Abstract
A series of stable Pt(IV) corrole complexes with the general formula PtIV[TpXPC](m/p-C6H4CN)(py), where TpXPC3– is the trianion of a tris(p-X-phenyl)corrole and X = CF3, H, and CH3, has been synthesized, affording key physicochemical data on a rare and elusive class of metallocorroles. Single-crystal X-ray structures of two of the complexes revealed very short equatorial Pt–N distances of 1.94–1.97 Å, an axial Pt–C distance of ∼2.03 Å, and an axial Pt–N distance of ∼2.22 Å. The complexes exhibit Soret maxima at ∼430 nm, which are essentially independent of the meso-aryl para substituents, and strong Q bands with the most intense peak at 595–599 nm. The substituent-independent Soret maxima are consistent with an innocent PtIV–corrole3– description for the complexes. The low reduction potentials (−1.45 ± 0.08 V vs saturated calomel reference electrode) also support a highly stable Pt(IV) ground state as opposed to a noninnocent corrole•2– description. The reductions, however, are irreversible, which suggests that they involve concomitant cleavage of the Pt–aryl bond. Unlike Pt(IV) porphyrins, two of the complexes, PtIV[TpXPC](m-C6H4CN)(py) (X = CF3 and CH3), were found to exhibit room-temperature near-IR phosphorescence with emission maxima at 813 and 826 nm, respectively. The quantum yield of ∼0.3% is comparable to those observed for six-coordinate Ir(III) corroles.
Introduction
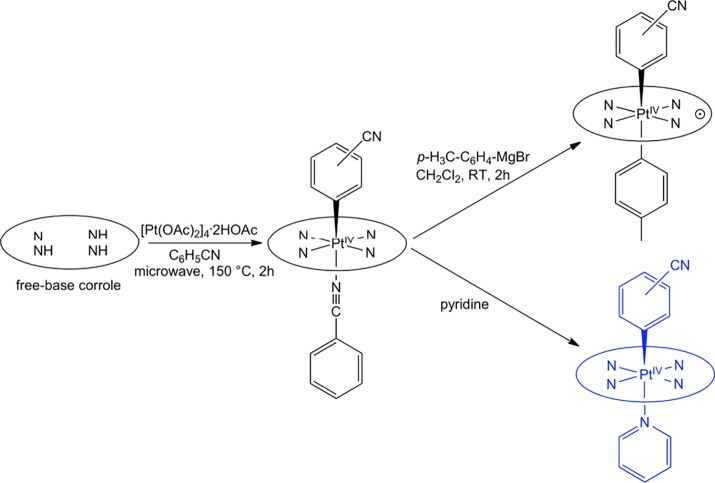
The 5d metallocorroles constitute a unique class of size-mismatched complexes that incorporate a large 5d transition-metal ion within a sterically constrained macrocyclic ligand.1 Despite a steric mismatch inherent in their structures, the majority of them exhibit remarkable chemical and photochemical stabilities. A number of them also exhibit room-temperature near-IR phosphorescence,2 which has led to applications as oxygen sensors3−5 and as photosensitizers in photodynamic therapy and dye-sensitized solar cells.6,7 Platinum(IV) corroles, of which there has been only a single report,8 are particularly intriguing because of their potential for axial reactivity. They are, however, only accessible via a low-yielding, serendipitously discovered reaction, which involves the interaction of a free-base corrole and Pt4(OAc)8·2HOAc in benzonitrile at high temperature. The initially formed Pt(IV) products, PtIV[TpXPC](m/p-C6H4CN)(PhCN), where TpXPC is the trianion of a meso-tris(para-X-phenyl)corrole (X = CF3, H, and CH3) and the m/p-C6H4CN group derives from the solvent (i.e., PhCN), proved unstable, but could be derivatized to stable, paramagnetic products PtIV[TpXPC•2–](m/p-C6H4CN)(Ar), which proved amenable to single-crystal X-ray structure determination.8 Here, we report that in situ exposure of the initially formed Pt(IV)-PhCN products to pyridine leads to a new class of stable, nonradical Pt(IV) corroles with the general formula PtIV[TpXPC](m/p-C6H4CN)(py), which have been variously characterized with single-crystal X-ray structure determination, electrochemical studies, and UV–vis–NIR absorption and emission spectroscopy (Figure 1). Although the results represent modest progress from a synthetic viewpoint, the physicochemical measurements afford significant insight into the electronic properties of a rare and elusive class of substances.
Figure 1.
Current status of Pt–corrole chemistry; the complexes prepared in the course of this study are schematically depicted in blue.
Results and Discussion
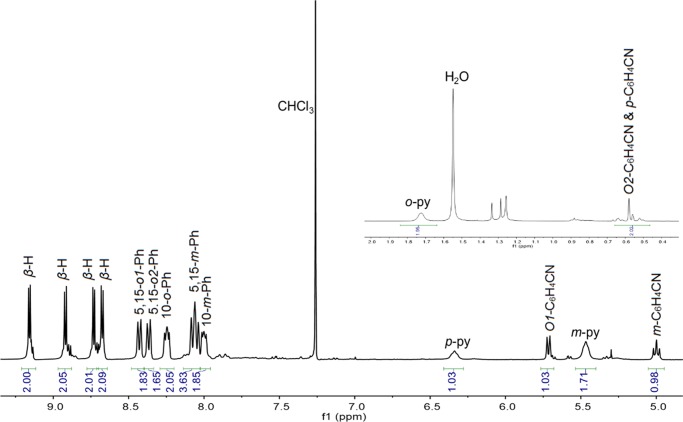
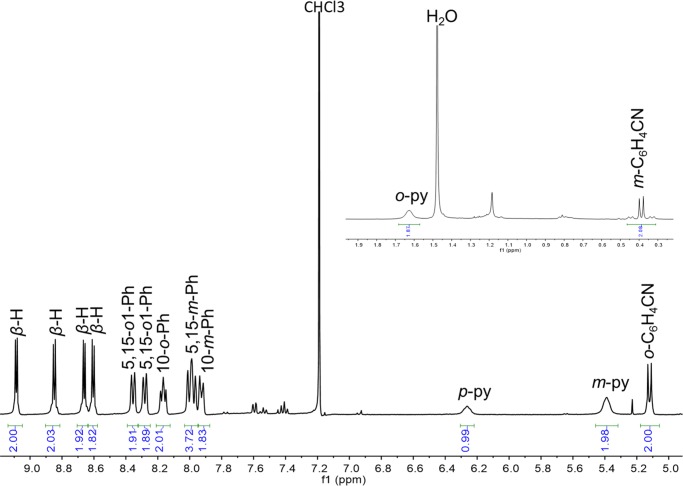
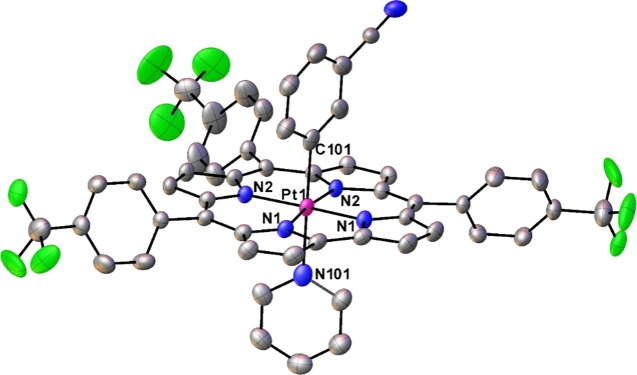
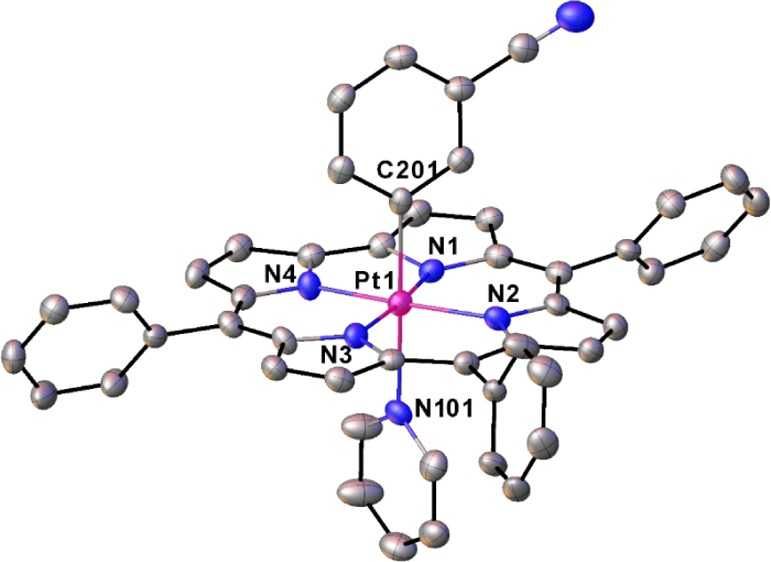
