Abstract
The gut microbiome is composed of a vast number of microbes in the gastrointestinal tract, which benefit host metabolism, aid in digestion, and contribute to normal immune function. Alterations in microbial composition can result in intestinal dysbiosis, which has been implicated in several diseases including obesity, inflammatory bowel disease, and liver diseases. Over the past several years, significant interactions between the intestinal microbiota and liver have been discovered, with possible mechanisms for the development as well as progression of liver disease and promising therapeutic targets to either prevent or halt the progression of liver disease. In this review the authors examine mechanisms of dysbiosis-induced liver disease; highlight current knowledge regarding the role of dysbiosis in nonalcoholic liver disease, alcoholic liver disease, and cirrhosis; and discuss potential therapeutic targets.
Keywords: microbiota, dysbiosis, liver disease, therapy
Chronic liver diseases leading to cirrhosis and hepatocellu- lar carcinoma are a major cause of morbidity and mortality worldwide.1 The burden of illness due to chronic liver diseases is linked to three major etiologies: viral hepatitis (B, C, and D), alcoholic liver disease, and nonalcoholic fatty liver disease (NAFLD).1,2 With the widespread use of vacci- nation for hepatitis B and the advent of more potent directly acting antiviral therapies against hepatitis C, the prevalence of end-stage liver disease (ESLD) due to viral hepatitis is expected to decline in the coming decades.1,3,4 Although there have been dramatic advances in antiviral therapies, treatment options remain limited for both alco- holic liver disease and NAFLD. In contrast, due to the rising rates of obesity and metabolic syndrome that are commonly associated risk factors for NAFLD, it is expected to be the leading cause of cirrhosis and ESLD in the coming decades, especially in the developed world.5,6 Over the past several years, significant interactions between the intestinal microbiota and liver have been discovered with possible mechanisms for the development as well as progression of liver disease; these discoveries have providedinvaluable insight into promising therapeutic targets to either prevent or halt the progression of liver disease.
Our understanding of human gut microbiome is still in its infancy, especially as it relates to liver disease. Greater than 1014 microorganisms live in the human gastroenterological tract, including bacteria, viruses, fungi, and archaea; this collective microbial community is known as the gut microbiome. Most of the bacteria are anaerobic, with varying numbers and composition according to the site in the gut, increasing from the stomach to the small intestine to the colon. Over 90% of the intestinal bacteria belong to the phyla Firmicutes and Bacteroidetes.7 Although these main phyla are consistently seen, the species present and their relative proportions vary considerably between individuals.8 Due to the presence of a large number of anaerobic, fastidious organisms, the analysis of gut microbiota has evolved beyond culture-based techniques to enable sequencing of whole genomes.9
The gut microbiota is beneficial for host metabolism, aids in digestion, and contributes to normal immune function, thereby creating a symbiotic relationship with the host.7 Intestinal dysbiosis is defined as a disruption in symbiosis due to an imbalance in the microbial composition. This can present as quantitative (intestinal bacterial overgrowth) and/or qualitative changes in the intestinal microbiota.10 The gut microbiota plays a critical role in the gut and systemic immune system and has been potentially implicated in several different diseases, including obesity, neurologic diseases, inflammatory bowel disease, cancer, and liver disease.8
Our aim here is to examine mechanisms of dysbiosis- induced liver disease; highlight current knowledge regarding the role of dysbiosis in NAFLD, alcoholic liver disease, and cirrhosis; and review potential therapeutic targets.
Mechanisms of Liver Disease in Dysbiosis
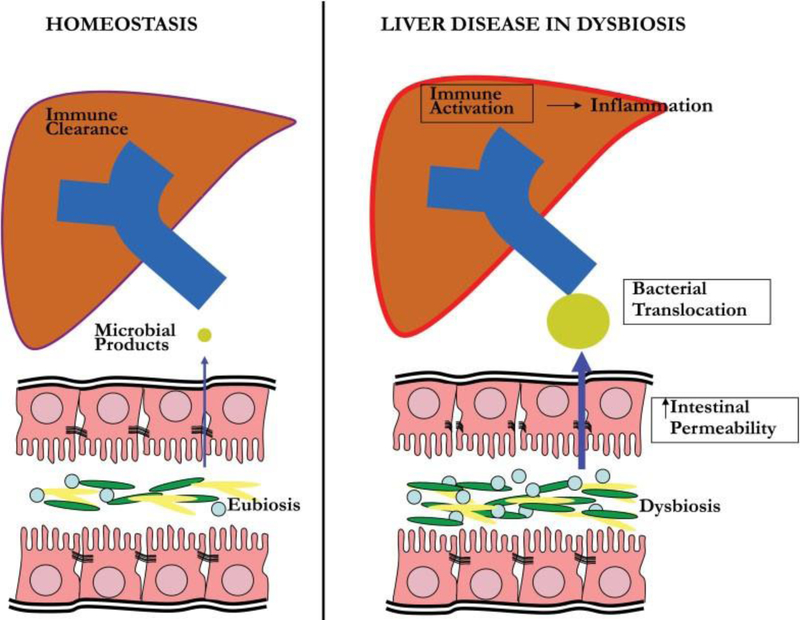
The liver is particularly susceptible to potential effects of dysbiosis. Greater than 70% of the liver’s blood supply is via the portal vein, resulting in frequent exposure of gut-derived toxins and microbial products. In normal conditions, small amounts of bacteria and bacterial metabolites enter the liver and are rapidly cleared. However, when the normal gut barrier is disrupted such as in intestinal dysbiosis, large amounts of bacteria and bacterial products enter the liver resulting in the activation of the immune cascade, production of proinflammatory cytokines, and subsequent liver dam-age.11 Mechanisms of dysbiosis-induced liver disease includeincreased intestinal permeability, bacterial/metabolite translocation, and immune activation (►Fig. 1). Increased intestinal permeability is attributed to tight junction disruption, possibly from metabolites or dysbiosis-induced inflamma-tion.12–15 Augmented gut permeability results in increasedtranslocation of bacteria, bacterial products (such as lipo- polysaccharide/endotoxin), and metabolites into the portal circulation. Metabolites such as trimethylamine (produced from bacterial enzymatic cleavage of dietary choline) and alcohol (produced by enteric bacteria) can have direct toxic effects on the liver, whereas bacterial products cause liverdamage through activation of the innate immune system.16 Foreign pathogens (such as bacteria, viruses, and fungi) are recognized by pathogen recognition receptors, such as toll- like receptors (TLRs) and inflammasomes. TLRs are expressed on cells in the hepatic sinusoids (including Kupffer cells and hepatic stellate cells) and recognize pathogen molecular patterns expressed on cell membranes.17,18 TLR-mediated signaling results in the production of inflammatory cytokines, which have antimicrobial effects. However, sustained production of these cytokines could cause or enhance hepatic injury.19 Toll-like receptor-4 (TLR-4) is the receptor for lipo- polysaccharide, the cell wall component of Gram-negative bacteria, and has been implicated in hepatic damage in NAFLD and alcoholic liver disease.20,21 Although TLRs recognize cell membrane components, inflammasomes are cytoplasmic multiprotein complexes that are critical in cytoplasmic surveillance of pathogen-associated molecular patterns and endogenous (noninfectious) damage-associated substances.22 Activation of inflammasomes results in the production of inflammatory cytokines that can cause hepatic injury.
Fig. 1.
Mechanisms of liver disease in dysbiosis. Under normal conditions, gut integrity is preserved with minimal entry of bacterial products into the portal circulation. In the liver, hepatocytes and Kupffer cells rapidly clear microbial products and maintain immunotolerance without inflammation. In dysbiosis, intestinal permeability is increased, resulting in increased translocation of bacteria, metabolites, and microbial products. Metabolites have direct toxic effects on the liver. Activation of the innate immune system via toll-like receptors and inflammasomes by bacteria and bacterial products produces large amounts of inflammatory cytokines and subsequent liver damage.
