Abstract
Lymphedema is an incurable, debilitating and progressive condition, leading to physical and psychosocial consequences for the patients, if left untreated. The Physical Medicine and Rehabilitation (PMR) specialist is responsible for the differential diagnosis and evaluation of the patient to tailor management and rehabilitation strategies. Therefore, the PMR specialist must have knowledge and education on the diagnosis of disease and possible complications as well as evaluation, treatment and follow-up of the patient. In this review, the pathophysiology, epidemiology, and diagnostic and therapeutic approaches of lymphedema as well as preventive strategies and follow-up strategies are discussed in the light of the current literature.
Keywords: Diagnosis, follow-up, lymphedema, physical medicine and rehabilitation specialist, treatment
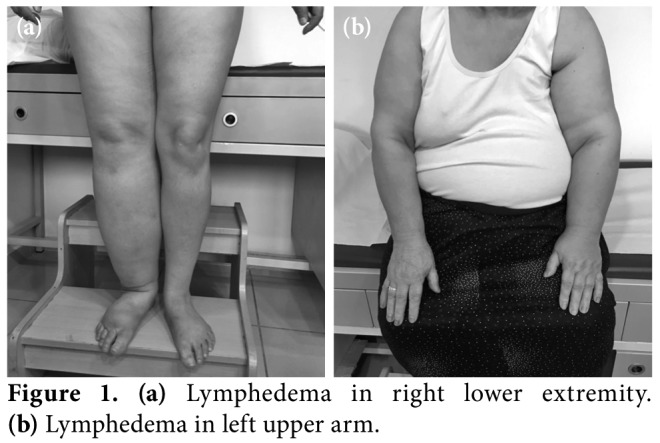
Lymphedema is an incurable, debilitating and progressive condition, characterized by persisted swelling of one or more parts of the body, due to impaired lymph transport. It is a major healthcare problem in both developed and non-developed countries. Lymphedema may be present in the extremities (Figures 1a, b), trunk, abdomen, head and neck, external genitalia, and internal organs. It is a serious concern due to its long-term physical and psychosocial consequences for the patient, if left untreated. The lack of awareness and medical expertise in the diagnosis and treatment of this condition and the tendency of clinicians to overlook lymphedema in patients who previously underwent cancer surgery, making this condition more problematic.[1,2]
Within this context, PMR specialists play a critical role in clinical care that their global approach of this problem, by integrating the physical disorders, psychological dimensions and quality of life (QoL) issues, provide the extensive management and rehabilitation of patients.[3]
The PMR specialist is responsible for the differential diagnosis and assessment of the patients to tailor management and rehabilitation strategies. Therefore, he/she should have a comprehensive knowledge about the pathophysiology, epidemiology, and diagnostic and preventive measures, as well as therapeutic approaches of lymphedema.
EPIDEMIOLOGY
The incidence of lymphedema has not been precisely estimated in Turkey and the estimated rates according to different areas vary widely in the literature. About 200 million cases of lymphedema have been estimated worldwide with filariasis, a parasitic infestation, being the most common cause in the world.[1,4]
Lymphedema may be primary due to the congenital dysfunction of the lymphatic system and can be diagnosed at different stages in life without an obvious cause. Secondary forms mostly develop after cancer surgery and/or radiation therapy for various cancers comprising breast, endometrium, cervix, ovary, melanoma, prostate, and bladder. The rate of breast cancer-related lymphedema (BCRL) has been estimated as 7 to 28% in small-scale studies conducted in Turkey.[5] In addition, the incidence of lower limb lymphedema related with cancer therapies is reported to be over 70% in some previous reports.[6] Although the true incidence of primary lymphedema is not clear, it is estimated to be 5 to 10% with slightly higher in females.[1,4]
Figure 1. (a) Lymphedema in right lower extremity. (b) Lymphedema in left upper arm.
PATHOPHYSIOLOGY AND CLINICAL MANIFESTATIONS
The lymphatic system involves an extensive network of vessels and the primary function of the lymphatic system is to transport lymph fluid which contains proteins, macromolecules, cells, and white blood cells throughout the body. The lymphatic system primarily consists of the lymphatic vessels, which are similar to the circulatory system's veins and capillaries.[7] The beginning of lymphatic vasculature is lymphatic capillaries, which are permeable to protein, fluid, and cells by the fenestrations in their basement membrane. At the level of the blood capillaries, the systemic circulation loses 2 to 4 L fluid and nearly 100 g protein into the interstitium daily. When this protein-rich fluid is absorbed by the lymphatic capillaries, it is named as lymph fluid. The lymphatic capillaries merge into collectors and, then, into the lymph nodes. The efferents from the lymph nodes generate into the truncus structures that ultimately drain into the thoracic duct which empties to the left subclavian vein on the left side, and into the right lymphatic truncus, which empties to the right subclavian vein on the right side. In a normal physiological state, the fluid enters into the interstitial space by arterioles; some returns to the venules, and the remainder (~10%) is taken up by the lymphatics. The amount of entrance and exit of the fluid is approximately equal in normal conditions. Lymphedema develops, when the volume of interstitial fluid increases, either from increased inflow or decreased outflow or both.[7,8] If lymphedema is left untreated, chronic lymph stasis stimulates fibroblasts, adipocytes, and keratinocytes and infiltration of neutrophils and collagen, leading to skin complications, lymphostatic fibrosis, hardening of the skin texture, papillomas, and deep skin folds.[8]
Lymphedema is classified as primary or secondary according to the underlying etiology. Primary lymphedema represents developmental lymphatic vascular deficiency which can be either congenital or hereditary. A number of mutations of genes involved in lymphatic development are associated with primary lymphedema (i.e., GJC2, FOXC2, CCBE1, VGFR-3, PTPN14, GATA2, and SOX18). Although developmental anomalies are present at birth, lymphedema may develop at some time period, later in life. Congenital lymphedema is clinically evident at birth or within the first two years of life. Many lymphatic malformations were defined as different diseases: Milroy disease is a familial congenital disease and appears at or soon after birth. It affects mainly the lower extremity. Meige disease develops at puberty or later, after a minor injury, and presents mostly with foot and ankle swelling. Girls are affected more than boys. If primary lymphedema presents at birth, but exists before the age of 35 years, it is named lymphedema praecox. This form is common and it frequently arises during puberty or pregnancy. Lymphedema tarda is relatively rare and develops after age of 35 years. Lymphangiomas are uncommon, congenital, benign, often cystic malformations of the lymphatics and may be associated with other vascular malformations.[4,7,8]
On the other hand, secondary lymphedema can be acquired and may arise as a result of cancer surgery, radiation therapy, chronic venous insufficiency, lipedema, trauma, infection, immobility, or underlying systemic diseases. The most common cause of lymphedema worldwide is filariasis; however, BCRL is the particular cause of secondary lymphedema in the Western countries.[4,8] Breast cancer is the most common cancer among the Turkish woman, similar to worldwide.[5] Improvements in early diagnosis and treatment of breast cancer have led a growing number of survivors, and the rates of treatment side effects, including BCRL, has increased in the last couple of decades.
DIAGNOSIS
Although lymphedema leads to physical and psychological problems and impairs QoL, it is underrecognized and undertreated.[1] The diagnosis of lymphedema is clinical and depends on taking detailed history and comprehensive physical examination. The PMR specialist takes the patient and family' history and performs clinical examination comprising inspection, palpation, ROM assessment and neurological examination. Additionally, the quantitative measurements for functional disability and QoL have to be performed and the psychological context of the disease (anxiety, depression, sleep disorders, fear of cancer recurrence, and sexuality problems) has also be addressed.[3,8,9]
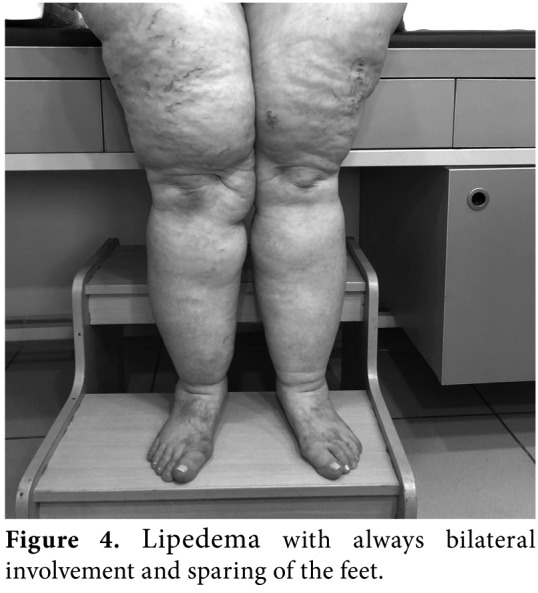
Lymphedema occurs not only in a pure form, but also in combination with lipedema (Figure 2), obesity and chronic venous insufficiency. Malignant lymphedema (impairment and blockage of lymph drainage caused by direct tumor infiltration and/ or lymph node metastases) (Figure 3), inflammatory conditions, chronic venous insufficiency (brown pigmentation at distal foot, eczema-like skin changes), deep venous thrombosis (DVT), hypoalbuminemia, inflammatory rheumatic disease, heart failure, renal insufficiency, drug-induced edema (i.e., chemotherapeutics, corticosteroids, hormones, antihypertensives, antivirals, non-steroidal anti- inflammatory drugs [NSAIDs]), lipedema (foot are spared, Stemmer sign negative) (Figure 4) have to be suggested in the differential diagnosis of lymphedema. Diagnostic laboratory tests (kidney, liver thyroid functions, D-dimer), chest X-ray, electrocardiography, echocardiogram, venous Doppler ultrasonography (US), magnetic resonance imaging (MRI), or computed tomography (CT) can be performed for the diagnosis of edema.[8,9]
Figure 2. Severe lipedema associated with obesity (note the skin folds at the ankles and skin texture of orange peel with large dimples).
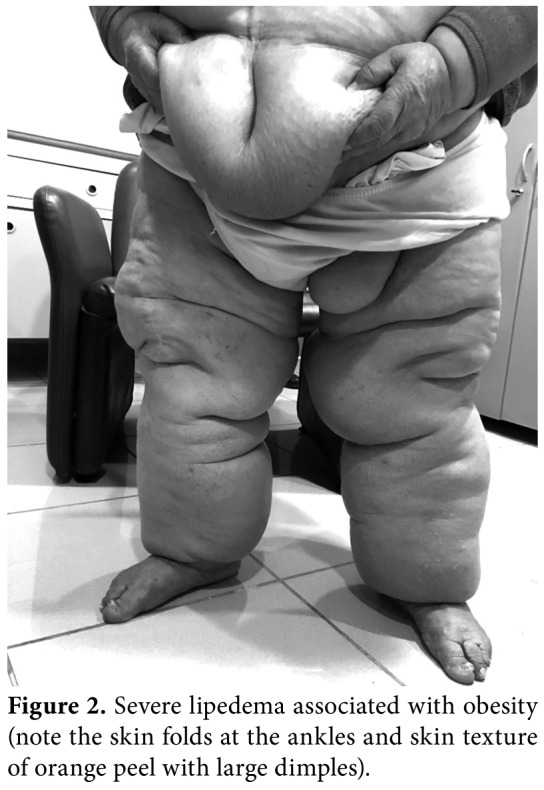
Figure 3. Malign lymphedema with rapid onset, fullness of supraclavicular fossa, more proximal/ central location and cyanotic skin developed in a patient with right breast cancer surgery.
