the present reaction proceeds through a directed electrophilic metalation pathway which is the slowest step. However, the oxidative addition may take place through either ionic (2e–) or radical (1e–) pathway to generate hypervalent Pd(IV) or Pd(III) intermediate, respectively. A facile reductive elimination from the hypervalent palladium complex furnishes the C–H arylation product under mild conditions. The carbamate directing group is easily removed from the product to obtain the corresponding ortho-arylated aniline, which is a precursor for plethora of carbazole alkaloids and other biologically active molecules. The reaction is scaled-up to gram scale to furnish the desired product in comparable yields. Finally, we have applied this C–H arylation methodology for the synthesis of series of carbazole alkaloids such as clausine V, clauszoline K, O-methoxymahanine, and O-methylmurrayamine-D.
1. Introduction
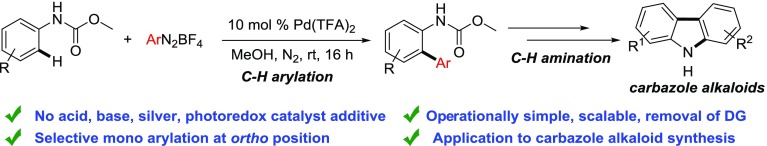
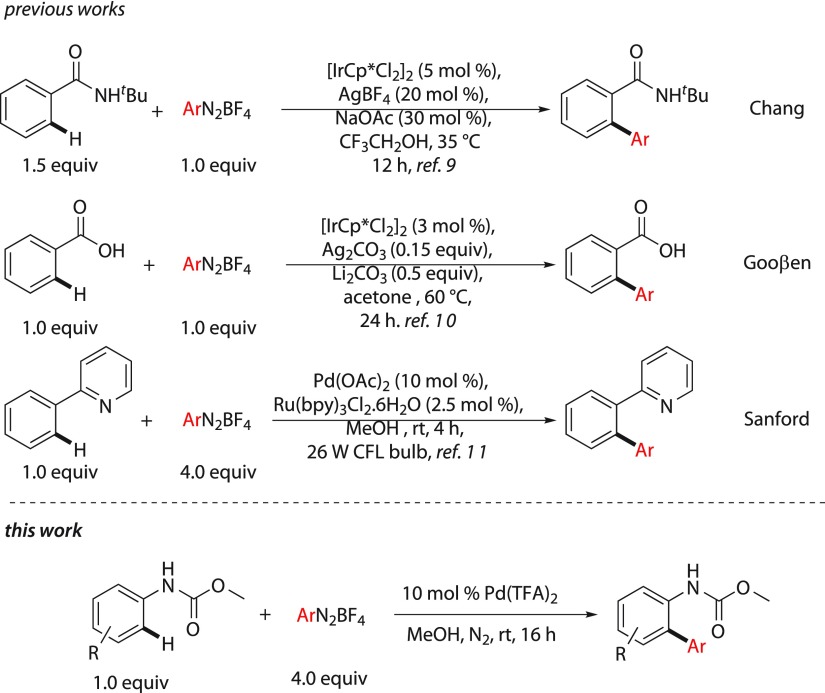
Aryldiazonium salts are a viable surrogate to the conventional aryl halides and oxygen-based electrophilic coupling partners.1 Although they have been used extensively in Heck–Matsuda arylation of alkenes, they have not been explored much in C–H arylations.2−6 This could be attributed due to the fact that oxidative addition of metal complex to the diazonium salts is extremely facile, whereas C–H insertion proceeds through a high energetic pathway at a sluggish reaction rate7,8 because the differentiation in the kinetics of deleterious side product such as homocoupling formation becomes predominant. However, the reactivity of the catalyst could be manipulated to obtain the selective cross-coupling product through C–H activation. Recently, the Chang group has reported an highly reactive iridium(III)-catalyzed C–H arylation of benzamides with aryldiazonium salts.9 The Gooßen group also reported an iridium(III)-catalyzed C–H arylation of carboxylic acids.10 The Sanford group introduced a dual catalysis concept for C–H arylation with diazonium salts by merging photoredox and palladium catalysis which proceeds through the radical pathway.11 Several other groups have also extended this concept to other molecular systems.12 Although palladium and photoredox dual catalysis has been explored, only palladium-catalyzed C–H arylation of aniline carbamates with aryldiazonium salt is not known. Previously, ortho C–H arylation of aniline derivatives was reported by the Lipshutz and the Jeganmohan group with arylboronic acids where stoichiometric metal or nonmetal oxidants were necessary.13 The Li group reported ortho-arylation of aniline carbamates with diaryliodonium salts.14 However, expensive iodonium salts, addition of strong acid additive, and requirement of moderate temperature are some of the limitations of this methodology. As a part of our research program in C–H functionalization, we have accomplished the synthesis of indoles and indolines from aryl urea through C–H activation at room temperature.15 On the other hand, we have performed chemo-, regio-, and stereoselective Heck–Matsuda reaction with allyl alcohol at room temperature.16 During Heck–Matsuda reaction, we observed that after oxidative addition to the aryldiazonium salts, a highly electrophilic palladium species is formed which undergoes alkene insertion rapidly.4e Keeping this in mind, we hypothesized that the similar electrophilic palladium may undergo a facile electrophilic C–H insertion to the electron-rich arenes under mild conditions. We report herein a palladium-catalyzed ortho C–H arylation of aniline carbamates with inexpensive and readily available aryldiazonium salts at room temperature. Interestingly, both coupling partners are derived from the inexpensive aniline derivatives where aniline carbamate acts as a nucleophile and aryldiazonium salts as an electrophile in this palladium catalysis (Scheme 1).
Scheme 1. Directed C–H Arylation with Diazonium Salts.
2. Results and Discussion
Initially, we started optimization of the reaction condition with acetanilide and phenyldiazonium salts using palladium(II) acetate. However, only a trace amount of arylation product was obtained. We realized that the ortho-position of acetanilide may not be electron-rich enough for electrophilic ortho-palladation. Therefore, we examined N,N-dimethyl phenyl urea as an aniline counterpart, which is more electron-rich than acetanilide. However, a mixture of mono- and diarylation product was obtained. Therefore, we anticipated that because of the electron-donating nature of the N,N-dimethyl group, the ortho-position becomes highly electron-rich for uncontrolled electrophilic palladation. Therefore, a less electron-rich substrate could be a better substrate for selective monoarylation. From the literature, the Li group has demonstrated aniline carbamates as a versatile and removable directing group for C–H arylation with expensive diaryliodonium salts.14 Thus, we decided to use this moderate electron-rich carbamate substrate for further optimization. As hypothesized, only monoarylation product was obtained from this aniline carbamate substrate. Subsequently, we examined several palladium catalysts, and electrophilic palladium(II) trifluoroacetate was found to be better than palladium(II) acetate (entry 4 vs 11, Table 1). Other palladium(0) catalysts provided homocoupling of the aryldiazonium salts via facile oxidative addition. Among different solvents tested, methanol was found to be optimal for this transformation. However, excess amount of diazonium salt was necessary because of the deleterious homocoupling product formation from the corresponding diazonium salts. Strikingly, we did not find any positive influence of photoredox catalysts under the irradiation of blue light-emitting diode (LED), green LED, or household CFL on this palladium-catalysis condition for this substrate. Any other oxidants, acids, bases, and so forth did not improve the yield of the reaction further. Thus, we decided to proceed for the substrate scope under this optimized condition.
Table 1. Optimization of the Reaction Conditionsa.
| entry | catalyst (10 mol %) | additive | x equiv | yield (%) of 3b |
|---|---|---|---|---|
| 1 | Pd(MeCN)4BF4 | 4 | 26 | |
| 2 | Pd(MeCN)2(OTf)2 | 4 | 27 | |
| 3 | Pd(MeCN)2(OTs)2 | 4 | 34 | |
| 4 | Pd(OAc)2 | 4 | 52 | |
| 5 | Pd(OAc)2 | Ru(bpy)3Cl2·H2O | 4 | 55 |
| 6 | Pd(TFA)2 | 4 | 58 | |
| 7 | Pd(TFA)2 | PTSA (2 equiv) | 4 | 64 |
| 8 | Pd(TFA)2 | AgOAc (1 equiv) | 4 | 50 |
| 9 | Pd(TFA)2 | Ag2CO3 (1 equiv) | 4 | 30 |
| 10 | Pd(TFA)2 | 2 | 38 | |
| 11c | Pd(TFA)2 | 4 | 68 | |
| 12d | Pd(TFA)2 | 4 | 15 | |
| 13e | Pd(TFA)2 | 4 | 20 | |
| 14 | Pd/C | 4 | <5% | |
| 15f | Pd2dba3 | 4 | <5% |
All reactions were carried out in 0.1 mmol scale.
Yields refer to here are isolated yields.
Two equiv of aryldiazonium salt added different of 30 min interval.
Isopropanol used as a solvent.
THF used as a solvent.
Homocoupling product was isolated.
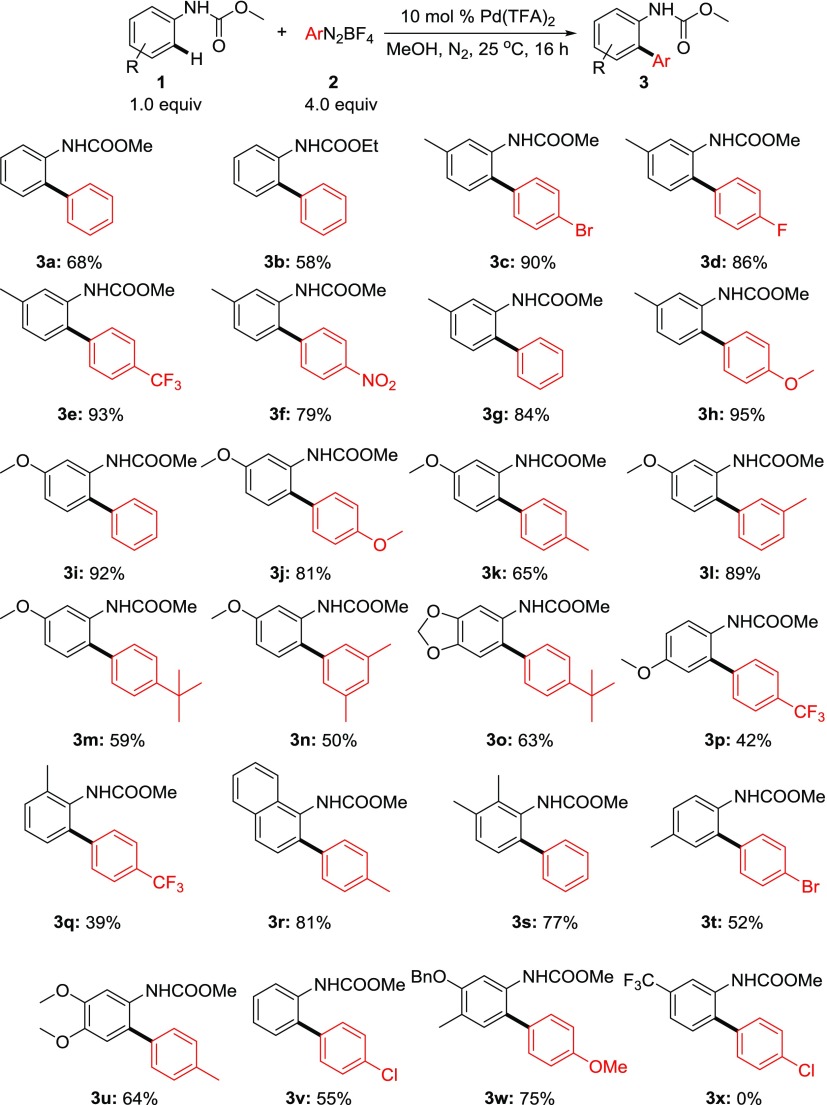
A series of aniline were converted to the corresponding methyl carbamate and examined the C–H arylation scope. Unsubstituted aniline carbamate provided the corresponding C–H arylation product in moderate to good yields (3a, 3b, and 3v, Scheme 2). However, electron-rich substrates provided the ortho C–H arylation products in excellent yields. Thus, methoxy- and methyl, dioxymethylene, 3,4-dimethoxy, and benzyloxy-substituted aniline carbamates reacted with a series of diazonium salts to furnish the desired monoarylation product. Diazonium salts containing electron-donating groups such as methoxy, methyl, tert-butyl, dimethyl groups, and so forth were an effective coupling partner (3h, 3j, 3k, 3l, 3m, 3n, 3o, 3r, 3u, and 3w, Scheme 2). In addition, electron-withdrawing groups such as trifluoromethyl, fluoro, or even nitro groups on diazonium were also proved to be an effective coupling partner for this transformation (3d, 3e, 3f, 3p, and 3q, Scheme 2). Halogens such as chloro- and bromo- on the diazonium counterpart were compatible which is useful for further cross-coupling reactions (3c, 3t, and 3v, Scheme 2). Although ortho-methyl-substituted carbamate provided moderate yield (3q and 3s, Scheme 2), sterically hindered naphthalene furnished high yield of the cross-coupling product selectively at the ortho-position where the peri C–H bond remains inert (3r, Scheme 2). Among all aniline carbamates examined meta-methoxy or meta-methyl carbamate were efficient substrates presumably because of electron donation and formation of highly nucleophilic center at the ortho-position for facile electrophilic palladation. A similar result was also observed by the Lipshutz group during C–H arylation of arylurea at room temperature using the cationic palladium catalyst.13a It was further supported by the fact that electron-withdrawing nitro-, chloro, fluoro-substituted aniline did not furnish any product. A combination of meta-methoxy carbamate and para-nitro-substituted diazonium provided the corresponding diazocoupling product because the electronic nature of the coupling partners favored diazocoupling over the oxidative addition. Similar to methyl carbamate, ethyl carbamate (3b, Scheme 2) also exhibited similar reactivity and provided the corresponding products in comparable yield. However, corresponding phenyl carbamates were found to be ineffective for this transformation. The interesting feature of this present reaction is that the anilines lacking ortho- or meta-substitution also provide the corresponding monoarylation product which are prone to undergo double arylation.
