Abstract
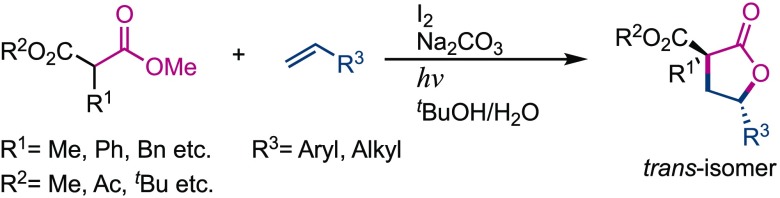
This study aims to develop an intermolecular lactonization reaction of alkenes with carbonyls mediated by visible light and molecular iodine. The one-step reaction involved the carboesterification of alkenes to produce the corresponding lactones in moderate to good yield. It was also revealed that it is possible to control the diastereoselectivity of the reaction by altering the base used and the reaction conditions. When water was added as a solvent, the reaction resulted in the formation of lactones with trans-selectivity. A mechanistic investigation was undertaken and it was found that the reaction requires the generation of an iodine radical from molecular iodine, driven by visible light irradiation, and proceeds via the formation of an iodine radical alkene adduct. The proposed reaction is an example of a rare-metal free intermolecular addition cyclization reaction, which is an environment-friendly chemical process that only uses molecular iodine. In addition, since diastereoselectivity was observed without the use of any specific reagents, the developed methodology is an example of a novel stereoselective transformation using only cost-effective reagents.
Introduction
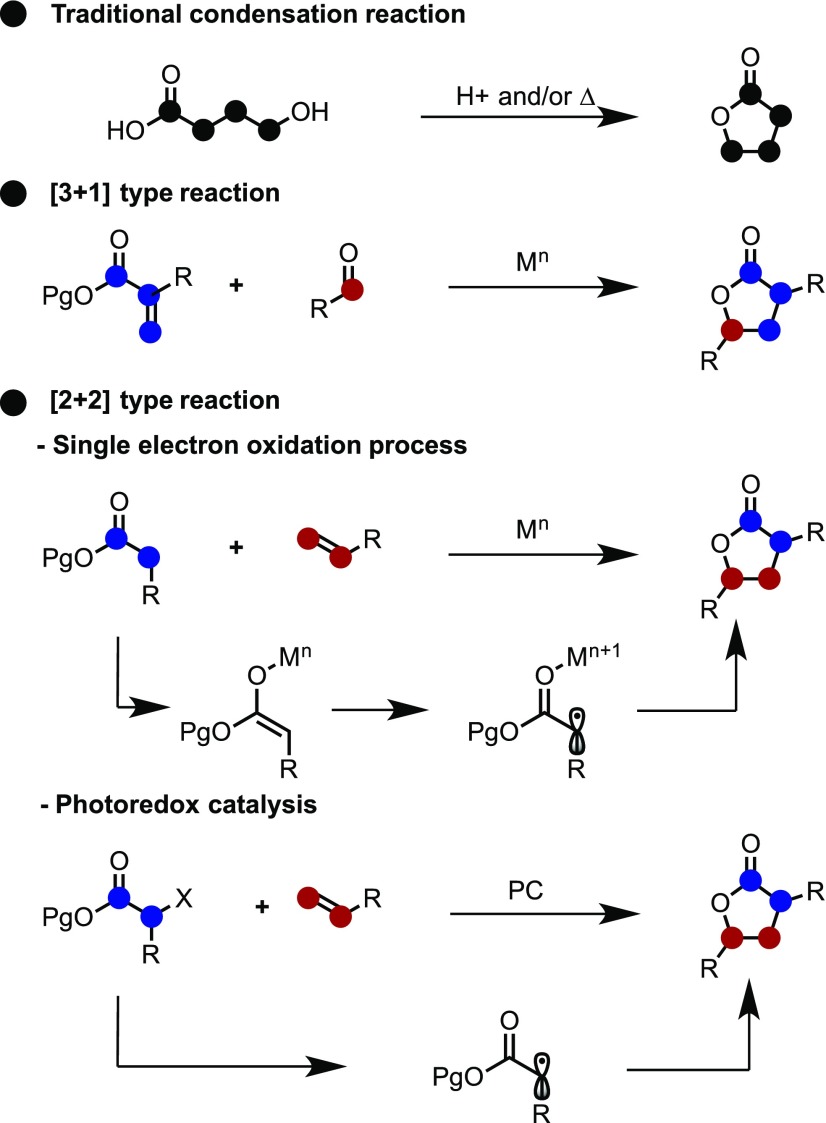
Oxygen-containing 5-membered ring systems such as γ-lactones are found in naturally occurring products and pharmaceuticals and are commonly used as building blocks in various fields of chemistry.1,2 Traditionally, the lactone skeleton is constructed through the intramolecular condensation reaction of a hydroxycarboxylic acid under Brønsted/Lewis acidic and thermal conditions (Scheme 1, top).3−6 However, this methodology is not suitable for the synthesis of some derivatives. Toward this aim, the intermolecular diversity-oriented synthesis of lactones has been developed.7 For example, a [3 + 1] type intermolecular cycloaddition reaction based on the generation of carbon radicals using a strong single-electron reductant such as SmI2 was reported by Fukuzawa and Procter (Scheme 1, middle).8 Furthermore, various intermolecular [2 + 2] type reactions such as the C–C/C–O bond formation of an alkene with an acetyl unit have also been investigated. Among them is a common and powerful method based on the generation of carbon radicals in a one-electron oxidation process using heavy metals (Scheme 1, bottom).9,10 Recently, a methodology using photo-redox catalysts has also been developed.11 However, in intermolecular reactions, it is difficult to control the diastereoselectivity by changing only the reaction conditions, and only a few examples of this have been reported in the literature.12
Scheme 1. Reported Methodology for Lactone Synthesis.
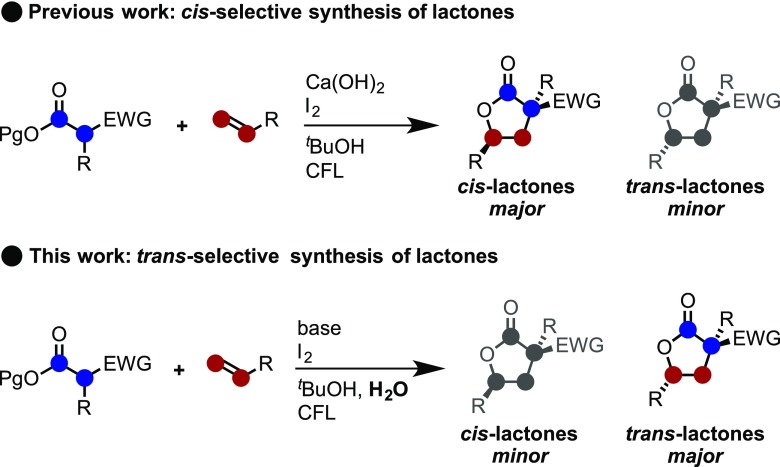
Previously, we developed the iodine/visible light-mediated carboesterification of styrene using carbonyls that led to the formation of cis-lactones through the photoinduced generation of an iodine radical as the key active species (Scheme 2; previous work).13,14 Over the course of this study, it was found that the opposite diastereomer could be formed without the use of any specific reagent by simply controlling the reaction conditions. In particular, it was effective for priory to trans-selectivity when water was added as a solvent. Herein, the trans-selective synthesis of γ-lactones is described via visible light/iodine-mediated intermolecular carboesterification of styrenes with carbonyls (Scheme 2; this work). A mechanistic investigation using stereochemical models is also described.
Scheme 2. Schematic Overview of the trans-Diastereoselective Synthesis of Lactones in This Work.
Results and Discussion
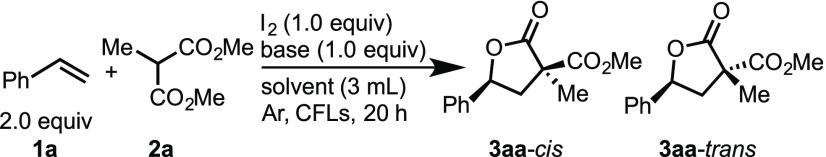
Previous studies have shown that using Ca(OH)2 as a base in combination with molecular iodine in the presence of alkenes and malonates always affords products with cis-diastereoselectivity as a result of an addition/cyclization process (Table 1, entry 1).15 Therefore, to develop a stereoselective iodine/visible light-mediated alkene carboesterification reaction that can be applied to carbonyls, various inorganic bases were explored. Upon screening of alkaline earth metal-based inorganic bases in the reaction of styrene 1a with malonate 2a in the presence of molecular iodine, the formation of cis-diastereomer 3aa-cis preferentially occurred when Ba(OH)2 was used (entry 3). Unfortunately, the reaction significantly decreased the yield of the product when the reaction employed other alkaline earth metal bases (entries 2, 4–6). On the other hand, upon the evaluation of alkaline metal-based inorganic bases, such as NaHCO3, Na2CO3, K2CO3, Cs2CO3, and K3PO4, a slight preference for the formation of trans-diastereomer 3aa-trans was observed (entries 7–12). The basicity of the base used was found to have a strong effect on the yield of product 3aa. For instance, the intermolecular addition reaction of styrene with malonate resulted in a significant decrease in the yield of the product in the presence of a weak base, such as NaHCO3 (entry 7). In contrast, the desired lactonization and hydrolysis of the ester proceeded competitively in the presence of strong bases, such as KOH and K3PO4 (entries 11 and 12). Gratifyingly, when using Na2CO3, a further variation in the reaction conditions resulted in 3aa-trans being generated in 69% yield with 25:75 diastereoselectivity (entry 13). Under the optimal conditions, further base screening was performed. Interestingly, the reaction in tBuOH with water resulted in an increase in trans-diastereoselectivity (entries 13–17), and even more interestingly, inverse diastereoselectivity was observed using bases such as Ca(OH)2, Ba(OH)2, and Sr(OH)2 under the same conditions (entry 1,3,4 vs 14–16).
Table 1. Optimization of the Intermolecular Lactonization of Styrene 1a with Malonate (2a)a.
| entry | base | solvent | 3 (%) | dr (cis:trans)b |
|---|---|---|---|---|
| 1 | Ca(OH)2 | tBuOH | 83 | 79:21 |
| 2 | Mg(OH)2 | tBuOH | trace | |
| 3 | Ba(OH)2 | tBuOH | 74 | 67:33 |
| 4 | Sr(OH)2 | tBuOH | 33 | 50:50 |
| 5 | BaCO3 | tBuOH | trace | |
| 6 | SrCO3 | tBuOH | trace | |
| 7 | NaHCO3 | tBuOH | 32 | 50:50 |
| 8 | Na2CO3 | tBuOH | 77 | 45:55 |
| 9 | K2CO3 | tBuOH | 53 | 40:60 |
| 10 | Cs2CO3 | tBuOH | 49 | 37:63 |
| 11 | KOH | tBuOH | 36 | 33:67 |
| 12 | K3PO4 | tBuOH | 26 | 30:70 |
| 13 | Na2CO3 | tBuOH/H2O (2 mL/1 mL) | 69 | 25:75 |
| 14 | Ca(OH)2 | tBuOH/H2O (2 mL/1 mL) | 61 | 40:60 |
| 15 | Ba(OH)2 | tBuOH/H2O (2 mL/1 mL) | 7 | 29:71 |
| 16 | Sr(OH)2 | tBuOH/H2O (2 mL/1 mL) | 29 | 42:58 |
| 17 | K2CO3 | tBuOH/H2O (2 mL/1 mL) | 60 | 26:74 |
| 18 | Cs2CO3 | tBuOH/H2O (2 mL/1 mL) | 56 | 28:72 |
| 19 | KOH | tBuOH/H2O (2 mL/1 mL) | trace | |
| 20 | K3PO4 | tBuOH/H2O (2 mL/1 mL) | 41 | 27:73 |
Reaction conditions: 1a (2 equiv), 2a (0.3 mmol), I2 (0.3 mmol), and base (0.3 mmol) in solvent (3 mL) were stirred at ambient temperature irradiated with four of compact fluorescent lamps (CFLs) for 20 h.
Diasteromeric ratios (dr) were determined by 1H NMR analysis of the crude reaction mixture.
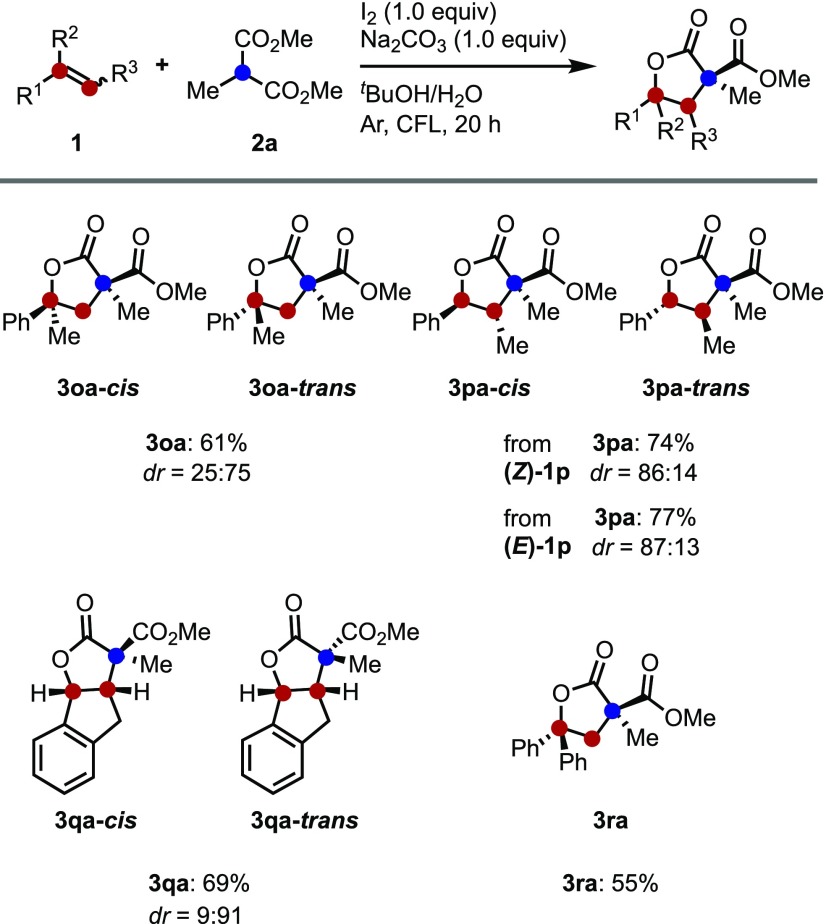
After the optimization study, the scope of the trans-diastereoselective synthesis of lactones was investigated by making 2-methyl dimethyl malonate 2a to react with a series of alkenes, 1 (Table 2). Generally, the controllable carboesterification reactions furnished trans-butyrolactones with good diastereoselectivities in the presence of water as a co-solvent. At first, trans-selective lactonization was investigated using Na2CO3 as a base under the optimized reaction conditions (Table 1, entry 13). Most of the reactions proceeded smoothly using the developed system to afford the corresponding butyrolactones 3 in good yield and trans-diastereoselectivity. As shown in Table 2, styrenes with various substituents, such as tert-butyl, 4-methyl, 3-methyl, and 2-methylstyrene (1b–1e), gave products in 61–72% isolated yield with moderate to good trans-diastereoselectivity. In addition, styrenes bearing halogen substituents (1f–1h) also furnished the desired products. The stereochemical assignments were corroborated using single-crystal X-ray diffraction analysis of the obtained trans-3ha adduct.16 Unfortunately, methyl-4-vinylbenzoate, 1i, did not work under the present conditions, even though good conversion could be achieved using a set of previous cis-selective conditions.13 This is probably due to the instability of the ester under these conditions. Electron-rich substrates such as 1j also did not react with malonate due to the rapid polymerization of styrene.17
Table 2. trans-Diastereoselective Synthesis of Lactones, 3, from Various Alkenes Using Malonate 2a.
| entry | R | 3 | yield (%) | dra (cis:trans) |
|---|---|---|---|---|
| 1 | Ph (1a) | 3aa | 69 | 25:75 |
| 2 | 4-tBu-C6H4 (1b) | 3ba | 65 | 33:67 |
| 3 | 4-Me-C6H4 (1c) | 3ca | 61 | 33:67 |
| 4 | 3-Me-C6H4 (1d) | 3da | 72 | 33:67 |
| 5 | 2-Me-C6H4 (1e) | 3ea | 66 | 26:74 |
| 6 | 4-F-C6H4 (1f) | 3fa | 28 | 20:80 |
| 7 | 4-Cl-C6H4 (1g) | 3ga | 60 | 29:71 |
| 8 | 4-Br-C6H4 (1h) | 3ha | 75 | 26:74 |
| 9 | 4-MeO2C-C6H4 (1i) | 3ia | nd | |
| 10 | 4-MeO-C6H4 (1j) | 3ja | trace | |
| 11 | 4-Ph-C6H4 (1k) | 3ka | 38 | 33:67 |
| 12 | 2-naph (1l) | 3la | 75 | 28:72 |
| 13 | 2-pyridyl (1m) | 3ma | 50 | 37:63 |
| 14 | C10H21 (1n) | 3na | 50 | 33:67 |
Diasteromeric ratios (dr) were determined by 1H NMR analysis of the crude reaction mixture.
