Abstract
In this article, we review signal transduction pathways through which acupuncture treats nervous system diseases. We electronically searched the databases, including PubMed, MEDLINE, clinical Key, the Cochrane Library, and the China National Knowledge Infrastructure from their inception to December 2018 using the following MeSH headings and keywords alone or in varied combination: acupuncture, molecular, signal transduction, genetic, cerebral ischemic injury, cerebral hemorrhagic injury, stroke, epilepsy, seizure, depression, Alzheimer's disease, dementia, vascular dementia, and Parkinson's disease. Acupuncture treats nervous system diseases by increasing the brain-derived neurotrophic factor level and involves multiple signal pathways, including p38 MAPKs, Raf/MAPK/ERK 1/2, TLR4/ERK, PI3K/AKT, AC/cAMP/PKA, ASK1-JNK/p38, and downstream CREB, JNK, m-TOR, NF-κB, and Bcl-2/Bax balance. Acupuncture affects synaptic plasticity, causes an increase in neurotrophic factors, and results in neuroprotection, cell proliferation, antiapoptosis, antioxidant activity, anti-inflammation, and maintenance of the blood-brain barrier.
1. Introduction
Acupuncture is a form of therapy practiced for more than 3000 years in Asia. Medical doctors practice acupuncture under the guidance of meridian theory to achieve “de qi” status [1]. To perform acupuncture, doctors use thin and sterile metal needles to penetrate specific stimulation points termed acupoints. Both manual and electroacupuncture (EA) are used in medical practice. Many studies have reported the benefits of acupuncture for treating diseases such as stroke, musculoskeletal disorders, chronic urticaria, irritable bowel syndrome, overactive bladder, cancer-related fatigue, and pain in humans [2–6]. Furthermore, few adverse effects have been observed when acupuncture is performed correctly, even in children and pregnant women [7, 8]. The widely known mechanism of acupuncture is that it results in the secretion of endorphins that exert an analgesic effect. With advances in understanding, more mechanisms of acupuncture have been determined, including the local segmental effect, somatoautonomic reflex, immune system regulation, neurotransmitter modulation, the neuroendocrine effect, and the functional connectivity neural network [9–11].
Nowadays, signal transduction has been applied for explaining acupuncture mechanisms. The signal transduction pathway of acupuncture has been mentioned with respect to many diseases, including neurological [12], cardiovascular [13], metabolic [14], and gynecological [15] diseases. Among the aforementioned diseases, nervous system diseases are the most common complaints in daily practice. When used to treat nervous system diseases, acupuncture enhances cell proliferation and neuroblast differentiation by increasing the levels of brain-derived neurotrophic factor (BDNF) and phosphorylated cyclic AMP response element-binding (CREB) protein [16]. Acupuncture was reported to exert a neuroprotective effect on dopaminergic neurons through anti-inflammatory and neurotrophic effects [17]. Other mechanisms, including antioxidation, antiapoptosis, and improved energy metabolism in the brain, have been reported [18–20]. Although many studies on the signal transduction pathway of acupuncture have been conducted, few reviews have been published on this topic. In the present review, we discuss the involvement of the signal transduction pathway as a mechanism underlying the effects of acupuncture when used for treating nervous system diseases.
2. Method
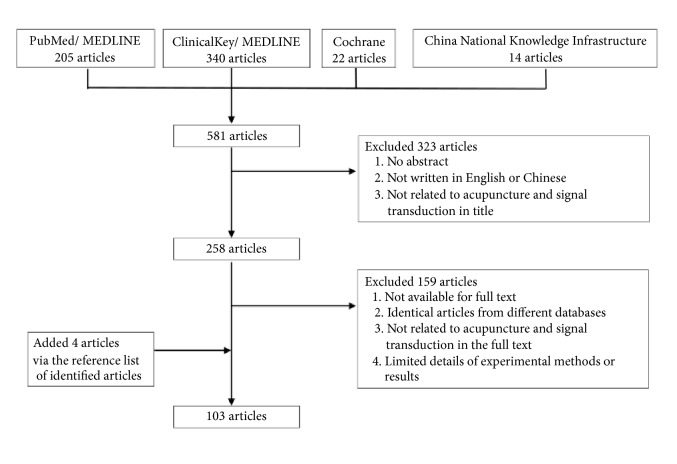
We electronically searched the databases, including PubMed, MEDLINE, clinical Key, the Cochrane Library, and the China National Knowledge Infrastructure from their inception to December 2018 using the following MeSH headings and keywords alone or in varied combination: acupuncture, molecular, signal transduction, genetic, cerebral ischemic injury, cerebral hemorrhagic injury, stroke, epilepsy, seizure, depression, Alzheimer's disease (AD), dementia, vascular dementia (VD), and Parkinson's disease (PD). In addition, we used Boolean operators (“not,” “and,” ”or”) to narrow or widen search results. All articles written in English or Chinese were manually screened, and relevant studies were identified. We included additional articles after performing a manual review of the reference lists of identified studies or review articles. Excluded articles included those with unavailable full text, those written in other languages, those not mainly related to the mechanism of the signal transduction pathway, or those with limited details of experimental methods or results. Flowchart of the search processes was as shown in Figure 1.
Figure 1.
Flow chart of the search processes. The 103 articles were summarized in Tables 1–7.
3. Cerebral Ischemic Injury
Ischemic injury of the brain, also known as cerebral infarction, is a crucial health issue in the modern world because of its associated disability and socioeconomic burden. Acupuncture has shown beneficial effects on ischemic stroke rehabilitation by exerting the antiapoptosis effect on the ischemic area, promoting neurogenesis and cell proliferation, and regulating cerebral blood flow [21, 22]. A retrospective cohort study reported that acupuncture was effective at reducing the stroke recurrence rate [23]. Ischemic stroke causes neural cell damage related to excitotoxicity, oxygen free radical injury, inflammatory status, and blood-brain barrier (BBB) damage [24]. Experimental pathways that can reverse apoptosis and improve cell proliferation and differentiation have been proposed.
Acupuncture causes an increase in the expression of neurotrophic factors, such as BDNF and glial-derived neurotrophic factor (GDNF), in the central nervous system (CNS), exerts a neuroprotective effect on hypoxic-ischemic insults, and results in neurogenesis after the reconstruction phase [25, 26]. In addition, acupuncture increased the vascular endothelial growth factor (VEGF) level in the hippocampus, promoting the proliferation and differentiation of neuronal stem cells [27]. Thus, acupuncture can be used to treat ischemic injury in the brain. Zhang et al. performed manual acupuncture on GV20 and Ex-HN 1 to increase GDNF and BDNF levels in a rat model [19]. The elevation of the BDNF level was related to the increased expression of BDNF/tyrosine receptor kinase B (TrkB) and the induction of neurogenesis [28].
The mitogen-activated protein kinase (MAPK) family includes ERK1/2, JNK, and p38 MAPK proteins. In animals, the MAPK family is triggered by growth factors, stress, or an inflammatory environment and regulates cell functions, such as proliferation, division, differentiation, survival, and apoptosis. EA can trigger the MAPK family. ERK is believed to mediate reperfusion injury by inhibiting inflammatory reactions and promoting cell proliferation and growth [29]. However, equivocal results have been reported concerning the protective effect of ERK on ischemic brain injury [30, 31]. Some studies have demonstrated that EA protects against ischemic brain injury by reducing infarct volumes and improving neurological outcomes through activation of the ERK1/2 signaling pathway [29, 32–34]. EA is reported to be effective in neuroprotection and neural cell proliferation. The chosen acupoints in EA include GV20, GV14, ST36, and LI11. The activation of the ERK pathway is combined with an increase in BDNF and p-ERK1/2 levels [34]. Some studies have demonstrated that the application of EA on LU5, LI4, ST36, and SP6 was effective in reducing neurogenic deficits and causing antiapoptosis in the brain cortex and hippocampus [35, 36].
Environmental stresses and inflammatory cytokines activate p38 MAPKs and induce apoptosis and inflammation [37]. In the acute phase of ischemic brain injury, the p38 MAPK signaling pathway induces neurotoxicity, whereas in the subacute phase, this pathway serves as a proinflammatory mediator in the neuroprotective antiapoptosis effect [38–40]. Some studies have reported that EA exerts the antiapoptosis effect on the peri-infarct cortex by modulating the ERK/JNK/p38 MAPK signaling pathway [41–44]. The chosen acupoints include GV14, GV20, GV24, GV26, LU5, LILI4, LI11, ST36, and SP6. Liu et al. reported that EA inhibits microglia-mediated neuroinflammation mediated by nuclear factor kappa-light-chain-enhancer of activated B (NF-κB) cells, p38 MAPK, and myeloid differentiation primary response 88 (MYD88), as well as simultaneously reducing cytokine tumor necrosis factor-alpha (TNF-α), interleukin-1 beta (IL-1β), and interleukin-6 (IL-6) levels [45].
The p38 MAPK pathway activates the expression of CREB protein and reduces the apoptosis of ischemic neural cells. Acupuncture on GV16, GV20, GV24, ST36, and HT7 also triggered the CREB pathway in the hippocampus and improved cognitive impairment in an animal model [46–51]. The CREB pathway is related to BDNF, p38 MAPK, and Ca2+/calmodulin-dependent protein kinase (CaMK) [46, 50, 52]. Lin et al. reported that EA exerted antioxidant and antiapoptosis effects by increasing superoxide dismutase and glutathione peroxidase levels and reducing the malondialdehyde level in the hippocampus and improved the learning and memory ability of rats [48]. A study reported that laser acupuncture on GV20 and HT7 for 14 days excited the cholinergic system and increased CREB, BDNF, and B-cell lymphoma 2 (Bcl-2) levels, thereby improving cognitive impairment in rats [51].
Being a cell cycle initiator, PI3K/AKT pathways are essential for cell survival [53]. However, interactions between transactivation of Raf/MAPK/ERK1/2 and PI3K/AKT systems were noted during ischemia and reperfusion phases. During ischemia, Akt reduces Raf/MAPK/ERK1/2 activity through phosphorylation of Raf-1. During reperfusion, abrupt reactive oxygen species (ROS) increases the phosphatase and tensin homolog and reactivates Raf/MAPK/ERK1/2 signaling [54]. For the modulation of the PI3K pathway, some studies have reported that EA on GV12, GV20, GV24, GV26, KI1, LI11, and ST36 activates the PI3K/AKT pathway and exerts antiapoptosis and neuroprotective effects [12, 55–60]. The effect of EA on the PI3K pathway can activate the downstream mTOR complex 1–UNC-51-like kinase 1 complex–Beclin-1 pathway, reduce caspase-3, caspase-8, and caspase-9 levels, and inhibit the autophagy process [61, 62]. EA also reduces nitric oxide (NO), neuronal NO synthase (nNOS), and inducible NO synthase (iNOS) levels by activating the PI3K pathway [58]. Xie et al. demonstrated that EA improved neurological deficit scores and increased the expression of p-AKT protein and bone marrow CD34+ endothelial progenitor cells in rats [63].
Because of the balance between Raf/MAPK/ERK1/2 and PI3K/AKT systems, some studies have included the pretreatment protocol [64, 65]. EA pretreatment in a rat model reduced the expression of p-Akt protein and prevented the downregulation of tight junction proteins, namely, claudin-5 and occludin, attenuating BBB disruption and brain edema [65].
NF-κB is another protein complex related to cell survival. Some studies have demonstrated that EA regulates the NF-κB-mediated apoptosis pathway and provides neuroprotection [66, 67].
Acupuncture improved neurogenic defects and cognitive impairment in a cerebral ischemic/reperfusion animal model. In summary, acupuncture not only increases the levels of neurotrophic factors but also modulates signaling pathways, such as Raf/MAPK/ERK1/2 and PI3K/AKT and downstream CREB and NF-κB. Therefore, acupuncture results in cell proliferation, antiapoptosis, neuroprotection, and BBB maintenance. The most frequently chosen acupoints include GV20, GV14, and ST36. The mechanisms and main results of identified articles are summarized in Table 1.
Table 1.
Signal transduction pathways of acupuncture in treating cerebral ischemic injury.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| Male, SD rats, MCAO | brain | GV20 | EA, 3mA, 2/20Hz | 30min, QOD for 14 days | increase expression of BDNF/TrkB | elevation of BDNF neuron proliferation |
Kim MW, et al. 2012[28] |
|
| |||||||
| Male, postnatal SD rats, MCAO | hippocampus | GV20, GV14 | EA, 2Hz | 20min, QD for 10 days | increase VEGF and BDNF levels | proliferation and differentiation of neuronal stem cells | Kim YR, et al. 2014[27] |
|
| |||||||
| Male, postnatal SD rats, CCAO | hippocampus | GV20, Ex-HN 1 | MA, 2Hz for 15 sec | 30min/time, 3 times | increase GDNF and BDNF levels | antiapoptosis | Zhang Y, et al. 2015[19] |
|
| |||||||
| Either sex, SD rats, CCAO combination with hypoxic treatment | cerebral cortex | MA: GV 20, GV 14, LI 11, KI 1 EA: GV 14, LI 11 |
MA and EA, 1mA, 1/20 Hz | 10 min, QD | activation of GDNF/RET/Akt pathway | neuroprotection | Xu T, et al. 2016[25] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20 | EA, 1 mA, 2/15 Hz | 30min | activation of ERK1/2 pathway | elevation of CB1 neuroprotection |
Du J et al. 2010[32] |
|
| |||||||
| Male, SD rats, MCAO | brain | ST36, LI11 | EA, 1/20 Hz | 30 min, QD | activation of the ERK pathway | elevation of Ras, cyclin D1 and CDK4 neural cell proliferation |
Xie G, et al. 2013[33] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV 20, GV14 | EA,2.7-3.0 mA, 5Hz | 25min, QD for 2 days | activation of MAPK/ERK kinase, ERK1/2 pathway | elevation of BDNF, pRaf-1, pp90RSK, pBad depression of caspase-3 protein neuroprotection |
Cheng CY, et al. 2014[34] |
|
| |||||||
| Male, SD rats, MCAO | brain | LI11, ST36 | EA, 1-20 Hz | 30min, QD for 3 days | activation of the ERK1/2 pathway | elevation of p21 or p27 depression of cyclin D1, CDK4, cyclin E and CDK2 neural cell proliferation |
Huang J, et al. 2014[29] |
|
| |||||||
| Male, SD rats, MCAO | hippocampus | LU5, LI4, ST36, SP6 | EA, 2mA, 2/15 Hz | 20 min, QD for 3 days | activation of the ERK pathway | antiapoptosis | Wu C, et al. 2015[35] |
|
| |||||||
| Male, SD rats, MCAO | brain | LU5, LI4, ST36, SP6 | EA,2 mA, 2/15Hz | 20min, QD for 3 days and 7 days | activation of ERK pathway | antiapoptosis | Wu C, et al. 2017[36] |
|
| |||||||
| Male, SD rats, ligation of common carotid artery and external carotid artery | hippocampus | LU5, LI4, ST36, SP6 | EA, 2/50 Hz | 20 min, QD | regulation of p38 MAPK signal pathway | depression of phosphorylated p38 MAPK antiapoptosis |
Lan X, et al. 2017[41] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20, GV14, GV26 | MA | 30min/time, 7 times | Inactivation of MAPK/ERK pathway | elevation of Bcl-2 depression of Bax anti-apoptosis |
Lin Y, et al. 2017[42] |
|
| |||||||
| Male, SD rats, MCAO | brain | LI11, ST36 | EA, 1mA, 4/20 Hz | 30min, QD for 3 days | modulation of ERK/JNK/p38 signal pathway | elevation of caspase-3, growth factor midkine depression of Bcl-2 anti-apoptosis |
Xing Y, et al. 2018[43] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20, GV24 | EA, 1mA, 1/20 Hz | 30 min, QD for 10 days | modulation of p38MAPK/ERK1/2/JNK pathway | elevation of ERK1/2, Bcl-2/Bax ratio depression of JNK, p38 MAPK anti-apoptosis |
Liu J, et al. 2018[44] |
|
| |||||||
| Male, SD rats, MCAO | sensorimotor cortex | LI11, ST36 | EA, 0.2mA, 1/20Hz | 30 min, QD for 3 days | inactivation of NF-κB, p38 MAPK and MYD88 pathway | depression of TNF-α, IL-1β, IL-6 inhibition of microglia-mediated neuroinflammation |
Liu W, et al. 2016[45] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20, GV16 | EA, 5 Hz and 25Hz | 25 min, QD | activation of p38 MAPK/CREB pathway | decrease reactive astrocytosis | Cheng CY, et al. 2015[46] |
|
| |||||||
| Male, Wistar rats, homologous blood emboli injection of internal carotid artery | hippocampus | ST36 | MA | QD for 14 days | activation of cAMP/PKA/CREB pathway | activation of long-term potentiation | Li QQ, et al. 2015[47] |
|
| |||||||
| Male, SD rats, MCAO | hippocampus | GV24, GV20 | EA, 1-3mA, 5/20Hz | 30min, QD | increase expression of p-CREB | elevation of superoxide dismutase and glutathione peroxidase, Bcl-2 depression of malondialdehyde, Bcl2-xl anti-oxidase anti-apoptosis |
Lin R, et al. 2015[48] |
|
| |||||||
| Male C57BL/6 mice, bilateral stenosis of the common carotid artery | corpus callosum | GV20, GV14 | EA, 2Hz | 20min, QD for 7 days | p-CREB pathway | oligodendrocyte regeneration | Ahn SM, et al. 2016[49] |
|
| |||||||
