Key Points
Question
Is bilateral voretigene neparvovec-rzyl cost-effective compared with standard care for treating RPE65-mediated inherited retinal disease in the United States over a lifetime horizon?
Findings
In this economic analysis of data from 70 patients with genetically confirmed RPE65-mediated inherited retinal disease, voretigene neparvovec-rzyl was associated with lower total costs and higher quality-adjusted life-years compared with standard care. In addition, voretigene neparvovec-rzyl was cost-effective over a lifetime horizon using a threshold of $50 000 to $150 000 per quality-adjusted life-year, assuming that the health states across patients in year 3 subsequently did not change over a lifetime.
Meaning
These findings, with their assumptions and using a lifetime horizon, suggest that bilateral voretigene neparvovec-rzyl is cost-effective given its efficacy and the quality-of-life decrement associated with the natural course of inherited retinal disease.
Abstract
Importance
Voretigene neparvovec-rzyl, the first gene therapy approved by the US Food and Drug Administration, was approved for the treatment for RPE65-mediated inherited retinal disease (IRD) in December 2017. This gene therapy is associated with high up-front costs and high efficacy, although of unknown duration, and its cost-effectiveness has not been assessed with RPE65 IRD-specific, longitudinal, patient-observation-level data.
Objective
To assess the incremental cost-effectiveness ratio (ICER) of voretigene neparvovec-rzyl compared with standard care for RPE65-mediated inherited retinal disease.
Design, Setting, and Participants
In this economic analysis, a health state transition model based on visual acuity and field with a lifetime horizon was developed to estimate the cost-effectiveness of voretigene neparvovec-rzyl. The model was populated with data from a clinical trial of voretigene neparvovec-rzyl to evaluate treatment outcome and a natural history study of RPE65-mediated IRD to examine disease progression. Direct costs were derived from the literature. Indirect costs, including educational attainment, productivity, caregiver burden, and governmental programs, were estimated using published literature and data analysis of public national surveys. A health utility vignette study specific to RPE65-mediated IRD was used for health utility inputs. The cost-effectiveness study described in this article was conducted from September 15, 2017, to August 23, 2018.
Exposures
Bilateral voretigene neparvovec-rzyl therapy or standard care.
Main Outcomes and Measures
Incremental cost-effectiveness ratio.
Results
The model population included 70 patients with RPE65-mediated IRD, with a mean age of 15 years; 42 of 70 patients (60%) were female. In the base case, voretigene neparvovec-rzyl compared with standard care was associated with lower total costs ($2.2 million vs $2.8 million) and higher quality-adjusted life-years (18.1 vs 8.6). Voretigene neparvovec-rzyl remains cost-effective if at least 8.8% of the long-term treatment effect continues after year 3 when including indirect costs and 43.3% when excluding indirect costs, assuming a cost threshold of $150 000 per quality-adjusted life-year.
Conclusions and Relevance
Results of this study suggest that voretigene neparvovec-rzyl is cost-effective compared with standard care when using a lifetime horizon, excluding indirect costs, and using a threshold of $150 000 per quality-adjusted life-year.
This economic analysis evaluates the incremental cost-effectiveness ratio for use of voretigene neparvovec-rzyl vs standard care in patients with RPE65-mediated inherited retinal disease.
Introduction
Biallelic RPE65 (OMIM *180069) mutation-associated inherited retinal disease (IRD) is an ultrarare, severely progressive retinal condition that affects approximately 1000 to 2000 patients in the United States.1,2,3,4,5,6,7,8 Because this disease has been considered medically untreatable, management has focused on psychological support and visual rehabilitation, including low vision aids.
Voretigene Neparvovec-rzyl
Voretigene neparvovec-rzyl is the first gene therapy approved by the FDA to treat an inherited genetic disease. The US Food and Drug Administration (FDA) approved voretigene neparvovec-rzyl, a gene therapy administered by subretinal injection, in December 2017 for patients with confirmed biallelic RPE65-mediated IRD and sufficient viable retinal cells. The phase 3 randomized clinical trial for voretigene neparvovec-rzyl found statistically significant improvements in the multiluminance mobility test (a test of functional vision, the primary end point), full-field light sensitivity test, and visual field (VF) end points for 20 patients who received voretigene neparvovec-rzyl compared with 9 patients serving as controls over a 1-year period.9 Directional improvement in visual acuity (VA) was observed in patients who receive voretigene neparvovec-rzyl, but was not statistically significant.
Average multiluminance mobility test, full-field light sensitivity test, VF, and VA measures have been maintained for 3 years in the original interventional participants of the phase 3 trial, with follow-up ongoing.10,11 Anecdotal evidence suggests that vision improvement was maintained in some clinical trial participants followed up to 9 years after treatment12 and persistent effects have been noted for up to 11 years in canine models.13 A long-term benefit may be expected, as recombinant adeno-associated viruses, used as vectors in voretigene neparvovec-rzyl, “mediate long-term gene expression in the absence of an immune or inflammatory response.”14[p263]
Objectives
The potential for significant quality-of-life gains and indirect cost offsets with voretigene neparvovec-rzyl is accompanied by a substantial 1-time cost at administration. A 2019 cost-effectiveness model for voretigene neparvovec-rzyl15 did not use patient-level data to estimate the natural history of the disease or voretigene neparvovec-rzyl treatment effect, RPE65-mediated IRD-specific health utilities, or appropriate indirect costs. The authors concluded that voretigene neparvovec-rzyl is not cost-effective at its current price. Under similar assumptions, we developed a model, using more disease-specific data and inputs, to evaluate the cost-effectiveness of the therapy.
Methods
Model Structure
The cost-effectiveness study described in this article was conducted from September 15, 2017, to August 23, 2018. This analysis is considered exempt from institutional review board review as the research involved only the assessment of existing data that had been previously deidentified.