The biphenyl 1k, 2-naphthyl 1l, and 2-pyridyl 1m derivatives were also found to be good substrates, resulting in the formation of trans-3ka, trans-3la, and trans-3ma in moderate to good yield with good diastereomeric ratios. Finally, the reaction of aliphatic substrates, such as undecene 1n with malonate 2a, proceeded to give product 3na in moderate yield. The diastereoselectivity did not decrease compared with other aromatic alkenes, gave trans-3na with good dr.
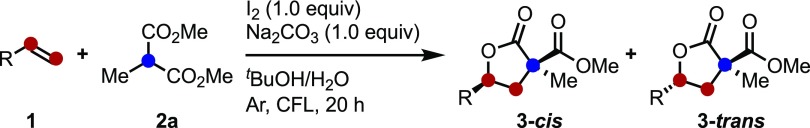
These reaction conditions for the iodine/visible light-mediated carboesterification of alkenes were then applied to the reaction of malonate 2a with disubstituted styrenes (1o–1r) (Scheme 3). The combination of α-methylstyrenes, 1o, with malonate 2a resulted in the formation of products in 61% yield with moderate diastereoselectivity. When cis (Z)-1p and trans-β-methylstyrene (E)-1p were used, the products 3pa were synthesized with the same diastereoselectivity regardless of the styrene isomer.18 Indene 1q afforded polycyclic lactone trans-3qa with excellent diastereoselectivity by 9:91. Also, the use of 1,1-diphenylethylene resulted in the formation of product 3ra in moderate yield.
Scheme 3. trans-Diastereoselective Synthesis of Lactones, 3, via the Reaction of Disubstituted Alkenes with Malonate 2a.
Diasteromeric ratios (dr) were determined by 1H NMR analysis of the crude reaction mixture.
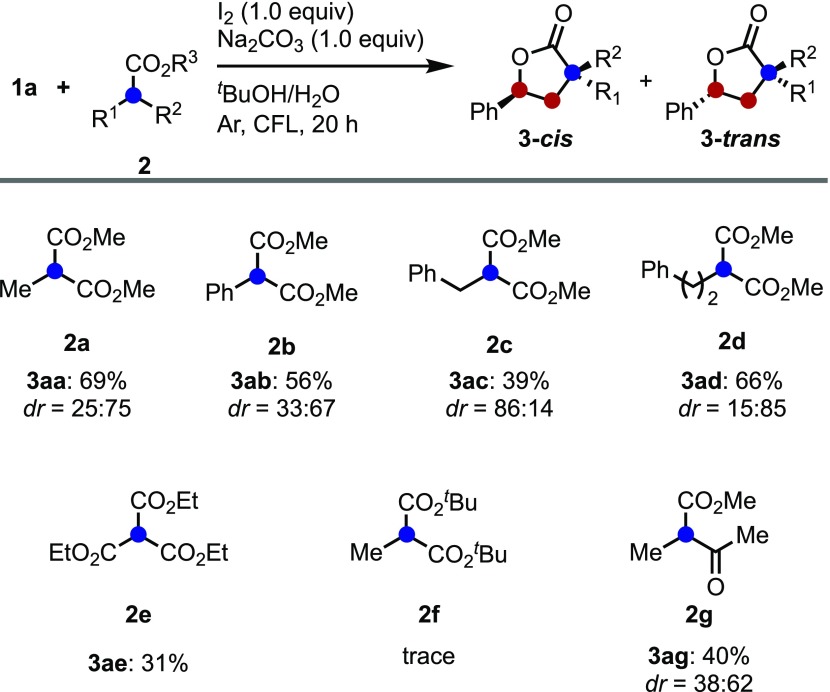
To further evaluate the reaction scope, the optimal conditions for the trans-diastereoselective synthesis of lactones were used in the reaction of styrene 1a with various carbonyls 2 (Scheme 4). The desired trans-lactones 3aa, 3ab, 3ac, and 3ad were obtained in moderate to good yield with diastereoselectivities ranging between ca. 2:1 and 5:1. Interestingly, carbonyl bearing benzyl group 2c gave cis-3ac lactone preferentially. The formation of trans-3ac was suppressed due to the steric repulsion between the phenyl group of styrene and benzyl group (see the cyclization mechanism details, Scheme 10). In addition, when the reaction used triester 2e, it resulted in the formation of lactone 3ae albeit in low yield. To probe other substituents, the ester substituent group was changed. Expectedly, the reaction using tert-butoxy ester 2f, it is possible to interfere with nucleophilic attack because of bulky ester could not be obtained the lactone 3af. When acetyl derivative 2g was used as a substrate, the desired product 3ag was obtained in moderate yield.
Scheme 4. trans-Diastereoselective Synthesis of Lactones, 3, from Styrene 1a with Various Carbonyls, 2.
Diasteromeric ratios (dr) were determined by 1H NMR analysis of the crude reaction mixture.
Scheme 10. Experiments of the Mechanism of Cyclization with H218O.
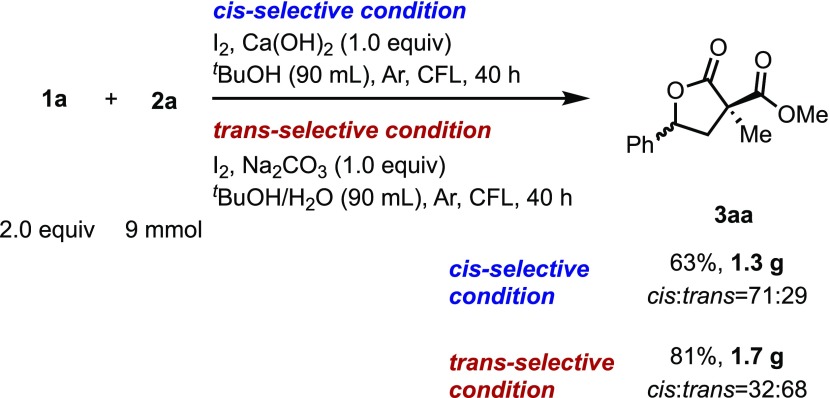
To test the scalability of the reactions, gram-scale reactions using malonate 2a and styrene 1a were performed under both sets of cis-13 and trans-selective conditions (Scheme 5). When scaled up in size by 30 times to 9 mmol, the reactions under both sets of conditions proceeded as expected to afford 63 and 81% isolated yields, respectively.
Scheme 5. Scale-up of the Intermolecular Lactonization Reactions.
Mechanistic Investigations
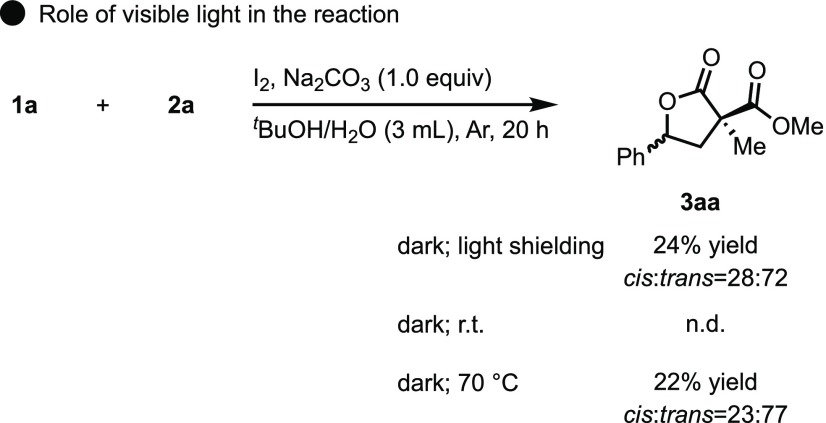
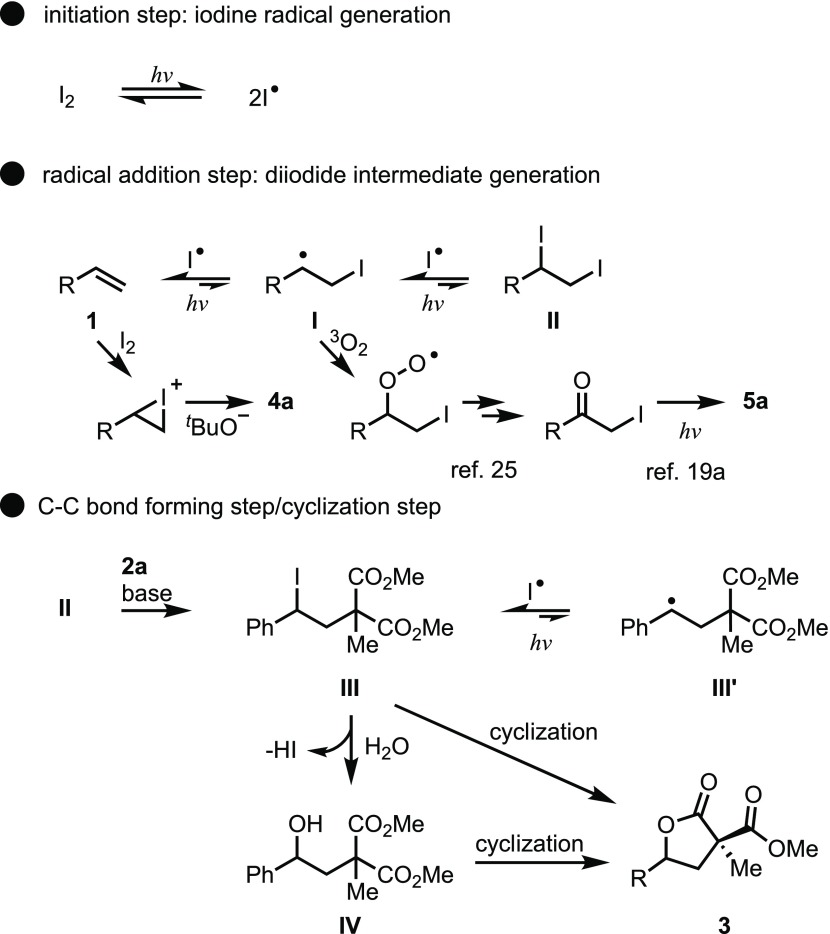
To gain mechanistic insight into the reactions, several control experiments were carried out. Initially, the role of visible light in the reaction was investigated (Scheme 6). As previously reported, we hypothesized that the iodine radical was formed by irradiating molecular iodine with visible light.19 Indeed, the yield of 3aa was found to greatly decrease to 24% under dark conditions shielded with aluminum foil sheets. Also, since the reaction could be significantly suppressed in the dark at room temperature, the heat from compact fluorescent light (CFL) bulbs did not affect the reaction. In addition, 3aa was obtained in a significantly lower yield under heating conditions at 70°. These results indicate that iodine was not thermally activated and that visible light activation of molecular iodine is necessary for the reaction to proceed.
Scheme 6. Investigation into the Role of Visible Light in the Reaction.
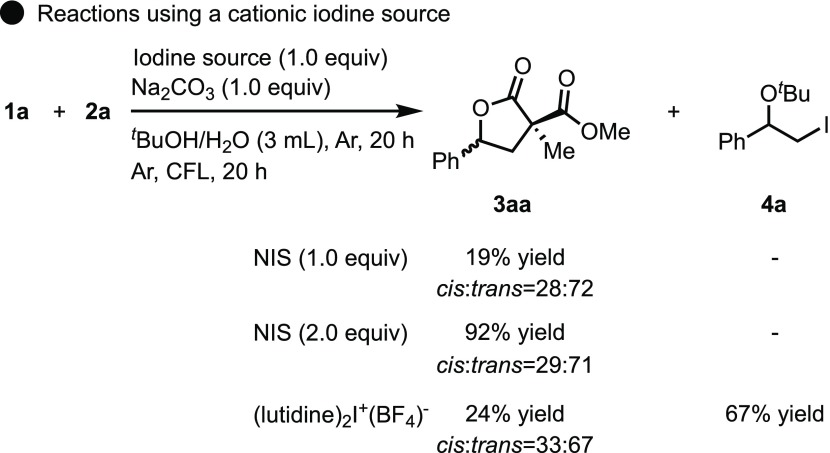
The iodonium cation is one of the reactive species that can be generated by the reaction of molecular iodine or cationic iodine with alkenes without being irradiated with visible light.19,20 This means that the reaction may proceed via an iodonium intermediate, whereby an alkene is electrophilically activated by cationic iodine.21 Therefore, we investigated the role of iodine in the reaction (Scheme 7). When reactions were carried out using a cationic iodine source, such as NIS or (lutidine)2I+BF4–, instead of molecular iodine under standard reaction conditions,22 a significant decrease in the yield of 3aa was observed. Interestingly, the formation of the iodoalkoxylation product 4a was observed in some cases. This product is thought to be generated through the solvolysis of an iodonium cation intermediate.23 However, 2 equiv of NIS produced a similar yield of the product. These observations revealed that alkene was not predominantly activated by a cationic form of iodine to form an iodonium intermediate.
Scheme 7. Reactions Using a Cationic Iodine Source.
