Abstract
In situ forming hydrogel shows enormous potential as a therapeutic implant or carrier in tissue repair and regeneration. It can perfectly seal or fill the defective tissue, consequently functioning as a cell/drug delivery vehicle. In this contribution, a new gelatin hydrogel with dual-enzymatic cross-linking of horseradish peroxidase (HRP) and galactose oxidase (GalOx) was developed, and the therapeutic effect of this hydrogel encapsulated with bone mesenchymal stem cells (BMSC) in dermal wound healing was investigated. This hydrogel possesses a quick gelation process within 5 min, a high water content, and a uniform three-dimensional (3D) porous network. The 3D cell culture study indicated that gelatin hydrogel matrix of HRP(5U):GalOx(1U) or HRP(2U):GalOx(1U) could provide a friendly 3D microenvironment for supporting the survival, proliferation, and spread of mouse bone mesenchymal stem cells (BMSC) with negligible cytotoxicity. Hematoxylin and eosin staining test suggested that this hydrogel has superior histocompatibility and minimized immune response in vivo. Furthermore, wound-healing studies on a C57 mouse model of excised wound demonstrated that BMSC-laden gelatin hydrogel could significantly accelerate the wound closure as compared to other groups. These data suggest that this dual-enzymatically cross-linked gelatin hydrogel loaded with BMSC has a great potential in wound healing and other tissue-regeneration applications.
1. Introduction
As the largest and the most exposed and vulnerable tissue of human body, skin tissue, once damaged, undergoes a repair process that appears to be very complex.1 Although most skin wound can be quickly and effectively healed within 1 or 2 weeks,2,3 the extensive full-thickness wounds are often hard to repair, which causes serious impact on the health and even threaten people’s life. The repair of wound is one of the most complex biological processes involving activation of numerous intracellular and intercellular pathways able to restore tissue integrity and homeostasis.4 Many studies have successfully used stem cells–seeded hydrogels to promote the wound-healing process.5−8 Stem cells have the potential of continuous proliferation and multidirectional differentiation.9 Hydrogels are three-dimensional (3D) cross-linked polymeric networks and have been used extensively as scaffold or cell/drug vehicle in tissue repair and regeneration.10−13 They have the appropriate structural properties, including high moisture, elasticity, porous structure, and good permeability to oxygen and metabolites, which make them suitable to mimic the natural extracellular matrix. Especially, in situ forming hydrogels exhibit many advantages, such as minimally invasive implantation, simple encapsulation of cells and/or biomolecules, and so on.14−16 Through injection- or spray-based approaches, in situ forming hydrogels enable the accurate filling of any irregular tissue defects. As the artificial scaffold or delivery vehicle for application in tissue repair and regeneration, these hydrogels should possess superior biocompatibility.
Recently, enzymatic cross-linking strategies have received considerable attention for the development of in situ forming hydrogels due to their quick gelation rate, high specificity, and mild reaction condition for cell and tissue.17 Specifically, gelation of phenol-rich polymer mediated by horseradish peroxidase (HRP) has been studied for wide range of applications, such as drug delivery, tissue repairing, and tissue engineering.18,19 For this cross-linking system, HRP catalyzes the cross-linking of phenol-rich polymer by consuming hydrogen peroxide (H2O2) as an oxidizing agent, and the most frequently used way of supplying H2O2 is the direct addition of H2O2 aqueous solution from an external environment. However, this kind of hydrogel has a limitation associated with compromising cytocompatibility and formation of heterogeneous hydrogel due to initially high localization H2O2 concentration.20,21 Recently, Sakai et al. proposed a method of HRP-mediated gelation through “indirect” addition of H2O2 generated by glucose oxidase (GOx) and d-glucose.22 This hydrogelation could be triggered by glucose-containing body fluids such as human blood23 and serum,24 which means that the use of the gel for in vivo applications may have a potential problem. As glucose is a common molecule in our bodies, superfluous GOx in the gel will further oxidize glucose molecules and generate undesired H2O2 in vivo. Therefore, development of other methods for gelation using a combination of various oxidases and HRP should lead to much more biocompatible processes suitable for widespread applications.
Unlike glucose, galactose is rarely found in mammals and can be oxidized by galactose oxidase (GalOx) to produce H2O2 and galactohexanose. Herein, we utilize GalOx as a H2O2-generating enzyme to indirectly supply H2O2 in HRP-mediated cross-linking reaction and successfully fabricate an injectable gelatin hydrogel with negligible cytotoxicity in vitro and immune response in vivo. In addition, this gelatin hydrogel loaded with bone mesenchymal stem cells (BMSC) significantly promoted the wound-closure process. Hence, this dual-enzymatically cross-linked gelatin hydrogel encapsulated with BMSC might have a great potential in dermal wound healing.
