Abstract
Combinatorial expression of Brn3 transcription factors is required for the development of cell specific morphologies in Retinal Ganglion Cells (RGCs). The molecular mechanisms by which Brn3s regulate RGC type specific features are largely unexplored. We previously identified several members of the Copine (Cpne) family of molecules as potential targets of Brn3 transcription factors in the retina. We now use in situ hybridization and immunohistochemistry to characterize Copine expression in the postnatal and adult mouse retina. We find that Cpne5, 6 and 9 are expressed in the Ganglion Cell Layer (GCL) and Inner Nuclear Layer (INL) in both amacrine cells and RGCs. Cpne4 expression is restricted to one amacrine cell population of the INL, but is specifically expressed in RGCs in the GCL. Cpne4 expression in RGCs is regulated by Brn3b both cell autonomously (in Brn3b+ RGCs) and cell non-autonomously (in Brn3b− RGCs). Copines exhibit a variety of subcellular distributions when overexpressed in tissue culture cells (HEK293), and can induce the formation of elongated processes reminiscent of neurites in these non-neuronal cells. Our results suggest that Copines might be involved in a combinatorial fashion in Brn3b dependent specification of RGC types. Given their expression profile and previously proven role as Ca2+ sensors, they may participate in the morphogenetic processes that shape RGC dendrite and axon formation at early postnatal ages.
Keywords: Copine, retina, retinal ganglion cells, Research Resource Identifiers (RRIDs): RRID: AB_10610096, RRID: AB_94166, RRID: AB_2079751, RRID: AB_1549585, RRID: AB_300798, AB_1542751, RRID: AB_528427, RRID: AB_2313552, RRID: AB_2492226
Graphical abstract description.
Copines are a family of Ca2+ and phospholipid binding proteins that can induce morphological changes reminiscent of neurite formation when overexpressed in tissue culture cells (shown). Within the retina, at least four family members, Copine 4, 5, 6 and 9 are expressed in Retinal Ganglion, Amacrine, and Bipolar Cells.
1. Introduction
RGCs are responsible for transmitting visual information received by photoreceptors to the visual processing areas in the brain. A diversity of cell types helps the retina compute different aspects of the visual stimuli before passing that information onto retinorecipient nuclei. For RGCs alone, there are at least 20 subtypes (and maybe upto 50) in the mouse retina as identified based on distinct soma size, dendritic arbor morphology and stratification in the inner plexiform layer (IPL). Combinatorial expression of Brn3 transcription factors- Brn3a, 3b and 3c is required for RGC fate and is linked to development of cell specific morphologies and dendritic stratification of the RGCs in the IPL. The absence of Brn3a and/or Brn3b leads to loss of several RGC subtypes in the mouse retina. The dendrite stratification of the RGCs is also altered. The axons fail to reach their brain targets in the absence of Brn3b (Badea, Cahill, Ecker, Hattar, & Nathans, 2009; Badea & Nathans, 2011), and can exhibit intraretinal and intra-thalamic axonal defects.
Each RGC type connects with specific presynaptic partners in the IPL. It is known that homophilic and heterophilic interactions between several cell adhesion molecules and their partners- Cadherins, Sidekicks-DSCAMs and Plexin-Semaphorins help establish cell specific synapses in the retina (Masai, 2003; Matsuoka et al., 2011; Yamagata & Sanes, 2008). But how cell specific morphologies develop is not well understood. One proposed mechanism is that visual input is important for refinement of the dendrites. For example, pruning of dendrites occurs in on-off bistratified cells during development and converts them into monostratified cells. This is an activity-dependent event that happens after eye opening (Bodnarenko, Jeyarasasingam, & Chalupa, 1995; Tian & Copenhagen, 2003). One of the signaling pathways that could mediate activity-dependent dendrite refinement is the BDNF-Trk retrograde signaling pathway (X. Liu et al., 2007). However, the molecules that link the combinatorial Brn3 expression to the activity-dependent and/or a retrograde signaling pathway-dependent pruning of dendrites are not known. Brn3a and 3b regulate the expression of several proteins in the mouse retina (Sajgo et al., 2017). Whether these molecules have a role in dendritic stratification, synapse formation and axon targeting to the brain by themselves or in conjunction with combinatorial Brn3 expression remains to be explored.
Amongst the protein families whose expression is regulated by Brn3s in the mouse retina, we focused on the Copines. Copines are conserved across various species such as Arabidopsis, Dictyostelium, C. Elegans, mice and humans. They were first identified as an intracellular, Ca2+ sensitive phospholipid binding protein in Paramecium (Creutz et al., 1998). The protein structure has two N terminal C2 domains- C2A and C2B, and a vonWillebrand domain A (vWA) domain at the C terminal (Creutz et al., 1998; Tomsig & Creutz, 2000). The C2 domains mediate Ca2+ - dependent binding to the internal surface of the plasma membrane phospholipids (P. Perestenko, Watanabe, Beusnard-Bee, Guna, & McIlhinney, 2015). C2A and C2B domains are also present in proteins such as some Synaptotagmins, Munc13s, Doc2 and Rabphilin 3A that are involved in Ca2+ dependent vesicle trafficking at the synapse. The vWA domain is found in several extracellular and a few intracellular proteins and is thought to be involved in protein-protein interaction and cell adhesion (Whittaker & Hynes, 2002). Thus, vWA domain might be the protein binding domain of Copine. There are nine Copines expressed in mammals- Cpne1 through 9 (Creutz et al., 1998; Maitra, Grigoryev, Kumar Bera, Pastan, & Lee, 2003; Nakayama, Yaoi, Yasui, & Kuwajima, 1998; Savino et al., 1999). They exhibit tissue specific distribution in mammals. Cpne1, 2 and 3 are ubiquitously expressed, whereas Cpne 4, 6, 7 and 9 are neuronally enriched. Cpne5 and 8 are expressed in central nervous system (CNS) and other tissues such as lungs, kidneys, testes and mammary glands etc (Cowland et al., 2003; Zhang et al., 2014).
Cpne6 has recently been shown to be required for dendritic spine formation associated with long term potentiation in hippocampal neurons. This happens by regulating the actin cytoskeleton via the Rac1-PAKLIMK1-Cofilin pathway in response to increases in intracellular calcium (Reinhard et al., 2016). Cpne6 also affects spine stability and plasticity by increasing the BDNF-TrkB signaling and recycling of the TrkB receptors on the post-synaptic membranes (Burk et al., 2018) and is required for spontaneous neurotransmitter release via it’s interaction with synaptobrevin in the SNARE (soluble N-ethylmaleimide-sensitive fusion protein attachment protein receptors) complex (P. Liu, Khvotchev, Li, Chanaday, & Kavalali, 2018). It is not known if Cpne6 or other Copines could also have similar functions in other parts of the CNS or specifically in the retina.
The expansion of the Copine protein family in mammals, their domain structure and biochemistry make them a likely candidate for mediating cell specific development of neuronal arbor morphologies. Similar to Cpne6 functions in cytoskeleton rearrangement in hippocampus, Copines in the retina could be involved in a Ca2+ dependent dendritic arbor rearrangement in the developing RGCs. Additionally, vWA domain, the protein interacting domain of Copines, might be involved in Ca2+ dependent trafficking of cargo or vesicles inside the RGC dendrites and/or axons. However, the presence of nine Copines in mammals led us to ask the question if they are simply redundant in expression and function or, like Synaptotagmins, could they have different sub-cellular expression (Südhof, 2002) that might lead to differential physiological functions and responses to Ca2+ levels in the RGCs.
In this study, we investigated the temporal/spatial expression patterns and Brn3b regulation of Cpne1–9 in the retina using in-situ hybridization (ISH) and immunohistochemistry (IHC). We also examined and compared the sub-cellular distribution of Copines in HEK293. We found that Copines, a molecular family sharing high protein sequence homology exhibit differential sub-cellular distribution in tissue culture and cell type distribution in the mouse retina.
2. Materials and Methods
2.1. Mouse lines
We used conventional (germline) Brn3b knockout and eye specific conditional Brn3b knock-in mice for both ISH and IHC experiments. The conventional Brn3b knockout (Brn3bKO/KO) is a complete knockout of the Brn3b allele and has been previously characterized (Gan et al., 1996). Age-matched Brn3bKO/WT or Brn3bWT/WT were used as the corresponding wild-type controls. We will use throughout the text Brn3bWT/WT to refer to both. Retina specific conditional Brn3b knockins with AP (Brn3bCKOAP) have been described previously (Badea et al., 2009). Specifically, Rax:Cre; Brn3bWT/KO males were crossed with BrnbCKOAP/CKOAP females. The four possible genotypes of the pups are: 1) Rax:Cre; Brn3bCKOAP/WT, 2) Rax:Cre; Brn3bCKOAP/KO, 3) Brn3bCKOAP/WT and 4) Brn3bCKOAP/KO Rax:Cre; Brn3bCKOAP/WT, and Rax:Cre; Brn3bCKOAP/KO were used as the controls and retina- specific knockouts for Brn3b, respectively. Both these genotypes had AP expression in the retinal ganglion cells that express Brn3b. All the experiments described in this study were carried out according to the guidelines of the National Eye Institute (NEI) animal care and user committee (animal study protocol #NEI-640).
2.2. Histology
Eyes were enucleated and fixed for 15 minutes in either 2% formaldehyde for IHC or 4% formaldehyde for ISH. They were then dissected to remove cornea and lens and fixed for an additional 30 minutes. The eyes were immersed in 30% sucrose in phosphate buffered saline (PBS) overnight at 4°C. and then mounted in Tissue-Tek O.C.T. media and flash frozen. Eyes from Brn3b knockouts (conventional or retina specific knockouts) and corresponding wild-type (WT) littermate controls were embedded in the same sectioning block for each age group. Duplicate cryosections of 14 μm thickness were collected on glass slides.
2.3. In situ hybridization
RNA probes for Cpne4, 5, 6, 8 and 9 were generated by PCR amplification from ES cell DNA of the Sv129 strain origin. The reverse primers contained the T3 promoter sequence. Probes were about 500 bases long with melting temperatures between 75–90°C and recognized the 3’UTR (untranslated regions) of the targeted genes. Probes were made using the Digoxigenin- RNA labeling kit (Millipore-Sigma – Roche subdivision-, Darmstadt, Germany) as described previously (Sajgo et al., 2017). Slides were washed with PBS for 10 minutes at room temperature and post fixed with 4% formaldehyde for 10 minutes, and then incubated in acetylation mix (triethanolamine, HCl and acetic anhydride in water), for 10 minutes at room temperature. Pre-hybridization was done in hybridization buffer (formamide, SSC, Denhardt, yeast RNA, fish sperm DNA in water) without probes overnight at room temperature. Hybridization buffer containing each probe for Cpne4, 5, 6, 8, 9 or Brn3b (positive control) was added to the respective slides. The slides were cover slipped and incubated overnight at 72°C, in a sealed humidifier chamber. Coverslips were removed in 5X SSC and washes done in 0.2X SSC at 72°C for one hour, followed by 0.2X SSC, 5 minutes at room temperature. Slides were equilibrated in B1 buffer (0.1 M Tris pH 7.5, 0.15 M NaCl) for 5 mins and blocked in B1 buffer containing 10% heat inactivated goat serum (HINGS) for 1 hour at room temperature. Alkaline Phosphatase coupled Anti-digoxygenin antibody was applied at 1:2000 in B1 buffer containing 1% HINGS and sections were incubated overnight at 4°C. The sections were then washed three times with B1 buffer and equilibrated with B3 buffer (0.1 M Tris pH 9.5, 0.1 M NaCl, 50 mM MgCl2) for 5 minutes. A mix of nitro blue tetrazolium (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (BCIP) in B3 buffer was added to the sections and incubated at room temperature for 1–24 hours until a visible signal appeared. The reaction was then stopped by removing the NBT-BCIP mix and washing three times with PBS. The slides were cover slipped in Fluoromount-G (Southern Biotech, Birmingham, AL). Brightfield images were acquired on the Zeiss Imager Z2 using the Axiovision software.
