Abstract
Purpose:
We aimed to described 25-hydroxyvitamin D [25(OH)D] levels in newly diagnosed colorectal cancer (CRC) patients and to re-revaluate levels after chemotherapy.
Methods:
Permanent residents of the San Francisco Bay Area with a new CRC diagnosis of any stage were recruited prior to any non-surgical therapy. Serum 25(OH)D levels were measured at time of diagnosis and six-month follow-up. Supplement use was not restricted. The primary endpoint was the frequency of vitamin D deficiency in patients with newly diagnosed CRC of all stages. Kruskal-Wallis and Spearman correlation tests were used to evaluate associations of patient characteristics with 25(OH)D levels.
Results:
Median 25(OH)D level at baseline was 27.0 ng/mL range: [7.2,59.0]; 65% of patients had insufficient levels (25(OH)D <30 ng/mL) (n=94). Race, disease stage, multivitamin use, vitamin D supplementation, and county of residence were associated with baseline 25(OH)D levels (P<0.05). The median change in 25(OH)D from baseline to six months was −0.7 ng/mL [−19.4,51.7] for patients treated with chemotherapy (n=58) and 1.6 ng/mL [−6.4,33.2] for patients who did not receive chemotherapy (n=19) (P=0.26). For patients who received vitamin D supplementation during chemotherapy, the median 25(OH)D change was 8.3 ng/mL [−7.6,51.7] versus −1.6 [−19.4,24.3] for chemotherapy patients who did not take vitamin D supplements (P=0.02).
Conclusion:
Among patients with a new diagnosis of CRC, most patients were found to have 25(OH)D levels consistent with either deficiency or insufficiency. In the subset of patients who received chemotherapy and took a vitamin D supplement, serum 25(OH)D levels increased, suggesting that vitamin D repletion is a feasible intervention during chemotherapy.
Keywords: colorectal cancer, vitamin D, chemotherapy, geographic variation, supplementation
BACKGROUND
Since the initial report that vitamin D deficiency may be associated with higher rates of colorectal cancer (CRC) at latitudes in the U.S. associated with lower solar UVB exposure [1], a growing body of literature has produced biochemical, animal model, and human trial data linking the vitamin D pathway to the pathogenesis of CRC. Epidemiological data substantiate that 25(OH)D levels are inversely related to the risk of developing CRC[1,2][1–3] and more recent data provide compelling evidence that vitamin D deficiency is associated with CRC-specific mortality [2,4–7]
Colon cancer cells ubiquitously express vitamin D receptors (VDR) [8,9] and 1-α-hydroxylase, which converts plasma 25(OH)D into 1,25(OH)2D [10]. Normal colon cells express a VDR that is highly sensitive to 1,25(OH)2D; when activated by 1,25(OH)2D, the VDR acts as a transcription factor that decreases epithelial cell proliferation and induces differentiation and apoptosis in colorectal neoplasia [11–13]. In a state of vitamin D deficiency, processes of cell proliferation [14], angiogenesis [15,16], and metastatic potential [17,18] are enhanced.
Accumulating data suggest that vitamin D deficiency is highly prevalent among patients with CRC. Among 515 subjects with advanced CRC accrued to a clinical trial throughout the United States and Canada, the median plasma 25(OH)D was 20 ng/mL (range, 2.3 to 75.4 ng/mL); 82% were vitamin D insufficient (<30 ng/mL) and 50% were deficient (<20 ng/mL) [5]. In CALGB 89803, which enrolled 1016 stage III CRC patients, the median predicted 25(OH)D level prior to adjuvant chemotherapy was 27.6 ng/mL (range, 16.0 −36.4 ng/mL) [7]. Inverse associations between 25(OH)D levels at time of diagnosis and overall survival have been consistently reported in cohorts of stage III and IV CRC patients [7]. However, the impact of treatment on 25(OH)D levels following diagnosis has not been evaluated although studies are underway to assess how vitamin D levels change with chemotherapy treatment [19,20].
Epidemiologic data indicate that age, race, UV dose, dietary vitamin D consumption, vitamin D supplement consumption, body mass index (BMI), post-menopausal hormone use, alcohol intake, and blood draw season are associated with 25(OH)D levels [21]. San Francisco’s maritime surroundings and sharp topography result in mild winters, summers of cool marine air and persistent coastal stratus and fog. Given the combined effects of the Bay Area’s racial-ethnic diversity and its unique weather patterns, we hypothesized that vitamin D deficiency is highly prevalent among patients with a new diagnosis of CRC from the San Francisco Bay Area. Furthermore, fluoropyrimidine-based chemotherapies are standard first line treatment for stage III-IV CRC [22] and patients on such treatment are cautioned to avoid sun exposure due to photosensitivity [23]. Additionally, patients undergoing fluoropyrimidine-based chemotherapies (e.g., in combination with irinotecan) may experience treatment-related gastrointestinal toxicities that could affect vitamin D absorption in ways that are not currently well understood. Our hypothesis that vitamin D levels would decrease during treatment is further supported by data from breast cancer patients undergoing chemotherapy, in which decreases in serum 25(OH)D during treatment have been ascribed to photoaversion and altered vitamin D absorption and metabolism from chemotherapy [24].
Herein, we describe baseline and following treatment vitamin D levels in a population with the unique sun exposure patterns and racial-ethnic diversity of the San Francisco Bay Area. We remeasured 25(OH)D levels at six months following diagnosis to examine the impact of therapy on 25(OH)D levels to test the hypothesis that patients on 5-FU would have lower vitamin D levels after treatment. Previous demonstration that vitamin D promotes a cytotoxic response to 5-FU in human colon carcinoma lines provides a plausible mechanistic explanation for a synergistic therapeutic relationship between vitamin D and 5-FU [25] and supports the importance of understanding vitamin D levels in CRC patients undergoing chemotherapy.
METHODS
Study population
We prospectively recruited patients evaluated at our institution for a new diagnosis of CRC of any stage between 2011 and 2015. Eligible patients were identified from review of pathology reports obtained following diagnosis of pathologically confirmed colorectal adenocarcinoma, and recruitment letters were mailed. Patients were also recruited in our colorectal surgery and medical oncology clinics. Patients were all permanent residents of the San Francisco Bay Area for at least twelve months prior to study registration. For the purposes of this study, the “Bay Area” was defined as residence in one of the nine counties that share coastline along the San Francisco Bay. Patients with diseases associated with altered vitamin D metabolism were excluded, including those with malnutrition (albumin < 3 g/dL), chronic kidney disease, secondary hyperparathyroidism, rickets, osteomalacia, a malabsorptive disorder, or current use of anti-convulsant medications or anti-tuberculosis medications; however, exclusions for these conditions were few.
