MOST was a multicenter observational study by the Veneto Oncology Network that monitored the diagnostic‐therapeutic pathway of nonsquamous EGFR mutant non‐small cell lung cancer patients, using one of three recommended agents: gefitinib, erlotinib, or afatinib. Results are reported here.
Keywords: Non‐small cell lung cancer, Epidermal growth factor receptor, Tyrosine kinase inhibitor, Real world
Abstract
Introduction.
Gefitinib, erlotinib, and afatinib represent the approved first‐line options for epidermal growth factor receptor (EGFR)‐mutant non‐small cell lung cancer (NSCLC). Because pivotal trials frequently lack external validity, real‐world data may help to depict the diagnostic‐therapeutic pathway and treatment outcome in clinical practice.
Methods.
MOST is a multicenter observational study promoted by the Veneto Oncology Network, aiming at monitoring the diagnostic‐therapeutic pathway of patients with nonsquamous EGFR‐mutant NSCLC. We reported treatment outcome in terms of median time to treatment failure (mTTF) and assessed the impact of each agent on the expense of the regional health system, comparing it with a prediction based on the pivotal trials.
Results.
An EGFR mutation test was performed in 447 enrolled patients, of whom 124 had EGFR mutation and who received gefitinib (n = 69, 55%), erlotinib (n = 33, 27%), or afatinib (n = 22, 18%) as first‐line treatment. Because erlotinib was administered within a clinical trial to 15 patients, final analysis was limited to 109 patients. mTTF was 15.3 months, regardless of the type of tyrosine kinase inhibitor (TKI) used. In the MOST study, the budget impact analysis showed a total expense of €3,238,602.17, whereas the cost estimation according to median progression‐free survival from pivotal phase III trials was €1,813,557.88.
Conclusion.
Good regional adherence and compliance to the diagnostic‐therapeutic pathway defined for patients with nonsquamous NSCLC was shown. mTTF did not significantly differ among the three targeted TKIs. Our budget impact analysis suggests the potential application of real‐world data in the process of drug price negotiation.
Implications for Practice.
The MOST study is a real‐world data collection reporting a multicenter adherence and compliance to diagnostic‐therapeutic pathways defined for patients with epidermal growth factor receptor‐mutant non‐small cell lung cancer. This represents an essential element of evidence‐based medicine, providing information on patients and situations that may be challenging to assess using only data from randomized controlled trials, e.g., turn‐around time of diagnostic tests, treatment compliance and persistence, guideline adherence, challenging‐to‐treat populations, drug safety, comparative effectiveness, and cost effectiveness. This study may be of interest to various stakeholders (patients, clinicians, and payers), providing a meaningful picture of the value of a given therapy in routine clinical practice.
摘要
介绍。吉非替尼、厄洛替尼和阿法替尼是表皮生长因子受体 (EGFR) ‐ 突变非小细胞肺癌 (NSCLC) 的获批一线药选择。由于关键性临床试验经常缺乏外部有效性,因此真实世界数据可能有助于描述临床实践中的诊疗途径和治疗效果。
方法。MOST 是威尼托肿瘤学网络 (Veneto Oncology Network) 推动的一项多中心观察研究,旨在监测非鳞状EGFR突变 NSCLC 患者的诊疗途径。我们根据中位至治疗失败时间 (mTTF) 报告了治疗效果,并评估了每种药剂对区域性卫生系统费用的影响,并将其与基于关键性临床试验的预测进行了对比。
结果。对 447 例入组患者进行 EGFR突变检测,其中 124 例发生EGFR突变,这些患者的一线治疗药物为吉非替尼(n = 69, 55%)、厄洛替尼(n = 33, 27%)或阿法替尼(n = 22, 18%)。由于厄洛替尼在 15 名患者的临床试验中使用,最终分析仅限于 109 名患者。 无论使用何种类型的酪氨酸激酶抑制剂 (TKI),mTTF 均为 15.3 个月。在 MOST 研究中,预算影响分析显示总费用为 € 3 238 602.17,而根据关键性 III 期试验的中位无进展生存期进行的成本估算为 €1 813 557.88。
结论。结果显示,非鳞状 NSCLC 患者具有良好的区域依从性和对诊疗途径的顺从性。mTTF 在三种目标 TKI 中无显著差异。我们的预算影响分析表明了真实世界数据在药品价格谈判过程中的潜在应用。
实践意义:MOST 研究是一种真实世界数据收集研究,报告了对表皮生长因子受体突变型非小细胞肺癌患者定义的诊疗途径的多中心依从性和顺从性。这是循证医学的一个基本要素,提供关于患者和病情的信息,这些信息可能很难仅使用随机对照试验的数据进行评估,例如诊断性测试的周转时间、治疗依从性和持久性、指南遵从性、治疗人群的挑战性、药物安全性、比较效果和成本效益。该研究可能会引起各种利益相关者(患者、临床医生和支付方)的兴趣,为常规临床实践中给定治疗的价值提供了有意义的图景。
Introduction
Systemic treatment of non‐small cell lung cancer (NSCLC) has experienced a remarkable evolution in the last 30 years. In particular, the launch of genomic sequencing programs has made further classification of each histologic subtype possible through the identification of molecular alterations at the basis of tumor growth and progression, which might be potential targets for treatment [1], [2], [3]. Most breakthroughs have been made in lung adenocarcinoma, where epidermal growth factor receptor (EGFR) mutations are the target for tyrosine kinase inhibitors (TKIs: gefitinib, erlotinib, afatinib), which, as first‐line treatment, have shown progression‐free survival (PFS) and response rate improvements compared with platinum‐based chemotherapy [4], [5], [6], [7], [8], [9], [10], [11]. The LUX‐Lung 7 phase II randomized clinical trial demonstrated longer PFS and time to treatment failure (TTF) in patients receiving afatinib compared with gefitinib, although the third coprimary endpoint of survival was not reached [12], [13]. No clear criteria for treatment selection among the three TKIs are available, although a different toxicity profile has been shown in recent meta‐analyses [14], [15]. Moreover, the results of published studies have some limitations in regard to transferability in the real‐world setting because white patients and patients with poor performance status were underrepresented.
