Introduction
Very early in the 20th century, Elie Metchnikoff, a Nobel Laureate and pioneer in immunology, hypothesized that bacteria residing in the colon were instrumental in initiating many diseases, including neurodegenerative diseases associated with aging. Metchnikoff believed some bacteria were harmful while other bacteria were beneficial and that longevity could be enhanced and senility delayed by ingesting beneficial bacteria (now known as probiotics), daily exercise, mental stimulation, and avoidance of alcohol. For much of the last century Metchnikoff’s ideas linking gut bacteria to host health and disease sat relatively idle. Although many of his hypotheses have required modification, his ideas have now come full circle with recent findings that gut bacteria and gut health have major implications in diseases affecting the entire body. This resurgence of interest was sparked by new sequencing technologies that have allowed rapid identification of bacteria and other microorganisms in our body to the genus or species level. Our goal in this review is to summarize the recent literature as it pertains to interaction of bacteria in the gut and host health, with an emphasis on stroke and stroke recovery.
The majority of bacteria, a major group of the microbiota residing in or on our bodies, are contained within the gastrointestinal (GI) tract. Under normal physiological conditions, the bacteria are instrumental in host immune development and control, act as a source for nutrients, and maintain metabolic homeostasis. Shifts in the makeup of these microbes, termed dysbiosis, can have significant pathological consequences for the host and has been linked to the development of numerous disease states.1–7 In this review, the term dysbiosis is reserved for shifts in the microbiota that are associated with a pathological state.
Microbiota-Gut-Brain Axis
Investigation of the extent and mechanisms of interaction between the gut microbiota and non-GI organs remains in its infancy. However, evidence suggests that bidirectional communication exists between the gut and its microbiota, and the brain. This bidirectional communication is referred to as the microbiota-gut-brain axis.
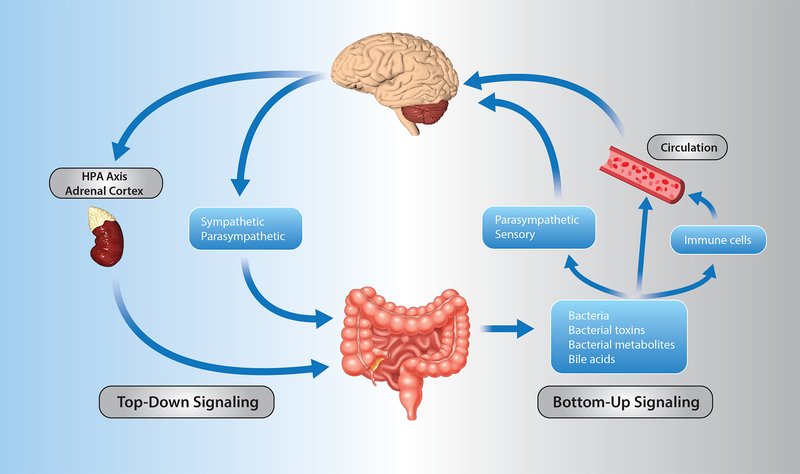
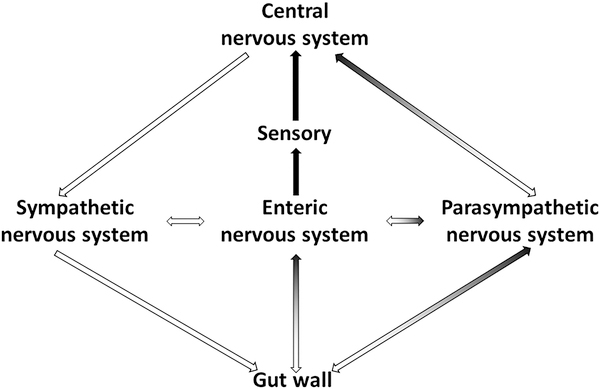
Signaling between the brain and the gut occur through both neuronal and non-neuronal mechanisms (Figures 1 and 2). For top-down signaling (brain → gut) the gut wall receives direct communication via parasympathetic and sympathetic nerve fibers, or indirectly following stimulation of the enteric nervous system, a highly developed system of neuronal connections located in the submucosa and myenteric plexi of the gut wall (Figure 2). These neuronal inputs influence gut motility, gut permeability, microbiota makeup, and resident immune cell activation. In addition to direct neural input, the hypothalamic-pituitary-adrenal axis is particularly important as a communication pathway in the response to stress (Figure 1).
Figure 1. Routes of bidirectional communication along the microbiota-gut-brain axis.
Left (blue) represents pathways for brain to gut signaling. Right (grey) represents pathways for gut to brain signaling.
Figure 2. Neural pathways of communication between brain and gut.
Black arrows represent afferent signals from gut to brain, white arrows represent efferent signals from brain to gut.
