Abstract
An amphiphilic block copolymer (BCP) which contains both photoresponsive and thermoresponsive blocks was synthesized by the atom transfer radical polymerization approach. Meanwhile, a new core/shell type of the upconversion nanoparticle (UCNP) LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 was successfully synthesized. By encapsulating UCNPs inside the micelles of the BCP and incorporating Nile red (NR) into the UCNP@BCP hybrid nanoparticles as a model drug, controlled release of the drug by the dual-stimuli BCP could be studied. After exposing the UCNP-loaded micellar solution to near-infrared (NIR) light, it was found that the UV light pumped from UCNPs could disrupt the polymer micelles and the fluorescence intensity of NR decreased with the increase of the irradiation time of the NIR light. The thermoresponsive study indicated that the fluorescence intensity of NR decreased with the increase of temperature of the micellar solution because of the release of NR into water arising from the contraction of the amphiphilic BCP.
1. Introduction
In recent years, stimuli-sensitive materials have attracted considerable attention in a vast variety of applications. Among them, stimuli-responsive polymers have gained practical and enhanced interests owing to their response to external stimuli such as variation of temperature, light, pH, pressure, magnetic and electric fields, and so forth.1−6 Multistimuli-responsive polymers have recently been paid more attention because of the variation in property control.7−10 To trigger the multistimuli action, the stimuli-responsive polymer must possess a block copolymer (BCP) structure with each block facilitating the response to the variation of environmental conditions such as temperature, light, and so forth. In addition, the BCP is often a type of amphiphilic micelle in aqueous media for biomedical applications. By incorporating appropriate photoresponsive moieties into a BCP, the polymeric micelles can be disrupted through photochemical reactions such as trans–cis isomerization, molecular dimerization, and bond cleavage. Alternatively, a temperature-sensitive polymer can be transferred from a hydrophilic state to a hydrophobic state when the solution temperature is raised above the lower critical solution temperature (LCST). Hence, this type of polymer is also frequently used as a stimulus-responsive material in a wide range of applications.11,12
However, all explored photoreactions require strong radiation, such as UV or visible light, which may cause a serious injury to organisms. In this regard, triggering the action of a photoresponsive polymeric micelle by near-infrared (NIR) light may be an appealing alternative because of the less detrimental radiation and deeper penetration compared to ultraviolet/visible (UV/vis) light. To achieve this goal, lanthanide-doped upconversion nanoparticles (UCNPs) for use in converting NIR radiation into UV/vis light have been recently developed.13,14 By employing this method, combining UCNPs and photoresponsive polymers in hybrid nanocapsules has become a widely applied strategy for use in NIR-triggered drug delivery.15−19
UCNPs are usually composed of lanthanide-doped transition metals and are dilute guest–host systems. Lanthanide ions are dispersed as a guest in a proper dielectric host lattice with a dimension of less than 100 nm. These Ln3+ ions are optically active centers and can exhibit sharp luminescence emissions via intra-4f or 4f–5d transitions.20,21 Through judicious selection of lanthanide dopants including the sensitizer and activator, UCNPs can display anti-Stokes emission such as NIR to shorter NIR, visible, or UV light by upconversion mechanisms. In addition, selection of appropriate host materials is crucial for high-efficiency upconversion emissions. As for the host materials, NaYF4 is one of the most studied material.15,21−24 To the best of our knowledge, LiYF4 is rarely studied to date. On the other hand, it has been indicated that the utilization of a core/shell structure can enhance the fluorescence intensity of UCNPs. There are three types of core/shell structures that have been utilized to enhance the photoluminescence (PL) intensity of UCNPs.25,26 In this study, we adopt the active core/active shell structure, in which the host material and the sensitizer of the shell are the same as those of the core materials.25−29 Owing to the sensitizer dopant in the shell, the active shell can not only protect the core from surface defects but also transfer absorbed NIR light from the pump source to the core UCNP. Therefore, the sensitizer-doped active shell can significantly enhance the fluorescence intensity of UCNPs.
This research aims to design and synthesize a dual-stimuli BCP in conjunction with the upconversion function of a new type of LiYF4 core/shell UCNP for application in controlled release of drugs. The strategy is based on that hydrophobic UCNPs and water insoluble drugs tend to be entrapped in the hydrophobic core of polymer micelles, while the hydrophilic polymer shell stabilizes the whole nanocapsule in aqueous solution. The dual-stimuli BCP consists of a photosensitive block and a temperature-sensitive block which are hydrophobic and hydrophilic, respectively. Upon encapsulation of a model drug, Nile red (NR) dye, into the diblock copolymer, the phenomenon of controlled release of drugs can be monitored by the variation of the temperature. When the temperature is raised above the LCST of the thermoresponsive block, the amphiphilic BCP will shrink and squeeze NR out of the BCP. Alternatively, when both NR and LiYF4 UCNPs are loaded into the amphiphilic BCP, the UV emission from UCNPs can cleave the photoresponsive block; thus, the hydrophobic core becomes hydrophilic. As a result, the polymeric micelles are disrupted and release the model drug (NR) into aqueous media.
Hence, by using the hybrid UCNP@BCP strategy, the controlled release of encapsulated molecules in the UCNP@BCP hybrid nanoparticles could be accomplished effectively by photostimulation for biomedical applications. On the other hand, to mimic the environmental change and enhance the function of drug delivery, incorporation of a temperature-sensitive segment into the polymeric micelles coupled with a photoresponsive segment could be a more effective approach for application in biotechnology.
