Abstract
Background
Device-detected atrial fibrillation (AF) is associated with increased risk of stroke. However, there are no clearly-defined thresholds of AF burden for which to initiate oral anticoagulation (OAC). We sought to describe OAC prescription practice variation in response to new device-detected AF and the association to outcomes.
Methods
We performed a retrospective cohort study using data from the Veterans Health Administration linked to remote monitoring data that included day-level AF burden. We included patients with cardiac implantable electronic devices (CIED) and remote monitoring from 2011–2014, CHA2DS2-VASc ≥2, and no prior stroke or OAC receipt in the preceding 2 years. We determined the proportion of patients prescribed OAC within 90-days following new device-detected AF across a range of AF thresholds (≥6 minutes to >24 hours), and examined site variation in OAC prescription. We used multivariable Cox proportional hazards regressions to determine the association of OAC prescription with stroke by device-detected AF burden.
Results
Among 10,212 patients with CIEDs, 4,570 patients (45%), 3,969 patients (39%), 3,263 patients (32%), and 2,469 patients (24%) had device-detected AF >6 minutes, >1 hour, >6 hours, and >24 hours, respectively. For device-detected AF >1 hour, 1,712 patients met inclusion criteria (72±10 years; 1.5% female; CHA2DS2-VASc 4.0±1.4; HAS-BLED 2.6±1.1). The proportion receiving OAC varied based on device-detected AF burden (≥6 minutes: 272/2,101 (13%); >1 hour: 273/1,712 (16%); >6 hours: 263/1,279 (21%); >24 hours: 224/818 (27%)). Across 52 sites (N=1,329 patients), there was substantial site-level variation in OAC prescription after device-detected AF >1 hour (median: 16%; range: 3%−67%; median odds ratio: 1.56 [95% credible interval 1.49–1.71]). In adjusted models, OAC prescription after device-detected AF >24 hours was associated with reduced stroke risk (HR 0.28, 95% CI 0.10–0.81, p=0.02), although the propensity-adjusted model was significant when AF lasted at least 6 minutes.
Conclusions
Among Veterans with CIEDs, device-detected AF is common. There is large practice variation in 90-day OAC initiation after new device-detected AF with low rates of treatment overall, even for episodes >24 hours. The strongest association of OAC with reduction in stroke was observed after device-detected AF >24 hours. Randomized trials are needed to confirm these observational findings.
Keywords: atrial fibrillation, cardiac implantable electronic device, stroke, anticoagulation
INTRODUCTION
In patients with clinical atrial fibrillation (AF), oral anticoagulation (OAC) has been shown to prevent stroke,1, 2 with current consensus statements recommending OAC prescription based on clinical risk factors independent of AF pattern (i.e., paroxysmal and non-paroxysmal) or burden.3, 4 However, post-hoc analyses of randomized trials indicate that persistent and permanent AF are associated with a higher risk of stroke than paroxysmal AF, suggesting AF pattern or burden may impact risk5–9, even transiently.10 Although atrial high rate episodes detected by cardiac implantable electronic devices (CIED) lasting 6 minutes or more have been associated with stroke11–13 and are common14, treatment thresholds for initiating OAC are unknown.11–13, 15, 16
Because of the lack of clear evidence on treatment thresholds3, 4, 17, 18 there is potential for practice variation in treatment. We therefore sought to examine practice variation in OAC prescription after device-detected AF, by linking datasets of CIED remote monitoring and data from the Veterans Health Administration, the largest integrated health care system in the United States. Additionally, we examined the association of OAC prescription to stroke by device-detected AF burden.
METHODS
We performed a retrospective cohort study using data from the Department of Veterans Affairs (VA) national health care system. The analytic cohort was created by linking two data sources: 1) administrative and electronic health record data representing the full denominator of VA users; 2) remote monitoring enrollment and daily AF burden data from the device manufacturer. The VA maintains a centralized CIED remote monitoring program, the National Cardiac Device Surveillance Program (NCDSP),19 which voluntarily enrolls patients implanted with CIEDs in the VA system. The NCDSP centralizes and coordinates CIED care for 150 participating VA centers and provides remote monitoring for approximately 50,000 patients. Clinical electronic health records and medical claims data included the VA National Patient Care Database,20 the VA Decision Support System national pharmacy extract,21 the VA Fee Basis Inpatient and Outpatient datasets, the VA Laboratory Decision Support System extract,22 Medicare inpatient and outpatient institutional claims data (part A, part B, and carrier files),23 and the VA Vital Status File that contains validated combined mortality data from VA, Medicare, and Social Security Administration sources.24, 25 Methods for linkage of VA administrative and electronic medical record data have been previously described in detail.26–28 The data, analytic methods, and study materials will not be made available to other researchers for purposes of reproducing the results.
Assessment of Device-Detected AF
We obtained CIED remote monitoring data from the manufacturer’s data warehouse (CareLink® Data Warehousing and Analytics Service; Medtronic plc, Mounds View, MN). Linkage was limited to this single device manufacturer for several reasons. Remote monitoring AF burden is expressed differently across different device manufacturers, remote monitoring services, and remote monitoring data management platforms. For the current analysis, our goal was to evaluate treatment response based on duration of AF episode, using day-level AF burden as a surrogate. The Medtronic CareLink® remote monitoring platform records day-level AF burden data (expressed as number of seconds of AF, and other atrial tachyarrhythmias such as atrial flutter, during a 24-hour calendar day) for the bradycardia and tachycardia platform devices. Day-level AF burden data were not available in most CIEDs from other manufacturers during the observation period. Also, the selected manufacturer represents the majority of CIEDs followed by the VA during the observation period. When quantifying example AF patterns as day-level AF burden, 7 discrete 1-hour episodes of AF during a calendar day are reported as > 6 hours of AF. A continuous 24-hour episode that begins at 10 pm on day 1 and ends at 10 pm on day 2 (i.e., crosses midnight) would be reported as > 1 hour of AF on day 1 and > 12 hours of AF on day 2.
We included patients with CIEDs with 1) enrollment in the VA NCDSP from January 1, 2011 through March 31, 2014, 2) a Medtronic CIED with an atrial lead capable of detecting and transmitting data on atrial tachyarrhythmias (pacemaker or defibrillator, with or without cardiac resynchronization [CRT]) or insertable cardiac monitor (ICM), and 3) at least one day with device-detected AF burden ≥ 6 minutes (atrial rate > 170 beats per minute in most devices). We excluded patients who, in the two years prior to index device-detected AF episode, had 1) a primary diagnosis of stroke (International Classification of Diseases, Ninth Revision [ICD-9]: 433.*, 434.*, 436.*, 437.1) associated with an inpatient VA encounter; 2) a baseline CHA2DS2-VASc score of 0 or 1 (less than class I recommendation for OAC in patients with clinical AF); or 3) OAC prescription (warfarin or non-vitamin K antagonist oral anticoagulants [NOAC]) prescription of at least 30 days.