As mentioned above, the Pt(IV) corroles PtIV[TpXPC](m/p-C6H4CN)(py) (X = CF3, H, and CH3) were obtained rather simply by the addition of pyridine to the reaction mixture at the end of the Pt insertion. For all compounds, purity and composition were established via thin-layer chromatography, high-resolution electrospray ionization mass spectrometry, and 1H NMR spectroscopy (Figures 2 and 3). Elemental analyses, however, could not be obtained because of the very small quantities available. Single-crystal X-ray structures could be obtained for two of the complexes, providing unambiguous proof of structure (Table 1). Both structures revealed a Pt atom located exactly or nearly exactly in the mean plane of a planar corrole ligand. For PtIV[TpCF3PC](m-C6H4CN)(py), the two axial ligands, m-C6H4CN and pyridine, were found to occupy symmetry-equivalent sites in the crystal, each with 50% occupancy, and were modeled such that the atoms of the two six-membered rings were superimposed (Figure 4). Accordingly, the axial Pt–C/N distances for this structure only represent an average of the “true” Pt–C and Pt–N distances. Fortunately, the second structure, PtIV[TPC](m-C6H4CN)(py) (TPC = triphenylcorrolato), was found to be fully ordered (Figure 5). The structures exhibit some of the shortest Pt–N distances known, which for the equatorial nitrogens are 1.955 ± 0.015 Å, reflecting the sterically constrained character of 5d metallocorroles. The axial Pt–C and Pt–N distances in the TPC complex are longer, 2.033(7) and 2.216(6) Å, respectively.
Figure 2.
Representative 1H NMR spectrum: Pt[TpCF3PC](m-C6H4CN)(py).
Figure 3.
Representative 1H NMR spectrum: Pt[TpCF3PC](p-C6H4CN)(py).
Table 1. X-ray Crystallographic Data for the Two Crystals Analyzeda.
| sample | Pt[TPC](Ar)(py) | Pt[TpCF3PC](Ar)(py) |
|---|---|---|
| chemical formula | C49H32N6Pt | C52H29F9N6Pt |
| formula mass | 899.89 | 1103.90 |
| crystal system | triclinic | monoclinic |
| space group | P1̅ | C2/c |
| λ (Å) | 0.7293 | 0.8857 |
| a (Å) | 9.4792(15) | 18.9584(10) |
| b (Å) | 12.0922(19) | 16.8577(8) |
| c (Å) | 16.675(3) | 14.0096(7) |
| α (deg) | 109.102(3) | 90 |
| β (deg) | 95.415(3) | 111.553(3) |
| γ (deg) | 90.850(3) | 90 |
| Z | 2 | 4 |
| V (Å3) | 1795.9(5) | 4164.3(4) |
| temperature (K) | 173(2) | 100(2) |
| density (g/cm3) | 1.664 | 1.761 |
| measured reflections | 47 230 | 20 649 |
| unique reflections | 11 271 | 4807 |
| parameters | 506 | 340 |
| restraints | 0 | 58 |
| Rint | 0.1151 | 0.0544 |
| θ range (deg) | 2.217–31.857 | 2.237–21.225 |
| R1, wR2 all data | 0.0583, 0.1336 | 0.0482, 0.0746 |
| S (GooF) all data | 1.033 | 1.037 |
| max/min res. dens. (e/Å3) | 3.436/–1.496 | 0.968/–0.872 |
Ar = m-C6H4CN.
Figure 4.
Thermal ellipsoid plot for PtIV[TpCF3PC](m-C6H4CN)(py). Selected distances (Å): Pt1–N1 1.950(3), Pt1–N2 1.971(3), and Pt1–C/N101 2.148(4).
Figure 5.
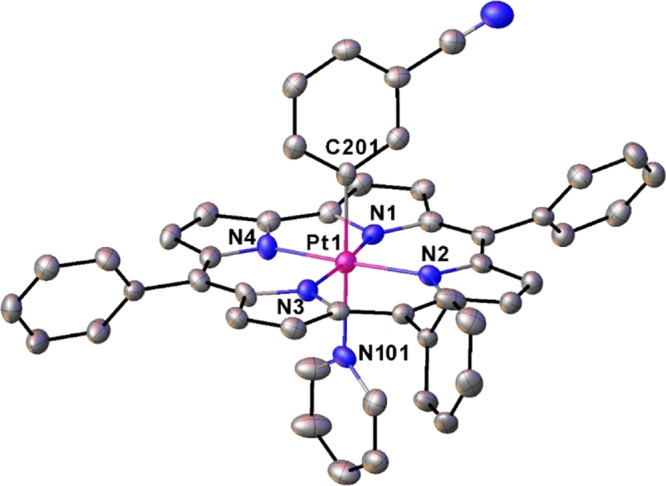
Selected distances (Å): Pt1–N1 1.944(5), Pt1–N2 1.966(5), Pt1–N3 1.955(6), Pt1–N4 1.944(5), Pt1–N101 2.216(6), and Pt1–C201 2.033(7).
All six complexes exhibit slightly split Soret bands (Table 2 and Figures 6 and 7), which are essentially unaffected by the para substituents on the meso-aryl groups as well as strong Q bands. Over a long series of studies, we have shown that such substituent-insensitive Soret maxima are indicative of an innocent, nonradical corrole macrocycle, which is typical of the great majority of stable 4d and 5d metallocorroles, including MoO,9 RuN,10 OsN,11 TcO,12 ReO,13 and Au14−17 corroles as well as Mo18 and W biscorroles.19 In contrast, the Soret maxima of the PtIV[TpXPC•2–](m/p-C6H4CN)(Ar) were found to redshift dramatically in response to increasing electron-donating character of the para substituent X,7 a phenomenon that is also observed for other noninnocent metallocorroles, such as MnCl,20,21 FeCl,22,23 FeNO,24,25 Fe2(μ-O),26 and Cu corroles.27−35
Table 2. Spectroscopic and Electrochemical Properties: UV–vis λmax (nm) and E1/2 Values (V) of Pt[TpXPC](m/p-C6H4CN)(py).
| complex | λmax (Soret) | λmax (Q) | E1/2(ox2) | E1/2(ox1) | E1/2(red1) | ΔE |
|---|---|---|---|---|---|---|
| Pt[TpCF3PC](m-C6H4CN)(py) | 430 | 569, 595 | 1.37 | 0.74 | –1.37 | 2.11 |
| Pt[TPC](m-C6H4CN)(py) | 427, 437 | 567, 596 | 1.12 | 0.61 | –1.49 | 2.10 |
| Pt[TpCH3PC](m-C6H4CN)(py) | 427, 438 | 567, 599 | 1.11 | 0.56 | –1.53 | 2.09 |
| Pt[TpCF3PC](p-C6H4CN)(py) | 430 | 571, 595 | ||||
| Pt[TPC](p-C6H4CN)(py) | 427, 437 | 568, 597 | ||||
| Pt[TpCH3PC](p-C6H4CN)(py) | 427, 438 | 567, 599 |
Figure 6.