A closed-cohort health-state transition model was developed containing 6 states reflecting the course of RPE65-mediated IRD and mortality: moderate visual impairment (VI) (health state [HS] 1); severe VI (HS2); profound VI (HS3); count fingers (HS4); hand motion, light perception, or no light perception (HS5); and death. Health states were defined by patient VF, measured as sum total degrees of Goldmann VF test using the III4e marker, and VA, based on logMAR measurements using the Holladay scale or clinician’s assessment of count fingers, hand motion, light perception, or no light perception VA.16 Visual field and VA measurements mapped patients into the 6 health states, the definitions of which align with the American Medical Association Guides to the Evaluation of Permanent Impairment (eTable 1 and eTable 2 in the Supplement provide details).17 The eMethods in the Supplement has additional information on methodology.
Clinical Data
Transition probabilities representing disease progression and treatment efficacy were derived from 3 data sources with at least annual VF and VA measures. Transition probabilities for standard care (SC) came from a retrospective natural history study of 70 (42 [60%] female) patients with genetically confirmed RPE65-mediated IRD who met treatment criteria.18 Voretigene neparvovec-rzyl and SC treatment outcomes came from the randomized clinical phase 3 trial AAV2-hRPE65v2-301,19 which has been described elsewhere (18 of 31 [58%] female) (eTable 3 in the Supplement).3 Longer term outcomes came from voretigene neparvovec-rzyl extension study data.10,11
Modeled Patient Population
Patients initiated the model at the mean (SD) baseline age (15 [10.9] years; 54 of 91 females) in the phase 3 trial. The modeled cohort was distributed across the health states per the distribution of VF and VA measures at baseline in the trial: HS1, 4 (19%); HS2, 5 (24%); HS3, 6 (29%); HS4, 6 (29%); and HS5, 0.
Natural History Model of Vision Loss
Disease progression during SC was modeled using data from the natural history study of patient visits in which VA and VF were recorded. A parametric multistate survival model was estimated on the basis of observed health state transitions to derive the annual transition probability of disease progression for patients who received SC (eTable 4 in the Supplement provides details).20 By construct, the multistate survival model allows progression from less to more severe health states but restricts patients from regressing to less visually impaired states, consistent with disease progression. Estimations were conducted using the msset and predictms commands in Stata, IC version 15 (StataCorp).
Voretigene Neparvovec-rzyl Treatment Effect
The mean treatment effect, defined as the change in outcomes for voretigene neparvovec-rzyl minus placebo, was 378.7 (95% CI, 145.5-612.0) sum total degrees for Goldmann VF and −0.16 (95% CI, −0.41 to 0.08) logMAR for VA at the end of year 1 in the phase 3 trial.9 This effect was applied in the model such that after year 1, patients receiving voretigene neparvovec-rzyl transitioned from the baseline distribution across health states to a distribution reflecting the association between mean VA and VF changes observed in the trial. Standard errors were used in the probabilistic sensitivity analysis.
The modeled treatment effect matched trial observations in the first 3 years, followed by a long-term treatment effect defined by the probability of avoiding SC disease progression (ie, a 20% reduction in the long-term treatment effect suggests that 20% of the voretigene neparvovec-rzyl–treated cohort would progress at the same rate as SC-treated patients from year 3 onward). The base-case analysis assumed that the distribution of patients across health states in year 3 was maintained over the lifetime, thus, showing a 0% reduction in the treatment effect after 3 years, which is consistent with the available data. Scenario analyses considered the outcome of varying the rate of reduction in long-term treatment effect after year 3.
Mortality
Annual age-specific probabilities of general population mortality were sourced from United States life tables.21 Christ et al22 reported excess mortality risk associated with VI, estimated as relative risks, which were used to adjust age-specific general population probabilities (eTable 5 in the Supplement). We tested this excess mortality risk in sensitivity analysis.
Health Utilities
Unlike other serious ophthalmologic conditions, which commonly affect elderly individuals and predominantly cause loss of VA or VF, RPE65-mediated IRD affects children, causes both VF loss and VA loss, as well as nyctalopia, and progresses to complete blindness. The health utility literature in ophthalmology primarily assesses VI only through VA, focuses on older patients with age-related macular degeneration, diabetic macular edema, or glaucoma while excluding patients with no light perception, thus limiting its generalizability to RPE65-mediated IRD. To our knowledge, only 1 study estimated health utility values for an RPE65-mediated IRD population and was aligned with the health states in this model.23 Health utility derivation in this study was based on vignettes describing the health states. Expert elicitation was then performed, during which these descriptions were valued by 6 clinical IRD experts using a standardized health utility instrument developed by the EuroQol Group, the EQ-5D-5L. This vignette and expert elicitation approach has been used for other rare diseases where health-related quality of life data were limited or nonexistent.24,25
As a sensitivity analysis, we used health utilities from a large study of patients with a variety of ophthalmologic disorders; these health utilities were derived from time trade-off research based solely on VA and included patients with count fingers, hand motion, light perception, and no light perception.26
Direct Medical Costs
We used estimates of direct ophthalmic medical costs (more details presented in the eMethods in the Supplement) and ophthalmic-related medical costs (ie, depression and trauma) by VA categories from a study of 200 patients with neovascular age-related macular degeneration.27 Costs were inflation adjusted to 2018 using the medical care consumer price index.28
The cost of voretigene neparvovec-rzyl was modeled as the wholesale acquisition cost of $425 000 per eye. We assumed that all patients received treatment for both eyes, as per label and because bilateral treatment is current SC in other progressive ocular disorders. The total cost of administration was $4535.33, based on Medicare’s 2018 national average reimbursement rates29; it was assumed that the surgeon would be reimbursed for Current Procedural Terminology 67036 (vitrectomy, mechanical, pars plana approach; $924.83) and the hospital would be reimbursed for comprehensive ambulatory payment classification for a level 2 intraocular procedure ($3610.50).