Bottom-up signaling (gut → brain) is thought to occur through a number of different mechanisms. First, the vagus nerve, composed of 80% afferent and 20% efferent fibers, serves a dual role in conveying signals between the gut and brain (Figure 2). These afferent fibers can be stimulated by microbial compounds and metabolites as well as hormones (e.g. serotonin, cholecystokinin, glucagon-like peptide-1, peptide YY) released from enteroendocrine cells of the gut epithelial layer to initiate bottom-up signaling (Figure 1). Stimulation of these afferent projections signals throughout the brain, including hypothalamic neurons that regulate pituitary secretions and the nucleus tractus solaritarius with its downstream projections. Second, immunogenic endotoxins from the microbiota, such as LPS, can induce neuroinflammation either directly or via activation of peripheral immune cells that can then migrate to the brain (Figure 1).6,7 Third, the microbiota generate or stimulate release of a number of metabolites such as neurotransmitters, short-chain fatty acids (SCFA, see paragraph below), indoles, and bile acids that are thought to enter the systemic blood and travel to the brain to modulate the function of neurons, microglia, astrocytes, and the blood brain barrier (Figure 1).8–10 Not only do microbiota produce neurotransmitters that are capable of influencing the host, but several studies have reported binding sites for neurotransmitters on bacteria that influence bacterial metabolism, proliferation, and virulence.
Short chain fatty acids (SCFA) such as acetate, butyrate and propionate, are major end products of bacterial fermentation of resistant starch and fiber. These SCFAs not only serve as an energy source for the host, but also have vasoactive properties. Additionally, SCFAs influence host cells through a variety of mechanisms including, altering histone acetylation and cell proliferation, and activation of G-protein coupled receptors. Loss of SCFA producing bacteria has been described in several cardiovascular and metabolic disease models including stroke, hypertension, obesity, and diabetes.1,3,4 SCFAs in relation to stroke and other disease states will be further discussed throughout this review.
Gut Dysbiosis Influences Stroke Risk Factors
Studies examining the role of the microbiota with the onset of stroke as the final outcome are limited. However, the gut microbiota is directly linked to a number of conditions that are themselves risk factors for stroke. This list includes hypertension, diabetes, atherosclerosis, aging, vascular dysfunction and obesity. It follows logically that if the gut microbiota is involved with the onset of these risk factors, then gut dysbiosis should indirectly increase stroke risk.
Dysbiosis and Hypertension
Hypertension in both animal models and humans correlate with gut dysbiosis as indicated by decreased diversity of the gut microbiota and/or increased ratio of the bacterial phyla, Firmicutes to Bacteroidetes.1–3,5 The gut microbiota in hypertensive and pre-hypertensive patients were reportedly similar but differed from that in normotensive subjects. The dysbiosis and metabolite profiles in pre-hypertensive patients suggest that dysbiosis precedes hypertension, rather than being a consequence of hypertension.
Both spontaneously hypertensive rats, a genetic model of hypertension, and rats rendered hypertensive by angiotensin II (AngII) infusion exhibited dysbiosis when compared to corresponding normotensive rats.3 Furthermore, AngII does not elicit hypertension in germ-free mice, which are born and raised under sterile conditions and lack any microbiota. This observation reinforces the idea that microbiota are required for the development of hypertension.
The strongest evidence to date for an underlying role of the gut microbiota in the development of hypertension involves studies using fecal microbiota transplantation (FMT). When normotensive Wistar Kyoto (WKY) rats were transplanted with the microbiota of a sub-strain of spontaneously hypertensive rats, systolic blood pressure significantly increased by 17mmHg compared to WKY rats transplanted with WKY microbiota.2 Thus, something in the transplant, presumably bacteria or bacterial products, was responsible for the induction of hypertension.
Similarly, a causal relationship exists between the gut microbiota and hypertension in a rat model of obstructive sleep apnea (OSA)-induced hypertension. Microbiota transplanted from hypertensive OSA donor rats into normotensive recipients, produced hypertension that otherwise would not have occurred; while microbiota transplants from normotensive sham rats (i.e., OSA device implanted but not activated) had no effect on blood pressure.1 In additional studies, OSA-induced hypertension was shown to be accompanied by significant decreases in cecal acetate, an important SCFA signaling molecule.5 Hypertension was prevented by restoring cecal acetate concentrations through oral prebiotics or probiotics, or direct infusion of acetate into the cecum.5
Dysbiosis, Obesity and Diabetes
Mounting evidence strongly suggests a cause and effect relationship between gut dysbiosis and metabolic diseases. For example, germ-free mice, which lack gut microbiota, exhibited reduced adiposity, improved glucose homeostasis, and were protected against high fat diet-induced glucose intolerance compared to conventionally raised mice. Transplanting gut microbiota from obese mice or humans into germ-free mice induced an obese phenotype.11 Finally, transplanting the gut microbiota from lean human donors into patients with metabolic syndrome improved insulin sensitivity. While these studies suggest a connection between the microbiota and obesity and diabetes, defining the underlying mechanisms has proven difficult.
Obesity and diabetes are multi-faceted metabolic disorders, and interestingly the microbiota has been shown to influence several pathways implicated in development of these disease states. The gut microbiota plays a key role in stimulating the release of gut hormones involved in satiety signaling, thermogenesis, and energy balance. Bile acids, which are regulated and modified by the gut microbiota, have been shown to influence appetite and energy expenditure. Finally, the gut microbiota strongly influences host immune cell maturation and activation. Obesity and diabetes are accompanied by increased plasma LPS, an endotoxin from the outer membrane of gram-negative bacteria. Reducing the bacterial sources responsible for the LPS with antibiotics not only decreased plasma LPS, but also decreased body weight gain, fat mass, inflammation of adipose tissue, and improved glucose tolerance and insulin sensitivity in obese mice. In summary, there is strong evidence that gut bacteria are directly involved with metabolic disorders.