Based upon previous assay results from use of the DiaSorin© RIA, we presumed a standard deviation of 3.0 ng/mL, and based upon results published from the NHANES cohort, we anticipated a desired width of the confidence interval measuring 2 ng/mL [26]. To report a median serum 25(OH)D level for this patient population with 99 percent confidence, we aimed to recruit at total of 74 subjects.
This study was approved by the UCSF Committee for Human Research (IRB Number 10–03402), all participants provided informed consent to participate. Serum 25(OH)D levels obtained for research purposes were not reported back to patients. However, measurement of 25(OH)D by clinical providers and vitamin D repletion and/or supplementation, according to individual care practices, were not restricted during the study period.
Serum biomarker measurement
The primary endpoint was the frequency of vitamin D deficiency in patients with newly diagnosed CRC of all stages. Secondary analyses included measurement of sociodemographic, clinical, and lifestyle variables and their association with serum 25(OH)D at baseline, and serum 25(OH)D changes from baseline to six months after some patients were treated with chemotherapy. Phlebotomy samples were collected from consenting participants at baseline and six-month follow-up. Baseline blood samples were drawn pre-operatively or at least four weeks after surgical resection. All patients were chemotherapy and radiation naïve at the time of initial laboratory assessment. Season of the baseline measurement was categorized as winter (December, January, February); spring (March, April, May); summer (June, July, August); or autumn (September, October, November).
Serum aliquots were stored in −80°C freezers. Batched samples were shipped on dry ice to Heartland Assays, Inc. (Ames, IA). The method for quantitative determination of 25(OH)D is a Food and Drug Administration (FDA) approved direct, competitive chemiluminescence immunoassay (CLIA) using the DiaSorin© LIASON 25-OH Vitamin D Total Assay [27]. The assay for a later batch of serum samples was LIASON XL, a comparable technique. The LIASON system was selected based upon its high sample throughput and equal recognition of circulating 25(OH)D2 and 25(OH)D3 to provide a total 25(OH)D value which is recognized as the clinically relevant value [28].
In order to minimize interassay variability, both baseline and six-month follow-up serum samples for each patient were analyzed in the same assay batch. To further ensure quality control, five percent of our total number of samples were submitted as blinded duplicates to verify reproducibility of assay results. The intraclass correlation coefficient was 0.98 (95% confidence interval = 0.91 −0.99).
Although there is no consensus regarding optimal physiologic levels, we defined vitamin D levels using definitions most commonly reported in the literature: vitamin D deficiency was defined as a serum 25(OH)D level <20 ng/mL, insufficiency as 20–29 ng/mL, and sufficiency as a level ≥30 ng/mL [29,30]. While the Institute of Medicine guidelines recommend repletion to 20 ng/mL for vitamin D sufficiency [31], evidence from pooling of 17 international cohorts of CRC patients and matched controls suggest that an optimal 25(OH)D range for lessening CRC risk is 30–40 ng/mL [32].
Questionnaire development and administration
At the time of study registration, participants completed questionnaires on geographic residence, race/ethnicity, exercise, sun exposure, dietary vitamin D intake, multivitamin use, and vitamin D supplement use. The dietary vitamin D questionnaire solicited consumption frequency of 13 common foods with high vitamin D contributions (milk, soy milk, yogurt, cheese, fortified orange juice, canned tuna fish, sardines, shellfish, dark meat fish, other fish, eggs, beef liver, and cold breakfast cereal) [33]. At six-month follow-up, participants completed questionnaires on oncologic treatment and current use of medications, supplements, and multivitamins.
Calculation of sun exposure
Cumulative UV Exposure Score (CUES) methodology was utilized as the indicator of sun exposure [34]. Self-reported sun exposure patterns such as sunscreen and protective clothing use were not incorporated into the sun exposure score due to lack of available validated methodology [35]. CUES was calculated as ∑ (hours of exposure x UV index) [34]. CUES is measured in units of (Watts*hour)/meter2, but should be considered a relative measure rather than an absolute dosimetry measure [34].
Hours of sun exposure were calculated by self-reported hours spent outside during weekdays, weekends, and vacations over the year prior to study registration. Subjects reported days spent on vacations at the beach or comparable outdoor locations; this time was considered eight hours of sun exposure per day, and these days were subtracted from the summer months. Average annual UV indices for 58 major U.S. cities were calculated using data from the National Oceanic and Atmospheric Administration (2009–2012) [34]. The UV index for San Francisco was used for all time spent in the San Francisco Bay Area. If subjects spent >1 month outside of the Bay Area in the year prior to registration, a separate CUES was calculated for the duration of that time with the UV index of the city nearest to the subject’s self-reported location. Subjects who spent greater than one month outside of the United States during the preceding year were excluded from the sun exposure analysis.
Calculation of dietary and supplemental vitamin D consumption
The baseline questionnaire solicited consumption frequency of thirteen common foods with high vitamin D contributions. Self-reported food frequency consumption was multiplied by IU of vitamin D per serving to generate a relative measurement of dietary vitamin D consumption with units of IU/month [33]. Additionally, subjects were asked whether they currently used a dedicated vitamin D supplement or multivitamins. Current supplement, multivitamin, and medication information was re-solicited from subjects at six-month follow-up.
Calculation of exercise score
A physical activity score was calculated by self-reported frequency of performing nine common exercise activities. This yielded units of activity-specific MET-hours per week.
Clinicopathologic data
Height and weight were measured at study intake and body mass index (BMI) was calculated as weight (kilograms)/height2 (meters2). Clinical and pathologic data were abstracted from the medical record, including location of the primary tumor in the colon (right, transverse, left, or rectum), disease stage at diagnosis, mismatch repair proficiency (pMMR) or deficiency (dMMR), and microsatellite stability (MSS) or microstatellite instability (MSI).
Statistical analyses
Comparison of the continuous variables among groups were tested using Kruskal-Wallis one-way analysis of variance. Pearson chi-square test was applied to evaluate for statistical association between two categorical variables. In addition, Cochran-Mantel-Haenszel test was applied for the ordinal categorical variable. Spearman correlation coefficient was used for testing the association between two continuous variables. The statistical significance was declared at p-value of <0.05. All analyses were performed using SAS® statistical computing software.