2.4. Cpne4 and Cpne9 antibody generation and purification
Peptides derived from N and C- terminal regions of Cpne4 (N-terminal peptide-KKMSNIYESAANTLGIFNS-C and C-terminal peptide- EVYESSRTLA-C) were co-injected into a single rabbit. Two peptides derived from Cpne9- one in the N-terminal region and the other an internal peptide close to the N- terminal region (N-terminal peptide- MSLSGASERSVPA-C and internal peptide- TQSRASQEWREFGR-C) were co-injected into a second rabbit. Peptide synthesis, conjugation to Keyhole limpet hemocyanin antigen and inoculation into rabbits was performed by Cocalico Biologicals (Stevens, PA). Rabbit antisera were checked for presence of either anti-Cpne9 or anti-Cpne4 antibodies by western blotting, and then affinity purified. To generate antibodies monospecific for each antigen, each peptide used for immunization (two each for Cpne4 and Cpne9) was individually cross-linked to a Sulfolink column (Sulfolink immobilization kit, Thermo Fisher Scientific, Waltham, MA), according to the manufacturer’s instructions. For each Copine, about 4.5 ml of rabbit serum was passed sequentially through the two columns, and, after washes, antibodies specific peptide were eluted separately with 8 ml elution buffer (0.1M glycine; pH2.0), collected directly into 7 ml saturated ammonium sulfate, mixed well and precipitated overnight at 4°C. The tubes were centrifuged at 4000 rpm for 20 minutes and the pellets were resuspended in PBS with 50% glycerol and 0.02% sodium azide.
2.5. Copine protein synthesis and Western blot
GST- tagged Cpne4, 5, 8 and His- tagged Cpne9 fusion proteins were synthesized by recombinant expression in E. coli for testing the Cpne4 and Cpne9 peptide antibodies and the commercial Cpne5 antibody. Cpne4, 5 and 8 cDNAs (Thermo Fisher Scientific) were each cloned into pGEX2T plasmid containing GST and transformed into BL21- Codon Plus competent cells (Agilent Technologies, Santa Clara, CA). The Copine protein synthesis was induced by adding Isopropyl-β-D-thiogalactoside (IPTG) to the bacterial culture for two hours at 37°C. The induced bacterial cells were then collected by centrifugation and lysis buffer was added (5mM DTT, 5mM EGTA, 1mM PMSF, 0.5% sarkosyl in PBS; pH=7). The cells were sonicated and centrifuged at 13000 rpm for 30 minutes at 4°C. The pellet was discarded and the supernatant was collected. Triton-x was added to a final concentration of 0.5% and the supernatant incubated for 2 minutes on ice. Glutathione-sepharose beads (GE Healthcare, Chicago, IL) were added to the supernatant and incubated at 4°C overnight. The beads were then washed with PBS and incubated with the elution buffer (50 mM Tris-HCl, 10 mM reduced glutathione, pH 8.0) for 30 minutes at room temperature. The beads were centrifuged and the supernatant collected for loading on the gel.
Cpne9 cDNA (Thermo Fisher Scientific) was fused to a N-terminal His- tag by cloning into the pET28a vector. His- Cpne9 protein synthesis was induced using IPTG as described above for the GST- tagged proteins. After induction, the bacterial pellet was resuspended in the lysis buffer and sonicated. The cell lysate was centrifuged at 13000 rpm for 30 minutes at 4°C and supernatant collected. EGTA, sarkosyl and DTT were removed from the supernanatant using the Slide-a-lyzer Dialysis cassette (Thermo Fisher Scientific). The NaCl concentration was adjusted to 0.5M and imidazole was added to a final concentration of 5mM. The Ni2+ chelated His-Trap FF column (GE Healthcare) was washed with distilled water and then with binding buffer (20 mM Tris, pH 8.0, 0.5 M NaCl, 5 mM imidazole) at a flow rate of ~1ml per minute. The supernatant was applied on the column using a syringe. The column was then washed with about 10 volumes of the binding buffer. For elution, 5 volumes of elution buffer (20 mM Tris, pH 8.0, 0.5 M NaCl, 500 mM imidazole) was applied to the column and the eluate collected.
The protein concentrations were measured using the BCA method. The purified GST-Cpne4, -Cpne5, -Cpne8 and His-Cpne9 were loaded on a 10% SDS-PAGE gel and transferred to PVDF membranes.
For western blotting, the membrane was washed with PBST (PBS, 0.1% Tween-20). Blocking solution (5% non-fat dry milk in PBST) was added to the membrane and incubated for 1 hour at room temperature. Primary antibody was added directly to the blocking solution and the membrane incubated overnight at 4°C. The primary antibodies used were N-term Cpne4, C-term Cpne4, Cpne5, N-term Cpne9 or internal-Cpne9 (refered to as pan Cpne 5/8/9 in rest of the text). Membranes were then washed three times with PBST and secondary antibody solution (HRP-conjugated anti-rabbit; 1:5000) was added to the membranes and incubated for one hour at room temperature. The membranes were washed again three times with PBST and incubated with the Super Signal Pico Chemiluminescence mix (Thermo Fisher Scientific) for 5 minutes at room temperature. Excess reagent was drained and the membranes were imaged on a BioRad (Hercules, CA) Chemidoc imager. The exposure times were adjusted to get an optimum signal at the expected molecular weight position.
2.6. HEK293 transfection
The cDNAs for Cpne4, 5, 6, 8 or 9 were cloned into pAAV-FLEX-HA-T2A-meGFP plasmid vector, such that the cDNA was in frame with the 3X HA (Sajgo et al., 2017). Copine plasmid constructs were transfected into the HEK293Cre line using Lipofectamine (Invitrogen, Carlsbad, CA). Transfection was performed as per the user manual for Lipofectamine. After 24–48 hours of transfection, the cells were fixed in 2% PFA for 10 minutes at room temperature and washed three times with PBS-triton-x.
2.7. Immunohistochemistry
IHC conditions were similar for retinal sections or coverslips containing transfected HEK293 cells. Slides or coverslips were washed with PBS and blocked for one hour in blocking solution (10% normal donkey serum, 1% bovine serum albumin and 0.5% Triton-X100 in PBS). Primary antibody mix (3% normal donkey serum, 1% bovine serum albumin and 0.5% Triton-X100 in PBS) was applied overnight at 4°C to retinal sections or one hour at room temperature for transfected HEK293 cells. The various primary antibodies used are listed in Table 1. The sections/cells were then washed three times with PBS containing 0.5% Triton-X100. Secondary antibody solution (3 % normal donkey serum, 1% bovine serum albumin and 0.5% Triton-X100 in PBS) was added and incubated for one hour at room temperature. Secondary antibodies raised in Donkey were Alexa fluor 488, 568 or 647 conjugates (Thermo Fisher Scientific) and used at 1/300 dilution. The sections/cells were washed three times with PBS/0.5%Triton X-100, mounted using Aquamount and coverslipped. Images were acquired on a Zeiss LSM 700 microscope using ZEN black software. Image import and processing was done using ImageJ (https://imagej.nih.gov/ij/) software, and cell counting on the images was done using the Cell counter plugin (contributed by Kurt De Vos) in Fiji (https://fiji.sc/).
Table1:
Primary antibodies and dilutions used for IHC and Western blotting
| Antigen | immunogen | Antibody details | Dilution used for IHC |
|---|---|---|---|
| N-terminal Cpne4 | KKMSNIYESAANTLGIFNS | made in house, rabbit polyclonal, | 1:400 (1:1000 for WB) |
| C- terminal Cpne4 | EVYESSRTLA | made in house, rabbit polyclonal | 1:2000 (1:1000 for WB) |
| Pan- Cpne 5/8/9 | TQSRASQEWREFGR | made in house, rabbit polyclonal | 1:5000 (1:4000 for WB) |
| Cpne 5 | SLSEFDSLAGSIPATKVEIT VSCRNLLDKDMFSKSDPL CVMYTQGMENKQ | Sigma-Aldrich, rabbit polyclonal, Cat# SAB2107277 | 1:2666 (1:5000 for WB) |
| Cpne 6 | Mouse Cpne6 aa 4–125 | Santa Cruz, mouse monoclonal, clone42, Cat# sc-136357, RRID:AB_10610096 | 1:100 |
| Brn3a | Human Brn3a aal86–224 protein 10 fusion (pGEMEX) | Millipore, mouse monoclonal, Cat# MAB1585, RRID:AB_94166 | 1:20 |
| Brn3b | Human Brn3b aal84–252 GST fusion | made in house, rabbit polyclonal | 1:20 |
| Brn3c | Human Brn3c aa 110–180 GST fusion | made in house, chicken polyclonal | 1:20 |
| ChAT | Human placental ChAT enzyme | Chemicon, goat polyclonal, Cat# AB144P, RRID:AB_2079751 | 1:100 |
| mouse HA | CYPYDVPDYASL | Covance, mouse monoclonal, clone 16B12, Cat# MMS-101R | 1:100 |
| rabbit HA | YPYDVPDYASL | Cell Signaling Technologies, rabbit polyclonal, Cat# 3724, RRID:AB_1549585 | 1:100 |
| GFP | Recombinant full length GFP | Abeam, Chicken polyclonal, Cat# ab 13970, RRID:AB_300798 | 1:700 |
| Sheep AP | Human placental Alkaline phophatase | American Research Products, sheep polyclonal, Cat# 13–2355, RRID:AB_1542751 | 1:500 |
| Mouse AP | Human placental Alkaline phophatase | VIB Gent, mouse monoclonal, E6 clone | 1:50 |
| Pax6 | Recombinant chick Pax6 aa 1–223 | DSHB hybridoma product, mouse monoclonal, Cat# pax6, RRID:AB_528427 | 1:100 |
| NF-H | Bovine Neurofilament heavy chain | Aves Labs, chicken polyclonal, Cat# NFH, RRID:AB_2313552 | 1:400 |
| RBPMS | N-terminal region of rat RBPMS | Phospho Solutions, guinea pig polyclonal, Cat#1832-RBPMS, RRID:AB_2492226 | 1:500 |
2.8. Primary Antibodies
N-terminal Cpne4, C- terminal Cpne4 and Pan Cpne5/8/9 were synthesized in-house. The detailed method of synthesis and purification has been described separately above.
Cpne5 antibody (Sigma Aldrich) was raised against the N-terminal of human Cpne5 protein. We tested the specificity of the Cpne5 antibody using Western blot and it was found to label the GST-Cpne5 (91.9kDa; Supp Fig.2) specifically and not GST-Cpne4, GST-Cpne8 or His-Cpne9.
Cpne6 antibody (Santa Cruz; RRID: AB_10610096) was raised against amino acids 4–125 of mouse Cpne6. The specificity of the antibody has been tested by the manufacturer on rat brain tissue and detects a 62kDa band on Western blot. It also labels parts of dentate gyrus and the CA regions in the hippocampus in mouse brain by immunohistochemistry, and gives negative reactions in brains of Cpne6KO/KO mice (Reinhard et al., 2016).
Brn3a antibody (Millipore; RRID: AB_94166) was raised against the amino acids 186–224 of human Brn3a fused to T7 gene of 10 protein. According to the manufacturer’s specifications, this antibody is specific for Brn3a and does not label Brn3b or Brn3c. It also has no reactivity to Brn3a knockout mouse (Xiang, Gan, Zhou, Klein, & Nathans, 1996).
Brn3b antibody was generated in-house. It has been described previously (Parmhans, Sajgo, Niu, Luo, & Badea, 2018; Xiang et al., 1995; Xiang et al., 1993). A GST fused recombinant peptide with amino acids 184–252 of human Brn3b was used to immunize the rabbits. The antibody was purified using Maltose Binding protein (MBP) fusion Brn3b protein. Using immunohistochemistry it was confirmed that the antibody did not label the Brn3b knockout tissues. The antibody labeled Brn3b and not Brn3a or Brn3c peptides on a Western blot.
Brn3c antibody was generated in-house and has been described previously in Xiang et al. (1993, 1995). A GST fused recombinant peptide with amino acids 110–180 of human Brn3c was used to immunize chickens. The antibody was purified using Maltose Binding protein (MBP) fusion Brn3c protein. The antibody labels Brn3c on Western blot and does not cross react with Brn3a or Brn3b.
