Abstract
The risk of incident hypertension associated with long-term exposure to fine particulate matter (PM2.5) was still unclear by studies conducted in North America and Europe, and this relationship has rarely been quantified at higher ambient concentrations typically found in developing countries. We aimed to investigate the association between PM2.5 and incident hypertension using the large-scale prospective cohorts in China. We included 59 456 participants without hypertension aged ≥18 years from the China-PAR (Prediction for Atherosclerotic Cardiovascular Disease Risk in China) project. Data on ambient PM2.5 at participants’ residential address were obtained during 2004 to 2015 using a satellite-based spatial-temporal model. Hazard ratios and 95% CIs were calculated for incident hypertension using stratified Cox proportional hazards models with adjustment of potential confounders. The findings indicated that average PM2.5 concentration from 2004 to 2015 at study participants’ address was 77.7 μg/m3. During the follow-up of 364 947 person-years, we identified 13 981 incident hypertension cases. Compared with the lowest quartile exposure of PM2.5, participants in the highest quartile had an increased risk of incident hypertension with a hazard ratio (95% CI) of 1.77 (1.56-2.00). Each 10 μg/m3 increment of PM2.5 concentration could increase 11% risk of hypertension (hazard ratio, 1.11; 95% CI, 1.05-1.17). This cohort study provided the first evidence from China that long-term exposure to PM2.5 was independently associated with incident hypertension at relatively high ambient concentrations. Stringent strategies on PM2.5 pollution control are warranted to improve the air quality and contribute to the reduction of disease burden of hypertension in China.
Keywords: air pollution, cohort study, hypertension, particulate matter, risk
Introduction
Many epidemiological studies have shown that exposure to fine particulate matter (PM2.5, particles with an aerodynamic diameter ≤2.5 μm) was related to higher risks of cardiovascular diseases (CVDs).1–4 However, the underlying mechanisms of the relation between PM2.5 exposure and CVD were complex. One potential mechanism is that PM2.5 exposure might elevate blood pressure (BP), the most important modifiable risk factor for CVD, thereby mediating adverse effect of PM2.5 on CVD.5–7
Previous studies have reported the association between long-term exposure to ambient PM2.5 and hypertension incidence, but their results were inconsistent.5–8 Moreover, most of the existing studies were conducted in North America or Europe, where the average PM2.5 levels were much lower than those in China. In 2015, the annual mean PM2.5 was <15 μg/m3 in European countries and the United States, but it was 58.4 μg/m3 in China.9 In the densely populated areas such as Beijing-Tianjin metropolitan region, the long-term PM2.5 concentrations from 2004 to 2013 were generally ≥ 100 μg/m3.10 Thus, the effect estimates of PM2.5 on incident hypertension from other countries might not be generalizable to the Chinese population. In addition, the prevalence of hypertension has been increasing continuously in China,11 and it is expected to reach ≈300 million patients with hypertension by 2025.12 Assessing the long-term effects of PM2.5 on incident hypertension is of great public health significance in China.
In the current study, using data from the China-PAR project (Prediction for Atherosclerotic Cardiovascular Disease Risk in China), we aimed to investigate the association between long-term exposure to PM2.5 and incident hypertension by incorporating 4 contemporary Chinese cohorts with multiple follow-up visits until 2015.
Methods
The authors declare that all supporting data are available within the article and its online-only Data Supplement.
Study Population
The China-PAR project included 4 prospective cohorts, including the China MUCA (1992-1994) (China Multi-Center Collaborative Study of Cardiovascular Epidemiology), China MUCA (1998), InterASIA (International Collaborative Study of Cardiovascular Disease in Asia), and CIMIC (Community Intervention of Metabolic Syndrome in China and Chinese Family Health Study). The detailed design and inclusion criteria for those studies have been published elsewhere.13 In brief, China MUCA (1992-1994), which was established from 1992 to 1994, used a cluster random sampling method to select participants aged 35 to 59 years from 14 clusters in China. This cohort was followed up every 2 years from 1996 to 2004, and then a recent follow-up survey was conducted from 2012 to 2015. China MUCA (1998) was established in 1998 with cluster random sampling, and about 1000 participants aged 35 to 59 years were recruited in each cluster among 15 clusters. The InterASIA study, which was established from 2000 to 2001, selected a nationally representative sample aged 35 to 74 years in China using a 4-stage stratified sampling method based on geographic region (northern versus southern China, divided by the Yangtze River) and urbanicity (urban versus rural). For both China MUCA (1998) and InterASIA cohorts, the first follow-up was conducted from 2007 to 2008, and the second follow-up was conducted from 2012 to 2015. The CIMIC study was a large, community-based cohort that was established from 2007 to 2008. A cluster random sampling method was used to select participants aged ≥18 years from 4 survey sites from Shandong, Henan and Jiangsu provinces based on different economic development levels and geographic regions in central and eastern China. The CIMIC study was followed up from 2012 to 2015.