2. Results and Discussion
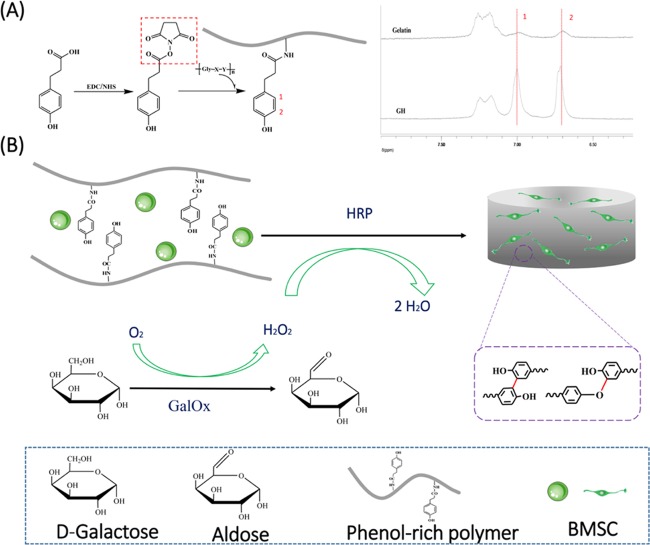
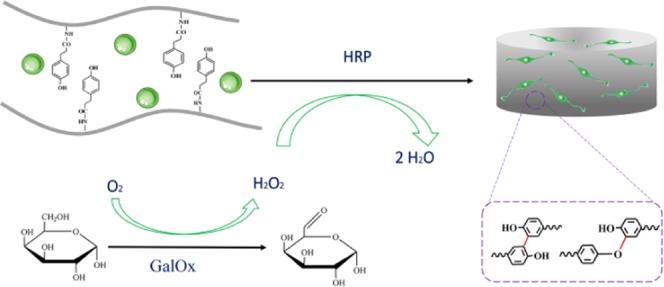
Gelatin, a hydrolysis product of collagen, has shown remarkable advantages for tissue engineering applications, including excellent biocompatibility, matrix metalloproteinase mediated degradability, and retaining natural cell adhesion motifs (e.g., Arg–Gly–Asp (RGD)).25,26 In addition, the digestion process confers gelatin low antigenicity and minimal inflammatory response in vivo. As a result, it has been broadly used in the pharmaceutical and biomedical area. However, the in vivo application of gelatin has been limited thus far due to its low upper critical solution temperature and quick enzymatic degradation.27 Many attempts were made to overcome these challenges, including a HRP-mediated cross-linkable gelatin hydrogel developed by conjugating enzymatically cross-linkable hydroxyphenyl propionic acid.28 However, this method needs H2O2, which creates an adverse microenvironment to the cells. Sakai et al. developed a new way by an indirect addition of H2O2 generated by GOx and d-glucose, which highly improved the cytocompatibility of gelatin hydrogel.22 Since glucose is a common molecule in our body, further oxidation of glucose molecules leads to the generation of undesired H2O2 in the body if GOx remains in the gel.21 Therefore, in this study, we developed a novel approach to supply H2O2 generated by GalOx, and the scheme is shown in Figure 1.
Figure 1.
(A) Synthetic scheme of gelatin–hydroxyphenyl conjugate and the 1H NMR spectra of the GH conjugate. (B) Synthetic scheme of BMSC-loaded GH hydrogel dual-enzymatically cross-linked by HRP and GalOx.
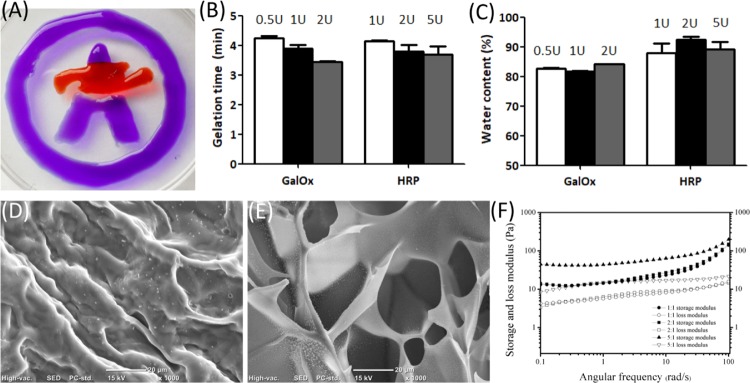
According to the 1H NMR and UV–vis spectra (data not shown) of GH and unmodified gelatin in Figure 1A, the successful conjugation of hydroxyphenyl to gelatin is confirmed,28 and the degree of substitution of hydrophenyl groups is 31.38 μM/g gelatin. Physical characteristics of GH hydrogels including injectability, gelation time, water content, external and internal micromorphology, and mechanical property were detected and analyzed. Result in Figure 2A suggest that this hydrogel can be injected and molded for in situ cross-linking for minimally invasive in vivo applications. The gelation time and water content of the GH hydrogels enzymatically cross-linked by different units of GalOx and HRP were also investigated and are shown in Figure 2B,C. With the increase of enzymatic concentration, the gelation process was accelerated. However, there is no significant difference among their gelation times. The water content of all samples exceeds 80% but displays a nonpositive relation between enzymatic content. High water content property might contribute to reducing the frictional irritation to the surrounding tissue.29,30
Figure 2.
