Abstract
Background
The long‐term effectiveness of atherectomy treatment for peripheral arterial disease is unknown. We studied 5‐year clinical outcomes by endovascular treatment type among patients with peripheral arterial disease.
Methods and Results
We queried the Medicare‐linked VQI (Vascular Quality Initiative) registry for endovascular interventions from 2010 to 2015. The exposure was treatment type: atherectomy (with or without percutaneous transluminal angioplasty [PTA]), stent (with or without PTA), or PTA alone. The outcomes were major amputation, any amputation, and major adverse limb event (major amputation or any reintervention). We used the center‐specific proportions of atherectomy procedures performed in the 12 months before a patient's procedure as the instruments to perform an instrumental‐variable Cox model analysis. Among 16 838 eligible patients (median follow‐up: 1.3–1.5 years), 11% underwent atherectomy, 40% received PTA alone, and 49% underwent stenting. Patients receiving atherectomy commonly underwent femoropopliteal artery treatment (atherectomy: 65%; PTA: 49%; stenting: 43%; P<0.001) and had worse disease severity (Trans‐Atlantic Inter‐Society Consensus score [TASC] B and greater; atherectomy: 77%; PTA: 68%; stenting: 67%; P<0.001). The 5‐year rate of major adverse limb events was 38% in patients receiving atherectomy versus 33% for PTA and 32% for stenting (log rank P<0.001). Controlling for unmeasured confounding using instrumental‐variable analysis, patients treated with atherectomy experienced outcomes similar to those of patients treated with PTA, except for a higher risk of any amputation (hazard ratio: 1.51; 95% CI, 1.08–2.13). However, compared with stenting, atherectomy patients had a higher risk of major amputation (hazard ratio: 3.66; 95% CI, 1.72–7.81), any amputation (hazard ratio: 2.73; 95% CI, 1.60–4.76), and major adverse limb event (hazard ratio: 1.61; 95% CI, 1.10–2.38).
Conclusions
Atherectomy is used to treat severe femoropopliteal and tibial peripheral arterial disease even though long‐term adverse outcomes occur more frequently after this treatment modality.
Keywords: angioplasty, atherectomy, outcomes, peripheral artery disease, stent
Subject Categories: Peripheral Vascular Disease, Treatment, Quality and Outcomes
Clinical Perspective
What Is New?
In our study of >16 000 patients who underwent lower extremity endovascular intervention, we found that 5‐year rates of amputation and major adverse limb events (major amputation or any reintervention) following atherectomy were inferior to stenting in unadjusted, multivariable, and instrumental‐variable analyses.
What Are the Clinical Implications?
The inferior outcomes and higher cost of atherectomy relative to other treatment options calls into question the ubiquitous use of atherectomy in clinical practice.
Percutaneous transluminal angioplasty and stenting should remain the first‐line endovascular treatments for peripheral arterial disease until the appropriate indications for atherectomy are identified.
Technological advances in the endovascular treatment of peripheral arterial disease (PAD) have spurred the rapid adoption of newer techniques, such as atherectomy, in clinical practice.1, 2, 3 In particular, atherectomy use grew disproportionately higher than use of other procedures in the outpatient setting from 2011 to 2014.4 Atherectomy, designed to treat advanced and heavily calcified lesions, is an attractive treatment option because it can remove atherosclerotic plaque from the vessel wall, thus acting as a stand‐alone treatment or being used to debulk a large plaque before percutaneous transluminal angioplasty (PTA) or stenting.1, 2, 5, 6, 7, 8, 9
Despite these theoretical advantages of atherectomy, its long‐term effectiveness remains unclear.1, 2, 8, 9, 10, 11, 12 Real‐world evidence varies, with reports of improved10, 13, 14, 15, 16 or equivalent8, 11, 12, 17 outcomes compared with traditional treatments such as PTA or stenting and higher rates of amputation noted by others.3 Randomized controlled trials of atherectomy6, 7, 18, 19, 20, 21, 22, 23, 24, 25, 26 lack long‐term outcome evaluation and are underpowered to appropriately evaluate atherectomy's performance against other endovascular treatments. Consequently, the long‐term durability of atherectomy remains unknown despite its widespread use.1, 2, 8, 9, 10, 11, 13
In this analysis, our objective was to examine long‐term amputation and major adverse limb event (MALE) rates after atherectomy compared with more traditional endovascular treatments. We studied patients within the peripheral vascular intervention (PVI) module of the Medicare‐linked VQI (Vascular Quality Initiative), a national quality improvement registry in which patients have been linked to Medicare claims for long‐term outcome assessment. Although traditional risk‐adjustment approaches adjust for measured confounders, we used instrumental‐variable (IV) methods to adjust for unmeasured and measured confounding. Specifically, we used a 2‐stage IV procedure designed for time‐to‐event outcomes.27, 28 We hypothesized that leveraging the strengths of the Medicare‐linked VQI PVI data set and the novel IV risk‐adjustment methods for time‐to‐event analysis might reveal new insights into the impact and role of atherectomy in treating PAD.
Methods
Data Source
Our study used data from the Medicare‐linked VQI PVI data set for procedures performed from January 1, 2010, through September 30, 2015. Utilizing patient‐level data collected as part of an Agency for Healthcare Research and Quality–listed Patient Safety Organization,29 the VQI prospectively collects patient and procedure variables for commonly performed vascular procedures at >500 centers in the United States and Canada.30 Through Medicare claims linkage, this data set also contains long‐term follow‐up until September 30, 2015, for linked patients. Prior publications have outlined our matching algorithms, codes, and success rates.31 The data and analytic methods for this project are available to other researchers on request, pending approval by the Research Advisory Committee at VQI. Our study was approved by the Center for the Protection of Human Subjects at Dartmouth College, and the informed consent requirement was waived.
Forming the Analytic Cohort
Between January 1, 2010, and September 30, 2015, the Medicare‐linked VQI PVI data set registered 35 458 PVI procedures that were eligible for outcomes analysis. We systematically applied our exclusion criteria to this cohort. Data on the artery, side, and indication treated were necessary for inclusion in the study, thus observations missing these values were dropped (n=2071). We omitted procedures that were not a primary procedure (eg, reintervention) or in which the aorta, aneurysmal pathology, asymptomatic indication, or acute ischemia (n=7680) was treated. We also excluded cases that used an ineligible treatment type (eg, not PTA, stent, or atherectomy). To apply this criterion, we retained artery‐level data (eg, treatment type, Trans‐Atlantic Inter‐Society Consensus [TASC] score) for only the most severely diseased artery. We defined this artery as whichever had the highest TASC score or indication per patient. After excluding cases that did not meet the artery‐level criteria and patients whose IV was based on ≤10 procedures (n=8869), our final analytic cohort included 16 838 patients eligible for analysis.