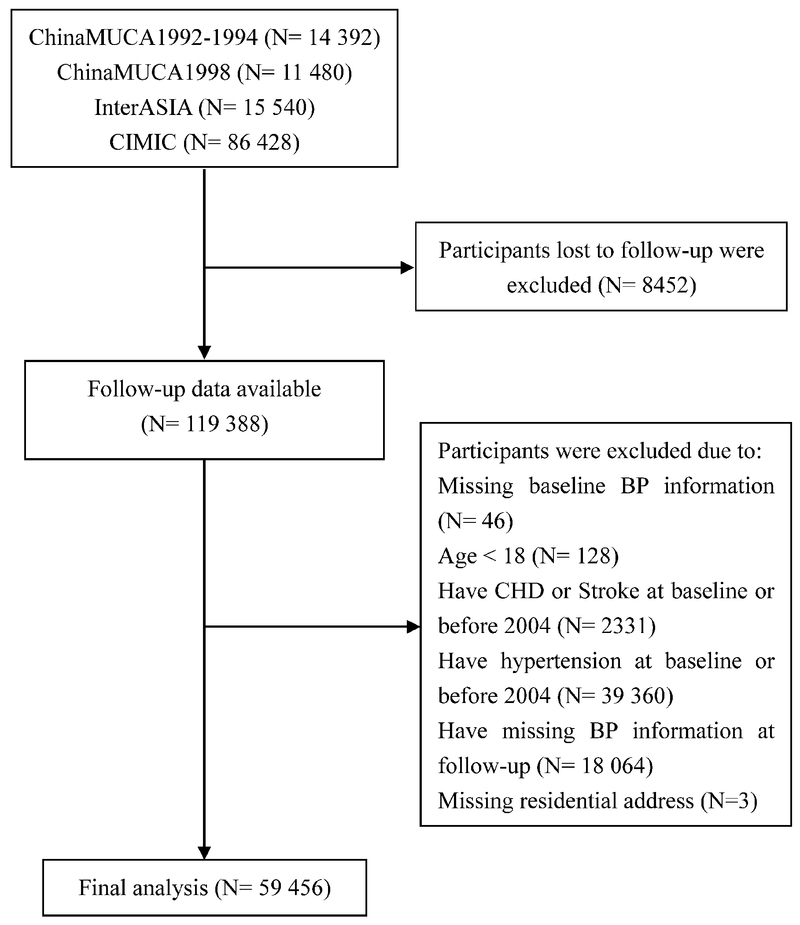
In total, 127 840 participants were enrolled in the 4 cohorts at baseline, and 119 388 participants (93.4%) were followed up successfully.
After excluding 46 participants with missing baseline BP information, 128 participants with baseline age <18 years, 2331 participants with coronary heart disease or stroke before 2004 from which PM2.5 concentrations were available, 39 360 ineligible participants with hypertension before 2004, 18 064 participants with missing BP information at follow-up, and 3 participants with missing residential address, finally, we included 59 456 participants for the final analysis (Figure 1).
Figure 1.
Flow chart of study participants included and excluded in the analyses. BP indicates blood pressure; CHD, coronary heart disease; China MUCA (1992-1994), the China Multi-Center Collaborative Study of Cardiovascular Epidemiology 1992-1994; China MUCA (1998), China Multi-Center Collaborative Study of Cardiovascular Epidemiology 1998; CIMIC, Community Intervention of Metabolic Syndrome in China and Chinese Family Health Study; and InterASIA, International Collaborative Study of Cardiovascular Disease in Asia.
Data Collection
At baseline, a standardized questionnaire was used by trained research staff under stringent quality control to collect information about demographic characteristics, medical history, and lifestyle risk factors. Smoking status was categorized as current, former, or never smoker by asking the participant whether he or she smoked at least 1 cigarette per day for 1 year or more and whether he or she was former or current smoker at the time of baseline survey. Alcohol consumption was defined as drinking alcohol at least once per week during the last year. Body weight and height were measured to the nearest 0.5 kg and 1 cm, respectively, with the participant in lightweight clothing and without shoes. Body mass index (BMI) was calculated as weight in kilograms divided by height in square meters (kg/m2). According to the criteria from World Health Organization,14 underweight, normal weight, and overweight were defined as BMI <18.5 kg/m2, 18.5-25.0 kg/m2, and ≥25.0 kg/m2, respectively. Trained investigators measured BP during the clinic or home visits generally in the morning, according to a common protocol recommended by American Heart Association. BP was measured with the participant in the sitting position after 5 minutes of rest. In addition, participants were advised to avoid alcohol, cigarette smoking, coffee/tea, and exercise for at least 30 minutes before their BP measurement. One of 4 cuff sizes (pediatric, regular adult, large, or thigh) was chosen on the basis of the circumference of participant’s arm. The cuff was placed on the participant’s right arm, and 3 BP measurements were obtained with a 30-second interval between each measurement. The average of the 3 BP measurements was used in the analysis. In addition, blood samples were drawn from participants after fasting for at least 10 hours to measure serum glucose and lipid levels.
