Abstract
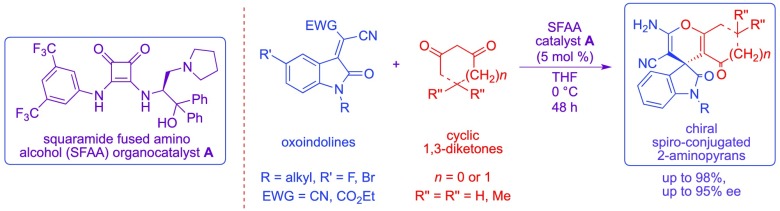
The new hybrid-type squaramide-fused amino alcohol containing both a Brønsted basic site and hydrogen-bonding sites in the molecule showed a high catalytic activity as an organocatalyst in the enantioselective domino Michael addition/cyclization reaction of oxoindolines with cyclic 1,3-diketones to afford the chiral spiro-conjugated oxindoles featuring 2-aminopyrans fusing with carbo-heterocyclic ring systems with excellent chemical yields (up to 98%) and enantioselectivities (up to 95% ee). The obtained chiral spiro-conjugated 2-aminopyrans bearing quaternary stereogenic carbon center could be used as synthetic precursors for several natural products that have a broad spectrum of fascinating biological activities.
Introduction
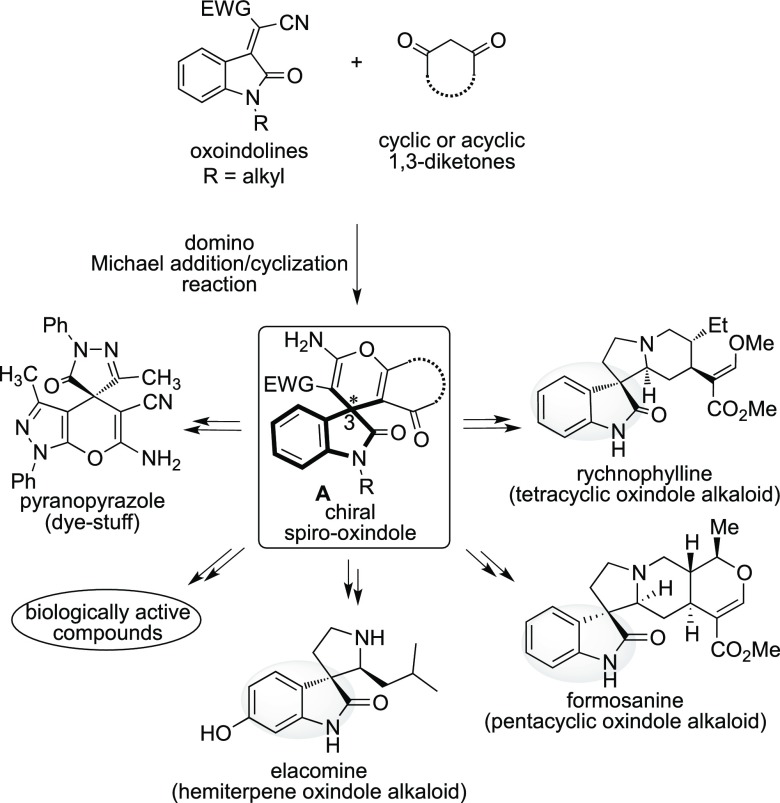
In the enormously progressing field of synthetic organic chemistry, the construction of carbon–carbon and a carbon–heteroatom bond is an important strategy for the construction of complex organic molecules.1 The domino approach is one of the major tools to construct biologically important complex molecules and its precursors from simple substrates in one-pot fashion, which is ecologically and economically benign.2 The enantioselective domino Michael addition/cyclization reaction of oxoindoline with cyclic or acyclic 1,3-diketones affords the synthetically valuable chiral carbo-heterocyclic spiro-oxindoles A, that are synthetic intermediates for preparing many biologically active molecules, such as rychnophylline (tetracyclic oxindole alkaloid), formosanine (pentacyclic oxindole alkaloid), elacomine (hemiterpene oxindole alkaloid), and pyranopyrazole (synthetic dye-stuff), in the field of synthetic organic chemistry (Scheme 1).3−6
Scheme 1. Biological Significance of Chiral Oxindole Incorporating Spiro-conjugated Amino Framework.
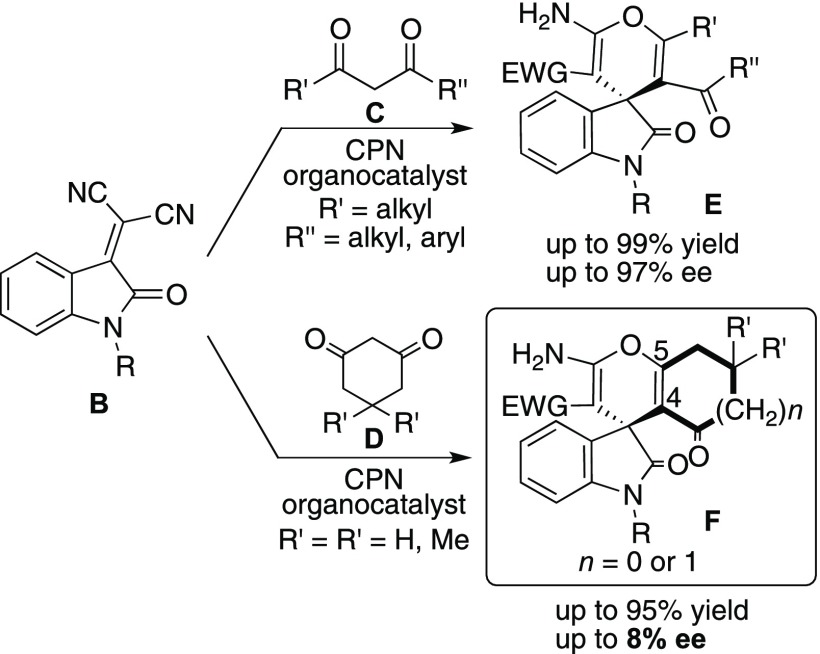
However, the methodology of preparation of vital spiro-oxindole A, incorporating a chiral spiro-conjugated aminopyran framework at C-3 position of oxindoles, is still limited. Recently, Yuan and his co-workers7 reported the first enantioselective domino Michael addition/cyclization reaction of oxoindolines B with acyclic 1,3-diketones C using cupreine (CPN) organocatalyst to afford chiral spiro-conjugated oxindoles with 2-aminopyrans framework E in both excellent chemical yield (up to 99%) and enantioselectivities (up to 97% ee) (Scheme 2). However, this CPN catalyst did not work efficiently for the reaction of cyclic 1,3-diketones D with oxoindolines to afford the chiral spiro-conjugated oxindoles F with 2-aminopyrans fusing carbo-heterocyclic ring systems at C-4 and C-5 positions in terms of enantioselectivity (up to 8% ee), in spite of the obtained products being useful for the synthesis of biologically important molecules from the point of view of drug discovery.8
Scheme 2. Cupreine-Catalyzed Enantioselective Domino Michael Addition/Cyclization.
Most recently, we developed the new hybrid-type squaramide-fused amino alcohol (SFAA) organocatalysts and the catalyst was successfully employed in enantioselective nitro-aldol and Diels–Alder reactions to afford the corresponding synthetically important chiral products in satisfactory chemical yields and enantioselectivities.9 On the basis of these reasons, we planned to apply our newly developed SFAA organocatalyst for enantioselective domino Michael addition/cyclization reaction of oxoindolines with cyclic 1,3-diketones, affording the chiral spiro-conjugated oxindoles with 2-aminopyrans fusing with carbo-heterocyclic ring systems.
