Abstract
Gangliosidoses, including GM1-gangliosidosis and GM2-gangliosidosis (Tay-Sachs disease and Sandhoff disease), are lysosomal disorders resulting from enzyme deficiencies and accumulation of gangliosides. Phenotypes of gangliosidoses range from infantile, late-infantile, juvenile, and to the adult form. The genotype-phenotype correlation is essential for prognosis and clinical care planning for patients with a gangliosidosis condition. Previously, we have developed a method to establish the genotype-phenotype correlation of another lysosomal disease, mucopolysaccharidosis type I, with in silico tools. This same method was applied to analyze the genotype and phenotype of 38 patients diagnosed with a gangliosidosis disease in the United States. Out of 40 mutations identified, 3 were novel, including p.Tyr192His and p.Phe556Ser of the GLB1 gene and p.Gly461Val of the HEXA gene. Furthermore, the mutant protein structure of all missense mutations was constructed by homology modeling. A systemic structural analysis of these models revealed the specific mechanisms of how each mutation may lead to the disease. In summary, the method developed in this study holds promise as a tool that can be broadly applicable to other lysosomal diseases and monogenic diseases.
Keywords: Genotype-phenotype correlation, In silico, Gangliosidosis, Disease subtype, Lysosomal disorder
1. Introduction
The gangliosidoses are inherited metabolic disorders resulting from the accumulation of gangliosides in the central nervous system, which leads to severe and progressive neurological impairment [1]. They are categorized into GM1-gangliosidosis and GM2-gangliosidosis, and both diseases are autosomal recessive. GM1-gangliosidosis (MIM #230500) is due to mutations in the GLB1 gene, leading to deficiency of lysosomal enzyme β-galactosidase and subsequent accumulation of GM1-gangliosides [1]. Notably, the mutations in the GLB1 gene can also lead to another lysosomal disease, mucopolysaccharidosis type IV B (MPS IVB, MIM # 253010), or Morquio syndrome type B. MPS IVB is mainly a skeletal disease due to the accumulation of keratan sulfate [2]. GM2-gangliosidoses, including Tay-Sachs disease (MIM #272800) and Sandhoff disease (MIM #268800), are due to mutations in the HEXA and HEXB genes encoding the α and β subunits, respectively, of lysosomal enzyme β-hexosaminidase A, resulting in accumulation of GM2 gangliosides [1].
Phenotypes of GM1- and GM2-gangliosidoses can be generally classified as infantile, late-infantile, juvenile, and adult. Patients with the infantile form exhibit symptoms during infancy, presenting with progressive neurological impairment and death in early childhood [3,4]. A late-infantile form has also been reported, in which patients exhibit symptoms between one and three years of age and may live into later childhood [4,5]. The onset of symptoms in the juvenile form is usually between three to five years of age, manifested as ataxia, dysarthria, hypotonia, and dysphagia [6,7]. The lifespan of the juvenile form ranges from late childhood to early adulthood [1]. In contrast, the adult (or late-onset) form exhibit symptoms in early or mid-adulthood. The symptoms include limb-girdle weakness, ataxia, neuromuscular weakness, and eventual loss of ability to ambulate independently. The symptoms include limb-girdle weakness, ataxia, neuromuscular weakness, and eventual loss of ability to ambulate independently [[8], [9], [10]]. In addition, difficulties with speech may develop. Patients may also develop psychiatric changes [4,6,7]. The lifespan of the adult form varies greatly [7]. There are no effective therapies for GM1- and GM2-gangliosidoses, with palliative measures being the current standard of care. There are many continuing efforts, however, to develop therapeutic protocols, which include establishment of novel animal models [11], enzyme replacement therapy [12], substrate reduction therapy [13], bone marrow transplantation [14], and gene therapy [15] in animal models.
At this time, understanding the genotype-phenotype correlation of gangliosidoses is critical in understanding the patient's prognosis and planning for different stages of palliative care. As the era of newborn screening emerges, the relationship between the genotype and phenotype will play a greater role in planning critical care, especially in light of increasing efforts towards developing effective treatments for patients with gangliosidoses. Newborn screening is being performed in an increasing number of lysosomal diseases. Newborn screening of gangliosidoses is not currently done but is anticipated to become routine as future therapies become available. One method of newborn screening in lysosomal disease is with assays of the defected enzyme. Another potential method is metabolomics profiling with reverse phase liquid chromatography (RPLC) [16]. However, it is still difficult to reliably predict phenotypes of the disease, such as late-infantile or juvenile versus adult phenotypes. Methods to identify the phenotypes of diseases are urgently lacking. Identification of the phenotypes is crucial because phenotypes with a worsened prognosis, such as infantile gangliosidoses, require more urgent and intensive interventions. Furthermore, the ability to predict phenotypes from genotypes would allow enrichment of disease subtypes in clinical trials, advancing the development of treatments.
In a previous study, we demonstrated a single amino acid mutation prediction (SAAMP) algorithm to predict whether a missense mutation is pathogenic or benign for MPS I disease [17]. This method integrates the prediction outcome of multiple bioinformatics tools and achieves a high sensitivity (94%) and specificity (80%). More recently, a SAAMP 2.0 algorithm, which has better sensitivity and specificity, has been developed. When assessed in a total of 13 lysosomal diseases, it yielded a further improved sensitivity (95%) and specificity (90%), which outperformed the mainstream bioinformatics tools evaluated [18]. In the study reported herein, the in silico method was application was expanded to analyze the genotype-phenotype correlation of patients with gangliosidoses. Additionally, 3D structural analysis was also conducted to elucidate the mechanisms of how each mutation can lead to the disease.
2. Methods and materials
2.1. Patients
This study was conducted under a clinical trial, The Natural History of Gangliosidoses (NCT00668187), of the Lysosomal Disease Network (U54NS0657698) which is part the National Institutes of Health (NIH) Rare Diseases Clinical Research Network (RDCRN). Patients participating in this study were enrolled in the natural history study. The study was conducted at the University of Minnesota with Institutional Review Board (IRB) approval and IRB-approved consent of the patients or patients' parents/legal guardians. The diagnosis of each patient was confirmed through genetic sequencing and biochemically by enzyme assays. The clinical course, and thus the phenotype, of each patient was documented through retrospective chart review and prospective clinical care.
2.2. Predicting functional impacts of missense mutations
A total of 7 bioinformatics tools were used as previously described [17]. These tools include: Sorting Intolerant From Tolerant (SIFT, http://sift.jcvi.org/) [19], Polymorphism Phenotyping (PolyPhen, http://genetics.bwh.harvard.edu/pph2/) [20], I-Mutant (http://gpcr2.biocomp.unibo.it/cgi/predictors/I-Mutant3.0/I-Mutant3.0.cgi) [21], PROtein Variation Effect ANalyzer (PROVEAN, http://provean.jcvi.org/index.php) [22], Protein ANalysis THrough Evolutionary Relationships (PANTHER, http://www.pantherdb.org/) [23], Single Nucleotide Polymorphism Database & Gene Ontology (SNPs&GO, http://snps.biofold.org/snps-and-go/snps-and-go.html) [24], Predictor of Human Deleterious Single Nucleotide Polymorphism (PHD-SNP, http://snps.biofold.org/phd-snp/phd-snp.html) [25].
Each bioinformatics tool predicts whether a single mutation is pathogenic or benign with an inherent index indicating the confidence of the prediction. SIFT focuses on predicting the effect of a single nucleotide polymorphism (SNP) through sequence preservation over the evolutionary time. PolyPhen utilizes a sequence and structure-based method to predict the possible impact of SNP. I-Mutant is a support vector machine (SVM) based predictor of protein stability changes introduced by a SNP. PROVEAN is a sequence-based predictor that estimates whether a SNP affects the protein function. SNPs&GO is a SVM based web server that combines protein structural/functional parameters and sequence analysis derived information. PHD-SNP is a SVM web server based on evolutionary information. PANTHER is a protein family and subfamily database that predicts the frequency of occurrence of an amino acid at a particular position in homologous sequences.
2.3. Homology modeling and 3D structural analysis
Iterative Threading ASSEmbly Refinement (I-TASSER, http://zhanglab.ccmb.med.umich.edu/I-TASSER/) was used to build the three-dimensional structure model with replica-exchanged Monte Carlo simulations [26]. The Swiss-PDB viewer was used to analyze the models generated by homology modeling. Additionally, Project Have yOur Protein Explained (HOPE; http://www.cmbi.ru.nl/hope/home) was used for analyzing the structural impacts of these mutations as previously described [17].