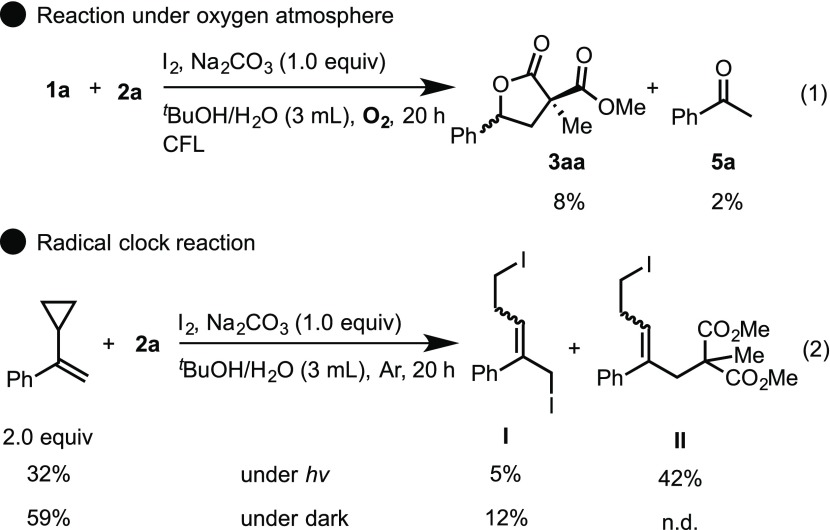
To determine the key intermediate, the reaction was conducted under an oxygen atmosphere (Scheme 8, eq 1). Acetophenone 5a was formed by dehalogenation of phenacyl iodide,19a,24 which was generated by the photooxidation of styrene via sequential addition of the iodine radical and triplet oxygen to styrene.25 To further investigate, we then attempted radical clock experiments (Scheme 8, eq 2). As a result, using 1-cyclopropyl-1-phenylethylene as a radical clock instead of styrene 1a under general conditions, the ring-opened products I and II were obtained significantly under visible light irradiation from CFL bulbs. In addition, it is indicated that the iodinated compound I was almost entirely consumed to form the nucleophilic substitution product II under visible light irradiation. Thus, these observations indicate that an iodine radical intermediate was generated and this radical sequentially reacted with an alkene to form a diiodide intermediate.
Scheme 8. Controlled Experiments to Elucidate the Nature of the Radical Intermediate.
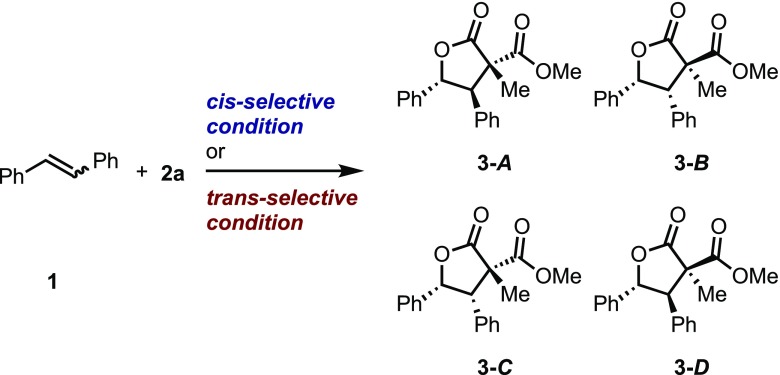
To confirm the formation of the above-mentioned diiodo intermediate, reactions employing cis- or trans-stilbene (Z)-1s and (E)-1s were conducted (Table 3). Under a set of cis-selective conditions, the reaction employing both stilbenes gave four possible diastereomers in moderate yield with diastereomeric ratios of approximately 50:10:20:20, respectively (entries 1 and 2).26 Furthermore, a similar diastereomeric ratio was observed under a set of trans-selective conditions (entries 3 and 4). For both sets of conditions, it was revealed that lactones 3-A–D were produced with similar diastereoselectivity without any influence from the structure of the stilbene itself. Therefore, it was observed that the reaction proceeded via a common intermediate, which could be the diiodo intermediate, as hypothesized previously.13
Table 3. Lactonization Reaction Using cis- or trans-Stilbenea,b.
| diastereomer |
|||||||
|---|---|---|---|---|---|---|---|
| entry | conditions | 1s | 3 (%) | A | B | C | D |
| 1 | cis-selective conditions | Z | 49 | 52 | 9 | 22 | 17 |
| 2 | E | 70 | 43 | 24 | 21 | 12 | |
| 3 | trans-selective conditions | Z | 41 | 52 | 31 | 0 | 17 |
| 4 | E | 73 | 53 | 35 | 0 | 18 | |
Reaction conditions: cis-selective conditions: 1 (2 equiv), 2a (0.3 mmol), I2 (0.3 mmol), and Ca(OH)2 (0.3 mmol) in tBuOH (3 mL) were stirred at ambient temperature and irradiated with a compact fluorescent lamp (CFL) for 20 h. trans-Selective conditions: 1 (2 equiv), 2a (0.3 mmol), I2 (0.3 mmol), and Na2CO3 (0.3 mmol) in tBuOH (2 mL) and H2O (1 mL) were stirred at ambient temperature and irradiated with compact fluorescent lamps (CFLs) for 20 h.
Diasteromeric ratios (dr) were determined by 1H NMR analysis of the crude reaction mixture.
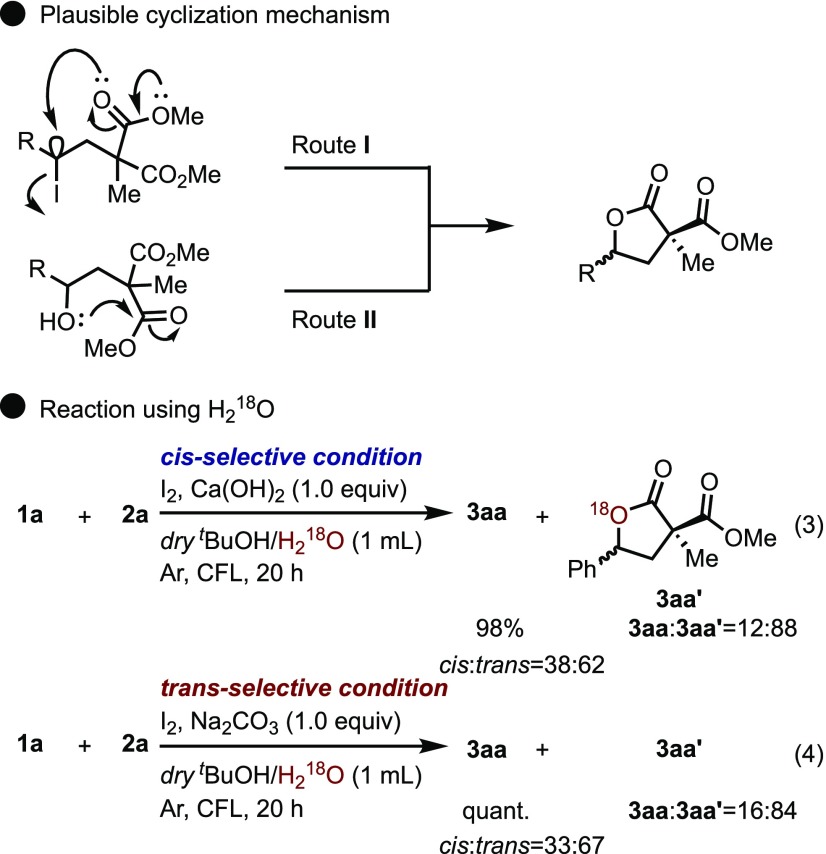
On the basis of the results of these investigations, a tentative mechanism is proposed in Scheme 9. Molecular iodine and the iodine radical are in equilibrium under visible light irradiation and the generated radical is sequentially added to styrene to yield II. vic-Diiodoalkanes, II, when generated in situ, are generally unstable and tend to revert back to iodine and the original alkene.27 In the absence of water, compound II reacts with malonate 2a to afford the corresponding product III and produces lactone 3 by the subsequent cyclization reaction. In the presence of water, compound III reacts with water to afford the corresponding product IV. Finally, cyclization of compound III or IV produces the desired lactone, 3.
Scheme 9. Plausible Mechanism of the Intermolecular Lactonization.
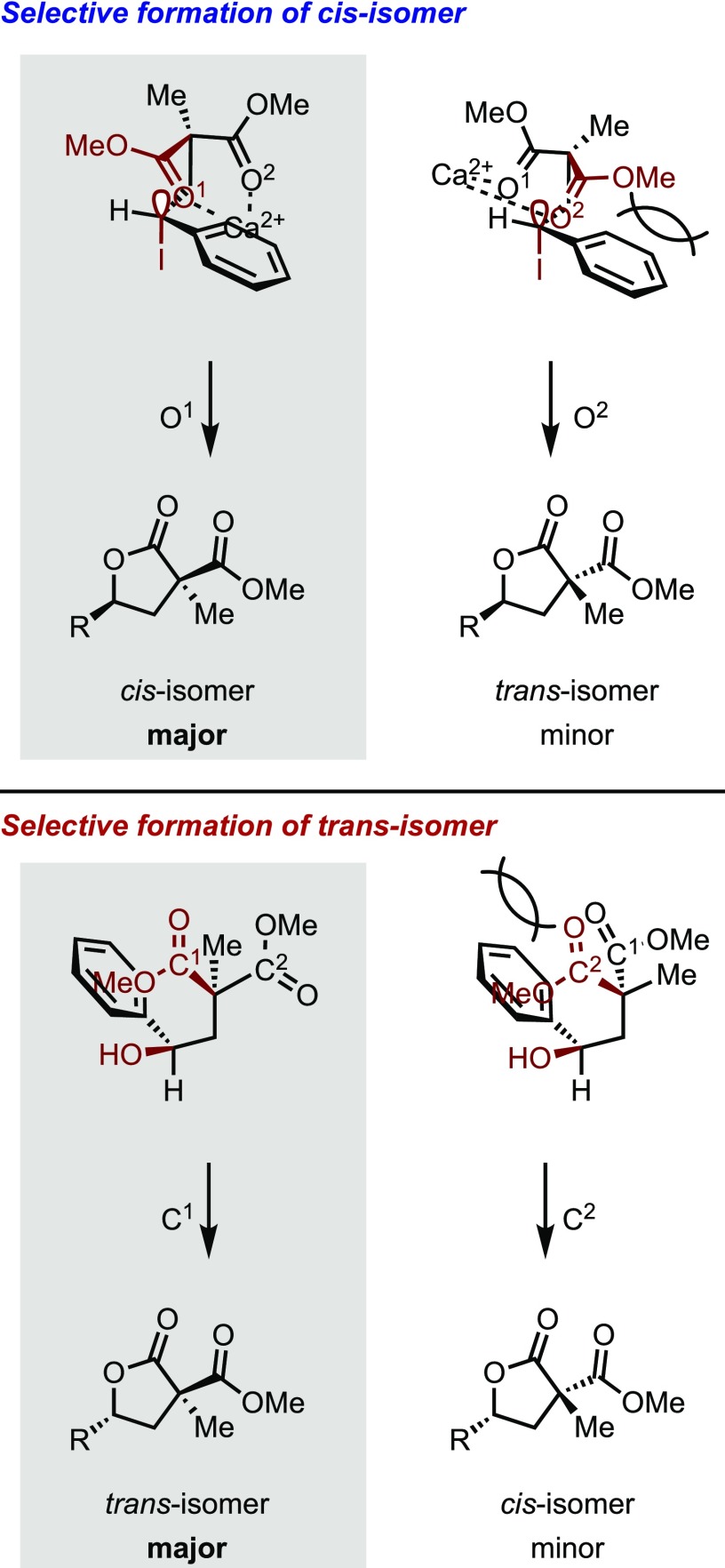
As previously described, we were able to successfully determine the reaction conditions that allowed the control of diastereoselectivity (in the absence of water and using Ca(OH)2 as base resulted in the formation of cis-isomers, and in the presence of water and using Na2CO3 as the base produced the opposite isomer). We then elucidated the stereo determining step. Two types of cyclization mechanisms can be considered, as shown in Scheme 10; route I: cyclization by the nucleophilic substitution reaction from a lone pair on the carbonyl oxygen of the malonate (SN1 or SN2) and route II: cyclization from the hydroxy group, which is generated as a result of the hydrolysis of the iodinated intermediate. A reaction employing 18O-labeled H218O was conducted to evaluate the preferred route (Scheme 10, eqs 3 and 4). As a result, 18O-labeled 3aa′ was mainly generated in good yield with moderate trans-selectivity in both cases.28 Therefore, this indicates that the cyclization reaction mainly proceeds via the route II mechanism in the presence of water.
On the basis of these investigations, the plausible cyclization mechanism and stereo determination are proposed in Scheme 11. Since cyclization is mainly through route I, the product gives the cis-isomer. This is because the less sterically hindered carbonyl oxygen O1 attacks the anti-bonding orbital of the C–I bond or carbocation to avoid steric repulsion of the phenyl group (or substituent R) between the ester. Furthermore, the cis-selectivity appears remarkably when Ca(OH)2 is used13 (Table 1, entry 1), it is conceivable that the metal cation forms chelate with two esters and contributes to the improvement of cis-selectivity by controlling the free rotation of esters.29
Scheme 11. Plausible Cyclization Mechanism and Base Effect.
On the other hand, it is considered that cyclization in trans-selective conditions, using water as a co-solvent, proceeds mainly according to route II. Thus, a trans-diastereomer was obtained when cyclization proceeds through the hydroxy group to carbonyl C1 having a small interference with the phenyl (or substituent R) and another ester.
Conclusions
In this study, we have reported the visible light-iodine-mediated trans-diastereoselective synthesis of γ-lactones using a broad range of alkenes and carbonyls. The molecular iodine used in the reaction is cost-effective, easy to handle, and can be easily removed from the reaction mixture. The selective synthesis of trans-isomer was controlled using water as the co-solvent. Furthermore, the selective formation routes of cis- and trans-isomers are identified. Notably, this method does not require any co-catalyst/reagent. 16 terminal alkenes and 5 internal alkenes were converted to the corresponding lactones under visible light irradiation from CFL bulbs in yields of up to 77% with moderate to good diastereoselectivity. Additionally, 7 lactones were prepared from various carbonyls with moderate to good diastereoselectivity.
Experimental Section
General Information
Unless otherwise noted, all reactants or reagents including dry solvents were obtained from commercial suppliers and used as received. Malonate 2a,302b,312c,322d,332f,3h styrene 1i,341k,351l,36 and 1-cyclopropyl-1-phenylethyrene37 were prepared according to the procedure reported.
The fluorescent lamp was ERF25ED/22-SP-F from Panasonic. Analytical thin-layer chromatography (TLC) was carried out using 0.25 mm commercial silica gel plates (Merck silica gel 60 F254). Flash column chromatography was performed with Kanto silica gel 60N (Spherical, Neutral, 40–50 mm). Visualization of the developed chromatogram was performed by a UV lamp (254 nm) and p-anisaldehyde or basic potassium permanganate stain. NMR spectra were recorded on a JEOL ECA 500 spectrometer (500 MHz for 1H NMR, 125 MHz for 13C NMR, and 470 MHz for 19F NMR), and are internally referenced to residual protio solvent signals or tetramethylsilane (TMS) (note: CDCl3 referenced at δ 7.26 and 77.0 ppm respectively, TMS referenced at δ 0 and 0 ppm respectively). Data for 1 H NMR are reported as follows: chemical shift (δ ppm), multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, m = multiplet, br = broad, dd = doublet of doublets, ddd = doublet of doublet of doublets, dq = doublet of quartets, and td = triplet of doublets), coupling constant (Hz), integration, and assignment. Data for 13C NMR are reported in terms of chemical shifts (δ ppm). IR spectra were recorded on a PerkinElmer Spectrum 100 FTIR spectrometer and are reported in terms of frequency of absorption (cm–1). High-resolution mass spectra (HRMS) were obtained on a JEOL JMS-T100TD or JEOL. The AccuTOF GC JMS-T100GC are reported as m/z (M + H+, relative intensity) or m/z (M, relative intensity). Melting points were measured on a Yanagimoto micro melting point apparatus without correlation. The ultraviolet-visible absorption spectra were recorded with SHIMADZU UV-3600 and are reported in terms of frequency of absorption.