| Female, SD rats, MCAO | hippocampus | GV20, GV24 | EA, 1/20Hz | 30min, QD for 7 days | inactivation of CaM-CaMKIV-CREB pathway | inactivation of CaM-CaMKIV-CREB pathway | Zhang Y, et al. 2016[50] |
|
| |||||||
| Male, SD rats, MCAO | hippocampus | GV20, HT7 | MA LA, 30 mW, 100Hz |
14 days | enhance cholinergic system | elevation of CREB, BDNF, and Bcl-2 depression of Bax anti-apoptosis |
Yun YC, et al. 2017[51] |
|
| |||||||
| Neonatal SD rats, CCAO | brain | GV20, ST36 | EA, 1mA, 2Hz | 20min | activation of CREB/BDNF pathway | oligodendrogenesis | Pak ME, et al. 2018[52] |
|
| |||||||
| Male, Wistar rats, MCAO | forebrain | GV20, GV26 | EA, 3mA, 3/20Hz | 60min | activation of Akt | depression of caspase-9 anti-apoptosis |
Wang SJ, et al. 2002[55] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV26, CV 24, | EA,1 mA, 4/16Hz | 30min | activation of PI3K pathway | neuroprotection | Sun N, et al. 2005[56] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV26, CV24 | EA, 4/16Hz | 30 min | activation of TrkA-PI3K pathway | neuroprotection | Zhao L, et al. 2007[57] |
|
| |||||||
| Rats, modified intravascular suture technique | hippocampus, cerebral cortex | GV26, CV 24 | acupuncture | - - | activation of TrkA/PI3K pathway | depression of NO, nNOS and iNOS | Chen SX, et al. 2011[58] |
|
| |||||||
| Male, SD rats, MCAO | brain | LI11, ST36 | EA, 1mA, 1/20 Hz | 30 min, QD | activation of PI3K/Akt pathway | elevation of BDNF, GDNF, Bcl-2/Bax ratio anti-apoptosis |
Chen A, et al. 2012[59] |
|
| |||||||
| SD rats, left common carotid artery (LCCA) ligation | cerebral cortex | GV 20, GV 14, LI 11, KI 1 | MA and EA | - - | activation of PI3K/Akt pathway | neuroprotection | Xu T, et al. 2014[60] |
|
| |||||||
| Male, SD rats, MCAO | brain | LI11, ST36 | EA, 4/20 Hz | 30 min, QD for 3 days | activation of PI3K/Akt pathway | elevation of PI3K, p-Akt, p-Bad and Bcl-2 depression of Bax, caspase-3-positive expression anti-apoptosis |
Xue X, et al. 2014[12] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20, CV6 | EA,1mA, 2Hz | 30min, BID | activation of PI3K/Akt pathway | depression of caspase-3, -8 and -9 anti-apoptosis |
Kim YR, et al. 2013[61] |
|
| |||||||
| Male, SD rats, MCAO | bone marrow | GV20, LI4, LR3 | EA, 3mA, 2/20Hz | 30min, QD | increase expression of p-Akt protein | elevation of CD 34+ endothelial progenitor cell | Xie CC, et al. 2014[63] |
|
| |||||||
| Male, SD rats, MCAO | brain | LI11, ST36 | EA, 0.2 mA, 1/20 Hz | 30 min, QD for 3 days | activation of mTORC1-ULK1 complex-beclin1 pathway | depression of microtubule-associated protein 1 light chain 3 beta II/I, ULK1, autophagy related gene 13 and Beclin1 anti-autophagy |
Liu W, et al. 2016[62] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20 | EA, 1mA, 2/15 Hz | 30 min, QD for 3 days | phosphorylation of GSK-3β | anti-apoptosis | Wei H, et al. 2014[64] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20 | EA, 1mA, 2/15Hz | 30min, QD for 5 days | decrease expression of p-Akt | elevation of claudin-5, occludin decrease blood-brain barrier disruption |
Zou R, et al. 2015[65] |
|
| |||||||
| Male, SD rats, MCAO | brain | GV20, GV24 | EA 1/20Hz | 30min, QD for 10 days | inhibition of NF-κB-mediated apoptosis pathway | depression of Bax and Fas anti-apoptosis |
Feng X, et al. 2013[66] |
|
| |||||||
| Male, SD rats, MCAO | brain | LI11, ST36 | EA,0.01mA, 1/20Hz | - - | regulation of TLR4/NF-κB pathway | depression of TNF-α, IL-1β and IL-6 neuroprotection |
Lan L, et al. 2013[67] |
Abbreviations
- -: not mentioned; Bax: Bcl-2 associated X; Bad: Bcl-2-associated death promoter; Bcl-2: B-cell lymphoma 2; BDNF: brain-derived neurotrophic factor; CaMK: Ca2+/calmodulin-dependent protein kinase; cAMP: cyclic adenosine monophosphate; CB1: cannabinoid receptor type 1; CCAO: occlusion of common carotid artery; CDK: cyclin-dependent kinase; CREB: phosphorylated cyclic AMP response element-binding protein; EA: electroacupuncture; ERK: extracellular signal-regulated kinase; GDNF: glial-derived neurotrophic factor; IL: interleukin; JNK: c-Jun N-terminal kinases; MA: manual acupuncture; MAPK: mitogen-activated protein kinases; MCAO: occlusion of MCA; mTOR: mammalian target of rapamycin; MYD88: myeloid differentiation primary response 88; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; p38 MAPKs: p38 mitogen-activated protein kinases; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PKA: protein kinase A; pp90RSK: phospho-90 kDa ribosomal S6 kinase; QD: daily; QOD: every other day; SD rat: Sprague Dawley rat; TLR4: Toll-like receptor 4; TNF-α: tumor necrosis factor-alpha; Trk: tyrosine receptor kinase; ULK: UNC-51-like kinase; VEGF: vascular endothelial growth factor.
4. Cerebral Hemorrhagic Injury
Hemorrhagic stroke is less common than ischemic stroke. The causes of hemorrhagic stroke include high blood pressure, brain trauma, aneurysms, arteriovenous malformations, and brain tumors. In cerebral hemorrhagic injury, blood vessel spasms and oxidative stress caused by ischemia and reperfusion cause an injury to neural cells. Acupuncture could improve the hypoperfusion status and hematoma absorption, reduce brain edema, and promote neurogenesis in the brain [68]. Thus, some studies have reported that acupuncture is beneficial for treating cerebral hemorrhage because it results in functional improvements [69, 70]. Acupuncture also regulates inflammatory factors, such as IL-6, IL-1β, and NF-κB, prevents apoptosis by reducing the expression of p53 protein, and promotes neurogenesis by increasing the levels of BDNF and nerve growth factors [71].
Acupuncture increased the expression of endogenous GDNF and inhibited the early expression of VEGF, thus regulating nerve remodeling after cerebral hemorrhagic injury [72]. At the level of molecular signal transduction, acupuncture exerts a neuroprotective effect by increasing the angiopoietin level and reducing TNF-α and NF-κB levels [73, 74]. Li et al. reported that EA on GV20 and GB7 could reduce BBB permeability and improve brain edema by activating the caveolin-1/matrix metalloproteinase pathway [75]. Antiapoptosis is also an important pathway for neural preservation. Zhu et al. and Li et al. have demonstrated that EA activated the Bcl-2 pathway to increase hematoma absorption and antiapoptosis. This effect is combined with the suppression of caspase-3 and Bcl-2-associated X (Bax) proteins [76, 77]. However, the chosen acupoints were heterogeneous, including ST36, GV14, GV20, GV26, GB7, and PC6.
Taken together, acupuncture could improve neurogenic disability and reduce brain edema by increasing caveolin-1/matrix metalloproteinase levels and inducing antiapoptosis through the activation of the Bcl-2 pathway in a cerebral hemorrhagic model. The mechanisms and main results of identified articles are summarized in Table 2.
Table 2.
Signal transduction pathways of acupuncture in treating cerebral hemorrhagic injury.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| Male, Wistar rats | brain | GV20, GB7 | MA | 30min, QD for 1,2,3,7,10 days | increase GDNF level and modulate VEGF level | elevation of GDNF, VEGF (early) depression of VEGF (late) modulate neuron remodeling |
Zhang GW, et al. 2012[72] |
|
| |||||||
| Male, SD rats, collagenase-induced ICH | right globus pallidus | ST36 | EA,2-20Hz | 30min, QD, 14 days | activation of Ang-1 and Ang-2 | elevation of Ang-1 and Ang-2 neuroprotection |
Zhou HJ, et al. 2014[73] |
|
| |||||||
| Male, SD rats, autologous blood-induced ICH | right caudate nucleus | GV20, GB7 | MA, 3-4Hz, 5min | 30min, QD, 7 days | inactivation of TNF pathway | depression of TNF-α and NF-κB anti-inflammation |
Liu H, et al. 2017[74] |
|
| |||||||
| Male, SD rats, collagenase-induced ICH | right caudate nucleus | GV20, GB7 | EA,0.2mA, 2Hz | 30min, QD, 1,3,7 days | activation of caveolin-1/matrix metalloproteinase/blood-brain barrier permeability pathway | elevation of caveolin-1, matrix metalloproteinase-2/9 reduce blood-brain barrier permeability |
Li HQ, et al. 2016[75] |
|
| |||||||
| Male, SD rats, collagenase and heparin-induced ICH | right caudate putamen | GV20, GV14 | EA,1mA, 3Hz | 10min, QD, 14 days | activation of Bcl-2 pathway | elevation of Bcl-2 protein depression of caspase-3 and Bax proteins increase absorption of hematoma and anti-apoptosis |
Zhu Y, et al. 2017[76] |
|
| |||||||
| Male, Wistar rats, autologous blood-induced ICH | caudate nucleus | PC6, GV26 | EA, 4Hz | 1min | balance of BCL-2 and Bax | elevation of BCL-2 mRNA depression of Bax mRNA anti-apoptosis |
Li Z, et al. 2017[77] |
Abbreviations
- -: not mentioned; Ang: Angiopoietin; Bax: Bcl-2 associated X; Bcl-2: B-cell lymphoma 2; EA: electroacupuncture; GDNF: glial-derived neurotrophic factor; ICH: intracranial hemorrhage; MA: manual acupuncture; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; QD: daily; SD rat: Sprague Dawley rat; TNF-α: tumor necrosis factor-alpha; VEGF: vascular endothelial growth factor.
5. Seizure
Seizure is an abrupt, spontaneous, excessive, or synchronous neuronal activity in the brain that leads to various uncontrolled shaking movements or loss of consciousness. Seizure attack affects 8%–10% of the general population in their lifetimes. The recurrence of seizure results in epileptic syndrome, which affects 2%–3% of the general population [78]. Epileptic seizures can be induced by metabolic imbalance, electrolyte imbalance, encephalitis, traumatic brain injury, brain tumor, stroke, and medication [78]. During the process of an epileptic seizure, changes occur in molecular, anatomical, or circuit development, including cell death, inflammatory cytokine production, and neurotransmitter dysregulation. This process is called epileptogenesis [79]. Involvement of BDNF–TrkB signaling, the mTOR pathway, and the repressor element 1-silencing transcription factor pathway was considered to be the underlying molecular mechanism [79].
In addition to the use of medication, some studies have reported that acupuncture reduced the frequency of seizures and improved the quality of life [80–82]. Some studies reported that acupuncture has effect on change of anatomical, neurotransmitter, inflammatory cytokines and molecular level. The augmentation of γ-aminobutyric acid neurotransmission, including the upregulation of glutamic acid decarboxylase 67 (GAD67), is a self-protective and anticonvulsive mechanism [83, 84]. Acupuncture reduced seizure attacks by enhancing GAD67 mRNA production in the dentate gyrus of epileptic rats [85]. Acupuncture changed the brain structure and reduced the mossy fiber sprouting in the dentate gyrus and exerted an antiepileptic effect [86]. Inflammation can increase neuronal excitability and result in the frequent onset of epilepsy, which is related to epileptogenesis [87]. Acupuncture also contributes to the antiepileptic effect accompanied by the anti-inflammatory effect of reducing IL-1β, TNF-α, and cyclooxygenase-2 (COX-2) levels in the hippocampus of an epileptic rat model [88, 89]. Wang et al. and Wang et al. have demonstrated that EA attenuated the seizure-induced increase in c-fos protein and preproenkephalin messenger ribonucleic acid (mRNA) levels in the hippocampus of a penicillin-induced seizure rat model [90, 91]. Yang et al. reported that EA on GV16 and GV8 exerted an anticonvulsant effect combined with a reduction in nNOS and iNOS levels [92].
With regard to molecular pathways, acupuncture on the auricular acupoint suppressed transient receptor potential ankyrin 1 (TRPA1) pathways by increasing the phosphorylated protein kinase C (pPKC)-α level and reducing pPKCε and pERk1/2 levels in a kainic acid-induced rat model [93]. Liao et al. used a similar rat model and reported that acupuncture exerted an antiepileptic effect by inactivating the Toll-like receptor 4 (TLR4) pathway, which was accompanied by a decrease in pCaMKIIα, pERK, pp38, pJNK, and pNFκB levels [94]. Yang et al. demonstrated that acupuncture on GV20 and GV14 reduced epileptic seizures by exerting a protective effect on the pyramidal cells of hippocampal CA 1 and CA 3. This effect was related to the activation of the PI3 K/Akt pathway [95]. The upregulation of glucose-regulated protein 78 (GRP78) and the downregulation of C/EBP homologous protein (CHOP) prevent neuronal cell death induced by endoreticulum stress. Acupuncture on GV20 and GV14 elevated the GRP78 level, reduced CHOP and caspase-12 levels, and exerted an antiapoptosis effect on the hippocampus, thus reducing epileptic seizure attacks [96, 97].
Taken together, acupuncture exerts the antiepileptic effect by changing anatomical, neurotransmitter, inflammatory cytokines and molecular level. With respect to signal transduction, acupuncture reduces seizure frequency by suppressing TRPA1/pERK and TLR4/ERK pathways and activating the PI3K/Akt pathway. Furthermore, acupuncture augments the antiapoptosis process and provides neuroprotection by increasing the GRP78 level and reducing the CHOP level. The mechanisms and main results of identified articles are summarized in Table 3.
Table 3.
Signal transduction pathways of acupuncture in treating seizure.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| Male, SD rats, lithium-pilocarpine injection | dentate gyrus | ST36 | EA, 1-20mA, 4/20Hz | 30min, QD for 30,45,60 days | activation of GAD 67 | elevation of GAD67 mRNA anti-epileptic |
Guo J, et al. 2008[85] |
|
| |||||||
| Male, SD rats, kainic acid injection | prefrontal cortex, hippocampus, and somatosensory cortex | auricular acupoint | Auricular EA, 2 and 15Hz | 20min, QD, 3 days/wk for 3 wks | inactivation of TLR 4 pathway | depression of pCaMKIIα, pERK, pp38, pJNK, pNFκB anti-epileptic |
Liao ET, et al. 2018[94] |
|
| |||||||
| Male, SD rats, intraperitoneal injection of pentylenetetrazol | hippocampal CA 1 and CA 3 | GV20, GV14 | MA | QD for 5 days | activation of PI3 K/Akt pathway | increase pyramidal cells | Yang, F, et al. 2013[95] |
|
| |||||||
| Male, SD rats, kainic acid injection | hippocampal CA1 areas | Auricular acupoint | EA, 2Hz | 20min, 3 days/wk for 6wks | Inactivation of TRPA1, pPKCα, pPKCε, and pERk1/2 pathways | elevation of PKCα depression of TRPA1, PKCε, pERK1/2 anti-epileptic |
Lin YW, et al. 2014[93] |
|
| |||||||
| Male, SD rats, intraperitoneal injection of pentylenetetrazol | hippocampal CA 1 region | GV20, GV14 | MA | 30min | balance of GRP78 and CHOP | elevation of GRP 78 protein depression of CHOP neuroprotection |
Yang F, et al. 2014[96] |
|
| |||||||
| Male, newly-born SD rats, pentylenetetrazol intraperitoneal injection | hippocampus | GV20, GV14 | MA | QD for 7 days | balance of GRP78 and CHOP | elevation of GRP 78 protein depression of CHOP, caspase-12 anti-apoptosis |
Zhang, H, et al. 2017[97] |
Abbreviations
Akt: protein kinase B; CaMK: Ca2+/calmodulin-dependent protein kinase; CHOP: C/-EBP homologous protein; COX: cyclooxygenase; EA: electroacupuncture; ERK: extracellular signal-regulated kinase; GAD67: glutamic acid decarboxylase 67; GRP78: glucose-regulated protein 78; IL: interleukin; JNK: c-Jun N-terminal kinases; MA: manual acupuncture; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; p38 MAPKs: p38 mitogen-activated protein kinases; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PKC: protein kinase C; QD: daily; QOD: every other day; SD rat: Sprague Dawley rat; TLR4: Toll-like receptor 4; TNF-α: tumor necrosis factor-alpha; TRPA: transient receptor potential ankyrin 1.
6. Depression
Depressive disorders are common psychiatric disorders that affect approximately 17% of people in their lifetimes. A study reported that 12%–20% of depressed patients experience treatment-resistant depression, resulting in a considerable social burden [98]. In addition to medication and psychosocial support, acupuncture serves as an alternative option for patients with depression that exhibits promising effects and fewer side effects [99]. The mechanism of depression includes dysregulation of neuroinflammatory cytokines, neurotransmitters, neuroplasticity, and the neuroendocrine system [100, 101]. At the molecular level, dysregulation of striatal-enriched tyrosine protein phosphatase inactivates the neuronal signaling pathway, including ERK1/2, p38, Src family tyrosine kinases, and glutamate receptors. This process attenuates the neurogenesis effect of BDNF and causes depression [102].
