Abstract
OBJECTIVE
To determine associations of a Mediterranean diet score (MeDS) with 2-year change in cognitive function by type 2 diabetes and glycemic control status and contrast it against other diet quality scores.
RESEARCH DESIGN AND METHODS
We used data from the longitudinal Boston Puerto Rican Health Study (n = 913; 42.6% with type 2 diabetes at 2 years). Glycemic control at baseline was categorized as uncontrolled (hemoglobin A1c ≥7% [53 mmol/mol]) versus controlled. Two-year change in glycemic control was defined as stable/improved versus poor/declined. We defined MeDS, Healthy Eating Index, Alternate Healthy Eating Index, and Dietary Approaches to Stop Hypertension scores. Adjusted mixed linear models assessed 2-year change in global cognitive function z score, executive and memory function, and nine individual cognitive tests.
RESULTS
Higher MeDS, but no other diet quality score, was associated with higher 2-year change in global cognitive function in adults with type 2 diabetes (β ± SE = 0.027 ± 0.011; P = 0.016) but not in those without (P = 0.80). Similar results were noted for Mini-Mental State Examination, word recognition, digit span, and clock drawing tests. Results remained consistent for individuals under glycemic control at baseline (0.062 ± 0.020; P = 0.004) and stable/improved over 2 years (0.053 ± 0.019; P = 0.007), but not for individuals with uncontrolled or poor/declined glycemic control. All diet quality scores were associated with higher 2-year memory function in adults without type 2 diabetes.
CONCLUSIONS
Both adhering to a Mediterranean diet and effectively managing type 2 diabetes may support optimal cognitive function. Healthy diets, in general, can help improve memory function among adults without type 2 diabetes.
Introduction
Consuming a healthy diet, such as fruit and vegetables rich in antioxidants and vitamins and healthy fats, protects against cognitive decline and Alzheimer disease (1–3), whereas diets high in saturated fat and sugar have been related to poor cognitive function (3,4). As nutritional epidemiology emphasizes the overall intake of foods as a contributing factor to disease, rather than single food groups or nutrients, various scores that comprise diet quality as a whole have been defined to assess the relationship with cognitive function. In particular, consuming foods and nutrients characteristic of the Mediterranean dietary pattern has been consistently associated with better cognitive function among adults and older adults (5–7). Other diet quality scores have also been assessed for association with cognitive outcomes, for example, the Healthy Eating Index (HEI), the Alternate Healthy Eating Index (AHEI), and the Dietary Approaches to Stop Hypertension (DASH); however, the evidence with these scores has been inconsistent (6,8–10), and the bulk of the evidence rests on the Mediterranean diet (11).
Adults with prevalent type 2 diabetes are more likely to have lower cognitive function than those without (12). Glycemic control in type 2 diabetes may further influence this, as a higher concentration of hemoglobin A1c has been associated with decline in global cognitive function and with lower memory and executive function (13,14). Vascular damage and metabolic disturbances in type 2 diabetes have been proposed as contributors to these negative outcomes (12). As consuming a Mediterranean diet has been associated with prevention and control of type 2 diabetes (15,16), this dietary pattern may have dual benefits for both type 2 diabetes and cognition. However, it is unclear if the pathways linking the Mediterranean dietary components to cognitive function are similar for individuals with versus without type 2 diabetes or by level of glycemic control, given the different vascular and metabolic underlying states. Furthermore, it is also unknown if the Mediterranean diet definition captures the dietary components associated with better cognitive function by type 2 diabetes status or if other diet quality scores comprise foods and nutrients that are also important for cognition depending on type 2 diabetes status.
We aimed to determine associations of a Mediterranean diet score (MeDS) with 2-year change in cognitive function by type 2 diabetes and glycemic control status and to contrast it against other diet quality scores. We hypothezised that diet quality is associated differentially with 2-year cognition, according to diet quality score, cognitive function test, and status of type 2 diabetes and glycemic control, as the dietary components of each score may operate differently on each cognitive function outcome, and underlying physiology may have further influence. Based on the evidence, we also hypothesized that the MeDS includes dietary components more relevant for cognitive function changes than the other diet quality scores.
We conducted this analysis in a cohort of middle-aged and older Puerto Rican adults living on the mainland U.S., for which both high prevalence of type 2 diabetes and cognitive impairment have been reported (17). In this population, the prevalence of cognitive impairment was higher in individuals with type 2 diabetes than without (18). Moreover, Puerto Ricans on the mainland U.S. have been shown to have poorer diet quality (19) and to be less likely to achieve glycemic control (20) than non-Hispanic whites or other U.S. Hispanics/Latinos. We have previously reported cross-sectional positive associations between MeDS and HEI and better cognitive function in this cohort (21), but we have also reported variations in association of diet quality scores with 2-year changes in cardiometabolic factors (22), with the MeDS being more strongly associated with these factors than other scores. Thus, contrasting diet quality scores in association with change in cognitive function may help us identify the most relevant diets associated with cognition, specifically by type 2 diabetes status.
Research Design and Methods
Study Participants
The Boston Puerto Rican Health Study is a longitudinal observational study of diet, psychosocial factors, and health among Puerto Rican adults at baseline visit (2004–2007) and follow-up at 2 and 5 years. Detailed recruitment strategies and protocols have been previously published (17). Recruitment was conducted using door-to-door enumeration in Hispanic-dense census blocks, supplemented with community recruitment strategies. The study recruited 1,499 adults aged 45–75 years who self-identified as Puerto Rican, lived in the Boston metro area at the time of enrollment, and were able to answer interview questions independently. Individuals were excluded if they were deemed to have severe cognitive impairment, as assessed with a screening Mini-Mental State Examination (MMSE) score ≤10. Participants provided written informed consent before having measurements and questionnaires obtained at their home by a trained interviewer. The study protocols were approved by the institutional review board at Tufts Medical Center and Northeastern University. The study has ClinicalTrials.gov registration no. NCT01231958.
Dietary Assessment
A validated food frequency questionnaire (FFQ) was used to assess traditional foods and beverages habitually consumed by Puerto Ricans, using portions and foods appropriate for this population (23). We excluded participants with energy intakes <600 or >4,800 kcal/day (<2,510 or >20,083 kJ/day), as these were deemed implausible, and/or >10 questions blank on the FFQ. The four diet quality scores analyzed were previously described in this cohort (21,22).