Dysbiosis and Vascular Dysfunction
Gut microbes can produce metabolites that influence the host cardiovascular system in either a beneficial or detrimental manner. As with hypertension and diabetes, gut microbiota can contribute to vascular dysfunction that, in turn, increases the risk for stroke and exacerbates severity and outcome once a stroke has occurred. Examples of bacterial metabolites that have effects on the cardiovascular system include SCFAs, nitrites, flavanol metabolites, tri-methyl amine N-oxide (TMAO), indoles, and H2S. Certain microbial metabolites such as SCFAs and H2S have been shown to have vasorelaxant properties, and bacterial species producing these compounds have been shown to be decreased in models of hypertension.1–3,5 The microbial metabolites, indole sulfate and TMAO, increase endothelial ROS production and impair endothelial mediated vasodilation. In the case of TMAO, a cause and effect relationship linking this microbial metabolite to atherosclerosis has been well established.
The gut microbiota may serve as a novel therapeutic target for the treatment of vascular dysfunction and associated pathologies. Selective probiotics prevented or restored endothelial dysfunction in rats, through downregulation of vascular NOX2 and improved eNOS coupling.
Dysbiosis and Aging
The GI tract and resident microbiota are susceptible to the progressive functional decline associated with aging. With aging there is a breakdown of the gut epithelial barrier, loss of enteric neurons, and altered mucosal immune function resulting in excessive production of proinflammatory cytokines. Concomitant with changes in GI physiology, aging is also associated with significant shifts in the makeup of the gut microbiota including decreased microbial richness and diversity and a decrease in bacteria with anti-inflammatory properties. These changes likely have a major role in the persistent low levels of inflammation, often referred to as “inflammaging”. This state of “inflammaging” decreases the ability of older individuals to cope with antigenic, toxic, physical and ischemic stress.
Microbiota transplant studies have been useful in demonstrating the detrimental and proinflammatory state of the aged microbiota. Germ-free mice receiving microbiota from aged mice (17 months) exhibited significant increases in pro-inflammatory gene expression in the ileum, increased TH1 and TH2 cells in spleen, and increased bacterial products in the systemic circulation when compared to germ-free mice transplanted with microbiota from young mice (7–10 weeks). Exchanging the gut microbiota between aged (~ 20 months) and young (~ 3 months) mice demonstrated that mice receiving an aged microbiota, regardless of chronological age, exhibited impaired motor strength and cognitive function when compared to mice receiving a young microbiota.4 Understanding of the role of dysbiosis with aging brings with it hope that altering the gut microbiota may prove effective in attenuating inflammaging and the associated etiologies. This becomes even more significant when considering that stroke is a disease that primarily affects the elderly.
Gut Dysbiosis and Stroke
As discussed above, communication along the microbiota-gut-brain axis is bidirectional. Accordingly, mounting evidence demonstrates that stroke alters the gut microbiota in top down signaling and the makeup of gut microbiota can influence stroke outcome in bottoms up signaling (Table 1). Thus, stroke may produce a feed-forward cycle for secondary damage to the brain.
Table 1:
Studies Examining Interactions between the Gut Microbiota and Stroke
| Study | Species | Key Findings |
|---|---|---|
| Spychala et al 2018 4 | Mice | • Young microbiota improved stroke recovery when transplanted into aged mice |
| Singh et al 2016 6 | Mice | • Stroke induces gut paralysis, barrier disruption, and dysbiosis |
| • Presence of a dysbiotic microbiota at time of stroke led to larger infarct and impaired recovery | ||
| • T cells, Th cells, and monocytes migrate from Peyer’s patches to peri-infarct brain region following stroke | ||
| Benakis et al 2016 7 | Mice | • Microbiota contributes to activation of IL-17 producing xδ T-cells after stroke |
| • Following stroke γδ T-cells migrate from gut to the brain meninges | ||
| Yamashiro et al 2017 12 | Human | • Stroke altered gut microbiota and decreased fecal acetic acid concentrations |
| Yin et al 2015 13 | Human | • Stroke and transient ischemic attack altered the gut microbiota, including increased abundance of pathogens and decreased beneficial commensals |
| Stanley et al 2018 14 | Mice | • Stroke altered the mucosa associated microbiota along the length of the intestine |
| Stanley et al 2016 15 | Human & Mice | • Bacteria originating from the small intestine contribute to post-stroke infection |
| Houlden et al 2016 16 | Mice | • Stroke induces cecal dysbiosis, reduced mucus production, and loss of goblet cells |
| • Increased sympathetic activity in gut wall following stroke | ||
| Crapser et al 2016 17 | Mice | • Stroke increased gut permeability and bacterial translocation in young and aged mice |
| Winek et al 2016 18 | Mice | • Antibiotic treatment prior to stroke did not affect infarct volume |
| • Stopping antibiotics three days prior to stroke significantly decreased survival | ||
| Singh et al 2018 19 | Mice | • GF mice have larger infarct compared to recolonized or specific pathogen free mice |
| • Gut bacterial load is important in stroke outcome |
Effects of stroke on the gut and gut microbiota
Although limited in number, existing studies in humans indicate that stroke alters the gut microbiota. Yamashiro et al analyzed the fecal gut microbiota and fecal organic acids in a Japanese cohort of stroke and control subjects. Ischemic stroke altered the abundance of several genera and species independent of age, type 2 diabetes, and hypertension.12 Increased abundance of Lactobacillus ruminis positively correlated with markers of inflammation in stroke patients. These microbiota changes were accompanied by a 13% decrease in total organic acids with the SCFAs, valeric and acetic acids, increasing and decreasing by 54% and 18% respectively in the stroke patients. Given that SCFAs are products of bacterial fermentation, it is presumed that the changes in SCFAs were a result of the altered microbiota after stroke. However, it is also possible that dietary changes in stroke patients following hospital admission may have contributed to the observed microbiota and metabolite differences.