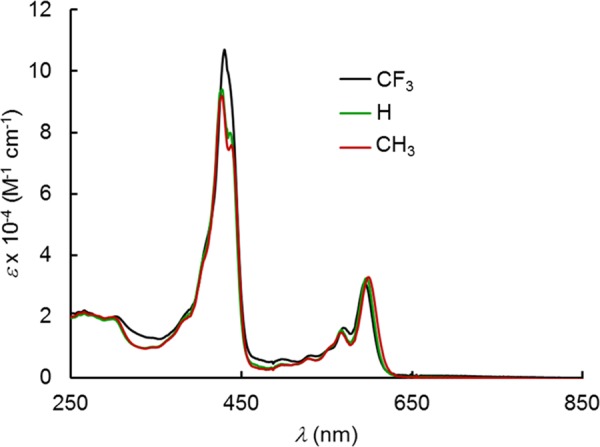
UV–vis spectra of Pt[TpXPC](m-C6H4CN)(py), X = CF3, H, and CH3.
Figure 7.
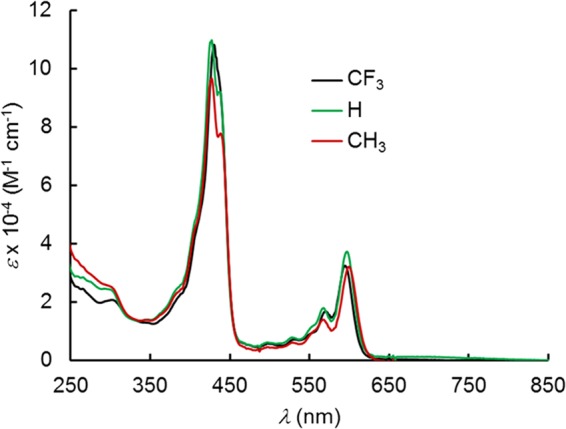
UV–vis spectra of Pt[TpXPC](p-C6H4CN)(py).
Cyclic voltammetry measurements were carried out for the meta-cyanophenyl series PtIV[TpXPC](m-C6H4CN)(py), which could be obtained in somewhat higher yields than the para series (Figure 8 and Table 2). Given the instability of the Pt(V) state, the oxidation potentials, which range from 0.56 V (for X = CH3) to 0.74 V (for X = CF3), may be safely assigned to corrole-centered oxidation. The low values of the reduction potentials, which range from −1.53 V (for X = CH3) to −1.37 V (for X = CF3), underscore the high stability of the PtIVAr–corrole unit toward reduction. That said, although the electrochemical HOMO-LUMO gap of 2.1 eV is typically indicative of a redox-inactive metal center and of ligand-centered oxidation and reduction,10−13,16,36 the fact that the reduction is irreversible suggests concomitant cleavage of the Pt–Ar bond.
Figure 8.
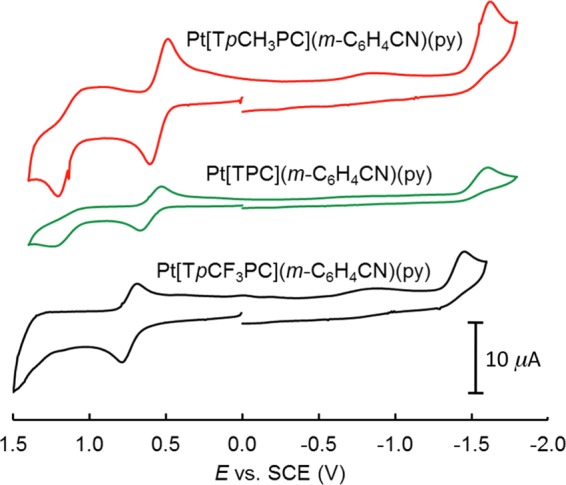
Cyclic voltammograms of Pt[TpXPC](m-C6H4CN)(py) (X = CF3, H, and CH3) in CH2Cl2 recorded at a scan rate of 100 mV/s.
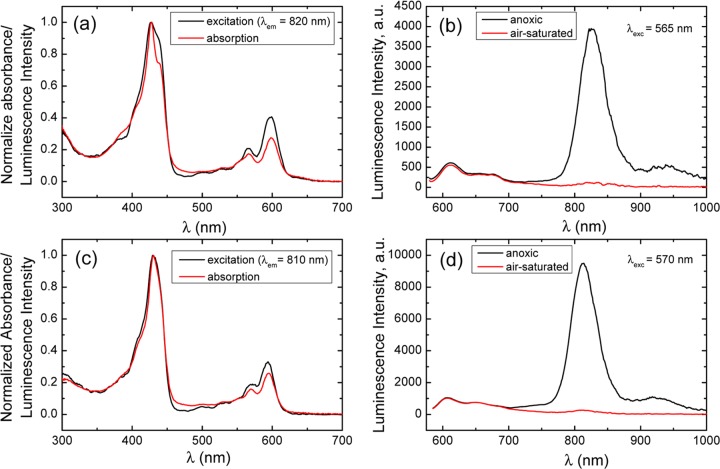
Photophysical measurements were carried out on two of the complexes, PtIV[TpXPC](m-C6H4CN)(py) for X = CF3 and CH3 (Table 3 and Figures 9 and 10). Both are clearly phosphorescent, which was confirmed by almost complete quenching of the emission in the presence of oxygen (Figure 9b,d), measurement of the decay time (Figure 10) and by acquisition of luminescence excitation spectra (Figure 9a,c). The latter are essentially identical to the absorption spectra; the small deviations are due to nonlinearities ascribable to strong absorption in the Soret region (the concentration used was necessary for obtaining high-quality emission spectra with excitation in the Q-band). The NIR phosphorescence is rather weak, but the quantum yields are in the same order of magnitude as those observed for Ir(III) corroles.2 This observation is interesting, considering that Pt(IV) porphyrins,37 in contrast to Pt(II) porphyrins,38−41 have been reported to be nonemissive.42 Weak red fluorescence (not quenchable by oxygen) was also clearly detected for the two compounds studied. The quantum yields for the fluorescence were estimated to be about an order of magnitude lower than those for the phosphorescence. Upconversion with a triplet annihilator, which proved feasible with OsN corroles,4 was found to be very weak due to the relatively low energy of the triplet state and the short triplet state decay times.
Table 3. Summary of Photophysical Properties Measured in Deoxygenated Toluene at 25 °C.
| complex | λmax,em (nm) | Φ (%) | decay time (μs) |
|---|---|---|---|
| PtIV[TpCF3PC](m-C6H4CN)(py) | 813 | 0.27 | 22.9 |
| PtIV[TpCH3PC](m-C6H4CN)(py) | 826 | 0.19 | 17.5 |
Figure 9.
Optical properties of Pt(IV) corroles: (a, c) absorption and luminescence excitation spectra of the PtIV[TpCH3PC](m-C6H4CN)(py) and PtIV[TpCF3PC](m-C6H4CN)(py), respectively, in toluene solution at 25 °C; (b, d) luminescence spectra of PtIV[TpCH3PC](m-C6H4CN)(py) and PtIV[TpCF3PC](m-C6H4CN)(py), respectively, in toluene under anoxic and air-saturated conditions at 25 °C.