The MeDS measured adherence to nine dietary components typical of a Mediterranean diet (21,24). One point was assigned to individuals above the population- and sex-specific median cutoff, adjusted for total energy by using the residual method, and no points were assigned to individuals at or below the median cutoff for the components of vegetables, fruits, nuts and legumes, whole grains, fish, and monounsaturated-to-saturated fats ratio. The components of meat and meat products and dairy and dairy products were reverse coded. For the alcohol component, one point was assigned to men with alcohol intake of two or less drinks per day and zero for men with either no alcohol intake or intake of more than two drinks per day; one point was assigned for women with alcohol intake of one or less drinks per day and zero for women with no intake or more than one drink per day. Points were summed, and total MeDS ranged from 0 (low adherence) to 9 (high adherence).
In brief, the HEI 2005 definition, corresponding to the dietary guidelines in place at the time of baseline data collection, includes 12 food and nutrient components, expressed per 1,000 kcal/day, according to procedures from the U.S. Department of Agriculture Center for Nutrition Policy and Promotion (25). The sum of the 12 components ranged from 0 to 100, with higher scores indicative of better adherence to the guidelines. The AHEI 2010 score included 11 food groups or nutrient components with consistent evidence of association with lower risk of chronic diseases (26). Each component was given a continuous score between 0 for minimal observance of the recommended intake and 10 points for maximal observance; intermediate values were prorated. The total added AHEI score for all components ranged from 0 (lowest diet quality) to 110 (highest diet quality). The DASH score was defined according to the definition from Fung et al. (27) by including eight food and nutrient components categorized into quintiles of intake. A point was assigned for each higher quintile of intake of healthful food groups (1–5). Unfavorable food groups were reverse coded. Components were added, and the overall DASH score ranged from 8 to 40.
Type 2 Diabetes Status
Participants were asked to fast for the previous 12 h before the morning blood draw in their home. Blood was drawn by a trained phlebotomist. Serum glucose was measured using an enzymatic, kinetic reaction on the Olympus AU400e with Olympus glucose reagents (Olympus America Inc., Melville, NY). Hemoglobin A1c was analyzed in a two-step process where the final value was determined by a ratio of hemoglobin A1c to hemoglobin along with a conversion factor. A whole blood hemolysate was analyzed by latex-enhanced immunoturbidimetric assay to determine hemoglobin A1c, and by colorimetric end point method for hemoglobin, on the Cobas FARA using the Roche Unimate hemoglobin A1c kit (Roche Diagnostics, Indianapolis, IN).
Type 2 diabetes was defined as having fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or self-reported use of diabetes medication (including insulin) (28). At baseline, glycemic control was defined as uncontrolled (hemoglobin A1c ≥7% [53 mmol/mol]) versus controlled (hemoglobin A1c <7% [53 mmol/mol]) (28). We defined 2-year change in glycemic control as those with stable or improved values (a reduction of ≤0.5% in hemoglobin A1c) versus poor or declined values (an increase in >0.5% hemoglobin A1c).
Cognitive Function
Specific areas of cognitive function were assessed using a comprehensive battery of seven neuropsychological examinations, administered in the participant’s preferred language by a neuropsychologist-trained research assistant. Tests included 1) the MMSE for general function (29) with a score ranging from 0 to 30 (lowest to highest function), 2) the 16-word list learning test (30) for verbal memory, 3) the Stroop test (30) (reading the color rather than the text of the words) for mental processing speed, 4) the digit span forward and backward test (30) for attention and working memory, 5) verbal fluency (30) (name as many words as possible that start with a given letter) for executive function with language, and 6) clock drawing (31) and 7) figure copying (32) for visuospatial (executive) function. Scores for the figure copying test were weighted for the complexity of the figure copied, one point for easy figures and four points for the most difficult.
We calculated a global cognitive function score by averaging the z scores for each of the 10 cognitive scores generated from the seven tests: MMSE, word list learning, word list recognition, percent retention, Stroop, letter fluency, digit span forward, digit span backward, clock drawing, and weighted figure copying. Additionally, cognitive function factors were derived through principal components analysis using the PROC FACTOR procedure in SAS (version 9.4; SAS Institute, Cary, NC) (33). Two factors were identified and labeled “executive” and “memory” functions. A score for each factor was calculated by summing the test scores weighted by the factor loading.
Assessment of Covariates
Trained research interviewers asked participants for information on age, sex, marital status, years of education, household income, and smoking history. A questionnaire on psychological attachment to U.S. or Puerto Rican culture was used to measure psychological acculturation. Physical activity was estimated as a physical activity score, based on a modified Paffenbarger questionnaire of the Harvard Alumni Activity Survey. Income-to-poverty ratio was computed using the poverty guidelines released each year by the U.S. Department of Health and Human Services (http://aspe.hhs.gov/poverty/index.shtml). Depressive symptomatology was assessed with the Center for Epidemiologic Studies Depression (CES-D) Scale. We used the U.S. Department of Agriculture 10-item adult food security survey module to assess food security, for the past 12 months, of the respondent and other adult household members. Affirmative responses were summed to create a food security score that corresponds with one of the following four categories of food security among adults: high, marginal, low, and very low.
Blood pressure was measured in duplicate at three time points during the interview with an electronic sphygmomanometer; the average of the last two measures was considered as the final value. Hypertension was defined as mean systolic blood pressure of ≥140 mmHg and/or mean diastolic blood pressure of ≥90 mmHg, or use of blood pressure medication. Body weight and standing height were measured in duplicate, according to standard protocol. BMI was calculated as weight (kg)/height (m)2. Total homocysteine in plasma was measured using an adaptation of the method described by Araki and Sako (34). The coefficient of variation for this assay in our laboratory is 6.0%. Serum hs-CRP was measured using the Immulite 1,000 High Sensitive CRP Kit (LKCRP1) on the Immulite 1,000 (Seimens Medical Solutions Diagnostics, Los Angeles, CA).