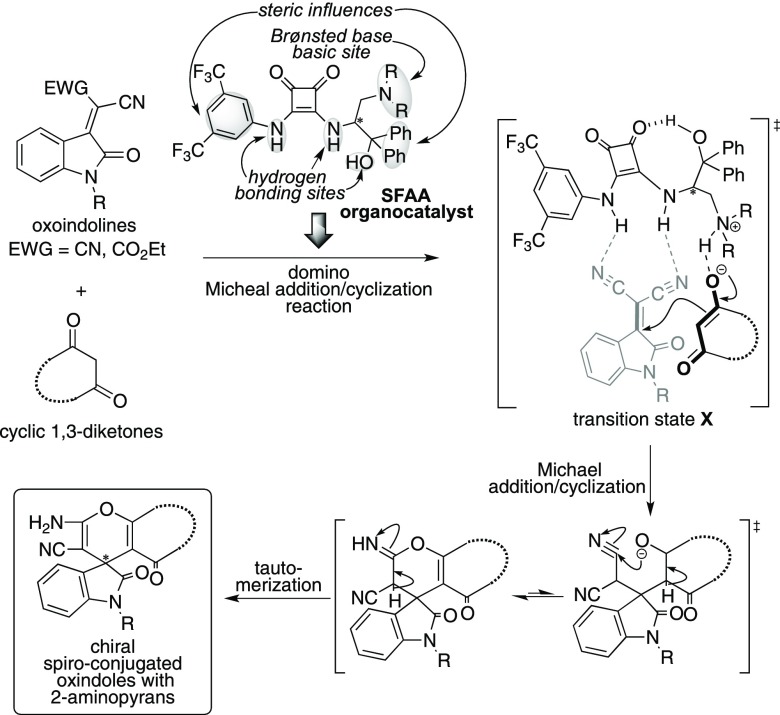
The SFAA organocatalyst possesses multiple functional sites, such as diamino groups on squaramide part for hydrogen-bonding with the substrate, tertiary amino group acting as a Brønsted base, and phenyl groups acting as steric influence site for recognition by substrates (Scheme 3). The enantioselective domino Michael addition/cyclization reaction of oxoindolines with cyclic 1,3-diketones using SFAA catalyst might proceed via transition state X, in which the hydroxy functional group on catalysts might participate in hydrogen-bonding interaction with squaramide carbonyl oxygen atom. Additionally, the enolate of cyclic 1,3-diketones might be fixed through hydrogen-bonding interactions with the ammonium site at the side chain of the catalyst part. Furthermore, two cyano groups of oxoindoline might be fixed through two hydrogen-bonding interactions with two amino hydrogen atoms of the squaramide unit of the catalyst. The activated enolate generated from 1,3-diketones might attack stereoselectively on electron-deficient olefin on oxoindolines from the less sterically hindered site of the fixed oxoindoline to afford the chiral Michael adduct, and subsequent intramolecular cyclization/tautomerization might afford the corresponding chiral spiro-conjugated oxindoles with 2-aminopyrans fusing carbo-heterocyclic ring systems in satisfactory chemical yield and enantioselectivity.
Scheme 3. Concept of Domino Michael Addition/Cyclization Reaction of Oxoindolines with Cyclic 1,3-Diketones Using SFAA Organocatalyst.
We report herein that the first domino Michael addition/cyclization reaction of cyclic 1,3-diketones with oxoindolines using SFAA catalyst afforded the chiral spiro-conjugated oxindoles with 2-aminopyrans fusing carbo-heterocyclic ring systems in excellent chemical yields (up to 98%) and with excellent enantioselectivities (up to 95% ee), although cupreine catalyst only afforded the spiro products in poor enantioselectivities (up to 8% ee) when cyclic 1,3-diketones were used.
Results and Discussion
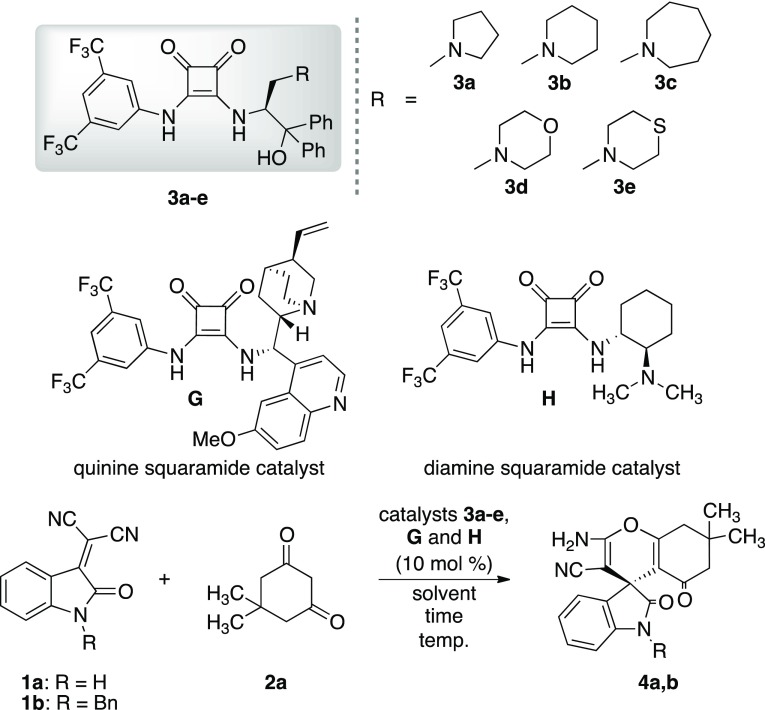
The catalysts 3a–e with different sizes of heterocyclic ring systems on the amino alcohol part were easily prepared according to our previously reported method using the condensation of squaramide with amino alcohols.9 At the outset of the study, the domino Michael addition/cyclization reaction of oxoindolines 1a,b with dimedone 2a was tried using SFAA catalysts 3a–e (10 mol %) in toluene or tetrahydrofuran (THF). The results are summarized in Table 1. First, the reaction using catalyst 3a (10 mol %) was conducted with oxoindoline 1a and dimedone 2a in toluene at room temperature (RT) for 24 h but only a trace amount of desired spiro-pyran 4a was obtained (entry 1). Next, the reactions using catalysts 3b and 3c were also carried out, respectively, and the corresponding 4a was afforded in good chemical yields (3b: 82%, 3c: 90%) but with poor enantioselectivities (3b: 7% ee, 3c: 9% ee) (entries 2 and 3). Furthermore, the reaction using catalyst 3b was also conducted in THF (polar aprotic solvent) but the reaction only afforded 4a as racemate, although good chemical yield (90%) was obtained (entry 4). On the basis of these results, we assumed that the use of sterically bulkier oxoindolines might be effective to control the enantioselectivity in this reaction.
Table 1. Optimization of the Michael Addition/Cyclization Reaction of Oxoindolines 1a,b with Dimedone 2a Using Catalysts 3a–e, G, and H.
| entrya | catalysts 3a–e, G, and H | oxoindolines 1a,b | solvent | time (h) | temp (°C) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|---|---|
| 1 | a | H | toluene | 24 | RT | trace | |
| 2 | b | H | toluene | 24 | RT | 82 | 9 |
| 3 | c | H | toluene | 24 | RT | 90 | 7 |
| 4 | b | H | THF | 24 | 0 | 90 | rac |
| 5 | b | Bn | toluene | 24 | RT | 95 | rac |
| 6 | b | Bn | THF | 48 | 0 | 89 | 77 |
| 7 | a | Bn | THF | 48 | 0 | 92 | 79 |
| 8 | c | Bn | THF | 48 | 0 | 95 | 75 |
| 9 | d | Bn | THF | 48 | 0 | 96 | 10 |
| 10 | e | Bn | THF | 48 | 0 | 97 | rac |
| 11 | G | Bn | THF | 48 | 0 | 86 | 70 |
| 12 | H | Bn | THF | 48 | 0 | 91 | 24 |
The reactions were carried out with 1a,b (0.1 mmol) and 2a (0.1 mmol) using catalysts 3a–e, G and H (10 mol %) in above-mentioned solvents (1.0 mL).
Isolated yields.
The ee were determined by chiral high-performance liquid chromatography (HPLC) using CHIRALCEL OD-H or CHIRALPAK AD-H columns.
