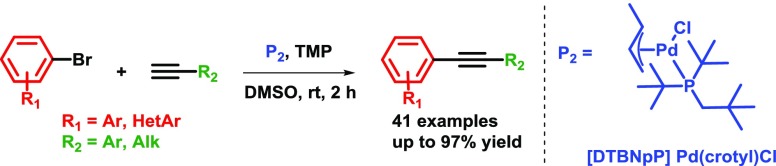
Abstract
A novel application of [DTBNpP] Pd(crotyl)Cl (DTBNpP = di-tert-butylneopentylphosphine) (P2), an air-stable, commercially available palladium precatalyst that allows rapid access to a monoligated state, has been identified for room-temperature, copper-free Sonogashira couplings of challenging aryl bromides and alkynes. The mild reaction conditions with TMP in dimethyl sulfoxide afford up to 97% yields, excellent functional group tolerability, and broad reaction compatibility with access to one-pot indole formation.
Introduction
The Sonogashira reaction, catalyzing C(sp)-C(sp2) bond formation, is a vital tool in academia and industry, for its ability to increase conjugation and rigidity in natural product synthesis, drug development, molecular electronics, nanoscale scaffolds, and heterocyclic chemistry.1−4 Historically, the use of copper co-catalysts with palladium allowed for mild reaction conditions due to transmetallation, as reported by Sonogashira.5 However, undesirable homocoupling of acetylenes, air sensitivity, moisture sensitivity, and difficulties in pharmaceutical purification processes prompted the need for well-refined, copper-free systems while broadening the scope for challenging substrates. Classic addition of a ligand to an air-stable Pd-source (such as Pd(OAc)2 or Pd2(dba)3) may encounter disruption from inadvertent ligand coordination, catalyst impurity, or delayed catalyst generation due to in situ formation.6−10 Even with the variety of palladium sources and ligands currently available, arguably the most popular conditions for Sonogashira reactions are Pd(PPh3)4, a catalyst lacking air stability, and Pd(PPh3)2Cl2. These reactions frequently require high temperatures, copper salts, or additional ligands that may be pyrophoric or noncommercial, limiting feasibility.11 Among newly published protocols some call for atypical solvents, additives, or catalysts, and many requiring long reaction times and show few examples of pharmaceutically relevant heteroaromatics.12−17 Although few attractive methods are available in the literature,18,19 it is still desirable to develop a more robust protocol broadly applicable for complex substrates.
Recently developed preformed palladacycles20 and palladium dimers21,22 allow for a defined ligand ratio and exhibit greater catalytic rates than their individual counterparts, exemplifying the utility of preformed palladium complexes for numerous carbon–carbon couplings. Additionally, these palladium precatalysts pose an improvement to traditional catalysts by exhibiting greater stability and feasibility in reaction set up while still providing the same active catalyst. Despite the coordinative potential of palladium, monoligated adducts (L1Pd0) frequently produce a more efficient catalyst and thus there is a desire for precatalysts capable of producing monoligated palladium in situ.23−25 The proposed mechanism for monoligated precatalysts begins by activation to the LPd0 state, utilizing bulky, electron-rich ligands to stabilize a reactive catalytic species that facilitates faster oxidative addition and efficient catalytic cycle.26,27 One of the prominent monoligated precatalyst backbones was introduced by Buchwald in 2007: air-stable palladacycles that are readily activated by deprotonation under mild reaction conditions to obtain the monoligated Pd0 complex via reductive elimination of the intramolecular amine–aryl group.28−32 Another successful, commercially available precatalysts synthesized in 2002 by Nolan and co-workers utilize an allyl-based palladium precatalyst that incorporates the NHC-carbene ligand. This monoligated precatalyst is air stable, room-temperature activated and capable of performing efficient Suzuki, Buchwald, and other cross-couplings.33,34 In 2010, Shaughnessy35 and Colacot36 found success replacing the NHC-ligand on Pd(allyl)Cl complexes with bulky phosphines or varying the π-allyl substituents in Suzuki, α-arylation, and amination reactions. These base-promoted, bench-stable precatalysts reductively eliminate a noninhibitory olefin byproduct while creating an active palladium complex proficient in a wide range of cross-coupling reactions, including Sonogashira.27,37−39
Results and Discussion
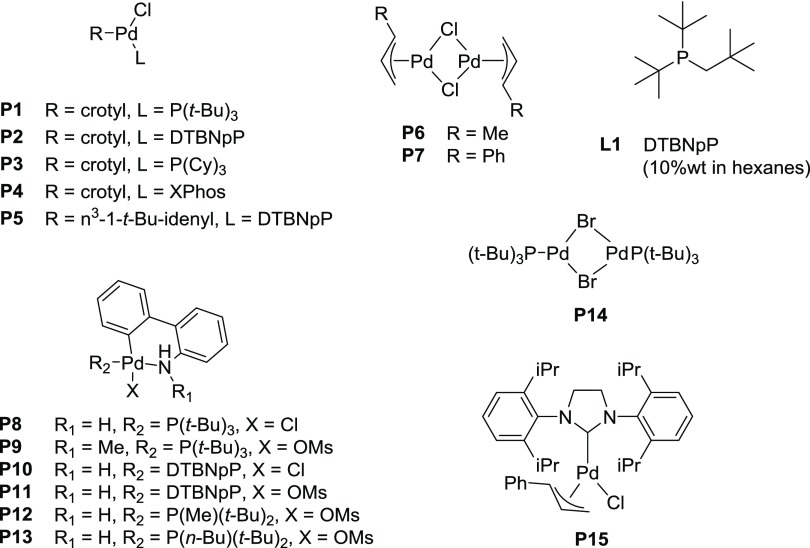
The Sonogashira coupling has become a prominent intermediate step and functional group addition for medicinal chemistry projects. A quick survey of prominent medicinal chemistry journals finds 18 references already this year and 75 in 2017 employing the alkyne coupling, yet, Pd(PPh3)4, Pd(PPh3)2Cl2, and palladium salts with copper-dominated catalyst conditions. During our medicinal chemistry efforts in synthesizing novel inhibitors of human lactate dehydrogenase, we required a reliable and facile Sonogashira coupling condition for library synthesis. These classical conditions were minimally successful with our diversely functionalized substrates and risked catalyst poisoning by incorporating nitrogen and sulfur containing aryl groups, especially when attempting scale-up procedures. We sought an improved catalyst and optimized condition applicable to a wide variety of functional groups for application in our work as well other medicinal chemistry projects that experienced limitations similar to those listed above. The initial catalyst search was based off the work of Soheili et al.,40 who performed Sonogashira couplings at room temperature with allyl palladium chloride and P(t-Bu)3, without copper, and hypothesized the formation of a monoligated active L1Pd0 catalyst using these substrates. In view of the success of Buchwald and allyl monoligated palladium catalysts in numerous cross-coupling reactions,41 we envisioned that preformed Buchwald and allyl monoligated palladium precatalysts with bulky, electron-rich phosphines, have the capability to be a bench-stable, monoligated precatalyst for copper-free Sonogashira couplings. These next generation catalysts would provide a powerful synthetic solution by expanding the scope, increasing catalytic rates, and eliminating the use of pyrophoric ligands. Herein, we report the catalytic efficiencies of several Buchwald palladacycles and allyl monoligated palladium catalysts (Figure 1) applied to the Sonogashira reaction.
Figure 1.
Currently established palladium precatalysts: P1–P4, P6–P15. Ligand for in situ catalyst: L1. P5 was synthesized in house.
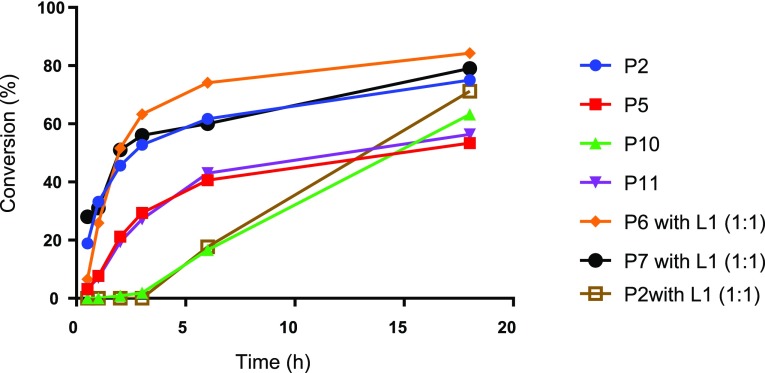
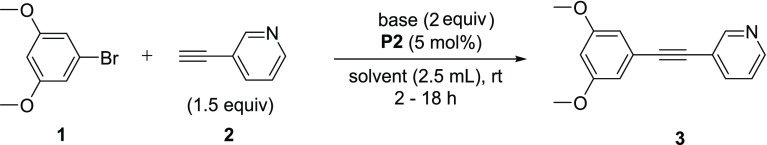
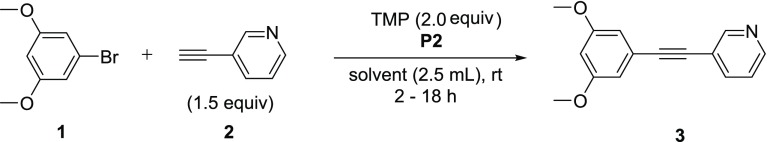
Initial efforts were focused on the screening of precatalysts P1–P14 using challenging coupling partners. The 3,5-dimethoxyphenyl bromide (1), which does not give any product under classical Sonogashira conditions40 (entry 1 in Table 1) due to hindered oxidative addition step, and an electron-deficient heteroaromatic alkyne (2), a representative for difficult heteroaromatic alkynes that are frequently employed in medicinal chemistry and pharmaceutical development, were coupled with base DABCO in tetrahydrofuran (THF), conditions similar to those used by Soheli et al. For comparison, several difficult electron-rich substrates, such as p-bromophenol (entry 5m), p-bromoaniline (entry 5n), or 3-bromothiophene (entry 5r), were coupled using our protocol. The π–allyl palladium-based catalyst (P1), which incorporated P(t-Bu)3 and a crotyl ligand, successfully produced coupling product 3, without copper, in moderate yield (52%, entry 2). In changing the phosphine ligand to DTBNpP (P2) and XPhos (P4) with the same palladium precatalyst, the P2 catalyst revealed to be a more efficient catalyst with a 75% yield (entries 3 and 5).42 However, the activity diminished dramatically when P(t-Bu)3 was replaced with P(Cy)3 (P3, entry 4). Attempting to improve yields with the η3-1-t-Bu-indenyl ligand (P5) (investigated by Hazari et al.)43 led to only 53% yield (entry 6). The original π–allyl palladium complex without a bulky phosphine ligand, unsurprisingly showed no appreciable product (entries 7 and 8). Switching to Buchwald type precatalysts, both the P8 and P9 catalysts that contain the P(t-Bu)3 ligand were able to produce the desired product, but much less effectively than P2 (23–27% yield, entries 9 and 10). However, P10 and P11 incorporating the DTBNpP ligand markedly increased the yields to 63 and 56%, respectively (entries 11 and 12). Furthermore, P12 and P13 catalysts with P(t-Bu)2(Me) and P(t-Bu)2(n-Bu) ligand, respectively, almost completely diminished catalyst activity (entries 13 and 14), proving the specificity of the DTBNpP ligand for this system. The catalyst P14, a precursor to L1Pd0 and successfully used in amination reactions,10 demonstrated negligible activity within these conditions (entry 15). Additionally, a commercially available NHC precatalyst, P15, with a cinnamyl and chloride ligand similar to the phosphine-based allyl precatalysts, was tested for comparison; however, it afforded no product (entry 16). After finding P2 as the most efficient precatalyst, we then compared its reactivity with the catalyst formed in situ. The catalyst formed from 1:1 (Pd/P) ratio of P6/L1 exhibits a slightly higher yield after 18 h compared to P2, but a lower yield within the first hour, likely due to formation of the active catalyst (entry 17). Exchanging the crotyl ligand for cinnamyl to form the in situ catalyst exhibited no significant improvement and provided comparable product formation over time (entry 18). The addition of L1 to P2 (1:1) significantly retarded P2 activity with almost no conversion observed during the first 3 h (entry 19). However, the activity progressed gradually to produce a similar yield as independent P2 after 18 h (Figure 2 and entry 17 vs entry 3). This could be due to the initial formation of the coordinately saturated 14-electron L2Pd0. The remainder of catalysts in Figure 2 tapered off their rate of product formation after 6 h, except for P10, which exhibited an increase in activity after 3 h possibly due to delayed release of the aromatic amine. A comparison of the DTBNpP-containing catalysts in Figure 2 demonstrates the efficiency of P2 compared to its individual ligand/palladium sources and over the Buchwald precatalysts for the Sonogashira reaction.