Acupuncture treats depression by regulating neurotransmitters, neuroinflammatory cytokines, the hypothalamus–pituitary–adrenal axis, and the hypothalamus–pituitary–sex gland axis [103]. Furthermore, acupuncture plays a role in molecular signaling pathways. Acupuncture elevated BDNF production and excitatory amino acid transporter levels and maintained neural regeneration of the hippocampus in a depressive rat model [104, 105]. The chosen acupoints include GV20, EX-HN3, and PC6 [104, 105]. Fan et al. demonstrated that acupuncture on LI4 and LR3 regulated the expression of soluble N-ethylmaleimide-sensitive factor attachment receptor protein, a fusion mediator, and promoted depression remission [106]. NO is a small molecule that freely diffuses across cell membranes and serves as a neurotransmitter in the CNS. NO initiates the NO-cyclic guanosine monophosphate (NO-cGMP) pathway and activates protein kinases. Acupuncture regulates the NO-cGMP pathway by increasing nNOS and cGMP levels, which contribute to its effect on depression relief [107]. Shao et al. demonstrated that acupuncture on GV20 and PC6 inhibited the proinflammatory pathway of depression by reducing NF-κB protein and COX-2 levels [108].
Antidepressants alleviate the symptoms of depression by activating the MAPK/ERK pathway, which increases ERK1/2 and p-ERK1/2 expression. Many studies have reported that acupuncture activates the MAPK/ERK pathway and downstream CREB pathway and elevates BDNF production [109–114]. The most commonly chosen acupoints include GV20 and GV29, followed by EX-HN3, GB34, and PC6. The MAPK/ERK pathway induces neurogenesis and antiapoptosis of hippocampal neurons and eliminates the depression state. EA on GV20 and EX-HN3 also enhances the p-p38MAPK pathway [111]. Some studies have reported that EA on GV20 and GV29 reduced the hippocampal neural apoptotic rate by downregulating the hippocampal p-JNK pathway in depression rat model [115, 116]. Acupuncture also activated the adenyl cyclase (AC)–cyclic adenosine monophosphate (cAMP)–protein kinase A (PKA)–CREB signaling pathway and elevated the BDNF level [117–120]. In the AC–cAMP–PKA–CREB signaling pathway, heterogeneous acupoints were chosen, including GV20, EX-HN1, EX-HN3, ST36, ST40, LI4, and LR3.
Molecular studies have reported that acupuncture plays a role in the neuroendocrine model of depression. Lu et al. demonstrated that acupuncture could relieve the symptoms of depression and increase cortisol, PKA, and PKC levels [117]. Oh et al. reported that acupuncture on HT8 elevated the serum corticosterone level and hippocampal mTOR phosphorylation, Akt, ERK, p70S6K, p4E-BP1, and CREB enhanced the effect of BDNF on neuroprotection and synaptic plasticity. Furthermore, acupuncture elevated the levels of synaptic proteins (e.g., PSD95, Syn1, and GluR1), which are crucial for neuronal synaptic plasticity [121].
The results of the Gene Ontology functional term and Kyoto Encyclopedia of Genes and Genomes database analysis indicated that the regulation of the Toll-like receptor signaling pathway, nucleotide-binding oligomerization domain-like receptor signaling pathway, MAPK/ERK pathway, PI3K/Akt pathway, neurotrophin signaling pathway, TNF pathway, and NF-κB pathway is the mechanism through which acupuncture treats depression. The aforementioned pathways cause cell survival, differentiation, antiapoptosis, and synaptic plasticity of neurons, thus alleviating depression symptoms and improving learning/memory dysfunction [122–124].
In summary, acupuncture can treat depression by upregulating MAPK/ERK and AC–cAMP–PKA–CREB pathways and downregulating JNK and NF-κB pathways. Because of the aforementioned mechanism, we observed an increase in neuron growth factor levels, neurogenesis, and antiapoptosis accompanied by the alleviation of depression symptoms. The mechanisms and main results of identified articles are summarized in Table 4.
Table 4.
Signal transduction pathways of acupuncture in treating depression.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| SD rats, CUMS | hippocampus, frontal cortex | GV20, EX-HN3, PC6 | - - | QOD for 28 days | activation of BDNF pathway | elevation of BDNF mRNA and protein neural regeneration |
Liang J, et al. 2012[104] |
|
| |||||||
| Male, SD rats, CUS | hippocampus | LI4, LR3 | EA | QD for 21 days | regulation of soluble N-ethylmaleimide-sensitive factor attachment receptor proteins | depression of SNAP25, VAMP1, VAMP2, VAMP7, and syntaxin1 | Fan L, et al. 2016[106] |
|
| |||||||
| SD rats, CUMS | hippocampus | GV20, EX-HN3 | EA, 0.6mA, 2Hz | 20min, QD for 21 days | activation of NO-cGMP pathway | elevation of nNOS, cGMP normalize activity of the NO/cGMP pathway |
Han YJ, et al. 2009[107] |
|
| |||||||
| Male, SD rats, CUS | hippocampus | GV20, PC6 | - - | QD for 28 days | Inactivation of NF-κB inflammatory pathway | depression of NF-κB, COX-2, prostaglandin inhibition of pro-inflammatory pathway |
Shao RH, et al. 2015[108] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus, prefrontal cortex | GV20, PC6 | MA, rotated 2Hz for 1 min and retained | 10min, QOD for 28 days | activation of ERK-CREB pathway | elevation of ratio of p-ERK1/2 to ERK1/2, ratio of p-CREB to CREB influence BDNF expression |
Lu J, et al. 2013[109] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, GB34 | EA, 0.3mA, 2/100Hz | 30min, QD for 14 days | activation of ERK pathway | elevation of p-ERK neural stem cells proliferation |
Yang L, et al. 2013[110] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, EX-HN3 | EA, 1-3mA, 2Hz | 15 min, QD for 14 days | modulation of the p-ERK1/2 and p-p38MAPK pathway | elevation of p-ERK1/2, p-p38 | Xu J, et al. 2015[111] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, GV29 | MA, 2Hz for 1min | 10min, QD for 21 days | activation of ERK pathway | elevation of -ERK1/2, CREB, and p-CREB neurotrophy and neurogenesis |
Zhang X, et al. 2016[112] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, GV29 | EA, 0.6mA, 2Hz | 20min, QD for 21 days | Activation of MAPK/ERK pathway | elevation of BDNF, ERK, pERK, ribosomal s6 kinase augmentation of BDNF pathway, neurogenesis, anti-apoptosis |
Li W, et al. 2017[113] |
|
| |||||||
| Male, specific pathogen-free SD rats, CRS | hippocampus | GV20, GV29 | EA, 1mA, 2Hz | pre-stress, 20min, QD for 28 days | modulation of MAPK/ERK pathway | elevation of MAPT depression of PKC inhibition of cell differentiation and proliferation |
Yang X, et al. 2017[114] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, GV29 | EA | 21 days | inactivation of JNK pathway | depression of p-JNK anti-apoptosis |
Dai W, et al. 2010[115] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, GV29 | acupuncture | 20 min, QD | inactivation of JNK pathway | depression of p-JNK protein, MKK 4, MKK 7 protein | Guo Y, et al. 2016[116] |
|
| |||||||
| Male, SD rats | hippocampus, serum | GV20, EX-HN1, ST36, ST40 | EA | QOD for 21 days | regulation of hypothalamus-pituitary-adrenal axis | elevation of cortisol, PKA, PKC | Lu F, et al. 2008[117] |
|
| |||||||
| Male, SD rats, chronic mild stress | hippocampus | LI4, LR3 | EA, 2/20 Hz | 30min, QOD for 42 days | activation of AC-cAMP-PKA pathway | activation of AC-cAMP-PKA pathway | Liu JH, et al. 2012[118] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, EX-HN3 | EA, 0.6mA, 2Hz | 30min, QD for 14, 28 days | activation of CREB and BDNF pathways | elevation of BDNF, TrkB, PKA, pCREB depression of CaMKII anti-apoptosis, neuroprotection |
Duan DM, et al. 2016[119] |
|
| |||||||
| Male, SD rats, CUMS | hippocampus | GV20, EX-HN3 | MA, | pre-stress, 30min for 21 days | Activation of PKA/CREB pathway | elevation of PKA-α and p-CREB | Jiang H, et al. 2017[120] |
|
| |||||||
| Male, SD rats, Single prolonged stress | Hippocampus, serum | HT8 | MA, rotate 2Hz for 30sec | QD | activation of mTOR pathway | elevation of corticosterone(serum), corticotropin-releasing factor, mTOR phosphorylation, Akt, ERK, p70S6K, p4E-BP-1, CREB, PSD95, Syn1, GluR1 increase synaptic plasticity |
Oh JY, et al. 2018[121] |
|
| |||||||
| Male, Wistar rats, CUMS | hippocampus and serum | GV20, EX-HN3 | EA, 1mA, 2Hz, pre-stress | 60min, QD for 28 days | Regulation of neurotrophin signaling pathway, MAPK/ERK pathway and PI3K/Akt pathway | depression of miR-383-5p and miR-764-5p activation of neurotrophy and inhibition of abnormal apoptosis |
Duan DM, et al. 2017[122] |
|
| |||||||
| Male, SD rats, CRS | hippocampus | GV20, EX-HN3 | not mentioned | 20min, QD for 28 days | down regulation of toll-like receptor signalling pathway and nucleotide-binding oligomerization domain-like receptor pathway | regulating inflammatory response, innate immunity and immune response | Wang Y, et al. 2017[123] |
|
| |||||||
| Male, SD rats, CRS | frontal cortex | GV20, GV29 | MA | pre-stress, 20min, QD for 28 days | Toll-like receptor pathway, TNF pathway, NF-κB pathway | inhibition of inflammatory process | Wang Y, et al. 2017[124] |
Abbreviations
AC: adenyl cyclase; Akt: protein kinase B; BDNF: brain-derived neurotrophic factor; CaMK: Ca2+/calmodulin-dependent protein kinase; cAM: cyclic adenosine monophosphate; cGMP: cyclic guanosine monophosphate; COX: cyclooxygenase; CREB: phosphorylated cyclic AMP response element-binding protein; CRS: chronic restraint stress; CUMS: chronic unpredictable mild stress; CUS: chronic unpredictable stress; EA: electroacupuncture; ERK: extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinases; MA: manual acupuncture; MAPK: mitogen-activated protein kinases; MAPT: microtubule-associated protein Tau; mRNA: messenger ribonucleic acid; mTOR: mammalian target of rapamycin; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; nNOS: neuronal nitric oxide synthase; NO: nitric oxide; p38 MAPKs: p38 mitogen-activated protein kinases; PKA: protein kinase A; PKC: protein kinase C; QD: daily; QOD: every other day; SD rat: Sprague Dawley rat; TrkB: tyrosine receptor kinase B; VAMP: vesicle-associated membrane protein.
7. Alzheimer's Disease
AD is a progressive neurodegenerative disease that is presented with dementia, memory loss, disorientation, personality disorder, mood swings, behavior disturbance, and language problems. Because of patients' cognitive decline, they withdraw from their family and society [125]. Risk factors for AD include genetic factors, a history of head trauma, depression, and hypertension [126]. The progression of AD is associated with the formation of amyloid plaques and neurofibrillary tangles in the brain [126]. Treatment of AD should be started immediately after the diagnosis to prevent cognitive decline. Both patients and their families are involved in administration of medication and psychosocial therapy for AD. Medication for AD includes cholinesterase inhibitors (donepezil, rivastigmine, and galantamine), N-methyl-D-aspartate receptor antagonists (memantine), atypical antipsychotics, antidepressants, and anticonvulsants [126].
In addition to medication, acupuncture has been reported to improve cognitive function and the global clinical status of patients with AD without causing major adverse effects [127, 128]. Mechanisms through which acupuncture improves cognitive impairment in AD include attenuation of Aβ deposits, upregulation of BDNF expression, and regulation of cell proliferation and neural plasticity in the brain [129–131]. Acupuncture also regulates cytokine and growth factor levels associated with survival, proliferation, and differentiation of neural stem cells in the brain to promote the repair of damaged cells [130, 132].
Aβ deposits in the brain disturb BDNF signaling pathways, such as Ras/ERK, PI3K/Akt, and PKA/cAMP, which regulate BDNF expression and cause AD development [133, 134]. Acupuncture on GV20 reduces Aβ deposits in the brain, elevates the BDNF level, and exerts a neuroprotective effect on CNS cells [135, 136]. Lin et al. reported that the signaling pathway of BDNF elevation is mediated by the BDNF–TrkB pathway, which exerts an antiapoptosis effect [136]. The central cholinergic pathway is important for learning acquisition and synaptic plasticity in the mammalian limbic system; thus, increasing the acetylcholine level is a type of treatment strategy for AD. Lee et al. reported that acupuncture enhances the cholinergic system–CREB–BDNF pathway and exerts a neuroprotective effect [135].
The p38 MAPKs are activated by environmental stresses and inflammatory cytokines and induce apoptosis and inflammation. In an AD animal model, acupuncture could improve cognitive impairment by reducing p38 MAPK levels, thus reducing neuroinflammation in the CNS [18, 137, 138]. Some studies have reported using Sanjiao acupuncture, which uses CV17, CV12, CV6, ST36, and SP10, as a standard regimen for AD [18, 139, 140]. A DNA microarray analysis demonstrated that Sanjiao acupuncture could reverse gene expression profiles related to aging in the hippocampus of senescence-accelerated mouse prone 10 (SAMP10) mice and reduce oxidative stress–induced damage [18]. Luo et al. reported that Sanjiao acupuncture attenuated cognitive deficits by regulating the G-protein/inositol triphosphate/Ca2+ amplitude pathway and signal homeostasis [140]. In an Aβ-induced AD model, acupuncture on GV20 and BL23 reduced the level of peroxisome proliferator-activated receptor-γ (PPAR-γ) level and the deposition of Tau protein, thus reducing neuroinflammation [138].
Acupuncture regulated cell cycle and aging in an AD model. N-myc downregulated gene 2 (NDRG2) encodes a cytoplasmic protein that may play a role in neurite outgrowth. Wang et al. demonstrated that EA on GV20 suppressed the astrocyte NDRG2 expression and glial fibrillary acidic protein level, thereby treating memory impairment of amyloid precursor protein/presenilin-1 double transgenic mice [141]. P130, known as retinoblastoma-like protein 2 (RBL2), is a protein encoded by the RBL2 gene in humans and serves as a tumor suppressor signal. Acupuncture on CV17, CV12, CV6, SP10, and ST36 elevated the p130 level, caused cell proliferation in the brain, and treated dementia and aging-related diseases in SAMP10 mice [139]. Telomerase is a critical enzyme involved in aging and apoptosis. Lin et al. demonstrated that acupuncture on ST35 of telomerase-deficient mice activated the BDNF–TrkB signaling pathway along with elevating BDNF, TrkB, Akt, and ERK1/2 levels, which resulted in an increase in telomerase activity [142]. Acupuncture also modulates the balance of Bcl-2/Bax to regulate the cell cycle of neurons. However, the chosen acupoints were heterogeneous, including LI20, EX-HN3, GV20, BL23, and KI1 [143–145].
Metabolic stress modulates β-secretase gene transcription and β-site amyloid precursor protein-cleaving enzyme 1 (BACE1) protein levels in AD through the sirtuin 1 (SIRT1)-PPARγ-proliferator-activated receptor γ coactivator 1 (PGC-1) pathway [146]. Aβ 25–35 suppresses mitochondrial biogenesis by inactivating the AMP-activated protein kinase (AMPK)–SIRT1–PGC-1α pathway in hippocampal neurons [147]. Therefore, brain energy metabolism impairment is considered an underlying pathogenesis of AD progression. Acupuncture on GV20 elevates glucose transporter (GLUT1 and GLUT3), p-AMPK, p-AKT, and mTOR levels in the hippocampus and cortex. Through regulation of brain energy metabolism, acupuncture has effect on decreasing Aβ deposits, suppressing autophagy process and relieving cognition deficits [148]. Acupuncture improved the spatial learning and memory ability of AD mice by increasing blood perfusion and glucose uptake in the bilateral amygdala, hippocampus, and left temporal lobe [149, 150]. For the molecular signaling pathway, Dong et al. demonstrated in two series studies that acupuncture in GV14 and BL23 exerted AMPK expression, activated SIRT1-PPARγ- PGC-1 pathway, and elevated ATP level. Because of the aforementioned mechanism, acupuncture balances brain metabolism and improves cognition impairment of AD mice [20, 151]. Furthermore, the upregulation of SIRT1–PPARγ–PGC-1 suppresses BACE1 expression, thus reducing Aβ production in the hippocampus and improving cognitive decline in SAMP8 mice [152].
In summary, acupuncture treats AD by regulating neurotransmitter release, elevating the neurotrophic factor level, and exerting anti-inflammatory effects. Thus, many molecular signaling pathways involved in acupuncture were reported in the AD model, including the BDNF–TrkB pathway, the cholinergic system–CREB–BDNF pathway, G-protein regulation, and the p38 MAPK family. The aforementioned pathways are believed to exert antiapoptosis and anti-inflammatory effects and reduce Aβ deposits in the brain, thereby improving learning ability and memory in AD models. The most commonly chosen acupoints were GV20 and the Sanjiao regimen (CV17, CV12, CV6, ST36, and SP10). Acupuncture regulates cell cycle and aging by modulating NDRG2 and P130 expression, telomerase activity, and Bcl-2/Bax balance. Many studies have reported that acupuncture on GV14 and BL23 modulates brain energy metabolism impairment and treats cognitive impairment. The mechanisms and main results of identified articles are summarized in Table 5.