Physical characterization of GH hydrogels including injectability (A), gelation time (B), water content (C), external and internal micromorphology (D, E), and mechanical property (F). GH (8% wt), d-galactose (50 mM), HRP (1, 2, and 5 U/mL), and GalOx (0.5, 1, and 2 U/mL). GH hydrogels of HRP(5U):GalOx(1U), HRP(2U):GalOx(1U), and HRP(1U):GalOx(1U) is 5:1, 2:1, and 1:1, respectively in (F).
In addition, the external and internal structures of the GH hydrogels were observed by scanning electron microscopy (SEM) (Figure 2D,E). It can be seen that hydrogels have a uniform porous network, and the internal pore sizes of the GH hydrogel range from hundreds to dozens of microns. The relatively large pore sizes of these hydrogels might make a great contribution to the permeation of nutrients, exchange of oxygen and carbon dioxide, discharge of metabolites, and so on, which will be beneficial to cell growth and proliferation encapsulated within the hydrogel.30
The mechanical properties of the hydrogel were evaluated by measuring Young’s modulus using small-amplitude oscillatory shear experiments. A strain sweep test (0–10%) was performed at an oscillatory frequency of 10 rad/s for each hydrogel, and the results shows that a strain of 1% is in the linear viscoelastic range for all of the tested hydrogels. Then, the linear viscoelastic behavior of the hydrogels was characterized by oscillatory frequency sweep measurements, and the data of storage modulus (G′) and loss modulus (G″) are shown in Figure 2F. Hydrogel formation was further confirmed because the storage modulus (G′) was greater than the loss modulus (G″). And the increase in G′ values ranged from 100 to 200 Pa at an angular frequency of 10 rad/s with the increase in HRP content. However, all G′ values of hydrogels show no significant difference.
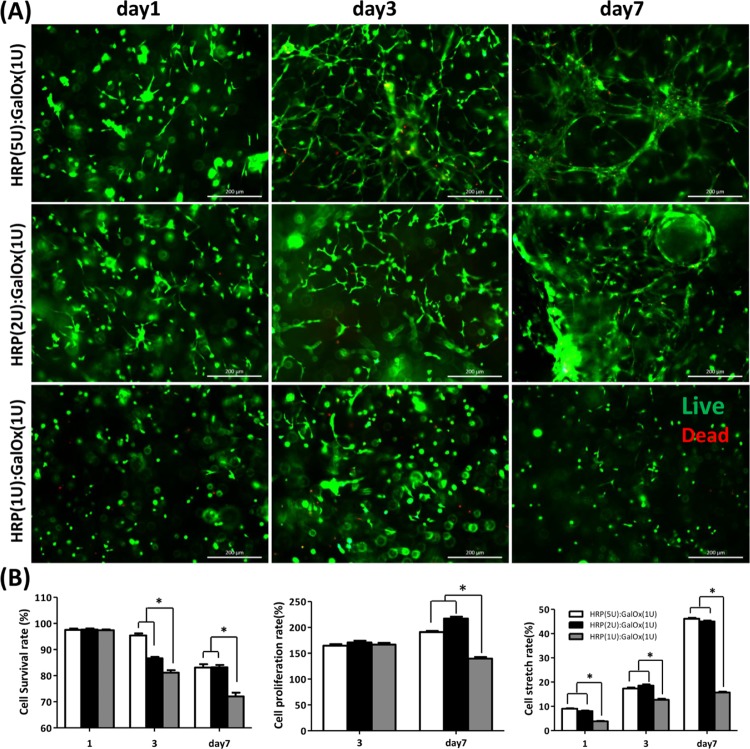
As a cellular scaffold, hydrogel should possess superior biocompatibility. The cytocompatibility was first evaluated by a 3D culture in vitro for 1, 3, and 7 days. Interestingly, the BMSC encapsulated within the hydrogels of HRP(5U):GalOx(1U) and HRP(2U):GalOx(1U) displays a significantly higher survival, proliferation, and stretch ratio compared with the BMSC encapsulated within the HRP(1U):GalOx(1U) hydrogel (Figure 3). More importantly, the BMSC within the hydrogels of HRP(5U):GalOx(1U) and HRP(2U):GalOx(1U) stretched extremely well. This phenomenon might be attributed to the slower consumption ratio of H2O2 under less HRP content condition. Long exposure time to H2O2 may lead to more cellular harm, which affects cellular proliferation and stretching. Hence, we chose the GH hydrogel of HRP(5U):GalOx(1U) as the scaffold to encapsulate BMSC for further research in vivo. In addition, the magnified views of BMSC culturing within the hydrogels for 1, 3, and 7 days are shown in Figures S1–S3.
