Leukotoxin (LtxA) (trade name, Leukothera) is a protein secreted by the oral bacterium Aggregatibacter actinomycetemcomitans. A. actinomycetemcomitans is an oral pathogen strongly associated with development of localized aggressive periodontitis. LtxA acts as a virulence factor for A. actinomycetemcomitans by binding to the β2 integrin lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) on white blood cells (WBCs) and causing cell death.
KEYWORDS: autoimmunity, CRISPR, cell death, death receptor, inflammation, integrins, leukemia, lymphoma, oral microbiology, periodontal disease
ABSTRACT
Leukotoxin (LtxA) (trade name, Leukothera) is a protein secreted by the oral bacterium Aggregatibacter actinomycetemcomitans. A. actinomycetemcomitans is an oral pathogen strongly associated with development of localized aggressive periodontitis. LtxA acts as a virulence factor for A. actinomycetemcomitans by binding to the β2 integrin lymphocyte function-associated antigen-1 (LFA-1; CD11a/CD18) on white blood cells (WBCs) and causing cell death. In addition, because of its specificity for malignant and activated WBCs, LtxA is being investigated as a therapeutic agent for treatment of hematological malignancies and autoimmune diseases. Here, we report the successful generation and characterization of Jurkat T lymphocytes with deletions in CD18, CD11a, and Fas that were engineered using CRISPR/Cas9 gene editing. Using these clones, we demonstrate the specificity of LtxA for cells expressing LFA-1. We also demonstrate the requirement of the cell death receptor Fas for LtxA-mediated cell death in T lymphocytes. We show that LFA-1 and Fas are early events in the LtxA-mediated cell death cascade as caspase activation and mitochondrial perturbation do not occur in the absence of either receptor. To our knowledge, LtxA is the first molecule, other than FasL, known to require the Fas death receptor to initiate cell death. Knowledge of the mechanism of cell death induced by LtxA will facilitate the understanding of LtxA as a bacterial virulence factor and development of it as a potential therapeutic agent.
INTRODUCTION
Leukotoxin (LtxA; trade name, Leukothera) is a bacterial protein produced by the oral bacterium Aggregatibacter actinomycetemcomitans (reviewed in reference 1). A. actinomycetemcomitans is an oral pathogen that is implicated as the causative agent of localized aggressive periodontitis. As a bacterial pathogen, A. actinomycetemcomitans produces an arsenal of virulence factors, including LtxA, which help with evasion of the host immune response. LtxA is an important virulence factor as it kills white blood cells (WBCs) via a specific interaction with lymphocyte function-associated antigen-1 (LFA-1) (2–4). LFA-1 is a β2 integrin composed of an α subunit, CD11a, and a β subunit, CD18. Expression of LFA-1 is required for LtxA to intoxicate cells. LFA-1 is exclusively expressed on WBCs and is a key adhesion molecule that aids in leukocyte migration via interaction with the intercellular adhesion molecules (ICAMs) (5). Clinically, patients who harbor mutations in CD18 suffer from leukocyte adhesion deficiency (LAD), which results in early death (6). LAD patients are unable to clear pathogens because immune cells cannot migrate to infected tissues.
LFA-1 exists in three conformational states. Resting leukocytes express the low-affinity, closed conformation of LFA-1, which is unable to bind to ICAM-1. When leukocytes become activated, LFA-1 changes conformation to the intermediate-affinity/extended conformation, which can weakly bind to ICAM-1, leading to signaling cascades that trigger a conformational change to the high-affinity/activated state where cells can strongly bind ICAM-1 and migrate to peripheral tissues (7, 8). Interaction of integrins with their ligands leads to enhanced cell survival, differentiation, and other immunological events (9, 10). The cells involved in many WBC diseases, such as leukemia, lymphoma, and autoimmune and inflammatory diseases, are known to overexpress the active conformation of LFA-1, leading to enhanced cell activity and migration (11–13). Given the physiological function of integrins, it is very intriguing that the interaction between LtxA and LFA-1 induces cell death.
We have shown that LtxA preferentially targets WBCs expressing the active conformation of LFA-1 (14, 15). Several cellular changes are induced by LtxA, depending on the immune cell subset. Initially, LtxA causes an increase in cytosolic Ca2+ levels via an unknown mechanism (16). This results in the activation of the calcium-dependent protease, calpain, which cleaves talin, allowing LFA-1 to cluster in the lipid raft compartment. In monocytes, LtxA binds to activated LFA-1 and is taken into the cell where it induces a novel lysosomal-mediated cell death pathway, in addition to triggering a secondary apoptotic cascade that involves the activation of caspase-1, phosphorylation of p38, and secretion of interleukin-1β (IL-1β) and IL-18 (14, 17, 18). After interacting with LFA-1, LtxA induces endocytosis of the LFA-1/LtxA complex where LtxA is shuttled to the lysosome, causing its rupture, release of cathepsin D, and acidification of the cytosol (14, 19). Lymphocytes, however, do not undergo the same mechanism of cell death as monocytes. Lymphocytes die via a caspase-8-dependent cell death pathway that may involve the death receptor Fas (CD95) (20). LFA-1 and Fas were found to colocalize on the cell surface. It is currently unknown what the role of Fas is in LtxA-mediated cell death.
Because LtxA specifically targets the cells that highly express LFA-1, which are the most activated, immunologically relevant subsets, LtxA is being developed as a therapeutic agent for WBC diseases (under the trade name Leukothera). LtxA has shown preclinical, therapeutic efficacy both in vitro and in vivo in humanized mouse models for leukemia (4, 21), lymphoma (20), allergic asthma (22), and psoriasis (23). Additionally, LtxA has been well tolerated in rodents, canines, and nonhuman primates (4, 24).
Here, we sought to determine the specific roles that LFA-1 and Fas contribute to the cell death signaling pathways that are activated by LtxA in malignant T lymphocytes. We report the first known human white blood cell lines with stable deletions of CD11a, CD18, and Fas generated with CRISPR/Cas9 gene editing. We report that caspase activation occurs downstream of LFA-1. Additionally, we report that Fas is required for LtxA-mediated cell death in T lymphocytes. Fas plays a role in downstream LtxA-mediated cell death signaling to activate caspases and permeabilize the mitochondrial membrane. This work not only contributes to the understanding of LtxA cell death mechanisms but also provides valuable tools for studying immunological events involving LFA-1 and Fas.
RESULTS
Generation and validation of CD18 and CD11a knockout cell lines using CRISPR/Cas9.