Indirect Costs
Indirect cost estimates were sourced from a study that used national surveys to estimate productivity loss, caregiver burden, and government program loss for patients with IRD.30 Productivity loss was assumed to be zero for patients younger than 18 years. For patients aged 18 years or older, we relied on average productivity loss associated with VI and blindness by educational attainment and weighted it by the relative percentage of patients likely to have achieved a college degree vs a high school diploma for the reported VA categories.30 The values for these inputs are in eTable 6 and eTable 7 in the Supplement. Caregiver burden was reported by VA categories for children and adults (eTable 8 in the Supplement). Estimated indirect costs were compared for various stages of VI relative to a normally sighted population.30 Government loss, defined as excess costs incurred from government programs for individuals with VI or blindness, was reported in the study for those with VA less than 1 and greater than 1 logMAR and by age groups: 0 to 19, 20 to 64, and 65 years or older (eTable 9 in the Supplement).
Outcomes
The model estimates costs and quality-adjusted life-years (QALYs) for voretigene neparvovec-rzyl and SC patients over a lifetime horizon. Costs and benefits were discounted at 3.0% annually.31 Costs, utilities, and incremental values, including the incremental cost-effectiveness ratio (ICER), were calculated. Deterministic probabilistic sensitivity analyses were undertaken (inputs reported in Table 1). The probabilistic sensitivity analyses used 1000 simulations in a Monte Carlo analysis; SC transition probabilities were output from Stata and randomly sampled in Excel (Microsoft Corp). Cost-effectiveness acceptability curves were estimated.
Table 1. Summary of Variables Applied in the Cost-effectiveness Model.
| Variable | Value (SD) [Range] | Distribution Assumed in PSA | Source |
|---|---|---|---|
| Total No. | 21 | NA | Russell et al,9 2017a |
| HS1: moderate VI | 19 | ||
| HS2: severe VI | 24 | ||
| HS3: profound VI | 29 | ||
| HS4: CF | 29 | ||
| HS5: HM/LP/NLP, % | 0 | ||
| Age, y | 15 (10.9) [4-44] | Uniform | Russell et al,9 2017 |
| Progression rate during SC (natural history) | 1 [2-1000] | Uniform | Chung et al, al,18 2019 |
| VA | −0.16 (0.13) [−0.41 to 0.08] | Normal | Russell et al,9 2017 |
| VF | 378.7 (119.0) [145.5-612.0] | Normal | Russell et al,9 2017 |
| Reduction in long-term treatment effect after year 3 (%) | Lifetime (0) [0-100] | NA | Assumption |
| VI mortality rate | Christ et al,22 2014 | ||
| HS1: moderate VI hazard rate | 1.08 [1.02-1.15] | ||
| HS2: severe VI hazard rate | 1.18 [1.05-1.32] | ||
| HS3: profound VI hazard rate | 1.18 [1.05-1.32] | ||
| HS4: CF hazard rate | 1.18 [1.05-1.32] | ||
| HS5: HM/LP/NLP hazard rate | 1.18 [1.05 to 1.32] | ||
| HS1: moderate VI | 0.71 (0.04) [0.63-0.78] | β | Lloyd et al,23 2019 |
| HS2: severe VI | 0.62 (0.02) [0.58-0.65] | β | |
| HS3: profound VI | 0.52 (0.03) [0.46-0.57] | β | |
| HS4: CF | 0.35 (0.03) [0.30-0.40] | β | |
| HS5: HM/LP/NLP | 0.15 (0.05) [0.06-0.24] | β | |
| HS1: moderate VI | 8818 (882) [7055-10 582] | γ | Brown et al,27 2016 |
| HS2: severe VI | 11 573 (1157) [9259-13 888] | γ | |
| HS3: profound VI | 12 655 (1266) [10 124-15 186] | γ | |
| HS4: CF | 13 737 (1374) [10 990-16 485] | γ | |
| HS5: HM/LP/NLP | 13 737 (1374) [10 990-16 485] | γ | |
| Age <18 y | Jensen et al,30 2018 | ||
| HS1: moderate VI | 10 005 (1000) [8004-12 006] | γ | |
| HS2: severe VI | 56 883 (5688) [45 507-68 260] | γ | |
| HS3: profound VI | 56 883 (5688) [45 507-68 260] | γ | |
| HS4: CF | 74 877 (7488) [59 901-89 852] | γ | |
| HS5: HM/LP/NLP | 74 877 (7488) [59 901-89 852] | γ | |
| Age ≥18 y | Jensen et al,30 2018 | ||
| HS1: moderate VI | 24 191 (2419) [19 353-29 029] | γ | |
| HS2: severe VI | 60 226 (6023) [48 181-72 271] | γ | |
| HS3: profound VI | 60 226 (6023) [48 181-72 271] | γ | |
| HS4: CF | 95 184 (9518) [76 147 -114 221] | γ | |
| HS5: HM/LP/NLP | 95 184 (9518) [76 147-114 221] | γ | |
| Discount rate, annual, costs, % | 3.0 | NA | Sanders et al,31 2016 |
| Discount rate, annual, utilities, % | 3.0 | NA | |
| Voretigene neparvovec-rzyl, price per injection, $b | 854 535 | NA |
Abbreviations: CF, count fingers; EQ-5D, EuroQol 5-dimension, 5-level standard for valuing quality of life; HM, hand motion; HS, health state; LP, light perception; NA, not applicable; NLP, no light perception; PSA, probabilistic sensitivity analyses; SC, standard care; VA, visual acuity; VF, visual field; VI, visual impairment.
Analysis of baseline visual acuity and visual field data from AAV2-hRPE65v2-301.19
Cost of voretigene neparvovec-rzyl based on published wholesale acquisition cost, cost of administration based on Medicare 2018 average national reimbursement for the Comprehensive Ambulatory Payment Classification for a level 2 intraocular procedure and Current Procedural Terminology 67036, vitrectomy, mechanical, pars plana approach.
