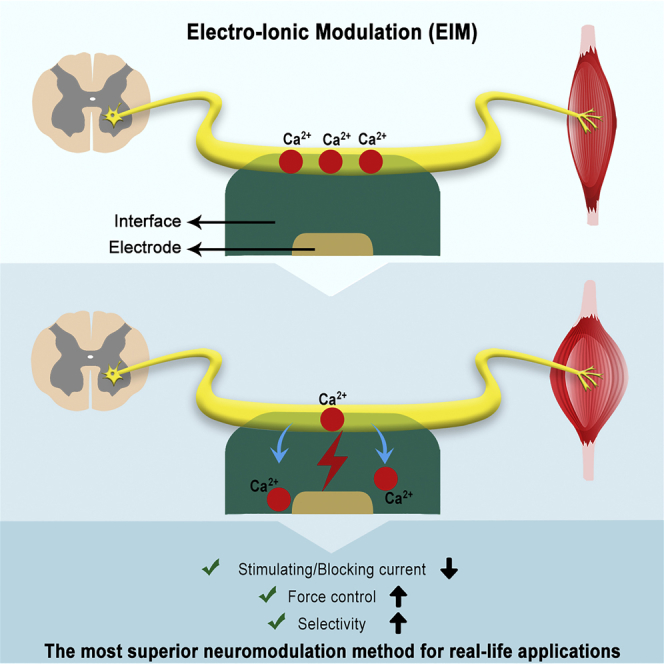
Summary
Theoretically, by controlling neural membrane potential (Vm) in vivo, motion, sensation, and behavior can be controlled. Until now, there was no available technique that can increase or decrease ion concentration in vivo in real time to change neural membrane potential. We introduce a method that we coin electro-ionic modulation (EIM), wherein ionic concentration around a nerve can be controlled in real time and in vivo. We used an interface to regulate the Ca2+ ion concentration around the sciatic nerve of a frog and thus achieved stimulation and blocking with higher resolution and lower current compared with electrical stimulation. As EIM achieves higher controllability of Vm, it has potential to replace conventional methods used for the treatment of neurological disorders and may bring a new perspective to neuromodulation techniques.
Subject Areas: Bioelectrical Engineering, Bioengineering, Biological Sciences, Techniques in Neuroscience
Graphical Abstract
Highlights
-
•
EIM regulates extracellular ion concentration in vivo in real time
-
•
EIM stimulates or blocks the nerve via Ca2+ ion depletion or enhancement
-
•
EIM achieves selective stimulation or blocking of large or small axons
-
•
EIM is the most superior neuromodulation method for real-life applications
Bioelectrical Engineering; Bioengineering; Biological Sciences; Techniques in Neuroscience
Introduction
During the evolutionary process, the neuromuscular system, which functions through the Vm change, is developed to make the organisms able to sense and move (Weiss, 1996, Campbell et al., 2015, Butler and Hodos, 2005). Therefore, in theory, if we manage to control Vm in vivo, we can control motion and sensation (Weiss, 1996). To control Vm, there are four known parameters that can be regulated: pH, temperature, extracellular ion concentration, and application of extrinsic current (Weiss, 1996). In literature, techniques that change only one of these parameters were generally used (Ho et al., 2014, Harris, 2008). For instance, functional electrical stimulation (FES) uses electrical stimulation method. FES has several clinical applications for restoring the neurological functions in paralyzed patients such as control of upper extremity (Memberg et al., 2014, Micera et al., 2010, Hochberg et al., 2012, Bouton et al., 2016, Peckham and Kilgore, 2013), lower extremity (Rohde et al., 2012, Triolo et al., 2012, Nataraj et al., 2012, Sadowsky et al., 2013), respiratory muscles (Elefteriades et al., 2002, Onders et al., 2004), posture (Wu et al., 2013, Triolo et al., 2013a, Triolo et al., 2013b), gait (Wenger et al., 2016, Mazurek et al., 2012), and prevention of pressure ulcers (Solis et al., 2011, Solis et al., 2012). However, because the stimulation current used in FES is too high, it may cause over-heating in tissues, leading to nerve damage (Ho et al., 2014). Also, because it cannot selectively stimulate or block the nerves, localization of signal propagation cannot be achieved. As a result, it activates the related sensory nerve fibers resulting in pain sensation (Peckham and Knutson, 2005). Another reason FES has a limited use in daily life is that nerve blockage cannot be achieved in a controlled manner (Peckham and Knutson, 2005). In addition, there are some direct clinical applications for blocking such as regaining of bladder control in neurologically disabled patients (Boger et al., 2012).
Another major approach to change Vm is using chemical methods to change ion concentration. Some pharmacological agents are used for this purpose. For example opiates, such as morphine and codeine, are commonly used in pain management (Kandel et al., 2013). However, they can lead to systemic side effects such as nausea, constipation, urinary retention, respiratory depression, and cardiac arrest (Peckham and Knutson, 2005, Kandel et al., 2013, Choi, 2016). These adverse effects cause patients to discontinue the medication (Gilron et al., 2015). To establish more control over Vm, extracellular ion concentration was changed and extrinsic current was applied, but there is no available technique that can simultaneously combine them. Electrochemical methods (van den Brand et al., 2012), in which extrinsic current stimulates the nerve after injecting some chemicals, are developed for controlling Vm. However, as they use the injection method for changing chemical concentration, they can only alter the neural membrane environment once, hence lacking the real-time modulation ability. Previously, in vitro ion concentration was changed by ion-selective membrane (ISM) (Song et al., 2011). This study first changes the neural membrane environment and then electrically stimulates the nerve; hence it changes ion concentration and applies current separately, which causes delay. Because of this one-minute-delay (Luan et al., 2014, Theogarajan, 2012) this method is not suitable for use in real-life applications such as prosthesis for motion and retina (Theogarajan, 2012). Another method targeting to control Vm is optogenetics (Boyden et al., 2005). This technique aims to change ion concentration around the nerve via light. To achieve this, optogenetics genetically modifies the neurons to make them sensitive to light. However, optogenetics is not suitable for daily-life applications and can only be used for research purposes because it genetically alters the neurons for modifying ion channels. In sum, until now there has been no technique that can change Vm in vivo in real time with high control and be applicable to real life. We have developed a method, electro-ionic modulation (EIM) that achieves neuromodulation via real-time regulation of extracellular ion concentration around the nerve in vivo and application of extrinsic current to the nerve at the same time by using an electrode and an interface on it. In this work we used ISM as the interface to change ion concentration, but any other interface can be used for the purpose of EIM such as uniform films, nanoparticles, peptides, or polymers. EIM would enable better regulation of Vm compared with the traditional approaches, which have high potential to affect a variety of neuromodolution applications.
Results
We first aimed to decrease the current needed for stimulation, and thus reduced power consumption and prevented nerve damage with EIM by using flexible electrodes coated with ISM. The fabrication procedure for flexible electrodes and ISM coating can be seen in the Transparent Methods section. As shown in Figure S1, we obtained the lowest threshold value with the flexible electrode compared with conventional cable method and the planar electrode, because flexible electrode increases the contact area between the nerve and the electrode surface. Therefore, we stimulated the nerve by using flexible electrode through all experiments.