In this study, we employed a single activator/sensitizer combination of Tm3+/Yb3+ to prepare a new kind of lanthanide-doped core/shell UCNP. This kind of UCNP has the active core/active shell structure, which can enhance the fluorescence intensity of the UCNP significantly. Upon excitation with a 980 nm NIR laser diode, the UCNPs can emit fluorescence spanning from the UV to NIR region. Because this kind of UCNP can emit fluorescence in the UV region, which can disrupt the photoresponsive polymeric micelles to release the encapsulated molecules, the BCP employed in the encapsulation of the loaded UCNPs and the model drug should contain the photosensitive segment. In addition, a thermoresponsive polymeric segment with a LCST lower than the temperature of human body (37 °C) is thought to be suitable as the temperature-sensitive block along with the photoresponsive block in the synthesis of the dual-stimuli BCP. In aqueous media, this diblock copolymer could self-assemble into polymeric micelles with the photosensitive segments as the hydrophobic core and the thermosensitive segments as the hydrophilic shell. Upon UV irradiation, the photosensitive segments perform the photolysis reaction and trigger the dissociation of the polymeric micelles. At the temperature above the LCST, the polymeric micelles shrink because of a decrease of the hydrophilicity of the thermosensitive segments; hence, the encapsulated molecules are squeezed out. Furthermore, because it has been indicated that UV/vis light can cleave chemical groups (called photocleavable/photoremovable/photolabile groups) such as o-nitrobenzyl- and coumarin-based groups30−32 and the UV light emitted from our synthesized UCNPs overlaps well with the absorption band of o-nitrobenzyl groups (around 362 nm), we designed an amphiphilic BCP composed of the hydrophobic block poly(o-nitrobenzyl acrylate) (PNBA) bearing the o-nitrobenzyl groups as the photosensitive block. On the other hand, because poly(N-alkyl (meth)acrylamide)s are perhaps the most heavily studied thermoresponsive polymers,33 we designed the amphiphilic BCP containing poly(N,N-diethylacrylamide) (PDEAAm) as the hydrophilic block. The as-synthesized amphiphilic BCP is designated as PDEAAm-b-PNBA.
2. Results and Discussion
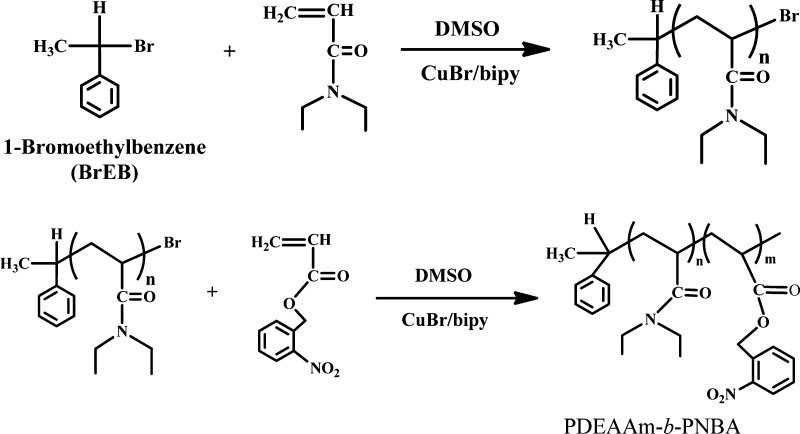
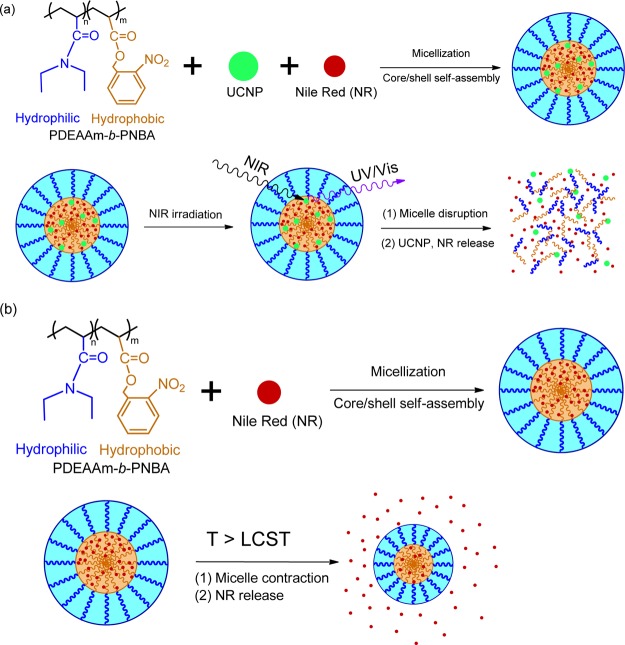
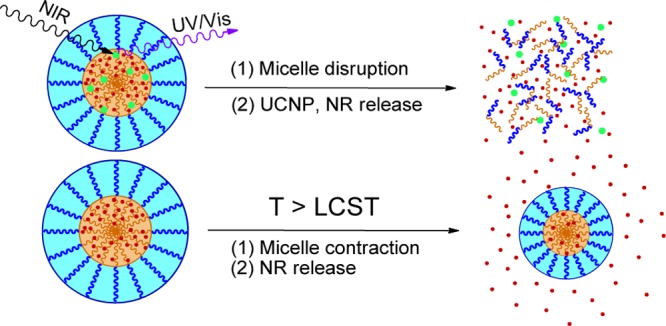
The new nanocomposite UCNP@BCP was prepared and NR was encapsulated to simulate the process of controlled release of drugs. In this work, a core/shell-type UCNP with emission of UV light at 361 nm has been synthesized. In addition, a new type of dual stimuli-responsive BCP composed of a photosensitive and temperature-sensitive block by atom transfer radical polymerization (ATRP) has been synthesized (Figure 1). To our knowledge, this kind of LiYF4:Yb3+,Tm3+@LiYF4:Yb3+ core/shell UCNP has not been studied in combination with the newly synthesized, dual stimuli-responsive BCP PDEAAm-b-PNBA. The controlled release triggered by the dual-stimuli approach is illustrated in Figure 2.
Figure 1.
Synthetic route for the dual-stimuli BCP PDEAAm-b-PNBA via ATRP.
Figure 2.
Schematic illustration of triggering dissociation of dual-stimuli polymeric micelles under (a) photochange or (b) temperature (UCNP unloaded)-change stimulations.