Scheme 2. Substrate Scope of Ortho C–H Arylation,
All reactions were carried out in 0.2 mmol scale.
Yields refer to the average of isolated yields.
3. Mechanistic Studies
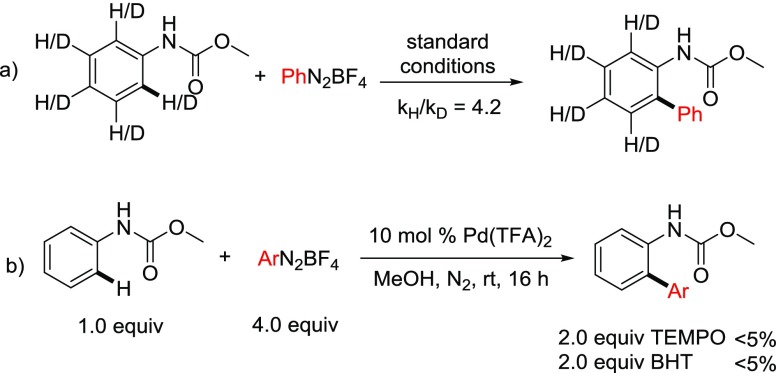
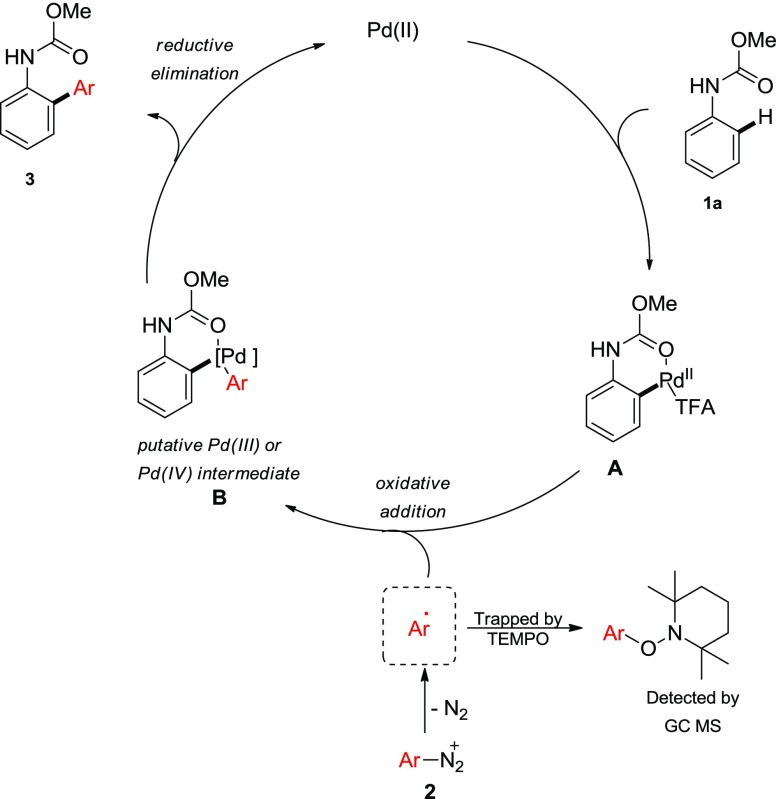
The present C–H arylation reaction at room temperature proceeds through three key steps: (1) ortho C–H activation by the cationic palladium(II) complex to form palladacycle; (2) oxidative addition of the palladacycle to aryldiazonium salt; and (3) reductive elimination to form the desired biaryl product. During optimization and substrate scope studies, we observed that N,N-dimethylanilineurea was highly reactive to provide a mixture of mono- and ortho-, ortho-diarylation product, whereas acetanilide furnishes trace amount of arylation product. Ultimately, methylcarbamate of aniline provided the ortho-selective monoarylation product. In addition, electron-rich aniline carbamates are better substrates for C–H arylation with respect to yield. Furthermore, electrophilic palladium(II) trifluoroacetate is a superior catalyst than palladium(II) acetate. However, other cationic palladium(II) complexes bearing strongly coordinating acetonitrile ligand were found to be inferior. This could be attributed due to the fact that although these complexes undergo facile electrophilic palladation, subsequent oxidative addition to aryldiazonium salts, ligand exchange, or reductive elimination steps may be inhibited by acetonitrile which is well precedented in the literature.17 A non-coordinating anion source, that is, tetrafluoroborate, is crucial for this reaction to occur. All of these data suggest that the electrophilic metalation mechanism might be operative instead of concerted metalation deprotonation. Presumably, the initial electrophilic ortho-palladation takes place with cationic palladium(II) trifluoroacetate. Subsequently, one equivalent tetrafluoroboric acid (HBF4) is generated which helps for the persistence of highly cationic palladium species without any external acid additive. Thereby, directed electrophilic ortho-palladation may take place to generate the palladacycle. Because the present arylation reaction takes place at room temperature, it is presumed that electrophilic palladation and oxidative addition steps are competitive. To determine the involvement of C–H activation in the rate-determining step, we prepared a d5-aniline carbamate (d5-1a) and subjected to the reaction conditions with equimolar quantities of aniline carbamate (1a). From this competitive experiment, the KH/KD value was determined as 4.2 (Scheme 3a). Therefore, the C–H activation step is slower than oxidative addition to aryldiazonium salts. It is further supported by the fact that a substantial amount of homocoupling product from the corresponding diazonium salt is formed through the faster oxidative addition. Typically, palladium(II)-catalyzed C–H arylation proceeds through the ionic pathway. Whereas, Sanford and others have demonstrated that a distinct single-electron process is evoke merging of palladium(II) and photoredox catalysts.11,12 When we performed the standard reaction in the presence of stoichiometric amount of radical scavengers such as TEMPO or BHT, the reaction was completely suppressed. We have also detected the TEMPO-adduct of the corresponding diazonium salt from gas chromatography mass spectrometry. However, these radical-scavenging experiments are not sufficient to propose the radical pathway at this moment and warrant further studies. In fact, recent studies revealed that a single palladium complex can also promote two distinct 2e– and 1e– processes simultaneously.18
Scheme 3. Control Experiments.
From control experiments and previous literature,11,12,13a,17 we speculate that the present C–H arylation at ambient condition initiates through the formation of arylpalladium(II) palladacycle. Subsequently, oxidative addition to the aryldiazonium salt may lead to the formation of cationic palladium(III) (via 1e– process with aryl radical from the diazonium salts) or palladium(IV) (via 2e– process) intermediate. The cationic palladium(III) species may disproportionate to generate corresponding palladium(IV) and palladium(II) species.18 The hypervalent palladium(IV) species then undergo rapid reductive elimination to provide a cross-coupling product and palladium(II) species for the subsequent runs. Thus, no external oxidant is necessary for this process (Scheme 4).
Scheme 4. Plausible Catalytic Cycle.
3.1. Gram-Scale Reaction and Deprotection
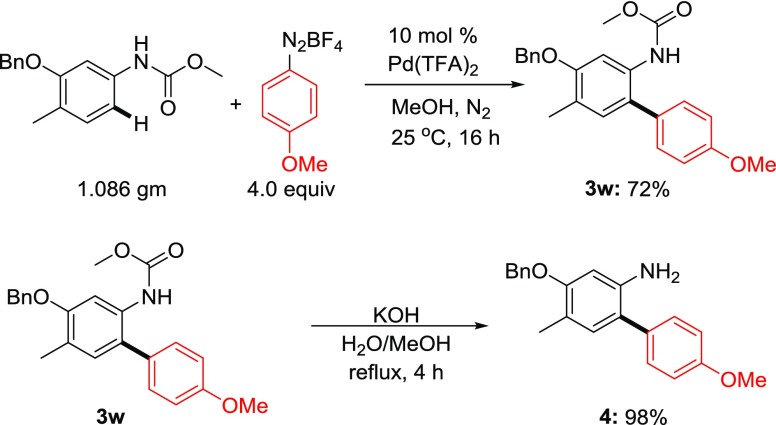
To demonstrate the synthetic utility of the present mild protocol, we have performed a C–H arylation reaction in gram scale. When carbamate 1w was subjected to the standard reaction conditions with para-methoxyphenyldiazonium salt, the corresponding cross-coupling product was obtained in comparable yields. Thus, the present reaction is reproducible in higher scale and useful for industrial process development. Further, refluxing the cross-coupling product 3w with potassium hydroxide in aqueous methanol furnished the corresponding amine in quantitative yields(Scheme 5).
Scheme 5. Gram-Scale Synthesis and Deprotection.
4. Application to the Synthesis of Carbazole Alkaloids
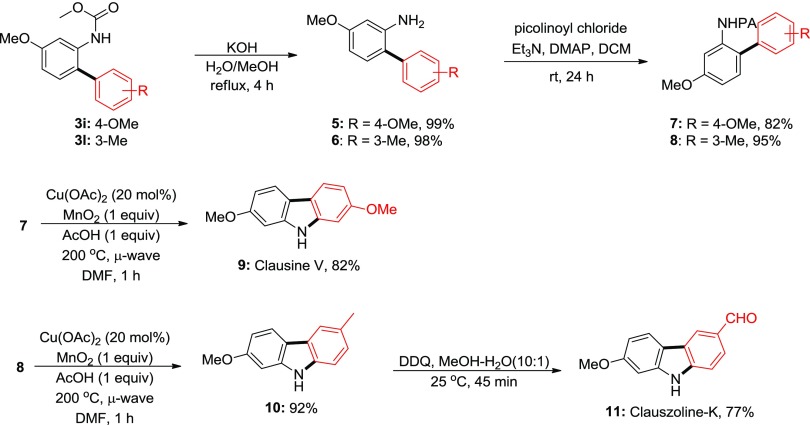
Owing to their diverse biological and photochemical properties, numerous classical and nonclassical methods have been developed for the synthesis of carbazole moiety.19 In recent years, two complementary approaches for their synthesis via C–H activation are (1) C–C bond formation to obtain ortho-aryl aniline followed by C–N bond formation20 and (2) C–N bond formation to obtain N,N-diarylaniline followed by C–C bond formation.21 Carbazole alkaloids isolated from diverse natural sources exhibit an impressive array of biological activities. Therefore, intrigued by nature, we turned our attention to apply this mild protocol for the synthesis of carbazole alkaloids. The present strategy for carbazole synthesis is the successive C–H amination of the C–H arylation product. Our initial efforts for the synthesis of carbazole directly from incipient carbamate protecting group were in vain. Therefore, we screened several other conditions for carbazole synthesis. Finally, we applied Miura’s condition for carbazole synthesis through C–H amination.22 The corresponding cross-coupling product was deprotected under alkaline condition to obtain the corresponding 2-arylaniline in quantitative yield. Subsequently, aniline was protected with 2-picolinamide, and the C–H amination reaction was performed under inexpensive copper catalysis. Gratifyingly, the picolinamide-protecting group is removed under the same reaction condition under microwave irradiation. Finally, clausine V was synthesized from the corresponding meta-anisidine as a carbamate and para-anidine as a diazonium counterpart in 4 steps, 54% overall yield, whereas Dash et al. reported in 2 steps, 51% yield.21a,23 Notably, both cross-coupling partners are originated from inexpensive anilines, which offers a practical route for carbazole alkaloid synthesis.
Subsequently, almost similar strategy was applied for the synthesis of clauszoline K.24 The C–H arylation between meta-anisidine carbamate and meta-toluidine diazonium salt provided the corresponding 3l in 89% yield. Subsequent deprotection–protection steps followed by the carbazole formation step afforded excellent yields in all steps. Finally, oxidation of the methyl group by DDQ furnished the desired clauszoline K in 77% yield and 59% overall yield in 5 steps, whereas Knölker et al. reported in 3 steps with 57% overall yield(Scheme 6).24c
Scheme 6. Synthesis of Clausine V and Clauszoline K.