EIM with Ca2+ Ion Depletion
We coated the flexible electrode with ISM for changing ion concentration around the nerve to obtain more excitable membrane environment. In literature, ISM is generally used in sensors to obtain selectivity (Gao et al., 2016, Plesha et al., 2006); however, we utilized ISM to actively change the neural membrane environment. To observe the effects of ion concentrations on nerve stimulation, we made separate in vivo manual ion injection experiments of PO43−, Ca2+, Na+, and K+ ions in the sciatic nerve of frogs. We used PO43− ion to observe the effect of the Ca2+ ion depletion. The manual ion injection procedures can be seen in the Transparent Methods section. The threshold values before and after the ion injection are summarized in Figure S2. We obtained the most efficient results with PO43−. Thus, we conclude that Ca2+ has a high impact on threshold regulation for nerve stimulation; hence we focused on changing the Ca2+ concentration.
The Ca2+ ion is critical for stimulation because it blocks the voltage-gated Na+ channels (Armstrong and Cota, 1999). Therefore, depletion of Ca2+ ions around the nerve makes the stimulation easier because opening voltage-gated Na+ channels facilitates the nerve stimulation (Kandel et al., 2013). The mechanism for the effect of Ca2+ ion on nerve stimulation is shown in Figure S3. Hence, we can conclude that the stimulation of nerve is easier with Ca2+ ion depletion around the nerve.
Stimulation Experiments with Ca2+ Ion Depletion
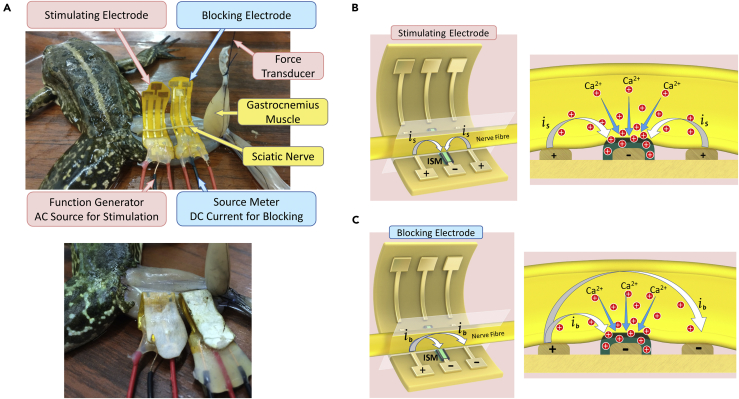
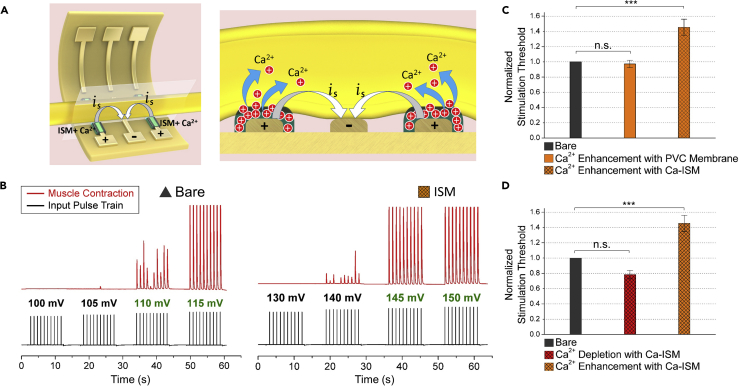
After exploring the effectiveness of the Ca2+ ion, we made in vivo EIM nerve stimulation experiments using flexible electrodes coated with ISM. In this section, we examined the nerve stimulation using EIM, which we coined electro-ionic stimulation (EIS). We compared the results of EIS and electrical stimulation in this section. Figure 1A (top) shows the experimental setup used for both stimulation and blocking experiments. Figure 1A (bottom) demonstrates the folded flexible electrodes used in stimulation and blocking experiments. As we applied enough force to fold our flexible electrodes, we did not use anything to hold the electrodes in folded state. Once the flexible electrodes are folded, they are held in folded state for days. We started the experiments by anesthetizing the frog. Details of anesthesia and frog preparation procedures are given in the Transparent Methods section. Subsequently, we stimulated the sciatic nerve of the frog in vivo with the stimulating electrode by pulsed DC signal applied from the function generator, as can be seen in Figure 1A (top). When the sciatic nerve was stimulated, the gastrocnemius muscle contracted and we measured the contractile force with the force transducer. Figure 1B (left) demonstrates the schematic drawing of the flexible electrode and illustrates the operating principle of the device. The electrode is polarized as (+/-/+) configuration, and ISM is coated on the negatively polarized middle pole. The stimulating current flows from positively polarized poles to negatively polarized pole, and the nerve is stimulated through the negative pole. As can be observed in the cross-sectional view of the sciatic nerve and flexible stimulating electrode shown in Figure 1B (right), the negatively polarized ISM attracts the positively charged Ca2+ ions from the nerve thereby depleting Ca2+ ions around the nerve. As a result, we obtained a more excitable membrane environment and increased the Vm and hence decreased the threshold for stimulation.
Figure 1.
Experimental Setup and Operating Principle of Electro-ionic Modulation with Ca2+ Ion Depletion
(A) Photograph of the in vivo EIM stimulation and blocking experiments (top) and of the folded flexible electrodes used in in vivo EIM stimulation and blocking experiments (bottom).
(B) Schematic drawing of the flexible stimulating electrode and the operating principle of the device (left); cross section of sciatic nerve and flexible stimulating electrode to demonstrate the operating principle of ion-selective membrane (ISM) (right).
(C) Schematic drawing of the flexible blocking electrode and the operating principle of the device (left); cross section of sciatic nerve and flexible blocking electrode to demonstrate the operating principle of ISM (right).
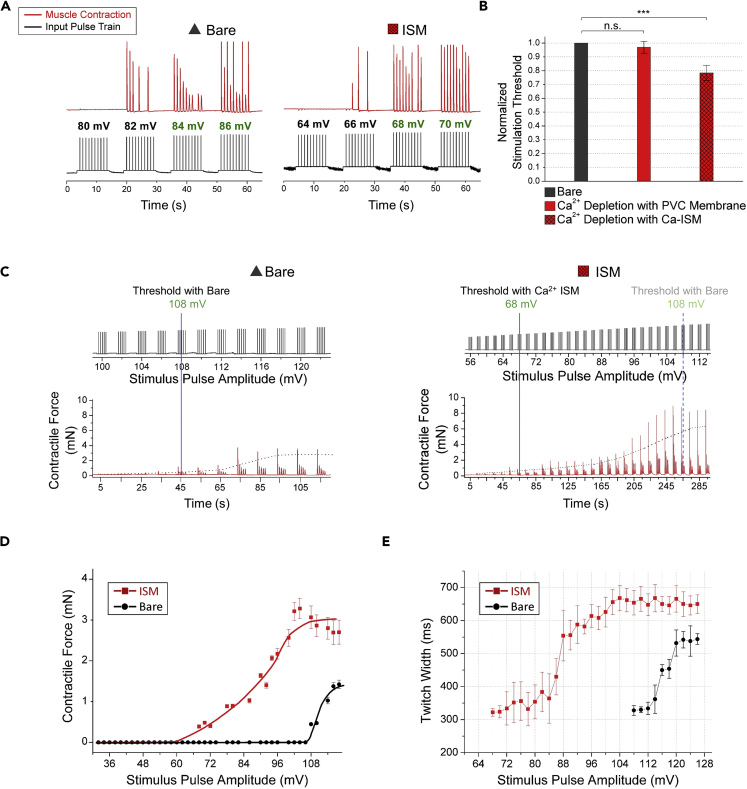
In our experiments, we applied an input pulse from the function generator and measured the output force using the force transducer. We first stimulated the sciatic nerve with bare side of the electrode and recorded the stimulation threshold. Then we put the nerve onto the ISM-coated side of the electrode and recorded the threshold for a comparative analysis. We accepted the recorded voltage as the threshold value, when at least 80% of the applied pulses induce muscle contraction. The results of stimulation experiments are summarized in Figure 2. As can be seen in Figure 2A, the stimulation threshold decreases from 84 to 68 mV on the ISM-coated electrode. We got an average decrease of 22% in the threshold for three experiments. In our experiments, we obtained stimulation threshold ranging from 68 to 90 mV using ISM and obtained 39% energy saving; however, in the literature, 10% energy saving was achieved by optimizing the electrical stimulator circuit in rat's sciatic nerve (Foutz et al., 2012).