Results
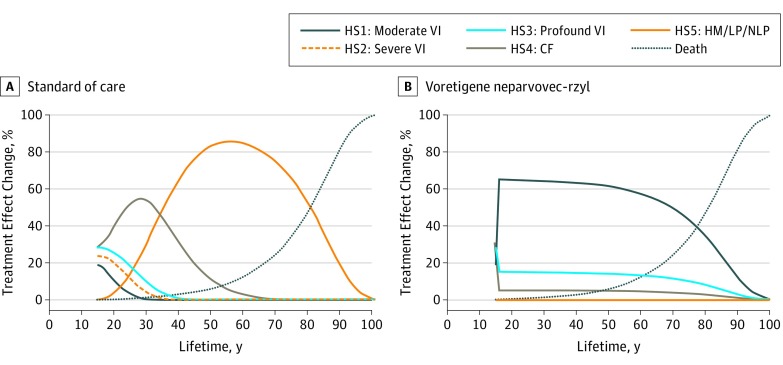
The model population included 70 patients with RPE65-mediated IRD, with a mean age of 15 years; 42 of 70 patients (60%) were female. The base case assumptions for the cost-effectiveness model are provided in Table 1. Voretigene neparvovec-rzyl had higher QALYs (18.1 vs 8.6) and lower total lifetime costs ($2.2 million vs $2.8 million) than SC (ie, was dominant) when including indirect costs, resulting in cost-savings and QALY increases. When indirect costs were excluded, the cost per QALY was $79 618. Table 2 contains results for the base case and scenario analyses varying the duration of treatment effect after year 3, evaluated with and without indirect costs (eFigure 1 in the Supplement shows continuous traces). The Markov traces for the base case scenario are presented in Figure 1 (additional scenarios in eFigure 2 and external validation discussed in eAppendix 1 in the Supplement). The model predicted that most untreated patients had progressed to VI of hand motion or worse by their early to mid-30s, which was consistent with expert opinion and the natural history analysis.18
Table 2. Results of the Base Case Analysis and Scenario Analyses Varying the Reduction in the Long-term Treatment Effect After Year 3.
| Variable | Cost Comparison, $a | ||
|---|---|---|---|
| Voretigene Neparvovec-rzyl | SC | Incremental Value (Voretigene Neparvovec-rzyl − SC) | |
| Base Case, Lifetime Treatment Effect | |||
| Direct medical costs, $ | 301 794 | 406 404 | − 104 610 |
| Drug | 854 535 | 0 | 854 535 |
| Indirect | 1 063 739 | 2 373 702 | −1 309 963 |
| Total | 2 220 069 | 2 780 106 | −560 038 |
| QALYs | 18.1 | 8.6 | 9.4 |
| ICER | |||
| With indirect costs | NA | NA | −59 458 Voretigene neparvovec-rzyl lower costs and higher QALYs |
| Without indirect costs | NA | NA | 79 618 |
| Voretigene Neparvovec-rzyl With 5% Reduction in Long-term Treatment Effect After Year 3 | |||
| Direct medical costs, $ | 305 780 | 406 404 | −100 625 |
| Drug | 854 535 | 0 | 854 535 |
| Indirect | 1 113 916 | 2 373 702 | −1 259 786 |
| Total | 2 274 232 | 2 780 106 | − 505 875 |
| QALYs | 17.7 | 8.6 | 9.1 |
| ICER | |||
| With indirect costs | NA | NA | − 55 869 |
| Without indirect costs | NA | NA | 83 262 |
| Voretigene Neparvovec-rzyl With 10% Reduction in Long-term Treatment Effect After Year 3 | |||
| Direct medical costs, $ | 309 763 | 406 404 | −96 641 |
| Drug | 854 535 | 0 | 854 535 |
| Indirect | 1 164 064 | 2 373 702 | − 1 209 638 |
| Total | 2 328 362 | 2 780 106 | −451 744 |
| QALYs | 17.3 | 8.6 | 8.7 |
| ICER | |||
| With indirect costs | NA | NA | −51 981 |
| Without indirect costs | NA | NA | 87 209 |
| Voretigene Neparvovec-rzyl With 50% Reduction in Long-term Treatment Effect After Year 3 | |||
| Direct medical costs, $ | 341 550 | 406 404 | −64 855 |
| Drug | 854 535 | 0 | 854 535 |
| Indirect | 1 564 180 | 2 373 702 | −809 522 |
| Total | 2 760 265 | 2 780 106 | −19 842 |
| QALYs | 14.4 | 8.6 | 5.8 |
| ICER | |||
| With indirect costs | −3429 | ||
| Without indirect costs | 136 452 | ||
| Voretigene Neparvovec-rzyl With 100% Reduction in Long-term Treatment Effect After Year 3 | |||
| Direct medical costs, $ | 381 087 | 406 404 | −25 318 |
| Drug | 854 535 | 0 | 854 535 |
| Indirect | 2 061 709 | 2 373 702 | −311 993 |
| Total | 3 297 331 | 2 780 106 | 517 225 |
| QALYs | 10.8 | 8.6 | 2.2 |
| ICER | |||
| With indirect costs | 237 140 | ||
| Without indirect costs | 380 185 | ||
Abbreviations: ICER, incremental cost-effectiveness ratio; QALY, quality-adjusted life-year; SC, standard care; TE, treatment effect; VI, visual impairment.
Values are discounted.
Figure 1. Base Case Analysis.
CF indicates count fingers; HM, hand motion; HS, health state; LP, light perception; NLP, no light perception; and VI, visual impairment.