Table 1. Catalyst Screening with 1-Bromo-3,5-dimethoxybenzene (1) and 3-Ethynylpyridine (2)a.
| entry | catalyst | 3 (yield, %)b |
|---|---|---|
| 1 | Pd(PPh3)2Cl2, CuI | 0c |
| 2 | P1 | 52 |
| 3 | P2 | 75 |
| 4 | P3 | 0 |
| 5 | P4 | 57 |
| 6 | P5 | 53 |
| 7 | P6 | 0 |
| 8 | P7 | 0 |
| 9 | P8 | 27 |
| 10 | P9 | 23 |
| 11 | P10 | 63 |
| 12 | P11 | 56 |
| 13 | P12 | 0 |
| 14 | P13 | 4 |
| 15 | P14 | 1 |
| 16 | P15 | 0 |
| 17 | P6 with L1 (1:1) | 84d |
| 18 | P7 with L1 (1:1) | 78d |
| 19 | P2 with L1 (1:1) | 71d |
| 20 | P2 | 2e |
Reaction conditions: 1 (0.5 mmol), 2 (0.8 mmol), cat. (.025 mmol), DABCO (1.0 mmol), THF (2.5 mL), rt for 18 h under argon atmosphere.
Yield was determined by liquid chromatography/mass spectrometry (LC/MS) with pyrene as internal standard.
Followed standard Sonogashira reaction procedure.
Catalyst and ligand stirred for 5 min prior to reagent addition.
Without degassing using argon or nitrogen.
Figure 2.
Conversion of 2 over an 18 h period using catalysts containing the DTBNpP ligand.
With the finding of efficient catalyst P2, the effects of base and solvent were next examined (Table 2). By using DABCO as the base, the reaction achieved less than 50% yield with nonpolar (e.g., DCM and MTBE) or polar protic solvents (e.g., MeOH and EtOH) (entries 1–4). In contrast, using polar aprotic solvents (e.g., THF, ACN, DMF, and DMSO) provided better conversions with 62–100% yield, DMSO being the best solvent in this reaction condition (entries 5, 7, 8, and 11). Several exceptions were also observed during the solvent screening. For instance, a good yield (74%) was observed by using the nonpolar 1,4-dioxane solvent (entry 6), but the reaction performed poorly in NMP, a polar solvent (40% yield, entry 9). Moderate yields were found by using Lipshutz’s Sonogoshira protocol in water using 3% nonionic amphiphile PTS (entry 10).44 DMSO was carried through the screening of various bases (entries 12–29). As expected, the lack of base afforded no product (entry 12). A wide variety of inorganic bases produced excellent yield over the course of 18 h (entries 13–16). Among them, NaOAc was the only one that reached a high yield (86%) in 2 h (entry 13). Although KHCO3 as the base is less effective, Cs2CO3 and TBAF were found to be totally ineffective (entries 17–19). Continuously, various organic bases were screened under the same conditions. Sterically hindered amines, such as TMP, t-BuNH2, (i-Pr)2NH, and Hunig’s base, reached high yields (>84%) in 2 h (entries 20–24). However, using Et2NH and Et3N as the bases only produced product in moderate yield (56–58%) after 18 h (entries 24 and 25). Some cyclic amines, e.g., pyrrolidine and piperidine, were also effective bases, but required 18 h to reach completion (entries 26 and 27). Morpholine and DBU were found to be much less effective (entries 28 and 29). Of the bases, (i-Pr)2NH and TMP afforded product 3 (100%) within 2 h, making them both attractive bases for this reaction. TMP was used in subsequent optimization due to faster product formation within 2 h; although, (i-Pr)2NH is a valuable cost-effective substitute.
Table 2. Optimization of Base and Solvent in the Coupling of 1 and 2a.
|
3 (yield, %)b |
||||
|---|---|---|---|---|
| entry | base | solvent | t = 2 h | t = 18 h |
| 1 | DABCO | MTBE | 42 | 54 |
| 2 | DABCO | DCM | 25 | 35 |
| 3 | DABCO | MeOH | 27 | 35 |
| 4 | DABCO | EtOH | 26 | 40 |
| 5 | DABCO | THF | 46 | 72 |
| 6 | DABCO | 1,4-dioxane | 40 | 74 |
| 7 | DABCO | ACN | 67 | 82 |
| 8 | DABCO | DMF | 42 | 62 |
| 9 | DABCO | NMP | 26 | 40 |
| 10 | DABCO | 3 wt % PTS in H2O | 33 | 50 |
| 11 | DABCO | DMSO | 91 | 100 |
| 12 | none | DMSO | 0 | 0 |
| 13 | NaOAc | DMSO | 86 | 100 |
| 14 | KOH | DMSO | 50 | 100 |
| 15 | K2CO3 | DMSO | 50 | 100 |
| 16 | K3PO4 | DMSO | 51 | 100 |
| 17 | KHCO3 | DMSO | 26 | 43 |
| 18 | Cs2CO3 | DMSO | 0 | 0 |
| 19 | TBAF | DMSO | 0 | 0 |
| 20 | t-BuNH2 | DMSO | 86 | 89 |
| 21 | iPr2NH | DMSO | 100 | 100 |
| 22 | Hunigs’ base | DMSO | 84 | 100 |
| 23 | TMP | DMSO | 100 | 100 |
| 24 | Et2NH | DMSO | 15 | 56 |
| 25 | Et3N | DMSO | 53 | 58 |
| 26 | pyrrolidine | DMSO | 27 | 100 |
| 27 | piperidine | DMSO | 42 | 100 |
| 28 | morpholine | DMSO | 20 | 58 |
| 29 | DBU | DMSO | 2 | 10 |
Reaction conditions: 1 (0.5 mmol), 2 (0.8 mmol), P2 (0.025 mmol, 5 mol %), base (1.0 mmol), solvent (2.5 mL), rt for 18 h under argon atmosphere.
Yield was determined by LC/MS with pyrene as internal standard.
TMP was re-evaluated with alternative and sustainable solvents45 to broaden the applicability of these conditions for purposes, such as process chemistry (Table S1). Although the THF/TMP combination was not as productive as DMSO/TMP (Table S1, entry 1), high yields were still obtained after 18 h (70%). Sustainable solvents, such as 2-MeTHF and sulfolane, were able to provide acceptable yields after 18 h if a greener solvent is desired (Table S1, entries 2 and 3). EtOAc, a solvent recommended for environmentally friendly chemistry and a top 10 solvent used in GSK pilot operations in 2005, was able to produce a 62% yield in 18 h that could likely be increased with heating (Table S1, entry 4). ACN was the most effective solvent from this table (Table S1, entry 5) with product formation similar to DMSO as the solvent, a positive result considering its use in process chemistry and recommended use in medicinal chemistry.
TMP and DMSO were chosen to evaluate catalyst loading (Table 3). The 5 mol % catalyst loading produced a 96% yield in only 0.5 h, whereas a 2.5 mol % achieved 77% (entries 1 and 2). Both conditions were highly effective, reaching 100% yield in 1.5 h. Decreasing loading to 1 or cot0.5 mol % resulted in significant product formation delays (48 and 42%, respectively), though both went to completion by 18 h (entries 3 and 4). Increasing the temperature to 60 and 100 °C allowed the catalyst load to be decreased to 0.5 mol % while still achieving 80 and 85% yield, respectively, in 0.5 h. For our purposes, a 2.5 mol % catalyst load was chosen to limit palladium usage while retaining rapid product formation at room temperature.
Table 3. Effect of Catalyst Loading in the Coupling of 1 and 2a.
|
3 (yield, %)b |
|||||
|---|---|---|---|---|---|
| entry | catalyst load (%) | temperature (°C) | t = 0.5 h | t = 1.5 h | t = 18 h |
| 1 | 5 | rt | 96 | 100 | 100 |
| 2 | 2.5 | rt | 77 | 100 (86)c | 100 |
| 3 | 1.0 | rt | 25 | 48 | 92 |
| 4 | 60 | 100 | 100 | 100 | |
| 5 | 100 | 100 | 100 | 100 | |
| 6 | 0.5 | rt | 15 | 42 | 88 |
| 7 | 60 | 80 | 97 | 100 | |
| 8 | 100 | 85 | 93 | 100 | |
| 9 | 0.1 | 60 | 15 | 33 | 56 |
| 10 | 100 | 25 | 26 | 39 | |
| 11 | 0.01 | 60 | 0 | 0 | 2 |
| 12 | 100 | 4 | 5 | 10 | |
Reaction conditions: 1 (0.5 mmol), 2 (0.8 mmol), [DTBNpP] Pd(crotyl)Cl, TMP (1.0 mmol), DMSO (2.5 mL), rt for 18 h under argon atmosphere.
Yield was determined by LC/MS with pyrene as internal standard.
Parentheses indicate an isolated yield.
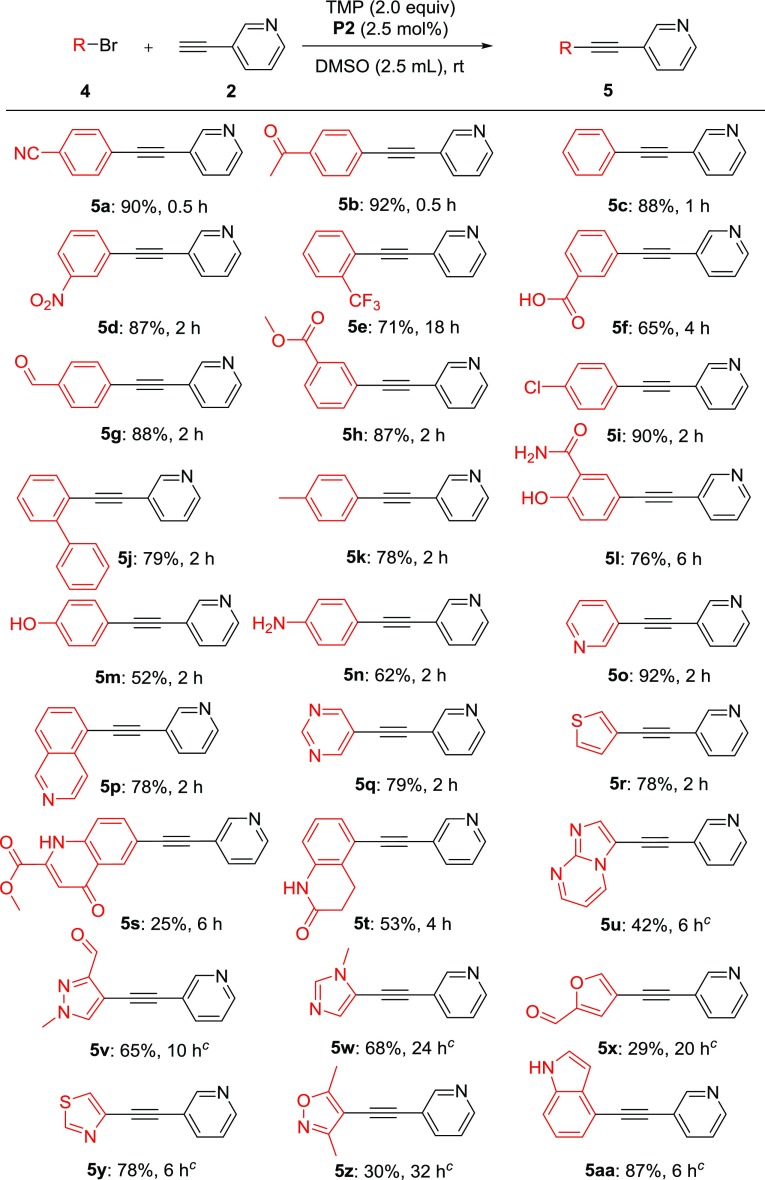
The attention was then shifted to exploration of coupling partner scope using the optimized reaction condition, namely, P2 (2.5 mol %), TMP, and DMSO. Various bromides were tested, and the results are summarized in Scheme 1. In general, electron-withdrawing group-substituted aryl bromides (5a–d and 5f–i) and heteroaryl bromides (5o–r) were completed within 2–4 h with high isolated yields (65–92%). The exception was the 2-CF3 substitution (5e), requiring 18 h to reach completion. Though the aryl bromides with electron-donating substitutions (5j–n) had slightly lower yields (52–78%), the electronic substitution effect seemed minimal. These examples also demonstrated excellent tolerability of functional groups, including nitrile, nitro group, ketone, carboxylic acid, ester, amide, as well as unprotected hydroxyl (5l, 5m) and amino (5n) groups. However, some heterocycles, such as 5t–aa, required slightly elevated temperature (60 °C) to push to completion with moderate to good yields (42–87%). Despite the challenging sterics of 4-bromo-3,5-dimethylisoxazole, it achieved 61% conversion over 32 h, however, difficulty in purification likely due to instability limited isolated yields (5z). In addition, an 87% yield was attained using 4-bromo-1H-indole in 6 h (5aa). The present protocol is not applicable for aryl chlorides, as exemplified by 5i, only exhibiting bromide coupling.