Nonalcoholic Fatty Liver Disease
Nonalcoholic fatty liver disease is defined as hepatic steatosis on either liver histology or imaging without any secondary causes of hepatic fat accumulation such as alcohol consumption.23 Nonalcoholic fatty liver disease can be broadly classified into two categories: nonalcoholic fatty liver (NAFL), which is considered to have minimal risk of liver disease progression; and nonalcoholic steatohepatitis (NASH), which is considered to have a substantially higher risk of liver disease progression.24 Nonalcoholic steatohepatitis is defined as the presence of hepatic steatosis typically in zone 3 with lobular inflammation and ballooning with or without peri- sinusoidal fibrosis.23 Obesity, type 2 diabetes, dyslipidemia, and metabolic syndrome are all risk factors for NAFLD.23,25 Although there are many animal and human studies demonstrating the effects of gut microbiota on energy absorption, obesity, and insulin resistance, in this review we will focus primarily on selected studies describing the association of dysbiosis with NAFLD.26,27
Recent studies have suggested that fibrosis progression is substantially higher in NASH versus NAFL; it is likely that the various stages of liver diseases may have the following steps: normal to NAFL to borderline NASH to definite NASH to cirrhosis.24 When patients develop cirrhosis, they may not show all the typical features that are diagnostic of NASH. Various hypotheses have been proposed, but it is commonly accepted that multiple hits may be needed to progress from NAFL to NASH.28–31 Lipotoxicity, oxidative stress, inflamma-tory cytokines, innate immune mechanisms, or toxic metabolites may all contribute in progression from NAFL to NASH.28,32 The intestinal microbiota has been implicated in both the development of hepatic steatosis and subsequent progression to NASH.
Multiple animal studies have investigated the first hit (i.e., the development of hepatic steatosis); intestinal microbiota greatly impact hepatic lipid metabolism. Donor mice on a diet-induced obesity protocol who develop microvesicular steatosis, hyperglycemia, and hyperinsulinemia can transmit this phenotype to germ-free mice.33 However, donor diet- induced obesity mice who did not develop dysmetabolism or hepatic steatosis while on a high-fat diet did not transmit their phenotype. The intestinal microbiota may promote hepatic steatosis through several mechanisms including al- terations in energy harvest and homeostasis,26,34 endogenous alcohol production,35,36 altered choline metabolism,37 and changes in bile acid metabolism.38
Additional animal studies have improved our understanding of the role of gut microbiota in development of NAFLD and its contributions toward the progression from NAFL to NASH. These studies have suggested multiple mechanisms for liver injury in steatohepatitis. One mechanism is that endotoxin production from gut microbiota results in the activation of Kupffer cells via the TLR-4 complex and subsequent release of inflammatory mediators that promote steatohepatitis.21 TLR-4-deficient mice and Kupffer cell-depleted mice have decreased steatohepatitis compared with wild-type mice. In addition, inflammasome plays an important role in preventing the progression of NAFL to NASH. Inflammasome- depleted mice develop dysbiosis that results in more severe steatohepatitis. This phenotype was mediated through an influx of TLR-4 and TLR-9 agonists into the portal vein, ultimately resulting in the activation of tumor necrosis factor α (TNF-α) in the liver.39
Nevertheless, the physiological phenomena observed in animal studies have been difficult to demonstrate in humans. Most human studies investigating the role of intestinal microbiota in NAFLD/NASH have instead focused on the compositional differences between those who have the disease (or trait), and those who do not. Initial real-time polymerase chain reaction analysis of the fecal microbiome among patients with NAFL, NASH, and healthy controls demonstrated a lower percentage of Bacteroidetes among NASH patients, a finding that was independent of body mass index (BMI) and diet.40 Subsequent studies, using 16S rRNA sequencing of the fecal microbiome, showed a more nuanced picture of the gut microbiome, with overrepresentation of several Firmicutes genera such as those belonging to Lactobacillus, Dorea, Rob- insoniella, and Roseburia, but underrepresentation of other Firmicutes genera such as Oscillibacter.41,42 Similar findings are reported in a pediatric population.35 ►Table 1 reviews changes in intestinal microbiota composition among human studies of NAFLD. However, these studies did not control for various factors that are now known to have an effect on the composition of the gut microbiome in humans, such as diet in the days immediate to fecal sample collection.43 Furthermore, there is ongoing debate as to whether bacteria residing in the mucous layer, where it more intimately interacts with the host cells, are more likely to affect host physiology than luminal bacteria, found in fecal samples. It should also be noted that Lactobacillus genera contains multiple members that are particularly adapted to be commensal gut species. Other studies have found that particular lactobacillus species can be protective against hepatic injury,44 hence the diversity of this genera, with both protective and disruptive member species, should be fully appreciated.
Table 1.
Changes in intestinal microbiota in human studies of nonalcoholic fatty liver disease
| Study | Study population | Comparison groupa | Intestinal microbiota changes in NAFLD groupb | Technique |
|---|---|---|---|---|
| Jiang et al, 201532 | Healthy adults (n ¼ 32) Clinical diagnosis or bi- opsy-proven NAFLD (n ¼ 53) | Healthy vs. NAFLD | “ Lentisphaerae; “ Lactobacillus (genus); “Anaerobacter (genus); “Escherichia (genus); “Streptococcus (genus); “Clostridium XI (genus); ↓Alistipes (genus); ↓Odoribacter; ↓Flavoni- fractor (genus); ↓Oscil- libacter (genus) | 16s rRNA gene sequencing stool sample |
| Mouzaki et al, 201325 | Healthy adults (n ¼ 17) Biopsy-proven simple steatosis (n ¼ 11) Biopsy-proven NASH (n ¼ 22) | Healthy vs. NASH | ↓ Bacteroidetes | Real-time PCR; stool sample |
| Steatosis vs. NASH | ↓ Bacteroidetes; “ Clostridium coccoides (Firmicutes phylum) | |||
| Raman et al, 201326 | Healthy adults (n ¼ 30) Clinically diagnosed NAFLD (n ¼ 30) | Healthy vs. NAFLD | No change at phylum level; “ Lactobacillaceae (Family); ↓Ruminococ- caceae (Family) | 16s rRNA gene pyrosequencing; stool sample |
| Zhu et al, 201321 | Healthy children (n ¼ 16) Obese children (n ¼ 25) Biopsy-proven NASH (n ¼ 22) | Healthy vs. obese | “ Bacteroidetes; ↓ Firmicutes | 16s rRNA gene pyrosequencing; stool sample |
| Healthy vs. NASH | “Bacteroidetes; ↓Firmi- cutes; ↓Actinobacteria; “Proteobacteria | |||
| Obese vs. NASH | “Proteobacteria |
Abbreviations: NAFLD, nonalcoholic fatty liver disease; NASH, nonalcoholic steatohepatitis; PCR, polymerase chain reaction.
Note: denotes increased in group B compared with group A. ↓ denotes decreased in group B compared with group A.
Comparison group A versus B.
Changes at the phylum level unless otherwise noted.