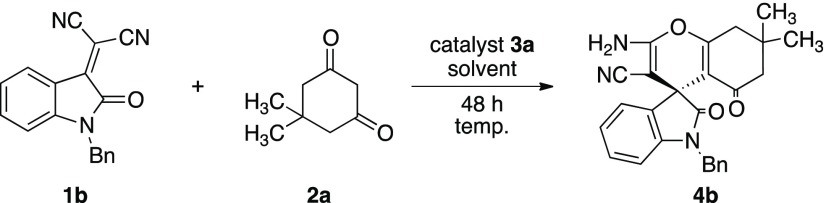
The reaction of bulkier N-Bn-oxoindoline 1b with 2a using catalyst 3b was carried out in toluene. Unfortunately, this reaction also afforded the racemic 4b, although the chemical yield was excellent (95%) (entry 5). Moreover, the same reaction using 3b was examined in THF. Surprisingly, the enantioselectivity was greatly increased to 77% ee with good chemical yield (89%) (entry 6). The reaction using 3a was also carried out in the same reaction conditions as those of 3b. Similarly, the catalyst 3a showed good catalytic activity to afford 4b in excellent chemical yield (92%) and with good enantioselectivity (79% ee) (entry 7). Furthermore, the use of catalyst 3c was also examined to afford the chiral 4a in excellent chemical yield (95%) with good enantioselectivity (75% ee) (entry 8). Encouraged by the results using THF as a solvent, the reactions using catalysts 3d and 3e were also carried out in THF, respectively. However, the reaction afforded 4b in poor enantioselectivities (entries 9 and 10). Furthermore, this reaction of 1b with 2a using well-known quinine G and diamine squaramide H catalysts was examined (entries 11 and 12), respectively. As a result, catalyst G afforded 4b in both good chemical yield (86%) and enantioselectivity (70% ee) (entry 11). On the other hand, catalyst H provided 4b in excellent chemical yield (91%) but with poor enantioselectivities (24% ee) (entry 12). By these results, it was found that catalyst 3a possessing pyrrolidine ring was the most effective in this reaction of 1b with 2a.
To optimize the reaction conditions using superior catalyst 3a, we next examined the molar ratio of catalyst, the effect of solvents, and the reaction temperatures (Table 2, entries 1–15). An increase in the catalyst loading amount from 10 to 20 mol % 3a resulted in a remarkable increase in chemical yield (96%) and unpredicted decrease in enantioselectivity (7% ee), although the reason was not clear (entry 1). On the other hand, the catalytic loading of 5 mol % 3a brought about both increase of chemical yield (95%) and enantioselectivity (83%) (entry 2). Furthermore, the reaction was also tried in the presence of 2 mol % 3a to afford the chiral product 4b in excellent chemical yield (92%) with good enantioselectivity (81% ee) (entry 3). To further improve the enantioselectivity of 4b, we also examined the catalytic activity of 3a at low temperatures (−10 to −80 °C). Under these conditions, good enantioselectivities (73–81% ee) were obtained with fairly good chemical yields (87–92%) (entries 4–6). Next, we examined the effects of different ethereal solvents, such as diethyl ether (Et2O), diisopropyl ether (DIPE), and 1,4-dioxane in this reaction using 5 mol % 3a. From the results, the enantioselectivities widely decreased in these solvents, although the chemical yields were good to excellent (entries 7–9). Moreover, the reactions were carried out in chlorinated solvents, such as dichloromethane (DCM), chloroform, and 1,2-dichloroethane (DCE). However, the chiral 4b was obtained in good-to-excellent chemical yield (89–96%) and only poor-to-moderate enantioselectivities (7–51% ee) (entries 10–12). Similarly, the uses of highly polar solvents, such as dimethylformamide (DMF), dimethylacetamide (DMA), and MeOH, also afforded racemic 4b with good chemical yields (89–92%) (entries 13–15). From these results, it turned out that the use of 5 mol % 3a in THF at 0 °C for 48 h was the optimal condition for this reaction. In general, enantiomeric excess in asymmetric reactions strongly depends on the solvent effect. It might be reasoned that THF coordinates in the reaction system include both the catalyst and substrates for forming a better transition state to afford satisfactory optical purity for this reaction.
Table 2. Optimization of Reaction Conditions for the Domino Michael Addition/Cyclization Reaction Using Catalyst 3a.
| entrya | catalyst 3a (mol %) | solvent | temp (°C) | yield (%)b | ee (%)c |
|---|---|---|---|---|---|
| 1 | 20 | THF | 0 | 96 | 7 |
| 2 | 5 | THF | 0 | 95 | 83 |
| 3 | 2 | THF | 0 | 92 | 81 |
| 4 | 5 | THF | –10 | 92 | 73 |
| 5 | 5 | THF | –50 | 90 | 81 |
| 6 | 5 | THF | –80 | 87 | 77 |
| 7 | 5 | Et2O | 0 | 82 | 67 |
| 8 | 5 | DIPE | 0 | 89 | 43 |
| 9d | 5 | 1,4-dioxane | RT | 98 | 5 |
| 10 | 5 | DCM | 0 | 96 | 7 |
| 11 | 5 | chloroform | 0 | 91 | 13 |
| 12 | 5 | DCE | 0 | 89 | 51 |
| 13 | 5 | DMF | 0 | 90 | rac |
| 14 | 5 | DMA | 0 | 89 | rac |
| 15 | 5 | MeOH | 0 | 92 | rac |
The reactions were carried out with 1b (0.1 mmol) and 2a (0.1 mmol) using catalyst 3a in above-mentioned solvents (0.1 mL).
Isolated yields.
The ee were determined by chiral HPLC using CHIRALPAK AD-H column.
The reaction was conducted at room temperature.
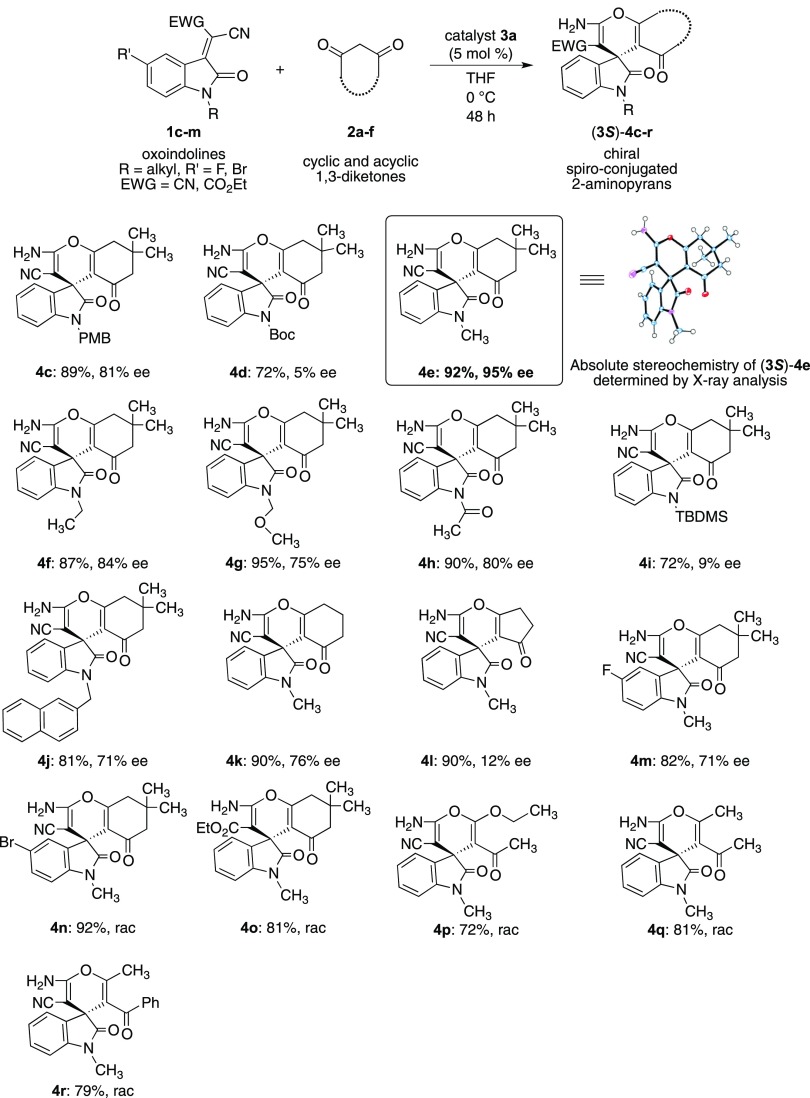
Under the optimal reaction conditions, the reactions using a wide range of oxoindolines 1c–m with cyclic 1,3-diketones 2a–c were examined in the domino Michael addition/cyclization reaction in the presence of superior catalyst 3a to afford the corresponding chiral spiro-conjugated 2-aminopyrans 4c–r. The results are summarized in Scheme 4. The reaction of N-para-methoxybenzyl-oxoindoline 1c with dimedone 2a afforded the corresponding spiro-pyran 4c in both good chemical yield (89%) and enantioselectivity (81% ee). However, the use of electron-deficient N-Boc-oxoindoline 1d gave 4d in good chemical yield (72%) but with poor enantioselectivity (5% ee). This failure might be due to the electronic characteristics of 1d, although the reason is not clear. The best result (92, 95% ee) was obtained in the reaction of N-methyl-oxoindoline 1e with 2a. The absolute configuration of the obtained chiral spiro-pyran (3S)-4e was determined by X-ray analysis (see the Supporting Information). The reactions of N-ethyl and N-methoxymethyl oxoindolines 1f,g with 2a afforded the corresponding chiral products 4f and 4g in good-to-excellent chemical yields (4f: 87%, 4g: 95%) and with moderate-to-good enantioselectivities (4f: 84% ee, 4g: 75% ee), respectively. Furthermore, the reactions were also conducted using N-acetyl oxoindoline 1h or silyl-protected N-tert-butyldimethylsilyl oxoindoline 1i with 2a, to afford the chiral spiro-pyrans 4h,i in moderate-to-good chemical yields (4h: 90%, 4i: 72%) and moderate-to-poor enantioselectivities (4h: 80% ee, 4i: 9% ee). The use of bulkier naphthyl-(2-methylene)-oxoindoline 1j also afforded the chiral 4j in good chemical yield (81%) with enantioselectivity (71% ee). Additionally, the domino Michael addition/cyclization reaction using N-methyl-oxoindoline 1e was extended to the reaction with other cyclic 1,3-diketones, such as 1,3-cyclohexadione 2b and 1,3-cyclopentadione 2c, using catalyst 3a. The reaction of 1e with 2b afforded the desired product 4k in both good chemical yield and enantioselectivity (4k: 90%, 76% ee). On the other hand, the reaction between 1e and 2c afforded product 4l in good chemical yield but with poor enantioselectivity (4l: 90%, 12% ee). The use of 5-fluoro-N-methyl-oxoindoline 1k also afforded the corresponding product 4m in both good chemical yield and enantioselectivity (4m: 72%, 71% ee).