In another study, patients with large-artery atherosclerotic stroke or transient ischemic attacks (TIA) were compared to asymptomatic controls with and without carotid atherosclerotic plaques.13 In asymptomatic controls, the gut microbiota was similar regardless of the presence or absence of carotid plaques. The makeup of the stroke/TIA gut microbiota exhibited increased relative abundance of several opportunistic pathogens and decreased abundance of commensal or beneficial genera; further demonstrating the adverse effects of stroke on the makeup of the gut microbiota.
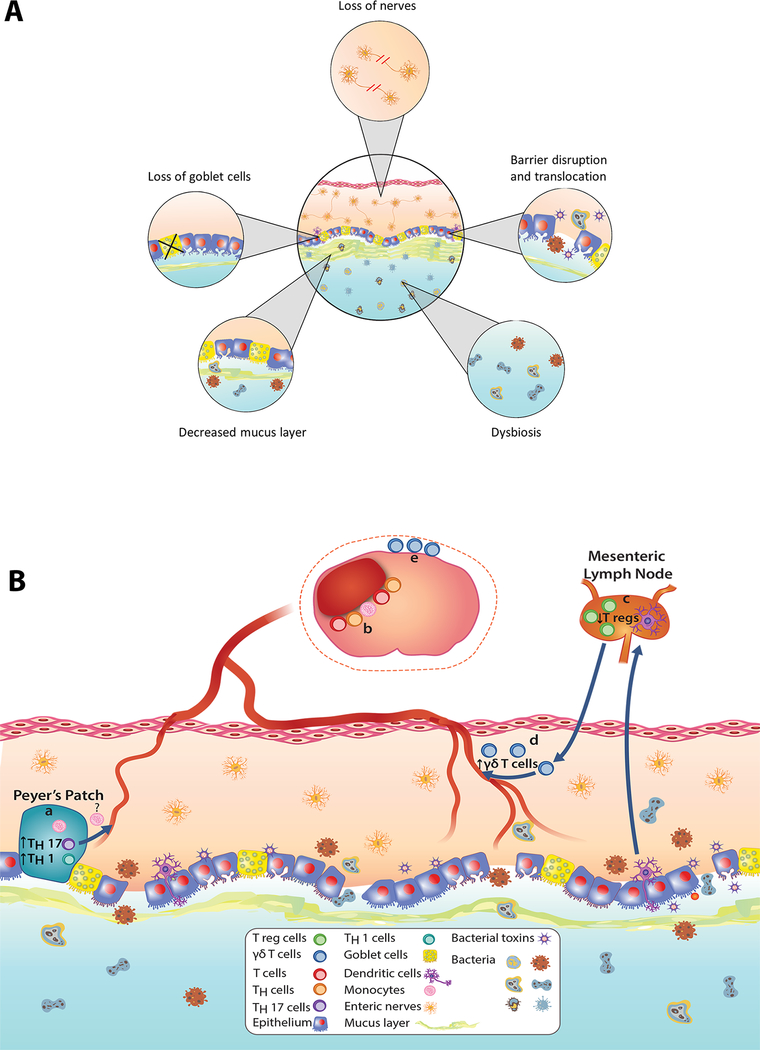
Studies involving animal models of stroke have provided further evidence of stroke-induced gut dysbiosis and helped identify the mechanisms through which stroke affects the gut and gut microbiota. Creating a large hemispheric lesion by occluding blood flow at the base of the middle cerebral (i.e., proximal) artery for 60 min produced gut dysbiosis, intestinal paralysis, increased gut permeability, a loss of cholinergic innervation in ileum, and increased sympathetic activity (Figure 3A).6,14,15 This loss of cholinergic signaling in favor of adrenergic signaling is known to promote inflammation in the gut. Increased adrenergic stimulation of the gut following proximal MCAO was also associated with reduced number of goblet cells in the cecum, and impaired production of mucin a major glycoprotein in GI mucus.16 The mucus layer, which acts as a protective barrier between the epithelium and gut lumen, is residence to bacteria that aid in communications between the host and the luminal microbiota. Following proximal MCAO, gut permeability and translocation of bacteria increased in both young and aged mice (Figure 3A).17 In addition, bacterial members that normally reside in the small intestine have been shown to colonize the lung post-stroke. In fact, 24 hours after proximal MCAO >60% of the lung microbiota community was predicted to have originated from the small intestine.15,20 However, increased bacterial translocation following stroke has not been observed in all studies.6 Overall, stroke-induced gut dysbiosis seems to initiate a cascade of events that lead to post-stroke infections, a major cause of prolonged hospitalizations and death following stroke.
