Abstract
Purpose
The carbohydrate Sialyl LewisX (sLeX) mediates cell adhesion, is critical in the normal function of immune cells, and is frequently over-expressed on cancer cells. We assessed the association, differential levels, and prognostic value of sLeX and inflammatory cytokines/chemokines in breast cancer sera.
Methods
We retrospectively measured sLeX and a panel of cytokines/chemokines in the sera of 26 non-invasive ductal carcinoma in situ (DCIS), 154 invasive non-metastatic breast cancer (non-MBC), 63 metastatic breast cancer (MBC) patients, and 43 healthy controls. Differences in sLeX and inflammatory cytokines among and between patient groups and healthy controls were assessed with nonparametric tests and we performed survival analysis for the prognostic potential of sLeX using a cut-off of 8 U/ml as previously defined.
Results
Median serum sLeX was significantly higher than controls for invasive breast cancer patients (MBC and non-MBC) but not DCIS. In univariate analysis, we confirmed patients with serum sLeX >8 U/ml have a significantly shorter progression free survival (PFS) (P=0.0074) and overall survival (OS (P=0.0003). Similarly, patients with high serum MCP-1 and IP-10 had shorter OS (P=0.001 and P<0.001, respectively) and PFS (P=0.010 and P<0.001, respectively). sLeX, MCP- 1 and IP-10 remained significant in multivariate survival analysis.
Conclusion
Elevated serum sLeX was associated with invasive cancer but not DCIS. High serum sLeX levels were associated with inflammatory mediators and may play a role in facilitating local invasion of breast tumor. Furthermore, serum MCP-1, IP-10 and sLeX may have prognostic value in breast cancer.
Keywords: sialyl LewisX (sLeX), inflammatory cytokines, breast cancer, serum
Introduction
The regulation of cell adhesion is a critical process throughout the development of metastatic cancer. Both heterotypic and homotypic cancer cell adhesion are mediated in part by specific interactions between cell surface lectins and their cognate carbohydrate ligands presented on glycoproteins and glycolipids[1–3]. Increased sialylation is a common feature in the glycoconjugates of malignant cells[4]. Sialyl Lewis X (sLeX) is a mucin-associated carbohydrate ligand on cancer cells that binds to its receptor E-selectin on endothelial cells to mediate adhesion between the cancer cells and the endothelium. E-selectin plays a pivotal role in the capture and rolling of cancer cells on the surface of endothelial cells. Surface expression of sLeX on cancer cells mediates their tethering and rolling along vascular endothelium expressing E-selectin, thereby facilitating migration and dissemination of cancer cells, potentially leading to metastasis. Aberrant expression of sLeX is associated with tumor formation and metastasis[5] and the level of sLeX was found to be elevated in the sera of patients with metastatic breast cancers (MBC) [6] and correlated with metastasis[7]. Furthermore, overexpression of sLeX was associated with higher tumor stage[8] poorer prognosis and malignant relapse [9].
Using an immunoassay (CSLEX; Nittobo Medical Co. Ltd., Japan), it is possible to measure sLeX in serum and it has been reported that the level is associated with clinical stage and response to treatment of breast cancer[10]. Using a cut-off of 8 U/ml for sLeX, serum sLeX in combination with CA15–3 (Cancer Antigen-Breast) was found to be more useful than carcinoembryonic antigen (CEA) and tumor marker CA15–3 combined in monitoring breast cancer patients[10]. Others have demonstrated that gene transcripts of sLeX are significantly increased in estrogen receptor alpha-negative (ER-negative) tumors compared with that of ER-positive ones. In this study serum sLeX level had no association with survival, irrespective of ER expression by their tumors. However, high expression of sLeX in ER-positive tumors is correlated with metastasis to the bone where the sLeX receptor, E-selectin, is constitutively expressed[11].
Typically, initial adhesion of leukocytes to a site of injury is mediated by E-selectin, the specific receptor for sLeX[12]. In tumors, E-selectin plays a pivotal role in recruiting leukocytes to the tumor microenvironment where immune-specific tumor lysis can trigger a storm of inflammatory cytokines inducing the over-expression of E-selectin on endothelial cells of blood vessels[13]. sLeX on the tumor cells interacts with E-selectin on endothelial cells to initiate motility by causing tumor cells to roll on the endothelium. Whereas sLeX binds to E-selectin to facilitate metastasis, inflammatory cytokines may enhance the metastatic potential of human cancer by upregulating the expression of E-selectin on endothelial cells.
Abnormal expression of sLeX has attracted much attention because of its function in cancer cell extravasation, mimicking a molecular mechanism involved in leukocyte extravasation[14–16]. In colorectal cancer, the high expression of sLeX in adenomas was correlated with a high degree of dysplasia[17]. In patients with small cell lung cancer, preoperative serum sLeX values were associated with pathological stages and survival after surgery[18].
In this study we evaluated the serum expression of sLeX and a panel of 39 inflammatory proteins. The primary aims were to compare differential serum levels of sLeX in patients with non-invasive ductal carcinoma in situ (DCIS), metastatic (MBC) and non-metastatic breast cancer (non-MBC) as well as healthy donors (HD) with the hypothesis that higher serum sLeX would correlate with higher disease stage, and second to confirm previous publications that serum sLex >8U/ml is correlated with decreased survival.
Methods
Patient characteristics
This retrospective study was approved by the MD Anderson Institutional Review Board (IRB) as LAB09–0347 for the evaluation of patients with non-invasive DCIS, invasive breast cancer (MBC), and healthy donors (HD) who have participated in previous diagnostic protocols at MDACC (IRB approved as LAB09–049, 2005–0243, ID02–052, 2006–1072, ID02–458, LAB03–0479, LAB05–0083, ID99–231, LAB08–0231, and LAB08–0199). A request for waiver of informed consent was approved for this retrospective study as it posed no more than minimal risk and would not adversely affect the rights or welfare of the patients. Serum samples were collected pre-treatment or at the start of a new line of therapy. Patients were classified as having hormone receptor (HR) positive tumors if estrogen receptor (ER) and/or progesterone receptor (PR) were expressed at >10% by immunohistochemistry (IHC) of the primary tumor. Likewise, patients were categorized as human epidermal growth factor receptor 2 (HER2) enriched if either the primary tumor had a fluorescent in situ hybridization (FISH) HER2:CEP 17 ratio > 2 or III+ by IHC if FISH was not performed.
Measurement of sLeX
Sera from 243 patients (26 with DCIS, 154 with non-MBC, 63 with MBC), and 43 HD were assayed for sLeX using a two-step sandwich enzyme immunoassay method (Nittobo Medical Co., Japan). Briefly, 20 μL of sera were incubated in a 96-well plate coated with anti-sLeX monoclonal antibody in a buffer solution for 2 hours at 37°C to enable the capture of sLeX. Thereafter, the plates were washed to remove unbound proteins. Next, horseradish peroxidase-labeled secondary antibody was added to the antibody-sLeX complex in buffer solution to form a sandwich complex of antibody-antigen-antibody and incubated for 2 hours at 37°C. Again, unbound proteins were washed out before the addition of substrate TMB (3,3,5,5′-tetramethylbenzidine). The absorbance was measured from the result of the enzymatic reaction. Because the strength of absorbance is closely related to the concentration of sLeX in the patient serum, the sLeX concentration was estimated using the standard curve.