Nevertheless, there have been observations in humans that indicate that the compositional differences observed in humans can play a role in the pathophysiology of NAFLD/NASH. For example, shifts in the fecal microbiome composition correlated with an increase in fecal ester volatile organic compounds among obese patients with NAFLD compared with healthy lean control patients.41 Volatile organic compounds are metabolic products that may have direct toxic effects on the liver via the portal circulation. In addition, there is an increase in small intestinal permeability and small intestinal bacterial overgrowth (SIBO) among patients with NAFLD compared with controls, which may influence the development of hepatic steatosis.12
Gut Microbiome and Bile Acid Signaling
The observed compositional changes in humans have also been reported in animal models of NAFLD. Mice with obesity, dysmetabolism, and hepatic steatosis also have a rise in Lactobacillus species and a decrease in Oscillibacter species.45,46 These compositional changes in the gut microbiome have been linked to altered luminal secondary bile acid profiles, specifically by an increase in bile acid hydrolases in Lactobacillus species.47,48 The modulation of the luminal bile acid profiles, in turn affect bile acid signaling by the farnesoid X receptor49 and the G protein-coupled bile acid receptor 1.50 Interestingly, agents that modulate bile acid signaling, such as obeticholic acid, are being vigorously investigated as novel treatments for NAFLD/NASH.51,52 Future NAFLD/NASH therapies that target the microbiome will likely mediate their affects by a combination of modulating host metabolism by bile acid signaling and host inflammation through TLR-4/inflammasome pathways.
In summary, animal and human studies have demonstrated differences in gut microbiota with NAFLD. Furthermore, there is emerging evidence to suggest that the gut microbiota may play a key role in the development of hepatic steatosis and progression from NAFL to NASH. However, whether the gut microbial dysbiosis is causally related to liver disease progression in patients with NASH cirrhosis remains to be elucidated.
Alcoholic Liver Disease
Alcoholic liver disease encompasses a spectrum of disease including hepatic steatosis, acute alcoholic hepatitis, chronic hepatitis with fibrosis, and cirrhosis. Development and progression of alcoholic liver disease is influenced by several factors including quantity of alcohol, consumption pattern, presence of obesity, gender, nutritional status, genetic poly- morphisms, and the presence of chronic viral hepatitis.53 Recently, data have emerged demonstrating an important role of intestinal microbiota in the development of alcoholic liver disease.
Alcohol use has both quantitative as well as qualitative effects on the gut microbiota. Alcohol use leads to decreased intestinal motility that can lead to the proliferation of intestinal bacteria.54 Several mouse and human studies have also demonstrated alterations in the composition of the intestinal microbiota with alcohol consumption. Chronic alcohol exposure in mice leads to significant changes in the intestinal microbiota.55 Chronic alcohol feeding resulted in a decline in Bacteroidetes and Firmicutes with an increase in Proteobacteria and Actinobacteria. Furthermore, the alcohol-fed mice had increased plasma endotoxin levels, higher fecal pH, and greater liver inflammation. Similar findings were observed in patients with chronic alcohol use. These patients had a lower quantity of Bacteroidetes and higher quantity of Proteobacteria when compared with control participants.56
In addition to alterations in intestinal microbiota, alcohol use has other effects on the gastrointestinal tract, which may contribute to liver toxicity such as increasing intestinal permeability.13 Acetaldehyde, a metabolic product of alcohol, induces increased gut permeability through the disruption of epithelial tight junctions and adherens junctions.57–60 Increased gut permeability has also been attributed to increased intestinal inflammation through the production of TNF-α in the lamina propria, which results in disruption of tight junctions.61,62
Overall, chronic alcohol use results in changes in the composition of intestinal microbiota and induces bacterial overgrowth. Furthermore, intestinal dysbiosis and metabolic products of alcohol promote gut hyperpermeability. As a result, bacteria and microbial products such as gut-derived lipopolysaccharides (endotoxin) can translocate into the portal circulation. Compared with control participants, patients with chronic alcohol use have an increase in plasma endotox- in levels in all stages of alcoholic liver disease. Furthermore, alcoholic cirrhotics also have higher levels of endotoxin when compared with patients who have cirrhosis from other causes.63,64 Endotoxins stimulate the innate immune system via TLR-4 and CD14, which activate hepatic stellate cells and Kupffer cells resulting in the release of proinflammatory mediators resulting in liver inflammation and damage. Mouse studies show TLR-4–deficient mice that are fed alcohol have less liver steatosis, inflammation, and cell death compared with wild-type mice.20
Cirrhosis
There are compositional and quantitative changes in gut microbiota of patients with cirrhosis. The fecal microbiome of these individuals have reduced Bacteroidetes and increased Proteobacteria and Fusobacteria at the phyla level when compared with that of healthy controls.65 On the family level, potentially pathogenic bacteria such as Enterobacter-iaceae and Streptococcaceae were increased and potentially beneficial populations such as Lachnospiraceae were diminished. Severity of liver disease was associated with greater changes in the microbiome. Comparison of the intestinal microbiota among control participants, compensated cirrhotics, and decompensated cirrhotics showed diminishing ratios of autochthonous and nonautochthonous taxa. This calculation, the cirrhosis dysbiosis ratio (CDR), significantly decreased with cirrhotic severity but was stable in compensated cirrhotics over a 4- to 6-month period. Metagenomic sequencing of the intestinal microbiota of cirrhotics and healthy controls revealed specific bacterial genes that are a signature of cirrhosis.66 Using 15 genes as biomarkers, Qin et al created a patient discrimination tool, which could accurately distinguish patients with cirrhosis from healthy individuals. Analysis of the intestinal microbiome among cirrhotics can be challenging due to many confounding variables such as differing etiologies of cirrhosis, frequent antibiotic exposure lactulose use, hospital admissions, and dietary effects.
In addition to qualitative changes, quantitative changes are common in the gut microbiota among cirrhotics. Many animal and human studies have demonstrated small intestinal bacterial overgrowth in patients with cirrhosis.67–69 Human studies utilizing jejunal aspirates and breath testing has estimated the prevalence of SIBO as 35% to 61% among cirrhotic patients, with increasing SIBO as severity of cirrhosis worsens.70–72
In addition to the proliferation of bacteria and compositional change of microbiota to potentially more pathogenic strains of bacteria, there is increased bacterial translocation with cirrhosis that may have clinical consequences.15 This was illustrated by comparing the bacterial cultures in mesenteric lymph nodes from noncirrhotics and cirrhotics, stratified by Childs-Pugh class.73 Enteric organisms grew from mesenteric lymph nodes in 30.8% of Childs C cirrhotics compared with 8.6% of noncirrhotics. Results were similar among noncirrhotics, Childs A cirrhotics and Childs B cirrhotics. Culture-based studies may underestimate true bacterial translocation due to the presence of fastidious anaerobic organisms, which are difficult to grow in a laboratory setting. Thus, more recent studies have measured bacterial DNA as an indicator of bacterial translocation. About 32% of patients with cirrhosis have detectable bacterial DNA in their blood and ascitic fluid, whereas no bacterial DNA was detected in the blood of healthy controls.74 Interestingly, all patients had nonneutrocytic sterile ascites and negative blood cultures. Factors promoting bacterial translocation in cirrhosis include increased intestinal permeability, SIBO, and host immunological alterations.15
Potential clinical consequences of dysbiosis and increased bacterial translocation in cirrhosis are worsened hyperdynamic circulation, infection, and hepatic encephalopathy.75 Patients with bacterial DNA in their ascites had significantly lower mean arterial pressure, lower systemic vascular resistance, and signs of worsened hepatic endothelial function compared with patients without ascitic fluid bacterial DNA.75 In terms of infection, it remains unclear if dysbiosis is a risk factor for the development of infections. However, patients with cirrhosis have an increased presence of potentially pathogenic species that are commonly involved in systemic infections with a decrease in commensal bacteria, raising the concern for an interaction of dysbiosis and infection. A comparison of 38 infected cirrhotic patients to uninfected cirrhotic patients found increased dysbiosis (reflected by lower CDR) and higher serum endotoxin levels among infected patients.63
Targeting Dysbiosis
The gut microbiome can be modulated in different ways, and targeting intestinal dysbiosis has been investigated as a way to combat liver disease. Therapies include prebiotics, probiotics, synbiotics, antibiotics, and fecal microbiota transplantation (►Table 2).76 Prebiotics are nondigestible carbohydrates that promote beneficial changes in the activity and composition of gastrointestinal microflora. Probiotics are living microorganisms (bacteria, fungi) that present a health benefit for the host. Synbiotics contain both prebiotics and probiotics.76,77
Table 2.