Figure 3. Effects of stroke on the microbiota-gut-brain axis.
A) Alterations to the gut and microbiota following stroke includes loss of enteric nerves, epithelial barrier breakdown, translocation of bacteria and bacterial toxins, loss of goblet cells, thinning of the mucus barrier, and gut dysbiosis. B) Two proposed mechanisms of gut immune cell activation and tracking to brain following stroke. (a) Following stroke, a pro-inflammatory microbiota leads to increased TH1 cells, TH17 cells and monocytes in Peyer’s Patches in the small intestine. (b) T cells, TH cells and monocytes migrate from Peyer’s Patches to the peri-infarct region. (c) In the presence of a pro-inflammatory microbiota, dendritic cells migrate to mesenteric lymph nodes to inhibit polarization of T cells into anti-inflammatory T reg cells. (d) In turn, decreased T reg cells migrating to the lamina propria favors γδ T cell differentiation. (e) Pro-inflammatory γδ T cells originating from the lamina propria of the small intestine migrate to the meninges and increase infarct size.
The makeup of the gut microbiota influences stroke outcome
Emerging literature demonstrates that the state of the gut microbiota has important effects on the development, severity, and persistence of a number of pathological conditions affecting the brain to include anxiety and depression, Parkinson’s disease, multiple sclerosis, Alzheimer’s disease, traumatic brain injury, and autism spectrum disorder.
Studies to date determining if the gut microbiota can influence stroke outcome have been conducted only in animal models. The preponderance of these studies has concluded that the gut microbiota can be manipulated in a manner to either improve or worsen stroke outcomes. In general, a gut microbiota that stabilizes the gut wall and maintains inflammation at bay is protective. On the other hand, conditions that produce inflammation in the gut tend to exacerbate injury and prolong or impair recovery.
Benakis et al demonstrated that an anti-inflammatory gut microbiome induced by antibiotics reduced infarct volume by 60% and better preserved sensory motor function for at least 1 week following proximal MCAO.7 In describing this study, we will refer to mice as having a proinflammatory microbiota or an anti-inflammatory microbiota. The anti-inflammatory microbiome utilized antibiotics to decrease species abundance and diversity and the pro-inflammatory microbiome was developed to be antibiotic resistant.
FMT from anti-inflammatory donors into naïve mice reduced the infarct volume by 54% in the recipient mice following MCAO.7 We point out to the reader that the term “dysbiosis” is used in different ways in the literature. Benakis et al used the term, “dysbiosis”, to mean simply a change in the gut microbiota. However, many investigators reserve the term, “dysbiosis”, only for those changes in the gut microbiota that are accompanied by a pathological change in the host. Thus, in the case of Benakis et al dysbiosis is protective while in other studies dysbiosis is detrimental by definition.
Singh et al also reported that the state of the gut microbiota can affect outcomes following stroke in mice. Germ-free mice received a microbiota transfer from either sham mice or mice after proximal MCAO. The proximal MCAO model used for these donor mice produced gut dysbiosis (see previous section “Top Down Signaling”).6 Metagenomics analysis of feces revealed that microbiota of the recipient mice resembled that of the donor mice. Following the microbiota transfers, recipient mice underwent permanent occlusion of the distal middle cerebral artery, a model that produced smaller cortical lesions and no dysbiosis when compared to transient occlusion at the origin of the MCA. Mice receiving microbiota transplant (3 days before distal MCAO) from mice that had been previously subjected to proximal transient MCAO had larger infarct volumes and showed enhanced functional impairment when compared to mice receiving transplants from sham mice.6 In addition, FMT from control mice, initiated the day of proximal MCAO, attenuated the gut dysbiosis associated with proximal MCAO and significantly decreased infarct volume.6
Winek et al examined lesion volumes following proximal MCAO in groups of mice with different dosing schedules of a quintuple antibiotic cocktail in the drinking water. Lesion volumes in all antibiotic groups were similar to the control group not receiving antibiotics.18 Of note, the lesion volumes were measured on day 1 after MCAO, a time when secondary injury from ongoing neuroinflammation may not be complete. The authors noted that severe colitis and increased mortality was observed in antibiotic-treated mice when the treatment was stopped prior to MCAO. The authors suggest that the increased mortality was the result of invading pathogens when antibiotics were discontinued. In humans, antibiotic treatment at the onset of stroke reduced the incidence of post-stroke infections but did not significantly decrease the rate of post-stroke pneumonia, change mortality, or alter functional outcomes in stroke patients. It is worth pointing out that different antibiotics and administration protocols have vastly different effects on the gut microbiota, likely contributing to differences among studies as well as complicating our understanding of the role of the microbiota in stroke recovery.