Serum cytokines and chemokines
A panel of 39 cytokines and chemokines including MCP-1 and IP-10 were measured in a 25μL sample using the Millipore Milliplex Human Cytokine/Chemokine Panel I, a multiplex assay kit (Millipore Corp, Billerica, MA), and a Luminex Analyzer 100 (Austin, TX), as previously described[19].
Statistical Analysis
The primary objective was to compare the serum level of sLeX in healthy individuals and patients with ductal carcinoma in situ (DCIS), non-metastatic breast cancer (non-MBC) and metastatic breast cancer (MBC). The secondary objective was to evaluate the prognostic value of sLex in patients with primary breast cancer (Stages I-III). The non-parametric Mann-Whitney U tests or Kruskal-Wallis H tests were used to determine the differences in sLeX, cytokine, and chemokines levels between/or among patient groups and HD. Spearman’s correlation was used to determine the correlation between the serum levels of sLeX and tumor-promoting inflammatory mediators. Overall survival time was calculated from the time of sample to death (event) and, progression free survival (PFS) time from sample time to progression or death whichever happens the first (event) or last follow-up time (censor). Only patients with Stage I ~ IV breast cancers (no DCIS) were included in the time to event analysis. OS and PFS times were estimated using the Kaplan-Meier method and compared between or among patients’ characteristics groups using log-rank test. A pre-determined cut-point of 8U/ml was used for sLeX, and for cytokines, a cutoff was established at the 95th percentile of the mean of the healthy donors. Cox proportional hazard models are applied to estimate the effect of covariates of interest on OS and PFS times. Stepwise selection method is used to choose the statistically significant covariates that are associated with OS and PFS times. All computations are carried out in SAS 9.3 (SAS Institute Inc., Cary, NC, USA) and Splus 8.2 (TIBCO Software Inc, Palo Alto, CA) with plots generated in R (R Core Team (2017), Vienna, Austria) and Graph Pad Prism (La Jolla, CA).
Results
Patient Characteristics
The study included a total of 243 breast cancer patients including 26 who presented with DCIS, 154 with primary, non-metastatic disease and 63 with metastatic disease. The median follow-up period for stage I ~IV breast cancer was 74.4 months (95% CI: 63.3 – 79.5 months). In addition, 43 healthy donors volunteered samples. Baseline patient characteristics are shown in Table 1. There were 166 patients with hormone receptor positive tumors (HR); 90 patients were HER2-enriched; 46 patients had triple negative breast cancer (TNBC). The 5 patients that had low or weak positive hormonal staining were classified TNBC (n=3) or HER2 (n=2). The clinic stages and subgroups of patients were listed in Table 2.
Table 1.
Patient Characteristics
| Count | Percentage | ||
|---|---|---|---|
| Type | DCIS (0) | 26 | 10.7% |
| non-MBC (I,II, III) | 154 | 63.4% | |
| MBC (IV) | 63 | 25.9% | |
| HD | 43 | ||
| Clinical Stage | DCIS (0) | 26 | 10.7% |
| I | 48 | 19.8% | |
| II | 47 | 19.3% | |
| III | 59 | 24.3% | |
| IV | 63 | 25.9% | |
| ER | Negative | 79 | 32.5% |
| Positive | 164 | 67.5% | |
| PR | Negative | 103 | 42.4% |
| Positive | 140 | 57.6% | |
| HR (ER or PR) | Negative | 77 | 31.7% |
| Positive | 166 | 68.3% | |
| Her2 | Negative | 153 | 62.9% |
| Positive | 90 | 37.1% | |
| Triple Negative | No | 197 | 81.1% |
| Yes | 46 | 18.9% |
Table 2.
Clinical stages and distribution
| CSLEX | ||||
|---|---|---|---|---|
| levels | High | Low | P value | |
| Breast Cancer Type | HD | 3(7%) | 40(93%) | .0106 |
| MBC | 21(33.3%) | 42(66.7%) | .1366* | |
| DCIS | 5(19.2%) | 21(80.8%) | ||
| nMBC | 32(20.8%) | 122(79.2%) | ||
| Clinical Stage | HD | 3(7%) | 40(93%) | .0202 |
| 0 | 5(19.2%) | 21(80.8%) | .1926* | |
| I | 8(16.7%) | 40(83.3%) | ||
| II | 8(17%) | 39(83%) | ||
| III | 16(27.1%) | 43(72.9%) | ||
| MBC | 21(33.3%) | 42(66.7%) | ||
| Hormone | Negative | 28(36.4%) | 49(63.6%) | .0019 |
| Positive | 30(18.1%) | 136(81.9%) | ||
| HER2 | Negative | 35(22.9%) | 118(77.1%) | .6361 |
| Positive | 23(25.6%) | 67(74.4%) | ||
| TNBC | Non-TNBC | 42(21.3%) | 155(78.7%) | .0538 |
| TNBC | 16(34.8%) | 30(65.2%) | ||
Excluding HD cases
sLeX and inflammatory cytokines elevated in breast cancer
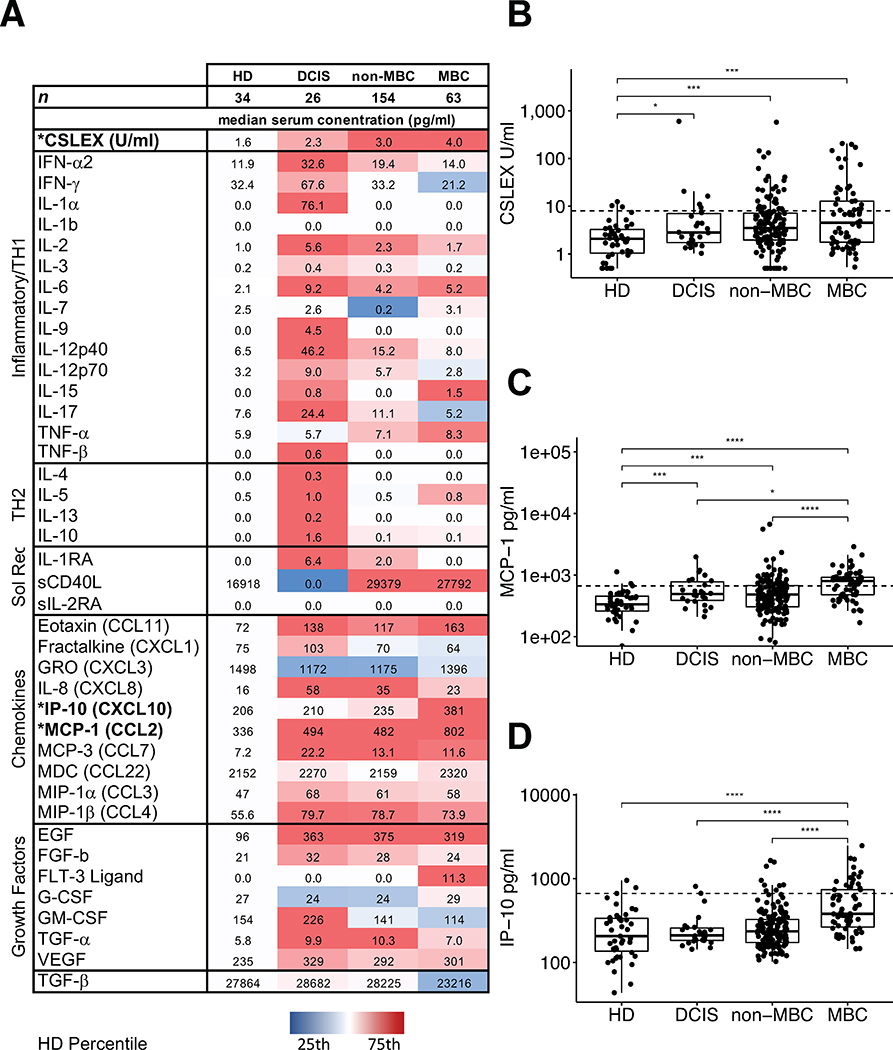
Compared with the median serum sLeX level in HD (1.6 U/mL), the median serum levels of sLeX of DCIS (2.3 U/ml) non-MBC (3.0 U/mL) and MBC (4.0 U/mL) were significantly higher (all P < 0.05) (Figure 1). In confirmation of previous reports that suggested using 8 U/ml as a cut-off for high levels of CSLEX[10], the 95th percentile of HD in the current study’s cohort was 9.2 U/ml, confirming 8.0 U/ml as a reasonable cut-off. Although not significant, patients with MBC were more likely than those with non-MBC to have serum sLeX levels above 8.0 U/ml (33.3% vs 20.8%, P=0.08).