Measures
The primary exposure was endovascular treatment type categorized as PTA alone, stenting, and atherectomy. The 2 comparisons of interest were PTA alone versus atherectomy and stenting versus atherectomy. Stenting procedures include self‐expanding stents, balloon‐expandable stents, and stent grafts. Atherectomy procedures include laser atherectomy, orbital atherectomy, and excisional atherectomy. Because PTA is commonly used in conjunction with other interventions, patients who underwent PTA in addition to atherectomy or stenting were included in the atherectomy or stenting groups, respectively. Patients (n=3314) who underwent a combination of other treatments (eg, stent plus atherectomy), were excluded from this analysis because our goal was to compare atherectomy, stent, and PTA treatment strategies.
The main outcomes for this study were major amputation (any above‐ankle amputation), any amputation (major or foot amputation), and MALE (major amputation or any reintervention). The Current Procedural Terminology (CPT) codes identifying these events in Medicare claims are included in Table S1. Because the procedure codes do not capture laterality, we cannot be certain that the amputations identified in Medicare claims are ipsilateral to where the intervention occurred. We conducted a sensitivity analysis to see how the unadjusted hazard ratio (HR) for any amputation changed as we varied the proportion of contralateral amputations for each treatment type from 0% to 50% to understand how this limitation might affect our results. Patient death was identified using the Social Security Death Index. We abstracted follow‐up data through September 30, 2015, at which point the patient was censored if he or she did not have an event (major amputation, any amputation, or MALE) or died.
Statistical Analysis
We used descriptive statistics (counts and percentages) and tests for statistical significance (χ2 tests or ANOVA) to explore demographic and clinical characteristics among patients receiving PTA, stent, or atherectomy. We set our threshold for statistical significance at a 2‐tailed P<0.05. Using the log‐rank test, we compared the unadjusted Kaplan–Meier cumulative event curve estimations for each outcome stratified by treatment type.
To estimate HRs for each outcome, we built 3 regression models: unadjusted Cox, multivariable Cox with a random‐effect factor for center, and a 2‐stage residual‐inclusion IV Cox model designed for time‐to‐event outcomes.27 This IV methodology specifically accounts for unmeasured confounding in Cox proportional hazards models. Please see Data S1 for a description and the code to implement our IV methods. For IV analysis, we assumed effect homogeneity, that is, the effect of atherectomy treatment on amputation and MALE is constant across our study population.32, 33 This assumption allows us to draw a more generalizable, causal inference from our IV‐based results.32, 33 To evaluate the sensitivity of our results, including the homogeneity assumption, we repeated these analyses in key clinical subgroups including patients with (1) only 1 artery treated, (2) femoropopliteal treatment, and (3) diabetes mellitus. All multivariable analyses are adjusted for patient and lesion characteristics including demographics, comorbidities, medication use, and symptom severity. PTA alone or stent served as the reference group for their respective comparisons. All statistical analyses were performed using R v3.3 (R Project for Statistical Computing).
The IV for analysis was the historical (12 months before patient procedure), center‐specific proportion of atherectomy procedures out of all atherectomy and stenting procedures or all atherectomy and PTA procedures, depending on the comparison. The IV was calculated for patients whose treatment center had performed at least 10 procedures in the 12 months before their case, thus adjusting for the relative procedure volume at each center. Our instrument capitalizes on the natural variation in facility treatment preferences and is commonly used in the medical literature.32 We visually inspected the strength of our instruments by identifying whether the proportion of patients receiving atherectomy varied at different levels of each instrument. This was supported by statistical confirmation using the F statistic adjusted for measured confounders, for which a value >10 indicates a strong instrument.34, 35 The other IV assumptions cannot be verified from the data; therefore, we relied on our expert's subject matter knowledge to assess the potential for an assumption violation (see Data S2 for details).
Results
Study Population
In this cohort of 16 838 patients, 11% underwent atherectomy, 40% received PTA, and 49% received stents (Table 1). The mean age of patients in the cohort was 72.5 years (SD: 9.9), and 43% were women. Patients receiving atherectomy are most commonly living independently (93%), white (81%), and male (61%), a pattern also seen among patients treated with stenting and PTA.
Table 1.
Demographics and Comorbidities of Patients Who Underwent Atherectomy, Stent, or PTA
| Characteristic | PTA, n=6718 | Stent, n=8229 | Atherectomy, n=1891 | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, y, mean (SD) | 72.9 (10.3) | 71.9 (9.4) | 73.1 (10.1) | 0.002 |
| Men | 3757 (55.9) | 4741 (57.1) | 1165 (61.1) | <0.001 |
| Race | ||||
| White | 5199 (77.4) | 7030 (85.4) | 1525 (80.6) | <0.001 |
| Black | 1136 (16.9) | 874 (10.6) | 277 (14.6) | <0.001 |
| Other | 383 (5.7) | 325 (3.9) | 89 (4.7) | <0.001 |
| Hispanic or Latino | 474 (7.1) | 360 (4.4) | 85 (4.5) | <0.001 |
| Transfer from rehabilitation | 418 (6.2) | 388 (4.7) | 86 (4.4) | <0.001 |
| Nursing home | 486 (7.2) | 366 (4.4) | 134 (7.1) | <0.001 |
| Comorbidities | ||||
| Smoking | ||||
| Never smoked | 2139 (31.9) | 1321 (16.1) | 569 (30.1) | <0.001 |
| Prior smoker | 3025 (45.0) | 4026 (48.9) | 894 (47.3) | <0.001 |
| Current smoker | 1554 (23.1) | 2882 (35.0) | 428 (22.6) | <0.001 |
| BMI (%) | ||||
| Underweight | 306 (4.6) | 377 (4.6) | 59 (3.1) | 0.015 |
| Normal | 2118 (31.5) | 2739 (33.3) | 539 (28.6) | <0.001 |
| Obese | 2012 (29.9) | 2367 (28.7) | 602 (31.8) | 0.021 |
| Overweight | 2282 (34.0) | 2746 (33.4) | 691 (36.5) | 0.032 |
| Hypertension | 6140 (91.4) | 7372 (89.6) | 1741 (92.1) | <0.001 |
| Diabetes mellitus | 4022 (59.9) | 3752 (45.6) | 1132 (59.9) | <0.001 |
| Insulin‐dependent diabetes mellitus | 2523 (37.6) | 2009 (24.4) | 707 (37.4) | <0.001 |
| Coronary disease | 2078 (30.9) | 2613 (31.8) | 639 (33.8) | 0.059 |
| Heart failure | 1665 (24.8) | 1510 (18.3) | 481 (25.4) | <0.001 |
| COPD | 1608 (23.9) | 2411 (29.3) | 471 (24.9) | <0.001 |
| Dialysis | ||||
| None | 5673 (84.4) | 7642 (92.9) | 1638 (86.6) | <0.001 |
| Functioning transplant | 106 (1.6) | 82 (1.0) | 17 (0.9) | 0.002 |
| On dialysis | 939 (14.0) | 505 (6.1) | 236 (12.5) | <0.001 |
| Prior leg bypass | 1095 (16.3) | 1092 (13.3) | 188 (9.9) | <0.001 |
| Prior PTA/stent | 2604 (38.8) | 2756 (33.5) | 772 (40.8) | <0.001 |
| Medications | ||||
| Aspirin | 4683 (69.7) | 5964 (72.5) | 1336 (70.6) | <0.001 |
| Antiplatelet | 2402 (35.7) | 2949 (35.8) | 791 (41.8) | <0.001 |
| β‐Blocker | 1115 (16.6) | 933 (11.3) | 265 (14.0) | <0.001 |
| Statin | 4401 (65.5) | 5732 (69.6) | 1263 (66.8) | <0.001 |
BMI indicates body mass index; COPD, chronic obstructive pulmonary disease; PTA, percutaneous transluminal angioplasty.