RESULTS
Patient Characteristics
Between January 2011 and December 2015, 94 patients with a new diagnosis of CRC were enrolled with completion of baseline 25(OH)D measurement and questionnaire. Of 94 enrolled cases, 10 patients were lost to follow-up prior to the six-month follow-up assessment, due to either death due to CRC (n=5) or other causes (n=5). All data from baseline and six-month follow-up were available for a total of 84 cases.
Baseline demographic and clinicopathologic characteristics of the 94 enrolled CRC patients are reported in Table 1. The median age of participants at study entry was 54 years (range: 36 to 88 years). Of these, 56% of participants were male (n=53), and 73% were Caucasian (n=69). All patients were permanent residents in one of nine counties included in the study catchment area, with 45% of patients residing in San Francisco County.
Table 1:
Clinicopathologic features and relationship to serum 25(OH)D at time of CRC diagnosis
| No. patients (%) (N=94) |
Median 25(OH)D, ng/mL (range) |
P* | |
|---|---|---|---|
| Gender | 0.25 | ||
| Female | 41 (44) | 27.2 [7.9, 59.0] | |
| Male | 53 (56) | 26.0 (7.2–47.6) | |
| Race | 0.03 | ||
| Caucasian | 69 (73) | 27.1 [8.1, 53.5] | |
| African-American | 2 (2) | 10.7 [7.9, 13.5] | |
| Asian | 14 (15) | 25.3 [7.2, 33.0] | |
| Unknown‡ | 9 (10) | 27.6 [17.4, 59.0] | |
| SF Bay Area County Group | 0.006 | ||
| Alameda-Santa Clara | 12 (13) | 18.1 [7.2, 43.6] | |
| Contra Costa | 13 (14) | 24.8 [11.9, 40.5] | |
| Marin-Napa-Sonoma | 15 (16) | 30.7 [23.1, 59.0] | |
| San Francisco | 42 (45) | 27.0 [8.1, 47.6] | |
| San Mateo | 12 (13) | 26.6 [11.3, 53.5] | |
| Age (years) | 0.89 | ||
| <50 | 36 (38) | 27.2 [10.4, 47.6] | |
| 50–59 | 16 (17) | 26.8 [13.7, 53.5] | |
| 60–69 | 25 (27) | 24.8 [8.1, 59.0] | |
| >70 | 17 (18) | 27.3 [7.2, 47.6] | |
| Body-mass index (BMI) | 0.51 | ||
| Underweight (BMI <18.5) | 2 (2) | 35.6 [27.0, 44.2] | |
| Normal (BMI ≥18.5 and <25) | 39 (41) | 28.6 [7.2, 53.5] | |
| Overweight (BMI ≥25 and <30) | 27 (29) | 27.2 [8.1, 43.6] | |
| Obese (BMI ≥30) | 26 (28) | 24.8 [10.5, 59.0] | |
| Disease stage | 0.13 | ||
| I | 14 (15) | 21.3 [11.3, 42.1] | |
| II | 10 (11) | 30.6 [20.5, 53.5] | |
| III | 50 (53) | 27.1 [7.9, 59.0] | |
| IV | 20 (21) | 23.2 [7.2, 47.6] | |
| Primary tumor location | 0.88 | ||
| Synchronous | 2 (2) | 17.9 [11.9, 23.8] | |
| Right colon | 24 (26) | 27.2 [7.9, 53.5] | |
| Transverse colon | 15 (16) | 24.8 [7.2, 49.3] | |
| Left colon | 22 (23) | 27.1 [8.1, 59.0] | |
| Rectum | 31 (33) | 27.0 [10.5, 47.6] | |
| DNA mismatch repair (MMR) or microsatellite status | 0.66 | ||
| pMMR / MSS | 71 (76) | 26.5 [7.2, 49.3] | |
| dMMR / MSI | 11 (12) | 30.0 [10.4, 53.5] | |
| Unknown or not tested‡ | 12 (13) | 28.7 [10.5, 59.0] | |
| Family history of CRC | 0.17 | ||
| Yes | 13 (14) | 26.5 [7.2, 31.0] | |
| No | 81 (86) | 27.2 [7.9, 59.0] | |
| Season of lab draw | 0.29 | ||
| Winter (D,J,F) | 30 (32) | 23.3 [8.1, 53.5] | |
| Spring (M,A,M) | 20 (21) | 24.9 [7.2, 59.0] | |
| Summer (J,J,A) | 21 (22) | 27.1 [8.9, 42.1] | |
| Autumn (S,O,N) | 23 (24) | 29.6 [7.9, 47.6] | |
| Multivitamin use | 0.04 | ||
| No | 60 (64) | 24.8 [7.2, 59.0] | |
| Currently | 33 (35) | 27.6 [11.3, 53.5] | |
| Unknown‡ | 1 (1) | 39.1 [39.1] | |
| Baseline vitamin D supplement use | <0.001 | ||
| No | 66 (70) | 24.5 [7.2, 47.6] | |
| Currently | 27 (29) | 32.8 [13.7, 59.0] | |
| Unknown‡ | 1 (1) | 39.1 [39.1] | |
P-value obtained by Kruskal-Wallis test
Unknown group not included in p-value calculation
Distribution of 25(OH)D Levels
The median baseline serum 25(OH)D level for all patients was 27.0 ng/mL (range: 7.2–59.0 ng/mL). At time of enrollment, 26% of patients were vitamin D deficient (serum 25(OH)D < 20 ng/mL), 39% were vitamin D insufficient (serum 25(OH)D ≥ 20 ng/mL and <30 ng/mL), and 35% were vitamin D sufficient (serum 25(OH)D > 30 ng/mL). Figure 1 demonstrates the distribution of serum 25(OH)D levels.