3. Results
3.1. Patients description
A total of 38 patients with infantile, late-infantile or juvenile gangliosidoses were enrolled and analyzed for correlations between genotypes and phenotypes. Patients with the infantile gangliosidoses are described in our previous paper [3]. Patients with the late-infantile or juvenile gangliosidoses are described in this paper (summarized in Table 1). Across both studies, there are patients with infantile GM1-gangliosidosis (n = 8), late-infantile GM1-gangliosidosis (n = 4), juvenile GM1-gangliosidosis (n = 6), infantile Tay-Sachs disease (n = 9), late-infantile Tay-Sachs disease (n = 1), juvenile Tay-Sachs disease (n = 3), infantile Sandhoff disease (n = 6), and juvenile Sandhoff disease (n = 1). Patients originated from different regions in the United States. Genotype-phenotype association was evaluated using a method previously reported [17,18], based on clinical and biochemical results of subjects enrolled in this study, as well as literature reports. There was one pair of siblings, and these patients had late-infantile GM1 (genotype p.Arg201Cys/p.Arg201Cys). A total of 40 mutations were identified, including 30 missense/nonsense mutations, 4 splicing mutations, and 6 insertions/deletions. A total of 3 novel mutations were identified in this study, which were the p.Tyr192His and p.Phe556Ser in the GLB1 gene, and p.Gly461Val in the HEXA gene.
Table 1.
Demographic information of late-infantile and juvenile GM1 and GM2 gangliosidoses patients. Abbreviations: F, female; M, male; mo, months old of age; MRI, magnetic resonance imaging; N/A, not available; yo, years old of age.
| Diagnosis | Gender | Race | Genotype | Age at clinical finding | Initial clinical finding leading to diagnosis | Age at diagnosis |
|---|---|---|---|---|---|---|
| Late-infantile GM1 | M | Caucasian | p.Arg201Cys/p.Lys578Arg | 1 yo | Abnormal gait noted around 1 year of age. Work -up at 2 years of age for muscular dystrophy | 22 mo |
| Late-infantile GM1 | M | French Canadian, Irish, Polish | c.75 + 2dupT/p.Phe556Ser | 1 yo | Difficulties with speech. Never learned to speak words | 21 mo |
| Late-infantile GM1 | M | Caucasian | p.Arg201Cys/p.Arg201Cys | 2 yo | Strabismus at 2 years of age. MRI at 2 years old showed demyelination and MRI at 4 years of age showed little to no change in myelination pattern. | 5 yo |
| Late-infantile GM1 | M | Caucasian | p.Arg201Cys/p.Arg201Cys | 2 yo | Speech and language delays. (Has older sibling diagnosed with late-infantile GM1) | 19 mo |
| Late-infantile GM1 | F | N/A | p.Leu228Pro/p.Asn669Lysfs*53 | 19 mo | Not walking | 18 mo |
| Juvenile GM1 | F | Korean, German, Irish | p.Leu155Arg/unknown | 4 yo | Falling down frequently and falling off chair | 5.5 yo |
| Juvenile GM1 | F | Irish, German, Korean | p.Arg148Ser/p.Tyr192His | 2.5 yo | Falling. Other symptoms noted after initial symptoms: dyspraxia, speech delays with poor articulation and limited vocabulary, dysmyelination on brain MRI, general developmental delays, inattention, hyperopia and astigmatism | 10 yo 5 mo |
| Juvenile GM1 | F | Caucasian | p.Arg201His/p.Arg351Ter | 4 yo | Developmental delays exhibited as loss of words at age 4 years | 12 yo |
| Juvenile GM1 | M | Caucasian | p.Arg201His/p.Ala301Val | 2 yo | Developmental delays in speech | 8 yo |
| Juvenile GM1 | F | N/A | p.Thr82Met/p.Gly123Arg | N/A | N/A | N/A |
| Late-infantile Tay-Sachs | F | Irish, Norwegian, German | c.570G > T/c.1273_1277dupATATC | 17 mo | Difficulty ambulating | 14 mo |
| Juvenile Tay-Sachs | F | Mexican, Italian, German, Native American and Dutch | c.1274_1277dupTATC/p.Gly461Val | N/A | N/A | 6 yo |
| Juvenile Tay-Sachs | F | African, French Canadian | p.Arg178His/p.Arg499Cys | N/A | N/A | 3 yo |
| Juvenile Tay-Sachs | F | N/A | c.77G > A (p.Trp26Stop)/p.Arg499His | N/A | N/A | 8 yo |
3.2. In silico prediction
As previously described, a total of seven bioinformatics tools were applied to predict the severity of the missense or nonsense mutations. As shown in our previous studies, the SAAMP 1.0 and SAAMP 2.0 algorithm can predict whether a mutation is pathogenic or benign by integrating the prediction outcomes of the aforementioned tools. The prediction outcomes of each individual tool and the SAAMP algorithm are shown in Table 2. All these mutations were predicted to be ‘pathogenic’ by the SAAMP algorithm, confirming the accuracy and reliability of this method.
Table 2.
Phenotype prediction by in silico tools. The prediction outcomes of each individual tool are listed in the table. ‘D' stands for ‘disease’ prediction, while ‘N' stands for ‘neutral’ prediction. The ‘P' stands for ‘pathogenic’ prediction by the SAAMP algorithm. The cut-off value of the SAAMP algorithm is set as 0.5. I, II and III represent infantile, late-infantile, and juvenile GM1 or GM2 gangliosidosis, respectively.
| Mutation | Phenotype | Bioinformatic Tool |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I-Mutant | PANTHER | SNP&GO | PROVEAN | PolyPhen | SIFT | PHD-SNP | SAAMP 2.0 | Pathogenic index | ||
| GLB1 | ||||||||||
| p.Arg68Trp | I | D | D | D | D | D | D | D | P | 1 |
| p.Thr82Met | III | N | D | D | D | D | N | D | P | 0.75 |
| p.Gly123Arg | III | D | D | D | D | D | D | D | P | 1 |
| p.Arg148Cys | I | D | D | D | D | D | D | D | P | 1 |
| p.Leu155Arg | III | D | D | D | D | D | D | D | P | 1 |
| p.Tyr192His | III | D | D | D | D | D | D | D | P | 1 |
| p.Arg201Cys | II | D | D | D | D | D | D | D | P | 1 |
| p.Leu228Pro | II or III | D | D | D | D | D | D | D | P | 1 |
| p.Tyr270Asp | I | D | D | D | D | D | D | D | P | 1 |
| p.His281Tyr | I | N | D | D | D | D | D | D | P | 1 |
| p.Ala301Val | III | D | D | N | D | D | D | D | P | 1 |
| p.Asn318Asp | I | D | D | D | D | D | D | D | P | 1 |
| p.Asp441Asn | I | D | D | D | D | D | D | D | P | 1 |
| p.Phe556Ser | II | D | N | N | D | D | D | D | P | 1 |
| p.Lys578Arg | I, II | D | D | D | D | D | D | D | P | 1 |
| HEXA | ||||||||||
| p.Leu127Arg | I | D | D | D | D | D | D | D | P | 1 |
| p.Arg170Trp | I | D | D | D | D | D | D | D | P | 1 |
| p.Arg178His | III | D | D | D | D | D | D | D | P | 1 |
| p.Gly461Val | III | D | D | D | D | D | D | D | P | 1 |
| p.Arg499Cys | III | D | D | D | D | D | D | D | P | 1 |
| p.Arg499His | III | D | D | D | D | D | D | D | P | 1 |
| HEXB | ||||||||||
| p.Gly301Arg | I | D | D | D | D | D | D | D | P | 1 |
| p.Val493Gly | I | D | D | D | D | D | D | D | P | 1 |
3.3. Establishment of 3D models
To analyze the 3D structural change introduced by the missense or nonsense mutations, structural analysis was performed by comparing the native and mutant protein structures. First, the native structure of GLB1 [27], HEXA, HEXB proteins were extracted from the Protein Data Bank (PDB, http://www.rcsb.org/). The mutant models were constructed by I-TASSER for further structural analysis. An illustration of detailed structural changes is shown in Fig. 1. The total energy of these models was calculated and shown in Table 3. Out of all 23 models, 11 had significantly higher energy than the native models, indicating that the stability of these mutants was severely affected. The remaining 12 mutant models had similar total energy to the native models. Therefore, these mutations may affect the protein by other mechanisms than affecting stability, which may include impairing the active site or binding abilities with other proteins or substrates.
