Neuroendocrine tumors (NETs), or carcinoids, comprise a spectrum of tumors that arise from neuroendocrine cells lining the gut and lung or that are present in the pancreas. Using a U.S. registry, this study examined the clinical utility of biomarker assays for management of neuroendocrine tumor disease. Diagnostic accuracy and relationship to clinical disease status in two cohorts was evaluated, and the results are reported in this article.
Keywords: Biomarker, Carcinoid, Liquid biopsy, NET transcripts, Neuroendocrine, Registry
Abstract
Background.
The clinical relevance of molecular biomarkers in oncology management has been recognized in breast and lung cancers. We evaluated a blood‐based multigene assay for management of neuroendocrine tumors (NETs) in a real‐world study (U.S. registry NCT02270567). Diagnostic accuracy and relationship to clinical disease status in two cohorts (treated and watch‐and‐wait) were evaluated.
Materials and Methods.
Patients with NETs (n = 100) were followed for 6–12 months. Patients’ primary tumors were gastroenteropancreatic (68%), lung 20%, and of unknown origin (12%). Characteristics included well‐differentiated, low‐grade tumors (97%), stage IV disease (96%); treatment with surgery (70%); and drug treatment (56%). NETest was measured at each visit and disease status determined by RECIST. Scores categorized as low (NETest 14%–40%) or high (≥80%) defined disease as stable or progressive. Multivariate analyses determined the strength of the association with progression‐free survival (PFS).
Results.
NETest diagnostic accuracy was 96% and concordant (95%) with image‐demonstrable disease. Scores were reproducible (97%) and concordant with clinical status (98%). The NETest was the only feature linked to PFS (odds ratio, 6.1; p < .0001). High NETest correlated with progressive disease (81%; median PFS, 6 months), and low NETest correlated with stable disease (87%; median PFS, not reached). In the watch‐and‐wait cohort, low NETest was concordant with stable disease in 100% of patients, and high NETest was associated with management changes in 83% of patients. In the treated cohort, all low NETest patients (100%) remained stable. A high NETest was linked to intervention and treatment stabilization (100%). Use of NETest was associated with reduced imaging (biannual to annual) in 36%–38% of patients.
Conclusion.
Blood NETest is an accurate diagnostic and can be of use in monitoring disease status and facilitating management change in both watch‐and‐wait and treatment cohorts.
Implications for Practice.
A circulating multigene molecular biomarker to guide neuroendocrine tumor (NET) management has been developed because current biomarkers have limited clinical utility. NETest is diagnostic (96%) and in real time defines the disease status (>95%) as stable or progressive. It is >90% effective in guiding treatment decisions in conjunction with diagnostic imaging. Monitoring was effective in watch‐and‐wait or treatment groups. Low levels supported no management change and reduced the need for imaging. High levels indicated the need for management intervention. Real‐time liquid biopsy assessment of NETs has clinical utility and can contribute additional value to patient management strategies and outcomes.
Introduction
Neuroendocrine tumors (NETs), or carcinoids, comprise a spectrum of tumors that arise from neuroendocrine cells lining the gut and lung or are present in the pancreas. They represent ∼2% of all neoplasia and have become increasingly common as awareness and techniques for identification have improved [1]. A diversity in presentation, variability in disease progression, the paucity of therapies that effectively impact on long‐term survival, and the limitations of imaging and biomarkers to accurately predict response to therapy make appropriate clinical management difficult [2].
The limitations of imaging—for example, tumor response to targeted therapies is rarely associated with shrinkage [3]—and biomarkers’ lack of sensitivity in determining early changes in disease, such as recurrence or progression, indicate the need for a NET biomarker that can monitor tumor activity and accurately assess tumor behavior [4]. One standard biomarker is chromogranin A (CgA). Although it is effective in histological assessments, it has inadequate clinical utility as a blood‐based tool for diagnosis or disease monitoring [5]. Although the U.S. National Comprehensive Cancer Network guidelines treat CgA circumspectly [6], the European guidelines include CgA for clinical management [7]. The confusion is compounded as CgA is included as a biomarker in current clinical studies [8], [9]. Several National Cancer Institute consensus conferences have confirmed the requirement of the development of a reliable biomarker to optimize NET management [10], [11]. This reflects the success of tissue‐ and blood‐based investigations in other neoplasia (breast, lung) that have demonstrated that measurements of molecular components of the tumor constituted an effective strategy for developing clinically effective predictive and prognostic tools [12], [13]. In neuroendocrine tumor disease, the absence of a clinically useful blood biomarker or liquid biopsy remains an important unmet need [14].
The morbidity and cost associated with certain therapies are major considerations, especially in patients who do not respond to a therapy, who have a low tumor burden, or who have little to no symptoms. The latter, whose survival may be substantial because much of NET disease is indolent, may be monitored for evidence of disease progression. Therapy may then be instituted at the point of clinically significant symptomatic disease or radiological progression [3]. The need to improve patient management and treatment outcome and the issue of optimizing resource utilization for NETs, particularly for advanced disease, are considerable in the U.S. [15]. Currently, the inability to accurately and noninvasively monitor NET disease is responsible for the use of substantial resources. These include increased physician visits, frequent exposure to isotopic imaging modalities, and the use of expensive cytotoxic therapies.