Table 5.
Signal transduction pathways of acupuncture in treating Alzheimer's disease.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| Male, SD rat, scopolamine injection | brain | GV20 | MA | pretreatment for 5 min, QD for 14 days | enhance cholinergic system-CREB-BDNF pathway | elevation of choline acetyltransferase, choline transporter 1, vesicular acetylcholine transporter, BDNF, CREB proteins neuroprotection |
Lee B, et al. 2014[135] |
|
| |||||||
| APP/PS1 mice | brain | GV20 | EA, 1/20 Hz | 30min, QD for 4 weeks | modulation of BDNF-TrkB pathway | elevation of BDNF/proBDNF ratio, p-TrkB depression of β-amyloid (1-42), p75 anti-apoptosis |
Lin R, et al. 2016[136] |
|
| |||||||
| Male, SAMP10 | hippocampus | CV17, CV12, CV6, ST36, SP10 | MA | QD | regulation of aging gene | elevation of p53, Mad related protein 2, Nucleoside diphosphate kinase B, AT motif-binding factor, Hsp84, Hsp86 depression of p38 MAPK, retinoblastoma-associated protein 1 anti-oxidation |
Ding X, et al. 2006[18] |
|
| |||||||
| SD rat, Aβ1-40 injection | hippocampus, frontal cortex | GV20, KI3, ST36 | EA, 1mA, 2Hz | 15min, QD for 12 days | inactivation of p38 MAPK pathway | depression of p-p38 MAPK protein, IL-1beta mRNA decrease neuroinflammation |
Fang JQ, et al. 2013[137] |
|
| |||||||
| Male, SD rat, Aβ1-40 injection | hippocampus CA1 | GV20, BL23 | EA, 2mA, 2-4V, 2Hz | 20min, QD, 6 days/ wk for 4 weeks | activation of PPAR-γ pathway | elevation of PPAR-γ depression of p-p38MAPK, Aβ, p-Tau Ser404 protein decrease neuroinflammation |
Zhang M, et al. 2017[138] |
|
| |||||||
| SAMP 10 mice | neocortex and hippocampus | CV17, CV12, CV6, SP10, ST36 | not mentioned | QD for 14 days | p 130 pathway | elevation of p130 cell proliferation |
Liu T, et al. 2008[139] |
|
| |||||||
| Male, SAMP8 mice | cortex and hippocampus | CV17, CV12, CV6, ST36, SP10 | MA, >2Hz | 30sec per acupoint, QD, 21 days | regulation of G protein/ IP3/ Ca2+ amplitude pathway | elevation of physiologically coupled activation rate and maximal coupled activation rate of Gαs and Gαi signal homeostasis |
Luo B, et al. 2017[140] |
|
| |||||||
| Male, APP/PS1 mice | brain | GV20 | EA, 1mA, 2/15Hz | 30min, QD, 5 days/wk for 4 weeks | suppression of astrocytic NDRG2 pathway | depression of Glial fibrillary acidic protein, NDRG2 increase astrocytic reactivity |
Wang F, et al. 2014[141] |
|
| |||||||
| telomerase-deficient mice(TERC-/-) mice | hippocampus and dentate gyrus | ST36 | MA | 30 min, QD for 4 days | activation of BDNF pathway | elevation of BDNF, TrkB, p75NTR, Akt, and ERK1/2 increase telomerase activity |
Lin D, et al. 2015[142] |
|
| |||||||
| SD rat, beta-amyloid (Abeta)(1-40) injection | hippocampal | LI20, EX-HN3 | EA, 1-3mA, 80-100Hz | 10min, QD, 5 days/wk for 6 weeks | regulation of Bcl-2/Bax | elevation of Bcl-2 depression of Bax anti-apoptosis |
Liu ZB, et al. 2011[143] |
|
| |||||||
| Male, SD rat, Aβ1-40 injection | hippocampus CA1 | GV20, BL23 | EA, <2mA, 20Hz | 30 min, QD, 6 days/ wk for 4 weeks | downregulation of Notch pathway | elevation of Bcl-2, synapsin-1, synaptophysin depression of Bax, Notch1 mRNA, Hes1 mRNA anti-apoptosis |
Guo HD, et al. 2015[144] |
|
| |||||||
| Male, APP/PS1 mice | hippocampus | GV20, KI1 | EA, 1mA, 2/100Hz | 15min, QD for 3 days | inactivation of caspase-3/ Bax pathway | elevation of Bcl-2/Bax ratio depression of caspase-3-positive cell number and Bax protein anti-apoptosis |
Li XY, et al. 2016[145] |
|
| |||||||
| APP/PS1 mice | hippocampus, cortex | GV20 | EA, 1/20Hz | 30min, QD, 5 days/wk for 4 weeks | regulation of AMPK/mTOR pathway | elevation of GLUT1, GLUT3, p-AMPK, p-Akt, mTOR decrease Aβ (1-42) deposition, decrease autophagy process |
Liu W, et al. 2017[148] |
|
| |||||||
| Male, SAMP8 mice | hippocampus CA1 | GV14, BL23 | EA, 1mA, 2Hz | 20min, QD, 8 days' treatment and 2 days' rest for 3 cycles | activation of AMPK pathway | elevation of p-AMPK balance energy metabolism and improved cognitive impairment |
Dong W, et al. 2015[20] |
|
| |||||||
| Male, SAMP8 mice | hippocampus and frontal cortex | GV14, BL23 | EA, 1mA, 2Hz | 20min, QD, 8 days' treatment and 2 days' rest for 3 cycles | activation of SIRT1-dependent PGC-1α expression pathway | elevation of ATP levels, SIRT1, PGC-1α depression of PGC-1α acetylation improved brain energy metabolism |
Dong W, et al. 2015[151] |
Abbreviations
Akt: protein kinase B; AMPK: AMP-activated protein kinase; APP/PS1: amyloid precursor protein (APP)/presenilin-1 (PS1) double transgenic; Bax: Bcl-2 associated X; Bcl-2: B-cell lymphoma 2; BDNF: brain-derived neurotrophic factor; CREB: phosphorylated cyclic AMP response element-binding protein; EA: electroacupuncture; ERK: extracellular signal-regulated kinase; GLUT: glucose transporter; IL: interleukin; IP3: Inositol triphosphate; MA: manual acupuncture; MAPK: mitogen-activated protein kinases; NDRG2: N-myc downregulated gene 2; NMDA: N-methyl-D-aspartate; PGC1: proliferator-activated receptor γ coactivator 1; PPAR-γ: peroxisome proliferator-activated receptors γ; QD: daily; QOD: every other day; RBL2: Retinoblastoma-like protein 2; SAMP: senescence-accelerated mouse prone; SD rat: Sprague Dawley rat; SIRT1: sirtuin 1; TrkB: tyrosine receptor kinase B.
8. Vascular Dementia
VD, which accounts for 15% of dementia cases, is the second most common cause of dementia after AD. Multiple and recurrent ischemia of the brain caused by ischemia or hemorrhage has been found to be the main causes of VD [169]. Although the pathophysiology of VD remains unclear, approximately 15%–30% of patients develop dementia three months after the occurrence of stroke. Furthermore, approximately 20%–25% of patients develop delayed dementia [170]. Because of intricate coordination in the brain and, sometimes, the presence of other brain damage causes, the cognitive changes and declines in VD can be variable, including impairment of attention, information processing, and executive function [169]. Few medications have been approved specifically for the prevention or treatment of VD. Thus, treatment strategies for VD are similar to those for AD and include the use of cholinesterase inhibitors and memantine and providing psychosocial support.
Acupuncture can improve the scores on the Mini-Mental Status Examination, the revised Hasegawa's dementia scale, and activities of daily living examination for VD patients [171, 172]. From the molecular viewpoint, acupuncture on GV20 and KI3 regulates the MAPK/ERK pathway by elevating the pERK level and reducing ionized calcium-binding adaptor molecule 1 (Iba-1), TLR4, and TNF-α levels [153]. Acupuncture reduced relevant proinflammatory factors, thus attenuating neuroinflammation and increasing neuronal synaptic plasticity.
Acupuncture exerted antioxidant and antiapoptosis effects in VD models. Zhu et al. reported that acupuncture on GV20 and ST36 inactivated the apoptosis signal-regulating kinase 1 (ASK1)–JNK/p38 pathway and elevated thioredoxin-1 and thioredoxin reductase-1 levels [154]. The p38 MAPK pathway activates the expression of CREB and reduces the apoptosis of ischemic neural cells. Some studies have reported that acupuncture activates the cAMP/PKA/CREB pathway and elevates the CREB level [47, 48, 50, 51]. The elevated CREB level upregulates Bcl-2 activity and downregulates Bcl-2xl and Bax activities, consequently preventing the apoptosis of neurons injured by vascular events [48, 51]. The most discussed acupoint was GV20, followed by GV24. Scalp and Sanjiao acupuncture techniques (CV17, CV12, CV6, ST36, and SP10) have been reported to affect the balance between Bcl-2 and Bax expression and antiapoptosis [155, 156]. VD rats had lower expression of mTOR and eukaryotic translation initiation factor 4E (eIF4E) in CA1 accompanied with decreased spatial memory [173]. Zhu et al. demonstrated that EA on GV20, GV14, and BL23 activates the mTOR pathway and increases mTOR and eIF4E levels, thus modulating cell growth, proliferation, and synaptic plasticity [157].
Taken together, acupuncture treats VD by activating MAPK/ERK and ASK1–JNK/p38 pathways; increasing CREB, mTOR, and Bcl-2 levels; and reducing the Bax level. In addition, through the aforementioned mechanism, acupuncture exerts an effect on antioxidant activity, antiapoptosis, and synaptic plasticity. The most commonly chosen acupoints were GV20, GV24, and ST36. The mechanisms and main results of identified articles are summarized in Table 6.
Table 6.
Signal transduction pathways of acupuncture in treating vascular dementia.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| Male, Wistar rats, homologous blood emboli injection of internal carotid artery | hippocampus | ST36 | MA | QD for 14 days | activation of cAMP/PKA/CREB pathway | activation of long-term potentiation | Li QQ, et al. 2015[47] |
|
| |||||||
| Male, SD rats, MCAO | hippocampus | GV24, GV20 | EA, 1-3mA, 5/20Hz | 30min, QD | increase expression of p-CREB | elevation of superoxide dismutase and glutathione peroxidase, Bcl-2 depression of malondialdehyde, Bcl2-xl anti-oxidase and anti-apoptosis |
Lin R, et al. 2015[48] |
|
| |||||||
| Female, SD rats, MCAO | hippocampus | GV20, GV24 | EA, 1/20Hz | 30min, QD for 7 days | inactivation of CaM-CaMKIV-CREB pathway | anti-apoptosis | Zhang Y, et al. 2016[50] |
|
| |||||||
| Male, SD rats, MCAO | hippocampus | GV20, HT7 | MA, LA, 30 mW, 100Hz | 14 days | enhance cholinergic system | elevation of CREB, BDNF and Bcl-2 depression of Bax anti-apoptosis |
Yun YC, et al. 2017[51] |
|
| |||||||
| Mongolian gerbils, CCAO | hippocampal CA1 | KI3, GV20 | EA, 1mA, 2Hz | 20 min, 4 times/ 2 days | regulate MAPK/ERK pathway | elevation of p-ERK depression of ionized calcium-binding adaptor molecule 1, TLR4, TNF-α decrease neuroinflammation, regulate the synaptic plasticity |
Yang EJ, et al. 2016[153] |
|
| |||||||
| Male Wistar rats, two-vessel occlusion model | hippocampus | GV20, ST36 | MA | QD for 14 days | inactivation of ASK1-JNK/p38 pathway | elevation of thioredoxin-1 and thioredoxin reductase-1 anti-oxidase and anti-apoptosis |
Zhu W, et al. 2018[154] |
|
| |||||||
| Male, Wistar rat, homoblood injection | hippocampal CA1 | CV17, CV12, CV6, ST36, SP10 | MA, 2Hz | 30sec for each acupoint, QD, 6 days/ wk for 3 weeks | balance Bcl-2 and Bax expression | elevation of Bcl-2 depression of Bax anti-apoptosis |
Wang T, et al. 2009[155] |
|
| |||||||
| Male, SD rat, using modified Pulsinelli 4-vessel-occlusion method | hippocampal CA1 | Scalp-acupuncture | MA | 30min, QD for 10 days | activation of Bcl-2 pathway | elevation of Bcl-2 anti-apoptosis of astrocytes |
Tian WJ, et al. 2015[156] |
|
| |||||||
| Female, SD rat, CCAO | hippocampus | GV20, GV14, BL23 | EA, 2mA, 4Hz | 30min, QD for 30 days | activation of mTOR pathway | elevation of mTOR and eIF4E modulates cell growth, proliferation and synaptic plasticity |
Zhu Y, et al. 2013[157] |
Abbreviations
ASK1: apoptosis signal-regulating kinase 1; Bax: Bcl-2 associated X; Bcl-2: B-cell lymphoma 2; BDNF: brain-derived neurotrophic factor; CaMK: Ca2+/calmodulin-dependent protein kinase; cAMP: cyclic adenosine monophosphate; CCAO: occlusion of common carotid artery; CREB: phosphorylated cyclic AMP response element-binding protein; EA: electroacupuncture; eIF4E: eukaryotic translation initiation factor 4E; ERK: extracellular signal-regulated kinase; JNK: c-Jun N-terminal kinases; MA: manual acupuncture; MAPK: mitogen-activated protein kinases; MCAO: occlusion of middle cerebral artery; mTOR: mammalian target of rapamycin; PKA: protein kinase A; QD: daily; TLR4: Toll-like receptor 4.
9. Parkinson's Disease
PD is a chronic neural degenerative disorder that mainly affects the motor system. Patients with PD experience shaking, rigidity, and walking difficulty. In advanced stages of the disease, behavioral disturbance, depression, poor sleep, and cognitive dysfunction are noted [174]. Treatments such as the administration of L-dopa, dopamine agonists, catechol-O-methyl transferase inhibitors, and monoamine oxidase inhibitor and deep brain stimulation are suggested for treating motor problems of patients with PD. However, dyskinesias and motor fluctuations that develop after a long-term use or high dose use of L-dopa and nonmovement-related symptoms, such as sleep disturbances and psychiatric problems, become problems for patients with PD [174].
Both manual acupuncture and EA help alleviate some motor symptoms in patients with PD and some nonmotor symptoms, such as psychiatric disorders, sleep disorders, and gastrointestinal symptoms. Acupuncture also improved the therapeutic efficacy of levodopa, lowering the necessary dosage [175–177]. Reducing dopaminergic neurons in the substantia nigra (SN) results in PD. Acupuncture has been reported to exert neuroprotective effects that increase the levels of endogenous neurotrophins and modulate the apoptosis and neuroinflammation of dopaminergic neurons in the SN [178, 179]. Neuroimaging findings of the human brain showed that acupuncture on GB34 and the scalp significantly increased glucose metabolism bilaterally in the frontal and occipital lobes and improved motor dysfunction in patients with PD [179, 180].
In light of signal transduction, EA at 2 Hz on GV16 and LR3 inactivate the ERK 1/2 signaling pathway and p38/MAPK signaling pathway, causing an increase in tyrosine hydroxylase–positive neurons and a decrease in COX-2, TNF-α, and IL-1β levels. The regulation of cytokines reduces the neuroinflammation of the SN and alleviates PD symptoms [158, 159]. Acupuncture also activates the PI3K/Akt pathway, which elevates the Bcl-2 level and reduces dopamine- and cAMP-regulated phosphoprotein of 32 kDa and Fos B. Through the activation of the PI3K/Akt pathway, acupuncture increases the dopamine turnover rate and availability in the synapse of the SN and striatum and regulates the tyrosine hydroxylase–positive cell cycle, thus improving motor function [160–162]. Lu et al. demonstrated that EA on KI3 inactivates pPKA/pPKC/CaMKIIα signaling pathways and reduces neuronal excitotoxicity in the hippocampus [163].
Rapamycin, an inhibitor of mTOR, is a potent inducer of autophagy and has an effect on PD [181]. However, rapamycin-based treatments for PD show adverse effects, including dyslipidemia, proliferative dysregulation, and renal dysfunction [182]. Acupuncture on GB34 affected the downstream autophagy–lysosome pathway through the m-TOR-independent pathway; this effect was comparable to that observed in the rapamycin treatment group [164]. Acupuncture induced autophagic clearance of α-syn, caused recovery of DA neurons in the SN, and improved motor function of an animal model without any notable adverse effect [164].
Oxidative stress and inflammation both contribute to the neural toxicity and development of PD [183]. Many studies have indicated the use of high-frequency EA for treating PD motor symptoms in animal models [184, 185]. Kim et al. reported that high-frequency EA on GB34 and GB39 increased tyrosine hydroxylase–positive neurons and cytochrome c oxidase subunit Vb and reduced cytosolic malate dehydrogenase, munc18-1, and hydroxyacylglutathione hydrolase levels, thus exerting an antioxidative effect on the SN [165]. Lv et al. demonstrated that EA at 100 Hz on ST36 and SP6 exerted a neuroprotective effect on PD mice and reversed the increase in the levels of Iba-1 and proinflammatory cytokines, including TNF-α, IL-6, and IL-1β, induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), thus suppressing the neuroinflammatory process [166]. The nuclear factor erythroid 2 -related factor 2 (Nrf2)–antioxidant response element (ARE) pathway regulates oxidative stress and inflammatory responses. EA enhances the Nrf2–ARE pathway and regulates the expression of antioxidants, such as the ARE-driven reporter gene, nicotinamide adenine dinucleotide phosphate quinone oxidoreductase, and heme oxygenase-1 (HO-1), thus relieving PD symptoms [166]. Similarly, Deng et al. reported that EA at 100 Hz on ST36 and SP6 elevated HO-1 and glutamate–cysteine ligase modifier subunits and reduced astrogliosis and neuroinflammation through the Nrf2–ARE pathway [167].