Fig 1. Levels of serum sLeX, IP-10, and MCP-1 in the study groups.
The heatmap (A) shows median values for each study group. Factors selected for survival analysis are in bold. The color code scale is set to the distribution of HD values for each target protein. Cytokines are loosely grouped into generally inflammatory and T-helper 1 (TH1) cellular immunity-related cytokines, TH2 humoral immunity-related cytokines, soluble cytokine receptors (Sol Rec), chemokines, and growth factors. (B) Compared to HD, median sLeX levels of DCIS (2.3 U/mL), non-MBC (2.9 U/mL), and MBC (4.0 U/mL) were significantly higher (all P < 0.05). MBC patients had a significantly higher levels of MCP-1 than DCIS (P = 0.019) or non-MBC (P < 0.0001, C); and higher levels of IP-10 than non-MBC, DCIS and HD (P < 0.0001, D). Dotted line shows cut-off for survival analysis.
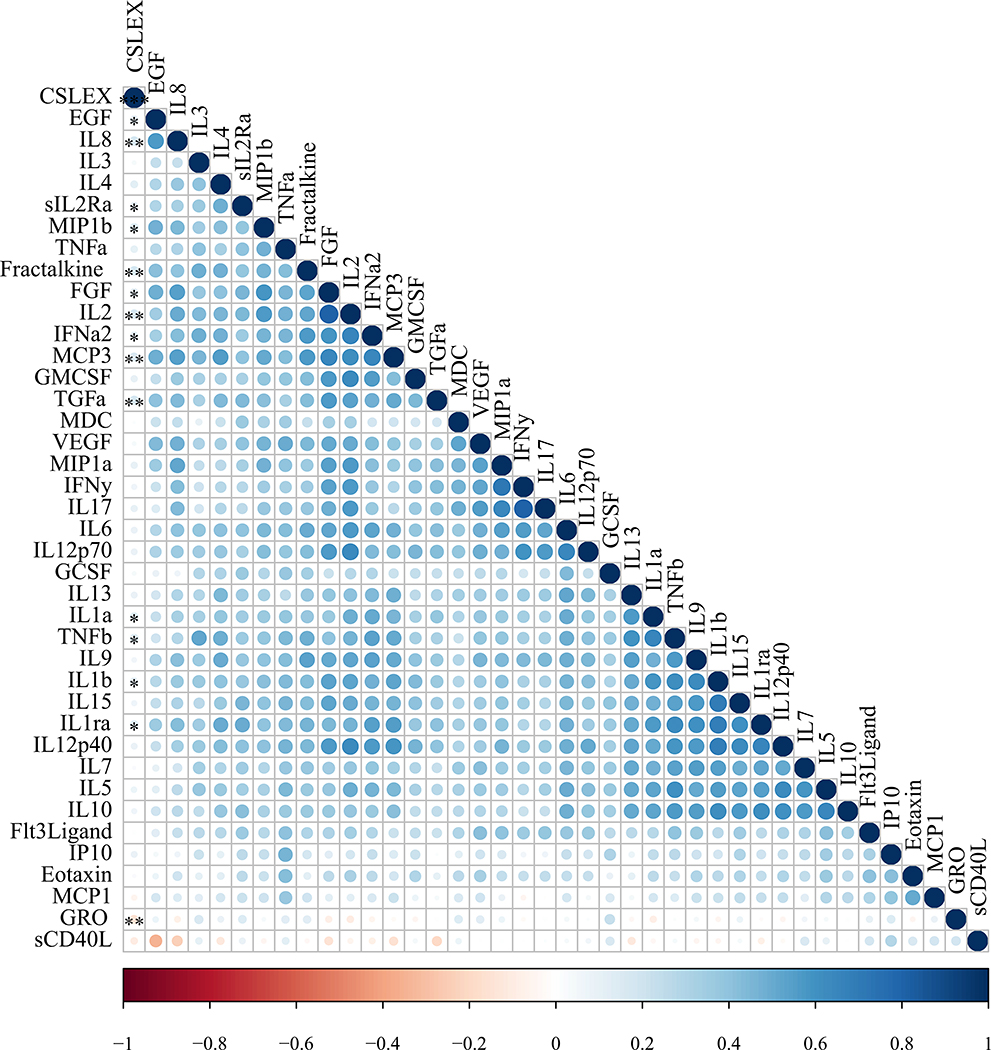
Compared with HD, DCIS or non-MBC patients had significantly higher median levels of epidermal growth factor (EGF), eotaxin (CCL11), fibroblast growth factor (FGF)-2, interferon (IFN)-α, interleukin (IL)-1 receptor antagonist (RA), IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-8, monocyte chemotactic protein (MCP)-1, MCP-3, macrophage inflammatory protein (MIP)-1β, and transforming growth factor (TGF)-α and lower growth-regulated oncogene (GRO). Samples from DCIS patients had significantly higher levels of IL-5, IL-9 and IL-12p40 than HD serum samples. MBC patients also had significantly higher serum levels of EGF, eotaxin, interferon gamma response protein (IP)-10, IL-1RA, IL-1β, IL-6, IL-8, IL-10, MCP-1, MCP-3, MIP-1β, sCD40L and tumor necrosis factor (TNF)-α than those of HD. Compared with DCIS or patients with non-MBC, those with MBC had a significantly higher level of IP-10 (P= 0.0001) and a higher level of MCP-1 (P<0.0001) (Figure 1) as well as higher levels TGF-α and lower IL-2 and TGF-β (not shown). Furthermore, from an analysis of all study samples there were positive correlations between the serum level of sLeX and the levels of growth factors EGF, and FGF, pro-inflammatory cytokines IL-1α, IL-1β, IL-1RA, IL-2, IL-2RA, IFN-α2, TGF-α, TNF-β, and chemokines IL-8 (CXCL8), fractalkine (CX3CL1), MIP-1β (CCL4) and MCP-3 (CCL7) and a negative correlation for GRO (CXCL1) (Spearman correlation, P < 0.05; Table 3, Figure 2). Additional summary statistics are provided in a supplemental table.