Figure 10.
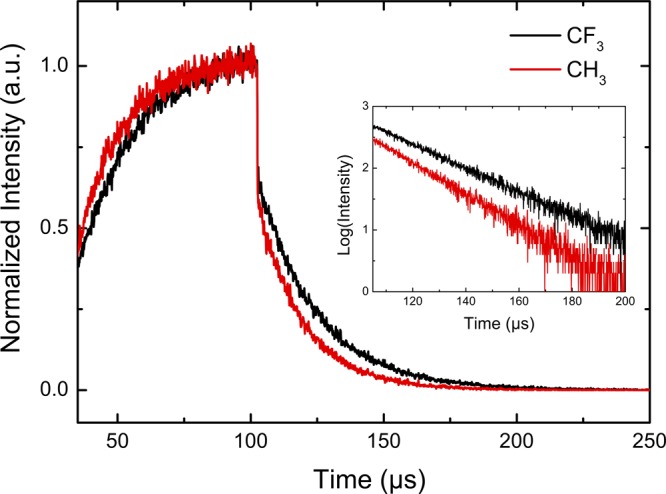
Phosphorescence decay for Pt(IV) corroles in anoxic toluene (25 °C, detected at 815 ± 7 nm).
Conclusions
In what is only the second report on platinum corroles, we have described the synthesis of the first set of stable Pt(IV) complexes, in which the corrole is thought to be an innocent ligand (i.e., without radical character). These have the general formula PtIV[TpXPC](m/p-C6H4CN)(py), where X = CF3, H, and CH3. Although the yields are low (typically <5%), the compounds could be characterized with the standard spectroscopic methods and in two cases single-crystal X-ray crystallography providing rare insight into an elusive class of molecules. The structures revealed short equatorial Pt–N distance of 1.94–1.97 Å, an axial Pt–C distance of ∼2.03 Å, and an axial Pt–N distance of ∼2.22 Å. The UV–vis spectra revealed Soret maxima at ∼430 nm, which are essentially independent of the meso-aryl para substituents and strong Q bands with the most intense peak at 595–599 nm. The substituent-independent Soret maxima are consistent with an innocent PtIV–corrole3– description for the new complexes. The low reduction potentials (−1.45 ± 0.08 V vs saturated calomel reference electrode (SCE)) also support a highly stable Pt(IV) ground state and rule out a corrole•2– description. The reductions, however, were found to be irreversible, which suggests that they involve concomitant cleavage of the Pt–aryl bond. Somewhat to our surprise and unlike Pt(IV) porphyrins, two of the complexes, PtIV[TpXPC](m-C6H4CN)(py) (X = CF3 and CH3), were found to exhibit room-temperature near-IR phosphorescence with emission maxima at 813 and 826 nm, respectively. The quantum yield of ∼0.3% is in the same order of magnitude as those of six-coordinate Ir(III) corroles.
Experimental Section
Materials
Free-base meso-triarylcorroles were synthesized according to a literature procedure.43 Platinum(II) chloride was purchased from Sigma-Aldrich and used to synthesize tetranuclear platinum(II) acetate, as described in the literature.44 Platinum insertion reactions were carried out in a Biotage microwave reactor using 20 mL of microwave vials. Silica gel 60 (0.04–0.063 mm particle size, 230–400 mesh, Merck) was used for flash chromatography, and silica gel 60 preparative thin-layer chromatography (PTLC) plates (20 cm × 20 cm, 0.5 mm thick, Merck) were used for final purification of all complexes.
Instrumental Methods
UV–visible–NIR spectra were recorded on an HP 8454 spectrophotometer. 1H NMR spectra were recorded on 400 MHz Bruker AVANCE III HD spectrometer equipped with a 5 mm BB/1H SmartProbe at 298 K in CDCl3 and referenced to residual CHCl3 at 7.26 ppm. Mass spectra were recorded on a Thermo Scientific LTQ Orbitrap XL spectrometer with an Ion-Max electrospray ion source.
Cyclic voltammetry was carried out at 298 K with an EG&G model 263A potentiostat having a three-electrode system: a glassy carbon working electrode, a platinum wire counter electrode, and a saturated calomel reference electrode (SCE). Anhydrous CH2Cl2 (Aldrich) was used as solvent and tetrakis(n-butyl)ammonium perchlorate, recrystallized twice from absolute ethanol and dried in a desiccator for at least 2 weeks, was used as the supporting electrolyte. The reference electrode was separated from the bulk solution using a fritted glass bridge filled with the solvent/supporting-electrolyte mixture. The electrolyte solution was purged with argon for at least 2 min, and all measurements were carried out under an argon blanket. All potentials were referenced to the SCE.
Emission and excitation spectra were acquired on a FluoroLog 3 spectrofluorometer (Horiba Scientific) equipped with a NIR-sensitive R2658 photomultiplier (Hamamatsu). Relative quantum yields at room temperature were estimated using a solution of Pt(II) tetraphenyltetrabenzoporphyrin in toluene as a reference (Φ = 21%).40 The dye solutions were deoxygenated in a screw-cap cuvette (Hellma, Mülheim, Germany) by bubbling argon through the solution for 12 min. Phosphorescence decay times were acquired in time domain on the FluoroLog 3 spectrofluorometer using a 390 nm SpectraLED (Horiba) as the excitation source.
General Procedure for the Synthesis of Pt[TpXPC](m/p-C6H4CN)(py), Where X = CF3, H, CH3
To a 20 mL microwave vial containing PhCN (5 mL) and a magnetic stirring bar were added a free-base corrole H3[TpXPC] (0.114 mmol) and Pt4(OAc)8·2HOAc (1 equiv). The vial was sealed and heated for 2 h at 150 °C under microwave irradiation. Upon completion of the reaction, pyridine (0.5 mL) was added and the contents of the vial were transferred to a round-bottom flask (50 mL) and evaporated to dryness. The resulting solid was dissolved in dichloromethane (5 mL) and loaded onto a silica gel column and eluted with a mixture of dichloromethane and n-hexane (the exact ratio of which is stated below for each case). All fractions containing Pt[TpXPC](m/p-C6H4CN)(py), characterized by a Soret λmax between 426 and 430 nm, were collected and evaporated to dryness. The product thus obtained was separated into the meta and para regioisomers with PTLC using a dichloromethane/n-hexane mixture as eluent, as indicated below.
Synthesis and Separation of Pt[TpCF3PC](m/p-C6H4CN)(py)
The crude reaction product was chromatographed on a silica gel column with 3:2 dichloromethane/n-hexane as eluent. The fractions with a UV–vis λmax of 430 nm were collected and evaporated to dryness, resulting in a combined yield of 3.69 mg (6.6%) for the Pt[TpCF3PC](m/p-C6H4CN)(py) regioisomers. PTLC with 1:1 dichloromethane/n-hexane as eluent was then used to separate the m- and p-isomers; the top band was identified as Pt[TpCF3PC](m-C6H4CN)(py) and the lower band as Pt[TpCF3PC](p-C6H4CN)(py) based on 1H NMR analysis. Dark purple X-ray quality crystals of the meta isomer were grown by slow evaporation of a dichloromethane/n-hexane solution of the complex over a period of 15 days. Spectroscopic characterization data for the two isomers are as follows (see also Figures 2 and 3).