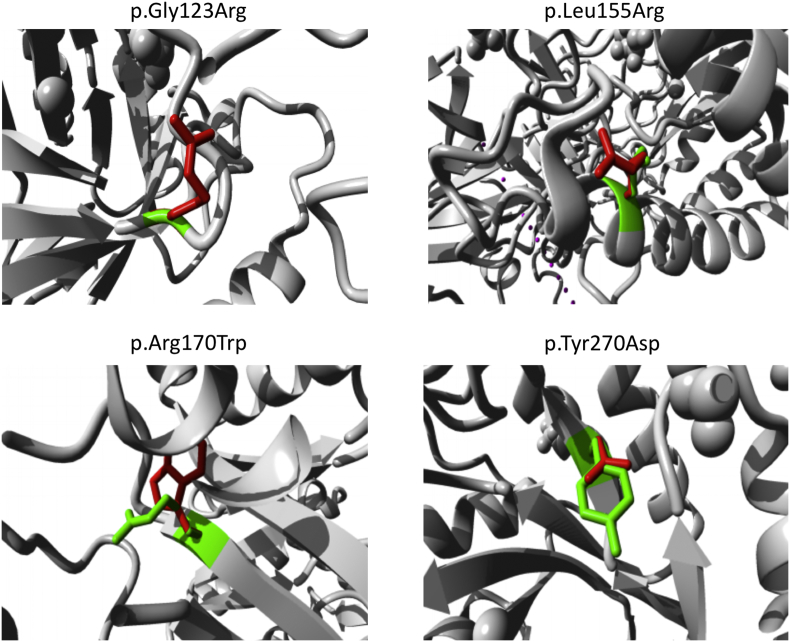
Fig. 1.
Close-up view of superimposed structure of native and mutant residues. The main protein core is shown in gray color while the wild-type and mutant residues are shown in red and green color, respectively. The following mutants were shown: p.Arg170Trp, p.Gly123Arg, p.Leu155Arg and p.Tyr270Asp. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Total energy of native and mutant protein structure models.
| Gene | AA change | Total energy after minimization (KJ/mol) |
|---|---|---|
| GLB1 | Native | −57,984 |
| p.Arg68Trp | −57,813 | |
| p.Thr82Met | −33,553 | |
| p.Gly123Arg | −33,956 | |
| p.Arg148Cys | −58,109 | |
| p.Leu155Arg | −58,777 | |
| p.Tyr192His | −33,389 | |
| p.Arg201Cys | −33,221 | |
| p.Leu228Pro | −32,189 | |
| p.Tyr270Asp | −57,033 | |
| p.His281Tyr | −33,559 | |
| p.Ala301Val | −58,629 | |
| p.Asn318Asp | −33,438 | |
| p.Asp441Asn | −33,753 | |
| p.Phe556Ser | −33,435 | |
| p.Lys578Arg | −33,351 | |
| HEXA | Native | −28,732 |
| p.Leu127Arg | −28,999 | |
| p.Arg170Trp | −28,413 | |
| p.Arg178His | −30,469 | |
| p.Gly461Val | −29,532 | |
| p.Arg499Cys | −28,442 | |
| p.Arg499His | −28,424 | |
| HEXB | Native | −59,199 |
| p.Gly301Arg | −56,720 | |
| p.Val493Gly | −29,294 |
3.4. Systemic structural analysis
To gain further insights into the impact of the missense or nonsense mutations, a systemic method was designed, which analyzes individual mutation in the following five features: amino acid properties (charge, size, and hydrophobic status), contacts, conservation, domain, and structure.
3.4.1. Amino acid properties
The size, charge, and hydrophobic status of the amino acids were evaluated in missense mutations.
3.4.1.1. Size change
In ten mutations, the mutant residue was larger than the wild-type residue. If the mutation in the wild-type residue was buried in the core of the protein, the larger mutant residue was sometimes too large to fit. This applied to the following mutations: six mutations of GLB1 (p.Arg68Trp, p.Thr82Met, p.Gly123Arg, p.Leu155Arg, p.Ala301Val, p.Lys578Arg), three mutations of HEXA (p.Leu127Arg, p.Arg170Trp, p.Gly461Val) and one mutation of HEXB (p.Gly301Arg).
In contrast, seven mutations resulted in smaller residues compared to the wild-type residues. If the residue was buried in the core, this mutation led to an empty space within the protein. This characteristic was found in five mutations of GLB1 (p.Arg148Cys, p.Tyr192His, p.Leu228Pro, p.Tyr270Asp, p.Phe556Ser), one mutation of HEXA (p.Arg178His), and one mutation of HEXB (p.Val493Gly).
For residues on the surface of proteins that were smaller or larger than the wild-type counterpart, four mutations were predicted to lead to loss of external interactions with other molecules or other residues of the protein. In the study populations, this type of external interaction defect was found with one mutation of GLB1 with a larger mutant residue (p.His281Tyr) and one mutation of GLB1 (p.Arg201Cys) with a smaller mutant residue, and two mutations of HEXA with smaller mutant residues (p.Arg499His and p.Arg499Cys).
3.4.1.2. Charge change
A positively or negatively charged wild-type residue was replaced with a neutral mutant residue in five mutations. Examples of these mutations are three mutations of GLB1 located in the core of the protein (p.Asp441Asn, p.Arg68Trp, p.Arg148Cys) and two mutations of HEXA (p.Arg170Trp, p.Arg178His). If a neutral mutant residue on the surface replaced a charged wild-type residue, a loss of normal interactions with other molecules was predicted. This applied to one mutation of GLB1 (p.Arg201Cys) and two mutations of HEXA (p.Arg499His and p.Arg499Cys).
A neutral wild-type residue was replaced by mutant residues with positive or negative charges in six mutations. When the mutation was in the core of the protein, the mutation introduced a charge in a buried residue, causing problems in protein folding. This situation applied to one mutation of HEXA (p.Leu127Arg), one mutation of HEXB (p.Gly301Arg), and four mutations of GLB1 (p.Leu155Arg, p.Gly123Arg, p.Tyr270Asp, p.Asn318Asp).
3.4.1.3. Hydrophobic status change
A wild-type residue which was more hydrophobic than the mutant residue was detected in ten mutations. This mutation caused a loss of hydrophobic interactions in the core of the protein, thereby disturbing correct protein folding. This situation applied to eight mutations of GLB1 (p.Arg68Trp, p.Thr82Met, p.Leu127Arg, p.Arg148Cys, p.Leu155Arg, p.Tyr192His, p.Tyr270Asp, p.Phe556Ser), one mutation of HEXA (p.Arg170Trp), and one mutation of HEXB (p.Val493Gly).
3.4.2. Contacts
Wild-type residues may form salt bridge and/or hydrogen bond with other residues (summarized in Table 4). The difference in charge between the wild-type and mutant residues is highly likely to disturb the ionic interaction (salt bridge). This applied to four mutations of GLB1 (p.Arg68Trp, p.Arg148Cys, p.Arg201Cys, p.Asp441Asn) and four mutations of HEXA (p.Arg170Trp, p.Arg178His, p.Arg499His, p.Arg499Cys). Moreover, the size difference between wild-type and mutant residues may place the new residue in an incorrect position to make the original hydrogen bond. This applied to eight mutations of GLB1 (p.Arg68Trp, p.Thr82Met, p.Arg148Cys, p.Tyr192His, p.Arg201Cys, p.Tyr270Asp, p.His281Tyr, p.Lys578Arg) and one mutation of HEXA (p.Arg170Trp). Additionally, the difference in hydrophobicity between the wild-type and mutant residues would affect hydrogen bond formation. Such was the case with six mutations of GLB1 (p.Thr82Met, p.Arg148Cys, p.Tyr192His, p.Arg201Cys, p.Tyr270Asp, p.His281Tyr), and one mutation of HEXA (p.Arg170Trp).
Table 4.
Interactions between the wildtype amino acid residues with other residues. The wildtype amino acids are involved in hydrogen bond and salt bridge with other residues, and the mutations lead to the loss of such interactions.
| Gene | Mutation | Hydrogen bond | Salt bridge |
|---|---|---|---|
| GLB1 | p.Asp441Asn | Arg457, Lys493, Met480, Lys493 | Arg457, Arg482, Lys493, Arg590 |
| GLB1 | p.Asp68Trp | Asp67 | Asp67, Asp342, Asp568 |
| GLB1 | p.Arg148Cys | Glu186 | Glu186, Glu188 and Asp221 |
| GLB1 | p.Arg201Cys | Asp198 | Asp 196, Asp198 |
| GLB1 | p.Tyr270Asp | Glu268 | |
| GLB1 | p.His281Tyr | Asp275 | |
| GLB1 | p.Lys578Arg | Tyr444 | Asp441, Glu478, Glu620 |
| GLB1 | p.Thr82Met | Ile55 | |
| GLB1 | p.Tyr192His | Leu147 | |
| HEXA | p.Arg170Trp | Phe167, Lys197 | Glu114 |
| HEXA | p.Arg178His | Asp175, Asp207, Asp208, Glu323, Glu462 | |
| HEXA | p.Arg499His, p.Arg499Cys | Glu482, Glu498, Glu506 |
As for p.Arg178His of HEXA, the wild-type residue has interactions with a ligand, (3aR,5R,6S,7R,7aR)-5-(hydroxymethyl)-2-methyl-5,6,7,7a-tetrahydro-3aH-pyrano[3,2-d][1,3]thiazole-6,7-diol. The different properties between the wild-type and mutant residues can result in loss of interactions with the ligand and impair the protein function. In both the PDB-file and in the Protein Interfaces Surfaces and Assemblies (PISA), this residue was found to be involved in a multimer contact. This confirmed that the residue contacting other proteins. Moreover, this mutation introduces a smaller residue at this position, which may be too small to make multimer contacts. As for p.Tyr270Asp of GLB1, the wild-type residue has interactions with a ligand annotated as (2R,3S,4R,5S)-2-(hydroxymethyl)piperidine-3,4,5-triol. The difference in properties between the wild-type and mutant residues can easily cause loss of interactions with the ligand, thereby disturbing the protein function. As for p.Thr82Met of GLB1 and p.Gly461Val of HEXA, the mutated residues are not in direct contact with a ligand. However, the mutation could affect the local stability, thereby affecting the ligand-contacts made by one of the neighboring residues.