Table 3.
Non-parametric correlation between sLeX and inflammatory cytokines
| Correlation with sLeX | ||
|---|---|---|
| Spearman’s rho | p | |
| IL-2 | 0.1749 | 0.0030 |
| TGF-a | 0.17156 | 0.0036 |
| IL-8 | 0.16728 | 0.0046 |
| Fractalkine | 0.16558 | 0.0050 |
| MCP-3 | 0.16121 | 0.0063 |
| GRO | −0.15812 | 0.0074 |
| TNF-b | 0.15055 | 0.0108 |
| MIP1-b | 0.14824 | 0.0121 |
| IL-1α | 0.14273 | 0.0157 |
| IL-1ra | 0.14117 | 0.0169 |
| IL-1b | 0.12844 | 0.0299 |
| IFN-a2 | 0.12497 | 0.0346 |
| EGF | 0.12027 | 0.0421 |
| FGF | 0.118 | 0.0462 |
| sIL-2Ra | 0.11787 | 0.0464 |
Fig 2. Non-parametric correlation between sLeX and inflammatory cytokines.
The correlation matrix shows Spearman correlation hierarchical clustering of sLeX (CSLEX assay, first column) with inflammatory cytokines in all samples. Color shows strength of correlation and stars in the CSLEX column indicate significance level of correlation with sLeX: * P<0.05, ** P< 0.01, *** P<0.001
Survival Analysis
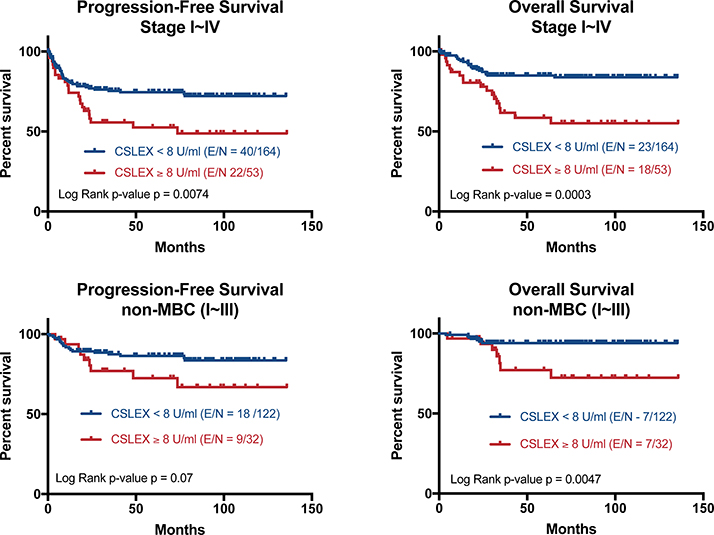
Progression-free survival (PFS) and overall survival (OS) were assessed in stage I ~ IV breast cancer patients based on sLeX stratification at 8 U/ml. The median follow-up time was 74.4 months and the median PFS and OS were not reached. For MBC patients, the median PFS was 8.5 months (95% CI 6.1 – 18.2) and median OS was 26.7 months (95% CI 13.8 - not reached). In univariate analysis, clinical pathological features including clinical stage, ER/PR negativity, TNBC status (but not HER2 alone) and sLeX status were all associated with decreased PFS and OS. Patients with a serum sLeX level above the predetermined level of 8 U/ml have a significantly shorter PFS (P=0.0074) and OS (P=0.0003) (Figure 3 top). Five-year OS is 84.9% in patients with serum sLeX < 8 U/ml group compared to 58.6% patients with sLeX ≥8 U/ml.
Fig 3. Elevated sLeX associated with decreased survival.
Top: Retrospective analysis of patients with stage I - IV breast cancer shows patients with high serum sLeX (above 8U/ml, n = 53) have a significantly shorter PFS (P=0.007) or OS (P<0.001) than those with low serum sLeX (n = 164). Bottom: In primary breast cancer (stages I – III including IBC), patients with high sLeX (n = 32) have a trend towards shorter PFS (p = 0.07) and significantly reduced OS (p = 0.0047) compared to patients with low sLeX (n = 122)
The primary end-point of the study was to determine the prognostic utility of CSLEX with a pre-defined cut-off of 8 U/ml in primary breast cancer (Stage I - III). The study included 154 non-MBC patients of whom 25 were diagnosed with inflammatory breast cancer (IBC). The full cohort of non-MBC patients (including IBC) with serum sLeX ≥ 8.0 U/ml had a trend towards shorter PFS (P = 0.07) and significantly shorter OS (P = 0.0047) (Figure 3 bottom) compared to patients with serum sLeX <8.0 U/ml. Excluding inflammatory breast cancer (not shown) there were 129 patients with non-MBC with an median follow-up of 81.1 months (range 6 to 135 months). There were 3 deaths, all in patients with serum sLeX ≥8.0 U/ml (log-rank P = 0.0003).
To characterize additional systemic inflammatory factors in breast cancer associated with breast cancer survival, a univariate Cox model indicates that, treating them as continuous variables, higher CSLEX and the chemokine IP-10 are associated with significantly shorter PFS time with hazard ratio of 1.004 and 1.002, respectively (p=0.0012 and p<.0001, respectively). In addition, higher CSLEX, IP10 and MCP1 are significantly associated with a shorter OS time with hazard ratios of 1.007, 1.002 and 1.0003, respectively (p<.0001, p<.0001 and p=0.002, respectively, not shown).
Multicovariate Cox models indicate that adjusted for the presence of metastasis (MBC vs non-MBC) and hormonal receptor status (positive vs negative), sLeX (>=8U/ml vs <8U/ml) was a significant factor associated with OS (HR=2.985, 95% CI 1.563 – 5.698, p=0.0009), as well as MCP-1 (HR=1.001, p=0.0003) and IP-10 (HR=1.001, p=0.0357, Table 4). Furthermore, adjusting for metastatic disease, sLeX (>=8U/ml vs <8U/ml) was associated with shorter PFS time (HR=1.653, 95% CI:0.977 – 2.795, p=0.0609), as well as IP-10 (HR=1.001, p=0.0017, Table 5).
Table 4.
Multivariate Cox regression model on overall survival
| Parameter | p-value | Hazard Ratio | 95% HR CI | ||
|---|---|---|---|---|---|
| Stage | MBC vs non-MBC | <.0001 | 8.787 | 4.314 | 17.895 |
| Hormone | Positive vs Negative | 0.012 | 0.429 | 0.222 | 0.83 |
| CSLEX_High | High vs Low | 0.0009 | 2.985 | 1.563 | 5.698 |
| MCP-1 | 0.0003 | 1.001 | 1 | 1.001 | |
| IP-10 | 0.0357 | 1.001 | 1 | 1.002 | |
Table 5.