Figure 1:
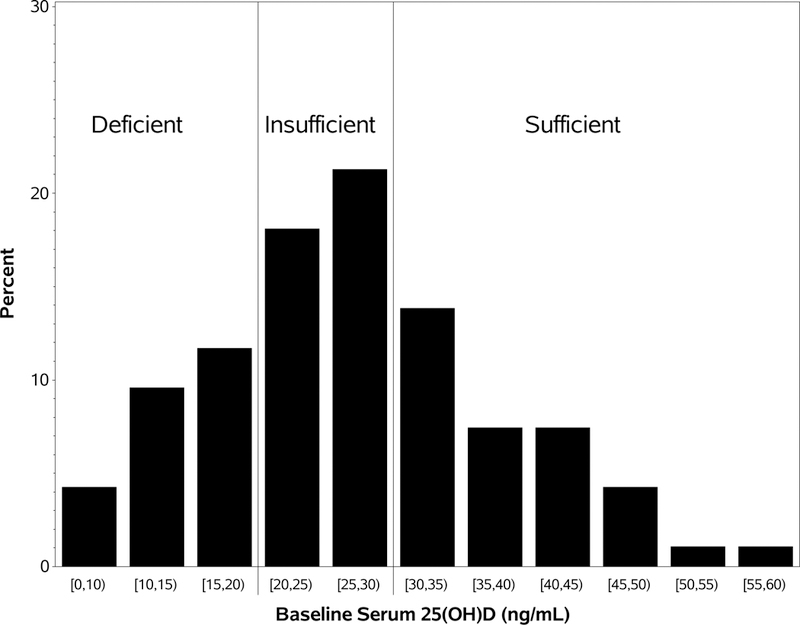
Distribution of serum 25(OH)D at baseline
Association of Baseline 25(OH)D Levels with Patient Characteristics
The relationships of demographic and clinicopathologic features to serum 25(OH)D at time of CRC diagnosis are summarized in Table 1. Baseline 25(OH)D levels were associated with race (P=0.03), multivitamin use (P=0.04), vitamin D supplement use (P<0.001), and county of permanent residence (P=0.006). Additionally, there was a significant positive correlation between vitamin D supplement dose and baseline 25(OH)D level (Spearmanr2 = 0.39, P<0.045). Calculated scores for dietary vitamin D, exercise, and UV exposure, were not significantly correlated with serum 25(OH)D levels. Although serum 25(OH)D levels vary by season in populations of other geographic regions [21], the lack of association seen here (Table 1) was not unexpected given the mild winters and summers of persistent coastal fog in the San Francisco Bay Area which result in a relatively stable UV index throughout the year [36].The median vitamin D status for each county in the San Francisco Bay Area is shown in Figure 3. Figure 3 demonstrates that counties with CRC patients that have higher rates of vitamin D deficiency do not tend to lie along coastal fogbanks as was originally hypothesized.
Figure 3:
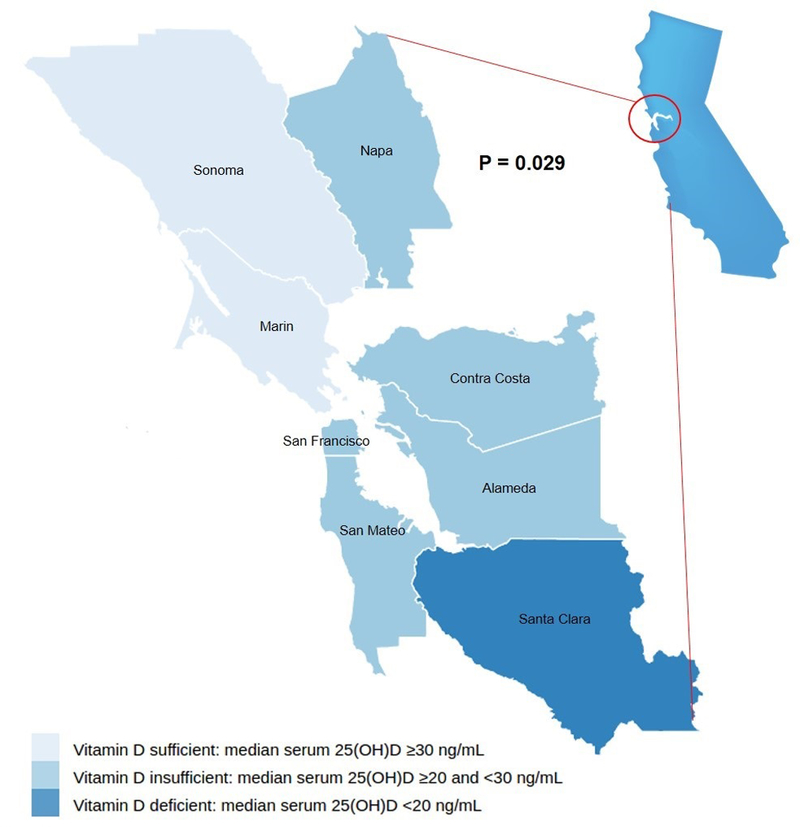
Median vitamin D status according to county of residence in the San Francisco Bay Area
In addition, we designated vitamin D status using three levels, deficient (25(OH)D <20 ng/mL), insufficient (20≤ 25(OH)D< 30 ng/mL), or sufficient (25(OH)D≥30 ng/mL), and tested for correlations by first using Pearson chi-square statistics then by using Cochran-Mantel-Haenszel chi-square statistics to treat the levels as ordered. Categorical but not ordinal vitamin D status was significantly associated with disease stage at diagnosis (P=0.02). The distribution of vitamin D status according to disease stage is shown in Figure 2. Ordinal vitamin D status was significantly linearly associated with reported vitamin D supplement use, showing a steadily increasing proportion who currently took supplement from the status deficient (8%) to insufficient (22%) and then to sufficient (53%) (P<0.001).
