Abstract
The conventional sodium dodecylbenzenesulfonate (NaDBS) has been converted into an efficient and nontoxic anionic surface-active ionic liquid, cholinium dodecylbenzenesulfonate (Cho[DBS]). We have investigated its self-assembling behavior, interaction with the enzyme cellulase, and ecotoxicity. The surface-active properties at the air–liquid interface and the aggregation behavior of Cho[DBS] in water have been determined using tensiometry, isothermal titration calorimetry (ITC), conductometry, and dynamic light scattering (DLS). The enzyme activity was observed using dinitro salicylic acid analysis. The enhanced enzyme activity was explained through active-site exfoliation and structural constancy of cellulase in the micellar medium using the results from fluorescence, circular dichroism, DLS, and ITC. The nontoxic nature was confirmed by toxicity analysis on the freshwater microalgae Scenedesmus sp.
1. Introduction
Ionic liquids (ILs) are considered as nontoxic and greener solvents.1 Owing to their versatile properties, they find applications in diverse fields of chemistry and biochemistry.2,3 In recent times, the conventional surfactants have been turned to IL surfactants commonly called surface-active ILs (SAILs) to achieve desired surface activity and dissolution in water. So far, most of these surfactants have imidazolium and ammonium as a cation.4−6 They have exhibited superior surface activity, are also capable of forming supramolecular nano- to giant aggregates viz., micelles, vesicles, wormlike micelles, and multilayered vesicles, and have found their uses in industrial, chemical, or pharmaceutical applications,7−16 but there are issues about their toxicities and biodegradabilities. Therefore, the choice of a bioderived countercation (choline- and amino acid-based) or counteranion (carboxylate and alkyl sulfate) would create more opportunities to introduce biodegradability in IL surfactants for wider practical utility.17,18 Moreover, the rise in concerns toward the toxicity of imidazolium-based ILs has further furnished the demand for more biodegradable IL surfactants for their efficient and biofriendly utilization.19−21 Seeking progress in this direction, herein, we have turned the commercial anionic surfactant sodium dodecylbenzenesulfonate NaDBS to a more eco-friendly and biodegradable surfactant “cholinium dodecylbenzenesulfonate Cho[DBS]”. In brief, we have synthesized Cho[DBS] as a potential nontoxic IL surfactant whose surface-active properties were analyzed by using tensiometry, conductivity, and isothermal titration calorimetry (ITC) and morphological/size analysis has been done using dynamic light scattering (DLS) and transmission electron microscopy (TEM).
Surface-active compounds find a wide range of applications in micellar catalysis, in DNA stability, in encapsulation of drugs, as a protein stabilizer, and in enzyme/protein stabilization in detergent formulations. Among these, the surfactant–protein interaction studies do hold a great relevance if needed to be utilized for practical applications.22,23 Therefore, we have explored the stabilization of cellulase (an important laundry enzyme) in micellar solution of Cho[DBS] for its suitability in enzymatic catalysis, protein stabilizer, and biodetergents (enzymes bearing detergents). So far, the enzymatic activities in direct micellar systems have occasionally been investigated, and only a few reports are there till date. It is assumed that organic substrates which have limited solubility in water are localized in the palisade layer or the hydrophobic core of the micelles, whereas the enzymes are dissolved and stabilized in the continuous aqueous phase. These essentially aqueous systems are more appropriate for potential industrial use in enzymatic conversion than colloidal systems of the inverse type.24 The term micellar enzymology has been introduced to investigate the kinetic properties of cytosolic enzymes in micellar systems resembling to mimic biological environments.25 Surfactants in their monomeric or aggregated form can act as potential crowding agents,26 and therefore the presence of surfactants may lead to either the activation27,28 or deactivation29,30 of enzyme activity. Recently in 2017, Mitra et al. demonstrated the five-fold α-chymotrypsin enzyme activity enhancement in the micellar medium of dodecyltrimethylammonium bromide (DTAB). They also investigated that DTAB does not produce noticeable changes in the protein structure before the postmicellar region.31 Verma et al. investigated the α-chymotrypsin activity presence of novel cationic amine-based gemini surfactants and observed the effect of the different spacers, chain lengths, and head group sizes with the comparison of cationic surfactants in aqueous buffer media.32 In an earlier study on cellulase enzymes with SAILs, [C8mim][C12OSO3], we found that SAILs can provide the structural and functional stability of the enzyme.33 Therefore, advancing the earlier studies, we have investigated here the structural stability and functional enzyme activity of cellulase and observed a significant enhancement in the activity of the enzyme in Cho[DBS].
Protection of the environment and replacement of toxic chemicals are key points of current research. Therefore, the surfactant was further subjected to toxicity analysis. The toxicity of Cho[DBS] in an aquatic ecosystem was studied by applying on freshwater microalgae Scenedesmus sp. at high concentrations (1000 μmol L–1). The nontoxic nature of Cho[DBS] also discloses its practical use in laundry industry where NaDBS is a key player in the detergent fixture.
2. Results and Discussion
2.1. Thermal Study of Cho[DBS]
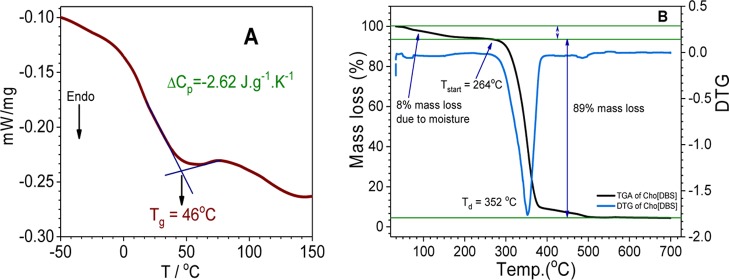
The glass-transition point of Cho[DBS] was determined from the differential scanning calorimetry (DSC) experiment (Figure 1A) and found near to 46 °C. According to the definition of ILs, we can include it in the category of ILs,34 and because of its amphiphilic nature, it will be considered as a SAIL. According to Kapustinskii eq S8 of Annexure 1, for inorganic ionic salts and can be applied on ILs also, lattice enthalpy is decreased with an increase in the ionic radius and leads to a markable decrease in the melting point.35 Depression in the melting point has been achieved by the incorporation of asymmetric large organic cholinium cations with the replacement of sodium cations. Here, the determination of ionic radii is more difficult because of the nonsymmetrical structure and smaller polarization effects of larger organic ions. In such compounds, the structural effects such as branching or lengthening of substituent tails will push down the melting point with the disruption of ideal crystal packing.36
Figure 1.
(A) DSC and (B) thermogravimetric analysis (TGA) profile of Cho[DBS].
Cho[DBS] has been synthesized as a nonhydrated stable low melting salt, and it is quite hygroscopic also. The Enthalpy of hydration depends on the interaction of water molecules to the organic ionic head8 and is much high for Cho[DBS] which makes it hygroscopic. The presence of water molecules can also be observed in TGA and NMR spectrum.
TGA analysis (Figure 1B) has been performed to evaluate the decomposition temperatures of Cho[DBS]. This IL surfactant starts to degrade at 264 °C and undergoes rapid degradation at 352 °C, indicating a reasonably good thermal stability.