3.4.3. Conservation
In general, mutations to highly conserved residues damage the protein.
All mutations analyzed were located near a highly conserved region. For mutations p.Arg68, p.Gly123, p.Tyr270 of GLB1, and p.Arg499 of HEXA, the region was 100% conserved. As for p.Arg201Cys, p.His281Tyr, and p.Phe556Ser of GLB1, and p.Arg170Trp of HEXA, neither the mutant residues nor another residue type with similar properties were observed at this position in other homologous sequences. Based on conservation scores, these mutations are highly likely damaging to the protein. As for p.Asp441Asn, p.Leu155Arg, p.Tyr192His, and p.Asn318Asp of GLB1 and p.Leu127Arg, p.Arg178His, and p.Gly461Val of HEXA, the mutant residues were not among the other residue types observed at this position in other homologous proteins. However, residues that have some properties in common with these mutated residues were observed. This indicates that, in some rare cases, these mutations might not be damaging to the protein. As for p.Leu228Pro, p.Arg148Cys, p.Thr82Met, p.Ala301Val, and p.Lys578Arg of GLB1, the mutant residues were among the residues at this position observed in other sequences. This indicates that homologous proteins exist with the same residue type as the mutant at this position, and these mutations may not be damaging to the protein.
3.4.4. Domain
All the mutations described in the previous paragraph were located in a certain domain that is crucial for the enzymatic activity. Therefore, these mutations can disturb the normal functions of these domains. Moreover, all these mutations were in contact with another domain that is also important for the activity. The interactions between these domains could be disturbed by these mutations, which likely affected the function of the protein.
3.4.5. Structure
The mutations with large structural impacts are discussed in detail as follows. For mutation p.Leu228Pro of GLB1, the wild-type residue is located in an α-helix. This mutant proline disrupts an α-helix since it is not located at one of the first three positions of that helix. Similarly, p.Leu155Arg of GLB1 was located in an α-helix, and the mutation converts the wild-type residue into a residue that does not conform to an α-helix. Therefore, this mutation would disturb the helix and thereby affect the structure of the protein. As for p.Thr82Met, p.Tyr192His, p.Tyr270Asp, and p.Phe556Ser of GLB1, the wild-type residue was predicted to be located in its preferred secondary structure, a β-strand. The mutant residue prefers to be in another secondary structure, therefore the local conformation may be slightly destabilized.
As for p.Gly123Arg of GLB1, p.Gly461Val of HEXA, and p.Gly301Arg of HEXB, the wild-type residue is a glycine, the most flexible of all residues. This flexibility might be necessary for the protein function, and mutation of this glycine can disturb this function. In addition, only glycine is flexible enough for unusual torsion angles. As a result, these mutations can force the local backbone into an incorrect conformation and thereby disturb the local structure. As for p.Val493Gly of HEXB, the mutant residue glycine is very flexible and may disturb the required rigidity of the protein at this position.
3.5. Disease subtype deduction for GM2-gangliosidoses
The SAAMP 2.0 algorithm is robust in predicting whether a mutation is pathogenic or benign, however, it is not sensitive enough to predict disease subtypes. Therefore, based on previous literature, the association between genotype and disease subtypes of GM2-gangliosidosis (infantile, juvenile or adult) was deducted in Table 5, Table 6. Three novel mutations were evaluated in this study: p.Gly461Val, p.Tyr192His, and p.Phe556Ser. The mutation is p.Gly461Val was found in a patient diagnosed with juvenile Tay-Sachs disease. The mutation was found in trans with c.1274_1277dupTATC (p.Tyr427Ilefs) in HEXA, which leads to a frameshift mutation and is predicted to lead to a complete loss of enzyme activity. The mutation p.Gly461Val is likely to be associated with the juvenile phenotype. The mutation p.Tyr192His was found in trans with p.Arg148Ser in a patient diagnosed with juvenile GM1-gangliosidosis. The mutation p.Arg148Ser was associated with the infantile phenotype in a previous paper, therefore p.Tyr192His is expected to be associated with the juvenile phenotype [17]. The mutation, p.Phe556Ser was found in a patient diagnosed with late-infantile GM1-gangliosidosis and was in trans with c.75 + 2dupT, a known mutation associated with the infantile phenotype. Therefore, p.Phe556Ser is likely to be associated with the late-infantile phenotype of GM1-gangliosidosis.
Table 5.
Deduction of phenotype severity through mutations in the HEXA gene through investigations of previous literature.
| Mutation | Phenotype | References | Mutation | Phenotype | References | Mutation | Phenotype | References |
|---|---|---|---|---|---|---|---|---|
| p.Met1Thr | Infantile | [28] | p.Ser210Phe | Infantile | [48] | p.Arg393Pro | Infantile | [34] |
| p.Met1Leu | Infantile | [29] | p.Phe211Ser | Infantile | [32] | p.tRP420cYS | Infantile | [40] |
| p.Met1Val | Infantile | [30] | p.Ser226Phe | Unknown | [35] | p.Phe434Leu | Juvenile or Adult | [41] |
| p.Pro25Ser | Adult | [28] | p.Ala246Thr | Infantile | [45] | P.Leu451Val | Unknown | [49] |
| p.Tyr37Asn | Juvenile | [31] | p.Gly250Ser | Unknown | [44] | p.Gly454Ser | Infantile | [32] |
| p.Leu39Arg | Infantile | [32] | p.Gly250Glu | Juvenile | [44] | p.Gly454Asp | Infantile | [57] |
| p.Cys58Tyr | Juvenile or Adult | [33] | p.Gly250Val | Unknown | [49] | p.Gly455Arg | Infantile | [50] |
| p.Glu114Lys | Infantile | [34] | p.Arg252His | Juvenile or Adult | [50] | p.Cys458Tyr | Infantile | [58] |
| p.Leu127Arg | Infantile | [27] | p.Arg252Leu | Infantile | [51] | p.Met459Val | Unknown | [29] |
| p.Leu127Phe | Infantile | [35] | p.Asp258His | Infantile | [52] | p.Gly461Val | Juvenile | This study |
| p.Arg166Gly | Juvenile | [36] | p.Thr259Ala | Unknown | [53] | p.Glu462Val | Infantile | [34] |
| p.Arg170Gln | Infantile | [37] | p.Pro260Ser | Unknown | [41] | p.Asp465Asn | Infantile | [59] |
| p.Arg170Trp | Infantile | [27] | p.Trp266Gly | Infantile | [41] | p.Trp474Cys | Juvenile | [60] |
| p.Arg178His | Juvenile | [38] | p.Gly269Ser | Adult | [54] | p.Gly478Arg | Infantile | [44] |
| p.Arg178Leu | Infantile | [39] | p.Gly269Asp | Infantile | [35] | p.Ala479Thr | Unknown | [29] |
| p.Arg178Cys | Infantile | [40] | p.Ser279Pro | Juvenile | [55] | p.Glu482Lys | Infantile | [39] |
| p.His179Arg | Unknown | [41] | p.Asn295Ser | Infantile | [51] | p.Leu484Pro | Infantile | [58] |
| p.His179Tyr | Infantile | [41] | p.Met301Arg | Infantile | [32] | p.Trp485Arg | Infantile | [61] |
| p.Tyr180His | Adult | [42] | p.Asp314Val | Unknown | [35] | p.Tyr497Cys | Unknown | [62] |
| p.Val192Leu | Infantile | [43] | p.Asp322Asn | Infantile | [34] | p.Arg499His | Juvenile | [32] |
| p.Asn196Ser | Unknown | [44] | p.Asp322Val | Unknown | [53] | p.Arg499Cys | Juvenile | [30] |
| p.Lys197Thr | Juvenile or Adult | [32] | p.Asp322Tyr | Infantile | [34] | p.Arg504His | Juvenile | [42] |
| p.Val200Met | Adult | [43] | p.Ile335Phe | Unknown | [56] | p.Arg504Leu | Infantile | [47] |
| p.Trp203Gly | Infantile | [45] | p.Gln336His | Unknown | [41] | p.Arg504Cys | Infantile | [32] |
| p.His204Arg | Infantile | [32] | p.Gln374Arg | Infantile | [45] | p.Phe521Leu | Juvenile or Adult | [63] |
| p.Asp207Glu | Infantile | [46] | p.Ile388Met | Unknown | [44] | |||
| p.Asp208Val | Infantile | [47] | p.Val391Met | Unknown | [54] |
Table 6.