Multivariate Cox regression model on progression-free survival
| Parameter | p-value | Hazard Ratio | 95% HR CI | ||
|---|---|---|---|---|---|
| Stage | MBC vs non-MBC | <.0001 | 7.661 | 4.371 | 13.428 |
| CSLEX | High vs Low | 0.0609 | 1.653 | 0.977 | 2.795 |
| IP-10 | 0.0017 | 1.001 | 1 | 1.002 | |
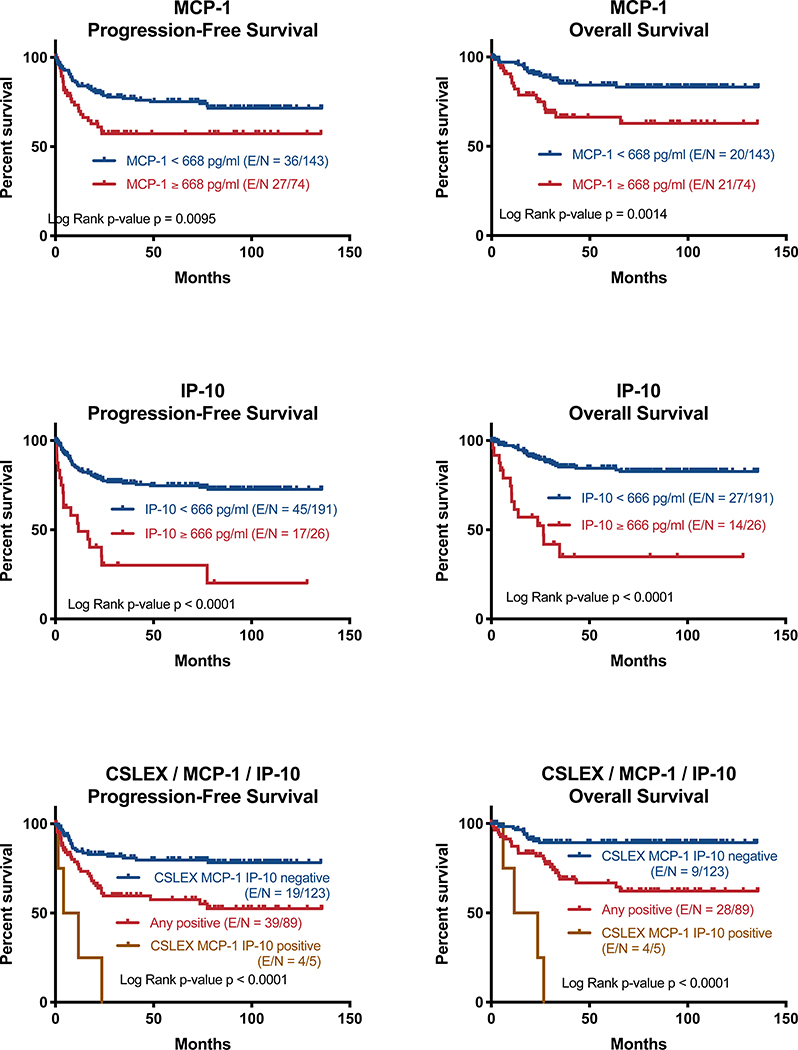
Dichotomizing MCP-1serum MCP-1 >= 668 pg/ml, the 95th percentile for HD, have a significantly shorter OS (P=0.0014) and PFS (P=0.0095) (Figure 4 top). Patients with serum IP-10 > 666 pg/ml, likewise the 95th percentile for HD, have significantly shorter OS and PFS (both P<0.0001, Figure 4 middle). Combining serum sLeX, MCP-1 and IP-10 into a single prognostic factor, patients with positive serum levels for all three had significantly worse OS and PFS compared to patients that were negative for all 3 or positive for any of the three (both P<0.0001, Figure 4 bottom).
Fig 4: Serum MCP-1 and IP-10 levels are predictive of PFS and OS in breast cancer.
Top: patients with a serum MCP-1 level above the 95 percentile of serum level of HD (668 pg/ml) have a significantly shorter PFS (P=0.0095) and OS (P=0.0014), respectively. MCP-1 high n = 74, MCP-1 low n = 143. Middle: patients with a serum IP-10 level above the 95 percentile of serum level of HD (666 pg/ml) have a significantly shorter PFS (P<0.0001) and OS (P<0.0001), respectively. IP-10 high n = 26, IP-10 low n = 191. Bottom: There is a significant survival advantage to low levels of sLeX, MCP-1 and IP-10. All low n = 123, any high n = 89, all high n = 5
Discussion
sLex is a tumor-associated carbohydrate that is used clinically for the management of lung, gastric and colorectal cancers in Japan [10]. Previous studies showed serum sLex > 8U/ml was a useful clinical marker in breast cancer patients. Here we report that serum sLeX levels tended to increase with advanced breast cancer stage and higher levels are correlated with decreased survival. Specifically, we found that MBC patients are more likely than non-MBC patients to have elevated serum sLeX levels >8 U/ml. In an earlier study, the expression of sLeX in paraffin-embedded tissue containing tumor was found to be highest in the primary tumor of patients with invasive mammary carcinoma that had already metastasized to the axillary lymph nodes[8]. Nevertheless, expression of sLeX was reduced in lymph node metastasis compared to the primary tumor. One possible explanation that could account for this difference between primary and metastatic tumor is the ability of natural killer (NK) cells to attack tumor cells that express high levels of sLeX[5] and could have broad implications for immunotherapy. It should be noted that among the primary cytokines regulating NK, serum IL-2 but neither IL-15 nor IL-12p70 were correlated with serum sLeX in the current study. However, despite our observation that the median serum sLeX level tended to increase with the stage of disease severity, sLeX was not significantly different by breast cancer stage.
We also observed that patients with a high serum sLeX have a significantly shorter survival (both PFS and OS) compared with patients with lower serum sLeX. Others have also assessed the prognostic value of sLeX expression in non-metastatic disease with a median follow-up of 140 months and found that the expression of sLeX antigen in breast cancer is not associated with breast cancer survival[20].
Cytokines and chemokines are known to be involved in tumor growth, metastasis, and progression of disease[21]. In particular, TNF-α, IL-1β or interferon-gamma (IFN-γ) are potential activators of expression of E-selectin, P-selectin, intercellular adhesion molecule 2 (ICAM-2) or vascular cell adhesion molecule on endothelial cells[22]. Further, the pro-inflammatory cytokine, IL-6 in serum is a negative prognosticator in breast tumor patients[23] and is predictive of inferior survival in MBC patients[24]. Moreover, the serum IL-8 level increases significantly with more advanced stages of disease and is associated with accelerated clinical progression, the presence of lymph node and liver metastasis[25] and represents a potential target for inhibiting breast cancer stem cells[26]. In our study, patients with DCIS, non-MBC, or MBC have significantly higher serum levels of IL-1RA, IL-1β, IL-6, and IL-8 than those of HD. Numerous studies have linked the increased serum levels of inflammatory cytokines to breast cancer progression[27]. High levels of serum IL-1β correlated with disease recurrence in breast cancer patients[28], and patients with higher levels of serum IL-6 were found to be less responsive to chemo-endocrine treatment than those with lower levels of IL-6[29]. In the current study, we found that there were positive correlations between the serum level of sLeX and inflammatory cytokines (IL-1β, IL-1RA, and IL-2, sIL-2Ra, IFN-α) and chemokines (IL-8 (CXCL8), Fractalkine (CXCL1), MCP-3 (CCL7) and MIP-1β (CCL4)). Although this study only measured serum levels with no quantification of tumor-stromal levels, it is possible that by working in concert sLeX, inflammatory mediators may facilitate local invasion of breast tumor.
