Abstract
BACKGROUND
Non-albicans Candida prosthetic joint infections (PJIs) are rare. Optimal treatment involves a two-stage revision surgery in combination with an antifungal agent. However, no clear guidelines have been developed regarding the agent or treatment duration. Hence, a broad range of antifungal and surgical treatments have been reported so far.
AIM
To clarify treatment of non-albicans Candida PJIs.
METHODS
A literature review of all existing non-albicans Candida PJIs cases through April 2018 was conducted. Information was extracted about demographics, comorbidities, responsible species, duration and type of antifungal treatment, type of surgical treatment, time between initial arthroplasty and symptom onset, time between symptom onset and definite diagnosis, outcome of the infection and follow-up.
RESULTS
A total of 83 cases, with a mean age of 66.3 years, were located. The causative yeast isolated in most cases was C. parapsilosis (45 cases; 54.2%), followed by C. glabrata (18 cases; 21.7%). The mean Charlson comorbidity index was 4.4 ± 1.5. The mean time from arthropalsty to symptom onset was 27.2 ± 43 mo, while the mean time from symptom onset to culture-confirmed diagnosis was 7.5 ± 12.5 mo. A two stage revision arthroplasty (TSRA), when compared to one stage revision arthroplasty, had a higher success rate (96% vs 73%, P = 0.023). Fluconazole was the preferred antifungal agent (59; 71%), followed by amphotericin B (41; 49.4%).
CONCLUSION
The combination of TSRA and administration of prolonged antifungal therapy after initial resection arthroplasty is suggested on the basis of limited data.
Keywords: Fungal prosthetic joint infection, Knee arthroplasty infection, Hip arthroplasty infection, Antifungal treatment, Non-albicans Candida prosthetic joint infections
Core tip: Non-albicans Candida prosthetic joint infections (PJIs) are rare, and no clear guidelines exist regarding the treatment of these infections. The purpose of this study was to clarify, by reviewing current published cases, the treatment options of non-albicans Candida PJIs and, possibly, to improve the medical and surgical care of such cases. A literature review of all existing non-albicans Candida PJIs cases through April 2018 was conducted. The combination of two stage revision arthroplasty and administration of prolonged antifungal therapy after initial resection arthroplasty is suggested on the basis of limited data.
INTRODUCTION
Prosthetic joint infection (PJI) is a severe complication of joint surgery, involving the joint prostheses and contiguous tissue, representing a main cause of total arthroplasty failure[1]. A plethora of microorganisms have been held responsible for these infections, such as Gram-positive and negative bacteria, while fungal microorganisms are considered rare causes. Fungal PJIs occur in about 1-2% of all cases, while the most common spread type seems to be hematogenous[2].
Optimal treatment is considered the two stage revision surgery in combination with an antifungal agent. However, no clear guidelines have yet been developed regarding the agent and treatment duration. Hence, a broad range of antifungal and surgical treatments have been reported so far[3,4].
Candida spp represent the most common fungal pathogens for PJIs, with Candida albicans being the most prevalent species. However, over the last years, the incidence of non-albicans Candida PJIs has increased[4,5]. The purpose of this study was to clarify, by reviewing current published cases, the treatment options of non-albicans Candida PJIs in order to potentially improve the medical and surgical care of such cases.
MATERIALS AND METHODS
A search of PubMed and MEDLINE databases was performed to identify all existing articles reporting the management of non-albicans Candida PJIs cases through April 2018. Isolated and combined terms of “fungal prosthetic joint infection”, “fungal arthroplasty infection”, “fungal hip arthroplasty infection”, “fungal knee arthroplasty infection”, “fungal shoulder arthroplasty infection”, as well as terms including each non-albicans Candida species (e.g., “candida glabrata joint infection”, “candida parapsilosis joint infection, etc) were used. The citations in each article were reviewed to locate additional references that were not retrieved during the initial search.
The present review is limited to papers published in English, peer-reviewed journals. Furthermore, cases without information about management and treatment were excluded. The data extracted from these studies included age, gender, affected joint, responsible non-albicans Candida species, duration and type of antifungal treatment, type of surgical treatment, time between initial arthroplasty and symptom onset, time between symptom onset and definite diagnosis (culture), outcome of the infection and follow-up of each case. Charlson Comorbidity score was calculated, when possible, by two independent investigators based on the information provided from each report.
Data were recorded and analyzed using Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington). Two-sided Fisher’s exact tests were used to compare success rates between groups. Statistical analyses were carried out at the 5% level of significance.
RESULTS
Table 1 highlights the findings from the electronic search, covering a 39 year period ending in 2018. A total of 83 non-albicans Candida PJIs were identified[4,6-47]. A total of 46 cases (55.4%) were female patients, thirty-six male (43.4%), while in one case the gender was not clarified. The mean age of the study population was 66.3 years [standard deviation (SD) = 10.2]. The affected joint was the knee in 52 cases (62.6%), the hip in 29 (35%) and the shoulder in two (2.4%). Two of the knee prosthetic joint cases were bilateral. The mean Charlson comorbidity index of patients was 4.4 (SD = 1.5).
Table 1.