The median follow-up durations were 19.2, 14.2, 12.5, and 5.9 years for China MUCA (1992-1994), China MUCA (1998), InterASIA, and CIMIC study, respectively. At follow-up survey, we used unified protocols and similar questionnaires for all cohorts in the China-PAR project. Study participants or their proxies were identified and interviewed to obtain disease status and other vital information. We measured BP and collected data on antihypertensive drugs at baseline and each follow-up visit for all cohorts. We used the same method of BP measurement recommended by American Heart Association, and BP measurements were taken 3× from each subject in the follow-up survey. In the current analysis, hypertension was defined as systolic BP ≥140 mm Hg or diastolic BP ≥90 mm Hg or use of antihypertensive medication within the past 2 weeks. The incident date of hypertension was identified as the date of first diagnosis or initial use of antihypertensive agents.
These preceding studies were all approved by the Institutional Review Board at Fuwai Hospital in Beijing. Written informed consent was obtained from each participant before data collection.
Exposure Assessment
A satellite-based spatiotemporal model was used to assess ambient PM2.5 exposure levels. The details of this model have been published elsewhere.10 In brief, a 2-stage spatial statistical model was developed to estimate ground-level PM2.5 concentrations at 10×10 km resolution in China based on aerosol optical depth retrieved by US National Aeronautics and Space Administration Moderate Resolution Imaging Spectroradiometer instruments, land use, and meteorology data. The model was validated using ground PM2.5 measurements from China Environmental Monitoring Center (http://www.cnemc.cn/). The overall model cross-validation R2 is 0.79 and validation analysis beyond the modeling period indicated that it can reliably estimate historical PM2.5 concentrations with little bias at the monthly (R2 = 0.73) and seasonal levels (R2 = 0.79). This model has been successfully applied in previous epidemiological studies.15,16
On the basis of this model, we obtained monthly PM2.5 concentrations from 2004 to 2015 in China. Each participant’s residential address was geocoded into latitude and longitude data. To account for the residential moving history, we calculated time-weighted average PM2.5 exposure from 2004 to 2015 with weights for each participant defined by the time spend at each of his/her residence. We similarly obtained the monthly air temperature data from National Aeronautics and Space Administration Modern-Era Retrospective analysis for Research and Applications version 2 data sets17 and calculated the time-weighted average temperature from 2004 to 2015 for each participant.
Statistical Analysis
The baseline characteristics of the included participants were presented as mean±SD or median values with interquartile range for continuous variables and as percentages for categorical variables.
In the current study, person-years of follow-up were calculated from January 1, 2004 until the date of incident hypertension, death, or the date of last follow-up, whichever occurred first. We used a random effects Cox proportional hazards model to assess the hazard ratios (HRs) for incident hypertension in relation to PM2.5. The random effects were added for the sampling sites, accounting for spatial dependence among study participants in the same sampling sites. Given the differences of baseline characteristics and follow-up durations in the 4 cohorts (Tables S1 and S2 in the online-only Data Supplement), a stratified Cox regression model was used with each cohort as the stratum to allow each cohort to have its own baseline hazard function. The participants were categorized into 4 groups according to the quartiles of PM2.5 (25th percentile: 71.9 μg/m3, 50th percentile: 73.7 μg/m3, 75th percentile: 82.2 μg/m3). When PM2.5 was considered as a continuous variable, HR of incident hypertension was reported for each 10 μg/m3 increment of PM2.5 concentrations. Covariates in the multivariate-adjusted analysis included age, sex, education level, smoking, drinking, work-related physical activity, BMI, systolic BP, diabetes mellitus, hypercholesterolemia, and ambient temperature. In addition, we calculated population attributable risk to reflect the hypertension burden attributable to ambient PM2.5. The cutoff point for PM2.5 level at the lowest quartile (<71.9 μg/m3) in our study population was used as the reference concentration.