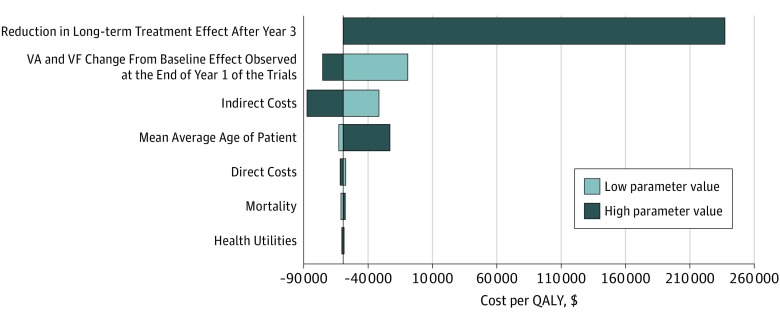
Assuming complete loss of the treatment effect immediately after the last trial observation in year 3 resulted in ICERs of $237 140 and $380 185 per QALY with and without consideration of indirect costs, respectively. Depending on the assumed rate of reversion to SC, the ICERs ranged between these values. Voretigene neparvovec-rzyl remains cost-effective if at least 8.8% of the long-term treatment effect continues after year 3 when including indirect costs and 43.3% when excluding indirect costs, assuming a cost per QALY threshold of $150 000 (eAppendix 2 in the Supplement provides more detail). The deterministic sensitivity analysis results appear in Figure 2. Consistent with Table 2, the largest individual driver of the model results involves the assumption around the reduction in the long-term treatment effect. Indirect costs were also an important determinant of the model. The deterministic sensitivity analysis reported a range based on variation of 20% in indirect costs, and the ICER remained negative for the lowest indirect cost values in the base case. Direct costs, as well as variation in the relative risk of death due to visual impairment, did not substantially influence the results (eTable 5, eTable 10, and eTable 11 in the Supplement).
Figure 2. Deterministic Sensitivity Analysis.
Reference incremental cost ratio, −$59 458. VA indicates visual acuity; VF, visual field; QALY, quality-adjusted life-year.
Health utilities had a small association with the ICER owing to relatively low SEs around the values. Selecting health utilities from other sources, such as Brown et al26 or Sharma et al,32 reduced the incremental QALYs gained by voretigene neparvovec-rzyl compared with SC to 6.7 and 3.2, respectively.
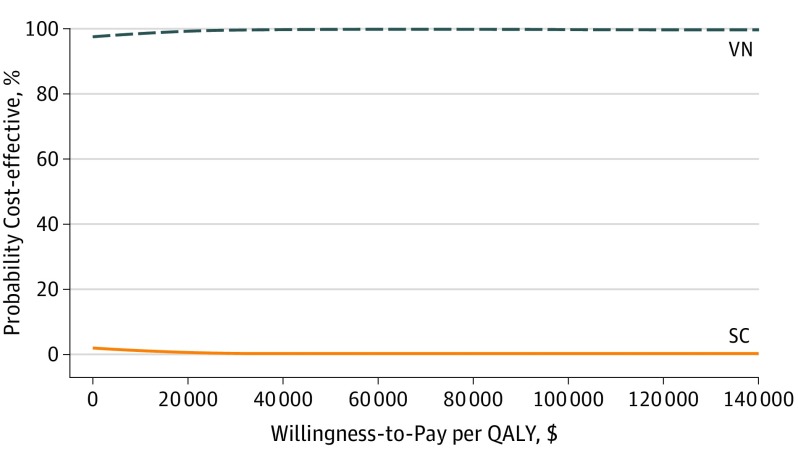
The probabilistic sensitivity analyses appear in Figure 3. Voretigene neparvovec-rzyl was cost-effective under typical ICER thresholds when assuming a lifetime treatment effect in the probabilistic sensitivity analyses. The cost-effectiveness acceptability curves for the sensitivities on the disease progression of patients after 3 years appear in eFigure 3 in the Supplement.
Figure 3. Probabilistic Sensitivity Analyses.
QALY indicates quality-adjusted life-year; SC, standard care; and VN, voretigene neparvovec-rzyl.
Discussion
Voretigene neparvovec-rzyl had lower costs and higher QALYs than SC for the treatment of RPE65-mediated IRD under base case assumptions. Our sensitivity analyses generally suggest that voretigene neparvovec-rzyl remains cost-effective at traditional thresholds after modification of model parameters, including sensitivity analysis of the treatment effect duration and excluding indirect costs. Results did not hold at traditional cost-per-QALY thresholds if the treatment effect was assumed to end at year 3. The assumption of immediate loss of the treatment effect is likely nonphysiologic but serves as a limit for sensitivity analysis. Voretigene neparvovec-rzyl restores vision such that even if progression begins anew, the therapy delivers QALY gains vs an untreated patient.
The indirect costs considered in this analysis were broad relative to most cost-effectiveness analyses, encompassing productivity, educational attainment, caregiver burden, and government programs. The disease usually presents in pediatric patients and rapidly and severely affects patients’ vision. Visual impairment is associated with reduced educational attainment and lower earnings.30 Caregiver burden was economically substantial for caregivers of both children and adults with VI. The cumulative effect of fewer educational options, lower educational achievement, reduced earnings across educational strata, and high caregiver needs, often from an early age, yielded a large lifetime indirect cost per patient.
The incremental QALY changes in the base case were the result of the high lifetime utility loss associated with RPE65-mediated IRD. The scale of the incremental QALY gain from the therapy is greater than that associated with most specialty drugs, but in line with other therapies for ultrarare pediatric conditions.33,34
The costs were driven by the price of the therapy, which, based on the current buy-and-bill practice in the United States, would be incurred in the first year of treatment. However, the benefits, including incremental QALY gains and indirect cost offsets associated with voretigene neparvovec-rzyl, would be distributed over patients’ lifetimes and driven largely by assumptions around the treatment effect duration. Uncertainty surrounding duration of treatment effect was the major driver of results in the assessment of cost-effectiveness for voretigene neparvovec-rzyl, and we expect that will be the case for future gene therapies developed for inherited conditions. We examined a range of scenarios up to and including stopping the therapeutic effect immediately for all patients at the end of year 3. The therapy continued to be cost-effective at a $150 000 per QALY threshold unless substantial reductions in efficacy were assumed. Some have argued that the threshold for ultrarare diseases and curative therapies should be higher than for other therapies (eAppendix 2 in the Supplement). The available evidence does not indicate that the voretigene neparvovec-rzyl treatment effect ends at year 3.12
The US Institute for Clinical and Economic Review (ICER-US) presented a base case cost-effectiveness model of voretigene neparvovec-rzyl from the modified societal perspective with an ICER of $480 130 for a patient aged 15 years.35 However, most of the ICER-US Comparative Effectiveness Public Advisory Council voted that voretigene neparvovec-rzyl provided “intermediate long-term value for money” despite the high ICER, likely owing to the wide range of ICER values in the sensitivity analyses. Zimmermann et al15 published the ICER model results. As noted by Brown and Brown,36 the choice of health utilities substantially affects results; specifically, the difference between the estimates presented by Zimmermann et al15 and those presented herein can be explained by choice of health utilities. Those described by Zimmermann et al15 did not account for the significant reduction in utility associated with complete blindness and were not sourced from a population of patients with RPE65-mediated IRD.