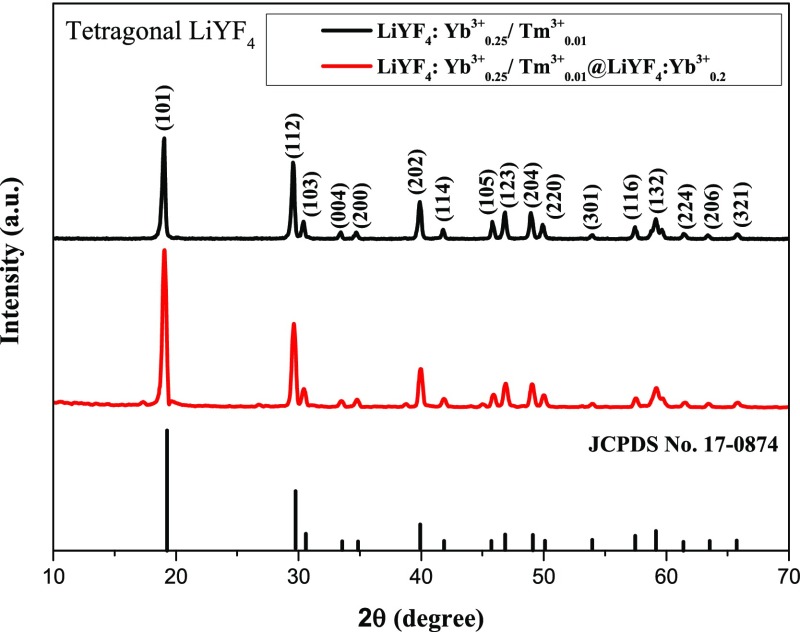
Wide-angle X-ray diffraction (XRD) was performed to investigate the crystal structure of the LiYF4:Yb3+0.25,Tm3+0.01 UCNP core and LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 core/shell UCNP. From the upper XRD pattern, the sharp and intense peaks from (101), (112), and (202) planes, as shown in Figure 3, the crystal structure of the core UCNP can be indexed as the tetragonal phase (JCPDS no. 17-0874). After coating the LiYF4:Yb3+ shell, it shows peaks that are similar to those of the core UCNP, as evident from the lower pattern of Figure 3. Because the shell composition is almost the same as that of the core, the XRD pattern of the core/shell UCNP is very similar to the pattern of its corresponding core UCNP.
Figure 3.
XRD patterns of the LiYF4:Yb3+0.25,Tm3+0.01 core UCNP and LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 core/shell UCNP.
Figure 4a,b depicts the high resolution transmission electron microscopy (HRTEM) images of the core and core/shell UCNP. As seen from these images, both the core UCNP and core/shell UCNP appear to be rhombus-shaped and monodispersed under high magnification (×500 K). The aspect ratio (length/breadth) of the diagonal is ca. 1.35 for the core UCNP and 1.64 for the core/shell UCNP. After shell coverage, both diagonal lengths of the core/shell UCNP increase significantly. This confirms the formation of the LiYF4 shell on the UCNP core and demonstrates why the XRD pattern of the core/shell type UCNP is very similar to that of the core UCNP. The d-spacing from HRTEM was determined to be 0.268 nm, which matches well with the distance between the (202) planes in LiYF4 (Figure 4c). The selected area electron diffraction (SAED) pattern in Figure 4d shows that the SAED pattern consists of many spots, which demonstrates that the LiYF4 core/shell UCNP has the single-crystal structure. The SAED pattern further illustrates the formation of the tetragonal phase of the core/shell UCNP and is indexed to the corresponding (hkl) planes according to the standard JCPDS no. 17-0874.
Figure 4.
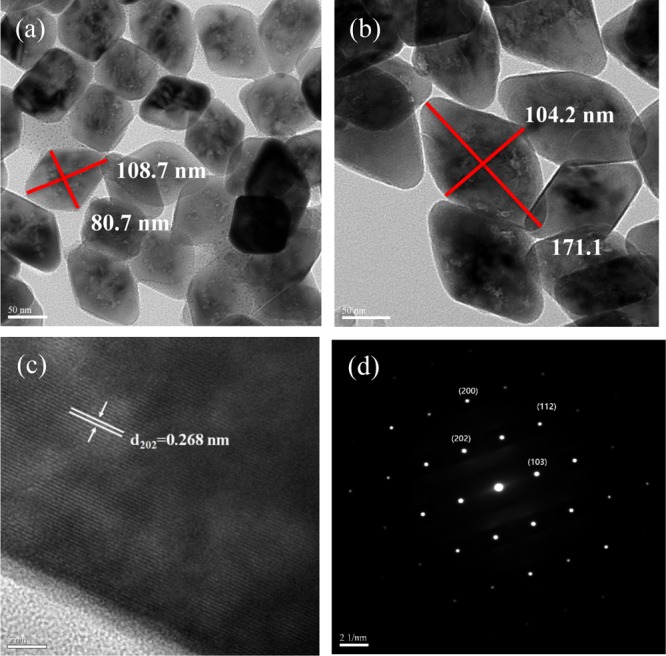
HRTEM images (×500 K) of (a) LiYF4:Yb3+0.25,Tm3+0.01 core UCNP and (b) LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 core/shell UCNP. (c) HRTEM image (×300 K) of the single-crystalline structure of the LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 core/shell UCNP. (d) SAED pattern of the LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 core/shell UCNP.
Because our research goal was to prepare a kind of UCNP with the function of emitting UV radiation to disrupt the micelles of the dual-stimuli BCP, the intensity of UV emission in relation to the doping concentrations of Yb3+ sensitizer and Tm3+ activator was studied. It was found that an optimum combination of Yb3+ and Tm3+ was 25 mol % Yb3+ and 1 mol % Tm3+ for the UCNP core. As for the shell of the UCNP, only Yb3+ sensitizer was needed to be used as the dopant of the LiYF4 host material, and the optimal ratio was around 20 mol %.