Statistical Analysis
Of the 1,499 Puerto Rican adults who completed the baseline visit, 1,246 (83.1%) completed the 2-year visit. We excluded from analysis participants with implausible energy intake (<600 kcal/day or >4,800 kcal/day) at baseline (n = 72), with incomplete cognitive tests at baseline (n = 185) or 2-year visit (n = 183), or with missing glucose values for type 2 diabetes classification at baseline (n = 52) or 2 years (n = 49). At baseline, there were 711 participants without type 2 diabetes and 465 with type 2 diabetes; at the 2-year visit, there were 657 participants without type 2 diabetes and 488 with type 2 diabetes. From the participants with type 2 diabetes, 457 had hemoglobin A1c data at baseline and 443 at 2 years. Final sample size for analysis of change in global cognitive function (primary outcome) as predicted by MeDS (primary exposure) by type 2 diabetes status was 913 participants and by glycemic control status was 334 participants.
Descriptive statistics were assessed for participants with versus without type 2 diabetes and for glycemic control versus no control at baseline using Student t test or ANOVA for continuous variables or χ2 for categorical variables. Repeated-measures linear mixed-effects models were fitted to assess 2-year change in global cognitive function z score, executive and memory function composite scores, and individual cognitive test scores. Exposures were the individual diet quality scores as continuous values. Models were adjusted for sex, age, marital status, income-to-poverty ratio, educational attainment, food security status, smoking status, psychological acculturation, physical activity score, depressive symptomatology score, hypertension status, homocysteine, CRP, BMI, baseline cognitive test score value, and time between measurements to evaluate change in outcomes by baseline dietary score. Models for AHEI and DASH were additionally adjusted for energy intake (MeDS and HEI are already energy-adjusted as part of the definition). Further adjustment for ApoE genotype, season of interview, or 2-year change in hemoglobin A1c, systolic blood pressure, or homocysteine did not change results (data not shown). Crude models adjusted for age, sex, baseline cognitive test score value, and time between measurements were also run. Based on a priori hypotheses, all models were stratified by type 2 diabetes status as well as by glycemic control status for the analysis of the primary exposure (MeDS) and primary outcome (global cognitive function). Adjustment for multiple comparisons was not conducted due to distinct a priori hypotheses. SAS version 9.4 was used for all analyses. P < 0.05 was deemed significant.
Results
At baseline, 39.5% of participants had type 2 diabetes, and at 2 years, 42.6% had type 2 diabetes. Of the participants with type 2 diabetes, 74.2% were not under glycemic control at baseline, and 67.9% had poor or declined hemoglobin A1c values at 2 years (vs. stable or improved) (data not shown). Participants with type 2 diabetes (vs. without) were older, had lower educational attainment and physical activity score, and had higher hypertension prevalence, BMI, CRP, glucose, and hemoglobin A1c at baseline (Table 1). They also had higher HEI score at baseline and lower cognitive function scores at 2 years. Participants with uncontrolled (vs. controlled) type 2 diabetes had higher CRP, glucose, and hemoglobin A1c at baseline and lower executive memory function at 2 years.
Table 1.
Characteristics of mainland U.S. Puerto Rican adults by type 2 diabetes and glycemic control status
| Type 2 diabetes status |
||||
|---|---|---|---|---|
| Without type 2 diabetes* (n = 711) | With type 2 diabetes* (n = 465) | Controlled type 2 diabetes† (n = 118) | Uncontrolled type 2 diabetes† (n = 339) | |
| Baseline | ||||
| Age (years) | 56.0 ± 7.7 | 58.9 ± 7.2# | 58.2 ± 6.8 | 59.1 ± 7.3 |
| Sex (% female) | 73.6 | 72.5 | 69.5 | 73.5 |
| Education ≤8th grade | 44.6 | 52.5# | 48.3 | 53.7 |
| Married/with partner | 34.6 | 31.0 | 31.4 | 31.1 |
| Under poverty line | 57.1 | 60.5 | 54.4 | 62.0 |
| Food insecure | 12.1 | 15.7 | 17.8 | 15.0 |
| Current smoker | 25.7 | 20.2 | 25.4 | 18.6 |
| Hypertension | 58.4 | 85.3# | 76.3 | 88.2 |
| Use of diabetes medication | 0 | 32.7# | 6.8 | 25.4¶ |
| Season of interview | ||||
| Winter | 18.6 | 18.1 | 20.3 | 17.4 |
| Spring | 24.3 | 21.1 | 26.3 | 19.5 |
| Summer | 30.4 | 35.3 | 28.8 | 37.2 |
| Fall | 26.7 | 25.6 | 24.6 | 26.0 |
| Physical activity score‡ | 31.8 ± 4.8 | 30.9 ± 4.3# | 31.1 ± 4.7 | 30.8 ± 4.1 |
| Psychological acculturation score‡ | 18.4 ± 6.6 | 18.0 ± 6.9 | 17.8 ± 7.3 | 18.1 ± 6.8 |
