Abstract
Obesity and metabolic syndrome display disparate prevalence and regulation between males and females. Human, as well as rodent, females with regular menstrual/estrous cycles exhibit protection from weight gain and associated chronic diseases. These beneficial effects are predominantly attributed to the female hormone estrogen, specifically l7β-estradiol(E2). E2 exerts its actions via multiple receptors, nuclear and extranuclear estrogen receptor (ER) α and ERβ, and the G-protein-coupled estrogen receptor (GPER, previously termed GPR30). The roles of GPER in metabolic homeostasis are beginning to emerge but are complex and remain unclear. The discovery of GPER-selective pharmacological agents (agonists and antagonists) and the availability of GPER knockout mice have significantly enhanced our understanding of the functions of GPER in normal physiology and disease. GPER action manifests pleiotropic effects in metabolically active tissues such as the pancreas, adipose, liver, and skeletal muscle. Cellular and animal studies have established that GPER is involved in the regulation of body weight, feeding behavior, inflammation, as well as glucose and lipid homeostasis. GPER deficiency leads to increased adiposity, insulin resistance, and metabolic dysfunction in mice. In contrast, pharmacologic stimulation of GPER in vivo limits weight gain and improves metabolic output, revealing a promising novel therapeutic potential for the treatment of obesity and diabetes.
Introduction: Obesity, Metabolism, and Sex Differences
Obesity represents a grave public health concern in the modern world. It is now recognized as a global epidemic with the number of obese individuals increasing drastically over the last two to three decades in both developed and more recently developing nations (Flegal et al. 2010; Guh et al. 2009). According to the latest demographic data from the Centre for Disease Control, it is estimated that in the United States alone, more than 65% of population is either overweight or obese (Center for Disease Control and Prevention: Adult Obesity Facts 2017). Obesity is not merely the presence of excessive body weight but represents a major risk factor for metabolic syndrome, a collection of conditions that includes high blood sugar, increased waist circumference, high blood pressure, and abnormal cholesterol (i.e., low high-density lipoprotein levels) or triglyceride levels (Han and Lean 2016; Keller and Lemberg 2003; Alberti et al. 2006; Mittendorfer 2011). Together, these conditions raise the risk of diabetes, heart disease, stroke, as well as certain forms of cancer (Eckel and Krauss 1998; Poirier et al. 2006; Kernan and Dearborn 2015; Basen-Engquist and Chang 2011). The socioeconomic burden of obesity is enormous, with current annual cost estimates in the United States alone ranging from $147 to 210 billion, and simultaneous reductions in the quality of life and life expectancy (Guh et al. 2009; Hammond and Levine 2010). Obesity can result from a multitude of factors ranging from genetic, behavioral to environmental (McAllister et al. 2009). However, the principal reasons for the recent obesity epidemic include increased consumption of calorie-dense foods, high in saturated fats and refined sugars, coupled with a sedentary lifestyle, resulting in a chronic energy imbalance. The precise mechanisms responsible for the development of obesity and metabolic dysfunction are complex and not yet fully understood. As a result, there is an urgent need to identify novel molecular targets and therapeutic agents capable of preventing or limiting the development of obesity and its resulting metabolic abnormalities.
Obesity, diabetes, and cardiovascular diseases exhibit a significant sexual dichotomy, with the incidence being lower in females in their premenopausal years compared to age-matched men or postmenopausal women (Kotani et al. 1994; Garaulet et al. 2002; Nakhjavani et al. 2014; Tandon et al. 2010; Regitz-Zagrosek et al. 2006). Furthermore, the quantity and site of fat distribution also varies between men and women, leading to differential health outcomes (Regitz-Zagrosek et al. 2006; Blaak 2001). Overall, men have less body fat than women; however, in men this fat is distributed in the upper body or abdominal area, reflecting an “android” pattern, whereas women display a “gynoid” pattern, with lower body or subcutaneous fat distribution. Properties of android and gynoid fat differ, with the former being more prone to lipolysis and secreting increased levels of pro-inflammatory cytokines and therefore associated with an increased risk of metabolic syndrome and cardiovascular disease (Kotani et al. 1994; Blaak 2001; Jensen 2008; Ritchie and Connell 2007; Monteiro et al. 2014; Shulman 2014). Typical sex-specific fat distributions in males and females suggest distinct regulatory mechanisms that control energy balance and adiposity. Obesity leads to altered secretory profiles of adipose-specific hormones and cytokines, increased levels of circulating glucose and lipids, and systemic inflammation, resulting in ectopic lipid deposition in peripheral metabolic tissues, such as the pancreas, liver, and skeletal muscle (Shulman 2014; Klop et al. 2013; Consitt et al. 2009; Bays et al. 2013; Shoelson et al. 2007; Tchernof and Despres 2013). The ensuing glucotoxicity, lipotoxicity, and inflammation result in dysfunction of these tissues, including defects in insulin production and secretion by the pancreas, inhibition of insulin-stimulated glucose uptake in skeletal muscle, and increases in glucose production by the liver (Consitt et al. 2009; Muoio and Neufer 2012; Mota et al. 2016; Brons and Vaag 2009; Poitout and Robertson 2008).
Differences also exist in insulin sensitivity between the sexes with premenopausal women being more insulin sensitive compared to age-matched men or postmenopausal women (Walton et al. 1993; Geer and Shen 2009; Lindheim et al. 1994; Mauvais-Jarvis 2011). Postmenopausal women experience a decline in metabolic health due to increased android fat deposition, reduced energy expenditure, insulin resistance, impaired glucose/lipid metabolism, and inflammation (Mauvais-Jarvis 2011; Poehlman et al. 1995; Lobo 2008; Abu-Taha et al. 2009; Godsland 2005; Bruns and Kemnitz 2004; Zhang et al. 2002; Andersson et al. 1997; Mauvais-Jarvis et al. 2013). The systemic loss of estrogen after menopause is linked to an elevated risk of age-related metabolic disease and cardiovascular disease in women (Tandon et al. 2010). Conversely, hormone replacement therapy in postmenopausal women improves multiple aspects of metabolism (Bonds et al. 2006; Gurney et al. 2014; Kanaya et al. 2003; Margolis et al. 2004; Pereira et al. 2015). With increases in overall life expectancy, it is critical to develop therapeutic agents that will ameliorate weight gain and the effects of metabolic dysfunction in women and improve the quality of life after menopause. As an experimental model, mice also display similar sex differences in weight gain and metabolism (Hong et al. 2009; Della Vedova et al. 2016). Male mice fed a high-fat diet exhibit more pronounced effects on adiposity, hormonal imbalance, and impaired glucose metabolism compared to females (Yang et al. 2014; Pettersson et al. 2012; El Akoum et al. 2011). In both human and rodent females, these protective metabolic effects are largely attributed to the female hormone estrogen. Although recognized for its effects on reproduction and development, the complex mechanisms of estrogen action on metabolic tissues remain incompletely understood.
Estrogen Action in Metabolic Tissues
Estrogen (most importantly 17β-estradiol, E2) is a steroid hormone critical for the development and function of reproductive organs as well as the development of secondary sex characteristics. In addition, E2 action is involved in the nervous, immune, vascular, muscular, skeletal, and endocrine systems, all of which contribute to multiple aspects of metabolism (Deroo and Korach 2006; Prossnitz et al. 2008a). The regulation of metabolic functions by E2 and its receptors has been a topic of great interest owing to the sexual dimorphisms that exist in body weight, food intake, glucose/lipid homeostasis, and insulin sensitivity (Mauvais-Jarvis 2011, 2015; Barros and Gustafsson 2011; Barros et al. 2006; Meyer et al. 2011). The decline of circulating E2 due to either natural or surgical menopause induces rapid changes in whole body metabolism, fat distribution, inflammation, and insulin action both in animals and humans (Abu-Taha et al. 2009; Mauvais-Jarvis et al. 2013; Lee et al. 2009; Straub 2007). Loss of E2 or its function increases the risk of central weight gain, abnormal lipid profiles, diabetes, and cardiovascular disease (Hewitt et al. 2005; Louet et al. 2004). Similarly, E2 insufficiency in male and female mice lacking aromatase, a key enzyme in the biosynthesis of E2 from testosterone, results in increased adiposity, higher circulating lipid and insulin levels, as well as reduction in lean mass (Jones et al. 2000). In rodent models of obesity, such as high-fat (aka Western) diet, leptin deficiency, or ovariectomy, supplementation with E2 or its mimetics attenuates weight gain and alleviates metabolic abnormalities (Stubbins et al. 2012; Lundholm et al. 2008; Shen et al. 2014). In postmenopausal women, although hormone replacement therapy is a viable treatment option to alleviate the symptoms of menopause (Kaunitz and Manson 2015), it is associated with oncogenic and cardiovascular risks (Hormone Replacement Therapy and Cancer 2001; Nabulsi et al. 1993).
One of the most widely used rodent models to study E2 action in vivo is ovariectomy, wherein surgical removal of the ovaries drastically reduces the levels of circulating endogenous E2 (Diaz Brinton 2012). The systemic loss of E2 in ovariectomized mice reveals that E2 is critical to several aspects of metabolism. E2 mediates certain metabolic effects via the central nervous system, as revealed by increased food intake, reduced energy expenditure, and weight gain in ovariectomized mice compared to ovary-intact littermates (Mauvais-Jarvis et al. 2013; Brown and Clegg 2010). In ovariectomized mice, the pancreas exhibits a loss of β-cell function and death (Louet et al. 2004; Le May et al. 2006). In contrast, supplementation with E2 promotes pancreatic β-cell survival in a proapoptotic environment (e.g., exposure to oxidative stress and/or pro-inflammatory cytokines), induces glucose-stimulated insulin secretion (GSIS), and suppresses lipogenesis, the latter by downregulating the expression of key transcription factors involved in lipid synthesis (Liu and Mauvais-Jarvis 2009; Liu et al. 2009; Balhuizen et al. 2010; Tiano et al. 2011; Tiano and Mauvais-Jarvis 2012). Furthermore, ovariectomy leads to aberrant glucose and lipid homeostasis resulting in increased fat mass, insulin resistance, glucose intolerance, dyslipidemia, and ectopic fat deposition (Vieira Potter et al. 2012; Yonezawa et al. 2012; Lin et al. 2013). Following ovariectomy, mice exhibit increases in visceral fat with larger adipocytes and increased expression of lipogenic and pro-inflammatory genes (Hong et al. 2009; Vieira Potter et al. 2012; Kamei et al. 2005; D’Eon et al. 2005). In addition, loss of E2 action increases susceptibility to oxidative stress and lowers fatty acid oxidation in multiple metabolic tissues (Muthusami et al. 2005; Paquette et al. 2009; Bokov et al. 2009). Rodent models of obesity and patients with type 2 diabetes also exhibit increased oxidative stress and inflammation (Furukawa et al. 2004; Fernandez-Sanchez et al. 2011; Wright et al. 2006; Folli et al. 2011; Domingueti et al. 2016). E2 supplementation in mice increases the expression of antioxidant enzymes and reduces inflammation (Monteiro et al. 2014; Strehlow et al. 2003; Borras et al. 2010). Finally, ovariectomized mice display increased susceptibility to the deleterious effects of HFD that can be reversed by supplementation with E2 at physiological concentrations (Stubbins et al. 2012; Litwak et al. 2014). Surprisingly, male mice fed a HFD also exhibit reduced body weight and improved glucose tolerance upon treatment with E2 or its mimetics (Huang et al. 2017; Vinayagam and Xu 2015; Kim et al. 2005).