Pt[TpCF3PC](m-C6H4CN)(py)
Yield 2.15 mg (3.52%). UV–vis (CH2Cl2) λmax (nm, ε × 10–4 M–1 cm–1): 430 (10.70), 498 (0.60), 531 (0.72), 569 (1.62), 595 (3.08). 1H NMR δ: 9.16 (d, 2H, 3JHH = 4.08 Hz, β-H); 8.92 (d, 2H, 3JHH = 4.60 Hz, β-H); 8.73 (d, 2H, 3JHH = 4.04 Hz, β-H); 8.68 (d, 2H, 3JHH = 5.12 Hz, β-H); 8.43 (d, 2H, 3JHH = 8.12 Hz, 5,15-o1-Ph); 8.37 (d, 2H, 3JHH = 7.60 Hz, 5,15-o2-Ph); 8.25 (overlapping doublets, 2H, 3JHH = 8.12 Hz, 10-o1-Ph and 10-o2-Ph); 8.07 (overlapping doublets, 4H, 3JHH = 7.60 Hz, 5,15-m1-Ph and 5,15-m2-Ph); 8.00 (overlapping doublets, 2H, 3JHH = 8.12 Hz, 10-m1-Ph and 10-m2-Ph); 6.34 (br s, 1H, 4-py); 5.72 (d, 1H, 3JHH = 7.44 Hz, C6H4CN ortho1); 5.47 (br s, 2H, 3,5-py); 5.00 (t, 1H, C6H4CN meta); 1.73 (br s, 2H, 2,6-py), 0.57 (d, 1H, C6H4CN para; overlapping with s, 1H, C6H4CN ortho2). High-resolution mass spectrometry (HRMS) (major isotopomer): M+ = 1103.2123 (expt), 1103.1952 (calcd for C52H29N6F9Pt).
Pt[TpCF3PC](p-C6H4CN)(py)
Yield 1.12 mg (1.83%). UV–vis (CH2Cl2) λmax (nm, ε × 10–4 M–1 cm–1): 430 (10.82), 498 (0.56), 531 (0.70), 571 (1.64), 595 (3.23). 1H NMR δ: 9.16 (d, 2H, 3JHH = 3.68 Hz, β-H); 8.92 (d, 2H, 3JHH = 5.20 Hz, β-H); 8.73 (d, 2H, 3JHH = 4.28 Hz, β-H); 8.68 (d, 2H, 3JHH = 5.02 Hz, β-H); 8.43 (d, 2H, 3JHH = 7.96 Hz, 5,15-o1-Ph); 8.35 (d, 2H, 3JHH = 7.96 Hz, 5,15-o2-Ph); 8.24 (overlapping doublets, 2H, 3JHH = 8.12 Hz, 10-o1-Ph and 10-o2-Ph); 8.06 (d, 4H, 3JHH = 8.12 Hz, 5,15-m1-Ph and 5,15-m2-Ph); 8.00 (overlapping doublets, 2H, 3JHH = 8 Hz, 10-m1-Ph and 10-m2-Ph); 6.33 (br s, 1H, 4-py); 5.46 (br s, 2H, 3,5-py); 5.19 (d, 2H, 3JHH = 6.88 Hz, C6H4CN ortho); 1.70 (br s, 2H, 2,6-py), 0.46 (d, 2H, 3JHH = 8.16 Hz, C6H4CN meta). HRMS (major isotopomer): M+ = 1103.2118 (expt), 1103.1952 (calcd for C52H29N6F9Pt).
Synthesis and Separation of Pt[TPC](m/p-C6H4CN)(py)
The crude reaction product was initially chromatographed on a silica gel column with 2:1 dichloromethane/n-hexane as eluent. The fractions with a λmax of 427 nm were collected and evaporated to dryness, resulting in combined yield of 3.59 mg (7.2%) for the Pt(TPC)(m/p-C6H4CN)(PhCN) regioisomers. PTLC with 3:2 dichloromethane/n-hexane as eluent was then used to separate the isomers; the top band was identified as Pt[TPC](m-C6H4CN)(py) and the lower band as Pt[TPC](p-C6H4CN)(py) based on 1H NMR analysis.
Pt[TPC](m-C6H4CN)(py)
Yield 2.1 mg (4.21%). UV–vis (CH2Cl2) λmax (nm, ε × 10–4 M–1 cm–1): 427 (9.41), 437 (7.99), 496 (0.46), 528 (0.64), 567 (1.56), 596 (3.25). 1H NMR δ: 9.09 (d, 2H, 3JHH = 4.88 Hz, β-H); 8.93 (d, 2H, 3JHH = 4.88 Hz, β-H); 8.73 (d, 2H, 3JHH = 4.88 Hz, β-H); 8.67 (d, 2H, 3JHH = 4.24 Hz, β-H); 8.32 (d, 2H, 3JHH = 6.80 Hz, 5,15-o1); 8.24 (d, 2H, 3JHH = 6.56 Hz, 5,15-o2); 8.10 (overlapping doublets, 2H, 3JHH = 8.12 Hz, 10-o1 and 10-o2); 7.68–7.83 (m, 9H, overlapping 5,10,15-m-Ph and 5,10,15-p-Ph); 6.29 (br s, 1H, 4-py); 5.70 (d, 1H, 3JHH = 6.92 Hz, C6H4CN ortho1); 5.43 (br s, 2H, 3,5-py); 4.99 (t, 1H, 3JHH = 6.92 Hz, C6H4CN meta); 1.79 (br d, 2H, 2,6-py), 0.68 (d, 1H, C6H4CN para overlapping with s, 1H, C6H4CN ortho2). HRMS (major isotopomer): M+ = 899.2327 (expt), 899.2331 (calcd for C49H32N6Pt).
Pt[TPC](p-C6H4CN)(py)
Yield 1.08 mg (2.16%). UV–vis (CH2Cl2) λmax (nm, ε × 10–4 M–1 cm–1): 427 (10.98), 437 (9.02), 496 (0.61), 528 (0.78), 568 (1.78), 597 (3.72). 1H NMR δ: 9.08 (d, 2H, 3JHH = 4.28 Hz, β-H); 8.92 (d, 2H, 3JHH = 4.92 Hz, β-H); 8.73 (d, 2H, 3JHH = 4.32 Hz, β-H); 8.66 (d, 2H, 3JHH = 4.28 Hz, β-H); 8.31 (d, 2H, 3JHH = 7.96 Hz, 5,15-o1-Ph); 8.24 (d, 2H, 3JHH = 6.88 Hz, 5,15-o2-Ph); 8.10 (overlapping doublets, 2H, 3JHH = 8.12 Hz, 10-o1-Ph and 10-o2-Ph); 7.83–7.68 (m, 9H, overlapping 5,10,15-m-Ph and 5,10,15-p-Ph); 6.30 (br s, 1H, 4-py); 5.43 (br s, 2H, 3-py); 5.19 (d, 2H, 3JHH = 6.80 Hz C6H4CN ortho); 1.77 (br s, 2H, 2,6-py); 0.56 (d, 2H, 3JHH = 6.08 Hz, C6H4CN meta). HRMS (major isotopomer): M+ = 899.2331 (expt), 899.2331 (calcd for C49H32N6Pt).
Synthesis and Separation of Pt[TpCH3PC](m/p-C6H4CN)(py)
The crude reaction product was chromatographed on a silica gel column with 3:1 dichloromethane/n-hexane as eluent. The fractions with a λmax of 427 nm were collected and evaporated to dryness, resulting in combined yield of 4.17 mg (8.0%) for the Pt[TpCH3PC](m/p-C6H4CN)(py) regioisomers. PTLC with 3:1 dichloromethane/n-hexane as eluent was then used to separate the isomers; the top band was identified as Pt[TpCH3PC](p-C6H4CN)(py) and the lower band as Pt[TpCH3PC](m-C6H4CN)(py) based on 1H NMR analysis.