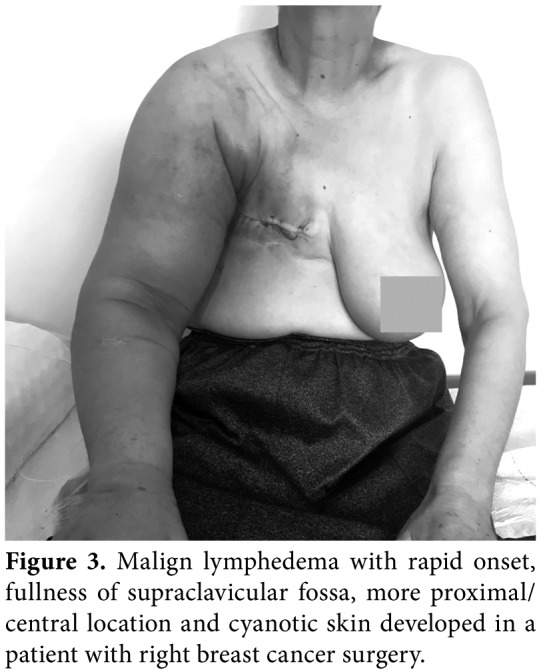
Figure 4. Lipedema with always bilateral involvement and sparing of the feet.
ANAMNESIS
The history taking of patients with lymphedema must include the causative or initiative factors (cancer, surgery, trauma, pregnancy, weight gaining), duration, initial side of edema (proximal vs distal), pain, paresthesia, progress time (slow vs rapid), and comorbid conditions (cardiac insufficiency, renal diseases, chronic venous insufficiency, rheumatic diseases, thyroid disease, and diabetes).
If the duration of swelling is short and an acute stage, DVT, cellulitis, trauma or reflex sympathetic dystrophy, and if the onset is gradual and progressive over a period of weeks or months, chronic venous insufficiency, lipedema, congestive heart failure, or kidney problems should be considered in the differential diagnosis of edema. In addition, cardiac and renal insufficiencies should not be overlooked, as complete decongestive therapy (CDT) is contraindicated in such conditions. Rarely, the initial sign of malignancy may be lymphedema, which can supported by weight loss, pain, fatigue, nerve lesions, and etc. Pain can be evaluated using the Visual Analog Scale. Family history and family support should be also searched.[9,10]
INSPECTION
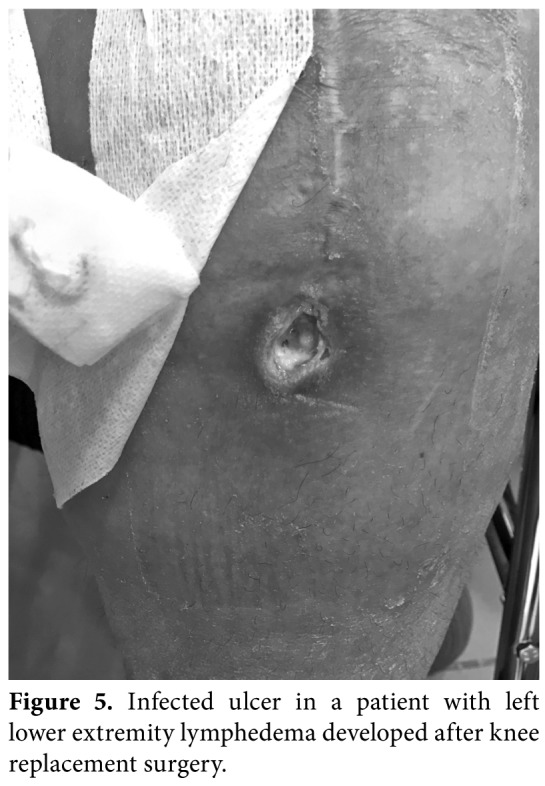
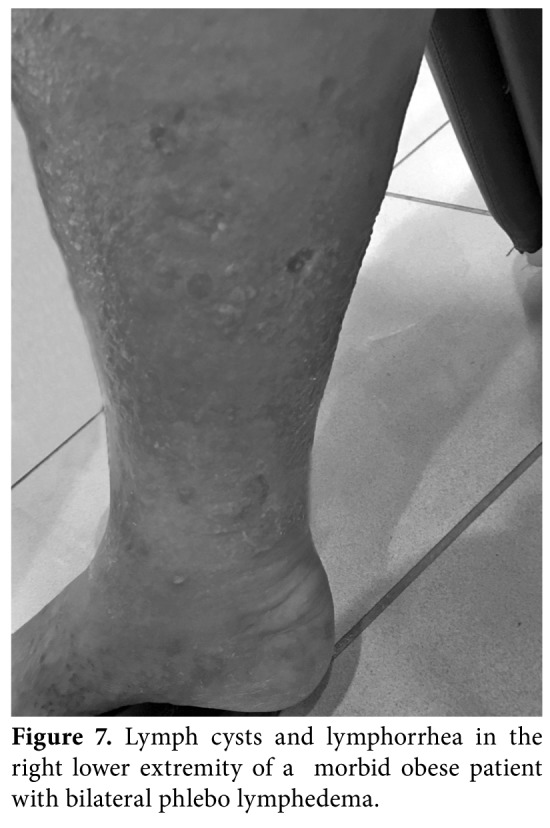
The posture, asymmetry, obesity, localization, and size of lymphedema, skin changes (skin color, ulcer (Figure 5), papillomas (Figure 6), skin fold (Figure 2), hyperemia, intertrigo), and atrophy of muscles must be noted. Any leaking skin change (i.e., Lymphorrhea) (Figure 7), wound or eruption must be also recorded. Truncal, genital or abdominal areas must be checked for the presence of combined edema in lymphedema of the extremities.[9-11]
Figure 5. Infected ulcer in a patient with left lower extremity lymphedema developed after knee replacement surgery.
PALPATION
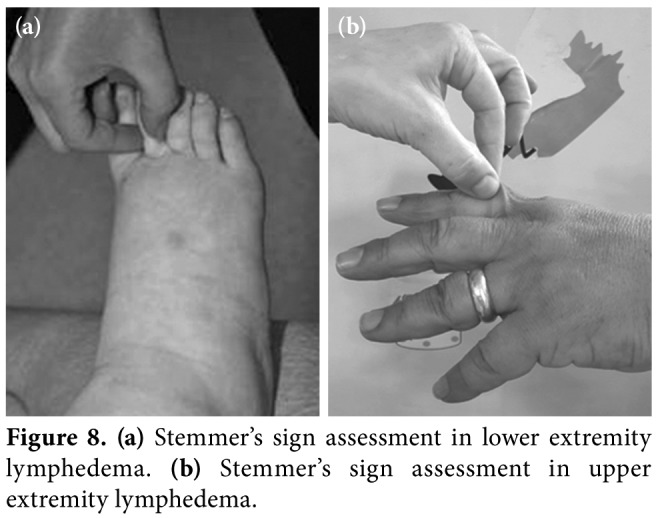
Subcutaneous connective tissue changes, tissue texture, temperature, and mechanical properties of the skin can be assessed by palpation. The thickness of dermis and fibrosis is checked by the Stemmer's sign. If the skin on dorsum of fingers or toes cannot be lifted easily, it is called as a positive Stemmer's sign (Figures 8a, b). The Stemmer's sign positivity indicates that fibrosis is present and the grade of lymphedema is at least 2. The negative Stemmer sign does not exclude lymphedema. Pitting of edema, pain on palpation, and consistency of regional lymph nodes should also be addressed.[10,11]
Complete neurological examination consisting muscle strength, sensation and deep tendon reflexes should be performed for the differential diagnosis of radiation plexopathy, entrapment neuropathy, or other neurological and metabolic diseases. Examination of sensation is important for compression therapies, as skin complications may develop in neuropathic conditions.[8-11]
Figure 6. Papilloma on the dorsum of the right toes developed in a pediatric patient with congenital lymphedema.
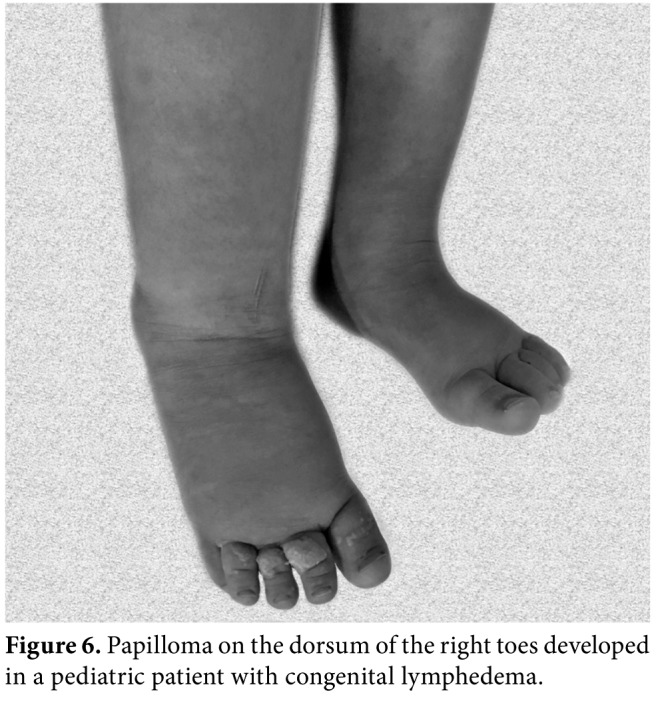
Figure 7. Lymph cysts and lymphorrhea in the right lower extremity of a morbid obese patient with bilateral phlebo lymphedema.
FUNCTIONAL DISABILITY AND QoL ASSESSMENTS
Lymphedema may have severe consequences in terms of patients' functional and psycho-social aspects of life, resulting in impaired QoL. Loss of function can be due to the heaviness, pain, and decreased range of motion (ROM) of affected limbs or due to infection, pain, and impaired wound healing. The Disabilities of Arm, Shoulder, and Hand (DASH) Questionnaire can be used to assess functional disability in upper limbs, while the Lower Extremity Functional Scale (LEFS) can be used for the assessment of lower limb involvement. These instruments have been also validated in the Turkish patients.[12,13]
Accurate information on health-related QoL outcomes among patients with lymphedema is critically needed to determine evidence-based decision making, indicating the impact of disease on survivors' lives. Due to the special symptoms and difficulties of the patients with lymphedema, it is important to use a questionnaire developed specifically for patients suffering from this chronic condition. There are several instruments including Upper Limb Lymphedema-27, Lymphedema Quality of Life Inventory, Lymphedema Functioning, Disability and Health Questionnaire, and Lymphedema Life Impact Scale;[14] however, only the Quality of Life Measure for Limb Lymphoedema (LYMQOL), which was developed by Keeley et al.[15] for upper and lower lymphedema, has been validated in Turkish population.[16,17]
Figure 8. (a) Stemmer's sign assessment in lower extremity lymphedema. (b) Stemmer's sign assessment in upper extremity lymphedema.