Potential therapies for dysbiosis
| Therapy | Effect on intestinal microbiota | Examples |
|---|---|---|
| Prebiotic | Complex carbohydrates; digested by colonic microbes to form short-chain fatty acids and lactate, which stimulate the growth of beneficial bacteria | Fructo-oligosaccharide (FOS) Galacto-oligosaccharide (GOS) Lactulose |
| Probiotic | Living microorganisms that confer a health benefit on their host through antimicrobial effects, enhancement of mucosal barrier integrity, and immunomodulation | Lactobacillus GG (LGG) Lactobacillus casei Lactobacillus plantarum Lactobacillus johnsonii Bifidobacterium lactis Saccharomyces boulardii VSL#3 |
| Synbiotic | Contain prebiotic and probiotic; augment the activity and prolong the survival of potentially beneficial probiotics | Bifidobacterium þ FOS Protexin |
| Antibiotic | Antimicrobial effects; changes in bacterial populations and composition; alterations in bacterial metabolic function and virulence | Rifaximin Norfloxacin Neomycin Metronidazole |
| Fecal microbiota transplant | Colonization resistance (limiting the colonization of patho- gens); modulation of bacterial metabolic function |
Prebiotics
Prebiotics are complex carbohydrates including lactulose, inulin, fructo-oligosaccharides (FOS), and galacto-oligosac- charides (GOS), which stimulate the growth of certain bacteria, most commonly Bifidobacteria and Lactobacilli.78 There are limited data describing the effects of other prebiotics in liver disease. Although prebiotics are nondigestible by the host, they are fermented by colonic microbes to form short- chain fatty acids (SCFAs) and lactate.79 The production of SCFAs has been shown to modulate cytokine production and may have immunomodulatory effects. Lactulose is probably the best-studied prebiotic in liver disease and is commonly used for the treatment of hepatic encephalopathy. However, studies have shown different compositional outcomes in patients with cirrhosis who are taking lactulose. In one study, lactulose administration resulted in decreased stool pH and increased Lactobacilli in the stool.80 In another study, however, there was greater dysbiosis, reflected by a decline in CDR, without a change in Lactobacilli.63 In the latter study, only seven patients were included and these results may be limited by a small sample size. In patients with alcoholic liver disease, there is strong evidence in animal models that prebiotics could play a protective role.81 Chronic alcohol consumption affects bacterial synthesis of saturated long-chain fatty acids (LCFAs). When LCFAs were added to the diet in mice models of alcoholic liver disease, there was an improvement in the function of the epithelial barrier and reduction in liver injury. Furthermore, changes were also noted in the composition of the gut microbiome with an increase in Lactobacilli species. Therefore, prebiotics that may augment LCFA production may be helpful in the treatment of alcoholic liver disease. Ran- domized, controlled clinical trials along with mechanistic studies are needed to better assess the role of prebiotics in improving alcoholic hepatitis and alcoholic cirrhosis.
Probiotics
Probiotics are living microorganisms that confer a health benefit on their host through antimicrobial effects, en- hancement of mucosal barrier integrity, and immunomo- dulation.76 Antimicrobial effects of probiotics are related to the production of antimicrobial products (such as bacteriocins and hydrogen peroxide), competitive colonization with other microbes, and production of organic acids that acidify the lumen, inhibiting growth and colonization of pathogenic bacteria.82 Enhancement of the mucosal barrier is accomplished through stimulation of mucin production and enhanced tight junction function through the actions of butyrate, a SCFA produced by probiotics.76,77,83 Immunomodulation by probiotics occurs through effects on epithelial cells, dendritic cells, Treg cells, natural killer (NK) T cells, and immunoglobulin-A-producing B cells.77 These immunomodulating interactions result in changes in cytokine production that can inhibit epithelial cell apoptosis.84 Commonly used and studied probiotics include Lactobacillus GG (LGG), Lactobacillus casei, Lactobacillus plantarum, Lactobacillus johnsonii, Bifidobacterium lactis, and Saccharomyces boulardii and VSL#3, which is a probiotic combination consisting of eight strains of Lactobacilli, Bifidobacteria, and Streptococcus.
Over the past few years, there have been an increasing number of studies evaluating the effect of probiotics in liver disease. Several small clinical trials have evaluated the effect of probiotics (predominantly Lactobacillus, VSL#3, Bifidobacterium) in patients with NAFLD with varying results.85–89 The main outcomes in these studies were improvement in liver enzymes (serum alanine [ALT] and aspartate [AST] aminotransferases), inflammatory markers, and anthropometric measurements. Two studies measured changes in steatosis via magnetic resonance spectroscopy but none of the studies assessed liver histological response to probiotics.87,89 A meta- analysis found significantly improved levels of ALT, AST, TNF- α, and cholesterol with probiotic use among patients with NAFLD but included two studies which used synbiotics.90 Overall, clinical data remain limited to recommend the use of probiotics as therapy for patients with NAFLD and larger randomized controlled trials with either resolution of NASH or improvement in hepatic fibrosis on histology as endpoint are needed
In alcoholic liver disease, animal studies have shown probiotics can reduce endotoxinemia and liver injury associated with alcohol consumption. A recent study has evaluated liver histology and serum endotoxin levels in rats fed alcohol compared with rats fed alcohol and LGG. The group treated with LGG had minimal histological liver damage and significantly lower serum endotoxin levels.91 In addition, LGG was associated with less liver injury and diminished gut leakiness in a steatohepatitis rat model.92 Administration of oats as a prebiotic or LGG as a probiotic prevented alcohol-induced dysbiosis in rats.93 There are a limited number of human studies evaluating the effects of probiotics on alcoholic liver disease. The effect of 5 days of Bifidobacterium bifidum and Lactobacillus plantarum 8PA3 has been investigated in an open-label randomized trial evaluating males with alcoholic psychosis in Russia.94 Probiotic-treated patients had significantly increased levels of Bifidobacteria and Lactobacilli and lower ALT and AST compared with patients treated with standard therapy.
Clinical studies of probiotics among cirrhotics show im- provements in hyperdynamic circulation, decreased dysbiosis, and possible improvement in liver function. Rincon et al evaluated systemic and hepatic hemodynamic changes in 12 cirrhotic patients with ascites treated with 6 weeks of VSL#3.95 Patients experienced a statistically significant re- duction in hepatic vein pressure gradient (21.8 to 19.6 mm Hg), reduction in cardiac index (4.6 to 4.2 L/min/m2), reduc- tion in heart rate (83 to 75 bpm) and increase in systemic vascular resistance (803 to 912 d ∙ s ∙ cm-5) with the use of probiotics. However, the clinical significance of these hemo-dynamic changes and whether they reduce morbidity ormortality is unclear. A recent study in 42 cirrhotic patients treated with Escherichia coli Nissle demonstrated an improvement from dysbiosis and trends toward decreased endotoxinemia and decreased Model for End-Stage Liver Disease (MELD) score.96 Furthermore, probiotics were investigated in a double-blind randomized control trial among cirrhotics with a recent episode of hepatic encephalopathy. Patients treated with VSL#3 experienced fewer hospitalizations for hepatic encephalopathy (19.7% vs. 42%) and fewer complications of cirrhosis (24% vs. 45%) compared with placebo at the end of the study (6 months).97 Furthermore, the MELD score improved significantly ( from 14 to 12) during the study period in the probiotic arm compared with the placebo group. Studies evaluating probiotics for hepatic encephalopathy date back to the 1960s.98,99 The theoretical benefit of probiotics in hepatic encephalopathy is from decreasing non- urease-producing bacteria, resulting in decreased ammonia production. There are now multiple randomized control trials evaluating the role of probiotics in overt and minimal hepatic encephalopathy. In 2011, a Cochrane Review evaluated seven clinical trials and found that probiotics reduce plasma am- monia levels, but concluded there is insufficient evidence to support efficacy in treating hepatic encephalopathy.100 The study highlighted methodological issues with concerns for bias and random error. In the past few years, additional clinical trials have been published. An open-label randomized trial evaluating the use of VSL#3 for the primary prophylaxis of hepatic encephalopathy found decreased hepatic enceph- alopathy (9% vs. 20%) in the probiotic group compared with the control group.101 In addition, a phase I randomized control trial evaluating the safety and tolerability of LGG among cirrhotic patients with minimal hepatic encephalopathy was recently performed.102 The LGG was well tolerated and associated with a reduction in endotoxemia and dysbiosis, but no change in cognition. Most studies have revealed no severe adverse reactions to the use of probiotics.