Non-albicans Candida prosthetic joint infection cases
| Ref. | Year | Fungus | Gender | Age | Joint | Charlson comorbidity index | Antifungal treatment | Surgical treatment | Treatment duration | Outcome | Follow-up in mo | Time from implantation to symptoms onset in mo | Time from symptoms onset to definite diagnosis by culture, in mo |
| Koutserimpas et al[6] | 2018 | C. glabrata | Female | 68 | Knee | 4 | Anidulafungin / Voriconazole | TSRA | 28 wk | Successful | - | 180 | 0.5 |
| Geng et al[7] | 2016 | C. glabrata | Male | 78 | Hip | 4 | Fluconazole / Amphotericin B / Caspofungin | Spacer implantation (failure)/RA | 26 wk | Successful | 11 | 34.8 | |
| Klatte et al[8] | 2014 | C. glabrata | Female | 81 | Hip | 4 | Flucytosine / Amphotericin. B / Fluconazole | OSRA | - | Successful | 8 | - | |
| Zhu et al[9] | 2014 | C. glabrata | Male | 44 | Hip | - | Amphotericin B / Voriconazole | No | 6 wk | Successful | 3 | - | 5 |
| Anagnostakos et al[10] | 2012 | C. glabrata | Female | 51 | Hip | 6 | Fluconazole | TSRA | 6 wk | Successful | 70 | - | - |
| Anagnostakos et al[10] | 2012 | C. glabrata | Male | 78 | Hip | 6 | Fluconazole | TSRA | 6 wk | Successful | 15 | - | - |
| Bartalesi et al[11] | 2012 | C. glabrata | Female | 60 | Hip | - | Voriconazole / Caspofungin + Amphotericin B | TSRA | 6 wk | Successful | 48 | - | - |
| Hall et al[12] | 2012 | C. glabrata | Female | 60 | Hip | 4 | Caspofungin | RA | 6 wk | - | - | < 0.5 | 0.2 |
| Dumaine et al[13] | 2008 | C. glabrata | - | 72 | Knee | 5 | Caspofungin + Flucytosine / Fluconazole + Flucytosine | Arthrodesis | 16 wk | Successful | 15 | - | - |
| Lejko-Zupanc et al[14] | 2005 | C. glabrata | Male | 74 | Hip | 5 | Amphotericin B + Fluconazole / Caspofungin | RA | → 3 wk | Successful | 36 | 72 | - |
| Fabry et al[15] | 2005 | C. glabrata | Female | 74 | Knee | 6 | Voriconazole | 2x Debridement | 32 wk | Death from unrelated causes while on therapy | 24 | 72 | - |
| Gaston et al[16] | 2004 | C. glabrata | Female | 42 | Knee | 4 | Voriconazole / Amphotericin B | Amputation (above knee) | 8 wk | Successful | 6 | 264 | - |
| Açikgöz et al[17] | 2002 | C. glabrata | Female | 70 | Knee | - | Fluconazole | Arthrodesis | - | Successful | 7.5 | 9 | 6 |
| Ramamohan et al[18] | 2001 | C. glabrata | Female | 65 | Hip | - | Amphotericin B + Flucytosine | TSRA | 7 wk | Successful | 24 | 48 | - |
| Selmon et al[19] | 1998 | C. glabrata | Female | 75 | Knee | 5 | Amphotericin B/Itraconazole + Fluconazole | OSRA | > 8 wk | Successful | 48 | 84 | - |
| Nayeri et al[20] | 1997 | C. glabrata | Female | 62 | Hip | 4 | Amphotericin B+ Flucytosine / Itraconazole + Flucytosine | OSRA | 11 wk | Successful | 22 | 60 | - |
| Darouiche et al[21] | 1989 | C. glabrata | Female | 69 | Hip | - | Amphotericin B | RA | 1 wk | Successful | - | 27 | 0.5 |
| Goodman et al[22] | 1983 | C. glabrata | Female | 69 | Hip | 3 | - | RA | - | Successful | 12 | 27 | - |
| Geng et al[7] | 2016 | C. papapsilosis | Male | 67 | Knee | 3 | Fluconazole | TSRA | 10 wk | Successful | 48 | 3 | - |
| Wang et al[23] | 2015 | C. parapsilosis | Female | 67 | Knee | 4 | Fluconazole | TSRA | 10 wk | Successful | 27 | - | - |
| Wang et al[23] | 2015 | C. parapsilosis | Male | 74 | Knee | 5 | Fluconazole | TSRA | 8 wk | Successful | 30 | - | - |
| Wang et al[23] | 2015 | C. pasapsilosis | Female | 71 | Knee | 4 | Fluconazole | TSRA | 6 wk | Successful | 62 | - | - |
| Klatte et al[8] | 2014 | C. parapsilosis | Male | 69 | Knee | 8 | Flucytosine+ Amphotericin. B | OSRAx3 (Failure the first 2 times) | - | Successful | 19 | 1 | - |
| Klatte et al[8] | 2014 | C. parapsilosis | Female | 82 | Knee | 4 | Flucytosine+ Amphotericin. B | OSRA | - | Successful | 2 | - | |
| Klatte et al[8] | 2014 | C. parapsilosis | Male | 46 | Knee | 1 | Flucytosine+ Amphotericin. B | OSRA | - | Successful | 6 | - | |
| Ueng et al[24] | 2013 | C. parapsilosis | Male | 62 | Knee | 4 | Fluconazole | TSRA | - | Successful | ≥ 24 | 4.5 | 1.5 |
| Ueng et al[24] | 2013 | C. parapsilosis | Male | 77 | Knee | - | Fluconazole | TSRA | - | Successful | ≥ 24 | 4 | 3 |
| Ueng et al[24] | 2013 | C. parapsilosis | Male | 66 | Hip | - | Fluconazole | TSRA | - | Successful | ≥ 24 | 2 | 1 |
| Ueng et al[24] | 2013 | C. parapsilosis | Male | 76 | Knee | 8 | Fluconazole | TSRA | - | Successful | ≥ 24 | 33 | 2 |
| Ueng et al[24] | 2013 | C. parapsilosis | Male | 36 | Knee | - | Fluconazole | TSRA | - | Successful | ≥ 24 | 74 | 2 |
| Ueng et al[24] | 2013 | C. parapsilosis | Male | 66 | Hip | 4 | Fluconazole | RA | - | - | - | 2 | 2 |
| Kuiper et al[25] | 2013 | C. parapsilosis | Male | 58 | Hip | - | None | RA/refused further treatment | - | Refused further treatment | 8 | - | - |
| Chiu et al[26] | 2013 | C. parapsilosis | Male | 71 | Hip | 5 | Fluconazole | RA | 40 wk | Successful | 24 | 48 | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 71 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 46 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 76 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 56 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 76 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 67 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 72 | Knee | - | Fluconazole, | TSRA | ≥ 6 wk | Successful | 73 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female1 | 611 | Bilateral Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 65 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female1 | 611 | Bilateral Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 46 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 67 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 65 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 68 | Knee | - | Fluconazole, | TSRA | ≥ 6 wk | Successful | 69 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 68 | Knee | - | Fluconazole, | TSRA | ≥ 6 wk | Successful | 42 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 67 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 49 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 60 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 41 | - | - |
| Hwang et al[4] | 2012 | C. parapsilosis | Female | 73 | Knee | - | Amphotericin B / Fluconazole | TSRA | ≥ 6 wk | Successful | 52 | - | - |
