Abstract
Objective:
Few randomized controlled trials have focused on the optimal management of patients with type 2 diabetes (T2D) during the transition from the inpatient to outpatient setting. This multicenter open-label study explored a discharge strategy based on admission hemoglobin A1c (HbA1c) to guide therapy in general medicine and surgery patients with T2D.
Methods:
Patients with HbA1c ≤7% (53 mmol/mol) were discharged on sitagliptin and metformin; patients with HbA1c between 7 and 9% (53–75 mmol/mol) and those >9% (75 mmol/mol) were discharged on sitagliptin-metformin with glargine U-100 at 50% or 80% of the hospital daily dose. The primary outcome was change in HbA1c at 3 and 6 months after discharge.
Results:
Mean HbA1c on admission for the entire cohort (N = 253) was 8.70 ± 2.3% and decreased to 7.30 ± 1.5% and 7.30 ± 1.7% at 3 and 6 months (P<.001). Patients with HbA1c <7% went from 6.3 ± 0.5% to 6.3 ± 0.80% and 6.2 ± 1.0% at 3 and 6 months. Patients with HbA1c between 7 and 9% had a reduction from 8.0 ± 0.6% to 7.3 ± 1.1% and 7.3 ± 1.3%, and those with HbA1c >9% from 11.3 ± 1.7% to 8.0 ± 1.8% and 8.0 ± 2.0% at 3 and 6 months after discharge (both P<.001). Clinically significant hypoglycemia (<54 mg/dL) was observed in 4%, 4%, and 7% among patients with a HbA1c <7%, 7 to 9%, and >9%, while a glucose <40 mg/dL was reported in <1% in all groups.
Conclusion:
The proposed HbA1c-based hospital discharge algorithm using a combination of sitagliptin-metformin was safe and significantly improved glycemic control after hospital discharge in general medicine and surgery patients with T2D.
INTRODUCTION
Patients with diabetes experience two- to threefold higher rates of hospital admissions compared to those without diabetes (1–3). The 2014 American Hospital Association annual survey data reported a total of 34,878,887 total number of admission in the United States (www.ahadataviewer.com), with approximately 25% of hospitalized patients with diabetes as a primary or secondary diagnosis. Patients with diabetes have longer lengths of hospital stay, more frequent complications, and more re-admissions compared to patients without diabetes (2,4). Several randomized controlled studies have reported successful algorithms for the management of inpatient hyperglycemia and diabetes and in reducing hospital complications in medicine and surgery patients with diabetes (5,6). Despite these findings, few prospective studies have reported the optimal process and impact of diabetes management from the inpatient to outpatient setting.
The transition of care from the hospital to the outpatient setting has been determined to be a priority in patients with diabetes (7,8). The Joint Commission National Patient Safety Goals document includes objectives and requirements for hospital discharge planning and transitional care. Hospital discharge represents an opportune time to address blood glucose (BG) control and adjust home diabetes therapy if necessary (9). It can also be used to address barriers to diabetes care and reinforce the importance of diabetes management and related issues (10). Several societies have developed patient centered care recommendations and algorithms for optimizing inpatient and outpatient diabetes care separately to help improve glycemic control, prevent hospital re-admissions, and reduce chronic complications of diabetes (11,12). To this effect, studies are needed to develop and evaluate safe and effective discharge algorithms for patients with diabetes (7).
The recent randomized Basal Plus trial (13) reported that the use of basal inulin as add-on to pre-admission diabetes therapy resulted in improved glycemic control along with hypoglycemia reported in over 30% of patients within 12 weeks after hospital discharge. These results indicated the need for utilizing drugs associated with low risk of hypoglycemia after hospital discharge. Because several studies have reported that dipeptidyl peptidase-4 (DPP-4) inhibitors are effective in the management of hospitalized medicine and surgery patients with type 2 diabetes (T2D) (14–16), we explored the efficacy and safety of sitagliptin-metformin combination, with or without glargine insulin based on the admission hemoglobin A1c (HbA1c), and frequent follow-up in general medicine and surgery patients with diabetes after hospital discharge.