“Me‐too” drugs may be a critical issue in oncology because of the limited incremental benefits in efficacy and tolerability and the negative impact on health expenditure [16].
On the other hand, molecular characterization of NSCLC is still suboptimal in many cancer centers, in terms of both proportion of patients with available molecular tests and time to molecular results before treatment starts [17], [18]. Diagnostic‐therapeutic pathways in oncology have been encouraged by scientific societies and improved across countries in recent years in order to promote quality and value in cancer care; to provide evidence‐based treatment protocols for specific clinical scenarios; and to balance efficacy, safety, toxicities, and cost. The availability and implementation of these pathways will hopefully provide a patient‐centric health care system, based on reproducibility and uniformity in health care service delivery, reduction of unscheduled events, information exchange, and role definition [19].
In this scenario, in order to address some of these issues, in 2014 the Veneto Oncology Network was established in Veneto, an Italian region where lung cancer incidence and prevalence account for about 3,300 and 7,600 cases yearly, respectively [20].
One of the aims of this network is to support observational real‐world and pragmatic clinical studies, which have the additional value of assessing safety and effectiveness of anticancer drugs in patients usually excluded from pivotal randomized clinical trials, often treated in smaller centers where resources and services are limited, and also allowing an active role in clinical research for community oncologists [21].
Patients and Methods
MOST is a real‐world multicenter observational study promoted by the Veneto Oncology Network, aiming at monitoring the diagnostic‐therapeutic pathway of patients with nonsquamous EGFR‐mutant NSCLC referred to oncology units of the Veneto region in Italy. The network covers a region of about 5 million people with 23 oncology units. In the first protocol version, enrollment duration was 12 months, followed by a 18‐month observation period; considering the heterogeneous time of activation among the oncology units, the protocol was subsequently amended to prolong enrollment in order to allow a minimum recruitment period of 6 months to all participating centers.
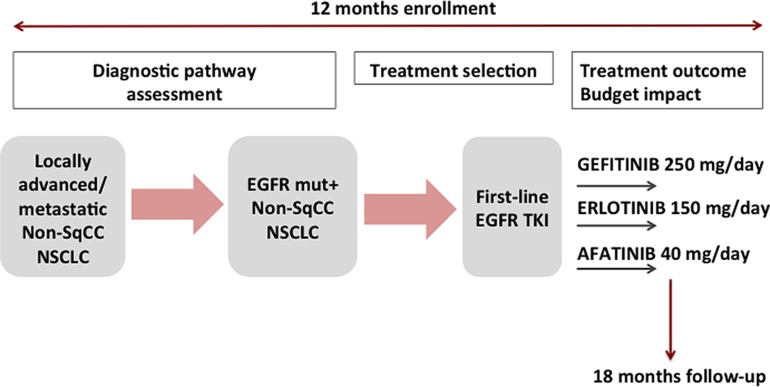
EGFR TKI first‐line treatment of patients with EGFR‐mutant NSCLC consisted of one of the three available recommended agents in this setting at the time of study conduction, i.e., oral daily administration of gefitinib 250 mg, erlotinib 150 mg, or afatinib 40 mg. A schematic representation of the study design is reported in Figure 1.
Figure 1.
Study design. Patients with nonsquamous NSCLC were included in the study. The recruitment lasted 12 months. The follow‐up time lasted 18 months from the inclusion of the last patient. Diagnostic pathway was monitored for all patients with nonsquamous NSCLC, whereas treatment outcome and budget impact analysis were assessed in patients with EGFR‐mutant NSCLC.
Abbreviations: EGFR, epidermal growth factor receptor; mut+, mutation positive; non‐sqCC, nonsquamous; NSCLC, non‐small cell lung cancer; TKI, tyrosine kinase inhibitor.