Relationship Between Diabetes Mellitus and Smoking in Choice of Treatment Type
Patients who underwent the 3 treatment types differed in the prevalence of key comorbidities: diabetes mellitus and smoking. Patients treated with atherectomy were less likely to be smokers than those receiving stents but were similar to those who underwent PTA (atherectomy and PTA: 23% smokers; stent: 35% smokers; P<0.001 for the difference across the 3 treatment groups). Diabetes mellitus, including insulin‐dependent diabetes mellitus, was equally common among patients receiving atherectomy or PTA but less common among patients receiving stents (diabetes mellitus: PTA, 60%; atherectomy, 60%; stent, 46%; P<0.001).
Relationship Between of Disease Severity and Choice of Treatment Type
We noted several differences in symptom severity and disease characteristics among the 3 treatment types (Table 2). Among patients treated with PTA, the femoropopliteal was the most commonly treated segment (49%), followed by the tibials (40%) and then the iliacs (11%). Patients who received stents, however, were more likely to receive them in the iliac arteries (53%), followed by the femoropopliteal arteries (43%) and rarely in the tibials (4%). In patients who underwent atherectomy, the femoropopliteal segment was most commonly treated (65%), followed by the tibials (33%) and then the iliacs (1%).
Table 2.
Disease Characteristics of Patients Who Underwent Atherectomy, Stent, or PTA
| Characteristic | PTA, n=6718 | Stent, n=8229 | Atherectomy, n=1891 | P Value |
|---|---|---|---|---|
| Ambulatory status | ||||
| Ambulatory | 4355 (64.8) | 6292 (76.4) | 1327 (70.2) | <0.001 |
| Ambulatory w/assistance | 1646 (24.5) | 1458 (17.7) | 360 (19.0) | <0.001 |
| Wheelchair | 615 (9.2) | 421 (5.1) | 188 (9.9) | <0.001 |
| Bedridden | 102 (1.5) | 58 (0.8) | 16 (0.9) | <0.001 |
| ASA class | ||||
| 1, normal/healthy | 78 (1.2) | 121 (1.5) | 17 (0.9) | 0.072 |
| 2, mild systemic disease | 1134 (16.9) | 1745 (21.2) | 340 (18.0) | <0.001 |
| 3, severe systemic disease | 4352 (64.8) | 5329 (64.8) | 1177 (62.2) | 0.096 |
| 4/5, disease is threat to life/moribund | 813 (12.1) | 745 (9.0) | 253 (13.4) | <0.001 |
| Urgency | ||||
| Elective | 5502 (81.9) | 7256 (88.2) | 1588 (84.0) | <0.001 |
| Urgent/emergent | 1216 (18.0) | 973 (11.8) | 303 (16.0) | <0.001 |
| Limb indication | ||||
| Claudication | 2286 (34.0) | 4608 (56.0) | 806 (42.6) | <0.001 |
| Rest pain | 927 (13.8) | 1291 (15.8) | 274 (14.4) | 0.003 |
| Tissue loss | 3505 (52.2) | 2322 (28.2) | 811 (42.9) | <0.001 |
| Number of arteries treated | ||||
| 1 | 1716 (25.5) | 2876 (34.9) | 519 (27.4) | <0.001 |
| 2 | 2593 (38.6) | 3190 (38.8) | 646 (34.2) | <0.001 |
| ≥3 | 2409 (35.9) | 2163 (26.3) | 726 (38.4) | <0.001 |
| Artery treated | ||||
| Iliac | 742 (11.0) | 4372 (53.1) | 27 (1.4) | <0.001 |
| Femoropopliteal | 3305 (49.1) | 3555 (43.2) | 1237 (65.4) | <0.001 |
| Tibial | 2671 (39.8) | 302 (3.7) | 627 (33.2) | <0.001 |
| TASC score‡ | ||||
| A | 2131 (31.7) | 2689 (32.7) | 439 (23.1) | <0.001 |
| B | 1303 (19.4) | 1952 (23.7) | 462 (24.4) | <0.001 |
| C | 1010 (15.0) | 1366 (16.6) | 373 (19.7) | <0.001 |
| D | 1095 (16.3) | 1044 (12.7) | 336 (17.8) | <0.001 |
| Occlusion length,‡ cm, median (IQR) | 1 (0–4) | 2 (0–6) | 2 (0–8) | <0.001 |
ASA indicates Association of Anesthesiologists; IQR, interquartile range; PTA, percutaneous transluminal angioplasty; TASC, Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease.