METHODS
This was a multicenter, prospective, nonrandomized, open-label clinical trial in 253 general medicine and surgery patients with T2D who participated in the Sitagliptin Hospital (SITA-Hospital) Trial (16). Patients with a known history of diabetes for >1 month, age between 18 and 80 years; treated at home with diet, any combination of OAD agents, or with or without insulin therapy at a daily dose ≤ 0.6 units/kg; and with a randomization glucose between 140 and 400 mg/dL were invited to participate in this trial at the time of hospital discharge. We excluded patients with a BG >400 mg/dL, history of diabetic ketoacidosis, treatment with a DPP-4 inhibitor or glucagon-like peptide 1 receptor analogs within the past 30 days, history of gastrointestinal obstruction or suction, cardiac surgery, history of pancreatitis or active gallbladder disease, corticosteroid therapy, estimated glomerular filtration rate (eGFR) <30 mL/min/1.73 m2, pregnancy, and any mental condition rendering the patient unable to give informed consent. For the SITA HOSPITAL trial, all admissions to participating medicine and surgery floors were evaluated daily to identify patients who met eligibility criteria. A minimum anticipated length of stay of 3 days was sought so the medication regimen could be evaluated for effect in the hospital.
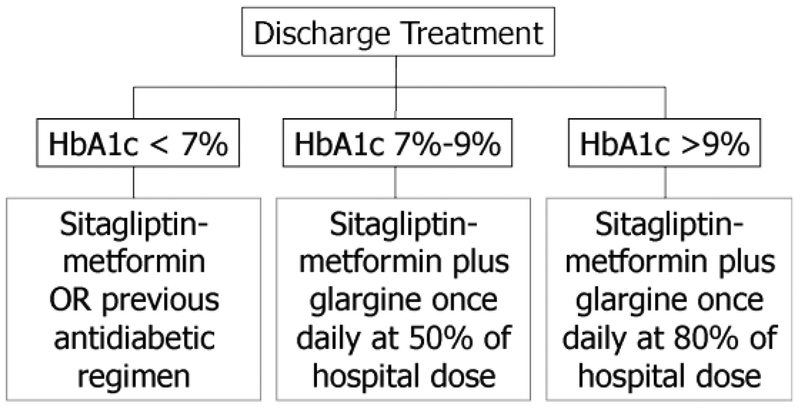
Pre-admission diabetes medications were discontinued at admission, and patients were switched to insulin with or without sitagliptin-metformin, as described in the SITA-HOSPITAL study (16). At discharge, sitagliptin/metformin with or without insulin was used as per the algorithm in Figure 1, based on HbA1c concentration measured during hospitalization. Insulin naïve patients with HbA1c ≤7% (53 mmol/mol) and no contra-indications to metformin were discharged on a combination of sitagliptin/metformin (Janumet®) or their pre-admission oral agents, and those on insulin pre-admission were discharged on metformin-sitagliptin plus 50% of the last inpatient basal insulin glargine U-100 daily dose. Patients with HbA1c between 7% (53 mmol/mol) and 9% (75 mmol/mol) were discharged on metformin-sitagliptin combination plus 50% of the hospital daily dose of glargine insulin. Patients with HbA1c >9% (75 mmol/mol) were discharged on metformin-sitagliptin plus glargine at 80% of the inpatient daily dose (Fig.1). For patients with previous intolerance or contra-indications to the use of metformin, sitagliptin was used alone or in combination with other pre-admission oral agents in all 3 groups.
Fig. 1.
Treatment algorithm upon hospital discharged based on HbA1c. HbA1c = hemoglobin A1c.
The starting metformin/sitagliptin dose was 50/500 mg twice daily if the eGFR was >50 mL/min/1.73 m2. If the eGFR was <50 mL/min/1.73 m2, 50 mg/day of sitagliptin was used alone without metformin. If the eGFR decreased to <30 mL/min/1.73 m2 during the study period, the dose of sitagliptin was reduced to 25 mg/day. Subjects taking metformin 1,000 mg twice daily at home were discharged on sitagliptin/metformin 1,000/50 mg twice daily. The used of other pre- admission antidiabetic agents was not protocolized, and their use was discouraged.
All patients received diabetes education on home glucose monitoring prior to discharge. This addressed the American Diabetes Association targets for fasting and premeal BG levels between 90 and 130 mg/dL. Use of glucose meters for home glucose self-monitoring (meters varied by institution) was demonstrated. Patients were shown how to keep BG records, and log-books were provided. Hypoglycemia recognition and management was reiterated. If needed, insulin administration was taught.