Two serum chemokines that did not significantly correlate with sLeX, MCP-1 and IP-10, showed independent prognostic value. Chemokines play a major role in recruiting leukocytes to the site of the tumor microenvironment. Monocyte chemoattractant protein-1 (MCP-1), also known as CCL2, is a ligand for CCR2 and CCR4. MCP-1 is a key member involved in the migration and infiltration of monocyte/macrophages from the bloodstream into the tumor microenvironment and has been shown to have properties that promote tumor progression[30,31] as well as to augment immune surveillance through recruitment of γδ T lymphocytes[32]. Others have found that MCP-1 expression was significantly associated with the number of tumor-associated macrophages and high expression levels of MCP-1 and VEGF were a significant indicator of early relapse[33]. In our study, sera of patients with DCIS, non-MBC, or MBC have significantly higher median levels of MCP-1, MCP-3, and MIP-1β than those of HD. Compared with DCIS or non-MBC patients, serum from MBC patients have a significantly higher level of MCP-1. Since breast tumor sites are enriched with inflammatory constituents and cytokines such as IL-1 and IFN-γ, potent stimulators of MCP-1, it is not surprising that serum level of MCP-1 is increased in MBC patients and further suggests a convergence of tumor promoting effects at the tumor site.
Similar to the results with serum sLeX, patients with elevated serum MCP-1 levels above the 95 percentile of serum level of HD have a significantly shorter OS (P=0.0014) and PFS (P=0.0095). This is consistent with previous reports that showed the expression of MCP-1 in extracts of primary tumors and infiltrating macrophages from patients with breast cancer was of a significant prognostic value for relapse free survival[33] and was significantly correlated with high grade tumor[34] and suggests that serum evaluation can also be useful.
IP-10 (interferon-gamma inducible protein 10 kDa), also known as CXCL10, is an ELR negative CXC ligand for CXCR3 induced by IFN-γ and LPS in a variety of cells including monocytes, T cells, keratinocytes, endothelial cells and fibroblasts. Like most chemokines, it has pleiotropic effects targeting numerous inflammatory pathways including cell adhesion through induction of integrins, natural killer and T-cell migration, and stimulation of monocytes, but does not attract neutrophils[35]. IP-10 (CXCL10) has been associated with osteolytic bone metastases[36] and although it inhibits both angiogenesis and bone marrow formation, high levels have been associated with advanced cancers[37] although high tumor levels have been positively associated with a doubling of overall survival in ovarian cancer[38]. In circulation, breast cancer patients have been found to have significantly higher CXCL10 than healthy controls[39].
For IP-10, we observed a small but significant hazard associated with overall survival and progression-free survival, both cases showing hazard ratios of non-transformed serum concentrations of 1.001. However, the range of IP-10 concentrations observed was quite large (44 pg/ml to 2,474 pg/ml), so a one-unit change is relatively small. Therefore, the hazard associated with a change on the order of the difference observed between mean non-MBC IP-10 (302 pg/ml) and MBC IP-10 (558 pg/ml) would be more substantive 1.292 (1.001∧256) and the hazard associated with a 1 mg/ml difference would be 2.7 or 2.8. For univariate analysis, we used the 95th percentile of healthy donors IP-10 serum levels at 666 pg/ml to establish a cutoff. This is slightly higher than most other publications but of the same order of magnitude. For example Toiyama used ROC analysis to establish a cut-off of 199 pg/ml in colorectal cancer[40] and Berres optimized survival analysis in hepatitis C with a cut-off of 220 pg/ml[41] whereas Piro used ROC analysis to set a cut-off of 2,958 pg/ml in pancreatic cancer[42]. As such, IP-10 serum concentrations above 200 – 2000 pg/ml are consistently related to poor survival in the literature, consistent with data presented here.
Patients with inflammatory breast cancer made up a disproportionate share of the non-MBC patients in this study. These patients have inferior prognosis in primary[43] and metastatic[44] disease. Interestingly, a reduced tissue expression of sLex has been proposed as a possible feature of IBC[45]. This may skew some results but was part of the initial study design. The association between inflammatory breast cancer and inflammatory serum proteins will be evaluated in further studies.
In addition to an oversampling of IBC patients, other study limitations include small sample size and patient heterogeneity and the limitation to a single institution. About half of the samples from patients with metastatic disease were collected post chemotherapy, which may alter the inflammatory profile. Because of the heterogeneity in sample times, survival times were calculated from the time of sample collection rather initial diagnosis and maybe shorter and not directly comparable to outcomes in the literature. Due to the small sample size for subgroup analyses along with the highly skewed distributions for cytokine expression with a subset of very high values, we used non-parametric Mann-Whitney U test to test for differences in cytokine levels between groups. This can report significantly different distributions in two populations with the same median[46] as observed for IL-1β, IL-1RA, sIL-2Ra. Although each of these soluble factors all have medians of 0 for MBC and HD, there is a tail of MBC patient samples with very high levels. It is worth noting that although the levels are significantly higher in MBC samples, this represents a minority of the population. Finally, recent studies have found that it may be possible to engineer IgG-based CSLEX ELISA assays that are less susceptible to the steric hindrance associated with the current IgM-based assays[47].
In conclusion, we found that serum sLeX levels tended to increase with severity of stage in breast cancer. Moreover, our study reporting commensurate increases in the levels of circulating sLeX and inflammatory mediators with severity of disease confirms previous reports that reported these factors separately[6,9,10,33,34,39]. Both serum MCP-1, IP-10 and sLeX may have prognostic value in breast cancer.
Supplementary Material
Acknowledgements
The authors would like to thank Meshaal Nadeem, Ken Tang, MingXi Yang, and Matthew Galland for their work analyzing samples for this work.
Funding
This work was supported by a grant from the State of Texas Rare and Aggressive Breast Cancer Research Program, which supports The University of Texas MD Anderson Morgan Welch Inflammatory Breast Cancer Research Program and Clinic, and the MD Anderson Cancer Center Support grant (NCI #CA16672), and by research support from Nittobo Medical Co., Japan.
Footnotes
Conflict of interest
Author MC has received research grants from Nittobi Medical, Co.
Authors TM, IK and JK are employed by Nittobi Medical, Co.
All other authors declare no conflicts of interest.