In a collaborative research program, to evaluate anticancer properties, we were interested to develop a scalable, concise methodology to synthesize (±)-mahanine and related carbazole alkaloids. Originally, (−)-mahanine, a dioxygenated and pyranocarbazole alkaloid, was isolated by Narasimhan et al. from an edible plant leaf of Murraya koenigii in 1970, and recent studies show a prominant anticancer activity.25 Therefore, a significant attention has been drawn for the efficient synthesis of mahanine.26
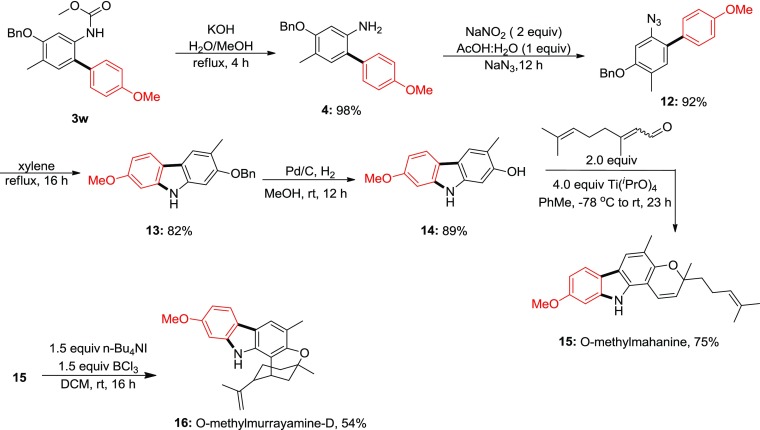
In our strategy, a substituted aniline carbamate, 3w, afforded the corresponding C–H arylation with para-methoxydiazonium salt in 75% yield. Next, the carbamate group was deprotected under alkaline condition to obtain the corresponding aniline in quantitative yield. Considering the scale-up issues under microwave irradiation, we redesigned our synthetic route for a practical synthesis of (±)-mahanine integrating conventional and robust methodology. In this approach, the aniline was converted to the corresponding azide via one-pot diazotization–azidation. Refluxing the azide compound in xylene at 140 °C afforded the corresponding carbazole via nitrene insertion to the C–H bond in 82% yield.26a The O-benzyl group was deprotected under Pd/C, H2 (balloon pressure) in methanol to obtain the corresponding phenol in 90% yield. The side chromene ring was constructed under titanium(IV) isopropoxide condition with citral to provide the desired (±)-O-methoxymahanine in 75% yield and 37% overall yield in 6 steps, whereas Knölker et al. reported in 5 steps and 57% overall yield.27 However, our all attempts to deprotect the −OMe group [BCl3 in dichloromethane (DCM), BBr3 in DCM, trimethyliodosilane in DCM, AlCl3 in benzene reflux, AlI3 pyridine in MeCN, for details; see the Supporting Information] were in vain because of the instability of mahanine under acidic condition. Interestingly, under one of the demethylation conditions with tetrabutylammonium iodide and boron trichloride, the (±)-O-methoxymahanine was converted to the O-methylmurrayamine-D in 54% yield and 7 steps, 20% overall yield (Scheme 7).
Scheme 7. Synthesis of O-Methoxymahanine and O-Methylmurrayamine-D.
5. Conclusions
In conclusion, we have developed a palladium(II)-catalyzed C–H arylation of aniline carbamates with diazonium salts from an inexpensive aniline precursor. By the judicial choice of directing group and catalyst, the present reaction proceeds smoothly at room temperature without any base, oxidant, silver, or photoredox catalyst to provide monoarylation at the ortho-position selectively. The present reaction proceeds via directed electrophilic metalation at the ortho-position to form a palladacycle which undergoes oxidative addition to the aryldiazonium salts. However, the mode of oxidative addition either via ionic (2e–) or radical (1e–) is not clear at this moment and warrants further studies. The present C–H arylation reaction is scalable, and the directing group is removed easily. The present protocol provides a direct access to carbazole alkaloids or their derivatives, such as clausine V, clauszoline K, O-methoxymahanine, and O-methylmurrayamine-D. We anticipate that this practical method will find many academic and industrial applications.
6. Experimental Section
6.1. General Information
All manipulations with air-sensitive reagents were carried out under dry nitrogen atmosphere. Unless otherwise stated, all commercial reagents were used without additional purification. Solvents were dried using standard methods and distilled before use. TLC was performed on silica gel plates (Merck silica gel 60, F254), and the spots were visualized with UV light (254 and 365 nm) or by charring the plate dipped in KMnO4 or vanillin charring solution. 1H NMR was recorded at 300, 400, and 600 MHz frequency, and 13C NMR spectra were recorded at 75, 100, and 150 MHz frequency in the CDCl3 solvent using TMS as the internal standard. 19F NMR spectra were recorded in the CDCl3 solvent using hexafluorobenzene as the internal standard. Chemical shifts were measured in parts per million (ppm) referenced to 0.0 ppm for tetramethylsilane. The following abbreviations were used to explain multiplicities: s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad. Coupling constants, J, were reported in hertz unit. HRMS (m/z) was measured using EI and ESI techniques (JEOL-JMS 700 and Q-Tof Micro mass spectrometer, respectively).
6.1.1. General Procedure for the Synthesis of Aniline Carbamates
Aniline carbamates were synthesized according to the reported procedure.14 In a 100 mL round-bottom flask, sodium carbonate (20 mmol) was dissolved in 25 mL of water. To this homogeneous mixture, 25 mL of 1,2-dichloroethane (DCE) was added to form a biphasic mixture. In this biphasic mixture, the corresponding aniline precursor (10 mmol) was added. To the reaction mixture, 15 mmol methyl chloroformate was added dropwise. Then, the reaction mixture was stirred for 12 h at room temperature. After reaction completion, water was added and extracted with ethyl acetate (3 × 50 mL). The combined organic layer was washed with 1 M HCl (2 × 50 mL) and brine (2 × 50 mL). The organic part was dried over Na2SO4 and evaporated under reduced pressure. The crude reaction mixture was purified by column chromatography using hexane ethyl acetate to yield corresponding aniline carbamate.
6.1.2. General Procedure for Aryldiazonium Tetrafluoroborates
Aniline (10 mmol) was taken in a 50 mL round-bottom flask and cooled in an ice bath, and tetrafluoroboric acid solution (48 wt % in H2O, 3.4 mL, 26 mmol) was added at 0 °C. A precipitate was formed which was dissolved in minimum amount of distilled water. Sodium nitrite (0.7 g, 10 mmol) in distilled water (2 mL) was added dropwise to the reaction mixture and allowed to stir for 0.5 h at 0 °C. Thick precipitate was formed and collected by filtration. The precipitate was washed with diethyl ether (40 mL) three times. The resulting precipitate was recrystallized with acetonitrile/diethyl ether to give desired aryldiazonium tetrafluoroborate as a crystalline solid.
6.1.3. General Procedure for Arylation of Aryl Carbamate
Aryl carbamate (0.2 mmol) and Pd(TFA)2 (10 mol %) were taken in an oven-dried 25 mL round-bottom flask containing a stir bar. Then, dry methanol (2 mL) was added followed by the addition of aryl diazonium salt (2 equiv). The round-bottom flask was purged by nitrogen, and the reaction mixture was stirred at room temperature for 30 min. The second fraction of aryl diazonium salt (2 equiv) was added and purged with nitrogen. The reaction mixture was stirred at room temperature for 16 h. The solvent was evaporated under reduced pressure. The crude mixture was purified by column chromatography using hexane/ethyl acetate to yield the corresponding arylated product.
6.1.3.1. Methyl [1,1′-Biphenyl]-2-ylcarbamate (3a), Scheme 2
The general procedures 6.1.3 were followed. Column chromatography (SiO2, eluting with 95:5 hexane/ethyl acetate) afforded the desired product as an oil (31 mg, 68% yield). 1H NMR (600 MHz, CDCl3): δ 8.16 (br s, 1H), 7.50 (t, J = 7.2 Hz, 2H), 7.44–7.41 (m, 1H), 7.40–7.37 (m, 3H), 7.23 (dd, J = 8.4 Hz, 1.8 Hz, 1H), 1.15 (td, J = 7.2 Hz, 1.2 Hz, 1H), 6.68 (br s, 1H), 3.73 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 153.9, 138.0, 134.8, 131.4, 130.1, 129.2, 129.1, 128.5, 127.9, 123.3, 119.5, 52.2; IR: 3421, 1736, 1523, 1217, 757; HRMS (EI, m/z): calcd for C14H13NO2 [M]+, 227.0946; found, 227.0943.
6.1.3.2. Ethyl [1,1′-Biphenyl]-2-carboxylate (3b), Scheme 2
The general procedures 6.1.3 were followed. Column chromatography (SiO2, eluting with 96:4 hexane/ethyl acetate) afforded the desired product as a yellow liquid (28 mg, 58% yield). 1H NMR (300 MHz, CDCl3): δ 8.15 (d, J = 8.4 Hz, 1H), 7.57–7.34 (m, 6H), 7.23–7.20 (m, 1H), 7.12 (dt, J = 7.2 Hz, 1.2 Hz, 1H), 6.63 (s, 1H), 4.21–4.14 (m, 2H), 1.26 (t, J = 2.0 Hz, 3H); 13C NMR (75 MHz, CDCl3): δ 153.6, 138.1, 134.9, 131.4, 130.2, 129.3, 129.1, 128.5, 127.9, 123.3, 119.6, 61.2, 14.5; IR: 3424, 2982, 1738, 1520, 1446, 1208, 1067, 754; HRMS (ESI, m/z): calcd for C15H15NO2 [M + Na]+, 264.1000; found, 264.1003.
6.1.3.3. Methyl (4′-Bromo-4-methyl-[1,1′-biphenyl]-2-yl)carbamate (3c), Scheme 2
The general procedures 6.1.3 were followed. Column chromatography (SiO2, eluting with 95:5 hexane/ethyl acetate) afforded the desired product as a yellow amorphous solid (58 mg, 90% yield, mp 102–104 °C). 1H NMR (300 MHz, CDCl3): δ 7.93 (s, 1H), 7.59 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.4 Hz, 2H), 7.07 (d, J = 7.8 Hz, 1H), 6.95 (dd, J = 7.8 Hz, 1.8 Hz, 1H), 6.49 (s, 1H), 3.71 (s, 3H), 3.40 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 153.9, 138.9, 137.0, 134.3, 132.2, 131.0, 129.7, 127.7, 124.5, 121.9, 120.6, 52.3, 21.5; IR: 3421, 2950, 1737, 1530, 1471, 1216, 1068; HRMS (ESI, m/z): calcd for C15H14BrNO2 [M + Na]+, 342.0106; found, 342.0105.
6.1.3.4. Methyl (4′-Fluoro-4-methyl-[1,1′-biphenyl]-2-yl)carbamate (3d), Scheme 2
The general procedures 6.1.3 were followed. Column chromatography (SiO2, eluting with 94:6 hexane/ethyl acetate) afforded the desired product as an off-white solid (45 mg, 86% yield, mp 80–82 °C). 1H NMR (300 MHz, CDCl3): δ 7.94 (br s, 1H), 7.33–7.26 (m, 2H), 7.15 (t, J = 8.7 Hz, 2H), 7.07 (d, J = 7.5 Hz, 1H), 6.95 (d, J = 7.8 Hz, 1H), 3.71 (s, 3H), 2.40 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 162.3 (d, J = 245.8 Hz), 154.0, 138.7, 134.5, 134.0 (d, J = 3.4 Hz), 131.0 (d, J = 8.0 Hz), 130.0, 127.8, 124.3, 120.4, 116.0 (d, J = 21.2 Hz), 52.3, 21.5; 19F NMR (377 MHz, CDCl3, C6F6 as internal standard): δ −117.44; IR: 3426, 2953, 1739, 1530, 1219, 1069, 816; HRMS (EI, m/z): calcd for C15H14FNO2 [M]+, 259.1009; found, 259.1003.
6.1.3.5. Methyl (4-Methyl-4′-(trifluoromethyl)-[1,1′-biphenyl]-2-yl)carbamate (3e), Scheme 2
The general procedures 6.1.3 were followed. Column chromatography (SiO2, eluting with 94:6 hexane/ethyl acetate) afforded the desired product as a white crystalline solid (57 mg, 93% yield, mp 90–92 °C). 1H NMR (300 MHz, CDCl3): δ 7.93 (br s, 1H), 7.72 (d, J = 8.1 Hz, 2H), 7.48 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 7.8 Hz, 1H), 6.99 (dd, J = 7.8 Hz, 1.8 Hz, 1H), 6.46 (s, 1H), 3.72 (s, 3H), 2.41 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 154.0, 142.0, 139.4, 134.3, 130.3, 130.2–129.5 (q, J = 32.3 Hz), 129.8, 129.7, 128.8, 126.7–121.3 (q, J = 270.5 Hz), 126.0–125.9 (q, J = 3.73 Hz), 124.7, 52.3, 21.5; 19F NMR (377 MHz, CDCl3, C6F6 as an internal standard): δ −62.75; IR: 3431, 2926, 1739, 1534, 1325, 1127, 1069; HRMS (ESI, m/z): calcd for C16H14F3NO2 [M + Na]+, 332.0874; found, 332.0879.
6.1.3.6. Methyl (4-Methyl-4′-nitro-[1,1′-biphenyl]-2-yl)carbamate (3f), Scheme 2
The general procedures 6.1.3 were followed. Column chromatography (SiO2, eluting with 98:2 hexane/acetone) afforded the desired product as a pale yellow amorphous solid (45 mg, 79% yield, mp 265–267 °C). 1H NMR (300 MHz, CDCl3): δ 8.33 (d, J = 8.7 Hz, 2H), 7.89 (br s, 1H), 7.56 (d, J = 8.7 Hz, 2H), 7.14 (d, J = 7.8 Hz, 1H), 7.03 (d, J = 7.8 Hz, 1H), 6.41 (s, 1H), 3.73 (s, 3H), 3.43 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 154.0, 147.1, 145.3, 140.0, 134.2, 130.2, 129.8, 127.4, 125.1, 124.2, 121.8, 52.4, 21.5; IR: 3384, 1735, 1520, 1347, 1217, 1068, 857; HRMS (EI, m/z): calcd for C15H14N2O4 [M]+, 286.0954; found, 286.0949.
6.1.3.7. Methyl 4-Methyl-[1,1′-biphenyl]-2-carboxylate (3g), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 95:5 hexane/ethyl acetate) afforded the desired product as a yellowish gummy liquid (41 mg, 84% yield). 1H NMR (300 MHz, CDCl3): δ 7.96 (s, 1H), 7.47–7.35 (m, 3H), 7.34–7.30 (m, 2H), 7.09 (d, J = 7.8 Hz, 1H), 6.93 (d, J = 7.8 Hz, 1H), 6.63 (s, 1H), 3.69 (s, 3H), 2.39 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 153.9, 138.4, 138.0, 134.0, 129.8, 129.2, 129.0, 128.6, 127.6, 124.1, 120.1, 52.1, 21.4; HRMS (ESI, m/z): calcd for C15H15NO2 [M + Na]+, 264.1000; found, 264.0999.