PD symptoms were relieved through the modification of TLR/NF-κB and Nrf2/HO-1 pathways [186]. EA on GV16 and LR3 upregulated NFκB protein expression and downregulated 26S proteasome protein expression in rotenone-induced PD rats [187]. P53 plays a role in DNA repair or cell death depending on the nature and extent of stress and damage [188]. P53 dysfunction was reported in neurodegenerative diseases and cancers [189]. Park et al. demonstrated that acupuncture on GB34 activated the p53 signaling pathway, protected dopaminergic neurons in the SN and striatum, and treated PD symptoms [168].
At the gene level, Choi et al. demonstrated that EA regulated gene expression in the striatum and exerted a neuroprotective effect on MPTP parkinsonism mice [190, 191]. Yeo et al. performed a microarray analysis study of acupuncture on GB34 and LR3 in an MPTP mouse model of parkinsonism and reported that acupuncture reversed the downregulation of five annotated genes and upregulation of three annotated genes through MPTP intoxication [192].
In summary, acupuncture improved motor dysfunction and memory of PD. These effects were accompanied by the regulation of gene expression. Acupuncture modulates neuroinflammation by inactivating ERK 1/2 and p38/MAPK signaling pathway and reduces neuronal excitotoxicity through the pPKA/pPKC/CaMKIIα signaling pathway. Acupuncture also regulates apoptosis by balancing the Bcl-2 and m-TOR-independent pathway. The most chosen acupoints include GB34, LR3, and GV16. Moreover, high-frequency EA (100 Hz) on ST36 and SP6 reduces neuroinflammation through the Nrf2–ARE pathway. The mechanisms and main results of identified articles are summarized in Table 7.
Table 7.
Signal transduction pathways of acupuncture in treating Parkinson's disease.
| Subjects | Location | Acupoint | Intervention | Time of intervention | Signal pathway | Main results | Author, reference |
|---|---|---|---|---|---|---|---|
| Male SD rats, rotenone injection | substantia nigra | GV16, LR3 | EA, 1mA, 2Hz | 20min, QD for 14 days | inactivation of p38-MAPK pathway | elevation of tyrosine hydroxylase-positive neuron depression of phosphorylated p38-MAPK, COX-2 decrease neuroinflammation |
Wang SJ, et al. 2013[158] |
|
| |||||||
| Male SD rats, rotenone injection | substantia nigra | GV16, LR3 | EA, 2mA, 2Hz | 20min, QD for 14 days | inactivation of ERK 1/2 pathway | elevation of tyrosine hydroxylase protein depression of p-ERK 1/2, TNF-α, IL-1β decrease neuroinflammation |
Wang SJ, et al. 2014[159] |
|
| |||||||
| Male C57BL/6 mice, MPTP injection | substantia nigra, striatum | GB34 | MA, 2Hz for 15sec | QD for 7 days | activation of PI3K/Akt pathway | elevation of pAkt prevents MPTP-induced dopaminergic neuron degeneration |
Kim SN, et al. 2011[160] |
|
| |||||||
| Male C57BL/6 mice, MPTP injection | substantia nigra pars compacta, striatum | GB34 | MA, 2Hz for 15sec | QD for 12 days | activation of PI3K/Akt pathway | elevation of dopamine depression of dopamine- and cAMP-regulated phosphoprotein of 32 kDa, Fos increase dopamine turnover rate |
Kim SN, et al. 2011[161] |
|
| |||||||
| Male, C57BL6 mice (MPTP intraperitoneal injection) and SD rats (Sigma-Aldrich injection into substantia nigra) | substantia nigra | GB34, LR3 | EA, 1mA, 50Hz | QD for 5(mice)/7(rats) days | activation of Akt pathway | elevation of BDNF, Bcl-2, tyrosine hydroxylase regulation of cell cycle |
Lin JG, et al. 2017[162] |
|
| |||||||
| Imprinting control region mouse pups, systemic 6- hydroxydopamine injection | hippocampus | KI3 | EA, 1mA, 2Hz | 15min, QD, 5 days/wk for 6wks | inactivation of pPKA/pPKC/CaMKIIα signaling pathways | depression of pNR1, pNR2B, pPKA, pPKC, pCaMKIIα, pERK, pCREB reduce neuronal excitotoxicity |
Lu KW, et al. 2017[163] |
|
| |||||||
| Male C57BL/6 mice, MPTP injection | substantia nigra par compacta | GB34 | MA, 2Hz for 15sec every 5min | 10min, QD for 7 days | m-TOR independent pathway | depression of α-synuclein induces autophagic clearance of α-syn, dopaminergic neurons protection |
Tian T, et al. 2016[164] |
|
| |||||||
| Male C57BL/6 mice, MPTP injection | substantia nigra, striatum | GB34, GB39 | EA, 1mA, 100Hz | 20min, QD for 12 days | regulation of glyoxalase system | elevation of tyrosine hydroxylase-positive neurons, cytochrome c oxidase subunit Vb depression of cytosolic malate dehydrogenase, munc18-1, hydroxyacylglutathione hydrolase anti-oxidative effect |
Kim ST, et al. 2010[165] |
|
| |||||||
| Male C57BL/6 mice, MPTP injection | midbrain, striatum | ST36, SP6 | EA, 1-1.4mA, 100Hz | 30min, QD for 12 days, except day 7 | activation of Nrf2-ARE pathway | elevation of tyrosine hydroxylase, ARE-driven reporter gene, NQO1, HO-1 depression of ionized calcium-binding adaptor molecule 1, TNF-α, IL-6, IL-1β anti-oxidative effect |
Lv E, et al. 2015[166] |
|
| |||||||
| GFAP-tTA/tetO-α-syn double transgenic mice | midbrain, striatum | ST36, SP6 | EA, 1-1.2mA, 100Hz | 30min, QD for 28 days | activation of Nrf2-ARE pathway | elevation of Nrf2, HO-1, glutamate-cysteine ligase modifier subunits depression of α-syn decrease astrogliosis and neuroinflammation |
Deng J, et al. 2015[167] |
|
| |||||||
| Male C57BL/6 mice, MPTP injection | striatum, substantia nigra | GB34 | MA, 2Hz, 15sec | QD for 12 days | activation of p53 signaling pathways | elevation of p53 dopaminergic neuron protection |
Park JY, et al. 2015[168] |
Abbreviations
Akt: protein kinase B; ARE: antioxidant response element; CaMK: Ca2+/calmodulin-dependent protein kinase; cAMP: cyclic adenosine monophosphate; COX: cyclooxygenase; CREB: phosphorylated cyclic AMP response element-binding protein; EA: electroacupuncture; ERK: extracellular signal-regulated kinase; HO-1: heme oxygenase-1; IL: interleukin; MA: manual acupuncture; MPTP: 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine; mTOR: mammalian target of rapamycin; NQO1: nicotinamide adenine dinucleotide phosphate quinone oxidoreductase; Nrf2: nuclear factor erythroid 2-related factor 2; p38 MAPKs: p38 mitogen-activated protein kinases; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PKA: protein kinase A; PKC: protein kinase C; pNR: phosphorylated N-methyl-D-aspartate receptor; QD: daily; SD rat: Sprague Dawley rat; TNF-α: tumor necrosis factor-alpha.
10. Conclusion
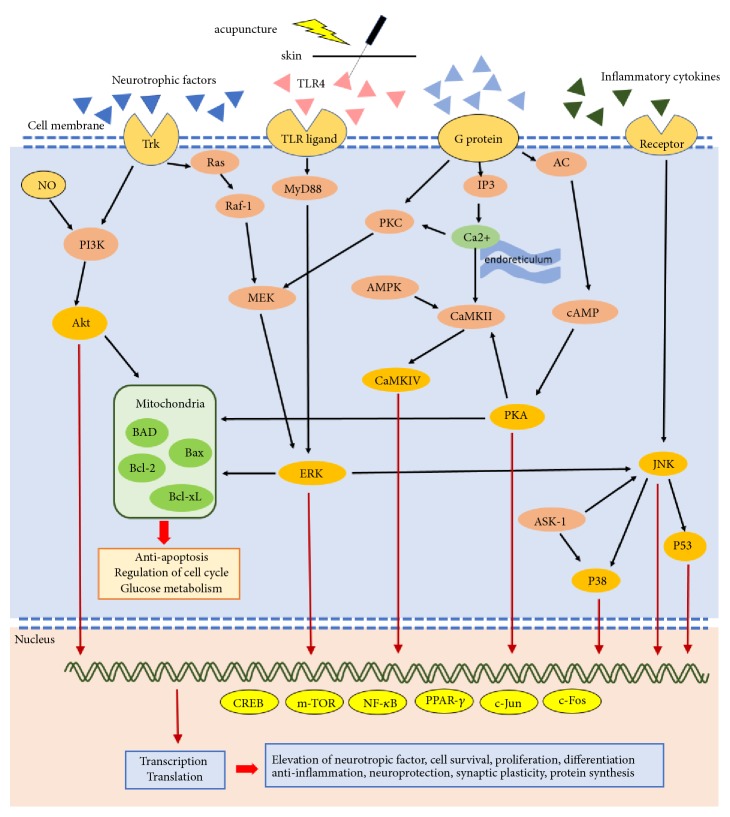
Acupuncture treats nervous system diseases through many signal transduction pathways. Besides increasing the neurotrophic factors level, acupuncture influences pathways including p38 MAPKs, Raf/MAPK/ERK1/2, TLR4/ERK, PI3K/AKT, AC/cAMP/PKA, ASK1–JNK/p38, and downstream CREB, JNK, m-TOR, NF-κB, and Bcl-2/Bax balance. We summarized the common signal transduction pathways through which acupuncture treats nervous system diseases (Figure 2). Through the aforementioned pathways, acupuncture affects synaptic plasticity, elevates neurotrophic factors, and results in neuroprotection, cell proliferation, antiapoptosis, antioxidant activity, anti-inflammation, and maintenance of the BBB.
Figure 2.
Summary of signal transduction pathways through which acupuncture treats nervous system diseases. Acupuncture is applied on acupoints and results in de qi, evoking excitation of cell membrane receptors, such as the Trk and TLR/ligand, and subsequently producing signal transduction. AC: adenyl cyclase; Akt: protein kinase B; AMPK: AMP-activated protein kinase; ASK-1: apoptosis signal-regulating kinase 1; Bad: Bcl-2-associated death promoter; Bax: Bcl-2 associated X; Bcl-2: B-cell lymphoma 2; Bcl2-xl: B-cell lymphoma-extralarge; CaMK: Ca2+/calmodulin-dependent protein kinase; cAMP: cyclic adenosine monophosphate; CREB: phosphorylated cyclic AMP response element-binding protein; ERK: extracellular signal-regulated kinase; IP3: inositol triphosphate; JNK: c-Jun N-terminal kinases; MEK: mitogen-activated protein kinase/extracellular signal-regulated kinase kinase; mTOR: mammalian target of rapamycin; MyD88: myeloid differentiation primary response 88; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NO: nitric oxide; PI3K: phosphatidylinositol-4,5-bisphosphate 3-kinase; PKA: protein kinase A; PKC: protein kinase C; PPAR-γ: peroxisome proliferator-activated receptor γ; TLR: Toll-like receptor; Trk: tyrosine receptor kinase.
Acknowledgments
This work was financially supported by the “Chinese Medicine Research Center, China Medical University” from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan (CMRC-CENTER-0). This study also was supported by grant DMR-108-176 from China Medical University Hospital.
Data Availability
The data in this study are available to other researchers upon request.
Conflicts of Interest
We declare that there are no conflicts of interest associated with this manuscript, and no significant financial support was received that would influence our findings.
Authors' Contributions
Hsiang-Chun Lai collected data and wrote the manuscript, Qwang-Yuen Chang participated in discussions and provided suggestions, and Ching-Liang Hsieh provided an informed opinion and revised the manuscript.
References
- 1.Zhou W., Benharash P. Effects and mechanisms of acupuncture based on the principle of meridians. Journal of Acupuncture and Meridian Studies. 2014;7(4):190–193. doi: 10.1016/j.jams.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 2.He X. R., Wang Q., Li P. P. Acupuncture and moxibustion for cancer-related fatigue: a systematic review and meta-analysis. Asian Pacific Journal of Cancer Prevention. 2013;14(5):3067–3074. doi: 10.7314/APJCP.2013.14.5.3067. [DOI] [PubMed] [Google Scholar]
- 3.Cox J., Varatharajan S., Côté P., Optima Collaboration Effectiveness of acupuncture therapies to manage musculoskeletal disorders of the extremities: a systematic review. Journal of Orthopaedic and Sports Physical Therapy. 2016;46(6):409–429. doi: 10.2519/jospt.2016.6270. [DOI] [PubMed] [Google Scholar]
- 4.Forde J. C., Jaffe E., Stone B. V., Te A. E., Espinosa G., Chughtai B. The role of acupuncture in managing overactive bladder; a review of the literature. International Urogynecology Journal. 2016;27(11):1645–1651. doi: 10.1007/s00192-015-2935-y. [DOI] [PubMed] [Google Scholar]
- 5.Yao Q., Li S., Liu X., Qin Z., Liu Z. The effectiveness and safety of acupuncture for patients with chronic urticaria: a systematic review. BioMed Research International. 2016;2016:7. doi: 10.1155/2016/5191729.5191729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan A. Y., Miller D. W., Bolash B., et al. Acupuncture's role in solving the opioid epidemic: evidence, cost-effectiveness, and care availability for acupuncture as a primary, non-pharmacologic method for pain relief and management–white paper 2017. Journal of Integrative Medicine. 2017;15(6):411–425. doi: 10.1016/S2095-4964(17)60378-9. [DOI] [PubMed] [Google Scholar]
- 7.Park J., Sohn Y., White A. R., Lee H. The safety of acupuncture during pregnancy: a systematic review. Acupuncture in Medicine. 2014;32(3):257–266. doi: 10.1136/acupmed-2013-010480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang C., Hao Z., Zhang L., Guo Q. Efficacy and safety of acupuncture in children: an overview of systematic reviews. Pediatric Research. 2015;78(2):112–119. doi: 10.1038/pr.2015.91. [DOI] [PubMed] [Google Scholar]
- 9.Wang K., Wu H., Wang G., Li M., Zhang Z., Gu G. The effects of electroacupuncture on Th1/Th2 cytokine mRNA expression and mitogen-activated protein kinase signaling pathways in the splenic t cells of traumatized rats. Anesthesia & Analgesia. 2009;109(5):1666–1673. doi: 10.1213/ANE.0b013e3181b5a234. [DOI] [PubMed] [Google Scholar]
- 10.Cheng K. J. Neurobiological mechanisms of acupuncture for some common illnesses: a clinician's perspective. Journal of Acupuncture and Meridian Studies. 2014;7(3):105–114. doi: 10.1016/j.jams.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 11.Cai R. L., Shen G. M., Wang H., Guan Y. Y. Brain functional connectivity network studies of acupuncture: a systematic review on resting-state fMRI. Journal of Integrative Medicine. 2018;16(1):26–33. doi: 10.1016/j.joim.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Xue X., You Y., Tao J., et al. Electro-acupuncture at points of Zusanli and Quchi exerts anti-apoptotic effect through the modulation of PI3K/Akt signaling pathway. Neuroscience Letters. 2014;558:14–19. doi: 10.1016/j.neulet.2013.10.029. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Wang L., Liang F., et al. Effects of electroacupuncture on electrocardiogram, myocardial pathological morphology and PI3K/Akt pathway in rats with chronic myocardial ischemia. Zhongguo Zhen Jiu. 2016;36(4):389–395. [PubMed] [Google Scholar]
- 14.Liao H., Sun M., Lin J., Chang S., Lee Y. Electroacupuncture plus metformin lowers glucose levels and facilitates insulin sensitivity by activating mapk in steroid-induced insulin-resistant rats. Acupuncture in Medicine. 2018;33(5):388–394. doi: 10.1136/acupmed-2014-010724. [DOI] [PubMed] [Google Scholar]
- 15.Zhang H., Qin F., Liu A., et al. Electro-acupuncture attenuates the mice premature ovarian failure via mediating PI3K/AKT/mTOR pathway. Life Sciences. 2019;217:169–175. doi: 10.1016/j.lfs.2018.11.059. [DOI] [PubMed] [Google Scholar]
- 16.Hwang I. K., Chung J. Y., Yoo D. Y., et al. Effects of electroacupuncture at Zusanli and Baihui on brain-derived neurotrophic factor and cyclic AMP response element-binding protein in the hippocampal dentate gyrus. Journal of Veterinary Medical Science. 2010;72(11):1431–1436. doi: 10.1292/jvms.09-0527. [DOI] [PubMed] [Google Scholar]
- 17.Liu X. Y., Zhou H. F., Pan Y. L., et al. Electro-acupuncture stimulation protects dopaminergic neurons from inflammation-mediated damage in medial forebrain bundle-transected rats. Experimental Neurology. 2004;189(1):189–196. doi: 10.1016/j.expneurol.2004.05.028. [DOI] [PubMed] [Google Scholar]
- 18.Ding X., Yu J., Yu T., Fu Y., Han J. Acupuncture regulates the aging-related changes in gene profile expression of the hippocampus in senescence-accelerated mouse (SAMP10) Neuroscience Letters. 2006;399(1-2):11–16. doi: 10.1016/j.neulet.2006.01.067. [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Lan R., Wang J., et al. Acupuncture reduced apoptosis and up-regulated BDNF and GDNF expression in hippocampus following hypoxia-ischemia in neonatal rats. Journal of Ethnopharmacology. 2015;172:124–132. doi: 10.1016/j.jep.2015.06.032. [DOI] [PubMed] [Google Scholar]
- 20.Dong W. G., Guo W. Q., Zheng X. H., et al. Electroacupuncture improves cognitive deficits associated with AMPK activation in SAMP8 mice. Metabolic Brain Disease. 2015;30(3):777–784. doi: 10.1007/s11011-014-9641-1. [DOI] [PubMed] [Google Scholar]
- 21.Lu L., Zhang X. G., Zhong L. L., et al. Acupuncture for neurogenesis in experimental ischemic stroke: a systematic review and meta-analysis. Scientific Reports. 2016;6 doi: 10.1038/srep19521.19521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chavez L. M., Huang S. S., MacDonald I., Lin J. G., Lee Y. C., Chen Y. H. Mechanisms of acupuncture therapy in ischemic stroke rehabilitation: a literature review of basic studies. International Journal of Molecular Sciences. 2017;18(11) doi: 10.3390/ijms18112270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shih C. C., Liao C. C., Sun M. F., et al. A Retrospective cohort study comparing stroke recurrence rate in ischemic stroke patients with and without acupuncture treatment. Medicine. 2015;94(39) doi: 10.1097/MD.0000000000001572.e1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang T., Sun S., Wang T., et al. Piperlonguminine is neuroprotective in experimental rat stroke. International Immunopharmacology. 2014;23(2):447–451. doi: 10.1016/j.intimp.2014.09.016. [DOI] [PubMed] [Google Scholar]