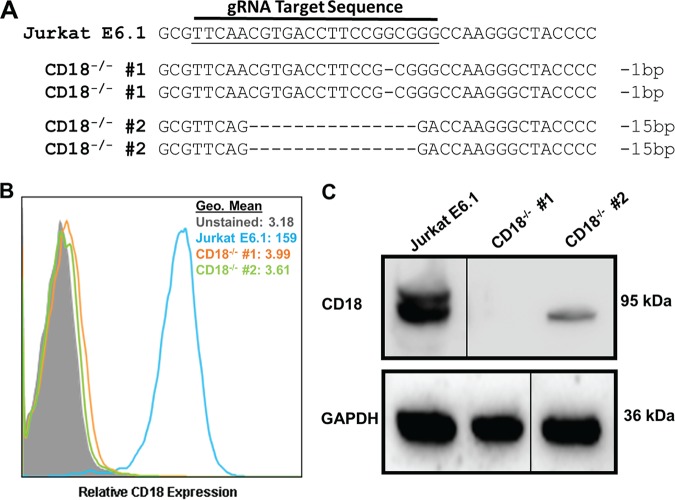
Previous studies have reported that LtxA binds to LFA-1 and induces cell death (2). Additionally, studies have shown that the N-terminal 128 amino acids in β-sheets 1 and 2 of the CD11a β-propeller domain, as well as the cysteine-rich tandem repeats encompassing the integrin-epidermal growth factor-like (I-EGF-like) domains 2, 3, and 4 of CD18, are critical for LtxA activity (3, 25). We sought to generate CD11a and CD18 knockout cells to assess the role of LFA-1 in cell death. Jurkat E6.1 T lymphocytes were transiently transfected with a CRISPR plasmid containing a guide RNA (gRNA) targeting exon 5 of the CD18 (ITGB2) gene locus. Exon 5 was chosen as a target because previous studies have shown that mutations in exon 5 of CD18 result in leukocyte adhesion deficiency (26). Two independent clones with deletions in CD18 were isolated. Sequencing analysis confirmed that gene editing occurred within the targeted gene locus, and, interestingly, within each clone, both alleles were found to contain the same genomic modification (Fig. 1A). To rule out the possibility that a large deletion resulted in loss of the primer binding sites, new primers were designed to amplify a larger region of DNA, and the same nucleotide deletions were again found on both alleles of each clone. To further validate the deletion of CD18 from Jurkat cells, protein analyses were performed. Flow cytometry revealed complete loss of CD18 from the surface of Jurkat cells (Fig. 1B). Deletion of CD18 was further confirmed via Western blot analysis (Fig. 1C). From these data, we conclude that we successfully generated Jurkat CD18−/− cells.
FIG 1.
Generation and validation of CD18 knockout in Jurkat cells. (A) DNA from the independent clones with mutations in CD18 was isolated, PCR amplified, cloned into a TOPO vector, and sequenced. Sequences were aligned to wild-type Jurkat E6.1 CD18 DNA. Any alteration in the DNA sequence was considered gene editing. Each dash indicates a single nucleotide deletion. The number of nucleotides deleted is indicated at the end of each sequence. (B) Flow cytometric analysis of Jurkat E6.1 cells and both Jurkat CD18−/− clones for surface expression of CD18. Cell populations are indicated according to the legend on the figure. (C) Western blot analysis to confirm deletion of CD18 and the GAPDH loading control. Gels were spliced for labeling purposes. Geo mean, geometric mean.
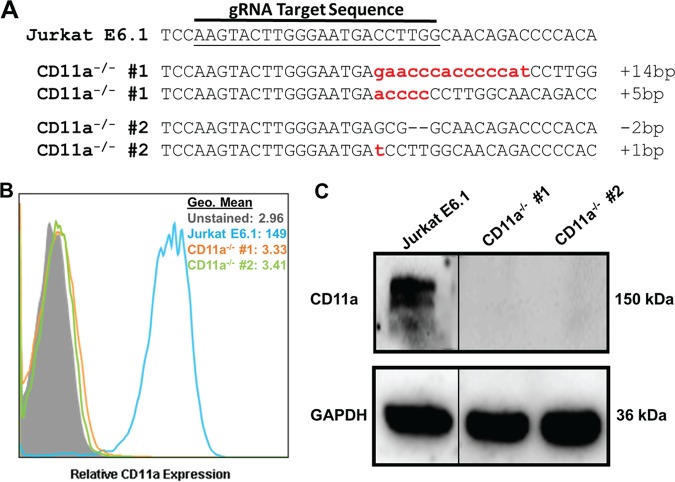
To generate knockouts of CD11a, Jurkat cells were transiently transfected with a CRISPR plasmid containing a gRNA targeting exon 4 of the CD11a (ITGAL) gene locus. Exon 4 was chosen because it codes for part of the β-propeller domain, previously shown to be important for LtxA cytotoxicity (25). Two independent clones with mutations in CD11a were isolated. Sequencing analysis confirmed that gene editing occurred in exon 4 of CD11a (Fig. 2A). Flow cytometry validated the knockout of CD11a from the surface of Jurkat cells (Fig. 2B). Western blot analysis further confirmed the deletion of CD11a from these clones (Fig. 2C). These data indicate the successful generation of Jurkat CD11a−/− cells.
FIG 2.
Generation and validation of CD11a knockout in Jurkat cells. (A) DNA from the independent clones with mutations in CD11a was isolated, PCR amplified, cloned into a TOPO vector, and sequenced. Sequences were aligned to wild-type Jurkat E6.1 CD11a DNA. Any alteration in the DNA sequence was considered gene editing. Each dash indicates a single nucleotide deletion; lowercase letters indicate nucleotide insertions. The number of nucleotides inserted or deleted is indicated at the end of each sequence. (B) Flow cytometric analysis of Jurkat E6.1 cells and both Jurkat CD11a−/− clones for surface expression of CD11a. Cell populations are indicated according to the legend on the figure. (C) Western blot analysis to confirm deletion of CD11a and the GAPDH loading control. Gels were spliced for labeling purposes.
Deletion of CD18 or CD11a renders Jurkat cells highly resistant to the effects of LtxA.
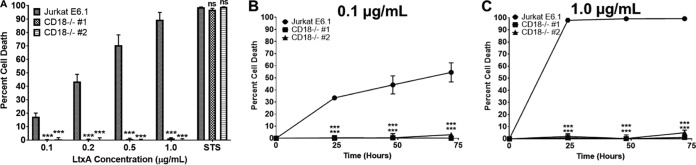
LFA-1 has previously been identified as the cellular receptor for LtxA (2). To determine the contribution of CD18 in LtxA-mediated cell death, Jurkat E6.1 and Jurkat CD18−/− cells were treated with various concentrations of LtxA, and after 24 h, cell death was measured via flow cytometry using viability stains annexin V and 7-amino-actinomycin-D (7-AAD). The percentage of cell death was defined as the total number of annexin V-positive/7-AAD-negative (annexin V+/7-AAD−) and annexin V-positive/7-AAD-positive (annexin V+/7-AAD+) cells. While Jurkat E6.1 cells died in a dose-dependent manner, both Jurkat CD18−/− clones were completely resistant to the effects of LtxA (Fig. 3A). Staurosporine was used as a positive control for cell death. To determine if LtxA could overcome the loss of CD18, Jurkat CD18−/− clones were treated with either 0.1 μg/ml or 1.0 μg/ml LtxA for 24, 48, or 72 h, and cell death was assessed. Jurkat E6.1 cells were killed in a dose- and time- dependent manner, while CD18 knockout cells were completely resistant to the effects of LtxA even after 72 h of treatment (Fig. 3B and C).
FIG 3.
Deletion of CD18 renders Jurkat cells resistant to LtxA-mediated cell death. (A) Jurkat E6.1 cells and both Jurkat CD18−/− clones were treated with LtxA for 24 h. Staurosporine (STS; 1 μM) was used as a positive control for cell death. (B and C) Jurkat E6.1 cells and Jurkat CD18−/− clones were treated with 0.1 μg/ml or 1.0 μg/ml LtxA for 24, 48, or 72 h, and cell death was assessed via flow cytometry. Cytotoxicity was determined by flow cytometry, and the percent cell death is defined as the sum of annexin V+/7-AAD− and annexin V+/7-AAD+ cells. Data represent the average from three independent experiments. Error bars represent SEM. The significance of differences between results for Jurkat E6.1 cells and those for each Jurkat CD18−/− clone was determined using Student's t test. ***, P ≤ 0.001; ns, not significant.