2.2. Aggregation Behavior and Interfacial Properties of Cho[DBS] in Aqueous Medium
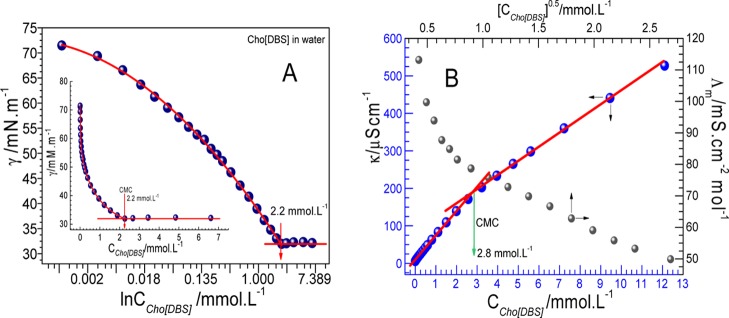
Surface tension (γ) has been measured as a function of concentration of Cho[DBS] (Figure 2A) for the evaluation of critical micelle concentration (CMC). Various parameters have also been derived from surface tension measurements using concerned equations (given in Annexure 1, Supporting Information) and are provided in Table 1. The CMC of Cho[DBS] in aqueous medium has been found around 2.2 mmol L–1 which is lower than that of its conventional analogue NaDBS (Figure S4, 2.9 mmol L–1). Low CMC was observed because of the replacement of a more hydrated Na+ ion with the less hydrated and hydrophobic large organic cholinium counterion ([Cho]+). The latter one is more effective in screening like-charge electrostatic repulsions between surface-active anions at the air–water interface and thereby more effective in decreasing the free energy of micellization.37
Figure 2.
(A) Surface tension profile and (B) conductivity (specific and molar) profile of water with the function of Cho[DBS] (the inset shown the exponential decrement).
Table 1. CMC, Surface Tension at CMC (γCMC), Effective Surface Tension Reduction (πCMC), Adsorption Efficiency (pC20), CMC/C20, Maximum Surface Excess Concentration (Γmax), and Area Occupied by a Single Molecule at the Air–Water Interface (Amin) of Cho[DBS] at 298.15 K.
| surfactant | CMC (mmol L–1) | γCMC (mN·m–1) | πCMC (mN·m–1) | pC20 | CMC/C20 | Γmax (μmol m–2) | Amin (Å2) |
|---|---|---|---|---|---|---|---|
| Cho[DBS] | 2.2 ± 0.1a | 31.9 ± 0.1 | 39.2 ± 0.1 | 4.2 ± 0.1 | 34.4 ± 0.1 | 2.6 ± 0.1 | 64.7 ± 0.1 |
| NaDBS | 2.9 ± 0.1a | 32.6 ± 0.1 | 38.5 ± 0.1 | 3.7 ± 0.1 | 13.7 ± 0.1 | 1.8 ± 0.1 | 94.2 ± 0.1 |
From surface tension. The measurements were performed in triplicate independently. The mean value of each measurement was given in the table. The errors (with two significant numbers) were estimated from the deviation of values from their mean for CMC and derived surface parameters.
Cho[DBS] has also shown lower CMC as compared to cholinium dodecyl carboxylate (25.5 mmol L–1)17 and cholinium dodecyl sulfate (6.0 mmol L–1)18 analogues because of a hydrophobic aromatic benzene ring.37 It can be seen that sulfonate groups have also reinforced the significant decrease in the CMC value as compared to the carboxylates.17 From Table 1, it is observed that the required surfactant concentration to reduce the surface γ of the solvent by 20 mN·m–1 (C20) is 0.064 × 10–3 mol L–1, whereas this value for NaDBS is found near to 0.212 × 10–3 mol L–1 which is quite more than that for Cho[DBS]. The value C20 is dominantly controlled by van der Waals volume which further related to the size of the cationic head group and interelectrostatic repulsions between the head groups at the air–solution interface in low concentration regimes.38 The CMC/C20 value which indicates the efficient performance toward the selectivity for adsorption over micellization39 is very high (34.37) as compared to that of its sodium analogue NaDBS (13.67). Other important parameters affecting the micellization are the effectiveness of surface tension reduction, πCMC, and the adsorption efficacy pC20. From Table 1, the values of these parameters are more near to those of its sodium analogue NaDBS. High Γmax and low Amin (from Table 1), which signify the efficient and compact packing of Cho[DBS] molecules at the air–solution interface, are also on the higher side than those of its sodium analogue.40
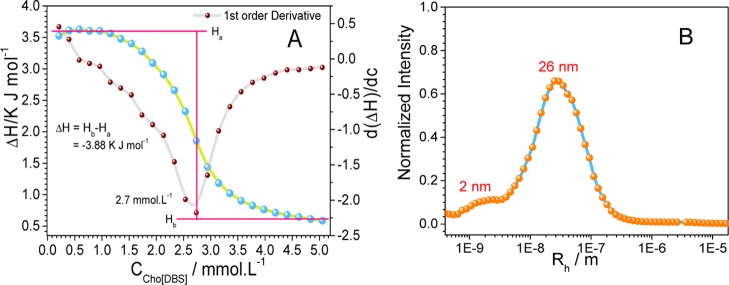
The inflection points in the curves for specific conductivity and molar conductivity represent the CMCs for Cho[DBS] (Figure 2B) and NaDBS (Figure S5). The ratio between the post- and preslope in the specific conductivity curve gives the degree of dissociation which is further used to evaluate the counterion binding and Gibbs free energy for the micellization process. In the molar conductivity curve, there are no maxima found which indicates the absence of preaggregates (aggregation below CMC). Thermodynamic parameters of aggregation, such as counterion binding, Gibbs free energy of aggregation (ΔGmico), and the entropy of micellization (ΔSmic), are calculated using well-known equations (Supporting Information). ITC experiment has been carried out to evaluate the thermodynamic parameters and CMC determination at a constant temperature of 298.15 K. For the ITC experiment, a concentrated solution was added into water and changes in enthalpy during the process were observed. Changes in enthalpy as a function of surfactant concentration are shown in Figure 3A along with the first-order differential curve.
Figure 3.
(A) ITC enthalpogram and (B) DLS profile of Cho[DBS] aggregation in water.
The enthalpy of the dilution profile can be explained by dividing it into three regimes: (i) below CMC where endothermic enthalpy value with constant changes leads to the dilution of micellar solution, demicellization, and hydration of surfactant molecules, (ii) gradual lowering in the endothermic enthalpy value at CMC leading to the reorganization of micelles, and (iii) exothermic enthalpy value after CMC leading to little changes in enthalpy due to less interaction between micelles and attained constancy onward. The enthalpy of micellization ΔHmico has been determined from the difference of the (ii) to the (i) regime, and accurate value of CMC has been determined from the first-order differential curve where minima of the curve indicate the CMC value. The summary of various thermodynamic parameters of micellization is given in Table 2. A negative value of ΔGmic indicates the favorable spontaneous micellization of Cho[DBS] in water. The negative values of ΔHmico and the positive values of ΔSmic for micellization collectively indicate the exothermic nature of micellization. A comparison of interfacial and thermodynamic parameters of Cho[DBS] with those of its analogue NaDBS reveals that there is no significant difference in the surface properties and micellization behavior, from Table 2.
Table 2. CMC, Counterion Binding (β), Gibbs Free Energy for Micellization (ΔGmic), Enthalpy for Micellization (ΔHmic), and Entropy during Micellization (ΔSmic) for Cho[DBS] and NaDBS in Water at 298.15 K.
| surfactant | CMC (mmol L–1) | β | ΔHmico (kJ mol–1) | ΔGmico (kJ mol–1) | ΔSmico (kJ mol–1 K–1) | ΔGadso (kJ mol–1) | |
|---|---|---|---|---|---|---|---|
| Cho[DBS] | 2.8 ± 0.1a | 2.7 ± 0.1b | 0.441 ± 0.07 | –3.9 ± 0.3 | –35.3 ± 0.1 | 0.105 ± 0.056 | –79.3 ± 0.1 |
| NaDBS | 3.4 ± 0.1a | 3.7c | 0.257 ± 0.09 | –0.6c | –30.2 ± 0.1 | 0.099 ± 0.061 | –52.0 ± 0.1 |
From conductivity.
