Abstract
The literatures on episodic memory for self-referential and emotional information have proceeded relatively independently, and most studies examining the effects of age on these memory processes have been interpreted within domain-specific frameworks. Yet there is increasing evidence for shared mechanisms that contribute to episodic memory benefits in these two domains. We review this evidence and propose a model that incorporates overlapping as well as domain-specific contributions to episodic memory encoding for self-referential and emotional material. We discuss implications for understanding older adults’ relatively intact memory for these classes of stimuli, and conclude with suggestions for future research to test key tenets and extensions of this shared-process model.
Keywords: self, emotion, encoding, memory, aging, socioemotional
Connection between Self and Emotion
Not all information is equally likely to be remembered, and extensive research has demonstrated that individuals are more likely to remember information that elicits emotion [1–4] or that is self-relevant [5–7]. Although some of these memory benefits may reflect memory storage processes that unfold over time, at least some of these memory benefits arise from the way that emotional or self-referential information is initially processed and encoded into memory [4, 7]. Here we propose that emotional or self-referential information engages overlapping processes that are beneficial to memory encoding, increasing the likelihood that information is retained in memory.
Many before us have written about the overlap between the experience of emotion and self-relevance [e.g., 8, 9–11]. It is hard to envision real-world scenarios in which emotion is elicited but the event contains no self-importance, and some theories of emotion dictate that self-relevance must be present in order for emotion to arise [e.g., 12]. The subjective self-relevance of information can dramatically affect both the magnitude of emotion experienced [13, 14] and the specific emotion that is experienced, and interactions between self-relevance and emotion are central to many appraisal theories of emotion [15]. Emotional states can also influence self-referential processing; for instance, depression reduces the positivity of one’s self-view and increases self-focus via rumination [16, 17]. Self-referencing and emotion additionally have been linked together in the study of conditioned fear and emotional learning, with the processes by which a neutral stimulus acquires emotional salience proposed to be facilitated when stimuli are self-relevant [see 18, 19].
While these theories propose links between the experience of emotion and self-relevance, they make no predictions about whether this overlap would be beneficial to memory, disruptive to memory, or have no effect on memory. Relatively less research has examined the links between emotion and self-relevance in episodic memory (see Glossary), or the ability for individuals to remember specific events in a detailed or context-rich manner, yet the extant research suggests that self and emotion can be tightly coupled in that realm as well. Autobiographical memories of events from our pasts provide one illustration of this overlap, as the memories we consider self-defining often include highly emotional experiences such as triumphs or times of distress, and these emotional events tend to be re-experienced more vividly than memories of other events [20]. Moreover, remembering these self-relevant past events can impact our current emotional experiences. For example, remembering positive autobiographical memories can be rewarding [21] and can boost one’s mood [22]; in depressed individuals, these beneficial effects are strongest when memories are in line with one’s current self-view [23].
One ambiguity with autobiographical memories is that it is challenging to separate the encoding of these events from their retrieval: typically, memories that are emotional or self-referential at the time of experience retain these characteristics at the time they are remembered. Yet, at each phase of memory, our goals and motivations shape when and how we allocate cognitive resources and can influence which events come to mind and which details we remember [20, 24]. As we outline below, recent research has suggested overlap in the processes engaged for the encoding of emotional and self-referential material into episodic memory. The processes that overlap are those that have strong ties to the ability to encode information into memory. Bolstered by this evidence, we propose a shared-processes model for the encoding of emotional and self-referential material into memory. This model proposes that not only are there processes that are shared between self-referential and emotional material but that these shared processes are instrumental for the encoding of this material into memory. Thus, these processes will be engaged more for information that will be successfully remembered than for information that will be subsequently forgotten. Especially over relatively short delays, these shared processes may predominate in explaining the detailed and accurate retrieval of memory for this material. We focus on encoding for a few reasons. First, in nearly all of the research that has been conducted, the self-relevance or emotionality of the material is introduced at encoding; rarely is self-relevance or emotion revealed at a later time-point. Second, even in paradigms in which self-relevance is not mentioned again at retrieval or neutral cues are used to trigger memories of emotional events, the memory effects remain, suggesting the importance of encoding processes engaged during the event.
Benefits of a Shared Process Model
Up until now, the literatures on how self-referential and emotional information is encoded into episodic memory have proceeded relatively independently. This separation may seem surprising; if self-relevance modulates an emotional response as proposed by appraisal theory, then it might be expected that self-relevance and emotion would be linked in processing from that point forward. While plausible, this perspective has not had a large influence on the emotional episodic memory literature [although see 14]. As has been typical in the episodic memory literature, terms like “emotional information” have been used as a shorthand to denote content in the environment that elicits a rapid change in the internal, affective state of the organism, whether or not it elicits a subjective feeling of emotion, and “emotional memory” has been used to refer to an individual’s ability to remember this information when explicitly asked to do so. Indeed, laboratory studies of episodic memory for self-referential or emotional material typically have attempted to force dissociations between the constructs—for example, asking participants to view emotional content void of autobiographical context, or to process non-emotional objects or words through a lens of self-relevance. This separate study of episodic memory for self-referential and emotional material has led to important discoveries and insights. For example, relating information to the self, such as by considering whether a word is descriptive of oneself, enhances memory compared to making the same decision in reference to another person [5]. These mnemonic benefits appear to be separable from semantic “depth of processing” effects [25], based on a dissociation in the regions of prefrontal cortex engaged during successful encoding under judgments of self-reference compared to other semantic conditions [6]. In the domain of emotion, arousing information tends to be remembered more than non-arousing information [1], especially over time [e.g., 2], and mechanistic accounts have revealed that high-arousal emotion triggers amygdala modulation of memory storage [3] and biases processing toward high-priority information [4]. There are also some unique aspects of the memory benefits to be gleaned from self-referential and emotional processing, as the motor component of moving an object closer to oneself is sufficient to induce self-reference benefits in memory [26] and the time-dependent role of the amygdala in modulating memory storage appears to be specific to high-arousal emotion [27]. While these discoveries have resulted from domain-specific study of self and emotion, there is a different perspective to be gained by taking a step back and considering these literatures together.
This broader approach may be particularly useful for understanding patterns of memory decline and stability with aging. Many theories of cognitive aging propose that age-related decline occurs as a result of changes in basic processes (e.g., sensory, speed-of-processing, cognitive control) that yield declines across many domains [e.g., 28, 29]. There has been less consideration of whether there is a set of shared processes that explain pockets of relative preservation with aging, such as those that occur in the realm of socioemotional episodic memory. Age-related declines in episodic memory are pronounced: As compared to younger adults, older adults have more difficulty remembering details of past events [30, 31], in part because they are less likely than younger adults to effectively encode information into memory [32, 33]. Yet just like younger adults, older adults are more likely to remember information that elicits emotion [34–36] or that is self-referential [37–40]. At least some of the time, the presence of emotion or self-relevance can also buffer against the age-related episodic memory deficits that would otherwise be revealed. We review this evidence and propose a contribution for shared processes to this relative preservation of memory.