A significant difference was noted in the distribution of disease severity across treatment types (P<0.001). PTA was generally used to treat tissue loss (52%), then claudication (34%) or rest pain (14%), and stents were mainly used to treat claudication (56%), then tissue loss (28%) or rest pain (16%). Atherectomy was equally used to treat claudication (43%) and tissue loss (43%) and then rest pain (14%). The pattern of atherectomy use for more severe disease was further noted when we examined the TASC score distribution, in which 77% of lesions treated with atherectomy were TASC type B and greater versus 68% for PTA and 67% for stent (P<0.001).
Outcome Rates at 5 Years by Treatment Type
Patients receiving PTA and atherectomy have similar rates of adverse outcomes, but patients receiving stents experience these outcomes less frequently (Figure 1A–1C). The 5‐year major amputation rate was highest for patients receiving PTA (11.1%) and atherectomy (9.9%). Patients who underwent stenting had the lowest 5‐year major amputation rate at 4.6%, which is significantly lower than PTA and atherectomy (log rank, P<0.001; Figure 1A).
Figure 1.
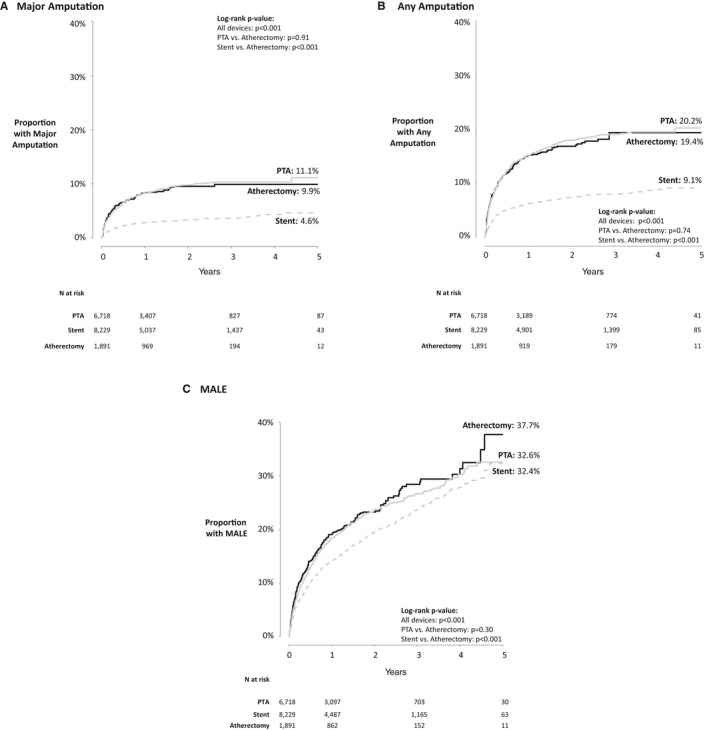
Unadjusted Kaplan–Meier hazard curves by treatment type for (A) major amputation, (B) any amputation, and (C) major adverse limb events in the overall population. For all graphs, the SEs are <0.10 (10%). MALE indicates major adverse limb event; PTA, percutaneous transluminal angioplasty.
A similar effect was seen in any amputation as an outcome, which included minor toe and forefoot amputations as well as major (above‐ and below‐knee) amputations (Figure 1B). At 5 years, 20.2% of patients who underwent PTA had an amputation compared with 19.4% of patients who underwent atherectomy and 9.1% of patients who underwent stenting (log rank, P<0.001; Figure 1B).
Differences in MALEs across the 3 treatment types were not as dramatic but still favored stenting over PTA and atherectomy (Figure 1C). Patients treated with atherectomy had the highest 5‐year incidence of MALEs, with 37.7% experiencing a major amputation or reintervention, compared with 32.6% of PTA patients and 32.4% of stent patients (log rank, P<0.001; Figure 1C).
Assessing the IVs
Instrument 1 was the center‐specific proportion of atherectomy among patients who underwent atherectomy or PTA in the 12 months before the patient's procedure. Looking at the distribution of patients receiving atherectomy by quintile of instrument 1, we can see that across quintiles, the proportion of atherectomy increases as the proportion of PTA decreases (Figure 2A). This result demonstrates the ability of the instrument to predict treatment type; the higher the value of the instrument, the more likely the patient will undergo atherectomy. The strength of our instrument is confirmed by the large F statistic F(1,8 608)=2 109.2.
Figure 2.
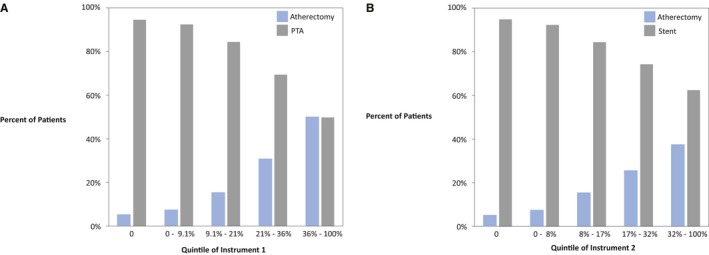
Proportion of patients receiving atherectomy or PTA by quintile of instrument 1 and atherectomy or stent by quintile of instrument 2. A, The distribution of treatment delivered to patients by quintile of instrument 1, which is the hospital‐specific proportion of atherectomy delivered among patients receiving atherectomy or PTA in the 12 months before their procedure. B, The distribution of treatment delivered to patients by quintile of instrument 2, which is the hospital‐specific proportion of atherectomy delivered among patients receiving atherectomy or stent in the 12 months before their procedure. PTA indicates percutaneous transluminal angioplasty.
Instrument 2 was the historical center‐specific proportion of atherectomy among patients who underwent atherectomy or stent in the 12 months before the patient's procedure. Across increasing quintiles of instrument 2, we see an increase in the proportion of atherectomy and a consequent decrease in stenting, just as we saw with instrument 1 (Figure 2B). Again, this result is confirmed by the F statistic for instrument 2 F(1,10 119)=1 764.4.
Adjusted HRs for Outcomes After Atherectomy Versus PTA
Patients treated with atherectomy and PTA generally had similar risks of major amputation and MALEs, even after adjusting for key observed covariates and using IV analysis (Table 3). Although multivariable Cox regression HRs revealed a 10% to 14% increased risk of adverse outcomes after atherectomy versus PTA, this finding was not statistically significant except for MALEs (HR: 1.14; 95% CI, 1.06–1.30). However, after IV risk adjustment, patients who underwent atherectomy were 51% more likely to have any amputation compared with patients treated with PTA (HR: 1.51; 95% CI, 1.08–2.13). In general, this effect remained similar in magnitude but was not statistically significant in each subgroup.