From ITC.
From ITC of ref (29). The measurements were performed in triplicate independently. The mean value of each measurement was given in the table. The errors (with two significant numbers except for β and ΔSmico) were estimated from the deviation of values from their mean for CMC and related thermodynamic parameters.
Size distribution (Figure 3B), TEM image (Figure 4), and autocorrelation plot (Figure S6) of Cho[DBS] aggregates at 5 mmol L–1 concentration collectively indicate that the Cho[DBS] aggregates are assembled as micelles with an average size of 2 nm and loose aggregates with an average size of 26 nm. The micellar sizes of both Cho[DBS] and NaDBS are nearly the same. Similar to Cho[DBS], NaDBS also forms micelles of Rh ≈ 4 nm and loose aggregates of Rh ≈ 100 nm at CMC and higher centrations.37 As habitual, most of the single long-chain surfactants and SAILs aggregate spontaneously into micelles.
Figure 4.

TEM images of Cho[DBS] showing micelles at 5 mmol L–1 concentration.
2.3. Functional Activity of Cellulase in a Colloidal Solution of Cho[DBS]
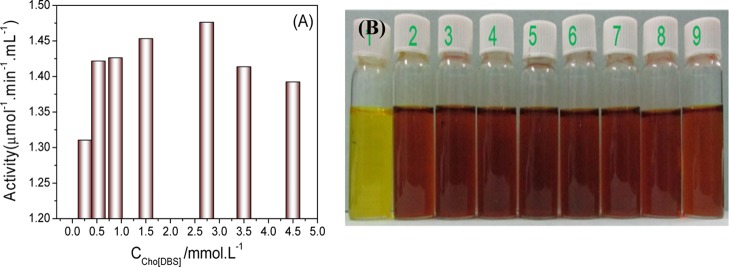
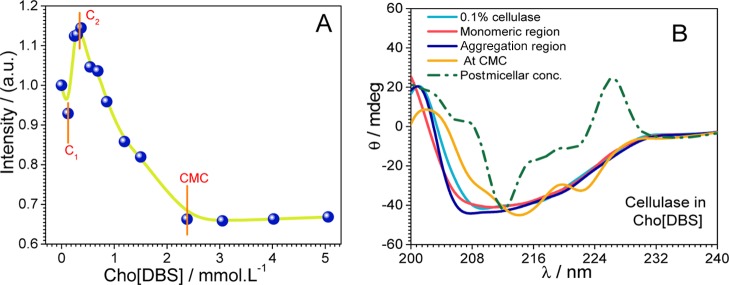
After investigating the surface activity and aggregation behavior, micelles were subjected to a further application. We looked into their effect (micelles of Cho[DBS]) on the structural stability and functional enzyme activity of the cellulase enzyme and excavated the potential utility of Cho[DBS] in enzymatic catalysis, protein storage, and detergency. Dinitro salicylic acid (DNS) sugar assay test has been applied to determine the functional activity of cellulase at different concentrations of Cho[DBS]. The enzyme activity profile of cellulase with Cho[DBS] concentrations is shown in Figure 5A,B. The absence of sugar impurities in the purchased enzymes has been ensured by carrying out blank DNS analysis in the absence of carboxymethyl cellulose. The absence of dark brown color in vial label number 1 (Figure 5B) indicates the absence of sugar impurities in cellulase. Rest of the vials (from 2 to 8) showing dark brown color at different concentrations of Cho[DBS] indicate that the enzyme is active in these colloidal solutions. Vial number 9 (absence of Cho[DBS]) served as a reference. From the enzyme activity profile (Figure 5A), it can be seen that the cellulase activity in surfactant solutions is enhanced than in the absence of surfactants. The enzyme activity increases from 0.2 to 1.5 mmol L–1 and has reached its highest value at 2.4 mmol L–1. It further decreases when moving to higher concentrations.
Figure 5.
(A) Enzyme activity (μmol mL–1 min–1) at different concentrations (mmol L–1) of Cho[DBS]. (B) Digital photographs of vials where vial numbers were mentioned at the top.
2.4. Plausible Mechanism for Enzyme Activity Enhancement via Physical Interactions
After establishing the structural stability and functional activity of cellulase, we looked into probable reasons for these effects. The enhancement in the enzyme activity compared to that in pure buffer or the absence of micelles is often referred to as “superactivity.” The enhancement is governed by several factors arising out of the heterogeneous environment created by the surfactant molecules around the enzyme.41 The theoretical model has to be assigned on the belief that the interaction between the micelle-bound enzyme and the substrate plays the key role behind the superactivity. Activity enhancement in the ionic surfactant-bound enzyme is probably the overall contribution of electrostatic interactions with the charged interfaces and noncovalent interactions with residues of proteins.42,43 However, there is no exact mechanism prescribed, but protein conformational changes, especially in the domain of active sites, are responsible for such a superactivity.28 A sequential discussion has been made by the results obtained from various complementary techniques.
2.4.1. Binding Enthalpogram for Cho[DBS] to Cellulase
Figure 6 shows the enthalpogram of Cho[DBS] aggregation in the buffer and cellulase solution and the binding behavior of the cellulase enzyme after subtraction at 298.15 K. The aggregation process of Cho[DBS] in the buffer is mostly endothermic and turned into exothermic after CMC (2.7 mmol L–1).
Figure 6.
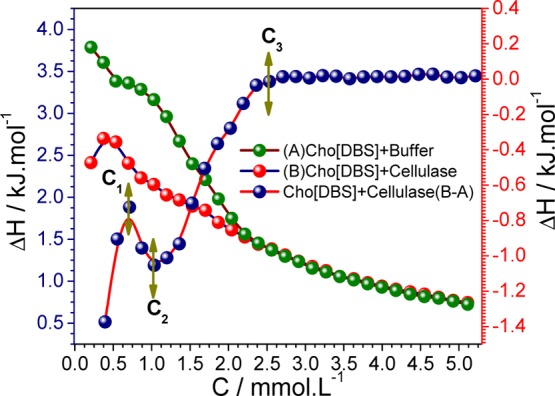
ITC enthalpogram of Cho[DBS] aggregation in the buffer (A) and cellulase solution (B) and subtracted binding isotherm (B – A) with the cellulase enzyme [right “Y axis” represents the enthalpy of the actual binding isotherm (B – A)].
Intermicellar interactions are responsible for these exothermic heat changes after micellization. The standard enthalpies of Cho[DBS] aggregation in the buffer and cellulase solution were found to be −2.64 and −1.96 kJ mol–1, respectively. According to Otzen report on surfactant–protein chemistry, the nature of heat changes can contribute to the electrostatic interaction that leads to exothermic changes and the hydrophobic interaction that relates to endothermic changes. After the subtraction of the Cho[DBS] aggregation from the Cho[DBS] aggregation in 0.1% cellulase solution, the actual enthalpy changes of Cho[DBS] binding to cellulase were determined. The subtracted binding enthalpogram can be divided into four regimes, that is, (i) from 0 to 0.53 mmol L–1 (C1) is the monomeric region, (ii) from 0.53 to 0.86 mmol L–1 (C1 → C2) is the aggregation region, (iii) from 0.86 to 2.39 mmol L–1 (C1 → C3) is the micellar region, and (iv) above 2.39 mmol L–1 (>C3) is the postmicellar region. In Figure 6, the constancy of the actual binding isotherm curve above 2.7 mM concentration of Cho[DBS] (>C3) indicates the stabilization of cellulase via hydrophobic interactions.