For the purposes of comparing self-referential and emotional material, we focus on effects that can be studied on individual trials, rather than based on prior experience or longer-term goals. Thus, we review studies that have examined emotion by presenting affectively valenced or arousing information, and we do not encompass studies focused on mood or emotion-regulation goals. We define self-relevance as relating information to the self in the moment, rather than through autobiographical memory or past experiences. Self-relevance is distinct from self-generation [e.g., 41], in that self-relevance can be invoked through simple judgments or association of information with oneself at one point in time, rather than actively generating rich cues that can aid retrieval of information at a later point in time. Self-relevant processes may plausibly facilitate encoding of other socioemotional information, such as socially-shared information beyond that which is self-referential, such as emotional faces or impressions of others [e.g., 42, 43]. This review focuses on processes unique to the self above and beyond control conditions, which often include judgments of another person, because there is not yet sufficient data for us to do more than speculate on the processes engaged by broader social conditions (see Outstanding Questions).
Outstanding Questions.
How much of the variance in socioemotional memory is accounted for by shared processes, and might this differ for self-referential and emotional processing? In the depicted model, we have lines of equal weights for all paths, but this assumption remains to be tested.
Are there other socioemotional domains that also benefit from these shared factors across the adult lifespan? While we focus on self-referential and emotional encoding, their benefits may extent to other socioemotional domains, such as when to-be-remembered information is socially relevant (e.g., faces or impressions of others) or associated with reward.
What is the full set of shared mechanisms? There may well be other processes beyond those reviewed here that enable effective socioemotional encoding across many classes of stimuli.
What is the specificity of anatomical overlap, across processes and across ages? Can the processes be dissociated in patients with MPFC lesions? It is plausible that there are content or age differences in the voxels that are most predictive of successful encoding or in the patterns of connectivity that correspond with subsequent memory. Whether such distinctions exist, and whether they reflect differences in the functions performed (e.g., neighboring voxels could respond to different classes of stimuli but perform comparable computations), remain important topics for future research.
To what extent do age-related compensatory patterns of reduced neural activity of some regions and over-recruitment of others extend to socioemotional networks? While these age-associated changes have been revealed in many cognitive domains including attention and memory, their extension to socioemotional domains is less clear. More generally, it has been under-investigated to what extent older adults can leverage the relatively preserved processes outlined here in a compensatory manner within domains typically associated with age-related decline.
Support for Overlap between Self-Referential and Emotional Processing
A number of lines of research point to overlap between the processing of self-referential and emotionally arousing information. As presented in Table 1, prioritized attention, sustained attention, elaboration, and refreshing of information have been demonstrated to be mechanisms that are engaged for self-referential and, in separate lines of work, emotionally arousing information. We purport that these are mechanisms that contribute directly to the successful encoding of that information. For example, both types of information capture attention, which enhances encoding processes. Self-related information has been proposed to impact the allocation of attention, with increased saliency granted toward self-relevant information [e.g., 44, 45]. Similarly, emotionally arousing information has been proposed to bias processing such that the arousing information gains priority [e.g., 4, 46, 47]. Relatedly, memory enhancements emerge for information that is emotionally arousing [e.g., 48, 49] or self-relevant [5, see 7 for a review] even when attention is divided [40, 50] [for an exception, see 51]. Moreover, self-referential and emotional material can be encoded even under conditions that should otherwise result in interference and forgetting [52, 53].
Table 1. Mechanisms of Stimulus Encoding.
The table presents the different stages of processing that have been linked to effective encoding and demonstrated, in separately literatures, to be enhanced by self-referencing and emotion.
| Evidence for Self-Referential Benefits | Evidence for Emotion Benefits | |
|---|---|---|
| Prioritized Attention | Self-referential information detected quickly [45; 118] and even when attention directed elsewhere or when presented briefly [119, 120] | Emotional stimuli detected even with divided attention or with damage to attentional networks [121–124] |
| Sustained attention | Self-relevant information is selected for further processing, as indexed by a P300 response [125] and narrowing of spatial attention around the self-relevant stimulus [126] | Emotional arousal biases attention toward high-arousal information and high-arousal emotional information is prioritized for conscious processing [4, 127–131] |
| Elaboration | Linking information to the self enables deep encoding; information judged in relation to, imagined with, or assigned ownership by the self, leads to enhanced memory [108, 109, 112] | Emotional information more likely to be linked to autobiographical or meaning-based concepts, enhancing depth of encoding [34, 132] |
| Refreshing of information | There is little research on this process in regards to episodic memory, though rumination can increase memory for negative, self-related information [133] | Emotional stimuli refreshed and rehearsed selectively, affecting retained content [134–136] |
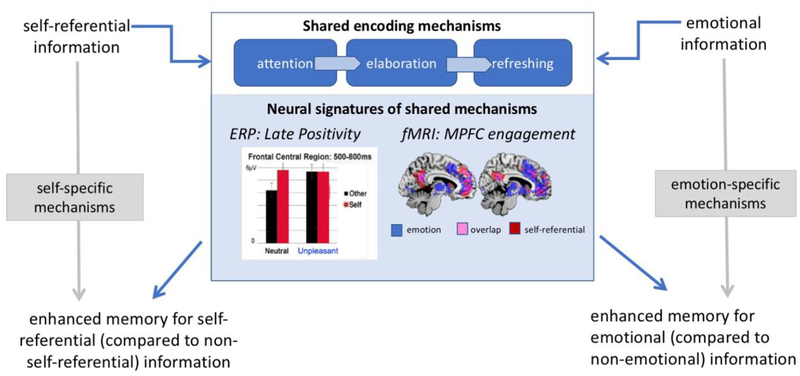
Another line of evidence for overlap in the encoding of emotional and self-relevant information stems from electrophysiological measures (EEG/ERP). The late positive potential (LPP) is an electrophysiological component thought to signify sustained attention and elaborative processing [54–56]. Although the LPP typically reflects a response to emotionally arousing information [54, 57], it also can occur when individuals process valenced [58–62] or even neutral [63] information with a self-referential encoding instruction. Thus, the late positive component is enhanced during the processing of both emotional information and self-referential information. Importantly, these effects may not be additive. For instance, in [63] the LPP was higher in a self-referential condition than in an other-person condition for sentences that contained neutral content, but when the sentences contained emotional content, there was no further modulation of the LPP based on self-relevance (see middle panel of Figure 1). Similarly, the LPP was enhanced for negative compared to neutral information that was not self-referential, but there was no emotional modulation when information was self-referential. These results are consistent with the proposal that the presence of either emotion or self-relevance is sufficient to trigger the shared encoding process measured by the LPP.
Figure 1 (Key Figure). Model of shared encoding mechanisms.
While much of the literature has focused on memory benefits that are proposed to be linked to self-referential processing (at left) or to emotional processing (at right), we propose that shared mechanisms (center panel; see Table 1) account for enhanced encoding of both of these types of information. We propose that neural signatures of these shared mechanisms include the Late Positivity (figure adapted from [63]) and MPFC engagement (figure from Neurosynth [74]).
A related line of evidence emerges from the fMRI literature. Activity in the medial prefrontal cortex (MPFC), a region broadly tied to the organization of information within a goal-relevant framework, also responds to the processing and encoding of self-referential [6, 64] and emotionally arousing information [65–67]. Data from separate studies from our laboratories suggest overlap between the prefrontal regions disproportionately engaged during the processing of emotional information [68–70] and self-referential information [71, 72], and a recent study suggested that MPFC was particularly engaged in encoding for emotional material tied to personal beliefs [73]. Another way of illustrating this overlap between self and emotion draws on the Neurosynth database [74]. This database contains coordinates from a number of fMRI studies. Searching for regions associated with ‘self referential’ processing and ‘emotional’ processing yields substantial overlap, particularly within regions of the MPFC and precuneus (see Figure 1, Key Figure).