Furthermore, subgroup analyses by sex, age, educational level, BMI, smoking status, and diabetes mellitus were conducted, and an interaction term with PM2.5 was tested in the multivariate-adjusted Cox regression model to investigate the potential effect modification. We also performed a sensitivity analysis by including CVD events before 2004 to assess the potential selection bias. All the statistical analyses were performed using SAS 9.4 (SAS Institute Inc, Cary, NC) and R software, version 3.4.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
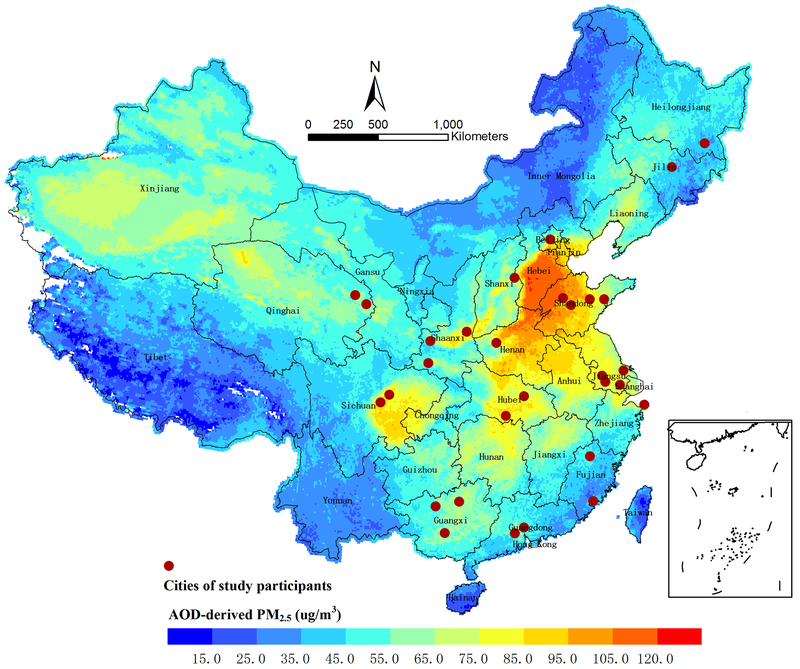
The cities in which study participants resided at baseline were shown in Figure 2. The average PM2.5 concentration during 2004 to 2015 at participants’ residential address was 77.7 μg/m3 with a range of 37.0 to 109.1 μg/m3. Table 1 presented the baseline characteristics of the study participants, overall and according to the quartiles of exposure to PM2.5. At baseline, the mean age of participants was 50 years in 2004, 39% were males, 23.6% were current smokers and 25.9% were overweight or obese. Participants in the highest PM2.5 exposure group (>75th percentile, Q4) tended to have higher prevalence of overweight or obesity, higher systolic BP and diastolic BP levels, and more often to have diabetes mellitus, compared with those with lower exposure levels.
Figure 2.
Average fine particulate matter (PM2.5) concentrations in China from 2004 to 2015. Solid circles denote the cities where the included study participants resided at baseline. AOD indicates aerosol optical depth. We did not estimate the PM2.5 concentrations over the South China Sea.
Table 1.
Baseline characteristics of the study participants in the entire cohort and according to the quartiles of PM2.5 concentrations.
| Variable | Entire Cohort | The quartile of PM2.5* | |||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | ||
| PM2.5 concentration (μg/m3) | 77.7±13.2 | 62.3±9.0 | 72.5±0.5 | 78.6±3.7 | 96.2±5.7 |
| Participants, n | 59 456 | 13 791 | 12 976 | 18 265 | 14 424 |
| Age at baseline, y | 48.4±11.3 | 48.7±10.0 | 50.7±12.1 | 46.7±11.3 | 48.2±11.3 |
| Age in 2004, y | 50.0±11.2 | 52.4±9.3 | 51.3±11.9 | 48.1±11.6 | 49.0±11.3 |
| Male | 39.0% | 42.5% | 34.4% | 40.8% | 37.4% |
| High school education and above | 15.4% | 21.2% | 9.7% | 13.6% | 17.3% |
| Medium-high physical activity | 59.0% | 53.4% | 65.1% | 57.5% | 60.9% |
| Smoking status | |||||
| Never | 73.3% | 69.2% | 77.7% | 69.0% | 78.6% |
| Former | 3.2% | 4.2% | 2.0% | 3.9% | 2.3% |
| Current | 23.6% | 26.6% | 20.3% | 27.1% | 19.1% |
| Alcohol drinkers | 18.0% | 23.6% | 19.2% | 14.7% | 15.7% |
| BMI (kg/m2) | |||||
| < 18.5 | 6.1% | 7.3% | 9.1% | 4.8% | 4.0% |
| 18.5-25.0 | 68.0% | 71.1% | 70.9% | 67.3% | 63.2% |
| ≥ 25.0 | 25.9% | 21.7% | 20.1% | 27.9% | 32.8% |
| SBP, mm Hg | 116.9±11.5 | 114.8±11.6 | 116.6±11.5 | 116.6±11.2 | 119.6±11.2 |
| DBP, mm Hg | 73.6±8.0 | 72.8±8.3 | 72.0±8.3 | 73.8±7.7 | 75.7±7.4 |
| Diabetes mellitus | 3.9% | 2.9% | 2.8% | 4.8% | 4.7% |
| Hypercholesterolemia | 3.5% | 5.6% | 1.8% | 2.6% | 4.2% |
| Average temperature, °C | 14.6±3.1 | 15.4±5.7 | 15.6±0.8 | 14.3±1.7 | 13.5±1.3 |
BMI indicates body mass index; DBP, diastolic blood pressure; PM2.5, fine particulate matter; and SBP, systolic blood pressure.