Figure 2.
Results of Stimulation Experiments with Ca2+ Ion Depletion
(A) Stimulation experiment results for bare electrode and ISM-coated electrode. The stimulation threshold decreases from 84 to 68 mV on the ISM-coated electrode.
(B) Comparison of stimulation threshold values for bare electrode, PVC-coated electrode, and ISM-coated electrode (n = 3). The values are normalized with respect to bare electrode. The threshold values for the bare electrode and the PVC-coated electrode are very similar, whereas there is 22% decrement with ISM-coated electrode. Vs = 10 mV→110 mV in 2-mV step (stimulus), tp = 1 ms (pulse width), f = 1 Hz (pulse frequency), n.s., not significant, ***p < 0.001, error bars represent 2 SD.
(C) Contractile force (mN) obtained for varying stimulus pulse amplitude (mV) for the bare electrode (left) and ISM-coated electrode (right). Higher dynamic range (higher maximum contractile force) is attained with ISM-coated electrode.
(D) Average and standard deviation of contractile force (mN) measured for varying stimulus pulse amplitude (mV) for the bare electrode and ISM-coated electrode obtained from the results in (C). Better resolution is acquired with ISM-coated electrode.
(E) Average and standard deviation of contractile force twitch width (ms) measured for varying stimulus pulse amplitude (mV) for bare electrode and ISM-coated electrode obtained from the results in (C). First large and then small axons are stimulated with ISM-coated electrode.
For validation of the working principle, we prepared a control experiment to prove that the decrement in the threshold is due to Ca2+ ion depletion around the nerve. For this experiment, we used polyvinyl chloride (PVC) membrane, which has the same content as ISM except for the Ca2+ ionophore, which is the part responsible for Ca2+ ion depletion (Wang et al., 2001, Kang and Hilgemann, 2004). When we compare the results given in Figure 2B, the threshold values in the bare electrode and the PVC-coated electrode are very similar, whereas there is a significant decrease with ISM-coated electrode. Hence we conclude that the reduction in the stimulation threshold using ISM-coated electrode originates from the Ca2+ ion depletion around the nerve.
We performed control experiments for Ca2+ ion modulation using ISM in Figure S4. First, we performed Ca2+ imaging experiments as additional control experiments to prove Ca2+ ion attraction via ISM. The schematic diagram of our experimental setup is given in Figure S4A. Using our planar electrodes coated with ISM, we applied 10 μA current for 65 s. The negatively charged ISM attracts positively charged Ca2+ ions. As a result, Ca2+ ions bind with Fluo-4 AM dye and Fluo-4 AM emission is increased as depicted in Figure S4B. The Fluo-4 AM fluorescence intensity is also increased as demonstrated in Figure S4D (left). We also calculated the Ca2+ ion concentration increase using the measured fluorescence intensity. The Ca2+ ion concentration after 10 μA current application for 5 s was 73.65 nM; however, Ca2+ ion concentration after 10 μA current application for 65 s was 715 nM. This experiment proves that we attracted Ca2+ ions using ISM. The Ca2+ imaging procedure and the details of Ca2+ ion concentration calculation can be seen in Transparent Methods section. The Ca2+ ion attraction experiment results are demonstrated in Video S1. In addition to the Ca2+ imaging experiments, we performed confocal imaging experiments of the sciatic nerve of a frog to prove the depletion of Ca2+ ions from the nerve using our device. Figure S4E demonstrates the results of the confocal imaging experiment. Figure S4E (left) exhibits the confocal imaging of the sciatic nerve before current application. Figure S4E (right) depicts another confocal image of the sciatic nerve after 20 μA current application for a minute. Because the negatively charged ISM attracts positively charged Ca2+ ions from the sciatic nerve after current application, we observe decrease in the fluorescence intensity of the Fluo-4 AM (Ca2+ reporter dye) in the sciatic nerve in Figure S4E (right). Therefore, Ca2+ ions were depleted from the sciatic nerve. This experiment demonstrates that we depleted Ca2+ ions from sciatic nerve of a frog using ISM. The details of the confocal imaging techniques can be seen in the Transparent Methods section.
Ca2+ ion attraction experiment results; 10 μA current was applied for 65 s, and a picture was taken in every 5 s. We created this video by compiling these pictures and demonstrating them at 500-ms intervals. Fluo-4 AM emission was increased with current application. This experiment proves that we attracted Ca2+ ions using ISM.
We also performed experiments to test the Ca2+ ion selectivity of our device in Figure S5. We measured the resistance of our test setup using different concentrations of Ca2+ solution in Figure S5A. We obtained lowest resistance value with highest Ca2+ concentration and highest resistance value with lowest Ca2+ concentration as demonstrated in Figure S5C. These results show the Ca2+ ion selectivity of our device. We also performed additional control experiments with different concentrations of NaCl solution using the same ISM (Figure S5B). As Ca2+ ions pass much more than Na+ ions through Ca2+ ISM (Wang et al., 2001), we measured much higher resistances with NaCl solutions as depicted in Figure S5C. These results validate the Ca2+ ion selectivity of our device. The details of Ca2+ ion selectivity experiments can be seen in the Transparent Methods section.
After decreasing the threshold for stimulation, we performed experiments to evaluate the control on stimulation using ISM. For this purpose, we first observed the maximum magnitude of the contractile force, which we define as dynamic range. As shown in Figure 2C, we increased the maximum contractile force with ISM-coated electrode in addition to decreasing the threshold. The underlying mechanism of dynamic range increase is the selective stimulation of axons. As large axons have lower resistance, they are stimulated more easily than small axons (Kandel et al., 2013). Because we decrease the threshold, we can stimulate both large and small axons with the ISM-coated electrode, whereas only large axons can be stimulated in bare electrode case. As higher number of axons are stimulated using ISM, we obtain higher contractile force and hence higher dynamic range. When dynamic range increases, stronger movements can be achieved, which can be helpful to improve the performance of neuroprostheses such as the ones used in correcting foot drop (Melo et al., 2015).