| Depressive symptomatology score‡ | 20.0 ± 13.3 | 20.0 ± 12.8 | 20.6 ± 13.1 | 19.8 ± 12.6 |
| BMI (kg/m2) | 31.2 ± 6.3 | 33.7 ± 6.9# | 33.4 ± 7.7 | 33.8 ± 6.7 |
| Homocysteine | 8.9 ± 4.7 | 9.2 ± 4.1 | 9.4 ± 5.3 | 9.1 ± 3.6 |
| C-reactive protein (mg/L) | 5.6 ± 7.2 | 7.2 ± 10.0# | 5.2 ± 5.8 | 7.9 ± 11.1¶ |
| Glucose (mg/dL) | 97.0 ± 10.9 | 154.6 ± 63.8# | 124.7 ± 46.5 | 165.1 ± 65.8¶ |
| Hemoglobin A1c (%) | 6.1 ± 0.7 | 8.3 ± 1.9# | 6.4 ± 0.4 | 9.0 ± 1.8¶ |
| MeDS§ | 4.4 ± 1.7 | 4.4 ± 1.6 | 4.2 ± 1.5 | 4.5 ± 1.7 |
| HEI-2005§ | 71.3 ± 9.7 | 73.2 ± 8.8# | 71.6 ± 8.5 | 73.9 ± 8.7 |
| AHEI-2010§ | 53.8 ± 9.1 | 54.4 ± 8.5 | 53.8 ± 7.9 | 54.6 ± 8.7 |
| DASH§ | 23.9 ± 4.1 | 24.2 ± 3.8 | 23.7 ± 3.8 | 24.4 ± 3.9 |
| 2-Year cognitive factors | ||||
| Global cognitive function‖ | 0.16 ± 0.54 | −0.06 ± 0.51# | −0.004 ± 0.44 | −0.08 ± 0.52 |
| Executive function‖ | 0.19 ± 1.0 | −0.21 ± 0.94# | 0.04 ± 0.89 | −0.29 ± 0.94¶ |
| Memory function‖ | 0.31 ± 0.88 | 0.03 ± 0.92# | −0.07 ± 0.92 | 0.06 ± 0.91 |
| MMSE | 23.6 ± 3.3 | 22.8 ± 3.4# | 22.7 ± 3.2 | 22.8 ± 3.4 |
| Word list learning | 39.0 ± 11.6 | 35.6 ± 10.6# | 35.5 ± 9.7 | 35.7 ± 10.9 |
| Word recognition | 30.9 ± 5.1 | 30.0 ± 5.9# | 30.1 ± 5.6 | 30.0 ± 6.1 |
| Stroop | 24.7 ± 11.2 | 20.8 ± 9.3# | 20.8 ± 9.6 | 20.9 ± 9.1 |
| Letter fluency | 26.3 ± 12.0 | 22.6 ± 10.2# | 24.1 ± 10.0 | 22.2 ± 10.2 |
| Digit span (forward) | 7.2 ± 1.9 | 7.0 ± 1.8# | 7.3 ± 1.9 | 6.9 ± 1.8 |
| Digit span (backward) | 3.4 ± 1.5 | 3.0 ± 1.5# | 3.2 ± 1.5 | 3.0 ± 1.4 |
| Clock drawing | 2.3 ± 1.0 | 2.0 ± 1.1# | 2.1 ± 1.1 | 1.9 ± 1.1 |
| Figure copying | 10.6 ± 8.0 | 8.0 ± 7.6# | 8.6 ± 7.7 | 7.8 ± 7.6 |
Data are mean ± SD or percent. *Type 2 diabetes was defined as having fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or self-reported use of diabetes medication (including insulin).
†Glycemic control was defined as uncontrolled (hemoglobin A1c ≥7% [53 mmol/mol]) vs. controlled (hemoglobin A1c <7% [53 mmol/mol]).
‡Psychological acculturation was assessed with a questionnaire on psychological attachment to U.S. or Puerto Rican culture; scores ranged from 0 to 50. Physical activity was estimated as a physical activity score, based on a modified Paffenbarger questionnaire of the Harvard Alumni Activity Survey. Depressive symptomatology was assessed with the CES-D Scale; scores ranged from 0 to 60.
§The MeDS was assessed based on adherence to nine foods or nutrients using sex-specific population-based median cutoffs; score ranges 0–9. The HEI-2005 was assessed based on dietary guideline recommendations for 12 foods or nutrients; score ranges 0–100. The AHEI-2010 was defined based on 11 food groups or nutrients components for chronic disease prevention; score ranges 0–110. DASH was defined based on eight food groups or nutrient components for hypertension prevention; score ranges 8–40. For all indices, a higher score is indicative of better diet quality.
‖Global cognitive function score was calculated by averaging the z scores for each of the 10 cognitive scores. Cognitive function factors were derived through principal components analysis that identified “executive” and “memory” functions.
#Significantly different from individuals without type 2 diabetes at P < 0.05 using Student t test or ANOVA for continuous variables or χ2 for categorical variables.
¶Significantly different from individuals with controlled type 2 diabetes at P < 0.05 using Student t test or ANOVA for continuous variables.
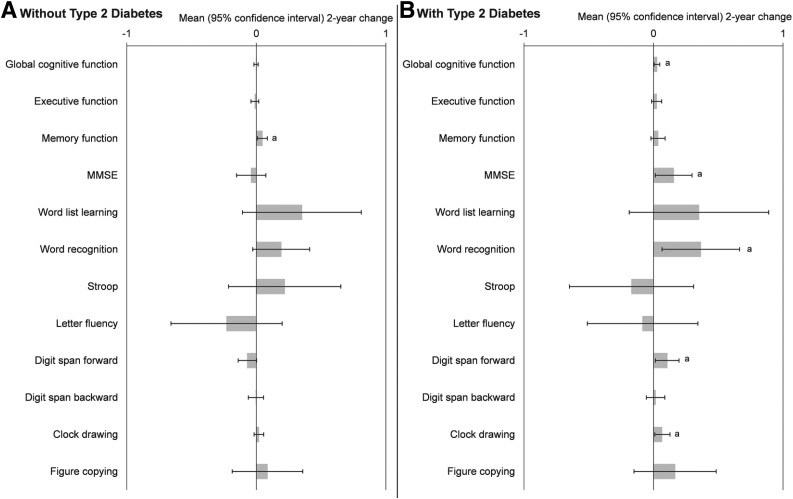
Among participants without type 2 diabetes, the MeDS was significantly and positively associated with 2-year change in memory function (β ± SE = 0.047 ± 0.020; P = 0.016), after adjustment for covariates, but was not associated with any other cognitive function score (Fig. 1A). MeDS explained 0.5% of the variability of the association with 2-year change in memory function among those without type 2 diabetes. In comparison, age at baseline explained 0.6% of the variability in the model. For participants with type 2 diabetes, higher MeDS was significantly associated with higher 2-year change in score of global cognitive function (0.027 ± 0.011; P = 0.016), MMSE (0.156 ± 0.072; P = 0.031), word recognition (0.365 ± 0.152; P = 0.017), digit span forward (0.106 ± 0.046; P = 0.023), and clock drawing (0.066 ± 0.028; P = 0.022) (Fig. 1B), after adjusting for baseline score. The MeDS was not significantly associated with remaining cognitive tests. MeDS explained 1.4% of the variability of the association with 2-year change in global cognitive function among participants with type 2 diabetes. In comparison, age at baseline explained 0.22% of the variability in the model.
Figure 1.