2.4.2. Changes in the Tertiary Structure of Cellulase
Variations in the intrinsic fluorescence of cellulase have been examined at various concentrations of Cho[DBS] at excitation (λex)/emission (λem) wavelengths of 280 nm/340 nm.44,45 Intrinsic fluorescence of cellulase appeared because of the fluorophore Trp, Phe, and Tyr domains present in the microenvironment of the protein. Cellulase absorbs ultraviolet light around 280 and 230 nm because of the n−π* transitions in aromatic amino acid residues and the π–π* transitions in the protein backbone, respectively.33,46 The effect coming from the absorption and emission of pure Cho[DBS] is negligible as compared to cellulase. Changes in the fluorescence intensity are indicated in Figure 7A. Initially, there is a slight decrease in the intensity up to the monomeric concentration (C1), which indicates the defolding of the tertiary structure and can state that the surfactant started to disturb the protein structure. Electrostatic interactions between Cho[DBS] and cellulase are likely to be responsible for such a defolding.47 It is followed by a marked increase in the intensity up to the aggregation concentration (C2). The increase in the fluorescence intensity from C1 to C2 is mainly due to the refolding of cellulase.48 After C2, the fluorescence intensity starts decreasing till CMC arrives which is due to the hydrophobic environment. However, if we see it in overall perspective, the total decrease in the fluorescence intensity is not more significant and the intensity becomes constant after CMC. Therefore, it can be concluded that the cellulase tertiary structure remains intact, even at CMC and high concentrations, which further explores the stabilizing effect of Cho[DBS] on the enzyme. In the postmicellar region, the Cho[DBS] micelles interact very less with cellulase, leading to stabilization.
Figure 7.
Variations in (A) the intrinsic fluorescence and (B) ellipticity of cellulase as a function of Cho[DBS] in mmol L–1.
2.4.3. Changes in the Secondary Structure of Cellulase
After alterations in the tertiary structure of cellulase, changes in the secondary structure of the protein need to be observed. Therefore, we have recorded far-UV circular dichroism (CD) spectra. The secondary structure of cellulase is composed of α-helix, β-sheet, and turn (Figure S1). The subunits of cellulase are well-organized in their secondary structure, and it is found that β-sheet and turn structures predominate in the N- and C-terminal regions, whereas α-helices have scarce distribution along the middle parts of cellulase.21,48,49 The CD spectra of native cellulase show a typical (α + β) secondary structural conformation. The CD spectra to observe the changes in the secondary structure of cellulase upon the addition of Cho[DBS] are shown in Figure 7B. With the addition of Cho[DBS], the α + β conformation remained intact up to 2.4 mmol L–1 concentration of Cho[DBS] (near to CMC). Above 2.4 mmol L–1 concentration of Cho[DBS], native CD band has been disturbed which is due to the defolding of cellulase with alterations in ellipticity. That time, active sites which are responsible for the enzyme activity have been exposed. Thus, exposed active sites enhanced the enzyme activity concerning the reference taken. The tertiary and secondary structure constancy in the presence of Cho[DBS] indicates its stability (cellulase) in between near and micelle concentration and vindicates its potential candidature for a protein stabilizer.
2.4.4. Variations in the Size of the Cellulase Enzyme
To understand the concept of defolding–refolding based on the protein hydrodynamic radius (Rh), we did DLS analysis of cellulase (0.1% (w/v)) as a function of Cho[DBS]. The variations in Rh of cellulase due to the unfolding and refolding of cellulase with the interaction or complexation with Cho[DBS] are shown in Figure S7. Native cellulase has Rh of 2.06 nm33 where DLS of aqueous Cho[DBS] at 5 mmol L–1 concentration showed 2 and 26 nm size distribution. The DLS curve for cellulase–Cho[DBS] can be divided into three regimes: (i) the monomeric regime (below C1, 0.53 mmol L–1) where an increase in the size of cellulase is due to defolding, (ii) the micellar regime (C2, 2.4 mmol L–1) where the size of cellulase started to decrease because of refolding, and (iii) the postmicellar pool (after 2.4 mmol L–1), where the size became constant, indicating the stabilization of cellulase. DLS measurements in support with fluorescence and CD confirmed that cellulase is structurally stable in a colloidal solution of Cho[DBS].
2.5. Toxicological Evaluation of Cho[DBS] on Microalgae
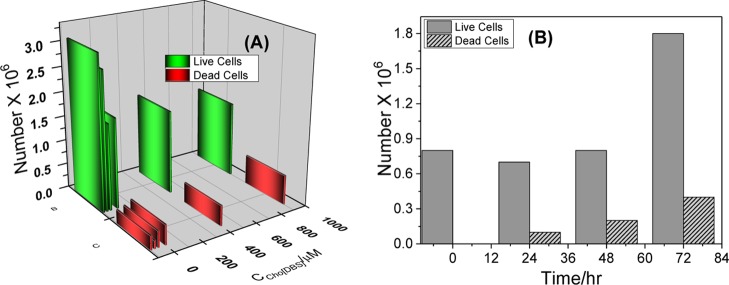
Toxicity of surfactants (in this case, SAILs) on live cells is influenced by certain structural properties of their monomeric cations and anions, including the type of the ions, length of the attached alkyl chains, size of cations, and presence and nature of functional groups. Their aggregation behavior also influences the toxicity in cells. Till now, only limited studies (mainly for carboxylate ILs) are available about the effect of the alkyl chains in IL anions on the toxicity of cells.50,51 The nontoxic nature of Cho[DBS] was, therefore, examined by carrying out toxicological evaluation on microalgae, freshwater strain Scenedesmus sp.20,52 The effects of Cho[DBS] on dead cells and live cells of the freshwater microalgae are shown in Figure 8A,B.
Figure 8.
(A) Number of live cells and dead cells of the fresh microalgae with various concentrations of Cho[DBS] at 72 h and (B) number of live and dead cells of the fresh microalgae with the time interval at 100 μM Cho[DBS].
The number of live cells of the freshwater microalgae in 0, 25, 50, 100, 500, and 1000 μM Cho[DBS] treatments was in the range of 1.5 to 3.1 × 106 after 72 h of exposure, indicating that a minimum number of dead cells (0.2 to 0.6 × 106) were produced when increasing the Cho[DBS] concentration. The effect was also observed with the increase in the exposure time from 0 to 72 h (at 100 μM Cho[DBS]), with an increase in the number of dead cells of the microalgae being countless (including natural death) in comparison with live cells, as observed in Figure 8B.53 At the monomeric concentration of Cho[DBS], a maximum number of live cells were unaffected in the presence of SAILs, indicating that neither the cation nor the anion part of SAILs produced any negative effect (negligible interaction or less penetration through the bilayered lipid membrane) on the metabolite rate of the microalgae.54 No toxic effect induced by the nonamphiphilic cholinium cation is likely due to its less interaction with the lipid membrane of the microalgae,55 whereas it has been shown that the amphiphilic cations normally have greater interaction toward the cell membrane, leading to higher toxicity.56 The literature also indicates that small cations are more aggressive than larger cations in inducing the toxicity when the anions are the same.57 Therefore, alkali cations (Na+ and Li+) are more toxic to living cells than the ammonium cation and the bigger cholinium cation ([Cho]+).