Figure 2:
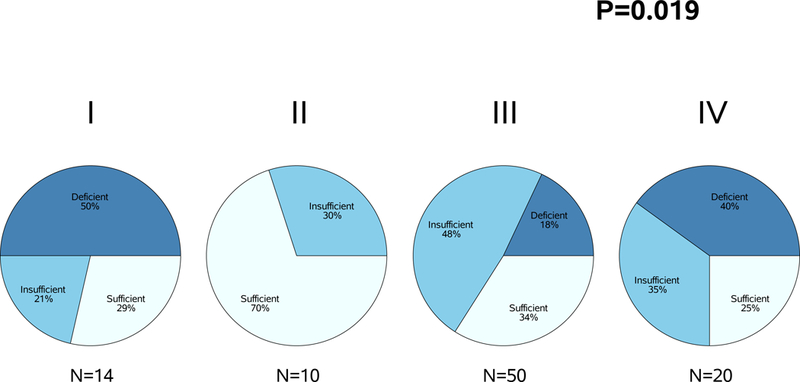
Association between vitamin D status and disease stage at diagnosis
Change in 25(OH)D Levels During Chemotherapy
A total of 58 patients received three or more cycles of fluoropyrimidine-based chemotherapy following the initial baseline blood draw. Among the 58 patients receiving chemotherapy, 13 (22%) patients reported taking a vitamin D supplement at baseline but not at the six-month follow-up and 4 (7%) patients reported not taking a vitamin D supplement at baseline but did report taking one at six-month follow-up. Among patients not receiving chemotherapy, two (10%) patients reported taking a vitamin D supplement at baseline but not at six-month follow-up, and four (21%) patients reported not taking a vitamin D supplement at baseline but did report taking one at six-month follow-up. Of note, median serum baseline 25(OH)D was 24.2 ng/mL among patients who started vitamin D supplements during the six month period, 25.8 mg/mL among those who had no change in their supplement use, and 36.1 among those who stopped taking vitamin D supplements. By linear regression of the change in serum 25(OH)D onto the exposures, vitamin D supplement usage at 6 months was significantly associated with the change and the effect estimate of 9.7 from the univariate model (P<0.001) was not materially changed by adding covariates (e.g., chemotherapy, supplement vitamin D at baseline, multivitamin usage, UV score, tumor stage, age, race, BMI, season of blood draw, dietary vitamin D, county of residence, and baseline serum 25(OH)D value.
Among the 58 patients who received chemotherapy, the median of serum 25(OH)D at baseline was 27.1 ng/mL (range: 7.2 to 59.0 ng/mL), the median 25(OH)D level at six-month follow-up was 25.1 ng/mL (range: 8.2 to 72.9 ng/mL), with a median change of −0.7 ng/mL (range: −19.4 to 51.7 ng/mL). Serum 25(OH)D changes from baseline to six months, according to chemotherapy administration and supplement use are summarized in Table 2. The crude difference in serum change comparing patients who did receive chemotherapy and those who did not was not significant (P=0.26). However, a subgroup analysis showed that among the 58 patients who received chemotherapy, the nine patients who reported taking a vitamin D supplement at six months had a significantly greater increase in serum D compared to the 49 who were not taking a vitamin D supplement (P=0.02), whereas among the 19 patients who did not receive chemotherapy, the change for the vitamin D supplement group and no supplement group was not statistically significant. However, the multivariate linear regression model with an interaction of chemotherapy receiving status and vitamin D supplement taking at six months didn’t show there was a significant interaction effect. Furthermore, we examined the effects of supplementation on 25(OH)D changes by season and found the effects consistent across seasons. Figure 4 compares 25(OH)D level changes between patients who did and did not report taking a vitamin D supplement at six months among patients treated with chemotherapy.
Table 2:
Serum 25(OH)D change from baseline to six-month follow-up by chemotherapy exposure and vitamin D supplement status
| Vitamin D supplement use at 6 months |
No. of patients (%) |
Median 25(OH)D Δ from baseline (range) |
P* | |
|---|---|---|---|---|
| 0.02 | ||||
| Chemotherapy (N=58) |
Yes | 9 (16%) | 8.3 [−7.6, 51.7] | |
| No | 49 (84%) | −1.6 [−19.4, 24.3] | ||
| 0.54 | ||||
| No chemotherapy (N=19) |
Yes | 6 (32%) | 1.5 [−6.2, 33.2] | |
| No | 13 (68%) | 1.6 [−6.4, 8.9] | ||
P-value obtained by Kruskal-Wallis test
Figure 4:
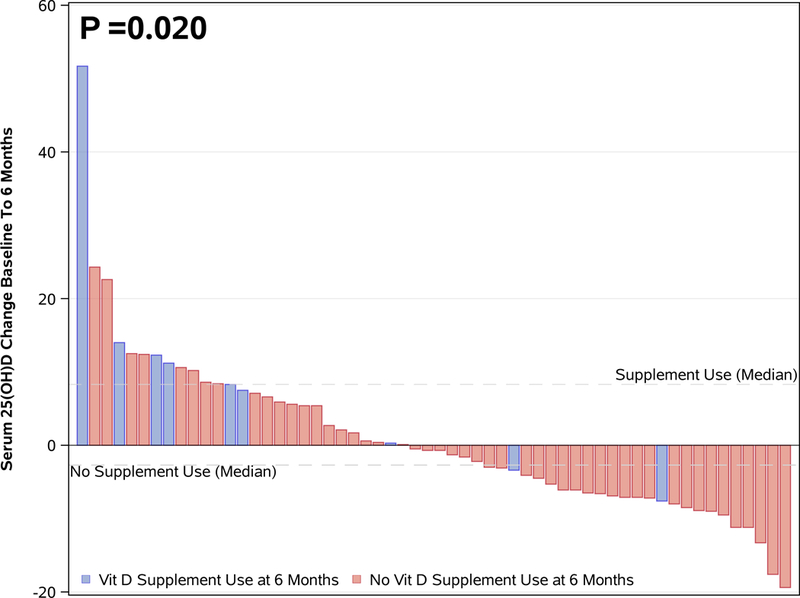
Serum 25(OH)D change from baseline to six months in patients receiving chemotherapy
DISCUSSION
This study evaluated serum 25(OH)D levels in CRC patients from the San Francisco Bay Area, following initial diagnosis and at six-month follow-up. Among the 94 patients, 65% had baseline 25(OH)D levels that were either insufficient or deficient. Among patients with metastatic disease, the proportion with vitamin D insufficiency (25(OH)D <30 ng/mL) was comparable to rates of vitamin D insufficiency seen in national metastatic CRC populations (75% vs. 82% respectively, P=0.39) [5]. Although we hypothesized vitamin D deficiency would be highly prevalent in patients from the San Francisco Bay Area due to both the maritime climate and racial-ethnic diversity, this suggests that our findings from our patient population may in fact be generalizable to other populations.
The frequency of vitamin D insufficiency in patients with early stage or locally advanced CRC has not been reported in larger studies. Our results suggest that 62% of patients with stage I-III CRC are vitamin D insufficient or deficient. Of interest, none of the stage II patients included in this cohort were deficient in 25(OH)D. As such, 25(OH)D results from the sufficient stage II patients drove the Pearson chi-square p-value. When stage I and II patients are combined for a Pearson chi-square analysis, these findings were no longer significant (P=0.17). As one plausible explanation, it has been speculated widely that stage II CRC harbors a unique tumor biology and thus these tumors may develop independently of the vitamin D pathway. This finding could also be attributable to random fluctuation magnified by the small sample size. If an inverse relationship did exist between disease stage and 25(OH)D levels, we would expect a significant trend test and stage I patients to have less vitamin D deficiency than was observed. Nevertheless, additional larger studies are needed to further evaluate whether stage at diagnosis is truly associated with vitamin D deficiency.