β-Coefficients for association of baseline MeDS and 2-year change in cognitive function for Puerto Rican adults with vs. without type 2 diabetes. Repeated-measures linear mixed-effects models predicting 2-year change in each cognitive function test by continuous MeDS were adjusted for sex, age, marital status, income-to-poverty ratio, educational attainment, food security status, smoking status, psychological acculturation, physical activity score, depressive symptomatology score, hypertension status, homocysteine, CRP, BMI, baseline value, and time. Results for participants without type 2 diabetes (n = 557) (A) and for participants with type 2 diabetes (n = 356) (B), defined as having fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or self-reported use of diabetes medication (including insulin). The MeDS was assessed based on adherence to nine foods or nutrients using sex-specific population-based median cutoffs; score ranges 0–9, with a higher score indicative of better diet quality. Global cognitive function score was calculated by averaging the z scores for each of the 10 cognitive scores. Cognitive function factors were derived through principal components analysis that identified “executive” and “memory” functions. a, significant change from baseline at P < 0.05.
Crude models, adjusted for age and sex, showed similar results as the fully adjusted model, except for significant associations between MeDS and 2-year change in word list learning (0.476 ± 0.222; P = 0.032) and figure copying (0.306 ± 0.135; P = 0.024) among participants without type 2 diabetes (Supplementary Fig. 1A); these associations were attenuated after covariate adjustment. Among participants with type 2 diabetes, 2-year change in word recognition was not significantly predicted by MeDS in the crude model (0.257 ± 0.153; P = 0.09) (Supplementary Fig. 1B).
The HEI was significantly associated with higher 2-year memory function (0.011 ± 0.003; P = 0.002) and word recognition (0.063 ± 0.020; P = 0.002) among participants without type 2 diabetes, as well as with higher word recognition (0.074 ± 0.030; P = 0.014) but with lower digit span forward score (−0.016 ± 0.007; P = 0.023) among participants with type 2 diabetes, after adjustment for baseline score (Table 2). The AHEI and DASH scores were significantly associated with cognitive outcomes among participants without type 2 diabetes only, but not in participants with type 2 diabetes. Specifically, higher AHEI was associated with higher 2-year memory function (0.012 ± 0.004; P = 0.001) and word recognition (0.062 ± 0.021; P = 0.004), and higher DASH score was associated with higher 2-year memory function (0.024 ± 0.008; P = 0.003), word list learning (0.224 ± 0.097; P = 0.021), and Stroop test score (0.271 ± 0.091; P = 0.003) among participants without type 2 diabetes, after adjusting for baseline measures. No other cognitive tests showed significant associations with the dietary indices evaluated.
Table 2.
β-Coefficients for association of baseline diet quality scores and 2-year change in cognitive function for Puerto Rican adults with versus without type 2 diabetes*
| HEI† |
AHEI† |
DASH† |
||||
|---|---|---|---|---|---|---|
| β ± SE | P value | β ± SE | P value | β ± SE | P value | |
| Without type 2 diabetes‡ | ||||||
| Global cognitive function§ | 0.001 ± 0.001 | 0.74 | 0.002 ± 0.002 | 0.25 | 0.002 ± 0.004 | 0.53 |
| Executive function§ | −0.287 ± 0.030 | 0.39 | 0.002 ± 0.003 | 0.42 | 0.003 ± 0.006 | 0.68 |
| Memory function§ | 0.011 ± 0.003 | 0.002 | 0.012 ± 0.004 | 0.001 | 0.024 ± 0.008 | 0.003 |
| MMSE | −0.012 ± 0.010 | 0.24 | 0.001 ± 0.011 | 0.94 | 0.003 ± 0.024 | 0.91 |
| Word list learning | 0.049 ± 0.042 | 0.25 | 0.066 ± 0.043 | 0.13 | 0.224 ± 0.097 | 0.021 |
| Word recognition | 0.063 ± 0.020 | 0.002 | 0.062 ± 0.021 | 0.004 | 0.081 ± 0.048 | 0.09 |
| Stroop | −0.004 ± 0.039 | 0.92 | 0.078 ± 0.041 | 0.06 | 0.271 ± 0.091 | 0.003 |
| Letter fluency | 0.002 ± 0.039 | 0.95 | −0.034 ± 0.042 | 0.42 | −0.064 ± 0.092 | 0.48 |
| Digit span forward | −0.011 ± 0.007 | 0.11 | 0.001 ± 0.007 | 0.89 | −0.012 ± 0.015 | 0.44 |
| Digit span backward | −0.003 ± 0.005 | 0.56 | 0.002 ± 0.006 | 0.73 | 0.012 ± 0.013 | 0.34 |
| Clock drawing | −0.001 ± 0.003 | 0.76 | 0.002 ± 0.004 | 0.51 | 0.001 ± 0.008 | 0.92 |
| Figure copying | 0.017 ± 0.025 | 0.50 | 0.026 ± 0.025 | 0.32 | 0.059 ± 0.058 | 0.31 |
| With type 2 diabetes‡ | ||||||
| Global cognitive function§ | 0.003 ± 0.002 | 0.18 | 0.001 ± 0.002 | 0.57 | 0.002 ± 0.005 | 0.65 |
| Executive function§ | 0.001 ± 0.004 | 0.74 | 0.002 ± 0.004 | 0.54 | −0.001 ± 0.008 | 0.90 |
| Memory function§ | 0.007 ± 0.005 | 0.19 | 0.007 ± 0.005 | 0.17 | 0.020 ± 0.012 | 0.09 |
| MMSE | 0.023 ± 0.014 | 0.09 | 0.003 ± 0.014 | 0.85 | 0.047 ± 0.032 | 0.14 |
| Word list learning | 0.012 ± 0.054 | 0.82 | 0.080 ± 0.054 | 0.14 | 0.128 ± 0.119 | 0.25 |
| Word recognition | 0.074 ± 0.030 | 0.014 | 0.033 ± 0.030 | 0.27 | 0.039 ± 0.067 | 0.56 |
| Stroop | −0.052 ± 0.046 | 0.26 | −0.021 ± 0.045 | 0.64 | 0.029 ± 0.100 | 0.78 |
| Letter fluency | 0.018 ± 0.041 | 0.67 | −0.019 ± 0.042 | 0.65 | −0.100 ± 0.092 | 0.28 |
| Digit span forward | 0.014 ± 0.009 | 0.13 | 0.008 ± 0.009 | 0.36 | −0.024 ± 0.020 | 0.23 |
| Digit span backward | -0.016 ± 0.007 | 0.023 | 0.0001 ± 0.007 | 0.98 | 0.007 ± 0.016 | 0.65 |
| Clock drawing | 0.007 ± 0.006 | 0.20 | 0.004 ± 0.006 | 0.50 | 0.006 ± 0.013 | 0.62 |
| Figure copying | −0.010 ± 0.031 | 0.74 | −0.040 ± 0.031 | 0.20 | −0.049 ± 0.071 | 0.49 |
Statistically significant associations at P < 0.05 are shown in boldface type.