- 25.Xu T., Xu N., Yang Z., Wan Y., Wu Q., Huang K. Neuroprotective effects of electroacupuncture on hypoxic-ischemic encephalopathy in newborn rats are associated with increased expression of GDNF-RET and protein kinase B. Chinese Journal of Integrative Medicine. 2016;22(6):457–466. doi: 10.1007/s11655-015-1972-1. [DOI] [PubMed] [Google Scholar]
- 26.Shin H. K., Lee S.-W., Choi B. T. Modulation of neurogenesis via neurotrophic factors in acupuncture treatments for neurological diseases. Biochemical Pharmacology. 2017;141:132–142. doi: 10.1016/j.bcp.2017.04.029. [DOI] [PubMed] [Google Scholar]
- 27.Kim Y. R., Kim H. N., Ahn S. M., Choi Y. H., Shin H. K., Choi B. T. Electroacupuncture promotes post-stroke functional recovery via enhancing endogenous neurogenesis in mouse focal cerebral ischemia. PLoS ONE. 2014;9(2) doi: 10.1371/journal.pone.0090000.e90000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim M., Kim M.-W., Chung Y. C., et al. Electroacupuncture enhances motor recovery performance with brain-Derived neurotrophic factor expression in rats with cerebral infarction. Acupuncture in Medicine. 2012;30(3):222–226. doi: 10.1136/acupmed-2011-010126. [DOI] [PubMed] [Google Scholar]
- 29.Huang J., Ye X., You Y., et al. Electroacupuncture promotes neural cell proliferation in vivo through activation of the ERK1/2 signaling pathway. International Journal of Molecular Medicine. 2014;33(6):1547–1553. doi: 10.3892/ijmm.2014.1702. [DOI] [PubMed] [Google Scholar]
- 30.Sawe N., Steinberg G., Zhao H. Dual roles of the MAPK/ERK1/2 cell signaling pathway after stroke. Journal of Neuroscience Research. 2008;86(8):1659–1669. doi: 10.1002/jnr.21604. [DOI] [PubMed] [Google Scholar]
- 31.Lai T. W., Zhang S., Wang Y. T. Excitotoxicity and stroke: identifying novel targets for neuroprotection. Progress in Neurobiology. 2014;115:157–188. doi: 10.1016/j.pneurobio.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Du J., Wang Q., Hu B., et al. Involvement of ERK 1/2 activation in electroacupuncture pretreatment via cannabinoid CB1 receptor in rats. Brain Research. 2010;1360:1–7. doi: 10.1016/j.brainres.2010.07.034. [DOI] [PubMed] [Google Scholar]
- 33.Xie G., Yang S., Chen A., et al. Electroacupuncture at Quchi and Zusanli treats cerebral ischemia-reperfusion injury through activation of ERK signaling. Experimental and Therapeutic Medicine. 2013;5(6):1593–1597. doi: 10.3892/etm.2013.1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng C. Y., Lin J. G., Su S. Y., Tang N. Y., Kao S. T., Hsieh C. L. Electroacupuncture-like stimulation at Baihui and Dazhui acupoints exerts neuroprotective effects through activation of the brain-derived neurotrophic factor-mediated MEK1/2/ERK1/2/p90RSK/bad signaling pathway in mild transient focal cerebral ischemia in rats. BMC Complementary and Alternative Medicine. 2014;14:p. 92. doi: 10.1186/1472-6882-14-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wu C., Wang J., Li C., et al. Effect of electroacupuncture on cell apoptosis and erk signal pathway in the hippocampus of adult rats with cerebral ischemia-reperfusion. Evidence-Based Complementary and Alternative Medicine. 2015;2015:10. doi: 10.1155/2015/414965.414965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu C., Li C., Zhou G., et al. Effects of electroacupuncture on the cortical extracellular signal regulated kinase pathway in rats with cerebral ischaemia/reperfusion. Acupuncture in Medicine. 2018;35(6):430–436. doi: 10.1136/acupmed-2016-011121. [DOI] [PubMed] [Google Scholar]
- 37.Johnson G. L., Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298(5600):1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 38.Yang S., Yuan Y., Jiao S., Luo Q., Yu J. Calcitonin gene-related peptide protects rats from cerebral ischemia/reperfusion injury via a mechanism of action in the MAPK pathway. Biomedical Reports. 2016;4(6):699–703. doi: 10.3892/br.2016.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cheng C.-Y., Tang N.-Y., Kao S.-T., Hsieh C.-L. Ferulic acid administered at various time points protects against cerebral infarction by activating p38 MAPK/p90RSK/CREB/Bcl-2 anti-apoptotic signaling in the subacute phase of cerebral ischemia-reperfusion injury in rats. PLoS ONE. 2016;11(5) doi: 10.1371/journal.pone.0155748.e0155748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cheng C.-Y., Ho T.-Y., Hsiang C.-Y., et al. Angelica sinensis exerts angiogenic and anti-apoptotic effects against cerebral ischemia-reperfusion injury by activating p38MAPK/HIF-1 α /VEGF-A signaling in rats. American Journal of Chinese Medicine. 2017;45(8):1683–1708. doi: 10.1142/S0192415X17500914. [DOI] [PubMed] [Google Scholar]
- 41.Lan X., Zhang X., Zhou G., Wu C., Li C., Xu X. Electroacupuncture reduces apoptotic index and inhibits p38 mitogen-activated protein kinase signaling pathway in the hippocampus of rats with cerebral ischemia/reperfusion injury. Neural Regeneration Research. 2017;12(3):409–416. doi: 10.4103/1673-5374.202944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin Y., Liu Q., Chen C., et al. Effect of acupuncture combined with hypothermia on MAPK/ERK pathway and apoptosis related factors in rats with cerebral ischemia reperfusion injury. Journal of Central South University. Medical Sciences. 2017;42(4):380–388. doi: 10.11817/j.issn.1672-7347.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 43.Xing Y., Yang S., Wang M., Dong F., Feng Y., Zhang F. Electroacupuncture alleviated neuronal apoptosis following ischemic stroke in rats via midkine and ERK/JNK/p38 signaling pathway. Journal of Molecular Neuroscience. 2018;66(1):26–36. doi: 10.1007/s12031-018-1142-y. [DOI] [PubMed] [Google Scholar]
- 44.Liu J., Wang Q., Yang S., et al. Electroacupuncture inhibits apoptosis of peri-ischemic regions via modulating p38, extracellular signal-regulated kinase (ERK1/2), and c-Jun N terminal kinases (jnk) in cerebral ischemia-reperfusion-injured rats. Medical Science Monitor. 2018;24:4395–4404. doi: 10.12659/MSM.908473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Liu W., Wang X., Yang S., et al. Electroacupunctre improves motor impairment via inhibition of microglia-mediated neuroinflammation in the sensorimotor cortex after ischemic stroke. Life Sciences. 2016;151:313–322. doi: 10.1016/j.lfs.2016.01.045. [DOI] [PubMed] [Google Scholar]
- 46.Cheng C.-Y., Lin J.-G., Tang N.-Y., Kao S.-T., Hsieh C.-L. Electroacupuncture at different frequencies (5Hz and 25Hz) ameliorates cerebral ischemia-reperfusion injury in rats: possible involvement of p38 MAPK-mediated anti-apoptotic signaling pathways. BMC Complementary and Alternative Medicine. 2015;15(1):p. 241. doi: 10.1186/s12906-015-0752-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li Q.-Q., Shi G.-X., Yang J.-W., et al. Hippocampal cAMP/PKA/CREB is required for neuroprotective effect of acupuncture. Physiology & Behavior. 2015;139:482–490. doi: 10.1016/j.physbeh.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 48.Lin R., Lin Y., Tao J., et al. Electroacupuncture ameliorates learning and memory in rats with cerebral ischemia-reperfusion injury by inhibiting oxidative stress and promoting p-CREB expression in the hippocampus. Molecular Medicine Reports. 2015;12(5):6807–6814. doi: 10.3892/mmr.2015.4321. [DOI] [PubMed] [Google Scholar]
- 49.Ahn S. M., Kim Y. R., Kim H. N., Shin Y., Shin H. K., Choi B. T. Electroacupuncture ameliorates memory impairments by enhancing oligodendrocyte regeneration in a mouse model of prolonged cerebral hypoperfusion. Scientific Reports. 2016;6(1) doi: 10.1038/srep28646.28646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y., Lin R., Tao J., et al. Electroacupuncture improves cognitive ability following cerebral ischemia reperfusion injury via CaM-CaMKIV-CREB signaling in the rat hippocampus. Experimental and Therapeutic Medicine. 2016;12(2):777–782. doi: 10.3892/etm.2016.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yun Y. C., Jang D., Yoon S. B., et al. Laser acupuncture exerts neuroprotective effects via regulation of Creb, Bdnf, Bcl-2, and Bax gene expressions in the hippocampus. Evidence-Based Complementary and Alternative Medicine. 2017;2017:11. doi: 10.1155/2017/7181637.7181637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pak M. E., Jung D. H., Lee H. J., et al. Combined therapy involving electroacupuncture and treadmill exercise attenuates demyelination in the corpus callosum by stimulating oligodendrogenesis in a rat model of neonatal hypoxia-ischemia. Experimental Neurology. 2018;300:222–231. doi: 10.1016/j.expneurol.2017.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Ishrat T., Sayeed I., Atif F., Hua F., Stein D. G. Progesterone is neuroprotective against ischemic brain injury through its effects on the phosphoinositide 3-kinase/protein kinase B signaling pathway. Neuroscience. 2012;210:442–450. doi: 10.1016/j.neuroscience.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou J., Du T., Li B., Rong Y., Verkhratsky A., Peng L. Crosstalk between MAPK/ERK and PI3K/AKT signal pathways during brain ischemia/reperfusion. ASN Neuro. 2015;7(5) doi: 10.1177/1759091415602463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang S. J., Omori N., Li F., et al. Potentiation of Akt and suppression of caspase-9 activations by electroacupuncture after transient middle cerebral artery occlusion in rats. Neuroscience Letters. 2002;331(2):115–118. doi: 10.1016/S0304-3940(02)00866-2. [DOI] [PubMed] [Google Scholar]
- 56.Sun N., Zou X., Shi J., Liu X., Li L., Zhao L. Electroacupuncture regulates NMDA receptor NR1 subunit expression via PI3-K pathway in a rat model of cerebral ischemia-reperfusion. Brain Research. 2005;1064(1-2):98–107. doi: 10.1016/j.brainres.2005.09.060. [DOI] [PubMed] [Google Scholar]
- 57.Zhao L., Wang Y., Sun N., Liu X., Li L., Shi J. Electroacupuncture regulates TRPM7 expression through the trkA/PI3K pathway after cerebral ischemia–reperfusion in rats. Life Sciences. 2007;81(15):1211–1222. doi: 10.1016/j.lfs.2007.08.034. [DOI] [PubMed] [Google Scholar]
- 58.Chen S.-X., Ding M.-C., Dai K.-Y. Effect of electroacupuncture on nitric oxide synthase in rats with cerebral ischemia-reperfusion injury. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2011;31(6):784–788. [PubMed] [Google Scholar]
- 59.Chen A., Lin Z., Lan L., et al. Electroacupuncture at the Quchi and Zusanli acupoints exerts neuroprotective role in cerebral ischemia-reperfusion injured rats via activation of the PI3K/Akt pathway. International Journal of Molecular Medicine. 2012;30(4):791–796. doi: 10.3892/ijmm.2012.1074. [DOI] [PubMed] [Google Scholar]
- 60.Xu T., Li W., Liang Y., et al. Neuroprotective effects of electro acupuncture on hypoxic-ischemic encephalopathy in newborn rats Ass. Pakistan Journal of Pharmaceutical Sciences. 2014;27(6, supplement):1991–2000. [PubMed] [Google Scholar]
- 61.Kim Y. R., Kim H. N., Jang J. Y., et al. Effects of electroacupuncture on apoptotic pathways in a rat model of focal cerebral ischemia. International Journal of Molecular Medicine. 2013;32(6):1303–1310. doi: 10.3892/ijmm.2013.1511. [DOI] [PubMed] [Google Scholar]
- 62.Liu W., Shang G., Yang S., et al. Electroacupuncture protects against ischemic stroke by reducing autophagosome formation and inhibiting autophagy through the mTORC1-ULK1 complex-Beclin1 pathway. International Journal of Molecular Medicine. 2016;37(2):309–318. doi: 10.3892/ijmm.2015.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xie C. C., Luo Y., Pang Y. S., et al. Effect of electroacupuncture on CD 34+ endothelial progenitor cell counts in bone marrow and peripheral blood in focal cerebral ischemia/reperfusion rats. Zhen Ci Yan Jiu. 2014;39(6):437–442. [PubMed] [Google Scholar]
- 64.Wei H., Yao X., Yang L., et al. Glycogen synthase kinase-3β is involved in electroacupuncture pretreatment via the cannabinoid CB1 receptor in ischemic stroke. Molecular Neurobiology. 2014;49(1):326–336. doi: 10.1007/s12035-013-8524-5. [DOI] [PubMed] [Google Scholar]
- 65.Zou R., Wu Z., Cui S. Electroacupuncture pretreatment attenuates blood-brain barrier disruption following cerebral ischemia/reperfusion. Molecular Medicine Reports. 2015;12(2):2027–2034. doi: 10.3892/mmr.2015.3672. [DOI] [PubMed] [Google Scholar]
- 66.Feng X., Yang S., Liu J., et al. Electroacupuncture ameliorates cognitive impairment through inhibition of NF-κB-mediated neuronal cell apoptosis in cerebral ischemia-reperfusion injured rats. Molecular Medicine Reports. 2013;7(5):1516–1522. doi: 10.3892/mmr.2013.1392. [DOI] [PubMed] [Google Scholar]
- 67.Lan L., Tao J., Chen A., et al. Electroacupuncture exerts anti-inflammatory effects in cerebral ischemia-reperfusion injured rats via suppression of the TLR4/NF-κB pathway. International Journal of Molecular Medicine. 2013;31(1):75–80. doi: 10.3892/ijmm.2012.1184. [DOI] [PubMed] [Google Scholar]
- 68.Liu Z., Guan L., Wang Y., Xie C.-L., Lin X.-M., Zheng G.-Q. History and mechanism for treatment of intracerebral hemorrhage with scalp acupuncture. Evidence-Based Complementary and Alternative Medicine. 2012;2012:9. doi: 10.1155/2012/895032.895032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li T., Zhao J.-G., Tian G.-J., Zhang L., Liu S.-J. Clinical observation on effect of acupuncture on nervous functions of the patient after operation of. hypertensive cerebral hemorrhage. Chinese Acupuncture & Moxibustion. 2006;26(4):247–249. [PubMed] [Google Scholar]
- 70.Wang H.-Q., Bao C.-L., Jiao Z.-H., Dong G.-R. Efficacy and safety of penetration acupuncture on head for acute intracerebral hemorrhage: A randomized controlled study. Medicine. 2016;95(48) doi: 10.1097/MD.0000000000005562.e5562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li H. Q., Li J. H., Liu A. J., Ye M. Y., Zheng G. Q. GV20-based acupuncture for animal models of acute intracerebral haemorrhage: a preclinical systematic review and meta-analysis. Acupuncture in Medicine. 2014;32(6):495–502. doi: 10.1136/acupmed-2014-010546. [DOI] [PubMed] [Google Scholar]
- 72.Zhang G.-W., Zou W., Liu F. Effects of the scalp acupuncture at baihui (DU20) through qubin (GB7) on the expressions of GDNF VEGF in the brain tissue of rats with acute intracerebral hemorrhage. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2012;32(9):1264–1270. [PubMed] [Google Scholar]
- 73.Zhou H., Tang T., Zhong J., et al. Electroacupuncture improves recovery after hemorrhagic brain injury by inducing the expression of angiopoietin-1 and -2 in rats. BMC Complementary and Alternative Medicine. 2014;14(1):p. 127. doi: 10.1186/1472-6882-14-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Liu H., Sun X., Zou W., et al. Scalp acupuncture attenuates neurological deficits in a rat model of hemorrhagic stroke. Complementary Therapies in Medicine. 2017;32:85–90. doi: 10.1016/j.ctim.2017.03.014. [DOI] [PubMed] [Google Scholar]
- 75.Li H. Q., Li Y., Chen Z. X., et al. Electroacupuncture exerts neuroprotection through caveolin-1 mediated molecular pathway in intracerebral hemorrhage of rats. Neural Plasticity. 2016;2016:8. doi: 10.1155/2016/7308261.7308261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhu Y., Deng L., Tang H., et al. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Research Bulletin. 2017;131:123–132. doi: 10.1016/j.brainresbull.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 77.Li Z., Zheng X., Li P., Itoua E. S., Moukassa D., Ndinga Andely F. Effects of acupuncture on mrna levels of apoptotic factors in perihematomal brain tissue during the acute phase of cerebral hemorrhage. Medical Science Monitor. 2017;23:1522–1532. doi: 10.12659/MSM.897689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Gavvala J. R., Schuele S. U. New-onset seizure in adults and adolescents: A review. Journal of the American Medical Association. 2016;316(24):2657–2668. doi: 10.1001/jama.2016.18625. [DOI] [PubMed] [Google Scholar]