Many natural or synthetic compounds with estrogenic activity can regulate endocrine signaling pathways, exerting either beneficial or adverse effects. Animal studies have revealed that prenatal exposure to endocrine disruptors, such as bisphenol A and diethylstilbestrol, causes abnormal programming of differentiating E2 target tissues that leads to the development of obesity later in life (Garcia-Arevalo et al. 2014; Liu et al. 2013; Newbold et al. 2007, 2009). On the other hand, dietary intake of genistein, an isoflavone that mimics certain actions of E2, exerts antidiabetic effects by improving hyperglycemia, glucose tolerance, and insulin levels in multiple mouse models of metabolic dysfunction (Lei et al. 2011; Liu et al. 2006). Taken together, these observations suggest that E2 exerts pleiotropic effects on multiple tissues involved in metabolism. Thus, a more complete understanding of the mechanisms underlying E2 action on metabolic tissues requires a thorough investigation of the roles of individual estrogen receptors.
Estrogen Receptors, Signaling, and GPER Selectivity
The actions of E2, as an important physiological modulator of the complex events that regulate body weight and metabolism in multiple tissues, are mediated by its multiple receptors. The classical genomic actions through nuclear estrogen receptors (ERα and β) involve dimerization upon ligand activation and eventual binding to ER response elements in the promoters of target genes to facilitate regulation of gene expression (Barkhem et al. 2004; Dahlman-Wright et al. 2006; Jia et al. 2015). In addition to genomic responses, E2 also elicits rapid non-genomic signaling through extranuclear ERs and the G-protein-coupled estrogen receptor (GPER, previously known as GPR30) (Prossnitz et al. 2008a, b, c; Levin 2015). Long-term effects of GPER activity, however, also involve transcriptional regulation of target genes (Pandey et al. 2009; Prossnitz and Barton 2014; Vivacqua et al. 2015). GPER was initially discovered as an orphan receptor (Owman et al. 1996) but has since been demonstrated to bind E2 and activate multiple non-genomic, as well as genomic, pathways (Prossnitz et al. 2008a; Barton et al. 2017; Prossnitz and Arterburn 2015; Revankar et al. 2005, 2007; Filardo et al. 2000, 2002). GPER is expressed in diverse cell types and tissues, including the reproductive tissues, pancreatic islets, adipose, liver, skeletal muscle, CNS, heart, intestine, and inflammatory cells (Prossnitz and Barton 2011). It has been functionally implicated in metabolic regulation (Prossnitz and Barton 2014; Sharma et al. 2013; Sharma and Prossnitz 2016; Martensson et al. 2009; Barton and Prossnitz 2015), immune regulation (Blasko et al. 2009; Brunsing et al. 2013; Brunsing and Prossnitz 2011), cardiovascular physiology (Haas et al. 2009; Meyer et al. 2014, 2015, 2016; Fredette et al. 2017), reproduction (Thomas et al. 2010; Wang et al. 2008), the nervous system (Srivastava and Evans 2013; Xu et al. 2009; Hazell et al. 2009), and cancer (Prossnitz and Barton 2011; Arias-Pulido et al. 2010; Lappano et al. 2014; Marjon et al. 2014; Petrie et al. 2013; Smith et al. 2007, 2009). Stimulation of GPER activates a multitude of cellular signaling pathways including MAPK, PKC, PI3K, adenylyl cyclase, eNOS, and Ca2+ mobilization (Prossnitz and Barton 2014; Prossnitz and Arterburn 2015; Revankar et al. 2005; Filardo et al. 2000, 2002).
Whereas the roles of nuclear ERs in metabolism are more established (Barros and Gustafsson 2011; Jia et al. 2015), the physiological or pathological roles of GPER in metabolic signaling are still emerging (Sharma and Prossnitz 2016; Nilsson et al. 2011; Sharma et al. 2017) (Fig. 1). The effects of E2 and its multiple receptors on metabolism, as in other systems (Hadjimarkou and Vasudevan 2017), may be direct or indirect and furthermore may exhibit synergism or antagonism that may impact overall metabolic status. Thus, to assess the mechanisms involved in these complex interactions, the specific contribution of individual receptors must be assessed. Interestingly, mice lacking either ERα or GPER share similarities in metabolic phenotypes to varying degrees, such as increased adiposity, decreased insulin sensitivity, defective glucose/lipid homeostasis, and inflammation (Sharma et al. 2013; Martensson et al. 2009; Davis et al. 2014; Heine et al. 2000; Ribas et al. 2010; Prossnitz and Hathaway 2015). This suggests that both receptors might act cooperatively to mediate metabolic effects through similar or alternatively distinct mechanistic pathways. It is important to note, however, that in the chronic absence of an individual receptor, compensatory effects may take place, masking the role of a given receptor in normal physiology or disease.
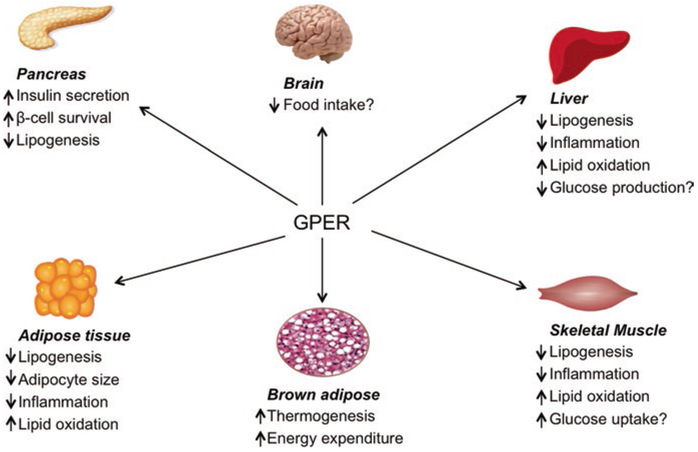
Fig. 1.
Schematic representation of the metabolic roles of GPER. GPER exerts pleiotropic effects on metabolically active tissues such as the pancreas, adipose, brown adipose, liver, and muscle. GPER controls body weight by regulating food intake and increasing energy expenditure as well as thermogenesis in brown adipose tissue. GPER activation in the pancreas promotes β-cell survival and insulin secretion. Stimulation of GPER in the pancreas, adipose, liver, and skeletal muscle reduces lipid deposition by inhibiting lipogenesis and promoting lipid oxidation. GPER activation also attenuates inflammation in multiple tissues. GPER-mediated prevention of lipotoxicity and inflammation in non-adipose tissues may improve glucose homeostasis by increasing insulin secretion, improving glucose uptake, and reducing hepatic glucose production in the pancreas, skeletal muscle, and liver, respectively. Effects not clearly known are indicated by. See text for details
Since E2 binds to multiple receptors, pharmacological and genetic approaches can be employed to discriminate the contributions of individual receptors. Through the use of GPER-selective pharmacological agents, such as the agonist G-1 or antagonists G15 and G36, it is now possible to investigate the specific functions of GPER compared with those of ERs in complex systems expressing multiple estrogen receptor types (Prossnitz and Arterburn 2015; Revankar et al. 2007; Prossnitz 2017; Bologa et al. 2006; Dennis et al. 2009, 2011). However, experiments employing selective estrogen receptor modulators (SERMs) and selective estrogen receptor downregulators (SERDs) should be carefully interpreted as multiple SERMs, such as tamoxifen and raloxifene, and the SERD fulvestrant have been shown to lack ER specificity, acting as GPER agonists (Prossnitz and Arterburn 2015; Revankar et al. 2005; Filardo et al. 2000; Petrie et al. 2013). In addition, the availability of various genetic tools such as GPER knockout (GPER KO) mice (Prossnitz and Hathaway 2015) and siRNA or shRNA directed against GPER has significantly advanced our knowledge of GPER function. GPER KO mice with a global gene deletion of GPER (of which four independent genetically modified strains have been developed as reviewed in Prossnitz and Hathaway 2015) have been employed to evaluate metabolic phenotypes, generally revealing weight gain and metabolic dysfunction (Sharma et al. 2013; Martensson et al. 2009; Davis et al. 2014). Pharmacological and genetic approaches have often complemented each other, wherein treatment of mice with the GPER-selective agonist G-1 results in the opposite effect observed in GPER KO mice (Prossnitz and Hathaway 2015; Sharma and Prossnitz 2011). Furthermore, treatment of GPER KO mice with G-1 lacks the stimulatory effect of G-1 in WT mice, thereby confirming the selectivity of this compound for GPER via the absence of off-target effects (Prossnitz and Hathaway 2015; Sharma and Prossnitz 2011). In many systems, the lack of effects upon stimulation of GPER KO mice with E2 further validates the importance of GPER signaling in the actions of E2 (Prossnitz and Hathaway 2015; Sharma and Prossnitz 2011). Thus, recent studies utilizing GPER-selective approaches have provided strong evidence of the contributions of GPER signaling to metabolic homeostasis.
GPER, Body Weight, and Energy Homeostasis
Multiple studies have examined whether GPER regulates overall body weight, fat content, and energy balance (Tables 1 and 2). In the first such study, in 2009, Barton and colleagues reported that both male and female mice lacking GPER exhibited increases in body weight and visceral adiposity compared to their WT counterparts (Haas et al. 2009). At about the same time, but contrary to these results, Leeb-Lundberg and coworkers reported that female GPER KO mice exhibited slightly lower body weights than the corresponding WT mice, whereas GPER deficiency in males had no effects on body weight (Martensson et al. 2009). In 2013, we reported increased adiposity in male GPER KO mice throughout life, from 6 to 24 months of age, compared to WT mice (Sharma et al. 2013). MRI analysis revealed an overall increase in fat content of GPER KO mice with increased fat deposition in subcutaneous depots as well as visceral fat depots, such as the epididymal and perirenal fat pads. The increase in adiposity of GPER KO mice occurred in the absence of altered food intake or locomotor activity. Furthermore, weight gain in male GPER KO mice was correlated with a significant increase in circulating levels of cholesterol, triglycerides, and LDL (Sharma et al. 2013), suggesting that GPER regulates key pathways involved in lipid homeostasis.
Table 1.
Effects of loss of GPER expression on metabolism. In vitro, ex vivo, and in vivo studies reveal that GPER regulates body weight, food intake, and energy expenditure. In addition, GPER modulates pancreatic cell survival and hormone secretion as well as glucose and lipid metabolism. Effects in GPER KO mice or islets are compared to WT control animals or islets. See the text for details
| Cells/tissues/ mice |
Treatment | Effect/(s) | Reference/year |
|---|---|---|---|
| GPER KO (F) mice | - | Slightly reduced body weight | Martensson et al. 2009 |
| Hyperglycemia and impaired glucose tolerance | |||
| OVX + E2 | No increase in serum insulin levels | ||
| GPER KO (F) islets | Basal | Reduced insulin and glucagon secretion | |
| E2 | No increase in insulin or decrease in glucagon secretion | ||
| GPER KO (M) mice | - | No changes in body weight and normal glucose homeostasis | |
| GPER KO (M) islets | Basal | Reduced insulin secretion but glucagon secretion unchanged | |
| E2 | No changes in insulin or glucagon secretion | ||
| GPER KO (F) mice | STZ | Increased incidence of diabetes (vs. WT mice + STZ) | Liu et al. 2009 |
| Lower insulin levels and pancreatic insulin content | |||
| GPER KO (M) mice | STZ | Incidence of diabetes similar to WT mice | |
| GPER KO islets | STZ+E2 | Increased pancreatic islet survival (possibly via ER) | |
| STZ+G-1 | No increase in pancreatic islet survival | ||
| GPER KO (F) islets | E2 or G-1 | Reduced insulin secretion under high glucose | Sharma and Prossnitz 2011 |
| GPER KO islets | E2 | No decrease in lipid accumulation | Tiano et al. 2011 |
| GPER KO (M) mice | - | Increased body weight, fat content, and dyslipidemia | Sharma et al. 2013 |
| Impaired glucose tolerance and insulin resistance | |||
| Increased fasting insulin | |||
| GPER KO (F) mice | - | Increased body weight | Davis et al. 2014 |
| Reduced energy expenditure and brown fat thermogenesis | |||
| OVX + E2 | No decrease in body weight or improvement in glucose tolerance | ||
| Leptin or CCK | No decrease in food intake | ||
| GPER KO (M) mice | - | Increased body weight | |
| Reduced energy expenditure and brown fat thermogenesis | |||
| Leptin or CCK | Reduction in food intake | ||
| GPER-lacZ (F) mice | HFD | No changes in body weight | Meoli et al. 2014 |
| Increased liver fat accumulation, decreased HDL | |||
| GPER-lacZ (M) mice | No changes in body weight, liver fat accumulation, or HDL levels |
Table 2.