6.1.3.8. Methyl (4′-Methoxy-4-methyl-[1,1′-biphenyl]-2-yl)carbamate (3h), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 94:6 hexane/ethyl acetate) afforded the desired product as a white solid (52 mg, 95% yield, mp 100–102 °C). 1H NMR (300 MHz, CDCl3): δ 7.98 (s, 1H), 7.28–7.25 (m, 2H), 7.09 (d, J = 7.8 Hz, 1H), 7.00 (d, J = 8.4 Hz, 2H), 6.94 (d, J = 7.2 Hz, 1H), 6.67 (s, 1H), 3.85 (s, 3H), 3.72 (s, 3H), 2.41 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.0, 153.9, 138.1, 134.6, 130.4, 130.1, 129.9, 128.3, 124.0, 119.9, 114.4, 55.2, 52.1, 21.4; IR: 3420, 2952, 1738, 1526, 1245, 1069, 814; HRMS (ESI, m/z): calcd for C16H17NO3 [M + Na]+, 294.1106; found, 294.1104.
6.1.3.9. Methyl-4-methoxy-[1,1′-biphenyl]-2-carboxylate (3i), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 94:6 hexane/ethyl acetate) afforded the desired product as an off-white crystalline solid (47 mg, 92% yield, mp 94–96 °C). 1H NMR (300 MHz, CDCl3): δ 7.87 (s, 1H), 7.51–7.34 (m, 5H), 7.14 (d, J = 8.4 Hz, 1H), 6.77 (s, 1H), 6.71 (dd, J = 8.4 Hz, 2.7 Hz, 1H), 3.86 (s, 3H), 3.74 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.7, 153.8, 137.9, 135.8, 130.8, 129.4, 129.1, 127.6, 123.6, 109.5, 104.3, 55.4, 52.2; IR: 3422, 2954, 1742, 1471, 1212, 1060, 767; HRMS (ESI, m/z): calcd for C15H15NO3 [M + Na]+, 280.0950; found, 280.0958.
6.1.3.10. Methyl (4,4′-Dimethoxy-[1,1′-biphenyl]-2-yl)carbamate (3j), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 90:10 hexane/ethyl acetate) afforded the desired product as a brown solid (47 mg, 81% yield, mp 78–80 °C). 1H NMR (300 MHz, CDCl3): δ 7.83 (br s, 1H), 7.24 (d, J = 8.7 Hz, 2H), 7.09 (d, J = 8.4 Hz, 1H), 6.99 (d, J = 8.7 Hz, 2H), 6.73 (br s, 1H), 6.66 (dd, J = 8.4 Hz, 2.7 Hz, 1H), 3.85 (br s, 3H), 3.851 (s, 3H), 3.71 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.5, 159.0, 153.8, 135.9, 130.8, 130.5, 129.9, 123.3, 114.5, 109.4, 104.1, 55.33, 55.27, 52.2; IR: 3419, 2953, 1738, 1522, 1472, 1243, 1056; HRMS (ESI, m/z): calcd for C16H17NO4 [M + Na]+, 310.1055; found, 310.1052.
6.1.3.11. Methyl (4-Methoxy-4′-methyl-[1,1′-biphenyl]-2-yl)carbamate (3k), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 93:7 hexane/ethyl acetate) afforded the desired product as a brown gummy liquid (35 mg, 65% yield). 1H NMR (300 MHz, CDCl3): δ 7.85 (s, 1H), 7.29–7.21 (m, 4H), 7.11 (d, J = 8.4 Hz, 1H), 6.77 (s, 1H), 6.68 (dd, J = 8.7 Hz, 2.7 Hz, 1H), 3.87 (s, 3H), 3.72 (s, 3H), 2.41 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.6, 153.8, 137.3, 135.8, 134.8, 130.8, 129.8, 129.3, 123.6, 109.4, 104.2, 55.3, 52.2, 21.1; IR: 3420, 2951, 1740, 1526, 1473, 1214, 1058, 810; HRMS (ESI, m/z): calcd for C16H17NO3 [M + Na]+, 294.1106; found, 294.1108.
6.1.3.12. Methyl (4-Methoxy-3′-methyl-[1,1′-biphenyl]-2-yl)carbamate (3l), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 96:4 hexane/ethyl acetate) afforded the desired product as a yellow gummy liquid (48 mg, 89% yield). 1H NMR (400 MHz, CDCl3): δ 7.83 (br s, 1H), 7.33 (t, J = 7.2 Hz, 1H), 7.17 (d, J = 7.6 Hz, 1H), 7.11–7.07 (m, 3H), 6.74 (br s, 1H), 6.65 (dd, J = 8.4, 2.4 Hz, 1H), 3.85 (s, 3H), 3.70 (s, 3H), 2.39 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 159.8, 153.9, 139.0, 137.9, 135.9, 130.8, 130.3, 129.1, 128.5, 126.5, 123.8, 109.5, 104.2, 55.5, 52.3, 21.5; IR: 3468, 3374, 2921, 1654, 1503, 1216, 736, 696; HRMS (ESI, m/z): calcd for C16H17NO3 [M + H]+, 272.1287; found, 272.1277.
6.1.3.13. Methyl (4′-(tert-Butyl)-4-methoxy-[1,1′-biphenyl]-2-yl)carbamate (3m), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 96:4 hexane/ethyl acetate) afforded the desired product as a brown crystalline solid (37 mg, 59% yield, mp 80–82 °C). 1H NMR (600 MHz, CDCl3): δ 7.86 (br s, 1H), 7.49 (d, J = 8.4 Hz, 2H), 7.28 (d, J = 8.4 Hz, 2H), 7.13 (d, J = 8.4 Hz, 1H), 6.82 (br s, 1H), 6.69 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 3.88 (s, 3H) 3.75 (s, 3H), 1.39 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 159.6, 153.9, 150.5, 139.7, 135.8, 134.8, 130.9, 129.0, 126.0, 109.5, 55.4, 52.2, 34.6, 31.3; IR: 3421, 2926, 1742, 1530, 1472, 1213, 1059; HRMS (EI, m/z): calcd for C19H23NO3 [M]+, 313.1678; found, 313.1671.
6.1.3.14. Methyl (4-Methoxy-3′,5′-dimethyl-[1,1′-biphenyl]-2-yl)carbamate (3n), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 97:3 hexane/ethyl acetate) afforded the desired product as a brown gummy liquid (29 mg, 50% yield). 1H NMR (300 MHz, CDCl3): δ 7.84 (s, 1H), 7.08 (d, J = 8.7 Hz, 1H), 7.01 (s, 1H), 6.92 (s, 2H), 6.79 (br s, 1H), 6.65 (dd, J = 8.4 Hz, 2.7 Hz, 1H), 3.86 (s, 3H), 3.72 (s, 3H), 2.37 (s, 6H); 13C NMR (75 MHz, CDCl3): δ 159.6, 153.8, 138.7, 137.7, 135.8, 130.7, 129.3, 127.1, 123.7, 109.3, 104.0, 55.4, 52.2, 21.3; IR: 3417, 2925, 1740, 1528, 1473, 1219, 1056; HRMS (ESI, m/z): calcd for C17H19NO3 [M + Na]+, 308.1263; found, 308.1261.
6.1.3.15. Methyl (6-(4-(tert-Butyl)phenyl)benzo[d][1,3]dioxol-5-yl)carbamate (3o), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 91:9 hexane/ethyl acetate) afforded the desired product as a brown solid (41 mg, 63% yield, mp 90–92 °C). 1H NMR (600 MHz, CDCl3): δ 7.65 (s, 1H), 7.46 (d, J = 8.4 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 6.69 (s, 1H), 6.57 (s, 1H), 5.99 (s, 2H), 3.72 (s, 3H), 1.36 (s, 9H); 13C NMR (150 MHz, CDCl3): δ 154.2, 150.7, 147.1, 143.6, 135.0, 129.0, 126.0, 120.2, 114.8, 109.7, 106.4, 101.3, 52.3, 34.6, 31.3; IR: 3423, 2961, 1737, 1527, 1218, 1040, 760; HRMS (ESI, m/z): calcd for C19H21NO4 [M + Na]+, 350.1368; found, 350.1369.
6.1.3.16. Methyl (5-Methoxy-4′-(trifluoromethyl)-[1,1′-biphenyl]-2-yl)carbamate (3p), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 92:8 hexane/ethyl acetate) afforded the desired product as a brown solid (27 mg, 42% yield, mp 91–93 °C). 1H NMR (600 MHz, CDCl3): δ 7.88 (s, 1H), 7.74 (d, J = 8.4 Hz, 2H), 7.50 (d, J = 7.8 Hz, 2H), 6.97 (dd, J = 9.0 Hz, 3.0 Hz, 1H), 6.79 (d, J = 2.4 Hz, 1H), 6.26 (s, 1H), 3.83 (s, 3H), 3.71 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 156.3, 154.6, 142.0, 133.0, 130.3–129.7 (q, J = 32.1 Hz), 129.5, 127.5, 126.7–121.3 (q, J = 270.6 Hz), 125.9–125.8 (q, J = 3.75 Hz), 123.4, 115.4, 114.2, 55.5, 52.3; 19F NMR (377 MHz, CDCl3, C6F6 as an internal standard): δ −65.73; IR: 3320, 2953, 1726, 1524, 1324, 1228, 1122; HRMS (ESI, m/z): calcd for C16H14F3NO3 [M + Na]+, 348.0823; found, 348.0831.
6.1.3.17. Methyl (3-Methyl-4′-(trifluoromethyl)-[1,1′-biphenyl]-2-yl)carbamate (3q), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 96:4 hexane/ethyl acetate) afforded the desired product as a yellow solid (24 mg, 39% yield, mp 138–140 °C). 1H NMR (600 MHz, CDCl3): δ 7.68 (d, J = 7.8 Hz, 2H), 7.46 (d, J = 8.4 Hz, 2H), 7.33–7.28 (m, 2H), 7.18 (d, J = 7.2 Hz, 1H), 5.99 (s, 1H), 3.68 (s, 3H), 2.36 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 155.0, 143.4, 138.6, 136.9, 132.2, 130.83, 129.7–129.1 (q, J = 32.4 Hz), 129.2, 128.0, 127.4, 126.9–121.5 (q, J = 269.8 Hz), 125.3–125.2 (q, J = 3.75 Hz), 125.1, 123.3, 52.6, 18.4; 19F NMR (377 MHz, CDCl3, C6F6 as an internal standard): δ −62.45; HRMS (ESI, m/z): calcd for C16H14F3NO2 [M + Na]+, 332.0874; found, 332.0879.
6.1.3.18. Methyl (2-(p-Tolyl)naphthalen-1-yl)carbamate (3r), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 95:5 hexane/ethyl acetate) afforded the desired product as a brown crystalline solid (50 mg, 81% yield, mp 205–207 °C). 1H NMR (300 MHz, CDCl3): δ 8.02 (d, J = 8.4 Hz, 1H), 7.87 (t, J = 9.0 Hz, 2H), 7.60–7.46 (m, 3H), 7.34–7.26 (m, 4H), 5.31 (s, 1H), 3.71 (s, 3H), 2.43 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 139.5, 137.2, 136.8, 136.6, 133.5, 131.0, 129.2, 129.1, 128.9, 128.1, 128.0, 127.8, 126.9, 126.1, 123.5, 52.7, 21.1; IR: 3277, 2924, 1710, 1501, 1348, 1237, 1075, 811; HRMS (EI, m/z): calcd for C19H17N2O2 [M]+, 291.1259; found, 291.1260.
6.1.3.19. Methyl (3,4-Dimethyl-[1,1′-biphenyl]-2-yl)carbamate (3s), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 95:5 hexane/ethyl acetate) afforded the desired product as a white solid (39 mg, 77% yield, mp 170–172 °C). 1H NMR (300 MHz, CDCl3): δ 7.44–7.29 (m, 5H), 7.18 (d, J = 7.8 Hz, 1H), 7.10 (d, J = 7.8 Hz, 1H), 6.00 (br s, 1H), 3.69 (s, 3H), 2.36 (s, 3H), 2.25 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 155.4, 149.8, 137.3, 135.1, 132.1, 128.9, 128.8, 128.7, 128.3, 127.2, 127.1, 52.5, 20.5, 14.7; IR: 3315, 2924, 1711, 1455, 1235; HRMS (ESI, m/z): calcd for C16H17NO2 [M + Na]+, 278.1157; found, 278.1166.
6.1.3.20. Methyl (4′-Bromo-5-methyl-[1,1′-biphenyl]-2-yl)carbamate (3t), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 96:4 hexane/ethyl acetate) afforded the desired product as a yellow gummy liquid (33 mg, 52% yield). 1H NMR (300 MHz, CDCl3): δ 7.94 (d, J = 6.9 Hz, 1H), 7.61 (d, J = 8.1 Hz, 2H), 7.28–7.19 (m, 3H), 7.02 (d, J = 2.1 Hz, 1H), 6.45 (s, 1H), 3.73 (s, 3H), 2.36 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 154.1, 137.2, 133.4, 132.1, 132.0, 130.9, 130.5, 129.4, 122.0, 120.5, 52.3, 20.7; IR: 3424, 2924, 1735, 1522, 1212, 1069, 825; HRMS (ESI, m/z): calcd for C15H14BrNO2 [M + Na]+, 342.0106; found, 342.0111.