General Procedure for Iodine-Mediated Lactonization
A Pyrex test tube (16.5 cm × 1.5 cm) containing a mixture of malonate 2 (1.0 equiv, 0.30 mmol), I2 (76 mg, 1.0 equiv, 0.30 mmol), calcium hydroxide (22 mg, 1.0 equiv, 0.30 mmol), and alkenes 1 (2.0 equiv, 0.60 mmol) in tButyl alcohol (2.0 mL) and H2O (1.0 mL) was degassed three times via freeze–pump–thaw (FPT) cycling and backfilled with Ar. The resulting solution was stirred at ambient temperature for 20 h. The reaction was quenched with sat. aq Na2S2O3 and extracted with Et2O (10 mL × 3). The combined organic layers were washed with brine, dried over Mg2SO4, filtered and concentrated in vacuo. The resulting mixture was purified by flash column chromatography on silica gel (nhexane/EtOAc) to give the desired product 3.
Synthesis of Methyl-2-carboxy-2-methyl-4-phenyl-4-butanolide (3aa)13
48 mg (0.21 mmol) of 3aa as a white solid (69% yield: dr = 25:75). 3aa-cis: TLC (SiO2): Rf = 0.56 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.43–7.36 (m, 5H), 5.52 (dd, J = 6.9 Hz, 9.1 Hz, 1H), 3.74 (s, 3H), 2.85 (dd, J = 9.1 Hz, 13.1 Hz, 1H), 2.56 (dd, J = 6.9 Hz, 13.1 Hz, 1H), 1.65 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.3, 170.9, 138.3, 128.7, 125.5, 78.7, 53.0, 51.4, 42.8, 19.6. 3aa-trans: TLC (SiO2): Rf = 0.63 (nhexane/ethyl acetate = 4:1); mp: 74.1–74.8 °C; 1H NMR: (500 MHz, CDCl3): δ 7.43–7.34 (m, 5H), 5.59 (dd, J = 6.3 Hz, 10.4 Hz, 1H), 3.84 (s, 3H), 3.11 (dd, J = 6.3 Hz, 13.5 Hz, 1H), 2.12 (dd, J = 10.4 Hz, 13.5 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.7, 170.6, 138.4, 128.7, 128.6, 125.3, 78.8, 53.2, 51.9, 43.6, 20.8.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-tert-butyl-phenyl)-4-butanolide (3ba)13
57 mg (0.20 mmol) of 3ba as a colorless oil (65% yield: dr = 33:67). 3ba-cis: TLC (SiO2): Rf = 0.42 (nhexane/ethyl acetate = 4:1); mp: 71.9–74.1 °C; 1H NMR: (500 MHz, CDCl3): δ 7.43 (d, J = 8.0 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 5.50 (dd, J = 6.9 Hz, 9.2 Hz, 1H), 3.74 (s, 3H), 2.86 (dd, J = 9.2 Hz, 13.2 Hz, 1H), 2.53 (dd, J = 6.9 Hz, 13.2 Hz, 1H), 1.64 (s, 3H), 1.32 (s, 9H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.4, 171.1, 152.0, 135.2, 125.7, 125.5, 78.8, 53.0, 51.5, 42.8, 34.6, 31.2, 19.5. 3ba-trans: TLC (SiO2): Rf = 0.45 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.42 (d, J = 8.5 Hz, 2H), 7.28 (d, J = 8.5 Hz, 2H), 5.56 (dd, J = 6.3 Hz, 10.1 Hz, 1H), 3.84 (s, 3H), 3.08 (dd, J = 6.3 Hz, 13.5 Hz, 1H), 2.13 (dd, J = 10.1 Hz, 13.5 Hz, 1H), 1.59 (s, 3H), 1.32 (s, 9H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.9, 152.0, 135.4, 125.8, 125.3, 79.0, 53.3, 52.0, 43.7, 34.6, 31.2, 20.9.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-methyl-butyl-phenyl)-4-butanolide (3ca)13
45 mg of 3ca as a colorless oil (61% yield: dr = 33:67). 3ca-cis: TLC (SiO2): Rf = 0.33 (nhexane/ethyl acetate = 4:1); mp: 78.6–79.8 °C; 1H NMR: (500 MHz, CDCl3): δ 7.26 (d, J = 7.5 Hz, 2H), 7.21 (dd, J = 8.0 Hz, 2H), 5.49 (dd, J = 6.8 Hz, 9.2 Hz, 1H), 3.75 (s, 3H), 2.83 (dd, J = 9.2 Hz, 13.6 Hz, 1H), 2.53 (dd, J = 6.8 Hz, 13.6 Hz, 1H), 2.37 (s, 3H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.4, 171.1, 138.8, 135.3, 129.5, 125.7, 78.8, 53.1, 51.5, 42.9, 21.1, 19.6. 3ca-trans: TLC (SiO2): Rf = 0.36 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.26 (d, J = 8.6 Hz, 2H), 7.22 (d, J = 8.6 Hz, 2H), 5.55 (dd, J = 6.3 Hz, 10.3 Hz, 1H), 3.84 (s, 3H), 3.07 (dd, J = 6.3 Hz, 13.6 Hz, 1H), 2.37 (s, 3H) 2.11 (dd, J = 10.3 Hz, 13.5 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.9, 138.7, 135.5, 129.5, 125.6, 79.0, 53.3, 52.1, 43.8, 21.1, 20.9.
Synthesis of Methyl-2-carboxy-2-methyl-4-(3-methyl-phenyl)-4-butanolide (3da)13
53 mg (0.22 mmol) of 3da as a yellow solid (72% yield: dr = 33:67). 3da-cis: TLC (SiO2): Rf = 0.29 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.28 (dd, J = 7.3 Hz, 11.5 Hz, 1H), 7.19–7.15 (m, 3H), 5.48 (dd, J = 6.3 Hz, 9.2 Hz, 1H), 3.75 (s, 3H), 2.83 (dd, J = 9.2 Hz, 13.2 Hz, 1H), 2.53 (dd, J = 6.3 Hz, 13.2 Hz, 1H), 2.38 (s, 3H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.4, 171.0, 138.6, 138.3, 129.5, 128.7, 126.2, 122.7, 78.8, 53.0, 51.5, 42.9, 21.3, 19.6. 3da-trans: TLC (SiO2): Rf = 0.38 (nhexane/ethyl acetate = 4:1); mp: 58.8–60.2 °C; 1H NMR: (500 MHz, CDCl3): δ 7.28 (dd, J = 7.6 Hz, 10.9 Hz, 1H), 7.17–7.12 (m, 3H), 5.55 (dd, J = 6.3 Hz, 10.3 Hz, 1H), 3.84 (s, 3H), 3.08 (dd, J = 6.3 Hz, 13.4 Hz, 1H), 2.37 (s, 3H), 2.11 (dd, J = 10.3 Hz, 13.4 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.9, 138.7, 138.5, 129.5, 128.8, 126.1, 122.5, 79.0, 53.3, 52.0, 43.8, 21.3, 20.9.
Synthesis of Methyl-2-carboxy-2-methyl-4-(2-methyl-phenyl)-4-butanolide (3ea)13
49 mg of 3ea as a colorless oil (66% yield: dr = 26:74). 3ea-cis: TLC (SiO2): Rf = 0.29 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.43 (dd, J = 3.8 Hz, 5.3 Hz, 1H), 7.27–7.25 (m, 2H), 7.19 (dd, J = 3.0 Hz, 3.8 Hz, 1H), 5.71 (dd, J = 6.8 Hz, 9.2 Hz, 1H), 3.76 (s, 3H), 2.77 (dd, J = 9.2 Hz, 13.0 Hz, 1H), 2.56 (dd, J = 6.8 Hz, 13.0 Hz, 1H), 2.35 (s, 3H), 1.66 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 171.1, 169.7, 136.6, 134.3, 130.8, 128.5, 126.5, 124.8, 76.4, 53.1, 51.3, 41.7, 19.9, 19.0. 3ea-trans: TLC (SiO2): Rf = 0.38 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.39 (dd, J = 2.9 Hz, 4.4 Hz, 1H), 7.27–7.24 (m, 2H), 7.18 (dd, J = 3.9 Hz, 4.4 Hz, 1H), 5.77 (dd, J = 6.3 Hz, 10.1 Hz, 1H), 3.85 (s, 3H), 3.14 (dd, J = 6.3 Hz, 13.3 Hz, 1H), 2.35 (s, 3H), 2.03 (dd, J = 10.1 Hz, 13.3 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.9, 136.8, 134.5, 130.8, 128.4, 126.6, 124.3, 76.8, 53.3, 51.9, 42.5, 21.0, 19.0.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-fluorophenyl)-4-butanolide (3fa)13
21 mg of 3fa as a yellow oil (28% yield: dr = 20:80). 3fa-cis: TLC (SiO2): Rf = 0.28 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.36 (d, J = 8.7 Hz, 2H), 7.10 (d, J = 8.7 Hz, 2H), 5.50 (dd, J = 6.7 Hz, 9.1 Hz, 1H), 3.76 (s, 3H), 2.83 (dd, J = 9.1 Hz, 13.0 Hz, 1H), 2.55 (dd, J = 6.7 Hz, 13.0 Hz, 1H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.2, 170.9, 163.0 (d, J = 274.4 Hz), 134.1, 127.6 (d, J = 8.2 Hz), 115.8 (d, J = 21.3 Hz), 78.1, 53.2, 51.4, 42.9, 19.6; 19F NMR: (470 MHz, CDCl3): δ −112.67. 3fa-trans: TLC (SiO2): Rf = 0.31 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.34 (d, J = 8.1 Hz, 2H), 7.10 (d, J = 8.1 Hz, 2H), 5.56 (dd, J = 5.9 Hz, 10.3 Hz, 1H), 3.84 (s, 3H), 3.09 (dd, J = 5.9 Hz, 13.2 Hz, 1H), 2.08 (dd, J = 10.3 Hz, 13.2 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.7, 170.7, 162.9 (d, J = 274.4 Hz), 134.2, 127.4 (d, J = 8.2 Hz), 115.9 (d, J = 21.3 Hz), 78.4, 53.3, 52.0, 43.8, 20.9; 19F NMR: (470 MHz, CDCl3): δ −112.70.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-chlorophenyl)-4-butanolide (3ga)13
49 mg of 3ga as a white solid (60% yield: dr = 29:71). 3ga-cis: TLC (SiO2): Rf = 0.30 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.38 (d, J = 8.5 Hz, 2H), 7.31 (d, J = 8.5 Hz, 2H), 5.49 (dd, J = 6.8 Hz, 9.2 Hz, 1H), 3.74 (s, 3H), 2.80 (dd, J = 9.2 Hz, 13.0 Hz, 1H), 2.56 (dd, J = 6.8 Hz, 13.0 Hz, 1H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.1, 170.8, 137.0, 134.6, 129.0, 127.0, 77.9, 53.1, 51.3, 42.7, 19.6. 3ga-trans: TLC (SiO2): Rf = 0.36 (nhexane/ethyl acetate = 4:1); mp: 66.6–72.7 °C; 1H NMR: (500 MHz, CDCl3): δ 7.38 (d, J = 8.6, 2H), 7.29 (d, J = 8.6 Hz, 2H), 5.55 (dd, J = 6.3 Hz, 10.3 Hz, 1H), 3.84 (s, 3H), 3.10 (dd, J = 6.3 Hz, 13.2 Hz, 1H), 2.06 (dd, J = 10.3 Hz, 13.2 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.6, 170.7, 137.1, 134.6, 129.1, 126.8, 78.2, 77.3, 76.7, 53.4, 52.0, 43.7, 20.9.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-bromophenyl)-4-butanolide (3ha)13
70 mg of 3ha as a crystalline solid (75% yield: dr = 26:74). 3ha-cis: TLC (SiO2): Rf = 0.30 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.54 (d, J = 8.7 Hz, 2H), 7.25 (d, J = 8.7 Hz, 2H), 5.47 (dd, J = 6.8 Hz, 9.1 Hz, 1H), 3.74 (s, 3H), 2.80 (dd, J = 9.2 Hz, 13.0 Hz, 1H), 2.56 (dd, J = 6.8 Hz, 13.0 Hz, 1H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.1, 170.8, 137.5, 132.0, 127.2, 122.7, 77.9, 53.1, 51.3, 42.7, 19.6. 3ha-trans: TLC (SiO2): Rf = 0.28 (nhexane/ethyl acetate = 4:1); mp: 76.8–80.0 °C; 1H NMR: (500 MHz, CDCl3): δ 7.54 (d, J = 8.5 Hz, 2H), 7.23 (d, J = 8.5 Hz, 2H), 5.54 (d, J = 6.3 Hz, 10.2 Hz, 1H), 3.84 (s, 3H), 3.11 (dd, J = 6.3 Hz, 13.2 Hz, 1H), 2.05 (dd, J = 10.2 Hz, 13.2 Hz, 1H), 1.68 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.6, 170.6, 137.6, 132.1, 127.1, 122.7, 78.2, 53.3, 51.9, 43.6, 20.8.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-methylcarboxyphenyl)-4-butanolide (3ia)13
No product was obtained. 3ia-cis: TLC (SiO2): Rf = 0.15 (nhexane/ethyl acetate = 4:1); mp: 86.1–88.6 °C; 1H NMR: (500 MHz, CDCl3): δ 8.08 (d, J = 8.2 Hz, 2H), 7.45 (d, J = 8.2 Hz, 2H), 5.58 (dd, J = 6.8 Hz, 8.5 Hz, 1H), 3.93 (s, 3H), 3.72 (s, 3H), 2.82 (dd, J = 8.5 Hz, 13.5 Hz, 1H), 2.61 (dd, J = 6.8 Hz, 13.5 Hz, 1H), 1.65 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.7, 166.6, 143.5, 130.5, 130.1, 125.3, 77.8, 53.1, 52.2, 51.1, 42.6, 19.7. 3ia-trans: TLC (SiO2): Rf = 0.18 (nhexane/ethyl acetate = 4:1); mp: 61.2–87.5 °C; 1H NMR: (500 MHz, CDCl3): δ 8.08 (d, J = 8.2 Hz, 2H), 7.43 (d, J = 8.2 Hz, 2H), 5.63 (dd, J = 6.1 Hz, 10.1 Hz, 1H), 3.93 (s, 3H), 3.85 (s, 3H), 3.16 (dd, J = 6.1 Hz, 13.3 Hz, 1H), 2.07 (dd, J = 10.1 Hz, 13.3 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.6, 170.6, 166.6, 143.6, 130.5, 130.2, 125.2, 78.2, 53.4, 52.2, 51.9, 43.6, 20.8.
Synthesis of Methyl-2-carboxy-2-methyl-4-(4-phenyl-phenyl)-4-butanolide (3ka)13
34 mg (0.12 mmol) of 3ka as a white solid (38% yield: dr = 33:67). 3ka-cis: TLC (SiO2): Rf = 0.34 (nhexane/ethyl acetate = 4:1); mp: 38.8–39.8 °C; 1H NMR: (500 MHz, CDCl3): δ 7.64–7.58 (m, 4H), 7.48–7.44 (m, 4H), 7.38 (t, J = 6.8 Hz, 1H), 5.56 (dd, J = 6.8 Hz, 9.1 Hz, 1H), 3.76 (s, 3H), 2.89 (dd, J = 9.1 Hz, 13.0 Hz, 1H), 2.58 (dd, J = 6.8 Hz, 13.0 Hz, 1H), 1.66 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.3, 171.0, 141.8, 140.4, 137.3, 128.9, 127.7, 127.5, 127.1, 126.1, 78.5, 53.1, 51.5, 42.8, 19.6. 3ka-trans: TLC (SiO2): Rf = 0.33 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.64–7.58 (m, 4H), 7.48–7.37 (m, 5H), 5.63 (dd, J = 6.3 Hz, 10.1 Hz, 1H), 3.85 (s, 3H), 3.14 (dd, J = 6.3 Hz, 13.0 Hz, 1H), 2.16 (dd, J = 10.1 Hz, 13.0 Hz, 1H), 1.61 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.9, 170.8, 141.8, 140.4, 137.4, 128.9, 127.8, 127.6, 127.1, 126.0, 78.8, 53.3, 52.0, 43.8, 20.9.