The primary outcome of interest was OAC prescription (warfarin or NOAC) within 90-days of index episode of device-detected AF with day-level burden > 1 hour. We chose this cutpoint as the primary outcome because of the lack of clear treatment thresholds and based on informal surveys with electrophysiologists and cardiologists regarding conceptual and face validity. We performed a sensitivity analysis on the primary outcome by burden of index device-detected AF episode (≥ 6 minutes, > 6 hours, > 24 hours), with the 6-hour cutpoint intended to replicate recently recommended European Heart Rhythm Association cutpoint of 5.5 hours, based on the TRENDS observational study.18 Patients included for an episode of device-detected AF who at a later date had an episode that exceeded a higher burden cutpoint, were included in analyses for both thresholds with different time 0.
To examine variation in OAC prescription across sites, we aggregated patients by site based on the location of clinical encounters within the 90-days following index device-detected AF > 1 hour. Site was determined according to a uniform set of numeric facility stop codes used to identify site of care across the entire VA health care system. If there was more than one encounter site, then site assignment was prioritized based on the following order: device management or device clinic, outpatient cardiology, inpatient cardiology, non-cardiology, and fee basis care. For the site variation analysis, we excluded patients that could not be mapped to a site, sites that managed < 7 patients with device-detected AF > 1 hour (median number of patients per site), and sites that did not prescribe OAC to any patients (which could be explained by data coding issues or unique exceptions to care processes). We also determined site variation of OAC prescription within 90-days of device-detected AF > 24 hours for sites included in the > 1 hour site variation analysis.
We also determined incidence rates of stroke (ICD-9 codes 433.*, 434.*, or 437.1 as primary diagnosis associated with an inpatient VA, VA fee basis care, or Medicare encounter) and death (using the VA Vital Status File24, 25) by OAC prescription status and device-detected AF burden.
Statistical Methods
For each device-detected AF burden threshold, we then determined the association of OAC prescription within 90 days after device-detected AF and stroke using Cox proportional hazard regression with 1) multivariable adjustment and 2) propensity score adjustment using inverse probability of treatment weights (IPTW). We also performed an interaction analysis between OAC prescription and device-detected AF burden.
For the multivariable analysis, we used Cox proportional hazards models with shared frailty (to adjust for site) and competing risk of death to determine the association of OAC prescription with stroke by burden of device-detected AF. Multivariable models included baseline variables with univariate associations with P < 0.10, including CIED type, age, clinical AF, hypertension, heart failure, myocardial infarction, diabetes, CHADS2 score, niacin/fibrates prescription, and statin prescription.
For the propensity score analysis, we fit shared frailty models (to adjust for site) with competing risk of death using IPTW. Propensity scores were calculated using logistic regression, with conditional probability of OAC prescription within 90 days after device-detected AF episode based on baseline covariates (excluding baseline medications to avoid overfitting). Model fit was assessed by the Hosmer-Lemeshow goodness-of-fit test and C-statistic. Covariate weights were calculated as the inverse of the estimated propensity score for OAC-treated patients and the inverse of one minus the estimated propensity score for untreated patients. Balance diagnostics were assessed using standardized difference in baseline covariates before and after IPTW. A standardized difference after IPTW less than 0.1 is acceptable.
For the interaction analysis between burden of device-detected AF and OAC prescription within 90 days, we used Cox proportional hazard regression with stroke as the dependent variable. We included all eligible device-detected AF events and clustered at the patient level to account for patients who met inclusion for multiple AF burden thresholds. We then determined the significance of the interaction term (device-detected AF and OAC) for each device-detected AF threshold with AF ≥ 6 minutes as the reference.
Baseline characteristics were determined using previously described methods28 and stratified by patient’s CIED type, implantable cardioverter defibrillator (ICD) (including CRT-defibrillator) versus pacemaker (including CRT-pacemaker) and ICM. Differences in baseline characteristic between OAC-treated and untreated patients were assessed with the chi-squared test and 2-sample t-test for categorical and continuous variables, respectively. Calculated HAS-BLED scores exclude labile international normalized ratio component as this is inapplicable across the whole cohort and also cannot be obtained at baseline prior to treatment.30 Time to OAC prescription was measured in days from the index AF episode. To determine which patient and site covariates were associated with OAC prescription after device-detected AF > 1 hour, we used a generalized linear mixed model with random effects, adjusting for age, sex, CHA2DS2-VASc score, and site volume. The median odds ratio was used to quantify the extent of variation across sites on a similar scale that covariate effects are usually expressed, which is determined by comparing OAC prescription for all possible pairs of patients with similar covariates treated at different sites.29
All analyses were performed using SAS, version 9.2 (Cary, NC) and STATA, version 11.0 (College Station, TX). The senior author had full access to all study data and takes responsibility for its integrity and the data analysis. This study was approved by local institutional review board (Stanford, CA) and the VA Research and Development Committee (Palo Alto, CA).
RESULTS
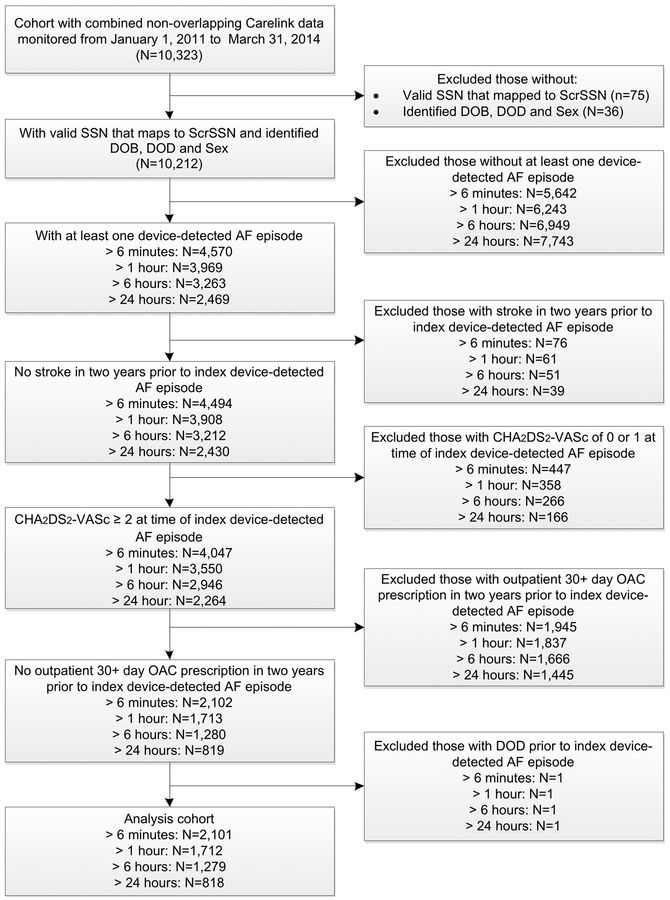
The full cohort included 10,212 patients with CIEDs of which 4,570 patients (45%), 3,969 patients (39%), 3,263 patients (32%), and 2,469 patients (24%) had at least one day where cumulative amount of device-detected AF (day-level burden) exceeded 6 minutes, 1 hour, 6 hours, and 24 hours, respectively. The primary analysis cohort, after exclusion criteria were applied, included 1,712 patients with device-detected AF episode > 1 hour (age 72±10 years; 1.5% female; CHA2DS2-VASc 4.0±1.4; HAS-BLED 2.6±1.1; 1,398 with ICDs; 272 with pacemakers; 42 with ICM) (Figure 1) (Table 1). CHA2DS2-VASc and HAS-BLED scores, by burden of device-detected AF, are reported in Table 2. Within 90-days of index device-detected AF episode > 1 hour, 273 out of 1,712 patients (16%) received OAC prescription, on average, 32±24 days after index device-detected AF episode. The proportion receiving OAC within 90-days of index device-detected AF episode varied by burden (≥ 6 minutes: 272/2,101 (13%); > 6 hours: 263/1,279 (21%); > 24 hours: 224/818 (27%), while average time to OAC prescription after index device-detected AF episode was greater than 30 days for all burdens (Table 2). For patients with device-detected AF > 1 hour, estimated bleeding risk was similar for OAC-treated and untreated patients (Table 1). Patients with and without a prior ICD-9 code diagnosis of AF had similar proportions receive OAC within 90-days of index device-detected AF (Supplemental Table 1).