To address this, NETest (Wren Laboratories, Branford, CT), a blood‐based multianalyte neuroendocrine specific transcript assay, has been developed. The assay is a liquid biopsy strategy designed to detect NET gene expression signatures in peripheral blood [16], [17]. The gene expression fingerprint provides a tumor activity score. Scoring stratifies patients into three groups: low, ≤40%; moderate/intermediate, 41%–79%; and high, ≥80%. This 51 gene assay is accurate for diagnosis (>95%) [18], [19] and differentiating stable from progressive disease (>90%) [20]. Prospective clinical studies demonstrate it predicts the effectiveness of surgery [21], [22], can monitor tumor progression during somatostatin analog (SSA) therapy [23], and has utility in watch‐and‐wait programs [20]. Expression of individual genes is also effective in predicting response to peptide receptor radiotherapy (PRRT) prior to therapy initiation [24].
In many instances, biomarker assays that exhibit utility in clinical academic trials do not effectively translate to real‐world settings [25], [26]. In this study, a U.S. registry was utilized to examine the clinical utility of the assay. NETest registry patients were evaluated from large U.S. referral practices, and their subsequent clinical data, including decision‐making, were interfaced with NETest data. The study addresses five principal questions: (a) What is the diagnostic accuracy of the NETest? (b) Does the NETest score accurately reflect the disease status? (c) Does it have clinical utility in decision‐making? (d) Can it alter the frequency and type of imaging? (e) Does the NETest have greater clinical utility than CgA?
Materials and Methods
The registry of neuroendocrine tumor patients used in this study comprises test results and clinical information collected for the study RegisterNET – A Registry of Neuroendocrine Tumors in the USA (ClinicalTrials.gov identifier NCT02270567), which was established by Wren Laboratories. The aim of this registry was to include clinical and biomarker data from patients enrolled by interested physicians who could then use it to answer specific clinical questions. Consent was obtained (WIRB: 20150174). Deidentified data was included in the database. Clinicians could use the NETest at their discretion for clinical management.
A total of 100 patients with pathological confirmation of a NET were enrolled over a period of 22 months (February 2015 to November 2017; Table 1). Follow‐up was median 6 months (range, 3–18 months). NETest was performed at enrollment. The assay has been described elsewhere in detail [17]. In brief, it involves measurement, by polymerase chain reaction (PCR), of 51 individual genes linked by discriminant classifier analyses to pathobiological behavior characteristics of the tumor, such as proliferation and growth factor signaling. Scores are generated from PCR results using a series of algorithms. Only molecular genomic data are used, and no clinical information is incorporated. The test is performed in a single laboratory (Clinical Laboratory Improvement Amendments certification number 07D2081388) using a standard methodology.
Table 1. Demographics of patients enrolled.
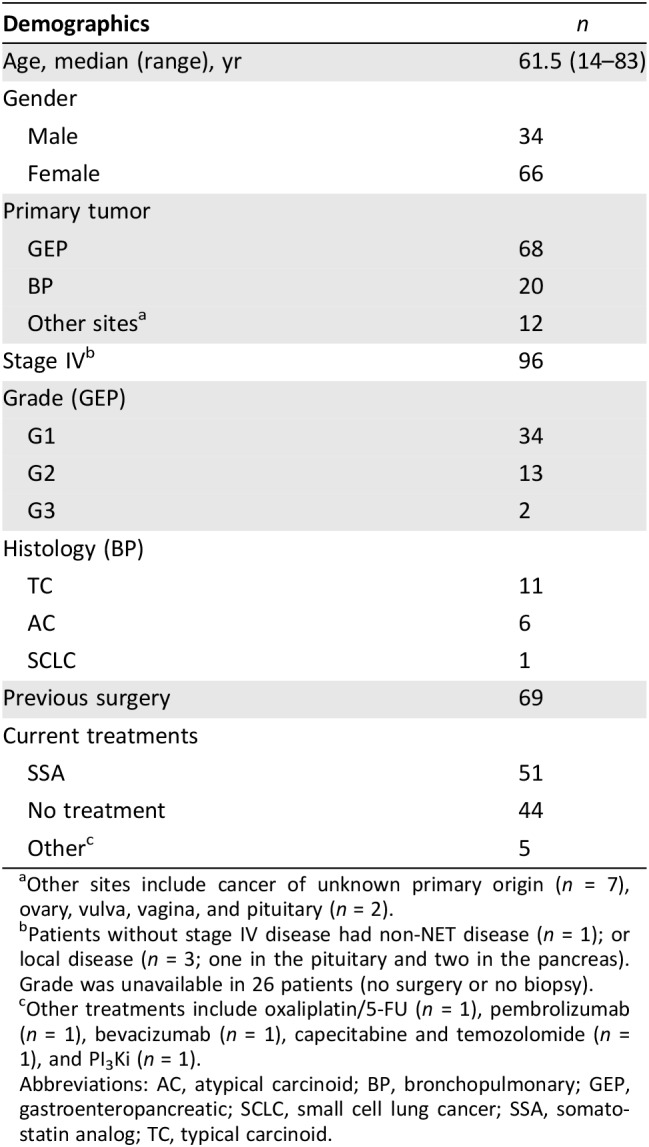
Other sites include cancer of unknown primary origin (n = 7), ovary, vulva, vagina, and pituitary (n = 2).
Patients without stage IV disease had non‐NET disease (n = 1); or local disease (n = 3; one in the pituitary and two in the pancreas). Grade was unavailable in 26 patients (no surgery or no biopsy).
Other treatments include oxaliplatin/5‐FU (n = 1), pembrolizumab (n = 1), bevacizumab (n = 1), capecitabine and temozolomide (n = 1), and PI3Ki (n = 1).
Abbreviations: AC, atypical carcinoid; BP, bronchopulmonary; GEP, gastroenteropancreatic; SCLC, small cell lung cancer; SSA, somatostatin analog; TC, typical carcinoid.