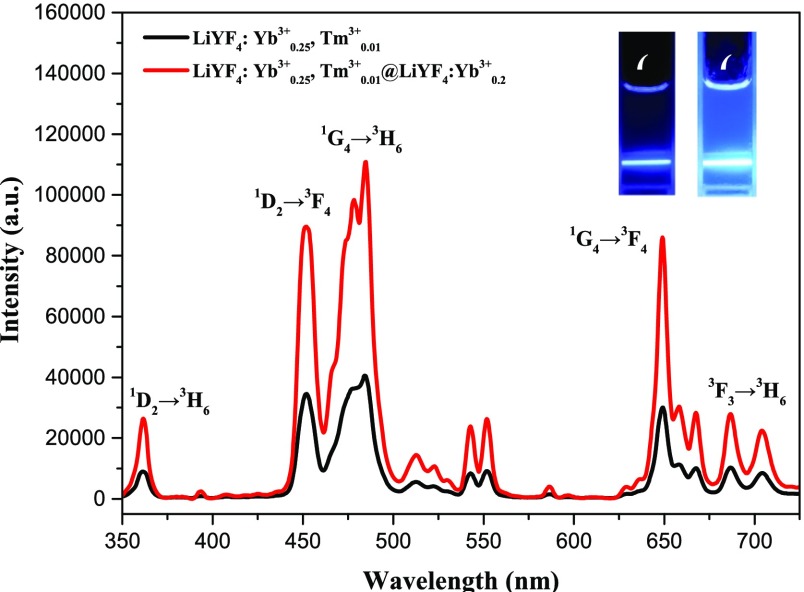
The PL spectra of the colloidal LiYF4:Yb3+,Tm3+ UCNP core and LiYF4:Yb3+,Tm3+@LiYF4:Yb3+ core/shell UCNP under laser excitation at 980 nm are shown in Figure 5. Several PL bands ranging from the UV to visible region are observed. For both core UCNP and core/shell UCNP, it can be found that their maxima are at 362, 451, 484, 649, and 687 nm, which correspond to 1D2 → 3H6, 1D2 → 3F4, 1G4 → 3H6, 1G4 → 3F4, and 3F3 → 3H6, respectively. The UV range before 350 nm is beyond the detection range of our spectrometer setup. It is striking that the intensities of all PL bands are much enhanced after overcoating the shell. This is very beneficial for the disruption of the BCP which contains photoresponsive segment units.
Figure 5.
PL spectra of the LiYF4:Yb3+0.25,Tm3+0.01 core UCNP and LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 core/shell UCNP under 980 nm NIR irradiation. The insets (from left to right) are the corresponding luminescence photographs of solutions of the core UCNP and core/shell UCNP, respectively.
In the preparation of the BCP
for controlled release of drugs via
the dual-stimuli function, we have synthesized an amphiphilic dual-stimuli
BCP composed of a thermoresponsive building block and a photoresponsive
building block. The thermoresponsive block was synthesized from N,N-diethylacrylamide (DEAAm) and functions
as the hydrophilic segment, while the photoresponsive block was synthesized
from 2-nitrobenzyl acrylate (NBA) and functions as the hydrophobic
segment. The as-synthesized dual-stimuli BCP was characterized by
proton nuclear magnetic resonance (1H NMR), IR, gel permeation
chromatography (GPC), and differential scanning calorimetry (DSC)
(Supporting Information). The number average
molecular weight (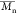 ) of the copolymer PDEAAm-b-PNBA
is shown in Figure S3 (Supporting Information). It is ca. 11 000 g/mol with a polydispersity index of 1.28.
Because the ratio of m and n of
the copolymer PDEAAm-b-PNBA obtained from the 1H NMR spectrum (Figure S1, Supporting Information) is about 1:3, the average values of m and n calculated are ca. 18.3 and 54.8, respectively.
) of the copolymer PDEAAm-b-PNBA
is shown in Figure S3 (Supporting Information). It is ca. 11 000 g/mol with a polydispersity index of 1.28.
Because the ratio of m and n of
the copolymer PDEAAm-b-PNBA obtained from the 1H NMR spectrum (Figure S1, Supporting Information) is about 1:3, the average values of m and n calculated are ca. 18.3 and 54.8, respectively.
NR is a common dye and was used as a model drug to simulate the process of controlled release of drugs. After UCNP and NR were encapsulated into the amphiphilic BCP, the NR-loaded UCNP@BCP hybrid was first dissolved in tetrahydrofuran (THF). Subsequently, the solution was poured into water and then THF was evaporated to form an amphiphilic micelle solution in aqueous media. The HRTEM images of the UCNP-loaded BCP micelles and the UCNP and NR co-loaded BCP micelles are shown in Figures S5 and S6 (Supporting Information). From both figures, it is apparent that UCNPs are loaded into the BCP micelles. Upon NIR irradiation at 980 nm, the nitrobenzyl groups on the PNBA block were cleaved because of the 362 nm UV radiation emitted by UCNPs. Photocleavage of the nitrobenzyl groups converted the PNBA block into the hydrophilic polyacrylic acid, which rendered the disruption of the micelles and the release of NR. On the other hand, after raising the temperature above the LCST of the amphiphilic BCP, the micelles contracted and the NR molecules in the micelles were squeezed out. Hence, the controlled release of drugs could be proceeded by two different ways via the dual-stimuli BCP.