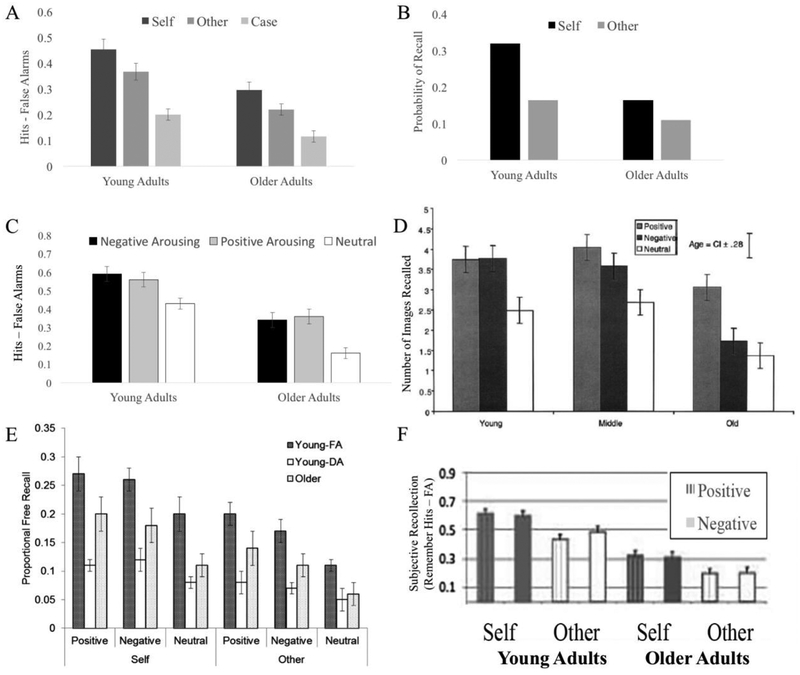
As shown in Figure 2, there are beneficial mnemonic outcomes that stem from relating information to oneself or from encountering information that is positively or negatively valenced. Self-referential processing and emotional valence enhance both recall and recognition and also lead to similar enhancements in memory qualities, most notably, vivid recollection (see example in Figure 2). Emotional memory enhancements often are stronger on tests of recall than recognition, and on tests of recognition, they tend to be reflected by increased recollection; a hallmark of emotional memory is the subjective vividness with which information is remembered [reviewed by 75]. Self-referencing similarly enhances recall and recollection: Tests of recall can show enhanced memory for positive information related to oneself, compared to tests of recognition [11] and encoding personality trait words by judging self-relevance increases recollection, compared to judging whether the word described another person [76, 77]. Although retrieval cues and controlled retrieval processes could account for these findings [11], such self-referential memory enhancements have been argued to reflect the rich integration of the information with existing knowledge structures in long-term memory [76, 77].
Figure 2. Self-referential or emotional processes enhance memory with age.
Older adults’ memories often benefit from self-referential encoding (top row) or from emotional arousal (middle row). These benefits exist when memory is tested via recognition (panels A [38] & C [87]) or recall (panels B [37] & D [137]). The benefits from self-reference and emotion extend when both are combined (panel E [40]) during full attention (FA) or divided attention (DA) for younger adults as well as older adults. Effects of self-reference extend to measures of recollection (panel F [86]) for both positive and negative information of younger and older adults. Note that while the shared process model predicts self x emotion interactions [sub-additivity] for the shared mechanisms themselves (e.g., LPP, MPFC engagement, sustained attention), because of the existence of domain-specific as well as shared processes to the memory enhancements, it does make predictions as to the additivity of self and emotion on the mnemonic outcomes.
Self-Referential and Emotional Memory Rely on Overlapping Processes
Although the literatures on memory for self-referential or emotional information often have proposed domain-specific mechanisms [see 64, 78], similarity in memory patterns suggests the possibility of shared mechanisms. Our Key Figure (Figure 1) presents a model depicting the stages of processing that are relevant to successful encoding and that have been demonstrated to be influenced by both emotion and self-referencing.
We propose that, over relatively short delays, the overlapping processes described above are key contributors to the memory enhancements for both self-referential and emotional information. Thus, we predict that neural signatures of self-referential and emotional processing—including the LPP and MPFC engagement—will not only be present during the encoding of self-referential and emotional material but also relate to memory performance for these stimuli. These shared markers could reflect a prioritized allocation of attention to goal-directed stimuli that is shared by emotional and self-relevant information. Indeed, some work [79] has proposed that the allocation of cognitive resources during encoding can explain many of the effects of emotion on memory over short-term delays. Here, we expand this view by proposing that self-referential and emotional information both capitalize on shared processes not otherwise engaged during event encoding. These processes may adjust the allocation of cognitive resources in favor of successful encoding of self-referential or emotional information. Although other frameworks consider the role of MPFC in appraisal of self-related information [80], to our knowledge this is the first framework to consider the separate and shared contributions of self and emotion as they relate to episodic memory.
Extension to Aging
Motivation has been recognized as an important factor in cognitive aging, with older adults more selective with when they will deploy cognitive resources or with effects of aging on attention and memory emerging in a context-dependent fashion [24, 81, 82]. In addition, successful encoding of emotional and self-relevant processes may rely on neural substrates distinct from the oft-studied processes that decline with aging. For instance, in contrast to robust age-related changes in lateral PFC recruitment during memory encoding, the MPFC processes engaged within socioemotional domains may show relative preservation with age [as reviewed in 83, 84] and may enable older adults to successfully encode these contents into memory. Several studies have demonstrated that enhancements in memory from emotional [34–36] or self-referential [37–40, 85, 86] processes are intact with age, even when older adults exhibit poorer memory overall (Figure 2). The similarity across younger and older adults includes enhanced recall and recollection of emotionally arousing [e.g., 87, 88] or self-referential [e.g., 37, 38] material as well as enhanced recollection of positive words studied self-referentially [86].
Less work has investigated the neural components underlying these effects with age. This is of particular interest, because there is little understanding of how aging affects networks implicated in social abilities. Thus far, fMRI findings indicate that similar neural regions contribute to benefits from self and emotion in younger and older adults. For example, younger and older adults engage MPFC when making judgments and encoding self-referential material into memory [71, 72], or when viewing and encoding valenced information [68–70]. In terms of ERP measures, a handful of ERP studies have investigated the response of the LPP to emotional information with age, finding that the LPP is modulated during younger and older adults’ viewing and encoding of emotional information [89–91], although there can be age differences in the emotional valence of information that is prioritized (see Box 1). To date, no work has integrated self-referential processing into examinations of how emotion and memory impact the LPP across the lifespan.
Box 1: Changing Priorities with Age.
Although the prioritization of emotional and self-referential information over other types of information may be maintained into older adulthood, age may affect the particular aspects of emotional experiences or of self-concept that are prioritized.