The diagnostic accuracy of the assay was assessed at enrollment by determining whether the NETest was positive (score >14%) or negative. Patients with image‐based evidence of disease (computed tomography [CT]/magnetic resonance imaging [MRI] or 68Ga‐SSA positron emission tomography [PET]/CT) were considered to have a NET. The correlation between low and high NETest scores and clinical status (i.e., stable or progressive disease) was examined.
At the time of the initial NETest, 45% of patients were under observation (watch‐and‐wait monitoring program), and 55% were in a treatment cohort. Each patient group was analyzed separately. Records were analyzed to determine if the NETest scores predicted subsequent clinical course and if the NETest results altered patient management. Disease was considered stable if no radiological or clinical progression was noted. Disease was considered progressive if sequential images met RECIST version 1.0 criteria for progression [3] or if significant changes in clinical symptoms relevant to progression were documented. The following questions were specifically evaluated: Did low test scores (≤40%) correlate with a patient remaining in a watch‐and‐wait program, and were they associated with a decrease in imaging? How did high test scores (≥80%) correlate with disease progression and alterations in clinical management? Kaplan‐Meier survival curves were generated, and the relationship between median progression‐free survival (mPFS) and low and high scores was assessed.
In the treatment group (patients receiving any therapeutic intervention at the time of the blood test; Table 1), NETest results were examined to evaluate if they stratified patients responding to treatment versus those who required alteration in treatment strategy. Correlations between score and treatment maintenance (or alteration) and image frequency were evaluated.
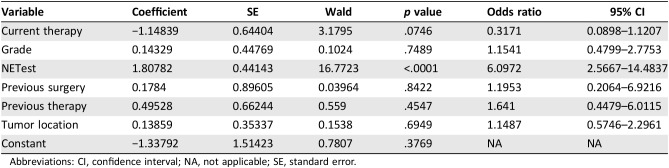
Multivariable analyses (multiple regression, Cox proportional, and logistic regression) were also undertaken to determine the strength of association between outcome (progression‐free survival; PFS) and the NETest score. Input variables included grade, tumor site, previous surgery, previous therapy, current therapy, and NETest score.
Chromogranin A results were available in 53 individuals. The diagnostic and predictive utility of CgA in this cohort was examined and directly compared with results of the NETest in the same patients. Comparisons were made using McNemar's test for paired samples (NETest/CgA) or Fisher's test (two‐tailed) for low/high scores or normal/abnormal CgA levels. A p value <.05 was considered significant.
Results
One hundred patients were included; demographic characteristics are shown in Table 1. The median age was 62 years (range, 20–83 years). The majority were female (67%). The primary site of the NET was known in 93% and was predominantly the gastrointestinal tract and the pancreas (n = 68). The histological types were grade 1 (n = 35), grade 2 (n = 31), and grade 3 (n = 2). There were 20 bronchopulmonary NETs, of which 11 were typical, 6 were atypical carcinoid, 1 was small cell lung cancer, and 2 had no data. Stage IV (metastatic) disease was present in 96% of patients, and 70% had undergone surgery before enrollment. The remaining 12 are included in Table 1. A single NETest was undertaken in the majority (n = 72); 18 patients had two NETests, 8 patients had three NETests, and 1 patient had four NETests performed (total 136 samples).
Diagnostic Efficacy
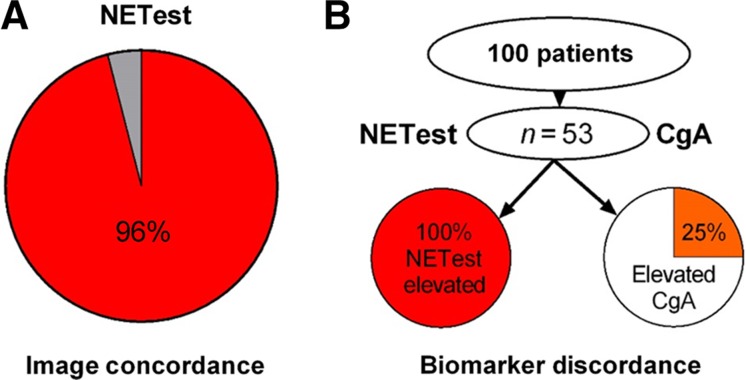
The NETest was positive in 135/136 (99%) samples. Image‐detectable disease was evident in 96% of patients with a positive NETest (Fig. 1A). Three individuals with no evidence of disease had a positive result. One of these patients had a gastrinoma with elevated gastrin levels after surgery (720 pg/mL) and negative 68Ga‐SSA‐PET/CT. Two patients had undergone extensive metastatic small bowel disease surgery. One patient with a bronchopulmonary NET (curative resection 4 years previously) presented with CT‐positive liver disease and was NETest negative. The diagnostic accuracy of the test was ≥96%.
Figure 1.
Diagnostic efficacy and concordance with imaging. (A): The NETest was concordant with image‐confirmed disease in 96% of patients. (B): CgA was ordered for 53 of the 100 patients. The NETest was positive in all 53 (100%). CgA was positive in 25%. NETest positivity was significantly greater than CgA (p = .0004).
Abbreviation: CgA, chromogranin A.
In those tested, CgA was not elevated in 40 patients (75%) despite documented clinical evidence of disease. The NETest was positive in all 53 patients (100%; Fig. 1B). The McNemar test indicated that the NETest was significantly more accurate (p = .0004; χ2, 12.76) than CgA.
Concordance with Disease Status and Reproducibility
RECIST criteria identified stable disease in 65% of patients and progressive disease in 35%. Of patients with stable disease, 54 (83%) had a low NETest score, 6 (9%) had intermediate scores, and 5 (8%) had high scores. The latter included two patients with small bowel NETs with extensive disease on SSAs. Of patients with progressive disease (n = 35), the NETest score was high in 21 (60%), and 6 (17%) had intermediate scores. Low scores were noted in 8 patients (23%), of whom two had high‐grade neuroendocrine carcinoma; one had progression based upon symptoms (gastrinoma, 720 pg/mL), and the second had a cancer of unknown primary origin (CUP; normal CgA), which was considered slowly progressive.