Figure 1: Cohort Selection Diagram.
Inclusion and exclusion criteria used to select analysis cohort. AF: atrial fibrillation; DOB: date of birth; DOD: date of death; OAC: oral anticoagulation; ScrSSN: Scrambled SSN; SSN: social security number
Table 1:
Baseline Characteristics For Patients with Device-Detected AF > 1 hour
| Total (N=1,712) |
ICD (N=1,398) |
PPM/ICM (N=314) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Demographics | No OAC (N=1,171) |
OAC (N=227) |
P-value* | No OAC (N=268) |
OAC (N=46) |
P-value* | |||
| Age (mean±SD) | 71.5 ± 9.6 | 71.1 ± 9.5 | 68.9 ± 8.4 | 0.001 | 75.1 ± 9.8 | 73.0 ± 8.0 | 0.18 | ||
| Male | 1,686 (98.5%) | 1,159 (99.0%) | 223 (98.2%) | 0.34 | 259 (96.6%) | 45 (97.8%) | 0.67 | ||
| Race | 0.21 | 0.57 | |||||||
| White | 1,467 (85.7%) | 985 (84.1%) | 199 (87.7%) | 243 (90.7%) | 40 (87.0%) | ||||
| Black | 155 (9.1%) | 117 (10.0%) | 21 (9.3%) | 13 (4.9%) | 4 (8.7%) | ||||
| Clinical AF† | 598 (34.9%) | 388 (33.1%) | 85 (37.4%) | 0.21 | 99 (36.9%) | 26 (56.5%) | 0.01 | ||
| Hypertension | 1,411 (82.4%) | 948 (81.0%) | 199 (87.7%) | 0.02 | 225 (84.0%) | 39 (84.8%) | 0.89 | ||
| Heart Failure | 1,239 (72.4%) | 952 (81.3%) | 203 (89.4%) | 0.0031 | 71 (26.5%) | 13 (28.3%) | 0.80 | ||
| Prior Stroke/TIA | 161 (9.4%) | 108 (9.2%) | 17 (7.5%) | 0.40 | 32 (11.9%) | 4 (8.7%) | 0.52 | ||
| Prior MI | 196 (11.5%) | 141 (12.0%) | 38 (16.7%) | 0.05 | 13 (4.9%) | 4 (8.7%) | 0.29 | ||
| Diabetes | 814 (47.6%) | 556 (47.5%) | 129 (56.8%) | 0.001 | 104 (38.8%) | 25 (54.4%) | 0.05 | ||
| Coronary Artery Disease | 1,267 (74.0%) | 933 (69.8%) | 185 (76.8%) | 0.03 | 147 (43.4%) | 25 (50.0%) | 0.38 | ||
| Peripheral Vascular Disease | 182 (10.6%) | 126 (9.4%) | 22 (9.1%) | 0.88 | 27 (8.0%) | 7 (14.0%) | 0.16 | ||
| Charlson Comorbidity Index (mean±SD) | 3.0 ± 1.7 | 3.1 ± 1.7 | 3.4 ± 1.6 | 0.04 | 2.4 ± 1.7 | 2.4 ± 1.9 | 0.99 | ||
| CHADS2 Score (mean±SD) | 2.5 ± 1.2 | 2.5 ± 1.1 | 2.6 ± 1.0 | 0.10 | 2.2 ± 1.2 | 2.3 ± 1.2 | 0.12 | ||
| CHADS2 Score Group | 0.02 | 0.91 | |||||||
| CHADS2 0–1 | 358 (20.9%) | 226 (19.3%) | 42 (17.4%) | 90 (33.6%) | 14 (30.4%) | ||||
| CHADS2 2–3 | 1,091 (63.7%) | 758 (64.7%) | 167 (73.6%) | 141 (52.6%) | 25 (54.4%) | ||||
| CHADS2 4–6 | 263 (15.4%) | 187 (16.0%) | 32 (14.1%) | 37 (13.8%) | 7 (15.2%) | ||||
| CHA2DS2-VASc Score (mean±SD) | 4.0 ± 1.4 | 4.0 ± 1.4 | 4.1 ± 1.3 | 0.42 | 3.7 ± 1.5 | 3.7 ± 1.5 | 0.91 | ||
| HAS-BLED Score (mean±SD)‡ | 2.6 ± 1.1 | 2.6 ± 1.0 | 2.6 ± 1.1 | 0.52 | 2.7 ± 1.0 | 2.8 ± 1.2 | 0.64 | ||
| Baseline Medications§ | |||||||||
| Aspirin | 385 (22.5%) | 257 (22.0%) | 54 (23.8%) | 0.54 | 64 (23.9%) | 10 (21.7%) | 0.75 | ||
| Clopidogrel | 218 (12.7%) | 149 (12.7%) | 28 (12.3%) | 0.87 | 36 (13.4%) | 5 (10.9%) | 0.63 | ||
| ACE-I/ARB | 929 (54.3%) | 632 (54.0%) | 126 (55.5%) | 0.67 | 146 (54.5%) | 25 (54.4%) | 0.99 | ||
| Diuretic | 821 (48.0%) | 561(47.9%) | 112 (49.3%) | 0.69 | 128 (47.8%) | 20 (43.5%) | 0.59 | ||
| Niacin/Fibrates | 157 (9.2%) | 107 (9.1%) | 13 (5.7%) | 0.09 | 34 (12.7%) | 3 (6.5%) | 0.23 | ||
| Statin | 1,000 (58.4%) | 919 (58.2%) | 218 (56.0%) | 0.43 | 152 (56.7%) | 24 (52.2%) | 0.57 | ||
| Class 1 AAD | 22 (1.3%) | 16(1.4%) | 0 | 0.08 | 5 (1.87%) | 1 (2.2%) | 0.89 | ||
| Class 3 AAD|| | 80 (4.7%) | 54 (4.6%) | 8 (3.5%) | 0.47 | 16 (6.0%) | 2 (4.4%) | 0.66 | ||
| Amiodarone | 97 (5.7%) | 66 (5.6%) | 9 (4.0%) | 0.31 | 17 (6.3%) | 5 (10.9%) | 0.27 | ||
| Beta Blockers | 1,041 (60.8%) | 710 (60.6%) | 141 (62.1%) | 0.68 | 165 (61.6%) | 25 (54.4%) | 0.35 | ||
| Calcium Channel Blockers# | 42 (2.5%) | 26 (2.2%) | 7 (3.1%) | 0.43 | 8 (3.0%) | 1 (2.0%) | 0.76 | ||
Differences between OAC-treated and untreated groups assessed using the chi-squared test and 2-sample t-test for categorical and continuous variables, respectively;
At least one encounter with an International Classification of Diseases, Ninth Revision (ICD-9) code for AF (427.31 or 427.32), in the primary or secondary position, in the 2 years prior to index device-detected AF;
Excludes labile international normalized ratio component;
90 days prior to index device-detected AF episode;
Excluding amiodarone;
Non-dihydropyridine