Patients were asked to measure glucose levels before meals and bedtime and were also asked to keep records of home self-monitoring of BG (SMBG). During follow up, the research team contacted patients every 2 weeks via telephone to discuss SMBG results to assess the need for adjusting therapy. The dose of sitagliptin/metformin was increased to 50/1,000 mg twice daily after 4 weeks or earlier if the fasting and premeal glucose concentrations were higher than 130 mg/dL. For patients treated with insulin, the daily insulin dose was adjusted following a prespecified insulin algorithm (17). Patients were asked to visit the diabetes research center at 1, 3, and 6 months after discharge. Glycemic data, treatment, and associated adherence and complication data were reviewed during each visit.
The primary outcome of the study was change in HbA1C from baseline at 3 and 6 months after hospital discharge. Secondary outcomes included change in fasting and mean daily glucose concentrations, number of hypoglycemic events <70 and <40 mg/dL (18), daily insulin requirements, use of oral antidiabetic agents, number of emergency room visits, and hospital re-admissions. An additional post hoc outcome measure of clinically important hypoglycemia (<54 mg/dL) was analyzed, as recently recommended by the International Hypoglycemia Study Group (18).
The study was conducted at 4 academic institutions including Emory University (Emory University Hospital and Grady Hospital), Atlanta; University of Michigan, Ann Arbor; Temple University, Philadelphia; and The Ohio State University, Columbus. The Institutional Review Board at each institution approved the protocol and consent forms.
Statistical Analysis
The primary aim of this study was to assess differences in HbA1c from baseline at 3 and 6 months after hospital discharge. Comparisons between different HbA1c groups were made with Wilcoxon tests (or Kruskal-Wallis tests) for continuous variables and χ2 tests (or Fisher exact tests) for discrete variables. The differences in BG or HbA1c from baseline to 3 months/6 months for overall and each HbA1c group were tested by Wilcoxon signed rank tests. The same methods were applied to the secondary outcomes. P<.05 was considered significant. The data are generally presented as means ± SD for continuous variables and count (percentage) for discrete variables. Statistical analyses were performed using SAS (version 9.2; Cary, NC).
RESULTS
A total of 253 general medicine and surgery patients were enrolled; of them, 68 patients (27%) had a baseline HbA1c <7% (53 mmol/mol), 98 patients (39%) had an HbA1c between 7% (53 mmol/mol) and 9% (75 mmol/mol), and 87 patients (34%) had an HbA1c >9% (75 mmol/mol). Demographic data for all groups are detailed in Table 1. Other than age, the groups were similar. The poorly controlled patients had the lowest average age (P<.01).
Table 1.
Baseline Clinical Characteristics
| Characteristic | All | HbA1c < 7% | HbA1c 7–9% | HbA1c >9% | P |
|---|---|---|---|---|---|