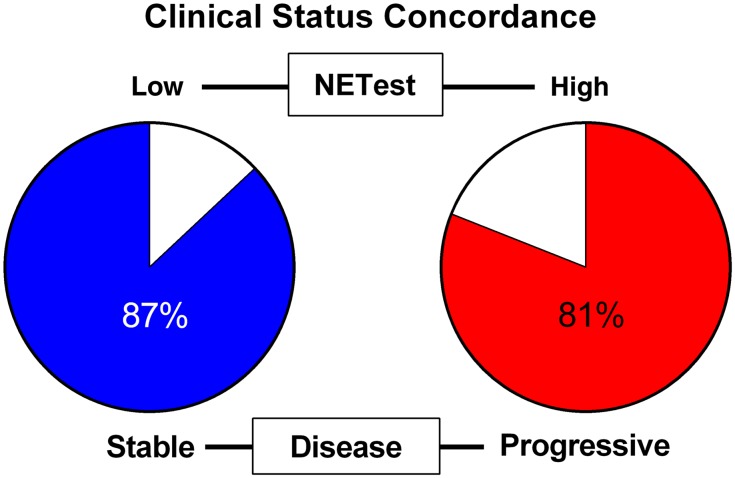
High NETest scores (n = 26) were associated with progressive disease in 21 patients (81%). The NETest was low in 62 patients, of whom 54 (87%) had stable disease (Fig. 2). The overall concordance between the two NETest categories and disease was 75/88 (88%).
Figure 2.
Concordance between NETest and clinical disease status. Stable disease was associated with a low NETest (≤40%) in 87% of patients (54/62); progressive disease was associated with a high score (≥80%) in 81% of patients (21/26).
Twenty‐eight patients underwent more than one blood test. In 17 patients, disease was either stable (at two time points, n = 12) or remained progressive (n = 5). Mean NETest levels for stable disease were 30% ±7% and 28% ±4%, at the two time‐points tested (p = .98). For progressive disease, levels were 82% ±7% and 85% ±7% (p = .5). The overall concordance was 97% (33/34); one stable patient (1/34 samples) had a score of 74% at the first time point; at the second time point this was 40%, consistent with stable disease. These data demonstrate the score is highly reproducible.
In the remaining 11 patients, 4 demonstrated progression of disease (NETest increased in 100%), 4 underwent surgery (all 4 exhibited a decrease in score), and 3 were placed on treatment with a subsequent decrease in score. Overall, the score was concordant in 22/22 samples. These data demonstrate that the concordance between the score and clinical status was 98% (55/56). Overall, this information suggests that the NETest score can play a role in effectively monitoring disease status.
Scores in the Watch‐and‐Wait Cohort (n = 45)
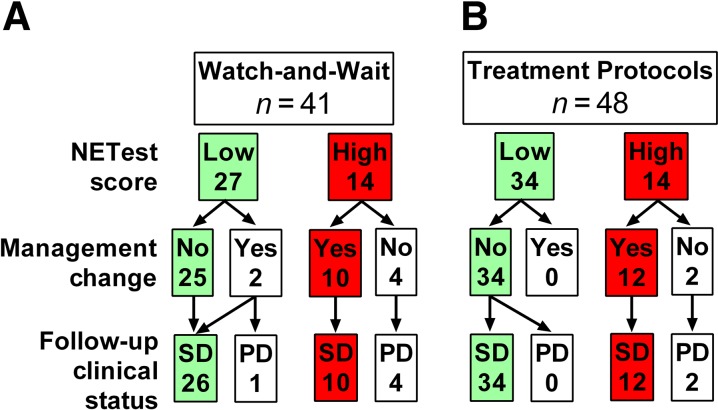
In the watch‐and‐wait cohort, a low NETest score was present in 27 patients (60%) an intermediate score in 4 and a high score in 14 patients. Of the 27 patients with a low score, 25 (93%) were managed conservatively and continued without treatment (Fig. 3). At follow‐up (6–12 months), all 25 (100%) remained stable clinically and by RECIST criteria. One patient with a low score started SSAs; another started Afinitor (everolimus, Novartis, Basel, Switzerland), subsequently developing progression. A high score was identified in 14 patients (30%); 10 underwent treatment interventions. Five underwent subsequent imaging to evaluate progression, which was confirmed in all five. Five additional patients exhibited clinical (symptomatic) progression and were not reimaged. Treatment interventions included surgery (40%), SSA (40%), Afinitor (10%), and PRRT (10%). At follow‐up (6 months), all 10 had disease stabilization (imaging or symptom diminution). Four with high scores who did not undergo a treatment intervention subsequently had progressive disease (confirmed by imaging). Of the three patients (10%) with an intermediate score, two underwent imaging. Both exhibited progression, and treatment intervention was initiated.
Figure 3.
Relationship between NETest score and clinical management. (A): In the watch‐and‐wait cohort, a low score was associated with no treatment intervention in 93% of patients. A high score led to a treatment intervention in 71%. (B): In the treatment cohort, a low score was associated with no change in treatment in 100% of patients. A high score led to a treatment modification in 86%.
Abbreviations: PD, progressive disease; SD, stable disease.