| Anagnostakos et al[10] | 2012 | C. parapsilosis | Male | 67 | Knee | - | Fluconazole | RA | 6 wk | - | - | - | - |
| Dutronc et al[27] | 2010 | C. parapsilosis | Male | 66 | Hip | 5 | Fluconazole | TSRA | 24 wk | Successful | - | 0 | - |
| Dutronc et al[27] | 2010 | C. parapsilosis | Female | 77 | Hip | 4 | Ampotericin B + fluorocytosine / Fluconazole | RA | 38 wk | Successful | - | 5 | - |
| Antony et al[28] | 2008 | C. parapsilosis | Female | 67 | Shoulder | 3 | Voriconazole / Fluconazole | TSRA | - | Successful | 6 | - | - |
| Antony et al[28] | 2008 | C. parapsilosis | Female | 67 | Hip | 3 | Fluconazole | TSRA | - | Successful | - | - | - |
| Yang et al[29] | 2001 | C. parapsilosis | Female | 68 | Knee | 3 | Fluconazole | TSRA | 10 wk | Successful | 48 | 0 | 16 |
| Bruce et al[30] | 2001 | C. parapsilosis | Female | 51 | Hip | - | Fluconazole | TSRA | - | Successful | 84 | 36 | 36 |
| Brooks[31] | 1998 | C. parapsilosis | Male | 64 | Knee | 5 | Amphotericin B/Fluconazole | Debridement | 28 wk | Successful | 24 | 15 | 4 |
| Wada et al[32] | 1998 | C. parapsilosis | Male | 77 | Knee | 4 | Fluconazole | Debridement | 28 wk | Successful | 36 | 0.5 | 0.5 |
| Cushing et al[33] | 1997 | C. parapsilosis | Female | 73 | Knee | - | Fluconazole | No | > 24 wk | Successful | 12 | 1 | - |
| Fukasawa et al[34] | 1997 | C. parapsilosis | Female | 80 | Knee | - | Fluconazole | Debridement | 53 wk | Successful | 24 | 2 | |
| White et al[35] | 1995 | C. parapsilosis | Female | 64 | Knee | - | Fluconazole/Ampotericin B / Itraconazole | RA | 20 wk | Successful | - | 0 | 9 |
| Tunkel et al[36] | 1993 | C. parapsilosis | Male | 37 | Knee | 6 | Amphotericin B / Ketoconazole / Fluconazole | TSRA (Failure)/Amputation (Successful) | - | Successful | 7 | 4 | 0 |
| Paul et al[37] | 1992 | C. pasapsilosis | Male | 63 | Knee | - | Amphotericin + fluorocytosine / ketoconazole | Arthrodesis | 9 wk | Successful | 24 | 3 | 4 |
| Lim et al[38] | 1986 | C. parapsilosis | Male | 35 | Knee | - | Fluorocytosine | Arthrodesis | - | - | - | 0 | 6 |
| Younkin et al[39] | 1984 | C. parapsilosis | Female | 75 | Hip | - | Fluorocytosine+ Amphotericin B | TSRA | 6 wk | Successful | 24 | 0 | 60 |
| Lichtman[40] | 1983 | C. parapsilosis | Male | 59 | Shoulder | - | Amphotericin B / ketoconazole | RA | Indefinite (> 58 d) | -- | - | 20 | 9 |
| MacGregor et al[41] | 1979 | C. parapsilosis | Male | 64 | Knee | - | Amphotericin B + Fluorocytosine | Non-surgical (failure)/ then RA | 21 wk | Successful | 12 | 27 | 5 |
| Sebastian et al[42] | 2017 | C. tropicalis | Male | 53 | Hip | 3 | Fluconazole | TSRA | 28 wk | Successful | NR | 24 | 0 |
| Reddy et al[43] | 2013 | C. tropicalis | Female | 62 | Knee | 3 | Fluconazole | TSRA | 30 wk | Successful | 24 | 22.5 | 1.5 |
| Ueng et al[24] | 2013 | C. tropicalis | Male | 67 | Hip | - | Fluconazole | RA | - | - | - | 15 | 1 |
| Lidder et al[44] | 2013 | C. tropicalis | Female | 76 | Hip | - | Amphotericin B | RA | 24 wk | Successful | 24 | 36 | 15 |
| Azam et al[45] | 2008 | C. tropicalis | Male | 73 | Hip | 6 | Caspofungin / Fluconazole | TSRA | ≥ 9 wk, (Caspofungin 1 wk, fluconazole ≥ 8 wk) | Successful | 12 | 108 | - |
| Wyman et al[46] | 2002 | C. tropicalis | Male | 62 | Knee | Fluconazole / Amphotericin B | TSRA | 18 wk | Successful | 36 | 0.25 | 6 | |
| Darouiche et al[21] | 1989 | C. tropicalis | Male | 72 | Hip | 4 | Amphotericin B / ketoconazole | Debridement (failure)/ RA | 12 wk | Successful | 36 | 0.5 | 1 |
| Lambertus et al[47] | 1988 | C. tropicalis | Male | 61 | Hip | 3 | Amphotericin B | RA | - | Successful | 24 | 6 | 6 |
| Lambertus et al[47] | 1988 | C. tropicalis | Male | 65 | Hip | - | Amphotericin B / ketoconazole | RA/ arthrodesis | >24 wk | Successful | 14 | 4 | 4 |
| 73.Goodman et al[30] | 1983 | C. tropicalis | Female | 59 | Knee | - | Amphotericin B | TSRA (failure)/ RA | 6 wk | Successful | 12 | 3 | 6 |
| Hwang et al[4] | 2012 | C. pelliculosa | Female | 67 | Knee | - | Fluconazole | TSRA | ≥ 6 wk | Successful | 56 | - | - |
| Hwang et al[4] | 2012 | C .pelliculosa | Female | 64 | Knee | - | Amphotericin B / fluconazole | TSRA | ≥ 6 wk | Successful | 35 | - | - |
| Hwang et al[4] | 2012 | C. pelliculosa | Male | 75 | Knee | - | Amphotericin B / fluconazole | TSRA | ≥ 6 wk | Successful | 34 | - | - |
| Klatte et al[8] | 2014 | C. lusitaniae | Male | 74 | Knee | 6 | Voriconazole | OSRA | - | Successful | 12 | - | |
| Hwang et al[4] | 2012 | C. lusitaniae | Female | 66 | Knee | - | Amphotericin B / fluconazole | TSRA | ≥ 6 wk | Successful | 43 | - | - |
| Hwang et al[4] | 2012 | C. famata | Female | 83 | Knee | - | Amphotericin B / fluconazole | TSRA | ≥ 6 wk | Successful | 33 | - | - |
| Anagnostakos et al[10] | 2012 | C. lipolytica | Male | 77 | Hip | 7 | Fluconazole | TSRA | 6 wk | Successful | 22 | - | - |
| Wang et al[23] | 2015 | C. utilis | Female | 56 | Knee | 2 | Fluconazole | TSRA | 6 wk | Successful | 24 | - | 5 |
| Dutronc et al[27] | 2010 | C. guillermondii | Male | 76 | Knee | 4 | Amphotericin B+ fluorocytosine / Fluconazole | None | 14 wk (2 wk AMB+5FC, then 3 mo FZ) | Failure | - | 0 | - |
| Geng et al[7] | 2016 | C. freyschussii | Female | 58 | Knee | 2 | Fluconazole / Caspofungin | TSRA | 10 wk | Successful | 55 | <0.25 |
Patient demographics, antifungal treatment, its duration, as well as infection outcome and follow-up period are presented.
Bilateral PJI in the same patient; -: Not reported or unclear. TSRA: Two stage revision arthroplasty; OSRA: One stage revision arthroplasty; RA: Resection arthroplasty.
The mean time from initial arthroplasty implantation surgery to symptom onset was 27.2 mo (SD = 43), while the mean time from symptom onset to culture-confirmed diagnosis was 7.5 mo (SD = 12.5). Regarding the causative non-albicans Candida species, the most frequently isolated one was C. parapsilosis, found in 45 cases (54.2%), followed by C. glabrata in 18 (21.7%), C. tropicalis in ten (12%), C pelliculosa in three (3.6%) and C. lusitanae in two (2.4%), while C. famata, C. lipolytica, C. utilis, C. guilliermondii and C. freyschussii had caused one case each (1.2%) (Figure 1).
Figure 1.
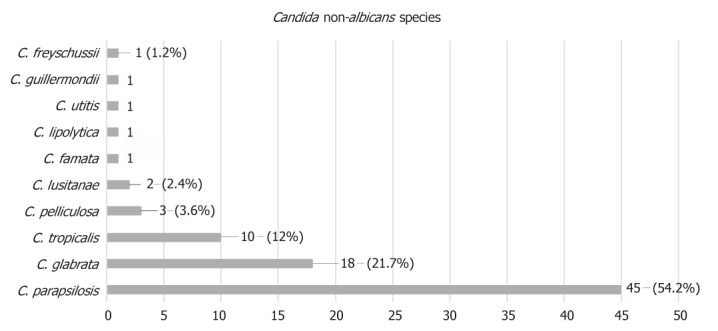
Prosthetic joint infection causative non-albicans Candida species.