6.1.3.21. Methyl (4,5-Dimethoxy-4′-methyl-[1,1′-biphenyl]-2-yl)carbamate (3u), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 89:11 hexane/ethyl acetate) afforded the desired product as a yellow gummy liquid (39 mg, 64% yield). 1H NMR (600 MHz, CDCl3): δ 7.80 (br s, 1H), 7.29 (d, J = 7.8 Hz, 2H), 7.25 (d, J = 8.4 Hz, 2H), 6.74 (br s, 1H), 6.59 (s, 1H), 3.96 (s, 3H) 3.86 (s, 3H), 3.72 (s, 3H), 2.43 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 154.2, 148.5, 144.9, 137.5, 135.0, 129.8, 129.2, 128.74, 128.70, 128.1, 113.1, 56.1, 56.0, 52.2, 21.2; IR: 3423, 3337, 2949, 1733, 1525, 1455, 1210, 1035; HRMS (ESI, m/z): calcd for C17H19NO4 [M + Na]+, 324.1212; found, 324.1188.
6.1.3.22. Methyl (4′-Chloro-[1,1′-biphenyl]-2-yl)carbamate (3v), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 95:5 hexane/ethyl acetate) afforded the desired product as a yellow gummy liquid (30 mg, 58% yield). 1H NMR (300 MHz, CDCl3): δ 8.13 (d, J = 8.4 Hz, 1H), 7.47 (d, J = 8.4 Hz, 2H), 7.40 (dt, J = 6.9 Hz, 1.8 Hz, 1H), 7.32 (d, J = 8.4 Hz, 2H), 7.22–7.12 (m, 2H), 6.50 (br s, 1H), 3.74 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 153.9, 136.4, 134.7, 134.0, 130.6, 130.0, 129.3, 128.8, 123.6, 120.0, 52.3; IR: 3424, 2927, 1738, 1524, 1217, 1074, 761; HRMS (EI, m/z): calcd for C14H12ClNO2 [M]+, 261.0557; found, 261.0546.
6.1.3.23. Methyl (4-(Benzyloxy)-4′-methoxy-5-methyl-[1,1′-biphenyl]-2-yl)carbamate (3w), Scheme 2
The same general procedure 6.1.3 was followed. Column chromatography (SiO2, eluting with 98:2 hexane/ethyl acetate) afforded the desired product as a yellow solid (57 mg, 75% yield, mp 96–98 °C). 1H NMR (300 MHz, CDCl3): δ 7.87 (s, 1H), 7.52–7.47 (m, 2H), 7.43–7.30 (m, 3H), 7.01–6.95 (m, 3H), 6.67 (s, 1H), 5.15 (s, 2H), 3.85 (s, 3H), 3.71 (s, 3H), 2.25 (s, 3H); 13C NMR (75 MHz, CDCl3): δ 159.0, 156.4, 154.0, 137.3, 133.4, 131.9, 130.5, 130.1, 128.4, 127.7, 127.3, 122.9, 121.7, 114.5, 103.0, 69.9, 55.3, 52.1, 15.7; IR: 3438, 2921, 1609, 1499, 1244, 1033; HRMS (EI, m/z): calcd for C23H23NO4 [M]+, 377.1627; found, 377.1628.
6.1.4. General Procedure for Gram-Scale Arylation of Aryl Carbamate
Aryl carbamate (1.086 g, 4 mmol) and Pd(TFA)2 (10 mol %) were taken in an oven-dried 250 mL round-bottom flask containing a stir bar. Then, dry methanol (40 mL) was added followed by the addition of aryl diazonium salt (2 equiv). The round-bottom flask was purged by nitrogen, and the reaction mixture was stirred at room temperature for 30 min. The second fraction of aryl diazonium salt (2 equiv) was added and purged with nitrogen. The reaction mixture was stirred at room temperature for 16 h. The solvent was evaporated under reduced pressure. The crude mixture was purified by column chromatography using hexane/ethyl acetate to yield the corresponding product.
6.1.5. General Procedure for Deprotection of Carbamate
Aryl carbamate (2.0 mmol) was dissolved in 30 mL of methanol, to which was added 15 mL of 40% aqueous KOH. The solution was then refluxed for 2 h. After reaction completion by TLC checking, the reaction mixture was diluted with water (100 mL) and extracted with ethyl acetate (4 × 50 mL). The organic fractions were combined, washed with brine, dried over anhydrous sodium sulfate, and evaporated under reduced pressure. The crude residue was then purified by column chromatography eluting with hexane/ethyl acetate to afford pure product amine.
6.1.6. General Procedure for the Synthesis of Picolinamide Derivate
Pyridine-2-carbonyl chloride hydrochloride (60 mg, 0.35 mmol) and N,N-dimethyl-4-aminopyridine (12 mg, 0.1 mmol) were placed in a 25 mL two-necked reaction flask, and the flask was flushed with nitrogen. DCM (6 mL), triethylamine (80 mg, 0.7 mmol), and corresponding aniline (0.3 mmol) were added. The reaction mixture was stirred at room temperature for 24 h. The resulting mixture was then quenched with water. The mixture was extracted with DCM (3 × 20 mL), and the combined organic layer was dried over anhydrous sodium sulfate and concentration under reduced pressure. The crude was purified by silica gel column purification eluting with hexane/ethyl acetate to provide corresponding amide derivative.
6.1.7. General Procedure for Cu-Catalyzed Intramolecular Coupling
To a solution of corresponding amide (0.25 mmol) in N,N-dimethylformamide (DMF, 1.5 mL) in a 5 mL microwave vessel, acetic acid (15 mg, 0.25 mmol), manganese dioxide (43 mg, 0.50 mmol), and Cu(OAc)2 (14 mg, 0.075 mmol) were added. The vessel was sealed with a cap, and the mixture was irradiated under microwave reactor conditions at 200 °C for 1 h. The resulting mixture was then quenched with water (10 mL) and ethylenediamine (1 mL). The reaction mixture was extracted with ethyl acetate (3 × 30 mL), and the combined organic layer was dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude mixture was purified by silica gel column purification eluting with hexane/ethyl acetate to afford pure product.
6.1.7.1. 4-(Benzyloxy)-4′-methoxy-5-methyl-[1,1′-biphenyl]-2-amine (4), Scheme 5
Compound 4 was synthesized according to the general procedure 6.1.5. Column chromatography (SiO2, eluting with 85:15 hexane/ethyl acetate) to afford pure product as a yellow crystalline solid (828 mg, 99% yield, mp 84–86 °C). 1H NMR (400 MHz, CDCl3): δ 7.44–7.33 (m, 7H), 6.96–6.91 (m, 3H), 6.34 (s, 1H), 5.05 (s, 2H), 3.82 (s, 3H), 3.61 (br s, 2H), 2.21 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 158.5, 157.0, 142.4, 137.7, 132.6, 131.9, 130.4, 128.6, 127.8, 127.2, 119.9, 117.2, 114.3, 100.1, 70.0, 56.4, 15.6; HRMS (ESI, m/z): calcd for C21H21NO2 [M + Na]+, 342.1470; found, 342.1473.
6.1.7.2. 4,4′-Dimethoxy-[1,1′-biphenyl]-2-amine (5), Scheme 6
Compound 5 was synthesized according to the general procedure 6.1.5. Column chromatography (SiO2, eluting with 91:9 hexane/ethyl acetate) to afford pure product as a yellow solid (80 mg, 99% yield, mp 102–104 °C). 1H NMR (600 MHz, CDCl3): δ 7.36 (d, J = 8.4 Hz, 2H), 7.04 (d, J = 8.4 Hz, 1H), 6.98 (d, J = 8.4 Hz, 2H), 6.42 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 6.36 (d, J = 2.4 Hz, 1H), 3.85 (s, 3H), 3.81 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 159.8, 158.5, 144.3, 131.4, 131.3, 130.3, 120.7, 114.1, 104.4, 101.2, 55.3, 55.2; HRMS (EI, m/z): calcd for C14H15NO2 [M]+, 229.1103; found, 229.1104.
6.1.7.3. 4-Methoxy-3′-methyl-[1,1′-biphenyl]-2-amine (6), Scheme 6
Compound 6 was synthesized according to the general procedure 6.1.5. Column chromatography (SiO2, eluting with 93:7 hexane/ethyl acetate) to afford pure product as a yellow liquid (80 mg, 99% yield). 1H NMR (400 MHz, CDCl3): δ 7.29 (t, J = 7.2 Hz, 1H), 7.22–7.19 (m, 2H), 7.13–7.10 (m, 1H), 7.03 (d, J = 8 Hz, 1H), 6.40 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 6.33 (d, J = 2.4 Hz, 1H), 3.78 (s, 3H), 2.37 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 160.1, 144.7, 139.3, 138.5, 131.4, 130.0, 128.7, 127.7, 126.3, 121.1, 104.3, 101.1, 55.3, 21.6; HRMS (ESI, m/z): calcd for C14H15NO [M + H]+, 214.1232; found, 214.1224.
6.1.7.4. N-(4,4′-Dimethoxy-[1,1′-biphenyl]-2-yl)picolinamide (7), Scheme 6
Compound 7 was synthesized according to the general procedure 6.1.6. Column chromatography (SiO2, eluting with 89:11 hexane/ethyl acetate) to afford pure product as a white crystalline solid (83 mg, 82% yield, mp 86–88 °C). 1H NMR (600 MHz, CDCl3): δ 10.38 (br s, 1H), 8.42–8.41 (m, 1H), 8.38 (d, J = 3.0 Hz, 1H), 8.26 (dt, J = 7.8 Hz, 1.2 Hz, 1H), 7.87 (td, J = 6.0 Hz, 1.8 Hz, 1H), 7.41–7.38 (m, 3H), 7.22 (d, J = 8.4 Hz, 1H), 7.04 (d, J = 8.4 Hz, 2H), 6.78 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 3.92 (s, 3H), 3.90 (s, 3H); 13C NMR (150 MHz, CDCl3): δ 162.1, 159.3, 159.0, 149.9, 148.0, 137.5, 135.8, 130.9, 130.8, 130.2, 126.2, 124.5, 122.1, 114.3, 110.7, 105.2, 55.5, 55.3; IR: 3336, 2835, 1690, 1524, 1175, 1043; HRMS (EI, m/z): calcd for C20H18N2O3[M]+, 334.1317; found, 334.1313.
6.1.7.5. N-(4-Methoxy-3′-methyl-[1,1′-biphenyl]-2-yl)picolinamide (8), Scheme 6
Compound 8 was synthesized according to the general procedure 6.1.6. Column chromatography (SiO2, eluting with 90:10 hexane/ethyl acetate) to afford pure product as a light yellow powder (110 mg, 95% yield, mp 216–218 °C). 1H NMR (400 MHz, CDCl3): δ 10.47 (br s, 1H), 8.40–8.37 (m, 2H), 8.24 (d, J = 8 Hz, 1H), 7.84 (dt, J = 7.6 Hz, 1.6 Hz, 1H), 7.37 (t, J = 8 Hz, 2H), 7.28–7.21 (m, 4H), 6.77 (dd, J = 8.4 Hz, 2.8 Hz, 1H), 3.91 (s, 3H), 2.41 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 162.1, 159.7, 150.1, 148.1, 138.7, 137.9, 137.6, 135.9, 130.9, 130.5, 128.9, 128.3, 126.8, 126.3, 124.1, 122.3, 110.9, 105.1, 55.6, 21.6; HRMS (ESI, m/z): calcd for C20H18N2O2 [M + H]+: 319.1447; found: 319.1431.
6.1.7.6. 2,7-Dimethoxy-9H-carbazole (9), Clausine V, Scheme 6
Compound 9 was synthesized according to the general procedure 6.1.7. Column chromatography (SiO2, eluting with 92:8 hexane/ethyl acetate) to afford pure product as a white solid (27 mg, 82% yield, mp 275–276 °C). 1H NMR (300 MHz, DMSO-d6): δ 10.95 (br s, 1H), 7.83 (d, J = 8.4 Hz, 2H), 6.94 (d, J = 2.1 Hz, 2H), 6.72 (dd, J = 8.4 Hz, 2.1 Hz, 2H), 3.81 (s, 6H); 13C NMR (75 MHz, DMSO-d6): δ 157.6, 141.1, 120.0, 116.5, 107.4, 94.7, 55.3; HRMS (EI, m/z): calcd for C14H13NO2 [M]+, 227.0946; found, 227.0937.
6.1.7.7. 2-Methoxy-6-methyl-9H-carbazole (10), Scheme 6
Compound 10 was synthesized according to the general procedure 6.1.7. Column chromatography (SiO2, eluting with 96:4 hexane/acetone) to afford pure product as a crystalline solid (60 mg, 95% yield, mp 226–228 °C). 1H NMR (400 MHz, CDCl3): δ 7.91 (d, J = 8.8 Hz, 1H), 7.87 (br s, 1H), 7.82–7.81 (m, 1H), 7.15–7.10 (m, 2H), 6.93 (d, J = 2.4 Hz 1H), 6.84 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 3.89 (s, 3H), 2.53 (s, 3H); 13C NMR (100 MHz, CDCL3): δ 159.0, 140.8, 138.9, 125.3, 123.1, 121.2, 119.8, 119.5, 117.9, 117.2, 108.2, 94.9, 55.7, 16.9; HRMS (ESI, m/z): calcd for C14H13NO [M + H]+, 212.1075; found, 212.1067.