Study participants were categorized into 4 groups based on the quartiles of PM2.5 concentrations. Minimum, 37.0 μg/m3; 25th percentile, 71.9 μg/m3; 50th percentile, 73.7 μg/m3; 75th percentile, 82.2 μg/m3; maximum, 109.1 μg/m3. Thus, Q1: <25th percentile; Q2: 25th-50th percentile; Q3: 50th-75th percentile; Q4: >75th percentile.
During the follow-up of 364 947 person-years, we identified 13 981 incident hypertension cases. Crude and multivariate-adjusted HRs and 95% CIs for the associations of hypertension incidence with PM2.5 exposure were presented in Table 2. In the crude model, higher PM2.5 exposure was significantly associated with increased risk of developing hypertension (P < 0.0001). Similar results were observed after multivariate adjustment. The HRs (95% CIs) for hypertension incidence in the fully adjusted model (adjusted model 3) were 1.27 (1.17-1.39), 1.44 (1.30-1.58), and 1.77 (1.56-2.00) for the participants in the second, third, and fourth quartiles of PM2.5 concentrations, respectively. Also, each 10 μg/m3 increase in PM2.5 was significantly associated with 11% increase in hypertension incidence (HR, 1.11; 95% CI, 1.05-1.17) after multivariate adjustment. Moreover, we estimated that the population attributable risk for ambient PM2.5 higher than the lowest quartile was 20.7% (95% CI, 13.9%-26.1%) in total population, 16.3% (95% CI, 8.2%-24.3%) in males, and 21.9% (95% CI, 15.2%-28.9%) in females (data not shown).
Table 2.
Hazard ratios and 95% confidence intervals for hypertension incidence across quartiles of PM2.5 in Chinese adults.
| Characteristics | PM2.5 | PM2.5 | ||||
|---|---|---|---|---|---|---|
| First Quartile | Second Quartile | Third Quartile | Fourth Quartile | P trend | Per 10-μg/m3 increment | |
| Person-years | 89 835 | 80 521 | 109 878 | 84 713 | … | … |
| Cases, n | 4293 | 2781 | 4084 | 2823 | … | … |
| Incidence rate (per 1000 person-years) | 47.8 | 34.5 | 37.2 | 33.3 | … | … |
| Crude model* | 1.00 | 1.14 (1.05-1.23) | 1.31 (1.19–1.44) | 1.62 (1.44–1.82) | <0.0001 | 1.12 (1.06–1.18) |
| Adjusted model 1† | 1.00 | 1.17 (1.08–1.27) | 1.39 (1.26–1.52) | 1.68 (1.49–1.89) | <0.0001 | 1.10 (1.04–1.16) |
| Adjusted model 2‡ | 1.00 | 1.17 (1.08–1.27) | 1.37 (1.24–1.50) | 1.66 (1.47–1.87) | <0.0001 | 1.10 (1.04–1.16) |
| Adjusted model 3§ | 1.00 | 1.27 (1.17–1.39) | 1.44 (1.30–1.58) | 1.77 (1.56–2.00) | <0.0001 | 1.11 (1.05–1.17) |
PM2.5 indicates fine particulate matter.
Crude model: Cox proportional hazard model, stratified by cohort, with random effects for the sampling sites and no adjustment.
Adjusted model 1: crude model + adjusted for age and sex.
Adjusted model 2: adjusted model 1 + smoking status, alcohol drinking, physical activity and education level.
Adjusted model 3: adjusted model 2 + body mass index, hypercholesterolemia, diabetes mellitus, systolic blood pressure and average temperature.
In addition, we conducted subgroup analyses to examine the potential effect modification (Table S3). Results were similar between strata of sex, age, educational level, smoking status, BMI, and diabetes mellitus. However, there might be a stronger association found in younger adults (HR, 1.15; 95% CI, 1.08-1.23) compared with the elderly (HR, 0.95; 95% CI, 0.86-1.04; P for interaction=0.08). Also, we observed stronger associations between PM2.5 and hypertension in normal weight and overweight individuals compared with underweight participants, although the interaction term did not reach statistical significance (P for interaction=0.423). The effect in overweight individuals appeared to be even stronger (HR, 1.09; 95% CI, 1.02-1.17) if overweight was defined as BMI ≥24.0 kg/m2 according to the guideline on prevention and control of overweight and obesity for Chinese adults.18
To assess the robustness of our results, we further performed a sensitivity analysis. The estimation for effect of PM2.5 exposure on incident hypertension did not change substantially after reserving those who have CVD at baseline or before 2004 (data not shown).