Figure 3.
(A) Live/Dead staining and viability results of BMSC loaded within the hydrogels of HRP(5U):GalOx(1U), HRP(2U):GalOx(1U), and HRP(1U):GalOx(1U) after culturing for 1, 3, and 7 days. Scale bar is 200 μm. (B) Statistical analysis of the cellular survival, proliferation, and stretching rate encapsulated within the GH hydrogel after 1, 3, and 5 days of culture (*p < 0.001).
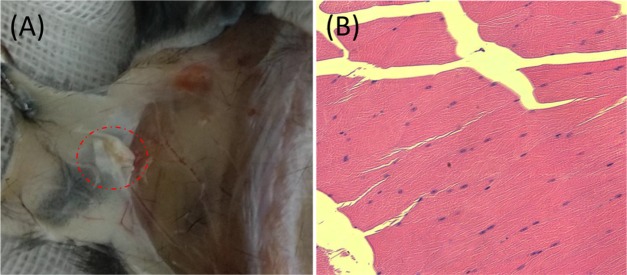
Moreover, the biocompatibility of the GH hydrogel in vivo was also assessed by subcutaneous experiment of the C57 mice . Hematoxylin and eosin (HE) staining was performed to investigate the immune response for the transplanted hydrogel, and the results are displayed in Figure 4. As shown in Figure 4B, the hydrogel clearly presents almost no inflammatory cells, which verify the low immunogenicity of the GH hydrogel enzymatically cross-linked by HRP and GalOx.
Figure 4.
(A) GH hydrogel (in red virtual box) under the skin of C57 mouse after 4 days. (B) HE staining of the muscular tissue around the GH hydrogel.
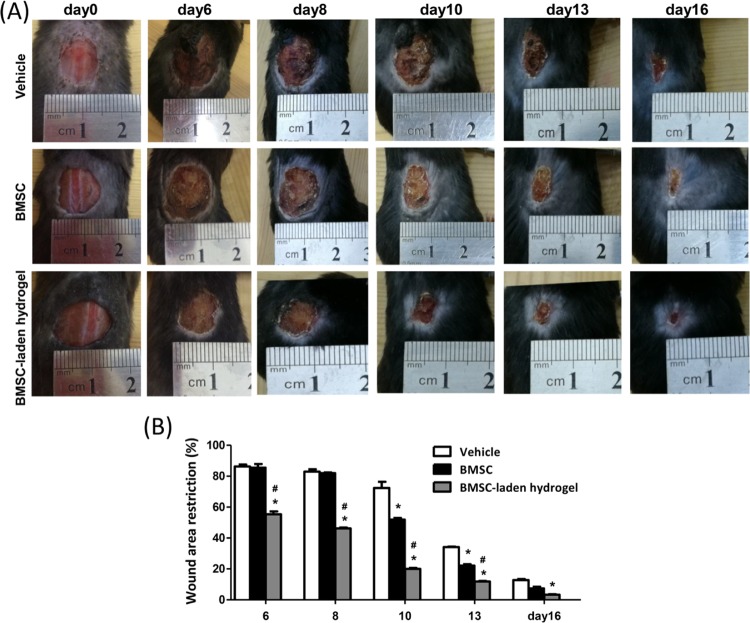
In recent years, studies have found that cell-laden or cytokine-encapsulated hydrogels could promote the wound-healing process. Therefore, in this study, we investigate the promoting capacity of BMSC-laden GH hydrogels on the dermal wound-healing process. As shown in Figure 5, BMSC-laden GH hydrogels group induced significant reduction of wound extension compared with the control group and BMSC group after 6 days of treatment. As we know, the ability of pure BMSC to repair is mainly determined by the paracrine effect. However, purely cell therapy is limited by the low survival rate and insufficient number of transplanted cells.31,32 The GH hydrogel could dramatically enhance the cellular survival and proliferation under 3D culture condition, thus possibly improving the survival rate and the number of transplanted cells. Therefore, the enhanced dermal wound-healing ability of the BMSC-laden GH hydrogels can be reasonably ascribed to the improved microenvironment supplied by the GH hydrogel for transplanted BMSC.