- 79.Goldberg E. M., Coulter D. A. Mechanisms of epileptogenesis: a convergence on neural circuit dysfunction. Nature Reviews Neuroscience. 2013;14(5):337–349. doi: 10.1038/nrn3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rong P., Liu A., Zhang J., et al. An alternative therapy for drug-resistant epilepsy: transcutaneous auricular vagus nerve stimulation. Chinese Medical Journal. 2014;127(2):300–304. [PubMed] [Google Scholar]
- 81.Cheuk D. K., Wong V. Acupuncture for epilepsy. Cochrane Database of Systematic Reviews. 2014;5 doi: 10.1002/14651858.CD005062.pub4.CD005062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li S., Liu Z., Zhao W., Jin B., Li N., Luo G. Scalp acupuncture for epileptiform discharges of children with cerebral palsy. Zhongguo Zhen Jiu. 2017;37(3):265–268. doi: 10.13703/j.0255-2930.2017.03.012. [DOI] [PubMed] [Google Scholar]
- 83.Sperk G., Wieselthaler-Holzl A., Pirker S., et al. Glutamate decarboxylase 67 is expressed in hippocampal mossy fibers of temporal lobe epilepsy patients. Hippocampus. 2012;22(3):590–603. doi: 10.1002/hipo.20923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gulcebi M., Akman O., Carcak N., Karamahmutoglu T., Onat F. Evaluation of GAD67 immunoreactivity in the region of substantia nigra pars reticulata in resistance to development of convulsive seizure in genetic absence epilepsy rats. Northern Clinics of İstanbul. 2017;3(3):161–167. doi: 10.14744/nci.2016.16056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Guo J., Liu J., Fu W., et al. The effect of electroacupuncture on spontaneous recurrent seizure and expression of GAD67 mRNA in dentate gyrus in a rat model of epilepsy. Brain Research. 2008;1188(1):165–172. doi: 10.1016/j.brainres.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 86.Guo J., Liu J., Fu W., et al. Effect of electroacupuncture stimulation of hindlimb on seizure incidence and supragranular mossy fiber sprouting in a rat model of epilepsy. The Journal of Physiological Sciences. 2008;58(5):309–315. doi: 10.2170/physiolsci.RP010508. [DOI] [PubMed] [Google Scholar]
- 87.Vezzani A. Epilepsy and inflammation in the brain: overview and pathophysiology. Epilepsy Currents. 2014;14(1):3–7. doi: 10.5698/1535-7511-14.s2.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim S., Doo A., Kim S., et al. Acupuncture suppresses kainic acid-induced neuronal death and inflammatory events in mouse hippocampus. The Journal of Physiological Sciences. 2012;62(5):377–383. doi: 10.1007/s12576-012-0216-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liao E.-T., Tang N.-Y., Lin Y.-W., Liang Hsieh C. Long-term electrical stimulation at ear and electro-acupuncture at ST36-ST37 attenuated COX-2 in the CA1 of hippocampus in kainic acid-induced epileptic seizure rats. Scientific Reports. 2017;7(1):p. 472. doi: 10.1038/s41598-017-00601-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang B. E., Cheng J. S. C-fos expression in rat brain during seizure and electroacupuncture. Zhongguo Yao Li Xue Bao. 1994;15(1):73–75. [PubMed] [Google Scholar]
- 91.Wang B., Yang R., Cheng J. Effect of electroacupuncture on the level of preproenkephalin mrna in rat during penicillin-induced epilepsy. Acupuncture & Electro-Therapeutics Research. 1994;19(2):129–140. doi: 10.3727/036012994816357277. [DOI] [PubMed] [Google Scholar]
- 92.Yang R., Huang Z. N., Cheng J. S. Anticonvulsion effect of acupuncture might be related to the decrease of neuronal and inducible nitric oxide synthases. Acupuncture & Electro-Therapeutics Research. 1999;24(3-4):161–167. doi: 10.3727/036012999816356327. [DOI] [PubMed] [Google Scholar]
- 93.Lin Y.-W., Hsieh C.-L. Auricular electroacupuncture reduced inflammation-related epilepsy accompanied by altered TRPA1, pPKCα, pPKCε, and pERk1/2 signaling pathways in kainic acid-treated rats. Mediators of Inflammation. 2014;2014:9. doi: 10.1155/2014/493480.493480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Liao E.-T., Lin Y.-W., Huang C.-P., Tang N.-Y., Hsieh C.-L. Electric stimulation of ear reduces the effect of toll-like receptor 4 signaling pathway on kainic acid-induced epileptic seizures in rats. BioMed Research International. 2018;2018:11. doi: 10.1155/2018/5407256.5407256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang F., Ang W.-P., Shen D.-K., Liu X.-G., Yang Y.-Q., Ma Y. PI 3 K/Akt signaling pathway contributed to the protective effect of acupuncture intervention on epileptic seizure-induced injury of hippocampal pyramidal cells in epilepsy rats. Zhen Ci Yan Jiu. 2013;38(1):20–25. [PubMed] [Google Scholar]
- 96.Yang F., Ma Y., Ang W.-P., et al. Effects of acupuncture intervention on expression of glucose-regulated protein 78 and C/EBP homologous protein in hippocampal CA 1 region in rats with hyperspasmia. Zhen Ci Yan Jiu. 2014;39(4):267–271. [PubMed] [Google Scholar]
- 97.Zhang H., Yang F., Wu X., et al. Protective effect of acupuncture serum derived from acute convulsion rats on cultured hip-pocampal neurons with seizure-like discharges by regulating expression of endoplasmic reticulum stress-inducible molecular chaperones. Acupuncture Research. 2017;42(2):95–101. [PubMed] [Google Scholar]
- 98.Mrazek D. A., Hornberger J. C., Altar C. A., Degtiar I. A review of the clinical, economic, and societal burden of treatment-resistant depression: 1996–2013. Psychiatric Services. 2014;65(8):977–987. doi: 10.1176/appi.ps.201300059. [DOI] [PubMed] [Google Scholar]
- 99.Haddad N. E., Palesh O. Acupuncture in the treatment of cancer-related psychological symptoms. Integrative Cancer Therapies. 2014;13(5):371–385. doi: 10.1177/1534735413520181. [DOI] [PubMed] [Google Scholar]
- 100.Bhattacharya A., Derecki N. C., Lovenberg T. W., Drevets W. C. Role of neuro-immunological factors in the pathophysiology of mood disorders. Psychopharmacology. 2016;233(9):1623–1636. doi: 10.1007/s00213-016-4214-0. [DOI] [PubMed] [Google Scholar]
- 101.Liu W., Ge T., Leng Y., et al. The role of neural plasticity in depression: from hippocampus to prefrontal cortex. Neural Plasticity. 2017;2017:11. doi: 10.1155/2017/6871089.6871089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kulikova E., Kulikov A. Striatal-enriched tyrosine protein phosphatase (STEP) in the mechanisms of depressive disorders. Current Protein & Peptide Science. 2017;18(11):1152–1162. doi: 10.2174/1389203718666170710121532. [DOI] [PubMed] [Google Scholar]
- 103.Hu L., Liang J., Jin S. Y., Han Y. J., Lu J., Tu Y. Progress of researches on mechanisms of acupuncture underlying improvement of depression in the past five years. Zhen Ci Yan Jiu. 2013;38(3):253–258. [PubMed] [Google Scholar]
- 104.Liang J., Lu J., Cui S. F., Wang J. R., Tu Y. Effect of acupuncture on expression of brain-derived neurotrophic factor gene and protein in frontal cortex and hippocampus of depression rats. Zhen Ci Yan Jiu. 2012;37(1):20–24. [PubMed] [Google Scholar]
- 105.Ji Q., Li Z.-G., Tang Y.-S., Mo Y.-P., Yao H.-J., Saiyin C.-K. Effect of electroacupuncture intervention on behavioral changes and hippocampal excitatory amino acid transporter mRNA expression in depression rats. Zhen Ci Yan Jiu. 2013;38(3):202–207, 219. [PubMed] [Google Scholar]
- 106.Fan L., Chen Z., Fu W., et al. Soluble N-ethylmaleimide-sensitive factor attachment receptor (SNARE) protein involved in the remission of depression by acupuncture in rats. Journal of Acupuncture and Meridian Studies. 2016;9(5):242–249. doi: 10.1016/j.jams.2016.04.002. [DOI] [PubMed] [Google Scholar]
- 107.Han Y. J., Li W. X., Jia B. H., Shi Y. J., Tu Y. Effect of electroacupuncture on hippocampal NO-cGMP signaling pathway in depression rats. Zhen Ci Yan Jiu. 2009;34(4):236–241. [PubMed] [Google Scholar]
- 108.Shao R. H., Jin S. Y., Lu J., Hu L., Tu Y. Effect of Acupuncture intervention on expression of NF-kappaB signal pathway in the hippocampus of chronic stress-induced depression rats. Zhen Ci Yan Jiu. 2015;40(5):368–372. [PubMed] [Google Scholar]
- 109.Lu J., Liang J., Wang J.-R., Hu L., Tu Y., Guo J.-Y. Acupuncture activates ERK-CREB pathway in rats exposed to chronic unpredictable mild stress. Evidence-Based Complementary and Alternative Medicine. 2013;2013:7. doi: 10.1155/2013/469765.469765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang L., Yue N., Zhu X., et al. Electroacupuncture upregulates ERK signaling pathways and promotes adult hippocampal neural progenitors proliferation in a rat model of depression. BMC Complementary and Alternative Medicine. 2013;13:p. 288. doi: 10.1186/1472-6882-13-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Xu J., She Y., Su N., Zhang R., Lao L., Xu S. Effects of electroacupuncture on chronic unpredictable mild stress rats depression-like behavior and expression of p-ERK/ERK and p-P38/P38. Evidence-Based Complementary and Alternative Medicine. 2015;2015:8. doi: 10.1155/2015/650729.650729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhang X., Song Y., Bao T., et al. Antidepressant-like effects of acupuncture involved the ERK signaling pathway in rats. BMC Complementary and Alternative Medicine. 2016;16(1):p. 380. doi: 10.1186/s12906-016-1356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li W., Zhu Y., Saud S. M., et al. Electroacupuncture relieves depression-like symptoms in rats exposed to chronic unpredictable mild stress by activating ERK signaling pathway. Neuroscience Letters. 2017;642:43–50. doi: 10.1016/j.neulet.2017.01.060. [DOI] [PubMed] [Google Scholar]
- 114.Yang X., Guo Z., Lu J., et al. The Role of MAPK and dopaminergic synapse signaling pathways in antidepressant effect of electroacupuncture pretreatment in chronic restraint stress rats. Evidence-Based Complementary and Alternative Medicine. 2017;2017:9. doi: 10.1155/2017/2357653.2357653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Dai W., Li W. D., Lu J. Effect of electroacupuncture on hippocampal apoptosis and JNK signal pathway in chronic stress depression rats. Zhen Ci Yan Jiu. 2010;35(5):330–334. [PubMed] [Google Scholar]
- 116.Guo Y., Xu K., Bao W. Y., et al. Effect of acupuncture intervention on c-jun N-terminal kinase signaling in the hippocampus in rats with forced swimming stress. Zhen Ci Yan Jiu. 2016;41(1):18–23. [PubMed] [Google Scholar]
- 117.Lu F., Zhu H. M., Xie J. J., Zhou H. H., Chen Y. L., Hu J. Y. Effects of electroacupuncture on behavior, plasma COR and expressions of PKA and PKC in hippocampus of the depression model rat. Zhongguo Zhen Jiu. 2008;28(3):214–218. [PubMed] [Google Scholar]
- 118.Liu J.-H., Wu Z.-F., Sun J., Jiang L., Jiang S., Fu W.-B. Role of AC-cAMP-PKA cascade in antidepressant action of electroacupuncture treatment in rats. Evidence-Based Complementary and Alternative Medicine. 2012;2012:7. doi: 10.1155/2012/932414.932414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duan D.-M., Tu Y., Liu P., Jiao S. Antidepressant effect of electroacupuncture regulates signal targeting in the brain and increases brain-derived neurotrophic factor levels. Neural Regeneration Research. 2016;11(10):1595–1602. doi: 10.4103/1673-5374.193238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jiang H., Zhang X., Wang Y., et al. Mechanisms underlying the antidepressant response of acupuncture via PKA/CREB signaling pathway. Neural Plasticity. 2017;2017:11. doi: 10.1155/2017/4135164.4135164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Oh J. Y., Kim Y. K., Kim S. N., et al. Acupuncture modulates stress response by the mTOR signaling pathway in a rat post-traumatic stress disorder model. Scientific Reports. 2018;8(1):p. 11864. doi: 10.1038/s41598-018-30337-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Duan D. M., Dong X., Tu Y., Liu P. A microarray study of chronic unpredictable mild stress rat blood serum with electro-acupuncture intervention. Neuroscience Letters. 2016;627:160–167. doi: 10.1016/j.neulet.2016.05.054. [DOI] [PubMed] [Google Scholar]
- 123.Wang Y., Jiang H., Meng H., et al. Genome-wide transcriptome analysis of hippocampus in rats indicated that TLR/NLR signaling pathway was involved in the pathogenisis of depressive disorder induced by chronic restraint stress. Brain Research Bulletin. 2017;134:195–204. doi: 10.1016/j.brainresbull.2017.07.021. [DOI] [PubMed] [Google Scholar]
- 124.Wang Y., Jiang H., Meng H., et al. Antidepressant mechanism research of acupuncture: insights from a genome-wide transcriptome analysis of frontal cortex in rats with chronic restraint stress. Evidence-Based Complementary and Alternative Medicine. 2017;2017:13. doi: 10.1155/2017/1676808.1676808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Burns A., Iliffe S. Alzheimer's disease. BMJ. 2009;338:p. b158. doi: 10.1136/bmj.b158. [DOI] [PubMed] [Google Scholar]
- 126.Ballard C., Gauthier S., Corbett A., Brayne C., Aarsland D., Jones E. Alzheimer's disease. The Lancet. 2011;377(9770):1019–1031. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]
- 127.Jia Y., Zhang X., Yu J., et al. Acupuncture for patients with mild to moderate Alzheimer's disease: a randomized controlled trial. BMC Complementary and Alternative Medicine. 2017;17(1):p. 556. doi: 10.1186/s12906-017-2064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhou S., Dong L., He Y., Xiao H. Acupuncture plus herbal medicine for alzheimer's disease: a systematic review and meta-analysis. American Journal of Chinese Medicine. 2017;45(7):1327–1344. doi: 10.1142/S0192415X17500732. [DOI] [PubMed] [Google Scholar]
- 129.Cheng H., Yu J., Jiang Z., et al. Acupuncture improves cognitive deficits and regulates the brain cell proliferation of SAMP8 mice. Neuroscience Letters. 2008;432(2):111–116. doi: 10.1016/j.neulet.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 130.Li X., Guo F., Zhang Q., et al. Electroacupuncture decreases cognitive impairment and promotes neurogenesis in the APP/PS1 transgenic mice. BMC Complementary and Alternative Medicine. 2014;14:p. 37. doi: 10.1186/1472-6882-14-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Xiao L. Y., Wang X. R., Yang Y., et al. Applications of acupuncture therapy in modulating plasticity of central nervous system. Neuromodulation. 2018;21(8):762–776. doi: 10.1111/ner.12724. [DOI] [PubMed] [Google Scholar]
- 132.Zhao L., Zhou C., Li L., et al. Acupuncture improves cerebral microenvironment in mice with alzheimer's disease treated with hippocampal neural stem cells. Molecular Neurobiology. 2017;54(7):5120–5130. doi: 10.1007/s12035-016-0054-5. [DOI] [PubMed] [Google Scholar]