Scheme 1. Scope of Bromides,,
Reaction conditions: 4 (0.5 mmol), 2 (0.8 mmol), P2 (2.5 mol %), TMP (1.0 mmol), DMSO (2.5 mL), rt under argon atmosphere.
Isolated yield.
Stir at rt for 3 h then increase temperature to 60 °C.
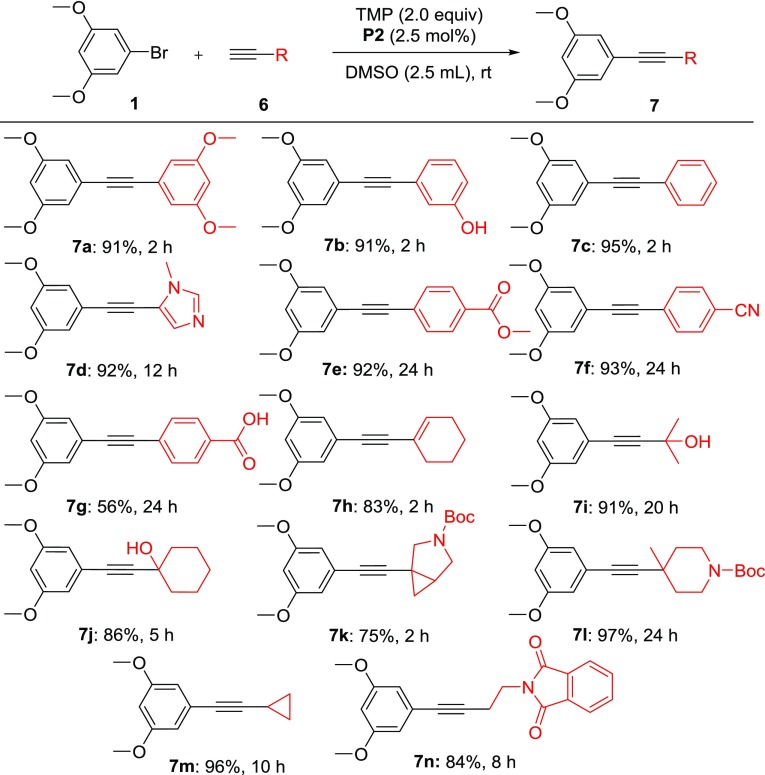
With the broad scope of bromides obtained, various aryl- and alkyl-substituted alkynes with electron-donating bromide 1 were then evaluated (Scheme 2). The phenylacetylene and aryl acetylene with electron-donating groups typically proceeded to completion in 2 h with excellent isolated yields (7a–c). The heteroaryl acetylene, such as N-methylated imidazole (7d), and aryl acetylene with electron-withdrawing substitutions (7e–g) required prolonged reaction time (12–24 h) to secure a good yield, but were still completed at room temperature. Finally, the alkenyl- (7h) and alkyl (7i–n)-substituted alkynes were efficiently coupled with 1 under similar conditions to produce the desired product in excellent yield (75–96%).
Scheme 2. Coupling of 1 with Aryl and Alkyl Acetylenes,,
Reaction conditions: 4 (0.5 mmol), 6 (0.8 mmol), P2 (2.5 mol %), TMP (1.0 mmol), DMSO (2.5 mL), rt with argon atmosphere.
Isolated yield.
Stir at rt for 3 h then increase temperature to 60 °C.
To test the feasibility in large-scale preparation, a 2 g scale reaction was performed on 1 and 2 using 2.5 mol % catalyst loading. To our gratification, the reaction reached 100% conversion in 2 h with 92% isolated yield that further confirmed the efficiency and effectiveness of this coupling protocol (Scheme 3).
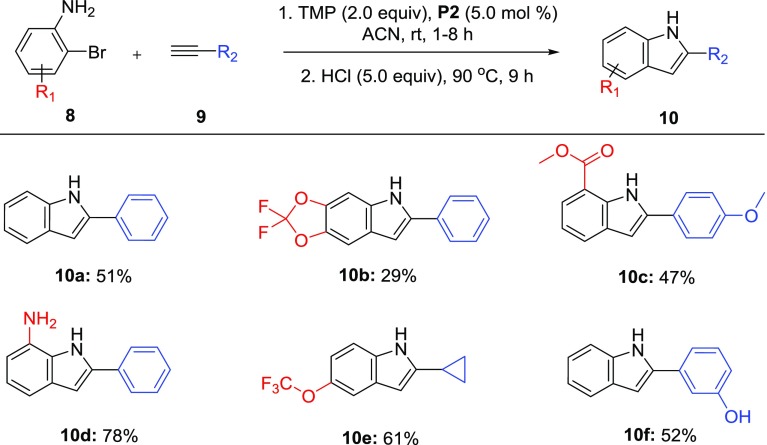
Scheme 3. Indole Synthesis via Sonogashira Coupling with 2-Bromoanilines,
Reaction conditions: 1. 8 (0.5 mmol), 9 (0.63 mmol), P2 (5.0 mol %), TMP (1.0 mmol), ACN (2.0 mL), rt under argon atmosphere. 2. HCl (2.5 mmol), 90 °C.
Isolated yield.
To further test the utility of these conditions, 2-bromoanilines were coupled with various alkynes in hopes of intramolecular cyclization to form the indole. Moderate yields were achieved using an unoptimized one-pot indole synthesis utilizing the Sonogashira protocol followed by refluxing in the presence of concentrated HCl. The method accommodated both electron-withdrawing and electron-donating functionalities on the bromide (10a–d), producing isolated yields up to 78%. Additionally, the cyclization tolerated alkynes containing an alkyl or phenol substituent while still maintaining yields around 55% (10e, 10f). With further optimization, we believe this one-pot approach may provide a rapid pathway toward diverse indole library synthesis.
Conclusions
In summary, a robust, copper-free Sonogashira coupling reaction is described. Through extensive optimization campaign, the homogeneous precatalyst P2, [DTBNpP] Pd(crotyl)Cl, was found to be the most effective catalyst. Together with optimized base (TMP) and solvent (DMSO), this condition provides a simple, mild, scalable, and versatile alternative for the coupling of variety of aryl bromides and alkynes. Additionally, this precatalyst provides rapid access to indoles via the one-pot method, further expanding on the utility of P2. We believe the preformed, air-stable P2 provides a reliable and more effective alternative to the commonly used catalysts, such as Pd(PPh3)4 and PdCl2(PPh3)2, by retaining or surpassing coupling efficiencies, bypassing in situ catalyst formation, forgoing usage of pyrophoric agents, and decreasing the likelihood of catalyst poisoning. A broad scope of both coupling partners together with high functional group tolerability and excellent isolated yield makes it an attractive addition to the existing Sonogashira coupling conditions for chemical library generation, medicinal chemistry, and with potential use in process chemistry.
Experimental Section
All commercial solvents and reagents were purchased from commercial sources and used without alteration. The modified water solvent (3% PTS in H2O) was created using a 15% PTS in H2O stock from Sigma-Aldrich and water from our lab, heating to achieve uniform consistency. Precatalysts were purchased from Strem Chemicals (46-0028 (P8), 46-0365 (P13), 46-0358 (P11), 46-0385 (P9), 46-0275 (P6), and 46-0295 (P7)), Johnson Matthey (Pd-162 (P1), Pd-163 (P2), Pd-170 (P4), Pd-178 (P3), and Pd-113 (P14)), and Sigma-Aldrich (RNI00185 (P10) and 794198 (P12)). Additional P2 was synthesized with procedures published by Seechurn et al.36 NMR data comparing the commercially available and homemade catalyst is provided in spectral data. Reaction monitoring was performed on the Agilent 1200 series LC/MS containing a Luna C18 (3 mm × 75 mm, 3 μm) reversed-phase column, utilizing UV detection at λ = 220 nm. The LC/MS ran a 3 min gradient spanning 4–100% acetonitrile in H2O modified by trifluoroacetic acid (0.05%) at a flow rate of 0.8 mL/min. Flash column chromatography was carried out on Teledyne Isco CombiFlash Rf+ systems using 24G Isco Silica Gel columns (230–400 mesh) and HPLC grade solvents. Products purified on 1H NMR and 13C NMR spectra were analyzed by the Varian 400 MHz in DMSO-d or CDCl3. 1H NMR spectra were referenced to 7.26 ppm for CDCl3 and 2.50 ppm for DMSO-d. 13C NMR were referenced to 77.23 ppm for CDCl3 and 39.5 ppm for DMSO-d6. 31P NMR was referenced externally to 0.00 ppm for H3PO4. Splitting patterns are reported as: singlet (s), doublet (d), triplet (t); quartet (q), septet (s), and other combinations of these patterns. Coupling constants are reported in Hertz. High-resolution mass spectrometry (HRMS) was obtained by the Agilent 6210 Time-of-Flight (TOF) LC/MS system. Proton and sodium adducts may be observed from the salt exposure in the purification and mass spectrometry instrument. In certain cases, heating was utilized.
General Procedure for Synthesis of (3), (5), and (7)
To an oven-dried Biotage microwave process vial (#355630), bromide (0.50 mmol, 1.00 equiv), alkyne(0.75 mmol, 1.50 equiv), 2,2,6,6-tetramethylpiperidine (169 μL, 1.00 mmol, 2.00 equiv), and P2 (5.17 mg, 0.013 mmol, 0.025 equiv) were added to DMSO (1.5 mL). Remaining DMSO (1 mL) was added to rinse the sides. The reaction vessel was sealed and bubbled with in-house argon for 5 min. The reaction stirred at room temperature for the designated time. Work up involved ammonium chloride, EtOAc, and brine. The organic layer was concentrated and purified on Isco silica gel columns to give the resulting product using a gradient of 0–100% for ethyl acetate/hexanes with a 0.1% NH4OH modifier, 0–20% for methanol/DCM with a 0.1% NH4OH modifier, or 0–100% water/ACN with a 0.1% TFA modifier.
3-((3,5-Dimethoxyphenyl)ethynyl)pyridine (3)
Tan solid. Yield: 97 mg, 85%. Time: 1.5 h. 1H NMR (400 MHz, DMSO-d6) δ 8.75 (dd,
J = 2.2, 1.0 Hz, 1H), 8.59 (dd, J = 4.9, 1.6 Hz, 1H), 7.97 (dt, J = 7.9, 1.9 Hz, 1H), 7.46 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H), 6.75 (d, J = 2.3 Hz, 2H), 6.59 (t, J = 2.3 Hz, 1H), 3.78 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 160.41, 151.59, 149.02, 138.51, 123.57, 123.07, 119.22, 109.17, 102.26, 92.29, 85.64, 55.40. HRMS (ESI+) in m/z: Expected 240.1019 [M + H+] (C15H14NO2+). Observed 240.1015.