VOLUME MEASUREMENTS
Volume measurement is essential to evaluate the size of the lymphedema, diagnosis, and treatment monitoring. It is of utmost importance that both the normal and affected limbs are measured in exactly the same way at all time points to eliminate natural volume variations. The normal arm volume variations are between 2 and 13% in healthy individuals, and the dominant arm is usually about 1.5% larger.[18] There are several methods to measure the volume of lymphedema, depending on the involvement sides, technological ability, and the size of edema.[11]
Circumferential measurements
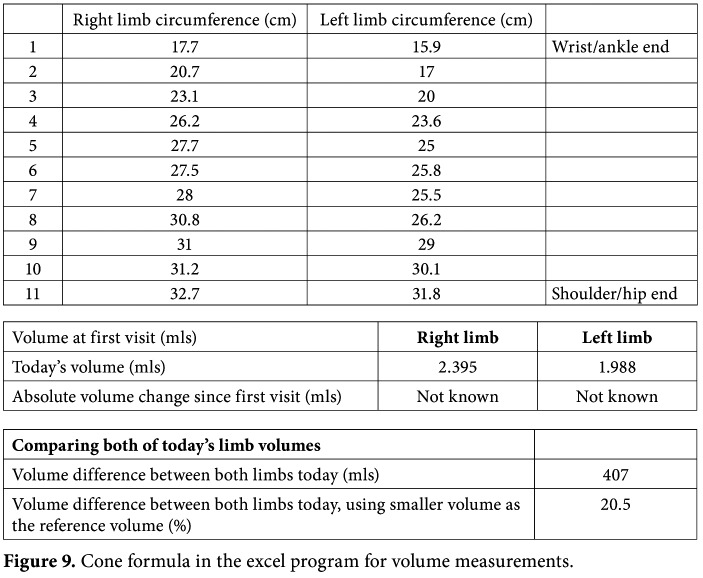
Circumferential measurements depend on well- defined distances along the limb, but hand and foot volumes cannot be calculated. This method also provides information on the localization of the swelling. In our clinical practice, the circumferences of the limb are measured every 4 cm along the limb from the wrist/ankle and up to the axilla/symphysis pubis and the volume is calculated according to the truncated formula in upper/lower extremity lymphedema (Figure 9):
V = 1/3 x π x h x (r12 + r22 + r1 x r2).
The excess volume of unilateral lymphedema is calculated as the difference between the edematous and the contralateral health extremity.[11,18] There is also an Excel® formula to provide volume measurements. The measurement programs for arms and legs can be downloaded from the following link: https://www. palliativedrugs.com/.../081218_limb_volume_c.
Water displacement method
The volume is quantified accurately by water displacement method, using specially designed arm and leg volume meters. The volume meter is fully filled with water, the limbs are drained and held in a specific position, and wait a few seconds to allow the water to flow-out. The drained water is collected in a plastic tray and the entire volume of the limb is directly measured. However, this method is not practical due to the difficulty in huge sized limbs, hygienic concerns, and consumed amount of water.[18]
Perometry
This is an optoelectronic measuring method using a square frame with multiple perpendicular light beams. The frame is moved along the limb, and the cross-sectional area is calculated continuously and, thereby, by volume. The equipment is expensive, but the method is accurate and fast.[18,19] The only perometer in Turkey belongs to the Anatolian Lymphedema Association and available in the Hacettepe Lymphedema Research and Practice Center. This tool can be used free for the measurements and for research purposes.
Bioelectrical impedance spectrometry (BIS)
The BIS is a causally related direct measure of lymph accumulation. It measures the opposition (impedance) of the body's tissues to the flow of an electrical current. It is a rapid, non-invasive, and relatively inexpensive method that can measure both unilateral and bilateral lymphedema of arms and legs. The method is particularly useful for diagnosing early lymphedema and has proven useful in the monitoring of various treatment interventions in BCRL. The contraindications for the use of this device include the presence of fitted cardiac pacemakers or other implantable medical devices.[18,20]
Criteria for lymphedema are primarily based on comparison between the at-risk/swelled and the contralateral arm. The difference between arms of >2 cm by tape circumference, >200 mL or >10% by volumetric measurements, or an impedance ratio between arms of >3 standard deviations from the normal range by BIS indicates lymphedema.[8,11,18]
Quantification of bilateral lymphedema is particularly challenging, since these patients lack a contralateral control arm for comparative purposes. Miller et al.[21] suggested a novel and validated method to quantify BCRL following bilateral breast surgery. This weight-adjusted volume change formula, which functions independently of the contralateral arm, accounts for changes in the arm size, according to the patient weight.[21]
Figure 9. Cone formula in the excel program for volume measurements.
STAGING AND GRADING
The transport capacity of normal lymphatic load has decreased in lymphedema which indicates mechanical insufficiency. This transport capacity in the damaged lymphatic vessels cannot be regenerated, and there is no cure for lymphedema. In early stages, edema may regress during nighttime, although lymphedema would gradually progress through its stages, if left untreated. The International Society of Lymphedema (ISL) has defined the stages of lymphedema between 0 and 3 as follows:[11]
Stage 0: Is characterized as latency period presenting with impaired lymphatic transport but no manifesting edema. This stage is of major importance in secondary prevention of lymphedema.
Stage 1: Presents with reversible pitting edema, without secondary tissue changes. In this stage, elevation reduces swelling.
Stage 2: Represents lymphostatic fibrosis, non- pitting, and irreversible edema with a positive Stemmer's sign. Frequent infections can be seen in this stage (Figures 1a, b).
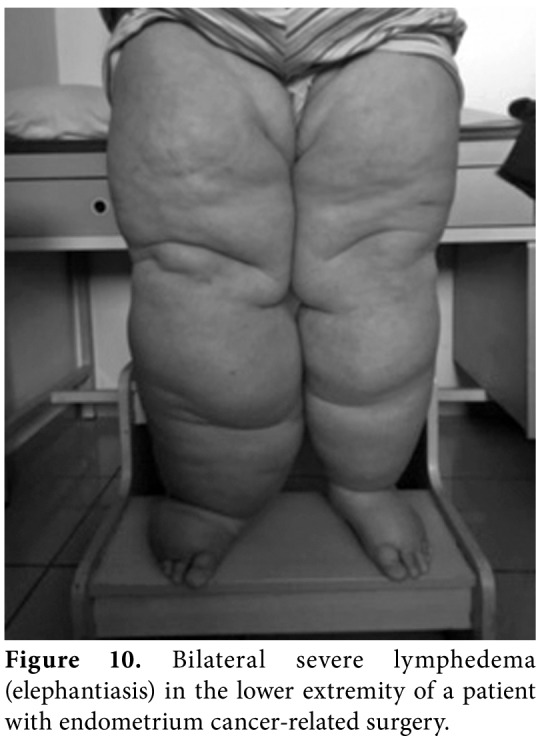
Stage 3: Results in lymphostatic elephantiasis with extreme increase in volume and typical skin changes (papillomatous outgrowths, hyperkeratosis, and skin folds). The Stemmer's sign is positive (Figure 10).
Extremity volume is not considered within different stages of lymphedema. But the severity of unilateral limb edema in regard to the volume differences with intact extremity, can be graded as listed in Table 1.[8,11]
Figure 10. Bilateral severe lymphedema (elephantiasis) in the lower extremity of a patient with endometrium cancer-related surgery.
IMAGING MODALITIES
Lymphoscintigraphy is the most optimal imaging procedure for lymphedema. A radioisotope-labelled colloid is injected into the web space between the first and second toe, and the colloid movement is measured, as it travels toward the proximal lymph nodes using a gamma camera. Hypoplasia of the peripheral lymphatics (slow progress of radioisotope), dermal back-flow, reflux (i.e., radioisotope escapes from the lymph channel into the skin) can be visualized via this method. On the other hand, lymphoscintigraphy has some disadvantages: it requires specific equipment, has irradiation, and is time-consuming. Lymphoscintigraphy has 92% sensitivity and 100% specificity for lymphedema. However, the results must always be interpreted considering patients' symptoms, history, and examination findings in this functional and morphological analysis of the lymphatic system.[11,22]
Ultrasonographic evaluation can be also used to rule out the cause of increased interstitial fluid formation due to systemic diseases (i.e., congestive heart failure, liver disease, or renal disease). In patients with lymphedema, gray scale images shows the thickening of the cutaneous, epifascial tissue compartments, and interstitial fluid accumulation and may allow the evaluation of the degree of fibrosis.[23] Recent studies have shown that decisions regarding skin and subcutaneous changes in patients with lymphedema (Marshall's cleft) and lipedema (snowstorm appearance) can be reached with the aid of this technique. Ultrasonographic evaluation of soft tissue thickness was reported to be useful as an outcome measure for therapeutic effects of CDT.[24]
Low-flow color Doppler US as a non-invasive, safe, and reproducible method which is helpful in the diagnosis of DVT and venous insufficiency. Therefore, it must be ordered to evaluate the cause of lower extremity lymphedema.[3,9]
In addition, lymphofluoroscopy, near-infrared imaging of the lymphatic system, can be used to visualize the lymph vessels surgery.[25] Other techniques useful for the investigation of a patient with edema include MRI and CT which can be used to distinguish between lymphedema and other swellings of the limb.[22]
Table 1. Severity of unilateral lymphedema based on volume difference between swollen and intact limbs.
| Severity | Volume increase |
| Minimal | <20% |
| Moderate | 20-40% |
| Severe | >40% |
COMPLICATIONS OF LYMPHEDEMA
The PMR specialist should be aware of the complications and conditions that may be associated with lymphedema.[3,26]
Cellulitis (sudden reddening, increased local temperature, worsening of edema, inflammation, pain, and high fever) is an emergency condition requiring antibiotherapy. If the patient is still under treatment, CDT is stopped. After the regression of the clinical picture, CDT can be re-started. Preventive strategies should be also taken for this condition.
Fungal infections of the skin (i.e., itching, whitish exudate, and moisture between toes) cause deterioration of edema, and pathogenic microorganisms can pass through cracks in the skin, leading to inflammation. Anti-fungal therapy and education on skin care is, therefore, needed.
Papillomatosis is the protrusion and wart-like growths on the skin of edematous tissue, caused by chronic congestion of the smallest lymphatic vessels (Figure 6). These papillomas regress with CDT.
Lymph cysts are lymph fluid-filled blisters or vesicles in the skin of lymphedematous limb, and lymph fistula is unnatural orifices from which lymph fluid leaks out (Figure 7). They can be treated and closed by CDT.