Pax6 antibody (RRID: AB_528427) developed by Kawakami A was obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242. It was raised against amino acids 1–223 of recombinant Pax6 protein and labels the neuronal progenitors in the developing brain. Pax6 is a transcription factor that is also involved in eye specification, development of different retinal cell types and is expressed in amacrine cells and RGCs in the adult retina (Ashery-Padan et al., 2000, Marquardt et al., 2001).
Hemaglutinin (HA): (a) Mouse HA antibody HA.11 (Covance) was raised against the twelve amino acid peptide CYPYDVPDYASL of the influenza hemagglutinin. (b) Rabbit HA antibody (Cell Signaling Technologies; RRID: AB_1549585) is also raised against the YPYDVPDYASL peptide of the influenza hemagglutinin. Both antibodies have been tested by the manufacturer to label HA- tag by Western blot as well as immunohistochemistry.
Alkaline Phosphatase (AP): (a) Sheep AP antibody (American Research Products; RRID: AB_1542751) was raised against human placental alkaline phosphatase (PLAP) and has been shown to co-label the cells that were genetically expressing AP (Wichterle, Turnbull, Nery, Fishell, & Alavarez-Buylla, 2001). (b) Mouse AP antibody (VIB, Gent, Belgium) is also raised against the human PLAP. It has been previously shown to co-label the transgenic lines expressing the AP reporter (Parmhans et al., 2018).
Green Fluorescent protein (GFP) antibody (Abcam; RRID: AB_300798) was raised against the full length recombinant GFP. According to manufacturer’s data, the antibody labels a 25kDa band on Western blot of the GFP transfected cell lysates.
Neurofilament Heavy chain (NF-H) antibody (Aves Labs; RRID: AB_2313552) was raised against full length bovine NF-H protein. According to the manufacturer, it specifically labels the neurons from neonatal mouse brain cell cultures.
Choline Acetyl Transferase (ChAT) antibody (Millipore; RRID: AB_2079751) was raised against the human placental ChAT. According to manufacturer’s data, the antibody labels a ~68–70kDa band on a Western blot of the mouse brain lysate. It labels the cholinergic (starburst) amacrine cells in the mouse retina (Elshatory et al., 2007).
RNA binding protein with multiple splicing (RBPMS) antibody (Phosopho Solutions; RRID: AB_2492226) was raised against a synthetic peptide corresponding to the KLH conjugated- N-terminal region of the rat RBPMS. This antibody has been previously shown to label RGCs specifically (Pérez de Sevilla Müller et al., 2017).
2.9. Statistical analysis
Statistical analysis for cell counting after IHC on retinal sections was done using t-test as well as Kolmogorov-Smirnov 2 (KS2) test. Number of images, animals and cells measured, average morphometric measurements or cell counts and p-values are given in the Supplementary tables 1, 2 and 3.
3. Results
3.1. Six of the nine Copine genes are differentially expressed in early postnatal mouse retina
To study and compare the expression profile of Copines both embryonically and post-nataly, we extracted transcript and gene level expression data for the nine members of the Copine family from our previously reported RNA sequencing data for RGCs, retina and the retino recipient brain areas (Sajgo et al., 2017) (Fig. 1 and Supp. Fig. 1). Briefly, in our previous work, we had purified by immuno-magnetic isolation genetically labeled RGCs expressing either Brn3a or Brn3b (Brn3aAP and Brn3bAP RGCs), and compared their RNA expression profile with expression profiles generated from age-matched (Postnatal day 3 – P3) whole retina or retinorecipient brain areas. Several Copine family members appeared as differentially regulated and/or expressed targets, and preliminary results in tissue culture showed a potential role for Cpne4 in cellular morphology and process extension. For details of the methods used for RNA sequencing please refer to Sajgo et al. (2017).
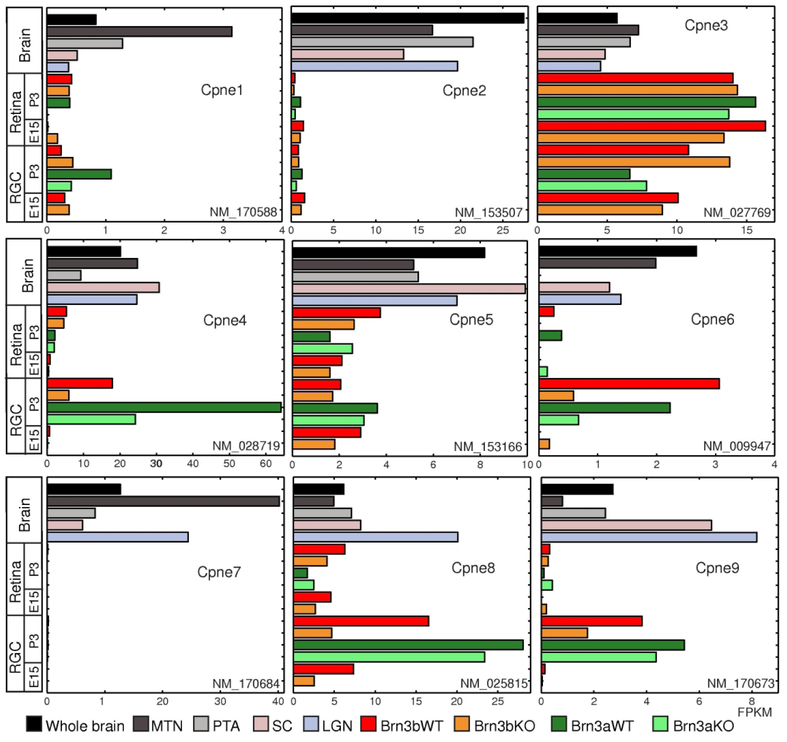
Figure 1. Several Copine family members are enriched in RGCs.
FPKM (Fragments Per Kilobase of transcript per Million mapped reads) values for Cpne1–9 (from top to bottom) for visual brain areas and rest of the ‘whole brain’ (Brain), retinas excluding RGCs (Retina) and RGCs. For each Copine gene, the detected RefSeq transcript is indicated (“NM” number). Brain samples are derived from WT P3 mice. Retina and RGC samples are derived from either P3 or E15 mice, as indicated. Genotypes for retina and RGC samples are as follows: “Brn3bWT” = Pax6α:Cre; Brn3bCKOAP/WT; “Brn3bKO” = Pax6α:Cre; Brn3bCKOAP/KO; “Brn3aWT” = Pax6α:Cre; Brn3aCKOAP/WT; “Brn3aKO” = Pax6α:Cre; Brn3aCKOAP/KO. The color codes indicate the brain areas and/or genotypes for all the samples. Values for the brain areas represent medians for three samples (LGN: lateral geniculate nucleas, SC: superior colliculus), two samples (whole brain) or individual samples pooled from three animals (MTN: medial terminal nucleas and PTA: pretectal area). Retina values represent samples derived from two pooled retinas, while RGC values are medians of two biological replicates each derived from 6–8 retinas.
Cpne1, Cpne2 and Cpne7 have very low expression in the retina and the RGCs. All three are however differentially enriched in retinorecipient brain areas. Cpne3 is expressed at higher levels in retina and RGCs compared to brain samples. Cpne5 is moderately enriched in RGCs but not regulated by either Brn3a or Brn3b. Cpne4, 6, 8 and 9 are enriched in both Brn3aAP and Brn3bAP RGCs during early postnatal development (P3), but are barely detectable in embryonic (E15) RGCs. While RGC expression of all four appears to depend on Brn3b (compare P3 Brn3bAP/KO - “KO” and Brn3bAP/WT - “WT” RGCs), only Cpne4 and Cpne6 expression is reduced in Brn3aAP/KO (“KO”) compared to Brn3aAP/WT (“WT”) RGCs. Thus, Cpne4, 5, 6, 8 and 9 appear to be preferentially expressed in RGCs at P3, a time of active development for dendrites and synaptogenesis both within the IPL and in the retinorecipient target regions.
3.2. Temporal differences in RNA expression of Copines in developing retina
We next used ISH to explore the postnatal expression profile of the five most interesting Copines from P0 onwards and assess their dependency on the transcription factor Brn3b (Fig. 2).
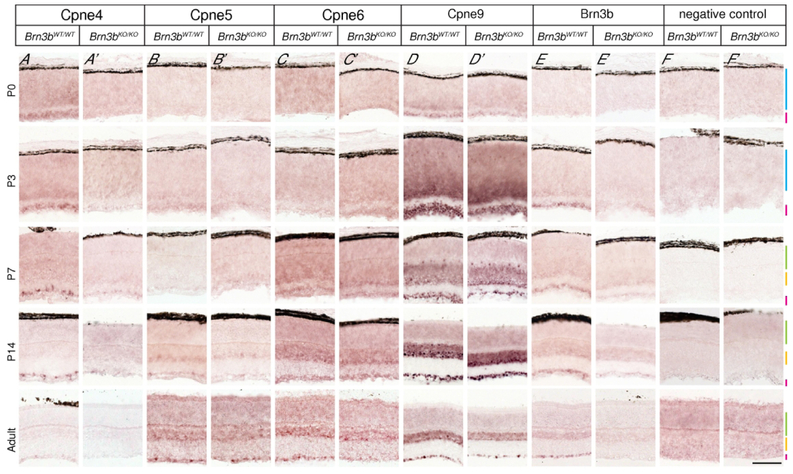
Figure 2. Temporal dynamics of Copine RNA expression in postnatal retina.
Panels show in situ hybridization with probes against the 3’UTRs of four Copines predicted to be enriched in RGCs (Cpne4 = A-A’, Cpne5 = B-B’ Cpne6 = C-C’, Cpne9 = D-D’), the Brn3b gene (E-E’) and a negative control (F-F’). Experiments were performed on retinas from Brn3bWT/WT (A-F) and Brn3bKO/KO (A’-F’) mice at four different postnatal ages (P0, P3, P7, P14) and adult (between 2 and 6 months old). Retinal layers color code: NBL- blue, ONL- green, INL- yellow, GCL- pink. Note that Cpne4 is selectively expressed in the GCL beginning with P3, and specifically lost from the Brn3bKO/KO retinas while Cpne5, Cpne6 and Cpne9 show varying degrees of enrichment in the GCL and INL, with Cpne9 being expressed at the highest levels, and essentially no regulation by Brn3b. Scale bar in F’ : 50μm. n=2–6 animals for each age for Brn3bKO/KO as well as the age matched Brn3bWT/WT.
Cpne4 expression was detectable in the GCL at P0-P3 and exhibited a sparse pattern of GCL labelling from P7 to adult in the Brn3bWT/WT retina (Fig. 2A–A’). Brn3bKO/KO retinas exhibited a steady decrease of Cpne4 signal through postnatal development and into the adult life, but residual signal was still present between P7 and P14. The other tested Copines had a broader expression in the INL and GCL. Cpne5 expression was visible in the GCL and inner aspect of the neuroblast layer (NBL) at P3 (Fig. 2B–B’). Beginning with P14, the expression became more punctate in both the GCL and the inner aspect of the INL. The expression pattern of Cpne5 was similar in Brn3bWT/WT and Brn3bKO/KO retinas throughout all stages of development, with only a modest reduction in expression in Brn3bKO/KO GCL compared to the Brn3bWT/WT (Fig. 2B, B’). Cpne6 started to express at P7 in the inner INL and GCL and increased at P14. Cpne6 expression pattern in Brn3bKO/KO was the same as Brn3bWT/WT at P7, P14 and adult ages. However, there was some differential expression in the Brn3bKO/KO as compared to the Brn3bWT/WT in the adult retina (Fig. 2C, C’). Cpne9 was first detectable at P0 and at P3 there was intense expression in the GCL as well as the inner part of the NBL and horizontal cells of the Brn3bWT/WT retina. Cpne9 expression appeared enriched in the INL and GCL in both Brn3bWT/WT and Brn3bKO/KO from P7 onwards and was maintained in the adult retina. Cpne9 expression in Brn3bKO/KO retinas was generally comparable to the WT except for some differential expression in the GCL of the adult retina (Fig. 2D, D’). We also tested for Cpne8 but failed to detect any Cpne8 expression in either Brn3bKO/KO or Brn3bWT/WT at any age (data not shown). The Brn3b positive control had Brn3b expression in the GCL from P3 onwards and no expression in the Brn3bKO/KO (Fig 2E, E’). The negative control had no expression at any of the ages (Fig. 2F, F’).