In addition to obtaining a higher dynamic range, we also achieved better resolution, which we define as the inverse of the slope of the contractile force over the stimulus amplitude, with ISM-coated electrode. Resolution is calculated as 7.35 mV/mN for bare electrode and 17.88 mV/mN for ISM-coated electrode from the results shown in Figure 2D. The rationale behind this increase in resolution is also related to stimulation of first large and then small axons with the ISM-coated electrode. At 60 mV, we stimulate large axons with ISM-coated electrode; however, no stimulation is observed in bare electrode. Around 108 mV, small axons are stimulated by ISM-coated electrode, whereas for bare electrode, threshold is just achieved and only large axons start to be stimulated. Besides, contractile force saturates nearly at the same stimulus for ISM-coated electrode and bare electrode. Therefore, we stimulate axons in a wider range of stimulus with ISM and hence acquire better resolution, which enables us to have better control over the nerve stimulation. Better resolution is important especially in terms of achieving fine motor skills, such as movement of fingers.
We can deduce that we first stimulate the large axons and then small axons with ISM-coated electrode by exploring the relation between the input pulse and the twitch width of the gastrocnemius muscle. It is known that the velocity of the signal propagation is proportional to the square root of the axon diameter (Weiss, 1996). Furthermore, as signal propagation along the axon gets slower, higher twitch width is obtained (Ridge, 1967). As can be seen in Figure 2E, when we stimulate the nerve with ISM-coated electrode, we first obtain low twitch width, which means that we first stimulate large axons. Then as we increase the input pulse, the twitch width increases, implying that we stimulate the small axons. This observation proves that we first stimulate large and then small axons with ISM-coated electrode.
Blocking Experiments with Ca2+ Ion Depletion
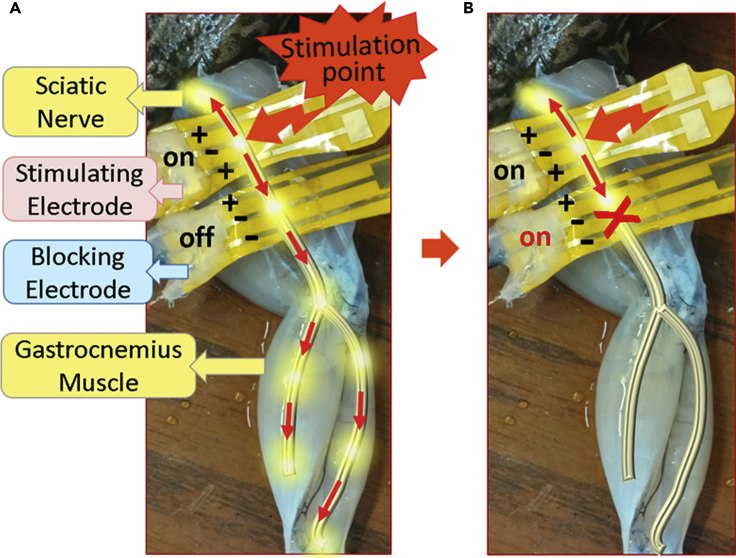
Following the selective stimulation with decreased stimulation threshold using ISM-coated electrode, we worked on blocking experiments and aimed to achieve controlled blocking with EIM. Figure 3 depicts the working principle of our blocking method. When the stimulation is applied and the blocking electrode is off, the signal propagates bidirectionally. When we turn on the blocking electrode, the signal stops to propagate downward. The signal cannot reach gastrocnemius muscle and contractions are terminated, hence we manage to localize the stimulation.
Figure 3.
The Working Principle of Our Blocking Method
(A) When the stimulation current is applied and the blocking electrode is off, the signal propagates bidirectionally.
(B) When blocking electrode is turned on, the signal stops to propagate downward as depicted in right.
We again make use of the flexible electrodes coated with ISM to control blocking of the nerves. We performed blocking experiments with conventional cable method, planar electrode, and flexible electrode. Unsurprisingly, we obtained the lowest blocking current values with flexible electrodes as a consequence of increased contact area, as shown in Figure S6.
In addition to using flexible electrodes, we utilized ISM and depleted Ca2+ ions around the nerve to facilitate blocking through DC current application. Pulsed DC current is applied for stimulation, whereas DC current is applied for blocking because inactivation gate of voltage-gated Na+ channels is closed with prolonged current application (Kandel et al., 2013). Na+ channels should be in open position to go into inactivation phase (Kandel et al., 2013). Moreover, Ca2+ ion blocks the voltage-gated Na+ channels (Armstrong and Cota, 1999). Therefore, depletion of Ca2+ ions around the nerve with DC current application makes the blocking easier. The mechanism for the effect of Ca2+ ion on nerve blocking is shown in Figure S7. Hence, we apply DC current with Ca2+ ion depletion to block the nerve more easily.
After exploring the effectiveness of the Ca2+ ion depletion, we made in vivo experiments for blocking the signal propagation along the nerve with ISM-coated flexible electrodes. The experimental setup used for blocking is shown in Figure 1A (top). In blocking experiments, we used an additional electrode and a second signal source for blocking when compared with the stimulation experiments. As in stimulation experiments, we first stimulated the sciatic nerve using stimulating electrode by applying pulsed DC current from function generator that contracts the gastrocnemius muscle. Then, we used blocking electrode by applying DC current with source meter. We measured the contractile force from force transducer and monitored it from the PC together with the input signals. Figure 1C (left) illustrates the operating principle of the flexible blocking electrode. We used (+/-/-) configuration for the blocking electrode and coated the ISM on the negatively polarized middle pole. When we apply the DC current, as negatively charged ISM attracts the positively charged Ca2+ ions, Ca2+ ion concentration around the nerve decreases as schematically shown in Figure 1C (right), which enables us to block the nerve more easily.
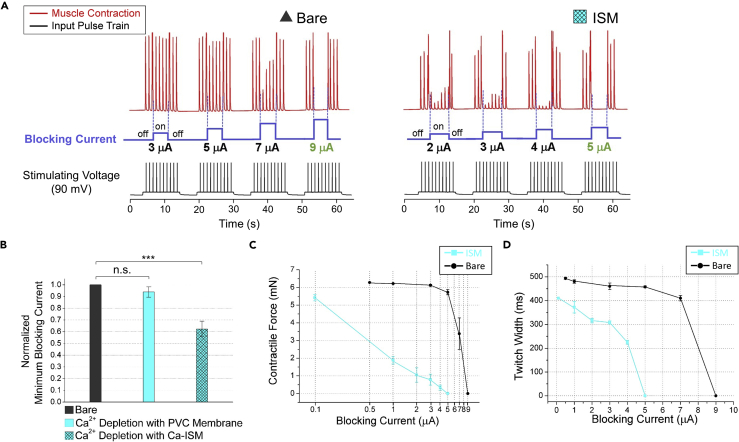
In blocking experiments, we first blocked the nerve with bare electrode and then repeated the same procedure with the ISM-coated electrode and compared the results. We applied incrementing input currents to the blocking electrode. We first observed partial blocking and then full blocking because the degree of the nerve blockage depends on the magnitude of current. The experimental results can be seen in Figure 4. Figure 4A presents that the current needed for full blocking decreases from 9 to 5 μA using ISM. We got an average decrease of 38% in full blocking current for three experiments. We obtained full blocking ranging from 5 to 60 μA in sciatic nerve of the frog in our experiments; however, in literature, 0.15–3.0 mA blocking current was used in sciatic nerve of the rat (Vrabec et al., 2015). Also when we turned off the blocking electrode, contractile force returned back to similar values before blocking current application as can be seen from Figure 4A. Therefore we can conclude that we achieved reversible blocking and hence had control on blocking.
Figure 4.