Figure 5.
Effects of BMSC-laden GH hydrogels on the healing process in a C57 mouse model of an excised wound. (A) A full-thickness wound was excised from the back of the rats (n = 6) using sterile scissors and left open. Wounds were treated with saline (vehicle), BMSC, and BMSC-laden GH hydrogels. (B) Wound area restriction on day6, day8, day10, day13, and day16 as compared to day0. *p < 0.001 vs control; #p < 0.001 vs BMSC.
3. Conclusions
A new GH hydrogel in situ enzymatically cross-linked by HRP and GalOx was developed and characterized. This hydrogel of HRP(5U):GalOx(1U) and HRP(2U):GalOx(1U) exhibits many superior physical and biological properties including injectability, quick gelation process within 5 min, and excellent cytocompatibility and histocompatibility. Significantly, BMSC-laden hydrogel composite of HRP(5U):GalOx(1U) could obviously promote the dermal wound-healing process. Therefore, it is anticipated that this BMSC-carrying hydrogel composite has a great application potential in dermal tissue repair and regeneration.
4. Materials and Methods
4.1. Materials
Gelatin (type A from porcine skin, less than 300 bloom), HRP (type VI, salt-free, 250–330 units/mg solid), 3-(4-hydroxy-phenyl) propionic acid (HPA), 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide (EDC), N-hydroxysuccinimide (NHS), GalOx, and d-galactose were purchased from Sigma-Aldrich (St. Louis, MO). Dimethylformamide (DMF) was obtained from Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The chemical reagents were used as obtained without purification. The dialysis membrane (molecular cutoff = 3500 Da) was purchased from Spectrum Laboratories (Rancho Dominguez, CA). Mouse bone mesenchymal stem cells (BMSC) were obtained from the Cell Bank, Type Culture Collection, Chinese Academy of Sciences, Shanghai, China.
4.2. Synthesis and Characterization of Gelatin–Hydroxyphenyl
Synthesis of gelatin–hydroxyphenyl (GH) has been described everywhere.28,33,34 Briefly, HPA was first activated with EDC and NHS in a co-solvent of water and DMF (volume ratio of 3:2) for 30 min. The activated HPA solution was then added to the preheated gelatin solution and stirred at 40 °C for 24 h. The resulting solution was transferred into a dialysis bag, dialyzed against deionized water at 45 °C for 3 days, filtered, and lyophilized to obtain the GH conjugates. GH was characterized by 1H NMR spectroscopy (AS400, OXFORD instruments, U.K.), and the phenolic contents of the conjugates were measured quantitatively at 275 nm using a UV–vis spectrophotometer (V-750 UV/vis/NIR, Jasco, Japan).
4.3. Fabrication and Characterization of GH Hydrogel
Eight weight percent GH was dissolved in Dulbecco’s modified Eagle medium (DMEM) media containing 50 mM d-galactose, followed by adding different amounts of HRP (1, 2, and 5 U/mL) and GalOx (0.5, 1, and 2 U/mL). The injectability of this GH hydrogel was also verified by a syringe. The gelation time of the GH hydrogels was calculated by the inverted tube test.
The water content of the GH hydrogels was calculated with the following formula: D (%) = [(W0 – W1)/W0] × 100, where D denotes the water content of the hydrogels, W0 denotes the wet weights of the hydrogels, and W1 denotes the dried weights after freeze-drying.
The surface and internal morphologies of the GH hydrogel was characterized by scanning electron microscopy (SEM, FEI Quanta200, Netherland) after lyophilizing, breakage, and gold spraying.
The rheological behavior of the GH hydrogels was evaluated by detecting their modulus of elasticity using a rheometer platform (Leica DHR2, Germany). The dynamic oscillation scanning frequency ranged from 1 to 100 Hz, and the temperature and strain were set as 37 °C and 1%, respectively.
4.4. Three-Dimensional Culture of BMSC within GH Hydrogel
BMSC were suspended in the 8% GH solution containing HRP (1, 2, and 5 U/mL) and d-galactose (50 mM) at a concentration of 1 × 10 6 cells/mL. Then, GalOx (1 U/mL) was added to the GH/HRP/d-galactose/BMSC solution to induce gelation at 37 °C for 10 min. The BMSC-loaded GH hydrogels were cultured using fresh DMEM/F12 complete medium (with 10% fetal bovine serum) at 37 °C in a humidified atmosphere containing 95% air and 5% CO2.