Discussion
To our knowledge, this is the first prospective cohort study in China to evaluate the association and effect strength of long-term exposure to ambient PM2.5 on incident hypertension at high ambient concentrations. We found that each 10 μg/m3 increase of PM2.5 concentrations raised the incidence risk of hypertension by 11% (HR, 1.11; 95% CI, 1.05-1.17). In addition, the effects seemed to be stronger among younger adults and among overweight individuals.
Previous studies have reported the association between long-term exposure to PM2.5 and incident hypertension.5–8 For example, 1 cohort study conducted in Canada with 35 303 adults without hypertension identified an increased risk of incident hypertension associated with every 10 μg/m3 increase of PM2.5 (HR, 1.13; 95% CI: 1.05-1.22), which was similar to our findings.6 However, the results reported from western countries were inconsistent. The study of 3236 black women selected from the BWHS (Black Women’s Health Study) and its extension analysis based on the full cohort (n=33 771) did not find significant associations of PM2.5 exposure with developing hypertension.7,19 The ESCAPE (European Study of Cohorts for Air Pollution Effects) project only found the significant association between PM2.5 and self-reported hypertension (relative risk, 1.22; 95% CI, 1.08-1.37), but not for measured hypertension (relative risk, 0.97; 95% CI, 0.80-1.17).8 The most recent meta-analysis including 5 cohorts in the West did not find any association between PM2.5 and hypertension (odds ratio, 1.08; 95% CI, 0.95-1.22) with high heterogeneity (I2=61%).20 Three of the 5 included cohorts recruited only women participants, and those cohort studies used various outcome assessment methods, including self-reported hypertension by telephone interview or mailed questionnaire, hypertension registry database, or measured hypertension. In addition, all of the cohorts included in meta-analysis were conducted in North America or Europe, where the annual average PM2.5 ranged from 6 to 18 μg/m3, much lower than that in China, which can reach >100 μg/m3 in some densely populated cities.20
The current study incorporated large population-based cohorts among the general Chinese adults, assessed hypertension outcome by standardized clinical measurements of BP, and used a recent validated high-accuracy satellite-based PM2.5 exposure model.10 Furthermore, our study participants were widely distributed across China, covering to a large range of PM2.5 levels ranging from 37 to 109 μg/m3. These strengths in design and methodology allowed us to identify a significant association between PM2.5 exposure and hypertension incidence, and the results remained significant in sensitivity analysis. Compared with studies conducted in North America or European countries,6,8 our study found similar or even lower HRs for each 10 μg/m3 increase of PM2.5 concentrations, although China had much higher ambient PM2.5 concentrations. In the future, more prospective studies among Chinese adults were needed to examine the risk of incident hypertension at higher PM2.5 concentrations.
In stratified analyses, we observed a possible effect modification by age, showing stronger associations between PM2.5 and hypertension risk in younger adults compared with elder people. This observation is consistent with findings from 2 previous studies reporting that younger individuals were more susceptible to incident hypertension after long-term exposure to ambient particulate matters.5,21 The possible mechanism for the effect modification by age may be partly related to the reduced responsiveness to autonomic nervous system stimuli among elder individuals.22
In addition, a higher effect estimation of PM2.5 on hypertension was observed among normal and overweight individuals compared with underweight individuals (Table S3). Previous findings from the Nurses’ Health Study and Women’s Health Initiative study have shown that US obese women were more susceptible to hypertension after exposure to PM2.5.5,23 In the panel study conducted among metabolic syndrome patients with overweight or obesity, cumulative PM2.5 exposure and black carbon exposure over the prior 1 to 7 days were also associated with systolic BP elevations.24,25 The potential mechanism may include enhancement of the associations between PM2.5 and systemic inflammation,26 as well as higher levels of oxidative stress and reactive oxygen species production among obese individuals.27 More epidemiological studies and experimental studies were needed to explain the physiological mechanisms by which long-term exposure to PM2.5 may lead to hypertension, especially among overweight or obese individuals.
Several limitations should be noted in this study. First, we did not collect the indoor air pollution data such as household use of solid fuels in our study. Previous studies have observed a high correlation between outdoor air pollution and indoor air pollution.28 Second, the spatial resolution of our PM2.5 prediction model is 10×10 km, which might make the exposure assessment subject to nondifferential misclassification and bias the effect estimates toward the null hypothesis. In addition, ambient PM2.5 levels fluctuated a lot depending on the distance to major pollution sources, density of buildings and types of land use. We are going to address these points in future analysis by incorporating more advanced PM2.5 prediction models and considering nearby roads and major pollution sources with relevant information available. Third, we have not yet obtained data on other air pollutants and were therefore unable to control them in our statistical models. Last, as an important risk factor of hypertension,29 the influence of excessive drinking was not fully considered in our study because of lack of drinking quantities in China MUCA (1992-1994) cohort, although we have adjusted the alcohol drinking status in the statistical models.