*Repeated-measures linear mixed-effects models predicting 2-year change in each cognitive function test by continuous diet quality score, adjusted for sex, age, marital status, income-to-poverty ratio, educational attainment, food security status, smoking status, psychological acculturation, physical activity score, depressive symptomatology score, hypertension status, homocysteine, CRP, BMI, baseline value, and time. Models for AHEI and DASH were additionally adjusted for energy intake.
†The HEI-2005 was assessed based on dietary guideline recommendations for 12 foods or nutrients; score ranges 0–100. The AHEI-2010 was defined based on 11 food groups or nutrient components for chronic disease prevention; score ranges 0–110. DASH was defined based on eight food groups or nutrient components for hypertension prevention; score ranges 8–40. For all indices, a higher score is indicative of better diet quality.
‡Type 2 diabetes defined as having fasting plasma glucose ≥126 mg/dL (7.0 mmol/L) or self-reported use of diabetes medication (including insulin) (n = 557) vs. without type 2 diabetes (n = 356).
§Global cognitive function score was calculated by averaging the z scores for each of the 10 cognitive scores. Cognitive function factors were derived through principal components analysis that identified “executive” and “memory” functions.
We stratified analysis of the MeDS and global cognitive function by glycemic status at baseline and for 2-year change. The significant associations observed for all participants with type 2 diabetes remained for individuals under glycemic control at baseline (0.062 ± 0.020; P = 0.004) or with stable/improved control over 2 years (0.053 ± 0.019; P = 0.007), but not for those uncontrolled at baseline (0.017 ± 0.012; P = 0.16) or with poor/declined glycemic control (0.021 ± 0.012; P = 0.10). MeDS explained 3.6% of the variability for participants under glycemic control at baseline and 3.3% for participants with stable/improved control over 2 years.
Conclusions
In a cohort of middle-aged and older Puerto Rican adults, adhering to a Mediterranean diet was associated with higher 2-year cognitive function among those with type 2 diabetes. Glycemic control further sustained these benefits, suggesting that both a healthy Mediterranean diet and effective diabetes management may help preserve optimal cognitive function. Other definitions of diet quality were not associated with cognitive function in adults with type 2 diabetes. Among adults without type 2 diabetes, following any healthy dietary pattern was associated with better memory function, including word list learning and recognition, stressing the importance of a healthy diet for memory functioning. Dietary recommendations for cognitive health may need to be tailored for individuals with versus without type 2 diabetes.
The observed associations between the Mediterranean diet and cognition were expected, given the strong evidence for this relationship (11). Furthermore, in this particular cohort of Puerto Ricans, we previously reported that the MeDS was cross-sectionally associated with higher MMSE score and lower likelihood of cognitive impairment (odds ratio 0.87 [95% CI 0.80, 0.94] for each additional point of MeDS) (21). A systematic review of longitudinal studies and prospective trials showed that the Mediterranean diet improved the specific cognitive domains of memory (delayed recognition and long-term and working memory), executive function, and visual constructs (7). That review did not stratify by diabetes status, but a meta-analysis of randomized control trials of Mediterranean diet among healthy adults only showed that the diet improved delayed recall, working memory, and global cognition, but not other cognitive function domains (5).
Contrary to our results, the Reasons for Geographic and Racial Differences in Stroke (REGARDS) Study showed that higher adherence to the Mediterranean diet was associated with a 20% lower likelihood of incident cognitive impairment in participants without type 2 diabetes, but the association was not significant in individuals with type 2 diabetes (35). The authors explained the different results by possible noncerebrovascular mechanisms linking type 2 diabetes to dementia, repeat episodes of hypoglycemia, and different dietary patterns between the groups. Our findings suggest that the Mediterranean diet benefits cognition among both individuals without type 2 diabetes and patients with type 2 diabetes, with the latter obtaining the most benefits. Furthermore, being under glycemic control (vs. uncontrolled) seems to amplify the benefits, which may explain null results in other studies if participants had uncontrolled diabetes.
For all individuals, the Mediterranean diet’s abundant antioxidants, vitamins, and unsaturated fatty acids may improve neurovascular health and reduce oxidative stress, metabolites, and chronic inflammation (5). Yet, for individuals with type 2 diabetes, an additional mechanism for improved cognition may be the favorable effects of the Mediterranean diet on glycemic control (36); hemoglobin A1c has been associated with multiple domains of cognitive function in individuals with type 2 diabetes in the Memory in Diabetes substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial (37). The authors suggested that poor glycemic control may lower cognitive function through higher occurrence of cardiovascular risk factors, metabolic oxidation products associated with hyperglycemia, or insufficient action or effect of insulin that has a role on cognitive processes.
Of note, the Mediterranean diet explained as much or more of the variability predicting changes in cognitive function in our study as age, especially for participants with type 2 diabetes under glycemic control. Furthermore, the associations did not change after adjustment for 2-year changes in hemoglobin A1c, blood pressure, and homocysteine, suggesting that changes in these biomarkers do not mediate improvements in cognitive function from the Mediterranean diet. Clinical and public health recommendations to follow this dietary pattern may provide more cognitive benefits than other modifiable and nonmodifiable factors.