4-(Pyridin-3-ylethynyl)benzonitrile (5a)
While solid. Yield: 92 mg, 90%. Time: 0.5 h. 1H NMR (400 MHz, DMSO-d6) δ 8.84–8.77 (m, 1H), 8.63 (dd, J = 4.9, 1.7 Hz, 1H), 8.03 (dt, J = 7.9, 1.9 Hz, 1H), 7.95–7.88 (m, 2H), 7.82–7.75 (m, 2H), 7.50 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.78, 149.60, 138.79, 132.62, 132.23, 126.51, 123.65, 118.59, 118.29, 111.39, 90.68, 89.96. HRMS (ESI+) in m/z: Expected 528.1904 [M + H+] (C14H9N2+). Observed 205.0758. 5a is a known compound.46
1-(4-(Pyridin-3-ylethynyl)phenyl)ethan-1-one (5b)
White solid. Yield: 102 mg, 92%. Time: 0.5 h. 1H NMR (400 MHz, DMSO-d6) δ 8.80 (dd, J = 2.3, 0.9 Hz, 1H), 8.62 (dd, J = 4.9, 1.6 Hz, 1H), 8.08–7.96 (m, 3H), 7.78–7.69 (m, 2H), 7.49 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H), 2.61 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 197.18, 151.71, 149.38, 138.68, 136.56, 131.69, 128.45, 126.17, 123.62, 118.87, 91.42, 88.97, 26.73. HRMS (ESI+) in m/z: Expected 222.0913 [M + H+] (C15H12NO+). Observed 222.0914. 5b is a known compound.47
3-(Phenylethynyl)pyridine (5c)
Tan solid. Yield: 79 mg, 88%. Time: 1 h. 1H NMR (400 MHz, DMSO-d6) δ 8.76 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 4.9, 1.6 Hz, 1H), 7.98 (dt, J = 7.9, 2.0 Hz, 1H), 7.59 (ddd, J = 6.7, 3.0, 1.6 Hz, 2H), 7.46 (tt, J = 6.2, 1.8 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 151.55, 148.96, 138.47, 131.43, 129.18, 128.77, 123.56, 121.64, 119.32, 92.21, 86.08. HRMS (ESI+) in m/z: Expected 202.0627 [M + Na+] (C13H9NNa+). Observed 202.0634. 5c is a known compound.48
3-((3-Nitrophenyl)ethynyl)pyridine (5d)
Tan solid. Yield: 99 mg, 87%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.83 (d, J = 2.1 Hz, 1H), 8.63 (dd, J = 4.9, 1.7 Hz, 1H), 8.39 (t, J = 1.9 Hz, 1H), 8.28 (ddd, J = 8.4, 2.5, 1.1 Hz, 1H), 8.04 (ddt, J = 7.6, 5.6, 1.6 Hz, 2H), 7.75 (t, J = 8.0 Hz, 1H), 7.55–7.44 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.82, 149.53, 147.89, 138.79, 137.54, 130.49, 125.97, 123.85, 123.64, 123.25, 118.62, 89.89, 88.20. HRMS (ESI+) in m/z: Expected 225.0659 [M + H+] (C13H9N2O2+). Observed 225.0657.
3-((2-(Trifluoromethyl)phenyl)ethynyl)pyridine (5e)
Yellow oil. Yield: 88 mg, 71%. Time: 18 h. 1H NMR (400 MHz, DMSO-d6) δ 8.74 (dd, J = 2.1, 1.0 Hz, 1H), 8.64 (dd, J = 4.9, 1.7 Hz, 1H), 7.97 (dt, J = 7.9, 1.9 Hz, 1H), 7.86 (dd, J = 7.6, 1.4 Hz, 2H), 7.80–7.71 (m, 1H), 7.66 (tdd, J = 8.6, 2.2, 1.1 Hz, 1H), 7.50 (ddd, J = 7.9, 4.9, 1.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.42, 149.59, 138.53, 134.03, 129.57, 123.74, 122.13, 119.45, 118.70, 91.26, 87.89. HRMS (ESI+) in m/z: Expected 248.0682 [M + H+] (C14H9F3N+). Observed 248.0675. 5e is a known compound.49
3-(Pyridin-3-ylethynyl)benzoic Acid (5f)
Tan solid. Yield: 69 mg, 65%. Time: 4 h. 1H NMR (400 MHz, DMSO-d6) δ 13.24 (s, 1H), 8.80 (dd, J = 2.2, 0.9 Hz, 1H), 8.61 (dd, J = 4.9, 1.6 Hz, 1H), 8.10 (t, J = 1.7 Hz, 1H), 8.01 (ddt, J = 13.2, 7.8, 1.7 Hz, 2H), 7.83 (dt, J = 7.7, 1.4 Hz, 1H), 7.59 (t, J = 7.8 Hz, 1H), 7.49 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 166.38, 151.61, 149.09, 138.74, 135.37, 132.08, 131.42, 129.79, 129.29, 123.63, 122.08, 119.08, 91.21, 86.83. HRMS (ESI+) in m/z: Expected 224.0706 [M + H+] (C14H10NO2+). Observed 224.0706.
4-(Pyridin-3-ylethynyl)benzaldehyde (5g)
Tan solid. Yield: 91 mg, 88%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 10.05 (s, 1H), 8.83–8.77 (m, 1H), 8.63 (dd, J = 4.9, 1.7 Hz, 1H), 8.03 (dt, J = 7.9, 2.0 Hz, 1H), 8.00–7.94 (m, 2H), 7.84–7.77 (m, 2H), 7.50 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 192.42, 151.75, 149.49, 138.74, 135.82, 132.13, 129.64, 127.44, 123.65, 118.77, 91.35, 89.54. HRMS (ESI+) in m/z: Expected 208.0757 [M + H+] (C14H10NO+). Observed 208.0755. 5g is a known compound.50
Methyl 3-(Pyridin-3-ylethynyl)benzoate (5h)
Tan solid. Yield: 103 mg, 87%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.80 (dd, J = 2.2, 1.0 Hz, 1H), 8.61 (dd, J = 4.9, 1.7 Hz, 1H), 8.14–8.08 (m, 1H), 8.05–7.97 (m, 2H), 7.86 (dt, J = 7.8, 1.4 Hz, 1H), 7.65–7.58 (m, 1H), 7.48 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H), 3.88 (s, 3H).13C NMR (101 MHz, DMSO-d6) δ 165.35, 151.71, 149.23, 138.65, 135.77, 131.87, 130.24, 129.59, 129.47, 123.58, 122.29, 118.97, 90.95, 87.07, 52.37. HRMS (ESI+) in m/z: Expected 238.0863 [M + H+] (C15H12NO2+). Observed 238.0851.
3-((4-Chlorophenyl)ethynyl)pyridine (5i)
White solid. Yield: 95 mg, 89%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.76 (d, J = 2.6 Hz, 1H), 8.60 (dd, J = 4.3, 2.2 Hz, 1H), 7.99 (dd, J = 8.0, 2.3 Hz, 1H), 7.66–7.57 (m, 2H), 7.52 (dd, J = 8.7, 2.2 Hz, 2H), 7.50–7.43 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.63, 149.19, 138.58, 133.97, 133.22, 129.00, 123.63, 120.56, 119.08, 91.06, 87.17. HRMS (ESI+) in m/z: Expected 214.0418 [M + H+] (C13H9ClN+). Observed 214.0413. 5i is a known compound.51
3-([1,1′-Biphenyl]-2-ylethynyl)pyridine (5j)
Yellow oil. Yield: 101 mg, 79%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.58–8.46 (m, 2H), 7.78–7.68 (m, 2H), 7.68–7.62 (m, 2H), 7.58–7.38 (m, 7H). 13C NMR (101 MHz, DMSO-d6) δ 151.20, 148.90, 143.44, 139.59, 138.05, 132.79, 129.64, 129.53, 129.07, 128.12, 127.79, 127.58, 123.62, 119.89, 119.48, 92.24, 88.64. HRMS (ESI+) in m/z: Expected 256.1121 [M + H+] (C19H14N+). Observed 256.1118. Anal. Calcd for C19H13N: C, 88.86; H, 5.39; N: 5.76. Found: C, 88.75; H, 5.14; N, 5.38.
3-(p-Tolylethynyl)pyridine (5k)
Tan solid. Yield: 75 mg, 78%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.74 (dd, J = 2.2, 1.0 Hz, 1H), 8.58 (dd, J = 4.9, 1.6 Hz, 1H), 7.96 (dt, J = 7.9, 1.9 Hz, 1H), 7.53–7.40 (m, 3H), 7.26 (d, J = 7.8 Hz, 2H), 2.35 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 151.48, 148.79, 139.05, 138.37, 131.35, 129.39, 123.54, 119.52, 118.63, 92.45, 85.51, 21.02. HRMS (ESI+) in m/z: Expected 194.0964 [M + H+] (C14H12N+). Observed 194.0958. 5k is known compound.52
2-Hydroxy-5-(pyridin-3-ylethynyl)benzamide (5l)
White solid. Yield: 90 mg, 76%. Time: 6 h. 1H NMR (400 MHz, DMSO-d6) δ 13.42 (s, 1H), 8.75–8.69 (m, 1H), 8.61–8.50 (m, 2H), 8.17 (d, J = 2.1 Hz, 1H), 8.05 (s, 1H), 7.94 (dt, J = 7.9, 1.9 Hz, 1H), 7.62 (dd, J = 8.6, 2.0 Hz, 1H), 7.46 (ddd, J = 8.0, 4.9, 0.9 Hz, 1H), 6.95 (d, J = 8.6 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 171.10, 161.73, 151.35, 148.73, 138.25, 136.89, 131.74, 123.60, 119.57, 118.21, 114.84, 111.58, 92.01, 84.57. HRMS (ESI+) in m/z: Expected 239.0815 [M + H+] (C14H11N2O2+). Observed 239.0808. Anal. Calcd for C14H10N2O2: C, 70.58; H, 4.23; N, 11.76. Found: C, 70.48; H, 4.31; N, 11.78.
4-(Pyridin-3-ylethynyl)phenol (5m)
Tan solid. Yield: 50 mg, 52%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 10.00 (s, 1H), 8.70 (d, J = 2.1 Hz, 1H), 8.54 (dd, J = 4.9, 1.6 Hz, 1H), 7.91 (dt, J = 7.9, 1.9 Hz, 1H), 7.48–7.36 (m, 3H), 6.85–6.77 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 158.40, 151.31, 148.42, 138.13, 133.16, 123.51, 119.96, 115.78, 111.78, 93.04, 84.18. HRMS (ESI+) in m/z: Expected 196.0759 [M + H+] (C13H10NO+). Observed 196.0757.
4-(Pyridin-3-ylethynyl)aniline (5n)
Tan solid. Yield: 60 mg, 62%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.65 (d, J = 2.1 Hz, 1H), 8.50 (dd, J = 4.9, 1.7 Hz, 1H), 7.86 (dt, J = 7.9, 2.0 Hz, 1H), 7.40 (dd, J = 7.9, 4.9 Hz, 1H), 7.28–7.19 (m, 2H), 6.63–6.52 (m, 2H), 5.63 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 151.07, 149.86, 147.92, 137.78, 132.70, 123.46, 120.48, 113.57, 107.33, 94.49, 83.35. HRMS (ESI+) in m/z: Expected 195.0917 [M + H+] (C13H11N2+). Observed 195.0919. 5n is a known compound.53
1,2-Di(pyridin-3-yl)ethyne (5o)
Tan solid. Yield: 83 mg, 92%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.80 (dd, J = 2.2, 1.0 Hz, 2H), 8.62 (dd, J = 4.9, 1.7 Hz, 2H), 8.02 (dt, J = 7.9, 1.9 Hz, 2H), 7.49 (ddd, J = 7.9, 4.9, 0.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 151.66, 149.37, 138.64, 123.62, 118.81, 89.05. HRMS (ESI+) in m/z: Expected 181.076 [M + H+] (C12H9N2+). Observed 181.0762. 5o is a known compound.54
5-(Pyridin-3-ylethynyl)isoquinoline (5p)
Tan solid. Yield: 90 mg, 78%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 9.42 (d, J = 1.0 Hz, 1H), 8.93 (dd, J = 2.2, 0.9 Hz, 1H), 8.71–8.61 (m, 2H), 8.27–8.19 (m, 2H), 8.15 (dt, J = 7.9, 2.0 Hz, 1H), 8.10 (dd, J = 7.2, 1.1 Hz, 1H), 7.75 (dd, J = 8.2, 7.2 Hz, 1H), 7.52 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 152.91, 151.80, 149.33, 144.30, 138.73, 134.94, 134.61, 129.08, 127.90, 127.22, 123.63, 119.06, 118.32, 118.09, 91.94, 88.69. HRMS (ESI+) in m/z: Expected 231.0919 [M + H+] (C16H11N2+). Observed 231.0917. Anal. Calcd for C16H10N2: C, 83.46; H, 4.38; N: 12.17. Found: C, 83.22; H, 4.53; N, 12.07.