Deduction of phenotype severity through mutations in the HEXB gene through investigations of previous literature.
| Mutation | Phenotype | References | Mutation | Phenotype | References |
|---|---|---|---|---|---|
| p.Trp57Cys | Juvenile | [64] | p.Cys360Arg | Unknown | [66] |
| p.Ser62Leu | Infantile | [65] | p.Pro417Leu | Adult | [4] |
| p.Ala97Pro | Unknown | [66] | p.Tyr456Ser | Adult | [71] |
| p.Cys137Tyr | Juvenile | [4] | p.Asp459Ala | Juvenile | [72] |
| p.Thr150Leu | Infantile | [4] | p.Gly483Ser | Infantile | [73] |
| p.Thr150Pro | Infantile | [47] | p.Gly484Glu | Infantile | [47] |
| p.Ile207Val | Unknown | [67] | p.Val493Gly | Infantile | [27] |
| p.Thr209Ile | Infantile | [47] | p.Asp494Gly | Juvenile or Adult | [74] |
| p.His212Asn | Infantile | [47] | p.Trp503Arg | Unknown | [66] |
| p.His235Tyr | Unknown | [68] | p.Pro504Ser | Adult | [4] |
| p.Ser255Arg | Unknown | [69] | p.Arg505Gln | Adult | [4] |
| p.Tyr266Asp | Infantile | [41] | p.Leu513Pro | Infantile | [75] |
| p.Gly282Glu | Infantile | [41] | p.Arg533His | Adult | [76] |
| p.Arg284Gln | Juvenile | [70] | p.Arg533Cys | Infantile | [47] |
| p.Thr295Arg | Unknown | [66] | p.Cys534Tyr | Infantile | [47] |
| p.Cys309Phe | Infantile | [47] | p.Arg539Cys | Unknown | [41] |
| p.Gly353Arg | Infantile | [4] | p.Ala543Thr | Unknown | [77] |
4. Discussion
GM1- and GM2-gangliosidoses have variable phenotypes (infantile, late-infantile, juvenile, and adult), like other lysosomal diseases. The wide variations in phenotype result in considerable differences in the urgency of initiating treatment. Residual enzyme activity provides a rough estimate on the phenotype. However, with currently available laboratory methods, there is no clear correlation between clinical presentation of patients with gangliosidoses and the percentage of the enzyme activity reported in leukocytes or fibroblasts [2,41]. Therefore, the residual activity is not a good indicator of the severity of the disease. Diagnosis is based on clinical evaluation and laboratory testing of respective enzyme activity; however, the average time from onset of symptoms to diagnosis is often greater than 5 years for the adult onset phenotype. In the infantile phenotype, onset of symptoms to diagnosis usually occurs well after the disease has caused severe neurological impairment and severe disability. [78,79].
As the era of newborn screening expands and more treatments become available for lysosomal diseases, improving understanding of genotype-phenotype correlation will become increasingly important. For variants of unknown significance (VUS), our SAAMP algorithm provides a powerful method to predict whether a missense mutation is ‘pathogenic’ or ‘benign'. This SAAMP algorithm provides unprecedented sensitivity and specificity when compared to the other individual bioinformatics tools tested [17,18].
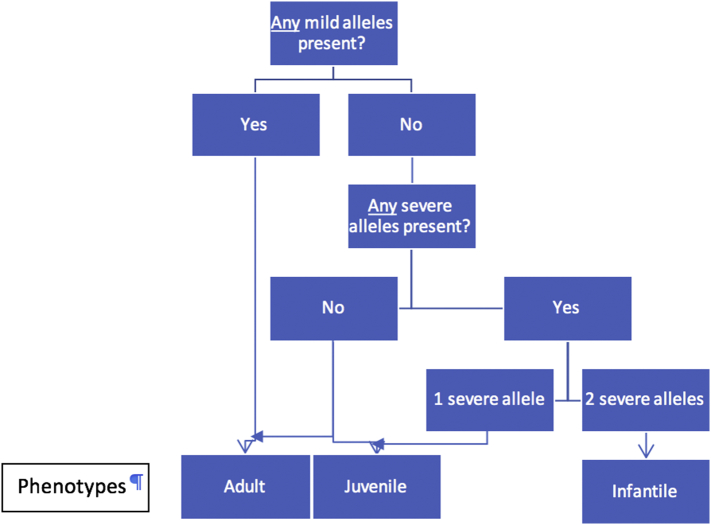
To determine the phenotype of a patient, mutations on both alleles need be considered. Predicting disease phenotypes based on genotypes is a major barrier in initiating appropriate treatment. In this study, phenotypes and mutations on both alleles of patients from other reports were analyzed and the phenotype of each mutation was deduced manually. We improved upon our previous work in MPS I [17] and GM1-gangliosidosis [18]. Establishment of such a database and making it readily accessible to clinicians and researchers will be remarkably beneficial. To predict the disease subtypes based on each mutation, four general assumptions are recommended for autosomal recessive diseases (Fig. 2). 1) The phenotype is infantile only if both alleles are severe. 2) The phenotype is adult if any of the alleles are mild. 3) The phenotype is juvenile if the alleles are intermediate and severe. 4) Juvenile or adult phenotypes can occur if both alleles are intermediate [17]. Notably, there may be heterogeneity in phenotype even among siblings with the same mutation due to genetic background. This phenomenon makes phenotype prediction more complicated.
Fig. 2.
Predicting disease subtypes based on mutations in autosomal recessive diseases. A general protocol for predicting disease subtypes of autosomal recessive diseases was proposed.
Based on the phenotypes of our patients and previously reported patients, we propose the effects of mutations on the allele functionality (Table 5, Table 6) in GM2-gangliosidosis. Clinicians can identify select the mutation in their patients from this table, determine its impact on the allele, then determine the predicted phenotype using Fig. 2. If a genotype present in our patients was also identified in previous reports, we cross-referenced the phenotype (p.Thr82Met, p.Arg148Cys, p.Arg148Ser, p.Arg201His, and p.Ala301Val) [[80], [81], [82]].