Regarding surgical treatment, in the majority of the described cases (44 cases; 53%) a two stage revision arthroplasty (TSRA) was performed, followed by resection arthroplasty (RA) (18 cases; 22%), one stage revision arthroplasty (OSRA) (eight cases; 9.6%), arthrodesis (five cases; 6%), debridement (three cases; 3.6%) and amputation (two cases; 2.4%). In three cases (3.6%), there was no surgical treatment (Figure 2). TSRA exhibited a success rate of 96%, RA a rate of ≥ 61% [in 7 cases information about the infection outcome was not provided (studies 8, 31, 32, 46, 60, 62, 66 in Table 1)], OSRA a rate of 73%, debridement a rate of 75%, amputation a rate of 100%, while no surgical treatment has shown a success rate of 60%. TSRA when compared to OSRA had a higher success rate (96% vs 73%; P-value = 0.023).
Figure 2.
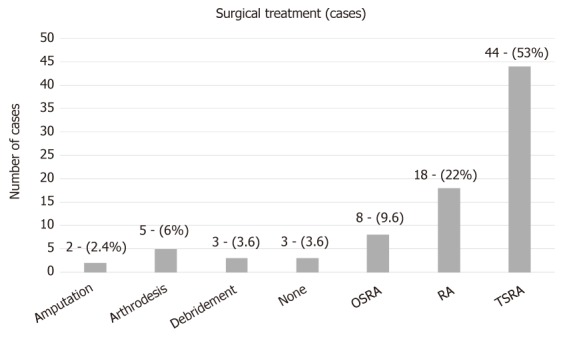
Surgical treatment for non-albicans Candida prosthetic joint infection. TSRA: Two stage revision arthroplasty; OSRA: One stage revision arthroplasty; RA: Resection arthroplasty.
Regarding the preferred antifungal agent, 38 cases (45.8%) were treated with a single drug, 29 (34.9%) with two, 12 (14.5%) with more than two, while one case did not receive antifungal treatment [(1.2%); case 17 in Table 1; the antifungal agent was not reported in case 32].
Fluconazole was used in most cases [59; (71%), in 31 of them (52.5%) as monotherapy], followed by amphotericin B [41; (49.4%), in 4 (9.8%) as monotherapy], flucytosine [13; (15.7%), in 1 (7.7%) as monotherapy], caspofungin [7; (8.4%), in 1 (14.3%) as monotherapy], voriconazole [7; (8.4%), in 2 (28.6%) as monotherapy], ketoconazole [5; (6%), none as monotherapy], itraconazole [3; (3.6%), none as monotherapy] and anidulafungin [l; (1.2%), not as monotherapy]. The final outcome was successful in 74 cases (89.2%).
The majority of patients with C. parapsilosis PJIs were treated with fluconazole [30 cases; (66.7%), in 22 (73.3%) as monotherapy], followed by amphotericin B [19; (42.2%), none as monotherapy], flucytosine [8; (17.8%), in 1 (12.5%) as monotherapy], ketoconazole [3; (6.7%), none as monotherapy], while voriconazole and itraconazole were used in 1 case each (none as monotherapy). Outcome was successful in 40 cases (88.9%).
The majority of cases with C. glabrata PJIs were treated with amphotericin B [8; (44.4%), in 1 (12.5%) as monotherapy], followed by fluconazole [7; (38.9%), in 3 (42.9%) as monotherapy]. Caspofungin and voriconazole were used in 5 cases (27.8%) each, in 1 (20%) case each as monotherapy. Furthermore, three (16.7%) patients received flucytosine (none as monotherapy), two (11.1%) itraconazole (none as monotherapy) and one (5.6%) anidulafungin (not as monotherapy). Outcome was successful in 17 cases (94.4%), while one patient (case number 11 in Table 1) passed away from unrelated causes.
The majority of C. tropicalis PJIs were treated with amphotericin B [6; (60%), in 3 (50%) as monotherapy], followed by fluconazole [5; (50%), in 3 (60%) as monotherapy], ketoconazole [2; (20%), none as monotherapy] and caspofungin [1; (10%), not as monotherapy]. Outcome was successful in 9 cases (90%; case number 66 in Table 1, treated with fluconazole as monotherapy, did not provide information about outcome).
All patients suffering from C. pelliculosa PJI received fluconazole [3 cases; (100%), 1 (33.3%) as monotherapy], while 2 of them (66.7%) received amphotericin B (none as monotherapy). All patients (100%) were treated successfully. Both patients suffering from C.lusitaniae were treated successfully. Voriconazole was used as monotherapy in 1 case (50%), while amphotericin B and fluconazole were used in the other.
One patient suffering from C. famata was successfully treated with amphotericin B and fluconazole, while fluconazole as monotherapy was successfully used for the treatment of each case of C. lipolytica and C. utilis PJI. One case of C. freyschussii was successfully treated with fluconazole and caspofungin. A case of C. guilliermondii infection received amphotericin B, fluorocytosine and fluconazole, however, the result was failure. The mean antifungal treatment duration was 12.8 wk (SD = 10.9), while the mean follow-up of these cases was 33.3 mo (SD = 19.6).
DISCUSSION
Fungal invasive infections, such as PJIs, due to Candida species have been acknowledged as a major cause of morbidity and mortality. These infections have been associated with progress in medical modalities, and is some cases have been considered iatrogenic[3,4]. Candida PJIs are relative rare, since only case reports or small case series have been reported so far. Candida albicans is the most prevalent species. However, the incidence of invasive candidiasis and PJIs due to non-albicans Candida is increasing[5,48]. Optimal treatment of these infections remains unclear, since no certain guidelines exist for the antifungal, as well as for the surgical treatments[48]. It is, therefore, of paramount importance to report such cases and to obtain a better understanding of treatment options and outcomes of these infections. The present study is an effort to review, in a systematic way, the non-albicans Candida PJI cases described in the literature. The study focuses on the preferred antifungal agent, the optimal surgical treatment, and the duration of therapy.
The electronic search has revealed a total of 83 patients with non-albicans Candida PJI. Their mean age was 66.3 years. Although Candida PJI is still considered rare, its incidence is expected to rise, due to the increasing number of joint arthroplasty surgeries performed worldwide[2,48].
Several risk factors have been identified for invasive candidiasis, such as immunosuppression, long-term antimicrobial use and systemic disease[3,4,5,48]. Such a patient profile has been illustrated by the relatively high mean Charlson comorbidity index of the present study’s population, found to be 4.4.
Candida PJI is most commonly considered of hematogenous origin[2,6,48]. The mean time between initial arthroplasty surgery and symptomatology onset in the study population was 27.2 mo, while it ranged from immediately after surgery to 264 mo. It is of note that in 13 cases [15.7%; cases 8, 23, 47, 51, 54, 55, 57, 60, 61, 69, 70, 82, 83 (Table 1)] this time was found to be less than 1 mo. Therefore, in these cases the spread should be considered perioperative.