Synthesis of Methyl-2-carboxy-2-methyl-4-(2-naphtyl)-4-butanolide (3la)13
64 mg (0.22 mmol) of 3la as a white solid (75% yield: dr = 28:72). 3la-cis: TLC (SiO2): Rf = 0.24 (nhexane/ethyl acetate = 4:1); mp: 93.3–95.8 °C; 1H NMR: (500 MHz, CDCl3): δ 7.90 (d, J = 8.6 Hz, 1H), 7.86–7.84 (m, 3H), 7.53–7.51 (m, 2H), 7.46 (dd, J = 1.7 Hz, 8.6 Hz, 1H), 5.68 (dd, J = 6.9 Hz, 9.3 Hz, 1H), 3.73 (s, 3H), 2.94 (dd, J = 9.3 Hz, 13.2 Hz, 1H), 2.62 (dd, J = 6.9 Hz, 13.2 Hz, 1H), 1.68 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.4, 171.0, 135.6, 133.3, 133.0, 129.0, 128.1, 127.8, 126.7, 126.6, 124.9, 122.9, 78.8, 53.1, 51.4, 42.8, 19.7. 3la-trans: TLC (SiO2): Rf = 0.33 (nhexane/ethyl acetate = 4:1); mp: 58.6–62.5 °C; 1H NMR: (500 MHz, CDCl3): δ 7.91–7.85 (m, 4H), 7.55–7.51 (m, 2H), 7.42 (d, J = 8.2 Hz, 1H), 5.76 (dd, J = 6.4 Hz, 10.2 Hz, 1H), 3.87 (s, 3H), 3.18 (dd, J = 6.4 Hz, 13.8 Hz, 1H), 2.20 (dd, J = 10.2 Hz, 13.8 Hz, 1H), 1.63 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.9, 135.8, 133.3, 133.1, 129.0, 128.1, 127.8, 126.7, 126.6, 124.7, 122.7, 79.1, 53.3, 52.0, 43.7, 20.9.
Synthesis of Ethyl-2-carboxy-2-methyl-4-(2-pyridyl)-4-butanolide (3ma)13
35 mg (0.15 mmol) of 3ma as a brown solid (50% yield: dr = 37:63). 3ma-cis: TLC (SiO2): Rf = 0.09 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 8.58 (d, J = 4.3 Hz, 1H), 7.78–7.74 (m, 1H), 7.48 (d, J = 7.7 Hz, 1H), 7.31–7.27 (m, 1H), 5.60 (dd, J = 7.2 Hz, 7.5 Hz, 1H), 3.65 (s, 3H), 3.03 (dd, J = 7.2 Hz, 13.0 Hz, 1H), 2.68 (dd, J = 7.5 Hz, 13.0 Hz, 1H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.3, 169.6, 158.0, 149.3, 137.0, 123.2, 120.1, 78.4, 52.9, 50.6, 40.7, 20.2. 3ma-trans: TLC (SiO2): Rf = 0.09 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 8.61 (dd, J = 4.3 Hz, 1H), 7.78–7.74 (m, 1H), 7.51 (d, J = 7.7 Hz, 1H), 7.31–7.27 (m, 1H), 5.66 (dd, J = 6.8 Hz, 9.7 Hz, 1H), 3.83 (s, 3H), 3.18 (dd, J = 6.8 Hz, 13.5 Hz, 1H), 2.38 (dd, J = 9.7 Hz, 13.5 Hz, 1H), 1.57 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 170.8, 157.6, 149.6, 137.2, 123.5, 120.6, 78.8, 53.2, 51.5, 41.3, 20.7.
Synthesis of Methyl-2-carboxy-2-methyl-4-decyl-4-butanolide (3na)13
45 mg (0.15 mmol) of 3na as a yellow oil (50% yield: dr = 33:67).
3na-cis: TLC (SiO2): Rf = 0.26 (nhexane/ethyl acetate = 10:1); 1H NMR: (500 MHz, CDCl3): δ 4.53–4.45 (m, 1H), 3.79 (s, 3H), 2.49 (dd, J = 9.2 Hz, 13.0 Hz, 1H), 2.22 (dd, J = 6.8 Hz, 13.0 Hz, 1H), 1.80–1.27 (m, 18H), 1.54 (s, 3H), 0.88 (t, J = 6.8 Hz, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.6, 171.4, 79.3, 53.0, 51.2, 40.3, 35.3, 31.8, 29.5, 29.43, 29.37, 29.2, 25.21, 22.6, 21.0, 20.0, 14.0. 3na-trans: TLC (SiO2): Rf = 0.38 (nhexane/ethyl acetate = 10:1); 1H NMR: (500 MHz, CDCl3): δ 4.53–4.45 (m, 1H), 3.78 (s, 3H), 2.78 (dd, J = 9.2 HZ, 13.0 Hz, 1H), 1.80–1.27 (m, 19H), 1.53 (s, 3H), 0.88 (t, J = 6.8 Hz, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.2, 171.2, 78.5, 53.1, 51.7, 41.4, 35.4, 31.8, 29.5, 29.43, 29.37, 29.2, 25.18, 22.6, 21.0, 20.0, 14.0.
Synthesis of Methyl-2-carboxy-2-methyl-4,4-methyl-phenyl-4-butanolide (3oa)
42 mg (0.18 mmol) of 3oa as a white solid (61% yield: dr = 25:75). 3oa-cis: TLC (SiO2): Rf = 0.35 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.36–7.27 (m, 5H), 3.26 (d, J = 13.2 Hz, 1H), 3.24 (s, 3H), 2.32 (d, J = 13.2 Hz, 1H), 1.73 (s, 3H), 1.57 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.8, 171.3, 143.4, 128.4, 127.8, 124.4, 84.5, 52.5, 51.5, 48.4, 31.2, 21.9 (one carbon atom was overlapped); HRMS: m/z (DART) calcd for C14H16O4 (M + H)+ 249.1121, found 249.1130 (mixture of diastereomers); Fourier transform infrared (FTIR): (neat): 2981, 2953, 1776, 1736, 1496, 1447, 1378, 1318, 1296, 1242, 1194, 1170, 1152, 1132, 1118, 1082, 1053, 856, 843, 767, 747, 701 cm–1 (mixture of diastereomers). 3oa-trans: TLC (SiO2): Rf = 0.38 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.39–7.27 (m, 5H), 3.83 (s, 3H), 3.10 (d, J = 13.0 Hz, 1H), 2.50 (d, J = 13.0 Hz, 1H), 1.74 (s, 3H), 1.35 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.0, 171.7, 145.3, 128.8, 127.7, 123.8, 84.7, 53.3, 52.0, 47.8, 30.6, 21.7 (one carbon atom was overlapped); HRMS: m/z (DART) calcd for C14H16O4 (M + H)+ 249.1121, found 249.1132 (mixture of diastereomers); FTIR: (neat): 2984, 1774, 1740, 1496, 1447, 1380, 1297, 1269, 1236, 1203, 1136, 1116, 1084, 1064, 1029, 857, 767, 702 cm–1 (mixture of diastereomers).
Synthesis of Methyl-2-carboxy-2-methyl-3-methyl-4-phenyl-4-butanolide (3pa)
Using (Z)-1p: 55 mg (0.22 mmol) of 3pa as a colorless oil (74% yield: dr = 86:14). using (E)-1p: 58 mg (0.23 mmol) of 3pa as a colorless oil (77% yield: dr = 87:13). 3pa-cis: TLC (SiO2): Rf = 0.47 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.44–7.36 (m, 5H), 4.92 (d, J = 10.3 Hz, 1H), 3.80 (s, 3H), 2.97 (dq, J = 10.3 Hz, 6.8 Hz, 1H), 1.50 (s, 3H), 1.01 (d, J = 6.8 Hz, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.1, 170.7, 136.5, 128.9, 128.6, 126.2, 85.2, 54.7, 52.9, 47.1, 13.6, 9.8 (one carbon atom was overlapped); HRMS: m/z (DART) calcd for C14H16O4 (M + H)+ 249.1121, found 249.1115; FTIR: (neat): 3036, 2955, 2850, 1773, 1732, 1496, 1456, 1435, 1389, 1332, 1295, 1285, 1250, 1224, 1188, 1132, 1123, 1097, 1074, 997, 936, 916, 891, 836, 794, 777, 757, 726, 698 cm–1. 3pa-trans: TLC (SiO2): Rf = 0.54 (nhexane/ethyl acetate = 4:1); mp: 85.5–86.7 °C; 1H NMR: (500 MHz, CDCl3): δ 7.41–7.33 (m, 5H), 5.15 (d, J = 10.2 Hz, 1H), 3.84 (s, 3H), 2.26 (dq, J = 10.2 Hz, 6.8 Hz, 1H), 1.53 (s, 3H), 1.01 (d, J = 6.8 Hz, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.2, 169.3, 136.9, 129.0, 128.8, 126.2, 85.3, 55.8, 52.7, 51.1, 19.1, 10.8 (one carbon atom was overlapped); HRMS: m/z (DART) calcd for C14H16O4 (M + H)+ 249.1121, found 249.1110; FTIR: (neat): 3036, 2957, 2937, 2850, 1774, 1741, 1720, 1498, 1455, 1435, 1381, 1345, 1331, 1298, 1282, 1261, 1209, 1174, 1148, 1125, 1105, 1076, 1031, 999, 936, 919, 882, 843, 801, 781, 753, 734, 699 cm–1.
Synthesis of Methyl-2-carboxy-2-methyl-3,4-2H-indeno-4-butanolide (3qa)
51 mg (0.21 mmol) of 3qa as a yellow oil (69% yield: dr = 9:91). 3qa-trans: TLC (SiO2): Rf = 0.30 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.53–7.27 (m, 4H), 5.86 (d, J = 6.5 Hz, 1H), 3.83 (s, 3H), 3.59 (dt, J = 6.5 Hz, 8.9 Hz, 16.3 Hz, 1H), 3.09 (ddd, J = 8.9 Hz, 16.3 Hz, 2H), 1.51 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.2, 171.6, 143.4, 138.0, 130.4, 127.6, 126.5, 124.9, 85.6, 54.4, 53.3, 47.4, 32.2, 17.0; HRMS: m/z (DART) calcd for C14H14O4 (M + H)+ 247.0965, found 247.0963; FTIR: (neat): 3475, 2953, 2923, 2852, 1770, 1736, 1480, 1460, 1435, 1381, 1346, 1327, 1250, 1224, 1212, 1179, 1150, 1135, 1099, 1036, 967, 893, 878, 749, 708 cm–1.
Synthesis of Methyl-2-carboxy-2-methyl-3,4-2H-indeno-4-butanolide (3ra)
51 mg (0.16 mmol) of 3ra as a white solid (55% yield). TLC (SiO2): Rf = 0.43 (nhexane/ethyl acetate = 4:1); mp: 94.1–95.0 °C; 1H NMR: (500 MHz, CDCl3): δ 7.45–7.25 (m, 10H), 3.67 (d, J = 13.4 Hz, 1H), 3.35 (s, 3H), 2.83 (d, J = 13.4 Hz, 1H), 1.47 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.3, 171.1, 143.6, 142.3, 128.6, 128.4, 127.9, 127.8, 125.4, 124.9, 87.1, 52.7, 51.7, 47.6, 21.3 (two carbon atoms were overlapped).; HRMS: m/z (DART) calcd for C19H19O4 (M + H)+ 311.1278, found 311.1285; FTIR: (neat): 3062, 3034, 2953, 1779, 1737, 1599, 1493, 1450, 1379, 1259, 1229, 1210, 1153, 1111, 1083, 1042, 978, 951, 918, 889, 748, 697, 667 cm–1.
Synthesis of Methyl-2-carboxy-2-methyl-4,3-diphenyl-4-butanolide (3A–D)
cis-Selective conditions (using (Z)-1s); 46 mg (0.15 mmol) mixture of 3A–D as a white solid (49% yield: dr = 52:9:22:17). trans-Selective conditions (using (Z)-1s): 38 mg (0.12 mmol) mixture of 3A–D as a white solid (41% yield: dr = 52:31:0:17). cis-Selective conditions (using (E)-1s): 65 mg (0.21 mmol) mixture of 3A–D as a white solid (70% yield: dr = 43:24:21:12). trans-Selective conditions (using (E)-1s): 68 mg (0.22 mmol) mixture of 3A–D as a white solid (73% yield: dr = 53:35:0:18). 3-A: TLC (SiO2): Rf = 0.60 (nhexane/ethyl acetate = 4:1); mp: 105.3–106.2 °C; 1H NMR: (500 MHz, CDCl3): δ 7.35–6.89 (m, 10H), 5.81 (d, J = 9.8 Hz, 1H), 4.37 (d, J = 9.8 Hz, 1H), 3.81 (s, 3H), 1.30 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.3, 170.5, 136.7, 133.1, 128.9, 128.6, 128.3, 126.1, 81.6, 57.1, 56.2, 53.2, 15.7 (three carbon atoms were overlapped); HRMS: m/z (DART) calcd for C19H19O4 (M + H)+ 311.1278, found 311.1276; FTIR: (neat): 3035, 2954, 2920, 2850, 1775, 1741, 1728, 1604, 1585, 1499, 1454, 1435, 1383, 1301, 1277, 1242, 1218, 1187, 1153, 1110, 1077, 1049, 1031, 997, 942, 913, 860, 809, 794, 756, 697, 661 cm–1. 3-B: TLC (SiO2): Rf = 0.57 (nhexane/ethyl acetate = 4:1); mp: 90.1–91.0 °C; 1H NMR: (500 MHz, CDCl3): δ 7.17–6.89 (m, 10H), 5.99 (d, J = 5.6 Hz, 1H), 4.31 (d, J = 5.6 Hz, 1H), 3.90 (s, 3H), 1.21 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.9, 171.5, 135.4, 133.8, 129.5, 128.3, 128.0, 127.6, 127.3, 125.2, 81.8, 57.5, 55.7, 53.7, 17.5 (two carbon atoms were overlapped); HRMS: m/z (DART) calcd for C19H19O4 (M + H)+ 311.1278, found 311.1275; FTIR: (neat): 3064, 3034, 2955, 2921, 2850, 1780, 1735, 1605, 1499, 1452, 1434, 1378, 1336, 1248, 1219, 1157, 1129, 1106, 1078, 1035, 1020, 1001, 959, 919, 898, 863, 788, 748, 710, 673 cm–1. 3-C: TLC (SiO2): Rf = 0.57 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.13–6.85 (m, 10H), 6.03 (d, J = 6.3 Hz, 1H), 3.89 (d, J = 6.3 Hz, 1H), 3.24 (s, 3H), 1.90 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.5, 169.2, 135.3, 134.5, 129.3, 128.0, 127.8, 127.5, 127.4, 125.5, 80.5, 58.1, 57.1, 52.1, 21.7. 3-D: TLC (SiO2): Rf = 0.42 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.39–7.18 (m, 10H), 6.09 (d, J = 10.6 Hz, 1H), 3.63 (s, 3H), 3.43 (d, J = 10.6 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.2, 168.7, 137.1, 133.0, 129.0, 128.6, 128.1, 125.8, 81.6, 61.9, 58.2, 52.6, 19.3 (three carbon atoms were overlapped); HRMS: m/z (DART) calcd for C19H19O4 (M + H)+ 311.1278, found 311.1282; FTIR: (neat): 2956, 2920, 2850, 1781, 1747, 1719, 1500, 1455, 1379, 1311, 1276, 1251, 1218, 1136, 1110, 1002, 940, 920, 899, 865, 820, 797, 749, 700 cm–1.