The evidence of association between the other three diet quality indices tested here (HEI, AHEI, and DASH) and cognitive function remains inconsistent (6,8–10). We showed that among participants without type 2 diabetes, only the HEI was significantly associated with word recognition and was inversely associated with digit span. In African American older adults, lower HEI was associated with poorer verbal learning and memory (9). AHEI and DASH showed null associations with cognitive outcomes in participants without type 2 diabetes. The Women’s Health Initiative Memory Study of postmenopausal older women also showed no associations between the four diet quality scores and cognitive function (8). Yet, consistent positive associations between HEI, AHEI, and DASH were observed in our study for memory function and word and Stroop tests only among individuals without type 2 diabetes. Higher adherence to the AHEI was associated with lower risk of cognitive decline among middle-aged and older adults from 40 countries (38), but not among predominantly white community-dwelling adults (39). In the Memory and Aging Project, the DASH score was associated with a slower rate of global cognitive decline to a similar extent as the Mediterranean diet (40). Furthermore, a combined Mediterranean-DASH diet (MIND) was associated with slower decline in global cognitive score and with five cognitive domains, including three functions of memory (41). Discrepancies across studies may be explained by specific types of foods (i.e., fish, meat, and fruit) consumed by a population, which may contribute different nutrient composition that influences cognition, or differences in cognitive testing.
As an observational study, our analysis is prone to residual confounding from factors not assessed in our models. Although we believe that the results are pertinent to other populations, the specific dietary and cultural context of the Puerto Rican participants may limit generalizability. Our study is strengthened by the use of a comprehensive and thoroughly measured battery of cognitive tests that assess various function areas, as well as the use of an FFQ developed and validated for the Puerto Rican population. Our statistical models were adjusted for multiple sociodemographic, economic, anthropometric, and biological confounders of the association between diet and cognition. Notably, we have previously shown that the MeDS defined here captures traditional foods of the Puerto Rican cuisine, such as vegetables and meats in homemade soups, orange juice, oatmeal, beans and legumes, fish, whole milk, corn oil, and beer (22). Thus, promoting a healthy Mediterranean diet for cognitive health for this population can be culturally accessible by using these familiar foods.
Supplementary Material
Article Information
Funding. This study was funded by the National Heart, Lung, and Blood Institute (grant P50-HL105185), the National Institute on Aging (grants P01-AG023394 and R01-AG055948 to K.L.T.), and a Mentored Career Development Award to Promote Faculty Diversity in Biomedical Research from the National Heart, Lung, and Blood Institute (K01-HL120951 to J.M.). Additional support was obtained from Harvard Catalyst of the Harvard University Clinical and Translational Science Center (National Center for Research Resources and National Center for Advancing Translational Sciences award UL1-TR001102), financial contributions from Harvard University and its affiliated academic health care centers, and the New York Regional Center for Diabetes Translation Research (DK111022).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. J.M. designed the research, performed statistical analysis, and wrote the manuscript. S.J.B., M.S.-P., T.S., and X.G. contributed to data analysis and to interpretation of results. K.L.T. is the principal investigator of the Boston Puerto Rican Health Study and contributed to research design and interpretation of results. All authors contributed meaningfully to this manuscript and approved the final version. J.M. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Parts of this work were presented in abstract form at the 78th Scientific Sessions of the American Diabetes Association, Orlando, FL, 22–26 June 2018.
Footnotes
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc19-0130/-/DC1.
References
- 1.Wu L, Sun D, Tan Y. Intake of fruit and vegetables and the incident risk of cognitive disorders: a systematic review and meta-analysis of cohort studies. J Nutr Health Aging 2017;21:1284–1290 [DOI] [PubMed] [Google Scholar]
- 2.Masana MF, Koyanagi A, Haro JM, Tyrovolas S. n-3 fatty acids, Mediterranean diet and cognitive function in normal aging: a systematic review. Exp Gerontol 2017;91:39–50 [DOI] [PubMed] [Google Scholar]
- 3.Cheung BH, Ho IC, Chan RS, Sea MM, Woo J. Current evidence on dietary pattern and cognitive function. Adv Food Nutr Res 2014;71:137–163 [DOI] [PubMed] [Google Scholar]
- 4.Yeomans MR. Adverse effects of consuming high fat-sugar diets on cognition: implications for understanding obesity. Proc Nutr Soc 2017;76:455–465 [DOI] [PubMed] [Google Scholar]
- 5.Loughrey DG, Lavecchia S, Brennan S, Lawlor BA, Kelly ME. The impact of the Mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv Nutr 2017;8:571–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solfrizzi V, Custodero C, Lozupone M, et al. Relationships of dietary patterns, foods, and micro- and macronutrients with Alzheimer’s disease and late-life cognitive disorders: a systematic review. J Alzheimers Dis 2017;59:815–849 [DOI] [PubMed] [Google Scholar]
- 7.Hardman RJ, Kennedy G, Macpherson H, Scholey AB, Pipingas A. Adherence to a Mediterranean-style diet and effects on cognition in adults: a qualitative evaluation and systematic review of longitudinal and prospective trials. Front Nutr 2016;3:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haring B, Wu C, Mossavar-Rahmani Y, et al. No association between dietary patterns and risk for cognitive decline in older women with 9-year follow-up: data from the Women's Health Initiative Memory Study. J Acad Nutr Diet 2016;116:921–930.e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wright RS, Waldstein SR, Kuczmarski MF, et al. Diet quality and cognitive function in an urban sample: findings from the Healthy Aging in Neighborhoods of Diversity across the Life Span (HANDLS) study. Public Health Nutr 2017;20:92–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tangney CC, Kwasny MJ, Li H, Wilson RS, Evans DA, Morris MC. Adherence to a Mediterranean-type dietary pattern and cognitive decline in a community population. Am J Clin Nutr 2011;93:601–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van de Rest O, Berendsen AA, Haveman-Nies A, de Groot LC. Dietary patterns, cognitive decline, and dementia: a systematic review. Adv Nutr 2015;6:154–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Biessels GJ, Strachan MW, Visseren FL, Kappelle LJ, Whitmer RA. Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2014;2:246–255 [DOI] [PubMed] [Google Scholar]