Scheme 4. Substrate Scope for the Domino Michael Addition/Cyclization Reaction Using Catalyst 3a.
Next, the reaction of 5-bromo-N-methyl-oxoindoline 1l with 2a was also carried out and the product 4n was obtained in excellent chemical yield with racemate, although the reason is not clear. Similarly, the reaction of 1m with 2a also afforded the anticipated product 4o obtained as racemate but with good chemical yield (4o: 81%). We also examined the reaction of N-methyl-oxoindoline 1e with acyclic 1,3-diketones 2d–f using catalyst 3a. However, the desired products 4p–r were obtained as racemates, proceeded with a good chemical yield (72–81%), respectively. The formation of racemates 4p–r may be for the unfavored interaction of substrates 2d–f with catalyst 3a, respectively, although the reason is not clear. These results of the reactions of acyclic 1,3-diketones 4p–r are in contrast to the previously reported results.8 To demonstrate the practical utility of catalyst 3a, the domino Michael addition/cyclization reaction between oxoindoline 1e and 2a was conducted on a gram scale (1e: 1 g) using optimized reaction conditions (3a: 5 mol %, THF, 0 °C, 48 h) and the corresponding product 4e was obtained in excellent chemical yield (96%). Unfortunately, the enantioselectivity of 4e was 35% ee (see the Supporting Information). This result indicated that a detailed examination of the reaction conditions should be necessary (on a small scale) for obtaining satisfactory optical purity of 4e for use on the gram scale.
On the basis of the observed enantiopurity (95% ee) of chiral spiro-pyran 4e that was obtained from the reaction of 1e and 2a using catalyst 3a, the enantioselective reaction course was proposed as follows (Scheme 5). The configuration of 3a might be fixed by the hydrogen-bonding interaction between the hydroxyl hydrogen atom on diphenyl hydroxyl part at side chain and the squaramide carbonyl group. The active methylene proton of dimedone 2a is abstracted by basic tertiary nitrogen in the pyrrolidine ring of 3a, and the generated enolate species forms hydrogen bond with ammonium site of 3a. Furthermore, the oxoindoline 1e is also fixed to catalyst 3a through two hydrogen-bonding interactions of cyano groups on 2a with two amino hydrogen atoms of the squaramide part of the catalyst. Among the two proposed transition states Ts-I and Ts-II, the Michael addition might proceed through Ts-II that has less steric interactions between the substrate and enolate species than those of Ts-I that has larger steric interactions between 1e and enolate species. Thus, enolate of dimedone 2a attacks the electron-deficient olefin site of oxoindoline 1e from the side below (si face) to afford the chiral Michael adduct X. Furthermore, subsequent intramolecular cyclization leads to the formation of intermediate Y. Finally, tautomerization of 2-iminopyran Y might afford the formation of (3S)-4e as the major enantiomer.
Scheme 5. Plausible Reaction Course for Domino Michael Addition/Cyclization Reaction of 1e with 2a Using Catalyst 3a.
Conclusions
We have demonstrated the first successful enantioselective domino Michael addition/cyclization reaction of cyclic 1,3-diketones using our new hybrid-type squaramide-fused amino alcohol (SFAA) organocatalysts 3a–e containing both the Brønsted basic site and hydrogen-bonding sites. In the used SFAA catalysts, the catalyst 3a especially showed excellent catalytic activity in the reaction of oxoindolines 1a–m with 2a–f to afford the quaternary stereogenic chiral spiro-conjugated oxindoles with 2-aminopyrans fusing carbo-heterocyclic ring systems 4a–r in excellent chemical yields (up to 98%) with satisfactory-to-excellent enantioselectivities (up to 95% ee). The obtained chiral spiro-conjugated oxindoles 4a–r bearing quaternary stereogenic chiral carbon center may work as efficient synthetic intermediates for the synthesis of chiral biologically active compounds and for drug discovery.
Experimental Section
General Information
All reagents and solvents used in this work were bought from commercial channels and used directly. Oxoindolines 1a–m were prepared according to the literature procedure.10 All reactions were placed under argon atmosphere in flame-dried 4 mL sample vials inserted with magnetic beads. Thin-layer chromatography (TLC) was performed on Merck silica gel 60 F254 plates, and the analytes were identified under UV light. Flash column chromatography was performed using silica gel pore size 60N (40–100 μm). Melting points were recorded with a micro-melting-point apparatus. IR spectra were recorded with a JASCO 4100 Fourier transform infrared spectrophotometer. 1H and 13C NMR spectroscopic data were recorded using JEOL JNM-ECA500 instrument with tetramethylsilane as the internal standard. X-ray diffraction data of 4e were collected at 93 K on a Rigaku R-AXIS RAPID diffractometer using multilayer mirror monochromated Cu Kα radiation. HPLC data were collected using TOSOH instrument equipped with (UV-8020, DP-8020, and SD-8022) detectors using CHIRALCEL OD-H and CHIRALPAK AD-H columns. Optical rotations were recorded using a JASCO DIP-360 digital polarimeter. High-resolution mass spectrometry (HRMS) data were collected by electron impact (EI) and fast atom bombardment (FAB) modes using Hitachi RMG-GMG and JEOL JNX-DX303 sector instruments.
General Procedure for the Synthesis of Compounds (4a–r)
To a 4 mL sample vial containing 1,3-diketones 2a–f (0.1 mmol) and catalyst 3a (5 mol %) was added anhydrous THF (1.0 mL). The reaction mixture was allowed to stir at 0 °C for 1 h, followed by addition of 3-dicyano-2-oxoindolines 1a–j (0.1 mmol) at the same temperature. The reaction mixture was allowed to be stirred at the same temperature for 48 h. After completion of reaction, indicated by TLC, the residue was purified by using flash column chromatography (ethyl acetate/hexane, 4:6). The enantiomeric excess was determined by chiral HPLC using CHIRALPAK AD-H or CHIRALCEL OD-H columns.