3.3. Copines have highly similar protein sequences
Our in situ hybridization analysis suggested GCL specific expression of Cpne4 and high levels of expression in both INL and GCL for Cpne9. We therefore sought to better understand the retinal expression profile of these two Copines by raising antibodies against them. Using TreeDyn198.3 dendrogram analysis (Chevenet, Brun, Bañuls, Jacq, & Christen, 2006), Copines can be clustered into three families based on sequence similarity- (1) Cpne1, 2 and 3, (2) Cpne4, 6 and 7, (3) Cpne5, 8 and 9 (Fig. 3B). They have highly conserved amino acid sequences (Fig. 3A), sharing three highly homologous domains, two C2 domains- C2A, C2B and a vonWillebrand A (vWA) domain. The N- and C- terminal regions, extending beyond the domain boundaries, are somewhat less homologous. We therefore chose peptides located in the regions of reduced homology, as antigens for raising anti-Cpne4 and Cpne9 antibodies (highlighted in Fig. 3A, and see Material and Methods).
Figure 3. Copine proteins are highly similar.
A, ClustalW alignment of the amino acid sequences for mouse Cpne1–9. Grey shaded letters indicate identical or similar amino acids. Consensus sequence is shown at the bottom, with Dissimilar regions represented by ‘X’ on the consensus sequence. The three domains- C2A (red), C2B (blue) and vWA (green) are indicated on the consensus sequence. The antigen sequences for generating Cpne4 (orange: N-term Cpne4, pink: C-term Cpne4) and Cpne9 (yellow: N-term Cpne9, light blue: pan Cpne 5/8/9) antibodies are indicated on the Cpne4 and Cpne9 sequences respectively. B, Phylogenetic dendrogram generated using TreeDyn198.3 for Cpne1–9 shows the clustering based on sequence similarity. Three subfamilies can be distinguished: i) Cpne1, Cpne2, Cpne3; ii) Cpne4, Cpne6, Cpne7; iii) Cpne5, Cpne8, Cpne9. C, Western blot images for affinity purified N-terminal Cpne4 (left), C-terminal Cpne4 (middle) and pan-Cpne 5/8/9 (right) antibodies tested against Cpne fusion proteins expressed in E. Coli and affinity purified. For each antibody, GSTCpne4 (88.7 kDa), GST- Cpne5 (91.9 kDa), GST- Cpne8 (88.7 kDa) and His- Cpne9 (65.4 kDa) were tested. The Cpne4 antibodies- N- terminal and C- terminal label GST-Cpne4 specifically and not Cpne5, 8 or 9. The pan Cpne 5/8/9 antibody labels Cpne5 in addition to Cpne9.
N-terminal and C-terminal antibodies both labeled a GST- Cpne4 fusion protein specifically on Western blots but not GST- Cpne5, -Cpne8 or His-Cpne9 (Fig. 3C). The N-terminal Cpne9 antibody failed to detect Cpne9 or any other Copines (data not shown) and was not used further. The Cpne9 antibody against the internal Cpne9 peptide labeled His-tagged-Cpne9 as well as GST-Cpne5 (Fig. 3C). It also detected GST-Cpne8 weakly at a higher antibody concentration (Supp. Fig. 2). We designate this antibody pan-Cpne 5/8/9 as it cross reacts with the closely related Cpne5 and 8 but not Cpne4 (Fig. 3C). We have also confirmed that the commercial anti-Cpne5 antibody we employed in this study is indeed monospecific for Cpne5 and does not cross react with its closest homologues Cpne8 and 9, nor does it react with Cpne4. (Supp. Fig. 2).
3.4. Copine family members exhibit differential subcellular localization and affect cellular morphology when expressed in HEK293 cells
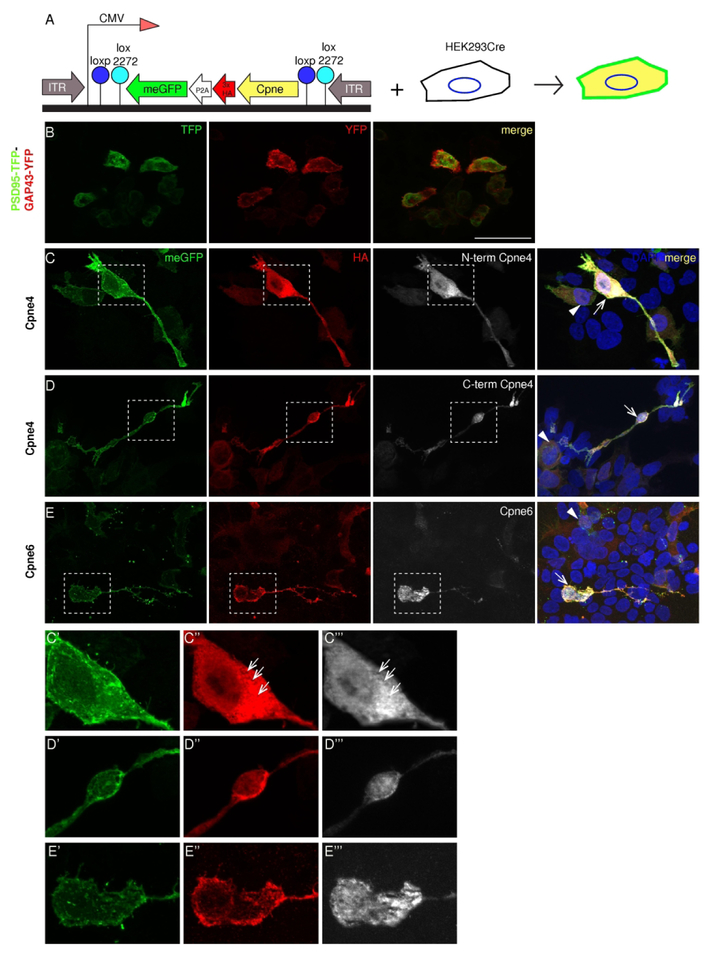
Are Copines entirely redundant or do they serve a variety of distinct functions within the same cell? As a first step towards understanding Copine function, we used commercial and custom made Copine antibodies to characterize their subcellular expression. We transfected HA-tagged Cpne4, 5, 6, 8 or 9 into HEK293-Cre cells and checked the colocalization of HA-tag and Copine proteins (Fig. 4A). HEK293 cells express low levels of some Copines endogenously (https://www.proteinatlas.org/humanproteome/cell; Cpne4 and Cpne5=0TPM, Cpne6=0.1TPM, Cpne7= 11.1TPM, Cpne8=10.3TPM, Cpne9= 0.6TPM). Negative controls were transfected with a bicistronic construct (PTPY) consisting of PSD95-TFP (green) and GAP43-YFP (red; Figure 4B). The outline of transfected cells, including the plasma membrane and filopodial extrusions was revealed by the co-expressed, membrane attached GFP (meGFP; green; Figs. 4, 5). Double labelling with anti-HA (red) and anti-Copine specific antibodies (white) showed good colocalization (Figs. 4, 5), confirming that the fusion proteins were expressed and that the Copine – specific antibodies were indeed detecting Copine protein expression in immunofluorescence experiments. Cpne4 had a diffuse distribution throughout the nucleus and the cytosol of the transfected cells, as indicated by HA and both the N-terminal and C-terminal Cpne4 antibodies (Fig. 4C). We noticed vacuole-like, Cpne4− structures in the cytosol (arrows), and somewhat larger accumulation of Cpne4 in the region adjacent to the nucleus (Fig. 4 C”, C’’’, D”, D’’’). Cpne6 had variable nuclear localization as seen both by HA as well as Cpne6 antibody and punctate expression in the cytosol (Fig. 4E, E”, E’’’).
Figure 4. Sub-cellular localization of Copines 4 and 6 in HEK293 cells.
A, Expression construct and strategy. The expressed cDNA consists of the Cpne gene tagged with 3x HA, self-cleaving P2A signal and eGFP coupled to a GAP43 membrane localization signal (meGFP). The entire cassette is placed in reverse orientation between the tandem inverted lox sites- loxp and lox 2272 downstream of a CMV promoter. Transfection into HEK293Cre cells leads to an irreversible inversion of the expression cassette and two fusion proteins are synthesized- meGFP which localizes on the cell membrane and Cpne-3xHA peptide. B, Control transfection with a bicistronic construct containing PSD95-TFP and GAP43-YFP shows the lack of colocalization of the two peptides. C-E, Constructs containing Cpne4 or 6 genes were transfected into the HEK293-Cre cells. Localization of the two fusion proteins is revealed by immunostaining for GFP (green, first column), HA (red, second column) and Cpne (white, third column) antibodies indicate their localization in the HEK293Cre cells. C’-E’’’, The dashed boxes in C, D and E are zoomed in to show the differences in sub-cellular distribution between Cpne4 and 6, respectively. The meGFP is localized to the cell membrane in all the transfected cells. HA immunostaining indicates the presence of Copines in the cytosol as well as the nuclei of the cells for Cpne4 and is mostly punctate and cytosolic for Cpne6. n= 4 each for Cpne4 and 6, n=2 for control. Scale bar in B: 50μm.
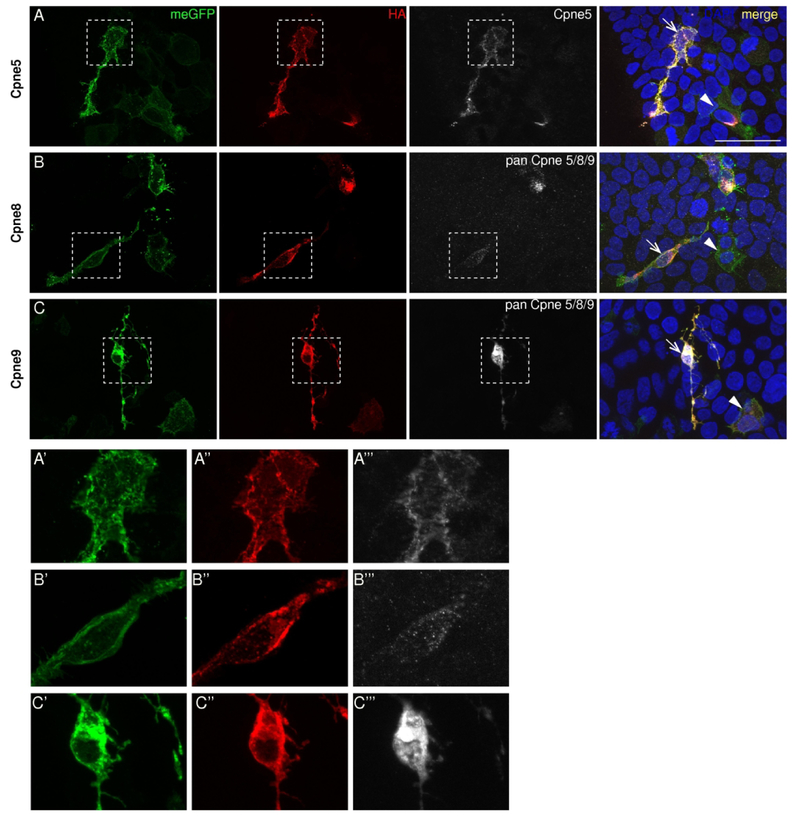
Figure 5. Sub-cellular localization of Copines 5, 8 and 9 in HEK293 cells.
A-C, Constructs containing Cpne5, 8 or 9 genes were transfected into the HEK293-Cre cells. Localization of the two fusion proteins is revealed by immunostaining for GFP (green, first column), HA (red, second column) and Cpne (white, third column) antibodies indicate their localization in the HEK293Cre cells. The meGFP is localized to the cell membrane in all the transfected cells. A’-C’’’, The dashed boxes in A, B and C are zoomed in to show the differences in sub-cellular distribution between Cpne5, 8 and 9, respectively. HA immunostaining indicates the presence of Copines mostly in the cytosol and an absence in the nuclei. Pan Cpne 5/8/9 antibody however has some localization in the nuclei of the cell (C, C’’’). n= 4 each. Scale bar in A: 50μm.