Table 3.
Effect of Atherectomy Versus PTA Treatment on Major Amputation, Any Amputation, and MALE Risk
| Outcome | Overall (n=8609) | 1 Artery Treated (n=2235) | Femoropopliteal Treated (n=4542) | Diabetic (n=5154) | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Major amputation | ||||||||
| Unadjusted | 0.99 (0.82–1.19) | 0.906 | 1.07 (0.74–1.56) | 0.718 | 0.87 (0.66–1.14) | 0.300 | 0.94 (0.76–1.16) | 0.549 |
| Multivariable+REa | 1.14 (0.93–1.39) | 0.200 | 1.18 (0.78–1.79) | 0.420 | 1.13 (0.85–1.52) | 0.400 | 1.08 (0.86–1.35) | 0.510 |
| IVb | 1.38 (0.86–2.22) | 0.180 | 1.91 (0.69–5.30) | 0.210 | 1.10 (0.59–2.05) | 0.770 | 1.19 (0.71–1.99) | 0.511 |
| Any amputation | ||||||||
| Unadjusted | 0.98 (0.85–1.12) | 0.744 | 0.93 (0.70–1.24) | 0.634 | 0.96 (0.79–1.18) | 0.709 | 0.95 (0.82–1.11) | 0.553 |
| Multivariable+REa | 1.10 (0.93–1.27) | 0.230 | 1.07 (0.79–1.45) | 0.670 | 1.21 (0.98–1.49) | 0.082 | 1.09 (0.91–1.28) | 0.390 |
| IVb | 1.51 (1.08–2.13) | 0.019 | 2.42 (1.15–5.10) | 0.019 | 1.32 (0.84–2.06) | 0.230 | 1.39 (0.96–2.01) | 0.077 |
| MALE | ||||||||
| Unadjusted | 1.07 (0.94–1.20) | 0.304 | 0.98 (0.78–1.24) | 0.882 | 1.00 (0.86–1.17) | 0.968 | 1.09 (0.94–1.27) | 0.246 |
| Multivariable+REa | 1.14 (1.06–1.30) | 0.041 | 1.09 (0.85–1.39) | 0.510 | 1.10 (0.93–1.30) | 0.250 | 1.15 (0.98–1.35) | 0.093 |
| IVb | 1.28 (0.95–1.75) | 0.097 | 1.12 (0.61–2.09) | 0.700 | 1.17 (0.81–1.67) | 0.410 | 1.41 (0.97–2.04) | 0.070 |
HR indicates hazard ratio; IV, instrumental variable; MALE, major adverse limb event; PTA, percutaneous transluminal angioplasty; RE, random effect.
*All HR estimates from Cox regression models. Unless specified (eg, unadjusted) models are adjusted for age, sex, race, ethnicity, transfer from rehabilitation, nursing home living, smoking, body mass index, hypertension, diabetes mellitus, insulin‐dependent diabetes mellitus, coronary disease, chronic obstructive pulmonary disease, congestive heart failure, dialysis, prior stent or PTA, prior bypass, aspirin, P2Y antagonist use, statin, ambulatory status, procedure urgency, limb indication, number of arteries treated, arterial location, and Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease (TASC) score.
Adjusted model includes random‐effects component for center.
Adjusted model incorporates instrument (proportion of atherectomy of all atherectomy and PTA procedures performed at center in the 12 months before patient's procedure).
Adjusted HRs for Outcomes After Atherectomy Versus Stent
Compared with stenting, patients who underwent atherectomy had a statistically significant increase in their risk of all studied adverse outcomes (Table 4). Though random‐effects Cox regression adjustment reduced the unadjusted effect size, atherectomy patients remained at increased risk for all outcomes. IV adjustment increased the HR for all outcomes when atherectomy was compared with stent, even in subgroup analyses. After accounting for unmeasured confounding, atherectomy patients were almost 4 times more likely than stent patients to have a major amputation (HR: 3.66; 95% CI, 1.72–7.81) and 3 times more likely to have any amputation (HR: 2.73; 95% CI, 1.60–4.76). They were also almost twice as likely to experience a MALE (HR: 1.61; 95% CI, 1.10–2.38) compared with patients receiving stents. The impact of stent versus atherectomy treatment was estimated to be of similar magnitude across the clinical subgroups as well.
Table 4.
Effect of Atherectomy Versus Stent Treatment on Major Amputation, Any Amputation, and MALE Risk
| Outcome | Overall (n=10 120) | 1 Artery Treated (n=3395) | Femoropopliteal Treated (n=4792) | Diabetic (n=4884) | ||||
|---|---|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Major amputation | ||||||||
| Unadjusted | 2.94 (2.38–3.57) | <0.001 | 2.86 (1.92–4.24) | <0.001 | 1.60 (1.19–2.12) | 0.001 | 2.27 (1.78–2.86) | <0.001 |
| Multivariable+REa | 1.49 (1.18–1.92) | 0.001 | 1.60 (1.00–2.63) | 0.052 | 1.50 (1.10–2.04) | 0.010 | 1.35 (1.02–1.79) | 0.033 |
| IVb | 3.66 (1.72–7.81) | <0.001 | 8.39 (2.10–33.60) | 0.003 | 2.32 (1.16–4.62) | 0.017 | 2.71 (1.21–6.07) | 0.015 |
| Any amputation | ||||||||
| Unadjusted | 2.44 (2.13–2.86) | <0.001 | 2.34 (1.74–3.15) | <0.001 | 1.34 (1.10–1.63) | 0.004 | 2.05 (1.72–2.44) | <0.001 |
| Multivariable+REa | 1.23 (1.03–1.47) | 0.019 | 1.24 (0.88–1.75) | 0.220 | 1.27 (1.02–1.59) | 0.033 | 1.20 (0.99–1.47) | 0.068 |
| IVb | 2.73 (1.60–4.76) | <0.001 | 4.48 (1.57–12.81) | 0.005 | 1.85 (1.15–2.99) | 0.012 | 2.79 (1.51–4.94) | <0.001 |
| MALE | ||||||||
| Unadjusted | 1.32 (1.16–1.47) | <0.001 | 1.13 (0.78–1.65) | 0.272 | 1.15 (0.98–1.35) | 0.077 | 1.30 (1.12–1.52) | <0.001 |
| Multivariable+REa | 1.21 (1.06–1.41) | 0.004 | 1.15 (0.90–1.47) | 0.280 | 1.14 (0.97–1.35) | 0.120 | 1.18 (0.99–1.41) | 0.065 |
| IVb | 1.61 (1.10–2.38) | 0.015 | 1.46 (0.75–2.86) | 0.260 | 1.43 (0.99–2.05) | 0.055 | 1.50 (0.92–2.45) | 0.100 |
HR indicates hazard ratio; IV, instrumental variable; MALE, major adverse limb event; PTA, percutaneous transluminal angioplasty; RE, random effect.