Pt[TpCH3PC](m-C6H4CN)(py)
Yield 2.3 mg (4.41%). UV–vis (CH2Cl2) λmax (nm, ε × 10–4 M–1 cm–1): 427 (9.19), 438 (7.59), 498 (0.41), 528 (0.62), 567 (1.47), 599 (3.28). 1H NMR δ: 9.06 (d, 2H, 3JHH = 4.40 Hz, β-H); 8.92 (d, 2H, 3JHH = 4.36 Hz, β-H); 8.72 (d, 2H, 3JHH = 4.44 Hz, β-H); 8.66 (d, 2H, 3JHH = 4.40 Hz, β-H); 8.21 (d, 2H, 3JHH = 7.96 Hz, 5,15-o1-Ph); 8.12 (d, 2H, 3JHH = 6.68 Hz, 5,15-o2-Ph); 7.98 (overlapping doublets, 2H, 3JHH = 8.24 Hz, 10-o1-Ph and 10-o2-Ph); 7.60 (d, 2H, 3JHH = 8.24 Hz, 5,15-m1-Ph); 7.57 (d, 2H, 3JHH = 7.96 Hz, 5,15-m2-Ph); 7.51 (overlapping doublets, 2H, 3JHH = 8.12 Hz, 10-m1-Ph and 10-m2-Ph); 6.28 (br s, 1H, 4-py); 5.69 (d, 1H, 3JHH = 6.84 Hz, C6H4CN ortho1); 5.42 (br s, 2H, 3-py); 4.98 (t, 1H, 3JHH = 8.36 Hz, C6H4CN meta); 2.69 (s, 6H, 5,15-p-CH3); 2.65 (s, 3H, 10-p-CH3); 1.79 (br s, 2H, 2,6-py), 0.69 (d, 1H, C6H4CN para and s, 1H, C6H4CN ortho2). HRMS (major isotopomer): M+ = 941.2897 (expt), 941.2800 (calcd for C52H38N6Pt).
Pt[TpCH3PC](p-C6H4CN)(py)
Yield 1.17 mg (2.22%). UV–vis (CH2Cl2) λmax (nm, ε × 10–4 M–1 cm–1): 427 (9.67), 438 (7.79), 496 (0.46), 528 (0.62), 567 (1.40), 599 (3.21). 1H NMR δ: 9.06 (d, 2H, 3JHH = 4.16 Hz, β-H); 8.92 (d, 2H, 3JHH = 3.80 Hz, β-H); 8.72 (d, 2H, 3JHH = 4.16 Hz, β-H); 8.66 (d, 2H, 3JHH = 5.20 Hz, β-H); 8.21 (d, 2H, 3JHH = 8.00 Hz, 5,15-o1-Ph); 8.12 (d, 2H, 3JHH = 8.68 Hz, 5,15-o2-Ph); 7.98 (overlapping doublets, 2H, 3JHH = 8.64 Hz, 10-o1-Ph and 10-o2-Ph); 7.60 (d, 2H, 3JHH = 8.64 Hz, 5,15-m1-Ph); 7.57 (d, 2H, 3JHH = 7.96 Hz, 5,15-m2-Ph); 7.51 (overlapping doublets, 2H, 3JHH = 7.96 Hz, 10-m1-Ph and 10-m2-Ph); 6.29 (br s, 1H, 4-py); 5.42 (br s, 2H, 3,5-py); 5.18 (d, 2H, 3JHH = 6.80 Hz, C6H4CN ortho); 2.69 (s, 6H, 5,15-p-CH3); 2.65 (s, 3H, 10-p-CH3); 1.76 (br s, 2H, 2,6-py), 0.57 (d, 2H, C6H4CN meta). HRMS (major isotopomer): M+ = 941.2897 (expt), 941.2800 (calcd for C52H38N6Pt).
X-ray Crystallographic Analyses
X-ray data for Pt[TPC](m-C6H4CN)(py) and Pt[TpCF3PC](m-C6H4CN)(py) were collected on beamline 11.3.1 at the Advanced Light Source, Lawrence Berkeley National Laboratory. Each crystal was mounted on a MiTeGen Kapton loop and placed in a nitrogen cold stream provided by an Oxford Cryostream 800 Plus low-temperature apparatus on the goniometer head of a Bruker D8 diffractometer. The diffractometer was equipped with a PHOTON 100 CMOS detector for Pt[TPC](m-C6H4CN)(py) and a PHOTONII CPAD detector for Pt[TpCF3PC](m-C6H4CN)(py), each operating in shutterless mode. Diffraction data were collected with synchrotron radiation monochromated using silicon (111) to a wavelength of 0.7293(1) Å for Pt[TPC](m-C6H4CN)(py) and 0.7749(1) Å for Pt[TpCF3PC](m-C6H4CN)(py). An approximate full sphere of data was collected on each crystal using a combination of ϕ and ω scans. The crystals of Pt[TPC](m-C6H4CN)(py) were found to be twinned, the components were separated using the CELL_NOW program.45 Absorption corrections were applied with SADABS46 for Pt[TpCF3PC](m-C6H4CN)(py) and with TWINABS47 for Pt[TPC](m-C6H4CN)(py). The structures were solved by intrinsic phasing (SHELXT)48 and refined by full-matrix least squares on F2 (SHELXL-2014)49 using the ShelXle GUI.50 All non-hydrogen atoms were refined anisotropically. Hydrogen atoms were geometrically calculated and refined as riding atoms.
The two axial ligands in Pt[TpCF3PC](m-C6H4CN)(py), pyridine and C6H4CN, were found to occupy symmetry-equivalent sites within the crystal, each with 50% occupancy, and were modeled such that the atoms of the two six-membered rings were superimposed. The C and N atoms that coordinate to the Pt center (C101 and N101) were constrained to have identical x, y, and z coordinates via the EXYZ command in SHELX and were refined under separate PART instructions. Each of the remaining five atoms of the aromatic ring was modeled as common to both orientations with full occupancies, since attempts to independently model the two rings were unsuccessful. The CN and H substituents bound to C105 were refined under the same PART instructions as C101 and N101, respectively. The disordered axial ligands led to disorder in the unique C6H4CF3 substituent, causing the CF3 group to be positionally disordered over two symmetry-equivalent sites. The atoms belonging to this CF3 group were refined with an occupancy of 0.5, but no attempt was made to model disorder in the aromatic ring of this substituent. Rotational disorder was also found for the CF3 groups on the other two C6H4CF3 substituents, and each CF3 group was accordingly modeled over two orientations with complementary occupancies. Equivalent disordered atoms (e.g., N101/C101) were constrained to have equal Uij values via the EADP command in SHELX. Additional crystallographic information has been summarized in Table 1, and full details can be found in the Crystallographic Information File provided as Supporting Information.
Acknowledgments
This work was supported by NANO2021 grant no. 262229 of the Research Council of Norway (A.G.) and used resources of the Advanced Light Source, which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01149.
The authors declare no competing financial interest.