GPER-mediated effects on metabolism. Selective pharmacological activation or genetic knockdown of GPER reveals that stimulation of GPER promotes human and murine pancreatic cell survival and insulin secretion, reduces lipid accumulation in adipocytes. In ovariectomized mice, GPER activation attenuates adiposity and improves glucose tolerance and lowers fasting glucose, insulin, and cholesterol
| Cells/tissues/mice | Treatment | Effect/(s) | Reference/year |
|---|---|---|---|
| αβERKO (F) mice | OVX + STZ + E2 | Lower incidence of diabetes (vs. OVX + STZ) (via non-ERs possibly GPER) | Liu et al. 2009 |
| Higher insulin levels and pancreatic insulin content | |||
| Mice | G-1 | Increased pancreatic islet cell survival | Liu et al. 2009,Balhuizen et al. 2010, and Kumar et al. 2011 |
| Human islets | |||
| MIN6 cells | |||
| WT mice (F) islets | G-1 | Increase in insulin secretion under high glucose | Balhuizen et al. 2010 |
| Decrease in glucagon secretion under low glucose | |||
| Decrease in somatostatin secretion under high glucose | |||
| Inhibition of pancreatic cell apoptosis | |||
| Human (F) islets | G-1 | Increase in insulin secretion under high glucose | Kumar et al. 2011 |
| Decrease in pancreatic cell apoptosis | |||
| MIN6 | E2 or G-1 | Increased insulin secretion | Sharma and Prossnitz 2011 |
| (± G15 or ± siRNA) | Inhibition of E2 or G-1-mediated insulin secretion | ||
| WT mice (F) islets | E2 or G-1 | Increased insulin secretion | |
| (± G15) | Inhibition of E2 or G-1-mediated insulin secretion | ||
| ZDF (M) islets | G-1 | Reduced lipid accumulation | Tiano et al. 2011 and Tiano and Mauvais-Jarvis 2012 |
| INS-1 cells | |||
| Human islets | |||
| 3T3-L1 | E2 or G-1 | Inhibition of Lipid accumulation | Zhu et al. 2013 |
| (± siRNA) | Reversal of GPER-mediated decrease in lipid deposition | ||
| WT mice | OVX+G-1 | Reduction in body weight and fat content | Unpublished data |
| Improved glucose tolerance | |||
| Reduced insulin resistance | |||
| Lower fasting glucose, insulin, and cholesterol levels | |||
| Increased fatty acid oxidation in metabolic tissues |
Consistent with these latter results, in a subsequent study in 2014, Clegg and colleagues observed that both male and female GPER KO mice demonstrated increased body weight and decreased energy expenditure in the absence of any changes in food intake (Davis et al. 2014). Furthermore, male and female GPER KO mice displayed a divergence in body weights compared to WT mice at different ages. Thus, while male GPER KO mice began to develop adiposity by 8 weeks of age, female GPER KO mice displayed detectable weight gain only at 14 weeks of age. Importantly, expression of two thermogenic genes, UCP1and β3-adrenergic receptor, was reduced in brown adipose tissue of GPER KO mice. Interestingly, although expression of the β3-adrenergic receptor was reduced in both male and female GPER KO mice, UCP1 expression was only decreased in male GPER KO mice. Furthermore, compared to WT mice, female GPER KO mice were less sensitive to the inhibitory effects of leptin on food intake and cholecystokinin (CCK) on satiety, whereas males did not reveal any differences. Interestingly, whereas E2 induced hypothalamic ERK activation in ovariectomized WT female mice, GPER KO mice failed to do so. Several studies have implicated hypothalamic ERK1/2 in the regulation of energy homeostasis (Rahmouni et al. 2009). Reduced E2-mediated ERK1/2 phosphorylation in hypothalamus may thus explain the diminished anorectic effects of leptin and CCK in female GPER KO mice. These findings strongly support the idea that the interaction of E2 with GPER is an important mediator of body weight regulation. Very recently and contrary to the multiple independent studies just outlined, Wang et al. reported that female GPER KO mice (the same as used in the previous studies by us and Clegg) were unexpectedly protected from diet-induced obesity, exhibiting lower body weight, decreased adipogenesis, and increased dark phase energy expenditure (Wang et al. 2016). The reasons behind these contradictory results are not clear. In general, factors such as chow, bedding, or environment can confound an observed phenotype. Furthermore, differences between studies could also arise as a result of the method used to generate GPER KO mice (e.g., homologous recombination of embryonic stem cells vs. cre/loxP, where chromosomal translocations are possible due to cryptic or pseudo-loxP sites) as recently reviewed (Prossnitz and Hathaway 2015).
Because a number of studies examining GPER KO mice reported that loss of GPER resulted in adiposity, we hypothesized that selective activation of GPER in a mouse model of obesity might attenuate weight gain and alleviate other chronic disease states arising from obesity. To this end, we tested the therapeutic potential of selective GPER agonism, employing G-1 in a mouse model of metabolic dysfunction, namely, ovariectomy. This model mimics menopause in women and results in adiposity and metabolic dysfunction due to loss of endogenous E2, as revealed by the reversal of metabolic dysfunction by E2 supplementation. In this model, treatment of ovariectomized mice with G-1 resulted in attenuation of overall weight gain and fat content as revealed by DEXA and MRI scans, without effects on bone mineral density, bone mineral content, or lean mass (Sharma and Prossnitz, unpublished data). In addition, a significant reduction in the mass of multiple fat depots was observed upon G-1 treatment, as well as increased energy expenditure and higher expression of UCP1 in brown adipose tissue. Although G-1 exerted similar actions to those of E2 on the regulation of body weight and fat deposition, unlike E2, it did not increase uterine weight, reflecting a lack of the potent feminizing effects of E2 (Dennis et al. 2009). Thus, activation of GPER may represent a novel strategy to counteract weight gain and fat deposition.
GPER and Glucose Homeostasis
GPER is emerging as a key player in glucose homeostasis (Tables 1 and 2) (Sharma and Prossnitz 2016; Sharma et al. 2017). Evidence for an in vivo role of GPER in the regulation of glucose metabolism first emerged from studies of ERα/β double knockout mice exposed to streptozotocin (STZ). These mice did not exhibit a further increase in the incidence of diabetes when compared to either ERα KO or ERβ KO mice alone (Liu et al. 2009). In addition, ovariectomy increased the severity of insulin-deficient diabetes in ERα/β double KO mice following STZ treatment, an effect that was reversed by E2 supplementation, suggesting the presence of an additional distinct response mechanism to E2. Taken together, these studies suggested that even in the absence of ERα and ERβ, E2 continues to exert antidiabetic actions, consistent with the possible involvement of another estrogen receptor, such as GPER. Roles for GPER in glucose homeostasis have been established by a number of groups through the study of GPER KO mice. In 2009, Leeb-Lundberg and colleagues reported that deletion of GPER in mice resulted in impaired glucose tolerance and hyperglycemia with differential effects on male and female mice (Martensson et al. 2009). Whereas female GPER KO mice exhibited impaired glucose tolerance, increased plasma glucose, and defective GSIS compared to their WT counterparts, males did not reveal any differences between WT and GPER KO genotypes. In ovariectomized mice, GPER deficiency completely abolished E2-mediated increases in serum insulin. Furthermore, Mauvais-Jarvis and colleagues revealed that only female but not male GPER KO mice, upon exposure to STZ, exhibited a higher propensity toward insulin-deficient diabetes, displaying higher blood glucose levels, loss of β-cells, and a decrease in pancreatic insulin content (Liu et al. 2009). However, in contrast to the above reports, our observations revealed that male GPER KO mice exhibit age-dependent effects on glucose tolerance and insulin resistance (Sharma et al. 2013). At 6 months, although GPER KO mice were already insulin resistant as revealed by insulin tolerance tests (ITTs), they did not display any differences in glucose tolerance tests (GTTs), the latter consistent with the previous study by Leeb-Lundberg and colleagues (Martensson et al. 2009). However, at 12 months of age, GPER KO mice exhibited a trend toward impaired glucose tolerance, which was statistically significant by 18 months of age, with a concomitant exacerbation of insulin resistance (Sharma et al. 2013). The detection of higher fasting plasma insulin levels in GPER KO mice, with normal fasting glucose levels, further confirmed the presence of insulin resistance, in which elevated insulin levels are required to maintain normal glucose levels (Abdul-Ghani and DeFronzo 2009).
Weight gain, specifically visceral adiposity, is linked to a chronic inflammatory state and a decrease in the serum levels of adiponectin, an insulin-sensitizing adipokine that also exhibits anti-inflammatory properties (Bastard et al. 2006; Mangge et al. 2010). Consistent with these observations, we observed that adiposity in GPER KO male mice was accompanied by increases in systemic markers of inflammation, such as TNFα, MCP1, IL-1β, and IL-6, along with a decrease in adiponectin levels (Sharma et al. 2013). The glucose intolerance present in aged GPER KO mice could have resulted from the cumulative effects of adiposity, insulin resistance, dyslipidemia, and inflammation. The existence of a pro-inflammatory state in GPER KO mice was subsequently confirmed by Davis et al., who demonstrated that both male and female GPER KO mice exhibit systemic increases in levels of the inflammatory marker SAA3 and a decrease in adiponectin levels compared to WT mice (Davis et al. 2014). Furthermore, treatment of ovariectomized GPER KO mice with E2 did not yield any improvements in glucose tolerance as in ovariectomized WT mice, indicating a definitive requirement for GPER in E2-mediated glucose metabolism. Taken together, these studies clearly indicate that both male and female GPER KO mice, in a sex-specific manner, exhibit regulation of glucose metabolism via GPER.
To determine whether selective activation of GPER could alleviate the symptoms of metabolic dysfunction with respect to insulin resistance and glucose tolerance, we treated ovariectomized mice with the GPER-selective agonist G-1 (Sharma and Prossnitz, unpublished data). Our results revealed that G-1 treatment led to a significant improvement in glucose tolerance in ovariectomized mice, with lower fasting glucose and insulin levels. In addition, G-1 treated mice exhibited improved insulin sensitivity and reduced the levels of circulating pro-inflammatory cytokines and hormones leptin and resistin. Lower fasting blood glucose and insulin levels in the ovariectomized mice suggest beneficial effects of G-1 on glucose homeostasis in both the liver and skeletal muscle, leading to speculation that G-1 may directly modulate glucose production in the liver and glucose uptake in skeletal muscle. However, as discussed above, since treatment with G-1 also prevents weight gain and visceral fat deposition in ovariectomized mice, an improvement in glucose homeostasis could be due to either direct or indirect effects of GPER-mediated signaling events in the tissues involved in metabolic regulation, which exhibit substantial cross talk with respect to glucose homeostasis (Samdani et al. 2015; Kim 2016).