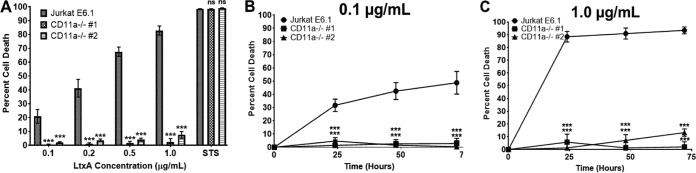
To determine the contribution of CD11a in LtxA-mediated cell death, Jurkat E6.1 and Jurkat CD11a−/− clones were treated with various concentrations of LtxA, and cell death was assessed after 24 h via flow cytometry. Jurkat E6.1 cells were killed in a dose-dependent manner, while both Jurkat CD11a−/− clones were completely resistant to the effects of LtxA (Fig. 4A). Staurosporine was used as a positive control for cell death. To determine if LtxA could overcome the loss of CD11a, Jurkat E6.1 and CD11a−/− clones were treated with either 0.1 μg/ml or 1.0 μg/ml LtxA for 24, 48, or 72 h, and cell death was assessed. Jurkat E6.1 cells were killed in a dose- and time-dependent manner, but both Jurkat CD11a−/− clones were completely resistant to the effects of LtxA (Fig. 4B and C). Together, these data suggest that in lymphocytes, both CD11a and CD18 are critical for LtxA to induce its toxic effects.
FIG 4.
CD11a is required for LtxA-mediated cell death. (A) Jurkat E6.1 cells and both Jurkat CD11a−/− clones were treated with LtxA for 24 h. Staurosporine (STS; 1 μM) was used as a positive control for cell death. (B and C) Jurkat E6.1 cells and Jurkat CD11a−/− clones were treated with 0.1 μg/ml or 1.0 μg/ml LtxA for 24, 48, or 72 h, and cell death was assessed via flow cytometry. Cytotoxicity was determined by flow cytometry, and the percent cell death is defined as the sum of annexin V+/7-AAD− and annexin V+/7-AAD+ cells. Data represent the average from three independent experiments. Error bars represent SEM. The significance of differences between results for Jurkat E6.1 cells and those for each Jurkat CD11a−/− clone was determined using Student's t test. ***, P ≤ 0.001; ns, not significant.
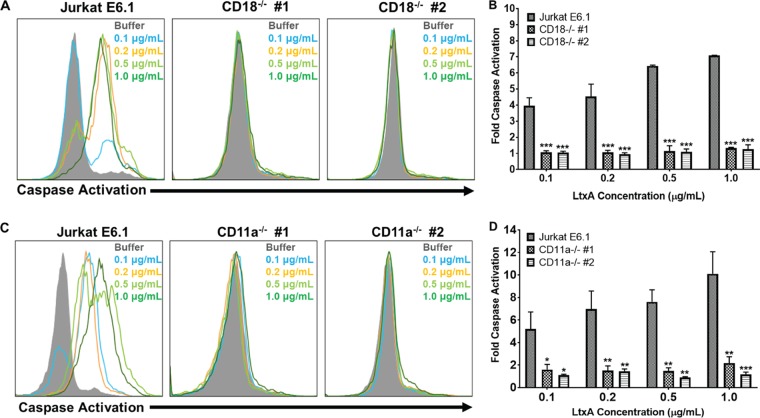
LtxA has been shown to kill lymphocytes via a caspase-dependent mechanism (18, 20, 27). Additionally, previous studies have suggested that LtxA can induce certain cellular events independent of LFA-1 expression (16, 28). To determine if LtxA is able to activate caspases in the absence of CD18 or CD11a, Jurkat E6.1 cells and Jurkat CD18−/− clones or Jurkat CD11a−/− clones were treated with various concentrations of LtxA for 24 h, and caspase activation was assessed using a fluorescent polycaspase reagent, FAM-VAD-FMK FLICA (where FLICA is fluorescent-labeled inhibitor of caspases). While Jurkat E6.1 cells had dose-dependent increases in caspase activation, both Jurkat CD18−/− clones (Fig. 5A and B) and Jurkat CD11a−/− clones (Fig. 5C and D) had no changes in caspase activation states from those of vehicle controls. These results suggest that both CD11a and CD18 are necessary for LtxA to trigger downstream activation of caspases.
FIG 5.
CD18 and CD11a are required for LtxA to activate caspases. (A) Jurkat E6.1 cells and Jurkat CD18−/− clones were treated with various concentrations of LtxA for 24 h. Caspase activation was assessed using a fluorescent polycaspase reagent and flow cytometry. Increases in fluorescent signal are directly related to the amount of active caspases within a cell. Representative images are shown. (B) Cells were gated on buffer-treated controls, and fold change in caspase activation was determined using FlowJo. (C) Jurkat E6.1 cells and both Jurkat CD11a−/− clones were treated with various concentrations of LtxA for 24 h, and caspase activation was assessed via flow cytometry. Representative images are shown. (D) Cells were gated on buffer controls and fold change in caspase activation was determined using FlowJo. Cell populations are indicated according to the legend on the figure. Data represent the average of at least three independent experiments. Error bars represent SEM. The significance of differences between results for Jurkat E6.1 cells and those of Jurkat CD18−/− clones or Jurkat CD11a−/− clones was determined using Student's t test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001.
We sought to further characterize these Jurkat CD18−/− cells and Jurkat CD11a−/− cells to determine why there were no observed differences in killing activity by LtxA. To determine if genomic alteration of CD18 affected expression of CD11a and/or Fas, further protein analyses of Jurkat CD18−/− cells were performed. Flow cytometric analysis of the Jurkat CD18−/− cells revealed that the genomic deletion of CD18 resulted in a complete loss of CD11a expression from the surface of these cells (see Fig. S1A in the supplemental material). Western blot analysis revealed that CD11a was still produced upon deletion of CD18, but the protein remained in the cytosol (Fig. S1B). Deletion of CD18 had no effect on surface expression of Fas (Fig. S1C). To determine if genomic alteration of CD11a affected expression of CD18 and/or Fas, flow cytometry and Western blotting were performed. Flow cytometric analysis of Jurkat CD11a−/− cells revealed that deletion of CD11a resulted in ablation of CD18 expression from the surface of these cells (Fig. S2A). Western blot analysis revealed that loss of CD11a prevented presentation of CD18 only on the surface of cells as cytosolic fractions were detected (Fig. S2B). Deletion of CD11a had no effect on the levels of Fas expression on the cell surface (Fig. S2C). Together, these results suggest that a genomic defect in either CD18 or CD11a leads to complete loss of surface expression of the other protein in Jurkat E6.1 cells. While genomic defects were created only in CD18 or CD11a, one chain of LFA-1 needs the other to be expressed on the surface of the cell (29) and, thus, both Jurkat CD18−/− and Jurkat CD11a−/− knockout cell lines are double LFA-1 mutants.
Generation and validation of Fas knockout cell lines using CRISPR/Cas9.