| 253 | 68 (27) | 98 (39) | 87 (34) | ||
| Sex, n (%) | .35 | ||||
| Male | 151 (60) | 38 (56) | 64 (65) | 49 (56) | |
| Female | 102 (40) | 30 (44) | 34 (35) | 38 (44) | |
| Age, years | 56.9 ± 11 | 59.7 ± 10 | 58.4 ± 10 | 52.9 ± 11 | <.001 |
| BMI, kg/m2 | 35.0 ± 10 | 34.3 ± 10 | 35.8 ± 12 | 34.6 ± 8 | .91 |
| Duration of diabetes, years | 10.5 ± 8 | 10.0 ± 8 | 10.2 ± 8 | 11.3 ± 7 | .18 |
| Hospital LOS, days | 6.1 ± 6 | 6.9 ± 8 | 6.4 ± 6 | 5.2 ± 4 | .34 |
| Admission service | .75 | ||||
| Medicine, n (%) | 212 (84) | 56 (82) | 81 (83) | 75 (86) | |
| Surgery, n (%) | 41 (16) | 12 (18) | 17 (17) | 12 (14) | |
| Admission HbAlc, % (mmol/mol) | 8.7 ± 2.372 | 6.3 ± 0.545 | 8.0 ± 0.664 | 11.3 ± 1.71 | <.001 |
| Admission glucose, mg/dL | 216 ±108 | 148 ± 53 | 195 ± 64 | 294 ±130 | <.001 |
| Admission diabetes therapy | .70 | ||||
| Diet alone, n (%) | 34 (13) | 8 (12) | 11 (11) | 15 (17) | |
| Oral agents, n (%) | 109 (43) | 34 (50) | 40 (41) | 35(40) | |
| Metformin | 23 | 36 | 29 | ||
| Sulfonylurea | 16 | 29 | 18 | ||
| Other | 0 | 0 | 1 | ||
| Insulin alone, n (%) | 62 (25) | 13 (19) | 28 (29) | 21 (24) | |
| Daily dose (units/kg) | 0.30 ± 0.19 | 0.33 ± 0.19 | 0.31 ± 0.18 | 0.27 ± 0.21 | .63 |
| Insulin and oral agents, n (%) | 48 (19) | 13 (19) | 19 (19) | 16 (18) | |
| Metformin | 9 | 19 | 16 | ||
| Sulfonylurea | 3 | 2 | 4 | ||
| Other | 1 | 0 | 0 | ||
| Basal | 13 | 19 | 15 | ||
| Discharge diabetes therapy | |||||
| Diet alone, n (%) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |
| Oral agents, n (%) | 97 (38) | 48 (71) | 44 (45) | 5 (6) | |
| Sitagliptin alone, n (%) | 29 | 16 | 12 | 1 | |
| Sitagliptin-metformin, n (%) | 68 | 32 | 32 | 4 | |
| Other, n (%) | 10 | 3 | 7 | 0 | |
| Insulin alone, n (%) | 4 (2) | 0 (0) | 2 (2) | 2 (2) | |
| Insulin and oral agents, n (%) | 152 (60) | 20 (29) | 52 (53) | 80 (92) | |
| Insulin dose units/day | 25.3 ± 18 | 17.3 ± 11 | 22.5 ± 17 | 29.1 ± 20 | <.001 |
| Insulin dose units/kg/day | 0.24 ± 0.2 | 0.16 ± 0.1 | 0.20 ± 0.1 | 0.29 ± 0.2 | <.001 |
Abbreviations: BMI = body mass index; HbA1c = hemoglobin A1c; LOS = length of stay
The discharge regimen of patients based on HbA1c is shown in Figure 1. Overall, 60% of the patients were discharged on insulin plus oral agents, 38% on oral agents alone, and a small number (2%) on insulin alone. According to protocol, oral agents were more common among patients with lower HbA1c, and insulin was more common among patients with higher HbA1c. A small number of patients in the >9% (75 mmol/mol) HbA1c group received either an oral agent or insulin at discharge but not both as per the algorithm. Reasons included changes in clinical condition or patient refusal to take insulin at discharge. Clinical situations included acute kidney injury with start of short-term hemodialysis and improvement in infections and high-dose steroids at discharge. All patients had consented to the discharge algorithm, but some changed their mind about the therapeutic options when they were ready to go home.