Acknowledgments
This work is supported by NIH grant P01HD032652. Dr. Li Ou is a fellow of the Lysosomal Disease Network (U54NS065768). The Lysosomal Disease Network is a part of the Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), and NCATS. This consortium is funded through a collaboration between NCATS, the National Institute of Neurological Disorders and Stroke (NINDS), and the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
References
- 1.Sarafoglou K., Hoffmann G.F., Roth K.S. McGraw-Hill Company; New York: 2009. Pediatric Endocrinology and Inborn Errors of Metabolism; pp. 738–739. (& 744–745) [Google Scholar]
- 2.Callahan J.W. Molecular basis of GM1 gangliosidosis and Morquio disease, type B. structure-function studies of lysosomal beta-galactosidase and the non-lysosomal beta-galactosidase-like protein. Biochim. Biophys. Acta. 1999;1455:85–103. doi: 10.1016/s0925-4439(99)00075-7. [DOI] [PubMed] [Google Scholar]
- 3.Jarnes Utz J.R., Kim S., King K., Ziegler R., Schema L., Redtree E.S., Whitley C.B. Infantile gangliosidoses: mapping a timeline of clinical changes. Mol. Genet. Metab. 2017;121(2):170–179. doi: 10.1016/j.ymgme.2017.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Regier D.S., Proia R.L., D'Azzo A., Tifft C.J. The GM1 and GM2 gangliosidoses: natural history and Progress toward therapy. Pediatr. Endocrinol. Rev. 2016 Jun;13(Suppl. 1):663–673. [PMC free article] [PubMed] [Google Scholar]
- 5.Maegawa G.H., Tropak M., Buttner J., Stockley T., Kok F., Clarke J.T., Mahuran D.J. Pyrimethamine as a potential pharmacological chaperone for late-onset forms of GM2 gangliosidosis. J. Biol. Chem. 2007 Mar 23;282(12):9150–9161. doi: 10.1074/jbc.M609304200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith N.J., Winstone A.M., Stellitano L., Cox T.M., Verity C.M. GM2 gangliosidosis in a UK study of children with progressive neurodegeneration: 73 cases reviewed. Dev. Med. Child Neurol. 2012 Feb;54(2):176–182. doi: 10.1111/j.1469-8749.2011.04160.x. [DOI] [PubMed] [Google Scholar]
- 7.Kannebley J.S., Silveira-Moriyama L., Bastos L.O., Steiner C.E. Clinical findings and natural history in ten unrelated families with juvenile and adult GM1 gangliosidosis. JIMD Rep. 2015;24:115–122. doi: 10.1007/8904_2015_451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neudorfer O., Kolodny E.H. Late-onset Tay-Sachs disease. Isr. Med. Assoc. J. 2004 Feb;6(2):107–111. [PubMed] [Google Scholar]
- 9.Frey L.C., Ringel S.P., Filley C.M. The natural history of cognitive dysfunction in late-onset GM2 gangliosidosis. Arch. Neurol. 2005 Jun;62(6):989–994. doi: 10.1001/archneur.62.6.989. [DOI] [PubMed] [Google Scholar]
- 10.Scarpelli M., Tomelleri G., Bertolasi L., Salviati A. Natural history of motor neuron disease in adult onset GM2-gangliosidosis: a case report with 25 years of follow-up. Mol Genet Metab Rep. 2014 Jul 2;1:269–272. doi: 10.1016/j.ymgmr.2014.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Przybilla M.J., Ou L., Tăbăran A.F., Jiang X., Sidhu R., Kell P.J., Ory D.S., O'Sullivan M.G., Whitley C.B. Comprehensive behavioral and biochemical outcomes of novel murine models of GM1-gangliosidosis and Morquio syndrome type B. Mol. Genet. Metab. 2019 Feb;126(2):139–150. doi: 10.1016/j.ymgme.2018.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Tsuji D., Akeboshi H., Matsuoka K., Yasuoka H., Miyasaki E., Kasahara Y., Kawashima I., Chiba Y., Jigami Y., Taki T., Sakuraba H., Itoh K. Highly phosphomannosylated enzyme replacement therapy for GM2 gangliosidosis. Ann. Neurol. 2011 Apr;69(4):691–701. doi: 10.1002/ana.22262. [DOI] [PubMed] [Google Scholar]
- 13.Maegawa G.H., Banwell B.L., Blaser S., Sorge G., Toplak M., Ackerley C., Hawkins C., Hayes J., Clarke J.T. Substrate reduction therapy in juvenile GM2 gangliosidosis. Mol. Genet. Metab. 2009 Sep-Oct;98(1–2):215–224. doi: 10.1016/j.ymgme.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs J., Willemsen M., Groot-Loonen J., Wevers R., Hoogerbrugge P. Allogeneic BMT followed by substrate reduction therapy in a child with subacute Tay-Sachs disease. Bone Marrow Transplant. 2005 Nov;36(10):925–926. doi: 10.1038/sj.bmt.1705155. [DOI] [PubMed] [Google Scholar]
- 15.Kyrkanides S., Miller J.H., Brouxhon S.M., Olschowka J.A., Federoff H.J. Beta-hexosaminidase lentiviral vectors: transfer into the CNS via systemic administration. Brain Res. Mol. Brain Res. 2005 Feb 18;133(2):286–298. doi: 10.1016/j.molbrainres.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Ou L., Przybilla M.J. Whitley CB. Untargeted metabolomics profiling reveals profound metabolic impairments in mice and patients with Sandhoff disease. Mol Genet Metab. 2019 Feb;126(2):151–156. doi: 10.1016/j.ymgme.2018.09.005. (pii: S1096-7192(18)30437-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ou L., Przybilla M.J., Whitley C.B. Phenotype prediction for mucopolysaccharidosis type I by in silico analysis. Orphanet J Rare Dis. 2017;12(1):125. doi: 10.1186/s13023-017-0678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ou L., Przybilla M.J., Whitley C.B. SAAMP 2.0: an algorithm to predict genotype-phenotype correlation of lysosomal storage diseases. Clin. Genet. 2018 Feb 2 doi: 10.1111/cge.13226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ng P.C., Henikoff S. Predicting deleterious amino acid substitutions. Genome Res. 2001;11:863–874. doi: 10.1101/gr.176601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adzhubei I.A., Schmidt S., Peshkin L. A method and server for predicting damaging missense mutations. Nat. Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Capriotti E., Fariselli P., Casadio R. I-Mutant 2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33:W306–W310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi Y., Sims G.E., Murphy S., Miller J.R., Chan A.P. Predicting the functional effect of amino acid substitutions and indels. PLoS One. 2012;7 doi: 10.1371/journal.pone.0046688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mi H., Guo N., Kejariwal A., Thomas P.D. PANTHER version 6: protein sequence and function evolution data with expanded representation of biological pathways. Nucleic Acids Res. 2007;35:D247–D252. doi: 10.1093/nar/gkl869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calabrese R., Capriotti E., Fariselli P., Martelli P.L., Casadio R. Functional annotations improve the predictive score of human disease-related mutations in proteins. Hum. Mutat. 2009;30:1237–1244. doi: 10.1002/humu.21047. [DOI] [PubMed] [Google Scholar]
- 25.Capriotti E., Fariselli P. PhD-SNPg: a webserver and lightweight tool for scoring single nucleotide variants. Nucleic Acids Res. 2017 Jul 3;45(W1):W247–W252. doi: 10.1093/nar/gkx369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J., Yan R., Roy A., Xu D., Poisson J., Zhang Y. The I-TASSER suite: protein structure and function prediction. Nat. Methods. 2015;12:7–8. doi: 10.1038/nmeth.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohto U., Usui K., Ochi T., Yuki K., Satow Y., Shimizu T. Crystal structure of human β-galactosidase: structural basis of Gm1 gangliosidosis and morquio B diseases. J. Biol. Chem. 2012 Jan 13;287(3):1801–1812. doi: 10.1074/jbc.M111.293795. (Epub 2011 Nov 28) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harmon D.L., Gardner-Medwin D., Stirling J.L. Two new mutations in a late infantile Tay-Sachs patient are both in exon 1 of the beta-hexosaminidase alpha subunit gene. J. Med. Genet. 1993 Feb;30(2):123–128. doi: 10.1136/jmg.30.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strom C.M., Park N.J., Morgan C., Lobo R., Crossley B., Sharma R., Bonilla-Guerrero R., Salazar D. Tay-Sachs carrier screening in the genomics age: gene sequencing versus enzyme analysis in non-Jewish individuals. Open J Genet. 2013;3:61–66. [Google Scholar]
- 30.Mules E.H., Hayflick S., Miller C.S., Reynolds L.W., Thomas G.H. Six novel deleterious and three neutral mutations in the gene encoding the alpha-subunit of hexosaminidase A in non-Jewish individuals. Am. J. Hum. Genet. 1992 Apr;50(4):834–841. [PMC free article] [PubMed] [Google Scholar]