*All HR estimates from Cox regression models. Unless specified (eg, unadjusted) models adjusted for age, sex, race, ethnicity, transfer from rehabilitation, nursing home living, smoking, body mass index, hypertension, diabetes mellitus, insulin‐dependent diabetes mellitus, coronary disease, chronic obstructive pulmonary disease, congestive heart failure, dialysis, prior stent or PTA, prior bypass, aspirin, P2Y antagonist use, statin, ambulatory status, procedure urgency, limb indication, number of arteries treated, arterial location, and Trans‐Atlantic Inter‐Society Consensus Document on Management of Peripheral Arterial Disease (TASC) score.
Adjusted model includes random‐effects component for center.
Adjusted model incorporates instrument (proportion of atherectomy of all atherectomy and stent procedures performed at center in the 12 months before patient's procedure).
Discussion
In our study of >16 000 patients who underwent lower extremity endovascular intervention, we found that atherectomy was used in >10% of patients treated in our national registry. However, the 5‐year rates of amputation and MALEs following atherectomy were poorer than those for stenting in unadjusted, multivariable, and IV‐adjusted analyses. These findings persisted even in our subgroup analysis limited to femoropopliteal lesions and among patients with diabetes mellitus. Overall, patients who underwent atherectomy were nearly 4 times more likely to undergo major amputation than those who underwent stenting, a finding that was consistent across several subgroup analyses. Although the 95% CIs were wider for the IV estimates, the lower bound was similar in estimated direction and magnitude to that reported for the non‐IV adjusted estimates. These results indicate that although emerging endovascular technologies may be popular in contemporary practice, the related increased risk of long‐term adverse outcomes may caution against widespread use.
Given the wide variability in the disease characteristics of patients treated with atherectomy, patient selection likely plays an important role in which individuals receive which treatment type. This patient selection can also be associated with a reduced risk of adverse outcomes. Typically, risk adjustment will mitigate an effect seen across interventions when treatment selection bias is present. In our analyses, however, we found that the HR point estimates for all outcomes and comparisons increased after IV adjustment. This means that unmeasured confounders in our study associated with the likelihood that a patient receives atherectomy are also associated with a reduced risk of adverse outcomes. By accounting for this unmeasured factor or factors, it is possible that we have identified associations between atherectomy use and the risk of major amputation, any amputation, or MALE, and this may more accurately represent the actual treatment effect of atherectomy compared with stenting or PTA.
Endovascular treatments continue to outnumber open procedures.2, 36, 37, 38 In light of their growing popularity and technological developments, it is imperative that thorough evaluation of long‐term outcomes be conducted for new treatments. Previous observational research and randomized controlled trials addressing this objective, including 2 meta‐analyses, do not offer sufficient evidence for the superiority of atherectomy compared with other established treatments such as PTA or stent.4, 6, 7, 8, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26 Nevertheless, these studies do not consider the long‐term effects of atherectomy, a key element in evaluating different treatment options. We used a large, national, clinical registry with up to 5 years of patient follow‐up and accounted for unmeasured confounding with an IV‐analysis methodology designed for time‐to‐event outcomes27 to address the limitations of the existing evidence, which spans a large series of papers evaluating atherectomy.1, 2, 6, 7, 8, 9, 10, 11, 13, 18, 19, 20, 21, 22, 23, 24, 25, 26 Our results echo emerging evidence suggesting that atherectomy can be more harmful than other endovascular treatments.3 These recent research efforts, combined with the higher cost of atherectomy relative to other treatment options, cause concern for the ubiquitous use of atherectomy in clinical practice.3, 4, 39 PTA and stenting should remain the primary endovascular treatments for PAD choices until further research efforts can identify the appropriate indications for atherectomy.
Despite our best efforts, this study has limitations. Our study population was limited to Medicare patients in the VQI; therefore, our results might not be generalizable to a younger or more racially diverse cohort of PAD patients. Given small sample sizes, we did not study any combinations of endovascular treatment modalities, of which combined stent and atherectomy use (512 eligible patients) was the largest and most relevant subgroup. We hope to revisit this question in future work. Because we included artery‐level data for only the most severe lesion, we cannot fully account for the interaction of multiple lesions with different severities in 1 patient and its impact on outcomes. We tried to accommodate this limitation by comparing our overall results with the subpopulation of patients who had only 1 artery treated. Even so, this approach does not fully address this weakness. Furthermore, we cannot distinguish whether an amputation was ipsilateral or contralateral to the primary intervention site, a weakness inherent in the International Classification of Diseases, Ninth Revision (ICD‐9) and CPT codes used to identify events in Medicare claims. From our sensitivity analyses, if we assumed that the proportion of contralateral amputations identified was the same for each treatment type, then our estimated HRs did not change because we saw the same relative decrease in the number of any amputations for both atherectomy (ie, numerator) and PTA or stent (ie, the denominator). If we assumed that the proportion of contralateral amputations was different for each treatment type, then we discovered that the proportion of contralateral amputations could vary in any combination from 0% to 50% for stent and atherectomy, and atherectomy use would still lead to an increased risk of any amputation. Consequently, these sensitivity analyses support the validity of our findings. Finally, although we thoroughly assessed the validity of our IV‐analysis assumptions and are confident in our instrument (Data S1), there is no way to unconditionally confirm that all assumptions are valid. Nevertheless, based on our results for all patients and key clinical subgroups, we remain assured of the face validity of our findings. Because of the strong treatment selection bias in atherectomy use and the lack of adequate randomized trials addressing this question, the advantages of IV analytic methods outweigh the potential risk of residual bias.
Conclusion
Among >16 000 patients who underwent lower extremity endovascular intervention, we found that 1 in 3 patients who underwent atherectomy had a MALE within 5 years. After IV adjustment, patients who underwent atherectomy were nearly 4 times more likely to undergo major amputation than those who underwent stenting. These findings call into question the long‐term utility of atherectomy for PAD and the role it should play in the management of patients being considered for lower extremity revascularization.