AAD: antiarrhythmic drug; ACE-I: angiotensin converting enzyme inhibitor; AF: atrial fibrillation; ARB: angiotensin receptor blocker; ICD: implantable cardioverter defibrillator; ICM: insertable cardiac monitor; MI: myocardial infarction; OAC: oral anticoagulation; PPM: permanent pacemaker; SD: standard deviation; TIA: transient ischemic attack
Table 2:
Proportion Receiving Oral Anticoagulation by Index Device-Detected AF Episode Burden
| Index Device-Detected AF Episode Burden | ||||
|---|---|---|---|---|
| > 6 Minutes | > 1 Hour | > 6 Hours | > 24 Hours | |
| Included Patients | 2,101 | 1,712 | 1,279 | 818 |
| CHA2DS2-VASc Score (mean±SD) | 3.9 ± 1.4 | 4.0 ± 1.4 | 4.0 ± 1.4 | 4.2 ± 1.4 |
| HAS-BLED Score* (mean±SD) | 2.7 ± 1.1 | 2.6 ± 1.1 | 2.7 ± 1.1 | 2.8 ± 1.1 |
| OAC Prescribed†,‡ | 272 (13.0%) | 273 (16.0%) | 263 (20.6%) | 224 (27.4%) |
| Warfarin Prescribed | 258 (94.9%) | 257 (94.1%) | 246 (93.5%) | 208 (92.9%) |
| NOAC Prescribed | 14 (5.1%) | 17 (6.2%) | 18 (6.8%) | 18 (8.0%) |
| Days from Device-Detected AF to OAC (mean±SD) | 31.2 ± 24.6 | 31.9 ± 24.4 | 30.7 ± 24.0 | 33.3 ± 24.1 |
Excludes labile international normalized ratio component;
Within 90 days of index AF episode;
Warfarin and NOAC prescription are not mutually exclusive within 90-days of index AF episode.
AF: atrial fibrillation; NOAC: non-vitamin K antagonist oral anticoagulants; OAC: oral anticoagulation; SD: standard deviation
In multivariable analysis, CHA2DS2-VASc score ≥4 (reference CHA2DS2-VASc 2–3) weakly trended towards an association with OAC receipt within 90-days of device-detected AF episode > 1 hour (OR 1.3; 95% CI 0.9–1.7; p = 0.14), while every year above the mean patient age was associated with 3% reduction in odds of OAC receipt (OR 0.97; 95% CI 0.95–0.99; p <0.001) (Supplemental Table 2).
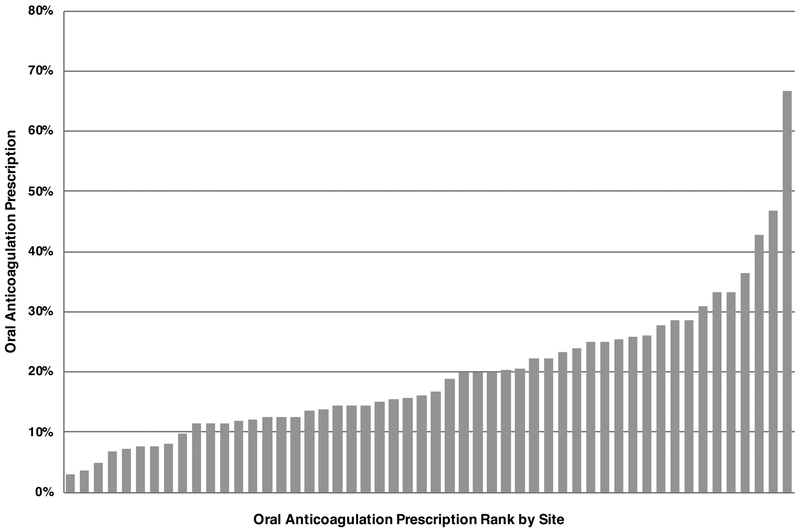
For the site level analysis, 52 sites met inclusion criteria, managing 1,329 patients with device-detected AF > 1 hour. Among these patients, 266 (20%) received OAC within 90-days of device-detected AF episode > 1 hour. The proportion of patients prescribed OAC by site ranged from 3% to 67% with a median odds ratio of 1.56 (95% credible interval 1.49–1.71). The median site OAC prescription proportion was 16% (IQR: 12%−25%), with ten percent of sites with an OAC prescription proportion less than 8% (Figure 2). For the 590 patients with AF > 24 hours managed at the 52 included sites, overall OAC prescription within 90-days of device-detected AF > 24 hours was 31% ranging by site from 0% to 60% (median: 33%; IQR: 19%−44%; 10% of sites < 9%) (Supplemental Figure 1). The median odds ratio for device-detected AF > 24 hours was 1.21 (95% credible interval 1.19–1.23).
Figure 2: Oral Anticoagulation Prescription for Device-Detected Atrial Fibrillation > 1 hour by Site.
Proportion of patients at a site with CHA2DS2-VASc ≥ 2 prescribed oral anticoagulation within 90 days of index device-detected atrial fibrillation > 1 hour ranked in order by sites’ oral anticoagulation prescription proportion (site number = 52).
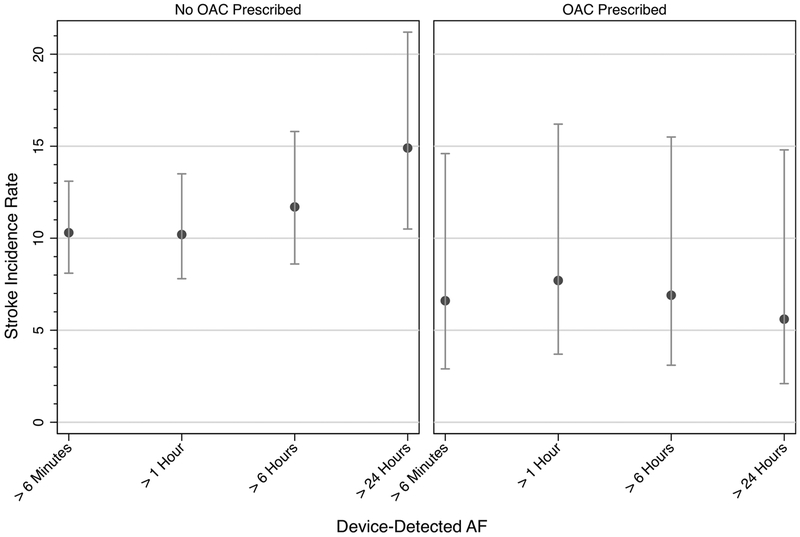
For the 1,712 patients with device-detected AF > 1 hour, the stroke incidence rates for patients who were and were not prescribed OAC were 7.7 (95% CI 3.7–16.2) and 10.2 (95% CI 7.8–16.2) per 1,000 person-years, respectively. With increasing burden of device-detected AF, the incidence rate for stroke numerically increased for patients not prescribed OAC and were similar for patients prescribed OAC (Figure 3) (Table 3). Cox proportional hazard regressions showed OAC prescription associated with greater numeric reductions in stroke risk with increasing burdens of device-detected AF, reaching significance in all models for device-detected AF > 24 hours (multivariable regression: HR 0.28, 95% CI 0.10–0.81, p = 0.02; propensity-adjusted with IPTW: HR 0.27, 95% CI 0.14–0.54, p = 0.0002). In the propensity-adjusted model with IPTW (model fit: p = 0.61, C statistic = 0.62, covariate standardized mean differences ≤ 0.1 after IPTW [Supplemental Table 3]), OAC prescription was also associated with reduced stroke risk when prompted by device-detected AF ≥ 6 minutes and > 6 hours (Table 4). In the analysis including all eligible device-detected AF events, the interaction term for device-detected AF and OAC was significant only when AF exceeded 24 hours. Incidence rates for stroke and death by CIED type are reported in Supplemental Table 4.