Cpne5 was primarily cytosolic with no expression in the nuclei of the transfected cells, and appeared to line the plasma membrane (Fig. 5A, A’, A”, A’’’). Like Cpne5, Cpne8 was also cytosolic and localized close to the cell membrane as indicated by the HA tag (Fig. 5B, B’, B”). Since we do not have a Cpne8 specific antibody, we used the Pan Cpne 5/8/9 antibody to detect Cpne8 in the transfected cells. It only labels Cpne8 in cells and structures with very high Cpne8-HA expression (Fig. 5B). Cpne9 had a cytosolic localization with some plasma membrane localization similar to Cpne5 and 8 (Fig. 5C, C’, C”, C’’’). In addition, the pan Cpne 5/8/9 antibody showed some nuclear localization, undetected by anti-HA staining (Fig. 5C, C”, C’’’).
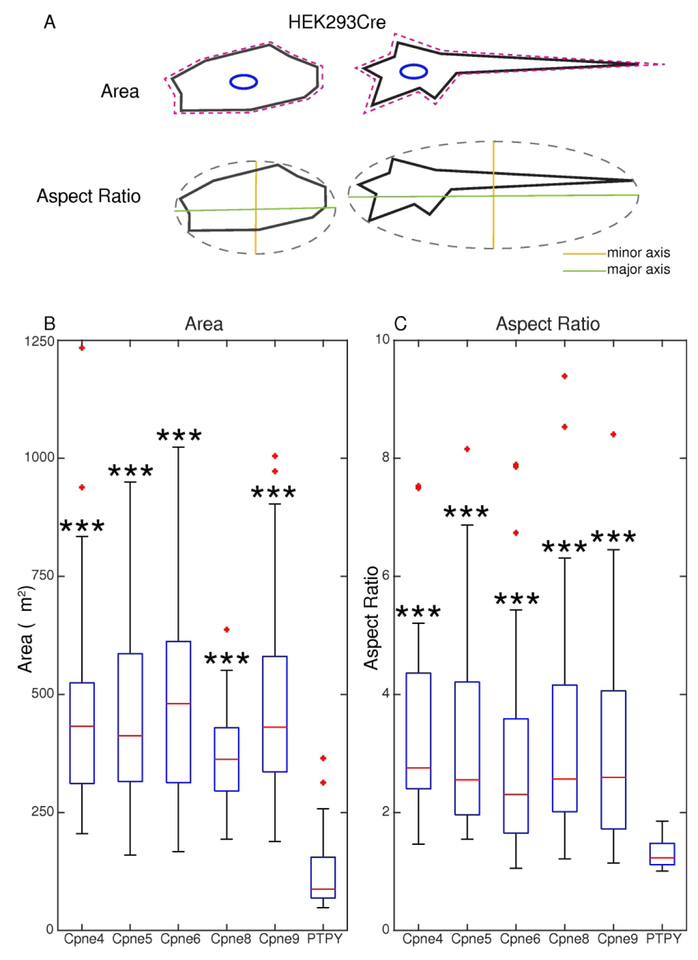
As described before, the domain structure of Copines and mutant phenotypes suggest that these proteins could mediate morphological changes involving cytoskeleton and plasma membrane. In addition, overexpression of Cpne4 in non-neural cells (HEK293) resulted in process extensions reminiscent of neurites. Interestingly we noticed that overexpression of Cpne4, 5, 6, 8 or 9 lead to morphological changes in HEK293 cells (Figs. 4–6), consisting of sprouting of filopodia and “spreading” of surface areas. These changes were especially pronounced in cells with high levels of transfection (compare high and low expressors indicated with arrows and arrowheads respectively in Figs. 4C, D, E and 5A, B, C), but were never noticed in the HEK293 control cells expressing just PSD95-tagged TFP and membrane localized-YFP regardless of expression levels (Fig. 4B). A morphometric analysis of transfected cells was performed to measure the changes in the area and the aspect ratios of the cells (Fig. 6A). Areas of all Copine-transfected cells were significantly increased compared to control (PTPY) (Fig. 6B). In a large fraction of Copine overexpressing cells, one or a few elongated processes emerged at opposing ends of the cell, resulting in a unipolar (e.g. Figs. 4E and 5A) or bipolar shape (e.g. Fig. 4C, D and Fig. 5B and C). The aspect ratio of Cpne4, 5, 6, 8 and 9 transfected cells were all significantly higher than the PTPY transfected cells (Fig. 6C). The areas, aspect ratios and statistical analysis for the measured cells are given in Supplementary table 1.
Figure 6. Morphometric analysis of Copine transfected HEK293 cells.
A, Areas and aspect ratios (AR) for transfected cells measured using ImageJ after drawing a freehand polygon (magenta) along the cell outlines. AR calulates the ratio of the major axis (green) to minor axis (orange) of an ellipse fitted to the outlined cell. B, The boxes represent the interquartile intervals, with the median values indicated in red. The whiskers indicate range of observations, while red crosses indicate outliers. Areas of all the Copine transfected cells are significantly higher to the PTPY (control) transfected HEK293 cells. C, AR of Cpne4, 5, 6, 8 and 9 transfected cells were all significantly higher than the PTPY transfected cells indicating an elongated major axis and hence a significant shift in morphology of the cells. Significance levels are compared for Cpne4, 5, 6, 8 and 9 to the PTPY control transfections and are *** = p< 0.001. Detailed meaurements and statistical comparisons are given in the Supplementary Table 1.
3.5. Copine protein expression in the retina
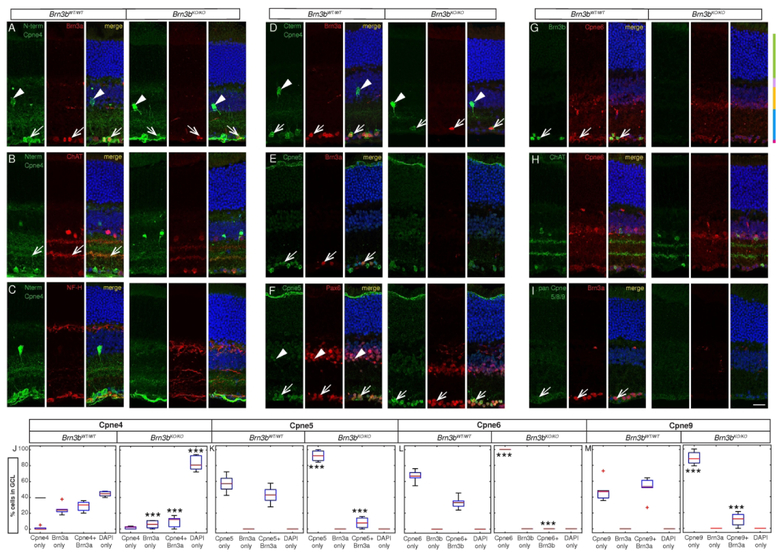
We used the custom made Cpne4 and Pan Cpne 5/8/9 antibodies and the commercially available Cpne5 and Cpne6 antibodies in conjunction with retinal neuronal markers to identify Copine expression in specific retinal cell populations. In the Brn3bWT/WT retina, N-terminal and C-terminal Cpne4 antibodies labeled mostly retinal ganglion cells in the GCL (marked by Brn3a, red, arrow, Fig. 7A, D, J) and a type of amacrine cells in the INL (Fig. 7A, D, arrowheads). However, in Brn3bKO/KO mice, most Cpne4+Brn3a+ double positive cells were lost, mirroring the loss in Cpne4−Brn3a+ RGCs (Fig. 7A, D, J). The relative ratio of Cpne4+Brn3a− in the GCL was not significantly changed as a result of Brn3b loss (Fig. 7A, D, J). The N-terminal antibody (green) also labeled two sub-laminae in the IPL in the Brn3bWT/WT retina (Fig 7B). The inner, GCL proximal Cpne4+ lamina, co-localized with the ChAT+ ON sub-lamina (Fig 7B, arrow) while the outer, Cpne4+ sub-lamina was placed roughly in the middle of the two ChAT+ bands. Of note, neither the intensely labelled Cpne4+ Amacrine cell bodies of the INL, nor any Cpne4+ cells in the GCL were ChAT+. In Brn3bKO/KO mice we observed only one diffuse Cpne4+ sublamina, which did not co-localize with ChAT, while the amacrine cells in INL were preserved (Fig. 7B). In the Brn3bWT/WT retina, N-terminal Cpne4 antibody (green) also labeled some fibers in the optic nerve fiber layer and some of these co-localized with NF-H (red) indicating that they could be ganglion cell axons (Fig. 7C).
Figure 7. Cpne proteins are expressed in the GCL and INL.
Vertical sections through retinas of adult Brn3bWT/WT (three left panels) and Brn3bKO/KO (three right panels) mice. A-F, I, For each genotype, immunostaining for Copines (left panel), various retinal cell type markers (middle panel), and merged image (right panel) are shown. G, H, For each genotype, immunostaining for various retinal cell type markers (left panel), Cpne6 (middle panel), and merged image (right panel) are shown. For all the sections, retinal nuclear layers are labelled with DAPI (blue). A-C Anti-Cpne4 N-terminal antibody (green) used in conjunction with a RGC marker (A, Brn3a, red), Amacrine cell marker (B, Choline Acetyl Transferase, ChAT, red) or a marker for RGC axons (C, Neurofilament Heavy chain, NFH, red). D, Staining for anti-Cpne4 C-terminal antibody (green) in conjunction with Brn3a (red). E-F, Cpne5 (green) double staining with either Brn3a (E, RGCs, red), or Pax6 (F, a marker for RGCs and amacrine cells, red). G-H, Cpne6 (red) stained in conjunction with an RGC marker (G, Brn3b, green) or ChAT (H, green). I, Staining for pan Cpne 5/8/9 (green) together with Brn3a (red). J-M, Box plot quantitation of co-expression of Copines with RGC cell markers Brn3a and Brn3b in the GCL of retinas from Brn3bWT/WT (top) and Brn3bKO/KO (bottom) mice. Data is represented as percent of single labeled (“Cpne only”, “Brn3 only”), double-labeled (“Cpne + Brn3”) or unlabeled (“DAPI only”) cells relative to all DAPI+ cells in the GCL. J, K and M are quantitations of stainings shown in D, E and I respectively, and show degree of overlap between the RGC marker Brn3a and Cpne4 (D, J, C-terminal antibody), Cpne5 (E, K) and Cpne9 (I, M, pan Cpne 5/8/9 antibody). L shows overlap of the RGC marker Brn3b and Cpne6 (G). The boxes are formatted as described in Figure 6. Significance levels in the bottom panels (Brn3bKO/KO) refer to comparisons with the homologous categories in the top panels, and are respectively * = p < 0.05, ** = p < 0.01, *** = p < 0.001. n= 4 animals for Brn3b WT for Cpne5; n=3 animals each for rest of the groups for both Brn3bWT/WT and Brn3bKO/KO. Detailed cell counts and statistical comparison is given in the Supplementary Table 2. Scale in J: 20μm.
The anti-Cpne5 antibody labeled intensely most cell bodies of the GCL, and to a lesser extent all cell bodies of the INL. Cpne5 (green) extensively co-localized with Pax6 (marking Amacrine cells and RGCs) both in the GCL and the INL (Fig. 7F, arrows). All Brn3a+ RGCs (red) of the GCL were also Cpne5+ (arrow), and their numbers were greatly reduced in Brn3bKO/KO compared to Brn3bWT/WT mice (Fig 7E, K). In the IPL, Cpne5 (green) was also expressed in the sublamina apposed to the GCL (Fig. 7E, F). Cpne6 (red) labeled all GCL cells of the WT retina, including RGCs (Brnb+, green), as well as cells in the inner half of the INL, most likely amacrine cells (Fig. 7G, arrow). Cpne6+ Brn3b− cells in the GCL likely included both Brn3b− RGCs and displaced amacrine cells. Cpne6 (red) colocalized with ChAT (green) in the WT as well as the KO retina (Fig. 7H). Pan Cpne 5/8/9 antibody (green) weakly labeled all Brn3a+ ganglion cells in the GCL in both WT and KO (Fig. 7I, arrow). There was no Cpne9 labeling in the INL or IPL (Fig.7I). The complete cell counts for Cpne4, 5, 6 and 9 in the GCL and statistical analysis are given in Supplementary Table 2.