6.1.7.8. 7-Methoxy-9H-carbazole-3-carbaldehyde (11), Clauszoline K, Scheme 6
Compound 10 (50 mg, 0.2 mmol) was dissolved in methanol–water (10:1), and DDQ (4.5 equiv) was added. The reaction mixture was stirred at room temperature for 45 min. After reaction completion, methanol was removed under reduced pressure and residue was extracted with ethyl acetate (3 × 20 mL). The combined organic layers were dried over anhydrous sodium sulfate and concentrated under reduced pressure. The crude mixture was purified by column chromatography eluting with 96:4 hexane/ethyl acetate to afford compound 11 as a yellow solid (35 mg, 77% yield, mp 184–186 °C). 1H NMR (400 MHz, CDCl3): δ 10.06 (br s, 1H), 8.48–8.49 (m, 1H), 8.30 (br s, 1H), 7.98 (d, J = 8.4 Hz, 1H), 7.88 (dd, J = 8.4 Hz, 1.6 Hz, 1H), 7.45 (d, J = 8.4 MHz, 1H), 6.94–6.91 (m, 2H), 3.89 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 192.0, 159.9, 152.9, 143.5, 141.5, 129.4, 123.9, 122.9, 121.6, 117.0, 110.6, 109.5, 95.3, 55.8; HRMS (ESI, m/z): calcd for C14H11NO2 [M + H]+, 226.0868; found, 226.0865.
6.1.7.9. 2-Azido-4-(benzyloxy)-4′-methoxy-5-methyl-1,1′-biphenyl (12), Scheme 7
To a solution of compound 4 (800 mg, 2.5 mmol) in HOAc–water (30 mL, v/v = 2/1), NaNO2 (175 mg, 2.5 mmol) was slowly added under stirring at 0 °C. After stirring for 30 min, NaN3 (165 mg, 2.5 mmol) was slowly added in portions at 0 °C. The reaction was stirred for 4 h, and the reaction was quenched by adding sodium carbonate till CO2 ceased, and the mixture was extracted with EtOAc (3 × 50 mL). The combined organic phase was washed with saturated brine (50 mL), dried with Na2SO4, and concentrated under reduced pressure. The crude was purified by column chromatography eluting with 91:9 hexane/ethyl acetate to afford the desired product as a yellow solid (795 mg, 92% yield, mp 88–90 °C). 1H NMR (400 MHz, CDCl3): δ 7.50–7.48 (m, 2H), 7.44–7.40 (m, 2H), 7.36–7.33 (m, 3H), 7.12–7.11 (m, 1H), 6.97–6.93 (m, 2H), 6.73 (s, 1H), 5.14 (s, 2H), 3.84 (s, 3H), 2.29 (s, 3H); 13C NMR (100 MHz, CDCl3): δ 158.8, 156.8, 136.9, 134.8, 133.1, 130.6, 128.8, 128.7, 128.1, 127.3, 125.9, 124.3, 113.7, 102.4, 70.4, 56.4, 16.0; IR: 2835, 2107, 1607, 1501, 1243, 1001, 832; HRMS (EI, m/z): calcd for C21H19N3O2 [M + Na]+, 368.1375; found, 368.1411.
6.1.7.10. 2-(Benzyloxy)-7-methoxy-3-methyl-9H-carbazole (13), Scheme 7
Compound 12 (500 mg, 1.5 mmol) was refluxed in xylenes under N2 overnight, and the mixture was concentrated to remove the solvent. The crude mixture was purified by silica gel column purification eluting with 99:1 DCM–methanol to give pure product as a brown crystalline solid (377 mg, 82% yield, mp 238–240 °C). 1H NMR (400 MHz, acetone-d6): δ 9.92 (br s, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.71 (s, 1H), 7.53–7.50 (m, 2H), 7.40–7.36 (m, 2H), 7.32–7.28 (m, 1H), 7.05 (s, 1H), 6.93 (d, J = 2.4 Hz, 1H), 6.72 (dd, J = 8.4, 2 Hz, 1H), 5.16 (s, 2H), 3.80 (s, 3H), 2.34 (s, 3H); 13C NMR (150 MHz, acetone-d6): δ 158.0, 155.0, 141.2, 139.6, 138.0, 128.4, 127.5, 127.2, 120.4, 119.6, 118.2, 117.0, 116.5, 107.3, 94.6, 94.2, 69.7, 54.7, 16.1; HRMS (EI, m/z): calcd for C21H19NO2 [M]+, 317.1416; found, 317.1415.
6.1.7.11. 7-Methoxy-3-methyl-9H-carbazol-2-ol (14), Scheme 7
To a solution of compound 13 (317 mg, 1.0 mmol) in 10 mL of MeOH, 10% Pd/C (50 mg) was added. The reaction mixture was subjected to hydrogenation by putting a hydrogen balloon, and the mixture was stirred at room temperature for 6 h. The reaction mixture was filtered through celite and washed with methanol until the filtrate became colorless. The filtrate was concentrated under reduced pressure to provide compound 7-methoxy-3-methyl-9H-carbazol-2-ol as a gray solid (200 mg, 89% yield, mp 234–236 °C decomposed). 1H NMR (400 MHz, acetone-d6): δ 9.78 (br s, 1H), 8.08 (br s, 1H), 7.75 (d, J = 8.4 MHz, 1H), 7.63 (s, 1H), 6.90–6.89 (m, 2H), 6.69 (dd, J = 8.8 Hz, 2.4 Hz, 1H), 3.80 (s, 3H), 2.29 (s, 3H); 13C NMR (150 MHz, acetone-d6): δ 157.9, 153.7, 141.2, 140.0, 120.5, 119.4, 117.4, 116.5, 116.2, 107.0, 96.3, 94.7, 54.8, 15.9; HRMS (EI, m/z): calcd for C14H13NO2 [M]+, 227.0946; found, 227.0943.
6.1.7.12. 9-Methoxy-3,5-dimethyl-3-(4-methylpent-3-en-1-yl)-3,11-dihydropyrano[3,2-a]carbazole (15), Methoxymahanine, Scheme 7
Citral (0.182 mL, 1 mmol) and compound 14 (100 mg, 0.5 mmol) were dissolved in toluene and cooled to −78 °C. Titanium(IV) isopropoxide (640 μL, 2 mmol) was added dropwise at −78 °C, and the reaction mixture was allowed to warm up at room temperature over a period of 23 h. After that, the reaction mixture was diluted with ethyl acetate and washed with water and brine. The aqueous layers were extracted with ethyl acetate (3 × 20 mL) and dried over anhydrous sodium sulfate. The combined organic layers were evaporated under reduced pressure. The residue was purified by flash column chromatography on silica gel using hexane/ethyl acetate (91:9) to afford O-methylmahanine, compound 15 as a light yellow amorphous solid, (115 mg, 75% yield, mp 179–181 °C). 1H NMR (600 MHz, acetone-d6): δ 10.20 (s, 1H), 7.80 (d, J = 8.4 Hz, 1H), 7.60 (s, 1H), 6.94 (d, J = 2.4 Hz, 1H), 6.92 (d, J = 9.6 Hz, 1H), 6.75 (dd, J = 8.4 Hz, 2.4 Hz, 1H), 5.74 (d, J = 9.6 Hz, 1H), 5.15–5.12 (m, 1H), 3.84 (s, 3H), 2.30 (s, 3H), 2.26–2.16 (m, 2H), 1.78–1.75 (m, 2H), 1.63 (s, 3H), 1.57 (s, 3H), 1.44 (s, 3H); 13C NMR (150 MHz, acetone-d6): δ 158.1, 148.6, 141.4, 135.3, 130.9, 128.1, 124.2, 120.1, 119.6, 118.1, 117.4, 116.9, 116.8, 107.3, 104.4, 94.8, 77.8, 54.8, 40.6, 25.2, 24.9, 22.6, 16.7, 15.3; IR: 3400, 2924, 1618, 1446, 1162, 820; HRMS (ESI, m/z): calcd for C24H27NO2 [M + H]+, 362.2120; found, 362.2103.
6.1.7.13. (1R,5R)-11-Methoxy-5,7-dimethyl-2-(prop-1-en-2-yl)-1,2,3,4,5,13-hexahydro-1,5-methanooxocino[3,2-a]carbazole (16), O-Methylmurrayamine-D, Scheme 7
To a solution of compound 15 (50 mg, 0.14 mmol) in DCM (3 mL), tetrabutylammonium iodide (1.5 equiv) and boron trichloride (1.5 equiv) were added at 0 °C and stirred for 16 h at room temperature. After reaction completion, DCM was evaporated under reduced pressure. The crude residue was purified by flash column chromatography on silica gel using hexane/ethyl acetate (93:7) to afford O-methylmurrayamine-D, compound 16 as a dark brownish crystalline solid (27 mg, 54% yield, mp 162–164 °C). 1H NMR (600 MHz, D6-acetone): δ 9.02 (br s, 1H), 7.74 (d, J = 8.4 Hz, 1H), 7.55 (s, 1H), 6.95 (d, J = 2.4 Hz, 1H), 6.70 (dd, J = 8.4, 2.4 Hz, 1H), 4.63 (t, J = 1.2 Hz, 1H), 4.45 (s, 1H), 3.81 (s, 3H), 3.59–3.57 (m, 1H), 2.57 (dt, J = 12.6, 3 Hz, 1H), 2.28 (s, 3H), 2.13 (dd, J = 10.2, 3 Hz, 1H), 2.03–2.00 (m, 1H), 1.84 (dt, J = 12.6, 3 Hz, 1H), 1.72 (td, J = 13.8, 5.4 Hz, 1H), 1.62 (s, 3H), 1.63–1.59 (m, 1H), 1.44–1.43 (m, 1H), 1.4 (s, 3H); 13C NMR (150 MHz, D6-acetone): δ 157.6, 152.5, 148.3, 140.9, 138.4, 119.1, 118.5, 117.7, 116.0, 114.7, 110.9, 107.0, 105.2, 94.7, 73.6, 54.7, 48.6, 39.9, 36.9, 34.4, 23.3, 21.4, 16.1; HRMS (ESI, m/z): calcd for C24H28NO2 [M + H]+, 362.2120; found, 362.2144.
6.1.7.14. Intermolecular Molecular Kinetic Isotopic Effect, Scheme 3a
1 (15.1 mg, 0.1 mmol), d5-1 (15.6 mg, 0.1 mmol), and Pd(TFA)2 (6.7 mg, 10 mol %) were taken in an oven-dried 25 mL round-bottom flask containing a stir bar. Then, dry methanol (2 mL) was added followed by the addition of phenyl diazonium salt (2 equiv). The round-bottom flask was purged by nitrogen, and the reaction mixture was stirred at room temperature for 30 min. The second fraction of phenyl diazonium salt (2 equiv) was added and purged with nitrogen. The reaction mixture was stirred at room temperature for 16 h. The solvent was evaporated under reduced pressure. The crude was purified by column chromatography on silica gel with a gradient of elution of pet ether and ethyl acetate to give the desired arylated product. The product was analyzed by 1H NMR (300 MHz, CDCl3).
Acknowledgments
This work was supported by DST, SERB, Govt. of India, Ramanujan fellowship; award no. SR/S2/RJN-97/2012 and extra mural research grant no. EMR/2014/000469. A.P. and K.V. thank CSIR and NIPER for their fellowships.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b02009.