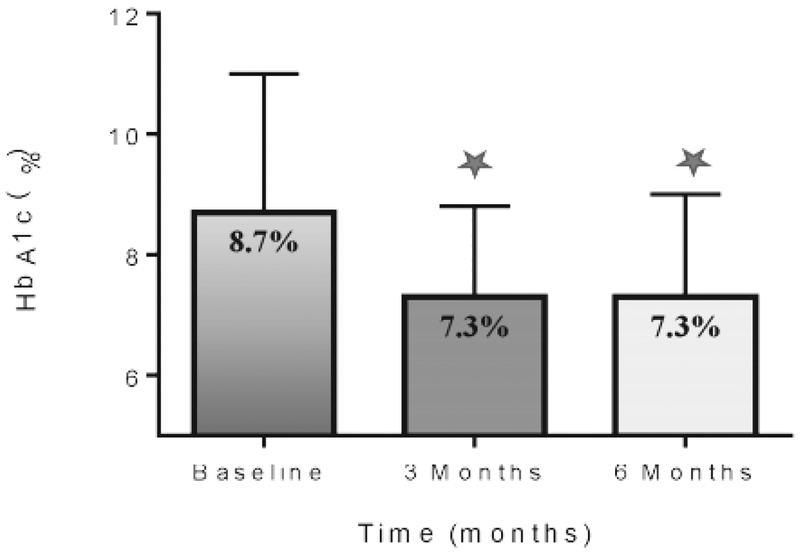
The admission HbA1c for the entire cohort was 8.70 (72 mmol/mol) ± 2.3% and decreased to 7.3 (56 mmol/mol)) ± 1.5% at 3 months and to 7.3 ± 1.7% at 6 months (both P<.001, Fig. 2). The mean changes in HbA1c from baseline to 3 and 6 months in each of the treatment groups are shown in Table 2. Patients with HbA1c <7% (53 mmol/mol) went from 6.3 (45 mmol/mol) ± 0.5% to 6.3 ± 0.80% and 6.2 (44 mmol/mol) ± 1.0% at 3 and 6 months. Patients with HbA1c between 7% (53 mmol/mol) and 9% (75 mmol/mol) had a reduction from 8.0 (64 mmol/mol) ± 0.6% to 7.3 (56 mmol/mol) ± 1.1% and 7.3 ± 1.3% (both P<.001), and those with HbA1c >9% (75 mmol/mol) had a reduction from 11.3 (100 mmol/mol) ± 1.7 to 8.0 (64 mmol/mol) ± 1.8% and 8.0 ± 2.0% at 3 and 6 months (both P<.001, Table 2).
Fig. 2.
Overall HbA1c concentration on admission and during follow-up. HbA1c = hemoglobin
Table 2.
Changes in HbA1c and Glucose After Hospital Discharge
| Hospital discharge | 3 Months | 6 Months | |
|---|---|---|---|
| All patients HbA1c% | 8.7 ± 2.3 | 7.3 ± 1.5a | 7.3 ± 1.7a |
| HbA1c <7% | 6.3 ± 0.5 | 6.3 ± 0.8 | 6.2 ± 1.0b |
| HbA1c 7–9% | 8.0 ± 0.6 | 7.3 ± 1.1a | 7.3 ± 1.3a |
| HbA1c >9% | 11.3 ± 1.7 | 8.0 ± 1.8a | 8.0 ± 2.0a |
| Mean daily BG (mg/dL) | |||
| All patients | 216 ±108 | 142±34a | 144±39a |
| Mean fasting BG (mg/dL) | |||
| All patients | 142 ± 39 | 134±33b | 133±40c |
| HbA1c <7% | 126 ± 28 | 122 ± 24 | 122 ± 37 |
| HbA1c 7–9% | 145 ± 39 | 141 ± 35 | 140 ± 38 |
| HbA1c >9% | 147 ± 41 | 135±33c | 134±42c |
Abbreviations: BG = blood glucose; HbA1c = hemoglobin A1c.
* P<.001 vs. HbA1c at hospital discharge
† P ≤05 vs. HbA1c at hospital discharge
§ P = .01 vs. HbA1c at hospital discharge
The mean daily glucose at enrollment was 216 ± 108 mg/dL and decreased to 142 ± 34 mg/dl at 3 months (P<.0001) and was 144 ± 39 mg/dL at 6 months of follow-up (P<.0001). Clinically important hypoglycemia (<54 mg/dL) was observed in 5% of the entire cohort with no significant differences among groups (4%, 4%, and 7% among patients with a HbA1c <7%, 7– 9%, and >9%, respectively). A BG <70 mg/dL was noted in about 20% of patients with no significant difference among the 3 groups. A BG <40 mg/dL was rare and reported in 2 (1%) patients in the >9% group (Table 2). The majority of hypoglycemic episodes occurred within the first month after discharge (Table 3). None of the hypoglycemic episodes required hospitalization or had any adverse complications.
Table 3.