Our results depended on a lifetime model horizon. A lifetime horizon is consistent with guidelines,37,38,39 for example, “the time horizon adopted in a comparative effectiveness assessment should be long enough to capture all relevant future effects of a health care intervention.”40[p109] Given the progressive nature of RPE65-IRD and the duration of voretigene neparvovec-rzyl treatment effect suggested by available data, future morbidity improvement is reasonable.
Aside from cost-effectiveness, other factors should guide payers’ decisions on covering voretigene neparvovec-rzyl. Approximately 1000 to 2000 individuals in the United States have RPE65-mediated IRD, yet it is unlikely that all would be eligible for voretigene neparvovec-rzyl therapy.9 Nonetheless, treating 2000 patients with RPE65-mediated IRD would be an expected 1-time cost of $1.7 billion, or $5.2 million for a private payer with 1 million enrollees. In addition, given the disproportionate value of insurance for low-probability, high-cost events, the value of insurance coverage of voretigene neparvovec-rzyl for this ultrarare condition is likely greater than the actual cost to a prospective enrollee.41
Limitations
This study has several limitations typical of ultrarare disease research, including sparse data on current practices, limited RPE65-mediated IRD-specific data, and small clinical trials. The health utility analysis was based on a vignette approach because health utility data were not collected in the clinical trials. This approach has been used previously in rare diseases.24,25 An additional limitation involves the direct medical cost estimates, which were sourced from patients with neovascular age-related macular degeneration, who are older and have more comorbidities than a RPE65-mediated IRD population. Furthermore, the estimates were only based on VA-defined levels of VI, and thus not directly analogous to the health states in our model. We assumed patients received bilateral voretigene neparvovec-rzyl treatment per FDA approval. Patients received bilateral injections in the voretigene neparvovec-rzyl clinical trials.9 The natural history study and health utilities research highlight the bilateral progressive nature of the disorder20,23 and suggest that a patient’s ability to compensate with her or his better-seeing eye is less likely than in other forms of VI.
Conclusions
Many ocular gene therapies in clinical trials have the potential to yield substantial QALY gains and indirect cost savings over patients’ lives but with an upfront treatment cost. Although there are challenges in valuing chronically administered medications, therapies that deliver long-lasting benefits in 1 administration may be more problematic from a cost-effectiveness perspective. We recommend analysis of treatment effect durability, appropriate selection of disease-specific utilities, and careful inclusion of indirect costs for future assessments of similar therapies.
eTable 1. Health State Cutpoints for Radius and Sum Total Degrees of the Patient’s Visual Field
eTable 2. Definition of Health States
eMethods. Further Details
eTable 3. Baseline Characteristics for Visual Impairment Analysis
eTable 4. Multistate Survival Model Coefficient Estimates From Natural History Analysis: Progression Estimates for SC Treated Patients
eTable 5. Relative Risk of Dying for Each Health State
eTable 6. Annual Productivity Loss for Visually Impaired and Blind Individuals, by Level of Educational Attainment
eTable 7. Completion Rates of High School and College, by VA Level
eTable 8. Caregiver Productivity Loss, by VA Range and Age Group of Their Patient
eTable 9. Annual Per-Patient Total Cost of Government Programs, by Age and VA Level
eFigure 1. Percentage Reduction in Long-term Treatment Effect After Year Three and ICER With and Without Indirect Costs
eFigure 2. Markov Traces for Sensitivity Analyses Varying Duration of Long-term Treatment Effect
eAppendix 1. External Validity of Natural History Analysis
eAppendix 2. Notes About Cost-Effectiveness Thresholds for Cost-Effectiveness Analysis Comparing Voretigene Neparvovec to Standard Care
eTable 10. Incremental Cost-effectiveness Ratio (ICER) of VN vs. SC in Two-Way Sensitivity Analysis of VN and SC Costs, Excluding Indirect Costs
eTable 11. Incremental Cost-effectiveness Ratio (ICER) of VN vs. SC in Two-Way Sensitivity Analysis of VN and SC Costs, Including Indirect Costs
eFigure 3. Cost-effectiveness Acceptability Curves for Sensitivity Analyses on the PSA
eReferences
References
- 1.Census US. US and world population clock. https://www.census.gov/popclock/. Published July 4, 2018. Accessed November 29, 2018.
- 2.Fahim A, Daiger S, Weleber R. Nonsyndromic retinitis pigmentosa overview. In: Pagon R, Adam M, Ardinger H, et al, eds. GeneReviews. Seattle, WA: University of Washington; 2017. http://www.ncbi.nlm.nih.gov/books/NBK1417/. Updated January 19, 2017. Accessed August 13, 2018.