Spychala et al exchanged gut microbial communities between young (~3 months) and aged mice (~20 months). Aging alone is known to produce gut dysbiosis.4 Having a young microbiota, regardless of mouse chronological age, decreased mortality, enhanced locomotor function and anxiety, and increased motor strength during the course of recovery from proximal MCAO.4 It is well established that MCAO lesion volumes of naïve aged mice (i.e., no FMT) are smaller than lesion volumes in young naïve mice. Despite having smaller lesion volumes, recovery from stroke is significantly impaired in the aged. Regardless of having a young or aged microbiota, the lesion volumes in young mice were similar and remained larger than in aged mice. Conversely, the lesion volumes in aged mice were similar regardless of the microbiota and remained smaller than that in the young mice.4 In a follow-up to this study, Lee et al demonstrated the same beneficial effect of a young microbiota on recovery occurs even when the FMTs were initiated 72 hours following proximal MCAO.21
Interestingly, the microbiota appears to have both a protective and a detrimental component. The infarct volume following distal MCAO was 50% and 300% greater in germ-free than in recolonized germ-free and specific pathogen free mice respectively.19 After examining microglia, peripheral and brain-infiltrating T cells in each of the groups, the authors concluded there is a “… lymphocyte-driven protective neuroinflammation after stroke under control of the microbiota”.19 The presence of bacteria, perhaps selected species, during maturation of the inflammatory system is required for a fully functional immune system including that of resident immune cells in brain.8 Since microbiota influence neuronal development myelination, it is not necessarily surprising that SPF mice have a smaller lesion volume than germ-free mice.19
Overall, conclusions from the literature support a role for the gut microbiota in influencing outcome after stroke. Depending on how the gut microbiota is manipulated, the outcome following stroke can be either harmful or protective. These studies in laboratory animals bring hope for altering the microbiota to limit the damage and hasten recovery from stroke in humans.
Mechanism of Bottom-Up Signaling
Bottom-up signaling following stroke is poorly understood. The only pathway implicated at this time consists of activation of immune cells in the gut which subsequently track to the brain by way of the circulation (Figure 1 and 3B). We do emphasize that other pathways for bottom-up signaling after stroke have not been ruled out, leaving open the possibility that multiple mechanisms are at play.
Benakis et al. provided evidence that the polarization of naïve T cells in the gut is dependent on the state of the gut microbiome.7 Mice with an anti-inflammatory microbiome showed more favorable outcomes after proximal MCAO when compared to mice with a pro-inflammatory microbiome (see above section for a description of the pro- and anti- inflammatory groups). The anti-inflammatory microbiome coax the polarization of naïve T cells in the lamina propria, a layer of cells on the serosal side of the of the gut epithelium, towards anti-inflammatory Tregs (Figure 3B). This polarization to Tregs involves dendritic cells, antigen-presenting cells in the gut wall, and the mesenteric lymph node.7 After stroke in mice with a pro-inflammatory microbiome, the polarization of naïve T cells to Tregs was impeded favoring the polarization towards pro-inflammatory IL-17+ γδ T cells. Once polarized in the lamina propria, the proinflammatory IL-17+ γδ T cells track primarily to the meninges in brain where they release IL-17. The IL-17 is proposed to increase chemokine production in the brain parenchyma attracting leukocytes (neutrophils being most prominent) and increase infarct size. (Figure 3B).7
Whereas the study by Benakis et al implicated activation of IL-17 producing γδ T cells in the lamina propria, Singh et al implicated activation of Th1 and Th17 cells possibly from Peyer’s patches of the small intestine (Figure 3B).6 Singh et al gavaged cecal contents, obtained from dysbiotic mice following proximal MCAO or from sham mice, into germ-free mice. These previously germ-free mice were subjected to distal MCAO, a milder form of stroke than the proximal MCAO. In mice receiving dysbiotic post-stroke cecal contents, behavioral performance was impaired, lesion volumes doubled, and mRNA of IFN-γ and IL-17 (markers of Th1 and Th17 T cells respectively) in brain were “massively” increased compared to mice receiving FMT from sham mice.6 In Peyer’s patches from the gut, proinflammatory Th1 (IFN-γ+) and Th17 (IL-17+) cells increased 3–4 fold in mice receiving the dysbiotic post-stroke cecal contents. In a separate experiment, the authors demonstrated migration of T cells, Th cells, and monocytes from the Peyer’s patches to the ischemic hemisphere of the brain (Figure 3B).6 Consistent with an important role for lymphocytes, Rag1−/− mice, which lack functional lymphocytes, showed similar lesion sizes regardless of the state of dysbiosis in the gut.6,19
Taken together these studies demonstrate that the composition of the gut microbiota plays a key role in stroke outcomes. Therefore, manipulating the gut microbiota may offer a promising approach in improving stroke therapy.