Figure 3: Stroke Incidence by Oral Anticoagulation Prescription and Burden of Device-Detected Atrial Fibrillation.
Stroke incidence rate per 1,000 person-years by 1) OAC prescription within 90-days of device-detected AF and 2) index device-detected AF episode (cumulative amount of AF over one day). Error bars represent 95% confidence intervals. AF: atrial fibrillation. OAC: oral anticoagulation.
Table 3:
Incidence of Stroke and Death in Patients with Device-Detected AF by AF Burden and OAC Prescription
| Total | No OAC‡ | OAC‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| N (%) | IR (95% CI) | N (%) | IR (95% CI) | N (%) | IR (95% CI) | P Value§ | |||
| AF > 6 minutes*,† | |||||||||
| Stroke | 72/2,101 (3.4%) | 9.9 (7.8–12.4) | 66/1,829 (3.6%) | 10.3 (8.1–13.1) | 6/272 (2.2%) | 6.6 (2.9–14.6) | 0.28 | ||
| Death | 587/2,101 (27.9%) | 92.5 (85.3–100.3) | 518/1,829 (28.3%) | 93.3 (85.6–101.7) | 69/272 (25.4%) | 87.1 (68.6–110.3) | 0.60 | ||
| AF > 1 hour*,† | |||||||||
| Stroke | 58/1,712 (3.4%) | 9.8 (7.6–12.7) | 51/1,439 (3.5%) | 10.2 (7.8–13.5) | 7/273 (2.6%) | 7.7 (3.7–16.2) | 0.50 | ||
| Death | 503/1,712 (29.4%) | 99.4 (91.1–108.5) | 429/1,439 (29.3%) | 100.4 (91.3–110.3) | 74/273 (27.1%) | 94.4 (75.1–118.5) | 0.63 | ||
| AF > 6 hours*,† | |||||||||
| Stroke | 47/1,279 (3.7%) | 10.7 (8.1–14.3) | 41/1,016 (4.0%) | 11.7 (8.6–15.8) | 6/263 (2.3%) | 6.9 (3.1–15.5) | 0.23 | ||
| Death | 395/1,279 (20.9%) | 106.1 (96.1–117.1) | 324/1,016 (31.9%) | 108.7 (97.5–121.2) | 7½63 (27.0%) | 95.8 (75.9–120.9) | 0.34 | ||
| AF > 24 hours*,† | |||||||||
| Stroke | 35/818 (4.3%) | 12.5 (9.0–17.4) | 31/594 (5.2%) | 14.9 (10.5–21.2) | 4/224 (1.8%) | 5.6 (2.1–14.8) | 0.04 | ||
| Death | 297/818 (36.3%) | 129.0 (115.1–144.5) | 234/594 (39.4%) | 139.3 122.5–158.3) | 63/224 (28.1%) | 101.1 (79.0–129.4) | 0.02 | ||
Device-detected;
Median follow-up time (interquartile range): > 6 minutes: 45.6 (20.9) months; > 1 hour: 44.9 (21.3) months; > 6 hours: 44.8 (21.2) months; > 24 hours: 44.3 (22.1) months;
Within 90 days of index AF episode;
IR differences between No OAC and OAC groups assessed using Fisher’s exact test, mid-P value reported.
AF: atrial fibrillation; CI: confidence interval; IR: incidence rate per 1,000 person-years; OAC: oral anticoagulation
Table 4:
Association of Oral Anticoagulation Prescription to Stroke by Burden of Device-Detected AF
| Unadjusted* | Multivariable Regression*,† | Propensity-Adjusted with IPTW*,‡ | ||||||
|---|---|---|---|---|---|---|---|---|
| Device-detected AF Burden | HR§ (95% CI) | P value | HR§ (95% CI) | P value | HR§ (95% CI) | P value | ||
| AF > 6 minutes | 0.63 (0.27–1.45) | 0.27 | 0.62 (0.26–1.43) | 0.29 | 0.67 (0.45–0.99) | 0.04 | ||
| AF > 1 hour | 0.77 (0.35–1.71) | 0.52 | 0.79 (0.35–1.77) | 0.57 | 0.88 (0.57–1.36) | 0.56 | ||
| AF > 6 hours | 0.57 (0.24–1.33) | 0.19 | 0.52 (0.22–1.25) | 0.14 | 0.59 (0.37–0.95) | 0.03 | ||
| AF > 24 hours | 0.34 (0.12–0.95) | 0.04 | 0.28 (0.10–0.81) | 0.02 | 0.27 (0.14–0.54) | 0.0002 | ||
Cox proportional hazards models with shared frailty (to adjust for site) and competing risk of death;
Multivariable model includes all baseline variables with univariate associations with P < 0.10 (cardiac implantable electronic device type, age, clinical AF, hypertension, heart failure, myocardial infarction, diabetes, CHADS2 score, niacin/fibrates prescription, statin prescription);
Propensity score model fit assessed by Hosmer-Lemeshow goodness-of-fit and C statistic (p = 0.61, C statistic = 0.62). Covariate standardized mean differences reported in Supplemental Table 3;
Risk of stroke when anticoagulation is prescribed within 90 days of device-detected index AF episode.
AF: atrial fibrillation; CI: confidence interval; HR: hazard ratio; IPTW: inverse probability of treatment weights
DISCUSSION
Using health care system data linked to CIED remote monitoring, we found that device-detected AF was common, with 39% of patients having at least one day where AF burden exceeded one hour. However, among patients with CHA2DS2-VASc ≥ 2, OAC prescription within 90-days of device-detected AF episodes was low overall, with substantial variation across sites and across thresholds of index AF episode burden. With increasing burden of device-detected AF, the incidence rate for stroke numerically increased for patients not prescribed OAC. Importantly, the strongest association of OAC with reduction in stroke was observed when treatment was prompted by AF episodes > 24 hours.