Hypoglycemia and Complications after Hospital Discharge
| All | HbA1c <7% | HbA1c 7–9% | HbA1c >9% | P | |
|---|---|---|---|---|---|
| # Patients with hypoglycemia | |||||
| BG <70 mg/dL, n (%) | 58 (23) | 17(25) | 20 (20) | 21(24) | .74 |
| BG <54 mg/dL, n (%) | 13 (5) | 3 (4) | 4 (4) | 6 (7) | .77 |
| BG <40 mg/dL, n (%) | 2 (1) | 0 (0) | 0 (0) | 2 (2) | .19 |
| Hypoglycemic events <70 mg/dL | |||||
| Hypoglycemia total events, n | 179 | 32 | 74 | 73 | |
| First month after discharge, n (%) | 123 | 27 | 46 | 50 | |
| Month 2–3 after discharge, n (%) | 36 | 5 | 20 | 11 | |
| Month 3–6 after discharge, n (%) | 20 | 0 | 8 | 12 | |
| Hypoglycemic events <54 mg/dL | |||||
| Hypoglycemia<54 total events, n | 18 | 3 | 7 | 8 | |
| First month after discharge, n (%) | 13 | 2 | 5 | 6 | |
| Month 2–3 after discharge, n (%) | 3 | 1 | 2 | 0 | |
| Month 3–6 after discharge, n (%) | 2 | 0 | 0 | 2 | |
| Emergency room visit, n (%) | 38 (15) | 12 (18) | 11 (11) | 15 (17) | 0.4 |
| Hospital re-admissions, n (%) | 54 (21) | 13 (19) | 23 (23) | 18 (21) | 0.78 |
| Mortality, n (%) | 3 (1) | 2 (3) | 1 (1) | 0 (0) | 0.27 |
Abbreviations: BG = blood glucose; HbA1c = hemoglobin A1c.
Rates of re-admissions, emergency room visits and complications after discharge were similar between groups (Table 3). There were no episodes of pancreatitis. Three patients died during the study: 2 patients in the HbA1c <7% (53 mmol/mol) group (1 with an intracranial hemorrhage and 1 after accidental fire at home) and 1 patient in the HbA1c between 7% (53 mmol/mol) and 9% (75 mmol/mol) (cardiovascular event). None of the deaths were attributed to study medication.
DISCUSSION
This multicenter study prospectively explored the safety and efficacy of a discharge strategy of co-administration of sitagliptin-metformin at hospital discharge based on admission HbA1c in patients with T2D. Our results indicate that the combination of sitagliptin-metformin, with or without basal insulin, significantly improved glycemic control during the transition from hospital to home. The overall reduction in HbA1c was 1.4% during the 3-month follow-up and was sustained up to 6 months. Reduction in BG was achieved with an acceptable risk of clinically significant hypoglycemia.
Few prospective studies have evaluated the efficacy and safety of treatment regimens during the transition from hospital to home. The Basal Plus trial (13) reported that the use of basal inulin as add-on to pre-admission diabetes therapy in patients with poorly controlled diabetes resulted in marked improvement in glycemic control with a reduction in HbA1c from 8.8% on admission to 7.3% at 12 weeks. However, a high rate of hypoglycemia (defined as glucose <70 mg/dL) was reported in more than 30% of patients within 12 weeks after hospital discharge in insulin-treated patients. In the present study, the observed reduction in HbA1c from baseline of 1.5% at 3 months and 1.4% at 6 months is consistent with the 1.5% HbA1c reduction observed at 3 months in the Basal Plus trial (13).
In the present study, we observed that the combined sitagliptin-metformin therapy, with low-dose glargine for patients with HbA1c >9% (75 mmol/mol), resulted in similar improvement in glycemic control with a low frequency (5%) of clinically significant hypoglycemia. This frequency is lower than those of the Basal Plus trial (13). An important observation in our study was the timing of hypoglycemic episodes. Most occurred within the first month after discharge. We postulate that this reflects the improving clinical condition of patients in addition to their increased mobility, which together lead to a reduction in insulin resistance due to illness. Doses of oral medications and insulin need to be tapered early in this period. These results underscore the importance of close follow-up after hospitalization and frequent titration of diabetes medications within weeks of discharge home.
The strengths of this study include the multicenter nature of the trial providing sufficient power to examine differences in outcomes across strata of baseline HbA1c levels. The treatment algorithm is widely applicable and uses a commonly prescribed oral diabetes combination therapy alone or in combination with glargine insulin, which is the current gold standard treatment in the hospital. The follow-up period of 6 months is sufficient to assess the impact of the medication regimen on long-term glycemic control. It also provides adequate time to overcome confounders in HbA1c measurement due to blood transfusions, acute stress, anemia, and glucose toxicity.