Synthesis of Methyl-2-carboxy-2-phenyl-4-phenyl-4-butanolide (3ab)13
50 mg (0.17 mmol) of 3ab as a colorless oil (56% yield: dr = 33:67). 3ab-cis: TLC (SiO2): Rf = 0.48 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.57–7.34 (m, 10H), 5.27 (dd, J = 5.3 Hz, 10.2 Hz, 1H), 3.74 (s, 3H), 3.24 (dd, J = 10.2 Hz, 13.2 Hz, 1H), 3.09 (dd, J = 5.3 Hz, 13.2 Hz, 1H); 13C{1H}NMR: (125 MHz, CDCl3): δ 172.4, 169.6, 137.6, 134.6, 129.2, 128.9, 128.8, 128.3, 127.2, 125.8, 78.6, 61.2, 53.5, 43.4. 3ab-trans: TLC (SiO2): Rf = 0.51 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.57–7.34 (m, 10H) 5.55 (dd, J = 5.3 Hz, 10.6 Hz, 1H), 3.84 (s, 3H), 3.61 (dd, J = 5.3 Hz, 13.0 Hz, 1H), 2.58 (dd, J = 10.6 Hz, 13.0 Hz, 1H); 13C{1H}NMR: (125 MHz, CDCl3): δ 172.0, 169.1, 138.0, 128.9, 128.7, 128.5, 126.9, 125.6, 78.7, 60.4, 53.9, 43.6 (two carbon atoms were overlapped).
Synthesis of Methyl-2-carboxy-2-benzyl-4-phenyl-4-butanolide (3ac)13
36 mg (0.12 mmol) of 3ac as a colorless oil (39% yield: dr = 86:14). 3ac-cis: TLC (SiO2): Rf = 0.51 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.33–7.25 (m, 6H), 7.20–7.17, (m, 2H), 6.96–6.94 (m, 2H), 5.45 (dd, J = 6.3 Hz, 10.1 Hz, 1H), 3.87 (s, 3H), 3.43 (d, J = 13.5 Hz, 2H), 3.34 (d, J = 13.5 Hz, 1H), 2.90 (dd, J = 6.3 Hz, 13.5 Hz, 1H), 2.20 (dd, J = 10.1 Hz, 13.5 Hz, 1H); 13C{1H}NMR: (125 MHz, CDCl3): δ 173.8, 169.8, 138.5, 135.4, 130.3, 128.8, 128.7, 127.5, 125.8, 79.7, 57.8, 53.4, 39.2, 39.1 (one carbon atom was overlapped).
Synthesis of Methyl-2-carboxy-2-benzyl-4-phenyl-4-butanolide (3ad)13
64 mg (0.20 mmol) of 3ad as a colorless oil (66% yield: dr = 12:88). 3ad-cis: TLC (SiO2): Rf = 0.51 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.43–7.17 (m, 10H), 5.52 (dd, J = 7.3 Hz, 10.5 Hz, 1H), 3.72 (s, 3H), 2.88 (dd, J = 8.2 HZ, 13.5 Hz, 1H), 2.87–2.69 (m, 3H), 2.41–2.25 (m, 2H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.2, 170.2, 140.5, 138.7, 128.9, 128.8, 128.7, 128.6, 128.4, 126.4, 125.5, 78.5, 77.3, 76.7, 55.2, 53.1, 40.3, 35.6, 31.1. 3ad-trans: TLC (SiO2): Rf = 0.54 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.44–7.22 (m, 10H), 5.56 (dd, J = 5.8 Hz, 10.4 Hz, 1H), 3.82 (s, 3H), 3.18 (dd, J = 5.8 Hz, 13.2 Hz, 1H), 2.71 (dd, J = 10.4 Hz, 13.2 Hz, 1H), 2.63–2.48 (m, 2H), 2.20–2.13 (m, 2H); 13C{1H}NMR: (125 MHz, CDCl3): δ 173.7, 169.7, 140.3, 138.6, 128.9, 128.8, 128.6, 128.4, 126.4, 125.5, 79.3, 56.4, 53.3, 40.9, 36.4, 31.2.
Synthesis of Ethyl-2-dicarboxy-4-phenyl-4-butanolide (3ae)
29 mg (0.09 mmol) of 3ae as a colorless oil (31% yield). 3ae: TLC (SiO2): Rf = 0.48 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.44–7.35 (m, 5H), 5.49 (dd, J = 6.3 Hz, 9.7 Hz, 1H), 4.38 (q, J = 7.2 Hz, 14.0 Hz, 2H), 4.27 (q, J = 7.3 Hz, 14.5 Hz, 2H), 3.27 (dd, J = 6.3 Hz, 13.5 Hz, 1H), 2.91 (dd, J = 9.7 Hz, 13.5 Hz, 1H), 1.36 (t, J = 7.2 Hz, 14.0 Hz, 3H), 1.29 (t, J = 7.3 Hz, 14.5 Hz, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 168.1, 165.4, 137.5, 129.0, 128.9, 125.7, 79.1, 63.4, 63.1, 39.9, 13.9, 13.8 (two carbon atoms were overlapped); HRMS: m/z (DART) calcd for C16H18O6 (M + H)+ 307.1176, found 307.1186; FTIR: (neat): 2923, 2853, 1788, 1731, 1452, 1368, 1254, 1215, 1193, 1164, 1117, 1092, 1072, 1048, 1020, 999, 860, 763, 698 cm–1.
Synthesis of tert-Butyl-2-carboxy-2-methyl-4-phenyl-4-butanolide (3af)13
No reaction. 3af-cis: TLC (SiO2): Rf = 0.61 (nhexane/ethyl acetate = 4:1); mp: 57.5–58.7 °C; 1H NMR: (500 MHz, CDCl3): δ 7.41–7.33 (m, 5H), 5.56 (dd, J = 6.3 Hz, 10.1 Hz, 1H), 3.04 (dd, J = 6.3 Hz, 13.5 Hz, 1H), 2.08 (dd, J = 10.1 Hz, 13.5 Hz, 1H), 1.52 (s, 9H), 1.48 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.4, 169.4, 138.9, 128.8, 128.6, 125.4, 83.2, 78.9, 52.8, 44.0, 29.6, 27.8, 20.7; HRMS: m/z (DART) calcd for C16H21O4 (M + H)+ 277.1434, found 277.1425; FTIR: (neat): 2980, 2936, 1775, 1734, 1498, 1456, 1394, 1369, 1330, 1270, 1259, 1211, 1150, 1101, 1076, 1022, 1001, 940, 893, 845, 760, 698 cm–1.
Synthesis of 2-Acetyl-2-methyl-4-phenyl-4-butanolide (3ag)13
26 mg (0.12 mmol) of 3ag as a colorless oil (40% yield: dr = 38:62). 3ag-cis: TLC (SiO2): Rf = 0.37 (nhexane/ethyl acetate = 4:1); mp: 63.3–66.7 °C; 1H NMR: (500 MHz, CDCl3): δ 7.42–7.33 (m, 5H), 5.38 (dd, J = 6.3 Hz, 10.4 Hz, 1H), 3.32 (dd, J = 6.3 Hz, 13.0 Hz, 1H), 2.38 (s, 3H), 1.95 (dd, J = 10.4 Hz, 13.0 Hz, 1H), 1.60 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 203.2, 175.5, 138.8, 128.8, 128.7, 125.4, 79.2, 77.3, 76.7, 59.2, 41.2, 25.5, 21.4. 3ag-trans: TLC (SiO2): Rf = 0.46 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.42–7.33 (m, 5H), 5.52 (dd, J = 6.6 Hz, 9.2 Hz, 1H), 2.86 (dd, J = 9.2 Hz, 13.5 Hz, 1H), 2.41 (dd, J = 6.6 Hz, 13.5 Hz, 1H), 2.38 (s, 3H), 1.63 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 203.5, 176.1, 138.4, 128.8, 128.7, 125.4, 78.3, 77.3, 76.7, 57.2, 40.2, 25.9, 20.5.
Radical Clock Experiments
A Pyrex test tube (16.5 cm × 1.5 cm) containing a mixture of dimethyl 2-methylmalonate 2a (44 mg, 0.30 mmol), I2 (76 mg, 1.0 equiv, 0.30 mmol), sodium carbonate (32 mg, 1.0 equiv, 0.30 mmol) and 1-cyclopropyl-1-phenylethyrene (86 mg, 2.0 equiv, 0.60 mmol) in tButyl alcohol (2.0 mL) and H2O (1.0 mL) was degassed three times via FPT cycling and backfilled with Ar. The resulting solution was stirred at ambient temperature for 2 h. The reaction was quenched with sat. aq Na2S2O3 and extracted with Et2O (10 mL × 3). The combined organic layers were washed with brine, dried over Mg2SO4, filtered and concentrated in vacuo. The resulting mixture was purified by flash column chromatography on silica gel (nhexane/EtOAc = 100:0–20:1) to give I as a colorless oil (5% yield) and II as a yellow oil (42% yield). I13: TLC (SiO2): Rf = 0.35 (nhexane); 1H NMR: (500 MHz, CDCl3): δ major 7.45–7.31 (m, 5H), 5.84 (t, J = 6.9 Hz, 1H), 4.25 (s, 2H), 3.31 (t, J = 7.5 Hz, 2H), 2.82 (dd, J = 6.9 Hz, 7.5 Hz, 2H). minor 7.45–7.31 (m, 3H), 7.10 (d, J = 6.9 Hz, 2H), 5.89 (t, J = 7.5 Hz, 1H), 4.20 (s, 2H), 3.08 (t, J = 6.9 Hz, 2H), 2.50 (q, J = 6.9 Hz, 2H); 13C{1H}NMR: (125 MHz, CDCl3): δ major 139.9, 139.2, 130.9, 128.5, 127.9, 126.1, 32.7, 2.8, 1.9. minor 140.9, 137.7, 129.9, 128.5, 128.4, 127.8, 33.0, 13.2, 4.2. II13: TLC (SiO2): Rf = 0.10 (nhexane/ethyl acetate = 20:1); 1H NMR: (500 MHz, CDCl3): δ major 7.30–7.11 (m, 5H), 5.56 (t, J = 6.9 Hz, 1H), 3.38 (s, 6H), 3.19 (s, 2H), 3.17 (t, J = 6.9 Hz, 2H), 2.78 (q, J = 6.9 Hz, 2H), 1.27 (s, 1H). 1H NMR: (500 MHz, CDCl3): δ minor 7.30–7.11 (m, 3H), 7.10 (d, J = 6.9 Hz, 2H), 5.48 (t, J = 6.9 Hz, 1H), 3.73 (s, 6H), 3.07 (t, J = 6.9 Hz, 2H), 3.04 (s, 2H), 2.48 (q, J = 6.9 Hz, 2H), 1.31 (s, 1H); 13C{1H}NMR: (125 MHz, CDCl3): δ major 172.1, 142.7, 137.9, 132.8, 127.9, 127. 2, 127.1, 53.1, 52.2, 34.9, 33.0, 19.8, 4.4. 13C{1H}NMR: (125 MHz, CDCl3): δ minor 170.5, 139.1, 138.0, 131.1, 128.7, 127.9, 127.1, 53.1, 52.2, 45.8, 44.3, 32.5, 5.4.
Reaction Using H218O Condition A
3aa′ as a yellow oil (98% yield: dr = 38:62, 3aa: 3aa′ = 12:88). 3aa′ as a yellow oil (quant.: dr = 33:67, 3aa: 3aa′ = 16:84). 3aa′-cis: TLC (SiO2): Rf = 0.35 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.43–7.36 (m, 5H), 5.52 (dd, J = 6.9 Hz, 9.1 Hz, 1H), 3.74 (s, 3H), 2.85 (dd, J = 9.1 Hz, 13.1 Hz, 1H), 2.56 (dd, J = 6.9 Hz, 13.1 Hz, 1H), 1.64 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 175.3, 170.9, 138.3, 128.8, 125.6, 78.7, 53.1, 51.5, 42.9, 19.7; HRMS: m/z (GC-MS) calcd for C13H14O318O (M) 236.0935, found 236.0924. 3aa′-trans: TLC (SiO2): Rf = 0.41 (nhexane/ethyl acetate = 4:1); 1H NMR: (500 MHz, CDCl3): δ 7.43–7.34 (m, 5H), 5.59 (dd, J = 6.3 Hz, 10.4 Hz, 1H), 3.84 (s, 3H), 3.11 (dd, J = 6.3 Hz, 13.5 Hz, 1H), 2.12 (dd, J = 10.4 Hz, 13.5 Hz, 1H), 1.59 (s, 3H); 13C{1H}NMR: (125 MHz, CDCl3): δ 174.8, 170.8, 138.5, 128.8, 128.7, 125.4, 78.9, 53.3, 52.0, 43.8, 20.9; HRMS: m/z (GC-MS) calcd for C13H14O318O (M) 236.0935, found 236.0926.
Acknowledgments
We thank Maruyama T. for assistance with the X-ray crystallographic studies.
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b00333.
Experimental procedures, optimization studies, NMR spectra for novel compounds; general reaction set up (Figure S1); wave length and spectral irradiance of fluorescent lamp (Figure S2); optimization of reaction conditions (Table S1); reaction with radical scavenger (Scheme S1) (PDF)
X-ray data for 3ha (CIF)
This work was financially supported by Kato Memorial Bioscience foundation.
The authors declare no competing financial interest.