OAC therapy is prescribed for 45 to 70% of patients with a clinical diagnosis of AF depending on the population studied,26, 27, 31 which is well below desired thresholds.32 However, in our cohort, device-detected AF episodes ranging from ≥ 6 minutes to > 24 hours resulted in OAC prescription rates that were substantially lower. Importantly, bleeding risk, based on modified HAS-BLED scores, were similar when comparing OAC-treated and untreated patients, suggesting that high bleeding risk did not drive low rates of OAC prescription in this cohort. There are several potential explanations for observed treatment variation. Consensus statements published in 2014 and 2016 progressed from stating a need for further evidence to a weak recommendation for OAC in rare patients with device-detected AF.3, 4 However, the European Heart Rhythm Association, citing observational data,11–13, 15, 16 recommends a device-detected AF threshold of >5.5 hours for OAC initiation (in patients with CHA2DS2-VASc ≥2).18 The observed treatment variation may fundamentally reflect the lack of high-quality evidence regarding when or at what threshold to treat device-detected AF. This is further confounded by considerations in patients with multiple comorbidities and concomitant use of antiplatelet therapy. The combination of limited overall evidence, variation in guidelines, and unique patient factors may all contribute to the high observed treatment variation.
Low or variable OAC prescription may be due to vulnerabilities in the health care system, such as breakdowns and delays in the communication loop, which includes 1) transmission of a device-detected event, 2) event receipt and review by the managing provider, and 3) delivery of recommendations to the patient. The frequency of scheduled transmissions and events that trigger an unscheduled transmission can be customized at the discretion of the managing provider. Additionally, some devices are not capable of automatic transmissions, with remote transmissions manually sent by the patient, usually in response to symptoms. With reimbursements for remote monitoring services limited to every 90 days by most payers, providers may be discouraged from selecting more frequent transmissions, resulting in clinically relevant delays in care, although this is not a likely factor in the VA system. Additionally, remote monitoring is not a closed loop system as the patient and device do not receive confirmation that the managing provider received and reviewed the transmission, specifically the patient’s burden of AF. Of note, in the VA system, the NCDSP is the designated managing provider for remote transmissions. Accordingly, all included episodes of new device-detected AF were reviewed and triaged by the NCDSP, with designated local site and set of clinicians then notified.
An additional barrier to treatment exists if expedited in-person or telehealth visits to discuss OAC initiation are not available. In our analysis, time from index device-detected AF episode (of any burden) to OAC prescription was on average greater than 30 days, suggesting opportunities to improve the speed of care delivery after index episode. For ICMs, which are underrepresented in our cohort, implanted to screen for actionable AF in real-time,33 these issues are particularly problematic.
Looking forward, recently released Bluetooth-enabled CIEDs are expected to use patients’ own smartphones to remotely transmit events in the near future. This may improve transmission frequency or compliance and could provide the patient confirmation as to whether the transmission was reviewed. This new transmission paradigm also has the potential to give patients their AF burden data, which may have the potential to improve patient engagement, patient-clinician communication, and improved decision making regarding OAC.
Rapid proliferation of wearable health technology, which can already accurately detect AF,34 has expanded remote monitoring beyond patients with a CIED and is now available on retail smartwatches and smartwatch accessories with an arrhythmia notification provided directly to the user. Given remote monitoring’s current lack of expedited closed-loop delivery of information to the patient, there is a potential role for wearables even in patients with CIEDs. Clinical trials of wearable technologies are ongoing.35
The optimal treatment threshold for device-detected AF remains a key evidence gap.7 In the ASSERT cohort, device-detected AF episodes > 24 hours were associated with stroke risk comparable to clinical AF,15 with OAC prescription for this burden associated with large reductions in stroke risk in our analysis. For lower burdens, we also detected reductions in stroke risk in the propensity-adjusted model, suggesting possible benefit to OAC at even lower burden thresholds than 24 hours. Event driven trials investigating optimal OAC strategies for device-detected AF are ongoing,36, 37 with feasibility of implementing CIED-guided NOAC strategies for AF already demonstrated.38
Our study has several limitations. The study is observational and is vulnerable to measured and unmeasured confounding by treatment selection, therefore limiting causal inference. The majority of devices were ICDs, which reflects the fact that daily AF burden was first implemented on the CIED vendor’s ICD platform and is only present in newer pacemaker models. Third, the number of stroke events in OAC-treated patients was low, leading to greater uncertainty in risk ratio point estimates. Fourth, this is a Veteran cohort that is overwhelmingly male and findings may not generalize to women or non-Veterans. Fifth, treatment effects may not be consistent 1) across CIEDs from other manufacturers, especially if there are systematic differences in calculation of AF burden; 2) in patients whose device’s atrial rate threshold for AF diagnosis has been lowered; and 3) when AF is described by duration, as opposed to day-level AF burden. Sixth, false positive device-detected AF episodes are described, particularly for lower burdens of AF, which may have contributed to low rates of OAC prescription. Finally, with an integrated health care system that utilizes a centralized CIED remote monitoring system, these results from the VA may not generalize the degree of variation that could be observed in other health care systems.
In conclusion, we found large practice variation in 90-day OAC initiation after new device-detected AF, even for episodes > 24 hours. Importantly, OAC was prescribed for device-detected AF substantially less often than previously reported for clinical AF. Across increasing burdens of AF, the risk of stroke in untreated patients numerically increased. The strongest association of OAC with reduction in stroke was observed after device-detected AF episodes > 24 hours. Randomized trials are needed to confirm these observational findings.
Supplementary Material
CLINICAL PERSPECTIVE
1). What is new?
Among Veterans with cardiac implantable electronic devices, device-detected atrial fibrillation is common.
There is large practice variation in 90-day oral anticoagulation initiation after new device-detected atrial fibrillation with substantially lower treatment rates as compared to clinical atrial fibrillation.
In patients not prescribed oral anticoagulation, stroke incidence increased as burden of device-detected atrial fibrillation increased.
The strongest association of oral anticoagulation with reduction in stroke was observed after device-detected atrial fibrillation > 24 hours.
2). What are the clinical implications?
Large variation in prescription of oral anticoagulation after device-detected atrial fibrillation highlights the uncertainty in optimal treatment strategies, failure to effectively utilize available clinical data sources, or both.
Higher stroke incidence with increasing burden of device-detected atrial fibrillation, in patients not anticoagulated, supports treating atrial fibrillation as a non-binary entity.
Although confirmation of treatment benefit awaits results from ongoing randomized studies, observational data supports initiation of oral anticoagulation after device-detected atrial fibrillation > 24 hours.
ACKNOWLEDGEMENTS
Jason P. Bentley Ph.D. (Quantitative Sciences Unit, Stanford University School of Medicine, USA) consulted on statistical methods.
FUNDING SOURCES
The work was supported by an American Heart Association Precision Medicine research grant.