Results of Blocking Experiments with Ca2+ Ion Depletion
(A) Blocking experiment results for bare electrode and ISM-coated electrode. The blocking current is applied after the third stimulation pulse for four or five pulse duration. The current needed for full blocking decreases from 9 to 5 μA using ISM-coated electrode.
(B) Comparison of blocking current values for bare electrode, PVC-coated electrode, and ISM-coated electrode (n = 3). The values are normalized with respect to bare electrode. The currents needed to obtain full blocking are very similar for the PVC-coated and the bare electrodes, whereas there is 38% decrease for the ISM-coated electrode. Vs = 90 mV (stimulus), Ib = 1 μA → 100 μA in 1-μA step (DC blocking), tp = 1 ms (pulse width), f = 1 Hz (pulse frequency), n.s., not significant, ***p < 0.001, error bars represent 2 SD.
(C) Average and standard deviation of contractile force (mN) measured for varying blocking current (μA) in log scale for bare electrode and ISM-coated electrode obtained from the results in (A).
(D) Average and standard deviation of contractile force twitch width (ms) measured for varying blocking current (μA) for the bare electrode and ISM-coated electrode obtained from the results in (A). First large and then small axons are blocked with ISM-coated electrode.
We performed a control experiment to prove that the decrement in the blocking current results from the Ca2+ ion depletion around the nerve. For this purpose, we used the Ca2+ ionophore lacking PVC-coated electrode. As shown in Figure 4B, the current needed for full blocking is very similar in the PVC-coated and the bare electrodes, whereas there is a significant decrease in the ISM-coated electrode. These results show that Ca2+ ion depletion around the nerve is responsible in the decrease of blocking current.
Electrical methods cannot be used to block the nerve in chronic clinical applications as they can damage the nerve (Whitwam and Kidd, 1975, Ravid and Prochazka, 2014). EIM uses lower blocking currents, hence does not cause nerve damage. Therefore, EIM has potential to take place in treatment procedure of several disorders caused by uncontrolled neural firing rates, such as chronic pain and epilepsy (Fridman and Della Santina, 2013).
In addition to decreasing the blocking current, we also explored the level of control on blocking using ISM. We studied the relationship between the contractile force of the gastrocnemius muscle and the magnitude of the blocking DC current and obtained the graph shown in Figure 4C. When we use bare electrode, we observe a very sharp decline in the contractile force of the muscle, hence limited control was achieved on partial blockage. However, with ISM-coated electrode, we observe a smooth decline in the contractile force until obtaining full blocking. These results demonstrate that we manage the blocking process in a well-controlled manner with ISM and achieve graded blocking. Graded blocking can be used in peripheral nerves to achieve voluntary movements in hypertonic conditions resulting from stroke (Bhadra and Chae, 2009).
Graded nerve blocking is related to selective blocking of axons. It is known that the velocity of the signal propagation is faster in large axons: the smaller the twitch width, the larger the axons that are activated, and vice versa. Hence, blocking large axons results in small decrease in compound twitch width. As can be seen in Figure 4D, because the large axons get blocked first, we first observe a small decrease from the compound twitch width. However, as we increase the blocking DC current, due to blockage of the small axons, we obtain full blocking. Therefore, we first block large axons and then small axons using ISM. There is, however, a sharp decrease in the twitch width with the bare electrode, because both the large and the small axons in the sciatic nerve are blocked simultaneously. Hence, we achieve selective blocking using ISM-coated electrode.
EIM with Ca2+ Ion Enhancement
The stimulation and blockage results given in Figures 2 and 4 show the usage of EIM for in vivo and real-time Ca2+ ion depletion. Through this modulation, we obtain a more excitable neural membrane environment; hence we increase the Vm of the nerve, which enables us to stimulate and block the signal propagation on the axon more easily in a more controlled way. Taking this control one step further, we aimed to decrease Vm, by creating a more resistant neural membrane environment. For this purpose, we designed a new experiment to increase the Ca2+ ion concentration around the sciatic nerve. Figure 5 shows the operating principle and experimental results of EIM for the Ca2+ enhancement around the nerve. Figure 5A (left) demonstrates the operating principle of the device for Ca2+ enhancement where we coat ISMs on the both sides of the electrode and then load Ca2+ ions to ISMs. Ca2+ ion loading procedure can be seen in the Transparent Methods section. To measure the stimulation threshold, we placed the sciatic nerve on the bare side of the flexible electrode. Similar to the stimulation experiments with Ca2+ ion depletion, we applied the pulsed DC current in (+/-/+) configuration and recorded the stimulation threshold value. For comparison, we placed the nerve on the Ca2+-loaded ISM-coated side of the electrode and repeated the same procedures. Figure 5A (right) demonstrates that positively polarized ISMs repel the positively charged Ca2+ ions into the sciatic nerve, thus enhancing the Ca2+ ion concentration around the nerve. Figure 5B illustrates that stimulation threshold was increased from 110 to 145 mV by using ISM-coated electrode. We obtained stimulation threshold ranging from 145 to 170 mV with Ca2+ enhancement using ISM and obtained an average increase of 45% in the stimulation threshold for three experiments. These results demonstrate that by enhancing Ca2+ ion concentration around the nerve, we decrease the Vm, obtain more resistant membrane environment, and increase the stimulation threshold.
Figure 5.
Operating Principle of EIM and Results of Stimulation Experiments with Ca2+ Ion Enhancement
(A) Operating principle of EIM with Ca2+ ion enhancement. Schematic drawing of the flexible stimulating electrode and the operating principle of the device (left); cross section of sciatic nerve and flexible stimulating electrode to demonstrate the operating principle of ion selective membrane (ISM) (right).
(B) Stimulation experiment results for bare electrode and ISM-coated electrode. Stimulation threshold is increased from 110 to 145 mV by using ISM-coated electrode.
(C) Comparison of stimulation threshold values for bare electrode, PVC-coated electrode, and ISM-coated electrode (n = 3). The values are normalized with respect to bare electrode. The stimulation thresholds are nearly the same for PVC-coated and bare electrodes; however, there is 45% increase for ISM-coated electrode. Vs = 30 mV→230 mV in 5-mV step (stimulus), tp = 1 ms (pulse width), f = 1 Hz (pulse frequency), n.s., not significant, ***p < 0.001, error bars represent 2 SD.
(D) Comparison of stimulation threshold values for bare electrode, ISM-coated electrode with Ca2+ ion depletion, and ISM-coated electrode with Ca2+ ion enhancement (n = 3). The values are normalized with respect to bare electrode. There is 22% decrease in stimulation threshold using ISM-coated electrode with Ca2+ ion depletion and 45% increase using ISM-coated electrode with Ca2+ ion enhancement. By depleting or enhancing Ca2+ ions around the nerve, we can either decrease or increase stimulation threshold. Vs = 60 mV→170 mV in 2-mV step (stimulus), tp = 1 ms (pulse width), f = 1 Hz (pulse frequency), n.s., not significant, ***p < 0.001, error bars represent 2 SD.
To show that the stimulation threshold increment results from the Ca2+ ion enhancement around the nerve, we performed control experiments using PVC-coated electrode. PVC-coated electrode is not capable of keeping the Ca2+ ions due to absence of the Ca2+ ionophore; hence we do not expect Ca2+ ion concentration enhancement around the nerve. We stimulated the sciatic nerve and recorded the thresholds with bare and PVC-coated electrodes. The results shown in the Figure 5C are consistent with our hypothesis: the stimulation thresholds are nearly the same in the PVC-coated and bare electrodes; however, there is an average increase of 45% in the stimulation threshold of ISM-coated electrode. Therefore, increment in stimulation threshold by using ISM-coated electrode results from the Ca2+ enhancement around the nerve.