Scores in the Treatment Cohort (n = 55)
In the treatment cohort, 34 patients (62%) had a low score, 14 (25%) had a high score, and 7 (13%) had intermediate scores (Fig. 3). Disease status and treatment results are known for 6 to 12 months after the NETest value. All 34 with low scores continued without any modification of therapy (86% were being treated with SSAs; Table 1); their disease remained stable. In the 14 (25%) with high scores (all SSAs), 12 (86%) underwent treatment modifications. In two of these patients, treatment was not altered. Cytoreductive surgery was undertaken in one patient after 6 months; the second patient exhibited inoperable lung disease and was maintained on analog treatment for symptomatic control. All with high NETest scores exhibited disease progression consistent with failure to respond to SSA. Among the 12 who underwent treatment modifications (dose increases) were undertaken in three; in four, lanreotide was substituted for octreotide. In the remainder, one underwent 90Y‐selective internal radiation therapy for progressive liver metastases; PRRT was undertaken in four patients. All 12 exhibited disease stabilization at follow‐up (6 months; image‐based confirmation). Of the seven patients (13%) with intermediate scores, the SSA dosage was increased in five. In two patients, dosage was not altered, and both subsequently developed RECIST‐confirmed disease progression within 6 months.
Scores and Progression‐Free Survival
The relationship between scores, other clinical variables, and PFS was evaluated in the entire group (Fig. 4).
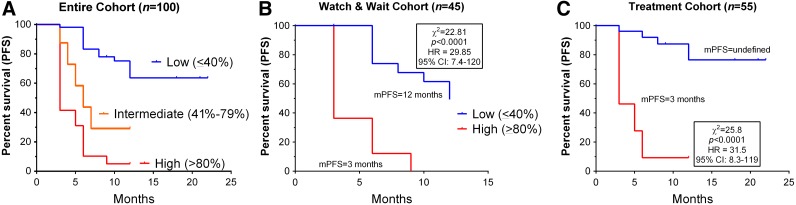
Figure 4.
Relationship between NETest score and PFS. (A): In the entire cohort (n = 100), NETest scores significantly differentiated mPFS. For patients with a low score, mPFS was not reached; an intermediate score was associated with an mPFS of 6 months, and a high score was associated with an mPFS of 3 months. This was highly significant (χ2, 59.9; p < .0001). The HR was 0 for a low score, 3.5 for an intermediate score, and 6.4 for a high score. (B): In the watch‐and‐wait cohort, a low score was associated with mPFS of 12 months. A high score was associated with a mPFS of 3 months. This difference was significant (HR, 29.9; p < .0001). (C): In the treatment cohort, a low score was associated with an mPFS not reached. A high score was associated with an mPFS of 3 months. This difference was significant (HR, 31.5; p < .0001).
Abbreviations: CI, confidence interval; HR, hazard ratio; mPFS, median PFS; PFS, progression‐free survival.
Multiple regression analysis indicated that none of the clinical variables was associated with PFS. This was further confirmed by logistic regression analysis. In contrast, the NETest was identified as the only variable associated with outcome (multiple regression: coefficient, 0.32; p < .001; logistic regression: odds ratio [OR], 6,1; p < .001). Analysis using Cox proportional modeling confirmed that the NETest was the only variable related to outcome (p < .0001; Table 2). These data indicate that clinical characteristics alone perform poorly as predictors of PFS as an outcome.
Table 2. Cox proportional model for progression‐free survival (χ2, 31.5; p < .0001).
Abbreviations: CI, confidence interval; NA, not applicable; SE, standard error.
In a separate analysis, the relationship between a low and high NETest and PFS in each of the two cohorts was determined. In the watch‐and‐wait cohort, a low score was associated with mPFS of 12 months. A high score was associated with a mPFS of 3 months. This difference was significant (χ2, 22.8; hazard ratio [HR], 29.9; 95% confidence interval [CI], 7.4–120; p < .0001). In the treatment cohort, mPFS was not reached for low scores. A high score was associated with a mPFS of 3 months (χ2, 25.7; HR, 31.5, 95% CI, 8.3–119; p < .0001).
Impact on Imaging
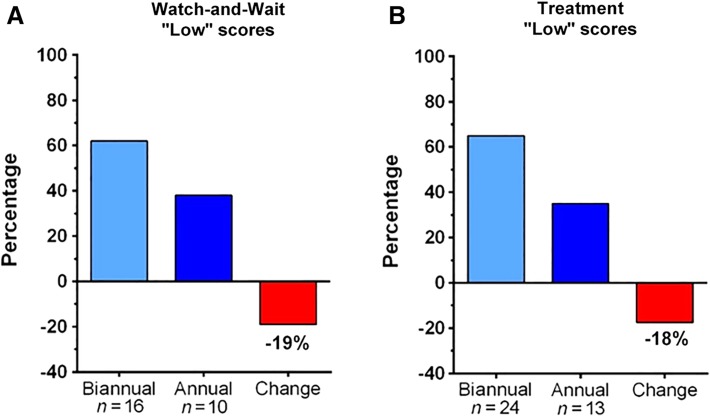
The question addressed was whether the NETest was associated with an alteration in the frequency of imaging studies in a real‐world scenario. In the watch‐and‐wait group, 26 patients had low scores and were managed conservatively. In 10 (38%), the proposed twice‐yearly CT/MRI imaging schedule was decreased to annual imaging. Only 42 of 52 projected scans were undertaken (19% decrease; Fig. 5A). In the 16 patients with high scores, there was no alteration in imaging frequency. In the treatment cohort, a low score was associated with decreased imaging in 13 (36%) of 37 patients. Imaging frequency was reduced to annual. Of the projected 74 scans, 61 were undertaken (18% decrease; Fig. 5B). A high score initiated earlier imaging (CT/MRI) in seven cases (58%). Overall, with a low score, a reduction in imaging of 23 patients (37%) was noted, irrespective of treatment or no treatment.