- 3.Stone EM. Leber congenital amaurosis—a model for efficient genetic testing of heterogeneous disorders: LXIV Edward Jackson Memorial Lecture. Am J Ophthalmol. 2007;144(6):791-811. doi: 10.1016/j.ajo.2007.08.022 [DOI] [PubMed] [Google Scholar]
- 4.Wang F, Wang H, Tuan HF, et al. Next generation sequencing-based molecular diagnosis of retinitis pigmentosa: identification of a novel genotype-phenotype correlation and clinical refinements. Hum Genet. 2014;133(3):331-345. doi: 10.1007/s00439-013-1381-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Simovich MJ, Miller B, Ezzeldin H, et al. Four novel mutations in the RPE65 gene in patients with Leber congenital amaurosis. Hum Mutat. 2001;18(2):164. doi: 10.1002/humu.1168 [DOI] [PubMed] [Google Scholar]
- 6.Morimura H, Fishman GA, Grover SA, Fulton AB, Berson EL, Dryja TP. Mutations in the RPE65 gene in patients with autosomal recessive retinitis pigmentosa or Leber congenital amaurosis. Proc Natl Acad Sci U S A. 1998;95(6):3088-3093. doi: 10.1073/pnas.95.6.3088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thompson DA, Gyürüs P, Fleischer LL, et al. Genetics and phenotypes of RPE65 mutations in inherited retinal degeneration. Invest Ophthalmol Vis Sci. 2000;41(13):4293-4299. [PubMed] [Google Scholar]
- 8.Astuti GD, Bertelsen M, Preising MN, et al. Comprehensive genotyping reveals RPE65 as the most frequently mutated gene in Leber congenital amaurosis in Denmark. Eur J Hum Genet. 2016;24(7):1071-1079. doi: 10.1038/ejhg.2015.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Russell S, Bennett J, Wellman JA, et al. Efficacy and safety of voretigene neparvovec (AAV2-hRPE65v2) in patients with RPE65-mediated inherited retinal dystrophy: a randomised, controlled, open-label, phase 3 trial. Lancet. 2017;390(10097):849-860. doi: 10.1016/S0140-6736(17)31868-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bennett J, Wellman J, Marshall KA, et al. Safety and durability of effect of contralateral-eye administration of AAV2 gene therapy in patients with childhood-onset blindness caused by RPE65 mutations: a follow-on phase 1 trial. Lancet. 2016;388(10045):661-672. doi: 10.1016/S0140-6736(16)30371-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Testa F, Maguire AM, Rossi S, et al. Three-year follow-up after unilateral subretinal delivery of adeno-associated virus in patients with Leber congenital amaurosis type 2. Ophthalmology. 2013;120(6):1283-1291. doi: 10.1016/j.ophtha.2012.11.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.67th Meeting of the Cellular, Tissue, and Gene Therapies Advisory Committee [transcript]. https://www.fda.gov/media/109384/download. October 12, 2017. Accessed June 14, 2019.
- 13.DiCarlo JE, Mahajan VB, Tsang SH. Gene therapy and genome surgery in the retina. J Clin Invest. 2018;128(6):2177-2188. doi: 10.1172/JCI120429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hildinger M, Auricchio A. Advances in AAV-mediated gene transfer for the treatment of inherited disorders. Eur J Hum Genet. 2004;12(4):263-271. doi: 10.1038/sj.ejhg.5201153 [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann M, Lubinga SJ, Banken R, et al. Cost utility of voretigene neparvovec for biallelic RPE65-mediated inherited retinal disease. Value Health. 2019;22(2):161-167. doi: 10.1016/j.jval.2018.09.2841 [DOI] [PubMed] [Google Scholar]
- 16.Holladay JT. Visual acuity measurements. J Cataract Refract Surg. 2004;30(2):287-290. doi: 10.1016/j.jcrs.2004.01.014 [DOI] [PubMed] [Google Scholar]
- 17.Cocchiarella L, Anderson GB. Guides to the Evaluation of Permanent Impairment. 5th ed. Chicago: American Medical Association; 2001. [Google Scholar]
- 18.Chung DC, Bertelsen M, Lorenz B, et al. The natural history of inherited retinal dystrophy due to biallelic mutations in the RPE65 gene. Am J Ophthalmol. 2019;199:58-70. doi: 10.1016/j.ajo.2018.09.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.ClinicalTrials.gov . Safety and Efficacy Study in Subjects With Leber Congenital Amaurosis. NCT00999609. https://clinicaltrials.gov/ct2/show/NCT00999609. Accessed June 6, 2019.
- 20.Crowther MJ, Lambert PC. Parametric multistate survival models: flexible modelling allowing transition-specific distributions with application to estimating clinically useful measures of effect differences. Stat Med. 2017;36(29):4719-4742. doi: 10.1002/sim.7448 [DOI] [PubMed] [Google Scholar]
- 21.Arias E, Heron M, Xu J. National Vital Statistics Report—United States Life Tables, 2014. Volume 66, Number 4, August 14, 2017. https://www.cdc.gov/nchs/data/nvsr/nvsr66/nvsr66_04.pdf. Accessed August 9, 2018. [PubMed]
- 22.Christ SL, Zheng DD, Swenor BK, et al. Longitudinal relationships among visual acuity, daily functional status, and mortality: the Salisbury Eye Evaluation Study. JAMA Ophthalmol. 2014;132(12):1400-1406. doi: 10.1001/jamaophthalmol.2014.2847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lloyd A, Piglowska N, Ciulla T, et al. Estimation of impact of RPE65-mediated inherited retinal disease on quality of life and the potential benefits of gene therapy. [published online January 18, 2019]. Br J Ophthalmol. 2019;bjophthalmol-2018-313089. doi: 10.1136/bjophthalmol-2018-313089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pastores G, Bracken J, Hughes J, et al. Estimation of resource use and quality of life in phenylketonuria (PKU) patients in Ireland [abstract 262]. J Inborn Errors Metab Screen. 2017;5(suppl):118. [Google Scholar]
- 25.Lloyd A, Tomazos I, Gallop K, Moseley S, Donato BMK, L’Italien G. Effect of asfotase alfa treatment on health states and ambulatory function in patients with hypophosphatasia [abstract SUO334]. In: 2017 Abstracts: 2017 Meeting of the American Society for Bone and Mineral Research, 2017:S282. [Google Scholar]
- 26.Brown MM, Brown GC, Sharma S, Landy J. Health care economic analyses and value-based medicine. Surv Ophthalmol. 2003;48(2):204-223. doi: 10.1016/S0039-6257(02)00457-5 [DOI] [PubMed] [Google Scholar]
- 27.Brown MM, Brown GC, Lieske HB, Tran I, Turpcu A, Colman S. Societal costs associated with neovascular age-related macular degeneration in the United States. Retina. 2016;36(2):285-298. doi: 10.1097/IAE.0000000000000717 [DOI] [PubMed] [Google Scholar]
- 28.FRED Economic Research. Consumer Price Index for All Urban Consumers: Medical Care [CPIMEDNS]. https://fred.stlouisfed.org/series/CPIMEDNS. Accessed June 19, 2018.