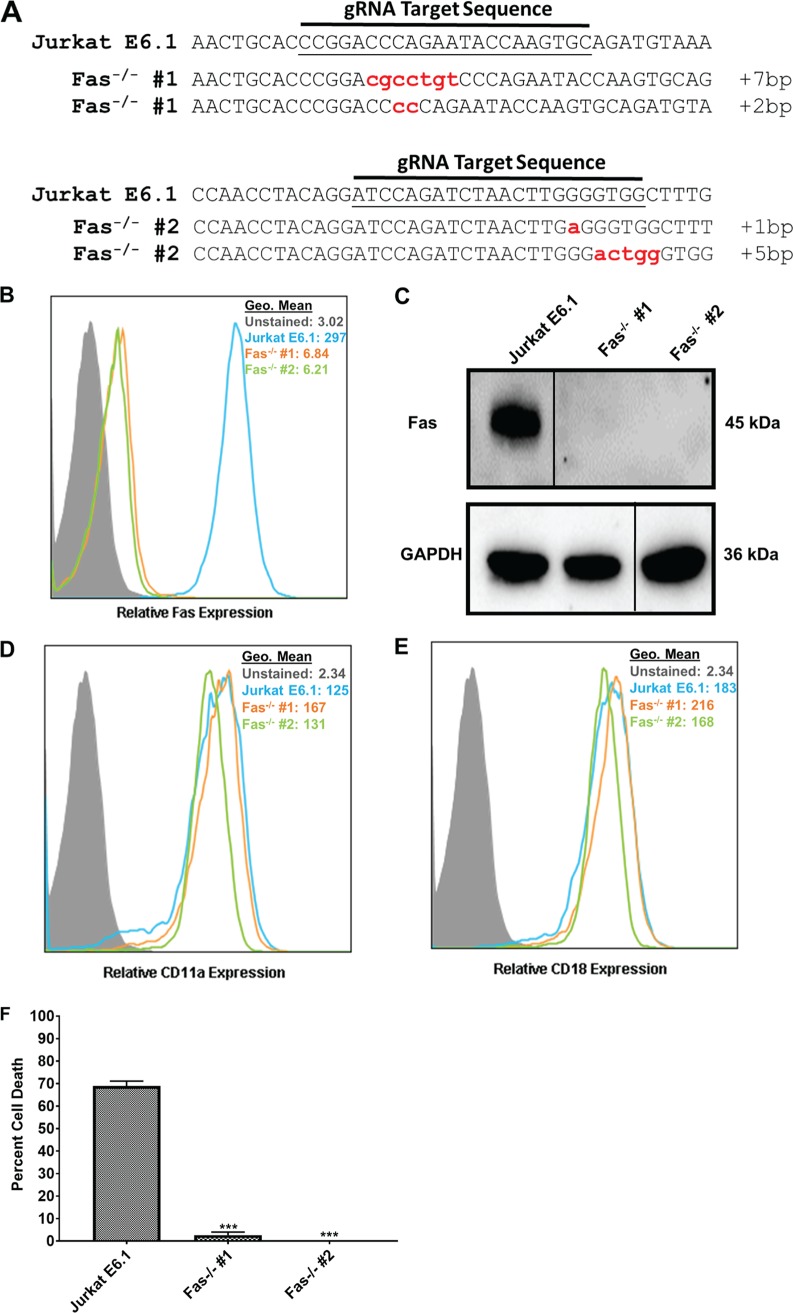
We previously reported that LtxA may intoxicate lymphocytes via a mechanism that requires both LFA-1 and Fas (20). Additionally, we showed that CD11a, the α-chain of LFA-1, and Fas colocalize more strongly than CD11a and CD18 (which are known to interact as LFA-1) on the surface of Jurkat E6.1 cells (20). However, another study proposed that death receptors were not involved in LtxA-mediated cytolysis in JY B cells (30). To determine if Fas played a role in LtxA-mediated cell death in lymphocytes, we sought to create Fas−/− cells in Jurkat E6.1 cells. Jurkat E6.1 cells were transiently transfected with CRISPR plasmids containing a gRNA targeting either exon 4 or exon 6 in the Fas (TNFRSF6; tumor necrosis factor [TNF] superfamily member 6) gene locus. Two independent clones (one with a deletion in each exon) were isolated. Sequencing analysis confirmed that gene editing occurred within the targeted region of Fas in each clone (Fig. 6A). To further validate the generation of Fas−/− clones, protein analyses were performed. Flow cytometry revealed a total loss of Fas from the surface of the isolated clones (Fig. 6B). Western blot analysis corroborated this finding (Fig. 6C). Because Fas was found to colocalize with LFA-1, we sought to determine if deletion of Fas affected expression of LFA-1. Flow cytometric analysis revealed that deletion of Fas had no effect on the surface expression of CD11a or CD18 (Fig. 6D and E).
FIG 6.
Characterization of Jurkat Fas knockout cells. (A) DNA from the independent clones with mutations in Fas was isolated, PCR amplified, cloned into a TOPO vector, and sequenced. Sequences were aligned to wild-type Jurkat E6.1 Fas DNA. Any alteration in the DNA sequence was considered gene editing. Each lowercase letter indicates a nucleotide insertion; the number of nucleotides inserted is indicated at the end of each sequence. Sequence of the clone with a deletion in Fas exon 4 (top) and of the clone with a deletion in Fas exon 6 (bottom). (B) Flow cytometric analysis of Jurkat E6.1 cells and both Jurkat Fas−/− clones for surface expression of Fas. Cell populations are indicated according to the legend on the figure. (C) Western blot analysis to confirm deletion of Fas and GAPDH as a loading control. Gels were spliced for labeling purposes. (D and E) Flow cytometric analysis for surface expression of CD11a (D) and CD18 (E) in Jurkat Fas−/− clones. Cell populations are indicated according to the legend on the figure. (F) Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with 10 ng/ml FasL for 24 h, and cell death was assessed via flow cytometry and annexin V/7-AAD staining. Data represent the average of three independent experiments. Error bars represent SEM. The significance of differences between results for Jurkat E6.1 cells and those for each Jurkat Fas−/− clone was determined using Student's t test. ***, P ≤ 0.001.
As a member of the TNF superfamily, Fas is involved in apoptosis via binding of its cognate ligand, FasL. To confirm that our knockouts had a functional deletion of Fas, Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with 10 ng/ml FasL for 24 h, and cell death was assessed via flow cytometry. Jurkat E6.1 cells were efficiently killed by FasL, while FasL had no effect on Jurkat Fas−/− clones (Fig. 6F). Together, these results indicate that we successfully generated Jurkat Fas−/− clones.
Fas is required for LtxA-mediated cell death.
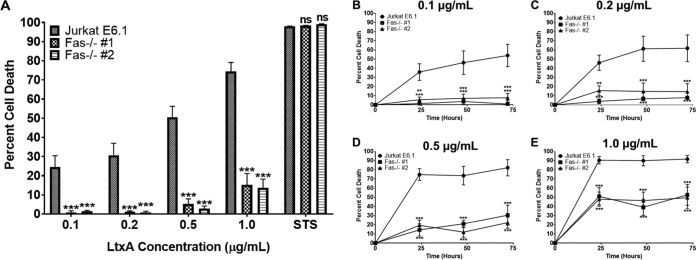
To determine if Fas plays a critical role in LtxA-mediated cell death in lymphocytes, Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24 h, and cell death was assessed using flow cytometry. Jurkat E6.1 cells were killed by LtxA in a dose-dependent manner, while both Jurkat Fas−/− clones were resistant to the effects of LtxA (Fig. 7A). Staurosporine was used as a positive control for cell death. These results suggest that Fas is required for LtxA to intoxicate Jurkat E6.1 cells.
FIG 7.
Fas is required for LtxA-mediated cell death. (A) Jurkat E6.1 cells and both Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24 h. Staurosporine (STS; 1 μM) was used as a positive control for cell death. (B to E) Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with LtxA at the indicated concentrations for 24, 48, or 72 h, and cell death was assessed via flow cytometry. Cytotoxicity was determined by annexin V/7-AAD staining and flow cytometry. Data represent the average from three independent experiments. Error bars represent SEM. The significance of differences between results for Jurkat E6.1 cells and those for each Jurkat Fas−/− clone was determined using Student's t test. **, P ≤ 0.01; ***, P ≤ 0.001; ns, not significant.