3. Conclusions
A potential nontoxic IL surfactant Cho[DBS] was synthesized and characterized. The study on its aggregation behavior indicates its superior surface-active properties and low CMC value compare to those of its competitor (NaDBS and other choline-based SAILs). An enhancement in enzyme activity was observed in the presence of Cho[DBS], which indicates that it showed excellent compatibility with cellulase. The results show that Cho[DBS] interacts with cellulase through the electrostatic and noncovalent interactions. The active sites of cellulase were exposed in Cho[DBS] micellar solutions during the interaction, thus increased its functional enzyme activity significantly. The slightly modified structure of cellulase after the interaction with Cho[DBS] was stabilized in the postmicellar regime. It is anticipated that the cholinium cation is a strong chaotropic cation (a structure breaker)58 and hence provides stability to the enzyme, whereas the Na+ cation is unable to provide such a stability to the enzyme because of its kosmotropic nature.59 The nontoxic effect of Cho[DBS] examined on microalgae reveals its excellent environmental friendly property and less toxicity for freshwater microalgae (Synedesmus sp.). The study explores the use of nontoxic Cho[DBS] in various colloidal applications. The stability of the cellulase enzyme in combination with Cho[DBS] indicates its practical utility for enzyme–detergent formulations.
4. Experimental Section
4.1. Materials
The cellulase enzyme from Trichoderma reesei ATCC 26921 (lyophilized powder, ≥1 unit/mg) and carboxymethyl cellulose (average Mw ≈ 9000) were purchased from Sigma-Aldrich. Sodium 4-dodecylbenzenesulfonate (>95% purity) was purchased from TCI Chemicals (India) Pvt. Ltd. Choline chloride (>97% purity) was procured from Sigma. Methanol, dichloromethane (DCM), and diethyl ether of analytical reagent (AR) grade were procured from S D Fine-Chem. Ltd., India. Evans blue and BG-11 medium were purchased from Himedia Pvt. Ltd., India. All chemicals were of AR grade and were used as received. Millipore grade water with a specific conductivity of 3 μS·cm–1 and a surface tension of 71 mN·m–1 was used during the whole study. The 0.1% cellulase solution with pH = 4.8 was prepared with AR-grade sodium acetate (99%) and acetic acid (99.7%) purchased from SRL, India. The stock solutions of cellulase were stored at 4 °C. The molecular model of cellulase is provided in the Supporting Information (Figure S1).
4.2. Synthesis of Cholinium Dodecylbenzenesulfonate
Cho[DBS] has been synthesized according to the previously reported procedure by our group.37,49 In brief, choline chloride (5 g, 0.036 mol) and sodium 4-dodecylbenzenesulfonate (12.48 g, 0.036 mol) were dissolved in 50 mL of methanol and made to volume 250 mL with DCM. The reaction mixture was kept overnight at constant stirring. The precipitate was filtered through Whatman paper, and the DCM solution was washed several times with Millipore water to extract the residual inorganic impurities. Complete removal of residual NaCl salt was checked by using alcoholic 0.1 M AgNO3 solution. After that, the DCM solution of the product was treated with activated charcoal overnight to remove color impurities. Suspended active charcoal was filtered off through the Whatman paper. After completion of all processes, the solvent was removed by vapor pressure distillation. The final white waxy product (93% yield) was dried and stored in a vacuum oven at 72 °C. CHNS analysis (theoretical values are in parentheses): % C = 63.82 (64.30), % H = 9.91 (10.09), % N = 4.01 (3.26), % O = 14.52 (14.89), and % S = 7.01 (7.46). LCMS: ESI+m/z value = 104.13 for [C5H14NO]+ and ESI–m/z value = 325.50 for [C18H29O3S]−. The moisture analysis shows the presence of water <200 ppm. The chloride content observed through turbidity was less than 5 ppm. The 1H NMR (200 MHz) data, NMR spectrum, and chemical structure of Cho[DBS] are given in the Supporting Information (Figures S2 and S3).
4.3. Methods
4.3.1. DSC and Thermal Gravimetric Analysis
The glass-transition temperature (Tg) of Cho[DBS] was determined from DSC measurements using a NETZSCH DSC 204F1 (Germany) thermal analyzer in a nitrogen atmosphere. The measurement was performed between −100 and 150 °C at a heating rate of 5 °C min–1. In a typical experiment, 2.41 mg of the sample taken in an alumina crucible was cooled to −100 °C using liquid nitrogen and maintained at −100 °C for 2 min. After an isothermal treatment of 2 min, the crucible was heated from −100 to 120 °C. An empty alumina crucible has been taken as a reference during the measurement. The degradation temperature (Td) was determined from a NETZSCH TG 209F1 Libra TGA209F1D-0105-L thermal analyzer under a nitrogen atmosphere. Cho[DBS] (10.6 mg) was placed in a crucible and kept at 25 °C. The temperature was increased from 25 to 700 °C at a heating rate of 10 °C per min.
4.3.2. Surface Tension Measurement from a Tensiometer
Surface tension measurements were performed using an Attension Force Sigma 700 tensiometer at 298.15 K. The Du Noüy ring method was applied for all experiments, and the values were taken directly from the provided software. Aliquots of Cho[DBS] stock solutions were added to Millipore grade water by incremental volume, and the inbuilt stirring was applied for about 5 min for complete homogenous solubilization and kept for 10 min for equilibrium. The average data were collected in triplicate with the accuracy within the limit of ±0.1 mN·m–1. The temperature of the sample cell was maintained with an instrument-connected JULABO water thermostat in the limit of ±0.1 °C error.
4.3.3. Conductometry
Specific conductivity experiment was carried out with a Eutech digital conductivity meter by applying unit cell constants. The temperature of the measurement cell was controlled with a JULABO water thermostat to within ±0.1 °C. The conductivity was measured after each aliquot addition of the Cho[DBS] stock solution to a sample containing Millipore water, a conductivity cell, and a temperature probe. Experiments were performed within a limit of an uncertainty <0.5%.
4.3.4. Isothermal Titration Calorimetry
Calorimetric titration was done with a MicroCal iTC200 microcalorimeter. The sample cell was filled with 200 μL of MQ water, 25 mM acetate buffer, and 0.1% cellulase solution according to the experiment. The reference cell was filled with 200 μL of 25 mM acetate buffer in all experiments related to cellulase where MQ water was used for the aggregation study. Both cells were stabilized at 298.15 K with a limit of ±0.001 °C. The Cho[DBS] stock solution (40 μL) which was prepared in 25 mM acetate buffer has been taken in an instrument-controlled Hamilton microsyringe injector. The aliquot stock solution (2 μL) was added into the sample cell containing 200 μL of acetate buffer and 0.1% cellulase solution in 25 mM acetate buffer with continuous stirring (at 500 rpm). The other parameters such as time of addition, volume, and duration between each addition were controlled by software provided with the instrument. The enthalpy change at each injection was measured and plotted against concentration by using MicroCal iTC200 analysis with Origin software.
4.3.5. TEM and DLS
The micelles and loose aggregates were digitally captured and investigated using a JEM-2100 transmission electron microscope at a working voltage of 200 kV at GNDU, Amritsar, India. For TEM analysis, the sample was prepared in MQ water, and a drop of the aqueous solution of Cho[DBS] was placed on a Lacey-coated copper grid (300 mesh). The sample was dried in a vacuum desiccator at room temperature for 24 h before the measurement.