- 133.Vitolo O. V., Sant'Angelo A., Costanzo V., Battaglia F., Arancio O., Shelanski M. Amyloid beta-peptide inhibition of the PKA/CREB pathway and long-term potentiation: reversibility by drugs that enhance cAMP signaling. Proceedings of the National Acadamy of Sciences of the United States of America. 2002;99(20):13217–13221. doi: 10.1073/pnas.172504199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Tong L., Balazs R., Thornton P. L., Cotman C. W. β-amyloid peptide at sublethal concentrations downregulates brain-derived neurotrophic factor functions in cultured cortical neurons. The Journal of Neuroscience. 2004;24(30):6799–6809. doi: 10.1523/JNEUROSCI.5463-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Lee B., Sur B., Shim J., Hahm D.-H., Lee H. Acupuncture stimulation improves scopolamine-induced cognitive impairment via activation of cholinergic system and regulation of BDNF and CREB expressions in rats. BMC Complementary and Alternative Medicine. 2014;14(1):p. 338. doi: 10.1186/1472-6882-14-338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Lin R., Chen J., Li X., et al. Electroacupuncture at the Baihui acupoint alleviates cognitive impairment and exerts neuroprotective effects by modulating the expression and processing of brain-derived neurotrophic factor in APP/PS1 transgenic mice. Molecular Medicine Reports. 2016;13(2):1611–1617. doi: 10.3892/mmr.2015.4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Fang J.-Q., Zhu S.-X., Zhang Y., Wang F., Zhu Q.-Y. Effect of electroacupuncture on expression of phosphorylated P 38 MAPK and IL-1beta in frontal lobe and hippocampus in rats with Alzheimer's disease. Zhen Ci Yan Jiu. 2013;38(1):35–39. [PubMed] [Google Scholar]
- 138.Zhang M., Xv G. H., Wang W. X., Meng D. J., Ji Y. Electroacupuncture improves cognitive deficits and activates PPAR-gamma in a rat model of Alzheimer's disease. Acupuncture in Medicine. 2017;35(1):44–51. doi: 10.1136/acupmed-2015-010972. [DOI] [PubMed] [Google Scholar]
- 139.Liu T., Yu J. C., Han J. X. Age-related changes of p130 expression in hippocampus and cerebral cortex and effects of acupuncture in SAMP 10. Zhen Ci Yan Jiu. 2008;33(4):223–244. [PubMed] [Google Scholar]
- 140.Luo B., Zhao L., Zhang X., et al. Acupuncture upregulates G protein coupled activity in SAMP8 mice. Acupuncture in Medicine. 2018;35(4):289–296. doi: 10.1136/acupmed-2016-011139. [DOI] [PubMed] [Google Scholar]
- 141.Wang F., Zhong H., Li X., et al. Electroacupuncture attenuates reference memory impairment associated with astrocytic NDRG2 suppression in APP/PS1 transgenic mice. Molecular Neurobiology. 2014;50(2):305–313. doi: 10.1007/s12035-013-8609-1. [DOI] [PubMed] [Google Scholar]
- 142.Lin D., Wu Q., Lin X., et al. Brain-derived neurotrophic factor signaling pathway: modulation by acupuncture in telomerase knockout mice. Alternative Therapies In Health And Medicine. 2015;21(6):36–46. [PubMed] [Google Scholar]
- 143.Liu Z.-B., Niu W.-M., Yang X.-B., Niu X.-M., Yuan W. Effect of 'Xiusanzhen' on expression of hippocampal Bcl-2 and Bax proteins in Alzheimer disease rats. Zhen Ci Yan Jiu. 2011;36(1):7–11. [PubMed] [Google Scholar]
- 144.Guo H. D., Tian J. X., Zhu J., et al. Electroacupuncture suppressed neuronal apoptosis and improved cognitive impairment in the ad model rats possibly via downregulation of notch signaling pathway. Evidence-Based Complementary and Alternative Medicine. 2015;2015:9. doi: 10.1155/2015/393569.393569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li X.-Y., Xu L., Liu C.-L., Huang L.-S., Zhu X.-Y. Electroacupuncture intervention inhibits the decline of learning-memory ability and overex- pression of cleaved caspase-3 and bax in hippocampus induced by isoflurane in APPswe/PS 1. Zhen Ci Yan Jiu. 2016;41(1):24–30. [PubMed] [Google Scholar]
- 146.Wang R., Li J. J., Diao S., et al. Metabolic stress modulates Alzheimer's β-secretase gene transcription via SIRT1-PPARγ-PGC-1 in neurons. Cell Metabolism. 2013;17(5):685–694. doi: 10.1016/j.cmet.2013.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dong W., Wang F., Guo W., et al. Abeta25-35 suppresses mitochondrial biogenesis in primary hippocampal neurons. Cellular and Molecular Neurobiology. 2016;36(1):83–91. doi: 10.1007/s10571-015-0222-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu W., Zhuo P., Li L., et al. Activation of brain glucose metabolism ameliorating cognitive impairment in APP/PS1 transgenic mice by electroacupuncture. Free Radical Biology & Medicine. 2017;112:174–190. doi: 10.1016/j.freeradbiomed.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 149.Lu Y., Huang Y., Tang C., et al. Brain areas involved in the acupuncture treatment of AD model rats: A PET study. BMC Complementary and Alternative Medicine. 2014;14(1):p. 178. doi: 10.1186/1472-6882-14-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Jiang J., Gao K., Zhou Y., et al. Electroacupuncture treatment improves learning-memory ability and brain glucose metabolism in a mouse model of alzheimer’s disease: using morris water maze and Micro-PET. Evidence-Based Complementary and Alternative Medicine. 2015;2015:7. doi: 10.1155/2015/142129.142129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Dong W., Guo W., Wang F., et al. Electroacupuncture upregulates SIRT1-dependent PGC-1α expression in SAMP8 Mice. Medical Science Monitor. 2015;21:3356–3362. doi: 10.12659/MSM.894864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Dong W.-G., Wang F., Chen Y., et al. Electroacupuncture reduces Aβ production and bace1 expression in SAMP8 mice. Frontiers in Aging Neuroscience. 2015;7:p. 148. doi: 10.3389/fnagi.2015.00148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang E. J., Cai M., Lee J. Neuroprotective effects of electroacupuncture on an animal model of bilateral common carotid artery occlusion. Molecular Neurobiology. 2016;53(10):7228–7236. doi: 10.1007/s12035-015-9610-7. [DOI] [PubMed] [Google Scholar]
- 154.Zhu W., Wang X., Du S., et al. Anti-oxidative and anti-apoptotic effects of acupuncture: role of thioredoxin-1 in the hippocampus of vascular dementia rats. Neuroscience. 2018;379:281–291. doi: 10.1016/j.neuroscience.2018.03.029. [DOI] [PubMed] [Google Scholar]
- 155.Wang T., Liu C. Z., Yu J. C., Jiang W., Han J. X. Acupuncture protected cerebral multi-infarction rats from memory impairment by regulating the expression of apoptosis related genes Bcl-2 and Bax in hippocampus. Physiology & Behavior. 2009;96(1):155–161. doi: 10.1016/j.physbeh.2008.09.024. [DOI] [PubMed] [Google Scholar]
- 156.Tian W. J., Huang L. N., Wang R. H., An J. M., Zhang M. Effects of scalp-acupuncture on astrocyte apoptosis in hippocampal CA 1 region in rats with vascular dementia. Zhen Ci Yan Jiu. 2015;40(1):6–12. [PubMed] [Google Scholar]
- 157.Zhu Y., Zeng Y., Wang X., Ye X. Effect of electroacupuncture on the expression of mTOR and eIF4E in hippocampus of rats with vascular dementia. Neurological Sciences. 2013;34(7):1093–1097. doi: 10.1007/s10072-012-1209-4. [DOI] [PubMed] [Google Scholar]
- 158.Wang S. J., Fang J. Q., Ma J., et al. Influence of electroacupuncture on p38-mitogen activated protein kinase in substantia nigra cells of rats with Parkinson disease model. Zhongguo Zhen Jiu. 2013;33(4):329–333. [PubMed] [Google Scholar]
- 159.Wang S. J., Ma J., Gong Y. X., et al. Effect of electroacupuncture intervention on ERK 1/2 signaling and TNF-alpha and IL-1beta protein levels in the substantia Nigra in rats with Parkinson's Disease. Zhen Ci Yan Jiu. 2014;39(6):456–460. [PubMed] [Google Scholar]
- 160.Kim S. N., Kim S. T., Doo A. R., et al. Phosphatidylinositol 3-kinase/Akt signaling pathway mediates acupuncture-induced dopaminergic neuron protection and motor function improvement in a mouse model of Parkinson's disease. International Journal of Neuroscience. 2011;121(10):562–569. doi: 10.3109/00207454.2011.591515. [DOI] [PubMed] [Google Scholar]
- 161.Kim S.-N., Doo A.-R., Park J.-Y., et al. Acupuncture enhances the synaptic dopamine availability to improve motor function in a mouse model of Parkinson's disease. PLoS ONE. 2011;6(11) doi: 10.1371/journal.pone.0027566.e27566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lin J. G., Chen C. J., Yang H. B., Chen Y. H., Hung S. Y. Electroacupuncture promotes recovery of motor function and reduces dopaminergic neuron degeneration in rodent models of Parkinson’s disease. International Journal of Molecular Sciences. 2017;18(9):p. 1846. doi: 10.3390/ijms18091846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lu K.-W., Yang J., Hsieh C.-L., Hsu Y.-C., Lin Y.-W. Electroacupuncture restores spatial learning and downregulates phosphorylated N-methyl-Daspartate receptors in a mouse model of Parkinson's disease. Acupuncture in Medicine. 2017;35(2):133–141. doi: 10.1136/acupmed-2015-011041. [DOI] [PubMed] [Google Scholar]
- 164.Tian T., Sun Y., Wu H., et al. Acupuncture promotes mTOR-independent autophagic clearance of aggregation-prone proteins in mouse brain. Scientific Reports. 2016;6 doi: 10.1038/srep19714.19714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kim S., Moon W., Chae Y., Kim Y. J., Lee H., Park H. The effect of electroaucpuncture for 1-methyl-4-phenyl-1,2,3,6- tetrahydropyridine-induced proteomic changes in the mouse striatum. The Journal of Physiological Sciences. 2010;60(1):27–34. doi: 10.1007/s12576-009-0061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Lv E., Deng J., Yu Y., et al. Nrf2-ARE signals mediated the anti-oxidative action of electroacupuncture in an MPTP mouse model of Parkinson's disease. Free Radical Research. 2015;49(11):1296–1307. doi: 10.3109/10715762.2015.1067696. [DOI] [PubMed] [Google Scholar]
- 167.Deng J., Lv E., Yang J., et al. Electroacupuncture remediates glial dysfunction and ameliorates neurodegeneration in the astrocytic α-synuclein mutant mouse model. Journal of Neuroinflammation. 2015;12(1):p. 103. doi: 10.1186/s12974-015-0302-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Park J.-Y., Choi H., Baek S., et al. P53 signalling mediates acupuncture-induced neuroprotection in Parkinson's disease. Biochemical and Biophysical Research Communications. 2015;460(3):772–779. doi: 10.1016/j.bbrc.2015.03.105. [DOI] [PubMed] [Google Scholar]
- 169.O'Brien J. T., Thomas A. Vascular dementia. The Lancet. 2015;386(10004):1698–1706. doi: 10.1016/S0140-6736(15)00463-8. [DOI] [PubMed] [Google Scholar]
- 170.Pendlebury S. T., Rothwell P. M. Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. The Lancet Neurology. 2009;8(11):1006–1018. doi: 10.1016/S1474-4422(09)70236-4. [DOI] [PubMed] [Google Scholar]
- 171.Yu J., Zhang X., Liu C., Meng Y., Han J. Effect of acupuncture treatment on vascular dementia. Neurological Research. 2006;28(1):97–103. doi: 10.1179/016164106X91951. [DOI] [PubMed] [Google Scholar]
- 172.Shi G. X., Li Q. Q., Yang B. F., et al. Acupuncture for vascular dementia: a pragmatic randomized clinical trial. The Scientific World Journal. 2015;2015:8. doi: 10.1155/2015/161439.161439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yang H., Shi O., Jin Y., et al. Functional protection of learning and memory abilities in rats with vascular dementia. Restorative Neurology and Neuroscience. 2014;32(5):689–700. doi: 10.3233/RNN-140409. [DOI] [PubMed] [Google Scholar]
- 174.Rizek P., Kumar N., Jog M. S. An update on the diagnosis and treatment of Parkinson disease. Canadian Medical Association Journal. 2016;188(16):1157–1165. doi: 10.1503/cmaj.151179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zeng B.-Y., Zhao K. Effect of acupuncture on the motor and nonmotor symptoms in parkinson's disease-a review of clinical studies. CNS Neuroscience & Therapeutics. 2016;22(5):333–341. doi: 10.1111/cns.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Noh H., Kwon S., Cho S. Y., et al. Effectiveness and safety of acupuncture in the treatment of Parkinson's disease: A systematic review and meta-analysis of randomized controlled trials. Complementary Therapies in Medicine. 2017;34:86–103. doi: 10.1016/j.ctim.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 177.Liu H., Chen L., Zhang Z., et al. Effectiveness and safety of acupuncture combined with madopar for parkinson—s disease: a systematic review with meta-analysis. Acupuncture in Medicine. 2017;35(6):404–412. doi: 10.1136/acupmed-2016-011342. [DOI] [PubMed] [Google Scholar]
- 178.Liang X.-B., Liu X.-Y., Li F.-Q., et al. Long-term high-frequency electro-acupuncture stimulation prevents neuronal degeneration and up-regulates BDNF mRNA in the substantia nigra and ventral tegmental area following medial forebrain bundle axotomy. Brain Research. Molecular Brain Research. 2002;108(1-2):51–59. doi: 10.1016/S0169-328X(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 179.Ghaffari B. D., Kluger B. Mechanisms for alternative treatments in Parkinson's disease: acupuncture, tai chi, and other treatments. Current Neurology and Neuroscience Reports. 2014;14(6):p. 451. doi: 10.1007/s11910-014-0451-y. [DOI] [PubMed] [Google Scholar]
- 180.Wang Z., Wan H., Li J., Zhang H., Tian M. Molecular imaging in traditional chinese medicine therapy for neurological diseases. BioMed Research International. 2013;2013:11. doi: 10.1155/2013/608430.608430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Li J., Kim S. G., Blenis J. Rapamycin: one drug, many effects. Cell Metabolism. 2014;19(3):373–379. doi: 10.1016/j.cmet.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kahan B. Toxicity spectrum of inhibitors of mammalian target of rapamycin in organ transplantation: etiology, pathogenesis and treatment. Expert Opinion on Drug Safety. 2011;10(5):727–749. doi: 10.1517/14740338.2011.579898. [DOI] [PubMed] [Google Scholar]
- 183.Niranjan R. The Role of inflammatory and oxidative stress mechanisms in the pathogenesis of parkinson's disease: focus on astrocytes. Molecular Neurobiology. 2014;49(1):28–38. doi: 10.1007/s12035-013-8483-x. [DOI] [PubMed] [Google Scholar]
- 184.Jia J., Sun Z., Li B., et al. Electro-acupuncture stimulation improves motor disorders in Parkinsonian rats. Behavioural Brain Research. 2009;205(1):214–218. doi: 10.1016/j.bbr.2009.06.024. [DOI] [PubMed] [Google Scholar]
- 185.Jia J., Li B., Sun Z.-L., Yu F., Wang X., Wang X.-M. Electro-acupuncture stimulation acts on the basal ganglia output pathway to ameliorate motor impairment in Parkinsonian model rats. Behavioral Neuroscience. 2010;124(2):305–310. doi: 10.1037/a0018931. [DOI] [PubMed] [Google Scholar]
- 186.Xu W., Zheng D., Liu Y., Li J., Yang L., Shang X. Glaucocalyxin B alleviates lipopolysaccharide-induced Parkinson’s disease by inhibiting TLR/NF-κB and activating Nrf2/HO-1 pathway. Cellular Physiology and Biochemistry. 2018;44(6):2091–2104. doi: 10.1159/000485947. [DOI] [PubMed] [Google Scholar]
- 187.Tu Q., Liang Y., Ma J., Wang S.-J., Shen F., Wang Y.-C. Effect of electroacupuncture on 26 S proteasome and nuclear factor kappa B in substantia nigra of rats with rotenone-induced Parkinson's disease. Zhen Ci Yan Jiu. 2015;40(4):259–264. [PubMed] [Google Scholar]
- 188.Li Z., Ni M., Li J., Zhang Y., Ouyang Q., Tang C. Decision making of the p53 network: Death by integration. Journal of Theoretical Biology. 2011;271(1):205–211. doi: 10.1016/j.jtbi.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Checler F., Alves da Costa C. p53 in neurodegenerative diseases and brain cancers. Pharmacology & Therapeutics. 2014;142(1):99–113. doi: 10.1016/j.pharmthera.2013.11.009. [DOI] [PubMed] [Google Scholar]
- 190.Choi Y., Yeo S., Hong Y., Kim S., Lim S. Changes of gene expression profiles in the cervical spinal cord by acupuncture in an MPTP-intoxicated mouse model: Microarray analysis. Gene. 2011;481(1):7–16. doi: 10.1016/j.gene.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 191.Choi Y.-G., Yeo S., Hong Y.-M., Lim S. Neuroprotective changes of striatal degeneration-related gene expression by acupuncture in an MPTP mouse model of Parkinsonism: microarray analysis. Cellular and Molecular Neurobiology. 2011;31(3):377–391. doi: 10.1007/s10571-010-9629-2. [DOI] [PubMed] [Google Scholar]
- 192.Yeo S., Choi Y., Hong Y., Lim S. Neuroprotective changes of thalamic degeneration-related gene expression by acupuncture in an MPTP mouse model of parkinsonism: microarray analysis. Gene. 2013;515(2):329–338. doi: 10.1016/j.gene.2012.12.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are available to other researchers upon request.