In larger multi-center studies, variation in 25(OH)D among counties within a particular geographical area has not been previously evaluated. Here, median 25(OH)D levels varied widely between the five county groups represented (P=0.006). Several counties had fewer than five residents represented in this patient population. Accordingly, Napa, Marin, and Sonoma counties were combined, and Alameda and Santa Clara were combined. A link between coastal cloud cover and lower 25(OH)D levels was not observed, and several coastal counties had higher median 25(OH)D levels. Of course, the fact that patients were enrolled in this study because they sought at least some care in San Francisco is a reminder that people, including cancer patients, are mobile and that a person’s place of residence is not a perfect surrogate for outdoor sun dose.
Because sufficient 25(OH)D levels may be associated with income-dependent factors such as consumption of vitamin D-rich foods (e.g., fatty fish) and supplement use, we considered whether socioeconomic factors could account for the disparate 25(OH)D levels between Bay Area counties. Data regarding household income were not collected from study participants; thus, the relationship between individual socioeconomic status and 25(OH)D level could not be examined. Further research is warranted into health disparities surrounding vitamin D status in CRC patients.
Our finding that the median 25(OH)D level improved in the subset of patients who received chemotherapy and reported taking a vitamin D supplement has not been previously reported. Although this finding is based on a small sample size, it is statistically significant and suggests that vitamin D supplementation during chemotherapy is a feasible intervention to achieve or maintain vitamin D sufficiency. Additionally, we found no difference in the 25(OH)D levels from baseline to six-month follow-up between patients who did and did not receive chemotherapy (P=0.26), suggesting that chemotherapy may not have the deleterious effects on 25(OH)D levels that we ascribed to increased photosensitivity and potential for gastrointestinal toxicities.
While other studies demonstrate that vitamin D deficiency is associated with poor response to chemotherapy [37,38], it is unclear how low vitamin D levels influences disease course independently from tumor burden in the setting of critical illness. Reverse causation is a commonly cited concern when discussing inverse associations between vitamin D levels and CRC mortality. It has been hypothesized that cancer patients with worse disease prognoses have higher tumor burden and inflammation that contribute to lowered vitamin D levels [39]. Although vitamin D levels are often low in critically ill patients, true hypercalcemia of malignancy and associated lowered 25(OH)D is rarely reported in the literature [40]. And furthermore, Song et al. have found that the associations between vitamin D deficiency and CRC in a large nested case-control study remain valid after adjustment for inflammatory markers [41]. Therefore investigation into a causative pathway from higher vitamin D levels to improved CRC outcomes continues with randomized controlled trials.
The possible interaction of vitamin D and chemotherapy is an important and timely issue, given the recently reported results of SUNSHINE trial [19]. This randomized phase II study reported a modest 1.9 month improvement in progression-free survival in metastatic CRC patients with high dose vitamin D supplementation combined with FOLFOX and bevacizumab compared to FOLFOX and bevacizumab alone [19]. The efficacy of vitamin D supplementation in improving progression free survival (PFS) may be dependent on vitamin D supplement dose, as a smaller randomized trial of supplementation with cholecalciferol 2000 IU/day in metastatic CRC patients showed lack of efficacy in improving PFS or overall survival [42].
Although we demonstrated that maintenance or improvement of a patient’s vitamin D status is feasible with chemotherapy and concurrent vitamin D supplementation, further investigation is needed to determine whether improvement of a patient’s vitamin D status from baseline translates to an improvement in overall survival. In addition to a possible survival benefit conferred by vitamin D sufficiency, limited data suggest that vitamin D supplementation in cancer patients receiving palliative care improves pain management and decreases infections [43]. Furthermore, investigation is underway to evaluate whether vitamin D repletion with supplements should be done with standard dosing or a treatment algorithm specific to baseline 25(OH)D; preliminary results suggest an algorithm adapted to baseline 25(OH)D levels is effective and well-tolerated in cancer patients [44–47].
Several limitations of our studies must be acknowledged. First, vitamin D supplement use was not controlled in this observational study, due to ethical concerns about not repleting patients with known 25(OH)D insufficiency, and some patients started or stopped taking vitamin D supplements between the two assessment time points. Indeed, emerging data regarding associations of vitamin D with outcomes in CRC patients [4–7] likely resulted in evolution of attitudes about the role of vitamin D in CRC amongst providers at our institution during the time of this study, and the inclusion of vitamin D measurements and repletion as part of routine clinical practice was likely inconsistent over time. Contrary to expectations, however, in patients receiving chemotherapy, more patients reported that they stopped taking vitamin D supplements than patients who reported starting taking vitamin D supplements. Due to our limited sample size, we do not know if this is pattern is generalizable to other CRC patients following initiation of chemotherapy.
In addition, this was a single-institution study with a modest sample size. Further research is needed to examine whether associations between chemotherapy and change in vitamin D levels exist in subgroups defined by disease stage, race, or geography. Due to the limitations of self-reported data we could not fully explore potential confounders such as socioeconomic status, heterogeneous microclimates within a county, and potential associations of supplement use with other healthy behaviors.
CONCLUSION
In conclusion, we observed that 65% of participants with a new diagnosis of CRC originating from the San Francisco Bay Area had vitamin D levels that were either insufficient or deficient (<30 ng/mL). Consistent with other studies, we found vitamin D supplementation, multivitamin use, and race to be associated with 25(OH)D levels at time of diagnosis. County of permanent residence was associated with 25(OH)D levels, although this did not seem to be correlated with UV dose in the different counties. There was no significant difference in change in serum 25(OH)D over six months between patients who did and did not receive chemotherapy. In the subset of patients who received chemotherapy and took a vitamin D supplement, serum 25(OH)D levels increased, suggesting that vitamin D repletion is a feasible intervention during chemotherapy.
Acknowledgments
Previous presentation: This work was previously presented in a poster session at the 2017 ASCO Gastrointestinal Cancers Symposium J Clin Oncol. 2017. 35 (4_suppl; abstr 793).
Footnotes
Disclosure statement: The authors have no disclosures.