The study has several weaknesses including a relatively small sample size and the loss of many patients during follow-up despite multiple efforts on the part of the dedicated research team, which reflects real-world patient compliance. The study results cannot be generalized to several subsets of patients that are commonly encountered in the hospital, such as those on steroids, patients undergoing solid organ transplants, and those fed enterally. Glucose meters were not standardized between sites. The lack of a control group limits the strength of conclusions that should be drawn from this study. However, given the currently limited literature in this area, these are valuable data to inform both clinical management and future research. Lastly, the patients had close phone follow-up, which could affect the outcome. Previous outpatient studies of frequent computer or telephone contact has shown that this process leads to an improvement in HbA1c compared with no other intervention. Although some of the glycemic improvement observed in the current study may be attributable to postdischarge contact with patients, the change in HbA1c was much larger than that shown by contact alone (0.7–3.3% vs.0.2–0.5%).
The results of the present oral agent-based study and our previous insulin-based hospital discharge regimen with appropriate follow up suggest that patients with T2D and mild to moderate hyperglycemia (HbA1c <9%) could be discharged on a combination of oral agents with a low risk of hypoglycemia. The use of insulin at discharge should be reserved for patients with poorly controlled T2D and in patients with pre-admission insulin therapy and should be used cautiously in insulin-naïve individuals with lower HbA1c as it results in higher rates of hypoglycemia (17,19). In addition, the daily basal insulin dose used in the hospital should be significantly reduced when oral agents are restarted at the time of discharge. Our studies indicate that giving 50% of the daily hospital dose for patients with HbA1c <9% (75 mmol/mol) and 80% of the dose in patients with HbA1c >9% (75 mmol/mol) is sufficient to maintain glycemic control after discharge. Most hospitalized patients at discharge are recovering from medical and surgical conditions and frequently have poor oral intake, limited physical activity, and experience a variety of social-financial changes after discharge that can increase the risks of hypoglycemia and hospital re-admissions (4,10,20,21).
CONCLUSION
In conclusion, the proposed discharge algorithm may help individualize diabetes therapy at hospital discharge. The algorithm allows the transition of inpatient diabetes therapy to an outpatient regimen with low-risk oral agents with or without basal insulin according to previous home therapy, admission HbA1c level, and inpatient insulin requirements.
Supplementary Material
ACKNOWLEDGMENT
This investigator-initiated study was supported by an investigator-initiated research grant from Merck Pharmaceutical. Some data from this trial were presented orally at the 2015 American Diabetes Association meeting in Boston, Massachusetts.
DISCLOSURE
R.G., J.I., I.A., P.G., S.L.P., and A.J. have nothing to declare. F.J.P. has received consulting fees from Merck, Sanofi, and Boehringer Ingelheim. P.V has received consulting fees from Boehringer Ingelheim. D.R. has received research funding from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health (K23DK102963), AstraZeneca, and Boehringer Ingelheim. K.D. is a consultant/advisory to Eli Lilly, GSK, Novo Nordisk, and Sanofi Aventis and has research funding from GSK, Novo Nordisk, Sanofi Aventis, and Astra Zeneca. I.H. is a cofounder of Hygieia INC and receives research funding from Boehringer Ingelheim and Pfizer. G.E.U. is supported in part by the National Center for Advancing Translational Sciences of the National Institutes of Health under Award Number UL1TR000454 and 1P30DK111024–01 from the National Institutes of Health and National Center for Research Resources. G.E.U. has received unrestricted research support for inpatient studies (to Emory University) from Merck, Novo Nordisk, AstraZeneca, Boehringer Ingelheim, and Sanofi.
Abbreviations:
- BG
blood glucose
- DPP-4
dipeptidyl peptidase-4
- eGFR
estimated glomerular filtration rate
- HbA1c
hemoglobin A1c
- T2D
type 2 diabetes
Footnotes
Publisher's Disclaimer: Rapid Electronic Articles in Press are preprinted manuscripts that have been reviewed and accepted for publication, but have yet to be edited, typeset and finalized. This version of the manuscript will be replaced with the final, published version after it has been published in the print edition of thejournal. The final, published version may differ from this proof.