GPER and Pancreatic Function
Pancreatic β-cells produce, store, and release insulin, the critical hormone in glucose homeostasis. GPER promotes the survival and function of multiple cell types in islets, particularly β-cells, the mechanisms of which have been examined in some detail and exhibit definite sex differences (Tables 1 and 2) (Liu et al. 2009; Mauvais-Jarvis 2016; Ropero et al. 2012). GPER expression was considerably higher in islets from females compared to males, both in mice and humans (Balhuizen et al. 2010; Kumar et al. 2011). Under basal conditions, islets isolated from male and female GPER KO mice exhibited reductions in insulin secretion compared to WT controls, as well as in the presence of glucose or tolbutamide, a potassium channel blocker that causes insulin secretion by blocking potassium channels in pancreatic β-cells (Martensson et al. 2009). Interestingly, with respect to their WT counterparts, islets isolated from female GPER KO mice exhibited a greater reduction in insulin secretion compared to islets from male GPER KO mice. Furthermore, islets from female GPER KO mice under basal conditions exhibited a decrease in pancreatic insulin content compared to islets from WT mice, which may have resulted from a defective E2 signaling in the absence of GPER. Similarly, islets isolated from both male and female GPER KO mice completely lacked the E2-stimulated insulin secretion present in islets from WT mice, despite the use of supraphysiological concentrations of E2 (5 μM). Although E2 treatment of ovariectomized GPER KO mice failed to increase serum insulin levels, islets from treated mice exhibited higher pancreatic insulin content (presumably via ERα) compared to WT controls, suggesting that GPER may be important for insulin secretion from the pancreas. Finally, G-1 stimulation of both human and murine islets modulated hormone secretion and exerted antidiabetic effects in a dose-dependent manner similar to E2, with both agents increasing insulin secretion while inhibiting glucagon and somatostatin secretion (Balhuizen et al. 2010; Kumar et al. 2011).
The mechanisms leading to insulin secretion upon GPER activation by E2 or G-1 involve increased signaling through the cAMP/PKA and PLC/IP3 pathways, as stimulation with both agents increased the formation of cAMP and IP3 in a dose-dependent manner in islets from human female donors (Kumar et al. 2011). Interestingly, G-1 was more potent in IP3 production, whereas E2 exhibited higher potency in cAMP generation. In cultured mouse insulinoma MIN6 cells, E2 and G-1 both stimulated insulin secretion that could be inhibited by pharmacologic GPER-selective antagonism with G15 and depletion of GPER by siRNA (Sharma and Prossnitz 2011). Similarly, insulin secretion in WT islets was inhibited by G15 in response to GPER activation by either E2 or G-1, both of which failed to induce insulin secretion in islets from GPER KO mice (Sharma and Prossnitz 2011). In MIN6 cells, stimulation of GPER results in intracellular calcium release as well as activation of the ERK and PI3K pathways (Sharma and Prossnitz 2011). As previously reported in some cancer and other cell lines, GPER-mediated ERK activation occurred via transactivation of the EGFR (Filardo et al. 2000; Sharma and Prossnitz 2011). Interestingly, whereas ERK activity exhibited a positive effect on insulin secretion, PI3K activity inhibited insulin secretion, as previously observed (Hagiwara et al. 1995; Longuet et al. 2005). Thus, whereas inhibition of either EGFR or ERK prevented E2- or G-1-induced increases in insulin secretion, inhibition of PI3K signaling led to an increase in insulin secretion compared to E2 and G-1 alone. These results suggest that E2- and G-1-mediated activation of the ERK and PI3K pathways oppose each other and may serve to balance the secretion of insulin in response to multiple signaling inputs.
Obesity and insulin resistance lead to the process of compensation in islets, increasing the biosynthesis and secretion of insulin as well as the number of β-cells to maintain normal blood glucose levels (Cerf 2013). Persistent cellular stresses associated with obesity and insulin resistance, such as cytokine-induced inflammation, mitochondrial dysfunction, oxidative stress, ER stress, and glucolipotoxicity, eventually lead to β-cell death and hyperglycemia. E2 is known to promote β-cell survival under these conditions (Prentki and Nolan 2006). Experimentally, activation of GPER by either E2 or G-1 promoted cell survival and counteracted apoptosis induced by pro-inflammatory cytokines and oxidative stress in both murine and human islets, as well as in MIN6 cells (Liu et al. 2009; Balhuizen et al. 2010; Kumar et al. 2011). In islets subjected to inflammatory injury, E2 or G-1 promoted islet cell survival via phosphorylation of pro-survival genes such as CREB, Akt, and ERK1/2 with concomitant suppression in the activity of stress proteins, such as SAPK/JNK and p38 (Kumar et al. 2011). Pretreatment with ER antagonists, ICI 182,780 or EM-652, did not inhibit the protective effects of E2, suggesting that E2 may function via GPER, consistent with the protective effects of G-1. In addition, islets from GPER KO mice exposed to oxidative stress lacked G-1-mediated protection, although survival mediated by E2 was maintained, suggesting a parallel effect of E2 via ERα (Liu et al. 2009). Interestingly, islets isolated from mice lacking both ERα and ERβ still exhibited protection against cell death when challenged with STZ, suggesting the involvement of GPER or another unknown ER in mediating this response (Liu et al. 2009). In addition to the mechanisms described above, studies on isolated islets and cultured cells have shown that GPER activation also inhibits lipid accumulation, by suppressing the expression of important transcription factors involved in lipogenesis, such as chSREBP and SREBP1 via STAT3, potentially leading to even greater antiapoptotic effects as a result of reduced lipotoxicity (Tiano et al. 2011; Tiano and Mauvais-Jarvis 2012).
An additional physiological stressor, pregnancy, also leads to the expansion of pancreatic β-cell mass in order to compensate for maternal insulin resistance (Rieck and Kaestner 2010; Ernst et al. 2011). Increases in β-cell mass during pregnancy result from an increase in proliferation and survival of β-cells through the downregulation of islet-specific microRNA mi-338-3p (Jacovetti et al. 2012). In cultured β-cells and dissociated islets, downregulation of miR-338-3p increased proliferation and protected cells against pro-inflammatory cytokine-induced apoptosis. GPER expression increased in rat islets during pregnancy, peaking at day 14, with E2 repressing mi-338-3p in rat islets via GPER through a cAMP-dependent pathway (Jacovetti et al. 2012). Activation of GPER in rat islets reduced the expression of mi-338-3p, an effect that was reversed by treatment with GPER-targeted siRNA. These results indicate that E2 signaling via GPER suppresses the expression of miR-338-3p, which may be critical for the increase in β-cell mass during pregnancy.
Effects of GPER in Peripheral Metabolic Tissues
Although roles for GPER in overall body weight regulation and glucose homeostasis have been observed (Sharma et al. 2013; Martensson et al. 2009; Davis et al. 2014), little is known regarding the effects of GPER in the individual peripheral tissues that are actively involved in metabolism, such as the adipose, liver, and skeletal muscle, all of which act in a coordinated manner to maintain metabolic homeostasis. Although GPER is widely expressed in multiple insulin-sensitive tissues such as the liver, adipose, and skeletal muscle, female mice exhibited higher GPER expression in white adipose tissue compared to males (Davis et al. 2014). GPER expression was localized predominantly to adipocytes with little expression in the stromal vascular fraction (Davis et al. 2014). GPER expression has also been reported in 3T3-L1 preadipocytes, where it was upregulated during differentiation of preadipocytes into adipocytes (Zhu et al. 2013). Treatment of 3T3-L1 preadipocytes with E2 or G-1 during differentiation inhibited lipid accumulation in adipocytes, an outcome that was reversed by GPER knockdown with siRNA (Zhu et al. 2013). During adipogenic differentiation, after initial mitotic clonal expansion, cells arrest at the G1 growth phase of the cell cycle and subsequently express adipogenic factors (Tang et al. 2003; Patel and Lane 2000). Treatment with G-1 in 3T3-L1 cells results in an aberrant differentiation process wherein most of the cells continue to divide even after 48 h of differentiation, whereas in the control group, the majority of cells arrested in the G0/G1 state by 24 h following the induction of differentiation (Zhu et al. 2013). Furthermore, GPER activation increased the expression of cell cycle-regulating factors, such as CDK4, CDK6, and cyclin D. Thus, GPER inhibited lipid accumulation in adipocytes at least in part by preventing cell cycle arrest and subsequent differentiation (Zhu et al. 2013).
As described above for multiple studies, mice lacking GPER exhibit an increase in overall adiposity with increased fat deposition in subcutaneous, perigonadal, and perirenal fat depots compared to their WT counterparts (Sharma et al. 2013; Haas et al. 2009; Davis et al. 2014). In the gonadal fat pads of GPER KO mice, adipocytes were larger compared to WT mice, as a result of increased lipid storage (Davis et al. 2014). Interestingly, in GPER-lacZ mice (a mouse mutant that harbors a β-galactosidase (lacZ) reporter within the Gper locus, disrupting Gper expression), only female GPER KO mice exhibited increased lipid accumulation in liver along with a decrease in circulating HDL levels compared to WT mice, whereas male mice displayed no such differences (Meoli et al. 2014). Consistent with GPER KO mice, a study of a human cohort of the Northern European descent has revealed that individuals carrying a hypofunctional P16L genetic variant of GPER have increased plasma LDL cholesterol (Hussain et al. 2015). These observations were further extended using HepG2 liver cells in which activation of GPER with G-1 increased the expression of the LDL receptor (Hussain et al. 2015). This upregulation was blocked by either GPER antagonist G15 or knockdown of GPER expression by shRNA. These results imply that GPER plays a crucial role in modulating central pathways involved in lipid metabolism in multiple tissues, suggesting that selective GPER activation might be beneficial in lowering lipid levels. Thus, we examined the effects of GPER stimulation on lipid homeostasis in vivo in an ovariectomized mouse model (Sharma and Prossnitz, unpublished data). G-1 treatment lowered the levels of circulating lipids, reduced the expression of lipogenic and pro-inflammatory genes, and increased the expression of genes involved in lipid oxidation in the adipose, liver, and skeletal muscle. Thus, GPER exerts pleiotropic effects in metabolic tissues leading to reductions in both lipid accumulation and inflammation.
Conclusions
It has now become clear that GPER regulates not only body weight but also multiple aspects of metabolism in numerous tissues throughout the body, such as the pancreas, adipose, liver, and skeletal muscle. However, mechanisms of GPER-mediated effects remain poorly understood and merit further study. Global GPER KO mice have been used by a number of groups to investigate the functions of GPER in vivo (Prossnitz and Hathaway 2015), but due to cross talk between metabolic tissues and the possibility of compensatory effects during development, conclusions from such studies must be interpreted with caution. The use of pharmacological approaches to modulate GPER activity has largely supported the conclusions from GPER KO studies (Prossnitz and Arterburn 2015). With the epidemic prevalence of obesity and metabolic dysfunction, it is more critical than ever to identify new therapeutic approaches to mimic the salutary effects of E2 without the feminizing and other side effects of estrogenic substances, particularly for men. The therapeutic targeting of GPER may represent one such approach to simultaneously treat multiple aspects of metabolic syndrome.