Extensive research has revealed that when older adults can engage controlled processes, they are more likely than younger adults to focus on positive rather than negative information and to remember positive rather than negative aspects of past experiences; these age-by-valence interactions have often been referred to as “positivity effects” [reviewed by 106]. There is corresponding electrophysiological evidence for age differences in the valence of emotional stimuli that elicit the largest LPP [89, 90] or the greatest MPFC response during encoding [68–70, 107]. These findings suggest that age affects the valence of stimuli most likely to elicit these shared mechanisms.
Age may also affect the aspects of self that are prioritized. Aging necessitates adaptation, which may lead to identity assimilation [108]. As one example, older adults may think of themselves more relationally, in comparison with others, than younger adults [72], perhaps reflecting the emphasis on personal relationships with age [109]. Yet despite these differences, both young and older adults tend to have positive self-concepts [110], and this can manifest in their cognitive performance. For instance, participants can be more likely to expect positive words in self-referential contexts than negative words [111], to remember positive adjectives processed self-referentially [11], and to self-generate positive personal memories [112]. The self-reference recollection effect also is greater for positively than negatively valenced words [86]. Such effects may emerge due to the tendency for people to hold positive views of the self, with a recent study suggesting this positive self-schema may be linked to representations in MPFC; when Transcranial Magnetic Stimulation (TMS) was used to disrupt MPFC, this disrupted the tendency for individuals to rate themselves more positively than others [113, 114].
Such findings provide indirect support for our claim of a shared mechanism that prioritizes the processing of emotional and self-referential information in cognition and memory. Establishing that the prioritized processing mechanism continues to operate across the lifespan, shared across self and emotion, is an important endeavor. Based on the results thus far, we expect the encoding benefits of emotion and self-referencing to operate in tandem across the lifespan, reflecting underlying shared mechanisms that may be preserved in aging and even in early stages of Alzheimer pathology (see Box 2). It is possible, however, that changes to cognition with age could cause a decoupling of these processes, or that limited processing resources could reveal differential prioritization of self or emotion with age. These alternatives will be adjudicated amongst by directly comparing the mnemonic effects of emotion and self-referencing on the LPP and MPFC activity in both younger and older adults in one unified dataset.
Box 2. Extension to aMCI and AD.
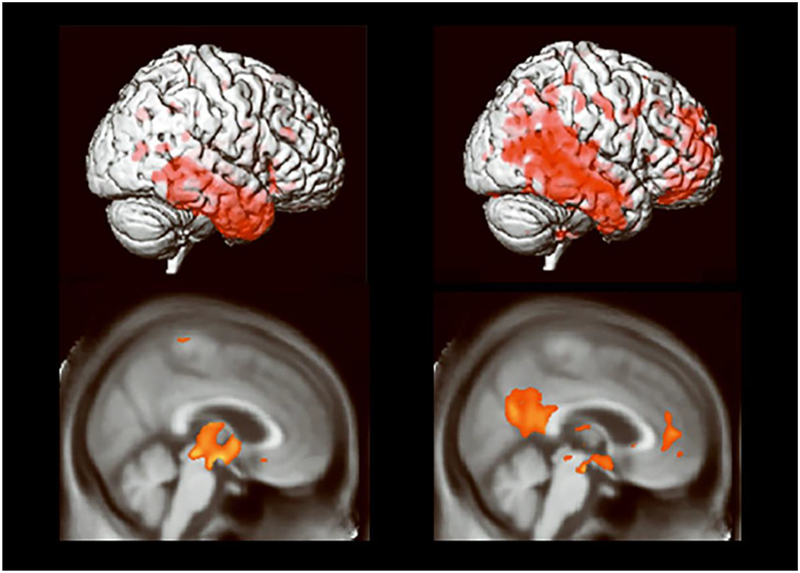
In late stages of Alzheimer’s disease (AD), memory, affective processes, and sense of self are profoundly disrupted. Yet in the early stages, such as aMCI, arousing and self-referential information enhance memory [115, 116]. The ability for aMCI patients to show these mnemonic benefits may be linked to their relatively preserved MPFC. As shown in Figure I, despite extensive cortical thinning in aMCI patients, MPFC does not show significant thinning relative to control participants (left panel; thinning shown by warm colors) and is especially resistant to thinning in those who do not progress quickly to AD (right panel compares those who progress to AD within 18 months to those who do not). Figure is adapted from Figures 4 and 5 in [117]. Indeed, when making judgments about oneself, MPFC is robustly activated by healthy controls and patients with aMCI [117].
The study of populations with memory disruptions allow for a strong test of the association between self-referencing and emotion. If the memory benefits are preserved, and correlated, even in the face of disease-related disruptions to the hippocampal episodic memory system, it provides further evidence for shared processes that dissociate from those typically engaged during episodic memory. Conversely, if pathology creates dissociations between memory for self-relevant and emotional information that are not otherwise seen, this can drive research to investigate the basis for those dissociations.
Concluding Remarks and Future Perspectives
Based on the reviewed evidence, we argue for the promise of considering shared prioritized processing that can enable the encoding of self-referential and emotional material. Markers such as memory enhancement, MPFC activity, and the LPP emerge in both of these domains, and preliminary evidence indicates that these processes may be interconnected in healthy aging as well as in early stages of pathological memory decline, such as in aMCI (amnestic mild cognitive impairment). Uniting the study of self and emotion may inform the broader debate about the role of ventromedial prefrontal cortex. This debate has centered around the extent to which the region contributes to affect valuation versus social cognition [92]; a single shared mechanism that encompasses self and emotion would necessitate reconceptualization of these processes. One caveat is that the neuroimaging results thus far may lack the resolution necessary to distinguish distinct subpopulations of voxels that respond to self or emotion. Applying multivariate methods (e.g., multi-voxel pattern analysis [MVPA]) to these questions will allow for finer-grain resolution of the extent to which young and older adults activate similar patterns of voxels within the broader swath of activation identified based on univariate methods. Even if distinct subpopulations of neurons respond to self or emotion, a high degree of co-localization may cause both processes to be impacted by effects of aging or pathology on the region.
To fully account for the shared mechanisms contributing to self-referencing and emotion effects in memory, it will be necessary to extend the study of the developmental trajectory to early childhood. Whether these mnemonic enhancements develop in tandem, with effects emerging at the same time points, or develop with one process proceeding the other will allow for important insights regarding the relationship between these domains. Similar insights have been gleaned from examining the emergence of self-concept and autobiographical memory in childhood, with this literature suggesting that these abilities are inherently linked [e.g., 93, 94]. Furthermore, adopting a life course perspective will help to illuminate whether these processes remain tightly coupled throughout the lifespan, during life periods important for the formation of self-identity [95] and maintenance of self-continuity [96] and over different developmental trajectories (e.g., the rate of development of neural regions). If memory for self and emotion track across the lifespan, this may reduce the plausibility of an explanation by which the processes occur in distinct but co-localized neural regions.
As we advance understanding of the contributions of shared versus domain-specific processes, extending this model to other memory domains may be illustrative. While some research [97] suggests that emotion and self make separate contributions in a perceptual matching task, revealing limits to their overlap, many of the processes we review may enhance other memory abilities, such as working memory or prospective memory [e.g., 98]. Given the role of MPFC in recollection of self-referenced information [99], and in emotional learning [100], it is also plausible that it can guide recollective processes in other motivationally-relevant domains, even though direct electrical stimulation of this region does not tend to elicit episodic reminiscence [101].