Although there are now multiple animal and human studies highlighting the promise of probiotics in liver disease, large well-designed randomized clinical trials with either reducing the risk of hospitalization or improving survival as an endpoint are needed to elucidate potential benefits of probiotics in patients with hepatic encephalopathy or decompensated cirrhosis. In addition to determining efficacy, there are major questions regarding the specific strains of effective probiotics, optimal dosing, duration of therapy, and long- term consequences of probiotic use.
Synbiotics
Synbiotics combine prebiotics and probiotics with the theoretical goal of augmenting the activity and prolonging the survival of potentially beneficial probiotics. There are limited clinical studies evaluating the use of synbiotics in liver disease. The effects of Protexin, a synbiotic capsule containing seven bacterial strains (mostly Lactobacilli, Streptococci, Bifi- dobacteria) and FOS, were recently investigated on patients with NAFLD (based on imaging and laboratory diagnosis).103 Patients in the synbiotic group had statistically significant declines in ALT, inflammatory markers, and fibrosis score (based on transient elastography) compared with placebo despite similar changes in BMI and waist-to-hip ratio in both groups. The effects of Bifidobacterium longum and FOS have also been evaluated on patients with biopsy-proven NASH. Patients treated with synbiotics had statistically significant declines in AST, inflammatory markers, serum endotoxin levels, and histological NASH activity score compared with a placebo despite similar declines in BMI in both groups.104 However, ALT was similar between the two groups at the end of the study.
Synbiotics have also been assessed in hepatic encephalopathy. The effects of Bifidobacterium and FOS have been compared with lactulose in a cohort of patients with mild- to-moderate hepatic encephalopathy.105 Patients in the synbiotic group had statistically significant declines in ammonia levels and improvements in psychometric testing compared with the lactulose-treated group. In addition, the effects of a synbiotic preparation, consisting of fermentable fiber (inulin, pectin, β glucan, and resistant starch) and four strains of bacteria (Pediacoccus pentoseceus, Leuconostoc mesenteroides, Lactobacillus paracasei, and Lactobacillus plantarum) have been evaluated on patients with minimal hepatic encephalopathy.106 Patients receiving synbiotics had statistically significant improvements in ammonia levels and reduction in endotoxin levels compared with placebo. Furthermore, 50% of patients in the synbiotic group experienced resolution of minimal hepatic encephalopathy based on psychometric tests compared with only 10% in the control group. Although these studies are promising, large well-designed clinical trials are needed to elucidate the efficacy of synbiotic use for patients with liver disease.
Antibiotics
Antibiotics have profound quantitative and qualitative effects on the intestinal microbiota, greatly affecting microbial bio- diversity.107,108 The class of antibiotic and mechanism of action greatly influence the ultimate effect on gut microbiota.107 In addition to changes in bacterial populations and composition, antibiotics also have significant effects on bacterial metabolic function and virulence.107,109 Patients with cirrhosis benefit from antibiotics in several settings such as prophylaxis for spontaneous bacterial peritonitis in high-risk patients, but it is unclear if these benefits are via modulation of gut microbiota.110 Rifaximin is a minimally absorbable oral antibiotic commonly used in the treatment of hepatic en- cephalopathy.111 An analysis of the effects of rifaximin on intestinal microbiota among patients with minimal hepatic encephalopathy found only minimal changes in microbial composition, but significant changes in microbial metabolic function.109 These findings suggest that rifaximin’s primary mechanism of action is altering metabolic function in the microbiota as opposed to promoting beneficial bacteria while decreasing harmful bacteria. Further studies are needed to elucidate the mechanisms by which antibiotics modulate gut microbiota and influence long-term outcomes in chronic liver diseases and decompensated cirrhosis.
Fecal Microbiota Transplant
Fecal microbiota transplant (FMT) has gained widespread acceptance as a highly effective therapy for the treatment of recurrent Clostridium difficile infection.112 A major mechanism by which FMT influences the microbiota is through limiting the colonization of pathogens, a concept known as colonization resistance. Studies have also shown FMT may also influence microbial metabolic function in addition to microbiota composition.113 Fecal microbiota transplant has not been evaluated in clinical liver disease, but a recent mouse study demonstrated proof of concept by successfully trans- planting a consortium of eight bacteria with minimal urease gene content to create an enduring new bacterial community that exhibited reduction in fecal urease activity.114
Conclusion
Preclinical and clinical studies show the emerging role of the gut microbiome in the development and progression of chronic liver diseases, particularly in NAFLD, alcoholic liver disease, and cirrhosis. Further research is needed to more clearly elucidate gut-liver homeostasis and mechanisms for dysbiosis induced liver injury in humans. Promising new therapies have emerged by which to modulate gut microbiota and potentially treat liver disease. However, current evidence is inadequate to recommend the use of probiotics, prebiotics, or synbiotics in liver disease. High-quality, well-designed, large, multicenter clinical trials are needed to determine the efficacy, optimal dosing, and duration of therapy of therapeutic agents that specifically modulate dysbiosis, and examine the influence of specific changes in gut microbiota on long-term clinical outcomes.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- BMI
body mass index
- CD
cluster of differentiation
- CDR
cirrhosis dysbiosis ratio
- ESLD
end-stage liver disease
- FMT
fecal microbiota transplant
- FOS
fructo-oligosaccharides
- GOS
galacto-oligosaccharides
- LCFA
long-chain fatty acid
- LGG
Lactobacillus GG
- MELD
Model for End-Stage liver Disease
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- NK
natural killer
- PCR
polymerase chain reaction
- rRNA
ribosomal ribonucleic acid
- SCFA
short-chain fatty acid
- SIBO
small intestinal bacterial overgrowth
- TLR
toll-like receptor
- TNF
tumor necrosis factor
References
- 1.Wang FS, Fan JG, Zhang Z, Gao B, Wang HY. The global burden of liver disease: the major impact of China. Hepatology 2014;60(6): 2099–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Loomba R, Sanyal AJ. The global NAFLD epidemic. Nat Rev Gastroenterol Hepatol 2013;10(11):686–690 [DOI] [PubMed] [Google Scholar]
- 3.Farrell GC, Wong VW, Chitturi S. NAFLD in Asia—as common and important as in the West. Nat Rev Gastroenterol Hepatol 2013; 10(5):307–318 [DOI] [PubMed] [Google Scholar]
- 4.Rinella ME, Loomba R, Caldwell SH, et al. Controversies in the Diagnosis and Management of NAFLD and NASH. Gastroenterol Hepatol (N Y) 2014;10(4):219–227 [PMC free article] [PubMed] [Google Scholar]