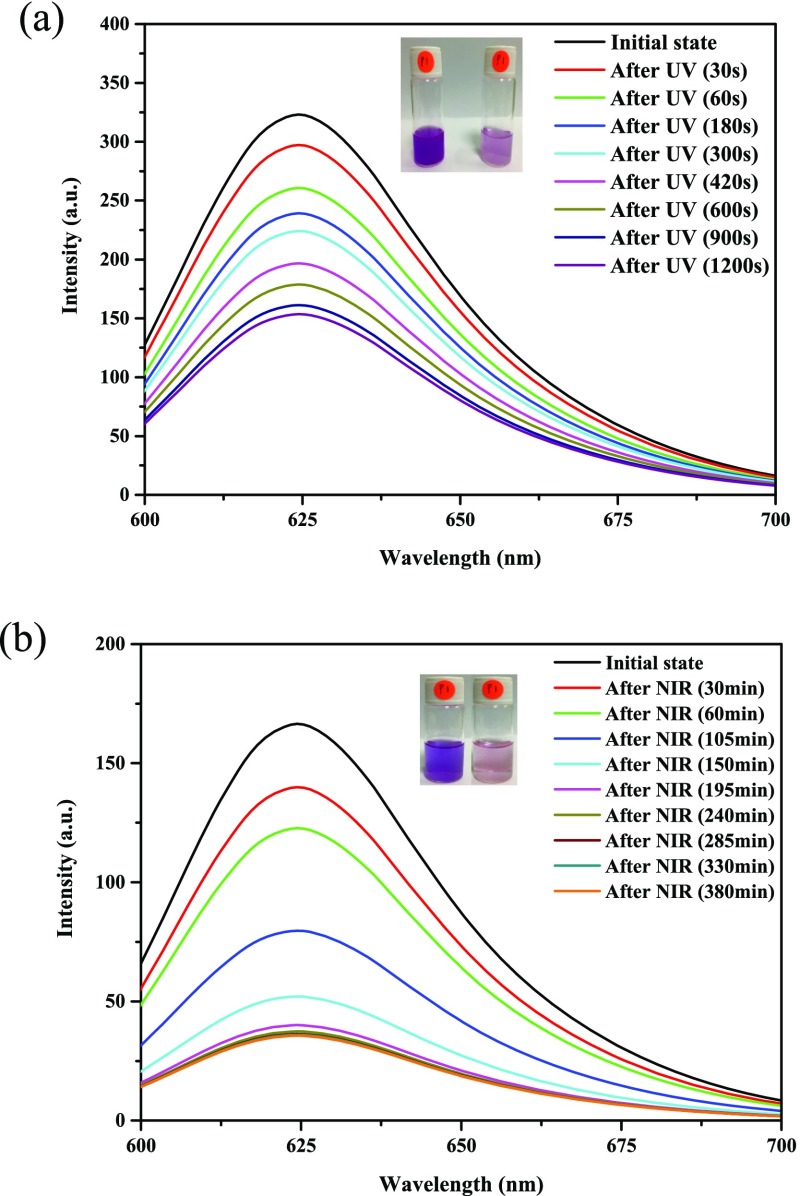
To illustrate the trigger function of drug release by using the micelle solution formed from the UCNP-loaded BCP, we further performed experiments to ensure the dissociation of micelles upon exposure of NR-loaded UCNP@BCP nanocarriers under irradiation of a 980 nm NIR laser diode. For comparison, the NR-loaded UCNP@BCP micellar solution was irradiated with UV and NIR light. Because NR is a hydrophobic dye and emits light around 625 nm, the emission intensity of the NR-loaded UCNP@BCP micellar solution in water under 365 nm UV or 980 nm NIR light irradiation was measured. As shown in Figure 6a, the PL intensity of NR decreases with the increase of irradiation time of UV light, indicating that more NR dye was released to water from the micelles. Because the NR dye was hydrophobic, if more NR dye remained in micelles, the PL intensity around 625 nm would be stronger. Hence, the decrease of PL intensity indicates that more of the o-nitrobenzyl moieties in the micelle core were cleaved by UV light and the PNBA block turned into the hydrophilic polyacrylic acid segment. To demonstrate the trigger function by the as-synthesized UCNP, the NR-loaded UCNP@BCP micellar solution was irradiated under NIR light; it was also found that the PL intensity of NR decreases with the increase of irradiation time (Figure 6b). Although the irradiation time is much longer than that of the micellar solution irradiated directly by UV light, this may be a beneficial factor for use in the drug delivery of an extended-release formulation. Moreover, as mentioned above, NIR irradiation is less detrimental and has deeper penetration to organisms compared to UV/vis light. The PL results strongly support that the core/shell UCNPs were encapsulated by the BCP and could emit UV light under NIR irradiation. The photos in the insets of Figure 6a,b display that after longer irradiation time, the purple color would turn into a light color because more NR molecules were released into water. This is a strong evidence to illustrate that our UCNP@BCP hybrid can be used to control the drug release.
Figure 6.
Emission spectra of NR (λexcitation = 550 nm) for the micellar solution with both UCNPs and NR loaded under (a) 365 nm UV irradiation or (b) 980 nm NIR light exposure. The insets (from left to right) are the corresponding photographs of the micellar solutions before and after UV or NIR irradiation.
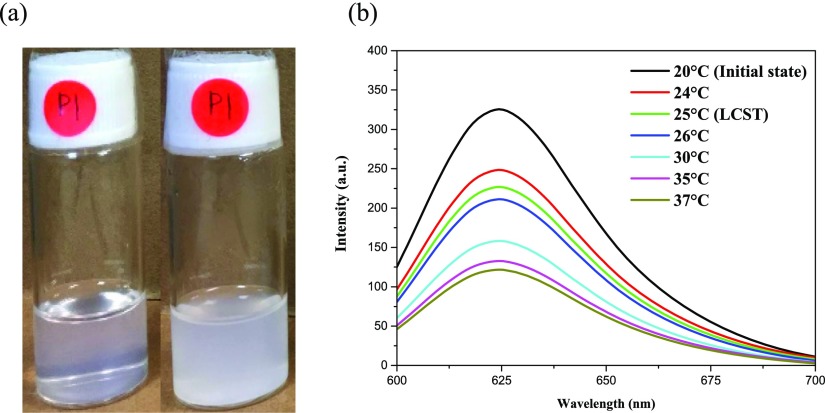
Furthermore, to test the function of the thermoresponsive block of the BCP, we carried out an experiment to detect the LCST of PDEAAm-b-PNBA. It was found that the polymeric solution turned turbid when the temperature was raised above 25 °C (Figure 7a). It indicated that the amphiphilic BCP turned hydrophobic in aqueous media because of the PDEAAm thermoresponsive segment and the LCST of PDEAAm-b-PNBA was around 25 °C. Subsequently, we studied the controlled-release process of the loaded NR molecules triggered by the thermoresponsive block. As shown in Figure 7b, it can be found that the emission intensity around 625 nm decreases with the increase of the temperature. We tested the temperature from 20 to 37 °C to simulate the drug release process in the human body. The result indicates that the NR dye has been squeezed out from the NR-loaded micelles because of the contraction of the polymeric micelles above the LCST of PDEAAm-b-PNBA.