We noted that high concentrations of LtxA (1.0 μg/ml) began to induce low levels of cell death in both of the Jurkat Fas−/− clones, suggesting that perhaps the kinetics of LtxA-mediated cell death were slower in the absence of Fas. To test this, Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24, 48, or 72 h, and cell death was assessed. Jurkat E6.1 cells experienced dose- and time-dependent increases in cell death following LtxA treatment (Fig. 7B to E). Low concentrations of LtxA (0.1 and 0.2 μg/ml) (Fig. 7B and C) had no effect on either Jurkat Fas−/− clone over the 72-h treatment. High concentrations of LtxA (0.5 and 1.0 μg/ml) (Fig. 7D and E) began to kill both Jurkat Fas−/− clones; however, there appeared to be a maximum threshold for killing by LtxA in the absence of Fas that does not increase over time. These results suggest that while LtxA can at least partially overcome the defect in Fas at high doses, Fas plays a crucial role in LtxA-mediated cell death.
Fas plays an important role in LtxA-induced caspase activation and permeabilization of the mitochondria.
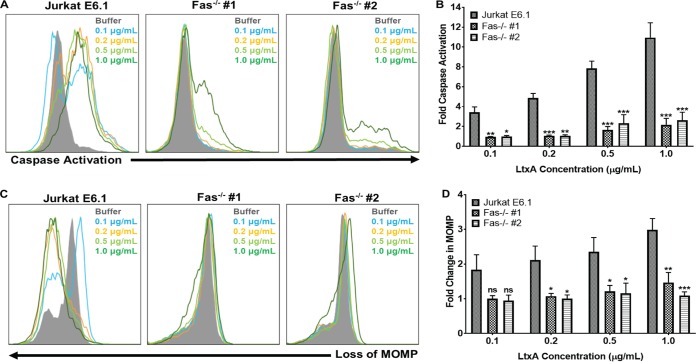
Previous studies have determined that in lymphocytes, LtxA causes activation of caspases and permeabilization of the mitochondrial membrane (20, 27, 30). To determine if Fas was required for caspase activation in response to LtxA, Jurkat E6.1 cells and both Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24 h, and caspase activation was assessed using a fluorescent polycaspase probe. Jurkat E6.1 cells had dose-dependent increases in caspase activation, while both Jurkat Fas−/− clones had little or no caspase activation in response to LtxA (Fig. 8A). Following treatment of both Jurkat Fas−/− clones with 1.0 μg/ml LtxA, the clones began to exhibit low levels of caspase activation, but it was significantly lower than that induced in Jurkat E6.1 cells (Fig. 8B).
FIG 8.
Fas mediates caspase activation and mitochondrial membrane permeabilization in response to LtxA. (A) Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24 h. Caspase activation was assessed using a fluorescent polycaspase reagent and flow cytometry. Increases in fluorescent signal are directly related to the amount of active caspases within a cell. Representative images are shown. (B) Cells were gated on buffer-treated controls, and fold change in caspase activation was determined using FlowJo software. (C) Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24 h, and mitochondrial membrane permeability was assessed via MitoTracker staining and flow cytometry. Decreases in fluorescent signal are associated with compromised mitochondrial membranes. Representative images are shown. MOMP, mitochondrial outer membrane protein. (D) Cells were gated on buffer controls, and fold change in mitochondrial membrane permeabilization was determined using FlowJo software. Cell populations are indicated according to the legend on the figure. Data represent the average of at least three independent experiments. Error bars represent SEM. The significance of differences between results for Jurkat E6.1 cells and those for each Jurkat Fas−/− clone was determined using Student's t test. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ns, not significant.
To determine if Fas was necessary for LtxA to induce permeabilization of the mitochondrial membrane, we utilized MitoTracker Red. MitoTracker is oxidized when it enters the mitochondria, resulting in a fluorescent signal, which decreases upon permeabilization of the mitochondrial membrane. Jurkat E6.1 cells and Jurkat Fas−/− clones were treated with various concentrations of LtxA for 24 h, and mitochondrial membrane permeabilization was assessed via flow cytometry. Jurkat E6.1 cells experienced dose-dependent changes in the integrity of the mitochondrial membrane, while Jurkat Fas−/− clones exhibited no changes in the mitochondrial membrane (Fig. 8C and D). Together, these results suggest that Fas plays an integral role in LtxA-mediated cell death upstream of caspase activation and mitochondrial disruption.
DISCUSSION
In this report, we demonstrate the successful generation and validation of knockout of CD11a, CD18, and Fas in Jurkat E6.1 cells using CRISPR/Cas9. All isolated CD18−/−, CD11a−/−, and Fas−/− clones had biallelic mutations in the targeted region of DNA. No donor DNA template was provided during gene editing. DNA cleavage by Cas9 activates the error-prone nonhomologous end joining (NHEJ) DNA repair pathway, which can result in random insertions or deletions in the targeted DNA sequence. Both CD11a−/− clones and both Fas−/− clones had different nucleotide insertions or deletions on each allele. Interestingly, within each CD18−/− clone, the same nucleotide deletion was found on both alleles. This was surprising, given the error-prone nature of NHEJ. Each CD18−/− clone was sequenced more than 40 times using two sets of primers. Therefore, either each clone randomly received the same DNA modification, a large deletion removed the primer binding sites for both sets of primers used to amplify CD18, or the frequency of detection for the second sequence in each clone is less than 1 in 40. Flow cytometric and Western blot analyses confirmed that genomic modifications led to protein knockout of CD18, CD11a, and Fas both on the cell surface and from the cytoplasm. It should be noted that in CD18−/− clone number 2, a small cytoplasmic CD18 protein band was detected. Genotypic analysis found that this clone had a 15-nucleotide deletion in both alleles, resulting in a frameshift mutation that is too small to resolve on a protein gel. Flow cytometric analysis for CD18 expression was carried out with multiple antibody clones, and CD18 was never detected on the cell surface. Additionally, there was a concomitant total loss of CD11a from the surface of the CD18−/− clones. These results suggest that this cytoplasmic fraction is turned over within the cell and never reaches the cell surface and/or that this region of the protein may be important for proper protein folding or function. To our knowledge, these are the first known white blood cell lines lacking CD11a, CD18, or Fas protein generated using CRISPR/Cas9 gene editing.
Using these knockout cell lines, we showed that both CD11a and CD18 are indispensable for LtxA-mediated cell death in T lymphocytes. We also show that both CD11a and CD18 are necessary for LtxA to activate caspases in the cell death cascade. Additionally, we demonstrated, through use of Fas−/− cells, that Fas is indeed required for LtxA to intoxicate Jurkat cells. Upon treatment with LtxA, Fas plays a critical role in the activation of caspases and mitochondrial membrane permeabilization that are known to be part of the cell death mechanism.
Previous studies identified LFA-1 (CD11a/CD18 heterodimer) as the cellular receptor for LtxA (2). Reports have been published demonstrating a critical role of each subunit of LFA-1 for toxin activity. One study reports that the N-terminal 128-amino-acid sequence encompassing the β-sheets 1 and 2 in the CD11a β-propeller domain are critical for cytotoxicity, while another report suggests that the cysteine-rich tandem repeats encompassing integrin-epidermal growth factor-like domains 2, 3, and 4 of CD18 are crucial for toxin activity (3, 25). Our results further support these claims that both CD11a and CD18 are required for toxin activity against Jurkat T cells. We along with others have shown that CD11a and CD18 are interdependent for surface expression (29) and, therefore, perhaps the toxin binds domains on both LFA-1 subunits, adding another layer of specificity of LtxA for WBCs to ensure killing of the appropriate target cells. While LFA-1 is known to be the receptor for LtxA, these are the first studies definitively demonstrating the requirement of LFA-1 by taking toxin-sensitive cells and rendering them completely resistant. This work further demonstrates the specificity of LtxA for LFA-1-expressing cells.