Supplementary Material
References
- a Hoffmann H. M. R.; Rabe J. Synthesis and Biological Activity of α-Methylene-γ-butyrolactones. Angew. Chem., Int. Ed. 1985, 24, 94–110. 10.1002/anie.198500941. [DOI] [Google Scholar]; b Picman A. K. Biological Activities of Sesquiterpene Lactones. Biochem. Syst. Ecol. 1986, 14, 255–281. 10.1016/0305-1978(86)90101-8. [DOI] [Google Scholar]
- a Nefkens G. H. L.; Thuring J. W. J. F.; Beenakkers M. F. M.; Zwanenburg B. J. Synthesis of a Phthaloylglycine-Derived Strigol Analogue and Its Germination Stimulatory Activity toward Seeds of the Parasitic Weeds Striga hermonthica and Orobanche crenata.; Asymmetric Synthesis of All Stereoisomers of the Strigol Analogue GR24. Dependence of Absolute Configuration on Stimulatory Activity of Striga hermonthica and Orobanche crenata Seed Germination. Agric. Food Chem. 1997, 45, 2273–2283. 10.1021/jf9604504. [DOI] [Google Scholar]; b Mangnus E. M.; Zwanenburg B. J. Tentative Molecular Mechanism for Germination Stimulation of Striga and Orobanche seeds by strigol and Its Synthetic Analogs. J. Agric. Food Chem. 1992, 40, 1066–1070. 10.1021/jf00018a032. [DOI] [Google Scholar]; c Fang B.; Xie X.; Zhao C.; Jing P.; Li H.; Wang Z.; Gu J.; She X. Asymmetric Total Synthesis of Fusarentin 6-Methyl Ether and Its Biomimetic Transformation into Fusarentin 6,7-Dimethyl Ether, 7-O-Demethylmonocerin, and (+)-Monocerin. J. Org. Chem. 2013, 78, 6338–6343. 10.1021/jo400760q. [DOI] [PubMed] [Google Scholar]
- Selected examples for Brønsted acid mediated cyclization:; a Nair V.; Prabhakaran J.; George T. G. A Facile Synthesis of Optically Active Lactones Using Benzyl-3,6-anhydro Glucofuranoside as Chiral Auxiliary. Tetrahedron 1997, 53, 15061–15068. 10.1016/S0040-4020(97)10005-9. [DOI] [Google Scholar]; b Taylor S. K. Spiro γ-Lactones via Aluminum Enolate-Spiroepoxide Openings. Synthesis 1998, 7, 1009–1014. 10.1055/s-1998-2108. [DOI] [Google Scholar]; c Ramachandran P. V.; Krzeminski M. P.; Reddy M. V. R.; Brown H. C. A Facile Synthesis of Chiral ω-Allyl- and ω-n-Propyllactones via Asymmetric Allylboration of Formyl Esters with B-Allyldiisopinocampheylborane. Tetrahedron: Asymmetry 1999, 10, 11–15. 10.1016/S0957-4166(98)00477-7. [DOI] [Google Scholar]; d Sibrian-Vazquez M.; Spivak D. A. Convenient Synthesis of 3-(S)-Amino-γ-butyrolactone. Synlett 2002, 7, 1105–1106. 10.1055/s-2002-32597. [DOI] [Google Scholar]; e Zhao J.; Burgess K. Aldol-Type Chirons from Asymmetric Hydrogenations of Trisubstituted Alkenes. Org. Lett. 2009, 11, 2053–2056. 10.1021/ol900308w. [DOI] [PubMed] [Google Scholar]; f Jha V.; Kondekar N. B. Enantioselective Synthesis of syn/anti-1,3-Amino Alcohols via Proline-Catalyzed Sequential α-Aminoxylation/α-Amination and Horner–Wadsworth–Emmons Olefination of Aldehydes. Org. Lett. 2010, 12, 2762–2765. 10.1021/ol100856u. [DOI] [PubMed] [Google Scholar]; g Qabaja G.; Wilent J. E.; Benavides A. R.; Bullard G. E.; Peterson K. S. Facile Synthesis of Versatile Enantioenriched α-Substituted Hydroxy Esters through a Brønsted Acid Catalyzed Kinetic Resolution. Org. Lett. 2013, 15, 1266–1269. 10.1021/ol400207t. [DOI] [PubMed] [Google Scholar]; h Wilent J.; Peterson K. S. Enantioselective Desymmetrization of Diesters. J. Org. Chem. 2014, 79, 2303–2307. 10.1021/jo402853v. [DOI] [PubMed] [Google Scholar]; i Jha V.; Kumar P. Organocatalytic Stereoselective Approach to the Total Synthesis of (-)-Halosaline. RSC Adv. 2014, 4, 3238–3244. 10.1039/C3RA44700F. [DOI] [Google Scholar]
- Selected examples for Lewis acid mediated cyclization:; a Yang C.-G.; Reich N. W.; Shi Z.; He C. Intramolecular Additions of Alcohols and Carboxylic Acids to Inert Olefins Catalyzed by Silver(I) Triflate. Org. Lett. 2005, 7, 4553–4556. 10.1021/ol051065f. [DOI] [PubMed] [Google Scholar]; b Yeh M.-C. P.; Lee Y.-C.; Young T.-C. A Facile Approach to the Synthesis of Allylic Spiro Ethers and Lactones. Synthesis 2006, 21, 3621–3624. 10.1055/s-2006-950294. [DOI] [Google Scholar]; c Toullec P. Y.; Genin E.; Antoniotti S.; Genêt J.-P.; Michelet V. Au2O3 as a Stable and Efficient Catalyst for the Selective Cycloisomerization of γ-Acetylenic Carboxylic Acids to γ-Alkylidene-γ-Butyrolactones. Synlett 2008, 5, 707–711. 10.1055/s-2008-1032108. [DOI] [Google Scholar]; d Gooßen L. J.; Ohlmann D. M.; Dierker M. Silver Triflate-Catalysed Synthesis of γ-Lactones from Fatty Acids. Green Chem. 2010, 12, 197–200. 10.1039/B916853B. [DOI] [Google Scholar]; e Valerio V.; Petkova D.; Madelaine C.; Maulide N. Direct Room-Temperature Lactonisation of Alcohols and Ethers onto Amides: An “Amide Strategy” for Synthesis. Chem. - Eur. J. 2013, 19, 2606–2610. 10.1002/chem.201203906. [DOI] [PubMed] [Google Scholar]; f Grover H. K.; Emmett M. R.; Kerr M. A. γ-Substituted Butanolides from Cyclopropane Hemimalonates: An Expedient Synthesis of Natural (R)-Dodecan-4-olide. Org. Lett. 2013, 15, 4838–4841. 10.1021/ol402252u. [DOI] [PubMed] [Google Scholar]; g Shu X.-Z.; Nguyen S. C.; He Y.; Oba F.; Zhang Q.; Canlas C.; Somorjai G. A.; Alivisatos A. P.; Toste F. D. Silica-Supported Cationic Gold(I) Complexes as Heterogeneous Catalysts for Regio-and Enantioselective Lactonization Reactions. J. Am. Chem. Soc. 2015, 137, 7083–7086. 10.1021/jacs.5b04294. [DOI] [PubMed] [Google Scholar]; h Zheng M.; Chen P.; Huang L.; Wu W.; Jiang H. Nucleo-Palladation-Triggering Alkene Functionalization: A Route to γ-Lactones. Org. Lett. 2017, 19, 5756–5759. 10.1021/acs.orglett.7b02688. [DOI] [PubMed] [Google Scholar]
- Selected examples for oxidative or reductive cyclization:; a Taylor S. K.; Chmiel N. H.; Simons L. J.; Vyvyan J. R. Conversion of Hydroxy Nitriles to Lactones Using Rhodococcus rhodochrous Whole Cells. J. Org. Chem. 1996, 61, 9084–9085. 10.1021/jo9616623. [DOI] [Google Scholar]; b Trend R. M.; Ramtohul Y. K.; Ferreira E. M.; Stolts B. M. Palladium-Catalyzed Oxidative Wacker Cyclizations in Nonpolar Organic Solvents with Molecular Oxygen: A Stepping Stone to Asymmetric Aerobic Cyclizations. Angew. Chem., Int. Ed. 2003, 42, 2892–2895. 10.1002/anie.200351196. [DOI] [PubMed] [Google Scholar]; c Tellitu I.; Serna S.; Herrero M. T.; Moreno I.; Domínguez E.; SanMartin R. Intramolecular PIFA-Mediated Alkyne Amidation and Carboxylation Reaction. J. Org. Chem. 2007, 72, 1526–1529. 10.1021/jo062320s. [DOI] [PubMed] [Google Scholar]; d Dohi T.; Takenaga N.; Goto A.; Maruyama A.; Kita Y. Direct Lactone Formation by Using Hypervalent Iodine(III) Reagents with KBr via Selective C–H Abstraction Protocol. Org. Lett. 2007, 9, 3129–3132. 10.1021/ol071315n. [DOI] [PubMed] [Google Scholar]; e Shu C.; Liu M.-Q.; Sun Y.-Z.; Ye L.-W. Efficient Synthesis of γ-Lactones via Gold-Catalyzed Tandem Cycloisomerization/Oxidation. Org. Lett. 2012, 14, 4958–4961. 10.1021/ol302323a. [DOI] [PubMed] [Google Scholar]; f Tada N.; Ishigami T.; Cui L.; Ban K.; Miura T.; Itoh A. Calcium Iodide Catalyzed Photooxidative Oxylactonization of Oxocarboxylic Acids Using Molecular Oxygen as Terminal Oxidant. Tetrahedron Lett. 2013, 54, 256–258. 10.1016/j.tetlet.2012.11.014. [DOI] [Google Scholar]; g Xie X.; Stahl S. S. Efficient and Selective Cu/Nitroxyl-Catalyzed Methods for Aerobic Oxidative Lactonization of Diols. J. Am. Chem. Soc. 2015, 137, 3767–3770. 10.1021/jacs.5b01036. [DOI] [PubMed] [Google Scholar]; h Duhamel T.; Muñiz K. Cooperative Iodine and Photoredox Catalysis for Direct Oxidative Lactonization of Carboxylic Acids. Chem. Commun. 2019, 55, 933–936. 10.1039/C8CC08594C. [DOI] [PubMed] [Google Scholar]
- Selected examples for cyclizative lactonization:; a Kishida A.; Nagaoka H. Samarium(II) Iodide-Induced Cascade Reaction for Tricyclic γ-Lactone Synthesis from Acyclic Keto Diesters. Tetrahedron Lett. 2008, 49, 6393–6397. 10.1016/j.tetlet.2008.08.105. [DOI] [Google Scholar]; b Murphy S. K.; Dong V. M. Enantioselective Ketone Hydroacylation Using Noyori’s Transfer Hydrogenation Catalyst. J. Am. Chem. Soc. 2013, 135, 5553–5556. 10.1021/ja4021974. [DOI] [PubMed] [Google Scholar]; c Zhang Q.-B.; Ban Y.-L.; Zhou D.-G.; Zhou P.-P.; Wu L.-Z.; Liu Q. Preparation of α-Acyloxy Ketones via Visible-Light-Driven Aerobic Oxo-Acyloxylation of Olefins with Carboxylic Acids. Org. Lett. 2016, 18, 5256–5259. 10.1021/acs.orglett.6b02560. [DOI] [PubMed] [Google Scholar]; d Sakai N.; Horikawa S.; Ogiwara Y. Gallium-Catalyzed Reductive Lactonization of γ-Keto Acids with a Hydrosilane. RSC Adv. 2016, 6, 81763–81766. 10.1039/C6RA19286F. [DOI] [Google Scholar]
- a Peterson J. R.; Do H. D.; Surjasasmita I. B. Manganese(III) Mediated Radical Cyclization. II. the Synthesis of Dihydro-5-Aryl-3,4-Di(Carboxymethyl)-2(3H)-Furanones by Substrate Selective Oxidation. Synth. Commun. 1988, 18, 1985–1993. 10.1080/00397918808068266. [DOI] [Google Scholar]; b Citterio A.; Sebastiano R.; Nicolini M.; Santi R. Synthesis of γ-Lactones by Iron(III) Perchlorate Oxidation of Malonic Esters in the Presence of Olefins. Synlett 1990, 42–43. 10.1055/s-1990-20980. [DOI] [Google Scholar]; c Peterson J. R.; Do H. D.; Rogers R. D. Anticancer Agent Development; 6. Application of the Heterocycle Annulation-Rearrangement Strategy in the Synthesis of a Podophyllotoxin Precursor. Synthesis 1991, 275–277. 10.1055/s-1991-26444. [DOI] [Google Scholar]; d Baciocchi E.; Ruzziconi R. Electronic and Steric Effects in the Addition of Electrophilic 1,3-Dicarbonylalkyl Radicals to Styrenes. J. Org. Chem. 1991, 56, 4772–4778. 10.1021/jo00015a037. [DOI] [Google Scholar]; e Allegretti M.; D’Annibale A.; Trogolo C. Lactonization of Olefins Mediated by Mn(OAc)3: A Sonochemical Approach. Tetrahedron 1993, 49, 10705–10714. 10.1016/S0040-4020(01)81559-3. [DOI] [Google Scholar]; f Nair V.; Mathew J. Cerium(IV) Ammonium Nitrate Mediated Addition of Dimethyl Malonate to Styrene: A Remarkable Reaction. J. Chem. Soc., Perkin Trans. 1 1995, 1881–1882. 10.1039/p19950001881. [DOI] [Google Scholar]; g Horikawa M.; Shirahama H. A Short Synthesis of Phenyl Kainoid. Synlett 1996, 95–96. 10.1055/s-1996-5333. [DOI] [Google Scholar]; h Nair V.; Mathew J.; Nair L. G. Oxidative Addition of Dimethyl Malonate to Styrenes Mediated by Cerium(IV) Ammonium Nitrate: Some Novel Observations. Synth. Commun. 1997, 27, 3053–3064. 10.1080/00397919708005011. [DOI] [Google Scholar]; i Fumagalli G.; Boyd S.; Greaney M. F. Exploiting Photoredox Catalysis for the Synthesis of Tetra- and Di-hydrofurans. Tetrahedron Lett. 2015, 56, 2571–2573. 10.1016/j.tetlet.2015.03.124. [DOI] [Google Scholar]
- a Fukuzawa S.; Nakanishi A.; Fujinami T.; Sakai S. Reductive Coupling of Ketones or Aldehydes with Electron-Deficient Alkenes Promoted by Samarium Di-Iodide. J. Chem. Soc., Chem. Commun. 1986, 624–625. 10.1039/c39860000624. [DOI] [Google Scholar]; b Fukuzawa S.; Seki K.; Tatsuzawa M.; Mutoh K. A Facile Synthesis of Chiral γ-Butyrolactones in Extremely High Enantioselectivity Mediated by Samarium(II) Iodide. J. Am. Chem. Soc. 1997, 119, 1482–1483. 10.1021/ja962965h. [DOI] [Google Scholar]; c Pham W.; Weissleder R.; Tung C.-H. A Practical Approach for the Preparation of Monofunctional Azulenyl Squaraine Dye. Tetrahedron Lett. 2003, 44, 3975–3978. 10.1016/S0040-4039(03)00819-0. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Kerrigan N. J.; Upadhyay T.; Procter J. D. The Samarium(II)-Mediated Intermolecular Couplings of Ketones and β-Alkoxyacrylates: A Short Asymmetric Synthesis of an Antifungal γ-Butyrolactone. Tetrahedron Lett. 2004, 45, 9087–9090. 10.1016/j.tetlet.2004.10.027. [DOI] [Google Scholar]; e Kim D. J.; B G.; Shilde U.; Linker T. New Radical Approaches to 3-Deoxy-D-oct-2-ulosonic Acids (KDO). Synthesis 2005, 9, 1507–1513. 10.1055/s-2005-865325. [DOI] [Google Scholar]; f Zhang Y.; Wang Y.; Dai W.-M. Efficient Remote Axial-to-Central Chirality Transfer in Enantioselective SmI2-Mediated Reductive Coupling of Aldehydes with Crotonates of Atropisomeric 1-Naphthamides. J. Org. Chem. 2006, 71, 2445–2455. 10.1021/jo0526486. [DOI] [PubMed] [Google Scholar]; g Vogel J. C.; Bulter R.; Procter D. J. An asymmetric, SmI2-Mediated Approach to γ-Butyrolactones Using a New, Fluorous-Tagged Auxiliary. Tetrahedron 2008, 64, 11876–11883. 10.1016/j.tet.2008.08.110. [DOI] [Google Scholar]; h Pinho V. D.; Procter D. J.; Burtoloso A. C. B. SmI2-Mediated Couplings of α-Amino Acid Derivatives. Formal Synthesis of (−)-Pumiliotoxin 251D and (±)-Epiquinamide. Org. Lett. 2013, 15, 2434–2437. 10.1021/ol400903n. [DOI] [PubMed] [Google Scholar]