It is also of interest that the mean time between symptomatology onset and definite (culture-based) diagnosis was 7.5 mo, ranging from immediately after onset to 60 mo. This could be attributed to the fact that the main symptoms of Candida PJI are non-specific, which mainly include pain and swelling[1-5,48]. The symptomatology onset may be mild, insidious and slowly progressive. Therefore, diagnosis may be delayed due to low suspicion index.
The most frequently isolated non-albicans Candida spp was C. parapsilosis, found in 45 cases (54.2%), followed by C. glabrata in 18 ( 21.7%), C. tropicalis in 10 (12%), C pelliculosa in 3 (3.6%), C. lusitanae in 2 ( 2.4%) and 1 case each (1.2%) of PJI caused by C. famata, C. lipolytica, C. utitis, C. guillermondii and C. freyschussii.
In the present study, C. parapsilosis was found to be the predominant pathogen causing PJIs, as compared to other non-albicans Candida species. C. parapsilosis prevalence has dramatically increased over the last 3 decades. Infections due to this pathogen are more frequently associated with prosthetic devices, indwelling catheters and hyperalimentation solutions. The pathogenesis of the infection depends on the expression of virulence factors, including adherence to host cells and tissues, biofilm formation and secretion of extracellular hydrolytic enzymes[5,49].
The treatment of C. parapsilosis PJIs has proven to be successful in 88.9% of the studied cases. This is probably due to the fact that echinocandines were not used for the treatment of such cases, taking into account that C. parapsilosis’ MICs are usually elevated to echinocandins, as compared to other Candida spp[50].
Treatment of C. glabrata PJIs has been successful in 94.4% of the studied cases. This is probably due to the fact that an azole compound has rarely been used for treatment and, in the limited number of cases when an azole was used, the drug has been given in combination with another antifungal, taking into account that C. glabrata is often resistant to azoles[50]. In most cases of C. tropicalis PJIs, treatment has been successful, since either single antifungal agents or combinations are known to be effective against this Candida spp.
All cases of C. pelliculosa PJIs have been treated successfully due to the use of effective agents. C. lusitaniae is intrinsically resistant to amphotericin B[50,51]. One case received this agent in combination with fluconazole, while the other one was treated with voriconazole as monotherapy. Hence, finally, the 2 cases caused by C. lusitanae were successfully treated.
The cases of C. famata, C. lypolitica and C. utilis were successfully treated with the antifungals given. It is of note that the single case of C. guillermondii, although treated with a combination of antifungals, resulted in failure. For successful treatment, it is of the utmost importance to carry out susceptibility testing to obtain accurate MIC values following Candida isolation, taking into account that different Candida species are characterized by intrinsic resistance to certain antifungal compounds. Regarding the preferred antifungal agent, fluconazole was used in most cases [59; (71%), in 31 of them (52.5%) as monotherapy], followed by amphotericin B [41; (49.4%), in 4 (9.8%) as monotherapy]. Fluconazole has been rarely associated with severe hepatotoxicity. Therefore, liver function tests should be performed regularly during prolonged fluconazole therapy[50,51]. Amphotericin B is an effective broad spectrum agent. However, it is relatively toxic and its side effects, including renal dysfunction, may restrict its long-term use, which is essential in PJI cases[6]. Echinocandines are the most recently developed anti-Candida agents. Although C. parapsilosis strains mostly exhibit high MICs, these agents can often be clinically effective due to their immunomodulatory properties and the fact that they successfully penetrate biofilms[52].
The mean duration of antifungal treatment in the study population was 12.8 wk, while it ranged from 1 to 53 wk. One case (case 32 in Table 1) did not receive any antifungal treatment. Guidelines for the treatment of osteoarticular infections from Candida spp exist. However, no clear recommendations are available for the treatment of such PJIs[6,48]. Therefore, the treatment duration is mainly based on the clinical and laboratory findings of each case.
Several options for surgical treatment have also been described. In the study population, in most cases (44 cases; 53%) a TSRA was performed, followed by RA (18 cases; 22%), OSRA (8 cases; 9.6%), arthrodesis (5 cases; 6%), debridement (3 cases; 3.6%) and amputation (2 cases; 2.4%).Three cases did not receive surgical treatment (3.6%; case 4, 55 and 82 in Table 1). RA, arthrodesis, amputation and debridement are usually considered alternative options to arthroplasty exchange. TSRA had a statistically significant higher success rate when compared to OSRA (96% vs 73%; P-value = 0.023). Therefore, it seems more proper that TSRA should be considered as the optimal surgical intervention.
The present review has shown that non-albicans Candida PJIs represent a dangerous reality. Optimal management consists of a combination of the proper medical antifungal treatment based on susceptibility testing and surgical intervention. Although there have been reports of successful treatment of such cases with OSRA and debridement only, TSRA should be strongly recommended. The combination of TSRA separated by 3–6 mo and a prolonged period of antifungal therapy is suggested on the basis of limited data. Additional issues, such as the duration of antifungal therapy after prosthesis implantation (second stage of the TSRA), as well as the role of antifungal-loaded cement spacers, need to be addressed in order to determine the optimal treatment combinations.
ARTICLE HIGHLIGHTS
Research background
Prosthetic joint infection (PJI) represents a severe complication of joint reconstruction surgery, causing total arthroplasty failure. Many pathogens have been identified in PJIs, such as Gram-positive and negative bacteria, while fungal microorganisms are considered rare causes, occurring in 1-2% of cases. Candida spp represent the most common fungal pathogens in these infections, with Candida albicans being the most prevalent species. However, the incidence of non-albicans Candida PJIs has increased over the last years. Hence, regarding non-albicans Candida PJIs, only case reports or small series have been reported so far. Optimal treatment is considered the two stage revision surgery in combination with an antifungal agent. However, no clear guidelines have yet been developed regarding the agent and treatment duration. Hence, a broad range of antifungal and surgical treatments has been reported so far. The present review article represents the first effort of evaluating the reported non-albicans Candida PJIs, aiming to clarify the treatment options of these infections and, possibly, to improve the medical and surgical care of such cases.
Research motivation
The absence of clear guidelines regarding fungal PJIs represents a primary issue in managing these infections in clinical practice. A broad range of antifungal and surgical treatments have been reported, while treatment duration remains unclear. Furthermore, due to the limited data regarding these infections, information about patient demographics, responsible non- albicans Candida species, time between initial arthroplasty and symptom onset, time between symptom onset and definite diagnosis (culture), and outcome of the infection has not been reported in a systematic way. Hence, it is of utmost importance in the future to report such cases in order to obtain a better understanding about this devastating arthroplasty complication.
Research objectives
The main objective of this study was to clarify, by systematically reviewing current published cases in the literature, the treatment options of non-albicans Candida PJIs and, possibly, to improve the medical and surgical care of such cases. During the process of reviewing the literature, it became apparent that information about patient demographics, fungal species, time between initial arthroplasty and symptom onset, time between symptom onset and definite diagnosis (culture), as well as outcome of the infection should also be reported, due to the absence of a systematic review regarding this topic.