5-(Pyridin-3-ylethynyl)pyrimidine (5q)
Tan solid. Yield: 72 mg, 90%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 9.23 (s, 1H), 9.06 (s, 2H), 8.82 (dd, J = 2.2, 1.0 Hz, 1H), 8.65 (dd, J = 4.9, 1.7 Hz, 1H), 8.04 (dt, J = 7.9, 1.9 Hz, 1H), 7.51 (ddd, J = 7.9, 4.9, 1.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 158.79, 157.14, 151.72, 149.77, 138.77, 123.69, 118.35, 118.33, 92.34, 85.79. HRMS (ESI+) in m/z: Expected 182.0713 [M + H+] (C11H10N3+). Observed 182.0713. 5q is a known compound.55
3-(Thiophen-3-ylethynyl)pyridine (5r)
Tan solid. Yield: 72 mg, 78%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 8.73 (dd, J = 2.2, 1.0 Hz, 1H), 8.58 (dd, J = 4.9, 1.6 Hz, 1H), 7.98–7.91 (m, 2H), 7.68 (ddd, J = 5.0, 2.9, 1.0 Hz, 1H), 7.45 (ddd, J = 7.9, 4.9, 1.0 Hz, 1H), 7.30 (dt, J = 5.0, 1.1 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.42, 148.83, 138.35, 130.65, 129.53, 127.10, 123.56, 120.53, 119.42, 87.83, 85.41. HRMS (ESI+) in m/z: Expected 186.0372 [M + H+] (C11H8NS+). Observed 186.0368. 5r is a known compound.56
Methyl 4-Oxo-6-(pyridin-3-ylethynyl)-1,4-dihydroquinoline-2-carboxylate (5s)
Yellow solid. Yield: 38 mg, 25%. Time: 6 h. 1H NMR (400 MHz, DMSO-d6) δ 12.30 (s, 1H), 8.86–8.71 (m, 1H), 8.60 (dd, J = 4.8, 1.7 Hz, 1H), 8.24 (d, J = 2.0 Hz, 1H), 8.05–7.95 (m, 2H), 7.87 (dd, J = 8.7, 2.0 Hz, 1H), 7.48 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H), 6.69 (s, 1H), 3.97 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 162.69, 151.71, 149.12, 138.68, 134.78, 128.32, 126.19–124.88 (m), 123.72, 120.13 (d, J = 157.9 Hz), 117.30, 110.62, 91.87, 86.68, 53.62, 29.03. HRMS (ESI+) in m/z: Expected 327.0752 [M + Na+] (C18H12N2O3Na+). Observed 327.0755.
5-(Pyridin-3-ylethynyl)-3,4-dihydroquinolin-2(1H)-one (5t)
White solid. Yield: 66 mg, 53%. Time: 4 h. 1H NMR (400 MHz, DMSO-d6) δ 8.76 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 4.9, 1.6 Hz, 1H), 7.98 (dt, J = 7.9, 2.0 Hz, 1H), 7.59 (ddd, J = 6.7, 3.0, 1.6 Hz, 2H), 7.46 (tt, J = 6.2, 1.8 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 169.90, 151.53, 149.04, 138.76, 138.47, 127.26, 125.40, 125.29, 123.58, 120.49, 119.29, 116.01, 90.28, 90.01, 29.80, 23.28. HRMS (ESI+) in m/z: Expected 249.1025 [M + H+] (C16H13N2O+). Observed 249.1022.
3-(Pyridin-3-ylethynyl)imidazo[1,2-a]pyrimidine (5u)
Tan solid. Yield: 46 mg, 42%. Time: 6 h, Temperature: 60 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.17 (dd, J = 6.8, 1.9 Hz, 1H), 8.90 (dd, J = 2.2, 0.9 Hz, 1H), 8.70 (dd, J = 4.1, 2.0 Hz, 1H), 8.62 (dd, J = 4.9, 1.7 Hz, 1H), 8.22 (s, 1H), 8.10 (dt, J = 7.9, 1.9 Hz, 1H), 7.51 (ddd, J = 8.0, 4.9, 1.0 Hz, 1H), 7.29 (dd, J = 6.8, 4.2 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.91, 151.34, 149.16, 148.33, 139.63, 138.15, 134.53, 123.55, 118.84, 110.17, 106.07, 95.71, 79.04. HRMS (ESI+) in m/z: Expected 221.0822 [M + H+] (C13H9N4+). Observed 221.0826.
1-Methyl-4-(pyridin-3-ylethynyl)-1H-pyrazole-3-carbaldehyde (5v)
Tan solid. Yield: 69 mg, 63%. Time: 20 h, Temperature: 60 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.98–9.93 (m, 1H), 8.71 (dd, J = 2.2, 1.0 Hz, 1H), 8.58 (dd, J = 4.9, 1.6 Hz, 1H), 8.31 (s, 1H), 7.93 (dt, J = 7.9, 1.9 Hz, 1H), 7.46 (ddd, J = 7.9, 4.9, 1.0 Hz, 1H), 4.00 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 184.92, 151.33, 149.42, 148.91, 138.30, 136.69, 123.59, 119.49, 102.13, 88.69, 82.95. HRMS (ESI+) in m/z: Expected 212.0818 [M + H+] (C12H10N3O+). Observed 212.0822.
3-((1-Methyl-1H-imidazol-5-yl)ethynyl)pyridine (5w)
Tan solid. Yield: 63 mg, 69%. Time: 24 h, 60 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.76 (dd, J = 2.2, 1.0 Hz, 1H), 8.59 (dd, J = 4.9, 1.6 Hz, 1H), 7.98 (dt, J = 7.9, 1.9 Hz, 1H), 7.81 (s, 1H), 7.47 (ddd, J = 7.9, 4.9, 0.9 Hz, 1H), 7.37 (d, J = 1.0 Hz, 1H), 3.73 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 151.22, 149.01, 139.85, 138.12, 134.44, 123.57, 119.03, 114.54, 92.74, 80.76, 31.73. HRMS (ESI+) in m/z: Expected 184.0869 [M + H+] (C11H10N3+). Observed 184.0868. 5w is a known compound.57
4-(Pyridin-3-ylethynyl)furan-2-carbaldehyde (5x)
Brown solid. Yield: 101 mg, 83%. Time: 20 h, Temperature: 60 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.64 (d, J = 0.9 Hz, 1H), 8.75 (dd, J = 2.2, 1.0 Hz, 1H), 8.61 (dd, J = 4.9, 1.6 Hz, 1H), 8.56 (d, J = 0.9 Hz, 1H), 7.98 (dt, J = 8.0, 1.9 Hz, 1H), 7.76 (d, J = 0.9 Hz, 1H), 7.48 (ddd, J = 7.9, 4.9, 1.0 Hz, 1H), 1.64 (s, 0H). 13C NMR (101 MHz, DMSO-d6) δ 178.61, 152.39, 151.86, 151.49, 149.30, 138.52, 123.62, 123.51, 118.79, 108.88, 88.69, 82.06. HRMS (ESI+) in m/z: Expected 198.055 [M + H+] (C12H8NO2+). Observed 198.0549.
4-(Pyridin-3-ylethynyl)thiazole (5y)
Tan solid. Yield: 73 mg, 78%. Time: 18 h, Temperature: 60 °C. 1H NMR (400 MHz, DMSO-d6) δ 9.20 (d, J = 1.9 Hz, 1H), 8.78 (dd, J = 2.2, 1.0 Hz, 1H), 8.62 (dd, J = 4.9, 1.6 Hz, 1H), 8.21 (d, J = 1.9 Hz, 1H), 8.01 (dt, J = 7.9, 1.9 Hz, 1H), 7.53–7.43 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 154.99, 151.59, 149.31, 138.63, 136.28, 125.41, 123.63, 118.71, 86.72, 85.38. HRMS (ESI+) in m/z: Expected 187.0324 [M + H+] (C10H7N2S+). Observed 187.0324.
3,5-Dimethyl-4-(pyridin-3-ylethynyl)isoxazole (5z)
Yellow oil. Yield: 30 mg, 30%. Time: 32 h, Temperature: 60 °C. 1H NMR (400 MHz, DMSO-d6) δ 8.76 (dd, J = 2.3, 1.0 Hz, 1H), 8.59 (dd, J = 4.9, 1.8 Hz, 1H), 7.98 (dq, J = 8.0, 1.9 Hz, 1H), 7.51–7.41 (m, 1H), 2.52 (d, J = 1.7 Hz, 4H), 2.30 (d, J = 1.7 Hz, 4H). 13C NMR (101 MHz, DMSO-d6) δ 172.12, 160.04, 151.54, 149.13, 138.46, 123.59, 119.08, 99.88, 90.96, 80.45, 11.72, 10.07. HRMS (ESI+) in m/z: Expected 199.0866 [M + H+] (C12H11N2O+). Observed 199.0860. Anal. Calcd for C12H10N2O: C, 72.71; H, 5.08; N, 14.13. Found: C, 72.96; H, 5.24; N, 13.85.
4-(Pyridin-3-ylethynyl)-1H-indole 9 (5aa)
Tan solid. Yield: 95 mg, 87%. Time: 6 h. 1H NMR (400 MHz, DMSO-d6) δ 11.39 (s, 1H), 8.85–8.79 (m, 1H), 8.58 (dt, J = 4.9, 1.4 Hz, 1H), 8.08–7.99 (m, 1H), 7.54–7.42 (m, 3H), 7.31–7.23 (m, 1H), 7.19–7.09 (m, 1H), 6.67 (ddt, J = 3.0, 1.9, 1.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 151.50, 148.59, 138.33, 135.50, 128.92, 126.60, 123.55, 122.88, 120.89, 119.97, 113.02, 112.42, 100.48, 91.91, 87.93. HRMS (ESI+) in m/z: Expected 219.0917 [M + H+] (C15H11N2+). Observed 219.0918.
1,2-Bis(3,5-dimethoxyphenyl)ethyne (7a)
Tan solid. Yield: 136 mg, 91%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 6.71 (dd, J = 2.4, 0.8 Hz, 4H), 6.56 (t, J = 2.3 Hz, 2H), 3.77 (d, J = 0.8 Hz, 12H). 13C NMR (101 MHz, DMSO-d6) δ 160.40, 123.57, 109.07, 101.99, 88.95, 55.41. HRMS (ESI+) in m/z: Expected 299.1278 [M + H+] (C18H19O4+). Observed 299.1268. 7a is a known compound.58
3-((3,5-Dimethoxyphenyl)ethynyl)phenol (7b)
Yellow solid. Yield: 116 mg, 91%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 9.71 (s, 1H), 7.21 (t, J = 7.9 Hz, 1H), 6.97 (dt, J = 7.6, 1.2 Hz, 1H), 6.91 (dd, J = 2.5, 1.5 Hz, 1H), 6.82 (ddd, J = 8.2, 2.5, 1.1 Hz, 1H), 6.70 (dd, J = 2.3, 1.1 Hz, 2H), 6.57–6.52 (m, 1H), 3.77 (d, J = 1.3 Hz, 6H). 13C NMR (101 MHz, DMSO-d6) δ 160.40, 157.37, 129.89, 123.74, 122.98, 122.24, 117.77, 116.38, 109.05, 101.84, 89.02, 88.79, 55.40. HRMS (ESI+) in m/z: Expected 255.1016 [M + H+] (C16H15O3+). Observed 255.1017. Anal. Calcd for C16H14O3: C, 75.57; H, 5.55; N, 0. Found: C, 75.53; H, 5.64; N, <0.02.
1,3-Dimethoxy-5-(phenylethynyl)benzene (7c)
Brown oil. Yield: 113 mg, 95%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 6.54–6.46 (m, 3H), 5.41 (s, 1H), 3.74 (s, 5H), 1.83 (dd, J = 12.0, 4.8 Hz, 2H), 1.75–1.36 (m, 9H), 1.30–1.16 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 160.41, 131.40, 128.87, 128.76, 123.68, 122.12, 109.05, 101.90, 89.39, 88.87, 55.40. HRMS (ESI+) in m/z: Expected 239.1067 [M + H+] (C14H15N2O2+). Observed 239.1072. 7c is a known compound.59
5-((3,5-Dimethoxyphenyl)ethynyl)-1-methyl-1H-imidazole (7d)
Yellow solid. Yield: 112 mg, 92%. Time: 12 h. 1H NMR (400 MHz, DMSO-d6) δ 7.78 (s, 1H), 7.31 (d, J = 1.1 Hz, 1H), 6.71 (d, J = 2.3 Hz, 2H), 6.56 (t, J = 2.3 Hz, 1H), 3.77 (s, 6H), 3.71 (s, 3H). 13C NMR (101 MHz, DMSO-d6) δ 160.43, 139.61, 134.02, 123.29, 108.73, 101.91, 95.89, 77.33, 55.44, 31.74. HRMS (ESI+) in m/z: Expected 243.1128 [M + H+] (C14H15N2O2+). Observed 243.1134. Anal. Calcd for C14H14N2O2: C, 69.41; H, 5.82; N, 11.56. Found: C, 69.34; H, 5.87; N, 11.37.
Methyl 4-((3,5-Dimethoxyphenyl)ethynyl)benzoate (7e)
Tan solid. Yield: 136 mg, 92%. Time: 24 h. 1H NMR (400 MHz, DMSO-d6) δ 8.02–7.97 (m, 2H), 7.74–7.65 (m, 2H), 6.75 (d, J = 2.3 Hz, 2H), 6.59 (t, J = 2.3 Hz, 1H), 3.87 (s, 4H), 3.78 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 165.60, 160.44, 131.71, 129.42, 129.36, 126.90, 123.10, 109.24, 102.36, 92.36, 87.95, 55.44, 52.34. HRMS (ESI+) in m/z: Expected 297.1135 [M + H+] (C18H17O4+). Observed 297.1135. Anal. Calcd for C18H16O4: C, 72.96; H, 5.44; N, 0. Found: C, 72.79; H, 5.48; N, <0.02.