- Selected examples for Mn-mediated oxidative lactonization:; a Bush J. B.; Finkbeiner H. Oxidation Reactions of Manganese(III) acetate. II. Formation of γ-Lactones from Olefins and Acetic Acid. J. Am. Chem. Soc. 1968, 90, 5903–5905. 10.1021/ja01023a048. [DOI] [Google Scholar]; b Heiba E. I.; Dessau R. M.; Koehl W. J. Jr Oxidation by Metal Salts. IV. A New Method for the Preparation of γ-Lactones by the Reaction of Manganic Acetate with Olefins. J. Am. Chem. Soc. 1968, 90, 5905–5906. 10.1021/ja01023a049. [DOI] [Google Scholar]; c Heiba E. I.; Dessau R. M.; Rodewald P. G. Oxidation by Metal Salts. X. One-Step Synthesis of γ-Lactones from Olefins. J. Am. Chem. Soc. 1974, 96, 7977–7981. 10.1021/ja00833a024. [DOI] [Google Scholar]; d Wang G.-W.; Li F.-B.; Zhang T.-H. [60]Fullerene-Fused Lactones: Manganese(III) Acetate-Mediated Synthesis and Novel Reductive Ring Opening. Org. Lett. 2006, 8, 1355–1358. 10.1021/ol060090y. [DOI] [PubMed] [Google Scholar]; e Demirhan H.; Mustafa A.; Mustafa Z.; Mustafa K. A Comparative Study in Oxidative Free Radical Reactions between 9-Benzylidene-9-H Fluorene Derivatives and β-Dicarbonyl Compounds in the Presence of Mn(OAc)3 and CAN. Lett. Org. Chem. 2011, 8, 488–494. 10.2174/157017811796504990. [DOI] [Google Scholar]; f Wu L.; Zhang Z.; Liao J.; Li J.; Wua W.; Jiang H. MnO2-Promoted Carboesterification of Alkenes with Anhydrides: A Facile Approach to γ-Lactones. Chem. Commun. 2016, 52, 2628–2631. 10.1039/C5CC08867D. [DOI] [PubMed] [Google Scholar]
- Selected examples for other metal-mediated oxidative lactonization:; a Ando T.; Kimura T.; Leveque J.-M.; Lorimer J. P.; Luche J.-L.; Mason T. J. Sonochemical Reactions of Lead Tetracarboxylates with Styrene. J. Org. Chem. 1998, 63, 9561–9564. 10.1021/jo981168u. [DOI] [Google Scholar]; b Huang L.; Jiang H.; Qi C.; Liu X. Copper-Catalyzed Intermolecular Oxidative [3 + 2] Cycloaddition between Alkenes and Anhydrides: A New Synthetic Approach to γ-Lactones. J. Am. Chem. Soc. 2010, 132, 17652–17654. 10.1021/ja108073k. [DOI] [PubMed] [Google Scholar]; c Ko T. Y.; Youn S. W. Cooperative Indium(III)/Silver(I) System for Oxidative Coupling/Annulation of 1,3-Dicarbonyls and Styrenes: Construction of 5-Membered Heterocycles. Adv. Synth. Catal. 2016, 358, 1934–1941. 10.1002/adsc.201600280. [DOI] [Google Scholar]; d Pan G.-H.; Song R.-J.; Li J. H. Radical-Mediated Synthesis of γ-Lactones by Copper-Catalyzed Intermolecular Carboesterification of Alkenes with α-Carbonyl Alkyl Bromides and H2O. Org. Chem. Front. 2018, 5, 179–182. 10.1039/C7QO00579B. [DOI] [Google Scholar]; e Iwasaki M.; Miki N.; Ikemoto Y.; Ura Y.; Nishihara Y. Regioselective Synthesis of γ-Lactones by Iron-Catalyzed Radical Annulation of Alkenes with α-Halocarboxylic Acids and Their Derivatives. Org. Lett. 2018, 20, 3848–3852. 10.1021/acs.orglett.8b01436. [DOI] [PubMed] [Google Scholar]
- Wei X.-J.; Yang D.-T.; Wang L.; Song T.; Wu L.-Z.; Liu Q. A Novel Intermolecular Synthesis of γ-Lactones via Visible-Light Photoredox Catalysis. Org. Lett. 2013, 15, 6054–6057. 10.1021/ol402954t. [DOI] [PubMed] [Google Scholar]
- León-Rayo D. F.; Morales-Chamorro M.; Cordero-Vargas A. A Formal Intermolecular Iodolactonization Reaction Based on a Radical-Ionic Sequence. Eur. J. Org. Chem. 2016, 1739–1750. 10.1002/ejoc.201600051. [DOI] [Google Scholar]
- Maejima S.; Yamaguchi E.; Itoh A. Intermolecular Tandem Addition/Esterification Reaction of Alkenes with Malonates Leading to γ-Lactones Mediated by Molecular Iodine under Visible Light Irradiation. Adv. Synth. Catal. 2017, 359, 3883–3887. 10.1002/adsc.201700809. [DOI] [Google Scholar]
- Recent examples for visible light/iodine photocatalysis.; a Liu Y.; Wang B.; Qiao X.; Tung C.-H.; Wang Y. Iodine/Visible Light Photocatalysis for Activation of Alkynes for Electrophilic Cyclization Reactions. ACS Catal. 2017, 7, 4093–4099. 10.1021/acscatal.7b00799. [DOI] [Google Scholar]; b Zhang H.; Muñiz K. Selective Piperidine Synthesis Exploiting Iodine-Catalyzed Csp3–H Amination under Visible Light. ACS Catal. 2017, 7, 4122–4125. 10.1021/acscatal.7b00928. [DOI] [Google Scholar]; c Becker P.; Duhamel T.; Stein C. J.; Reiher M.; Muñiz K. Cooperative Light-Activated Iodine and Photoredox Catalysis for the Amination of C–H Bonds. Angew. Chem., Int. Ed. 2017, 56, 8004–8008. 10.1002/anie.201703611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Full detail of the optimization study; see Tables S1 and S2.
- ORTEP representation of 3ha was shown in Figure S7.
- a Goto A.; Ohno K.; Fukuda T. Mechanism and Kinetics of Iodide-Mediated Polymerization of Styrene. Maclomolecules 1998, 31, 2809–2814. 10.1021/ma9712007. [DOI] [Google Scholar]; b Wright T.; Chirowodza H.; Pasch H. NMR Studies on the Mechanism of Reverse Iodine Transfer Polymerization of Styrene. Macromolecules 2012, 45, 2995–3003. 10.1021/ma3003888. [DOI] [Google Scholar]; c Tonnar J.; Lacroix-Desmazes P. Controlled Radical Polymerization of Styrene by Iodine Transfer Polymerization (ITP) in ab initio Emulsion Polymerization. Polymer 2016, 106, 267–274. 10.1016/j.polymer.2016.08.031. [DOI] [Google Scholar]
- Relative configuration was determined by 1D-NOE NMR analysis of 3pa. And detail was shown in Figures S4 and S5.
- a Nobuta T.; Hirashima S.; Tada N.; Miura T.; Itoh A. One-Pot Metal-Free Syntheses of Acetophenones from Styrenes through Aerobic Photo-oxidation and Deiodination with Iodine. Org. Lett. 2011, 13, 2576–2579. 10.1021/ol200681k. [DOI] [PubMed] [Google Scholar]; b Yamaguchi E.; Sudo Y.; Tada N.; Itoh A. Rare Metal-Free Photo-Aerobic Intramolecular Dehydrogenative Cyclization Reaction towards Polycyclic Heteroarenes. Adv. Synth. Catal. 2016, 358, 3191–3195. 10.1002/adsc.201600291. [DOI] [Google Scholar]
- O’Broin C. Q.; Fernández P.; Martínez C.; Muñiz K. N-Iodosuccinimide-Promoted Hofmann–Löffler Reactions of Sulfonimides under Visible Light. Org. Lett. 2016, 18, 436–439. 10.1021/acs.orglett.5b03476. [DOI] [PubMed] [Google Scholar]
- Achar T. K.; Maiti S.; Mal P. PIDA–I2 Mediated Direct Vicinal Difunctionalization of Olefins: Iodoazidation, Iodoetherification and Iodoacyloxylation. Org. Biomol. Chem. 2016, 14, 4654–4663. 10.1039/C6OB00532B. [DOI] [PubMed] [Google Scholar]
- a Evans R. D.; Schauble J. H. Syntheses of α-lodocarbonyl Compounds Using Bis(sym-collidine)lodine(I) Tetrafluoroborate/Dimethyl Sulfoxide. Synthesis 1986, 9, 727–730. 10.1055/s-1986-31757. [DOI] [Google Scholar]; b Noort D.; Veeneman G. H.; Boons G.-J. P.; Marel G. A.; Mulder G. J.; Boom J. H. Iodonium Ion Promoted Reactions at the Anomeric C-2 Center of 1-Methylene Sugars. Synlett 1990, 4, 205–206. 10.1055/s-1990-21034. [DOI] [Google Scholar]; c Romanov-Michailidis F.; Romanov-Michailidis M.; Pupier M.; Alexakis A. Enantioselective Halogenative Semi-Pinacol Rearrangement: Extension of Substrate Scope and Mechanistic Investigations. Chem. - Eur. J. 2015, 21, 5561–5583. 10.1002/chem.201406133. [DOI] [PubMed] [Google Scholar]
- a Sanseverino A. M.; Mattos M. C. S. An Improved Synthesis of β-Iodo Ethers and Iodohydrins from Alkenes. Synthesis 1998, 1584–1586. 10.1055/s-1998-2187. [DOI] [Google Scholar]; b Das B.; Venkateswarlu K.; Damodar K.; Suneel Rare-Earth Metal Triflates Catalyzed Three-Component Coupling of Aldehydes, Ketones or Ketoesters and Benzyl Carbamate: An Efficient One-Pot Stereoselective Synthesis of Cbz-Protected β-Amino Carbonyl Compounds. J. Mol. Catal. A: Chem. 2007, 269, 17–21. 10.1016/j.molcata.2006.12.041. [DOI] [Google Scholar]; c Villegas R. A. S.; Santo J. L. E.; Mattos M. C. S.; Aguiar M. R. M. P.; Guarino A. W. S. Natural Brazilian clays: Efficient green catalysts for coiodination of styrene. Catal. Commun. 2007, 8, 97–100. 10.1016/j.catcom.2006.05.028. [DOI] [Google Scholar]; d Agrawal M. K.; Adimurtyy S.; Ganguly B.; Ghosh P. K. Comparative Study of the Vicinal Functionalization of Olefins with 2:1 Bromide/Bromate and Iodide/Iodate Reagents. Tetrahedron 2009, 65, 2791–2797. 10.1016/j.tet.2009.01.095. [DOI] [Google Scholar]
- a Mandal A. K.; Nijasure A. M. Iodide Ion Catalysed Dehalogenation of 2-Halo Ketones, Acid Derivatives and Nitriles. Synlett 1990, 554 10.1055/s-1990-21165. [DOI] [Google Scholar]; b Penso M.; Mottadelli S.; Albanese D. Reductive Dehalogenation of α-Haloketones Promoted by Hydroiodic Acid and Without Solvent. Synth. Commun. 1993, 23, 1385–1391. 10.1080/00397919308011227. [DOI] [Google Scholar]
- Nobuta T.; Hirashima S.; Tada N.; Miura T.; Itoh A. Facile Aerobic Photo-Oxidative Synthesis of Phenacyl Iodides and Bromides from Styrenes Using I2 or Aqueous HBr. Synlett 2010, 2335–2339. 10.1055/s-0030-1258022. [DOI] [Google Scholar]
- Relative configuration was determined by 1D NOE NMR analysis of 3A–D. And details were shown in Figure S6.
- a Zanger M.; Rabinowitz J. L. Nuclear Magnetic Resonance Investigation of the Iodination of 1,2-Disubstituted Ethylenes. Evidence for a Trans Addition-Cis Elimination. J. Org. Chem. 1975, 40, 248–250. 10.1021/jo00890a022. [DOI] [Google Scholar]; b Barluenga J.; Rodriguez M. A.; Campos P. J.; Asensio G. A General and Useful Copper(II)-Promoted Iodofunctionalization of Unsaturated Systems. J. Chem. Soc., Chem. Commun. 1987, 1491–1492. 10.1039/C39870001491. [DOI] [Google Scholar]
- Furthermore, we investigated that the influences on stereoselectivity under general condition (see Table 1, entry 13), by reducing 3a–I instead of plausible intermediate IV in Scheme S3. Because this reaction is considered equally of Route II cyclization reaction, it expected when N- or K-selectride as reducing agent, the product 3aa gave trans-isomer selectivity. Since the diastereoselectivity is equally to general condition and H218O experiment (see Scheme 10), the stereo determination of trans-isomer would depend on the cyclization from intermediate IV.
- a Cook D. Infrared Spectra of Xanthones: Lewis Acid Complexes. Can. J. Chem. 1963, 41, 522–526. 10.1139/v63-072. [DOI] [Google Scholar]; b Hatano M.; Horibe T.; Ishihara K. Magnesium(II)-Binaphtholate as a Practical Chiral Catalyst for the Enantioselective Direct Mannich-Type Reaction with Malonates. Org. Lett. 2010, 12, 3502–3505. 10.1021/ol101353r. [DOI] [PubMed] [Google Scholar]; c Tsubogo T.; Shimizu S.; Kobayashi S. Chiral Calcium Iodide for Asymmetric Mannich-type Reactions of Malonates with Imines Providing β-Aminocarbonyl Compounds. Chem. - Asian J. 2013, 8, 872–876. 10.1002/asia.201300102. [DOI] [PubMed] [Google Scholar]
- Huntington K. M.; Yi T.; Wei Y.; Pei D. Synthesis and Antibacterial Activity of Peptide Deformylase Inhibitors. Biochemistry 2000, 39, 4543–4551. 10.1021/bi992452y. [DOI] [PubMed] [Google Scholar]
- Shintani R.; Murakami M.; Hayashi T. γ-Methylidene-δ-valerolactones as a Coupling Partner for Cycloaddition: Palladium-Catalyzed [4 + 3] Cycloaddition with Nitrones. J. Am. Chem. Soc. 2007, 129, 12356–12357. 10.1021/ja073997f. [DOI] [PubMed] [Google Scholar]
- Khumtaveeporn K.; Ulman A.; Matsumoto K.; Davis B. G.; Jones J. B. Expanding the Utility of Proteases in Synthesis: Broadening the Substrate Acceptance in Non-Coded Amide Bond Formation Using Chemically Modified Mutants of Subtilisin. Tetrahedron: Asymmetry 2001, 12, 249–261. 10.1016/S0957-4166(01)00024-6. [DOI] [Google Scholar]
- Subba Reddy B. V.; Kumar H.; Borkar P.; Yadav J. S.; Srodhar B. The Prins Cascade Cyclization Reaction for the Synthesis of Angularly-Fused Tetrahydropyran and Piperidine Derivatives. Eur. J. Org. Chem. 2013, 10, 1993–1999. 10.1002/ejoc.201201387. [DOI] [Google Scholar]
- Pawar G. G.; Singh G.; Tiwari V. K.; Kapur M. Dehydrogenative Heck Reaction (Fujiwara–Moritani Reaction) of Unactivated Olefins with Simple Dihydropyrans under Aprotic Conditions. Adv. Synth. Catal. 2013, 355, 2185–2190. 10.1002/adsc.201300370. [DOI] [Google Scholar]
- Zou Y.; Qin L.; Ren X.; Lu Y.; Li Y.; Zhou J. Selective Arylation and Vinylation at the α Position of Vinylarenes. Chem. - Eur. J. 2013, 19, 3540–3511. 10.1002/chem.201203646. [DOI] [PubMed] [Google Scholar]
- Liu Y.; Ma X. W.; Liu P.; Xie J. W.; Dai B. Synthesis of 4-Vinylbiphenyl Derivatives by Pd(II)-1,2-Diaminocyclohexane Complex Catalyzed Suzuki-Miyaura Reaction. Asian J. Chem. 2014, 26, 8022–8024. 10.14233/ajchem.2014.16972. [DOI] [Google Scholar]
- Li J.; Chen J.; Jiao W.; Wang G.; Li Y.; Cheng X.; Li G. Difluoroalkylation/C–H Annulation Cascade Reaction Induced by Visible-Light Photoredox Catalysis. J. Org. Chem. 2016, 81, 9992–10001. 10.1021/acs.joc.6b01825. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.