The primary endpoint of the study was to assess the adherence of the participating centers to the diagnostic‐therapeutic pathways and treatment recommendations defined by the Veneto Oncology Network. In particular, we evaluated (a) the proportion of nonsquamous NSCLC with available EGFR mutation test at diagnosis, (b) the time frame between the date of diagnostic biopsy reception at the pathology unit and histology report (including EGFR mutation test), (c) the proportion of EGFR mutation analyses performed autonomously by the pathologist (reflex test) or after physician request, and (d) the proportion of EGFR‐mutant patients receiving first‐line EGFR TKI, including selection criteria for treatment and time to treatment start.
The secondary endpoint of the study was to evaluate, in a real‐world setting, treatment outcome and budget impact (BI) of first‐line EGFR TKIs.
We also explored the reliability of real‐world data, matching them with health‐administrative data sets, with particular reference to TKI consumption.
Quality of life, budget impact analysis of adverse event management, overall survival, and postprogression diagnostic‐therapeutic pathway will be the objects of separate publications.
Eligibility criteria were age of ≥18 years, new histological and/or cytological diagnosis of advanced or metastatic nonsquamous NSCLC (7th edition of TNM staging system), and no previous oncology treatment for advanced or metastatic NSCLC.
The study was approved by the Veneto Oncology Network Ethical Committee (internal code 2016/03) and by the ethical committees of each participating center; every patient signed an informed consent form.
Information about molecular diagnosis and first‐line treatment of patients with EGFR‐mutant NSCLC were described in electronic case report forms.
The proportion of patients with nonsquamous NSCLC undergoing EGFR mutation analysis at the diagnosis time (expressed as percentage of all nonsquamous NSCLC diagnoses) and the proportion of EGFR‐mutant patients receiving each EGFR TKI (as percentage of the patients who received a first‐line EGFR TKI) were calculated.
The time frame between the date of diagnostic biopsy reception at the pathology unit and histology report (including EGFR mutation test) was also calculated, as the time elapsed before treatment start from diagnostic biopsy and histology report.
The monitoring of the diagnostic‐therapeutic pathway was performed through specific indicators aiming at evaluating its quality and adequacy (supplemental online Table 1).
Evidence‐based recommendation on afatinib use was expressed by the Working Group on Innovative Drugs of the Veneto Oncology Network. The assessment was based on clinical benefit shown by pivotal trials, quality of evidence assessed through the GRADE (Grading of Recommendations Assessment, Development, and Evaluation) system, alternative therapeutic options, and budget impact analysis.
Selection criteria of first‐line treatment choice were assessed through a questionnaire filled in by the medical oncologist at the time of first clinical evaluation and treatment (supplemental online Table 2).
Treatment outcome was reported in terms of TTF: from the first day of treatment to treatment discontinuation for any reason, including disease progression, adverse events (AEs), patient preference, or death. TTF curves of all EGFR‐mutant patients and for each treatment groups were estimated by Kaplan‐Meier method, and curves comparison among the three TKIs was performed by log‐rank test.
The pharmacoeconomic impact of the three agents was assessed through a BI analysis according to different reimbursement mechanisms: gefitinib, payment by result at 3 months; erlotinib, cost sharing (50% discount on the first two packs); afatinib, payment by result at 6 months.
Payment by result and cost sharing are different “managed entry agreements” between the Italian drug regulatory agency and the pharmaceutical companies. Payment by result implies a 100% reimbursement of the drug cost in those cases of therapeutic failure at different time points; cost sharing implies a discount percentage on the first treatment cycles for all patients eligible to a specific treatment.
The impact of each agent on the expense of the regional health system in the clinical practice (real BI) was calculated by multiplying the monthly cost per patient by the number of treated patients and the median TTF (mTTF) from the MOST study and finally subtracting the costs of patients who interrupted gefitinib after 3 months or afatinib after 6 months, or subtracting half of the price of the first two packs of erlotinib. According to the methodology adopted by our regional pharmaceutical service, the budget impact prediction (theoretical BI) was calculated multiplying for median PFS from the pivotal trials [7], [8], [10] and subtracting the costs of patients who interrupted gefitinib after 3 months or afatinib after 6 months in the clinical trials [7], [10] or subtracting half of the price of the first two packs of erlotinib. Finally, the difference between the two budget impact results was calculated (BI gap).
Health‐administrative data sets from hospital drug dispensing service were used to verify drug consumption and regional health system expense in participating centers during the same time frame of the study. Patients who received a first administration of gefitinib, erlotinib, or afatinib between January 2016 and December 2017 were selected by the drug distribution regional database. Patients who received one of the three TKIs after a different systemic treatment were excluded if such treatment was received within 1 year before TKI administration. For each center, we finally selected the drugs distributed in the same time of the MOST study.
Results
Eighteen oncology centers of the Veneto region participated to the clinical study; 17 of them recruited at least one patient.
Recruitment started on March 1, 2016, and enrollment time was prolonged until December 31, 2017.
There were 447 patients with nonsquamous NSCLC included in the study; a molecular test assessing EGFR mutation status was performed in all 447 patients (benchmark 100%; supplemental online Table 1).
Wild‐type EGFR was shown in 321 (72%) cases, and mutant EGFR was shown in 126 (28%).