4-((3,5-Dimethoxyphenyl)ethynyl)benzonitrile (7f)
White solid. Yield: 123 mg, 93%. 1H NMR (400 MHz, DMSO-d6) δ 7.95–7.85 (m, 2H), 7.79–7.67 (m, 2H), 6.76 (d, J = 2.3 Hz, 2H), 6.60 (t, J = 2.3 Hz, 1H), 3.78 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 160.45, 132.61, 132.18, 127.03, 122.84, 118.42, 111.03, 109.31, 102.54, 93.34, 87.45, 55.47. HRMS (ESI+) in m/z: Expected 286.0838 [M + Na+] (C17H14NO2Na+). Observed 286.0834.
4-((3,5-Dimethoxyphenyl)ethynyl)benzoic Acid (7g)
Yellow solid. Yield: 79 mg, 56%. 1H NMR (400 MHz, DMSO-d6) δ 13.16 (s, 1H), 7.97 (d, J = 8.1 Hz, 2H), 7.67 (d, J = 8.1 Hz, 2H), 6.75 (d, J = 2.3 Hz, 2H), 6.59 (t, J = 2.3 Hz, 1H), 3.78 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 166.67, 164.03, 160.44, 131.57, 130.62, 129.56, 126.46, 123.19, 109.22, 102.32, 92.03, 88.13, 55.45, 40.43, −1.92. HRMS (ESI+) in m/z: Expected 283.0978 [M + H+] (C17H15O4+). Observed 283.0973. Anal. Calcd for C17H14O4: C, 72.33; H, 5.00; N, 0. Found: C, 71.88; H, 4.95; N, <0.02.
1-(Cyclohex-1-en-1-ylethynyl)-3,5-dimethoxybenzene (7h)
Orange oil. Yield: 101 mg, 83%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 6.55 (d, J = 2.3 Hz, 2H), 6.49 (t, J = 2.3 Hz, 1H), 6.19 (tt, J = 3.8, 1.6 Hz, 1H), 3.74 (s, 6H), 2.13 (dtdd, J = 9.6, 5.8, 4.1, 2.3 Hz, 4H), 1.69–1.49 (m, 4H). 13C NMR (101 MHz, DMSO-d6) δ 160.29, 135.51, 124.25, 119.88, 108.78, 101.30, 90.81, 86.80, 55.28, 28.68, 25.18, 21.77, 20.94. HRMS (ESI+) in m/z: Expected 243.138 [M + H+] (C16H19O2+). Observed 243.1376.
4-(3,5-Dimethoxyphenyl)-2-methylbut-3-yn-2-ol (7i)
Yellow oil. Yield: 100 mg, 91%. Time: 20 h. 1H NMR (400 MHz, DMSO-d6) δ 6.57–6.39 (m, 3H), 5.45 (d, J = 1.2 Hz, 1H), 3.74 (d, J = 1.3 Hz, 6H), 1.45 (s, 6H). 13C NMR (101 MHz, DMSO-d6) δ 160.31, 124.09, 108.82, 101.35, 95.67, 80.37, 63.57, 55.32, 31.57. HRMS (ESI+) in m/z: Expected 221.1172 [M + H+] (C13H17O3+). Observed 221.1171. 7i is a known compound.60
1-((3,5-Dimethoxyphenyl)ethynyl)cyclohexan-1-ol (7j)
White solid. Yield: 112 mg, 86%. Time: 5 h. 1H NMR (400 MHz, DMSO-d6) δ 6.50 (dt, J = 6.5, 2.3 Hz, 3H), 5.41 (s, 1H), 3.74 (s, 6H), 1.83 (dd, J = 12.5, 4.8 Hz, 2H), 1.71–1.58 (m, 2H), 1.58–1.41 (m, 5H), 1.29–1.18 (m, 1H). 13C NMR (101 MHz, DMSO-d6) δ 160.32, 124.19, 108.87, 101.29, 94.60, 82.56, 66.87, 55.32, 39.63, 24.90, 22.74. HRMS (ESI+) in m/z: Expected 261.1485 [M + H+] (C16H21O3+). Observed 261.1481. Anal. Calcd for C16H20O3: C, 73.82; H, 7.74; N, 0. Found: C, 73.9; H, 7.95; N, <0.02.
tert-Butyl 1-((3,5-Dimethoxyphenyl)ethynyl)-3-azabicyclo[3.1.0]hexane-3-carboxylate (7k)
Clear oil. Yield: 128 mg, 75%. Time: 2 h. 1H NMR (400 MHz, DMSO-d6) δ 6.56–6.52 (m, 2H), 6.48 (dd, J = 2.7, 1.7 Hz, 1H), 3.73 (d, J = 1.1 Hz, 6H), 3.68 (dd, J = 10.1, 5.6 Hz, 0H), 3.54–3.29 (m, 3H), 2.02–1.93 (m, 1H), 1.47–1.32 (m, 9H), 1.22 (dd, J = 8.2, 4.8 Hz, 1H), 0.74 (t, J = 5.0 Hz, 1H). 13C NMR (101 MHz, DMSO-d6) δ 160.28, 153.77, 123.99, 109.05, 101.39, 78.89, 55.31, 51.13, 50.81, 47.58, 47.33, 28.05, 26.05, 25.21, 17.64. HRMS (ESI+) in m/z: Expected 366.1687 [M + Na+] (C20H25NO4Na+). Observed 366.169. Anal. Calcd for C20H25NO4: C, 69.95; H, 7.34; N, 4.08. Found: C, 70.05; H, 7.49; N, 4.04.
tert-Butyl 4-((3,5-Dimethoxyphenyl)ethynyl)-4-methylpiperidine-1-carboxylate (7l)
Clear oil. Yield: 174 mg, 97%. Time: 24 h. 1H NMR (400 MHz, DMSO-d6) δ 6.55 (d, J = 2.3 Hz, 2H), 6.48 (t, J = 2.3 Hz, 1H), 3.88 (d, J = 13.1 Hz, 2H), 3.73 (s, 5H), 3.04 (s, 3H), 1.72–1.64 (m, 2H), 1.40 (s, 11H), 1.28 (s, 4H). 13C NMR (101 MHz, DMSO-d6) δ 160.28, 153.78, 124.27, 109.06, 101.31, 93.85, 82.77, 78.64, 55.33, 31.43, 28.95, 28.09. HRMS (ESI+) in m/z: Expected 382.1989 [M + Na+] (C21H29NO4Na+). Observed 382.1985. Anal. Calcd for C21H29NO4: C, 70.17; H, 8.13; N, 3.90. Found: C, 70.45; H, 8.34; N, 3.93.
1-(Cyclopropylethynyl)-3,5-dimethoxybenzene (7m)
Orange oil. Yield: 97 mg, 96%. Time: 10 h. 1H NMR (400 MHz, DMSO-d6) δ 6.50 (d, J = 2.3 Hz, 2H), 6.45 (t, J = 2.3 Hz, 1H), 3.72 (s, 6H), 1.52 (tt, J = 8.3, 5.0 Hz, 1H), 0.93–0.82 (m, 2H), 0.76–0.66 (m, 2H). 13C NMR (101 MHz, DMSO-d6) δ 160.25, 124.64, 108.98, 100.93, 93.42, 75.67, 55.26, 8.34. HRMS (ESI+) in m/z: Expected 203.1067 [M + H+] (C13H15O2+). Observed 203.1060. 7m is a known compound.61
2-(4-(3,5-Dimethoxyphenyl)but-3-yn-1-yl)isoindoline-1,3-dione (7n)
White solid. Yield: 141 mg, 84%. Time: 8 h. 1H NMR (400 MHz, DMSO-d6) δ 7.95–7.88 (m, 2H), 7.86 (dt, J = 5.0, 3.4 Hz, 2H), 6.45 (t, J = 2.3 Hz, 1H), 6.39 (d, J = 2.3 Hz, 2H), 3.82 (t, J = 6.9 Hz, 2H), 3.68 (s, 6H), 2.78 (t, J = 6.9 Hz, 2H). 13C NMR (101 MHz, DMSO-d6) δ 167.66, 160.23, 134.57, 131.50, 124.03, 123.13, 108.86, 101.15, 86.55, 81.91, 55.24, 36.20, 18.44. HRMS (ESI+) in m/z: Expected 336.123 [M + H+] (C20H18NO4+). Observed 336.1229. Anal. Calcd for C20H17NO4: C, 71.63; N, 5.11; H, 4.18. Found: C, 71.65; H, 5.19; N, 4.13.
General Procedure for Synthesis of (10)
To an oven-dried Biotage microwave process vial (#355630), 2-bromoaniline (0.50 mmol, 1.00 equiv), alkyne (0.63 mmol, 1.25 equiv), 2,2,6,6-tetramethylpiperidine (169 μL, 1.00 mmol, 2.00 equiv), and P2 (10.33 mg, 0.025 mmol, 0.05 equiv) were added to ACN (1.0 mL). Remaining ACN (1.0 mL) was added to rinse the sides. The reaction vessel was sealed and bubbled with in-house argon for 5 min. The reaction was stirred at room temperature for the designated time. Concentrated HCl (2.50 mmol, 5.0 equiv) was added via syringe directly to the reaction mixture and stirred at 90 °C for 9 h. Work up involved ammonium chloride, EtOAc, and brine. The organic layer was concentrated and purified on Isco silica gel columns to give the resulting product using a gradient of 0–100% for ethyl acetate/hexanes.
2-Phenyl-1H-indole (10a)
Tan solid. Yield: 49 mg, 51%. Time: 1. 1 h; 2. 9 h. 1H NMR (400 MHz, chloroform-d) δ 8.31 (s, 1H), 7.71–7.63 (m, 3H), 7.46 (t, J = 7.7 Hz, 2H), 7.41 (d, J = 8.1 Hz, 1H), 7.34 (t, J = 7.5 Hz, 1H), 7.26–7.18 (m, 1H), 7.15 (t, J = 7.4 Hz, 1H), 6.85 (d, J = 2.2 Hz, 1H). 13C NMR (101 MHz, chloroform-d) δ 138.02, 136.96, 132.52, 129.42, 129.16, 127.85, 125.30, 122.50, 120.81, 120.42, 111.03, 100.15. HRMS (ESI+) in m/z: Expected 194.0964 [M + H+] (C14H12N+). Observed 194.0967. 10a is a known compound.62
2,2-Difluoro-6-phenyl-5H-[1,3]dioxolo[4,5-f]indole (10b)
Yellow solid. Yield: 40 mg, 29%. Time: 1. 3 h; 2. 9 h. 1H NMR (400 MHz, chloroform-d) δ 8.37 (s, 1H), 7.66–7.57 (m, 2H), 7.45 (t, J = 7.6 Hz, 2H), 7.37–7.30 (m, 1H), 7.22 (s, 1H), 7.08 (s, 1H), 6.79 (dd, J = 2.3, 1.1 Hz, 1H). 13C NMR (101 MHz, chloroform-d) δ 140.90, 139.50, 138.20, 131.89, 131.86, 129.10, 127.82, 124.86, 124.24, 100.35, 100.25, 92.61. HRMS (ESI+) in m/z: Expected 274.0686 [M + H+] (C15H10F2NO2+). Observed 274.0682.
Methyl 2-(4-Methoxyphenyl)-1H-indole-7-carboxylate (10c)
Tan solid. Yield: 66 mg, 47%. Time: 1. 1 h; 2. 9 h. 1H NMR (400 MHz, chloroform-d) δ 10.04 (s, 1H), 7.92–7.73 (m, 2H), 7.73–7.63 (m, 2H), 7.14 (t, J = 7.8 Hz, 1H), 7.03–6.94 (m, 2H), 6.75 (d, J = 2.3 Hz, 1H), 4.01 (d, J = 1.2 Hz, 3H), 3.86 (d, J = 1.2 Hz, 3H). 13C NMR (101 MHz, chloroform-d) δ 168.25, 159.77, 139.19, 136.93, 130.70, 126.83, 125.92, 124.78, 123.89, 119.48, 114.62, 112.20, 98.42, 55.50, 51.98. HRMS (ESI+) in m/z: Expected 282.1125 [M + H+] (C17H16NO3+). Observed 282.1133.