REFERENCES
- 1.Aro S, Kangas T, Reunanen A, Salinto M, Koivisto V. Hospital use among diabetic patients and the general population. Diabetes Care. 1994;17:1320–1329. [DOI] [PubMed] [Google Scholar]
- 2.De Berardis G, D’Ettorre A, Graziano G, et al. The burden of hospitalization related to diabetes mellitus: a population-based study. Nutr Metab Cardiovasc Dis. 2012;22:605–612. [DOI] [PubMed] [Google Scholar]
- 3.Bo S, Ciccone G, Grassi G, et al. Patients with type 2 diabetes had higher rates of hospitalization than the general population. J Clin Epidemiol. 2004;57:1196–1201. [DOI] [PubMed] [Google Scholar]
- 4.Rubin DJ. Hospital readmission of patients with diabetes. Curr Diab Rep. 2015;15:17. [DOI] [PubMed] [Google Scholar]
- 5.Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care. 2011;34:256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care. 2007;30:2181–2186. [DOI] [PubMed] [Google Scholar]
- 7.Draznin B, Gilden J, Golden SH, et al. Pathways to quality inpatient management of hyperglycemia and diabetes: a call to action. Diabetes Care. 2013;36:1807–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.http://www.jointcommission.org/standardsinformation/npsgs.aspx. Accessed Janaury 27, 2017.
- 9.Umpierrez GE, Hellman R, Korytkowski MT, et al. Management of hyperglycemia in hospitalized patients in non-critical care setting: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2012;97:16–38. [DOI] [PubMed] [Google Scholar]
- 10.Dungan K, Lyons S, Manu K, et al. An individualized inpatient diabetes education and hospital transition program for poorly controlled hospitalized patients with diabetes. Endocr Pract. 2014;20:1265–1273. [DOI] [PubMed] [Google Scholar]
- 11.Garber AJ, Moghissi ES, Bransome ED Jr, et al. American College of Endocrinology position statement on inpatient diabetes and metabolic control. Endocr Pract.2004;10 Suppl 2:4–9. [DOI] [PubMed] [Google Scholar]
- 12.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes, 2015: a patient-centered approach: update to a position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2015;38:140–149. [DOI] [PubMed] [Google Scholar]
- 13.Umpierrez GE, Reyes D, Smiley D, et al. Hospital discharge algorithm based on admission HbA1c for the management of patients with type 2 diabetes. Diabetes Care. 2014;37:2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Umpierrez GE, Gianchandani R, Smiley D, et al. Safety and efficacy of sitagliptin therapy for the inpatient management of general medicine and surgery patients with type 2 diabetes: a pilot, randomized, controlled study. Diabetes Care. 2013;36:3430–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pasquel FJ, Powell W, Peng L, et al. A randomized controlled trial comparing treatment with oral agents and basal insulin in elderly patients with type 2 diabetes in long-term care facilities. BMJ Open Diabetes Res Care. 2015;3:e000104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pasquel FJ, Gianchandani R, Rubin DJ, et al. Efficacy of sitagliptin for the hospital management of general medicine and surgery patients with type 2 diabetes (Sita- Hospital): a multicentre, prospective, open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2017;5:125–133. [DOI] [PubMed] [Google Scholar]
- 17.Umpierrez GE, Reyes D, Smiley D, et al. Hospital Discharge Algorithm Based on Admission HbA1c for the Management of Patients With Type 2 Diabetes. Diabetes Care. 2014;37:2934–2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.International Hypoglycaemia Study Group. Glucose concentrations of less than 3.0 mmol/l (54 mg/dl) should be reported in clinical trials: A joint position statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2017;40:155–157. [DOI] [PubMed] [Google Scholar]
- 19.Pasquel FJ, Gomez-Huelgas R, Anzola I, et al. Predictive Value of Admission Hemoglobin A1c on Inpatient Glycemic Control and Response to Insulin Therapy in Medicine and Surgery Patients With Type 2 Diabetes. Diabetes Care. 2015;38:e202–e203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Healy SJ, Black D, Harris C, Lorenz A, Dungan KM. Inpatient diabetes education is associated with less frequent hospital readmission among patients with poor glycemic control. Diabetes Care. 2013; 36:2960–2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei NJ, Nathan DM, Wexler DJ. Glycemic control after hospital discharge in insulintreated type 2 diabetes: a randomized pilot study of daily remote glucose monitoring. Endocr Pract. 2015;21:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.