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Ethical approval
This work complies with regulations governing ethical standards. The project was approved by the Institutional Review Board of The University of Texas MD Anderson Cancer Center.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM (2000) Interactions between cancer cells and the endothelium in metastasis. J Pathol 190 (3):310–329. doi: [DOI] [PubMed] [Google Scholar]
- 2.Daniotti JL, Lardone RD, Vilcaes AA (2015) Dysregulated Expression of Glycolipids in Tumor Cells: From Negative Modulator of Anti-tumor Immunity to Promising Targets for Developing Therapeutic Agents. Front Oncol 5:300. doi: 10.3389/fonc.2015.00300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannagi R, Izawa M, Koike T, Miyazaki K, Kimura N (2004) Carbohydrate-mediated cell adhesion in cancer metastasis and angiogenesis. Cancer Sci 95 (5):377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang Z, Wuhrer M, Holst S (2018) Serum sialylation changes in cancer. Glycoconj J 35 (2):139–160. doi: 10.1007/s10719-018-9820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohyama C, Tsuboi S, Fukuda M (1999) Dual roles of sialyl Lewis X oligosaccharides in tumor metastasis and rejection by natural killer cells. The EMBO journal 18 (6):1516–1525. doi: 10.1093/emboj/18.6.1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Matsuura N, Narita T, Mitsuoka C, Kimura N, Kannagi R, Imai T, Funahashi H, Takagi H (1997) Increased level of circulating adhesion molecules in the sera of breast cancer patients with distant metastases. Japanese journal of clinical oncology 27 (3):135–139 [DOI] [PubMed] [Google Scholar]
- 7.Fujita T, Murayama K, Hanamura T, Okada T, Ito T, Harada M, Komatsu A, Koyama H, Kanai T, Maeno K, Mochizuki Y, Hama Y, Ito K, Amano J, Fujimori M (2011) CSLEX (Sialyl Lewis X) is a useful tumor marker for monitoring of breast cancer patients. Japanese journal of clinical oncology 41 (3):394–399. doi: 10.1093/jjco/hyq190 [DOI] [PubMed] [Google Scholar]
- 8.Jeschke U, Mylonas I, Shabani N, Kunert-Keil C, Schindlbeck C, Gerber B, Friese K (2005) Expression of sialyl lewis X, sialyl Lewis A, E-cadherin and cathepsin-D in human breast cancer: immunohistochemical analysis in mammary carcinoma in situ, invasive carcinomas and their lymph node metastasis. Anticancer research 25 (3A):1615–1622 [PubMed] [Google Scholar]
- 9.Nakagoe T, Fukushima K, Itoyanagi N, Ikuta Y, Oka T, Nagayasu T, Ayabe H, Hara S, Ishikawa H, Minami H (2002) Expression of ABH/Lewis-related antigens as prognostic factors in patients with breast cancer. J Cancer Res Clin Oncol 128 (5):257–264. doi: 10.1007/s00432-002-0334-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kurebayashi J, Nomura T, Hirono M, Okubo S, Udagawa K, Shiiki S, Ikeda M, Nakashima K, Tanaka K, Sonoo H (2006) Combined measurement of serum sialyl Lewis X with serum CA15–3 in breast cancer patients. Japanese journal of clinical oncology 36 (3):150–153. doi: 10.1093/jjco/hyi235 [DOI] [PubMed] [Google Scholar]
- 11.Julien S, Ivetic A, Grigoriadis A, QiZe D, Burford B, Sproviero D, Picco G, Gillett C, Papp SL, Schaffer L, Tutt A, Taylor-Papadimitriou J, Pinder SE, Burchell JM (2011) Selectin ligand sialyl-Lewis x antigen drives metastasis of hormone-dependent breast cancers. Cancer research 71 (24):7683–7693. doi: 10.1158/0008-5472.CAN-11-1139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Silva Z, Tong Z, Cabral MG, Martins C, Castro R, Reis C, Trindade H, Konstantopoulos K, Videira PA (2011) Sialyl Lewisx-dependent binding of human monocyte-derived dendritic cells to selectins. Biochemical and biophysical research communications 409 (3):459–464. doi: 10.1016/j.bbrc.2011.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Narita T, Kawakami-Kimura N, Matsuura N, Hosono J, Kannagi R (1995) Corticosteroids and medroxyprogesterone acetate inhibit the induction of E-selectin on the vascular endothelium by MDA-MB-231 breast cancer cells. Anticancer research 15 (6B):2523–2527 [PubMed] [Google Scholar]
- 14.Barthel SR, Gavino JD, Descheny L, Dimitroff CJ (2007) Targeting selectins and selectin ligands in inflammation and cancer. Expert opinion on therapeutic targets 11 (11):1473–1491. doi: 10.1517/14728222.11.11.1473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laubli H, Borsig L (2010) Selectins as mediators of lung metastasis. Cancer microenvironment : official journal of the International Cancer Microenvironment Society 3 (1):97–105. doi: 10.1007/s12307-010-0043-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Laubli H, Borsig L (2010) Selectins promote tumor metastasis. Seminars in cancer biology 20 (3):169–177. doi: 10.1016/j.semcancer.2010.04.005 [DOI] [PubMed] [Google Scholar]
- 17.Portela SV, Martin CV, Romay LM, Cuevas E, Martin EG, Briera AF (2011) sLea and sLex expression in colorectal cancer: implications for tumourigenesis and disease prognosis. Histology and histopathology 26 (10):1305–1316 [DOI] [PubMed] [Google Scholar]
- 18.Iwata T, Nishiyama N, Nagano K, Izumi N, Tsukioka T, Chung K, Hanada S, Inoue K, Kaji M, Suehiro S (2012) Preoperative serum value of sialyl Lewis X predicts pathological nodal extension and survival in patients with surgically treated small cell lung cancer. Journal of surgical oncology 105 (8):818–824. doi: 10.1002/jso.23002 [DOI] [PubMed] [Google Scholar]
- 19.Ferrajoli A, Lee BN, Schlette EJ, O’Brien SM, Gao H, Wen S, Wierda WG, Estrov Z, Faderl S, Cohen EN, Li C, Reuben JM, Keating MJ (2008) Lenalidomide induces complete and partial remissions in patients with relapsed and refractory chronic lymphocytic leukemia. Blood 111 (11):5291–5297. doi: 10.1182/blood-2007-12-130120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sozzani P, Arisio R, Porpiglia M, Benedetto C (2008) Is Sialyl Lewis x antigen expression a prognostic factor in patients with breast cancer? International journal of surgical pathology 16 (4):365–374. doi: 10.1177/1066896908324668 [DOI] [PubMed] [Google Scholar]
- 21.Culig Z (2011) Cytokine disbalance in common human cancers. Biochim Biophys Acta 1813 (2):308–314. doi: 10.1016/j.bbamcr.2010.12.010 [DOI] [PubMed] [Google Scholar]
- 22.Walzog B, Gaehtgens P (2000) Adhesion Molecules: The Path to a New Understanding of Acute Inflammation. News in physiological sciences : an international journal of physiology produced jointly by the International Union of Physiological Sciences and the American Physiological Society 15:107–113 [DOI] [PubMed] [Google Scholar]
- 23.Knupfer H, Preiss R (2007) Significance of interleukin-6 (IL-6) in breast cancer (review). Breast cancer research and treatment 102 (2):129–135. doi: 10.1007/s10549-006-9328-3 [DOI] [PubMed] [Google Scholar]