2-Phenyl-1H-indol-7-amine (10d)
Brown solid. Yield: 81 mg, 78%. Time: 1. 8 h; 2. 8 h. 1H NMR (400 MHz, chloroform-d) δ 8.20 (s, 1H), 7.76–7.59 (m, 2H), 7.49–7.41 (m, 2H), 7.36–7.30 (m, 1H), 7.19 (d, J = 8.0 Hz, 1H), 6.97 (td, J = 7.7, 2.0 Hz, 1H), 6.82 (d, J = 2.1 Hz, 1H), 6.61 (d, J = 7.3 Hz, 1H), 3.66 (s, 2H). 13C NMR (101 MHz, DMSO-d6) δ 136.25, 133.66, 132.52, 129.25, 128.86, 127.07, 126.42, 124.66, 120.56, 108.51, 105.21, 99.24. HRMS (ESI+) in m/z: Expected 209.1073 [M + H+] (C14H13N2+). Observed 209.1077. 10d is a known compound but no analytical data can be found online.
2-Cyclopropyl-5-(trifluoromethoxy)-1H-indole (10e)
Brown solid. Yield: 73 mg, 61%. Time: 1. 1 h; 2. 8 h. 1H NMR (400 MHz, chloroform-d) δ 8.01 (s, 1H), 7.34 (d, J = 2.5 Hz, 1H), 7.23 (d, J = 8.7 Hz, 1H), 7.03–6.91 (m, 1H), 6.21–6.06 (m, 1H), 1.96 (tt, J = 8.4, 5.1 Hz, 1H), 1.07–0.94 (m, 2H), 0.84–0.72 (m, 2H). 13C NMR (101 MHz, chloroform-d) δ 144.00, 143.28, 134.15, 129.12, 122.26, 119.72, 114.97, 112.31, 110.72, 98.35, 9.04, 7.64. HRMS (ESI+) in m/z: Expected 242.0787 [M + H+] (C12H11F3NO+). Observed 242.0790.
3-(1H-Indol-2-yl)phenol (10f)
Tan solid. Yield: 54 mg, 52%. Time: 1. 1 h; 2. 9 h. HRMS (ESI+) in m/z: 1H NMR (400 MHz, chloroform-d) δ 8.30 (s, 1H), 7.63 (d, J = 7.8 Hz, 1H), 7.40 (d, J = 8.1 Hz, 1H), 7.31 (t, J = 7.8 Hz, 1H), 7.25–7.17 (m, 1H), 7.16–7.10 (m, 2H), 6.82–6.76 (m, 2H), 4.85 (s, 1H). 13C NMR (101 MHz, chloroform-d) δ 156.19, 137.59, 136.94, 134.22, 130.45, 129.31, 122.66, 120.88, 120.48, 117.89, 114.87, 112.29, 111.06, 100.48. Expected 210.0913 [M + H+] (C14H12NO+). Observed 210.0915. 10f is a known compound, but no analytical data can be found online.
Acknowledgments
We thank Shyh-Ming Yang, Patrick Morris, Dan Jansen, Samarjit Patnaik, and Sara Kearney for valuable suggestions; Dingyin Tao and Yuhong Fang for analytical chemistry support. The authors gratefully acknowledge funding by the Intramural Research Program, National Center for Advancing Translational Sciences (NCATS), National Institutes of Health (NIH).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01868.
Catalyst and compound characterization data (PDF)
Author Present Address
† UAB School of Medicine, 1670 University Blvd, Birmingham, Alabama 3523, United States (B.T.M.).
Author Present Address
‡ Nexus Discovery Advisorsm 7820B Wormans Mill Road, Suite 208, Frederick, Maryland 21701, United States (D.J.M.).
The authors declare no competing financial interest.
Supplementary Material
References
- Chinchilla R.; Najera C. Recent advances in Sonogashira reactions. Chem. Soc. Rev. 2011, 40, 5084–5121. 10.1039/c1cs15071e. [DOI] [PubMed] [Google Scholar]
- Chinchilla R.; Najera C. The Sonogashira reaction: a booming methodology in synthetic organic chemistry. Chem. Rev. 2007, 107, 874–922. 10.1021/cr050992x. [DOI] [PubMed] [Google Scholar]
- Jenny N. M.; Mayor M.; Eaton T. R. Phenyl-Acetylene Bond Assembly: A Powerful Tool for the Construction of Nanoscale Architectures. Eur. J. Org. Chem. 2011, 2011, 4965–4983. 10.1002/ejoc.201100176. [DOI] [Google Scholar]
- Wang D.; Gao S. Sonogashira coupling in natural product synthesis. Org. Chem. Front. 2014, 1, 556. 10.1039/C3QO00086A. [DOI] [Google Scholar]
- Sonogashira K.; Tohda Y.; Hagihara N. A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett. 1975, 16, 4467–4470. 10.1016/S0040-4039(00)91094-3. [DOI] [Google Scholar]
- Fairlamb I. J. S.; Kapdi A. R.; Lee A. F. η2-dba Complexes of Pd(0): The Substituent Effect in Suzuki–Miyaura Coupling. Org. Lett. 2004, 6, 4435–4438. 10.1021/ol048413i. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Oldenhuis N. J.; Buchwald S. L. Mild and general conditions for negishi cross-coupling enabled by the use of palladacycle precatalysts. Angew. Chem., Int. Ed. 2013, 52, 615–619. 10.1002/anie.201207750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amatore C.; Jutand A. Role of dba in the reactivity of palladium(0) complexes generated in situ from mixtures of Pd(dba)(2) and phosphines. Coord. Chem. Rev. 1998, 178–180, 511–528. 10.1016/S0010-8545(98)00073-3. [DOI] [Google Scholar]
- Carole W. A.; Bradley J.; Sarwar M.; Colacot T. J. Can Palladium Acetate Lose Its “Saltiness”? Catalytic Activities of the Impurities in Palladium Acetate. Org. Lett. 2015, 17, 5472–5475. 10.1021/acs.orglett.5b02835. [DOI] [PubMed] [Google Scholar]
- Johansson Seechurn C. C.; Sperger T.; Scrase T. G.; Schoenebeck F.; Colacot T. J. Understanding the Unusual Reduction Mechanism of Pd(II) to Pd(I): Uncovering Hidden Species and Implications in Catalytic Cross-Coupling Reactions. J. Am. Chem. Soc. 2017, 139, 5194–5200. 10.1021/jacs.7b01110. [DOI] [PubMed] [Google Scholar]
- Thomas A. M.; Sujatha A.; Anilkumar G. Recent advances and perspectives in copper-catalyzed Sonogashira coupling reactions. RSC Adv. 2014, 4, 21688–21698. 10.1039/C4RA02529F. [DOI] [Google Scholar]
- Handa S.; Smith J. D.; Zhang Y.; Takale B. S.; Gallou F.; Lipshutz B. H. Sustainable HandaPhos-ppm Palladium Technology for Copper-Free Sonogashira Couplings in Water under Mild Conditions. Org. Lett. 2018, 20, 542–545. 10.1021/acs.orglett.7b03621. [DOI] [PubMed] [Google Scholar]
- Gallop C. W.; Chen M. T.; Navarro O. Sonogashira couplings catalyzed by collaborative (N-heterocyclic carbene)-copper and -palladium complexes. Org. Lett. 2014, 16, 3724–3727. 10.1021/ol501540w. [DOI] [PubMed] [Google Scholar]
- Das S. K.; Sarmah M.; Bora U. An ambient temperature Sonogashira cross-coupling protocol using 4-aminobenzoic acid as promoter under copper and amine free conditions. Tetrahedron Lett. 2017, 58, 2094–2097. 10.1016/j.tetlet.2017.04.005. [DOI] [Google Scholar]
- Dewan A.; Sarmah M.; Bora U.; Thakur A. J. A green protocol for ligand, copper and base free Sonogashira cross-coupling reaction. Tetrahedron Lett. 2016, 57, 3760–3763. 10.1016/j.tetlet.2016.07.021. [DOI] [Google Scholar]
- Karami K.; Haghighat Naeini N. Copper-free Sonogashira cross-coupling reactions catalyzed by an efficient dimeric C,N-palladacycle in DMF/H2O. Turk. J. Chem. 2015, 39, 1199–1207. 10.3906/kim-1502-86. [DOI] [Google Scholar]
- Dehimat Z. I.; Yaşar S.; Tebbani D.; Özdemir İ. Sonogashira cross-coupling reaction catalyzed by N-heterocyclic carbene-Pd(II)-PPh3 complexes under copper free and aerobic conditions. Inorg. Chim. Acta 2018, 469, 325–334. 10.1016/j.ica.2017.09.048. [DOI] [Google Scholar]
- Lee D.-H.; Kwon Y.-J.; Jin M.-J. Highly Active Palladium Catalyst for the Sonogashira Coupling Reaction of Unreactive Aryl Chlorides. Adv. Synth. Catal. 2011, 353, 3090–3094. 10.1002/adsc.201100747. [DOI] [Google Scholar]
- Kolli M. K.; Shaik N. M.; Chandrasekar G.; Chidara S.; Korupolu R. B. Pd-PEPPSI-IPentCl: a new highly efficient ligand-free and recyclable catalyst system for the synthesis of 2-substituted indoles via domino copper-free Sonogashira coupling/cyclization. New J. Chem. 2017, 41, 8187–8195. 10.1039/C7NJ01544E. [DOI] [Google Scholar]
- Zim D.; Buchwald S. L. An air and thermally stable one- component catalyst for the amination of aryl chlorides. Org. Lett. 2003, 5, 2413–2415. 10.1021/ol034561h. [DOI] [PubMed] [Google Scholar]
- Stambuli J. P.; Kuwano R.; Hartwig J. F. Unparalleled rates for the activation of aryl chlorides and bromides: coupling with amines and boronic acids in minutes at room temperature. Angew. Chem., Int. Ed. 2002, 41, 4746–4748. 10.1002/anie.200290036. [DOI] [PubMed] [Google Scholar]
- Christmann U.; Pantazis D. A.; Benet-Buchholz J.; McGrady J. E.; Maseras F.; Vilar R. Experimental and theoretical investigations of new dinuclear palladium complexes as precatalysts for the amination of aryl chlorides. J. Am. Chem. Soc. 2006, 128, 6376–6390. 10.1021/ja057825z. [DOI] [PubMed] [Google Scholar]
- Aranyos A.; Old D. W.; Kiyomori A.; Wolfe J. P.; Sadighi J. P.; Buchwald S. L. Novel electron-rich bulky phosphine ligands facilitate the palladium-catalyzed preparation of diaryl ethers. J. Am. Chem. Soc. 1999, 121, 4369–4378. 10.1021/ja990324r. [DOI] [Google Scholar]
- Wei C. S.; Davies G. H.; Soltani O.; Albrecht J.; Gao Q.; Pathirana C.; Hsiao Y.; Tummala S.; Eastgate M. D. The impact of palladium(II) reduction pathways on the structure and activity of palladium(0) catalysts. Angew. Chem., Int. Ed. 2013, 52, 5822–5826. 10.1002/anie.201210252. [DOI] [PubMed] [Google Scholar]
- Hartwig J. F.; Kawatsura M.; Hauck S. I.; Shaughnessy K. H.; Alcazar-Roman L. M. Room-Temperature Palladium-Catalyzed Amination of Aryl Bromides and Chlorides and Extended Scope of Aromatic C-N Bond Formation with a Commercial Ligand. J. Org. Chem. 1999, 64, 5575–5580. 10.1021/jo990408i. [DOI] [PubMed] [Google Scholar]
- Christmann U.; Vilar R. Monoligated palladium species as catalysts in cross-coupling reactions. Angew. Chem., Int. Ed. Engl. 2005, 44, 366–374. 10.1002/anie.200461189. [DOI] [PubMed] [Google Scholar]
- Hruszkewycz D. P.; Balcells D.; Guard L. M.; Hazari N.; Tilset M. Insight into the efficiency of cinnamyl-supported precatalysts for the Suzuki-Miyaura reaction: observation of Pd(I) dimers with bridging allyl ligands during catalysis. J. Am. Chem. Soc. 2014, 136, 7300–7316. 10.1021/ja412565c. [DOI] [PubMed] [Google Scholar]
- Barder T. E.; Biscoe M. R.; Buchwald S. L. Structural Insights into Active Catalyst Structures and Oxidative Addition to (Biaryl)phosphine–Palladium Complexes via Density Functional Theory and Experimental Studies. Organometallics 2007, 26, 2183–2192. 10.1021/om0701017. [DOI] [Google Scholar]