Acknowledgments
The authors were supported by research grants from the National Institutes of Health (NIH R01 CA127731, CA163890, and CA194496 to ERP), Dialysis Clinic, Inc. (to ERP), and by the UNM Comprehensive Cancer Center (P30 CA118100).
Footnotes
Competing Interests GS and ERP are inventors on a US patent application for the therapeutic use of compounds targeting GPER. ERP is an inventor on US patent Nos. 7,875,721 and 8,487,100 for GPER-selective ligands and imaging agents.
Contributor Information
Geetanjali Sharma, Division of Molecular Medicine, Department of Internal Medicine, University of New Mexico Health Sciences Center, Albuquerque, NM, USA.
Eric R. Prossnitz, Division of Molecular Medicine, Department of Internal Medicine, and Autophagy, Inflammation and Metabolism Center of Biomedical Research Excellence, University of New Mexico Health Sciences Center, Albuquerque, NM, USA; University of New Mexico Comprehensive Cancer Center, University of New Mexico Health Sciences Center, Albuquerque, NM, USA
References
- Abdul-Ghani MA, & DeFronzo RA (2009). Pathophysiology of prediabetes. Current Diabetes Reports, 9, 193–199. [DOI] [PubMed] [Google Scholar]
- Abu-Taha M, Rius C, Hermenegildo C, Noguera I, Cerda-Nicolas JM, Issekutz AC, Jose PJ, Cortijo J, Morcillo EJ, & Sanz MJ (2009). Menopause and ovariectomy cause a low grade of systemic inflammation that may be prevented by chronic treatment with low doses of estrogen or losartan. Journal of Immunology, 183, 1393–1402. [DOI] [PubMed] [Google Scholar]
- Alberti KG, Zimmet P, & Shaw J (2006). Metabolic syndrome – a new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabetic Medicine: A Journal of the British Diabetic Association, 23, 469–480. [DOI] [PubMed] [Google Scholar]
- Andersson B, Mattsson LA, Hahn L, Marin P, Lapidus L, Holm G, Bengtsson BA, & Bjorntorp P (1997). Estrogen replacement therapy decreases hyperandrogenicity and improves glucose homeostasis and plasma lipids in postmenopausal women with noninsulin-dependent diabetes mellitus. The Journal of Clinical Endocrinology and Metabolism, 82, 638–643. [DOI] [PubMed] [Google Scholar]
- Arias-Pulido H, Royce M, Gong Y, Joste N, Lomo L, Lee SJ, Chaher N, Verschraegen C, Lara J, Prossnitz ER, & Cristofanilli M (2010). GPR30 and estrogen receptor expression: New insights into hormone dependence of inflammatory breast cancer. Breast Cancer Research and Treatment, 123, 51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balhuizen A, Kumar R, Amisten S, Lundquist I, & Salehi A (2010). Activation of G protein-coupled receptor 30 modulates hormone secretion and counteracts cytokine-induced apoptosis in pancreatic islets of female mice. Molecular and Cellular Endocrinology, 320, 16–24. [DOI] [PubMed] [Google Scholar]
- Barkhem T, Nilsson S, & Gustafsson JA (2004). Molecular mechanisms, physiological consequences and pharmacological implications of estrogen receptor action. American Journal of Pharmacogenomics, 4, 19–28. [DOI] [PubMed] [Google Scholar]
- Barros RP, & Gustafsson JA (2011). Estrogen receptors and the metabolic network. Cell Metabolism, 14, 289–299. [DOI] [PubMed] [Google Scholar]
- Barros RP, Machado UF, & Gustafsson JA (2006). Estrogen receptors: New players in diabetes mellitus. Trends in Molecular Medicine, 12, 425–431. [DOI] [PubMed] [Google Scholar]
- Barton M, & Prossnitz ER (2015). Emerging roles of GPER in diabetes and atherosclerosis. Trends in Endocrinology and Metabolism: TEM, 26, 185–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton M, Filardo EJ, Lolait SJ, Thomas P, Maggiolini M, & Prossnitz ER (2017). Twenty years of the G protein-coupled estrogen receptor GPER: Historical and personal perspectives. The Journal of Steroid Biochemistry and Molecular Biology. 10.1016/j.jsbmb.2017.03.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basen-Engquist K, & Chang M (2011). Obesity and cancer risk: Recent review and evidence. Current Oncology Reports, 13, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Lagathu C, Kim MJ, Caron M, Vidal H, Capeau J, & Feve B (2006). Recent advances in the relationship between obesity, inflammation, and insulin resistance. European Cytokine Network, 17, 4–12. [PubMed] [Google Scholar]
- Bays HE, Toth PP, Kris-Etherton PM, Abate N, Aronne LJ, Brown WV, Gonzalez-Campoy JM, Jones SR, Kumar R, La Forge R, & Samuel VT (2013). Obesity, adiposity, and dyslipidemia: A consensus statement from the National Lipid Association. Journal of Clinical Lipidology, 7, 304–383. [DOI] [PubMed] [Google Scholar]
- Blaak E (2001). Gender differences in fat metabolism. Current Opinion in Clinical Nutrition and Metabolic Care, 4, 499–502. [DOI] [PubMed] [Google Scholar]
- Blasko E, Haskell CA, Leung S, Gualtieri G, Halks-Miller M, Mahmoudi M, Dennis MK, Prossnitz ER, Karpus WJ, & Horuk R (2009). Beneficial role of the GPR30 agonist G-1 in an animal model of multiple sclerosis. Journal of Neuroimmunology, 214, 67–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokov AF, Ko D, & Richardson A (2009). The effect of gonadectomy and estradiol on sensitivity to oxidative stress. Endocrine Research, 34, 43–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bologa CG, Revankar CM, Young SM, Edwards BS, Arterburn JB, Kiselyov AS, Parker MA, Tkachenko SE, Savchuck NP, Sklar LA, Oprea TI, & Prossnitz ER (2006). Virtual and biomolecular screening converge on a selective agonist for GPR30. Nature Chemical Biology, 2, 207–212. [DOI] [PubMed] [Google Scholar]
- Bonds DE, Lasser N, Qi L, Brzyski R, Caan B, Heiss G, Limacher MC, Liu JH, Mason E, Oberman A, O’Sullivan MJ, Phillips LS, Prineas RJ, & Tinker L (2006). The effect of conjugated equine oestrogen on diabetes incidence: The Women’s Health Initiative randomised trial. Diabetologia, 49, 459–468. [DOI] [PubMed] [Google Scholar]
- Borras C, Gambini J, Lopez-Grueso R, Pallardo FV, & Vina J (2010). Direct antioxidant and protective effect of estradiol on isolated mitochondria. Biochimica et Biophysica Acta, 1802, 205–211. [DOI] [PubMed] [Google Scholar]
- Brons C, & Vaag A (2009). Skeletal muscle lipotoxicity in insulin resistance and type 2 diabetes. The Journal of Physiology, 587, 3977–3978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown LM, & Clegg DJ (2010). Central effects of estradiol in the regulation of food intake, body weight, and adiposity. The Journal of Steroid Biochemistry and Molecular Biology, 122, 65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns CM, & Kemnitz JW (2004). Sex hormones, insulin sensitivity, and diabetes mellitus. ILAR Journal, 45, 160–169. [DOI] [PubMed] [Google Scholar]
- Brunsing RL, & Prossnitz ER (2011). Induction of interleukin-10 in the T helper type 17 effector population by the G protein coupled estrogen receptor (GPER) agonist G-1. Immunology, 134, 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunsing RL, Owens KS, & Prossnitz ER (2013). The G protein-coupled estrogen receptor (GPER) agonist G-1 expands the regulatory T-cell population under TH17-polarizing conditions. Journal of Immunotherapy, 36, 190–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Center for Disease Control and Prevention: Adult Obesity Facts. (2017). https://www.cdc.gov/obesity/data/adult.html
- Cerf ME (2013). Beta cell dysfunction and insulin resistance. Frontiers in Endocrinology, 4, 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Consitt LA, Bell JA, & Houmard JA (2009). Intramuscular lipid metabolism, insulin action, and obesity. IUBMB Life, 61, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Eon TM, Souza SC, Aronovitz M, Obin MS, Fried SK, & Greenberg AS (2005). Estrogen regulation of adiposity and fuel partitioning. Evidence of genomic and non-genomic regulation of lipogenic and oxidative pathways. The Journal of Biological Chemistry, 280, 35983–35991. [DOI] [PubMed] [Google Scholar]
- Dahlman-Wright K, Cavailles V, Fuqua SA, Jordan VC, Katzenellenbogen JA, Korach KS, Maggi A, Muramatsu M, Parker MG, & Gustafsson JA (2006). International union of pharmacology. LXIV. Estrogen receptors. Pharmacological Reviews, 58, 773–781. [DOI] [PubMed] [Google Scholar]
- Davis KE, Carstens EJ, Irani BG, Gent LM, Hahner LM, & Clegg DJ (2014). Sexually dimorphic role of G protein-coupled estrogen receptor (GPER) in modulating energy homeostasis. Hormones and Behavior, 66, 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Vedova MC, Munoz MD, Santillan LD, Plateo-Pignatari MG, Germano MJ, Rinaldi Tosi ME, Garcia S, Gomez NN, Fornes MW, Gomez Mejiba SE, & Ramirez DC (2016). A mouse model of diet-induced obesity resembling most features of human metabolic syndrome. Nutrition and Metabolic Insights, 9, 93–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Burai R, Ramesh C, Petrie WK, Alcon SN, Nayak TK, Bologa CG, Leitao A, Brailoiu E, Deliu E, Dun NJ, Sklar LA, Hathaway HJ, Arterburn JB, Oprea TI, & Prossnitz ER (2009). In vivo effects of a GPR30 antagonist. Nature Chemical Biology, 5, 421–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis MK, Field AS, Burai R, Ramesh C, Petrie WK, Bologa CG, Oprea TI, Yamaguchi Y, Hayashi S, Sklar LA, Hathaway HJ, Arterburn JB, & Prossnitz ER (2011). Identification of a GPER/GPR30 antagonist with improved estrogen receptor counter-selectivity. The Journal of Steroid Biochemistry and Molecular Biology, 127, 358–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroo BJ, & Korach KS (2006). Estrogen receptors and human disease. The Journal of Clinical Investigation, 116, 561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz Brinton R (2012). Minireview: Translational animal models of human menopause: Challenges and emerging opportunities. Endocrinology, 153, 3571–3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domingueti CP, Dusse LM, Carvalho M, de Sousa LP, Gomes KB, & Fernandes AP (2016). Diabetes mellitus: The linkage between oxidative stress, inflammation, hypercoagulability and vascular complications. Journal of Diabetes and its Complications, 30, 738–745. [DOI] [PubMed] [Google Scholar]
- Eckel RH, & Krauss RM (1998). American Heart Association call to action: Obesity as a major risk factor for coronary heart disease. AHA Nutrition Committee. Circulation, 97, 2099–2100. [DOI] [PubMed] [Google Scholar]
- El Akoum S, Lamontagne V, Cloutier I, & Tanguay JF (2011). Nature of fatty acids in high fat diets differentially delineates obesity-linked metabolic syndrome components in male and female C57BL/6J mice. Diabetology & Metabolic Syndrome, 3, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst S, Demirci C, Valle S, Velazquez-Garcia S, & Garcia-Ocana A (2011). Mechanisms in the adaptation of maternal beta-cells during pregnancy. Diabetes Management, 1, 239–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Sanchez A, Madrigal-Santillan E, Bautista M, Esquivel-Soto J, Morales-Gonzalez A, Esquivel-Chirino C, Durante-Montiel I, Sanchez-Rivera G, Valadez-Vega C, & Morales-Gonzalez JA (2011). Inflammation, oxidative stress, and obesity. International Journal of Molecular Sciences, 12, 3117–3132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Bland KI, & Frackelton AR Jr. (2000). Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Molecular Endocrinology, 14, 1649–1660. [DOI] [PubMed] [Google Scholar]