3.6. Cpne4 is expressed in several RGC subpopulations and is partially regulated by Brn3b
Since all GCL neurons are positive for Cpne5, Cpne6 and Cpne9 (Figure 2 L–N, Brn3bWT/WT panels), we can conclude that these three Copine family members are expressed in both Amacrines and RGCs of the GCL. However, Cpne4 show an almost complete overlap with Brn3a, suggesting its GCL expression might be restricted to RGCs. We therefore performed Cpne4 staining in Rax:Cre; Brn3bCKOAP/WT and Rax:Cre; Brn3bCKOAP/KO retinas, in which AP was expressed from the Brn3b locus, in the presence of either a WT or KO allele (Brn3bAP/WT - “WT” or Brn3bAP/KO – “KO”). We assessed the overlap with the other two Pou4f transcription factors, Brn3a and Brn3c. In the Rax:Cre; Brn3bCKOAP/WT retina, Cpne4 (green) was either expressed alone or co-expressed with AP (red), Brn3c (white) or both in the GCL, suggesting that Cpne4 is expressed in at least three subpopulations of RGCs, and a fourth category that are either Amacrine cells or Brn3b−Brn3c− RGCs. Interestingly, the ratio of Brn3bAP+Brn3c+Cpne4+ cells did not dramatically change between the Brn3bAP/KO vs. Brn3bAP/WT RGCs, suggesting that in this particular subpopulation, neither Brn3c nor Cpne4 are under direct Brn3b control. Cpne4+AP+ cells were significantly reduced in the Brn3bAP/KO (Fig. 8A, B) suggesting cell autonomous regulation of this cell population by Brn3b, while loss of both Cpne4+ and Brn3c+Cpne4+ cells in the Brn3bAP/KO signifies non-cell autonomous regulation of these cell fates by Brn3b (Fig. 8A, B).
Figure 8. Brn3b regulates Cpne4 expression.
A, C, E Sections through retinas from adult Rax:Cre; Brn3bCKOAP/WT (left four panels) and Rax:Cre; Brn3bCKOAP/KO (right four panels) mice. For each genotype, panels represent in order from left to right: staining for Cpne4 (C-terminal antibody, green), AP (red), Brn3c (A, white), Brn3a (B, yellow) or RBPMS (C, yellow), and merge. Scale in E: 20μm. B, D, F Box plot quantitations for experiments illustrated in A, C and E, respectively, formatted as in Figure 6. Data is represented as percentage of single labeled (“Cpne4 only”, “Brn3a only”, “Brn3c only”, “RBPMS only”, “AP only”), double-labeled (“Brn3a+Cpne4 “, “Brn3c+Cpne4”, “RBPMS+Cpne4”, “AP+Cpne4”, “Brn3a+AP”, “Brn3c+AP”), triple positive (“Brn3a+AP+Cpne4”, “Brn3c+AP+Cpne4”, “RBPMS+AP+Cpne4”) or unlabeled (“DAPI only”) cells relative to all DAPI+ cells in the GCL. Statistical significance on the Rax:Cre; Brn3bCKOAP/KO panels refers to comparisons between the the abundance of the indicated cell populations in Rax:Cre; Brn3bCKOAP/KO to the corresponding Rax:Cre; Brn3bCKOAP/WT mice, and are * = p < 0.05, ** = p < 0.01, *** = p < 0.001. n= 3 animals each for all the groups for both the Rax:Cre; Brn3bCKOAP/WT and Rax:Cre; Brn3bCKOAP/KO. Detailed cell counts and statistical comparison is given in the Supplementary Table 3.
Interestingly, the entire Cpne4+ cell population of the GCL is composed of Brn3a+Cpne4+, Brn3bAP+Cpne4+ and Brn3bAP+Brn3a+Cpne4+ cells, all likely RGCs. Therefore Cpne4 is indeed exclusively expressed in RGCs. As was previously reported for Brn3b−Brn3c+ RGCs (Shi et al., 2013), loss of Brn3b has both cell autonomous and non-cell autonomous effects on Cpne4 RGC populations, since both Brn3bAP+Brn3a+Cpne4+ and Brn3a+Cpne4+ are significantly reduced in Brn3bAP/KO retinas (Fig.8C, D). However, Brn3bAP+Cpne4+ are preserved in the KO, showing that Cpne4 is not under Brn3b control in all RGC populations, as seen for Brn3bAP+Brn3c+Cpne4+ above.
To further test whether retinal Cpne4 expression is restricted to RGCs of the GCL, we also checked for colocalisation with RBPMS, a global RGC marker (Rodriguez, de Sevilla Müller, & Brecha, 2014). In WT retina, Cpne4 perfectly overlapped with RBPMS, and about 80 % of Cpne4+RBPMS+ cells were also Brn3bAP+. Interestingly, in the KO retina, besides a significant decrease in the RBPMS+Cpne4+Brn3bAP+ and RBPMS+Cpne4+ cells, a new sub-population of cells appeared that was Brn3bAP+Cpne4+RBPMS− (Figure 8E, F, Supplementary Table 3). These cells which express AP from the Brn3b locus, but no longer express Brn3b itself, as both alleles are knocked out, may have lost RBPMS expression as RBPMS itself is regulated by Brn3b (Sajgo et al., 2017), however they are still expressing Cpne4. Since RBPMS+Cpne4+Brn3bAP+ RGCs are significantly reduced but not completely ablated in Rax:Cre; Brn3bCKOAP/KO (essentially retinal Brn3b – KO/KO mice) RBPMS and Cpne4 are differentially regulated by Brn3b in distinct RGC populations. It is possible that Brn3a, Brn3c or other transcription factors substitute for Brn3b in those cells.
4. Discussion
Cell-intrinsic molecules and pathways that determine the arbor morphology and stratification of RGCs have not been studied extensively. It is well established that the combinatorial expression of Brn3 transcription factors is important for cell specific morphologies and stratification in the IPL. But what are the Brn3-dependent molecules that mediate the development of cell-specific morphologies? We had previously reported combinatorial codes of transcription factors, cell surface and intracellular molecules that are enriched in distinct RGC populations or regulated by different Brn3 transcription factors (Muzyka, Brooks, & Badea, 2018; Sajgo et al., 2017). Among these putative targets, we now focus on a protein family – Copines comprising nine members, of which six are expressed in the retina, five exhibit some level of enrichment in RGCs at early postnatal ages, and one (Cpne4) appears to be RGC specific. Four of the Copine family members (Cpne 4, 5, 6, and 9) show progressive increase in expression during postnatal development, coincident with extensive dendritic arbor growth, axonal arbor maturation and synapse formation in both retinal circuits and projection territories of RGCs. These results suggest that Copines could be implicated in Ca2+ dependent morphogenetic processes associated with the maturation of retinal synaptic architecture.
4.1. Cpne5, 6 and 9 have a broad expression in the GCL and INL
Data generated by RNA sequencing identified Cpne4, 5, 6, 8 and 9 as potential effectors mediating Brn3 effects on RGC differentiation. Cpne1, 2 and 7 appear highly enriched in brain samples, while Cpne3 seems to be ubiquitously expressed. However, these data represent two developmental snapshots, at E15 and P3. With the exception of Cpne5, our candidate Copines (4, 6, 8 and 9) are overwhelmingly induced postnatally (P3), when RGC dendritic arbors develop and synaptic connections are made in the retina and retinorecipient areas, and have only minimal expression in embryonic samples (E15) an active period for RGC genesis and axon guidance. In order to explore the expression dynamics of retina-specific Copines we focused our ISH survey on postnatal ages and the adult. Overall we find that RNA expression increases during postnatal development and peaks at P14 for the Copines studied indicating a higher requirement soon after eye opening. This is the first extensive survey of Copine expression in the retina, and only limited data is available for other Copines throughout the CNS. Cpne5 is expressed in various brain areas in mouse embryos. (Ding et al., 2008), whereas Cpne6 has been shown to express in post-natal mouse brains, and plays a functional role in the hipocampus (Nakayama, Yaoi, & Kuwajima, 1999; Nakayama et al., 1998).
Cpne9 was previously reported to be expressed in a subtype of bipolar cells in the adult mouse retina by single cell RNASeq and in-situ hybridisation (Shekhar et al., 2016). However, Figures 5B and 5C in Shekhar et al. also show intense staining in the GCL. The ISH pattern we observe shows intense staining in the GCL and throughout the INL, but some particularly intensely labelled cell bodies are visible at the outer limit of the future INL beginning at P7. It is therefore possible that Shekhar et al picked up the highest expression level observed in the bipolars and RGCs and failed to detect the less strong levels observed in the rest of the INL.
Cpne5, 6 and 9 are also expressed at the protein level in all GCL cells, in both Brn3b WT and KO retinas. The ratios of Cpne5 and Cpne6 RGCs (as labeled by Brn3a) are drastically reduced, but all remaining cells still express Cpne5, 6 and 9. This shift could be explained by the massive RGC depletion characteristic of the Brn3b loss of function phenotype, but some of the Cpne+Brn3a− cells in the GCL (besides the displaced amacrine cells) could still represent RGCs that have lost Brn3a expression. The late appearance and broad expression of Cpne5, 6 and 9 in the retina allow for potential functional redundancy. Cpne1, which is expressed throughout the embryonic brain, has early, essential functions during neural stem cell differentiation (Ding et al., 2008; Kim et al., 2018). In contrast, Cpne6, originally known as N-copine, is specifically expressed in the hippocampus and the olfactory bulbs post-natally and has recently been shown to be required for late, mature functions such as long term potentiation (LTP) in the hippocampus (Burk et al., 2018; Reinhard et al., 2016). Although Copines have a high sequence homology, they differ in their sensitivities to intracellular Ca2+ concentration (Ilacqua et al., 2018; P. V. Perestenko et al., 2010). This might lead to nuanced functional differences, despite cellular overlap of expression.
We note that our ISH probe failed to detect Cpne8 (data not shown), despite the high levels predicted by RNASeq. This might be because of low binding efficiency of the Cpne8 probe, or some contamination in the RNASeq sample. Unigene data, based on EST library enrichment shows some enrichment for Cpne8 in the eye (https://www.ncbi.nlm.nih.gov/UniGene/, Mm.290991), while the curated collection of microarray data collected by Genevestigator shows enrichment in the optic nerve head (optic disk, https://genevisible.com/tissues/MM/Gene%20Symbol/Cpne8).
While expression profiling by Deep Sequencing is a powerful discovery tool, the huge PCR amplification involved at several stages in the process of library generation and probe detection can induce a good number of false positives. This is a well-known fact in the field, and in fact, at least for our dataset, both Sajgo et al., 2017 and Muzyka et al., 2018 describe In Situ Hybridization validation rates of about 60 % when compared to RNASeq results. This not uncommon for such comparisons, making the point that careful validation work is required when drawing conclusions from Deep Sequencing data. In this study, out of the five Copines analyzed, four had fairly consistent results between ISH and RNASeq, which is in keeps with our previous experience. One should emphasize that each detection technique has its drawbacks. For instance the amplification artefact together with even small contaminations from other sources will increase the likelihood of false positives in Deep Sequencing, while ISH could be affected by probe peculiarities, and the relative abundance of the messages in subpopulations of cells investigated by RNASeq. Whereas for the in situ hybridization specific RNA probes were generated against the 3’UTR of the various Copines, for the RNA sequencing PCR amplification was done using random primers from the total RNA extracted from dissociated RGCs. We find that, typically ISH is less sensitive than RNASeq, but again this may be a case by case situation. While RNA sequencing is a powerful screening method for the detection of possible genes being expressed in the tissue, one needs to validate the actual expression using methods such as in-situ hybridization.