Blood-Brain Barrier
After stroke, as in other neurodegenerative diseases, the integrity of the blood-brain barrier (BBB) is an important consideration for brain protection. The major components of the BBB consist of cerebrovascular endothelial cells, astrocytes, and pericytes. A number of tight junction proteins between endothelial cells prevent the paracellular diffusion of various molecules in the blood to the brain and its extracellular fluid. The gut microbiota has a crucial role in regulating BBB integrity.22 Braniste et al showed that germ-free mice displayed increased BBB permeability when compared to SPF mice. The increased permeability was associated with decreased expression of tight junction proteins, occludin and claudin-5. Transplantation of germ-free mice with the microbiota of SPF mice decreased BBB permeability and increased the expression of tight junction proteins.22 Given that SCFAs help to maintain integrity of the gut epithelial barrier through tight junctions, the authors reasoned that the same could be true for the BBB. Gavage of Clostridium butyricum, a butyrate producer, Bacteroides thetaiotaomicron, an acetate and propionate producer, or sodium butyrate into adult germ-free mice enhanced BBB integrity.22 While this study demonstrated that SCFAs could decrease BBB permeability in germ-free mice, it is not known if a decrease in SCFAs once the BBB is matured is disruptive to the BBB. This role of SCFAs is notable since the microbiota of aged mice is associated with decreased fecal acetate and propionate concentrations.4
Sun et al demonstrated that Clostridium butyricum treatment pre-stroke resulted in improved neurological deficit scores and reduced hippocampal apoptosis following bilateral common carotid artery occlusion.9 The protective effects of C. butyricum were attributed to increased butyrate and reduced oxidative stress in brain.9 In a transient focal ischemia model (proximal MCAO) sodium butyrate attenuated BBB permeability and decreased the activity of matrix metalloproteinase 9.23 It is still uncertain if SCFA protection in stroke is altering the gut barrier, the BBB, both, or through a yet unidentified mechanism.
Summary and Conclusions
Over the past decade, it has become apparent that the gut microbiota has become very relevant to the develpoment and progression of a number of neurological diseases including autism spectrum disorder, anxiety, depression, Alzheimer’s disease, Parkinson’s disease, vascular cognitive impairment, experimental autoimmune encephalomyelitis (EAE) mouse model of multiple sclerosis (MS), and stroke. The microbiota-gut-brain axis, a relatively generalized phrase describing a bi-directional communication system between the gut and the brain, represents the focal point for the gut and brain interactions (Figure 1). While we have gained some knowledge of this axis, we do not fully understand the communication mechanisms involved.
We have reviewed the gut microbiota and the microbiota-gut-brain axis as it relates to stroke. It is important to note that dysbiosis is a potentially modifiable risk factors for hypertension, vascular dysfunction, diabetes, and obesity. It therefore stands to reason that dysbiosis must have at least some underlying relationship to the onset of stroke through initiation and progression of these stroke risk factors. Aging is another important risk factor for stroke; unfortunately, we are not currently able to prevent the majority of the adverse effects of aging. However, it is reasonable to view age related gut dysbiosis as being modifiable through a combination of diet, probiotics, prebiotics, and/or other healthy lifestyles (i.e., exercise). Note that these behaviors, for all practicle purposes, are lifestyle changes recommended by Elie Metchnikoff over 100 years ago. Maintaining a youthful or healthy gut microbiota in aging could reduce the incidence of stroke through reduction of the risk factors mentioned above. Furthermore, a youthful microbiota appears to be very beneficial during the recovery from stroke as noted in our previous work. Not only should a youthful microbiota have profound benefits involving stroke but it should also impede or reduce the inflammaging process in general. These tentative conclusions rely solely on studies conducted in laboratory animals. As a scientific community, we have learned that conclusions derived from animal studies do not necessarily translate to the human disease state. Several hurdles must be overcome in order to translate these findings in laboratory animals to humans. We must fully understand the microbiota community in the gut. Furthermore, dysbiosis or an unheathly shift in the gut microbiota is likely not an alteration in one or a few bacterial species. Rather, the dysbiotic gut must be viewed as a ecological community acting together to affect the host in a beneficial or detrimental manner. Considering that the human gut microbiota consists of over 1,000 species of bacteria (not to mention other types of microbes present), dysbiosis producing the same pathological effects could be accomplished by many different shifts in the microbiota community. A good example of this idea are the bacterially-derived short chain fatty acids (SCFAs), generally considered beneficial to the host. Since many bacterial species have the capacity to generate SCFAs, loss of one or a few of these species may have little to no effect on the concentration and types of SCFAs in the gut. It might take the loss of a critical number of these SCFA-producing bacteria before pathological decreases in SCFAs occur. Alternatively, the generation and concentraions of individual SCFAs might not be the responsibility of a single bacterial species but rather a function of the bacterial community as a whole where different bacterial species contribute to different steps in the synthetic pathway.
In order to effectively translate these provocative studies in animal models, to the human disease state, we must first fully understand the gut microbial community and how they interact with the host. Given that there is large inter-individual variations in the gut microbiota of humans, a need for personalized medicine becomes imperative. It could be envisioned that the gut microbiota can be effectively analyzed with the knowledge and ability to bring this dysbiotic state back to a healthy state. While this task may seem formidable, we as authors of this review fully believe that it is acheivable.
Supplementary Material
Acknowledgments
Grant Support: R01 HL134838 and 16SDG29970000 (DJD), R01 NS102594 (RMB), RO1 AG058463-01 (LDM, RMB), R01 NS103592 (RMB, LDM).