- Filardo EJ, Quinn JA, Frackelton AR Jr., & Bland KI (2002). Estrogen action via the G protein-coupled receptor, GPR30: Stimulation of adenylyl cyclase and cAMP-mediated attenuation of the epidermal growth factor receptor-to-MAPK signaling axis. Molecular Endocrinology, 16, 70–84. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, & Curtin LR (2010). Prevalence and trends in obesity among US adults, 1999–2008. JAMA, 303, 235–241. [DOI] [PubMed] [Google Scholar]
- Folli F, Corradi D, Fanti P, Davalli A, Paez A, Giaccari A, Perego C, & Muscogiuri G. (2011). The role of oxidative stress in the pathogenesis of type 2 diabetes mellitus micro- and macrovascular complications: Avenues for a mechanistic-based therapeutic approach. Current Diabetes Reviews, 7, 313–324. [DOI] [PubMed] [Google Scholar]
- Fredette NC, Meyer MR, & Prossnitz ER (2017). Role of GPER in estrogen-dependent nitric oxide formation and vasodilation. The Journal of Steroid Biochemistry and Molecular Biology. 10.1016/j.jsbmb.2017.05.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M, & Shimomura I (2004). Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation, 114, 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garaulet M, Perez-Llamas F, Baraza JC, Garcia-Prieto MD, Fardy PS, Tebar FJ, & Zamora S (2002). Body fat distribution in pre-and post-menopausal women: Metabolic and anthropometric variables. The Journal of Nutrition, Health & Aging, 6, 123–126. [PubMed] [Google Scholar]
- Garcia-Arevalo M, Alonso-Magdalena P, Rebelo Dos Santos J, Quesada I, Carneiro EM, & Nadal A (2014). Exposure to bisphenol-A during pregnancy partially mimics the effects of a high-fat diet altering glucose homeostasis and gene expression in adult male mice. PLoS One, 9, e100214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geer EB, & Shen W (2009). Gender differences in insulin resistance, body composition, and energy balance. Gender Medicine, 6(Suppl 1), 60–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godsland IF (2005). Oestrogens and insulin secretion. Diabetologia, 48, 2213–2220. [DOI] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, & Anis AH (2009). The incidence of co-morbidities related to obesity and overweight: A systematic review and meta-analysis. BMC Public Health, 9, 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney EP, Nachtigall MJ, Nachtigall LE, & Naftolin F (2014). The Women’s Health Initiative trial and related studies: 10 years later: A clinician’s view. The Journal of Steroid Biochemistry and Molecular Biology, 142, 4–11. [DOI] [PubMed] [Google Scholar]
- Haas E, Bhattacharya I, Brailoiu E, Damjanovic M, Brailoiu GC, Gao X, Mueller-Guerre L, Marjon NA, Gut A, Minotti R, Meyer MR, Amann K, Ammann E, Perez-Dominguez A, Genoni M, Clegg DJ, Dun NJ, Resta TC, Prossnitz ER, & Barton M (2009). Regulatory role of G protein-coupled estrogen receptor for vascular function and obesity. Circulation Research, 104, 288–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjimarkou MM, & Vasudevan N (2017). GPER1/GPR30 in the brain: Crosstalk with classical estrogen receptors and implications for behavior. The Journal of Steroid Biochemistry and Molecular Biology. 10.1016/j.jsbmb.2017.04.012 [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Sakurai T, Tashiro F, Hashimoto Y, Matsuda Y, Nonomura Y, & Miyazaki J (1995). An inhibitory role for phosphatidylinositol 3-kinase in insulin secretion from pancreatic B cell line MIN6. Biochemical and Biophysical Research Communications, 214, 51–59. [DOI] [PubMed] [Google Scholar]
- Hammond RA, & Levine R (2010). The economic impact of obesity in the United States. Diabetes, Metabolic Syndrome and Obesity: Targets and Therapy, 3, 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han TS, & Lean ME (2016). A clinical perspective of obesity, metabolic syndrome and cardiovascular disease. JRSM Cardiovascular Disease, 5, 2048004016633371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazell GG, Yao ST, Roper JA, Prossnitz ER, O’Carroll AM, & Lolait SJ (2009). Localisation of GPR30, a novel G protein-coupled oestrogen receptor, suggests multiple functions in rodent brain and peripheral tissues. The Journal of Endocrinology, 202, 223–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine PA, Taylor JA, Iwamoto GA, Lubahn DB, & Cooke PS (2000). Increased adipose tissue in male and female estrogen receptor-alpha knockout mice. Proceedings of the National Academy of Sciences of the United States of America, 97, 12729–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hewitt SC, Harrell JC, & Korach KS (2005). Lessons in estrogen biology from knockout and transgenic animals. Annual Review of Physiology, 67, 285–308. [DOI] [PubMed] [Google Scholar]
- Hong J, Stubbins RE, Smith RR, Harvey AE, & Nunez NP (2009). Differential susceptibility to obesity between male, female and ovariectomized female mice. Nutrition Journal, 8, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hormone Replacement Therapy and Cancer. (2001). Gynecological Endocrinology: The Official Journal of the International Society of Gynecological Endocrinology, 15, 453–465. [PubMed] [Google Scholar]
- Huang G, Xu J, Lefever DE, Glenn TC, Nagy T, & Guo TL (2017). Genistein prevention of hyperglycemia and improvement of glucose tolerance in adult non-obese diabetic mice are associated with alterations of gut microbiome and immune homeostasis. Toxicology and Applied Pharmacology, 332, 138–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain Y, Ding Q, Connelly PW, Brunt JH, Ban MR, McIntyre AD, Huff MW, Gros R, Hegele RA, & Feldman RD (2015). G-protein estrogen receptor as a regulator of low-density lipoprotein cholesterol metabolism: Cellular and population genetic studies. Arteriosclerosis, Thrombosis, and Vascular Biology, 35, 213–221. [DOI] [PubMed] [Google Scholar]
- Jacovetti C, Abderrahmani A, Parnaud G, Jonas JC, Peyot ML, Cornu M, Laybutt R, Meugnier E, Rome S, Thorens B, Prentki M, Bosco D, & Regazzi R (2012). MicroRNAs contribute to compensatory beta cell expansion during pregnancy and obesity. The Journal of Clinical Investigation, 122, 3541–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD (2008). Role of body fat distribution and the metabolic complications of obesity. The Journal of Clinical Endocrinology and Metabolism, 93, S57–S63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia M, Dahlman-Wright K, & Gustafsson JA (2015). Estrogen receptor alpha and beta in health and disease. Best Practice & Research. Clinical Endocrinology & Metabolism, 29, 557–568. [DOI] [PubMed] [Google Scholar]
- Jones ME, Thorburn AW, Britt KL, Hewitt KN, Wreford NG, Proietto J, Oz OK, Leury BJ, Robertson KM, Yao S, & Simpson ER (2000). Aromatase-deficient (ArKO) mice have a phenotype of increased adiposity. Proceedings of the National Academy of Sciences of the United States of America, 97, 12735–12740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei Y, Suzuki M, Miyazaki H, Tsuboyama-Kasaoka N, Wu J, Ishimi Y, & Ezaki O (2005). Ovariectomy in mice decreases lipid metabolism-related gene expression in adipose tissue and skeletal muscle with increased body fat. Journal of Nutritional Science and Vitaminology, 51, 110–117. [DOI] [PubMed] [Google Scholar]
- Kanaya AM, Herrington D, Vittinghoff E, Lin F, Grady D, Bittner V, Cauley JA, Barrett-Connor E, & Heart, Estrogen/progestin Replacement, S. (2003). Glycemic effects of postmenopausal hormone therapy: The heart and estrogen/progestin replacement study. A randomized, double-blind, placebo-controlled trial. Annals of Internal Medicine, 138, 1–9. [DOI] [PubMed] [Google Scholar]
- Kaunitz AM, & Manson JE (2015). Management of menopausal symptoms. Obstetrics and Gynecology, 126, 859–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KB, & Lemberg L (2003). Obesity and the metabolic syndrome. American Journal of Critical Care: An Official Publication, American Association of Critical-Care Nurses, 12, 167–170. [PubMed] [Google Scholar]
- Kernan WN, & Dearborn JL (2015). Obesity increases stroke risk in young adults: Opportunity for prevention. Stroke, 46, 1435–1436. [DOI] [PubMed] [Google Scholar]
- Kim JB (2016). Dynamic cross talk between metabolic organs in obesity and metabolic diseases. Experimental & Molecular Medicine, 48, e214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Sohn I, Lee YS, & Lee YS (2005). Hepatic gene expression profiles are altered by genistein supplementation in mice with diet-induced obesity. The Journal of Nutrition, 135, 33–41. [DOI] [PubMed] [Google Scholar]
- Klop B, Elte JW, & Cabezas MC (2013). Dyslipidemia in obesity: Mechanisms and potential targets. Nutrients, 5, 1218–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotani K, Tokunaga K, Fujioka S, Kobatake T, Keno Y, Yoshida S, Shimomura I, Tarui S, & Matsuzawa Y (1994). Sexual dimorphism of age-related changes in whole-body fat distribution in the obese. International Journal of Obesity and Related Metabolic Disorders: Journal of the International Association for the Study of Obesity, 18, 207–202. [PubMed] [Google Scholar]
- Kumar R, Balhuizen A, Amisten S, Lundquist I, & Salehi A (2011). Insulinotropic and antidiabetic effects of 17beta-estradiol and the GPR30 agonist G-1 on human pancreatic islets. Endocrinology, 152, 2568–2579. [DOI] [PubMed] [Google Scholar]
- Lappano R, Pisano A, & Maggiolini M (2014). GPER function in breast cancer: An overview. Frontiers in Endocrinology, 5, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le May C, Chu K, Hu M, Ortega CS, Simpson ER, Korach KS, Tsai MJ, & Mauvais-Jarvis F (2006). Estrogens protect pancreatic beta-cells from apoptosis and prevent insulin-deficient diabetes mellitus in mice. Proceedings of the National Academy of Sciences of the United States of America, 103, 9232–9237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CG, Carr MC, Murdoch SJ, Mitchell E, Woods NF, Wener MH, Chandler WL, Boyko EJ, & Brunzell JD (2009). Adipokines, inflammation, and visceral adiposity across the menopausal transition: A prospective study. The Journal of Clinical Endocrinology and Metabolism, 94, 1104–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei H, Lu F, Dong H, Xu L, Wang J, Zhao Y, & Huang Z (2011). Genistein reverses free fatty acid-induced insulin resistance in HepG2 hepatocytes through targeting JNK. Journal of Huazhong University of Science and Technology. Medical Sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban, 31, 185–189. [DOI] [PubMed] [Google Scholar]
- Levin ER (2015). Extranuclear steroid receptors are essential for steroid hormone actions. Annual Review of Medicine, 66, 271–280. [DOI] [PubMed] [Google Scholar]
- Lin SE, Huang JP, Wu LZ, Wu T, & Cui L (2013). Prevention of osteopenia and dyslipidemia in rats after ovariectomy with combined aspirin and low-dose diethylstilbestrol. Biomedical and Environmental Sciences: BES, 26, 249–257. [DOI] [PubMed] [Google Scholar]