Patients’ characteristics are described in Table 1.
Table 1. Patient characteristics.
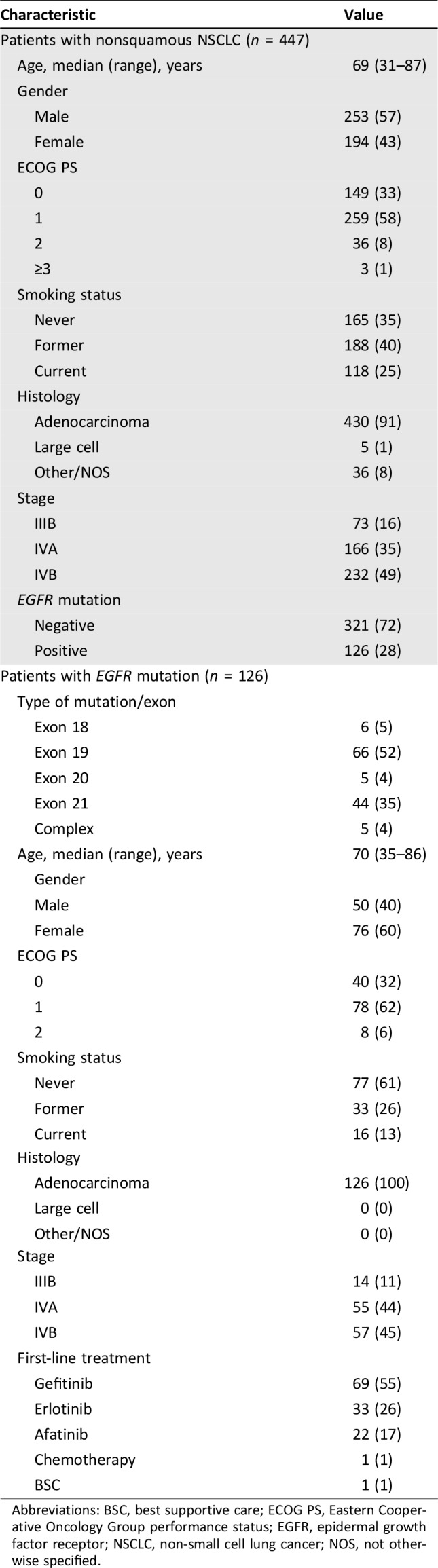
Abbreviations: BSC, best supportive care; ECOG PS, Eastern Cooperative Oncology Group performance status; EGFR, epidermal growth factor receptor; NSCLC, non‐small cell lung cancer; NOS, not otherwise specified.
EGFR mutations were in exon 18 in 6 patients (5%), exon 19 in 66 (52%), exon 20 in 5 (4%), and exon 21 in 44 (35%); 5 (4%) cases harbored a complex mutation.
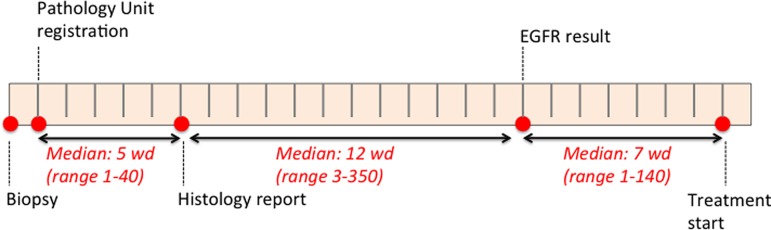
Median time for availability of EGFR mutation analysis result was 18 working days (WDs) from biopsy and 12 WDs from pathology report (benchmark 10 WDs; supplemental online Table 1; Fig. 2). Reflex test was performed in 60% of cases. More than 50% of EGFR mutation tests were not performed in house in seven centers; the most used molecular test was reverse transcription‐polymerase chain reaction.
Figure 2.
Time gap between diagnostic procedure and histological and molecular report and between molecular results and treatment start.
Abbreviatons: EGFR, epidermal growth factor receptor; wd, working day.
Among EGFR‐mutant patients, 124 (98%) received an EGFR TKI as first‐line treatment (benchmark: at least 90%; supplemental online Table 1). One patient harboring exon 20 mutation received first‐line chemotherapy, whereas the other patient did not receive any treatment because of sudden performance status deterioration. Among patients treated with first‐line EGFR TKIs, 69 (55%) received gefitinib, 33 (27%) received erlotinib, and 22 (18%) received afatinib (benchmark for afatinib: 10%–30%; supplemental online Table 1; Fig. 3A). Patients received erlotinib within a clinical trial in 45% of the cases; these patients were excluded from the final analysis on mTTF and budget impact.
Figure 3.
Percentage of use of first‐line EGFR TKIs. (A): Across participating centers. (B): According to physicians’ selection criteria.
Abbreviations: EGFR, epidermal growth factor receptor; PS, performance status, TKI, tyrosine kinase inhibitor.
The most used selection criterion for treatment choice was age for gefitinib, the availability of a clinical trial for erlotinib, and type of sensitizing EGFR mutation for afatinib (Fig. 3B).