Footnotes
Disclosures: None
References
References most relevant to microbiota and stroke have been included. Additional references can be found in the online Supplemental Material.
- (1).Durgan DJ, Ganesh BP, Cope JL, Ajami NJ, Phillips SC, Petrosino JF, et al. Role of the Gut Microbiome in Obstructive Sleep Apnea-Induced Hypertension. Hypertension. 2016;67:469–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM Jr, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Spychala MS, Venna VR, Jandzinski M, Doran SJ, Durgan DJ, Ganesh BP, et al. Age-related changes in the gut microbiota influence systemic inflammation and stroke outcome. Ann Neurol. 2018;84(1):23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Ganesh BP, Nelson JW, Eskew JR, Ganesan A, Ajami NJ, Petrosino JF, et al. Prebiotics, Probiotics, and Acetate Supplementation Prevent Hypertension in a Model of Obstructive Sleep Apnea. Hypertension. 2018;72:1141–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Singh V, Roth S, Llovera G, Sadler R, Garzetti D, Stecher B, et al. Microbiota Dysbiosis Controls the Neuroinflammatory Response after Stroke. J Neurosci. 2016;36:7428–7440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Benakis C, Brea D, Caballero S, Faraco G, Moore J, Murphy M, et al. Commensal microbiota affects ischemic stroke outcome by regulating intestinal gammadelta T cells. Nat Med. 2016;22:516–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).Erny D, Hrabe de Angelis AL, Jaitin D, Wieghofer P, Staszewski O, David E, et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat Neurosci. 2015;18:965–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (9).Sun J, Ling Z, Wang F, Chen W, Li H, Jin J, et al. Clostridium butyricum pretreatment attenuates cerebral ischemia/reperfusion injury in mice via anti-oxidation and anti-apoptosis. Neurosci Lett. 2016;613:30–35. [DOI] [PubMed] [Google Scholar]
- (10).Sun J, Wang F, Ling Z, Yu X, Chen W, Li H, et al. Clostridium butyricum attenuates cerebral ischemia/reperfusion injury in diabetic mice via modulation of gut microbiota. Brain Res. 2016;1642:180–188. [DOI] [PubMed] [Google Scholar]
- (11).Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. [DOI] [PubMed] [Google Scholar]
- (12).Yamashiro K, Tanaka R, Urabe T, Ueno Y, Yamashiro Y, Nomoto K, et al. Gut dysbiosis is associated with metabolism and systemic inflammation in patients with ischemic stroke. PLoS One. 2017;12:e0171521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Yin J, Liao SX, He Y, Wang S, Xia GH, Liu FT, et al. Dysbiosis of Gut Microbiota With Reduced Trimethylamine-N-Oxide Level in Patients With Large-Artery Atherosclerotic Stroke or Transient Ischemic Attack. J Am Heart Assoc. 2015;4:1. 10.1161/JAHA.115.002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Stanley D, Moore RJ, Wong CHY. An insight into intestinal mucosal microbiota disruption after stroke. Sci Rep. 2018;8:568–017-18904–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Stanley D, Mason LJ, Mackin KE, Srikhanta YN, Lyras D, Prakash MD, et al. Translocation and dissemination of commensal bacteria in post-stroke infection. Nat Med. 2016;22:1277–1284. [DOI] [PubMed] [Google Scholar]
- (16).Houlden A, Goldrick M, Brough D, Vizi ES, Lenart N, Martinecz B, et al. Brain injury induces specific changes in the caecal microbiota of mice via altered autonomic activity and mucoprotein production. Brain Behav Immun. 2016;57:10–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Crapser J, Ritzel R, Verma R, Venna VR, Liu F, Chauhan A, et al. Ischemic stroke induces gut permeability and enhances bacterial translocation leading to sepsis in aged mice. Aging (Albany NY). 2016;8:1049–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Winek K, Engel O, Koduah P, Heimesaat MM, Fischer A, Bereswill S, et al. Depletion of Cultivatable Gut Microbiota by Broad-Spectrum Antibiotic Pretreatment Worsens Outcome After Murine Stroke. Stroke. 2016;47:1354–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Singh V, Sadler R, Heindl S, Llovera G, Roth S, Benakis C, et al. The gut microbiome primes a cerebroprotective immune response after stroke. J Cereb Blood Flow Metab. 2018:0271678X18780130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Wen SW, Wong CHY. An unexplored brain-gut microbiota axis in stroke. Gut Microbes. 2017;8:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Lee J, Ganesh B, Spychala M, Putluri N, Ajami N, Durgan D, et al. Short chain fatty acids mediate the beneficial effects of young microbiome on recovery in aged mice after ischemic stroke. Stroke. 2019;49. [Google Scholar]
- (22).Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Toth M, et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med. 2014;6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Wang Z, Leng Y, Tsai LK, Leeds P, Chuang DM. Valproic acid attenuates blood-brain barrier disruption in a rat model of transient focal cerebral ischemia: the roles of HDAC and MMP-9 inhibition. J Cereb Blood Flow Metab. 2011;31:52–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.