(3S)-2-Amino-1-benzyl-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4b)
White solid. 92 mg, 98% yield. m.p. 250–251 °C. [α]D24 = −22 (c = 0.18, MeOH). IR (neat): cm–1 = 2962, 2189, 1706, 1632, 1492, 1470. 1H NMR (500 MHz, dimethyl sulfoxide (DMSO)-d6, ppm): δ 7.55 (d, J = 7.00 Hz, 2H), 7.40–7.30 (m, 5H), 7.18 (t, J = 7.50 Hz, 1H), 7.02 (t, J = 8.00 Hz, 1H), 6.74 (d, J = 8.00 Hz, 1H), 4.96 (q, J = 16.50, 29.50 Hz, 2H), 2.66 (q, J = 17.50, 25.00 Hz, 2H), 2.27 (d, J = 16.50 Hz, 1H), 2.18 (d, J = 16.50 Hz, 1H), 1.10 (s, 3H), 1.07 (s, 3H) ppm. 13C NMR (125 MHz, DMSO-d6, ppm): 195.2, 176.8, 164.6, 159.0, 142.6, 136.2, 133.6, 128.48, 128.40, 127.2, 127.1, 123.0, 122.7, 117.5, 110.7, 109.0, 57.2, 50.0, 46.6, 43.4, 32.0, 27.7, 27.12. MS (EI): m/z = 425 [M]+, HRMS (EI): calcd for C26H23N3O3m/z 425.1748; found: 425.1745. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/isopropyl alcohol (IPA); flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 32.02 min (major) and 41.46 min (minor), ee = 83%).
(3S)-2-Amino-1-(4-methoxybenzyl)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4c)
Brown solid. 70 mg, 89% yield. m.p. 147–149 °C. [α]D24 = −21 (c = 0.18, MeOH). IR (neat) cm–1: 2972, 2189, 1707, 1470, 1370. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.41 (d, J = 8.50 Hz, 2H), 7.33 (s, 2H), 7.18–7.06 (m, 2H), 6.94 (t, J = 10.00 Hz, 1H), 6.86 (d, J = 5.00 Hz, 2H), 6.69 (d, J = 8.00 Hz, 1H), 4.83 (q, J = 20.00, 23.50 Hz, 2H), 3.71 (s, 3H), 2.60 (q, J = 17.50, 29.00 Hz, 2H), 2.15 (dd, J = 16.00, 28.00 Hz, 2H), 1.04 (s, 3H), 1.00 (s, 3H). 13C NMR (125 MHz, DMSO-d6, ppm): 195.0, 176.6, 164.5, 158.9, 158.4, 142.6, 133.6, 128.9, 128.5, 128.2, 122.9, 122.5, 117.4, 113.7, 110.6, 109.0, 57.2, 55.0, 49.9, 46.5, 42.7, 32.0, 27.6, 27.0. MS (EI): m/z = 455 [M]+, HRMS (EI): calcd for C27H25N3O3m/z 455.1834; found: 455.1838. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 48.74 min (major) and 92.45 min (minor), ee = 81%).
(3S)-2-Amino-1-(tert-butyloxy)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4d)
Pale yellow solid. 57 mg, 72% yield. m.p. 135–136 °C. [α]D24 = −10 (c = 0.18, MeOH). IR (neat): cm–1 = 3309, 3179, 2189, 1720, 1715, 1610, 1407, 1309. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.87 (d, J = 8.00 Hz, 1H), 7.30 (t, J = 10.00 Hz, 1H), 7.12 (t, J = 7.50 Hz, 1H), 7.02 (d, J = 6.50 Hz, 1H), 4.99 (s, 2H), 2.56–2.46 (m, 2H), 2.23–2.13 (m, 2H), 1.65 (s, 12H), 1.11 (s, 3H), 1.06 (s, 3H). 13C NMR (125 MHz, DMSO-d6, ppm): 195.0, 175.5, 163.9, 158.3, 149.1, 139.8, 131.4, 129.4, 125.0, 123.2, 116.3, 115.5, 112.2, 84.6, 50.4, 40.9, 32.4, 28.6, 28.3, 27.9. MS (FAB): m/z = 436 [M]+, HRMS (FAB): calcd for C24H26N3O5m/z 436.1845; found: 436.1863. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 47.78 min (major) and 53.24 min (minor), ee = 5%).
(3S)-2-Amino-1-methyl-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4e)
White solid. 86 mg, 92% yield. m.p. 195–196 °C. [α]D24 = −23 (c = 0.18, MeOH). IR (neat): cm–1 = 3308, 3176, 2189, 1720, 1715, 1612, 1432, 1402. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.29 (s, 1H), 7.25 (t, J = 8.00 Hz, 1H), 7.04 (d, J = 7.50 Hz, 1H), 7.00–6.96 (m, 2H), 3.12 (s, 3H), 2.56 (d, J = 4.00 Hz, 2H), 2.11 (q, J = 16.00, 32.50 Hz, 2H), 1.02 (s, 3H), 0.98 (s, 3H). 13C NMR (125 MHz, DMSO-d6, ppm): 194.9, 176.6, 164.3, 158.9, 143.5, 137.4, 133.5, 128.9, 128.2, 125.3, 122.8, 122.4, 117.2, 110.7, 108.2, 57.0, 49.9, 32.0, 27.5, 27.0, 26.4, 21.1. MS (EI): m/z = 349 [M]+, HRMS (FAB): calcd for C20H19N3O3m/z 349.3814; found: 349.3820. The enantiomeric excess was determined by chiral HPLC; CHIRALCEL OD-H column (solvent system 80:20, hexane/IPA; flow rate, 0.5 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 95.54 min (major) and 48.69 min (minor), ee = 95%).
(3S)-2-Amino-1-(ethyl)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4f)
White solid. 56 mg, 87% yield. m.p. 280 °C. [α]D12 = −33 (c = 0.18, DCM). IR (neat): cm–1 = 3312, 3305, 2193, 1674, 1458. 1H NMR (500 MHz, CDCl3, ppm): δ 7.31–7.24 (m, 1H), 7.08–6.96 (m, 2H), 6.89 (d, J = 8.0 Hz, 1H), 4.81 (s, 2H), 3.92–3.76 (m, 2H), 2.63–2.41 (m, 2H), 2.29–2.0 (m, 2H), 1.39–1.30 (m, 3H), 1.12 (s, 3H), 1.06 (s, 3H). 13C NMR (125 MHz, CDCl3, ppm): δ 195.0, 176.2, 163.8, 158.4, 142.7, 133.0, 129.2, 123.2, 122.9, 116.3, 111.9, 108.8, 62.1, 50.6, 41.0, 35.4, 32.4, 28.7, 27.7, 12.2. MS (EI): m/z = 363 [M]+, HRMS (EI): calcd for C21H21N3O3m/z 363.1511; found: 363.1585. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 15.23 min (major) and 35.11 min (minor), ee = 83%).
(3S)-2-Amino-1-(monomethoxymethyl)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4g)
White solid. 49 mg, 95% yield. m.p. 109 °C. [α]D12 = −105 (c = 0.12, DCM). IR (neat): cm–1 = 3402, 3317, 2938, 2182, 1711, 1684. 1H NMR (500 MHz, CDCl3, ppm): δ 7.32–7.25 (m, 1H), 7.14–7.00 (m, 3H), 5.26–5.16 (m, 2H), 4.92 (s, 2H), 3.46 (s, 3H), 2.62–2.42 (m, 2H), 2.28–2.09 (m, 2H), 1.12 (s, 3H), 1.07 (s, 3H). 13C NMR (125 MHz, CDCl3, ppm): δ 195.17, 177.32, 164.00, 158.41, 142.00, 132.25, 129.41, 123.66, 123.18, 116.52, 111.86, 110.07, 72.29, 61.71, 60.59, 56.63, 50.55, 47.34, 41.01, 32.41, 28.68, 27.74, 21.24, 14.34. MS (EI): m/z = 379 [M]+, HRMS (EI): calcd for C21H21N3O4m/z 379.1512; found: 379.1537. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 20.7 min (major) and 31.48 min (minor), ee = 75%).