Elephantiasis is steadily increasing swelling of the affected extremities with areas of induration and skin changes, caused by untreated lymphedema (Figure 10). Obesity may associate and CDT may last months for the treatment of this condition.[26]
Stewart-Treves Syndrome is a rare, but lethal angiosarcoma arising from longstanding lymphedematous extremity and characterized by suddenly appeared multiple purplish or purple, painless, macular lesions which may be dismissed (Figure 11). The underlying pathophysiology is still debated. Diagnosis is made on skin biopsy and the prognosis is poor, when radical surgery is not performed. Of note, CDT is contraindicated in this condition.[26,27]
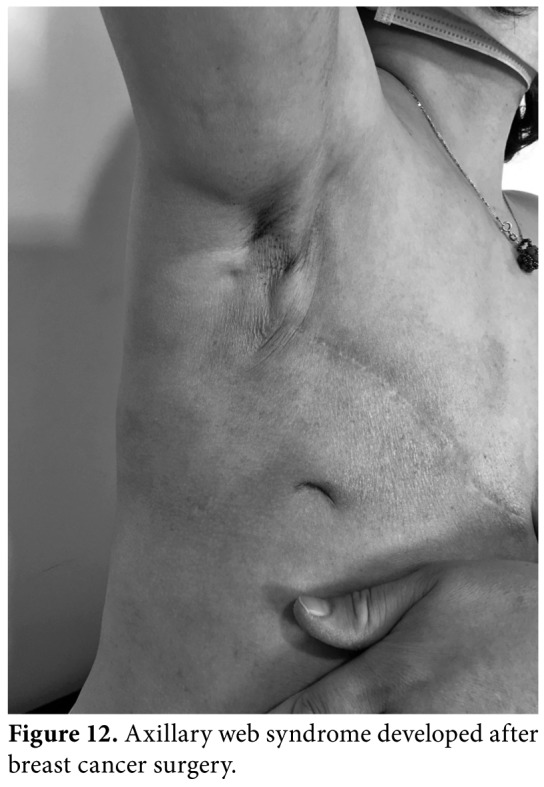
Axillary web syndrome (AWS) is a postoperative condition characterized by abnormal scarring of the subcutaneous tissue from the axilla down the medial arm, and over the lateral border of pectoralis major (Figure 12). Recent literature on AWS has addressed into the evidence regarding the palpable or visible tensioned cords being lymphatic in origin. However, several studies have demonstrated an association with Mondor's disease and venous involvement. The incidence of AWS varies due to the lack of certain definition, understanding of the pathophysiology and extent of anatomical structures involved in the clinical presentation. The mainstay of therapy is gentle stretching exercises and scar tissue massage. However, consensus on timing and efficacy of treatment is still lacking. In addition, a novel treatment Xiaflex® (United States Food and Drug Administration-approved collagenase-derived from bacterium clostridium histolyticum) injection into the cord and percutaneous needle cord disruption with autologous fat grafting have been also published in the literature.[28]
Figure 11. Stewart Treves syndrome in a patient with breast cancer-related lymphedema.
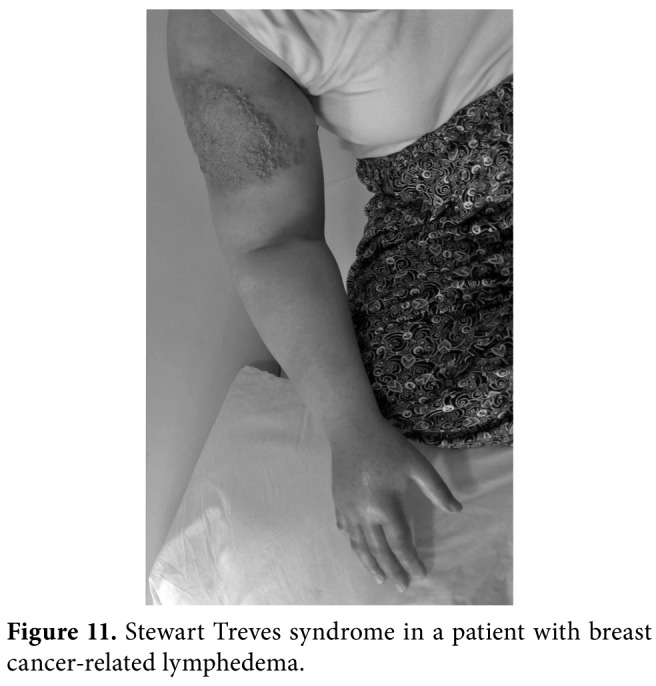
Figure 12. Axillary web syndrome developed after breast cancer surgery.
SPECIAL FORMS OF LYMPHEDEMA
Head and neck lymphedema
Patients with locally advanced head and neck cancer and/or cancer surgery are at risk of head and neck lymphedema (HNL). Progressive lymphedema leads to the sequence of conditions from swelling to hardening of fibrotic tissues. The literature has published that 75% of head and neck cancer patients may develop some degree of lymphedema three months after the surgery.[29] As HNL is associated with devastating functional impairment and decreased QoL, early and aggressive therapy is needed. On the other hand, accurate and quantitative techniques for the measurement of HNL are lacking. Circumferential measurements, ultrasonography for skin changes and soft tissue edema and shear-wave elastography can be used for the assessment of HNL. Neck and mandibular ROM must be also examined. Skin and soft tissue abnormalities can be graded using the Head and Neck External Lymphedema Fibrosis Grading Criteria.[30] Current gold standard therapy for HNL is CDT and includes education, skin care, manual lymphatic drainage (MLD), compression bandages and garments, and kinesiotaping, which can be chosen according to the condition of the individual patients. Protective self-management and adherence are also important in the long-term care.[29,30]
GENITAL LYMPHEDEMA
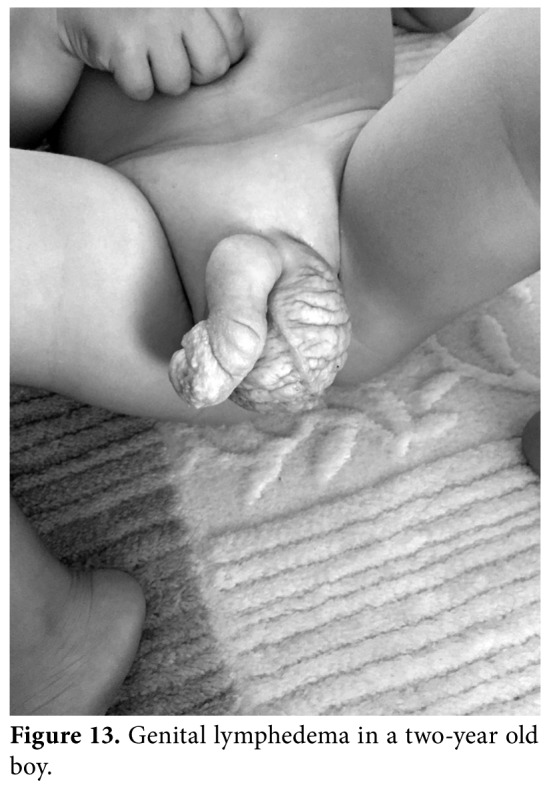
Genital lymphedema is a rare, but troublesome condition, which is often associated with lower extremity lymphedema. Genital lymphedema impairs the QoL, as well as functional and emotional well- being. The psychosocial consequences of disfigurement should not be underestimated, particularly in adolescents. Genital lymphedema usually affects the skin and subcutaneous tissue of the scrotum, penis (Figure 13), labia, and hypogastrium. The etiology of genital lymphedema may be primary or secondary to abdominopelvic organ cancer surgery or urogenital infections. It can be a part of inherited disease, such as Noonan or Milroy disease. Although most patients develop symptoms in infancy or early childhood, the diagnosis is often delayed for several years. Genital lymphedema develops relatively quickly due to loose subcutaneous tissues of genital organs. Chronic inflammation in external genitalia may also lead to skin changes, papillomas, lymphorrhea, hematuria, cellulitis, and fibrosis.[31]
History taking, physical examination, and imaging (lymphoscintigraphy, US, MRI) when needed, are used for diagnosis. Education of the patient and family, and skin care are essential. Daily washing of the genitalia and application of moisturizing agents are needed to prevent skin breakdown. Families should be educated regarding the signs and symptoms of cellulitis, a life-threatening complication.[32] The mainstay of treatment is CDT combined with MLD and compression therapies.
The latter is reported to resolve vulvar edema faster than expected.[33] In our clinical practice, we educate genital lymphedema patients for self-MLD at the initial visit. Due to the shape and location of genital lymphedema, wrapping bandages and compression garments can be difficult to apply. Therefore, we use CobanTM 2 layer bandages (3M Deutschland GmbH Health Care Business, Neuss Germany) in our male genital lymphedema patients. It is easy to wrap and comfortable than conventional short stretch bandages and is also effective in reducing the genital size. Compression garments with elastic shorts are needed to support and compress the external genitalia in the maintenance phase of CDT.[32,33]
Figure 13. Genital lymphedema in a two-year old boy.
PEDIATRIC LYMPHEDEMA
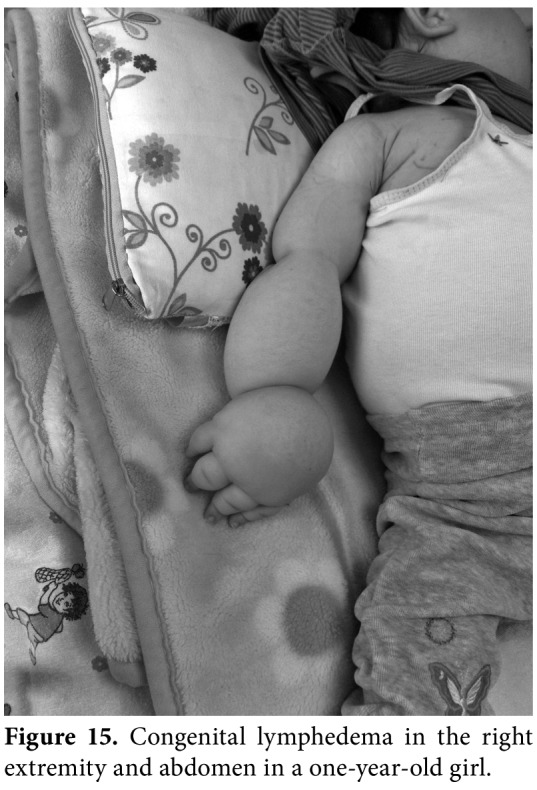
Primary lymphedema, which results from a non- syndromic inherited condition or as a part of a syndromic disorder (i.e., Turner, Noonan, Prader- Willi, Klippel-Trenaunay, or vascular malformations [Figure 14]), is the most common type in children. In most cases, edema is present from birth (Figure 15), but may also develop later in some cases. There are several forms of primary lymphedema, but the two main causes in children are Milroy disease and lymphedema distichiasis. The age of onset (congenital or pubertal), family history, location of swelling, associated conditions, and dysmorphic features, and underlying genetic causes are critical in the differential diagnosis of pediatric lymphedema. Diagnosis is mainly based on clinical presentation, while physicians must take complete anamnesis and perform a meticulous physical examination for coexisting systemic involvement and secondary causes. Physical examination must also cover the assessment of skin changes (i.e., subcutaneous thickening, fibrosis, hyperkeratosis, or papillomatosis), pitting edema, Stemmer's sign, and lymphangiectasia. If systemic involvement is present, ascites, pleural effusion and intestinal lymphangiectasia may occur. Lymphoscintigraphy is the gold standard for lymphatic system imaging in primary lymphedema, lymphangiography is avoided because of the technical difficulties in small children and potential serious side effects. Abdominal US, venous Doppler US, MRI, X-ray, immune function markers, serum albumin and genetic testing can be helpful in the differential diagnosis of pediatric lymphedema.[34]
Early and accurate diagnosis is critical. There is no specific guideline for the management of pediatric lymphedema, and consensus documents for adults can be adapted for children. Bandages must not interfere with normal growth and restrict activities. Toes are not wrapped and delicate tissues of children require more padding. Education of families about the skin care for the prevention of skin changes and infection, self-management (MLD by families), compression, and exercises are essential. Custom- made compression garments are not recommended for children younger than one year old. In addition, compression garments should not exceed 20 to 30 mmHg of compression in children younger than four years of age.[35] Psychosocial intervention for both children and their families should be taken into consideration.[34,35]
Figure 14. Left upper extremity lymphedema associated with vascular malformations in a eight- year-old boy.