We previously reported that Fas was important for LtxA-mediated cell death in lymphocytes (20). However, it was unclear if Fas was required for cell death or upstream signaling events. Here, we have conclusively shown the necessity of the Fas death receptor in LtxA-mediated cell death in T lymphocytes. We have demonstrated that Fas plays a role in mediating the downstream signals that result in caspase activation and mitochondrial membrane permeabilization, events previously shown to occur in lymphocytes (20, 27, 30).
An important question raised by our results is the relationship between LtxA, LFA-1, and Fas. Given the close proximity of LFA-1 and Fas, it is possible that they communicate either intracellularly or extracellularly or that LtxA interacts with both molecules, either simultaneously or at different times. LtxA is known to interact with the cysteine-rich tandem repeats in CD18 (3) and, of note, Fas also contains cysteine-rich domains, which serve as important binding sites for FasL (31, 32). It is also noteworthy that LtxA is posttranslationally modified at internal lysine residues with fatty acyl chains (33, 34). The only other molecules in biology known to have this fatty acylation at internal lysine residues are cytokines in the tumor necrosis family (TNF), which includes FasL (35, 36). Canonically, FasL binds to Fas, resulting in receptor trimerization, clustering of intracellular death domains, and recruitment of the adaptor protein Fas-associated protein with death domain (FADD) and subsequently of caspase-8 (37). If adequate caspase-8 is activated, cells can then die via type I extrinsic apoptosis. If low levels of caspase-8 are activated, cell death ensues via type II extrinsic apoptosis, which proceeds through the mitochondria (38). LtxA has been shown to require Fas, activate caspase-8, and perturb the mitochondrial membrane when intoxicating lymphocytes (20, 27, 30). Therefore, it is plausible that the fatty acyl chains on LtxA may resemble FasL, thus allowing LtxA to interact with the cysteine-rich domains on Fas, exploit the mammalian cell death machinery, and activate similar signaling pathways. To our knowledge, this is the first time a molecule other than FasL has been shown to require Fas to initiate cell death.
It is important that Jurkat Fas−/− clones began to undergo low levels of cell death when treated with high concentrations of LtxA. We demonstrated that LFA-1 does not inherently require Fas for its structure and/or function as deletion of Fas had no effect on surface expression of LFA-1. This was unsurprising since THP-1 monocytes express little or no Fas, express abundant levels of LFA-1, and remain highly susceptible to LtxA (4, 14, 39). Thus, it is possible that the reason Jurkat Fas−/− cells began to undergo cell death at high doses of LtxA is due to signaling via LFA-1. Given the colocalization of Fas and LFA-1 in Jurkat cells, it is possible that the reason Fas is required for LtxA-mediated cell death is to recruit proapoptotic machinery to close proximity of the β-tail of LFA-1, thus allowing LFA-1 to activate cell death upon LtxA ligation. However, when Fas is deleted, perhaps caspase-8 is still recruited, albeit less efficiently (in a process similar to integrin-mediated death), to the tail of LFA-1, which may explain why low levels of cell death are observed only at high concentrations of LtxA. This indicates that LtxA may be initiating the cell death signal directly via a noncanonical Fas-mediated apoptosis cascade that requires both LFA-1 and Fas. Therefore, because of this colocalization, LtxA may usurp LFA-1 to signal via Fas to more efficiently eliminate T lymphocytes.
In summary, this work expands on our knowledge of the mechanism of LtxA-mediated cell death in lymphocytes and demonstrates a need for Fas, in addition to LFA-1, in T lymphocytes. We have further supported the specific nature of LtxA for cells expressing LFA-1. We have conclusively demonstrated that LFA-1 and Fas are early, upstream events in the LtxA-mediated cell death pathway. We have also discovered that a bacterial virulence factor can usurp the Fas death receptor signaling pathway to initiate cell death.
MATERIALS AND METHODS
Cell culture.
Jurkat E6.1 cells were purchased from the American Type Culture Collection (ATCC). CD11a, CD18, and Fas knockout cell lines were generated on the Jurkat E6.1 background (described below). All cells were maintained in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal bovine serum (FBS) (Life Technologies) at 37°C in 5% CO2.
Purification of LtxA.
LtxA was purified from culture supernatants of A. actinomycetemcomitans strain NJ4500 using a modification of a previously described method (40). Briefly, an A. actinomycetemcomitans strain NJ4500 clone capable of growing in animal-free medium was isolated, and LtxA was purified from culture supernatants using glycomacropeptide (GMP) purification methods developed at Paragon Bioservices (Baltimore, MD).
Inhibitors, antibodies, and reagents.
Cell death was measured using annexin V-fluorescein isothiocyanate (FITC) and 7-amino-actinomycin-D (7-AAD) (BioLegend). When appropriate, soluble SuperFas ligand (10 ng/ml; Enzo Life Sciences) or staurosporine (1 μM; Santa Cruz Biotechnology) was used as a positive control for cell death. Anti-human CD11a-phycoerythrin (PE) (clone TS2/4; BioLegend), anti-human CD18-PE (clone TS1/18; BioLegend), and anti-human Fas-PE (clone DX2; BioLegend) were used for flow cytometric analysis of surface expression of CD11a, CD18, and Fas. Anti-human CD11a (clone MAB3595; R&D Systems) (1:500 dilution), anti-human CD18 (D4N5Z; Cell Signaling Technology) (1:1,000 dilution), anti-human Fas (clone 4C3; Cell Signaling Technology) (1:1,000 dilution) and anti-glyceraldehyde-3-phosphate dehydrogenase conjugated to horseradish peroxidase (GAPDH-HRP; 1:2,500 dilution) (clone 1E6D9; ProteinTech) were used as primary antibodies for Western blot analyses. The secondary antibody used to detect CD11a and Fas was goat anti-mouse-HRP (ThermoFisher), and the secondary antibody used to detect CD18 was goat anti-rabbit-HRP (Invitrogen).
Generation of CRISPR knockouts.
pCMV-GFP (where CMV is cytomegalovirus and GFP is green fluorescent protein) CRISPR plasmids with guide RNAs (gRNAs) targeting specific exons in CD11a, CD18, or Fas were purchased (Sigma-Aldrich). Plasmids were transiently transfected into Jurkat E6.1 cells using Fugene transfection reagent (Promega), according to the recommended protocol. GFP-positive (GFP+) populations were collected via fluorescence-activated cell sorting (FACS), and independent clones were isolated. Sequencing analysis of independent clones to confirm gene editing was performed at the Rutgers New Jersey Medical School Molecular Resource Facility. Flow cytometry and Western blot analysis (described below) were also used to confirm gene knockout.
Cell death assays.
To evaluate cell death, 106 cells/ml were treated with various concentrations of LtxA. Cell death was measured via staining with annexin V-FITC and 7-AAD (BioLegend) and flow cytometry. Samples were analyzed on a FACSCalibur (BD Biosciences). Ten thousand events per sample were recorded. Cytotoxicity was defined as the sum of cells staining annexin V+/7-AAD− and annexin V+/7-AAD+. In some experiments, staurosporine (1 μM) or soluble FasL (10 ng/ml) was used as a positive control.