Extending this shared process model to other groups may also elucidate conditions under which the shared-process model accounts for less of the variance and dissociable processes predominate. For example, depression or anxiety may alter the ways in which emotional valence intersects with self-referencing [e.g., 102]. It is also possible that our model may best characterize Western cultures, as they tend to prioritize the self and conceptualize it as an independent entity, separate from others. In contrast, Eastern cultures tend to be more interdependent, conceptualizing of the self as interconnected with others in their social networks [103]. These differences in self-construal could indicate that self-referencing would invoke prioritized processing in Westerners more than Easterners, or that both self and close others would enhance processing in Easterners. Some evidence supports these suggestions [e.g., 104, 105], but the cross-cultural relationships between emotion and self-referencing, particularly in the domain of memory, have not been thoroughly examined.
As few process models exist in socioemotional domains, we hope our working model will encourage research on this important topic (see Outstanding Questions), fleshing out the model and elaborating the contributions of shared processes within socioemotional domains. We think this approach holds promise not only for understanding socioemotional processing more generally but also for specifically advancing our understanding of age-related and disease-related memory changes. An understanding of cognitive aging has been greatly aided by the appreciation of domain-general changes, and neuroscience methods have further emphasized the importance of domain-general changes with aging for cognitive function. The model we propose here may suggest interventions that can be implemented in healthy older adults and those with signs of pathological aging, enabling them to capitalize on socioemotional processes that may circumvent some of the early age-associated loss that occurs within many cognitive domains.
Figure I.
Cortical thinning in aMCI patients.
Acknowledgements
This work was supported by the National Institute on Aging of the National Institutes of Health under award numbers AG051853 and AG055791. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Glossary
- Alzheimer’s disease (AD):
disease associated with progressive deterioration of the brain, with pronounced impairments to memory. Although the neuropathology is distinct from typical aging processes, it affects 10% of adults over the age of 65.
- amnestic mild cognitive impairment (aMCI):
an early stage of age-related memory impairment. Although individuals can live independently at this stage, in some cases it will progress to Alzheimer’s disease.
- amygdala:
region of the brain associated with emotion.
- arousal:
one dimension of an affective response, capturing whether there is a stimulated physiological state such as excitement or anger, as opposed to calmness or boredom.
- depth of processing:
the finding that processing information more deeply, such as focused on its meaning, will lead to better memory.
- encoding:
the set of processes that transform an experience into a representation that can be stored in memory.
- episodic memory:
memories of prior events from one’s life, including laboratory tasks (e.g. studying a list of words).
- hippocampus:
region of the brain associated with long-term memory for prior episodes and facts.
- late positive potential (LPP):
an electrophysiological component that signifies sustained attention and elaborative processing.
- medial prefrontal cortex (MPFC):
a brain region along the midline of the frontal lobes that is associated with self-related processes as well as social cognition and retrieval of memories.
- multi-voxel pattern analysis (MVPA).
An analytical approach used with fMRI data that considers the pattern of activity across a region, as opposed to whether a region activates or not.
- positivity effects:
findings that older adults may attend to and remember positive information better than negative information, or have less pronounced negativity effects than young adults.
- recall:
when memories are retrieved based on internal, self-generated cues.
- recognition:
when memories are retrieved in response to an external cue (e.g., responding to, “did you see this photograph previously?”).
- recollection:
the experience of re-living a prior episode, including being able to retrieve specific details (e.g., for a laboratory study, thoughts or feelings from when the word was encoded). In contrast to familiarity, or a sense of having encountered information previously but lacking the rich re-experiencing.
- socioemotional:
intrapersonal or interpersonal processes that relate to individuals’ emotions, self-concept, and relationships with others.
- retrieval:
when memories are brought to conscious awareness. This encompasses both recognition and recall tests of memory.
- valence:
a dimension of an affective response, capturing whether something is positive or negative.
References
- 1.Eysenck MW (1976) Arousal, Learning, and Memory. Psychological Bulletin 83 (3), 389–404. [PubMed] [Google Scholar]
- 2.Bradley MM et al. (1992) Remembering pictures - Pleasure and arousal in memory. Journal of Experimental Psychology-Learning Memory and Cognition 18 (2), 379–390. [DOI] [PubMed] [Google Scholar]
- 3.McGaugh JL (2004) The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annual Review of Neuroscience 27, 1–28. [DOI] [PubMed] [Google Scholar]
- 4.Mather M and Sutherland MR (2011) Arousal-Biased Competition in Perception and Memory. Perspectives on Psychological Science 6 (2), 114–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogers T et al. (1977) Self-reference and the encoding of personal information. Journal of Personality and Social Psychology 35 (9), 677–688. [DOI] [PubMed] [Google Scholar]
- 6.Kelley WM et al. (2002) Finding the self? An event-related fMRI study. Journal of Cognitive Neuroscience 14 (5), 785–794. [DOI] [PubMed] [Google Scholar]
- 7.Symons CS and Johnson BT (1997) The self-reference effect in memory: A meta-analysis. Psychological Bulletin 121 (3), 371–394. [DOI] [PubMed] [Google Scholar]
- 8.Fossati P et al. (2004) Distributed self in episodic memory: neural correlates of successful retrieval of self-encoded positive and negative personality traits. Neuroimage 22 (4), 1596–1604. [DOI] [PubMed] [Google Scholar]
- 9.Herbert C et al. (2011) Emotional self-reference: brain structures involved in the processing of words describing one’s own emotions. Neuropsychologia 49 (10), 2947–56. [DOI] [PubMed] [Google Scholar]
- 10.Skowronski JJ et al. (2015) Changing the working self alters the emotions prompted by recall. Memory 23 (2), 254–267. [DOI] [PubMed] [Google Scholar]
- 11.D’Argembeau A et al. (2005) Affective valence and the self-reference effect: Influence of retrieval conditions. British Journal of Psychology 96, 457–466. [DOI] [PubMed] [Google Scholar]
- 12.Lazarus RS (1991) Progress on a cognitive-motivational-relational theory of emotion. American Psychologist 46 (8), 819–834. [DOI] [PubMed] [Google Scholar]
- 13.Grezes J et al. (2013) Self-relevance appraisal influences facial reactions to emotional body expressions. PLoS One 8 (2), e55885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezdek K (2003) Event Memory and Autobiographical Memory for the Events of September 11, 2001. Applied Cognitive Psychology 17 (9), 1033–1045. [Google Scholar]