Sources of Funding
This work was supported by a Patient‐Centered Outcomes Research Institute (PCORI) Award ME‐1503‐28 261. All statements in this article, including its findings and conclusions, are solely those of the authors and do not necessarily represent the views of PCORI, its board of governors or its methodology committee.
Disclosures
None.
Supporting information
Data S1. Two‐Stage Instrumental Variable Methodology.
Data S2. Instrumental Variable Assumption Assessment.
Table S1. Current Procedural Terminology (CPT) Codes Used to Identify Outcomes in Medicare Claims
(J Am Heart Assoc. 2019;8:e012081 DOI: 10.1161/JAHA.119.012081.)
References
- 1. Franzone A, Ferrone M, Carotenuto G, Carbone A, Scudiero L, Serino F, Scudiero F, Izzo R, Piccolo R, Saviano S, Amato B, Perrino C, Trimarco B, Esposito G. The role of atherectomy in the treatment of lower extremity peripheral artery disease. BMC Surg. 2012;12:S13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mohan S, Flahive JM, Arous EJ, Judelson DR, Aiello FA, Schanzer A, Simons JP; Vascular Quality I . Peripheral atherectomy practice patterns in the United States from the vascular quality initiative. J Vasc Surg. 2018;68:1806–1816. [DOI] [PubMed] [Google Scholar]
- 3. Mukherjee D, Contos B, Emery E, Collins DT, Black JH III. High reintervention and amputation rates after outpatient atherectomy for claudication. Vasc Endovascular Surg. 2018:52:427–433. [DOI] [PubMed] [Google Scholar]
- 4. Mukherjee D, Hashemi H, Contos B. The disproportionate growth of office‐based atherectomy. J Vasc Surg. 2017;65:495–500. [DOI] [PubMed] [Google Scholar]
- 5. Shafique S, Nachreiner RD, Murphy MP, Cikrit DF, Sawchuk AP, Dalsing MC. Recanalization of infrainguinal vessels: Silverhawk, laser, and the remote superficial femoral artery endarterectomy. Semin Vasc Surg. 2007;20:29–36. [DOI] [PubMed] [Google Scholar]
- 6. Dattilo R, Himmelstein SI, Cuff RF. The compliance 360degree trial: a randomized, prospective, multicenter, pilot study comparing acute and long‐term results of orbital atherectomy to balloon angioplasty for calcified femoropopliteal disease. J Invasive Cardiol. 2014;26:355–360. [PubMed] [Google Scholar]
- 7. Shammas NW, Lam R, Mustapha J, Ellichman J, Aggarwala G, Rivera E, Niazi K, Balar N. Comparison of orbital atherectomy plus balloon angioplasty vs. Balloon angioplasty alone in patients with critical limb ischemia: results of the calcium 360 randomized pilot trial. J Endovasc Ther. 2012;19:480–488. [DOI] [PubMed] [Google Scholar]
- 8. Engelberger S, van den Berg JC. Atherectomy in complex infrainguinal lesions: a review. J Cardiovasc Surg (Torino). 2015;56:43–54. [PubMed] [Google Scholar]
- 9. Quevedo HC, Arain SA, Ali G, Abi Rafeh N. A critical view of the peripheral atherectomy data in the treatment of infrainguinal arterial disease. J Invasive Cardiol. 2014;26:22–29. [PubMed] [Google Scholar]
- 10. Doshi R, Shlofmitz E, Meraj P. Utilization and in‐hospital outcomes associated with atherectomy in the treatment of peripheral vascular disease: an observational analysis from the national inpatient sample. Vascular. 2018;26:464–471. [DOI] [PubMed] [Google Scholar]
- 11. Diamantopoulos A, Katsanos K. Atherectomy of the femoropopliteal artery: a systematic review and meta‐analysis of randomized controlled trials. J Cardiovasc Surg (Torino). 2014;55:655–665. [PubMed] [Google Scholar]
- 12. Ambler GK, Radwan R, Hayes PD, Twine CP. Atherectomy for peripheral arterial disease. Cochrane Database Syst Rev. 2014;Issue 3:CD006680. [DOI] [PubMed] [Google Scholar]
- 13. Katsanos K, Spiliopoulos S, Reppas L, Karnabatidis D. Debulking atherectomy in the peripheral arteries: is there a role and what is the evidence? Cardiovasc Intervent Radiol. 2017;40:964–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Laird JR, Zeller T, Gray BH, Scheinert D, Vranic M, Reiser C, Biamino G. Limb salvage following laser‐assisted angioplasty for critical limb ischemia: results of the LACI multicenter trial. J Endovasc Ther. 2006;13:1–11. [DOI] [PubMed] [Google Scholar]
- 15. Panaich SS, Arora S, Patel N, Patel NJ, Patel SV, Savani C, Singh V, Jhamnani S, Sonani R, Lahewala S, Thakkar B, Patel A, Dave A, Shah H, Bhatt P, Jaiswal R, Ghatak A, Gupta V, Deshmukh A, Kondur A, Schreiber T, Grines C, Badheka AO. In‐hospital outcomes of atherectomy during endovascular lower extremity revascularization. Am J Cardiol. 2016;117:676–684. [DOI] [PubMed] [Google Scholar]
- 16. Das T, Mustapha J, Indes J, Vorhies R, Beasley R, Doshi N, Adams GL. Technique optimization of orbital atherectomy in calcified peripheral lesions of the lower extremities. Catheter Cardiovasc Interv. 2014;83:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Todd KE, Ahanchi SS, Maurer CA, Kim JH, Chipman CR, Panneton JM. Atherectomy offers no benefits over balloon angioplasty in tibial interventions for critical limb ischemia. J Vasc Surg. 2013;58:941–948. [DOI] [PubMed] [Google Scholar]
- 18. Rastan A, McKinsey JF, Garcia LA, Rocha‐Singh KJ, Jaff MR, Noory E, Zeller T. One‐year outcomes following directional atherectomy of infrapopliteal artery lesions: subgroup results of the prospective, multicenter definitive le trial. J Endovasc Ther. 2015;22:839–846. [DOI] [PubMed] [Google Scholar]
- 19. Zeller T, Krankenberg H, Steinkamp H, Rastan A, Sixt S, Schmidt A, Sievert H, Minar E, Bosiers M, Peeters P, Balzer JO, Gray W, Tübler T, Wissgott C, Schwarzwälder U, Scheinert D. One‐year outcome of percutaneous rotational atherectomy with aspiration in infrainguinal peripheral arterial occlusive disease: the multicenter pathway PVD trial. J Endovasc Ther. 2009;16:653–662. [DOI] [PubMed] [Google Scholar]