After culturing for 1, 3 and 7 days, the BMSC-loaded hydrogels were stained with cell Live/Dead kit (Calcein-AM/PI) at 37 °C for 10 min and then observed using fluorescence microscopy (Leica DFC7000T, Germany). The viability of the BMSC was determined by counting and calculating from at least three fields of view for each sample.
4.5. Biocompatibility of GH Hydrogel in Vivo
To evaluate the biocompatibility of the GH hydrogel, 8% GH/HRP/GalOx/d-galactose mixed solution was injected subcutaneously in the C57 mouse. After 4 days, the surrounding tissues were sectioned and HE staining was used to evaluate the biocompatibility of the GH hydrogel in vivo.
4.6. In Vivo Wound-Healing Study
To monitor the process of wound closure, a full-thickness excision wound model was used. Male C57 mice weighing 22–26 g were used. All manipulations were carried out under aseptic condition. First, all of the animals were anesthetized with chloral hydrate (10% wt, 0.35 mL/100 g body weight) by intraperitoneal administration. Then, the dorsal region of the mice was chosen to avoid animals’ access to their own wound, and the dorsal hair was shaved. Whereafter, the shaved area was disinfected with Betadine R 10%, and a full-thickness wound with a diameter of 1.5 cm was excised from the back of the mice using sterile scissors. The mice’s respiration and heartbeat were monitored, and they were kept warm until they were completely awake and the vital signs were stable. The mice were housed one per box to prevent cross-access to the lesions. The C57 mice (n = 6) were divided into three groups and subcutaneously injected with normal saline (100 μL), BMSC (100 μL, 106 cells), and BMSC-laden GH solution (100 μL, 106 cells) around the wounds, respectively. The wound size of the mice on day0, day6, day8, day10, day13, and day16 was measured using ImageJ software. The rate of wound closure representing the percentage of wound reduction from the original wound size was calculated using the following formula: [wound area day0 – wound area (day6 or day8, day10, day13, day16)]/wound area day0 × 100. Values are expressed as percentage of the healed wounds ±SD.
4.7. Statistical Analysis
Error bars represent the mean ± SD of biological replicates. Statistical comparisons were made by two-way ANOVA for multiple comparisons.
Acknowledgments
We acknowledge funding from the Joint Fund of the National Natural Science Foundation of China and Henan province (U1804198), National Natural Science Foundation of China (31700820), China Postdoctoral Science Foundation (2017M612420), Key Scientific Research Projects of higher education institutions in Henan province (18A180003).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00878.
Live/Dead staining and viability ratio results of BMSC loaded in the hydrogels of HRP(5U):GalOx(1U), HRP(2U):GalOx(1U), and HRP(1U):GalOx(1U) after culturing for 1 day; scale bar is 400 μm (Figure S1); Live/Dead staining and viability ratio results of BMSC loaded in the hydrogels of HRP(5U):GalOx(1U), HRP(2U):GalOx(1U), and HRP(1U):GalOx(1U) after culturing for 3 days; scale bar is 400 μm (Figure S2); Live/Dead staining and viability ratio results of BMSC loaded in the hydrogels of HRP(5U):GalOx(1U), HRP(2U):GalOx(1U), and HRP(1U):GalOx(1U) after culturing for 7 days; scale bar is 400 μm (Figure S3) (PDF)
Author Contributions
† M.Y. and J.Z. are equal contributors.
The authors declare no competing financial interest.
Supplementary Material
References
- van Zelm R. T.; Clark M.; Haalboom J. R. E.. The Development, Dissemination, and Use of Pressure Ulcer Guidelines. Science and Practice of Pressure Ulcer Management; Springer: London, 2006; p 175. [Google Scholar]
- Sorg H.; Tilkorn D. J.; Hager S.; Hauser J.; Mirastschijski U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. Eur. Surg. Res. 2017, 58, 81. 10.1159/000454919. [DOI] [PubMed] [Google Scholar]