- 15.Scherer KR et al. , eds. (2001) Series in affective science Appraisal processes in emotion: Theory, methods, research, Oxford University Press. [Google Scholar]
- 16.Mezulis AH et al. (2004) Is There a Universal Positivity Bias in Attributions? A Meta-Analytic Review of Individual, Developmental, and Cultural Differences in the Self-Serving Attributional Bias. Psychological Bulletin 130 (5), 711–747. [DOI] [PubMed] [Google Scholar]
- 17.Nolen-Hoeksema S (1991) Responses to depression and their effects on the duration of depressive episodes. Journal of Abnormal Psychology 100 (4), 569–582. [DOI] [PubMed] [Google Scholar]
- 18.Sander D et al. (2003) The human amygdala: an evolved system for relevance detection. Rev Neurosci 14 (4), 303–16. [DOI] [PubMed] [Google Scholar]
- 19.Stussi Y et al. (2015) Learning to fear depends on emotion and gaze interaction: The role of self-relevance in fear learning. Biol Psychol 109, 232–8. [DOI] [PubMed] [Google Scholar]
- 20.Holland AC and Kensinger EA (2010) Emotion and autobiographical memory. Phys Life Rev 7 (1), 88–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Speer ME et al. (2014) Savoring the past: positive memories evoke value representations in the striatum. Neuron 84 (4), 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rusting CL and DeHart T (2000) Retrieving positive memories to regulate negative mood: Consequences for mood-congruent memory. Journal of Personality and Social Psychology 78 (4), 737–752. [DOI] [PubMed] [Google Scholar]
- 23.Werner-Seidler A et al. (2017) The vicissitudes of positive autobiographical recollection as an emotion regulation strategy in depression. Clinical Psychological Science 5 (1), 26–36. [Google Scholar]
- 24.Hess TM (2005) Memory and aging in context. Psychological Bulletin 13 (3), 383–406. [DOI] [PubMed] [Google Scholar]
- 25.Craik FIM and Tulving E (1975) Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General 104 (3), 268–294. [Google Scholar]
- 26.Truong G et al. (2016) Mine in Motion: How Physical Actions Impact the Psychological Sense of Object Ownership. Journal of Experimental Psychology-Human Perception and Performance 42 (3), 375–385. [DOI] [PubMed] [Google Scholar]
- 27.Manns JR and Bass DI (2016) The Amygdala and Prioritization of Declarative Memories. Current Directions in Psychological Science 25 (4), 261–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tucker-Drob EM (2011) Global and Domain-Specific Changes in Cognition Throughout Adulthood. Developmental Psychology 47 (2), 331–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Salthouse TA (2010) Selective review of cognitive aging. J Int Neuropsychol Soc 16 (5), 754–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kausler D (1994) Learning and memory in normal aging, Academic Press. [Google Scholar]
- 31.Koutstaal W and Schacter D (1997) Gist-based false recognition of pictures in older and younger adults. Journal of Memory and Language 37 (4), 555–583. [Google Scholar]
- 32.Craik FIM and McDowd JM (1987) Age differences in recall and recognition. Journal of Experimental Psychology: Learning, Memory, & Cognition 13, 474–479. [Google Scholar]
- 33.Logan JM et al. (2002) Under-recruitment and nonselective recruitment: dissociable neural mechanisms associated with aging. Neuron 33 (5), 827–40. [DOI] [PubMed] [Google Scholar]
- 34.Kensinger EA (2009) How emotion affects older adults’ memories for event details. Memory 17 (2), 208–19. [DOI] [PubMed] [Google Scholar]
- 35.Mather M and Carstensen LL (2005) Aging and motivated cognition: the positivity effect in attention and memory. Trends in Cognitive Sciences 9 (10), 496–502. [DOI] [PubMed] [Google Scholar]
- 36.May CP et al. (2005) Aging, source memory, and emotion. Psychology and Aging 20 (4), 571–578. [DOI] [PubMed] [Google Scholar]
- 37.Mueller JH et al. (1986) Self-referent processing of age-specific material. Psychology and Aging 1 (4), 293–9. [DOI] [PubMed] [Google Scholar]
- 38.Gutchess AH et al. (2007) Ageing and the self-reference effect in memory. Memory 15 (8), 822–37. [DOI] [PubMed] [Google Scholar]
- 39.Glisky E and Marquine M (2009) Semantic and self-referential processing of positive and negative adjectives in older adults. Memory 17 (2), 144–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L et al. (2012) The effects of aging and divided attention on the self-reference effect in emotional memory: Spontaneous or effortful mnemonic benefits? Memory 20, 596–607. [DOI] [PubMed] [Google Scholar]
- 41.Wheeler RL and Gabbert F (2017) Using Self-Generated Cues to Facilitate Recall: A Narrative Review. Front Psychol 8, 1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bublatzky F et al. (2014) Social and emotional relevance in face processing: happy faces of future interaction partners enhance the late positive potential. Front Hum Neurosci 8, 493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cassidy BS and Gutchess AH (2012) Social relevance enhances memory for impressions in older adults. Memory 20 (4), 332–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sui J and Humphreys GW (2017) The ubiquitous self: what the properties of self-bias tell us about the self. Annals of the New York Academy of Sciences 1396 (1), 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sui J and Humphreys GW (2015) The Integrative Self: How Self-Reference Integrates Perception and Memory. Trends Cogn Sci 19 (12), 719–28. [DOI] [PubMed] [Google Scholar]
- 46.Pessoa L (2009) How do emotion and motivation direct executive control? Trends in Cognitive Sciences 13 (4), 160–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vuilleumier P (2015) Affective and motivational control of vision. Current Opinion in Neurology 28 (1), 29–35. [DOI] [PubMed] [Google Scholar]
- 48.Kensinger EA and Corkin S (2004) Two routes to emotional memory: Distinct neural processes for valence and arousal. Proceedings of the National Academy of Sciences of the United States of America 101 (9), 3310–3315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Buchanan TW et al. (2006) The influence of autonomic arousal and semantic relatedness on memory for emotional words. Int J Psychophysiol 61 (1), 26–33. [DOI] [PubMed] [Google Scholar]
- 50.Tacikowski P et al. (2017) Goal-directed processing of self-relevant information is associated with less cognitive interference than the processing of information about other people. Journal of Experimental Social Psychology 68, 93–100. [Google Scholar]
- 51.Turk DJ et al. (2013) Divided attention selectively impairs memory for self-relevant information. Mem Cognit 41 (4), 503–10. [DOI] [PubMed] [Google Scholar]
- 52.Mizrak E and Oztekin I (2016) Relationship Between Emotion and Forgetting. Emotion 16 (1), 33–42. [DOI] [PubMed] [Google Scholar]
- 53.Yang WJ et al. (2013) Directed Forgetting of Negative Self-Referential Information Is Difficult: An fMRI Study. Plos One 8 (10). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cuthbert BN et al. (2000) Brain potentials in affective picture processing: covariation with autonomic arousal and affective report. Biol Psychol 52 (2), 95–111. [DOI] [PubMed] [Google Scholar]
- 55.Sabatinelli D et al. (2007) Emotional perception: correlation of functional MRI and event-related potentials. Cerebral Cortex 17 (5), 1085–91. [DOI] [PubMed] [Google Scholar]
- 56.Schupp HT et al. (2000) Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology 37 (2), 257–61. [PubMed] [Google Scholar]
- 57.Hajcak G et al. (2010) Event-related potentials, emotion, and emotion regulation: an integrative review. Dev Neuropsychol 35 (2), 129–55. [DOI] [PubMed] [Google Scholar]