In general, antibody staining data are in good agreement with the ISH results. One exception is the pan Cpne 5/8/9 antibody which recognizes Cpne9, Cpne5 and to a lesser degree Cpne8 on western blots for bacterially expressed and affinity purified proteins. In retinal stains this pan Cpne 5/8/9 antibody did not detect any cells in the INL, even though there was an intense Cpne9 RNA expression in the INL by ISH. This could be assigned to low titer, poor specificity or increased Cpne 5, 8 and 9 expression in the GCL compared to the INL.
4.2. Cpne4 selectively labels RGCs in the GCL and is regulated by Brn3b
Among the different Copines studied here, Cpne4 is an exclusive RGC marker for the GCL layer and is regulated by Brn3b. It is also expressed by one INL Amacrine cell type.
To study Cpne4 dependency on Brn3b, we compared Cpne4 expression in Brn3bAP/WT and Brn3bAP/KO RGCs. By visualizing RGCs in which both copies of Brn3b are missing, but the Brn3b “fate” is still marked by AP expression from the Brn3b locus (Brn3bAP/KO), we can differentiate between loss of Cpne4 as a result of RGC loss or as a result of missing Brn3b transcriptional activity. Using co-labelling with Brn3a and Brn3bAP, Cpne4+ cells can be subdivided in three subpopulations: 1 = Brn3a+, 2 = Brn3b+ or 3 = Brn3a+Brn3b+, while no Cpne4 only cells were observed. In other words, all GCL Cpne4+ cells are either Brn3a and/or Brn3b positive and hence are RGCs. Loss of Brn3b results in apparent non-cell autonomous ablation of Brn3a+Cpne4+ RGCs and a cell-autonomous reduction in Brn3a+Cpne4+Brn3bAP+ RGCs, while Cpne4+Brn3bAP+ RGCs were only mildly affected. Although this result is partially explained by RGC loss in Brn3b KO retinas, it suggests a complex picture in which Cpne4 is Brn3b dependent in some RGCs but not in others. This is consistent with the pattern seen in the RNASeq data in which Cpne4 FPKM values in Brn3bAP/KO RGCs is reduced to about one third compared to Brn3bAP/WT RGCs, but not completely lost. Similar insights can be derived from the co-labelling with anti-Brn3c antibodies and Brn3bAP, in which the Brn3c+Cpne4+Brn3bAP+ and Cpne4+Brn3bAP+ populations are not affected by Brn3b loss, while the Brn3c+Cpne4+ and Cpne4+ populations are drastically reduced, in an apparent non-cell autonomous fashion. Similarly, using the pan-RGC marker RBPMS (marks all RGCs), we see not only a decline in AP+Cpne4+RBPMS+ cells in the KO, there is a significant reduction in the APCpne4+RBPMS+ cells, revealing a non-cell autonomous regulation of Cpne4 by Brn3b. However, when invoking non-cell autonomous effects, one has to remember that Brn3b is expressed earlier than Brn3a and Brn3c during RGC development, and that the relative numbers of Brn3b+ cells tend to decline towards the adult age. This in turn opens the possibility that Brn3b is transiently expressed and required in RGC populations that are Brn3b negative in the adult (see also Shi et al., 2013). Be it as it may, Cpne4 expression is under Brn3b control in some but not all RGCs.
Both Cpne4 antibodies detected a sparse amacrine cell population (TH−, nNOS−, VGluT3−, data not shown) in the INL, which was not detected by the ISH. The Cpne4 expressing amacrine cell population in the INL was not affected by removing Brn3b suggesting a different transcriptional control of Cpne4 in the amacrine cells. The morphology and the dendritic lamination suggest it might be one of the wide-field amacrine cells. However, we did not do any experiments to confirm that.
Transcription factors have been known to regulate the dendrite and axon refinements both directly and indirectly through specific effector molecules. Recently, Tbr1, a transcription factor in the retina has been shown to regulate the dendritic stratification of certain Off RGCs and On-Off RGCs into the Off sub-lamina of the IPL. At least in one sub-type of these RGCs- J-RGCs, Tbr1 mediated regulation of cell adehesion molecules Cdh8 and Sorcs3 in these cells (J. Liu et al., 2018). Brn3a similarly has been shown to regulate several types of molecules including cell adhesion molecules, cytoskeletal proteins and others (Muzyka et al., 2018), some of which could potentially be important for specific morphologies of the Brn3a positive RGCs. If Brn3b regulated Cpne4 can play a role in determing the morphologies of the retinal ganglion cells during development remains to be studied.
4.3. Copine sub-cellular localization and implications for cell morphology
Copines were identified based on their ability to bind to cell membranes in a Ca2+ dependent manner, via their tandem C2 domains. This in turn could potentially recruit other proteins to the membrane, via the vWA domain, in a manner analogous to that seen in Doc2 and Rabphilin, or assist vesicle trafficking events as seen in the case of Synaptotagmins or Munc13 (reviewed in (Nalefski & Falke, 1996; Pinheiro, Houy, & Sørensen, 2016; Rizo & Südhof, 1998). Many C2 domain containing proteins are involved in calcium-triggered exocytosis, display low Ca2+ affinities, and induce rapid fusion of vesicles to the plasma membrane. In particular, Synaptotagmins, which provide the major Ca2+ sensor for synaptic release, and consist of 13 family members, are present on both the vesicle and synaptic membranes, and show some level of co-expression in the same neuronal populations and even in the same cellular compartments. Their distinct Ca2+ sensitivities could provide differential dynamics of vesicle release in different neurons, synapses or release sites (Südhof, 2002).
Our in depth analysis of retina-specific Copines shows that at least three (Cpne5, 6 and 9) or four (Cpne4, 5, 6 and 9) family members could be expressed in the same neuron (Amacrines and RGCs respectively). It is therefore critical to understand whether they also function in the same cellular compartments or segregated in different organelles. In HEK293 cells, Cpne1, 2, 3, 6 and 7 are distributed diffusely in the cytoplasm and nucleus. Influx of extracellular Ca2+ and/or release of intracellular Ca2+ stores induces this subset of Copines to translocate to the plasma and/or nuclear membrane (P. V. Perestenko et al., 2010). In addition, Ca2+ influx results in increased association of Cpne6 to clathrin-coated endosomes in HEK293 cells (P. Perestenko et al., 2015).
Our HEK293 experiments show that Cpne4 and Cpne6 have indeed both cytoplasmic and nuclear localization, but that Cpne4 tends to be more diffusely expressed, while Cpne6 exhibits punctate cytoplasmic patterns. Cpne5, 6, 8 and 9 and not Cpne4 labelled the plasma membrane just inside the meGFP expression. Assuming that the intracellular Ca2+ concentration was comparable in the imaged cells, this could indicate a basic level of plasma membrane binding of these Copines at normal physiological levels of Ca2+. This indicates that Cpne4 might have a different sensitivity to Ca2+ and hence plasma membrane binding as compared to Cpne5, 6, 8 and 9. In addition, Cpne4 staining revealed unstained vacuole-like structures in the cytosol and relatively higher levels of nuclear expression. Cpne6 had a more punctate expression in the cytosol similar to that previously observed by P. V. Perestenko et al. (2010). The significance of these findings is still to be explored but they might be suggestive of functional differences between the Copines.
In hippocampal neurons stimulated with NMDA, Cpne6 is localized to plasma membrane and dendritic spines, associates with Rac1, and mediates cytoskeletal changes and spine head plasticity in response to LTP inducing stimulation protocols. Cpne6 KO mice exhibit defects in learning and memory and lose the ability for LTP induction (Reinhard et al., 2016). However, a recent study involves Cpne6 in supression of spontaneous synaptic release and demonstrates its association with the SNARE complex (P. Liu et al., 2018). We had previously reported that overexpression of Cpne4 in HEK293 cells can lead to changes in cell morphology and extension of processes reminiscent of neurites (Sajgo et al., 2017). In this study, overexpression of Cpne4, 5, 6, 8 or 9 resulted in extension of neurite-like processes in non-neural cells, and somewhat elongated cellular shapes. Of note, this was not the case in HEK293s expressing moderate amounts of the proteins, or in controls expressing just the membrane label and a TFP-tagged PSD95 fusion protein. This could be a result of excessive recruitment of cytoskeletal elements (e.g. Rac), and may indicate roles for Copines in mediating membrane – cytoskeleton interactions.
Thus, a combination of Ca2+ sensitive C2 domains and a protein interacting vWA domain in the retina, specifically in the RGCs suggests a Brn3b dependent, Ca2+ dependent, trafficking of other proteins or vesicles during different physiological states of the RGCs or a cytoskeletal rearrangement to define the arbor morphology and/or stratification during the retinal development.
Conclusion
Previous studies have shown a variety of functions for different Copines. These are related to cytoskeletal rearrangements such as activity dependent alteration in spine morphology during long term potentiation in mouse hippocampus (Reinhard et al., 2016), myofilament stability in C.Elegans (Warner et al., 2013) and plant growth in Arabidopsis (Hua, Grisafi, Cheng, & Fink, 2001). Copines were also associated with signaling pathways and trafficking such as contractile vacuole function and cytokinesis in Dictyostelium in response to cAMP signaling (Damer et al., 2007; Ilacqua et al., 2018) and recycling TrkB receptors to promote BDNF-TrkB signaling in mouse hippocampus (Burk et al., 2018). From this study we conclude that Copines exhibit differential cellular expression in the mouse retina. Additionally, they are also different in their sub-cellular distribution. Despite of their different localizations, they produce a consistent morphological change in the transfected HEK293 cells. We are therefore interested to explore their role in regulating cell morphology, specifically in RGCs and whether they have a role in the activity dependent development of the inner retina circuit as well as synapses on the RGC axon targets in the brain.
Supplementary Material
Supplementary Figure1- Differential gene level RNA expression of Copines- CPM (Counts Per Million mapped reads) values indicate the gene level expression abundance for Copines for the same postnatal day 3 retina and RGC samples as shown in Figure 1.
Supplementary Figure2- Western blots for Cpne5 and pan Cpne 5/8/9 antibodies A, Western blot for the Cpne5 antibody shows that it labels GST-Cpne5 protein specifically and not GST-Cpne4, GST-Cpne8 or His-Cpne9. B, Western blot for the pan Cpne 5/8/9 antibody at a 1:1000 dilution (four times higher concentration as compared to that in Figure 3) shows a weak label for GST-Cpne8. The GST-Cpne5 and His-Cpne9 signals are saturated at this concentration whereas GST-Cpne4 is not labeled.
Acknowledgements
This study was funded by NIH intramural funding support to Tudor C. Badea (EY000504) and Tiansen Li (EY000490). We want to thank Nadia Parmhans for assistance with genotyping.
Footnotes
Data Availability Statement: All data are available upon request.