DLS measurements were carried out at 298.15 K by using a NaBiTecSpectro-Size300 light scattering instrument (NaBiTec, Germany) with a source of a He–Ne laser (633 nm, 4 mW). Cho[DBS] solutions (5 mmol L–1) were filtered directly into the quartz cell using a membrane syringe filter of 0.45 μm pore size. The temperature of the each measurement was controlled with an accuracy of ±0.1 °C. The data evaluation of the DLS measurements was performed with the inbuilt CONTIN algorithm and replotted with Origin 8.5 software. The error observed in the size of the micelles was ±1 nm.
4.3.6. DNS Sugar Assay Test for the Functional Activity of Cellulase in a Colloidal Solution
A DNS sugar assay test60 was applied to investigate the functional activity of cellulase enzyme at different colloidal concentrations (0, 0.26, 0.53, 0.88, 1.5, 2.74, 3.5, and 4.5 mmol L–1) of Cho[DBS]. Carboxymethyl cellulose has been used as the reference sugar substrate. For this analysis, 1% of cellulase (100 μL) and 900 μL of carboxymethyl cellulose solutions with different concentrations of Cho[DBS] were incubated for 20 min at 45 °C. The reaction was quenched by the addition of 1 mL of the DNS reagent. The reaction mixtures were boiled at 90 °C for 15 min in a water bath until the appearance of a deep red color which is due to the nitro group-to-amino group reductive interconversion by sugar released during the hydrolysis of carboxymethyl cellulose. After that, 0.33 mL of a 40% solution of Rochelle salt (Na+ and K+ tartrate) was added to this mixture to stabilize the deep red color. The solutions were cooled in a water bath and diluted up to 10 mL. The optical density of each sample was measured at a characteristic λmax of 546 nm. The measured optical density was correlated with a predetermined standard calibration curve (optical density against known glucose concentration) to calculate the sugar concentration in each sample. The enzyme activity was calculated using the standard eq S7 given in Annexure 1 of the Supporting Information.
4.3.7. Far-UV CD Spectrometer
Successive structural changes in the secondary structure (β-sheet) of cellulase enzyme upon the interaction with Cho[DBS] were monitored through a JASCO J-815 CD spectrometer with a controlled thermostat at 298.15 K in the far-UV spectrum (λ = 200–250 nm). The spectra were collected in a 1 mm path length quartz cuvette at a scan rate of 100 nm/min, and the sensitivity was set at 100 mdeg. The response time and the bandwidth were 2 s and 0.2 nm, respectively. The smoothed CD spectra were replotted against wavelength at different concentrations of Cho[DBS] using the Origin 8.5 software.
4.3.8. Spectrofluorometer for Fluorescence Study
Successive structural changes in the tertiary structure of cellulase enzyme upon the interaction with Cho[DBS] have been analyzed through a Fluorolog (HORIBA Jobin Yvon) spectrofluorometer. Changes in the intrinsic fluorescence of cellulase at various concentrations of Cho[DBS] were analyzed with determining parameters such as excitation/emission wavelengths (λex/λem) of 280 nm/340 nm, a slit width of 1 nm, and a path length of the quartz cuvette of 1 cm. Each experiment has a repeat, and average maximal values of these measurements have been considered. The smooth fluorescence spectra were replotted against wavelength at different concentrations of Cho[DBS] using the Origin 8.5 software.
4.3.9. Ecotoxicity on Microalgae
The toxicity of Cho[DBS] was evaluated on the selected strain of freshwater. The freshwater strain Scenedesmus sp. was grown in a BG-11 medium. The experiment was carried out in culture tubes containing 15 mL of culture and Cho[DBS] at concentrations of 5, 25, 50, 100, 500, and 1000 μM. The culture without the addition of Cho[DBS] served as a control. The tubes were incubated at 25 ± 1 °C under a light intensity of 150 μmol m–2 s–1, a light/dark period of 12:12 h, and a shaker speed of 120 rpm. The algal cell viability was assessed every 24 h. The culture (1 mL) was mixed with 20 μL of 1% (w/v) Evans blue and incubated for 3 h. The cell count was performed using a Neubauer chamber under a light microscope. Dead cells appeared blue as the Evans blue solution diffused in the protoplasm region and stained the cells blue.
Acknowledgments
The authors thank the Department of Science and Technology (DST), Government of India, for the financial support for this work (no. SB/S1/PC-104/2012). P.S.G. is thankful to UGC for Senior Research Fellowship. The authors are thankful to Dr. Tejwant Singh Kang (GNDU, Amritsar, India) for TEM analysis and valuable suggestions for TEM images. Central instrumentation facility of CSIR-CSMCRI is also acknowledged for its support in the sample characterization. BDIM is acknowledged for providing PRIS number CSIR-CSMCRI—135/2017.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.7b01291.
Standard equations used in the paper for the calculation of various parameters; cellulase model; chemical structure, NMR spectrum, surface tension, and conductivity of NaDBS; autocorrelation function of Cho[DBS]; and variation in the size of cellulase (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Rogers R. D.; Seddon K. R. Ionic Liquids—Solvents of the Future?. Science 2003, 302, 792–793. 10.1126/science.1090313. [DOI] [PubMed] [Google Scholar]
- Armand M.; Endres F.; MacFarlane D. R.; Ohno H.; Scrosati B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. 10.1038/nmat2448. [DOI] [PubMed] [Google Scholar]
- Giernoth R. Task-specific ionic liquids. Angew. Chem., Int. Ed. 2010, 49, 2834–2839. 10.1002/anie.200905981. [DOI] [PubMed] [Google Scholar]
- Bhadani A.; Misono T.; Singh S.; Sakai K.; Sakai H.; Abe M. Structural diversity, physicochemical properties and application of imidazolium surfactants: Recent advances. Adv. Colloid Interface Sci. 2016, 231, 36–58. 10.1016/j.cis.2016.03.005. [DOI] [PubMed] [Google Scholar]
- Dong B.; Li N.; Zheng L.; Yu L.; Inoue T. Surface adsorption and micelle formation of surface active ionic liquids in aqueous solution. Langmuir 2007, 23, 4178–4182. 10.1021/la0633029. [DOI] [PubMed] [Google Scholar]
- Yin T.; Wu J.; Wang S.; Shen W. Structural rearrangement in the aqueous solution of surface active ionic liquid 1-butyl-3-methylimidazolium bis(2-ethylhexyl)sulfosuccinate. Soft Matter 2015, 11, 4717–4722. 10.1039/c5sm00629e. [DOI] [PubMed] [Google Scholar]