(3S)-2-Amino-1-(acetyl)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4h)
White solid. 59 mg, 90% yield. m.p. 242 °C. [α]D14 = −99 (c = 0.15, DCM). IR (neat): cm–1 = 3379, 3305, 2939, 2200, 1712, 1619, 1472. 1H NMR (500 MHz, CDCl3, ppm): δ 8.26 (d, J = 8.6 Hz, 1H), 7.33 (t, J = 7.4 Hz, 1H), 7.17 (t, J = 7.7 Hz, 1H), 7.04 (d, J = 7.4 Hz, 1H), 4.97 (s, 2H), 2.69 (s, 3H), 2.60–2.45 (m, 2H), 2.35–2.11 (m, 3H), 1.13 (s, 3H), 1.07 (s, 3H). 13C NMR (125 MHz, CDCl3, ppm): δ 195.2, 177.7, 171.0, 164.0, 158.2, 140.0, 131.5, 129.6, 125.7, 123.0, 116.8, 116.1, 112.2, 68.7, 61.7, 53.6, 50.3, 47.6, 40.9, 32.5, 28.5, 27.8, 26.7, 22.3. MS (EI): m/z = 377 [M]+, HRMS (EI): calcd for C21H19N3O4m/z 377.1321; found: 377.1373. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 37.09 min (major) and 28.67 min (minor), ee = 80%).
(3S)-2-Amino-1-(tert-butyldimethylsilyl)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4i)
Yellow solid. 32 mg, 72% yield. m.p. 119 °C. [α]D14 = −18 (c = 0.21, DCM). IR (neat): cm–1 = 3345, 3309, 2829, 2215, 1725, 1470, 1719, 1677. 1H NMR (500 MHz, CDCl3, ppm): δ 7.26 (tt, J = 8.7, 3.4 Hz, 1H), 7.06–7.00 (m, 1H), 7.00–6.91 (m, 2H), 4.69 (d, J = 10.3 Hz, 2H), 2.58–2.40 (m, 2H), 2.29–2.09 (m, 2H), 1.12 (s, 3H), 1.07 (s, 12H), 0.56 (s, 3H), 0.52 (s, 3H). 13C NMR (125 MHz, CDCl3, ppm): δ 194.1, 183.7, 163.5, 157.8, 146.1, 134.9, 128.7, 123.2, 122.5, 116.3, 113.1, 112.4, 50.5, 40.9, 32.3, 28.8, 27.5, 26.4, 19.9, −3.2, −3.6. MS (EI): m/z = 449 [M]+, HRMS (EI): calcd for C25H31N3O3Si m/z 449.2145; found: 449.2137. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 4.2 min (major) and 5.99 min (minor), ee = 9%).
(3S)-2-Amino-1-(methylenenaphthyl)-7,7-dimethyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4j)
White solid. 42 mg, 81% yield. m.p. 188–190 °C. [α]D24 = −11 (c = 0.18, solvent). IR (neat): cm–1 = 3395, 3196, 2996, 2189, 1782, 1676, 1610. 1H NMR (500 MHz, DMSO-d6, ppm): δ 8.03 (s, 1H), 7.90–7.87 (m, 2H), 7.79–7.77 (m, 1H), 7.61 (d, J = 8.50 Hz, 1H), 7.51–7.42 (m, 2H), 7.38 (s, 2H), 7.36 (s, 1H), 7.10 (t, J = 7.50 Hz, 2H), 6.96 (t, J = 7.50 Hz, 1H), 6.74 (d, J = 8.00 Hz, 1H), 5.08 (dd, J = 16.50, 68.00 Hz, 2H), 2.62 (q, J = 17.50, 28.00 Hz, 2H), 2.19 (dd, J = 16.00, 41.00 Hz, 2H), 1.06 (s, 3H), 1.02 (s, 3H). 13C NMR (125 MHz, DMSO-d6, ppm): 195.1, 176.8, 164.6, 159.0, 142.6, 133.7, 133.6, 132.9, 132.2, 128.3, 128.0, 127.6, 127.5, 126.2, 125.8, 125.5, 125.2, 123.0, 122.6, 110.7, 108.9, 57.1, 50.0, 46.7, 43.4, 32.0, 27.6, 27.0. MS (EI): m/z = 475 [M]+, HRMS (FAB): calcd for C30H25N3O3m/z 475.1823; found: 475.1894. The enantiomeric excess was determined by chiral HPLC; CHIRALCEL OD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 45.82 min (major) and 66.26 min (minor), ee = 71%).
(3S)-2-Amino-1-methyl-2,5-doxo-tetrahydrospiro(chromene-4,3-indoline)-3-carbonitrile (4k)
White solid. 68 mg, 90% yield. m.p. 267–268 °C. [α]D24 = −130 (c = 0.1, DCM). IR (neat): cm–1 = 3302, 2187, 1723, 1671, 1629, 1293. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.30–7.22 (m, 3H), 7.08–7.05 (m, 1H), 7.00–6.95 (m, 2H), 3.12 (s, 3H), 2.70–2.61 (m, 2H), 2.25–2.14 (m, 2H), 1.936–1.88 (m, 2H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 195.2, 176.7, 166.3, 158.8, 143.5, 133.7, 128.4, 123.0, 122.5, 117.3, 111.8, 108.2, 57.0, 46.6, 36.4, 26.8, 26.4, 19.8. MS (EI): m/z = 321 [M]+, HRMS (EI): calcd for C18H15N3O3m/z 321.3312; found: 321.1145. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 12.81 min (major) and 28.44 min (minor), ee = 76%).
(S)-2-Amino-1′-methyl-2′,5-dioxo-6,7-dihydro-5H-spiro[cyclopenta-pyran-4,3′-indoline]-3-carbonitrile (4l)
White solid. 31 mg, 90% yield. m.p. 288 °C. [α]D14 = −33 (c = 0.16, DCM). IR (neat): cm–1 = 3345, 3216, 2945, 2200, 1723, 1678, 1478. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.12 (s, 2H), 6.92–6.78 (m, 1H), 6.78–6.64 (m, 1H), 6.64–6.47 (m, 2H), 2.70 (s, 3H), 2.44–2.29 (m, 2H), 1.96–1.82 (m, 2H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 199.9, 177.8, 175.2, 160.8, 143.5, 131.3, 129.1, 124.0, 122.9, 117.5, 114.8, 108.6, 67.1, 56.0, 46.3, 33.2, 26.5, 25.2, 25.0. MS (EI): m/z = 307 [M]+, HRMS (EI): calcd for C17H13N3O3m/z 307.0917; found: 307.0966. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 13.15 min (major) and 21.42 min (minor), ee = 12%).
(3S)-2-Amino-5′-fluoro-1′,7,7-trimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (4m)
White solid. 64 mg, 71% yield. m.p. 211 °C. [α]D24 = −34 (c = 0.23, DCM). IR (neat): cm–1 = 3308, 3176, 2189, 1720, 1715, 1612, 1432, 1402. 1H NMR (500 MHz, CD3OD, ppm): δ 7.04 (td, J = 8.9, 2.9 Hz, 1H), 6.98 (q, J = 4.2 Hz, 1H), 6.92 (dd, J = 7.7, 2.6 Hz, 1H), 3.25 (s, 3H), 2.68–2.55 (m, 2H), 2.29–2.22 (m, 2H), 1.07 (s, 6H). 13C NMR (125 MHz, CD3OD, ppm): δ 197.7, 179.5, 167.0, 162.2, 161.1, 160.2, 140.9, 117.9, 116.0, 115.8, 112.1, 111.9, 110.5, 51.3, 41.5, 33.2, 28.4, 27.0. MS (EI): m/z = 367 [M]+, HRMS (FAB): calcd for C20H18FN3O3m/z 367.1312; found: 367.1336. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 32.51 min (major) and 29.39 min (minor), ee = 71%).