References
- Badea TC, Cahill H, Ecker J, Hattar S, & Nathans J (2009). Distinct roles of transcription factors brn3a and brn3b in controlling the development, morphology, and function of retinal ganglion cells. Neuron, 61(6), 852–864. doi: 10.1016/j.neuron.2009.01.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badea TC, & Nathans J (2011). Morphologies of mouse retinal ganglion cells expressing transcription factors Brn3a, Brn3b, and Brn3c: analysis of wild type and mutant cells using genetically-directed sparse labeling. Vision Res, 51(2), 269–279. doi: 10.1016/j.visres.2010.08.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnarenko SR, Jeyarasasingam G, & Chalupa LM (1995). Development and Regulation of Dendritic Stratification in Retinal Ganglion Cells by Glutamate-Mediated Afferent Activity. The Journal of Neuroscience, 15(11), 7037–7045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk K, Ramachandran B, Ahmed S, Hurtado-Zavala JI, Awasthi A, Benito E, … Dean C (2018). Regulation of Dendritic Spine Morphology in Hippocampal Neurons by Copine-6. Cereb Cortex, 28(4), 1087–1104. doi: 10.1093/cercor/bhx009 [DOI] [PubMed] [Google Scholar]
- Chevenet F, Brun C, Bañuls AL, Jacq B, & Christen R (2006). TreeDyn: towards dynamic graphics and annotations for analyses of trees. BMC Bioinformatics, 10. doi: 10.1186/1471-2105-7-439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowland JB, Carter D, Bjerregaard MD, Johnsen AH, Borregaard N, & Lollike K (2003). Tissue expression of copines and isolation of copines I and III from the cytosol of human neutrophils. Journal of Leukocyte Biology, 74(3), 379–388. doi: 10.1189/jlb.0203083 [DOI] [PubMed] [Google Scholar]
- Creutz CE, Tomsig JL, Snyder SL, Gautier MC, Skouri F, Beisson J, & Cohen J (1998). The copines, a novel class of C2 domain-containing, calcium-dependent, phospholipid-binding proteins conserved from Paramecium to humans. J Biol Chem, 273(3), 1393–1402. doi: 10.1074/jbc.273.3.1393 [DOI] [PubMed] [Google Scholar]
- Damer CK, Bayeva M, Kim PS, Ho LK, Eberhardt ES, Socec CI, … Naliboff LC (2007). Copine A is required for cytokinesis, contractile vacuole function, and development in Dictyostelium. Eukaryot Cell, 6(3), 430–442. doi: 10.1128/EC.00322-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X, Jin Y, Wu Y, Wu Y, Wu H, Xiong L, … Fan M (2008). Localization and cellular distribution of CPNE5 in embryonic mouse brain. Brain Res, 1224, 20–28. doi: 10.1016/j.brainres.2008.05.051 [DOI] [PubMed] [Google Scholar]
- Elshatory Y, Everhart D, Deng M, Xie X, Barlow RB, & Gan L (2007). Islet-1 controls the differentiation of retinal bipolar and cholinergic amacrine cells. J Neurosci, 27(46), 12707–12720. doi: 10.1523/JNEUROSCI.3951-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua J, Grisafi P, Cheng SH, & Fink GR (2001). Plant growth homeostasis is controlled by the Arabidopsis BON1 and BAP1 genes. Genes Dev, 15(17), 2263–2272. doi: 10.1101/gad.918101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilacqua AN, Price JE, Graham BN, Buccilli MJ, McKellar DR, & Damer CK (2018). Cyclic AMP signaling in Dictyostelium promotes the translocation of the copine family of calcium-binding proteins to the plasma membrane. BMC Cell Biol, 19(13). doi: 10.1186/s12860-018-0160-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TH, Sung SE, Cheal Yoo J, Park JY, Yi GS, Heo JY, … Lee DY (2018). Copine1 regulates neural stem cell functions during brain development. Biochem Biophys Res Commun, 495(1), 168–173. doi: 10.1016/j.bbrc.2017.10.167 [DOI] [PubMed] [Google Scholar]
- Liu J, Reggiani JDS, Laboulaye MA, Pandey S, Chen B, Rubenstein JLR, … Sanes JR (2018). Tbr1 instructs laminar patterning of retinal ganglion cell dendrites. Nat Neurosci, 21(5), 659–670. doi: 10.1038/s41593-018-0127-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu P, Khvotchev M, Li YC, Chanaday NL, & Kavalali ET (2018). Copine-6 Binds to SNAREs and Selectively Suppresses Spontaneous Neurotransmission. J Neurosci, 38(26), 5888–5899. doi: 10.1523/JNEUROSCI.0461-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Grishanin RN, Tolwani RJ, Renteria RC, Xu B, Reichardt LF, & Copenhagen DR (2007). Brain-derived neurotrophic factor and TrkB modulate visual experience-dependent refinement of neuronal pathways in retina. J Neurosci, 27(27), 7256–7267. doi: 10.1523/JNEUROSCI.0779-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra R, Grigoryev DN, Kumar Bera T, Pastan IH, & Lee B (2003). Cloning, molecular characterization, and expression analysis of Copine 8. Biochemical and Biophysical Research Communications, 303(3), 842–847. doi: 10.1016/s0006-291x(03)00445-5 [DOI] [PubMed] [Google Scholar]
- Masai I (2003). N-cadherin mediates retinal lamination, maintenance of forebrain compartments and patterning of retinal neurites. Development, 130(11), 2479–2494. doi: 10.1242/dev.00465 [DOI] [PubMed] [Google Scholar]
- Matsuoka RL, Chivatakarn O, Badea TC, Samuels IS, Cahill H, Katayama K, … Kolodkin AL (2011). Class 5 transmembrane semaphorins control selective Mammalian retinal lamination and function. Neuron, 71(3), 460–473. doi: 10.1016/j.neuron.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muzyka VV, Brooks M, & Badea TC (2018). Postnatal developmental dynamics of cell type specification genes in Brn3a/Pou4f1 Retinal Ganglion Cells. doi: 10.1186/s13064-018-0110-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama T, Yaoi T, & Kuwajima G (1999). Localization and subcellular distribution of N-copine in mouse brain. J Neurochem, 72(1), 373–379. doi: 10.1046/j.1471-4159.1999.0720373.x [DOI] [PubMed] [Google Scholar]
- Nakayama T, Yaoi T, Yasui M, & Kuwajima G (1998). N-copine: a novel two C2-domain-containing protein with neuronal activity-regulated expression. FEBS Letters, 428(1–2), 80–84. doi: 10.1016/S0014-5793(98)00497-9 [DOI] [PubMed] [Google Scholar]
- Nalefski EA, & Falke JJ (1996). The C2 domain calcium-binding motif: Structural and functional diversity. Protein Science, 5(12), 2375–2390. doi: 10.1002/pro.5560051201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parmhans N, Sajgo S, Niu J, Luo W, & Badea TC (2018). Characterization of retinal ganglion cell, horizontal cell, and amacrine cell types expressing the neurotrophic receptor tyrosine kinase Ret. J Comp Neurol, 526(4), 742–766. doi: 10.1002/cne.24367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perestenko P, Watanabe M, Beusnard-Bee T, Guna P, & McIlhinney J (2015). The second C2-domain of copine-2, copine-6 and copine-7 is responsible for their calcium-dependent membrane association. FEBS J, 282(19), 3722–3736. doi: 10.1111/febs.13370 [DOI] [PubMed] [Google Scholar]
- Perestenko PV, Pooler AM, Noorbakhshnia M, Gray A, Bauccio C, & Jeffrey McIlhinney RA (2010). Copines-1, −2, −3, −6 and −7 show different calcium-dependent intracellular membrane translocation and targeting. FEBS J, 277(24), 5174–5189. doi: 10.1111/j.1742-4658.2010.07935.x [DOI] [PubMed] [Google Scholar]
- Pérez de Sevilla Müller L, Solomon A, Sheets K, Hapukino H, Rodriguez AR, & Brecha NC (2017). Multiple cell types form the VIP amacrine cell population. J Comp Neurol, 527(1), 133–158. doi: 10.1002/cne.24234 [DOI] [PubMed] [Google Scholar]
- Pinheiro PS, Houy S, & Sørensen JB (2016). C2-domain containing calcium sensors in neuroendocrine secretion. Journal of Neurochemistry, 139, 943–958. doi: 10.1111/jnc.13865 [DOI] [PubMed] [Google Scholar]
- Reinhard JR, Kriz A, Galic M, Angliker N, Rajalu M, Vogt KE, & Ruegg MA (2016). The calcium sensor Copine-6 regulates spine structural plasticity and learning and memory. Nat Commun, 7, 11613. doi: 10.1038/ncomms11613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizo J, & Südhof TC (1998). C2-domains, structure and function of a universal Ca2+-binding domain. Journal of Biological Chemistry, 273(26), 15879–15882. [DOI] [PubMed] [Google Scholar]
- Rodriguez AR, de Sevilla Müller LP, & Brecha NC (2014). The RNA binding protein RBPMS is a selective marker of ganglion cells in the mammalian retina. J Comp Neurol, 522(6), 1411–1443. doi: 10.1002/cne.23521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajgo S, Ghinia MG, Brooks M, Kretschmer F, Chuang K, Hiriyanna S, … Badea TC (2017). Molecular codes for cell type specification in Brn3 retinal ganglion cells. Proc Natl Acad Sci U S A, 114(20), E3974–E3983. doi: 10.1073/pnas.1618551114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savino M, d’Apolito M, Centra M, van Beerendonk HM, Cleton-Jansen AM, Whitmore SA, … Savoia A (1999). Characterization of copine VII, a new member of the copine family, and its exclusion as a candidate in sporadic breast cancers with loss of heterozygosity at 16q24.3. Genomics, 61(2), 219–226. doi: 10.1006/geno.1999.5958 [DOI] [PubMed] [Google Scholar]
- Shekhar K, Lapan SW, Whitney IE, Tran NM, Macosko EZ, Kowalczyk M, … Sanes JR (2016). Comprehensive Classification of Retinal Bipolar Neurons by Single-Cell Transcriptomics. Cell, 166(5), 1308–1323 e1330. doi: 10.1016/j.cell.2016.07.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi M, Kumar SR, Motajo O, Kretschmer F, Mu X, & Badea TC (2013). Genetic interactions between Brn3 transcription factors in retinal ganglion cell type specification. PLoS One, 8(10), e76347. doi: 10.1371/journal.pone.0076347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof TC (2002). Synaptotagmins: why so many? J Biol Chem, 277(10), 7629–7632. doi: 10.1074/jbc.R100052200 [DOI] [PubMed] [Google Scholar]
- Tian N, & Copenhagen DR (2003). Visual Stimulation Is Required for Refinement of ON and OFF Pathways in Postnatal Retina. Neuron, 39(1), 85–96. doi: 10.1016/s0896-6273(03)00389-1 [DOI] [PubMed] [Google Scholar]
- Tomsig JL, & Creutz CE (2000). Biochemical characterization of copine: a ubiquitous Ca2+ - dependent, phospholipid-binding protein. Biochemistry, 39(51), 16163–16175. doi: 10.1021/bi0019949 [DOI] [PubMed] [Google Scholar]
- Warner A, Xiong G, Qadota H, Rogalski T, Vogl AW, Moerman DG, & Benian GM (2013). CPNA-1, a copine domain protein, is located at integrin adhesion sites and is required for myofilament stability in Caenorhabditis elegans. Mol Biol Cell, 24(5), 601–616. doi: 10.1091/mbc.E12-06-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker CA, & Hynes RO (2002). Distribution and Evolution of von Willebrand/Integrin A Domains: Widely Dispersed Domains with Roles in Cell Adhesion and Elsewhere. Mol Biol Cell, 13(10), 3369–3387. doi: 10.1091/mbc.E02-05-0259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, & Alavarez-Buylla A (2001). In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development, 128, 3759–3771. [DOI] [PubMed] [Google Scholar]
- Xiang M, Gan L, Zhou L, Klein WH, & Nathans J (1996). Targeted deletion of the mouse POU domain gene Brn-3a causes selective loss of neurons in the brainstem and trigeminal ganglion, uncoordinated limb movement, and impaired suckling. Proc Natl Acad Sci U S A, 93(21), 11950–11955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SHC, Eddy RL, … Nathans J (1995). The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. The Journal of Neuroscience, 15(7), 4762–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Peng YW, Eddy RL, Shows TB, & Nathans J (1993). Brn-3b: a POU domain gene expressed in a subset of retinal ganglion cells. Neuron, 11(4), 689–701. [DOI] [PubMed] [Google Scholar]
- Yamagata M, & Sanes JR (2008). Dscam and Sidekick proteins direct lamina-specific synaptic connections in vertebrate retina. Nature, 451(7177), 465–469. doi: 10.1038/nature06469 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen K, Sloan SA, Bennett ML, Scholze AR, O’Keeffe S, … Wu JQ (2014). An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci, 34(36), 11929–11947. doi: 10.1523/JNEUROSCI.1860-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure1- Differential gene level RNA expression of Copines- CPM (Counts Per Million mapped reads) values indicate the gene level expression abundance for Copines for the same postnatal day 3 retina and RGC samples as shown in Figure 1.
Supplementary Figure2- Western blots for Cpne5 and pan Cpne 5/8/9 antibodies A, Western blot for the Cpne5 antibody shows that it labels GST-Cpne5 protein specifically and not GST-Cpne4, GST-Cpne8 or His-Cpne9. B, Western blot for the pan Cpne 5/8/9 antibody at a 1:1000 dilution (four times higher concentration as compared to that in Figure 3) shows a weak label for GST-Cpne8. The GST-Cpne5 and His-Cpne9 signals are saturated at this concentration whereas GST-Cpne4 is not labeled.