Research methods
A meticulous electronic search of PubMed and MEDLINE databases was performed to identify all articles reporting the management of non-albicans Candida PJIs cases through April 2018 by two independent investigators. The citations in each article were reviewed to locate additional references that were not retrieved during the initial search. The evaluated parameters were patient demographics and comorbidities, affected joints, responsible non- albicans Candida species, duration and type of antifungal treatment, type of surgical treatment, time between initial arthroplasty and symptom onset, time between symptom onset and definite diagnosis (culture), and outcome of the infection. Data were recorded and analyzed using Microsoft Excel 2010 (Microsoft Corporation, Redmond, Washington). Two-sided Fisher’s exact tests were used to compare success rates between groups. Statistical analyses were carried out at the 5% level of significance.
Research results
A total of 83 non-albicans Candida PJIs were located, with a mean age of 66.3 years (SD = 10.2). The knee was the affected joint in 52 cases (62.6%), the hip in 29 (35%) and the shoulder in 2 (2.4%). The mean time from arthroplasty to symptoms onset was found to be 27.2 mo (SD = 43), while the mean time from symptoms onset to culture-confirmed diagnosis was 7.5 mo (SD = 12.5). The most commonly isolated non-albicans Candida species was C. parapsilosis, found in 45 cases (54.2%), followed by C. glabrata in 18 (21.7%), C. tropicalis in 10 (12%), C pelliculosa in 3 (3.6%) and C. lusitanae in 2 (2.4%), while C. famata, C. lipolytica, C. utilis, C. guilliermondii and C. freyschussii had caused one case each (1.2%). A two stage revision arthroplasty (TSRA) was performed in most cases (44 cases; 53%), followed by RA (18 cases; 22%), OSRA (8 cases; 9.6%), arthrodesis (5 cases; 6%), debridement (3 cases; 3.6%) and amputation (2 cases; 2.4%), while 3 cases (3.6%) received no surgical treatment. TSRA when compared to OSRA had a higher success rate (96% vs 73%; P-value = 0.023). Fluconazole was used in most cases as antifungal treatment [59; (71%), in 31 of them (52.5%) as monotherapy], followed by amphotericin B [41; (49.4%), in 4 (9.8%) as monotherapy], flucytosine [13; (15.7%), in 1 (7.7%) as monotherapy], caspofungin [7; (8.4%), in 1 (14.3%) as monotherapy], voriconazole [7; (8.4%), in 2 (28.6%) as monotherapy], ketoconazole [5; (6%), none as monotherapy], itraconazole [3; (3.6%), none as monotherapy] and anidulafungin [l; (1.2%), none as monotherapy]. The final outcome was successful in 74 cases (89.2%). The mean antifungal treatment duration was 12.8 wk (SD = 10.9), while the mean follow-up of these cases was 33.3 mo (SD = 19.6). The present review has shown that the optimal management of non-albicans Candida consists of a combination of the proper medical antifungal treatment and surgical intervention. Although there have been reports of the successful treatment of such cases with OSRA and debridement only, TSRA should be strongly recommended. The combination of TSRA and a prolonged period of antifungal therapy based on susceptibility testing is suggested on the basis of limited data. Additional issues, such as the duration of antifungal therapy after prosthesis implantation (second stage of the TSRA) and the role of antifungal-loaded cement spacers need to be addressed in order to determine an optimal treatment combination.
Research conclusions
The present study is an effort to review, in a systematic way, the non-albicans Candida PJI cases described in the literature. The study focuses on the preferred antifungal agent, the optimal surgical treatment, and the duration of therapy. C. parapsilosis was found to be the predominant pathogen causing PJIs, as compared to other non-albicans Candida species. For successful management of non-albicans Candida PJI, susceptibility testing to obtain accurate MIC values should always be performed following the Candida isolation, considering that different Candida species are characterized by intrinsic resistance to certain antifungal compounds. The mean duration of antifungal treatment in the present review was 12.8 wk, while it ranged from 1 to 53 wk. Although, guidelines for the treatment of osteoarticular infections from Candida spp are available, no clear recommendations exist for the treatment of such PJIs. Therefore, the treatment duration is mostly based upon clinical and laboratory findings. In most cases (44 cases; 53%) a TSRA was performed, followed by RA (18 cases; 22%), OSRA (8 cases; 9.6%), arthrodesis (5 cases; 6%), debridement (3 cases; 3.6%) and amputation (2 cases; 2.4%). Three cases did not receive surgical treatment (3.6%). RA, arthrodesis, amputation and debridement are usually considered alternative options to arthroplasty exchange. TSRA when compared to OSRA had a statistically significant higher success rate (96% vs 73%; P-value= 0.023). Therefore, it seems more proper that TSRA should be considered as the optimal surgical intervention. The present review has shown that the optimal management of non-albicans Candida PJIs consists of a combination of the proper medical antifungal treatment based on susceptibility testing and a surgical intervention, while TSRA should be strongly recommended. The combination of TSRA separated by 3–6 mo, in addition to a prolonged period of antifungal therapy, is suggested.
Research perspectives
Non-albicans Candida PJIs represent a dangerous reality. The combination of TSRA separated by 3–6 mo and a prolonged period of antifungal therapy is suggested on the basis of limited data. It is of paramount importance to report the treatment of such cases, even the failed ones, in order to obtain a better understanding of these infections and to determine the optimum treatment combination.
Footnotes
Conflict-of-interest statement: The authors have no conflicts of interest to declare.
PRISMA 2009 Checklist statement: PRISMA 2009 Checklist statement is provided.
Manuscript source: Invited manuscript
Peer-review started: January 23, 2019
First decision: March 10, 2019
Article in press: April 19,2019
Specialty type: Medicine, research and experimental
Country of origin: Greece
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Li J S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
Contributor Information
Christos Koutserimpas, Department of Orthopaedics and Traumatology, “251” Hellenic Air Force General Hospital of Athens, Athens 11525, Greece. chrisku91@hotmail.com.
Stylianos G Zervakis, Department of Internal Medicine, University Hospital of Heraklion, Crete, Heraklion 71110, Greece.
Sofia Maraki, Department of Clinical Microbiology and Microbial Pathogenesis, University Hospital of Heraklion, Crete, Heraklion 71110, Greece.
Kalliopi Alpantaki, Department of Materials Science and Technology, University of Crete, Heraklion, Heraklion 71110, Greece.
Argyrios Ioannidis, Department of General Surgery, “Sismanoglion” General Hospital of Athens, Athens 15126, Greece.
Diamantis P Kofteridis, Department of Internal Medicine, University Hospital of Heraklion, Crete, Heraklion 71110, Greece.
George Samonis, Department of Internal Medicine, University Hospital of Heraklion, Crete, Heraklion 71110, Greece.