- Pereira R. F.; Bartolo P. J. Traditional Therapies for Skin Wound Healing. Adv. Wound Care 2016, 5, 208. 10.1089/wound.2013.0506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner G. C.; Werner S.; Barrandon Y.; Longaker M. T. Wound repair and regeneration. Nature 2008, 453, 314. 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Lee P. Y.; Cobain E.; Huard J.; Huang L. Thermosensitive hydrogel PEG–PLGA–PEG enhances engraftment of muscle-derived stem cells and promotes healing in diabetic wound. Mol. Ther. 2007, 15, 1194. 10.1038/sj.mt.6300156. [DOI] [PubMed] [Google Scholar]
- Xu Q.; A S.; Gao Y.; Guo L.; Creagh-Flynn J.; Zhou D.; Greiser U.; Dong Y.; Wang F.; Tai H.; et al. A hybrid injectable hydrogel from hyperbranched PEG macromer as a stem cell delivery and retention platform for diabetic wound healing. Acta Biomater. 2018, 75, 63. 10.1016/j.actbio.2018.05.039. [DOI] [PubMed] [Google Scholar]
- Dong Y.; A S.; Rodrigues M.; Li X.; Kwon S. H.; Kosaric N.; Khong S.; Gao Y.; Wang W.; Gurtner G. C. Injectable and Tunable Gelatin Hydrogels Enhance Stem Cell Retention and Improve Cutaneous Wound Healing. Adv. Funct. Mater. 2017, 27, 1606619 10.1002/adfm.201606619. [DOI] [Google Scholar]
- Burdick J. A.; Mauck R. L.; Gerecht S. To Serve and Protect: Hydrogels to Improve Stem Cell-Based Therapies. Cell Stem Cell 2016, 18, 13. 10.1016/j.stem.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Wu Y.; Chen L.; Scott P. G.; Tredget E. E. Mesenchymal stem cells enhance wound healing through differentiation and angiogenesis. Stem Cells 2007, 25, 2648. 10.1634/stemcells.2007-0226. [DOI] [PubMed] [Google Scholar]
- Qi C.; Liu J.; Jin Y.; Xu L.; Wang G.; Wang Z.; Wang L. Photo-crosslinkable, injectable sericin hydrogel as 3D biomimetic extracellular matrix for minimally invasive repairing cartilage. Biomaterials 2018, 163, 89. 10.1016/j.biomaterials.2018.02.016. [DOI] [PubMed] [Google Scholar]
- Wadley P. Pure spin currents find the off switch. Nat. Mater. 2018, 17, 566. 10.1038/s41563-018-0125-2. [DOI] [PubMed] [Google Scholar]
- Zhang K.; Shi Z.; Zhou J.; Xing Q.; Ma S.; Li Q.; Zhang Y.; Yao M.; Wang X.; Li Q.; et al. Potential application of an injectable hydrogel scaffold loaded with mesenchymal stem cells for treating traumatic brain injury. J. Mater. Chem. B 2018, 6, 2982. 10.1039/C7TB03213G. [DOI] [PubMed] [Google Scholar]
- Li X.; Sun Q.; Li Q.; Kawazoe N.; Chen G. Functional Hydrogels With Tunable Structures and Properties for Tissue Engineering Applications. Front. Chem. 2018, 6, 499. 10.3389/fchem.2018.00499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimatteo R.; Darling N. J.; Segura T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Delivery Rev. 2018, 127, 167. 10.1016/j.addr.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S. H.; Lee S. H.; Lee J. E.; Park S. J.; Kim K.; Kim I. S.; Lee Y. S.; Hwang N. S.; Kim B. G. Tissue adhesive, rapid forming, and sprayable ECM hydrogel via recombinant tyrosinase crosslinking. Biomaterials 2018, 178, 401. 10.1016/j.biomaterials.2018.04.057. [DOI] [PubMed] [Google Scholar]
- Xu G.; Wang X.; Deng C.; Teng X.; Suuronen E. J.; Shen Z.; Zhong Z. Injectable biodegradable hybrid hydrogels based on thiolated collagen and oligo(acryloyl carbonate)-poly(ethylene glycol)-oligo(acryloyl carbonate) copolymer for functional cardiac regeneration. Acta Biomater. 2015, 15, 55. 10.1016/j.actbio.2014.12.016. [DOI] [PubMed] [Google Scholar]
- Teixeira L. S.; Feijen J.; van Blitterswijk C. A.; Dijkstra P. J.; Karperien M. Enzyme-catalyzed crosslinkable hydrogels: emerging strategies for tissue engineering. Biomaterials 2012, 33, 1281. 10.1016/j.biomaterials.2011.10.067. [DOI] [PubMed] [Google Scholar]
- Bae J. W.; Choi J. H.; Lee Y.; Park K. D. Horseradish peroxidase-catalysed in situ-forming hydrogels for tissue-engineering applications. J. Tissue Eng. Regener. Med. 2015, 9, 1225. 10.1002/term.1917. [DOI] [PubMed] [Google Scholar]