- 24.Salgado R, Junius S, Benoy I, Van Dam P, Vermeulen P, Van Marck E, Huget P, Dirix LY (2003) Circulating interleukin-6 predicts survival in patients with metastatic breast cancer. International journal of cancer Journal international du cancer 103 (5):642–646. doi: 10.1002/ijc.10833 [DOI] [PubMed] [Google Scholar]
- 25.Benoy IH, Salgado R, Van Dam P, Geboers K, Van Marck E, Scharpe S, Vermeulen PB, Dirix LY (2004) Increased serum interleukin-8 in patients with early and metastatic breast cancer correlates with early dissemination and survival. Clinical cancer research : an official journal of the American Association for Cancer Research 10 (21):7157–7162. doi: 10.1158/1078-0432.CCR-04-0812 [DOI] [PubMed] [Google Scholar]
- 26.Singh JK, Simoes BM, Howell SJ, Farnie G, Clarke RB (2013) Recent advances reveal IL-8 signaling as a potential key to targeting breast cancer stem cells. Breast Cancer Res 15 (4):210. doi: 10.1186/bcr3436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldberg JE, Schwertfeger KL (2010) Proinflammatory cytokines in breast cancer: mechanisms of action and potential targets for therapeutics. Current drug targets 11 (9):1133–1146 [DOI] [PubMed] [Google Scholar]
- 28.Nicolini A, Carpi A, Rossi G (2006) Cytokines in breast cancer. Cytokine & growth factor reviews 17 (5):325–337. doi: 10.1016/j.cytogfr.2006.07.002 [DOI] [PubMed] [Google Scholar]
- 29.Zhang GJ, Adachi I (1999) Serum interleukin-6 levels correlate to tumor progression and prognosis in metastatic breast carcinoma. Anticancer research 19 (2B):1427–1432 [PubMed] [Google Scholar]
- 30.Soria G, Yaal-Hahoshen N, Azenshtein E, Shina S, Leider-Trejo L, Ryvo L, Cohen-Hillel E, Shtabsky A, Ehrlich M, Meshel T, Keydar I, Ben-Baruch A (2008) Concomitant expression of the chemokines RANTES and MCP-1 in human breast cancer: a basis for tumor-promoting interactions. Cytokine 44 (1):191–200. doi: 10.1016/j.cyto.2008.08.002 [DOI] [PubMed] [Google Scholar]
- 31.Soria G, Ben-Baruch A (2008) The inflammatory chemokines CCL2 and CCL5 in breast cancer. Cancer letters 267 (2):271–285. doi: 10.1016/j.canlet.2008.03.018 [DOI] [PubMed] [Google Scholar]
- 32.Lança T, Costa MF, Gonçalves-Sousa N, Rei M, Grosso AR, Penido C, Silva-Santos B (2013) Protective Role of the Inflammatory CCR2/CCL2 Chemokine Pathway through Recruitment of Type 1 Cytotoxic γδ T Lymphocytes to Tumor Beds. The Journal of Immunology 190 (12):6673–6680. doi: 10.4049/jimmunol.1300434 [DOI] [PubMed] [Google Scholar]
- 33.Ueno T, Toi M, Saji H, Muta M, Bando H, Kuroi K, Koike M, Inadera H, Matsushima K (2000) Significance of macrophage chemoattractant protein-1 in macrophage recruitment, angiogenesis, and survival in human breast cancer. Clinical cancer research : an official journal of the American Association for Cancer Research 6 (8):3282–3289 [PubMed] [Google Scholar]
- 34.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissiere F, Laune D, Roques S, Lazennec G (2007) Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res 9 (1):R15. doi: 10.1186/bcr1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tokunaga R, Zhang W, Naseem M, Puccini A, Berger MD, Soni S, McSkane M, Baba H, Lenz HJ (2018) CXCL9, CXCL10, CXCL11/CXCR3 axis for immune activation - A target for novel cancer therapy. Cancer Treat Rev 63:40–47. doi: 10.1016/j.ctrv.2017.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lee JH, Kim HN, Kim KO, Jin WJ, Lee S, Kim HH, Ha H, Lee ZH (2012) CXCL10 promotes osteolytic bone metastasis by enhancing cancer outgrowth and osteoclastogenesis. Cancer research 72 (13):3175–3186. doi: 10.1158/0008-5472.CAN-12-0481 [DOI] [PubMed] [Google Scholar]
- 37.Liu M, Guo S, Stiles JK (2011) The emerging role of CXCL10 in cancer (Review). Oncol Lett 2 (4):583–589. doi: 10.3892/ol.2011.300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bronger H, Singer J, Windmuller C, Reuning U, Zech D, Delbridge C, Dorn J, Kiechle M, Schmalfeldt B, Schmitt M, Avril S (2016) CXCL9 and CXCL10 predict survival and are regulated by cyclooxygenase inhibition in advanced serous ovarian cancer. Br J Cancer 115 (5):553–563. doi: 10.1038/bjc.2016.172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jafarzadeh A, Fooladseresht H, Nemati M, Assadollahi Z, Sheikhi A, Ghaderi A (2016) Higher circulating levels of chemokine CXCL10 in patients with breast cancer: Evaluation of the influences of tumor stage and chemokine gene polymorphism. Cancer Biomark 16 (4):545–554. doi: 10.3233/CBM-160596 [DOI] [PubMed] [Google Scholar]
- 40.Toiyama Y, Fujikawa H, Kawamura M, Matsushita K, Saigusa S, Tanaka K, Inoue Y, Uchida K, Mohri Y, Kusunoki M (2012) Evaluation of CXCL10 as a novel serum marker for predicting liver metastasis and prognosis in colorectal cancer. Int J Oncol 40 (2):560–566. doi: 10.3892/ijo.2011.1247 [DOI] [PubMed] [Google Scholar]
- 41.Berres ML, Trautwein C, Schmeding M, Eurich D, Tacke F, Bahra M, Neuhaus P, Neumann UP, Wasmuth HE (2011) Serum chemokine CXC ligand 10 (CXCL10) predicts fibrosis progression after liver transplantation for hepatitis C infection. Hepatology 53 (2):596–603. doi: 10.1002/hep.24098 [DOI] [PubMed] [Google Scholar]
- 42.Piro G, Simionato F, Carbone C, Frizziero M, Malleo G, Zanini S, Casolino R, Santoro R, Mina MM, Zecchetto C, Merz V, Scarpa A, Bassi C, Tortora G, Melisi D (2017) A circulating TH2 cytokines profile predicts survival in patients with resectable pancreatic adenocarcinoma. Oncoimmunology 6 (9):e1322242. doi: 10.1080/2162402X.2017.1322242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dawood S, Ueno NT, Valero V, Woodward WA, Buchholz TA, Hortobagyi GN, Gonzalez- Angulo AM, Cristofanilli M (2012) Identifying factors that impact survival among women with inflammatory breast cancer. Ann Oncol 23 (4):870–875. doi: 10.1093/annonc/mdr319 [DOI] [PubMed] [Google Scholar]
- 44.Fouad TM, Barrera AMG, Reuben JM, Lucci A, Woodward WA, Stauder MC, Lim B, DeSnyder SM, Arun B, Gildy B, Valero V, Hortobagyi GN, Ueno NT (2017) Inflammatory breast cancer: a proposed conceptual shift in the UICC-AJCC TNM staging system. Lancet Oncol 18 (4):e228–e232. doi: 10.1016/S1470-2045(17)30192-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alpaugh ML, Tomlinson JS, Ye Y, Barsky SH (2002) Relationship of sialyl-Lewis(x/a) underexpression and E-cadherin overexpression in the lymphovascular embolus of inflammatory breast carcinoma. Am J Pathol 161 (2):619–628. doi: 10.1016/S0002-9440(10)64217-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Conroy RM (2012) What hypotheses do “nonparametric” two-group tests actually test? Stata J 12 (2):182–190. doi:Doi [DOI] [Google Scholar]
- 47.Yamashita J, Kobayashi I, Tatematsu K, Sezutsu H, Noda K, Ishihara H (2016) Sandwich ELISA Using a Mouse/Human Chimeric CSLEX-1 Antibody. Clin Chem 62 (11):1516–1523. doi: 10.1373/clinchem.2016.260968 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.