1H and 13C NMR spectra for all synthesized compounds (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- a Mo F.; Dong G.; Zhang Y.; Wang J. Recent applications of arene diazonium salts in organic synthesis. Org. Biomol. Chem. 2013, 11, 1582–1593. 10.1039/c3ob27366k. [DOI] [PubMed] [Google Scholar]; b Taylor J. G.; Moro A. V.; Correia C. R. D. Evolution and Synthetic Applications of the Heck-Matsuda Reaction: The Return of Arenediazonium Salts to Prominence. Eur. J. Org. Chem. 2011, 2011, 1403–1428. 10.1002/ejoc.201001620. [DOI] [Google Scholar]; c Felpin F.-X.; Nassar-Hardy L.; Le Callonnec F.; Fouquet E. Recent advances in the Heck-Matsuda reaction in heterocyclic chemistry. Tetrahedron 2011, 67, 2815–2831. 10.1016/j.tet.2011.02.051. [DOI] [Google Scholar]
- a Lucks S.; Brunner H. In Situ Generated Palladium on Aluminum Phosphate as Catalytic System for the Preparation of β,β-Diarylated Olefins by Matsuda-Heck Reaction. Org. Process Res. Dev. 2017, 21, 1835–1842. 10.1021/acs.oprd.7b00279. [DOI] [Google Scholar]; b Kattela S.; Heerdt G.; Correia C. R. D. Non-Covalent Carbonyl-Directed Heck-Matsuda Desymmetrizations: Synthesis of Cyclopentene-Fused Spirooxindoles, Spirolactones, and Spirolactams. Adv. Synth. Catal. 2017, 359, 260–267. 10.1002/adsc.201600946. [DOI] [Google Scholar]; c Schmidt B.; Wolf F.; Brunner H. Styrylsulfonates and -Sulfonamides through Pd-Catalysed Matsuda-Heck Reactions of Vinylsulfonic Acid Derivatives and Arenediazonium Salts. Eur. J. Org. Chem. 2016, 2016, 2972–2982. 10.1002/ejoc.201600469. [DOI] [Google Scholar]; d Oliveira C. C.; Pfaltz A.; Correia C. R. D. Quaternary Stereogenic Centers through Enantioselective Heck Arylation of Acyclic Olefins with Aryldiazonium Salts: Application in a Concise Synthesis of (R)-Verapamil. Angew. Chem., Int. Ed. 2015, 54, 14036–14039. 10.1002/anie.201507927. [DOI] [PubMed] [Google Scholar]; e Angnes R. A.; Oliveira J. M.; Oliveira C. C.; Martins N. C.; Correia C. R. D. Stereoselective Synthesis of Aryl Cyclopentene Scaffolds by Heck-Matsuda Desymmetrization of 3-Cyclopentenol. Chem.—Eur. J. 2014, 20, 13117–13121. 10.1002/chem.201404159. [DOI] [PubMed] [Google Scholar]; f Schmidt B.; Elizarov N.; Berger R.; Hölter F. Scope and limitations of the Heck-Matsuda-coupling of phenol diazonium salts and styrenes: a protecting-group economic synthesis of phenolic stilbenes. Org. Biomol. Chem. 2013, 11, 3674–3691. 10.1039/c3ob40420j. [DOI] [PubMed] [Google Scholar]; g Soldi C.; Moro A. V.; Pizzolatti M. G.; Correia C. R. D. Heck-Matsuda Arylation as a Strategy to Access Kavalactones Isolated from Polygala sabulosa, Piper methysticum, and Analogues. Eur. J. Org. Chem. 2012, 2012, 3607–3616. 10.1002/ejoc.201200308. [DOI] [Google Scholar]; h Ibarguren O.; Zakri C.; Fouquet E.; Felpin F.-X. Heterogeneous palladium multi-task catalyst for sequential Heck-reduction-cyclization (HRC) reactions: influence of the support. Tetrahedron Lett. 2009, 50, 5071–5074. 10.1016/j.tetlet.2009.06.084. [DOI] [Google Scholar]; i Felpin F.-X.; Fouquet E.; Zakri C. Heck Cross-Coupling of Aryldiazonium Tetrafluoroborate with Acrylates Catalyzed by Palladium on Charcoal. Adv. Synth. Catal. 2008, 350, 2559–2565. 10.1002/adsc.200800539. [DOI] [Google Scholar]; j Schmidt B. Heck arylation of cyclic enol ethers with aryldiazonium salts: regio- and stereoselective synthesis of arylated oxacycles. Chem. Commun. 2003, 1656–1657. 10.1039/b305142k. [DOI] [Google Scholar]
- a Schmidt B.; Wolf F. Synthesis of Phenylpropanoids via Matsuda-Heck Coupling of Arene Diazonium Salts. J. Org. Chem. 2017, 82, 4386–4395. 10.1021/acs.joc.7b00447. [DOI] [PubMed] [Google Scholar]; b de Oliveira Silva J.; Angnes R. A.; Menezes da Silva V. H.; Servilha B. M.; Adeel M.; Braga A. A. C.; Aponick A.; Correia C. R. D. Intermolecular Noncovalent Hydroxy-Directed Enantioselective Heck Desymmetrization of Cyclopentenol: Computationally Driven Synthesis of Highly Functionalized cis-4-Arylcyclopentenol Scaffolds. J. Org. Chem. 2016, 81, 2010–2018. 10.1021/acs.joc.5b02846. [DOI] [PubMed] [Google Scholar]; c Schmidt B.; Elizarov N.; Riemer N.; Hölter F. Acetamidoarenediazonium Salts: Opportunities for Multiple Arene Functionalization. Eur. J. Org. Chem. 2015, 2015, 5826–5841. 10.1002/ejoc.201500795. [DOI] [Google Scholar]; d Oger N.; Le Callonnec F.; Jacquemin D.; Fouquet E.; Le Grognec E.; Felpin F.-X. Heck-Matsuda Arylation of Olefins Through a Bicatalytic Approach: Improved Procedures and Rationalization. Adv. Synth. Catal. 2014, 356, 1065–1071. 10.1002/adsc.201301144. [DOI] [Google Scholar]; e Canto K.; da Silva Ribeiro R.; Biajoli A. F. P.; Correia C. R. D. Expeditious Synthesis of the Marine Natural Products Prepolycitrin A and Polycitrins A and B through Heck Arylations. Eur. J. Org. Chem. 2013, 2013, 8004–8013. 10.1002/ejoc.201301108. [DOI] [Google Scholar]; f Nassar-Hardy L.; Fabre S.; Amer A. M.; Fouquet E.; Felpin F.-X. Synthesis of indanones by sequential Heck-reduction-cyclization-alkylation (HRCA) reactions. Tetrahedron Lett. 2012, 53, 338–341. 10.1016/j.tetlet.2011.11.042. [DOI] [Google Scholar]; g Felpin F.-X.; Miqueu K.; Sotiropoulos J.-M.; Fouquet E.; Ibarguren O.; Laudien J. Room-Temperature, Ligand- and Base-Free Heck Reactions of Aryl Diazonium Salts at Low Palladium Loading: Sustainable Preparation of Substituted Stilbene Derivatives. Chem.—Eur. J. 2010, 16, 5191–5204. 10.1002/chem.200903050. [DOI] [PubMed] [Google Scholar]
- a Schmidt B.; Elizarov N.; Schilde U.; Kelling A. Dual Role of Acetanilides: Traceless Removal of a Directing Group through Deacetylation/Diazotation and Palladium-Catalyzed C-C-Coupling Reactions. J. Org. Chem. 2015, 80, 4223–4234. 10.1021/acs.joc.5b00272. [DOI] [PubMed] [Google Scholar]; b El Bakouri O.; Fernández M.; Brun S.; Pla-Quintana A.; Roglans A. A simple catalytic system based on PdCl2(CH3CN)2 in water for cross-coupling reactions using diazonium salts. Tetrahedron 2013, 69, 9761–9765. 10.1016/j.tet.2013.09.010. [DOI] [Google Scholar]; c Biajoli A. F. P.; da Penha E. T.; Correia C. R. D. Palladium catalysed regioselective arylation of indoles, benzofuran and benzothiophene with aryldiazonium salts. RSC Adv. 2012, 2, 11930–11935. 10.1039/c2ra22213b. [DOI] [Google Scholar]; d Susperregui N.; Miqueu K.; Sotiropoulos J.-M.; Le Callonnec F.; Fouquet E.; Felpin F.-X. Sustainable Heck-Matsuda Reaction with Catalytic Amounts of Diazonium Salts: An Experimental and Theoretical Study. Chem.—Eur. J. 2012, 18, 7210–7218. 10.1002/chem.201200444. [DOI] [PubMed] [Google Scholar]; e Werner E. W.; Sigman M. S. Operationally simple and Highly (E)-Styrenyl-Selective Heck Reactions of Electronically Nonbiased Olefins. J. Am. Chem. Soc. 2011, 133, 9692–9695. 10.1021/ja203164p. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Schmidt B.; Hölter F.; Kelling A.; Schilde U. Pd-Catalyzed Arylation Reactions with Phenol Diazonium Salts: Application in the Synthesis of Diarylheptanoids. J. Org. Chem. 2011, 76, 3357–3365. 10.1021/jo2002787. [DOI] [PubMed] [Google Scholar]; g Raduán M.; Padrosa J.; Pla-Quintana A.; Parella T.; Roglans A. Functionalization of the 3-Position of Thiophene and Benzo[b]thiophene Moieties by Palladium-Catalyzed C-C Bond Forming Reactions using Diazonium Salts. Adv. Synth. Catal. 2011, 353, 2003–2012. 10.1002/adsc.201100226. [DOI] [Google Scholar]; h Felpin F.-X.; Coste J.; Zakri C.; Fouquet E. Preparation of 2-Quinolones by Sequential Heck Reduction-Cyclization (HRC) Reactions by Using a Multitask Palladium Catalyst. Chem.—Eur. J. 2009, 15, 7238–7245. 10.1002/chem.200900583. [DOI] [PubMed] [Google Scholar]
- a Schmidt B.; Wolf F.; Ehlert C. Systematic Investigation into the Matsuda-Heck Reaction of α-Methylene Lactones: How Conformational Constraints Direct the β-H-Elimination Step. J. Org. Chem. 2016, 81, 11235–11249. 10.1021/acs.joc.6b02207. [DOI] [PubMed] [Google Scholar]; b Kutonova K. V.; Trusova M. E.; Stankevich A. V.; Postnikov P. S.; Filimonov V. D. Matsuda-Heck reaction with arenediazonium tosylates in water. Beilstein J. Org. Chem. 2015, 11, 358–362. 10.3762/bjoc.11.41. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Oliveira C. C.; Angnes R. A.; Correia C. R. D. Intermolecular Enantioselective Heck-Matsuda Arylations of Acyclic Olefins: Application to the Synthesis of β-Aryl-γ-lactones and β-Aryl Aldehydes. J. Org. Chem. 2013, 78, 4373–4385. 10.1021/jo400378g. [DOI] [PubMed] [Google Scholar]; d Salabert J.; Sebastián R. M.; Vallribera A.; Roglans A.; Nájera C. Fluorous aryl compounds by Matsuda-Heck reaction. Tetrahedron 2011, 67, 8659–8664. 10.1016/j.tet.2011.09.046. [DOI] [Google Scholar]; e Schmidt B.; Hölter F.; Berger R.; Jessel S. Mizoroki-Heck Reactions with 4-Phenoldiazonium Salts. Adv. Synth. Catal. 2010, 352, 2463–2473. 10.1002/adsc.201000493. [DOI] [Google Scholar]; f Laudien J.; Fouquet E.; Zakri C.; Felpin F.-X. A Multi-Task Palladium Catalyst Involved in Heck-Reduction-Cyclization Sequences for the Preparation of 4-Benzyl-1,2-dihydroisoquinolin-3-ones: An Unusual Homogeneous-Heterogeneous Sustainable Approach. Synlett 2010, 2010, 1539–1543. 10.1055/s-0029-1219926. [DOI] [Google Scholar]; g Felpin F.-X.; Ibarguren O.; Nassar-Hardy L.; Fouquet E. Synthesis of Oxindoles by Tandem Heck-Reduction-Cyclization (HRC) from a Single Bifunctional, in Situ Generated Pd/C Catalyst. J. Org. Chem. 2009, 74, 1349–1352. 10.1021/jo802467s. [DOI] [PubMed] [Google Scholar]
- For C–H arylations see:; a Liang Y.-F.; Steinbock R.; Yang L.; Ackermann L. Continuous Visible-Light Photoflow Approach for a Manganese-Catalyzed (Het)Arene C–H Arylation. Angew. Chem., Int. Ed. 2018, 57, 10625–10629. 10.1002/anie.201805644. [DOI] [PubMed] [Google Scholar]; b Reay A. J.; Hammarback L. A.; Bray J. T. W.; Sheridan T.; Turnbull D.; Whitwood A. C.; Fairlamb I. J. S. Mild and Regioselective Pd(OAc)2-Catalyzed C–H Arylation of Tryptophans by [ArN2]X, Promoted by Tosic Acid. ACS Catal. 2017, 7, 5174–5179. 10.1021/acscatal.6b03121. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Crisýstomo F. P.; Martin T.; Carrillo R. Ascorbic Acid as an Initiator for the Direct C-H Arylation of (Hetero)arenes with Anilines Nitrosated In Situ. Angew. Chem., Int. Ed. 2014, 53, 2181–2185. [DOI] [PubMed] [Google Scholar]; d Honraedt A.; Raux M.-A.; Grognec E. L.; Jacquemin D.; Felpin F.-X. Copper-catalyzed free-radical C-H arylation of pyrroles. Chem. Commun. 2014, 50, 5236–5238. 10.1039/c3cc45240a. [DOI] [PubMed] [Google Scholar]; e Prediger P.; da Silva A. R.; Correia C. R. D. Construction of 3-arylpropylamines using Heck arylations. The total synthesis of cinacalcet hydrochloride, alverine, and tolpropamine. Tetrahedron 2014, 70, 3333–3341. 10.1016/j.tet.2013.10.014. [DOI] [Google Scholar]; f Hari D. P.; Schroll P.; König B. Metal-Free, Visible-Light-Mediated Direct C-H Arylation of Heteroarenes with Aryl Diazonium Salts. J. Am. Chem. Soc. 2012, 134, 2958–2961. 10.1021/ja212099r. [DOI] [PubMed] [Google Scholar]; g Wetzel A.; Ehrhardt V.; Heinrich M. R. Synthesis of Amino- and Hydroxybiphenyls by Radical Chain Reaction of Arenediazonium Salts. Angew. Chem., Int. Ed. 2008, 47, 9130–9133. 10.1002/anie.200803785. [DOI] [PubMed] [Google Scholar]; h Gomberg M.; Bachmann W. E. The synthesis of biaryl compounds by means of the diazo reaction. J. Am. Chem. Soc. 1924, 46, 2339–2343. 10.1021/ja01675a026. [DOI] [Google Scholar]; i Pschorr R. Neue Synthese des Phenanthrens und seiner Derivate. Ber. Dtsch. Chem. Ges. 1896, 29, 496–501. 10.1002/cber.18960290198. [DOI] [Google Scholar]