Activation of caspases.
A total of 106 cells/ml was plated and treated with various concentrations of LtxA for 24 h. Following treatment, cells were harvested and washed with phosphate-buffered saline (PBS). Cells were stained with polycaspase FAM-VAD-FMK (ThermoFisher) according to the recommended protocol. Activation of caspases was monitored using a FACSCalibur flow cytometer. Ten thousand events were recorded per sample. The mean fluorescence intensity (MFI) and percent caspase activation were determined using FlowJo software (BD Biosciences).
Loss of mitochondrial membrane potential.
A total of 106 cells/ml was plated and treated with various concentrations of LtxA. Cells were harvested at 24 h posttreatment and washed with PBS. Cells were stained with 75 nM MitoTracker Red CMXRos (Molecular Probes) according to the recommended protocol. Changes in mitochondrial membrane potential (MMP) were determined by flow cytometry. Ten thousand events were recorded per sample. Mean fluorescence intensity (MFI) and percent loss of MMP were determined using FlowJo software.
Western blot analysis.
Cells (2 × 106) were lysed in mammalian protein extraction reagent (m-PER; ThermoFisher) containing protease and phosphatase inhibitors (ThermoFisher). Samples were resuspended in SDS dye and separated via SDS-PAGE. Proteins were transferred to a nitrocellulose membrane using an iBlot dry blotting transfer system (Life Technologies). Membranes were probed with the appropriate primary antibodies (described above) overnight. Secondary antibodies conjugated to HRP were used to detect primary antibodies. For all Western blotting, 30 μg of protein was loaded into each well.
Flow cytometry.
A total 106 cells/ml was washed with PBS and stained with the appropriate fluorochrome-conjugated antibody. Samples were incubated on ice in the dark for 25 to 30 min. Following incubation, samples were washed with PBS twice and analyzed on a FACSCalibur (BD Biosciences). Ten thousand events were collected per sample, and the results were analyzed on FlowJo software (BD Biosciences).
Statistical analysis.
For statistical analysis, data were subjected to an unpaired Student's t test, with a P value os <0.05 considered to be statistically significant. Error bars represent the standard errors of the means (SEM). All calculations were performed using GraphPad Prism software.
Supplementary Material
ACKNOWLEDGMENTS
B.A.V., B.A.B., and S.C.K. designed the experiments and analyzed data. B.A.V., L.T.S., and T.K. performed the experiments. B.A.V. and S.C.K. wrote the manuscript.
This work was supported by a small business grant from the National Cancer Institute (R41CA173900) to B.A.B. Additionally, B.A.V. was supported by NIAID (T32AI125185).
We thank the New Jersey Medical School Molecular Resource Facility for assistance with sequencing analysis and Sukhwinder Singh and Tammy Galenkamp for technical assistance with flow cytometry.
S.C.K. declares competing interests in the form of stock ownership in the company (Actinobac Biomed, Inc.) that has licensed the use of leukotoxin. In addition, S.C.K. has received consulting fees from this company. B.A.B. is an employee of Actinobac Biomed, Inc., and owns stock in this company. There are no other conflicts of interest to declare.
This paper is dedicated to the memory of Edward T. Lally, who discovered that LFA-1 is the receptor for leukotoxin.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/IAI.00309-19.
REFERENCES
- 1.Kachlany SC. 2010. Aggregatibacter actinomycetemcomitans leukotoxin: from threat to therapy. J Dent Res 89:561–570. doi: 10.1177/0022034510363682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lally ET, Kieba IR, Sato A, Green CL, Rosenbloom J, Korostoff J, Wang JF, Shenker BJ, Ortlepp S, Robinson MK, Billings PC. 1997. RTX toxins recognize a 2 integrin on the surface of human target cells. J Biol Chem 272:30463–30469. doi: 10.1074/jbc.272.48.30463. [DOI] [PubMed] [Google Scholar]
- 3.Dileepan T, Kachlany SC, Balashova NV, Patel J, Maheswaran SK. 2007. Human CD18 is the functional receptor for Aggregatibacter actinomycetemcomitans leukotoxin. Infect Immun 75:4851–4856. doi: 10.1128/IAI.00314-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kachlany SC, Schwartz AB, Balashova NV, Hioe CE, Tuen M, Le A, Kaur M, Mei Y, Rao J. 2010. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk Res 34:777–785. doi: 10.1016/j.leukres.2009.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kinashi T. 2005. Intracellular signalling controlling integrin activation in lymphocytes. Nat Rev Immunol 5:546–559. doi: 10.1038/nri1646. [DOI] [PubMed] [Google Scholar]
- 6.Fischer A, Lisowska-Grospierre B, Anderson DC, Springer TA. 1988. Leukocyte adhesion deficiency: molecular basis and functional consequences. Immunodefic Rev 1:39–54. [PubMed] [Google Scholar]
- 7.Hogg N, Harvey J, Cabanas C, Landis RC. 1993. Control of leukocyte integrin activation. Am Rev Respir Dis 148:S55–9. doi: 10.1164/ajrccm/148.6_Pt_2.S55. [DOI] [PubMed] [Google Scholar]
- 8.Hogg N, Smith A, McDowall A, Giles K, Stanley P, Laschinger M, Henderson R. 2004. How T cells use LFA-1 to attach and migrate. Immunol Lett 92:51–54. doi: 10.1016/j.imlet.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 9.Damiano JS, Hazlehurst LA, Dalton WS. 2001. Cell adhesion-mediated drug resistance (CAM-DR) protects the K562 chronic myelogenous leukemia cell line from apoptosis induced by BCR/ABL inhibition, cytotoxic drugs, and gamma-irradiation. Leukemia 15:1232–1239. doi: 10.1038/sj.leu.2402179. [DOI] [PubMed] [Google Scholar]
- 10.de la Fuente MT, Casanova B, Moyano JV, Garcia-Gila M, Sanz L, Garcia-Marco J, Silva A, Garcia-Pardo A. 2002. Engagement of α4β1 integrin by fibronectin induces in vitro resistance of B chronic lymphocytic leukemia cells to fludarabine. J Leukoc Biol 71:495–502. [PubMed] [Google Scholar]
- 11.Bechter OE, Eisterer W, Dirnhofer S, Pall G, Kuhr T, Stauder R, Thaler J. 1999. Expression of LFA-1 identifies different prognostic subgroups in patients with advanced follicle center lymphoma (FCL). Leuk Res 23:483–488. doi: 10.1016/S0145-2126(99)00036-3. [DOI] [PubMed] [Google Scholar]
- 12.Desgrosellier JS, Cheresh DA. 2010. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer 10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inghirami G, Wieczorek R, Zhu BY, Silber R, Dalla-Favera R, Knowles DM. 1988. Differential expression of LFA-1 molecules in non-Hodgkin's lymphoma and lymphoid leukemia. Blood 72:1431–1434. [PubMed] [Google Scholar]