Perspectives
The current study provided the first evidence from China that long-term exposure to ambient PM2.5 was independently associated with an increased risk of incident hypertension at relatively high concentrations. Our study is more representative of the general Chinese adults covering to a broad range of PM2.5 levels. The findings help advance our understanding of the deleterious effects of PM2.5 on hypertension at high ambient levels. Given the great public health implications for prevention and control of hypertension and its targeted organ damages, our findings call for joint efforts of the whole society to improve air quality in China, including reduction of industrial emission, development of green transportation, and enforcement of national regulations or actions on air quality control.
Supplementary Material
Novelty and Significance.
What Is New?
This is the first large-scale, population-based prospective cohort from China to quantify the risk of incident hypertension associated with long-term exposure to fine particulate matter (PM2.5) at relatively high ambient concentrations. The study participants were widely distributed across China, covering to a broad range of PM2.5 exposures ranging from 37.0 to 109.1 μg/m3.
What Is Relevant?
The current study identified that each 10 μg/m3 increment of ambient PM2.5 was associated with 11% higher risk of incident hypertension among Chinese adults.
Summary
Long-term exposure to ambient PM2.5 is an independent risk factor for hypertension development among Chinese adults. Stringent strategies on PM2.5 pollution control are warranted to improve the air quality and contribute to the reduction of disease burden of hypertension in China.
Acknowledgements
The authors acknowledge the staffs and participants of the China-PAR (Prediction for Atherosclerotic Cardiovascular Disease Risk in China) project for their important participation and contribution.
Sources of Funding
This work was supported by the National Key R&D Program of China (2017YFC0211703), the Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (2017-I2M-1-004), National Natural Science Foundation of China (91643208), and the China Medical Board (15–220). The work of Y. Liu was supported by the National Institutes of Health (grant no. R01ES027892) and assistance agreement no. 83586901 awarded by the US Environmental Protection Agency (EPA) (principle investigator: Y. Liu). It has not been formally reviewed by EPA. The views expressed in this document are solely those of the authors and do not necessarily reflect those of the Agency. EPA does not endorse any products or commercial services mentioned in this publication.
Footnotes
Disclosures
None.
Reference
- 1.Pope CA 3rd, Turner MC, Burnett RT, Jerrett M, Gapstur SM, Diver WR, Krewski D and Brook RD. Relationships between fine particulate air pollution, cardiometabolic disorders, and cardiovascular mortality. Circ Res. 2015;116:108–15. [DOI] [PubMed] [Google Scholar]
- 2.Chi GC, Hajat A, Bird CE, Cullen MR, Griffin BA, Miller KA, Shih RA, Stefanick ML, Vedal S, Whitsel EA and Kaufman JD. Individual and neighborhood socioeconomic status and the association between air pollution and cardiovascular Disease. Environ Health Perspect. 2016;124:1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cesaroni G, Forastiere F, Stafoggia M, et al. Long term exposure to ambient air pollution and incidence of acute coronary events: prospective cohort study and meta-analysis in 11 European cohorts from the ESCAPE Project. BMJ. 2014;348:f7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pope CA, Muhlestein JB, Anderson JL, Cannon JB, Hales NM, Meredith KG, Le V and Horne BD. Short-term exposure to fine particulate matter air pollution is preferentially associated with the risk of ST-Segment elevation acute coronary events. J Am Heart Assoc. 2015;4:e002506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Laden F, Forman JP and Hart JE. Long-term exposure to particulate matter and self-reported hypertension: a prospective analysis in the Nurses’ Health Study. Environ Health Perspect. 2016;124:1414–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen H, Burnett RT, Kwong JC, Villeneuve PJ, Goldberg MS, Brook RD, van Donkelaar A, Jerrett M, Martin RV, Kopp A, Brook JR and Copes R. Spatial association between ambient fine particulate matter and incident hypertension. Circulation. 2014;129:562–9. [DOI] [PubMed] [Google Scholar]
- 7.Coogan PF, White LF, Jerrett M, Brook RD, Su JG, Seto E, Burnett R, Palmer JR and Rosenberg L. Air Pollution and Incidence of Hypertension and Diabetes Mellitus in Black Women Living in Los Angeles. Circulation. 2012;125:767–772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fuks KB, Weinmayr G, Basagana X, et al. Long-term exposure to ambient air pollution and traffic noise and incident hypertension in seven cohorts of the European study of cohorts for air pollution effects (ESCAPE). Eur Heart J. 2017;38:983–90. [DOI] [PubMed] [Google Scholar]