Figure 7.
(a) Photographs show the BCP aqueous solutions below (left) and above (right) the LCST of PDEAAm-b-PNBA. (b) Emission spectra of NR (λexcitation = 550 nm) for the micellar solution with only NR loaded under the temperature change.
3. Conclusions
In summary, an amphiphilic photoresponsive and thermoresponsive BCP which contains both thermoresponsive N-alkyl-substituted acrylamide group and photoresponsive o-nitrobenzyl group has been successfully synthesized. To achieve the design goal of using this dual-stimuli BCP in controlled release of drugs under photostimulation conditions, a core/shell UCNP with the structure of LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2 and anti-Stokes emission of UV light was prepared and loaded into the polymeric micelles. Upon NIR light exposure, the BCP displayed an extended release of the model drug NR because of the disruption of the BCP in the aqueous solution. Alternatively, in the case of the BCP in which only NR was loaded, the behavior of the controlled release could be observed when the temperature was raised above the LCST of the BCP. Both results confirmed that the synthesized PDEAAm-b-PNBA BCP in conjunction with the LiYF4 core/shell UCNP could be applied as an effective nanocarrier for controlled release of drugs under the stimulation of photochange or temperature change.
4. Experimental Section
4.1. Synthesis of the Core UCNP LiYF4:Yb3+0.25,Tm3+0.01
UCNPs of LiYF4 doped with 25% of Yb3+ and 1% Tm3+ ions were synthesized by the thermal decomposition approach. At first, thulium and lithium trifluoroacetate precursors in the presence of oleic acid coordinating ligands and non-coordinating 1-octadecene solvent molecules were prepared. First, 0.1094 g (1.48 mmol) of Li2CO3, 0.1671 g of Y2O3 (0.74 mmol), 0.0985 g (0.25 mmol) of Yb2O3, and 0.0039 g (0.01 mmol) of Tm2O3 were dissolved in 10 mL of 50% (v/v % in water) concentrated trifluoroacetic acid in a three-necked 100 mL flask and heated at 90 °C for 30 min. Next, 15 mL of oleic acid and 15 mL of 1-octadecene were added into the three-necked flask. The resulting solution was then heated at 120 °C under vacuum for 30 min with magnetic stirring to remove water and oxygen. The light yellow solution was then heated to 300 °C at a rate of about 30 °C per minute under argon gas protection and kept at this temperature under vigorous stirring for about 1 h. The reaction mixture was cooled to room temperature and precipitated in ethanol and collected by centrifugation at 8000 rpm for 10 min. The precipitate was dissolved in hexane and then reprecipitated in ethanol. The procedure was repeated two times, and the nanoparticles were dispersed in 10 mL of hexane for further synthesis steps.
4.2. Synthesis of the Core/Shell UCNP LiYF4:Yb3+0.25,Tm3+0.01@LiYF4:Yb3+0.2
The procedure for the growth of the shell on the core UCNP is similar to that of the synthesis of the core UCNP. First, 0.1182 g (1.60 mmol) of Li2CO3, 0.1806 g of Y2O3 (0.80 mmol), and 0.0788 g (0.20 mmol) of Yb2O3 were dissolved in 10 mL of 50% (v/v % in water) concentrated trifluoroacetic acid in a three-necked 100 mL flask and heated at 90 °C for 30 min. Next, 15 mL of oleic acid and 15 mL of 1-octadecene were added into the three-necked flask. The resulting solution was then heated at 120 °C under vacuum for 30 min. At this time, the core solution was poured into the flask. The reaction was continued until the solution turned light yellow to remove hexane and water. The reaction mixture was then heated to 300 °C at a rate of about 30 °C per minute under argon gas protection and kept at this temperature under vigorous stirring for about 1 h. The reaction mixture was cooled to room temperature, and the product was precipitated and purified according to the procedures as mentioned above. Thus, the active-core/active-shell structured UCNPs were obtained.
4.3. Synthesis of the BCP PDEAAm-b-PNBA
NBA was prepared according to the reported procedure.34 The amphiphilic BCP of PDEAAm-b-PNBA was synthesized by ATRP. At first, DEAAm (2.3 g, 18 mmol) was dissolved in 8 mL dimethyl sulfoxide (DMSO). The solution was degassed three times using the freeze–pump–thaw procedure under the protection of an argon atmosphere. Next, the purified CuBr (0.015 g, 0.11 mmol) and bipyridine (0.02 g, 0.13 mmol) were added sequentially. After the initiator, 1-bromoethyl benzene (0.12 g, 0.6 mmol), was added, the reaction mixture was heated at 80 °C for 3 h. The synthesized PDEAAm was used as a macroinitiator agent to polymerize with NBA. The NBA (0.38 g, 1.8 mmol) in 1 mL DMSO was also degassed three times using the freeze–pump–thaw procedure and sealed under vacuum. Thereafter, the NBA monomer was added into the flask and the reaction was continued for 3 h at 80 °C. Then, the mixture was exposed to air and cooled to room temperature to quench the polymerization. Afterward, the flask was removed from the oil bath and the reaction mixture was diluted with THF. The solution was filtered through a column with neutral alumina to remove the catalyst. The final BCP was recovered by precipitation in methanol, filtering, and then drying under vacuum for 48 h.