Median time to treatment start from EGFR result was 7 working days (Fig. 2).
Median follow‐up time was 14.5 months. At the time of last follow‐up (June 30, 2018), 95 (77%) EGFR‐mutant patients were still alive, 59 (48%) patients were still receiving an EGFR TKI, and 65 (52%) had stopped first‐line treatment.
The main reason for treatment discontinuation was progression or death in 63 (97%) and AE in 2 (3%) patients (both treated with afatinib).
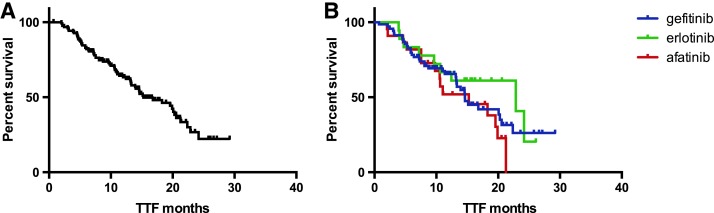
mTTF was 15.3 months in the overall EGFR‐mutant population receiving upfront EGFR TKI (n = 109; Fig. 4A). When we assessed mTTF in each treatment subgroup, we observed 14.6, 22.9, and 15.3 months in patients treated with gefitinib, erlotinib, and afatinib, respectively (p = .3; Fig. 4B).
Figure 4.
TTF of patients receiving an epidermal growth factor receptor TKI as upfront treatment. (A): Overall treatment population. (B): Subgroups of patients treated with gefitinib, erlotinib or afatinib. Median TTF in the overall population was 15.25 months; median TTF in patients treated with gefitinib, erlotinib, and afatinib was 14.6, 22.9, and 15.3 months, respectively (p = .3).
Abbreviation: TTF, time to treatment failure.
Median survival was not reached at the time of data cutoff (79% of censored subjects).
Considering that a remarkable percentage of patients received erlotinib within a clinical trial in which this TKI was often supplied by the study sponsor and prescribed in combination with a second biological agent, we limited the budget impact analysis to only those patients who received erlotinib as standard of care.
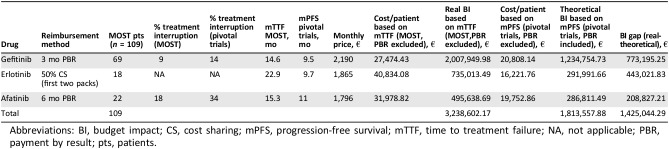
Budget impact analysis showed an expense estimation of €3,238,602.17 according to median TTF of the 109 patients treated with an EGFR TKI within the MOST study, whereas the cost estimation according to median progression‐free survival from pivotal phase III trials [7], [8], [10] was €1,813,557.88. The estimated budget impact gap of €1,425,044.29 was mainly due to longer treatment duration and lower percentage of patients who discontinued TKI treatment within the reimbursement time frame in the MOST study. Detailed budget impact analysis of the two treatment groups is reported in Table 2.
Table 2. Budget impact analysis of gefitinib, erlotinib, and afatinib according to different reimbursement methods and percentage of patients who interrupted treatment within reimbursement time in the MOST study compared with pivotal trials.
Abbreviations: BI, budget impact; CS, cost sharing; mPFS, progression‐free survival; mTTF, time to treatment failure; NA, not applicable; PBR, payment by result; pts, patients.
When we matched data from the MOST study with data of drug dispensation in the same time frame of study enrollment, we observed that participating centers included in the study 64% of all (n = 195) EGFR‐mutant cases receiving one of the three EGFR TKIs.
The difference between patients actually treated with EGFR TKIs and patients enrolled in the MOST study is reported in Figure 5.
Figure 5.
Number of patients with EGFR‐mutant NSCLC treated with upfront EGFR TKI according to the MOST study and to the DRD.
Abbreviations: DRD, drug distribution regional database; EGFR, epidermal growth factor receptor; m+, mutation positive; NSCLC, non‐small cell lung cancer; TKI, tyrosine kinase inhibitor.
Discussion
Our results show a high adherence of participant centers to the diagnostic‐therapeutic pathway defined for patients with nonsquamous NSCLC; in particular, all enrolled patients received the EGFR mutation analysis, and positive cases accounted for 28% of all patients with nonsquamous NSCLC. This percentage is higher than expected from literature data, in which EGFR‐mutant cases are described as about 15% of all patients with NSCLC [2], [3], and this could represent a selection bias of enrolled patients.
According to epidemiological data [20], we estimated an incidence of about 1,000 new cases of advanced nonsquamous NSCLC per year in Veneto. Patients enrolled in the MOST study (n = 447) account for about 45% of all estimated cases. Likely, patients not considered eligible for active treatments and patients treated at small oncology units represent the majority of the missing patient population.
Turnaround time for EGFR testing was 12 working days from histological diagnosis, which is slightly longer than the recommended 10 working days. The main reason for this result is the heterogeneity of diagnostic‐molecular flow across centers, with particular reference to reflex test execution and the availability of in‐house EGFR mutation test.