References
- 1.Pulido L, Ghanem E, Joshi A, Purtill JJ, Parvizi J. Periprosthetic joint infection: the incidence, timing, and predisposing factors. Clin Orthop Relat Res. 2008;466:1710–1715. doi: 10.1007/s11999-008-0209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schoof B, Jakobs O, Schmidl S, Klatte TO, Frommelt L, Gehrke T, Gebauer M. Fungal periprosthetic joint infection of the hip: a systematic review. Orthop Rev (Pavia) 2015;7:5748. doi: 10.4081/or.2015.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Azzam K, Parvizi J, Jungkind D, Hanssen A, Fehring T, Springer B, Bozic K, Della Valle C, Pulido L, Barrack R. Microbiological, clinical, and surgical features of fungal prosthetic joint infections: a multi-institutional experience. J Bone Joint Surg Am. 2009;91 Suppl 6:142–149. doi: 10.2106/JBJS.I.00574. [DOI] [PubMed] [Google Scholar]
- 4.Hwang BH, Yoon JY, Nam CH, Jung KA, Lee SC, Han CD, Moon SH. Fungal peri-prosthetic joint infection after primary total knee replacement. J Bone Joint Surg Br. 2012;94:656–659. doi: 10.1302/0301-620X.94B5.28125. [DOI] [PubMed] [Google Scholar]
- 5.Deorukhkar SC, Saini S, Mathew S. Virulence Factors Contributing to Pathogenicity of Candida tropicalis and Its Antifungal Susceptibility Profile. Int J Microbiol. 2014;2014:456878. doi: 10.1155/2014/456878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koutserimpas C, Samonis G, Velivassakis E, Iliopoulou-Kosmadaki S, Kontakis G, Kofteridis DP. Candida glabrata prosthetic joint infection, successfully treated with anidulafungin: A case report and review of the literature. Mycoses. 2018;61:266–269. doi: 10.1111/myc.12736. [DOI] [PubMed] [Google Scholar]
- 7.Geng L, Xu M, Yu L, Li J, Zhou Y, Wang Y, Chen J. Risk factors and the clinical and surgical features of fungal prosthetic joint infections: A retrospective analysis of eight cases. Exp Ther Med. 2016;12:991–999. doi: 10.3892/etm.2016.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Klatte TO, Kendoff D, Kamath AF, Jonen V, Rueger JM, Frommelt L, Gebauer M, Gehrke T. Single-stage revision for fungal peri-prosthetic joint infection: a single-centre experience. Bone Joint J. 2014;96-B:492–496. doi: 10.1302/0301-620X.96B4.32179. [DOI] [PubMed] [Google Scholar]
- 9.Zhu Y, Yue C, Huang Z, Pei F. Candida glabrata infection following total hip arthroplasty: A case report. Exp Ther Med. 2014;7:352–354. doi: 10.3892/etm.2013.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anagnostakos K, Kelm J, Schmitt E, Jung J. Fungal periprosthetic hip and knee joint infections clinical experience with a 2-stage treatment protocol. J Arthroplasty. 2012;27:293–298. doi: 10.1016/j.arth.2011.04.044. [DOI] [PubMed] [Google Scholar]
- 11.Bartalesi F, Fallani S, Salomoni E, Marcucci M, Meli M, Pecile P, Cassetta MI, Latella L, Bartoloni A, Novelli A. Candida glabrata prosthetic hip infection. Am J Orthop (Belle Mead NJ) 2012;41:500–505. [PubMed] [Google Scholar]
- 12.Hall RL, Frost RM, Vasukutty NL, Minhas H. Candida glabrata: an unusual fungal infection following a total hip replacement. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr-2012-006491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dumaine V, Eyrolle L, Baixench MT, Paugam A, Larousserie F, Padoin C, Tod M, Salmon D. Successful treatment of prosthetic knee Candida glabrata infection with caspofungin combined with flucytosine. Int J Antimicrob Agents. 2008;31:398–399. doi: 10.1016/j.ijantimicag.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 14.Lejko-Zupanc T, Mozina E, Vrevc F. Caspofungin as treatment for Candida glabrata hip infection. Int J Antimicrob Agents. 2005;25:273–274. doi: 10.1016/j.ijantimicag.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 15.Fabry K, Verheyden F, Nelen G. Infection of a total knee prosthesis by Candida glabrata: a case report. Acta Orthop Belg. 2005;71:119–121. [PubMed] [Google Scholar]
- 16.Gaston G, Ogden J. Candida glabrata periprosthetic infection: a case report and literature review. J Arthroplasty. 2004;19:927–930. doi: 10.1016/j.arth.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Açikgöz ZC, Sayli U, Avci S, Doğruel H, Gamberzade S. An extremely uncommon infection: Candida glabrata arthritis after total knee arthroplasty. Scand J Infect Dis. 2002;34:394–396. doi: 10.1080/00365540110080232. [DOI] [PubMed] [Google Scholar]
- 18.Ramamohan N, Zeineh N, Grigoris P, Butcher I. Candida glabrata infection after total hip arthroplasty. J Infect. 2001;42:74–76. doi: 10.1053/jinf.2000.0763. [DOI] [PubMed] [Google Scholar]
- 19.Selmon GP, Slater RN, Shepperd JA, Wright EP. Successful 1-stage exchange total knee arthroplasty for fungal infection. J Arthroplasty. 1998;13:114–115. doi: 10.1016/s0883-5403(98)90086-9. [DOI] [PubMed] [Google Scholar]
- 20.Nayeri F, Cameron R, Chryssanthou E, Johansson L, Söderström C. Candida glabrata prosthesis infection following pyelonephritis and septicaemia. Scand J Infect Dis. 1997;29:635–638. doi: 10.3109/00365549709035912. [DOI] [PubMed] [Google Scholar]
- 21.Darouiche RO, Hamill RJ, Musher DM, Young EJ, Harris RL. Periprosthetic candidal infections following arthroplasty. Rev Infect Dis. 1989;11:89–96. doi: 10.1093/clinids/11.1.89. [DOI] [PubMed] [Google Scholar]
- 22.Goodman JS, Seibert DG, Reahl GE, Jr, Geckler RW. Fungal infection of prosthetic joints: a report of two cases. J Rheumatol. 1983;10:494–495. [PubMed] [Google Scholar]
- 23.Wang QJ, Shen H, Zhang XL, Jiang Y, Wang Q, Chen YS, Shao JJ. Staged reimplantation for the treatment of fungal peri-prosthetic joint infection following primary total knee arthroplasty. Orthop Traumatol Surg Res. 2015;101:151–156. doi: 10.1016/j.otsr.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 24.Ueng SW, Lee CY, Hu CC, Hsieh PH, Chang Y. What is the success of treatment of hip and knee candidal periprosthetic joint infection? Clin Orthop Relat Res. 2013;471:3002–3009. doi: 10.1007/s11999-013-3007-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuiper JW, van den Bekerom MP, van der Stappen J, Nolte PA, Colen S. 2-stage revision recommended for treatment of fungal hip and knee prosthetic joint infections. Acta Orthop. 2013;84:517–523. doi: 10.3109/17453674.2013.859422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chiu WK, Chung KY, Cheung KW, Chiu KH. Candida parapsilosis total hip arthroplasty infection: case report and literature review. J Orthop Trauma. 2013;17:33–36. [Google Scholar]