- 58.Shestyuk AY and Deldin PJ (2010) Automatic and strategic representation of the self in major depression: trait and state abnormalities. Am J Psychiatry 167 (5), 536–44. [DOI] [PubMed] [Google Scholar]
- 59.Herbert C et al. (2011) His or mine? The time course of self-other discrimination in emotion processing. Soc Neurosci 6 (3), 277–88. [DOI] [PubMed] [Google Scholar]
- 60.Herbert C et al. (2011) Self-reference modulates the processing of emotional stimuli in the absence of explicit self-referential appraisal instructions. Soc Cogn Affect Neurosci 6 (5), 653–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fields EC and Kuperberg GR (2016) Dynamic Effects of Self-Relevance and Task on the Neural Processing of Emotional Words in Context. Frontiers in Psychology 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhou HY et al. (2017) Self-Reference Emerges Earlier than Emotion during an Implicit Self-Referential Emotion Processing Task: Event-Related Potential Evidence. Frontiers in Human Neuroscience 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fields EC and Kuperberg GR (2012) It’s All About You: an ERP study of emotion and self-relevance in discourse. Neuroimage 62 (1), 562–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Macrae CN et al. (2004) Medial prefrontal activity predicts memory for self. Cerebral Cortex 14 (6), 647–654. [DOI] [PubMed] [Google Scholar]
- 65.Dolcos F et al. (2004) Dissociable effects of arousal and valence on prefrontal activity indexing emotional evaluation and subsequent memory: an event-related fMRI study. Neuroimage 23 (1), 64–74. [DOI] [PubMed] [Google Scholar]
- 66.Kensinger EA and Schacter DL (2006) Processing emotional pictures and words: Effects of valence and arousal. Cognitive Affective & Behavioral Neuroscience 6 (2), 110–126. [DOI] [PubMed] [Google Scholar]
- 67.Phan KL et al. (2003) Activation of the medial prefrontal cortex and extended amygdala by individual ratings of emotional arousal: a fMRI study. Biol Psychiatry 53 (3), 211–5. [DOI] [PubMed] [Google Scholar]
- 68.Leclerc CM and Kensinger EA (2011) Neural processing of emotional pictures and words: a comparison of young and older adults. Dev Neuropsychol 36 (4), 519–38. [DOI] [PubMed] [Google Scholar]
- 69.Leclerc CM and Kensinger EA (2010) Age-related valence-based reversal in recruitment of medial prefrontal cortex on a visual search task. Social Neuroscience 5 (5–6), 560–76. [DOI] [PubMed] [Google Scholar]
- 70.Leclerc CM and Kensinger EA (2008) Age-related differences in medial prefrontal activation in response to emotional images. Cogn Affect Behav Neurosci 8 (2), 153–64. [DOI] [PubMed] [Google Scholar]
- 71.Gutchess AH et al. (2007) Aging, self-referencing, and medial prefrontal cortex. Social Neuroscience 2 (2), 117–133. [DOI] [PubMed] [Google Scholar]
- 72.Gutchess AH et al. (2015) Age differences in self-referencing: Evidence for common and distinct encoding strategies. Brain Res 1612, 118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wing EA et al. (2018) Neural mechanisms underlying subsequent memory for personal beliefs:An fMRI study. Cogn Affect Behav Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yarkoni T et al. (2011) Large-scale automated synthesis of human functional neuroimaging data. Nat Methods 8 (8), 665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kensinger EA and Schacter DL (2016) Memory and emotion In Handbook of emotion (4th edn) (Lewis M et al. eds), pp. 564–578, Guilford Press. [Google Scholar]
- 76.Conway MA and Dewhurst SA (1995) The self and recollective experience. Applied Cognitive Psychology 9 (1), 1–19. [Google Scholar]
- 77.Conway MA et al. (2001) The self and recollection reconsidered: How a ‘failure to replicate’ failed and why trace strength accounts of recollection are untenable. Applied Cognitive Psychology 15 (6), 673–686. [Google Scholar]
- 78.Hamann S (2001) Cognitive and neural mechanisms of emotional memory. Trends in Cognitive Sciences 5 (9), 394–400. [DOI] [PubMed] [Google Scholar]
- 79.Talmi D (2013) Enhanced Emotional Memory: Cognitive and Neural Mechanisms. Current Directions in Psychological Science 22 (6), 430–436. [Google Scholar]
- 80.Dixon ML et al. (2017) Emotion and the prefrontal cortex: An integrative review. Psychol Bull 143 (10), 1033–1081. [DOI] [PubMed] [Google Scholar]
- 81.Hess TM et al. (2013) Information content moderates positivity and negativity biases in memory. Psychol Aging 28 (3), 853–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Growney CM and Hess TM (in press) Affective influences on older adults’ attention to relevant negative information. Journals of Gerontology: Psychological Sciences. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kensinger EA and Gutchess AH (2015) Memory for emotional and social information in adulthood and old age In The Wiley Handbook on The Cognitive Neuroscience of Human Memory (Duarte A et al. eds), pp. 393–414, Wiley Blackwell. [Google Scholar]
- 84.Kensinger EA and Gutchess AH (2017) Cognitive Aging in a Social and Affective Context: Advances Over the Past 50 Years. J Gerontol B Psychol Sci Soc Sci 72 (1), 61–70. [DOI] [PubMed] [Google Scholar]
- 85.Hamami A et al. (2011) Self-referencing processing and memory specificity with age. Psychology and Aging 26, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Leshikar ED et al. (2015) Self-referencing enhances recollection in both young and older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 22 (4), 388–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kensinger EA (2008) Age differences in memory for arousing and nonarousing emotional words. J Gerontol B Psychol Sci Soc Sci 63 (1), P13–8. [DOI] [PubMed] [Google Scholar]
- 88.Mickley KR and Kensinger EA (2009) Phenomenological characteristics of emotional memories in younger and older adults. Memory 17 (5), 528–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kisley MA et al. (2007) Looking at the sunny side of life: age-related change in an event-related potential measure of the negativity bias. Psychol Sci 18 (9), 838–43. [DOI] [PubMed] [Google Scholar]
- 90.Wood S and Kisley MA (2006) The negativity bias is eliminated in older adults: age-related reduction in event-related brain potentials associated with evaluative categorization. Psychology and Aging 21 (4), 815–20. [DOI] [PubMed] [Google Scholar]
- 91.Langeslag SJ and Van Strien JW (2009) Aging and emotional memory: the cooccurrence of neurophysiological and behavioral positivity effects. Emotion 9 (3), 369–77. [DOI] [PubMed] [Google Scholar]
- 92.Delgado MR et al. (2016) Viewpoints: Dialogues on the functional role of the ventromedial prefrontal cortex. Nat Neurosci 19 (12), 1545–1552. [DOI] [PubMed] [Google Scholar]
- 93.Wang Q (2004) The emergence of cultural self-constructs: autobiographical memory and self-description in European American and Chinese children. Dev Psychol 40 (1), 3–15. [DOI] [PubMed] [Google Scholar]
- 94.Fivush R (2011) The development of autobiographical memory. Annual Review of Psychology 62, 559–582. [DOI] [PubMed] [Google Scholar]
- 95.Rathbone CJ et al. (2008) Self-centered memories: The reminiscence bump and the self. Memory & Cognition 36 (8), 1403–1414. [DOI] [PubMed] [Google Scholar]
- 96.Wolf T and Zimprich D (2016) The distribution and the functions of autobiographical memories: Why do older adults remember autobiographical memories from their youth? Eur J Ageing 13 (3), 241–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stolte M et al. (2017) Dissociating biases towards the self and positive emotion. Q J Exp Psychol (Hove) 70 (6), 1011–1022. [DOI] [PubMed] [Google Scholar]