- 5.Younossi ZM, Stepanova M, Afendy M, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroenterol Hepatol 2011;9(6):524–530.e1, quiz e60 [DOI] [PubMed] [Google Scholar]
- 6.Bazick J, Donithan M, Neuschwander-Tetri BA, et al. Clinical model for NASH and advanced fibrosis in adult patients with diabetes and NAFLD: guidelines for referral in NAFLD. Diabetes Care 2015;38(7):1347–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tremaroli V, Bäckhed F. Functional interactions between the gut microbiota and host metabolism. Nature 2012;489(7415):242–249 [DOI] [PubMed] [Google Scholar]
- 8.Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R. Diversity, stability and resilience of the human gut microbiota. Nature 2012;489(7415):220–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Minemura M, Shimizu Y. Gut microbiota and liver diseases. World J Gastroenterol 2015;21(6):1691–1702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer 2013;13(11):800–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Seo YS, Shah VH. The role of gut-liver axis in the pathogenesis of liver cirrhosis and portal hypertension. Clin Mol Hepatol 2012; 18(4):337–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miele L, Valenza V, La Torre G, et al. Increased intestinal perme- ability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 2009;49(6):1877–1887 [DOI] [PubMed] [Google Scholar]
- 13.Endotoxemia Rao R. and gut barrier dysfunction in alcoholic liver disease. Hepatology 2009;50(2):638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giorgio V, Miele L, Principessa L, et al. Intestinal permeability is increased in children with non-alcoholic fatty liver disease, and correlates with liver disease severity. Dig Liver Dis 2014;46(6): 556–560 [DOI] [PubMed] [Google Scholar]
- 15.Bellot P, Francés R, Such J. Pathological bacterial translocation in cirrhosis: pathophysiology, diagnosis and clinical implications. Liver Int 2013;33(1):31–39 [DOI] [PubMed] [Google Scholar]
- 16.Obesity Arslan N., fatty liver disease and intestinal microbiota. World J Gastroenterol 2014;20(44):16452–16463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szabo G, Dolganiuc A, Mandrekar P. Pattern recognition receptors: a contemporary view on liver diseases. Hepatology 2006; 44(2):287–298 [DOI] [PubMed] [Google Scholar]
- 18.Nakamoto N, Kanai T. Role of toll-like receptors in immune activation and tolerance in the liver. Front Immunol 2014;5:221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miura K, Ohnishi H. Role of gut microbiota and Toll-like receptors in nonalcoholic fatty liver disease. World J Gastroenterol 2014; 20(23):7381–7391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll- like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology 2008;48(4): 1224–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol 2007;47(4):571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henao-Mejia J, Elinav E, Thaiss CA, Licona-Limon P, Flavell RA. Role of the intestinal microbiome in liver disease. J Autoimmun 2013;46:66–73 [DOI] [PubMed] [Google Scholar]
- 23.Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of non-alcoholic fatty liver disease: practice guide-line by the American Association for the Study of Liver Diseases, American College of Gastroenterology, and the American Gastroenterological Association. Hepatology 2012;55(6):2005–2023 [DOI] [PubMed] [Google Scholar]
- 24.Singh S, Allen AM, Wang Z, Prokop LJ, Murad MH, Loomba R. Fibrosis progression in nonalcoholic fatty liver vs nonalcoholic steatohepatitis: a systematic review and meta-analysis of paired- biopsy studies. Clin Gastroenterol Hepatol 2015;13(4):643–54. e1, 9, quiz e39–e40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Loomba R, Abraham M, Unalp A, et al. ; Nonalcoholic Steatohepatitis Clinical Research Network. Association between diabetes, family history of diabetes, and risk of nonalcoholic steatohepatitis and fibrosis. Hepatology 2012;56(3):943–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci U S A 2004;101(44):15718–15723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Festi D, Schiumerini R, Eusebi LH, Marasco G, Taddia M, Colecchia A. Gut microbiota and metabolic syndrome. World J Gastroenterol 2014;20(43):16079–16094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malhi H, Gores GJ. Molecular mechanisms of lipotoxicity in nonalcoholic fatty liver disease. Semin Liver Dis 2008;28(4): 360–369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroen- terology 1998;114(4):842–845 [DOI] [PubMed] [Google Scholar]
- 30.Farrell GC, Larter CZ. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 2006;43(2, Suppl 1): S99–S112 [DOI] [PubMed] [Google Scholar]
- 31.Loomba R, Quehenberger O, Armando A, Dennis EA. Polyunsatu- rated fatty acid metabolites as novel lipidomic biomarkers for noninvasive diagnosis of nonalcoholic steatohepatitis. J Lipid Res 2015;56(1):185–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Feldstein AE, Werneburg NW, Canbay A, et al. Free fatty acids promote hepatic lipotoxicity by stimulating TNF-alpha expression via a lysosomal pathway. Hepatology 2004;40(1): 185–194 [DOI] [PubMed] [Google Scholar]
- 33.Le Roy T, Llopis M, Lepage P, et al. Intestinal microbiota deter- mines development of non-alcoholic fatty liver disease in mice. Gut 2013;62(12):1787–1794 [DOI] [PubMed] [Google Scholar]
- 34.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with in- creased capacity for energy harvest. Nature 2006;444(7122): 1027–1031 [DOI] [PubMed] [Google Scholar]
- 35.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology 2013;57(2): 601–609 [DOI] [PubMed] [Google Scholar]
- 36.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology 2000;119(5):1340–1347 [DOI] [PubMed] [Google Scholar]
- 37.Dumas ME, Barton RH, Toye A, et al. Metabolic profiling reveals a contribution of gut microbiota to fatty liver phenotype in insulin- resistant mice. Proc Natl Acad Sci U S A 2006;103(33):12511–12516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Swann JR, Want EJ, Geier FM, et al. Systemic gut microbial modulation of bile acid metabolism in host tissue compartments. Proc Natl Acad Sci U S A 2011;108(Suppl 1):4523–4530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Henao-Mejia J, Elinav E, Jin C, et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012;482(7384):179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mouzaki M, Comelli EM, Arendt BM, et al. Intestinal microbiota in patients with nonalcoholic fatty liver disease. Hepatology 2013; 58(1):120–127 [DOI] [PubMed] [Google Scholar]
- 41.Raman M, Ahmed I, Gillevet PM, et al. Fecal microbiome and volatile organic compound metabolome in obese humans with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol 2013; 11(7):868–75.e1, 3 [DOI] [PubMed] [Google Scholar]
- 42.Jiang W, Wu N, Wang X, et al. Dysbiosis gut microbiota associated with inflammation and impaired mucosal immune function in intestine of humans with non-alcoholic fatty liver disease. Sci Rep 2015;5:8096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David LA, Maurice CF, Carmody RN, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014; 505(7484):559–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nardone G, Compare D, Liguori E, et al. Protective effects of Lactobacillus paracasei F19 in a rat model of oxidative and metabolic hepatic injury. Am J Physiol Gastrointest Liver Physiol 2010;299(3):G669–G676 [DOI] [PubMed] [Google Scholar]