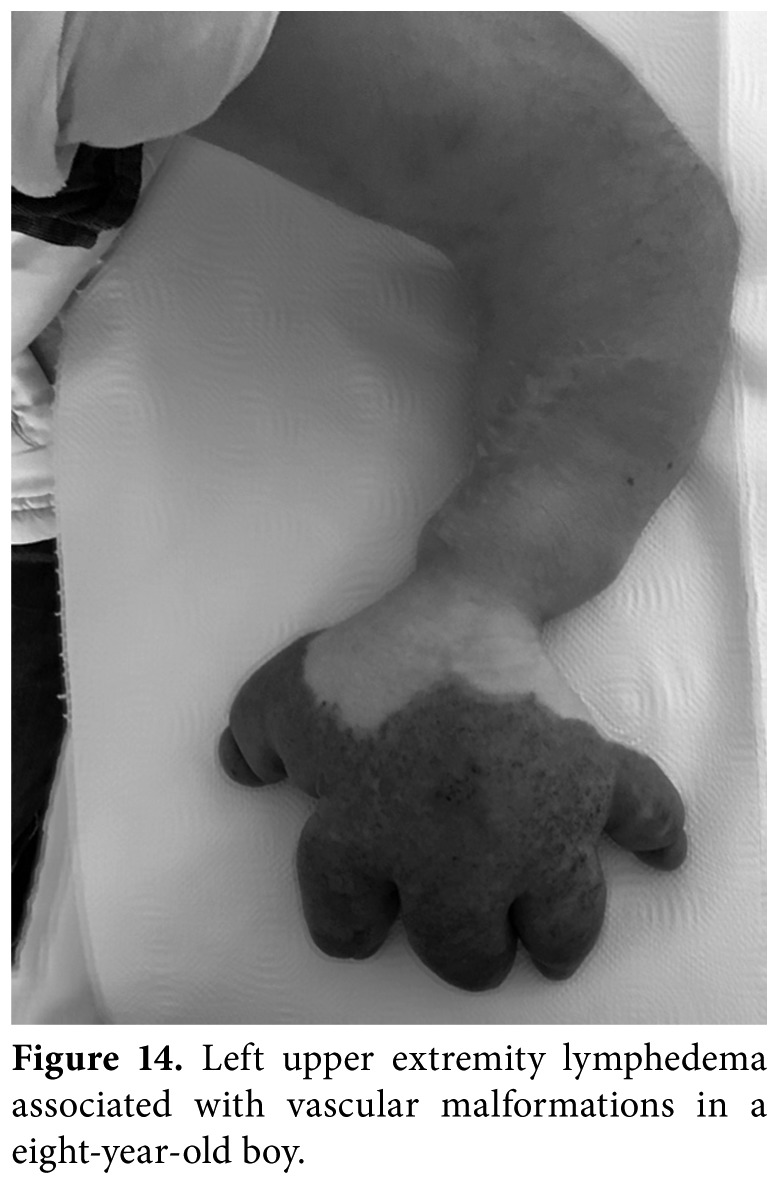
Figure 15. Congenital lymphedema in the right extremity and abdomen in a one-year-old girl.
TREATMENT OF LYMPHEDEMA
A multidisciplinary approach in a tailored program that would address the special needs of each patient should be considered in the treatment of lymphedema. In an ideal setting of an outpatient management program, the team should be composed of a PMR specialist as a leader and coordinator, a physiotherapist, nurse, psychologist, dermatologist, dietician, and vascular and/or plastic surgeon, working together in the assessment and management of all aspects of lymphedema. All management approaches should focus on the improvement of the QoL of patients and prevention of lymphedema in high-risk patients.[3,36,37]
There is no cure for lymphedema. However, with proper diagnosis and management, its progression and potential complications may be limited. Management guidelines and international consensus documents have been issued by the ISL.[11]
COMPLETE DECONGESTIVE THERAPY
Complete decongestive therapy is the recommended treatment which consists of intensive and long-term management phases. The intensive phase, which aims to reduce swelling and normalize the tissue pressure, consists of education and skin care, MLD, multilayer short stretch bandages, and lymphedema exercises. After the volume of the limb is stabilized, the patient enters the maintenance phase in which she/he continues to skin care, self-drainage, compression garments, and exercises. Complete decongestive therapy should be performed by educated and mastered lymphedema therapists or lymphedema specialists.[36,37] Systematic reviews have demonstrated that CDT is effective in reducing lymphedema when combined with MLD and compression bandages.[37,38] Some previous studies have also indicated that compression garments have similar effects on improvement on lymphedema with MLD and bandaging, but most of the patients has Grade 1 lymphedema in their study.[39] Long-term effects of CDT are well-maintained for 24 months in patients with BCRL.[37-39]
Education and skin care
Education and skin care are critical for both prevention and treatment of lymphedema and should be performed in both phases of CDT. Patients with lymphedema are advised to moisturize and protect their limb from incidental trauma. They are counseled not to walk barefoot in areas where there are risks for trauma, protect from mosquito bites, cover their limbs with clothing in gardening and walking through bushes, and wear gloves in cleaning and washing. They are also advised to avoid venesection and blood collecting from the affected arm, heat therapy, thermal spa and sauna use. The lymphedematous extremity has a significantly increased risk for infection. Dried and cracked skin can lead to cellulitis. Mild, soap free, pH-neutral cleansing lotion for everyday use and shower oils that replenish the skin's texture should be used. Before CDT, any skin infections including fungal diseases should be treated. Patients should avoid vigorous use of the affected extremity. Nail care is also mandatory for lymphedema patients.[37,40]
Manual lymphatic drainage
Manual lymphatic drainage is a specific manual technique which is frequently used as a part of CDT. Gentle stroking movements, directed proximally, are applied. The main goal of MLD is to increase the lymphokinetic activity and stimulate the functional units of lymph vessels. It usually lasts 30 to 45 min or even longer. Classically, it begins with manual stimulation of central lymph nodes (neck, superficial and deep abdomen) intact lymph nodes in adjacent drainage regions (axilla, groin), and areas of anastomosis (thorax, back, lateral trunk). This is followed by MLD of the limb from proximal to distal regions. The strokes should never be painful or unpleasant for the patient. Manual lymphatic drainage is not considered a stand-alone treatment and recommended before the compression therapy. Self-MLD is advised for maintenance phase of the CDT.[36,39]
Before beginning MLD, contraindications must be ruled out. Cardiac edema, cellulitis, and DVT are the major ones. Manual lymphatic drainage is safe and may offer additional benefits to compression bandaging for swelling reduction. It also decreases symptoms such as pain and heaviness and patients report feeling better regardless of which treatment they received. Previously, MLD has been shown to effectively prevent arm lymphedema after breast cancer surgery.[40] Self-MLD is also suggested for the prevention of lymphedema early after breast cancer surgery.[11,37] The effects of MLD on transport of radiotracers in lymphatic collectors have been shown by lymphoscintigraphic evaluation.[41] In a systematic review including randomized-controlled trials, the impact of MLD on the health-related QoL of adults with lymphedema or mixed edema was evaluated and the effect of MLD on the QoL of patients with chronic edema was reported to be unclear.[42]
Multilayer bandaging
Bandaging is the cornerstone of CDT. A correctly applied compression bandage would result in reduced ultrafiltration and improvement of lymph transport, as well as increase the effect of muscle and joint pump. Low stretch bandages are wrapped in multiple layers after covering the affected limb with padding composed of foam and/or cotton batting. Low stretch bandages have a low resting pressure and bring about a high working pressure. Bandages are applied progressively, starting from the distal extremity and gradually placed more proximally until reaching the axilla or groin. It may be kept in place for 24h and reapplied every day. Arterial occlusive disease, cardiac edema, DVT, and cellulitis are the main contraindications for bandaging.[37,43]
After the necessary skin care, tubular bandage is worn to prevent any direct contact between the cushioning material and the skin. Bandaging begins at the fingers and ends at the upper limb. After applying elastic gauze bandages to the fingers or toe, padding with cotton layers or foam is performed and multilayer bandaging beginning from the hand/foot with short- stretch bandages follow this procedure.[44]
The 3MTM CobanTM 2 layer compression system is designed to be applied without padding to achieve a thin and comfortable system allowing the patient a high degree of independence and mobility. This system has two layers and bandages are changed twice weekly. Previous studies have shown good edema reduction in both arms and legs and improvements in symptoms associated with lymphedema.[45,46]
Lymphedema exercises
Physical activity and exercises enhance physical and emotional well-being, increase fitness and vitality and improve health-related QoL outcomes. They are considered another major component of CDT. Different types of exercises have been devised: against/without resistance, isometric and aerobic, as repetitive, progressive and therapist- guided movements. These are commonly associated with abdominal breathing and posture correction exercises.[37,39,44] There may be a concern that exercise may have an adverse effect on lymphedema risk. Recent scientific evidence has indicated that weight training exercises do not appear to increase lymphedema risk under structured conditions. The initiation of a tailored and graduated strength training exercises may improve signs and symptoms of lymphedema. Exercises are intended to facilitate lymph resorption ideally within short-stretch bandages or compression garments, as compression provides a firm resistance for the musculature and muscle and joint pumps work more effectively.[37,44]
Resistance exercise programs have been shown to have a positive effect on arm function and muscular strength without increasing the arm volume in BCRL patients.[47] Although some small-scale studies have demonstrated an absence of positive and negative effect from compression use during exercise on lymphedema,[48] some authors have suggested the use of pressure garments during exercise.[37,44]
Compression garments
The most important factor to maintain the effect of Phase 1 therapy is the use of compression garments. They are also used at the onset of symptoms to possibly prevent the development of lymphedema in patients at risk. Compression garments must be prescribed by an educated lymphedema specialist and must be individualized. Compression class, material of the garment, and size and design of the garments must be ordered according each patient's lymphedema status.[11,37,44] The manufacturers provide different types of garments with different pressures (Figures 16a, b). Compression classes are listed in Table 2. Although standard ready-made garments are cheap, they may not fit to all widths and lengths, and models and materials are limited. Custom- made garments have optimal fit, but need extensive measuring and have long delivery time and high cost. The patient's ability to take on/off the garment should be checked and care instructions should be taught.[49,50] The pressure garments are worn throughout the day during daytime. The reimbursement of the pressure garments vary depending on grading of lymphedema and type of the garment. Partial reimbursement is available in Turkey with a medical report signed by three physicians in a university or tertiary state hospital setting.[50]
Figure 16. (a) Pressure garment examples for lower extremity lymphedema. (b) Pressure garment for upper extremity lymphedema.