Supplementary Material
References
- Ghosh A. Electronic Structure of Corrole Derivatives: Insights from Molecular Structures, Spectroscopy, Electrochemistry, and Quantum Chemical Calculations. Chem. Rev. 2017, 117, 3798–3881. 10.1021/acs.chemrev.6b00590. [DOI] [PubMed] [Google Scholar]
- Palmer J. H.; Durrell A. C.; Gross Z.; Winkler J. R.; Gray H. B. Near-IR Phosphorescence of Iridium(III) Corroles at Ambient Temperature. J. Am. Chem. Soc. 2010, 132, 9230–9231. 10.1021/ja101647t. [DOI] [PubMed] [Google Scholar]
- Sinha W.; Ravotto L.; Ceroni P.; Kar S. NIR-Emissive Iridium(III) Corrole Complexes as Efficient Singlet Oxygen Sensitizers. Dalton Trans. 2015, 44, 17767–17773. 10.1039/C5DT03041B. [DOI] [PubMed] [Google Scholar]
- Borisov S. M.; Alemayehu A.; Ghosh A. Osmium-Nitrido Corroles as NIR Indicators for Oxygen Sensors and Triplet Sensitizers for Organic Upconversion and Singlet Oxygen Generation. J. Mater. Chem. C 2016, 4, 5822–5828. 10.1039/C6TC01126H. [DOI] [Google Scholar]
- Lemon C. M.; Powers D. C.; Brothers P. J.; Nocera D. G. Gold Corroles as Near-IR Phosphors for Oxygen Sensing. Inorg. Chem. 2017, 56, 10991–10997. 10.1021/acs.inorgchem.7b01302. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Day N. U.; Mani T.; Rudine A. B.; Thomas K. E.; Gederaas O. A.; Vinogradov S. A.; Wamser C. C.; Ghosh A. Gold Tris(carboxyphenyl)corroles as Multifunctional Materials: Room Temperature Near-IR Phosphorescence and Applications to Photodynamic Therapy and Dye-Sensitized Solar Cells. ACS Appl. Mater. Interfaces 2016, 8, 18935–18942. 10.1021/acsami.6b04269. [DOI] [PubMed] [Google Scholar]
- Teo R. D.; Hwang J. Y.; Termini J.; Gross Z.; Gray H. B. Fighting Cancer with Corroles. Chem. Rev. 2016, 117, 2711–2729. 10.1021/acs.chemrev.6b00400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alemayehu A. B.; Vazquez-Lima H.; Beavers C. M.; Gagnon K. J.; Bendix J.; Ghosh A. Platinum Corroles. Chem. Commun. 2014, 50, 11093–11096. 10.1039/C4CC02548B. [DOI] [PubMed] [Google Scholar]
- Johansen I.; Norheim H.-K.; Larsen S.; Alemayehu A. B.; Conradie J.; Ghosh A. Substituent Effects on Metallocorrole Spectra: Insights from Chromium-Oxo and Molybdenum-Oxo Triarylcorroles. J. Porphyrins Phthalocyanines 2011, 15, 1335–1344. 10.1142/S1088424611004270. [DOI] [Google Scholar]
- Alemayehu A. B.; Vazquez-Lima H.; Gagnon K. J.; Ghosh A. Stepwise Deoxygenation of Nitrite as a Route to Two Families of Ruthenium Corroles: Group 8 Periodic Trends and Relativistic Effects. Inorg. Chem. 2017, 56, 5285–5294. 10.1021/acs.inorgchem.7b00377. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Gagnon K. J.; Terner J.; Ghosh A. Oxidative Metalation as a Route to Size-Mismatched Macrocyclic Complexes: Osmium Corroles. Angew. Chem., Int. Ed. 2014, 53, 14411–14414. 10.1002/anie.201405890. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Vazquez-Lima H.; Gagnon K. J.; Ghosh A. Stepwise Deoxygenation of Nitrite as a Route to Two Families of Ruthenium Corroles: Group 8 Periodic Trends and Relativistic Effects. Inorg. Chem. 2017, 56, 5285–5294. 10.1021/acs.inorgchem.7b00377. [DOI] [PubMed] [Google Scholar]
- Einrem R. F.; Gagnon K. J.; Alemayehu A. B.; Ghosh A. Metal-Ligand Misfits: Facile Access to Rhenium-Oxo Corroles by Oxidative Metalation. Chem. – Eur. J. 2016, 22, 517–520. 10.1002/chem.201504307. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Ghosh A. Gold Corroles. J. Porphyrins Phthalocyanines 2011, 15, 106–110. 10.1142/S1088424611003045. [DOI] [Google Scholar]
- Rabinovich E.; Goldberg I.; Gross Z. Gold(I) and Gold(III) Corroles. Chem. – Eur. J. 2011, 17, 12294–12301. 10.1002/chem.201102348. [DOI] [PubMed] [Google Scholar]
- Thomas K. E.; Alemayehu A. B.; Conradie J.; Beavers C.; Ghosh A. Synthesis and Molecular Structure of Gold Triarylcorroles. Inorg. Chem. 2011, 50, 12844–12851. 10.1021/ic202023r. [DOI] [PubMed] [Google Scholar]
- Thomas K. E.; Vazquez-Lima H.; Fang Y.; Song Y.; Gagnon K. J.; Beavers C. M.; Kadish K. M.; Ghosh A. Ligand Noninnocence in Coinage Metal Corroles: A Silver Knife-Edge. Chem. – Eur. J. 2015, 21, 16839–16847. 10.1002/chem.201502150. [DOI] [PubMed] [Google Scholar]
- Alemayehu A.; Vazquez-Lima H.; McCormick L. J.; Ghosh A. Relativistic effects in metallocorroles: comparison of molybdenum and tungsten biscorroles. Chem. Commun. 2017, 53, 5830–5833. 10.1039/C7CC01549F. [DOI] [PubMed] [Google Scholar]
- Alemayehu A.; Vazquez-Lima H.; Gagnon K. J.; Ghosh A. Tungsten Biscorroles: New Chiral Sandwich Compounds. Chem. – Eur. J. 2016, 22, 6914–6920. 10.1002/chem.201504848. [DOI] [PubMed] [Google Scholar]
- Steene E.; Wondimagegn T.; Ghosh A. Resonance Raman Spectroscopy and Density Functional Theoretical Calculations of Manganese Corroles. A Parallelism between High-Valent Metallocorroles and Metalloporphyrins, Relevant to Horseradish Peroxidase and Chloroperoxidase Compound I and II Intermediates. J. Inorg. Biochem. 2002, 88, 113–118. 10.1016/S0162-0134(01)00396-8. [DOI] [PubMed] [Google Scholar]
- Ganguly S.; McCormick L. J.; Conradie J.; Gagnon K. J.; Sarangi R.; Ghosh A.. Electronic Structure of Manganese Corroles Revisited: X-ray structures, Optical and X-ray Absorption Spectroscopies, and Electrochemistry as Probes of Ligand Noninnocence. Inorg. Chem. 2018. 10.1021/acs.inorgchem.8b00537. [DOI] [PubMed] [Google Scholar]
- a Steene E.; Wondimagegn T.; Ghosh A. Electrochemical and Electronic Absorption Spectroscopic Studies of Substituent Effects in Iron(IV) and Manganese(IV) Corroles. Do the Compounds Feature High-Valent Metal Centers or Noninnocent Corrole Ligands? Implications for Peroxidase Compound I and II Intermediates. J. Phys. Chem. B 2001, 105, 11406–11413. 10.1021/jp012037r. [DOI] [Google Scholar]; Addition/correction:; b Steene E.; Wondimagegn T.; Ghosh A. Electrochemical and Electronic Absorption Spectroscopic Studies of Substituent Effects in Iron(IV) and Manganese(IV) Corroles. Do the Compounds Feature High-Valent Metal Centers or Noninnocent Corrole Ligands? Implications for Peroxidase Compound I and II. J. Phys. Chem. B 2002, 106, 5312. 10.1021/jp014407h. [DOI] [Google Scholar]