- 20. Zeller T, Langhoff R, Rocha‐Singh KJ, Jaff MR, Blessing E, Amann‐Vesti B, Krzanowski M, Peeters P, Scheinert D, Torsello G, Sixt S, Tepe G; DEFINITIVE AR Investigators . Directional atherectomy followed by a paclitaxel‐coated balloon to inhibit restenosis and maintain vessel patency: twelve‐month results of the DEFINITIVE AR study. Circ Cardiovasc Interv. 2017;10:e004848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dippel EJ, Makam P, Kovach R, George JC, Patlola R, Metzger DC, Mena‐Hurtado C, Beasley R, Soukas P, Colon‐Hernandez PJ, Stark MA, Walker C; EXCITE ISR Investigators . Randomized controlled study of excimer laser atherectomy for treatment of femoropopliteal in‐stent restenosis: initial results from the EXCITE ISR trial (EXCImer Laser Randomized Controlled Study for Treatment of FemoropopliTEal In‐Stent Restenosis). JACC Cardiovasc Interv. 2015;8:92–101. [DOI] [PubMed] [Google Scholar]
- 22. Roberts D, Niazi K, Miller W, Krishnan P, Gammon R, Schreiber T, Shammas NW, Clair D. Effective endovascular treatment of calcified femoropopliteal disease with directional atherectomy and distal embolic protection: final results of the definitive Ca++ trial. Catheter Cardiovasc Interv. 2014;84:236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Shammas NW, Coiner D, Shammas GA, Dippel EJ, Christensen L, Jerin M. Percutaneous lower‐extremity arterial interventions with primary balloon angioplasty versus silverhawk atherectomy and adjunctive balloon angioplasty: randomized trial. J Vasc Interv Radiol. 2011;22:1223–1228. [DOI] [PubMed] [Google Scholar]
- 24. Ott I, Cassese S, Groha P, Steppich B, Hadamitzky M, Ibrahim T, Kufner S, Dewitz K, Hiendlmayer R, Laugwitz KL, Schunkert H, Kastrati A, Fusaro M. Randomized comparison of paclitaxel‐eluting balloon and stenting versus plain balloon plus stenting versus directional atherectomy for femoral artery disease (ISAR‐STATH). Circulation. 2017;135:2218–2226. [DOI] [PubMed] [Google Scholar]
- 25. Vroegindeweij D, Kemper FJ, Tielbeek AV, Buth J, Landman G. Recurrence of stenoses following balloon angioplasty and simpson atherectomy of the femoro‐popliteal segment. A randomised comparative 1‐year follow‐up study using colour flow duplex. Eur J Vasc Surg. 1992;6:164–171. [DOI] [PubMed] [Google Scholar]
- 26. Nakamura S, Conroy RM, Gordon IL, Deutsch LS, Maheswaran B, Antone CS, Tobis JM. A randomized trial of transcutaneous extraction atherectomy in femoral arteries: intravascular ultrasound observations. J Clin Ultrasound. 1995;23:461–471. [DOI] [PubMed] [Google Scholar]
- 27. Martinez‐Camblor P, Mackenzie T, Staiger DO, Goodney PP, O'Malley AJ. Adjusting for bias introduced by instrumental variable estimation in the Cox proportional hazards model. Biostatistics. 2019;20:80–96. [DOI] [PubMed] [Google Scholar]
- 28. Iwashyna TJ, Kennedy EH. Instrumental variable analyses. Exploiting natural randomness to understand causal mechanisms. Ann Am Thorac Soc. 2013;10:255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. AHRQ . Society for vascular surgery patient safety organization, llc. Federally‐Listed PSOs. 2018.
- 30. Cronenwett JL, Kraiss LW, Cambria RP. The society for vascular surgery vascular quality initiative. J Vasc Surg. 2012;55:1529–1537. [DOI] [PubMed] [Google Scholar]
- 31. Hoel AW, Faerber AE, Moore KO, Ramkumar N, Brooke BS, Scali ST, Sedrakyan A, Goodney PP. A pilot study for long‐term outcome assessment after aortic aneurysm repair using vascular quality initiative data matched to Medicare claims. J Vasc Surg. 2017;66:751–759.e751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lousdal ML. An introduction to instrumental variable assumptions, validation and estimation. Emerg Themes Epidemiol. 2018;15:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hernan MA, Robins JM. Causal Inference. CRC: Boca Raton, FL; 2018. [Google Scholar]
- 34. Martens EP, Pestman WR, de Boer A, Belitser SV, Klungel OH. Instrumental variables: application and limitations. Epidemiology. 2006;17:260–267. [DOI] [PubMed] [Google Scholar]
- 35. Staiger D, Stock JH. Instrumental variables regression with weak instruments. Econometrica. 1997;65:557–586. [Google Scholar]
- 36. Siracuse JJ, Menard MT, Eslami MH, Kalish JA, Robinson WP, Eberhardt RT, Hamburg NM, Farber A. Comparison of open and endovascular treatment of patients with critical limb ischemia in the vascular quality initiative. J Vasc Surg. 2016;63:958–965.e951. [DOI] [PubMed] [Google Scholar]
- 37. Goodney PP, Beck AW, Nagle J, Welch HG, Zwolak RM. National trends in lower extremity bypass surgery, endovascular interventions, and major amputations. J Vasc Surg. 2009;50:54–60. [DOI] [PubMed] [Google Scholar]
- 38. Thomas MP, Jung Park Y, Grey S, Schreiber TL, Gurm HS, Leffler D, Davis TP, Henke P, Michael Grossman P. Temporal trends in peripheral arterial interventions: observations from the blue cross blue shield of Michigan cardiovascular consortium (BMC2 PVI). Catheter Cardiovasc Interv. 2017;89:728–734. [DOI] [PubMed] [Google Scholar]
- 39. O'Brien‐Irr MS, Harris LM, Dosluoglu HH, Dayton M, Dryjski ML. Lower extremity endovascular interventions: can we improve cost‐efficiency? J Vasc Surg. 2008;47:982–987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Two‐Stage Instrumental Variable Methodology.
Data S2. Instrumental Variable Assumption Assessment.
Table S1. Current Procedural Terminology (CPT) Codes Used to Identify Outcomes in Medicare Claims