DISCLOSURES
A.C. Perino: None. J. Fan: None. M. Askari: None. P.A. Heidenreich: None. E. Keung: None. M. Raitt: None. J. Piccini: Research Grant; Significant; Abbott, ARCA biopharma, Boston Scientific, Gilead, and Janssen Pharmaceuticals. Consulting; Significant; GSK, Medtronic plc., and Phillips. Consulting; Modest: Abbott, Allergan, ARCA biopharma, Bayer, Biotronik, Motif Bio, and Sanofi. P.D. Ziegler: Employee and Shareholder: Medtronic plc. M. Turakhia: Research Grant; Significant; Janssen Pharmaceuticals, Medtronic plc., AstraZeneca, Veterans Health Administration, Cardiva Medical Inc. Other Research Support; Modest; AliveCor Inc., Amazon, Zipline Medical plc., iBeat Inc., iRhythm Technologies Inc. Honoraria; Significant; Abbott. Honoraria; Modest; Medtronic plc., Boehringer Ingelheim, Precision Health Economics, iBeat Inc., Akebia, Cardiva Medical Inc., Medscape/theheart.org.
REFERENCES
- 1.Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L, Committee R-LS and Investigators. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. [DOI] [PubMed] [Google Scholar]
- 2.Go AS, Hylek EM, Chang Y, Phillips KA, Henault LE, Capra AM, Jensvold NG, Selby JV, Singer DE. Anticoagulation therapy for stroke prevention in atrial fibrillation: how well do randomized trials translate into clinical practice? JAMA. 2003;290:2685–2692. [DOI] [PubMed] [Google Scholar]
- 3.January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW, ACC/AHA Task Force Members. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation. 2014;130:e199–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P, Agewall S, Camm J, Baron Esquivias G, Budts W, Carerj S, Casselman F, Coca A, De Caterina R, Deftereos S, Dobrev D, Ferro JM, Filippatos G, Fitzsimons D, Gorenek B, Guenoun M, Hohnloser SH, Kolh P, Lip GY, Manolis A, McMurray J, Ponikowski P, Rosenhek R, Ruschitzka F, Savelieva I, Sharma S, Suwalski P, Tamargo JL, Taylor CJ, Van Gelder IC, Voors AA, Windecker S, Zamorano JL, Zeppenfeld K. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893–2962. [DOI] [PubMed] [Google Scholar]
- 5.Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M, Avezum A, Diaz R, Hohnloser SH, Lewis BS, Shestakovska O, Wang J, Connolly SJ. Risk of ischaemic stroke according to pattern of atrial fibrillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J. 2015;36:281–287a. [DOI] [PubMed] [Google Scholar]
- 6.Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Garcia D, Ezekowitz J, Alings M, Yang H, Alexander JH, Flaker G, Hanna M, Granger CB. Outcomes of apixaban vs. warfarin by type and duration of atrial fibrillation: results from the ARISTOTLE trial. Eur Heart J. 2013;34:2464–2471. [DOI] [PubMed] [Google Scholar]
- 7.Chen LY, Chung MK, Allen LA, Ezekowitz M, Furie KL, McCabe P, Noseworthy PA, Perez MV, Turakhia MP, American Heart Association Council on Clinical Cardiology, Council on Cardiovascular and Stroke Nursuing, Council on Quality of Care and Outcomes Research, and Stroke Council. Atrial Fibrillation Burden: Moving Beyond Atrial Fibrillation as a Binary Entity: A Scientific Statement From the American Heart Association. Circulation. 2018;137:e623–e644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Link MS, Giugliano RP, Ruff CT, Scirica BM, Huikuri H, Oto A, Crompton AE, Murphy SA, Lanz H, Mercuri MF, Antman EM, Braunwald E, ENGAGE AF-TIMI 48 Investigators. Stroke and Mortality Risk in Patients With Various Patterns of Atrial Fibrillation: Results From the ENGAGE AF-TIMI 48 Trial (Effective Anticoagulation With Factor Xa Next Generation in Atrial Fibrillation-Thrombolysis in Myocardial Infarction 48). Circ Arrhythm Electrophysiol. 2017;10:pii: e004267. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJ, Becker RC, Singer DE, Halperin JL, Hacke W, Nessel CC, Berkowitz SD, Mahaffey KW, Fox KA, Califf RM, Piccini JP, ROCKET-AF Steering Committee and Investigators. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF Trial. Eur Heart J. 2015;36:288–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turakhia MP, Ziegler PD, Schmitt SK, Chang Y, Fan J, Than CT, Keung EK, Singer DE. Atrial Fibrillation Burden and Short-Term Risk of Stroke: Case-Crossover Analysis of Continuously Recorded Heart Rhythm From Cardiac Electronic Implanted Devices. Circ Arrhythm Electrophysiol. 2015;8:1040–1047. [DOI] [PubMed] [Google Scholar]
- 11.Healey JS, Connolly SJ, Gold MR, Israel CW, Van Gelder IC, Capucci A, Lau CP, Fain E, Yang S, Bailleul C, Morillo CA, Carlson M, Themeles E, Kaufman ES, Hohnloser SH; ASSERT Investigators. Subclinical atrial fibrillation and the risk of stroke. N Engl J Med. 2012;366:120–129. [DOI] [PubMed] [Google Scholar]
- 12.Glotzer TV, Daoud EG, Wyse DG, Singer DE, Ezekowitz MD, Hilker C, Miller C, Qi D, Ziegler PD. The relationship between daily atrial tachyarrhythmia burden from implantable device diagnostics and stroke risk: the TRENDS study. Circ Arrhythm Electrophysiol. 2009;2:474–480. [DOI] [PubMed] [Google Scholar]
- 13.Glotzer TV, Hellkamp AS, Zimmerman J, Sweeney MO, Yee R, Marinchak R, Cook J, Paraschos A, Love J, Radoslovich G, Lee KL, Lamas GA, MOST Investigators. Atrial high rate episodes detected by pacemaker diagnostics predict death and stroke: report of the Atrial Diagnostics Ancillary Study of the MOde Selection Trial (MOST). Circulation. 2003;107:1614–1619. [DOI] [PubMed] [Google Scholar]
- 14.Healey JS, Alings M, Ha A, Leong-Sit P, Birnie DH, de Graaf JJ, Freericks M, Verma A, Wang J, Leong D, Dokainish H, Philippon F, Barake W, McIntyre WF, Simek K, Hill MD, Mehta SR, Carlson M, Smeele F, Pandey AS, Connolly SJ, ASSERT-II Investigators. Subclinical Atrial Fibrillation in Older Patients. Circulation. 2017;136:1276–1283. [DOI] [PubMed] [Google Scholar]
- 15.Van Gelder IC, Healey JS, Crijns H, Wang J, Hohnloser SH, Gold MR, Capucci A, Lau CP, Morillo CA, Hobbelt AH, Rienstra M, Connolly SJ. Duration of device-detected subclinical atrial fibrillation and occurrence of stroke in ASSERT. Eur Heart J. 2017;38:1339–1344. [DOI] [PubMed] [Google Scholar]
- 16.Capucci A, Santini M, Padeletti L, Gulizia M, Botto G, Boriani G, Ricci R, Favale S, Zolezzi F, Di Belardino N, Molon G, Drago F, Villani GQ, Mazzini E, Vimercati M, Grammatico A, Italian AT500 Registry Investigators. Monitored atrial fibrillation duration predicts arterial embolic events in patients suffering from bradycardia and atrial fibrillation implanted with antitachycardia pacemakers. J Am Coll Cardiol. 2005;46:1913–1920. [DOI] [PubMed] [Google Scholar]