We performed Ca2+ imaging experiments as further control experiments to prove Ca2+ ion repulsion using ISM. We reversed the polarity of the experiment demonstrated in Figure S4B, where the Ca2+ ion bound with Fluo-4 AM dye, and applied 10μA current for 80 s. The positively charged ISM pushes the positively charged Ca2+ ions. Therefore, Ca2+ ion concentration is decreased. As a result, Fluo-4 AM emission is decreased as shown in Figure S4C. The Fluo-4 AM fluorescence intensity is also decreased as depicted in Figure S4D (right). We also calculated the Ca2+ ion concentration decrease using the measured fluorescence intensity. The Ca2+ ion concentration decreased from 715 to 37.96 nM after 10 μA current application for 80 s. This experiment demonstrates that we repulsed Ca2+ ions using ISM. The Ca2+ ion repulsion experiment results are demonstrated in Video S2.
Ca2+ ion repulsion experiment results; 10 μA current was applied for 80 s, and a picture was taken in every 5 s. We created this video by compiling these pictures and demonstrating them at 500-ms intervals. Fluo-4 AM emission was decreased with current application. This experiment proves that we repulsed Ca2+ ions using ISM.
Stimulation experiment results of EIM for Ca2+ ion depletion and enhancement are shown in Figure 5D. When we deplete Ca2+ ions around the nerve, we increase the Vm and hence decrease the threshold for stimulation. In contrast, when we enhance Ca2+ ions around the nerve, we decrease the Vm and therefore increase the threshold for stimulation. Thus we can totally control the Vm with EIM.
Discussion
Comparison of EIM with other neuromodulation methods reveals that electrical methods, optogenetics, and EIM have the capability of working in real time, but chemical and electrochemical methods do not have this capability because they work with latency. Electrical methods, chemical methods, and EIM have the potential to be used in real-life applications. However; electrochemical methods work with delay in every single usage; optogenetics genetically alters the neurons and exhibits side effects. Hence, electrochemical methods and optogenetics are not suitable for daily-life applications. Even though chemical methods have the capability of being used in real-life applications, they work with latency and exhibit side effects, which cause termination of the treatment. Electrical methods, optogenetics, and EIM exhibit high temporal resolution. On the other hand, electrical methods cause high power consumption, which is not desirable for the nerve and the patient. EIM is the only method capable of achieving selective stimulation and blocking of large and small axons. Thus, it provides improved stimulation or blocking resolution and more accurate force control. Optogenetics is capable of stimulation of single neuron. By considering all the features mentioned above, it can be said that EIM and optogenetics present the best neuromodulation properties. However, because optogenetics is not suitable for daily-life applications, EIM is superior to optogenetics. Therefore, EIM is the most superior neuromodulation method for real-life applications among all these techniques. Properties of the neuromodulation methods are listed in Table 1.
Table 1.
Properties of Neuromodulation Methods
| Neuromodulation Methods |
||||||
|---|---|---|---|---|---|---|
| Electrical | Chemical | Electrochemical | Optogenetics | EIM | ||
| Properties | Real time | ✓ | ✗ | ✗ | ✓ | ✓ |
| Real-life applicability | ✓ | ✓ | ✗ | ✗ | ✓ | |
| Selective stimulation/blocking of large/small axons | ✗ | ✗ | ✗ | ✗ | ✓ | |
| Stimulation of single neuron | ✗ | ✗ | ✗ | ✓ | ✗ | |
| Latency (delay) | ✗ | ✓ | ✓ | ✗ | ✗ | |
| Side effects | ✗ | ✓ | ✗ | ✓ | ✗ | |
| High-power consumption | ✓ | ✗ | ✗ | ✗ | ✗ | |
| High temporal resolution | ✓ | ✗ | ✗ | ✓ | ✓ | |
In addition to the differences demonstrated in Table 1, another fundamental novelty of EIM is its working principle. Electrical methods apply AC and perform just electrical modulation, chemical methods utilize pharmacological agents for chemical modulation, and optogenetics executes chemical modulation via light. Electrochemical methods separate electrical and chemical modulation. These methods apply DC for chemical modulation and AC for electrical modulation. EIM does not separate electrical and chemical modulation. EIM applies pulsed DC and performs electrical and chemical modulation at the same time. In addition to the working principle, EIM also exhibits properties that the other neuromodulation methods do not demonstrate. Electrochemical methods only presented ion concentration depletion. EIM is the only method that demonstrated ion concentration depletion and enhancement around the nerve practically.
In conclusion, we developed a technique, EIM, where we used an interface to both deplete and enhance the Ca2+ ion concentration around the sciatic nerve in vivo in real time and changed the excitability of the neural membrane environment using flexible electrodes. We achieved better control over Vm compared with electrical stimulation and hence decreased the current needed for stimulating or blocking the nerve. Thus, we obtained more precise force control and better selectivity; thereby EIM could open new avenues in the field of neuromodulation. The discovery of Hodgkin and Huxley (Hodgkin and Huxley, 1952) action potential is a result of the ion concentration change around the nerve and has not been directly applied to daily life due to lack of technology. By utilizing their discovery, EIM has the potential to be a paradigm-shifting method for treatment of neurological disorders. EIM may be used in the development of neuroprosthetic devices for paralysis (Ho et al., 2014), deep brain stimulation for Parkinson disease (De Hemptinne et al., 2015), stimulating devices for inducing nerve regeneration (van den Brand et al., 2012), and inhibition of nerve activation for migraine (Akerman et al., 2006) and may also play a role in therapy of other neurological disorders. If one achieves the use of EIM in other parts of the nervous system, such as in the brain, holistic control over the motion, sensation, and behavior can be obtained.
Limitations of the Study
In this work, we demonstrated a neuromodulation method, EIM, which is the most superior neuromodulation method for real-life applications. We performed in vivo experiments, but we did not observe EIM experiment subjects for months. Future studies should be focused on long-term observations to prepare EIM method for clinical research.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by The Scientific and Technological Research Council of Turkey (TUBITAK) 113S081 grant. We thank to Y. Tatar, A. Şahin, and F. Tokgöz for their help in experiments. We also thank to U. Bağcı for critical reading of the manuscript.
Author Contributions
R.M. conceived the idea, designed, and supervised the project. Z.S., S.Ş., B.A., Ç.E., and R.M. designed experiments. Z.S., S.Ş., F.M.B.E., U.Ç.E., E.N.Ş., B.Ş., and M.M. performed experiments. Z.S., S.Ş., and R.M. analyzed data. R.M wrote the manuscript and Z.S, S.Ş, F.M.B.E., U.Ç.E., and Ç.E. contributed to its editing.
Declaration of Interests
R.M. has a pending patent application (TR Patent: 2019/08,576) related to this work. The other authors declare no conflict of interest.
Published: July 26, 2019
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2019.06.038.
Data and Code Availability
Data generated in this study will be provided upon reasonable request to the lead contact, Rohat Melik (rmelik@etu.edu.tr).
LabVIEW was accessed at http://www.ni.com/sv-se/shop/labview.html (identifier: RRID:SCR_014325); Origin at https://www.originlab.com; and LabScribe at https://www.iworx.com/research/software/labscribe/.