- 98.Grilli MD and McFarland CP (2011) Imagine that: Self-imagination improves prospective memory in memory-impaired individuals with neurological damage. Neuropsychological Rehabilitation 21 (6), 847–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bergstrom ZM et al. (2015) Reflections of Oneself: Neurocognitive Evidence for Dissociable Forms of Self-Referential Recollection. Cereb Cortex 25 (9), 2648–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guhn A et al. (2014) Medial prefrontal cortex stimulation modulates the processing of conditioned fear. Front Behav Neurosci 8, 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Curot J et al. (2017) Memory scrutinized through electrical brain stimulation: A review of 80 years of experiential phenomena. Neuroscience and Biobehavioral Reviews 78, 161–177. [DOI] [PubMed] [Google Scholar]
- 102.Auerbach RP et al. (2015) Self-referential processing in depressed adolescents: A high-density event-related potential study. J Abnorm Psychol 124 (2), 233–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Markus HR and Kitayama S (1991) Culture and the self: Implications for cognition, emotion, & motivation. Psychological Review 98, 224–253. [Google Scholar]
- 104.Zhu Y et al. (2007) Neural basis of cultural influence on self-representation. Neuroimage 34 (3), 1310–6. [DOI] [PubMed] [Google Scholar]
- 105.Sparks S et al. (2016) Culture modulates implicit ownership-induced self-bias in memory. Cognition 153, 89–98. [DOI] [PubMed] [Google Scholar]
- 106.Mather M (2012) The emotion paradox in the aging brain. Annals of the New York Academy of Sciences 1251 (1), 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Cassidy BS et al. (2013) Valence-based age differences in medial prefrontal activity during impression formation. Social Neuroscience 8 (5), 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sneed JR and Whitbourne SK (2005) Models of the Aging Self. Journal of Social Issues 61 (2), 375–388. [Google Scholar]
- 109.Carstensen LL (1995) Evidence for a life-span theory of socioemotional selectivity. Current Directions in Psychological Science 4 (5), 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Taylor SE and Brown JD (1988) Illusion and well-being: a social psychological perspective on mental health. Psychol Bull 103 (2), 193–210. [PubMed] [Google Scholar]
- 111.Fields EC and Kuperberg GR (2015) Loving yourself more than your neighbor: ERPs reveal online effects of a self-positivity bias. Soc Cogn Affect Neurosci 10 (9), 1202–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hitchcock C et al. (2017) Autobiographical episodic memory-based training for the treatment of mood, anxiety and stress-related disorders: A systematic review and meta-analysis. Clin Psychol Rev 52, 92–107. [DOI] [PubMed] [Google Scholar]
- 113.Kwan VS et al. (2007) Assessing the neural correlates of self-enhancement bias: a transcranial magnetic stimulation study. Exp Brain Res 182 (3), 379–85. [DOI] [PubMed] [Google Scholar]
- 114.Gruberger M et al. (2015) I think therefore I am: Rest-related prefrontal cortex neural activity is involved in generating the sense of self. Conscious Cogn 33, 414–21. [DOI] [PubMed] [Google Scholar]
- 115.Waring JD et al. (2014) Memory for the 2008 presidential election in healthy ageing and mild cognitive impairment. Cognition & Emotion 28 (8), 1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rosa NM et al. (2016) Source Memory for Self and Other in Patients With Mild Cognitive Impairment due to Alzheimer’s Disease. Journals of Gerontology Series B-Psychological Sciences and Social Sciences 71 (1), 59–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Whitwell JL et al. (2008) MRI patterns of atrophy associated with progression to AD in amnestic mild cognitive impairment. Neurology 70 (7), 512–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tong F and Nakayama K (1999) Robust representations for faces: evidence from visual search. J Exp Psychol Hum Percept Perform 25 (4), 1016–35. [DOI] [PubMed] [Google Scholar]
- 119.Moray N (1959) Attention in Dichotic-Listening - Affective cues and the influence of instructions. Quarterly Journal of Experimental Psychology 11 (1), 56–60. [Google Scholar]
- 120.Postman L et al. (1948) Personal values as selective factors in perception. Journal of Abnormal and Social Psychology 43 (2), 142–154. [DOI] [PubMed] [Google Scholar]
- 121.Vuilleumier P and Righart R (2011) Attention and automaticity in processing facial expressions In The Oxford handbook of face perception (Calder AJ et al. eds), pp. 799–820, Oxford University Press. [Google Scholar]
- 122.Christianson SA (1992) Emotional stress and eyewitness memory: a critical review. Psychol Bull 112 (2), 284–309. [DOI] [PubMed] [Google Scholar]
- 123.Pessiglione M et al. (2008) Subliminal instrumental conditioning demonstrated in the human brain. Neuron 59 (4), 561–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pourtois G et al. (2013) Brain mechanisms for emotional influences on perception and attention: What is magic and what is not. Biological Psychology 92 (3), 492–512. [DOI] [PubMed] [Google Scholar]
- 125.Gray HM et al. (2004) P300 as an index of attention to self-relevant stimuli. Journal of Experimental Social Psychology 40 (2), 216–224. [Google Scholar]
- 126.Turk DJ et al. (2011) When “it” becomes “mine”: attentional biases triggered by object ownership. J Cogn Neurosci 23 (12), 3725–33. [DOI] [PubMed] [Google Scholar]
- 127.Amting JM et al. (2010) Multiple mechanisms of consciousness: The neural correlates of emotional awareness. The Journal of Neuroscience 30 (30), 10039–10047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Choi JM et al. (2012) Impact of state anxiety on the interaction between threat monitoring and cognition. NeuroImage 59 (2), 1912–1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Markovic J et al. (2014) Tuning to the significant: Neural and genetic processes underlying affective enhancement of visual perception and memory. Behavioural Brain Research 259, 229–241. [DOI] [PubMed] [Google Scholar]
- 130.Pessoa L (2013) The cognitive-emotional brain: From interactions to integration, MIT Press. [Google Scholar]
- 131.Schupp HT et al. (2006) Emotion and attention: event-related brain potential studies. Prog Brain Res 156, 31–51. [DOI] [PubMed] [Google Scholar]
- 132.Talmi D and Moscovitch M (2004) Can semantic relatedness explain the enhancement of memory for emotional words? Mem Cognit 32 (5), 742–51. [DOI] [PubMed] [Google Scholar]
- 133.Moulds ML et al. (2007) The impact of rumination on memory for self-referent material. Memory 15 (8), 814–21. [DOI] [PubMed] [Google Scholar]
- 134.Liu TL et al. (2017) Selective rehearsal is affected by the emotionality of the encoding context in item-method directed forgetting: An event-related potential study. Biol Psychol 123, 15–24. [DOI] [PubMed] [Google Scholar]
- 135.Van Damme I et al. (2017) Emotion and false memory: How goal-irrelevance can be relevant for what people remember. Memory 25 (2), 201–213. [DOI] [PubMed] [Google Scholar]
- 136.Bernblum R and Mor N (2010) Rumination and emotion-related biases in refreshing information. Emotion 10 (3), 423–32. [DOI] [PubMed] [Google Scholar]
- 137.Charles ST et al. (2003) Aging and emotional memory: The forgettable nature of negative images for older adults. Journal of Experimental Psychology-General 132 (2), 310–324. [DOI] [PubMed] [Google Scholar]