- Ganguly S.; Giles L. J.; Thomas K. E.; Sarangi R.; Ghosh A. Ligand Noninnocence in Iron Corroles: Insights from Optical and X-ray Absorption Spectroscopies and Electrochemical Redox Potentials. Chem. – Eur. J. 2017, 23, 15098–15106. 10.1002/chem.201702621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez-Lima H.; Norheim H. K.; Einrem R. F.; Ghosh A. Cryptic Noninnocence: FeNO Corroles in a New Light. Dalton Trans. 2015, 44, 10146–10151. 10.1039/C5DT01495F. [DOI] [PubMed] [Google Scholar]
- Norheim H.-K.; Capar J.; Einrem R. F.; Gagnon K. J.; Beavers C. M.; Vazquez-Lima H.; Ghosh A. Ligand Noninnocence in FeNO Corroles: Insights from β-Octabromocorrole Complexes. Dalton Trans. 2016, 45, 681–689. 10.1039/C5DT03947A. [DOI] [PubMed] [Google Scholar]
- Ganguly S.; Vazquez-Lima H.; Ghosh A. Wolves in Sheep’s Clothing: μ-Oxo-Diiron Corroles Revisited. Chem. – Eur. J. 2016, 22, 10336–10340. 10.1002/chem.201601062. [DOI] [PubMed] [Google Scholar]
- Wasbotten I. H.; Wondimagegn T.; Ghosh A. Electronic Absorption, Resonance Raman, and Electrochemical Studies of Planar and Saddled Copper(III) Meso-Triarylcorroles. Highly Substituent-Sensitive Soret Bands as a Distinctive Feature of High-Valent Transition Metal Corroles. J. Am. Chem. Soc. 2002, 124, 8104–8116. 10.1021/ja0113697. [DOI] [PubMed] [Google Scholar]
- Ou Z.; Shao J.; Zhao H.; Ohkubo K.; Wasbotten I. H.; Fukuzumi S.; Ghosh A.; Kadish K. M. Spectroelectrochemical and ESR Studies of Highly Substituted Copper Corroles. J. Porphyrins Phthalocyanines 2004, 08, 1236–1247. 10.1142/S1088424604000593. [DOI] [Google Scholar]
- Bröring M.; Brégier F.; Tejero E. C.; Hell C.; Holthausen M. C. Revisiting the Electronic Ground State of Copper Corroles. Angew. Chem., Int. Ed. 2007, 46, 445–448. 10.1002/anie.200603676. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Gonzalez E.; Hansen L. K.; Ghosh A. Copper Corroles Are Inherently Saddled. Inorg. Chem. 2009, 48, 7794–7799. 10.1021/ic900744v. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Hansen L. K.; Ghosh A. Nonplanar, Noninnocent, and Chiral: A Strongly Saddled Metallocorrole. Inorg. Chem. 2010, 49, 7608–7610. 10.1021/ic1008736. [DOI] [PubMed] [Google Scholar]
- Thomas K. E.; Wasbotten I. H.; Ghosh A. Copper β-Octakis(Trifluoromethyl)Corroles: New Paradigms for Ligand Substituent Effects in Transition Metal Complexes. Inorg. Chem. 2008, 47, 10469–10478. 10.1021/ic801101k. [DOI] [PubMed] [Google Scholar]
- Alemayehu A. B.; Conradie J.; Ghosh A. A First TDDFT Study of Metallocorrole Electronic Spectra: Copper meso-Triarylcorroles Exhibit Hyper Spectra. Eur. J. Inorg. Chem. 2011, 12, 1857–1864. 10.1002/ejic.201001026. [DOI] [Google Scholar]
- Berg S.; Thomas K. E.; Beavers C. M.; Ghosh A. Undecaphenylcorroles. Inorg. Chem. 2012, 51, 9911–9916. 10.1021/ic301388e. [DOI] [PubMed] [Google Scholar]
- Thomas K. E.; Vazquez-Lima H.; Fang Y.; Song Y.; Gagnon K. J.; Beavers C. M.; Kadish K. M.; Ghosh A. Ligand Noninnocence in Coinage Metal Corroles: A Silver Knife-Edge. Chem. – Eur. J. 2015, 21, 16839–16847. 10.1002/chem.201502150. [DOI] [PubMed] [Google Scholar]
- Fang Y.; Ou Z.; Kadish K. M. Electrochemistry of Corroles in Nonaqueous Media. Chem. Rev. 2017, 117, 3377–3419. 10.1021/acs.chemrev.6b00546. [DOI] [PubMed] [Google Scholar]
- Aartsma T. J.; Gouterman M.; Jochum C.; Kwiram A. L.; Pepich B. V.; Williams L. D. Porphyrins. 43. Triplet Sublevel Emission of Platinum Tetrabenzoporphyrin by Spectrothermal Principal Component Decomposition. J. Am. Chem. Soc. 1982, 104, 6278–6283. 10.1021/ja00387a021. [DOI] [Google Scholar]
- Baldo M. A.; O’Brien D. F.; You Y.; Shoustikov A.; Sibley S.; Thompson M. E.; Forrest S. R. Highly efficient phosphorescent emission from organic electroluminescent devices. Nature 1998, 395, 151–154. 10.1038/25954. [DOI] [Google Scholar]
- Kwong R. C.; Sibley S.; Dubovoy T.; Baldo M.; Forrest S. R.; Thompson M. E. Efficient, Saturated Red Organic Light Emitting Devices Based on Phosphorescent Platinum(II) Porphyrins. Chem. Mater. 1999, 11, 3709–3713. 10.1021/cm9906248. [DOI] [Google Scholar]
- Zach P. W.; Freunberger S. A.; Klimant I.; Borisov S. M. Electron-Deficient Near-Infrared Pt(II) and Pd(II) Benzoporphyrins with Dual Phosphorescence and Unusually Efficient Thermally Activated Delayed Fluorescence: First Demonstration of Simultaneous Oxygen and Temperature Sensing with a Single Emitter. ACS Appl. Mater. Interfaces 2017, 9, 38008–38023. 10.1021/acsami.7b10669. [DOI] [PubMed] [Google Scholar]
- Borisov S. M.; Nuss G.; Haas W.; Saf R.; Schmuck M.; Klimant I. New NIR-emitting complexes of platinum(II) and palladium(II) with fluorinated benzoporphyrins. J. Photochem. Photobiol., A 2009, 201, 128–135. 10.1016/j.jphotochem.2008.10.003. [DOI] [Google Scholar]
- Cebrián C.; Mauro M. Recent advances in phosphorescent platinum complexes for organic light-emitting diodes. Beilstein J. Org. Chem. 2018, 14, 1459–1481. 10.3762/bjoc.14.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koszarna B.; Gryko D. T. Efficient Synthesis of meso-Substituted Corroles in a H2O-MeOH Mixture. J. Org. Chem. 2006, 71, 3707–3717. 10.1021/jo060007k. [DOI] [PubMed] [Google Scholar]
- Basato M.; Biffis A.; Martinati G.; Tubaro C.; Venzo A.; Ganis P.; Benetollo F. Reaction of platinum acetate with phosphines and molecular structure of trans-[Pt(OAc)2(PPh3)2]. Inorg. Chim. Acta 2003, 355, 399–403. 10.1016/S0020-1693(03)00314-1. [DOI] [Google Scholar]
- CELL_NOW: Index Twins and Other Problem Crystals, version 2008/4; Bruker, 2016.
- Krause L.; Herbst-Irmer R.; Sheldrick G. M.; Stalke D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. 10.1107/S1600576714022985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TWINABS: Bruker AXS Scaling for Twinned Crystals, version 2012/1; Bruker, 2016.
- Sheldrick G. M. SHELXT - Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr., Sect. A: Found. Adv. 2015, 71, 3–8. 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldrick G. M. Crystal Structure Refinement with SHELXL. Acta Crystallogr., Sect. C: Struct. Chem. 2015, 71, 3–8. 10.1107/S2053229614024218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hübschle C. B.; Sheldrick G. M.; Dittrich B. ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Crystallogr. 2011, 44, 1281–1284. 10.1107/S0021889811043202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.