Gefitinib was the most used TKI in EGFR mutant cases, probably considering the preferable safety profile for elderly patients, who are well represented in this study population (Table 1). Different safety profiles have been described by two prospective randomized trials [13], [22] and recent meta‐analyses [14], [15] showing higher incidence of hypertransaminasemia in patients treated with gefitinib, diarrhea in patients treated with afatinib, and skin toxicity in patients treated with afatinib and erlotinib. Moreover, along the study conduction, the advent of liquid biopsy for T790 M‐acquired resistance mutation detection at the time of disease progression to first‐ and second‐generation EGFR TKI, and the following development of the third‐generation osimertinib, could have influenced the choice of a less toxic first‐line TKI within the overall treatment strategy.
We observed that medical oncologists choose erlotinib when a clinical trial was available in about 50% of cases, and afatinib according to the sensitizing EGFR mutation.
Although the higher sensitizing effect of exon 19 deletion compared with exon 21 EGFR mutation was already shown in post hoc and retrospective subgroup analysis [8], [23], the combined analysis of LUX‐Lung 3 and LUX‐Lung 6 trials prospectively demonstrated significant OS improvement in patients harboring EGFR exon 19 deletion treated with afatinib compared with chemotherapy [24]. However, considering that afatinib was the third drug “in class,” our regional oncology network panel recommended its use in selected cases. This recommendation corresponded to an expected use of the drug in 10% to 30% of EGFR‐positive cases. Scientific evidence quality was considered low because of the inadequate comparator in the control arm (chemotherapy) and the lack of evidence for patients with worse Eastern Cooperative Oncology Group performance status and of white race.
Although EGFR TKIs are usually continued up to disease progression or unacceptable toxicity, treatment duration in the clinical practice may often differ from that of randomized clinical trials. Oligoprogression frequently occurs in cases of oncogene‐addicted NSCLC, and the benefit of TKI continuation during local ablative treatment on the sites of disease progression has emerged over the years [25], [26], [27].
Moreover, continuation of EGFR TKI after progression in asymptomatic patients might delay treatment switch, providing some benefit in the overall strategy, as confirmed by some observational studies and the phase II ASPIRATION trial [28], [29].
This might explain why TTF in patients who received an EGFR TKI within the observational MOST study is longer than PFS achieved in phase III trials, where RECIST progression called for treatment discontinuation. On the other hand, patients enrolled in the MOST study might have been selected, thus explaining their better outcome; indeed, the number of EGFR‐mutant patients who received an EGFR TKI within the MOST study (n = 109) was lower than the number of patients actually treated in the same time frame of study enrollment (n = 195), as confirmed by health‐administrative data sets.
Time to EGFR TKI treatment failure actually determines the regional health service expense for such agents, whereas price negotiation is defined on the basis of median PFS (or percentage of patients progressing at different time points) shown in phase III pivotal trials.
In order to estimate the treatment duration and subsequent cost per patient of a drug (theoretical budget impact), our Drug Regional Service adopted a methodology based on the estimation of the number of patients eligible for that treatment and the cost of the specific treatment. The drug cost is calculated on median treatment duration for those treatments administered until progression. When real‐life data about median treatment exposure are lacking, median progression‐free survival achieved with the specific drug in pivotal clinical trials remains a reliable measure of median treatment duration, even though it may be subjected to some bias because of poststudy progression treatments.
Our budget impact analysis, based on reimbursement criteria and different treatment duration between the MOST study and pivotal trials, showed a €1,425,044.29 gap, thus suggesting the potential application of real‐world data in the process of drug price negotiation and cost estimation.
Conclusion
The importance of real‐world clinical and administrative health data collection to improve scientific evidence about safety and effectiveness of medical treatments has been recently highlighted by regulatory agencies and scientific societies [30].
Recently, both the European Medicines Agency and the U.S. Food and Drug Administration have promoted the collection of real‐world data in postmarketing drug monitoring, regulatory, and approval flow [31]. Randomized clinical trials frequently lack external validity because they usually include selected patients who account for 2% to 4% of the overall cancer population; indeed, these trials are under‐representative of some patient categories, such as elderly patients or patients with poor performance status, who are eligible for treatment in clinical practice [32]. These critical issues might be solved by real‐world studies, in which data collection from medical records reflects the experience of most patients with cancer. In our work, we observed a selection bias in patient enrollment within the MOST study; in this context, electronic health data sets are useful to be matched with study data in order to map all patients treated with a specific drug. On the other hand, administrative data are usually anonymized; thus they cannot capture the safety and effectiveness of a specific therapeutic pathway or deep biological and genomic data. These two data sources may complement each other in order to collect quality, complete, and reliable data, thus improving scientific evidence from randomized trials in a modern drug development model [33].
See http://www.TheOncologist.com for supplemental material available online.
Acknowledgments
We wish to thank Dr. Laura McMahon for study coordination and quality monitoring, Dr. Claudia Pupo for data entry, Dr. Sandro Tognazzo for epidemiological data extraction, and Dr. Paola Del Bianco and Gianluca De Salvo of the Veneto Oncology Network trial office for electronic case report form set‐up and data validation. This work was partially supported by Regional Health Research grants RP 2014/00000421 and RSF 2017/00000557 and by Health Ministry Research grant NET 2016/02363853.