- 17.Martin DT, Bersohn MM, Waldo AL, Wathen MS, Choucair WK, Lip GY, Ip J, Holcomb R, Akar JG, Halperin JL, IMPACT Investigators. Randomized trial of atrial arrhythmia monitoring to guide anticoagulation in patients with implanted defibrillator and cardiac resynchronization devices. Eur Heart J. 2015;36:1660–1668. [DOI] [PubMed] [Google Scholar]
- 18.Gorenek B, Bax J, Boriani G, Chen SA, Dagres N, Glotzer TV, Healey JS, Israel CW, Kudaiberdieva G, Levin LA, Lip GYH, Martin D, Okumura K, Svendsen JH, Tse HF, Botto GL, ESC Scientific Document Group. Device-detected subclinical atrial tachyarrhythmias: definition, implications and management-an European Heart Rhythm Association (EHRA) consensus document, endorsed by Heart Rhythm Society (HRS), Asia Pacific Heart Rhythm Society (APHRS) and Sociedad Latinoamericana de Estimulacion Cardiaca y Electrofisiologia (SOLEACE). Europace. 2017;19:1556–1578. [DOI] [PubMed] [Google Scholar]
- 19.Sung RK, Massie BM, Varosy PD, Moore H, Rumsfeld J, Lee BK, Keung E. Long-term electrical survival analysis of Riata and Riata ST silicone leads: National Veterans Affairs experience. Heart Rhythm. 2012;9:1954–1961. [DOI] [PubMed] [Google Scholar]
- 20.Cowper DC, Hynes DM, Kubal JD, Murphy PA. Using administrative databases for outcomes research: select examples from VA Health Services Research and Development. J Med Syst. 1999;23:249–259. [DOI] [PubMed] [Google Scholar]
- 21.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60:92S–123S. [DOI] [PubMed] [Google Scholar]
- 22.VA Information Resource Center. VIReC Research User Guide: VHA Decision Support System Clinical National Data Extracts. 2nd ed. Hines, IL: U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2009. [Google Scholar]
- 23.Hynes DM, Koelling K, Stroupe K, Arnold N, Mallin K, Sohn MW, Weaver FM, Manheim L, Kok L. Veterans’ access to and use of Medicare and Veterans Affairs health care. Medical care. 2007;45:214–223. [DOI] [PubMed] [Google Scholar]
- 24.Arnold N, Sohn M-W, Maynard C, Hynes DM. VIReC Technical Report 2: VANDI Mortality Data Merge Project. VA Information Resource Center: Hines, IL: 2006. [Google Scholar]
- 25.Sohn MW, Arnold N, Maynard C, Hynes DM. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4: doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perino AC, Fan J, Schmitt SK, Askari M, Kaiser DW, Deshmukh A, Heidenreich PA, Swan C, Narayan SM, Wang PJ, Turakhia MP. Treating Specialty and Outcomes in Newly Diagnosed Atrial Fibrillation: From the TREAT-AF Study. J Am Coll Cardiol. 2017;70:78–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Turakhia MP, Hoang DD, Xu X, Frayne S, Schmitt S, Yang F, Phibbs CS, Than CT, Wang PJ, Heidenreich PA. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165:93–101. [DOI] [PubMed] [Google Scholar]
- 28.Turakhia MP, Santangeli P, Winkelmayer WC, Xu X, Ullal AJ, Than CT, Schmitt S, Holmes TH, Frayne SM, Phibbs CS, Yang F, Hoang DD, Ho PM, Heidenreich PA. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol. 2014;64:660–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health: integrating random and fixed effects in multilevel logistic regression. Am J Epidemiol. 2005;161:81–88. [DOI] [PubMed] [Google Scholar]
- 30.Hellyer JA, Azarbal F, Than CT, Fan J, Schmitt SK, Yang F, Frayne SM, Phibbs CS, Yong C, Heidenreich PA, Turakhia MP. Impact of Baseline Stroke Risk and Bleeding Risk on Warfarin International Normalized Ratio Control in Atrial Fibrillation (from the TREAT-AF Study). Am J Cardiol. 2017;119:268–274. [DOI] [PubMed] [Google Scholar]
- 31.Hsu JC, Maddox TM, Kennedy KF, Katz DF, Marzec LN, Lubitz SA, Gehi AK, Turakhia MP, Marcus GM. Oral Anticoagulant Therapy Prescription in Patients With Atrial Fibrillation Across the Spectrum of Stroke Risk: Insights From the NCDR PINNACLE Registry. JAMA Cardiol. 2016;1:55–62. [DOI] [PubMed] [Google Scholar]
- 32.Gilstrap LG, Joynt KE. MACRA and Cardiology-The Devil Is in the Details. JAMA Cardiol. 2017;2:1177–1178. [DOI] [PubMed] [Google Scholar]
- 33.Sanna T, Diener HC, Passman RS, Di Lazzaro V, Bernstein RA, Morillo CA, Rymer MM, Thijs V, Rogers T, Beckers F, Lindborg K, Brachmann J, CRYSTAL AF Investigators. Cryptogenic stroke and underlying atrial fibrillation. N Engl J Med. 2014;370:2478–2486. [DOI] [PubMed] [Google Scholar]
- 34.Chan PH, Wong CK, Poh YC, Pun L, Leung WW, Wong YF, Wong MM, Poh MZ, Chu DW, Siu CW. Diagnostic Performance of a Smartphone-Based Photoplethysmographic Application for Atrial Fibrillation Screening in a Primary Care Setting. J Am Heart Assoc. 2016;5:pii: e003428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Apple Heart Study: Assessment of Wristwatch-Based Photoplethysmography to Identify Cardiac Arrhythmias. NCT03335800. U.S. National Library of Medicine: ClinicalTrials.gov. Novemeber 8, 2017. Availailable at https://clinicaltrials.gov/ct2/show/NCT03335800. Accessed July 27, 2018. [Google Scholar]
- 36.Lopes RD, Alings M, Connolly SJ, Beresh H, Granger CB, Mazuecos JB, Boriani G, Nielsen JC, Conen D, Hohnloser SH, Mairesse GH, Mabo P, Camm AJ and Healey JS. Rationale and design of the Apixaban for the Reduction of Thrombo-Embolism in Patients With Device-Detected Sub-Clinical Atrial Fibrillation (ARTESiA) trial. Am Heart J. 2017;189:137–145. [DOI] [PubMed] [Google Scholar]
- 37.Kirchhof P, Blank BF, Calvert M, Camm AJ, Chlouverakis G, Diener HC, Goette A, Huening A, Lip GYH, Simantirakis E, Vardas P. Probing oral anticoagulation in patients with atrial high rate episodes: Rationale and design of the Non-vitamin K antagonist Oral anticoagulants in patients with Atrial High rate episodes (NOAH-AFNET 6) trial. Am Heart J. 2017;190:12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Waks JW, Passman RS, Matos J, Reynolds M, Thosani A, Mela T, Pederson D, Glotzer TV, Zimetbaum P. Intermittent anticoagulation guided by continuous atrial fibrillation burden monitoring using dual-chamber pacemakers and implantable cardioverter-defibrillators: Results from the Tailored Anticoagulation for Non-Continuous Atrial Fibrillation (TACTIC-AF) pilot study. Heart Rhythm. 2018;15:1601–1607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.