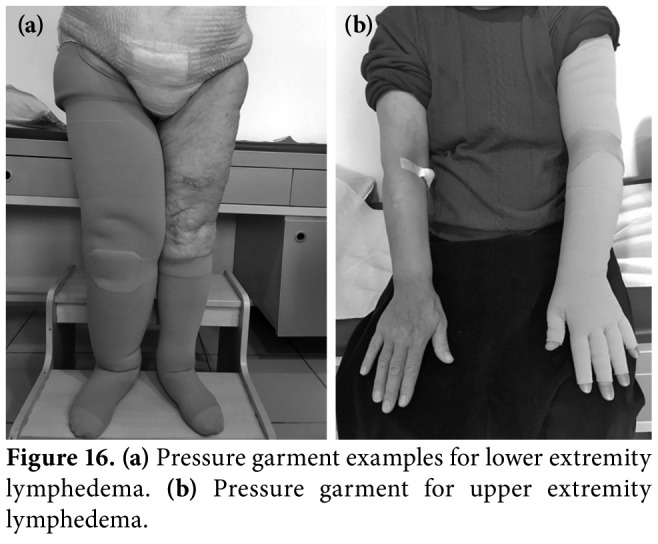
Table 2. Compression classes in regard to seamless (circular-knit) and seamed (flat-knit) compression garments.
| Compression class | Circular-knit | Flat-knit |
| CCL1 | 20-30 mmHg | 18-21 mmHg |
| CCL 2 | 30-40 mmHg | 23-32 mmHg |
| CCL 3 | 40-50 mmHg | 34-46 mmHg |
| CCL 4 | > 50 mmHg | |
| CCL: Compression class. | ||
SELF-MANAGEMENT
Lymphedema is a chronic disease that requires a life-long management. Although randomized trials on self-management are sorely lacking, self-MLD, self- bandaging, and exercises along with meticulous skin care are recommended in the maintenance phase.[51,52] In the author's clinic, education about self-management techniques (skin care, self-MLD and exercises) is given at the initial visit of lymphedema patients with printed and visual materials (www.lenfodem.hacettepe.edu.tr) and social organizations (www. lenfodemdernegi.org.tr) which support lymphedema patients are recommended to self-manage their chronic pathology and maintain long-term motivation.[50]
Other therapeutic options include pneumatic compression pumps (PCPs), physical therapy modalities, stellate ganglion block, negative pressure therapy, pharmaceutical options, alternative methods, kinesiotaping, and surgical approaches.[37,43,44]
PNEUMATIC COMPRESSION PUMPS
Pneumatic compression pumps offer intermittent compression and are hypothesized to help in delivering retained fluid to functional lymphatics and to facilitate reducing edema. Previous reports have indicated symptom relief, improvement in control of edema and in QoL in patients with lower extremity lymphedema.[53] It has been shown to be less effective in patients with increased subcutaneous fibrosis, leading to poor tissue compliance. The 2013 Consensus document of the ISL supports the use of intermittent pneumatic compression as an adjunct modality[11] and systematic reviews have suggested that PCP may provide an acceptable home-based treatment modality in selected patients, in addition to wearing compression garments.[54] Therapy may range from 30 min to several hours depending on the device and health status of the patient. The pressure of the device can be between 25 and 60 mmHg according to the site and grade of lymphedema and associated comorbidities. Devices that produce excessive pressure to the skin surface may be harmful on superficial lymphatic structure and, therefore, multi-chamber devices with trunk apparatus are recommended to avoid genital and trunk lymphedema.[53,55]
PHYSICAL THERAPY MODALITIES
Laser
Low-level laser therapy (LLLT) has been suggested as a useful treatment for lymphedema. It promotes lymphangiogenesis and stimulates lymphatic motility with no significant changes in the tissue architecture. It also improves overall lymphatic flow and reduce interstitial fibrosis which accompanies lymphatic stasis. However, there is no consensus on the timing of laser application, number of treatment sessions, energy settings, power density and dose. The LLLT have yielded favorable results in improving lymphedema, compared to placebo, PCPs and no intervention. A meta-analysis revealed moderate-to-strong evidence supporting the use of LLT in the management of BCRL with clinically relevant reductions in arm volume and pain immediately after the procedure. Reductions were higher in treatments with the use of LLLT than non-LLLT.[55] On the other hand, systematic reviews are unable to claim that laser treatment is the most effective modality in BCRL. In addition, as there is no study evaluating the hypothesis that the LLLT can increase the risk of recurrence or metastasis, questions about the safety of this procedure in cancer patients still remain.[56,57]
Far-infrared radiation (FIR)
Among the different frequencies using the infrared spectrum, far-infrared rays are the most beneficial for human beings. This treatment has an action similar to hyperthermia, which has three main biological effects: radiation, vibrational, and thermal. The effectiveness and safety of FIR as a promising treatment modality of lymphedema were evaluated in a group of Grade 2-3 lymphedema patients and a significant reduction in the limb size and improvement in QoL were recorded.[58,59] Another study reported similar findings and highlighted the treatment effect of FIR on tissue fibrosis and skin elasticity in patients with lymphedema.[58] This modality has been found to be safe and suggested to be used as an alternative monotherapy or a useful adjunctive to the conservative or surgical lymphedema procedures,[59] although further studies are needed to confirm these limited results.
Extracorporeal shock wave therapy (ESWT)
Extracorporeal Shock Wave Therapy has been widely used in PMR. Previous studies have concluded that ESWT promotes angiogenesis and decreases inflammation in humans and helps lymphangiogenesis in animal models. Bae and Kim[60] performed ESWT (0.056-0.068 Mj/mm2, 2000 impulses) four times over two weeks in seven patients with Stage 3 BCRL and demonstrated the effectiveness of ESWT (with or without other conventional treatments) in reducing the circumference and thickness of arms. This non- invasive procedure was suggested to provide clinically favorable outcome to patients with BCRL.[44]
STELLATE GANGLION BLOCK (SGB)
The therapeutic use of SGB for treatment of lymphedema was introduced considering the theory that the block itself interferes with sympathetic nervous system and, thus, relaxes the veins, thereby reducing the post-capillary resistance and leading to release of accumulated interstitial fluid into the venous system.[61] The effectiveness of SGB has been shown in intractable upper limb of BCRL patients. The SGB was performed with 5 mL of 0.5% mepivacaine by palpating the anterior transverse process from the C6 vertebra. Each process was repeated after four days, seven days and, then, three times a week with a two-week interval.[61] The SGB of the lumbar region has been also used in the same manner for the treatment of lower extremity lymphedema after gynecologic cancer surgery.[62]
NEGATIVE PRESSURE THERAPY
Conservative methods of lymphedema comprising pressure garments, bandaging, manual lymphatic drainage, and pneumatic compression devices represent examples of positive pressure therapy and technology. Despite these efforts, some patients progress to develop significant and morbid secondary skin changes (i.e., cellulitis or fatty tissue deposition), disability, and impaired QoL. Negative pressure therapy is a new therapy option whereby a pulling and opening force is applied to the tissues. Treatment can be targeted to specific areas of scars, radiation- induced fibrosis or wounds. This technology can be performed by kinesiological taping, deep breathing, and negative pressure massage devices (Lymphatouch®, Endermologia®) and can be used as an adjunct to MLD.[63] These novel and innovative methods can potentially improve complex lymphedema therapy outcomes, although further research studies are needed for strong evidence of their efficacy.[44,63]
PHARMACOLOGICAL TREATMENT
There is no drug that increases lymphatic activity. The use of diuretics is not indicated for lymphedema, as only the water load, not the protein load in the tissue, is affected by the medication. The pharmacological treatment is needed in complications of lymphedema such as cellulitis (antibiotics), erysipelas, fungal infections (antifungal agents), ulceration, and lymphorrhea (topical antibiotic ointments).[37,44]
ALTERNATIVE METHODS
The majority of lymphedema patients submit to the most contemporary evidence-based treatments, but they may also seek complementary and alternative medicine (CAM) treatments, as these approaches are viewed more humanistic and holistic. The effects of natural products including coumarin, Ginkor-Fort, horse chestnut complex, and vitamin E on lymphedema have been investigated. Despite the potential beneficial effects of herbal medicines, as indicated in small-scale, non-qualified studies, no firm evidence to support the effectiveness of herbal medicines in treating lymphedema was found.[64]
In a previous study, the effect of acupuncture on lymphedema reduction and rehabilitation in patients with BCRL was examined and encouraging results associated with acupuncture as preventive and therapeutic treatment for BCRL patients were demonstrated.[65] However, further controlled long-term clinical trials are needed to determine the potential risks and benefits of acupuncture on lymphedema treatment. Sodium selenite also showed promise as a cost-effective, non-toxic, and anti-inflammatory agent causing a spontaneous reduction in lymphedema volume and increasing the efficacy of physical therapy.[66] In addition, a small pilot trial demonstrated the positive effects of yoga on women with Stage 1 BCRL. Eight-week yoga intervention did not increase arm volume and decreased tissue induration on the affected arm with improved QoL scores.[67]
We recommend that PMR specialists should be aware of risks and benefits of CAM therapies and educate patients about the potential effects of CAM, particularly in patients with cancer-related lymphedema.
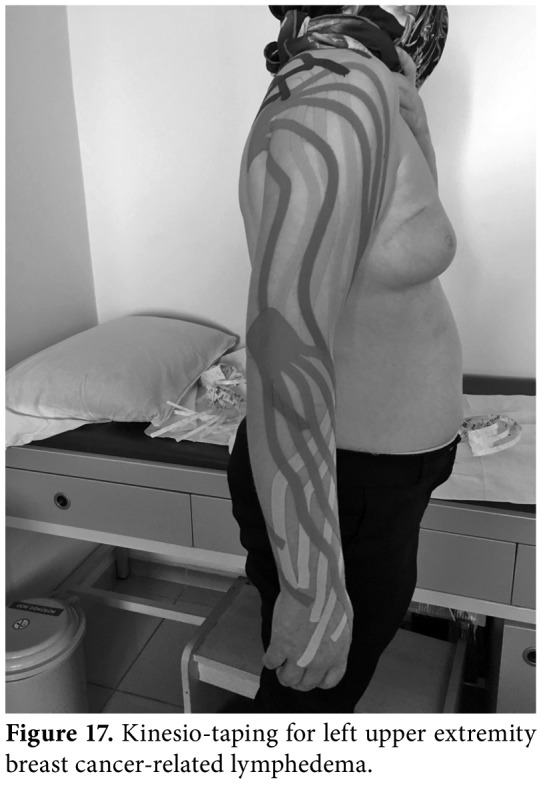
KINESIOTAPING
Kinesiotaping®, as a lymphatic correction method, helps to remove edema by directing the fluid toward a less congested lymphatic pathway and lymph nodes. The elasticity and lifting effects of kinesiotaping decrease the pressure on the superficial skin, open initial lymphatics, and creates a massage effect during the active motion. It also improves the transport of deeper lymphatics by allowing maximum contraction and relaxation of a muscle (Figure 17). Previous studies have yielded improved effects of kinesiotaping on decreasing lymphedema, when performed in addition to CDT.[68]
Figure 17. Kinesio-taping for left upper extremity breast cancer-related lymphedema.