- 13.Huang L, Yang L, Shen X, Yan S. Relationship between glycated hemoglobin A1c and cognitive function in nondemented elderly patients with type 2 diabetes. Metab Brain Dis 2016;31:347–353 [DOI] [PubMed] [Google Scholar]
- 14.Gatlin PK, Insel KC. Severity of type 2 diabetes, cognitive function, and self-care. Biol Res Nurs 2015;17:540–548 [DOI] [PubMed] [Google Scholar]
- 15.Ajala O, English P, Pinkney J. Systematic review and meta-analysis of different dietary approaches to the management of type 2 diabetes. Am J Clin Nutr 2013;97:505–516 [DOI] [PubMed] [Google Scholar]
- 16.Esposito K, Giugliano D. Mediterranean diet and type 2 diabetes. Diabetes Metab Res Rev 2014;30(Suppl. 1):34–40 [DOI] [PubMed] [Google Scholar]
- 17.Tucker KL, Mattei J, Noel SE, et al. The Boston Puerto Rican Health Study, a longitudinal cohort study on health disparities in Puerto Rican adults: challenges and opportunities. BMC Public Health 2010;10:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CE, Tucker KL, Scott TM, et al. Apolipoprotein C3 polymorphisms, cognitive function and diabetes in Caribbean origin Hispanics. PLoS One 2009;4:e5465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattei J, Sotres-Alvarez D, Daviglus ML, et al. Diet quality and its association with cardiometabolic risk factors vary by Hispanic and Latino ethnic background in the Hispanic Community Health Study/Study of Latinos. J Nutr 2016;146:2035–2044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Getaneh A, Light LS, Brillon DJ, et al. Diabetes control among Hispanics in the Action to Control Cardiovascular Risk in Diabetes trial. J Gen Intern Med 2012;27:1499–1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye X, Scott T, Gao X, Maras JE, Bakun PJ, Tucker KL. Mediterranean diet, healthy eating index 2005, and cognitive function in middle-aged and older Puerto Rican adults. J Acad Nutr Diet 2013;113:276–281.e1-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mattei J, Sotos-Prieto M, Bigornia SJ, Noel SE, Tucker KL. The Mediterranean diet score is more strongly associated with favorable cardiometabolic risk factors over 2 years than other diet quality indexes in Puerto Rican adults. J Nutr 2017;147:661–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tucker KL, Bianchi LA, Maras J, Bermudez OI. Adaptation of a food frequency questionnaire to assess diets of Puerto Rican and non-Hispanic adults. Am J Epidemiol 1998;148:507–518 [DOI] [PubMed] [Google Scholar]
- 24.Trichopoulou A, Costacou T, Bamia C, Trichopoulos D. Adherence to a Mediterranean diet and survival in a Greek population. N Engl J Med 2003;348:2599–2608 [DOI] [PubMed] [Google Scholar]
- 25.Guenther PM, Reedy J, Krebs-Smith SM. Development of the Healthy Eating Index-2005. J Am Diet Assoc 2008;108:1896–1901 [DOI] [PubMed] [Google Scholar]
- 26.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr 2012;142:1009–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fung TT, Chiuve SE, McCullough ML, Rexrode KM, Logroscino G, Hu FB. Adherence to a DASH-style diet and risk of coronary heart disease and stroke in women. Arch Intern Med 2008;168:713–720 [DOI] [PubMed] [Google Scholar]
- 28.American Diabetes Association Standards of Medical Care in Diabetes—2018. Diabetes Care 2018;41:S1–S159 [DOI] [PubMed] [Google Scholar]
- 29.Karno M, Burnam A, Escobar JI, Hough RL, Eaton WW. Development of the Spanish-language version of the National Institute of Mental Health Diagnostic Interview Schedule. Arch Gen Psychiatry 1983;40:1183–1188 [DOI] [PubMed] [Google Scholar]
- 30.Artiola Fortuny L, Romo HD, Heaton RK, Pardee RE. Manual de Normas y Procedimientos para la Batería Neuropsicológica en Español. Lisse, the Netherlands, Swets & Zeitlinger, 2000 [Google Scholar]
- 31.Wolf-Klein GP, Silverstone FA, Levy AP, Brod MS. Screening for Alzheimer’s disease by clock drawing. J Am Geriatr Soc 1989;37:730–734 [DOI] [PubMed] [Google Scholar]
- 32.Beery K. The Developmental Test of Visual-Motor Integration Manual. Revised ed. Cleveland, OH, Modern Curriculum Press, 1989 [Google Scholar]
- 33.Bigornia SJ, Scott TM, Harris WS, Tucker KL. Prospective associations of erythrocyte composition and dietary intake of n-3 and n-6 PUFA with measures of cognitive function. Nutrients 2018;10:pii:E1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Araki A, Sako Y. Determination of free and total homocysteine in human plasma by high-performance liquid chromatography with fluorescence detection. J Chromatogr 1987;422:43–52 [DOI] [PubMed] [Google Scholar]
- 35.Tsivgoulis G, Judd S, Letter AJ, et al. Adherence to a Mediterranean diet and risk of incident cognitive impairment. Neurology 2013;80:1684–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sleiman D, Al-Badri MR, Azar ST. Effect of Mediterranean diet in diabetes control and cardiovascular risk modification: a systematic review. Front Public Health 2015;3:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cukierman-Yaffe T, Gerstein HC, Williamson JD, et al.; Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) Investigators . Relationship between baseline glycemic control and cognitive function in individuals with type 2 diabetes and other cardiovascular risk factors: the Action to Control Cardiovascular Risk in Diabetes-Memory in Diabetes (ACCORD-MIND) trial. Diabetes Care 2009;32:221–226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Smyth A, Dehghan M, O’Donnell M, et al.; ONTARGET and TRANSCEND Investigators . Healthy eating and reduced risk of cognitive decline: a cohort from 40 countries. Neurology 2015;84:2258–2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richard EL, Laughlin GA, Kritz-Silverstein D, Reas ET, Barrett-Connor E, McEvoy LK. Dietary patterns and cognitive function among older community-dwelling adults. Nutrients 2018;10:pii:E1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tangney CC, Li H, Wang Y, et al. Relation of DASH- and Mediterranean-like dietary patterns to cognitive decline in older persons. Neurology 2014;83:1410–1416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris MC, Tangney CC, Wang Y, et al. MIND diet slows cognitive decline with aging. Alzheimers Dement 2015;11:1015–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.