- Lindheim SR, Buchanan TA, Duffy DM, Vijod MA, Kojima T, Stanczyk FZ, & Lobo RA (1994). Comparison of estimates of insulin sensitivity in pre- and postmenopausal women using the insulin tolerance test and the frequently sampled intravenous glucose tolerance test. Journal of the Society for Gynecologic Investigation, 1, 150–154. [DOI] [PubMed] [Google Scholar]
- Litwak SA, Wilson JL, Chen W, Garcia-Rudaz C, Khaksari M, Cowley MA, & Enriori PJ (2014). Estradiol prevents fat accumulation and overcomes leptin resistance in female high-fat diet mice. Endocrinology, 155, 4447–4460. [DOI] [PubMed] [Google Scholar]
- Liu S, & Mauvais-Jarvis F (2009). Rapid, nongenomic estrogen actions protect pancreatic islet survival. Islets, 1, 273–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Zhen W, Yang Z, Carter JD, Si H, & Reynolds KA (2006). Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes, 55, 1043–1050. [DOI] [PubMed] [Google Scholar]
- Liu S, Le May C, Wong WP, Ward RD, Clegg DJ, Marcelli M, Korach KS, & Mauvais-Jarvis F (2009). Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes, 58, 2292–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Yu P, Qian W, Li Y, Zhao J, Huan F, Wang J, & Xiao H (2013). Perinatal bisphenol A exposure and adult glucose homeostasis: Identifying critical windows of exposure. PLoS One, 8, e64143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo RA (2008). Metabolic syndrome after menopause and the role of hormones. Maturitas, 60, 10–18. [DOI] [PubMed] [Google Scholar]
- Longuet C, Broca C, Costes S, Hani EH, Bataille D, & Dalle S (2005). Extracellularly regulated kinases 1/2 (p44/42 mitogen-activated protein kinases) phosphorylate synapsin I and regulate insulin secretion in the MIN6 beta-cell line and islets of Langerhans. Endocrinology, 146, 643–654. [DOI] [PubMed] [Google Scholar]
- Louet JF, LeMay C, & Mauvais-Jarvis F (2004). Antidiabetic actions of estrogen: Insight from human and genetic mouse models. Current Atherosclerosis Reports, 6, 180–185. [DOI] [PubMed] [Google Scholar]
- Lundholm L, Bryzgalova G, Gao H, Portwood N, Falt S, Berndt KD, Dicker A, Galuska D, Zierath JR, Gustafsson JA, Efendic S, Dahlman-Wright K, & Khan A (2008). The estrogen receptor {alpha}-selective agonist propyl pyrazole triol improves glucose tolerance in ob/ob mice; potential molecular mechanisms. The Journal of Endocrinology, 199, 275–286. [DOI] [PubMed] [Google Scholar]
- Mangge H, Almer G, Truschnig-Wilders M, Schmidt A, Gasser R, & Fuchs D (2010). Inflammation, adiponectin, obesity and cardiovascular risk. Current Medicinal Chemistry, 17, 4511–4520. [DOI] [PubMed] [Google Scholar]
- Margolis KL, Bonds DE, Rodabough RJ, Tinker L, Phillips LS, Allen C, Bassford T, Burke G, Torrens J, Howard BV, & Women’s Health Initiative I (2004). Effect of oestrogen plus progestin on the incidence of diabetes in postmenopausal women: Results from the Women’s Health Initiative Hormone Trial. Diabetologia, 47, 1175–1187. [DOI] [PubMed] [Google Scholar]
- Marjon NA, Hu C, Hathaway HJ, & Prossnitz ER (2014). G protein-coupled estrogen receptor regulates mammary tumorigenesis and metastasis. Molecular Cancer Research: MCR, 12, 1644–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martensson UE, Salehi SA, Windahl S, Gomez MF, Sward K, Daszkiewicz-Nilsson J, Wendt A, Andersson N, Hellstrand P, Grande PO, Owman C, Rosen CJ, Adamo ML, Lundquist I, Rorsman P, Nilsson BO, Ohlsson C, Olde B, & Leeb-Lundberg LM (2009). Deletion of the G protein-coupled receptor 30 impairs glucose tolerance, reduces bone growth, increases blood pressure, and eliminates estradiol-stimulated insulin release in female mice. Endocrinology, 150, 687–698. [DOI] [PubMed] [Google Scholar]
- Mauvais-Jarvis F (2011). Estrogen and androgen receptors: Regulators of fuel homeostasis and emerging targets for diabetes and obesity. Trends in Endocrinology and Metabolism: TEM, 22, 24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F (2015). Sex differences in metabolic homeostasis, diabetes, and obesity. Biology of Sex Differences, 6, 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F (2016). Role of sex steroids in beta cell function, growth, and survival. Trends in Endocrinology and Metabolism: TEM, 27, 844–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mauvais-Jarvis F, Clegg DJ, & Hevener AL (2013). The role of estrogens in control of energy balance and glucose homeostasis. Endocrine Reviews, 34, 309–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister EJ, Dhurandhar NV, Keith SW, Aronne LJ, Barger J, Baskin M, Benca RM, Biggio J, Boggiano MM, Eisenmann JC, Elobeid M, Fontaine KR, Gluckman P, Hanlon EC, Katzmarzyk P, Pietrobelli A, Redden DT, Ruden DM, Wang C, Waterland RA, Wright SM, & Allison DB (2009). Ten putative contributors to the obesity epidemic. Critical Reviews in Food Science and Nutrition, 49, 868–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meoli L, Isensee J, Zazzu V, Nabzdyk CS, Soewarto D, Witt H, Foryst-Ludwig A, Kintscher U, & Noppinger PR (2014). Sex- and age-dependent effects of Gpr30 genetic deletion on the metabolic and cardiovascular profiles of diet-induced obese mice. Gene, 540, 210–216. [DOI] [PubMed] [Google Scholar]
- Meyer MR, Clegg DJ, Prossnitz ER, & Barton M (2011). Obesity, insulin resistance and diabetes: Sex differences and role of oestrogen receptors. Acta Physiologica, 203, 259–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Fredette NC, Howard TA, Hu C, Ramesh C, Daniel C, Amann K, Arterburn JB, Barton M, & Prossnitz ER (2014). G protein-coupled estrogen receptor protects from atherosclerosis. Scientific Reports, 4, 7564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Fredette NC, Barton M, & Prossnitz ER (2015). G protein-coupled estrogen receptor inhibits vascular prostanoid production and activity. The Journal of Endocrinology, 227, 61–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer MR, Fredette NC, Daniel C, Sharma G, Amann K, Arterburn JB, Barton M, & Prossnitz ER (2016). Obligatory role for GPER in cardiovascular aging and disease. Science Signaling, 9, ra105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittendorfer B (2011). Origins of metabolic complications in obesity: Adipose tissue and free fatty acid trafficking. Current Opinion in Clinical Nutrition and Metabolic Care, 14, 535–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro R, Teixeira D, & Calhau C (2014). Estrogen signaling in metabolic inflammation. Mediators of Inflammation, 2014, 615917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota M, Banini BA, Cazanave SC, & Sanyal AJ (2016). Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism: Clinical and Experimental, 65, 1049–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muoio DM, & Neufer PD (2012). Lipid-induced mitochondrial stress and insulin action in muscle. Cell Metabolism, 15, 595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A, & Narasimhan S (2005). Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clinica Chimica Acta; International Journal of Clinical Chemistry, 360, 81–86. [DOI] [PubMed] [Google Scholar]
- Nabulsi AA, Folsom AR, White A, Patsch W, Heiss G, Wu KK, & Szklo M (1993). Association of hormone-replacement therapy with various cardiovascular risk factors in postmenopausal women. The Atherosclerosis Risk in Communities Study Investigators. The New England Journal of Medicine, 328, 1069–1075. [DOI] [PubMed] [Google Scholar]
- Nakhjavani M, Imani M, Larry M, Aghajani-Nargesi A, Morteza A, & Esteghamati A (2014). Metabolic syndrome in pre-menopausal and postmenopausal women with type 2 diabetes: Loss of protective effects of pre-menopausal status. Journal of Diabetes and Metabolic Disorders, 13, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, Snyder RJ, & Jefferson WN (2007). Perinatal exposure to environmental estrogens and the development of obesity. Molecular Nutrition & Food Research, 51, 912–917. [DOI] [PubMed] [Google Scholar]
- Newbold RR, Padilla-Banks E, & Jefferson WN (2009). Environmental estrogens and obesity. Molecular and Cellular Endocrinology, 304, 84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson BO, Olde B, & Leeb-Lundberg LM (2011). G protein-coupled oestrogen receptor 1 (GPER1)/GPR30: A new player in cardiovascular and metabolic oestrogenic signalling. British Journal of Pharmacology, 163, 1131–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owman C, Blay P, Nilsson C, & Lolait SJ (1996). Cloning of human cDNA encoding a novel heptahelix receptor expressed in Burkitt’s lymphoma and widely distributed in brain and peripheral tissues. Biochemical and Biophysical Research Communications, 228, 285–292. [DOI] [PubMed] [Google Scholar]
- Pandey DP, Lappano R, Albanito L, Madeo A, Maggiolini M, & Picard D (2009). Estrogenic GPR30 signalling induces proliferation and migration of breast cancer cells through CTGF. EMBO Journal, 28, 523–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette A, Chapados NA, Bergeron R, & Lavoie JM (2009). Fatty acid oxidation is decreased in the liver of ovariectomized rats. Hormone and metabolic Research = Hormon- und Stoffwechselforschung = Hormones et Metabolisme, 41, 511–515. [DOI] [PubMed] [Google Scholar]
- Patel YM, & Lane MD (2000). Mitotic clonal expansion during preadipocyte differentiation: Calpain-mediated turnover of p27. The Journal of Biological Chemistry, 275, 17653–17660. [DOI] [PubMed] [Google Scholar]
- Pereira RI, Casey BA, Swibas TA, Erickson CB, Wolfe P, & Van Pelt RE (2015). Timing of estradiol treatment after menopause may determine benefit or harm to insulin action. The Journal of Clinical Endocrinology and Metabolism, 100, 4456–4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie WK, Dennis MK, Hu C, Dai D, Arterburn JB, Smith HO, Hathaway HJ, & Prossnitz ER (2013). G protein-coupled estrogen receptor-selective ligands modulate endometrial tumor growth. Obstetrics and Gynecology International, 2013, 472720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson US, Walden TB, Carlsson PO, Jansson L, & Phillipson M (2012). Female mice are protected against high-fat diet induced metabolic syndrome and increase the regulatory T cell population in adipose tissue. PLoS One, 7, e46057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poehlman ET, Toth MJ, & Gardner AW (1995). Changes in energy balance and body composition at menopause: A controlled longitudinal study. Annals of Internal Medicine, 123, 673–675. [DOI] [PubMed] [Google Scholar]
- Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH, & American Heart A, Obesity Committee of the Council on Nutrition, P. A., Metabolism. (2006). Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation, 113, 898–918. [DOI] [PubMed] [Google Scholar]