- 29.CMS.gov . License for Use of Current Procedural Terminology, Fourth Edition (“CPT”). https://www.cms.gov/apps/physician-fee-schedule/search/search-results.aspx?Y=1&T=0&HT=0&CT=3&H1=67036&M=5. Accessed June 14, 2019.
- 30.Jensen IS, Zacherle E, Nonne J, Blanchette C. Estimating the life-time indirect costs of vision impairment (VI) in inherited retinal degeneration (IRD): economic impact on education, government benefit programs, productivity and tax loss for patients and caregiver burden. Presented at: American Academy of Ophthalmology; October 28, 2018.
- 31.Sanders GD, Neumann PJ, Basu A, et al. Recommendations for conduct, methodological practices, and reporting of cost-effectiveness analyses: second panel on cost-effectiveness in health and medicine. JAMA. 2016;316(10):1093-1103. doi: 10.1001/jama.2016.12195 [DOI] [PubMed] [Google Scholar]
- 32.Sharma S, Oliver-Fernandez A, Bakal J, Hollands H, Brown GC, Brown MM. Utilities associated with diabetic retinopathy: results from a Canadian sample. Br J Ophthalmol. 2003;87(3):259-261. doi: 10.1136/bjo.87.3.259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chambers JD, Thorat T, Pyo J, Chenoweth M, Neumann PJ. Despite high costs, specialty drugs may offer value for money comparable to that of traditional drugs. Health Aff (Millwood). 2014;33(10):1751-1760. doi: 10.1377/hlthaff.2014.0574 [DOI] [PubMed] [Google Scholar]
- 34.Towse A, Garau M. Appraising ultra-orphan drugs: is cost-per-qaly appropriate? a review of the evidence. Office of Health Economics. https://www.ohe.org/system/files/private/publications/468%20-%20Appraising%20ultra-orphan%20drugs.pdf. Published March 2018. Accessed May 3, 2019.
- 35.Institute for Clinical and Economic Review . A look at vorentigene neparvovec. https://icer-review.org/wp-content/uploads/2018/02/MWCEPAC_Voretigene_RAAG_021418.pdf. Published February 2018. Accessed July 26, 2018.
- 36.Brown G, Brown M. Comment on voretigene neparvovec for biallelic RPE65-mediated retinal disease: effectiveness and value. Center for Value-Based Medicine. Draft Evidence Report, November 13, 2017. https://icer-review.org/wp-content/uploads/2017/06/MWCEPAC_VORETIGENE_DRAFT_EVIDENCE_REPORT_11152017.pdf. Published December 11, 2017. Accessed June 14, 2019.
- 37.Caro JJ, Briggs AH, Siebert U, Kuntz KM; ISPOR-SMDM Modeling Good Research Practices Task Force . Modeling good research practices—overview: a report of the ISPOR-SMDM Modeling Good Research Practices Task Force-1. Med Decis Making. 2012;32(5):667-677. doi: 10.1177/0272989X12454577 [DOI] [PubMed] [Google Scholar]
- 38.Briggs A, Sculpher M, Claxton K. Decision Modelling for Health Economic Evaluation. New York, NY: Oxford University Press; 2006. [Google Scholar]
- 39.Basu A, Maciejewski ML. Choosing a time horizon in cost and cost-effectiveness analyses. JAMA. 2019;321(11):1096-1097. doi: 10.1001/jama.2019.1153 [DOI] [PubMed] [Google Scholar]
- 40.Neumann PJ, Sanders GD, Russell LB, Siegel JE, Ganiats TG, eds. Cost-effectiveness in Health and Medicine, Second Edition. New York, NY: Oxford University Press; 2016. [Google Scholar]
- 41.Lakdawalla D, Malani A, Reif J. The insurance value of medical innovation. J Public Econ. 2017;145:94-102. doi: 10.1016/j.jpubeco.2016.11.012 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Health State Cutpoints for Radius and Sum Total Degrees of the Patient’s Visual Field
eTable 2. Definition of Health States
eMethods. Further Details
eTable 3. Baseline Characteristics for Visual Impairment Analysis
eTable 4. Multistate Survival Model Coefficient Estimates From Natural History Analysis: Progression Estimates for SC Treated Patients
eTable 5. Relative Risk of Dying for Each Health State
eTable 6. Annual Productivity Loss for Visually Impaired and Blind Individuals, by Level of Educational Attainment
eTable 7. Completion Rates of High School and College, by VA Level
eTable 8. Caregiver Productivity Loss, by VA Range and Age Group of Their Patient
eTable 9. Annual Per-Patient Total Cost of Government Programs, by Age and VA Level
eFigure 1. Percentage Reduction in Long-term Treatment Effect After Year Three and ICER With and Without Indirect Costs
eFigure 2. Markov Traces for Sensitivity Analyses Varying Duration of Long-term Treatment Effect
eAppendix 1. External Validity of Natural History Analysis
eAppendix 2. Notes About Cost-Effectiveness Thresholds for Cost-Effectiveness Analysis Comparing Voretigene Neparvovec to Standard Care
eTable 10. Incremental Cost-effectiveness Ratio (ICER) of VN vs. SC in Two-Way Sensitivity Analysis of VN and SC Costs, Excluding Indirect Costs
eTable 11. Incremental Cost-effectiveness Ratio (ICER) of VN vs. SC in Two-Way Sensitivity Analysis of VN and SC Costs, Including Indirect Costs
eFigure 3. Cost-effectiveness Acceptability Curves for Sensitivity Analyses on the PSA
eReferences