Figure 5.
Impact on imaging of a low NETest score. (A): In the watch‐and‐wait cohort, 10 patients (38%) switched from biannual to annual imaging with an overall decrease in imaging events of 19%. (B): In the treatment cohort, a low score resulted in 13 patients (36%) changing from biannual to annual imaging, for an overall reduction of 18%.
Clinical Utility Comparison with CgA
The clinical utility of CgA in the management of 53 patients with NETs was compared with the NETest. In the 40 patients with a normal CgA level, changes in management were made in 12 (30%). In the 13 with elevated CgA levels, management changes were made in 4 (31%). Neither normal nor elevated CgA affected clinical management decision‐making (p = 1.0).
The NETest was positive in all 53 patients. Of the 18 with a high NETest, 13 (72%) were normal, and 5 had elevated CgA. Of the 28 with a low NETest, CgA levels were elevated in 8 (29%). In patients with a low NETest (n = 27), changes in management were made in only one patient (4%). This patient with ovarian NET carcinomatosis had elevated CgA and, despite being considered stable, was placed on an SSA. In patients with a high NETest (n = 18), 14 (78%) underwent a management change. In the four patients with a high NETest for whom no changes were made, one developed carcinoid heart disease, two developed recurrent liver metastatic disease, and one became inoperable. The different NETest scores (high vs. low) were significantly associated with clinical management decision‐making (p < .0001) in this cohort (n = 53). The concordance between the NETest (high vs. low) and clinical management was 89% (40/45). Conversely, elevated CgA levels and management changes occurred in 6% (4/53; p < .00002).
Multiple regression analysis demonstrated that CgA (normal vs. abnormal) had no significant relationship to PFS (Cox proportional modeling, p = .899).
Discussion
This registry study demonstrated the utility of the NETest in the clinical management of NETs. The diagnostic accuracy of the NETest was ≥96%, and scores were highly reproducible (97%). There was a significant correlation with initial NETest levels and clinical status (stable vs. progressive disease, 93%), and levels were strongly associated with clinical outcomes (93%–100%) in both watch‐and‐wait programs and treatment protocols. Multivariate analyses identified the NETest as the only variable (including clinical characteristics) significantly related to PFS. A high NETest (≥80%) identified disease progression or treatment failure and was associated with a shorter PFS, and a low NETest (≤40%) indicated stable disease. Moreover, a low NETest score was associated with and prompted a reduction in imaging in 40%. This was corroborated by outcome. Comparison with CgA demonstrated that it was of little clinical value in decision‐making.
The blood‐based test was concordant with image‐based disease detection in 96% of patients. In three image‐negative patients, the NETest was positive. One patient had a biochemically positive gastrinoma (gastrin, 720 pg/mL) with elevated CgA levels 6 months after surgery. The positive NETest (73% score) likely reflects the presence of residual/recurrent disease as evidenced by the elevated gastrin levels [27], [28]. Two others (treated with SSA after surgery) had had extensive metastatic small bowel disease. These low but positive NETest scores (27%) could be the result of stable, residual disease not identified on imaging [23].
We next investigated whether high (≥80%) and low (≤40%) scores were related to progressive or stable disease at the time of the blood test. The results were 85% concordant. Two patients with small bowel NETs with extensive disease, both treated with a 20 mg dose of SSA, had high scores. In a previous study, a high score predicted SSA failure in 100% of cases [23]. It is likely that treatment modification may be required (i.e., increase in dosage).
Four patients had progressive disease and low (≤40%) NETest scores. These included two patients with high‐grade neuroendocrine carcinomas (human papillomavirus‐positive, rectal, grade 3 tumor [Ki67: 70%] and gastric grade 3 tumor [Ki67: 50%]), one patient with a gastrinoma, and one patient with a CUP with liver metastases reported as slow growing. The NETest identifies grade 3 lesions [19], [20], but no rigorous data regarding stratification of this group are available. The reason for the low gastrinoma result is unclear; the slow growth of liver lesions in the CUP may be reflected in the low NETest value.
We also evaluated whether scores were clinically useful in a watch‐and‐wait program. The NETest was identified as 100% clinically useful because patients with a low score (≤40%; n = 27) in whom no intervention was undertaken maintained stable disease. Low scores also associated with decreased imaging frequency in ∼40% of patients. The authors were comfortable using the NETest level to postpone imaging in these patients. This provides potential molecular support for the basis for a physician to safely continue a watch‐and‐wait program. In contrast, all patients with a high NETest (≥80%; n = 14) required treatment intervention or developed progression requiring an intervention. All of those in whom an intervention was undertaken exhibited clinically stable disease at a 6‐month follow‐up time point. Two patients did not undergo a treatment intervention, one of whom, a woman aged 41 years with low‐grade (grade 1) small bowel NET and carcinoid syndrome, subsequently developed carcinoid heart disease and underwent valve replacement surgery.
In the treatment program, the NETest was clinically useful because 100% of patients with a low score exhibited stable disease at 6–12 months. This result may support a physician to continue treatment programs in the knowledge that the therapy is likely to be effective. Of relevance was the observation that imaging frequency decreased in 36% of cases with a low NETest. This suggests that the blood biomarker measurement may provide added confidence in patient management. In patients with a high NETest, the impetus provided to treatment modification resulted in disease stabilization in 100%. Conversely, failure to intervene proved problematic. Two patients in whom no treatment modification was instituted despite high scores subsequently developed progressive disease requiring subsequent interventions (cytoreductive hepatic surgery and increased SSA for unresectable lung disease).