- 9.Cohen AJ, Brauer M, Burnett R, et al. Estimates and 25-year trends of the global burden of disease attributable to ambient air pollution: an analysis of data from the Global Burden of Diseases Study 2015. The Lancet. 2017;389:1907–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ma Z, Hu X, Sayer AM, Levy R, Zhang Q, Xue Y, Tong S, Bi J, Huang L and Liu Y. Satellite-based spatiotemporal trends in PM2.5 concentrations: China, 2004–2013. Environ Health Perspect. 2016;124:184–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.He J. Hypertension in China: a large and increasing public health challenge. J Hypertens. 2016;34:29–31. [DOI] [PubMed] [Google Scholar]
- 12.Kearney PM, Whelton M, Reynolds K, Muntner P, Whelton PK and He J. Global burden of hypertension: analysis of worldwide data. The Lancet. 2005;365:217–23. [DOI] [PubMed] [Google Scholar]
- 13.Yang X, Li J, Hu D, et al. Predicting the 10-year risks of atherosclerotic cardiovascular disease in Chinese population: The China-PAR Project (Prediction for ASCVD Risk in China). Circulation. 2016;134:1430–1440. [DOI] [PubMed] [Google Scholar]
- 14.Reynolds K, Gu D, Whelton PK, Wu X, Duan X, Mo J and He J. Prevalence and risk factors of overweight and obesity in China. Obesity (Silver Spring). 2007;15:10–8. [DOI] [PubMed] [Google Scholar]
- 15.Liu C, Yang C, Zhao Y, Ma Z, Bi J, Liu Y, Meng X, Wang Y, Cai J, Kan H and Chen R. Associations between long-term exposure to ambient particulate air pollution and type 2 diabetes prevalence, blood glucose and glycosylated hemoglobin levels in China. Environ Int. 2016;92-93:416–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen K, Zhou L, Chen X, Ma Z, Liu Y, Huang L, Bi J and Kinney PL. Urbanization level and vulnerability to heat-related mortality in Jiangsu Province, China. Environ Health Perspect. 2016;124:1863–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gelaro R, McCarty W, Suárez MJ, et al. The modern-era retrospective analysis for research and applications, version 2 (MERRA-2). Journal of Climate. 2017;30:5419–5454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ministry of Health of the People’s Republic of China. Guideline on the prevention and control of overweight and obesity for Chinese Adults. Beijing, China: People’s Medical Publishing House; 2006. [Google Scholar]
- 19.Coogan PF, White LF, Yu J, Burnett RT, Seto E, Brook RD, Palmer JR, Rosenberg L and Jerrett M. PM2.5 and diabetes and hypertension incidence in the Black Women’s Health Study. Epidemiology. 2016;27:202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK and Dong GH. Global association between ambient air pollution and blood pressure: a systematic review and meta-analysis. Environ Pollut. 2018;235:576–588. [DOI] [PubMed] [Google Scholar]
- 21.Bai L, Chen H, Hatzopoulou M, Jerrett M, Kwong JC, Burnett RT, van Donkelaar A, Copes R, Martin RV, Van Ryswyk K, Lu H, Kopp A and Weichenthal S. Exposure to ambient ultrafine particles and nitrogen dioxide and incident hypertension and diabetes. Epidemiology. 2018;29:323–332. [DOI] [PubMed] [Google Scholar]
- 22.Cohen L, Curhan GC and Forman JP. Influence of age on the association between lifestyle factors and risk of hypertension. J Am Soc Hypertens. 2012;6:284–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Honda T, Eliot MN, Eaton CB, Whitsel E, Stewart JD, Mu L, Suh H, Szpiro A, Kaufman JD, Vedal S and Wellenius GA. Long-term exposure to residential ambient fine and coarse particulate matter and incident hypertension in post-menopausal women. Environ Int. 2017;105:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brook RD, Sun Z, Brook JR, et al. Extreme air pollution conditions adversely affect blood pressure and insulin resistance: the air pollution and cardiometabolic disease study. Hypertension. 2016;67:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhao X, Sun Z, Ruan Y, et al. Personal black carbon exposure influences ambulatory blood pressure: air pollution and cardiometabolic disease (AIRCMD-China) study. Hypertension. 2014;63:871–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubowsky SD, Suh H, Schwartz J, Coull BA and Gold DR. Diabetes, obesity, and hypertension may enhance associations between air pollution and markers of systemic inflammation. Environ Health Perspect. 2006;114:992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, Nakajima Y, Nakayama O, Makishima M, Matsuda M and Shimomura I. Increased oxidative stress in obesity and its impact on metabolic syndrome. Journal of Clinical Investigation. 2004;114:1752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cyrys J, Pitz M, Bischof W, Wichmann HE and Heinrich J. Relationship between indoor and outdoor levels of fine particle mass, particle number concentrations and black smoke under different ventilation conditions. J Expo Anal Environ Epidemiol. 2004;14:275–83. [DOI] [PubMed] [Google Scholar]
- 29.Beilin LJ and Puddey IB. Alcohol and hypertension: an update. Hypertension. 2006;47:1035–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.