- 45.Joyce SA, MacSharry J, Casey PG, et al. Regulation of host weight gain and lipid metabolism by bacterial bile acid modification in the gut. Proc Natl Acad Sci U S A 2014;111(20):7421–7426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zarrinpar A, Chaix A, Yooseph S, Panda S. Diet and feeding pattern affect the diurnal dynamics of the gut microbiome. Cell Metab 2014;20(6):1006–1017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sayin SI, Wahlström A, Felin J, et al. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab 2013; 17(2):225–235 [DOI] [PubMed] [Google Scholar]
- 48.Li F, Jiang C, Krausz KW, et al. Microbiome remodelling leads to inhibition of intestinal farnesoid X receptor signalling and de- creased obesity. Nat Commun 2013;4:2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Matsubara T, Li F, Gonzalez FJ. FXR signaling in the enterohepatic system. Mol Cell Endocrinol 2013;368(1–2):17–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pols TW, Noriega LG, Nomura M, Auwerx J, Schoonjans K. The bile acid membrane receptor TGR5 as an emerging target in metabolism and inflammation. J Hepatol 2011;54(6): 1263–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mudaliar S, Henry RR, Sanyal AJ, et al. Efficacy and safety of the farnesoid X receptor agonist obeticholic acid in patients with type 2 diabetes and nonalcoholic fatty liver disease. Gastroenterology 2013;145(3):574–82.e1 [DOI] [PubMed] [Google Scholar]
- 52.Zarrinpar A, Loomba R. Review article: the emerging interplay among the gastrointestinal tract, bile acids and incretins in the pathogenesis of diabetes and non-alcoholic fatty liver disease. Aliment Pharmacol Ther 2012;36(10):909–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.O’Shea RS, Dasarathy S, McCullough AJ. Practice Guideline Com- mittee of the American Association for the Study of Liver diseases; Practice Parameters Committee of the American College of Gastroenterology. Alcoholic liver disease. Hepatology 2010; 51(1):307–328 [DOI] [PubMed] [Google Scholar]
- 54.Addolorato G, Montalto M, Capristo E, et al. Influence of alcohol on gastrointestinal motility: lactulose breath hydrogen testing in orocecal transit time in chronic alcoholics, social drinkers and teetotaler subjects. Hepatogastroenterology 1997;44(16): 1076–1081 [PubMed] [Google Scholar]
- 55.Bull-Otterson L, Feng W, Kirpich I, et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS ONE 2013;8(1):e53028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mutlu EA, Gillevet PM, Rangwala H, et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol 2012;302(9):G966–G978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao RK. Acetaldehyde-induced barrier disruption and paracellular permeability in Caco-2 cell monolayer. Methods Mol Biol 2008;447:171–183 [DOI] [PubMed] [Google Scholar]
- 58.Seth A, Basuroy S, Sheth P, Rao RK. L-Glutamine ameliorates acetaldehyde-induced increase in paracellular permeability in Caco-2 cell monolayer. Am J Physiol Gastrointest Liver Physiol 2004;287(3):G510–G517 [DOI] [PubMed] [Google Scholar]
- 59.Sheth P, Seth A, Atkinson KJ, et al. Acetaldehyde dissociates the PTP1B-E-cadherin-beta-catenin complex in Caco-2 cell mono- layers by a phosphorylation-dependent mechanism. Biochem J 2007;402(2):291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheth P, Seth A, Thangavel M, Basuroy S, Rao RK. Epidermal growth factor prevents acetaldehyde-induced paracellular per- meability in Caco-2 cell monolayer. Alcohol Clin Exp Res 2004; 28(5):797–804 [DOI] [PubMed] [Google Scholar]
- 61.Fleming S, Toratani S, Shea-Donohue T, Kashiwabara Y, Vogel SN, Metcalf ES. Pro- and anti-inflammatory gene expression in themurine small intestine and liver after chronic exposure to alco- hol. Alcohol Clin Exp Res 2001;25(4):579–589 [PubMed] [Google Scholar]
- 62.Chen P, Stärkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumor necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology 2015; 61(3):883–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bajaj JS, Heuman DM, Hylemon PB, et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol 2014;60(5):940–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Parlesak A, Schäfer C, Schütz T, Bode JC, Bode C. Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol- induced liver disease. J Hepatol 2000;32(5):742–747 [DOI] [PubMed] [Google Scholar]
- 65.Chen Y, Yang F, Lu H, et al. Characterization of fecal microbial communities in patients with liver cirrhosis. Hepatology 2011; 54(2):562–572 [DOI] [PubMed] [Google Scholar]
- 66.Qin N, Yang F, Li A, et al. Alterations of the human gut microbiome in liver cirrhosis. Nature 2014;513(7516):59–64 [DOI] [PubMed] [Google Scholar]
- 67.Guarner C, Runyon BA, Young S, Heck M, Sheikh MY. Intestinal bacterial overgrowth and bacterial translocation in cirrhotic rats with ascites. J Hepatol 1997;26(6):1372–1378 [DOI] [PubMed] [Google Scholar]
- 68.Bauer TM, Schwacha H, Steinbrückner B, et al. Small intestinal bacterial overgrowth in human cirrhosis is associated with systemic endotoxemia. Am J Gastroenterol 2002;97(9): 2364–2370 [DOI] [PubMed] [Google Scholar]
- 69.Bauer TM, Steinbrückner B, Brinkmann FE, et al. Small intestinal bacterial overgrowth in patients with cirrhosis: prevalence and relation with spontaneous bacterial peritonitis. Am J Gastroenterol 2001;96(10):2962–2967 [DOI] [PubMed] [Google Scholar]
- 70.Gupta A, Dhiman RK, Kumari S, et al. Role of small intestinal bacterial overgrowth and delayed gastrointestinal transit time in cirrhotic patients with minimal hepatic encephalopathy. J Hepatol 2010;53(5):849–855 [DOI] [PubMed] [Google Scholar]
- 71.Pande C, Kumar A, Sarin SK. Small-intestinal bacterial over- growth in cirrhosis is related to the severity of liver disease. Aliment Pharmacol Ther 2009;29(12):1273–1281 [DOI] [PubMed] [Google Scholar]
- 72.Dukowicz AC, Lacy BE, Levine GM. Small intestinal bacterial overgrowth: a comprehensive review. Gastroenterol Hepatol (N Y) 2007;3(2):112–122 [PMC free article] [PubMed] [Google Scholar]
- 73.Cirera I, Bauer TM, Navasa M, et al. Bacterial translocation of enteric organisms in patients with cirrhosis. J Hepatol 2001; 34(1):32–37 [DOI] [PubMed] [Google Scholar]
- 74.Such J, Francés R, Muñoz C, et al. Detection and identification of bacterial DNA in patients with cirrhosis and culture-negative, nonneutrocytic ascites. Hepatology 2002;36(1):135–141 [DOI] [PubMed] [Google Scholar]
- 75.Bellot P, García-Pagán JC, Francés R, et al. Bacterial DNA translocation is associated with systemic circulatory abnormalities and intrahepatic endothelial dysfunction in patients with cirrhosis. Hepatology 2010;52(6):2044–2052 [DOI] [PubMed] [Google Scholar]
- 76.Patel R, DuPont HL. New approaches for bacteriotherapy: pre- biotics, new-generation probiotics, and synbiotics. Clin Infect Dis 2015;60(Suppl 2):S108–S121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Frei R, Akdis M, O’Mahony L. Prebiotics, probiotics, synbiotics, and the immune system: experimental data and clinical evidence. Curr Opin Gastroenterol 2015;31(2):153–158 [DOI] [PubMed] [Google Scholar]
- 78.Cummings JH, Macfarlane GT. Gastrointestinal effects of pre- biotics. Br J Nutr 2002;87(Suppl 2):S145–S151 [DOI] [PubMed] [Google Scholar]