- Dong B.; Zhang J.; Zheng L.; Wang S.; Li X.; Inoue T. Salt-induced viscoelastic wormlike micelles formed in surface active ionic liquid aqueous solution. J. Colloid Interface Sci. 2008, 319, 338–343. 10.1016/j.jcis.2007.11.040. [DOI] [PubMed] [Google Scholar]
- Brown P.; Butts C. P.; Eastoe J.; Fermin D.; Grillo I.; Lee H.-C.; Parker D.; Plana D.; Richardson R. M. Anionic Surfactant Ionic Liquids with 1-Butyl-3-methyl-imidazolium Cations: Characterization and Application. Langmuir 2012, 28, 2502–2509. 10.1021/la204557t. [DOI] [PubMed] [Google Scholar]
- Wang H.; Zhang L.; Wang J.; Li Z.; Zhang S. The first evidence for unilamellar vesicle formation of ionic liquids in aqueous solutions. Chem. Commun. 2013, 49, 5222–5224. 10.1039/c3cc41908h. [DOI] [PubMed] [Google Scholar]
- Feng Q.; Wang H.; Zhang S.; Wang J. Aggregation behavior of 1-dodecyl-3-methylimidazolium bromide ionic liquid in non-aqueous solvents. Colloids Surf., A 2010, 367, 7–11. 10.1016/j.colsurfa.2010.05.032. [DOI] [Google Scholar]
- Blesic M.; Marques M. H.; Plechkova N. V.; Seddon K. R.; Rebelo L. P. N.; Lopes A. Self-aggregation of ionic liquids: micelle formation in aqueous solution. Green Chem. 2007, 9, 481–490. 10.1039/b615406a. [DOI] [Google Scholar]
- Rao V. G.; Ghosh S.; Ghatak C.; Mandal S.; Brahmachari U.; Sarkar N. Designing a new strategy for the formation of IL-in-oil microemulsions. J. Phys. Chem. B 2012, 116, 2850–2855. 10.1021/jp2110488. [DOI] [PubMed] [Google Scholar]
- Ezrahi S.; Tuval E.; Aserin A. Properties, main applications and perspectives of worm micelles. Adv. Colloid Interface Sci. 2006, 128, 77–102. 10.1016/j.cis.2006.11.017. [DOI] [PubMed] [Google Scholar]
- Dwars T.; Paetzold E.; Oehme G. Reactions in Micellar Systems. Angew. Chem., Int. Ed. 2005, 44, 7174–7199. 10.1002/anie.200501365. [DOI] [PubMed] [Google Scholar]
- Yang P.; Lipowsky R.; Dimova R. Nanoparticle formation in giant vesicles: synthesis in biomimetic compartments. Small 2009, 5, 2033–2037. 10.1002/smll.200900560. [DOI] [PubMed] [Google Scholar]
- Kumar G. P.; Rajeshwarrao P. Nonionic surfactant vesicular systems for effective drug delivery—an overview. Acta Pharm. Sin. B 2011, 1, 208–219. 10.1016/j.apsb.2011.09.002. [DOI] [Google Scholar]
- Klein R.; Touraud D.; Kunz W. Choline carboxylate surfactants: biocompatible and highly soluble in water. Green Chem. 2008, 10, 433–435. 10.1039/b718466b. [DOI] [Google Scholar]
- Klein R.; Kellermeier M.; Touraud D.; Müller E.; Kunz W. Choline alkylsulfates—New promising green surfactants. J. Colloid Interface Sci. 2013, 392, 274–280. 10.1016/j.jcis.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Liu T.; Zhu L.; Wang J.; Wang J.; Zhang J.; Sun X.; Zhang C. Biochemical toxicity and DNA damage of imidazolium-based ionic liquid with different anions in soil on Vicia faba seedlings. Sci. Rep. 2015, 5, 18444. 10.1038/srep18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X.; Tong Z.-H.; Li L.-L.; Yu H.-Q. Toxic effects of imidazolium-based ionic liquids on Caenorhabditis elegans: the role of reactive oxygen species. Chemosphere 2013, 93, 2399–2404. 10.1016/j.chemosphere.2013.08.040. [DOI] [PubMed] [Google Scholar]
- Matzke M.; Stolte S.; Arning J.; Uebers U.; Filser J. Imidazolium based ionic liquids in soils: effects of the side chain length on wheat (Triticum aestivum) and cress (Lepidium sativum) as affected by different clays and organic matter. Green Chem. 2008, 10, 584–591. 10.1039/b717811e. [DOI] [Google Scholar]
- Goddard E. D.; Ananthapadmanabhan K. P.. Interactions of Surfactants with Polymers and Proteins; CRC Press, 1993; pp 1–427. [Google Scholar]
- Jones M. N. Surfactant interactions with biomembranes and proteins. Chem. Soc. Rev. 1992, 21, 127–136. 10.1039/cs9922100127. [DOI] [Google Scholar]
- Meziani A.; Touraud D.; Zradba A.; Pulvin S.; Pezron I.; Clausse M.; Kunz W. Comparison of enzymatic activity and nanostructures in water/ethanol/Brij 35 and water/1-pentanol/Brij 35 systems. J. Phys. Chem. B 1997, 101, 3620–3625. 10.1021/jp963024u. [DOI] [Google Scholar]
- Martinek K.; Klyachko N. L.; Kabanov A. V.; Khmelnitsky Y. L.; Levashov A. V. Micellar enzymology: its relation to membranology. Biochim. Biophys. Acta, Biomembr. 1989, 981, 161–172. 10.1016/0005-2736(89)90024-2. [DOI] [PubMed] [Google Scholar]
- Malik A.; Kundu J.; Mukherjee S. K.; Chowdhury P. K. Myoglobin unfolding in crowding and confinement. J. Phys. Chem. B 2012, 116, 12895–12904. 10.1021/jp306873v. [DOI] [PubMed] [Google Scholar]
- Abuin E.; Lissi E.; Duarte R. Kinetics of N-glutaryl-L-phenylalanine p-nitroanilide hydrolysis catalyzed by α-chymotrypsin in aqueous solutions of dodecyltrimethylammonium bromide. J. Colloid Interface Sci. 2005, 283, 539–543. 10.1016/j.jcis.2004.08.177. [DOI] [PubMed] [Google Scholar]
- Celej M. S.; D’andrea M. G.; Campana P. T.; Fidelio G. D.; Bianconi M. L. Superactivity and conformational changes on alpha-chymotrypsin upon interfacial binding to cationic micelles. Biochem. J. 2004, 378, 1059–1066. 10.1042/bj20031536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savelli G.; Spreti N.; Di Profio P. Enzyme activity and stability control by amphiphilic self-organizing systems in aqueous solutions. Curr. Opin. Colloid Interface Sci. 2000, 5, 111–117. 10.1016/s1359-0294(00)00043-1. [DOI] [Google Scholar]
- Viparelli P.; Alfani F.; Cantarella M. Experimental validation of a model for α-chymotrypsin activity in aqueous solutions of surfactant aggregates. J. Mol. Catal. B: Enzym. 2001, 15, 1–8. 10.1016/s1381-1177(00)00250-2. [DOI] [Google Scholar]
- Patra A.; Samanta N.; Das D. K.; Mitra R. K. Enhanced Catalytic Activity of α-Chymotrypsin in Cationic Surfactant Solutions: The Component Specificity Revisited. J. Phys. Chem. B 2017, 121, 1457–1465. 10.1021/acs.jpcb.6b10472. [DOI] [PubMed] [Google Scholar]
- Verma S. K.; Ghritlahre B. K.; Ghosh K. K.; Verma R.; Verma S.; Zhao X. Influence of Amine-Based Cationic Gemini Surfactants on Catalytic Activity of α-Chymotrypsin. Int. J. Chem. Kinet. 2016, 48, 779–784. 10.1002/kin.21032. [DOI] [Google Scholar]
- Bharmoria P.; Mehta M. J.; Pancha I.; Kumar A. Structural and functional stability of cellulase in aqueous-biamphiphilic ionic liquid surfactant solution. J. Phys. Chem. B 2014, 118, 9890–9899. 10.1021/jp506211b. [DOI] [PubMed] [Google Scholar]