Author Contributions
Conception/design: Giulia Pasello, PierFranco Conte
Provision of study material or patients: Giulia Pasello, Giovanni Vicario, Fable Zustovich, Francesco Oniga, Stefania Gori, Francesco Rosetti, Andrea Bonetti, Adolfo Favaretto, Silvia Toso, Roberta Redelotti, Antonio Santo, Daniele Bernardi, Petros Giovanis, Cristina Oliani, Lorenzo Calvetti, Carlo Gatti, Giovanni Palazzolo, Zora Baretta, Laura Bonanno, Stefano Frega
Collection and/or assembly of data: Giulia Pasello, Marco Basso, Donatella Da Corte, Stefano Frega
Data analysis and interpretation: Giulia Pasello, Alberto Bortolami, Stefano Frega, PierFranco Conte
Manuscript writing: Giulia Pasello, Jessica Menis, Valentina Guarneri, PierFranco Conte
Final approval of manuscript: Giulia Pasello, Giovanni Vicario, Fable Zustovich, Francesco Oniga, Stefania Gori, Francesco Rosetti, Andrea Bonetti, Adolfo Favaretto, Silvia Toso, Roberta Redelotti, Antonio Santo, Daniele Bernardi, Petros Giovanis, Cristina Oliani, Lorenzo Calvetti, Carlo Gatti, Giovanni Palazzolo, Zora Baretta, Alberto Bortolami, Laura Bonanno, Marco Basso, Jessica Menis, Donatella Da Corte, Stefano Frega, Valentina Guarneri, PierFranco Conte
Disclosures
Valentina Guarneri: Eli Lilly & Co. (SAB), Roche (RF), Novartis, AstraZeneca (H). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
References
- 1.Li T, Kung HJ, Mack PC et al. Genotyping and genomic profiling of non‐small‐cell lung cancer: Implications for current and future therapies. J Clin Oncol 2013;31:1039–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Cancer Genome Atlas Research . Comprehensive genomic characterization of squamous cell lung cancers. Nature 2012;489:519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Cancer Genome Atlas Research . Comprehensive molecular profiling of lung adenocarcinoma. Nature 2014;511:543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han JY, Park K, Kim SW et al. First‐SIGNAL: First‐line single‐agent iressa versus gemcitabine and cisplatin trial in never‐smokers with adenocarcinoma of the lung. J Clin Oncol 2012;30:1122–1128. [DOI] [PubMed] [Google Scholar]
- 5.Maemondo M, Inoue A, Kobayashi K et al. Gefitinib or chemotherapy for non‐small‐cell lung cancer with mutated EGFR. N Engl J Med 2010;362:2380–2388. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y et al. Gefitinib versus cisplatin plus docetaxel in patients with non‐small‐cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): An open label, randomised phase 3 trial. Lancet Oncol 2010;11:121–128. [DOI] [PubMed] [Google Scholar]
- 7.Mok TS, Wu YL, Thongprasert S et al. Gefitinib or carboplatin‐paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009;361:947–957. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R et al. Erlotinib versus standard chemotherapy as first‐line treatment for European patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (EURTAC): A multicentre, open‐label, randomised phase 3 trial. Lancet Oncol 2012;13:239–246. [DOI] [PubMed] [Google Scholar]
- 9.Zhou C, Wu YL, Chen G et al. Erlotinib versus chemotherapy as first‐line treatment for patients with advanced EGFR mutation‐positive non‐small‐cell lung cancer (OPTIMAL, CTONG‐0802): A multicentre, open‐label, randomised, phase 3 study. Lancet Oncol 2011;12:735–742. [DOI] [PubMed] [Google Scholar]
- 10.Sequist LV, Yang JC, Yamamoto N et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013;31:3327–3334. [DOI] [PubMed] [Google Scholar]
- 11.Wu YL, Zhou C, Hu CP et al. Afatinib versus cisplatin plus gemcitabine for first‐line treatment of Asian patients with advanced non‐small‐cell lung cancer harbouring EGFR mutations (LUX‐Lung 6): An open‐label, randomised phase 3 trial. Lancet Oncol 2014;15:213–222. [DOI] [PubMed] [Google Scholar]
- 12.Park K, Tan EH, O'Byrne K et al. Afatinib versus gefitinib as first‐line treatment of patients with EGFR mutation‐positive non‐small‐celllung cancer (LUX‐Lung 7): A phase 2B, open‐label, randomised controlled trial. Lancet Oncol. 2016;17:577–589. [DOI] [PubMed] [Google Scholar]
- 13.Paz‐Ares L, Tan EH, O'Byrne K et al. Afatinib versus gefitinib in patients with EGFR mutation‐positive advanced non‐small‐cell lung cancer: Overall survival data from the phase IIb LUX‐Lung 7 trial. Ann Oncol 2017;28:270–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haspinger ER, Agustoni F, Torri V et al. Is there evidence for different effects among EGFR‐TKIs? Systematic review and meta‐analysis of EGFR tyrosine kinase inhibitors (TKIs) versus chemotherapy as first‐line treatment for patients harboring EGFR mutations. Crit Rev Oncol Hematol 2015;94:213–227. [DOI] [PubMed] [Google Scholar]