The majority of patients (92%) were treated with an SSA. Identification of a low NETest supported continuation of SSA therapy (100%). Demonstration of a high NETest value resulted in escalation of SSA therapy or addition of an alternative agent (100%), which is consistent with other studies [20], [23]. The NETest may therefore likely allow physicians to identify SSA‐treated patients who will develop progressive disease and require treatment changes.
CgA is the current biomarker used to aid clinical decision‐making and is widely used in clinical studies. There is, however, ambivalence about use [6], particularly because 40%–50% of all NETs do not have elevated CgA levels [14], and it is therefore not always ordered by physicians [5], [14]. It is, however, reimbursed by Medicare. The output of the assay is reported as normal or high with no scale related to clinical application [5], [14]. Actionable events are linked to high levels [5]. In this study, physicians only chose to order CgA in 53% of cases. In these, CgA was elevated in only 25%. CgA was noninformative in 75% and was of no value in clinical decision‐making.
Resource utilization in NETs is a difficult and understudied area. One publication [15] identified SSAs to be used in the highest proportion (∼80%). Hospitalization (∼60%), imaging (∼72%), and laboratory testing (∼65%) were common. Progression of disease was strongly associated with increased use of imaging and physician visits [15]. In our study, a low NETest score was associated with a decreased imaging frequency in ∼40% of patients, irrespective of whether they were in the watch‐and‐wait or treatment group. It seems likely that using the NETest as an adjunctive diagnostic has cost utilization implications and radiation exposure considerations. High scores provided an accurate (100%) assessment of disease progression. Although this resulted in earlier imaging in some cases, the subsequent intervention or modification in therapy significantly improved well‐being. In the four instances in which the elevated NETest was not used to inform clinical decision‐making, significant outcome issues as well as financial costs resulted (and the need for additional extensive surgery).
Conclusion
Clinical decision‐making is typically undertaken on the basis of integration of grade, imaging, symptomatology, tumor location, and an estimation of where the patient falls within the natural history of the disease [6], [29]. This registry study indicated that none of these clinical characteristics were strongly associated with outcome (PFS). Current therapy was the only variable associated with outcome (OR, 0.3; p = .07). The NETest, in contrast, was significantly associated with PFS (OR, 6.1; p < .0001). This multivariate analysis strongly suggests that NETest results should be included in clinical evaluation of the patient. Using the NETest value in its simplest form, a low NETest can be used to confirm stability or stabilization of disease, whereas a high score can be used to recommend intervention or treatment modification.
Registry studies are information‐rich entities that provide the ability to identify associations between clinical variables and different outcomes. They do, however, have limitations [30]. These may include the quality of the data (i.e., completeness and accuracy) and limitations in regard to how survival (e.g., PFS) is measured. Determining causative connections is also difficult unless the study protocol is specifically designed to evaluate such events. Multivariate analyses, however, represent powerful unbiased tools to interrogate such data and accurately identify individual factors that exhibit the most weight in this regard.
This study focuses on the clinical utility of the NETest when examined as either a high (≥80%) or low (≤40%) score). A consideration of scores that fall between these two parameters is important. Nine patients (n = 9) had intermediate scores (41%–79%). Four underwent disease progression during follow‐up, two were stable, and the dose of SSAs were increased in three. We are of the opinion that patients with an intermediate score represent those who are entering a progressive disease phenotype. Further study over time is needed to rigorously assess this.
Author Contributions
Conception/design: Lisa Bodei
Provision of study material or patients: Eric Liu, Scott Paulson, Anthony Gulati, Jon Freudman, William Grosh, Sheldon Kafer, Prasanna C. Wickremesinghe, Ronald R. Salem, Lisa Bodei
Collection and/or assembly of data: Eric Liu, Scott Paulson, Anthony Gulati, Jon Freudman, William Grosh, Sheldon Kafer, Prasanna C. Wickremesinghe, Ronald R. Salem, Lisa Bodei
Data analysis and interpretation: Eric Liu, Scott Paulson, Anthony Gulati, Jon Freudman, William Grosh, Sheldon Kafer, Prasanna C. Wickremesinghe, Ronald R. Salem, Lisa Bodei
Manuscript writing: Eric Liu, Scott Paulson, Anthony Gulati, Jon Freudman, William Grosh, Sheldon Kafer, Prasanna C. Wickremesinghe, Ronald R. Salem, Lisa Bodei
Final approval of manuscript: Eric Liu, Scott Paulson, Anthony Gulati, Jon Freudman, William Grosh, Sheldon Kafer, Prasanna C. Wickremesinghe, Ronald R. Salem, Lisa Bodei
Disclosures
Scott Paulson: Ipsen, Taiho, Merrimack, Advanced Accelerator Applications, Bristol‐Myers Squibb (C/A). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Hallet J, Law CH, Cukier M et al. Exploring the rising incidence of neuroendocrine tumors: A population‐based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2014;121:589–597. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Asa SL, Dey C et al. Diagnosis and management of gastrointestinal neuroendocrine tumors: An evidence‐based Canadian consensus. Cancer Treat Rev 2016;47:32–45. [DOI] [PubMed] [Google Scholar]
- 3.de Mestier L, Dromain C, d'Assignies G et al. Evaluating digestive neuroendocrine tumor progression and therapeutic responses in the era of targeted therapies: State of the art. Endocr Relat Cancer 2014;21:R105–R120. [DOI] [PubMed] [Google Scholar]