- 79.Bouhnik Y, Flourié B, D’Agay-Abensour L, et al. Administration of transgalacto-oligosaccharides increases fecal bifidobacteria and modifies colonic fermentation metabolism in healthy humans. J Nutr 1997;127(3):444–448 [DOI] [PubMed] [Google Scholar]
- 80.Riggio O, Varriale M, Testore GP, et al. Effect of lactitol and lactulose administration on the fecal flora in cirrhotic patients. J Clin Gastroenterol 1990;12(4):433–436 [DOI] [PubMed] [Google Scholar]
- 81.Chen P, Torralba M, Tan J, et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology 2015; 148(1):203–214.e16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kailasapathy K, Chin J. Survival and therapeutic potential of probiotic organisms with reference to Lactobacillus acidophilus and Bifidobacterium spp. Immunol Cell Biol 2000;78(1):80–88 [DOI] [PubMed] [Google Scholar]
- 83.Lewis K, Lutgendorff F, Phan V, Söderholm JD, Sherman PM, McKay DM. Enhanced translocation of bacteria across metabolically stressed epithelia is reduced by butyrate. Inflamm Bowel Dis 2010;16(7):1138–1148 [DOI] [PubMed] [Google Scholar]
- 84.Yan F, Polk DB. Probiotic bacterium prevents cytokine-induced apoptosis in intestinal epithelial cells. J Biol Chem 2002;277(52): 50959–50965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Loguercio C, De Simone T, Federico A, et al. Gut-liver axis: a new point of attack to treat chronic liver damage? Am J Gastroenterol 2002;97(8):2144–2146 [DOI] [PubMed] [Google Scholar]
- 86.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol 2005;39(6):540–543 [DOI] [PubMed] [Google Scholar]
- 87.Solga SF, Buckley G, Clark JM, Horska A, Diehl AM. The effect of a probiotic on hepatic steatosis. J Clin Gastroenterol 2008;42(10): 1117–1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aller R, De Luis DA, Izaola O, et al. Effect of a probiotic on liver aminotransferases in nonalcoholic fatty liver disease patients: a double blind randomized clinical trial. Eur Rev Med Pharmacol Sci 2011;15(9):1090–1095 [PubMed] [Google Scholar]
- 89.Wong VW, Won GL, Chim AM, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol 2013;12(2):256–262 [PubMed] [Google Scholar]
- 90.Ma YY, Li L, Yu CH, Shen Z, Chen LH, Li YM. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol 2013;19(40):6911–6918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease). Proc Soc Exp Biol Med 1994;205(3):243–247 [DOI] [PubMed] [Google Scholar]
- 92.Forsyth CB, Farhadi A, Jakate SM, Tang Y, Shaikh M, Keshavarzian A. Lactobacillus GG treatment ameliorates alcohol-induced intestinal oxidative stress, gut leakiness, and liver injury in a rat model of alcoholic steatohepatitis. Alcohol 2009;43(2):163–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mutlu E, Keshavarzian A, Engen P, Forsyth CB, Sikaroodi M, Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res 2009;33(10):1836–1846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kirpich IA, Solovieva NV, Leikhter SN, et al. Probiotics restore bowel flora and improve liver enzymes in human alcohol-in- duced liver injury: a pilot study. Alcohol 2008;42(8):675–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rincón D, Vaquero J, Hernando A, et al. Oral probiotic VSL#3 attenuates the circulatory disturbances of patients with cirrhosis and ascites. Liver Int 2014;34(10):1504–1512 [DOI] [PubMed] [Google Scholar]
- 96.Lata J, Novotný I, Príbramská V, et al. The effect of probiotics on gut flora, level of endotoxin and Child-Pugh score in cirrhotic patients: results of a double-blind randomized study. Eur J Gastroenterol Hepatol 2007;19(12):1111–1113 [DOI] [PubMed] [Google Scholar]
- 97.Dhiman RK, Rana B, Agrawal S, et al. Probiotic VSL#3 reduces liver disease severity and hospitalization in patients with cirrhosis: a randomized, controlled trial. Gastroenterology 2014;147(6): 1327–37.e3 [DOI] [PubMed] [Google Scholar]
- 98.MacBeth WA, Kass EH, McDermott WV Jr. Treatment of hepatic encephalopathy by alteration of intestinal flora with lactobacillus acidophilus. Lancet 1965;1(7382):399–403 [DOI] [PubMed] [Google Scholar]
- 99.Read AE, McCarthy CF, Heaton KW, Laidlaw J. Lactobacillus acidophilus (enpac) in treatment of hepatic encephalopathy. BMJ 1966;1(5498):1267–1269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.McGee RG, Bakens A, Wiley K, Riordan SM, Webster AC. Probiotics for patients with hepatic encephalopathy. Cochrane Database Syst Rev 2011;(11):CD008716. [DOI] [PubMed] [Google Scholar]
- 101.Lunia MK, Sharma BC, Sharma P, Sachdeva S, Srivastava S. probiotics prevent hepatic encephalopathy in patients with cirrhosis: a randomized controlled trial. Clin Gastroenterol Hepatol 2014;12(6):1003–8.e1 [DOI] [PubMed] [Google Scholar]
- 102.Bajaj JS, Heuman DM, Hylemon PB, et al. Randomised clinical trial: Lactobacillus GG modulates gut microbiome, metabolome and endotoxemia in patients with cirrhosis. Aliment Pharmacol Ther 2014;39(10):1113–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Eslamparast T, Poustchi H, Zamani F, Sharafkhah M, Malekzadeh R, Hekmatdoost A. Synbiotic supplementation in nonalcoholic fatty liver disease: a randomized, double-blind, placebo-con- trolled pilot study. Am J Clin Nutr 2014;99(3):535–542 [DOI] [PubMed] [Google Scholar]
- 104.Malaguarnera M, Vacante M, Antic T, et al. Bifidobacterium longum with fructo-oligosaccharides in patients with non alcoholic steatohepatitis. Dig Dis Sci 2012;57(2):545–553 [DOI] [PubMed] [Google Scholar]
- 105.Malaguarnera M, Gargante MP, Malaguarnera G, et al. Bifidobacterium combined with fructo-oligosaccharide versus lactulose in the treatment of patients with hepatic encephalopathy. Eur J Gastroenterol Hepatol 2010;22(2):199–206 [DOI] [PubMed] [Google Scholar]
- 106.Liu Q, Duan ZP, Ha DK, Bengmark S, Kurtovic J, Riordan SM. Synbiotic modulation of gut flora: effect on minimal hepatic encephalopathy in patients with cirrhosis. Hepatology 2004; 39(5):1441–1449 [DOI] [PubMed] [Google Scholar]
- 107.Pérez-Cobas AE, Artacho A, Knecht H, et al. Differential effects of antibiotic therapy on the structure and function of human gut microbiota. PLoS ONE 2013;8(11):e80201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Macfarlane S Antibiotic treatments and microbes in the gut. Environ Microbiol 2014;16(4):919–924 [DOI] [PubMed] [Google Scholar]
- 109.Bajaj JS, Heuman DM, Sanyal AJ, et al. Modulation of the metabiome by rifaximin in patients with cirrhosis and minimal hepatic encephalopathy. PLoS ONE 2013;8(4):e60042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Runyon BA. AASLD Practice Guidelines Committee. Management of adult patients with ascites due to cirrhosis: an update. Hepatology 2009;49(6):2087–2107 [DOI] [PubMed] [Google Scholar]
- 111.Bass NM, Mullen KD, Sanyal A, et al. Rifaximin treatment in hepatic encephalopathy. N Engl J Med 2010;362(12):1071–1081 [DOI] [PubMed] [Google Scholar]
- 112.van Nood E, Vrieze A, Nieuwdorp M, et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med 2013; 368(5):407–415 [DOI] [PubMed] [Google Scholar]
- 113.Seekatz AM, Aas J, Gessert CE, et al. Recovery of the gut microbiome following fecal microbiota transplantation. MBio 2014; 5(3):e00893–e14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Shen TC, Albenberg L, Bittinger K, et al. Engineering the gut microbiota to treat hyperammonemia. J Clin Invest 2015;125(7): 2841–2850 [DOI] [PMC free article] [PubMed] [Google Scholar]