- 31.Paciorkowski A.R., Sathe S., Zeng B.J., Torres P., Rosengren S.S., Kolodny E. Juvenile-onset G(M2)-gangliosidosis in an African-American child with nystagmus. Pediatr. Neurol. 2008 Apr;38(4):284–286. doi: 10.1016/j.pediatrneurol.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 32.Akli S., Chomel J.C., Lacorte J.M., Bachner L., Kahn A., Poenaru L. Ten novel mutations in the HEXA gene in non-Jewish Tay-Sachs patients. Hum. Mol. Genet. 1993 Jan;2(1):61–67. doi: 10.1093/hmg/2.1.61. [DOI] [PubMed] [Google Scholar]
- 33.Najmabadi H., Hu H., Garshasbi M., Zemojtel T., Abedini S.S., Chen W., Hosseini M., Behjati F., Haas S., Jamali P., Zecha A., Mohseni M., Püttmann L., Vahid L.N., Jensen C., Moheb L.A., Bienek M., Larti F., Mueller I., Weissmann R., Darvish H., Wrogemann K., Hadavi V., Lipkowitz B., Esmaeeli-Nieh S., Wieczorek D., Kariminejad R., Firouzabadi S.G., Cohen M., Fattahi Z., Rost I., Mojahedi F., Hertzberg C., Dehghan A., Rajab A., Banavandi M.J., Hoffer J., Falah M., Musante L., Kalscheuer V., Ullmann R., Kuss A.W., Tzschach A., Kahrizi K., Ropers H.H. Deep sequencing reveals 50 novel genes for recessive cognitive disorders. Nature. 2011 Sep 21;478(7367):57–63. doi: 10.1038/nature10423. [DOI] [PubMed] [Google Scholar]
- 34.Mistri M., Tamhankar P.M., Sheth F., Sanghavi D., Kondurkar P., Patil S., Idicula-Thomas S., Gupta S., Sheth J. Identification of novel mutations in HEXA gene in children affected with Tay Sachs disease from India. PLoS One. 2012;7(6) doi: 10.1371/journal.pone.0039122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Akerman B.R., Natowicz M.R., Kaback M.M., Loyer M., Campeau E., Gravel R.A. Novel mutations and DNA-based screening in non-Jewish carriers of Tay-Sachs disease. Am. J. Hum. Genet. 1997 May;60(5):1099–1106. [PMC free article] [PubMed] [Google Scholar]
- 36.Peleg L., Meltzer F., Karpati M., Goldman B. GM2 gangliosidosis B1 variant: biochemical and molecular characterization of hexosaminidase A. Biochem. Mol. Med. 1995 Apr;54(2):126–132. doi: 10.1006/bmme.1995.1018. [DOI] [PubMed] [Google Scholar]
- 37.Nakano T., Nanba E., Tanaka A., Ohno K., Suzuki Y., Suzuki K. A new point mutation within exon 5 of beta-hexosaminidase alpha gene in a Japanese infant with Tay-Sachs disease. Ann. Neurol. 1990 May;27(5):465–473. doi: 10.1002/ana.410270503. [DOI] [PubMed] [Google Scholar]
- 38.Ohno K., Suzuki K. Mutation in GM2-gangliosidosis B1 variant. J. Neurochem. 1988 Jan;50(1):316–318. doi: 10.1111/j.1471-4159.1988.tb13266.x. [DOI] [PubMed] [Google Scholar]
- 39.Triggs-Raine B.L., Akerman B.R., Clarke J.T., Gravel R.A. Sequence of DNA flanking the exons of the HEXA gene, and identification of mutations in Tay-Sachs disease. Am. J. Hum. Genet. 1991 Nov;49(5):1041–1054. [PMC free article] [PubMed] [Google Scholar]
- 40.Tanaka A., Punnett H.H., Suzuki K. A new point mutation in the beta-hexosaminidase alpha subunit gene responsible for infantile Tay-Sachs disease in a non-Jewish Caucasian patient (a Kpn mutant) Am. J. Hum. Genet. 1990 Sep;47(3):568–574. [PMC free article] [PubMed] [Google Scholar]
- 41.Gort L., de Olano N., Macías-Vidal J., Coll M.A., Spanish GM2 Working Group GM2 gangliosidoses in Spain: analysis of the HEXA and HEXB genes in 34 Tay-Sachs and 14 Sandhoff patients. Gene. 2012 Sep 10;506(1):25–30. doi: 10.1016/j.gene.2012.06.080. [DOI] [PubMed] [Google Scholar]
- 42.Paw B.H., Moskowitz S.M., Uhrhammer N., Wright N., Kaback M.M., Neufeld E.F. Juvenile GM2 gangliosidosis caused by substitution of histidine for arginine at position 499 or 504 of the alpha-subunit of beta-hexosaminidase. J. Biol. Chem. 1990 Jun 5;265(16):9452–9457. [PubMed] [Google Scholar]
- 43.Ainsworth P.J., Coulter-Mackie M.B. A double mutation in exon 6 of the beta-hexosaminidase alpha subunit in a patient with the B1 variant of Tay-Sachs disease. Am. J. Hum. Genet. 1992 Oct;51(4):802–809. [PMC free article] [PubMed] [Google Scholar]
- 44.Triggs-Raine B., Richard M., Wasel N., Prence E.M., Natowicz M.R. Mutational analyses of Tay-Sachs disease: studies on Tay-Sachs carriers of French Canadian background living in New England. Am. J. Hum. Genet. 1995 Apr;56(4):870–879. [PMC free article] [PubMed] [Google Scholar]
- 45.Montalvo A.L., Filocamo M., Vlahovicek K., Dardis A., Lualdi S., Corsolini F., Bembi B., Pittis M.G. Molecular analysis of the HEXA gene in Italian patients with infantile and late onset Tay-Sachs disease: detection of fourteen novel alleles. Hum. Mutat. 2005 Sep;26(3):282. doi: 10.1002/humu.9363. [DOI] [PubMed] [Google Scholar]
- 46.Giraud C., Dussau J., Azouguene E., Feillet F., Puech J.P., Caillaud C. Rapid identification of HEXA mutations in Tay-Sachs patients. Biochem. Biophys. Res. Commun. 2010 Feb 19;392(4):599–602. doi: 10.1016/j.bbrc.2010.01.088. [DOI] [PubMed] [Google Scholar]
- 47.Zampieri S., Cattarossi S., Oller Ramirez A.M., Rosano C., Lourenco C.M., Passon N., Moroni I., Uziel G., Pettinari A., Stanzial F., de Kremer R.D., Azar N.B., Hazan F., Filocamo M., Bembi B., Dardis A. Sequence and copy number analyses of HEXB gene in patients affected by Sandhoff disease: functional characterization of 9 novel sequence variants. PLoS One. 2012;7(7) doi: 10.1371/journal.pone.0041516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Akli S., Chelly J., Lacorte J.M., Poenaru L., Kahn A. Seven novel Tay-Sachs mutations detected by chemical mismatch cleavage of PCR-amplified cDNA fragments. Genomics. 1991 Sep;11(1):124–134. doi: 10.1016/0888-7543(91)90109-r. [DOI] [PubMed] [Google Scholar]
- 49.Karpati M., Peleg L., Gazit E., Akstein E., Goldman B. A novel mutation in the HEXA gene specific to Tay-Sachs disease carriers of Jewish Iraqi origin. Clin. Genet. 2000 May;57(5):398–400. doi: 10.1034/j.1399-0004.2000.570512.x. [DOI] [PubMed] [Google Scholar]
- 50.Ribeiro M.G., Sonin T., Pinto R.A., Fontes A., Ribeiro H., Pinto E., Palmeira M.M., Sá Miranda M.C. Clinical, enzymatic, and molecular characterisation of a Portuguese family with a chronic form of GM2-gangliosidosis B1 variant. J. Med. Genet. 1996 Apr;33(4):341–343. doi: 10.1136/jmg.33.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tanaka A., Hoang L.T., Nishi Y., Maniwa S., Oka M., Yamano T. Different attenuated phenotypes of GM2 gangliosidosis variant B in Japanese patients with HEXA mutations at codon 499, and five novel mutations responsible for infantile acute form. J. Hum. Genet. 2003;48(11):571–574. doi: 10.1007/s10038-003-0080-9. [DOI] [PubMed] [Google Scholar]
- 52.Fernandes M., Kaplan F., Natowicz M., Prence E., Kolodny E., Kaback M., Hechtman P. A new Tay-Sachs disease B1 allele in exon 7 in two compound heterozygotes each with a second novel mutation. Hum. Mol. Genet. 1992 Dec;1(9):759–761. doi: 10.1093/hmg/1.9.759. [DOI] [PubMed] [Google Scholar]
- 53.Park N.J., Morgan C., Sharma R., Li Y., Lobo R.M., Redman J.B., Salazar D., Sun W., Neidich J.A., Strom C.M. Improving accuracy of Tay Sachs carrier screening of the non-Jewish population: analysis of 34 carriers and six late-onset patients with HEXA enzyme and DNA sequence analysis. Pediatr. Res. 2010 Feb;67(2):217–220. doi: 10.1203/PDR.0b013e3181c6e318. [DOI] [PubMed] [Google Scholar]
- 54.Navon R., Proia R.L. The mutations in Ashkenazi Jews with adult GM2 gangliosidosis, the adult form of Tay-Sachs disease. Science. 1989 Mar 17;243(4897):1471–1474. doi: 10.1126/science.2522679. [DOI] [PubMed] [Google Scholar]
- 55.Drucker L., Hemli J.A., Navon R. Two mutated HEXA alleles in a Druze patient with late-infantile Tay-Sachs disease. Hum. Mutat. 1997;10(6):451–457. doi: 10.1002/(SICI)1098-1004(1997)10:6<451::AID-HUMU6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 56.Tomczak J., Grebner E.E. Three novel beta-hexosaminidase A mutations in obligate carriers of Tay-Sachs disease. Hum. Mutat. 1994;4(1):71–72. doi: 10.1002/humu.1380040112. [DOI] [PubMed] [Google Scholar]