DIET
There is no special diet for the management of lymphedema. The only condition on which diet has an impact is lymphostatic enteropathy. A healthy and well-balanced diet is needed for general health. Diets reducing fluid intake or low-protein diets must be avoided.[37] Previous studies reported a higher risk of lymphedema among women who were obese (body mass index [BMI] >30 kg/m2).[69] Weight gain after breast cancer has been also suggested as a risk factor in BCRL.[70] Weight loss interventions produced a significant reduction in upper extremity lymphedema volume in patients with BCRL.[69,70] Therefore, patients must be advised to maintain a normal BMI and weight control, as obesity is associated with inf lammation and worsening of lymphedema.[11,52]
SURGERY FOR LYMPHEDEMA
Although non-surgical conservative managements remain the first-line standard of care, safe surgical alternatives are available which can provide effective and long-term improvements in selected lymphedema patients.[36] Previous studies have demonstrated that best results can be achieved, when surgery is performed as a part of comprehensive treatment incorporating individualized CDT before and after surgery.[71-73] Modern lymphedema surgeries such as vascularized lymph node transfer (VLNT), lymphaticovenous anastomosis (LVA), lymphoticolymphatic bypass (LB), and suction-assisted protein lipectomy (SAPL) are less invasive than previous radical excisional debulking procedures, such as Charles procedure, which involves aggressive removal of the skin and deeper tissues. Such invasive techniques are now reserved only for extreme cases involving thickened, pendulous, and inflamed skin and tissues.[37,71]
The best candidates for lymphedema surgery are patients who have failed one or more courses of CDT. Good candidates are patients who would continue to CDT before and after therapy, although it is often possible to reduce CDT and compression garment requirements after a successful surgery. Obesity and morbid obesity produce poor surgical outcomes. Meaningful weight loss should be provided prior to surgical procedures. As different lymphedema surgeries address different aspects of swelling, proper patient and surgery type selection is critical to the success of any surgery. Of note, LVA and VLNT best address the fluid portion of swelling which typically is more prevalent during the early stages of lymphedema. In addition, SAPL best removes the solid component of the swelling, which is usually found in later chronic disease.[11,73]
The lymphedema surgeon must work closely with a PMR specialist and lymphedema therapist to ensure that the best conservative therapy is given both before and after any surgical procedure. This is particularly important for the success of SAPL procedure. Combined and staged lymphedema surgeries can be also performed to increase effectiveness.[71,73]
Follow-up
Both primary and secondary lymphedema are chronic diseases that require constant monitoring. Clinical follow-ups by six months need to be scheduled but complications of lymphedema may require close monitoring. In each visit, physical examination with circumferential measurements and weight control must be performed. The pressure garments need to be renewed by 6-9 month intervals. The self- management procedures, exercise interventions and compliance of the patient and/or families should also be addressed.[43,44,50]
PREVENTATION OF LYMPHEDEMA
Prevention of lymphedema can be primary and secondary prevention. Primary prevention is intended for all individuals at risk of lymphedema. Some health and diet guidelines are recommended for these patients including keeping body weight under control, skin care, balanced and controlled daily physical activity, exercises, low-pressure garments as a preventive measure at flights, or avoiding heavy physical efforts at work or at home.[43,44,52]
Secondary prevention is aimed to prevent the complications of lymphedema, particularly the infections such as cellulitis. Meticulous skin care and compliance in wearing pressure garments and self-management are crucial for preventing the worsening of edema and development of dystrophic complications.[43,44,50]
In conclusion, lymphedema is a chronic progressive condition which requires lifelong multidisciplinary treatment strategies under the supervision and responsibility of PMR specialists. The PMR specialist must have knowledge and education for the diagnosis of disease and complications, as well as evaluation, treatment and follow-up of the patient. The PMR physician sets up strategies for the management and rehabilitation of the patients with lymphedema. The point of view and global approach of PMR specialists are, therefore, important in clinical care of this suffering and debilitating condition.
Footnotes
Conflict of Interest: The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
Financial Disclosure: The authors received no financial support for the research and/or authorship of this article.
References
- 1.Keast DH, Despatis M, Allen JO, Brassard A. Chronic oedema/lymphoedema: under-recognised and under- treated. Int Wound J. 2015;12:328–333. doi: 10.1111/iwj.12224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borman P, Yaman A, Yasrebi S, Özdemir O. The Importance of Awareness and Education in Patients with Breast Cancer-Related Lymphedema. J Cancer Educ. 2017;32:629–633. doi: 10.1007/s13187-016-1026-1. [DOI] [PubMed] [Google Scholar]
- 3.Fialka-Moser V, Korpan M, Varela E, Ward A, Gutenbrunner C, Casillas JM, et al. The role of physical and rehabilitation medicine specialist in lymphoedema. Ann Phys Rehabil Med. 2013;56:396–410. doi: 10.1016/j.rehab.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Greene AK, Slavin SA, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. Greene AK Epidemiology and morbidity of lymphedema. pp. 33–50. [Google Scholar]
- 5.Ozaslan C, Kuru B. Lymphedema after treatment of breast cancer. Am J Surg. 2004;187:69–72. doi: 10.1016/j.amjsurg.2002.12.003. [DOI] [PubMed] [Google Scholar]
- 6.Hayes SC, Janda M, Ward LC, Reul-Hirche H, Steele ML, Carter J, et al. Lymphedema following gynecological cancer: Results from a prospective, longitudinal cohort study on prevalence, incidence and risk factors. Gynecol Oncol. 2017;146:623–629. doi: 10.1016/j.ygyno.2017.06.004. [DOI] [PubMed] [Google Scholar]
- 7.Ridner SH. Pathophysiology of lymphedema. Semin Oncol Nurs. 2013;29:4–11. doi: 10.1016/j.soncn.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Michelini S. Lymphedema etiology, epidemiology and clinical staging. In: Michelini S, Failla A, Moneta G, Cardone M, editors. Compression therapy in lymphatic Insufficiency. Milano: Cizeta-Medicali; 2010. pp. 14–18. [Google Scholar]
- 9.Simonian SJ, Morgan CL, Tretbar LL, Blondeau B. Differential diagnosis of lymphedema. In: Tretbar LL, Morgan CL, Lee BB, Simonian SJ, Blondeau B, editors. Lymphedema Diagnosis and Treatment. Chapter 2. London: Springer; 2008. pp. 12–20. [Google Scholar]
- 10.Greene AK. History and Physical Examination. In: Greene AK, Slavin SA, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. pp. 107–114. [Google Scholar]
- 11.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 Consensus Document of the International Society of Lymphology. Lymphology. 2013;46:1–11. [PubMed] [Google Scholar]
- 12.Koldas Dogan S, Ay S, Evcik D, Baser O. Adaptation of Turkish version of the questionnaire Quick Disability of the Arm, Shoulder, and Hand (Quick DASH) in patients with carpal tunnel syndrome. Clin Rheumatol. 2011;30:185–191. doi: 10.1007/s10067-010-1470-y. [DOI] [PubMed] [Google Scholar]
- 13.Citaker S, Kafa N, Hazar Kanik Z, Ugurlu M, Kafa B, Tuna Z. Translation, cross-cultural adaptation and validation of the Turkish version of the Lower Extremity Functional Scale on patients with knee injuries. Arch Orthop Trauma Surg. 2016;136:389–395. doi: 10.1007/s00402-015-2384-6. [DOI] [PubMed] [Google Scholar]
- 14.Pusic AL, Cemal Y, Albornoz C, Klassen A, Cano S, Sulimanoff I, et al. Quality of life among breast cancer patients with lymphedema: a systematic review of patient- reported outcome instruments and outcomes. J Cancer Surviv. 2013;7:83–92. doi: 10.1007/s11764-012-0247-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keeley V, Crooks S, Locke J, Veigas D, Riches K, Hilliam R. A quality of life measure for limb lymphedema (LYMQOL) J Lymphoedema. 2010;5:26–37. [Google Scholar]
- 16.Borman Yaman A, Denizli M, Karahan S, Özdemir O. The reliability and validity of lymphedema quality of life questionnaire-Arm in Turkish Patients with upper limb lymphedema. 12th National Lymphedema Network. 12th National Lymphedema Network International Conference Dallas, TX US, August 31, September 4, 2016. Available from: https://cme.uchicago.edu/NLNIC16.Brochure.pdf. [Google Scholar]
- 17.Borman P, Denizli M, Yaman A, Karahan S. The reliability and validity of lymphedema quality of life questionnaire- Leg (LYMQOL-Leg) in Turkish Patients with lower limb lymphedema. 8th International Lymphedema Framework Conference, Rotterdam, Netherlands, 6-9 June 2018, Programme and Abstract Book, 2018. p. 43. Available from: https://2018ilfconference.org. [Google Scholar]
- 18.Brorson H, Svensson B, Ohlin K. Volume measurements and follow-up. In: Greene AK, Slavin SA, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. pp. 115–122. [Google Scholar]
- 19.Sharkey AR, King SW, Kuo RY, Bickerton SB, Ramsden AJ, Furniss D. Measuring limb volume: Accuracy and reliability of tape measurement versus perometer measurement. Lymphat Res Biol. 2018;16:182–186. doi: 10.1089/lrb.2017.0039. [DOI] [PubMed] [Google Scholar]
- 20.Ward LC. Bioelectrical impedance spectrometry for the assessment of lymphedema: Principles and practice. In: Greene AK, Slavin SA, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. pp. 123–132. [Google Scholar]
- 21.Miller CL, Specht MC, Horick N, Skolny MN, Jammallo LS, O'Toole J, et al. A novel, validated method to quantify breast cancer-related lymphedema (BCRL) following bilateral breast surgery. Lymphology. 2013;46:64–74. [PubMed] [Google Scholar]
- 22.Bourgeois P. Lymphoscintigraphy and other imaging methods. In: Greene AK, Slavin SA, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. pp. 185–208. [Google Scholar]
- 23.Johnson KC, DeSarno M, Ashikaga T, Dee J, Henry SM. Ultrasound and Clinical Measures for Lymphedema. Lymphat Res Biol. 2016;14:8–17. doi: 10.1089/lrb.2015.0001. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Shin BW, Jeong HJ, Kim GC, Kim DK, Sim YJ. Ultrasonographic evaluation of therapeutic effects of complex decongestive therapy in breast cancer-related lymphedema. Ann Rehabil Med. 2013;37:683–689. doi: 10.5535/arm.2013.37.5.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O'Donnell TF Jr, Rasmussen JC, Sevick-Muraca EM. New diagnostic modalities in the evaluation of lymphedema. J Vasc Surg Venous Lymphat Disord. 2017;5:261–273. doi: 10.1016/j.jvsv.2016.10.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pritschow H, Schuchhardt C. Clinical symptoms of edema. In: Pritschow H, Schuchhardt C, editors. Lymphedema Management and Complete Physical Decongestive Therapy: A manual for treatment. Cologne: ViavitalVerlag; 2010. pp. 40–61. [Google Scholar]