- Roglans A.; Pla-Quintana A.; Moreno-Mañas M. Diazonium Salts as Substrates in Palladium-Catalyzed Cross-Coupling Reactions. Chem. Rev. 2006, 106, 4622–4643. 10.1021/cr0509861. [DOI] [PubMed] [Google Scholar]
- a Crabtree R. H.; Lei A. Introduction: CH Activation. Chem. Rev. 2017, 117, 8481–8482. 10.1021/acs.chemrev.7b00307. [DOI] [PubMed] [Google Scholar]; b Lyons T. W.; Sanford M. S. Palladium-Catalyzed Ligand-Directed C–H Functionalization Reactions. Chem. Rev. 2010, 110, 1147–1169. 10.1021/cr900184e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin K.; Park S.-W.; Chang S. Cp*Ir(III)-Catalyzed Mild and Broad C–H Arylation of Arenes and Alkenes with Aryldiazonium Salts Leading to the External Oxidant-Free Approach. J. Am. Chem. Soc. 2015, 137, 8584–8592. 10.1021/jacs.5b04043. [DOI] [PubMed] [Google Scholar]
- Huang L.; Hackenberger D.; Gooßen L. J. Iridium-Catalyzed ortho-Arylation of Benzoic Acids with Arenediazonium Salts. Angew. Chem., Int. Ed. 2015, 54, 12607–12611. 10.1002/anie.201505769. [DOI] [PubMed] [Google Scholar]
- Kalyani D.; McMurtrey K. B.; Neufeldt S. R.; Sanford M. S. Room-Temperature C-H Arylation: Merger of Pd-Catalyzed C-H Functionalization and Visible-Light Photocatalysis. J. Am. Chem. Soc. 2011, 133, 18566–18569. 10.1021/ja208068w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Sahoo M. K.; Midya S. P.; Landge V. G.; Balaraman E. A unified strategy for silver-, base-, and oxidant-free direct arylation of C-H bonds. Green Chem. 2017, 19, 2111–2117. 10.1039/c6gc03438a. [DOI] [Google Scholar]; b Jiang J.; Zhang W.-M.; Dai J.-J.; Xu J.; Xu H.-J. Visible-Light-Promoted C-H Arylation by Merging Palladium Catalysis with Organic Photoredox Catalysis. J. Org. Chem. 2017, 82, 3622–3630. 10.1021/acs.joc.7b00140. [DOI] [PubMed] [Google Scholar]; c Khan R.; Boonseng S.; Kemmitt P. D.; Felix R.; Coles S. J.; Tizzard G. J.; Williams G.; Simmonds O.; Harvey J.-L.; Atack J.; Cox H.; Spencer J. Combining Sanford Arylations on Benzodiazepines with the Nuisance Effect. Adv. Synth. Catal. 2017, 359, 3261–3269. 10.1002/adsc.201700626. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Sahoo M. K.; Rana J.; Subaramanian M.; Balaraman E. Room-Temperature Direct Arylation of Anilides under External Oxidant-Free Conditions Using CO2-Derived Dimethyl Carbonate (DMC) as a ’Green’ Solvent. ChemistrySelect 2017, 2, 7565–7569. 10.1002/slct.201701108. [DOI] [Google Scholar]
- a Chinnagolla R. K.; Jeganmohan M. Ruthenium-catalyzed ortho-arylation of acetanilides with aromatic boronic acids: an easy route to prepare phenanthridines and carbazoles. Chem. Commun. 2014, 50, 2442–2444. 10.1039/c3cc49398a. [DOI] [PubMed] [Google Scholar]; b Nishikata T.; Abela A. R.; Huang S.; Lipshutz B. H. Cationic Palladium(II) Catalysis: C–H Activation/Suzuki–Miyaura Couplings at Room Temperature. J. Am. Chem. Soc. 2010, 132, 4978–4979. 10.1021/ja910973a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlig N.; Li C.-J. Aniline Carbamates: A Versatile and Removable Motif for Palladium-Catalyzed Directed C-H Activation. Chem.—Eur. J. 2014, 20, 12066–12070. 10.1002/chem.201403712. [DOI] [PubMed] [Google Scholar]
- Manna M. K.; Hossian A.; Jana R. Merging C-H Activation and Alkene Difunctionalization at Room Temperature: A Palladium-Catalyzed Divergent Synthesis of Indoles and Indolines. Org. Lett. 2015, 17, 672–675. 10.1021/ol5036968. [DOI] [PubMed] [Google Scholar]
- Chaudhari T. Y.; Hossian A.; Manna M. K.; Jana R. Chemo-, regio-, and stereoselective Heck-Matsuda arylation of allylic alcohols under mild conditions. Org. Biomol. Chem. 2015, 13, 4841–4845. 10.1039/c5ob00235d. [DOI] [PubMed] [Google Scholar]
- Nishikata T.; Abela A. R.; Huang S.; Lipshutz B. H. Cationic Pd(II)-catalyzed C-H activation/cross-coupling reactions at room temperature: synthetic and mechanistic studies. Beilstein J. Org. Chem. 2016, 12, 1040–1064. 10.3762/bjoc.12.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu Y.-C.; Wang V. C.-C.; Au-Yeung K.-C.; Tsai C.-Y.; Chang C.-C.; Lin B.-C.; Chan Y.-T.; Hsu C.-P.; Yap G. P. A.; Jurca T.; Ong T.-G. One-Pot Tandem Photoredox and Cross-Coupling Catalysis with a Single Palladium Carbodicarbene Complex. Angew. Chem., Int. Ed. 2018, 57, 4622–4626. 10.1002/anie.201800951. [DOI] [PubMed] [Google Scholar]
- For a review of the synthesis of carbazoles and related nitrogen heterocycles through transition metal-catalyzed C–H functionalization reactions, see:; a Yoshikai N.; Wei Y. Synthesis of Pyrroles, Indoles, and Carbazoles through Transition-Metal-Catalyzed C-H Functionalization. Asian J. Org. Chem. 2013, 2, 466–478. 10.1002/ajoc.201300016. [DOI] [Google Scholar]; b Schmidt A. W.; Reddy K. R.; Knölker H.-J. Occurrence, Biogenesis, and Synthesis of Biologically Active Carbazole Alkaloids. Chem. Rev. 2012, 112, 3193–3328. 10.1021/cr200447s. [DOI] [PubMed] [Google Scholar]
- a Suzuki C.; Hirano K.; Satoh T.; Miura M. Direct Synthesis of N-H Carbazoles via Iridium(III)-Catalyzed Intramolecular C-H Amination. Org. Lett. 2015, 17, 1597–1600. 10.1021/acs.orglett.5b00502. [DOI] [PubMed] [Google Scholar]; b Stokes B. J.; Jovanović B.; Dong H.; Richert K. J.; Riell R. D.; Driver T. G. Rh2(II)-Catalyzed Synthesis of Carbazoles from Biaryl Azides. J. Org. Chem. 2009, 74, 3225–3228. 10.1021/jo9002536. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Jordan-Hore J. A.; Johansson C. C. C.; Gulias M.; Beck E. M.; Gaunt M. J. Oxidative Pd(II)-Catalyzed C–H Bond Amination to Carbazole at Ambient Temperature. J. Am. Chem. Soc. 2008, 130, 16184–16186. 10.1021/ja806543s. [DOI] [PubMed] [Google Scholar]; d Tsang W. C. P.; Zheng N.; Buchwald S. L. Combined C–H Functionalization/C–N Bond Formation Route to Carbazoles. J. Am. Chem. Soc. 2005, 127, 14560–14561. 10.1021/ja055353i. [DOI] [PubMed] [Google Scholar]
- a Chakraborti G.; Paladhi S.; Mandal T.; Dash J. ″On Water’’ Promoted Ullmann-Type C-N Bond-Forming Reactions: Application to Carbazole Alkaloids by Selective N-Arylation of Aminophenols. J. Org. Chem. 2018, 83, 7347–7359. 10.1021/acs.joc.7b03020. [DOI] [PubMed] [Google Scholar]; b Gensch T.; Richter N.; Theumer G.; Kataeva O.; Knölker H.-J. Synthesis of Stable Diarylpalladium(II) Complexes: Detailed Study of the Aryl-Aryl Bond-Forming Reductive Elimination. Chem.—Eur. J. 2016, 22, 11186–11190. 10.1002/chem.201602849. [DOI] [PubMed] [Google Scholar]; c Gensch T.; Rönnefahrt M.; Czerwonka R.; Jäger A.; Kataeva O.; Bauer I.; Knölker H.-J. Snapshot of the Palladium(II)-Catalyzed Oxidative Biaryl Bond Formation by X-ray Analysis of the Intermediate Diaryl Palladium(II) Complex. Chem.—Eur. J. 2012, 18, 770–776. 10.1002/chem.201103576. [DOI] [PubMed] [Google Scholar]; d Liegault B.; Lee D.; Huestis M. P.; Stuart D. R.; Fagnou K. Intramolecular Pd(II)-Catalyzed Oxidative Biaryl Synthesis Under Air: Reaction Development and Scope. J. Org. Chem. 2008, 73, 5022–5028. [DOI] [PubMed] [Google Scholar]; e Liu Z.; Larock R. C. Synthesis of Carbazoles and Dibenzofurans via Cross-Coupling ofo-Iodoanilines ando-Iodophenols with Silylaryl Triflates. Org. Lett. 2004, 6, 3739–3741. 10.1021/ol048564l. [DOI] [PubMed] [Google Scholar]
- Takamatsu K.; Hirano K.; Satoh T.; Miura M. Synthesis of Carbazoles by Copper-Catalyzed Intramolecular C-H/N-H Coupling. Org. Lett. 2014, 16, 2892–2895. 10.1021/ol501037j. [DOI] [PubMed] [Google Scholar]
- Ito C.; Katsuno S.; Ohta H.; Omura M.; Kajiura I.; Furukawa H. Constituents of Clausena excavata. Isolation and Structural Elucidation of New Carbazole Alkaloids. Chem. Pharm. Bull. 1997, 45, 48–52. 10.1248/cpb.45.48. [DOI] [Google Scholar]
- a Lin W.; Wang Y.; Lin S.; Li C.; Zhou C.; Wang S.; Huang H.; Liu P.; Ye G.; Shen X. Induction of cell cycle arrest by the carbazole alkaloid Clauszoline-I from Clausena vestita D. D. Tao via inhibition of the PKCδ phosphorylation. Eur. J. Med. Chem. 2012, 47, 214–220. 10.1016/j.ejmech.2011.10.047. [DOI] [PubMed] [Google Scholar]; b Birari R.; Roy S. K.; Singh A.; Bhutani K. Pancreatic lipase inhibitory alkaloids of Murraya koenigii leaves. Nat. Prod. Commun. 2009, 4, 1089–1092. [PubMed] [Google Scholar]; c Krahl M. P.; Jäger A.; Krause T.; Knölker H.-J. First total synthesis of the 7-oxygenated carbazole alkaloids clauszoline-K, 3-formyl-7-hydroxycarbazole, clausine M, clausine N and the anti-HIV active siamenol using a highly efficient palladium-catalyzed approach siamenol using a highly efficient palladium-catalyzed approach. Org. Biomol. Chem. 2006, 4, 3215–3219. 10.1039/b607792g. [DOI] [PubMed] [Google Scholar]
- a Amin K. S.; Jagadeesh S.; Baishya G.; Rao P. G.; Barua N. C.; Bhattacharya S.; Banerjee P. P. A Naturally Derived Small Molecule Disrupts Ligand-Dependent and Ligand-Independent Androgen Receptor Signaling in Human Prostate Cancer Cells. Mol. Cancer Ther. 2014, 13, 341–352. 10.1158/1535-7163.mct-13-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Agarwal S.; Amin K. S.; Jagadeesh S.; Baishay G.; Rao P. G.; Barua N. C.; Bhattacharya S.; Banerjee P. P. Mahanine restores RASSF1A expression by down-regulating DNMT1 and DNMT3B in prostate cancer cells. Mol. Cancer 2013, 12, 99. 10.1186/1476-4598-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Samanta S. K.; Dutta D.; Roy S.; Bhattacharya K.; Sarkar S.; Dasgupta A. K.; Pal B. C.; Mandal C.; Mandal C. Mahanine, A DNA Minor Groove Binding Agent Exerts Cellular Cytotoxicity with Involvement of C-7-OH and −NH Functional Groups. J. Med. Chem. 2013, 56, 5709–5721. 10.1021/jm400290q. [DOI] [PubMed] [Google Scholar]; d Bhattacharya K.; Samanta S. K.; Tripathi R.; Mallick A.; Chandra S.; Pal B. C.; Shaha C.; Mandal C. Apoptotic effects of mahanine on human leukemic cells are mediated through crosstalk between Apo-1/Fas signaling and the Bid protein and via mitochondrial pathways. Biochem. Pharmacol. 2010, 79, 361–372. 10.1016/j.bcp.2009.09.007. [DOI] [PubMed] [Google Scholar]; e Narasimhan N. S.; Paradkar M. V.; Kelkar S. L. Alkaloids of Murraya koenigii: structures of mahanimbine, koenimbine, (-)-mahanine, koenine, koenigine, koenidine & (+)-isomahanimbine. Indian J. Chem. 1970, 8, 473. [Google Scholar]
- a Hou S.; Liu Y.; Kong Y.; Brown M. L. Total Synthesis of 7-Hydroxymurrayazolinine, Murrayamine D, and Mahanine via m-Nitro Group Activated Pyran Annulation. Org. Lett. 2015, 17, 2298–2301. 10.1021/acs.orglett.5b00422. [DOI] [PubMed] [Google Scholar]; b Julich-Gruner K. K.; Kataeva O.; Schmidt A. W.; Knölker H.-J. Total synthesis of 7- and 8-oxygenated pyrano[3,2-a]carbazole and pyrano[2,3-a]carbazole alkaloids via boronic acid-catalyzed annulation of the pyran ring. Chem.—Eur. J. 2014, 20, 8536–8540. 10.1002/chem.201403143. [DOI] [PubMed] [Google Scholar]
- Hesse R.; Jäger A.; Schmidt A. W.; Knölker H.-J. Palladium(II)-catalysed total synthesis of naturally occurring pyrano[3,2-a]carbazole and pyrano[2,3-b]carbazole alkaloids. Org. Biomol. Chem. 2014, 12, 3866–3876. 10.1039/c4ob00367e. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.