- 14.DiFranco KM, Gupta A, Galusha LE, Perez J, Nguyen TV, Fineza CD, Kachlany SC. 2012. Leukotoxin (Leukothera®) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J Biol Chem 287:17618–17627. doi: 10.1074/jbc.M111.314674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hioe CE, Tuen M, Vasiliver-Shamis G, Alvarez Y, Prins KC, Banerjee S, Nadas A, Cho MW, Dustin ML, Kachlany SC. 2011. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA). PLoS One 6:e23202. doi: 10.1371/journal.pone.0023202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fong KP, Pacheco CM, Otis LL, Baranwal S, Kieba IR, Harrison G, Hersh EV, Boesze-Battaglia K, Lally ET. 2006. Actinobacillus actinomycetemcomitans leukotoxin requires lipid microdomains for target cell cytotoxicity. Cell Microbiol 8:1753–1767. doi: 10.1111/j.1462-5822.2006.00746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kelk P, Abd H, Claesson R, Sandstrom G, Sjostedt A, Johansson A. 2011. Cellular and molecular response of human macrophages exposed to Aggregatibacter actinomycetemcomitans leukotoxin. Cell Death Dis 2:e126. doi: 10.1038/cddis.2011.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kelk P, Johansson A, Claesson R, Hanstrom L, Kalfas S. 2003. Caspase 1 involvement in human monocyte lysis induced by Actinobacillus actinomycetemcomitans leukotoxin. Infect Immun 71:4448–4455. doi: 10.1128/iai.71.8.4448-4455.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balashova N, Dhingra A, Boesze-Battaglia K, Lally ET. 2016. Aggregatibacter actinomycetemcomitans leukotoxin induces cytosol acidification in LFA-1 expressing immune cells. Mol Oral Microbiol 31:106–114. doi: 10.1111/omi.12136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.DiFranco KM, Johnson-Farley N, Bertino JR, Elson D, Vega BA, Belinka BA Jr, Kachlany SC. 2015. LFA-1-targeting leukotoxin (LtxA; Leukothera®) causes lymphoma tumor regression in a humanized mouse model and requires caspase-8 and Fas to kill malignant lymphocytes. Leuk. Res 39:649–656. doi: 10.1016/j.leukres.2015.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta A, Le A, Belinka BA, Kachlany SC. 2011. In vitro synergism between LFA-1 targeting leukotoxin (Leukothera) and standard chemotherapeutic agents in leukemia cells. Leuk Res 35:1498–1505. doi: 10.1016/j.leukres.2011.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Gupta A, Espinosa V, Galusha LE, Rahimian V, Miro KL, Rivera-Medina A, Kasinathan C, Capitle E, Aguila HA, Kachlany SC. 2014. Expression and targeting of lymphocyte function-associated antigen 1 (LFA-1) on white blood cells for treatment of allergic asthma. J Leukoc Biol doi: 10.1189/jlb.5HI0414-196R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stenderup K, Rosada C, Dam TN, Salerno E, Belinka BA, Kachlany SC. 2011. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J Invest Dermatol 131:2033–2039. doi: 10.1038/jid.2011.161. [DOI] [PubMed] [Google Scholar]
- 24.DiFranco KM, Kaswala RH, Patel C, Kasinathan C, Kachlany SC. 2013. Leukotoxin kills rodent WBC by targeting leukocyte function associated antigen 1. Comp Med 63:331–337. [PMC free article] [PubMed] [Google Scholar]
- 25.Kieba IR, Fong KP, Tang HY, Hoffman KE, Speicher DW, Klickstein LB, Lally ET. 2007. Aggregatibacter actinomycetemcomitans leukotoxin requires beta-sheets 1 and 2 of the human CD11a beta-propeller for cytotoxicity. Cell Microbiol 9:2689–2699. doi: 10.1111/j.1462-5822.2007.00989.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Allende LM, Hernandez M, Corell A, Garcia-Perez MA, Varela P, Moreno A, Caragol I, Garcia-Martin F, Guillen-Perales J, Olive T, Espanol T, Arnaiz-Villena A. 2000. A novel CD18 genomic deletion in a patient with severe leucocyte adhesion deficiency: a possible CD2/lymphocyte function-associated antigen-1 functional association in humans. Immunology 99:440–450. doi: 10.1046/j.1365-2567.2000.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korostoff J, Yamaguchi N, Miller M, Kieba I, Lally ET. 2000. Perturbation of mitochondrial structure and function plays a central role in Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Microb Pathog 29:267–278. doi: 10.1006/mpat.2000.0390. [DOI] [PubMed] [Google Scholar]
- 28.Nice JB, Balashova NV, Kachlany SC, Koufos E, Krueger E, Lally ET, Brown AC. 2018. Aggregatibacter actinomycetemcomitans leukotoxin is delivered to host cells in an LFA-1-indepdendent manner when associated with outer membrane vesicles. Toxins (Basel) 10:e414. doi: 10.3390/toxins10100414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weber KS, York MR, Springer TA, Klickstein LB. 1997. Characterization of lymphocyte function-associated antigen 1 (LFA-1)-deficient T cell lines: the alphaL and beta2 subunits are interdependent for cell surface expression. J Immunol 158:273–279. [PubMed] [Google Scholar]
- 30.Yamaguchi N, Kieba IR, Korostoff J, Howard PS, Shenker BJ, Lally ET. 2001. Maintenance of oxidative phosphorylation protects cells from Actinobacillus actinomycetemcomitans leukotoxin-induced apoptosis. Cell Microbiol 3:811–823. doi: 10.1046/j.1462-5822.2001.00161.x. [DOI] [PubMed] [Google Scholar]
- 31.Schneider P, Bodmer JL, Holler N, Mattmann C, Scuderi P, Terskikh A, Peitsch MC, Tschopp J. 1997. Characterization of Fas (Apo-1, CD95)-Fas ligand interaction. J Biol Chem 272:18827–18833. doi: 10.1074/jbc.272.30.18827. [DOI] [PubMed] [Google Scholar]
- 32.Orlinick JR, Vaishnaw A, Elkon KB, Chao MV. 1997. Requirement of cysteine-rich repeats of the Fas receptor for binding by the Fas ligand. J Biol Chem 272:28889–28894. doi: 10.1074/jbc.272.46.28889. [DOI] [PubMed] [Google Scholar]
- 33.Balashova NV, Shah C, Patel JK, Megalla S, Kachlany SC. 2009. Aggregatibacter actinomycetemcomitans LtxC is required for leukotoxin activity and initial interaction between toxin and host cells. Gene 443:42–47. doi: 10.1016/j.gene.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 34.Fong KP, Tang HY, Brown AC, Kieba IR, Speicher DW, Boesze-Battaglia K, Lally ET. 2011. Aggregatibacter actinomycetemcomitans leukotoxin is post-translationally modified by addition of either saturated or hydroxylated fatty acyl chains. Mol Oral Microbiol 26:262–276. doi: 10.1111/j.2041-1014.2011.00617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Stanley P, Koronakis V, Hughes C. 1998. Acylation of Escherichia coli hemolysin: a unique protein lipidation mechanism underlying toxin function. Microbiol Mol Biol Rev 62:309–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stanley P, Packman LC, Koronakis V, Hughes C. 1994. Fatty acylation of two internal lysine residues required for the toxic activity of Escherichia coli hemolysin. Science 266:1992–1996. doi: 10.1126/science.7801126. [DOI] [PubMed] [Google Scholar]
- 37.Kischkel FC, Hellbardt S, Behrmann I, Germer M, Pawlita M, Krammer PH, Peter ME. 1995. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J 14:5579–5588. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME. 1998. Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liles WC, Kiener PA, Ledbetter JA, Aruffo A, Klebanoff SJ. 1996. Differential expression of Fas (CD95) and Fas ligand on normal human phagocytes: implications for the regulation of apoptosis in neutrophils. J Exp Med 184:429–440. doi: 10.1084/jem.184.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Diaz R, Ghofaily LA, Patel J, Balashova NV, Freitas AC, Labib I, Kachlany SC. 2006. Characterization of leukotoxin from a clinical strain of Actinobacillus actinomycetemcomitans. Microb Pathog 40:48–55. doi: 10.1016/j.micpath.2005.10.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.