REFERENCES
- [1].CF Garland FC Garland. Do sunlight and vitamin D reduce the likelihood of colon cancer? International journal of epidemiology. 35 (2006) 217–220. [DOI] [PubMed] [Google Scholar]
- [2].Dou R, Ng K, Giovannucci EL, Manson JE, Qian ZR, Ogino S. Vitamin D and colorectal cancer: molecular, epidemiological and clinical evidence, The British Journal of Nutrition. 115 (2016) 1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Garland CF, Gorham ED. Dose-response of serum 25-hydroxyvitamin D in association with risk of colorectal cancer: A meta-analysis, J. Steroid Biochem. Mol. Biol. 168 (2017) 1–8. [DOI] [PubMed] [Google Scholar]
- [4].Ng K, Wolpin BM, Meyerhardt JA, Wu K, Chan AT, Hollis BW, et al. Prospective study of predictors of vitamin D status and survival in patients with colorectal cancer, British Journal of Cancer. 101 (2009) 916–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ng K, Sargent DJ, Goldberg RM, Meyerhardt JA, Green EM, Pitot HC, et al. Vitamin D Status in Patients With Stage IV Colorectal Cancer: Findings From Intergroup Trial N9741, Journal of Clinical Oncology. 29 (2011) 1599–1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Ng K, Venook AP, Sato K, Hollis BW, Niedzwiecki D, Ye C, et al. Vitamin D status and survival of metastatic colorectal cancer patients: Results from CALGB/SWOG 80405 (Alliance), Journal of Clinical Oncology. 33 (2015) 507. [Google Scholar]
- [7].Fuchs MA, Yuan C, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson S, Atienza D, Messino M, Kindler H, Venook AP, Innocenti F, Warren RS, Bertagnolli MM, Ogino S, Giovannucci EL, Horvath E, Meyerhardt JA, Ng K. Predicted Vitamin D Status and Colon Cancer Recurrence and Mortality in CALGB 89803 (Alliance), Annals of Oncology. (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Vandewalle B, Adenis A, Hornez L, Revillion F, Lefebvre J. 1,25-dihydroxyvitamin D3 receptors in normal and malignant human colorectal tissues, Cancer letters. 86 (1994) 67–73. [DOI] [PubMed] [Google Scholar]
- [9].Lointier P, Meggouh F, Dechelotte P, Pezet D, Ferrier C, Chipponi J, et al. 1,25-Dihydroxyvitamin D3 receptors and human colon adenocarcinoma, The British journal of surgery. 78 (1991) 435–439. [DOI] [PubMed] [Google Scholar]
- [10].Zehnder D, Bland R, Williams MC, McNinch RW, Howie AJ, Stewart PM, et al. Extrarenal Expression of 25-Hydroxyvitamin D3–1α-Hydroxylase1, The Journal of Clinical Endocrinology & Metabolism. 86 (2001) 888–894. [DOI] [PubMed] [Google Scholar]
- [11].Miller EA, Keku TO, Satia JA, Martin CF, Galanko JA, Sandler RS. Calcium, Vitamin D, and Apoptosis in the Rectal Epithelium, Cancer Epidemiology Biomarkers & Prevention. 14 (2005) 525–528. [DOI] [PubMed] [Google Scholar]
- [12].Xue L, Lipkin M, Newmark H, Wang J. Influence of dietary calcium and vitamin D on diet-induced epithelial cell hyperproliferation in mice, Journal of the National Cancer Institute. 91 (1999) 176–181. [DOI] [PubMed] [Google Scholar]
- [13].Diaz GD, Paraskeva C, Thomas MG, Binderup L, Hague A. Apoptosis Is Induced by the Active Metabolite of Vitamin D3 and Its Analogue EB1089 in Colorectal Adenoma and Carcinoma Cells: Possible Implications for Prevention and Therapy, Cancer Research. 60 (2000) 2304. [PubMed] [Google Scholar]
- [14].Scaglione-Sewell BA, Bissonnette M, Skarosi S, Abraham C, Brasitus TA. A vitamin D3 analog induces a G1-phase arrest in CaCo-2 cells by inhibiting cdk2 and cdk6: roles of cyclin E, p21Waf1, and p27Kip1, Endocrinology. 141 (2000) 3931–3939. [DOI] [PubMed] [Google Scholar]
- [15].Fernandez-Garcia NI, Palmer HG, Garcia M, Gonzalez-Martin A, del Rio M, Barettino D, et al. 1alpha,25-Dihydroxyvitamin D3 regulates the expression of Id1 and Id2 genes and the angiogenic phenotype of human colon carcinoma cells, Oncogene. 24 (2005) 6533. [DOI] [PubMed] [Google Scholar]
- [16].Iseki K, Tatsuta M, Uehara H, Iishi H, Yano H, Sakai N, et al. Inhibition of angiogenesis as a mechanism for inhibition by 1alpha-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 of colon carcinogenesis induced by azoxymethane in Wistar rats, International journal of cancer. 81 (1999) 730–733. [DOI] [PubMed] [Google Scholar]
- [17].Evans S, Shchepotin E, Young H, Rochon J, Uskokovic M, Shchepotin IB. 1,25-dihydroxyvitamin D3 synthetic analogs inhibit spontaneous metastases in a 1,2-dimethylhydrazine-induced colon carcinogenesis model, International Journal of Oncology. 16 (2000) 1249–1303. [DOI] [PubMed] [Google Scholar]
- [18].Lamprecht SA, Lipkin M. Cellular Mechanisms of Calcium and Vitamin D in the Inhibition of Colorectal Carcinogenesis, Annals of the New York Academy of Sciences. 952 (2001) 73–87. [DOI] [PubMed] [Google Scholar]
- [19].Ng K, Nimeiri HS, McCleary NJ, Abrams TA, Yurgelun MB, Cleary JM, et al. SUNSHINE: Randomized double-blind phase II trial of vitamin D supplementation in patients with previously untreated metastatic colorectal cancer, Journal of Clinical Oncology. 35 (2017) 3506. [Google Scholar]
- [20].Anderson EC, Effectiveness of High Dose Vitamin D Supplementation in Stage III Colorectal Cancer, 2017 (2017). [Google Scholar]
- [21].Bertrand KA, Giovannucci E, Liu Y, Malspeis S, Eliassen AH, Wu K, et al. Determinants of plasma 25-hydroxyvitamin D and development of prediction models in three U.S. cohorts, Br.J.Nutr. 108 (2012) 1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Benson AB, Venook AP, Clinical Practice Guidelines in Oncology (NCCN Guidelines) Colon Cancer Version 2.2016, 2017 (2015). [Google Scholar]