- Khanmohammadi M.; Dastjerdi M. B.; Ai A.; Ahmadi A.; Godarzi A.; Rahimi A.; Ai J. Horseradish peroxidase-catalyzed hydrogelation for biomedical applications. Biomater. Sci. 2018, 6, 1286. 10.1039/C8BM00056E. [DOI] [PubMed] [Google Scholar]
- Kim B. Y.; Lee Y.; Son J. Y.; Park K. M.; Park K. D. Dual Enzyme-Triggered In Situ Crosslinkable Gelatin Hydrogels for Artificial Cellular Microenvironments. Macromol. Biosci. 2016, 16, 1570. 10.1002/mabi.201600312. [DOI] [PubMed] [Google Scholar]
- Nakahata M.; Gantumur E.; Furuno K.; Sakai S.; Taya M. Versatility of hydrogelation by dual-enzymatic reactions with oxidases and peroxidase. Biochem. Eng. J. 2018, 131, 1. 10.1016/j.bej.2017.12.003. [DOI] [Google Scholar]
- Sakai S.; Komatani K.; Taya M. Glucose-triggered co-enzymatic hydrogelation of aqueous polymer solutions. RSC Adv. 2012, 2, 1502. 10.1039/C1RA01060C. [DOI] [Google Scholar]
- Sakai S.; Tsumura M.; Inoue M.; Koga Y.; Fukano K.; Taya M. Polyvinyl alcohol-based hydrogel dressing gellable on-wound via a co-enzymatic reaction triggered by glucose in the wound exudate. J. Mater. Chem. B 2013, 1, 5067. 10.1039/c3tb20780c. [DOI] [PubMed] [Google Scholar]
- Sakai S.; Ueda K.; Taya M. Peritoneal adhesion prevention by a biodegradable hyaluronic acid-based hydrogel formed in situ through a cascade enzyme reaction initiated by contact with body fluid on tissue surfaces. Acta Biomater. 2015, 24, 152. 10.1016/j.actbio.2015.06.023. [DOI] [PubMed] [Google Scholar]
- Mũnoz Z.; Shih H.; Lin C.-C. Gelatin hydrogels formed by orthogonal thiol–norbornene photochemistry for cell encapsulation. Biomater. Sci. 2014, 2, 1063. 10.1039/C4BM00070F. [DOI] [PubMed] [Google Scholar]
- Lim K. S.; Schon B. S.; Mekhileri N. V.; Brown G. C. J.; Chia C. M.; Prabakar S.; Hooper G. J.; Woodfield T. B. F. New Visible-Light Photoinitiating System for Improved Print Fidelity in Gelatin-Based Bioinks. ACS Biomater. Sci. Eng. 2016, 2, 1752. 10.1021/acsbiomaterials.6b00149. [DOI] [PubMed] [Google Scholar]
- Bertlein S.; Brown G.; Lim K. S.; Jungst T.; Boeck T.; Blunk T.; Tessmar J.; Hooper G. J.; Woodfield T. B. F.; Groll J. Thiol-Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404 10.1002/adma.201703404. [DOI] [PubMed] [Google Scholar]
- Lee S. H.; Lee Y.; Chun Y. W.; Crowder S. W.; Young P. P.; Park K. D.; Sung H. J. In Situ Crosslinkable Gelatin Hydrogels for Vasculogenic Induction and Delivery of Mesenchymal Stem Cells. Adv. Funct. Mater. 2014, 24, 6771. 10.1002/adfm.201401110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balakrishnan B.; Banerjee R. Biopolymer-based hydrogels for cartilage tissue engineering. Chem. Rev. 2011, 111, 4453. 10.1021/cr100123h. [DOI] [PubMed] [Google Scholar]
- Drury J. L.; Mooney D. J. Hydrogels for tissue engineering: scaffold design variables and applications. Biomaterials 2003, 24, 4337. 10.1016/S0142-9612(03)00340-5. [DOI] [PubMed] [Google Scholar]
- Shen L. H.; Li Y.; Chen J.; Cui Y.; Zhang C.; Kapke A.; Lu M.; Savant-Bhonsale S.; Chopp M. One-year follow-up after bone marrow stromal cell treatment in middle-aged female rats with stroke. Stroke 2007, 38, 2150. 10.1161/STROKEAHA.106.481218. [DOI] [PubMed] [Google Scholar]
- Guan J.; Zhu Z.; Zhao R. C.; Xiao Z.; Wu C.; Han Q.; Chen L.; Tong W.; Zhang J.; Han Q.; et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials 2013, 34, 5937. 10.1016/j.biomaterials.2013.04.047. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Bae J. W.; Oh D. H.; Park K. M.; Chun Y. W.; Sung H.-J.; Park K. D. In situ forming gelatin-based tissue adhesives and their phenolic content-driven properties. J. Mater. Chem. B 2013, 1, 2407. 10.1039/c3tb00578j. [DOI] [PubMed] [Google Scholar]
- Lee Y.; Son J. Y.; Kang J. I.; Park K. M.; Park K. D. Hydrogen Peroxide-Releasing Hydrogels for Enhanced Endothelial Cell Activities and Neovascularization. ACS Appl. Mater. Interfaces 2018, 10, 18372. 10.1021/acsami.8b04522. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.