- Biscoe M. R.; Fors B. P.; Buchwald S. L. A new class of easily activated palladium precatalysts for facile C-N cross-coupling reactions and the low temperature oxidative addition of aryl chlorides. J. Am. Chem. Soc. 2008, 130, 6686–6687. 10.1021/ja801137k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinzel T.; Zhang Y.; Buchwald S. L. A New Palladium Precatalyst Allows for the Fast Suzuki–Miyaura Coupling Reactions of Unstable Polyfluorophenyl and 2-Heteroaryl Boronic Acids. J. Am. Chem. Soc. 2010, 132, 14073–14075. 10.1021/ja1073799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno N. C.; Tudge M. T.; Buchwald S. L. Design and Preparation of New Palladium Precatalysts for C-C and C-N Cross-Coupling Reactions. Chem. Sci. 2013, 4, 916–920. 10.1039/C2SC20903A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno N. C.; Niljianskul N.; Buchwald S. L. N-substituted 2-aminobiphenylpalladium methanesulfonate precatalysts and their use in C-C and C-N cross-couplings. J. Org. Chem. 2014, 79, 4161–4166. 10.1021/jo500355k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viciu M. S.; Germaneau R. F.; Navarro-Fernandez O.; Stevens E. D.; Nolan S. P. Activation and Reactivity of (NHC)Pd(allyl)Cl (NHC = N-Heterocyclic Carbene) Complexes in Cross-Coupling Reactions. Organometallics 2002, 21, 5470–5472. 10.1021/om020804i. [DOI] [Google Scholar]
- Marion N.; Navarro O.; Mei J.; Stevens E. D.; Scott N. M.; Nolan S. P. Modified (NHC)Pd(allyl)Cl (NHC = N-Heterocyclic Carbene) Complexes for Room-Temperature Suzuki–Miyaura and Buchwald–Hartwig Reactions. J. Am. Chem. Soc. 2006, 128, 4101–4111. 10.1021/ja057704z. [DOI] [PubMed] [Google Scholar]
- Hill L. L.; Crowell J. L.; Tutwiler S. L.; Massie N. L.; Hines C. C.; Griffin S. T.; Rogers R. D.; Shaughnessy K. H.; Grasa G. A.; Johansson Seechurn C. C. C.; Li H.; Colacot T. J.; Chou J.; Woltermann C. J. Synthesis and X-ray Structure Determination of Highly Active Pd(II), Pd(I), and Pd(0) Complexes of Di(tert-butyl)neopentylphosphine (DTBNpP) in the Arylation of Amines and Ketones. J. Org. Chem. 2010, 75, 6477–6488. 10.1021/jo101187q. [DOI] [PubMed] [Google Scholar]
- Johansson Seechurn C. C.; Parisel S. L.; Colacot T. J. Air-stable Pd(R-allyl)LCl (L = Q-Phos, P(t-Bu)3, etc.) systems for C-C/N couplings: insight into the structure-activity relationship and catalyst activation pathway. J. Org. Chem. 2011, 76, 7918–7932. 10.1021/jo2013324. [DOI] [PubMed] [Google Scholar]
- Melvin P. R.; Balcells D.; Hazari N.; Nova A. Understanding Precatalyst Activation in Cross-Coupling Reactions: Alcohol Facilitated Reduction from Pd(II) to Pd(0) in Precatalysts of the Type (η3-allyl)Pd(L)(Cl) and (η3-indenyl)Pd(L)(Cl). ACS Catal. 2015, 5, 5596–5606. 10.1021/acscatal.5b01291. [DOI] [Google Scholar]
- DeAngelis A. J.; Gildner P. G.; Chow R.; Colacot T. J. Generating Active “L-Pd(0)” via Neutral or Cationic pi-Allylpalladium Complexes Featuring Biaryl/Bipyrazolylphosphines: Synthetic, Mechanistic, and Structure-Activity Studies in Challenging Cross-Coupling Reactions. J. Org. Chem. 2015, 80, 6794–6813. 10.1021/acs.joc.5b01005. [DOI] [PubMed] [Google Scholar]
- Pu X.; Li H.; Colacot T. J. Heck alkynylation (copper-free Sonogashira coupling) of aryl and heteroaryl chlorides, using Pd complexes of t-Bu2(p-NMe2C6H4)P: understanding the structure-activity relationships and copper effects. J. Org. Chem. 2013, 78, 568–581. 10.1021/jo302195y. [DOI] [PubMed] [Google Scholar]
- Soheili A.; Albaneze-Walker J.; Murry J. A.; Dormer P. G.; Hughes D. L. Efficient and general protocol for the copper-free sonogashira coupling of aryl bromides at room temperature. Org. Lett. 2003, 5, 4191–4194. 10.1021/ol035632f. [DOI] [PubMed] [Google Scholar]
- Gildner P. G.; Colacot T. J. Reactions of the 21st Century: Two Decades of Innovative Catalyst Design for Palladium-Catalyzed Cross-Couplings. Organometallics 2015, 34, 5497–5508. 10.1021/acs.organomet.5b00567. [DOI] [Google Scholar]
- Hill L. L.; Smith J. M.; Brown W. S.; Moore L. R.; Guevera P.; Pair E. S.; Porter J.; Chou J.; Wolterman C. J.; Craciun R.; Dixon D. A.; Shaughnessy K. H. Neopentylphosphines as effective ligands in palladium-catalyzed cross-couplings of aryl bromides and chlorides. Tetrahedron 2008, 64, 6920–6934. 10.1016/j.tet.2008.02.037. [DOI] [Google Scholar]
- Melvin P. R.; Nova A.; Balcells D.; Dai W.; Hazari N.; Hruszkewycz D. P.; Shah H. P.; Tudge M. T. Design of a Versatile and Improved Precatalyst Scaffold for Palladium-Catalyzed Cross-Coupling: (η3-1-tBu-indenyl)2(μ-Cl)2Pd2. ACS Catal. 2015, 5, 3680–3688. 10.1021/acscatal.5b00878. [DOI] [Google Scholar]
- Lipshutz B. H.; Chung D. W.; Rich B. Sonogashira couplings of aryl bromides: room temperature, water only, no copper. Org. Lett. 2008, 10, 3793–3796. 10.1021/ol801471f. [DOI] [PubMed] [Google Scholar]
- Byrne F. P.; Jin S.; Paggiola G.; Petchey T. H. M.; Clark J. H.; Farmer T. J.; Hunt A. J.; McElroy C. R.; Sherwood J. Tools and techniques for solvent selection: green solvent selection guides. Sustainable Chem. Processes 2016, 4, 1–24. 10.1186/s40508-016-0051-z. [DOI] [Google Scholar]
- Wang Z.; Lin W.; Jiang C.; Guo Q. An improved coupling reaction for the preparation of pyridylethynyl benzonitrile compounds. Chin. Sci. Bull. 2001, 46, 1606–1608. 10.1007/BF02900616. [DOI] [Google Scholar]
- Park S.; Kim M.; Koo D. H.; Chang S. Use of Ruthenium/Alumina as a Convenient Catalyst for Copper-Free Sonogashira Coupling Reactions. Adv. Synth. Catal. 2004, 346, 1638–1640. 10.1002/adsc.200404189. [DOI] [Google Scholar]
- Li T.; Sun P.; Yang H.; Zhu Y.; Yan H.; Lu L.; Mao J. Copper-catalyzed decarboxylative coupling of aryl halides with alkynyl carboxylic acids performed in water. Tetrahedron 2012, 68, 6413–6419. 10.1016/j.tet.2012.06.003. [DOI] [Google Scholar]
- Mori S.; Yanase T.; Aoyagi S.; Monguchi Y.; Maegawa T.; Sajiki H. Ligand-Free Sonogashira Coupling Reactions with Heterogeneous Pd/C as the Catalyst. Chem. – Eur. J. 2008, 14, 6994–6999. 10.1002/chem.200800387. [DOI] [PubMed] [Google Scholar]
- Sørensen U. S.; Pombo-Villar E. Copper-free palladium-catalyzed sonogashira-type coupling of aryl halides and 1-aryl-2-(trimethylsilyl)acetylenes. Tetrahedron 2005, 61, 2697–2703. 10.1016/j.tet.2005.01.032. [DOI] [Google Scholar]
- Gil-Moltó J.; Nájera C. Direct Coupling Reactions of Alkynylsilanes Catalyzed by Palladium(II) Chloride and a Di(2-pyridyl)methylamine-Derived Palladium(II) Chloride Complex in Water and in NMP. Adv. Synth. Catal. 2006, 348, 1874–1882. 10.1002/adsc.200606033. [DOI] [Google Scholar]
- Yang F.; Wu Y. Facile Synthesis of Substituted Alkynes by Cyclopalladated Ferrocenylimine Catalyzed Cross-Coupling of Arylboronic Acids/Esters with Terminal Alkynes. Eur. J. Org. Chem. 2007, 2007, 3476–3479. 10.1002/ejoc.200700065. [DOI] [Google Scholar]
- Bolliger J. L.; Frech C. M. Highly Convenient, Clean, Fast, and Reliable Sonogashira Coupling Reactions Promoted by Aminophosphine-Based Pincer Complexes of Palladium Performed under Additive- and Amine-Free Reaction Conditions. Adv. Synth. Catal. 2009, 351, 891–902. 10.1002/adsc.200900112. [DOI] [Google Scholar]
- Negishi E.; Xu C.; Tan Z.; Korta M. Direct synthesis of heteroarylethynes via palladium-catalyzed coupling of heteroaryl halides with ethynylzinc halides. Its application to an efficient synthesis of a thiophenelactone from Chamaemelum nobile L.. Heterocycles 1997, 46, 209. 10.3987/COM-97-S79. [DOI] [Google Scholar]
- Yu H.; Li J.; Kou Z.; Du X.; Wei Y.; Fun H.-K.; Xu J.; Zhang Y. Photoinduced Tandem Reactions of Isoquinoline-1,3,4-trione with Alkynes To Build Aza-polycycles. J. Org. Chem. 2010, 75, 2989–3001. 10.1021/jo100218w. [DOI] [PubMed] [Google Scholar]
- Novak I.; Ng S.-C.; Mok C.-Y.; Huang H.-H.; Fang J.; Wang K. K.-T. New organic polymer precursors: synthesis and electronic structure of thienylpyridines and thienylethynylpyridines. J. Chem. Soc., Perkin Trans. 2 1994, 1771–1775. 10.1039/p29940001771. [DOI] [Google Scholar]
- Yang Y.; Chew X.; Johannes C. W.; Robins E. G.; Jong H.; Lim Y. H. A Versatile and Efficient Palladium–meta-Terarylphosphine Catalyst for the Copper-Free Sonogashira Coupling of (Hetero-)Aryl Chlorides and Alkynes. Eur. J. Org. Chem. 2014, 2014, 7184–7192. 10.1002/ejoc.201402699. [DOI] [Google Scholar]
- Wu Y.-T.; Kuo M.-Y.; Chang Y.-T.; Shin C.-C.; Wu T.-C.; Tai C.-C.; Cheng T.-H.; Liu W.-S. Synthesis, Structure, and Photophysical Properties of Highly Substituted 8,8a-Dihydrocyclopenta[a]indenes. Angew. Chem., Int. Ed. 2008, 47, 9891–9894. 10.1002/anie.200802560. [DOI] [PubMed] [Google Scholar]
- Truong T.; Daugulis O. Transition-Metal-Free Alkynylation of Aryl Chlorides. Org. Lett. 2011, 13, 4172–4175. 10.1021/ol2014736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulog L.; Körner B.; Heinze J.; Yang J. Synthesis and electrochemical properties of 4-phenyl-1-buten-3-yne-1,1,2-tricarbonitriles and tricyanoacrylates. Liebigs Ann. 1995, 1995, 1663–1671. 10.1002/jlac.1995199509231. [DOI] [Google Scholar]
- Li C.-W.; Pati K.; Lin G.-Y.; Sohel S. M. A.; Hung H.-H.; Liu R.-S. Gold-Catalyzed Oxidative Ring Expansions and Ring Cleavages of Alkynylcyclopropanes by Intermolecular Reactions Oxidized by Diphenylsulfoxide. Angew. Chem., Int. Ed. 2010, 49, 9891–9894. 10.1002/anie.201004647. [DOI] [PubMed] [Google Scholar]
- Chaisan N.; Kaewsri W.; Thongsornkleeb C.; Tummatorn J.; Ruchirawat S. PtCl4-catalyzed cyclization of N-acetyl-2-alkynylanilines: A mild and efficient synthesis of N-acetyl-2-substituted indoles. Tetrahedron Lett. 2018, 59, 675–680. 10.1016/j.tetlet.2018.01.014. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.