- 27.Farhat MM, Le Guern A, Peugniez C, Dabouz F, Quinchon JF, Modiano P. Angiosarcoma in primary lymphoedema: A rare complication. Ann Dermatol Venereol. 2018;145:266–269. doi: 10.1016/j.annder.2018.02.001. [DOI] [PubMed] [Google Scholar]
- 28.Piper M, Guajardo I, Denkler K, Sbitany H. Axillary Web Syndrome: Current Understanding and New Directions for Treatment. Ann Plast Surg. 2016;76:227–231. doi: 10.1097/SAP.0000000000000767. [DOI] [PubMed] [Google Scholar]
- 29.Deng J, Fleischer A, Niermann K, Byram B, Murphy B. Head and neck lymphedema and fibrosis: a case study. J Lymphology. 2018;13:24–28. [Google Scholar]
- 30.Deng J, Ridner SH, Aulino JM, Murphy BA. Assessment and measurement of head and neck lymphedema: state-of-the-science and future directions. Oral Oncol. 2015;51:431–437. doi: 10.1016/j.oraloncology.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Olszewski LW. Genital lymphedema. In: Lee BB, Simonian SJ, Blondeau B, Tretbar LL, Morgan CL, editors. Lymphedema. London: Springer-Verlag; 2011. pp. 307–312. [Google Scholar]
- 32.Borman P. Genital lenfödem. In: Alper S, Akalın E, Gündüz B, editors. Lenfödem Tanı ve Tedavi. İzmir: O'Tıp Kitabevi; 2017. pp. 171–178. [Google Scholar]
- 33.Pinto e Silva MP, Bassani MA, Miquelutti MA, Marques Ade A, do Amaral MT, de Oliveira MM, et al. Manual lymphatic drainage and multilayer compression therapy for vulvar edema: a case series. Physiother Theory Pract. 2015;31:527–531. doi: 10.3109/09593985.2015.1038375. [DOI] [PubMed] [Google Scholar]
- 34.International Lymphedema Framework Care of Children with Lymphedema. Focus Document. 2010 Available from: https://www.lympho.org/portfolio/care-of-children-with-lymphoedema/ [Google Scholar]
- 35.Zuther JE. Pediatric lymphedema. Treatment. In: Zuther JE, Norton S, editors. The comprehensive guide for practitioners. 3rd ed. Sttuttgard: Thieme Verlag KG; 2013. pp. 208–211. [Google Scholar]
- 36.Papadopoulou MC, Tsiouri I, Salta-Stankova R, Drakou A, Rousas N, Roussaki-Schulze AV, et al. Multidisciplinary lymphedema treatment program. Int J Low Extrem Wounds. 2012;11:20–27. doi: 10.1177/1534734612438436. [DOI] [PubMed] [Google Scholar]
- 37.Zuther JE, Norton S, editors. Lymphedema Management: The Comprehensive Guide for Practitioners. 3rd ed. Sttuttgard: Thieme Verlag KG; 2013. pp. 165–342. [Google Scholar]
- 38.Lasinski BB, McKillip Thrift K, Squire D, Austin MK, Smith KM, Wanchai A, et al. A systematic review of the evidence for complete decongestive therapy in the treatment of lymphedema from 2004 to 2011. PM R. 2012;4:580–601. doi: 10.1016/j.pmrj.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 39.Vignes S. Greene AK, Slavin SA, Brorson H. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. Complex decongestive therapy; pp. 227–236. [Google Scholar]
- 40.Ezzo J, Manheimer E, McNeely ML, Howell DM, Weiss R, Johansson KI, et al. Manual lymphatic drainage for lymphedema following breast cancer treatment. Cochrane Database Syst Rev. 2015;5:CD003475–CD003475. doi: 10.1002/14651858.CD003475.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Godoy JM, Santana KR, Godoy Mde F. Lymphoscintigraphic evaluation of manual lymphatic therapy: the Godoy & Godoy technique. Phlebology. 2015;30:39–44. doi: 10.1177/0268355513506574. [DOI] [PubMed] [Google Scholar]
- 42.Müller M, Klingberg K, Wertli MM, Carreira H. Manual lymphatic drainage and quality of life in patients with lymphoedema and mixed oedema: a systematic review of randomised controlled trials. Qual Life Res. 2018;27:1403–1414. doi: 10.1007/s11136-018-1796-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Michelini S, Failla A, Moneta G, Cardone M, editors. Compression therapy in lymphatic insufficiency. 2nd ed. Milano: Cizeta Medicali; 2011. pp. 66–67. [Google Scholar]
- 44.Pritschow H, Schuchhardt C. Lymphedema management. In: Pritschow H, Schuchhardt C, editors. Lymphedema Management and Complete Physical Decongestive Therapy: A manual for treatments. Cologne: ViavitalVerlag; 2010. pp. 68–75. [Google Scholar]
- 45.Franks PJ, Moffatt CJ, Murray S, Reddick M, Tilley A, Schreiber A. Evaluation of the performance of a new compression system in patients with lymphoedema. Int Wound J. 2013;10:203–209. doi: 10.1111/j.1742-481X.2012.00958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yaman A, Borman P, Pınar A, Kul F, Özçakar L, Karahan S. Comparison of the efficacy of different bandaging methods in treatment of patients with breast cancer-related lymphedema. International Lymphedema Framework Conference. 2017 Siracusa, Italy, 21-24 June 2017, Oral Presentation. [Google Scholar]
- 47.Do JH, Kim W, Cho YK, Lee J, Song EJ, Chun YM, et al. Effects of resistance exercises and complex decongestive therapy on arm function and muscular strength in breast cancer related lymphedema. Lymphology. 2015;48:184–196. [PubMed] [Google Scholar]
- 48.Singh B, Buchan J, Box R, Janda M, Peake J, Purcell A, et al. Compression use during an exercise intervention and associated changes in breast cancer-related lymphedema. Asia Pac J Clin Oncol. 2016;12:216–224. doi: 10.1111/ajco.12471. [DOI] [PubMed] [Google Scholar]
- 49.Ohlin K, Svensson B, Brorson H. Controlled compression therapy and compression garments. In: Greene AK, Svensson B, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. pp. 213–225. [Google Scholar]
- 50.Borman P. Beyazova M, Gökçe Kutsal Y. Fiziksel Tıp veRehabilitasyon. 3. Baskı. Ankara: Güneş Tıp Kitapevleri; 2016. Lenfödem rehabilitasyonu. Bölüm 94, Kısım: 5. Özel rehabilitasyon alanları; pp. 1263–1280. [Google Scholar]
- 51.Ridner SH, Fu MR, Wanchai A, Stewart BR, Armer JM, Cormier JN. Self-management of lymphedema: a systematic review of the literature from 2004 to 2011. Nurs Res. 2012;61:291–299. doi: 10.1097/NNR.0b013e31824f82b2. [DOI] [PubMed] [Google Scholar]
- 52.National Lymphedema Network. Lymphedema Risk reduction practices updated. May 2013-2. Available from: http://www.lymphnet.org/assets/docs/position_papers/Risk.Reduction.pdf. [Google Scholar]
- 53.Blumberg SN, Berland T, Rockman C, Mussa F, Brooks A, Cayne N, et al. Pneumatic compression improves quality of life in patients with lower-extremity lymphedema. Ann Vasc Surg. 2016;30:40–44. doi: 10.1016/j.avsg.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 54.Feldman JL, Stout NL, Wanchai A, Stewart BR, Cormier JN, Armer JM. Intermittent pneumatic compression therapy: a systematic review. Lymphology. 2012;45:13–25. [PubMed] [Google Scholar]
- 55.Maclellan RA. Pneumatic compression. In: Greene AK, Slavin SA, Brorson H, editors. Lymphedema Presentation, Diagnosis and Treatment. Switzerland: Springer; 2015. pp. 237–242. [Google Scholar]
- 56.E Lima MT, E Lima JG, de Andrade MF, Bergmann A. Low-level laser therapy in secondary lymphedema after breast cancer: systematic review. Lasers Med Sci. 2014;29:1289–1295. doi: 10.1007/s10103-012-1240-y. [DOI] [PubMed] [Google Scholar]
- 57.Smoot B, Chiavola-Larson L, Lee J, Manibusan H, Allen DD. Effect of low-level laser therapy on pain and swelling in women with breast cancer-related lymphedema: a systematic review and meta-analysis. J Cancer Surviv. 2015;9:287–304. doi: 10.1007/s11764-014-0411-1. [DOI] [PubMed] [Google Scholar]
- 58.Li K, Zhang Z, Liu NF, Feng SQ, Tong Y, Zhang JF, et al. Efficacy and safety of far infrared radiation in lymphedema treatment: clinical evaluation and laboratory analysis. Lasers Med Sci. 2017;32:485–494. doi: 10.1007/s10103-016-2135-0. [DOI] [PubMed] [Google Scholar]
- 59.Li K, Zhang Z, Liu NF, Sadigh P, Evans VJ, Zhou H, et al. Far-Infrared Radiation Thermotherapy Improves Tissue Fibrosis in Chronic Extremity Lymphedema. Lymphat Res Biol. 2018;16:248–257. doi: 10.1089/lrb.2016.0057. [DOI] [PubMed] [Google Scholar]
- 60.Bae H, Kim HJ. Clinical outcomes of extracorporeal shock wave therapy in patients with secondary lymphedema: a pilot study. Ann Rehabil Med. 2013;37:229–234. doi: 10.5535/arm.2013.37.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J, Park HS, Cho SY, Baik HJ, Kim JH. The effect of stellate ganglion block on intractable lymphedema after breast cancer surgery. Korean J Pain. 2015;28:61–63. doi: 10.3344/kjp.2015.28.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo JH, Park HS, Kim SC, Kim YH. The effect of lumbar sympathetic ganglion block on gynecologic cancer-related lymphedema. Pain Physician. 2013;16:345–352. [PubMed] [Google Scholar]
- 63.Gott FH, Ly K, Piller N, Mangion A. Negative pressure therapy in the management of lymphedema. J Lymphedema. 2018;13:43–48. [Google Scholar]
- 64.Wanchai A, Armer JM, Stewart BR. Complementary and alternative medicine and lymphedema. Semin Oncol Nurs. 2013;29:41–49. doi: 10.1016/j.soncn.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 65.Kim TH, Kang JW, Lee MS. Current evidence of acupuncture for symptoms related to breast cancer survivors: A PRISMA- compliant systematic review of clinical studies in Korea. Medicine (Baltimore) 2018;97:e11793–e11793. doi: 10.1097/MD.0000000000011793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Pfister C, Dawzcynski H, Schingale FJ. Sodium selenite and cancer related lymphedema: Biological and pharmacological effects. J Trace Elem Med Biol. 2016;37:111–116. doi: 10.1016/j.jtemb.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 67.Loudon A, Barnett T, Piller N, Immink MA, Williams AD. Yoga management of breast cancer-related lymphoedema: a randomised controlled pilot-trial. BMC Complement Altern Med. 2014;14:214–214. doi: 10.1186/1472-6882-14-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gatt M, Willis S, Leuschner S. A meta-analysis of the effectiveness and safety of kinesiology taping in the management of cancer-related lymphoedema. Eur J Cancer Care (Engl) 2017;26 doi: 10.1111/ecc.12510. [DOI] [PubMed] [Google Scholar]
- 69.Penn IW, Chang YC, Chuang E, Chen CM, Chung CF, Kuo CY, et al. Risk factors and prediction model for persistent breast-cancer-related lymphedema: a 5-year cohort study. Support Care Cancer. doi: 10.1007/s00520-018-4388-6. 2018 Aug 14. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Demark-Wahnefried W, Campbell KL, Hayes SC. Weight management and its role in breast cancer rehabilitation. Cancer. 2012;118:2277–2287. doi: 10.1002/cncr.27466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Granzow JW. The current state of surgery for lymphedema. Surgery for Lymphedema. National Lymphedema Network (NLN) Lymph Link. 2015;28:3–6. [Google Scholar]
- 72.Gallagher K, Marulanda K, Gray S. Surgical Intervention for Lymphedema. Surg Oncol Clin N Am. 2018;27:195–215. doi: 10.1016/j.soc.2017.08.001. [DOI] [PubMed] [Google Scholar]
- 73.Granzow JW, Soderberg JM, Kaji AH, Dauphine C. Review of current surgical treatments for lymphedema. Ann Surg Oncol. 2014;21:1195–1201. doi: 10.1245/s10434-014-3518-8. [DOI] [PubMed] [Google Scholar]