Supplemental Information
References
- Akerman S., Holland P.R., Goadsby P.J. Cannabinoid (CB1) receptor activation inhibits trigeminovascular neurons. J. Pharmacol. Exp. Ther. 2006;320:64–71. doi: 10.1124/jpet.106.106971. [DOI] [PubMed] [Google Scholar]
- Armstrong C.M., Cota G. Calcium block of Na+ channels and its effect on closing rate. Proc. Natl. Acad. Sci. U S A. 1999;96:4154–4157. doi: 10.1073/pnas.96.7.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhadra N., Chae J. Implantable neuroprosthetic technology. NeuroRehabilitation. 2009;25:69–83. doi: 10.3233/NRE-2009-0500. [DOI] [PubMed] [Google Scholar]
- Boger A.S., Bhadra N., Gustafson K.J. High frequency sacral root nerve block allows bladder voiding. Neurourol. Urodyn. 2012;31:677–682. doi: 10.1002/nau.21075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton C.E., Shaikhouni A., Annetta N.V., Bockbrader M.A., Friedenberg D.A., Nielson D.M., Sharma G., Sederberg P.B., Glenn B.C., Mysiw W.J. Restoring cortical control of functional movement in a human with quadriplegia. Nature. 2016;533:247–250. doi: 10.1038/nature17435. [DOI] [PubMed] [Google Scholar]
- Boyden E.S., Zhang F., Bamberg E., Nagel G., Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005;8:1263–1268. doi: 10.1038/nn1525. [DOI] [PubMed] [Google Scholar]
- van den Brand R., Heutschi J., Barraud Q., DiGiovanna J., Bartholdi K., Huerlimann M., Friedli L., Vollenweider I., Moraud E.M., Duis S. Restoring voluntary control of locomotion after paralyzing spinal cord injury. Science. 2012;336:1182–1185. doi: 10.1126/science.1217416. [DOI] [PubMed] [Google Scholar]
- Butler A.B., Hodos W. Second Edition. John Wiley & Sons; 2005. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. [Google Scholar]
- Campbell N.A., Reece J.B., Urry L.A., Cain M.L., Wasserman S.A., Minorsky P.V., Jackson R.B. Tenth Edition. Essex: Pearson Education Limited; 2015. Biology A Global Approach. [Google Scholar]
- Choi C.Y. Chronic pain and opiate management. Dis. Mon. 2016;62:334–345. doi: 10.1016/j.disamonth.2016.05.013. [DOI] [PubMed] [Google Scholar]
- Elefteriades J.A., Quin J.A., Hogan J.F., Holcomb W.G., Letsou G.V., Chlosta W.F., Glenn W.W. Long-term follow-up of pacing of the conditioned diaphragm in quadriplegia. Pacing Clin. Electrophysiol. 2002;25:897–906. doi: 10.1046/j.1460-9592.2002.00897.x. [DOI] [PubMed] [Google Scholar]
- Foutz T.J., Ackermann D.M., Jr., Kilgore K.L., McIntyre C.C. Energy efficient neural stimulation: coupling circuit design and membrane biophysics. PLoS One. 2012;7:e51901. doi: 10.1371/journal.pone.0051901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridman G.Y., Della Santina C.C. Safe direct current stimulation to expand capabilities of neural prostheses. IEEE Trans. Neural Syst. Rehabil. Eng. 2013;21:319–328. doi: 10.1109/TNSRE.2013.2245423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao W., Emaminejad S., Nyein H.Y.Y., Challa S., Chen K., Peck A., Fahad H.M., Ota H., Shiraki H., Kiriya D. Fully integrated wearable sensor arrays for multiplexed in situ perspiration analysis. Nature. 2016;529:509–514. doi: 10.1038/nature16521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilron I., Baron R., Jensen T. Neuropathic pain: principles of diagnosis and treatment. Mayo Clin. Proc. 2015;90:532–545. doi: 10.1016/j.mayocp.2015.01.018. [DOI] [PubMed] [Google Scholar]
- Harris J.D. Management of expected and unexpected opioid-related side effects. Clin. J. Pain. 2008;24:S8–S13. doi: 10.1097/AJP.0b013e31816b58eb. [DOI] [PubMed] [Google Scholar]
- De Hemptinne C., Swann N.C., Ostrem J.L., Ryapolova-Webb E.S., San Luciano M., Galifianakis N.B., Starr P.A. Therapeutic deep brain stimulation reduces cortical phase-amplitude coupling in Parkinson's disease. Nat. Neurosci. 2015;18:779–786. doi: 10.1038/nn.3997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho C.H., Triolo R.J., Elias A.L., Kilgore K.L., DiMarco A.F., Bogie K., Vette A.H., Audu M.L., Kobetic R., Chang S.R. Functional electrical stimulation and spinal cord injury. Phys. Med. Rehabil. Clin. N. Am. 2014;25:631–654. doi: 10.1016/j.pmr.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochberg L.R., Bacher D., Jarosiewicz B., Masse N.Y., Simeral J.D., Vogel J., Haddadin S., Liu J., Cash S.S., van der Smagt P. Reach and grasp by people with tetraplegia using a neurally controlled robotic arm. Nature. 2012;485:372–375. doi: 10.1038/nature11076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin A.L., Huxley A.F.A. Quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel E.R., Schwartz J.H., Jessell T.M., Siegelbaum S.A., Hudspeth A.J. Fifth Edition. McGraw-Hill; 2013. Principles of Neural Science. [Google Scholar]
- Kang T.M., Hilgemann D.W. Multiple transport modes of the cardiac Na+/Ca2+ exchanger. Nature. 2004;427:544–548. doi: 10.1038/nature02271. [DOI] [PubMed] [Google Scholar]
- Luan S., Williams I., Nikolic K., Constandinou T.G. Neuromodulation: present and emerging methods. Front. Neuroeng. 2014;7:27. doi: 10.3389/fneng.2014.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazurek K.A., Holinski B.J., Everaert D.G., Stein R.B., Etienne-Cummings R., Mushahwar V.K. Feed forward and feedback control for over-ground locomotion in anaesthetized cats. J. Neural Eng. 2012;9:026003. doi: 10.1088/1741-2560/9/2/026003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melo P.L., Silva M.T., Martins J.M., Newman D.J. Technical developments of functional electrical stimulation to correct drop foot: sensing, actuation and control strategies. Clin. Biomech. 2015;30:101–113. doi: 10.1016/j.clinbiomech.2014.11.007. [DOI] [PubMed] [Google Scholar]
- Memberg W.D., Polasek K.H., Hart R.L., Bryden A.M., Kilgore K.L., Nemunaitis G.A., Hoyen H.A., Keith M.W., Kirsch R.F. Implanted neuroprosthesis for restoring arm and hand function in people with high level tetraplegia. Arch. Phys. Med. Rehabil. 2014;95:1201–1211.e1. doi: 10.1016/j.apmr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Micera S., Keller T., Lawrence M., Morari M., Popovic D.B. Wearable neural prostheses. IEEE Eng. Med. Biol. 2010;29:64–69. doi: 10.1109/MEMB.2010.936547. [DOI] [PubMed] [Google Scholar]
- Nataraj R., Audu M.L., Triolo R.J. Comparing joint kinematics and center of mass acceleration as feedback for control of standing balance by functional neuromuscular stimulation. J. Neuroeng. Rehabil. 2012;9:25. doi: 10.1186/1743-0003-9-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onders R.P., DiMarco A.F., Ignagni A.R., Aiyar H., Mortimer J.T. Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery. 2004;136:819–826. doi: 10.1016/j.surg.2004.06.030. [DOI] [PubMed] [Google Scholar]