2-Amino-5′-bromo-1′,7,7-trimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carbonitrile (4n)
Brown solid. 57 mg, 92% yield. m.p. 242 °C. [α]D24 = −10 (c = 0.30, MeOH). IR (neat): cm–1 = 3310, 3146, 2199, 1721, 1715, 1612, 1432, 1402. 1H NMR (500 MHz, CD3OD) δ 7.34–7.27 (m, 1H), 7.14–7.08 (m, 1H), 6.99 (d, J = 7.7 Hz, 1H), 3.23 (s, 3H), 2.62 (q, J = 17.6 Hz, 2H), 2.27–2.13 (m, 2H), 1.11 (s, 3H), 1.09 (s, 3H). 13C NMR (125 MHz, CD3OD, ppm): δ 197.7, 179.3, 167.1, 161.2, 143.7, 136.7, 129.8, 129.6, 124.5, 117.9, 111.8, 110.8, 51.3, 41.5, 33.2, 28.1, 27.0. MS (EI): m/z = 428 [M]+, HRMS (FAB): calcd for C20H18BrN3O3m/z 428.0523; found: 428.0592. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 31.27 min (minor) and 82.45 min (major), racemate).
Ethyl 2-Amino-1′,7,7-trimethyl-2′,5-dioxo-5,6,7,8-tetrahydrospiro[chromene-4,3′-indoline]-3-carboxylate (4o)
White solid. 65 mg, 81% yield. m.p. 233 °C. [α]D24 = −10 (c = 0. 12, MeOH). IR (neat): cm–1 = 3415, 3322, 3293, 2198, 1725, 1674, 1483. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.86 (s, 2H), 7.29 (s, 2H), 7.08 (s, 1H), 6.83–6.61 (m, 2H), 3.03 (s, 3H), 2.53–2.44 (m, 4H), 2.07–1.85 (m, 2H), 0.93 (s, 3H), 0.86 (s, 3H), 0.65 (s, 3H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 194.9, 178.3, 167.6, 162.7, 159.3, 145.3, 135.0, 128.4, 127.6, 122.1, 121.4, 113.0, 107.0, 75.9, 58.9, 50.6, 46.1, 31.7, 27.8, 26.7, 26.2, 13.6. MS (EI): m/z = 396 [M]+, HRMS (EI): calcd for C22H24N2O5m/z 396.1611; found: 396.1683. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 41.65 min (major) and 14.95 min (minor), racemate).
3′-Acetyl-6′-amino-2′-ethoxy-1-methyl-2-oxospiro[indoline-3,4′-pyran]-5′-carbonitrile (4p)
White solid. 31 mg, 72% yield. m.p. 228 °C. [α]D24 = −22 (c = 0.11, MeOH). IR (neat): cm–1 = 3420, 3324, 3290, 2198, 1724, 1645, 1479. 1H NMR (500 MHz, CDCl3, ppm): δ 7.29 (td, J = 7.7, 1.2 Hz, 1H), 7.15–7.09 (m, 1H), 7.09–7.02 (m, 1H), 6.84 (t, J = 6.9 Hz, 1H), 4.80 (s, 2H), 3.94–3.68 (m, 2H), 3.25 (s, 3H), 2.40 (s, 3H), 0.86 (t, J = 7.50 Hz, 3H). 13C NMR (125 MHz, CDCl3, ppm): δ 177.4, 164.7, 159.7, 158.5, 143.5, 133.3, 129.3, 123.6, 123.3, 116.6, 108.3, 105.1, 60.8, 26.7, 19.2, 13.5. MS (EI): m/z = 339 [M]+, HRMS (EI): calcd for C18H17N3O4m/z 339.1256; found: 339.1224. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 10.1 min (minor) and 11.72 min (major), racemate).
3′-Acetyl-6′-amino-1,2′-dimethyl-2-oxospiro[indoline-3,4′-pyran]-5′-carbonitrile (4q)
White solid. 48 mg, 81% yield. m.p. 180 °C. [α]D24 = −21 (c = 0.13, MeOH). IR (neat): cm–1 = 3379, 3305, 2939, 2200, 1712, 1619, 1471. 1H NMR (500 MHz, DMSO-d6, ppm): δ 7.26 (td, J = 7.7, 1.1 Hz, 1H), 7.18 (s, 2H), 7.08 (dd, J = 7.4, 1.1 Hz, 1H), 7.02–6.95 (m, 2H), 3.12 (s, 3H), 2.31 (s, 3H), 2.08 (s, 3H). 13C NMR (125 MHz, DMSO-d6, ppm): δ 197.3, 177.1, 159.3, 157.0, 143.5, 133.5, 128.7, 123.0, 122.6, 117.5, 114.9, 108.4, 56.4, 49.0, 31.4, 26.4, 19.6. MS (EI): m/z = 309 [M]+, HRMS (EI): calcd for C17H15N3O3m/z 309.1112; found: 309.1112. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 13.66 min (major) and 16.72 min (minor), racemate).
2′-Amino-5′-benzoyl-1,6′-dimethyl-2-oxospiro[indoline-3,4′-pyran]-3′-carbonitrile (4r)
White solid. 48 mg, 79% yield. m.p. 180 °C. [α]D24 = −11 (c = 0.22, MeOH). IR (neat): cm–1 = 3371, 3315, 2929, 2200, 1712, 1619, 1481. 1H NMR (500 MHz, CD3OD, ppm): δ 7.78–7.62 (m, 2H), 7.56 (t, J = 7.4 Hz, 1H), 7.49–7.28 (m, 3H), 7.24 (t, J = 7.7 Hz, 1H), 6.99 (t, J = 7.4 Hz, 1H), 6.91 (d, J = 8.0 Hz, 1H), 3.23 (s, 3H), 1.77 (s, 3H). 13C NMR (125 MHz, CD3OD, ppm): δ 195.9, 162.5, 156.7, 139.9, 134.6, 130.6, 130.2, 130.0, 125.5, 124.6, 109.9, 27.1, 20.0. MS (EI): m/z = 371 [M]+, HRMS (EI): calcd for C22H17N3O3m/z 371.2780; found: 371.2783. The enantiomeric excess was determined by chiral HPLC; CHIRALPAK AD-H column (solvent system 80:20, hexane/IPA; flow rate, 1.0 mL/min; temp, 25 °C; detection UV, 254 nm; retention time, 25.46 min (minor) and 28.12 min (major), racemate).
Acknowledgments
We thank the Adaptable & Seamless Technology Transfer Program through Target-Driven R&D of the Japan Science and Technology (JST) (AS231Z01382G and AS221Z01186D) for partial financial support of this research.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b01271.
The authors declare no competing financial interest.