- Wilkes J. S.Introduction. Ionic Liquids in Synthesis; Wiley-VCH Verlag GmbH & Co. KGaA, 2003; pp 1–6. [Google Scholar]
- Kapustinskii A.Lattice Energy of Ionic Crystals; Quarterly Reviews, Chemical Society, 1956; Vol. 10, pp 283–294. [Google Scholar]
- Brown P.; Butts C.; Dyer R.; Eastoe J.; Grillo I.; Guittard F.; Rogers S.; Heenan R. Anionic surfactants and surfactant ionic liquids with quaternary ammonium counterions. Langmuir 2011, 27, 4563–4571. 10.1021/la200387n. [DOI] [PubMed] [Google Scholar]
- Rao K. S.; Gehlot P. S.; Gupta H.; Drechsler M.; Kumar A. Sodium Bromide Induced Micelle to Vesicle Transitions of Newly Synthesized Anionic Surface Active Ionic Liquids Based on Dodecylbenzenesulfonate. J. Phys. Chem. B 2015, 119, 4263–4274. 10.1021/jp512805e. [DOI] [PubMed] [Google Scholar]
- Kamboj R.; Bharmoria P.; Chauhan V.; Singh G.; Kumar A.; Singh S.; Kang T. S. Effect of cationic head group on micellization behavior of new amide-functionalized surface active ionic liquids. Phys. Chem. Chem. Phys. 2014, 16, 26040–26050. 10.1039/c4cp04054f. [DOI] [PubMed] [Google Scholar]
- Rosen M. J.; Mathias J. H.; Davenport L. Aberrant Aggregation Behavior in Cationic Gemini Surfactants Investigated by Surface Tension, Interfacial Tension, and Fluorescence Methods. Langmuir 1999, 15, 7340–7346. 10.1021/la9904096. [DOI] [Google Scholar]
- Quagliotto P.; Barbero N.; Barolo C.; Artuso E.; Compari C.; Fisicaro E.; Viscardi G. Synthesis and properties of cationic surfactants with tuned hydrophylicity. J. Colloid Interface Sci. 2009, 340, 269–275. 10.1016/j.jcis.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Viparelli P.; Alfani F.; Cantarella M. Models for enzyme superactivity in aqueous solutions of surfactants. Biochem. J. 1999, 344, 765–773. 10.1042/bj3440765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen K. K.; Otzen D. E. How chain length and charge affect surfactant denaturation of acyl coenzyme A binding protein (ACBP). J. Phys. Chem. B 2009, 113, 13942–13952. 10.1021/jp905553h. [DOI] [PubMed] [Google Scholar]
- Tanaka A.; Hoshino E. Thermodynamic and activation parameters for the hydrolysis of amylose with Bacillus α-amylases in a diluted anionic surfactant solution. J. Biosci. Bioeng. 2002, 93, 485–490. 10.1016/s1389-1723(02)80096-2. [DOI] [PubMed] [Google Scholar]
- Otzen D. Protein–surfactant interactions: a tale of many states. Biochim. Biophys. Acta, Proteins Proteomics 2011, 1814, 562–591. 10.1016/j.bbapap.2011.03.003. [DOI] [PubMed] [Google Scholar]
- Schmid F.Optical spectroscopy to characterize protein conformation and conformational changes. Proofs for Protein Structure: A Practical Approach; IRL Press: Oxford, UK, 1997; pp 259–295. [Google Scholar]
- Markus G.; Love R. L.; Wissler F. C. Mechanism of protection by anionic detergents against denaturation of serum albumin. J. Biol. Chem. 1964, 239, 3687–3693. [PubMed] [Google Scholar]
- Deep S.; Ahluwalia J. C. Interaction of bovine serum albumin with anionic surfactants. Phys. Chem. Chem. Phys. 2001, 3, 4583–4591. 10.1039/b105779k. [DOI] [Google Scholar]
- Ohmiya K.; Sakka K.; Karita S.; Kimura T. Structure of cellulases and their applications. Biotechnol. Genet. Eng. Rev. 1997, 14, 365–414. 10.1080/02648725.1997.10647949. [DOI] [PubMed] [Google Scholar]
- Bharmoria P.; Rao K. S.; Trivedi T. J.; Kumar A. Biamphiphilic ionic liquid induced folding alterations in the structure of bovine serum albumin in aqueous medium. J. Phys. Chem. B 2014, 118, 115–124. 10.1021/jp4102042. [DOI] [PubMed] [Google Scholar]
- Rengstl D.; Kraus B.; Van Vorst M.; Elliott G. D.; Kunz W. Effect of choline carboxylate ionic liquids on biological membranes. Colloids Surf., B 2014, 123, 575–581. 10.1016/j.colsurfb.2014.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petkovic M.; Ferguson J. L.; Gunaratne H. Q. N.; Ferreira R.; Leitão M. C.; Seddon K. R.; Rebelo L. P. N.; Pereira C. S. Novel biocompatible cholinium-based ionic liquids—toxicity and biodegradability. Green Chem. 2010, 12, 643–649. 10.1039/b922247b. [DOI] [Google Scholar]
- Lewis M. A. Chronic toxicities of surfactants and detergent builders to algae: a review and risk assessment. Ecotoxicol. Environ. Saf. 1990, 20, 123–140. 10.1016/0147-6513(90)90052-7. [DOI] [PubMed] [Google Scholar]
- Pavlić Ž.; Vidaković-Cifrek Ž.; Puntarić D. Toxicity of surfactants to green microalgae Pseudokirchneriella subcapitata and Scenedesmus subspicatus and to marine diatoms Phaeodactylum tricornutum and Skeletonema costatum. Chemosphere 2005, 61, 1061–1068. 10.1016/j.chemosphere.2005.03.051. [DOI] [PubMed] [Google Scholar]
- Sánchez L.; Mitjans M.; Infante M. R.; García M. T.; Manresa M. A.; Vinardell M. P. The biological properties of lysine-derived surfactants. Amino Acids 2007, 32, 133–136. 10.1007/s00726-006-0318-x. [DOI] [PubMed] [Google Scholar]
- Rantamäki A. H.; Ruokonen S.-K.; Sklavounos E.; Kyllönen L.; King A. W. T.; Wiedmer S. K. Impact of Surface-Active Guanidinium-, Tetramethylguanidinium-, and Cholinium-Based Ionic Liquids on Vibrio Fischeri Cells and Dipalmitoylphosphatidylcholine Liposomes. Sci. Rep. 2017, 7, 46673. 10.1038/srep46673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo J. Y.; Praveenkumar R.; Kim B.; Seo J.-C.; Park J.-Y.; Na J.-G.; Jeon S. G.; Park S. B.; Lee K.; Oh Y.-K. Downstream integration of microalgae harvesting and cell disruption by means of cationic surfactant-decorated Fe3O4 nanoparticles. Green Chem. 2016, 18, 3981–3989. 10.1039/c6gc00904b. [DOI] [Google Scholar]
- Maugras M.; Infante M. R.; Gerardin C.; Selve C.; Vinardell M. P. Possible effects of counterions on biological activities of anionic surfactants. Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol. 2001, 128, 541–545. 10.1016/s1532-0456(01)00174-0. [DOI] [PubMed] [Google Scholar]
- Naushad M.; ALOthman Z. A.; Khan A. B.; Ali M. Effect of ionic liquid on activity, stability, and structure of enzymes: a review. Int. J. Biol. Macromol. 2012, 51, 555–560. 10.1016/j.ijbiomac.2012.06.020. [DOI] [PubMed] [Google Scholar]
- Ishai P. B.; Mamontov E.; Nickels J. D.; Sokolov A. P. Influence of Ions on Water Diffusion—A Neutron Scattering Study. J. Phys. Chem. B 2013, 117, 7724–7728. 10.1021/jp4030415. [DOI] [PubMed] [Google Scholar]
- Miller G. L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. 10.1021/ac60147a030. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.