- Peckham P.H., Kilgore K.L. Challenges and opportunities in restoring function after paralysis. IEEE Trans. Biomed. Eng. 2013;60:602–609. doi: 10.1109/TBME.2013.2245128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peckham P.H., Knutson J.S. Functional electrical stimulation for neuromuscular applications. Annu. Rev. Biomed. Eng. 2005;7:327–360. doi: 10.1146/annurev.bioeng.6.040803.140103. [DOI] [PubMed] [Google Scholar]
- Plesha M.A., Van Wie B.J., Mullin J.M., Kidwell D.A. Measuring quaternary ammonium cleaning agents with ion selective electrodes. Anal. Chim. Acta. 2006;570:186–194. doi: 10.1016/j.aca.2006.04.024. [DOI] [PubMed] [Google Scholar]
- Ravid E., Prochazka A. Controlled nerve ablation with direct current: parameters and mechanisms. IEEE Trans. Neural Syst. Rehabil. Eng. 2014;22:1172–1185. doi: 10.1109/TNSRE.2014.2307756. [DOI] [PubMed] [Google Scholar]
- Ridge R.M.A.P. The differentiation of conduction velocities of slow twitch and fast twitch muscle motor innervations in kittens and cats. Q. J. Exp. Physiol. Cogn. Med. Sci. 1967;52:293–304. doi: 10.1113/expphysiol.1967.sp001915. [DOI] [PubMed] [Google Scholar]
- Rohde L.M., Bonder B.R., Triolo R.J. Exploratory study of perceived quality of life with implanted standing neuroprostheses. J. Rehabil. Res. Dev. 2012;49:265–278. doi: 10.1682/jrrd.2010.08.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowsky C.L., Hammond E.R., Strohl A.B., Commean P.K., Eby S.A., Damiano D.L., Wingert J.R., Bae K.T., McDonald J.W. Lower extremity functional electrical stimulation cycling promotes physical and functional recovery in chronic spinal cord injury. J. Spinal Cord Med. 2013;36:623–631. doi: 10.1179/2045772313Y.0000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis L.R., Gyawali S., Seres P., Curtis C.A., Chong S.L., Thompson R.B., Mushahwar V.K. Effects of intermittent electrical stimulation on superficial pressure, tissue oxygenation, and discomfort levels for the prevention of deep tissue injury. Ann. Biomed. Eng. 2011;39:649–663. doi: 10.1007/s10439-010-0193-1. [DOI] [PubMed] [Google Scholar]
- Solis L.R., Liggins A., Uwiera R.R.E., Poppe N., Pehowich E., Seres P., Thompson R.B., Mushahwar V.K. Distribution of internal pressure around bony prominences: implications to deep tissue injury and effectiveness of intermittent electrical stimulation. Ann. Biomed. Eng. 2012;40:1740–1759. doi: 10.1007/s10439-012-0529-0. [DOI] [PubMed] [Google Scholar]
- Song Y.A., Melik R., Rabie A.N., Ibrahim A.M.S., Moses D., Tan A., Han J., Lin S.J. Electrochemical activation and inhibition of neuromuscular systems through modulation of ion concentrations with ion-selective membranes. Nat. Mater. 2011;10:980–986. doi: 10.1038/nmat3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theogarajan L. Strategies for restoring vision to the blind: current and emerging technologies. Neurosci. Lett. 2012;519:129–133. doi: 10.1016/j.neulet.2012.02.001. [DOI] [PubMed] [Google Scholar]
- Triolo R.J., Bailey S.N., Miller M.E., Rohde L.M., Anderson J.S., Davis J.A., Jr., Abbas J.J., DiPonio L.A., Forrest G.P., Gater D.R., Jr. Longitudinal performance of a surgically implanted neuroprosthesis for lower-extremity exercise, standing, and transfers after spinal cord injury. Arch. Phys. Med. Rehabil. 2012;93:896–904. doi: 10.1016/j.apmr.2012.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo R.J., Bailey S.N., Miller M.E., Lombardo L.M., Audu M.L. Effects of stimulating hip and trunk muscles on seated stability, posture, and reach after spinal cord injury. Arch. Phys. Med. Rehabil. 2013;94:1766–1775. doi: 10.1016/j.apmr.2013.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triolo R.J., Bailey S.N., Lombardo L.M., Miller M.E., Foglyano K., Audu M.L. Effects of intramuscular trunk stimulation on manual wheelchair propulsion mechanics in 6 subjects with spinal cord injury. Arch. Phys. Med. Rehabil. 2013;94:1997–2005. doi: 10.1016/j.apmr.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vrabec T., Bhadra N., Wainright J., Bhadra N., Franke M., Kilgore K. Characterization of high capacitance electrodes for the application of direct current electrical nerve block. Med. Biol. Eng. Comput. 2015;54:191–203. doi: 10.1007/s11517-015-1385-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E., Erdahl W.L., Hamidinia S.A., Chapman C.J., Taylor R.W., Pfeiffer D.R. Transport properties of the calcium ionophore ETH-129. Biophys. J. 2001;81:3275–3284. doi: 10.1016/S0006-3495(01)75961-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss T.F. Vol. 2. MIT press; 1996. (Cellular Biophysics: Electrical Properties). [Google Scholar]
- Wenger N., Moraud E.M., Gandar J., Musienko P., Capogrosso M., Baud L., Le Goff C.G., Barraud Q., Pavlova N., Dominici N. Spatiotemporal neuromodulation therapies engaging muscle synergies improve motor control after spinal cord injury. Nat. Med. 2016;22:138–145. doi: 10.1038/nm.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitwam J.G., Kidd C. The use of direct current to cause selective block of large fibres in peripheral nerves. Br. J. Anaesth. 1975;47:1123–1132. doi: 10.1093/bja/47.11.1123-b. [DOI] [PubMed] [Google Scholar]
- Wu G.A., Lombardo L., Triolo R.J., Bogie K.M. The effects of combined trunk and gluteal neuromuscular electrical stimulation on posture and tissue health in spinal cord injury. PM R. 2013;5:688–696. doi: 10.1016/j.pmrj.2013.03.025. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Ca2+ ion attraction experiment results; 10 μA current was applied for 65 s, and a picture was taken in every 5 s. We created this video by compiling these pictures and demonstrating them at 500-ms intervals. Fluo-4 AM emission was increased with current application. This experiment proves that we attracted Ca2+ ions using ISM.
Ca2+ ion repulsion experiment results; 10 μA current was applied for 80 s, and a picture was taken in every 5 s. We created this video by compiling these pictures and demonstrating them at 500-ms intervals. Fluo-4 AM emission was decreased with current application. This experiment proves that we repulsed Ca2+ ions using ISM.
Data Availability Statement
Data generated in this study will be provided upon reasonable request to the lead contact, Rohat Melik (rmelik@etu.edu.tr).
LabVIEW was accessed at http://www.ni.com/sv-se/shop/labview.html (identifier: RRID:SCR_014325); Origin at https://www.originlab.com; and LabScribe at https://www.iworx.com/research/software/labscribe/.