Supplementary Material
References
- a Galliford C. V.; Scheidt K. A. Pyrrolidinyl-spirooxindoles Natural Products as Inspiration for the Development of Potential Therapeutic Agents. Angew. Chem., Int. Ed. 2007, 46, 8748–8758. [DOI] [PubMed] [Google Scholar]; b Trost B. M.; Chunhui J. Catalytic Enantioselective Construction of All-Carbon Quaternary Stereocenters. Synthesis 2006, 369–396. 10.1055/s-2006-926302. [DOI] [Google Scholar]; c Marti C.; Carreira E. M. Construction of Spiro[pyrrolidine-3,3′-oxindoles]-Recent Applications to the Synthesis of Oxindole Alkaloids. Eur. J. Org. Chem. 2003, 2209–2219. 10.1002/ejoc.200300050. [DOI] [Google Scholar]; d Cano R.; Ramón D. J.; Yus M. Transition-Metal-Free O-, S-, and N-Arylation of Alcohols, Thiols, Amides, Amines, and Related Heterocycles. J. Org. Chem. 2011, 76, 654–660. 10.1021/jo1022052. [DOI] [PubMed] [Google Scholar]; e Bisht S.; Rani R.; Peddinti R. K. Regioselective synthesis of Bicyclic and Polycyclic Systems by Cycloaddition Reactions of Alkenyl p-Benzoquinones. J. Org. Chem. 2018, 83, 75–84. 10.1021/acs.joc.7b02377. [DOI] [PubMed] [Google Scholar]; f Williams R. M.; Cox R. J. Paraherquamides, Brevianamides, and Asperparalines: Laboratory Synthesis and Biosynthesis. An Interim Report. Acc. Chem. Res. 2003, 36, 127–139. 10.1021/ar020229e. [DOI] [PubMed] [Google Scholar]; g Kumar M.; Chauhan P.; Valkonen A.; Rissanen K.; Enders D. Asymmetric Synthesis of Functionalized Tricyclic Chromanes via an organocatalytic Triple Domino Reaction. Org. Lett. 2017, 19, 3025–3028. 10.1021/acs.orglett.7b01322. [DOI] [PubMed] [Google Scholar]
- a Tietze L. F. Domino Reactions in Organic Synthesis. Chem. Rev. 1996, 96, 115–136. 10.1021/cr950027e. [DOI] [PubMed] [Google Scholar]; b Bisht S.; Peddinti R. K. FeCl3-Mediated domino Reaction of Benzoxazinones with Aroylmethylidene Malonates: Synthesis to Functionalized Pyrrolobenzoxazines. J. Org. Chem. 2017, 82, 13617–13625. 10.1021/acs.joc.7b02207. [DOI] [PubMed] [Google Scholar]; c Zhang J.; Yin G.; Du Y.; Yang Z.; Li Y.; Chen L. Michael-Michael Addition Reactions Promoted by Secondary Amine-Thiourea: Stereocontrolled constructions of Barbiturate-Fused Tetrahydropyrano Scaffolds and Pyranocoumarins. J. Org. Chem. 2017, 82, 13594–13601. 10.1021/acs.joc.7b01902. [DOI] [PubMed] [Google Scholar]; d Chauhan P.; Mahajan S.; Enders D. Achieving Molecular Complexity via Stereoselective Multiple Domino Reactions Promoted by a Secondary Amine Organocatalyst. Acc. Chem. Res. 2017, 50, 2809–2821. 10.1021/acs.accounts.7b00406. [DOI] [PubMed] [Google Scholar]; e Chaudhari P. D.; Hong B. C.; Lee G. H. Organocatalytic Enantioselective Michael-Michael-Michael-Aldol Condensation Reactions: Control of Six Streocenters in a quadruple-Cascade Asymmetric Synthesis of Polysubstituted Spirocyclic Oxindoles. Org. Lett. 2017, 19, 6112–6115. 10.1021/acs.orglett.7b02962. [DOI] [PubMed] [Google Scholar]
- a Bindra J. S. In The Alkaloids: Chemistry and Physiology; Manske R. H. F., Ed.; Academic Press: New York, 1973; Vol. 14, pp 83–121. [Google Scholar]; b Antonchick A. P.; Lopez-Tosco S.; Parga J.; Sievers S.; Schuermann M.; Preut H.; Susanne S.; Hans R.; Sterneckert J.; Rauh D.; Waldmann H. Highly Enantioselective Catalytic Synthesis of Neurite Growth-Promoting Secoyohimbanes. Chem. Biol. 2013, 20, 500–509. 10.1016/j.chembiol.2013.03.011. [DOI] [PubMed] [Google Scholar]; c Amat M.; Ramos C.; Pérez M.; Molins E.; Florindo P.; Santos M. M. M.; Bosch J. Enantioselective Formal synthesis of ent-Rhynchophylline and ent-Isorhynchophylline. Chem. Commun. 2013, 49, 1954–1956. 10.1039/c2cc38540f. [DOI] [PubMed] [Google Scholar]
- a Jossang A.; Jossang P.; Hadi H. A.; Sevenet T.; Bodo B. Horsfiline, an oxindole alkaloid from Horsfieldia superba. J. Org. Chem. 1991, 56, 6527–6530. 10.1021/jo00023a016. [DOI] [Google Scholar]; b Ban Y.; Taga N.; Oishi T. the synthesis of 3-spirooxindole derivatives: Total syntheses of dl-formosanine, dl-isoformosanine, dl-mitraphylline and dl-isomitraphylline. Tetrahedron Lett. 1974, 15, 187–190. 10.1016/S0040-4039(01)82169-9. [DOI] [Google Scholar]; c Beecham A. F.; Hart N. K.; Johns S. R.; Lamberton J. A. Stereochemistry of oxindole alkaloids. Uncarines A, B (Formosanine), C (Pteropodine), D (Speciophylline), E (Isopteropodine), and F. Aust. J. Chem. 1968, 21, 491–504. 10.1071/CH9680491. [DOI] [Google Scholar]
- a White J. D.; Li Y.; Ihle D. C. Tandem Intramolecular Photocycloaddition-Retro-Mannich Fragmentation as a route to spiro[pyrrolidine-3-3′-oxindoles]. Total synthesis of (±)-Coreulescine, (±)-Horsfiline, (±)-Elacomine, and (±)-6-Deoxyelacomine. J. Org. Chem. 2010, 75, 3569–3577. 10.1021/jo1002714. [DOI] [PubMed] [Google Scholar]; b Cui C. B.; Kakeya H.; Osada H. Spirotryprostatin B, a Novel Mammalian Cell Cycle Inhibitor Produced by Aspergillus fumigatus. J. Antibiot. 1996, 49, 832–835. 10.7164/antibiotics.49.832. [DOI] [PubMed] [Google Scholar]; c Miyake F. Y.; Yakushijin K.; Horne D. A. Preparation and Synthetic Applications of 2-Halotryptamines: Synthesis of Elacomine and Isoelacomine. Org. Lett. 2004, 6, 711–713. 10.1021/ol030138x. [DOI] [PubMed] [Google Scholar]; d Borschberg H. R. New Strategies for The Synthesis of Monoterpene indole Alkaloids. Curr. Org. Chem. 2005, 9, 1465–1491. 10.2174/138527205774370522. [DOI] [Google Scholar]
- a Kumar A.; Lohan P.; Aneja D. K.; Gupta G. K.; Kaushik D.; Prakash O. Design, synthesis, Computational and Biological Evaluation of Some New Hydrazino Derivatives of DHA and Pyranopyrazoles. Eur. J. Med. Chem. 2012, 50, 81–89. 10.1016/j.ejmech.2012.01.042. [DOI] [PubMed] [Google Scholar]; b Pellegrini C.; Weber M.; Borchbeg H.-J. Total Synthesis of (±)-Elacomine and (-)-Isoelcacomine, Two Hitherto Unnamed Oxindole Alkaloids from Elaeagnus commutata. Helv. Chim. Acta 1996, 79, 151–168. 10.1002/hlca.19960790116. [DOI] [Google Scholar]
- Chen W. B.; Wu Z. J.; Pei Q. L.; Cun L. F.; Zhang X. M.; Yuan W. C. Highly Enantioselective Construction of Spiro[4H-pyran-3-3′-oxindoles] Through a Domino Knoevenagel/Michael/Cyclization sequence Catalyzed by Cupreine. Org. Lett. 2010, 12, 3132–3135. 10.1021/ol1009224. [DOI] [PubMed] [Google Scholar]
- Birch A. J.; Russell R. A. Studies in relation to biosynthesis-XLIV: Structural elucidations of brevianamides-B, -C, -D and -F. Tetrahedron 1972, 28, 2999–3008. 10.1016/0040-4020(72)80014-0. [DOI] [Google Scholar]
- a Chennapuram M.; Reddy U. V. S.; Seki C.; Okuyama Y.; Kwon E.; Uwai K.; Tokiwa M.; Takeshita M.; Nakano H. Hybrid-Type Squaramide-Fused Amino Alcohol Organocatalysts for Enantioselective Nitro-Aldol Reaction of Nitromethane with Isatins. Eur. J. Org. Chem. 2017, 1638–1646. 10.1002/ejoc.201700138. [DOI] [Google Scholar]; b Chennapuram M.; Reddy U. V. S.; Seki C.; Okuyama Y.; Kwon E.; Uwai K.; Tokiwa M.; Takeshita M.; Nakano H. Hybrid-Type Squaramide-Fused Amino Alcohol Organocatalysts for Enantioselective Diels-Alder Reactions of 3-Hydroxy-2-pyridones with Maleimides. Eur. J. Org. Chem. 2017, 4633–4641. 10.1002/ejoc.201700830. [DOI] [Google Scholar]
- He T.; Zeng Q. Q.; Yang D. C.; He Y. H.; Guan Z. Biocatalytic one-pot three-component synthesis of 3-3′-disubstitued oxindoles and spirooxindole pyrans using α-amylase from hog pancreas. RSC Adv. 2015, 5, 37843–37852. 10.1039/C4RA16825A. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.