4.4. Characterization
The XRD patterns of the core UCNP and core/shell UCNP were obtained by using a Bruker D8 ADVANCE diffractometer, using Cu Kα radiation with a step size of 0.05° and a scanning speed of 4°/min. TEM images were obtained with a JEOL JEM-1230 transmission electron microscope. UV/vis spectra were collected on a PerkinElmer Lambda 35 UV–vis spectrophotometer. PL spectra were recorded on a Hitachi F-7000 fluorescence spectrophotometer. Upconversion PL measurements were performed on a Ray Lead Tech. spectrofluorometer. A 980 nm laser diode (SDL-980-LM-5000T-Diode Laser) with a power of 3 W/cm2 was employed as the excitation source. Infrared spectra of the samples were obtained with a Bio-Rad FTS 165 Fourier transform infrared spectrometer over the frequency range of 4000–400 cm–1 at a resolution of 4 cm–1. 1H NMR spectra were recorded on a Bruker AVANCE 400 FT-NMR spectrometer with tetramethylsilane as the internal standard. The molecular weight of the BCP was determined by GPC using a Young Lin Acme 9000 liquid chromatograph equipped with a 410 refractive index detector and μ-Styragel columns with THF as the carrier solvent and polystyrene standards used for calibration.
Acknowledgments
We gratefully acknowledge the support of the Ministry of Science and Technology in Taiwan through Grant MOST 105-2221-E-390-029.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.8b03414.
1H NMR spectrum, IR spectrum, GPC curve and data, and DSC thermogram of PDEAAm-b-PNBA and HRTEM images of UCNP-loaded BCP micelles and UCNP and NR co-loaded BCP micelles (PDF)
The authors declare no competing financial interest.
Supplementary Material
References
- Masci G.; Giacomelli L.; Crescenzi V. Atom Transfer Radical Polymerization ofN-Isopropylacrylamide. Macromol. Rapid Commun. 2004, 25, 559–564. 10.1002/marc.200300140. [DOI] [Google Scholar]
- Sugihara S.; Kanaoka S.; Aoshima S. Double thermosensitive diblock copoly-mers of vinyl ethers with pendant oxyethylene groups: Unique physical gelation. Macromolecules 2005, 38, 1919–1927. 10.1021/ma048409m. [DOI] [Google Scholar]
- Liu S.; Armes S. P. Synthesis and aqueous solution behavior of a pH-responsive schizophrenic diblock copolymer. Langmuir 2003, 19, 4432–4438. 10.1021/la020951l. [DOI] [Google Scholar]
- Yusa S.-i.; Shimada Y.; Mitsukami Y.; Yamamoto T.; Morishima Y. pH-Responsive Micellization of Amphiphilic Diblock Copolymers Synthesized via Reversible Addition–Fragmentation Chain Transfer Polymerization. Macromolecules 2003, 36, 4208–4215. 10.1021/ma030065x. [DOI] [Google Scholar]
- Shimoboji T.; Ding Z. L.; Stayton P. S.; Hoffman A. S. Photoswitching of Ligand Association with a Photoresponsive Polymer–Protein Conjugate. Bioconjugate Chem. 2002, 13, 915–919. 10.1021/bc010057q. [DOI] [PubMed] [Google Scholar]
- Zhu Z.; Xu J.; Zhou Y.; Jiang X.; Armes S. P.; Liu S. Effect of salt on the micellization kinetics of pH-responsive abc triblock copolymers. Macromolecules 2007, 40, 6393–6400. 10.1021/ma070978h. [DOI] [Google Scholar]
- Schattling P.; Jochum F. D.; Theato P. Multi-stimuli responsive polymers - the all-in-one talents. Polym. Chem. 2014, 5, 25–36. 10.1039/c3py00880k. [DOI] [Google Scholar]
- Zhuk A.; Mirza R.; Sukhishvili S. Multiresponsive clay-containing layer-by-layer films. ACS Nano 2011, 5, 8790–8799. 10.1021/nn202812a. [DOI] [PubMed] [Google Scholar]
- He Z.; Satarkar N.; Xie T.; Cheng Y.-T.; Hilt J. Z. Remote controlled multishape polymer nanocomposites with selective radiofrequency actuations. Adv. Mater. 2011, 23, 3192–3196. 10.1002/adma.201100646. [DOI] [PubMed] [Google Scholar]
- Jordão N.; Gavara R.; Parola A. J. Flavylium-supported poly(N-isopropylacrylamide): a class of multistimuli responsive polymer. Macromolecules 2013, 46, 9055–9063. 10.1021/ma401497q. [DOI] [Google Scholar]
- Yang F.; Cao Z.; Wang G. Micellar assembly of a photo- and temperature-responsive amphiphilic block copolymer for controlled release. Polym. Chem. 2015, 6, 7995–8002. 10.1039/c5py01435b. [DOI] [Google Scholar]
- Liras M.; González-Béjar M.; Peinado E.; Francés-Soriano L.; Pérez-Prieto J.; Quijada-Garrido I.; García O. Thin amphiphilic polymer-capped upconversion nanoparticles: enhanced emission and thermoresponsive properties. Chem. Mater. 2014, 26, 4014–4022. 10.1021/cm501663n. [DOI] [Google Scholar]
- Wang F.; Liu X. Recent advances in the chemistry of lanthanide-doped upconversion nanocrystals. Chem. Soc. Rev. 2009, 38, 976–989. 10.1039/b809132n. [DOI] [PubMed] [Google Scholar]
- Chen Z.; Chen H.; Hu H.; Yu M.; Li F.; Zhang Q.; Zhou Z.; Yi T.; Huang C. Versatile Synthesis Strategy for Carboxylic Acid–functionalized Upconverting Nanophosphors as Biological Labels. J. Am. Chem. Soc. 2008, 130, 3023–3029. 10.1021/ja076151k. [DOI] [PubMed] [Google Scholar]