- Poitout V, & Robertson RP (2008). Glucolipotoxicity: Fuel excess and beta-cell dysfunction. Endocrine Reviews, 29, 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prentki M, & Nolan CJ (2006). Islet beta cell failure in type 2 diabetes. The Journal of Clinical Investigation, 116, 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER (2017). GPER modulators: Opportunity Nox on the heels of a class Akt. The Journal of Steroid Biochemistry and Molecular Biology. 10.1016/j.jsbmb.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, & Arterburn JB (2015). International Union of Basic and Clinical Pharmacology. XCVII. G protein-coupled estrogen receptor and its pharmacologic modulators. Pharmacological Reviews, 67, 505–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, & Barton M (2011). The G-protein-coupled estrogen receptor GPER in health and disease. Nature Reviews Endocrinology, 7, 715–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, & Barton M (2014). Estrogen biology: New insights into GPER function and clinical opportunities. Molecular and Cellular Endocrinology, 389, 71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, & Hathaway HJ (2015). What have we learned about GPER function in physiology and disease from knockout mice? The Journal of Steroid Biochemistry and Molecular Biology, 153, 114–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Arterburn JB, Smith HO, Oprea TI, Sklar LA, & Hathaway HJ (2008a). Estrogen signaling through the transmembrane G protein-coupled receptor GPR30. Annual Review of Physiology, 70, 165–190. [DOI] [PubMed] [Google Scholar]
- Prossnitz ER, Oprea TI, Sklar LA, & Arterburn JB (2008b). The ins and outs of GPR30: A transmembrane estrogen receptor. Journal of Steroid Biochemistry and Molecular Biology, 109, 350–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prossnitz ER, Sklar LA, Oprea TI, & Arterburn JB (2008c). GPR30: A novel therapeutic target in estrogen-related disease. Trends in Pharmacological Sciences, 29, 116–123. [DOI] [PubMed] [Google Scholar]
- Rahmouni K, Sigmund CD, Haynes WG, & Mark AL (2009). Hypothalamic ERK mediates the anorectic and thermogenic sympathetic effects of leptin. Diabetes, 58, 536–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regitz-Zagrosek V, Lehmkuhl E, & Weickert MO (2006). Gender differences in the metabolic syndrome and their role for cardiovascular disease. Clinical Research in Cardiology: Official Journal of the German Cardiac Society, 95, 136–147. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Cimino DF, Sklar LA, Arterburn JB, & Prossnitz ER (2005). A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science, 307, 1625–1630. [DOI] [PubMed] [Google Scholar]
- Revankar CM, Mitchell HD, Field AS, Burai R, Corona C, Ramesh C, Sklar LA, Arterburn JB, & Prossnitz ER (2007). Synthetic estrogen derivatives demonstrate the functionality of intracellular GPR30. ACS Chemical Biology, 2, 536–544. [DOI] [PubMed] [Google Scholar]
- Ribas V, Nguyen MT, Henstridge DC, Nguyen AK, Beaven SW, Watt MJ, & Hevener AL (2010). Impaired oxidative metabolism and inflammation are associated with insulin resistance in ERalpha-deficient mice. American Journal of Physiology. Endocrinology and Metabolism, 298, E304–E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieck S, & Kaestner KH (2010). Expansion of beta-cell mass in response to pregnancy. Trends in Endocrinology and Metabolism: TEM, 21, 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie SA, & Connell JM (2007). The link between abdominal obesity, metabolic syndrome and cardiovascular disease. Nutrition, Metabolism, and Cardiovascular Diseases: NMCD, 17, 319–326. [DOI] [PubMed] [Google Scholar]
- Ropero AB, Pang Y, Alonso-Magdalena P, Thomas P, & Nadal A (2012). Role of ERbeta and GPR30 in the endocrine pancreas: A matter of estrogen dose. Steroids, 77, 951–958. [DOI] [PubMed] [Google Scholar]
- Samdani P, Singhal M, Sinha N, Tripathi P, Sharma S, Tikoo K, Rao KV, & Kumar D (2015). A comprehensive inter-tissue crosstalk analysis underlying progression and control of obesity and diabetes. Scientific Reports, 5, 12340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, & Prossnitz ER (2011). Mechanisms of estradiol-induced insulin secretion by the G protein-coupled estrogen receptor GPR30/GPER in pancreatic beta-cells. Endocrinology, 152, 3030–3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, & Prossnitz ER (2016). GPER/GPR30 knockout mice: Effects of GPER on metabolism. Methods in Molecular Biology, 1366, 489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Hu C, Brigman JL, Zhu G, Hathaway HJ, & Prossnitz ER (2013). GPER deficiency in male mice results in insulin resistance, dyslipidemia, and a proinflammatory state. Endocrinology, 154, 4136–4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma G, Mauvais-Jarvis F, & Prossnitz ER (2017). Roles of G protein-coupled estrogen receptor GPER in metabolic regulation. The Journal of Steroid Biochemistry and Molecular Biology. 10.1016/j.jsbmb.2017.02.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Kumar SP, & Shi H (2014). Estradiol regulates insulin signaling and inflammation in adipose tissue. Hormone Molecular Biology and Clinical Investigation, 17, 99–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoelson SE, Herrero L, & Naaz A (2007). Obesity, inflammation, and insulin resistance. Gastroenterology, 132, 2169–2180. [DOI] [PubMed] [Google Scholar]
- Shulman GI (2014). Ectopic fat in insulin resistance, dyslipidemia, and cardiometabolic disease. The New England Journal of Medicine, 371, 2237–2238. [DOI] [PubMed] [Google Scholar]
- Smith HO, Leslie KK, Singh M, Qualls CR, Revankar CM, Joste NE, & Prossnitz ER (2007). GPR30: A novel indicator of poor survival for endometrial carcinoma. American Journal of Obstetrics and Gynecology, 196, 386. e1–11. [DOI] [PubMed] [Google Scholar]
- Smith HO, Arias-Pulido H, Kuo DY, Howard T, Qualls CR, Lee SJ, Verschraegen CF, Hathaway HJ, Joste NE, & Prossnitz ER (2009). GPR30 predicts poor survival for ovarian cancer. Gynecologic Oncology, 114, 465–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava DP, & Evans PD (2013). G-protein oestrogen receptor 1: Trials and tribulations of a membrane oestrogen receptor. Journal of Neuroendocrinology, 25, 1219–1230. [DOI] [PubMed] [Google Scholar]
- Straub RH (2007). The complex role of estrogens in inflammation. Endocrine Reviews, 28, 521–574. [DOI] [PubMed] [Google Scholar]
- Strehlow K, Rotter S, Wassmann S, Adam O, Grohe C, Laufs K, Bohm M, & Nickenig G (2003). Modulation of antioxidant enzyme expression and function by estrogen. Circulation Research, 93, 170–177. [DOI] [PubMed] [Google Scholar]
- Stubbins RE, Holcomb VB, Hong J, & Nunez NP (2012). Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. European Journal of Nutrition, 51, 861–870. [DOI] [PubMed] [Google Scholar]
- Tandon VR, Mahajan A, Sharma S, & Sharma A (2010). Prevalence of cardiovascular risk factors in postmenopausal women: A rural study. Journal of Mid-life Health, 1, 26–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang QQ, Otto TC, & Lane MD (2003). Mitotic clonal expansion: A synchronous process required for adipogenesis. Proceedings of the National Academy of Sciences of the United States of America, 100, 44–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchernof A, & Despres JP (2013). Pathophysiology of human visceral obesity: An update. Physiological Reviews, 93, 359–404. [DOI] [PubMed] [Google Scholar]
- Thomas P, Alyea R, Pang Y, Peyton C, Dong J, & Berg AH (2010). Conserved estrogen binding and signaling functions of the G protein-coupled estrogen receptor 1 (GPER) in mammals and fish. Steroids, 75, 595–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiano JP, & Mauvais-Jarvis F (2012). Molecular mechanisms of estrogen receptors’ suppression of lipogenesis in pancreatic beta-cells. Endocrinology, 153, 2997–3005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiano JP, Delghingaro-Augusto V, Le May C, Liu S, Kaw MK, Khuder SS, Latour MG, Bhatt SA, Korach KS, Najjar SM, Prentki M, & Mauvais-Jarvis F (2011). Estrogen receptor activation reduces lipid synthesis in pancreatic islets and prevents beta cell failure in rodent models of type 2 diabetes. The Journal of Clinical Investigation, 121, 3331–3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, Obin MS, & Greenberg AS (2012). Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology, 153, 4266–4277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinayagam R, & Xu B (2015). Antidiabetic properties of dietary flavonoids: A cellular mechanism review. Nutrition & Metabolism, 12, 60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivacqua A, De Marco P, Santolla MF, Cirillo F, Pellegrino M, Panno ML, Abonante S, & Maggiolini M (2015). Estrogenic GPER signaling regulates mir144 expression in cancer cells and cancer-associated fibroblasts (CAFs). Oncotarget, 6, 16573–16587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton C, Godsland IF, Proudler AJ, Wynn V, & Stevenson JC (1993). The effects of the menopause on insulin sensitivity, secretion and elimination in non-obese, healthy women. European Journal of Clinical Investigation, 23, 466–473. [DOI] [PubMed] [Google Scholar]
- Wang C, Prossnitz ER, & Roy SK (2008). G protein-coupled receptor 30 expression is required for estrogen stimulation of primordial follicle formation in the hamster ovary. Endocrinology, 149, 4452–4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A, Luo J, Moore W, Alkhalidy H, Wu L, Zhang J, Zhen W, Wang Y, Clegg DJ, Bin X, Cheng Z, McMillan RP, Hulver MW, & Liu D (2016). GPR30 regulates diet-induced adiposity in female mice and adipogenesis in vitro. Scientific Reports, 6, 34302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright E Jr., Scism-Bacon JL, & Glass LC (2006). Oxidative stress in type 2 diabetes: The role of fasting and postprandial glycaemia. International Journal of Clinical Practice, 60, 308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Qin S, Carrasco GA, Dai Y, Filardo EJ, Prossnitz ER, Battaglia G, Doncarlos LL, & Muma NA (2009). Extra-nuclear estrogen receptor GPR30 regulates serotonin function in rat hypothalamus. Neuroscience, 158, 1599–1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Smith DL Jr., Keating KD, Allison DB, & Nagy TR (2014). Variations in body weight, food intake and body composition after long-term high-fat diet feeding in C57BL/6J mice. Obesity, 22, 2147–2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonezawa R, Wada T, Matsumoto N, Morita M, Sawakawa K, Ishii Y, Sasahara M, Tsuneki H, Saito S, & Sasaoka T (2012). Central versus peripheral impact of estradiol on the impaired glucose metabolism in ovariectomized mice on a high-fat diet. American Journal of Physiology - Endocrinology and Metabolism Published 15 August 2012, 303(4), E445–E456. 10.1152/ajpendo.00638.2011 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Howard BV, Cowan LD, Yeh J, Schaefer CF, Wild RA, Wang W, & Lee ET (2002). The effect of estrogen use on levels of glucose and insulin and the risk of type 2 diabetes in American Indian postmenopausal women : The strong heart study. Diabetes Care, 25, 500–504. [DOI] [PubMed] [Google Scholar]
- Zhu P, Yuen JM, Sham KW, & Cheng CH (2013). GPER mediates the inhibitory actions of estrogen on adipogenesis in 3T3-L1 cells through perturbation of mitotic clonal expansion. General and Comparative Endocrinology, 193, 19–26 [DOI] [PubMed] [Google Scholar]