- 4.Oberg K, Krenning E, Sundin A et al. A Delphic consensus assessment: Imaging and biomarkers in gastroenteropancreatic neuroendocrine tumor disease management. Endocr Connect 2016;5:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kidd M, Bodei L, Modlin I M. Chromogranin A: Any relevance in neuroendocrine tumors? Curr Opin Endocrinol Diabetes Obes 2015;30:28–37. [DOI] [PubMed] [Google Scholar]
- 6.Kulke MH, Shah MH, Benson AB 3rd et al. Neuroendocrine tumors, version 1.2015. J Natl Compr Canc Netw 2015;13:78–108. [DOI] [PubMed] [Google Scholar]
- 7.Oberg K, Couvelard A, Delle Fave G et al. ENETS consensus guidelines for standard of care in neuroendocrine tumours: Biochemical markers. Neuroendocrinology 2017;105:201–211. [DOI] [PubMed] [Google Scholar]
- 8.Kulke MH, Ruszniewski P, Van Cutsem E et al. A randomized, open‐label, phase 2 study of everolimus in combination with pasireotide LAR or everolimus alone in advanced, well‐differentiated, progressive pancreatic neuroendocrine tumors: COOPERATE‐2 trial. Ann Oncol 2017;28:1309–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Strosberg J, El‐Haddad G, Wolin E et al. Phase 3 trial of 177Lu‐dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Modlin IM, Moss SF, Chung DC et al. Priorities for improving the management of gastroenteropancreatic neuroendocrine tumors. J Natl Cancer Inst 2008;100:1282–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kulke MH, Siu LL, Tepper JE et al. Future directions in the treatment of neuroendocrine tumors: Consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol 2011;29:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krop I, Ismaila N, Andre F et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early‐stage invasive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol 2017;35:2838–2847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna N, Johnson D, Temin S et al. Systemic therapy for stage IV non‐small‐cell lung cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 2017;35:3484–3515. [DOI] [PubMed] [Google Scholar]
- 14.Oberg K, Modlin I, De Herder W et al. Consensus on biomarkers for neuroendocrine tumor disease. Lancet Oncol 2015;16:e435–e446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Strosberg J, Casciano R, Stern L et al. United States‐based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol 2013;19:2348–2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Modlin I, Drozdov I, Kidd M. The identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. PLoS One 2013;8:e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidd M, Drozdov I, Modlin I. Blood and tissue neuroendocrine tumor gene cluster analysis correlate, define hallmarks and predict disease status. Endocr Relat. Cancer 2015;22:561–575. [DOI] [PubMed] [Google Scholar]
- 18.Modlin I, Drozdov I, Alaimo D et al. A multianalyte PCR blood test outperforms single analyte ELISAs for neuroendocrine tumor detection. Endocr Relat. Cancer 2014;21:615–628. [DOI] [PubMed] [Google Scholar]
- 19.Modlin IM, Kidd M, Bodei L et al. The clinical utility of a novel blood‐based multi‐transcriptome assay for the diagnosis of neuroendocrine tumors of the gastrointestinal tract. Am J Gastroenterol 2015;110:1223–1232. [DOI] [PubMed] [Google Scholar]
- 20.Pavel M, Jann H, Prasad V et al. NET blood transcript analysis defines the crossing of the clinical Rubicon: When stable disease becomes progressive. Neuroendocrinology 2017;104:170–182. [DOI] [PubMed] [Google Scholar]
- 21.Modlin IM, Frilling A, Salem RR et al. Blood measurement of neuroendocrine gene transcripts defines the effectiveness of operative resection and ablation strategies. Surgery 2016;159:336–347. [DOI] [PubMed] [Google Scholar]
- 22.Filosso P, Kidd M, Roffinella M et al. The utility of blood neuroendocrine gene transcript measurement in the diagnosis of bronchopulmonary neuroendocrine tumors (BPNET) and as a tool to evaluate surgical resection and disease progression. European J Cardiothoracic. Surgery 2018;53:631–639. [DOI] [PubMed] [Google Scholar]
- 23.Ćwikła JB, Bodei L, Kolasinska‐Ćwikła A et al. Circulating transcript analysis (NETest) in GEP‐NETs treated with somatostatin analogs defines therapy. J Clin Endocrinol Metab 2015;100:E1437–E 1445. [DOI] [PubMed] [Google Scholar]
- 24.Bodei L, Kidd M, Modlin IM et al. Measurement of circulating transcripts and gene cluster analysis predicts and defines therapeutic efficacy of peptide receptor radionuclide therapy (PRRT) in neuroendocrine tumors. Eur J Nucl Med Mol Imaging 2016;43:839–851. [DOI] [PubMed] [Google Scholar]
- 25.Ginsburg GS, Kuderer NM. Comparative effectiveness research, genomics‐enabled personalized medicine, and rapid learning health care: A common bond. J Clin Oncol 2012;30:4233–4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik SM, Pazdur R, Abrams JS et al. Consensus report of a joint NCI thoracic malignancies steering committee: FDA workshop on strategies for integrating biomarkers into clinical development of new therapies for lung cancer leading to the inception of “master protocols” in lung cancer. J Thorac Oncol 2014;9:1443–1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sutliff VE, Doppman JL, Gibril F et al. Growth of newly diagnosed, untreated metastatic gastrinomas and predictors of growth patterns. J Clin Oncol 1997;15:2420–2431. [DOI] [PubMed] [Google Scholar]
- 28.Norton JA, Jensen RT. Resolved and unresolved controversies in the surgical management of patients with Zollinger‐Ellison syndrome. Ann Surg 2004;240:757–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strosberg JR, Halfdanarson TR, Bellizzi AM et al. The North American Neuroendocrine Tumor Society consensus guidelines for surveillance and medical management of midgut neuroendocrine tumors. Pancreas 2017;46:707–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Okuyama A, Shibata A, Nishimoto H. Critical points for interpreting patients’ survival rate using cancer registries: A literature review. J Epidemiol 2018;28:61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]