- Carling C.-J.; Nourmohammadian F.; Boyer J.-C.; Branda N. R. Remote-Control Photorelease of Caged Compounds Using Near-Infrared Light and Upconverting Nanoparticles. Angew. Chem., Int. Ed. 2010, 49, 3782–3785. 10.1002/anie.201000611. [DOI] [PubMed] [Google Scholar]
- Yan B.; Boyer J.-C.; Branda N. R.; Zhao Y. Near-infrared light-triggered dissociation of block copolymer micelles using upconverting nanoparticles. J. Am. Chem. Soc. 2011, 133, 19714–19717. 10.1021/ja209793b. [DOI] [PubMed] [Google Scholar]
- Xiang J.; Tong X.; Shi F.; Yan Q.; Yu B.; Zhao Y. Near-infrared light-triggered drug release from UV-responsive diblock copolymer-coated upconversion nanoparticles with high monodispersity. J. Mater. Chem. B 2018, 6, 3531–3540. 10.1039/c8tb00651b. [DOI] [PubMed] [Google Scholar]
- Zhao T.; Wang P.; Li Q.; Al-Khalaf A. A.; Hozzein W. N.; Zhang F.; Li X.; Zhao D. Near-infrared triggered decomposition of nanocapsules with high tumor accumulation and stimuli responsive fast elimination. Angew. Chem., Int. Ed. 2018, 57, 2611–2615. 10.1002/anie.201711354. [DOI] [PubMed] [Google Scholar]
- Chen G.; Jaskula-Sztul R.; Esquibel C. R.; Lou I.; Zheng Q.; Dammalapati A.; Harrison A.; Eliceiri K. W.; Tang W.; Chen H.; Gong S. Neuroendocrine Tumor-Targeted Upconversion Nanoparticle-Based Micelles for Simultaneous NIR-Controlled Combination Chemotherapy and Photodynamic Therapy, and Fluorescence Imaging. Adv. Funct. Mater. 2017, 27, 1604671. 10.1002/adfm.201604671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F.; Banerjee D.; Liu Y.; Chen X.; Liu X. Upconversion nanoparticles in biological labeling, imaging, and therapy. Analyst 2010, 135, 1839–1854. 10.1039/c0an00144a. [DOI] [PubMed] [Google Scholar]
- Gnach A.; Bednarkiewicz A. Lanthanide-doped up-converting nanoparticles: merits and challenges. Nano Today 2012, 7, 532–563. 10.1016/j.nantod.2012.10.006. [DOI] [Google Scholar]
- Carling C.-J.; Boyer J.-C.; Branda N. R. Remote-control photoswitching using NIR Light. J. Am. Chem. Soc. 2009, 131, 10838–10839. 10.1021/ja904746s. [DOI] [PubMed] [Google Scholar]
- Boyer J.-C.; Carling C.-J.; Gates B. D.; Branda N. R. Two-way photoswitching using one type of near-infrared light, upconverting nanoparticles, and changing only the light intensity. J. Am. Chem. Soc. 2010, 132, 15766–15772. 10.1021/ja107184z. [DOI] [PubMed] [Google Scholar]
- Qian H.-S.; Zhang Y. Synthesis of Hexagonal-Phase Core–Shell NaYF4Nanocrystals with Tunable Upconversion Fluorescence. Langmuir 2008, 24, 12123–12125. 10.1021/la802343f. [DOI] [PubMed] [Google Scholar]
- Chen G.; Qiu H.; Prasad P. N.; Chen X. Upconversion nanoparticles: design, nanochemistry, and applications in theranostics. Chem. Rev. 2014, 114, 5161–5214. 10.1021/cr400425h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang D.; Ma P. a.; Hou Z.; Cheng Z.; Li C.; Lin J. Current advances in lanthanide ion (Ln3+)-based upconversion nanomaterials for drug delivery. Chem. Soc. Rev. 2015, 44, 1416–1448. 10.1039/c4cs00155a. [DOI] [PubMed] [Google Scholar]
- Huang X.; Lin J. Active-core/active-shell nanostructured design: an effective strategy to enhance Nd3+/Yb3+ cascade sensitized upconversion luminescence in lanthanide-doped nanoparticles. J. Mater. Chem. C 2015, 3, 7652–7657. 10.1039/c5tc01438g. [DOI] [Google Scholar]
- Wang X.; Xu T.; Bu Y.; Yan X. Giant enhancement of upconversion emission in NaYF4:Er3+@NaYF4:Yb3+ active-core/active-shell nanoparticles. RSC Adv. 2016, 6, 22845–22851. 10.1039/c5ra25826j. [DOI] [Google Scholar]
- Du X.; Wang X.; Meng L.; Bu Y.; Yan X. Enhance the Er3+ Upconversion Luminescence by Constructing NaGdF4:Er3+@NaGdF4:Er3+ Active-Core/Active-Shell Nanocrystals. Nanoscale Res. Lett. 2017, 12, 163. 10.1186/s11671-017-1929-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klán P.; Šolomek T.; Bochet C. G.; Blanc A.; Givens R.; Rubina M.; Popik V.; Kostikov A.; Wirz J. Photoremovable protecting groups in chemistry and biology: reaction mechanisms and efficacy. Chem. Rev. 2012, 113, 119–191. 10.1021/cr300177k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelliccioli A. P.; Wirz J. Photoremovable protecting groups: reaction mechanisms and applications. Photochem. Photobiol. Sci. 2002, 1, 441–458. 10.1039/b200777k. [DOI] [PubMed] [Google Scholar]
- Furuta F.Coumarin-4-ylmethyl Phototriggers. In Dynamic Studies in Biology: Phototriggers, Photoswitches and Caged Biomolecules; Goeldner M., Givens R., Eds.; Wiley-VCH: Weinheim, 2005; pp 29–55. [Google Scholar]
- Roy D.; Brooks W. L. A.; Sumerlin B. S. New directions in thermoresponsive polymers. Chem. Soc. Rev. 2013, 42, 7214–7243. 10.1039/c3cs35499g. [DOI] [PubMed] [Google Scholar]
- Jiang X.; Lavender C. A.; Woodcock J. W.; Zhao B. Multiple Micellization and Dissociation Transitions of Thermo- and Light-Sensitive Poly(ethylene oxide)-b-poly(ethoxytri(ethylene glycol) acrylate-co-o-nitrobenzyl acrylate) in Water. Macromolecules 2008, 41, 2632–2643. 10.1021/ma7028105. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.