- 15.Takeda M, Okamoto I, Nakagawa K. Pooled safety analysis of EGFR‐TKI treatment for EGFR mutation‐positive non‐small cell lung cancer. Lung Cancer 2015;88:74–79. [DOI] [PubMed] [Google Scholar]
- 16.Tabernero J; ESMO Executive Board. Proven efficacy, equitable access, and adjusted pricing of anti‐cancer therapies: No ‘sweetheart’ solution. Ann Oncol 2015;26:1529–1531. [DOI] [PubMed] [Google Scholar]
- 17.Lim C, Tsao MS, Le LW et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall‐cell lung cancer. Ann Oncol 2015;26:1415–1421. [DOI] [PubMed] [Google Scholar]
- 18.Gobbini E, Galetta D, Tiseo M et al. Molecular profiling in Italian patients with advanced non‐small‐cell lung cancer: An observational prospective study. Lung Cancer 2017;111:30–37 [DOI] [PubMed] [Google Scholar]
- 19.Gesme DH, Wiseman M. Strategic use of clinical pathways. J Oncol Practice 2011;7:54–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.I numeri del Cancro in Italia 2018. 8th ed. Associazione Italiana di Oncologia Medica (AIOM), Associazione Italiana Registri Tumoria (AIRTUM), Fondazione AIOM, Progressi delle Aziende Sanitarie per la Salute in Italia (PASSI); 2018.
- 21.Khozin S, Blumenthal GM, Pazdur R. Real‐world data for clinical evidence generation in oncology. J Natl Cancer Inst 2017;109(11). [DOI] [PubMed] [Google Scholar]
- 22.Soria JC, Felip E, Cobo M et al. Afatinib versus erlotinib as second‐line treatment of patients with advanced squamous cell carcinoma of the lung (LUX‐Lung 8): An open‐label randomised controlled phase 3 trial. Lancet Oncol 2015;16:897–907. [DOI] [PubMed] [Google Scholar]
- 23.Fukuoka M, Wu YL, Thongprasert S et al. Biomarker analyses and final overall survival results from a phase III, randomized, open‐label, first‐line study of gefitinib versus carboplatin/paclitaxel in clinically selected patients with advanced non‐small‐cell lung cancer in Asia (IPASS). J Clin Oncol 2011;29:2866–2874. [DOI] [PubMed] [Google Scholar]
- 24.Yang JC, Sequist LV, Geater SL et al. Clinical activity of afatinib in patients with advanced non‐small‐cell lung cancer harbouring uncommon EGFR mutations: A combined post‐hoc analysis of LUX‐Lung 2, LUX‐Lung 3, and LUX‐Lung 6. Lancet Oncol 2015;16:830–838. [DOI] [PubMed] [Google Scholar]
- 25.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol 2014;11:473–481. [DOI] [PubMed] [Google Scholar]
- 26.Weickhardt AJ, Scheier B, Burke JM et al. Local ablative therapy of oligoprogressive disease prolongs disease control by tyrosine kinase inhibitors in oncogene addicted non‐small‐cell lung cancer. J Thorac Oncol 2012;7:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gan GN, Weickhardt AJ, Scheier B et al. Stereotactic radiation therapy can safely and durably control sites of extra‐central nervous system oligoprogressive disease in anaplastic lymphoma kinase‐positive lung cancer patients receiving crizotinib. Int J Radiat Oncol Biol Phys 2014;88:892–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Goto Y, Tanai C, Yoh K et al. Continuing EGFR‐TKI beyond radiological progression in patients with advanced or recurrent, EGFR mutation‐positive non‐small‐cell lung cancer: An observational study. ESMO Open 2017;2:e000214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Park K, Yu CJ, Kim SW et al. First‐line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation‐positive non‐small‐cell lung cancer: The ASPIRATION study. JAMA Oncol 2016;2:305–312. [DOI] [PubMed] [Google Scholar]
- 30.Platt R, Lieu T. Data enclaves for sharing information derived from clinical and administrative data. JAMA 2018;320:753–754. [DOI] [PubMed] [Google Scholar]
- 31.Kesselheim AS, Avorn J. New “21st century cures” legislation: Speed and ease vs science. JAMA 2017;317:581–582. [DOI] [PubMed] [Google Scholar]
- 32.Skovlund E, Leufkens HGM, Smyth JF. The use of real‐world data in cancer drug development. Eur J Cancer 2018;101:69–76. [DOI] [PubMed] [Google Scholar]
- 33.Agarwala V, Khozin S, Singal G et al. Real‐world evidence in support of precision medicine: Clinico‐genomic cancer data as a case study. Health Aff (Millwood) 2018;37:765–772. [DOI] [PubMed] [Google Scholar]