- 57.Ozkara H.A., Navon R. At least six different mutations in HEXA gene cause Tay-Sachs disease among the Turkish population. Mol. Genet. Metab. 1998 Nov;65(3):250–253. doi: 10.1006/mgme.1998.2742. [DOI] [PubMed] [Google Scholar]
- 58.Tanaka A., Sakazaki H., Murakami H., Isshiki G., Suzuki K. Molecular genetics of Tay-Sachs disease in Japan. J. Inherit. Metab. Dis. 1994;17(5):593–600. doi: 10.1007/BF00711597. [DOI] [PubMed] [Google Scholar]
- 59.Alvarez-Rodríguez A., Triggs-Raine B., Barros-Núñez P., Lozano C.M. A novel HEXA mutation [1393G>A (D465N)] in a Mexican Tay-Sachs disease patient. Hum. Mutat. 2001 May;17(5):437. doi: 10.1002/humu.1128. [DOI] [PubMed] [Google Scholar]
- 60.Petroulakis E., Cao Z., Clarke J.T., Mahuran D.J., Lee G., Triggs-Raine B. W474C amino acid substitution affects early processing of the alpha-subunit of beta-hexosaminidase A and is associated with subacute G(M2) gangliosidosis. Hum. Mutat. 1998;11(6):432–442. doi: 10.1002/(SICI)1098-1004(1998)11:6<432::AID-HUMU3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 61.Akalin N., Shi H.P., Vavougios G., Hechtman P., Lo W., Scriver C.R., Mahuran D., Kaplan F. Novel Tay-Sachs disease mutations from China. Hum. Mutat. 1992;1(1):40–46. doi: 10.1002/humu.1380010107. [DOI] [PubMed] [Google Scholar]
- 62.Chin E., Bean L., Coffee B., Hegde M.R. Novel human pathological mutations. Gene symbol: HEXA. Disease: Tay-Sachs disease. Hum. Genet. 2009 Aug;126(2):329. doi: 10.1007/s00439-009-0717-7. [DOI] [PubMed] [Google Scholar]
- 63.Stendel C., Gallenmüller C., Peters K., Bürger F., Gramer G., Biskup S., Klopstock T. Paranoid delusion as lead symptom in two siblings with late-onset Tay-Sachs disease and a novel mutation in the HEXA gene. J. Neurol. 2015;262(4):1072–1073. doi: 10.1007/s00415-015-7729-0. [DOI] [PubMed] [Google Scholar]
- 64.Gaignard P., Fagart J., Niemir N., Puech J.P., Azouguene E., Dussau J., Caillaud C. Characterization of seven novel mutations on the HEXB gene in French Sandhoff patients. Gene. 2013 Jan 10;512(2):521–526. doi: 10.1016/j.gene.2012.09.124. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z.X., Wakamatsu N., Akerman B.R., Mules E.H., Thomas G.H., Gravel R.A. A second, large deletion in the HEXB gene in a patient with infantile Sandhoff disease. Hum. Mol. Genet. 1995 Apr;4(4):777–780. doi: 10.1093/hmg/4.4.777. [DOI] [PubMed] [Google Scholar]
- 66.Sobek A.K., Evers C., Dekomien G. Integrated multiplex ligation dependent probe amplification (MLPA) assays for the detection of alterations in the HEXB, GM2A and SMARCAL1 genes to support the diagnosis of Morbus Sandhoff, M. Tay-Sachs variant AB and Schimke immuno-osseous dysplasia in humans. Mol. Cell. Probes. 2013 Feb;27(1):32–37. doi: 10.1016/j.mcp.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 67.Zhang W., Zeng H., Huang Y., Xie T., Zheng J., Zhao X., Sheng H., Liu H., Liu L. Clinical, biochemical and molecular analysis of five Chinese patients with Sandhoff disease. Metab. Brain Dis. 2016 Aug;31(4):861–867. doi: 10.1007/s11011-016-9819-9. [DOI] [PubMed] [Google Scholar]
- 68.Yamada K., Takado Y., Kato Y.S., Yamada Y., Ishiguro H., Wakamatsu N. Characterization of the mutant β-subunit of β-hexosaminidase for dimer formation responsible for the adult form of Sandhoff disease with the motor neuron disease phenotype. J. Biochem. 2013 Jan;153(1):111–119. doi: 10.1093/jb/mvs131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fujimaru M., Tanaka A., Choeh K., Wakamatsu N., Sakuraba H., Isshiki G. Two mutations remote from an exon/intron junction in the beta-hexosaminidase beta-subunit gene affect 3′-splice site selection and cause Sandhoff disease. Hum. Genet. 1998 Oct;103(4):462–469. doi: 10.1007/s004390050851. [DOI] [PubMed] [Google Scholar]
- 70.Wortmann S.B., Lefeber D.J., Dekomien G., Willemsen M.A., Wevers R.A., Morava E. Substrate deprivation therapy in juvenile Sandhoff disease. J. Inherit. Metab. Dis. 2009 Dec;32(Suppl. 1):S307–S311. doi: 10.1007/s10545-009-1261-2. [DOI] [PubMed] [Google Scholar]
- 71.Banerjee P., Siciliano L., Oliveri D., McCabe N.R., Boyers M.J., Horwitz A.L., Li S.C., Dawson G. Molecular basis of an adult form of beta-hexosaminidase B deficiency with motor neuron disease. Biochem. Biophys. Res. Commun. 1991 Nov 27;181(1):108–115. doi: 10.1016/s0006-291x(05)81388-9. [DOI] [PubMed] [Google Scholar]
- 72.Wang S.Z., Cachón-González M.B., Stein P.E., Lachmann R.H., Corry P.C., Wraith J.E., Cox T.M. A novel HEXB mutation and its structural effects in juvenile Sandhoff disease. Mol. Genet. Metab. 2008 Dec;95(4):236–238. doi: 10.1016/j.ymgme.2008.08.007. [DOI] [PubMed] [Google Scholar]
- 73.Beker-Acay M., Elmas M., Koken R., Unlu E., Bukulmez A. Infantile type Sandhoff disease with striking brain MRI findings and a novel mutation. Pol. J. Radiol. 2016 Mar 3;81:86–89. doi: 10.12659/PJR.895911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Santoro M., Modoni A., Sabatelli M., Madia F., Piemonte F., Tozzi G., Ricci E., Tonali P.A., Silvestri G. Chronic GM2 gangliosidosis type Sandhoff associated with a novel missense HEXB gene mutation causing a double pathogenic effect. Mol. Genet. Metab. 2007 May;91(1):111–114. doi: 10.1016/j.ymgme.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 75.Lee H.F., Chi C.S., Tsai C.R. Early cardiac involvement in an infantile Sandhoff disease case with novel mutations. Brain and Development. 2017 Feb;39(2):171–176. doi: 10.1016/j.braindev.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 76.Bolhuis P.A., Ponne N.J., Bikker H., Baas F., Vianney de Jong J.M. Molecular basis of an adult form of Sandhoff disease: substitution of glutamine for arginine at position 505 of the beta-chain of beta-hexosaminidase results in a labile enzyme. Biochim. Biophys. Acta. 1993 Sep 8;1182(2):142–146. doi: 10.1016/0925-4439(93)90134-m. [DOI] [PubMed] [Google Scholar]
- 77.Narkis G., Adam A., Jaber L., Pennybacker M., Proia R.L., Navon R. Molecular basis of heat labile hexosaminidase B among Jews and Arabs. Hum. Mutat. 1997;10(6):424–429. doi: 10.1002/(SICI)1098-1004(1997)10:6<424::AID-HUMU2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 78.Okada S., O'Brien J.S. Generalized gangliosidosis: β-galactosidase deficiency. Science. 1968;160:1002–1004. doi: 10.1126/science.160.3831.1002. [DOI] [PubMed] [Google Scholar]
- 79.Caciotti A., Garman S.C., Rivera-Colón Y., Procopio E., Catarzi S., Ferri L., Guido C., Martelli P., Parini R., Antuzzi D., Battini R., Sibilio M., Simonati A., Fontana E., Salviati A., Akinci G., Cereda C., Dionisi-Vici C., Deodato F., d'Amico A., d'Azzo A., Bertini E., Filocamo M., Scarpa M., di Rocco M., Tifft C.J., Ciani F., Gasperini S., Pasquini E., Guerrini R., Donati M.A., Morrone A. GM1 gangliosidosis and Morquio B disease: an update on genetic alterations and clinical findings. Biochim. Biophys. Acta. 2011 Jul;1812(7):782–790. doi: 10.1016/j.bbadis.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kaye E.M., Shalish C., Livermore J., Taylor H.A., Stevenson R.E., Breakefield X.O. beta-Galactosidase gene mutations in patients with slowly progressive GM1 gangliosidosis. J. Child Neurol. 1997 Jun;12(4):242–247. doi: 10.1177/088307389701200404. [DOI] [PubMed] [Google Scholar]
- 81.Morrone A., Bardelli T., Donati M.A., Giorgi M., Di Rocco M., Gatti R., Parini R., Ricci R., Taddeucci G., D'Azzo A., Zammarchi E. beta-Galactosidase gene mutations affecting the lysosomal enzyme and the elastin-binding protein in GM1-gangliosidosis patients with cardiac involvement. Hum. Mutat. 2000;15(4):354–366. doi: 10.1002/(SICI)1098-1004(200004)15:4<354::AID-HUMU8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 82.Hofer D., Paul K., Fantur K., Beck M., Roubergue A., Vellodi A., Poorthuis B.J., Michelakakis H., Plecko B., Paschke E. Phenotype determining alleles in GM1 gangliosidosis patients bearing novel GLB1 mutations. Clin. Genet. 2010 Sep;78(3):236–246. doi: 10.1111/j.1399-0004.2010.01379.x. [DOI] [PubMed] [Google Scholar]