- 27.Dutronc H, Dauchy FA, Cazanave C, Rougie C, Lafarie-Castet S, Couprie B, Fabre T, Dupon M. Candida prosthetic infections: case series and literature review. Scand J Infect Dis. 2010;42:890–895. doi: 10.3109/00365548.2010.498023. [DOI] [PubMed] [Google Scholar]
- 28.Antony S, Dominguez D C, Jackson J, Misenheimer G. Evaluation and treatment of candida species in prosthetic joint infections. Infect Dis Clin Pract. 2008;16:354–359. [Google Scholar]
- 29.Yang SH, Pao JL, Hang YS. Staged reimplantation of total knee arthroplasty after Candida infection. J Arthroplasty. 2001;16:529–532. doi: 10.1054/arth.2001.21458. [DOI] [PubMed] [Google Scholar]
- 30.Bruce AS, Kerry RM, Norman P, Stockley I. Fluconazole-impregnated beads in the management of fungal infection of prosthetic joints. J Bone Joint Surg Br. 2001;83:183–184. doi: 10.1302/0301-620x.83b2.11444. [DOI] [PubMed] [Google Scholar]
- 31.Brooks DH, Pupparo F. Successful salvage of a primary total knee arthroplasty infected with Candida parapsilosis. J Arthroplasty. 1998;13:707–712. doi: 10.1016/s0883-5403(98)80017-x. [DOI] [PubMed] [Google Scholar]
- 32.Wada M, Baba H, Imura S. Prosthetic knee Candida parapsilosis infection. J Arthroplasty. 1998;13:479–482. doi: 10.1016/s0883-5403(98)90019-5. [DOI] [PubMed] [Google Scholar]
- 33.Cushing RD, Fulgenzi WR. Synovial fluid levels of fluconazole in a patient with Candida parapsilosis prosthetic joint infection who had an excellent clinical response. J Arthroplasty. 1997;12:950. doi: 10.1016/s0883-5403(97)90166-2. [DOI] [PubMed] [Google Scholar]
- 34.Fukasawa N, Shirakura K. Candida arthritis after total knee arthroplasty--a case of successful treatment without prosthesis removal. Acta Orthop Scand. 1997;68:306–307. doi: 10.3109/17453679708996709. [DOI] [PubMed] [Google Scholar]
- 35.White A, Goetz MB. Candida parapsilosis prosthetic joint infection unresponsive to treatment with fluconazole. Clin Infect Dis. 1995;20:1068–1069. doi: 10.1093/clinids/20.4.1068. [DOI] [PubMed] [Google Scholar]
- 36.Tunkel AR, Thomas CY, Wispelwey B. Candida prosthetic arthritis: report of a case treated with fluconazole and review of the literature. Am J Med. 1993;94:100–103. doi: 10.1016/0002-9343(93)90127-b. [DOI] [PubMed] [Google Scholar]
- 37.Paul J, White SH, Nicholls KM, Crook DW. Prosthetic joint infection due to Candida parapsilosis in the UK: case report and literature review. Eur J Clin Microbiol Infect Dis. 1992;11:847–849. doi: 10.1007/BF01960889. [DOI] [PubMed] [Google Scholar]
- 38.Lim EV, Stern PJ. Candida infection after implant arthroplasty. A case report. J Bone Joint Surg Am. 1986;68:143–145. [PubMed] [Google Scholar]
- 39.Younkin S, Evarts CM, Steigbigel RT. Candida parapsilosis infection of a total hip-joint replacement: successful reimplantation after treatment with amphotericin B and 5-fluorocytosine. A case report. J Bone Joint Surg Am. 1984;66:142–143. [PubMed] [Google Scholar]
- 40.Lichtman EA. Candida infection of a prosthetic shoulder joint. Skeletal Radiol. 1983;10:176–177. doi: 10.1007/BF00357775. [DOI] [PubMed] [Google Scholar]
- 41.MacGregor RR, Schimmer BM, Steinberg ME. Results of combined amphotericin B-5-fluorcytosine therapy for prosthetic knee joint infected with Candida parapsilosis. J Rheumatol. 1979;6:451–455. [PubMed] [Google Scholar]
- 42.Sebastian S, Malhotra R, Pande A, Gautam D, Xess I, Dhawan B. Staged Reimplantation of a Total Hip Prosthesis After Co-infection with Candida tropicalis and Staphylococcus haemolyticus: A Case Report. Mycopathologia. 2018;183:579–584. doi: 10.1007/s11046-017-0177-x. [DOI] [PubMed] [Google Scholar]
- 43.Reddy KJ, Shah JD, Kale RV, Reddy TJ. Fungal prosthetic joint infection after total knee arthroplasty. Indian J Orthop. 2013;47:526–529. doi: 10.4103/0019-5413.118213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lidder S, Tasleem A, Masterson S, Carrington RW. Candida tropicalis: diagnostic dilemmas for an unusual prosthetic hip infection. J R Army Med Corps. 2013;159:123–125. doi: 10.1136/jramc-2013-000053. [DOI] [PubMed] [Google Scholar]
- 45.Azam A, Singh PK, Singh VK, Siddiqui A. A Rare Case of Candida Tropicalis Infection of a Total Hip Arthroplasty: A Case Report and Review of Literature. MOJ. 2008;2:43–46. [Google Scholar]
- 46.Wyman J, McGough R, Limbird R. Fungal infection of a total knee prosthesis: successful treatment using articulating cement spacers and staged reimplantation. Orthopedics. 2002;25:1391–4; discussion 1394. doi: 10.3928/0147-7447-20021201-19. [DOI] [PubMed] [Google Scholar]
- 47.Lambertus M, Thordarson D, Goetz MB. Fungal prosthetic arthritis: presentation of two cases and review of the literature. Rev Infect Dis. 1988;10:1038–1043. doi: 10.1093/clinids/10.5.1038. [DOI] [PubMed] [Google Scholar]
- 48.Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, Reboli AC, Schuster MG, Vazquez JA, Walsh TJ, Zaoutis TE, Sobel JD. Executive Summary: Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;62:409–417. doi: 10.1093/cid/civ1194. [DOI] [PubMed] [Google Scholar]
- 49.Trofa D, Gácser A, Nosanchuk JD. Candida parapsilosis, an emerging fungal pathogen. Clin Microbiol Rev. 2008;21:606–625. doi: 10.1128/CMR.00013-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sanguinetti M, Posteraro B, Lass-Flörl C. Antifungal drug resistance among Candida species: mechanisms and clinical impact. Mycoses. 2015;58 Suppl 2:2–13. doi: 10.1111/myc.12330. [DOI] [PubMed] [Google Scholar]
- 51.Pasternak B, Wintzell V, Furu K, Engeland A, Neovius M, Stephansson O. Oral Fluconazole in Pregnancy and Risk of Stillbirth and Neonatal Death. JAMA. 2018;319:2333–2335. doi: 10.1001/jama.2018.6237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dimopoulou D, Hamilos G, Tzardi M, Lewis RE, Samonis G, Kontoyiannis DP. Anidulafungin versus caspofungin in a mouse model of candidiasis caused by anidulafungin-susceptible Candida parapsilosis isolates with different degrees of caspofungin susceptibility. Antimicrob Agents Chemother. 2014;58:229–236. doi: 10.1128/AAC.01025-13. [DOI] [PMC free article] [PubMed] [Google Scholar]