- [23].Naughton M, Take Precautions During Cancer Treatment in Warmer Weather, 2017 (2017). [Google Scholar]
- [24].Charehbili A, Hamdy NaT, Smit VTHBM, Kessels L, van Bochove A, van Laarhoven HW, et al. Vitamin D (25–0H D3) status and pathological response to neoadjuvant chemotherapy in stage II/III breast cancer: Data from the NEOZOTAC trial (BOOG 10–01), Breast. 25 (2016) 69–74. [DOI] [PubMed] [Google Scholar]
- [25].Liu G, Xi Hu, Chakrabarty S. Vitamin D mediates its action in human colon carcinoma cells in a calcium-sensing receptor-dependent manner: downregulates malignant cell behavior and the expression of thymidylate synthase and survivin and promotes cellular sensitivity to 5-FU, International journal of cancer. 126 (2010) 631–639. [DOI] [PubMed] [Google Scholar]
- [26].Ginde AA, Liu MC, Camargo CA. Demographic Differences and Trends of Vitamin D Insufficiency in the US Population, 1988–2004, Archives of Internal Medicine. 169 (2009) 626–632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Wagner D, Hanwell HEC, Vieth R. An evaluation of automated methods for measurement of serum 25-hydroxyvitamin D, Clinical Biochemistry. 42 (2009) 1549–1556. [DOI] [PubMed] [Google Scholar]
- [28].Ersfeld DL, Rao DS, Body J, Sackrison JL, Miller AB, Parikh N, et al. Analytical and clinical validation of the 25 OH vitamin D assay for the LIAISON® automated analyzer, Clinical Biochemistry. 37 (2004) 867–874. [DOI] [PubMed] [Google Scholar]
- [29].Bischoff-Ferrari HA, Giovannucci E, Willett WC, Dietrich T, Dawson-Hughes B. Estimation of optimal serum concentrations of 25-hydroxyvitamin D for multiple health outcomes, The American journal of clinical nutrition. 84 (2006) 18. [DOI] [PubMed] [Google Scholar]
- [30].Holick MF. Vitamin D Status: Measurement, Interpretation, and Clinical Application, Annals of Epidemiology. 19 (2009) 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Dietary Reference Intakes for Calcium and Vitamin D,, National Academies Press (US), Washington (DC), 2011. [PubMed] [Google Scholar]
- [32].McCullough ML, Zoltick ES, Weinstein SJ, Fedirko V, Wang M, Cook NR, et al. Circulating Vitamin D and Colorectal Cancer Risk: An International Pooling Project of 17 Cohorts, J. Natl. Cancer Inst. (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ahuja JKC, Haytowitz DB, Pehrsson PR, Roseland JM, USDA National Nutrient Database for Standard Reference, Release 28, 2016 (2015). [Google Scholar]
- [34].Zhu GA, Raber I, Sakshuwong S, Li S, Li AS, Tan C, et al. Estimation of individual cumulative ultraviolet exposure using a geographically-adjusted, openly-accessible tool, BMC dermatology. 16 (2016) 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].McCarty CA. Sunlight exposure assessment: can we accurately assess vitamin D exposure from sunlight questionnaires? The American Journal of Clinical Nutrition. 87 (2008) 1101S. [DOI] [PubMed] [Google Scholar]
- [36].San Francisco Monthly Climate Averages, 2018. [Google Scholar]
- [37].Chiba A, Raman R, Thomas A, Lamy P, Viala M, Pouderoux S, et al. Serum Vitamin D Levels Affect Pathologic Complete Response in Patients Undergoing Neoadjuvant Systemic Therapy for Operable Breast Cancer, Clin. Breast Cancer. 18 (2018) 144–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ma K, Xu W, Wang C, Li B, Su K, Li W. Vitamin D deficiency is associated with a poor prognosis in advanced non-small cell lung cancer patients treated with platinum-based first-line chemotherapy, Cancer Biomark. 18 (2017) 297–303. [DOI] [PubMed] [Google Scholar]
- [39].Autier P, Boniol M, Pizot C, Mullie P. Vitamin D status and ill health: a systematic review, The Lancet Diabetes & Endocrinology. 2 (2014) 76–89. [DOI] [PubMed] [Google Scholar]
- [40].Galindo RJ, Romao I, Valsamis A, Weinerman S, Harris YT. Hypercalcemia of Malignancy and Colorectal Cancer, World J Oncol. 7 (2016) 5–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Song M, Wu K, Chan AT, Fuchs CS, Giovannucci EL. Plasma 25-hydroxyvitamin D and risk of colorectal cancer after adjusting for inflammatory markers, Cancer Epidemiol. Biomarkers Prev. 23 (2014) 2175–2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Antunac Golubić Z, Baršić I, Librenjak N, Pleština S. Vitamin D Supplementation and Survival in Metastatic Colorectal Cancer, Nutr Cancer. 70 (2018) 413–417. [DOI] [PubMed] [Google Scholar]
- [43].Helde-Frankling M, Höijer J, Bergqvist J, Björkhem-Bergman L. Vitamin D supplementation to palliative cancer patients shows positive effects on pain and infections-Results from a matched case-control study, PLoS ONE. 12 (2017) e0184208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Cummings LC, Thota PN, Willis JE, Chen Y, Cooper GS, Furey N, et al. A nonrandomized trial of vitamin D supplementation for Barrett’s esophagus, PLoS ONE. 12 (2017) e0184928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].WB Grant BJ Boucher. Randomized controlled trials of vitamin D and cancer incidence: A modeling study, PLoS ONE. 12 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Jacot W, Firmin N, Roca L, Topart D, Gallet S, Durigova A, et al. Impact of a tailored oral vitamin D supplementation regimen on serum 25-hydroxyvitamin D levels in early breast cancer patients: a randomized phase III study, Ann. Oncol. 27 (2016) 1235–1241. [DOI] [PubMed] [Google Scholar]
- [47].Young J, Welin E, Braeutigam C, Gilger E, Lane A, Salloum R. Impact of a Vitamin D Replacement Algorithm in Children and Young Adults With Acute Lymphoblastic Leukemia, J. Pediatr. Hematol. Oncol. (2018). [DOI] [PubMed] [Google Scholar]


