Abstract
Aim
Prophylactic human papillomavirus (HPV) vaccines are highly effective at preventing pre–cancerous cervical lesions when given in a three–dose schedule. Some post–hoc trial data suggest that one dose prevents HPV infection. If one dose could prevent pre–cancerous cervical lesions, then global cervical cancer prevention would be greatly facilitated. We assessed the effectiveness of quadrivalent HPV vaccine by number of doses against cervical intraepithelial neoplasia (CIN) 2 or 3/adenocarcinoma–in–situ (AIS)/cancer in Australia up to seven years post vaccination.
Methods
We linked registry data from all 8 jurisdictional cervical screening registers, with the national HPV vaccination register, death index and cancer registers for all Australian women aged 15 or under when eligible for vaccine who screened between April 2007 (when vaccination commenced) and 31 December 2014. We performed Cox proportional hazard regression, adjusted a priori for age, socioeconomic status, and area of residence, to estimate hazard ratios of histologically confirmed CIN2/CIN3/AIS/cancer.
Results
We included 250,648 women: 48,845 (19·5%) unvaccinated, 174,995 (69·8%) had received three doses, 18,190 (7·3%) two doses and 8,618 (3·4%) one dose. The adjusted hazard ratio was significantly lower for all dose groups compared to unvaccinated women (1 dose 0·65 (95%CI 0·52–0·81), 2 doses 0·61 (0·52–0·72) and 3 doses 0·59 (0·54–0·65).) With adjustment for age at vaccination amongst the vaccinated group, the adjusted hazard ratios for one dose and two dose recipients were comparable to three dose recipients (one dose 1.01 (95%CI 0.81–1.26), two doses 1.00 (0.85–1.17).) Multiple sensitivity analyses, including use of different dose assignment methods, produced consistent findings. Comparison with a historical cohort of age matched women showed that the result was not due to herd protection alone.
Conclusions
One dose had comparable effectiveness as two or three doses in preventing high–grade disease in a high coverage setting. These findings support the hypothesis that one dose vaccination may be a viable strategy when working towards the global elimination of cervical cancer.
Keywords: Human papillomavirus, Vaccination, Effectiveness, Cervical intraepithelial neoplasia, Australia
1. Introduction
Oncogenic types of human papillomavirus are the cause of cervical cancer [1]. Prophylactic HPV vaccines have been in use since 2006 following demonstration in clinical trials that, when given as a three–dose schedule in women without previous evidence of HPV type specific infection, they prevent targeted type HPV infection and high–grade cervical intraepithelial neoplasia (CIN) [2]. Titres of vaccine induced antibodies, elicited by any of the three HPV vaccines (bivalent protecting against the most oncogenic types 16/18, quadrivalent protecting against 6/11/16/18, nonavalent protecting against 6/11/16/18/31/33/45/52/58) greatly exceed those produced following natural infection [3]. This may be due to the inherent immunogenicity of the virus–like particles used in the vaccine, which display the viral outer coat protein L1 in a conformation mirroring that of the virus [4]. The vaccines are used globally, although programs predominate in high income countries, despite the disproportionate burden of cervical cancer in low income countries [5]. Globally only an estimated 6·1% of females aged 10–20 years have received 1 or more vaccine doses [6].
The vaccines were initially trialled in a three–dose schedule with doses spaced at 0, 1–2 and 6 months. However, observed higher titres in adolescent immunological bridging studies led to studies of the equivalence of a two–dose prime–boost strategy using more widely spaced dosing (0,6–12 months) in young adolescents [7]. In 2014, WHO recommended use of a routine two–dose schedule in immunocompetent girls aged 14 and under. Intriguingly a post–hoc analysis of bivalent HPV vaccine trials suggested equivalent efficacy of one dose of vaccine against persistent HPV infection [8,9]. Data from a prematurely ended quadrivalent vaccine trial in India, whereby some participants only received one dose, also suggest equivalent effectiveness against infection from one dose [10]. However, to date, data from population based usage of vaccine, whilst indicating high effectiveness against infection, warts and CIN [11], have not demonstrated one dose effectiveness equivalent to three doses [12]. This may be due to an underlying bias, given that the characteristics of those who fail to complete the vaccine course appear to be different to those who do and may be associated with an increased risk of HPV infection [12,13]. Additionally analyses of the impact of the vaccine amongst women attending cervical screening have been of women who were too old to have been predominantly HPV naïve at vaccination [12,13]. Here we present a national analysis of quadrivalent HPV vaccine effectiveness by number of doses against high–grade CIN+ (all cause, non-HPV type specific, histologically confirmed) in a screening cohort of young women who were vaccinated at an age at which they were predominantly HPV naïve.
2. Methods
2.1. Data sources and linkage
In Australia, from 1991 to 30 November 2017, the National Cervical Screening Program recommended two-yearly cervical cytology screening using Papanicolaou (or ‘Pap’) tests for sexually active women aged 18 (or two years after sexual debut, whichever was later) to 69. Cervical screening registries from all eight jurisdictions in Australia supported the Program and each systematically recorded cervical screening results, including histopathology inclusive of cancer, for participants. Women could ‘opt off’ a registry but opt off rates were low (<1%) [14]. Quality of cytology was high, with close quality and safety monitoring of the program down to the individual laboratory level through the registries [15]. Participation in screening was high, with 73·5% of women aged 20–24 having at least one screen in the period 2010–2014 [15].
Data from 1 January 2000–31 December 2014 from all eight registries was provided to the Data Linkage Unit, Australian Institute of Health and Welfare (AIHW), for linkage to data from the same time period obtained from the Australian Cancer Database (national data available to 31 December 2012) (to ascertain any cervical cancers missed by screening registers), National Death Index (to confirm vital status to allow censoring of follow up) and National HPV Vaccination Program Register (NHVPR). The NHVPR recorded individual HPV vaccination doses given in Australia. Quadrivalent vaccine was given in three doses from 2007 to 2017. Between 2007 and 2009, all females 12–26 years were offered vaccination with high coverage (Dose 1/2/3 coverage 12–17 years 83/78/70%, 18–26 years 55/45/32%) [16,17], and thereafter girls were vaccinated at age 12–13 years in school and boys from 2013.
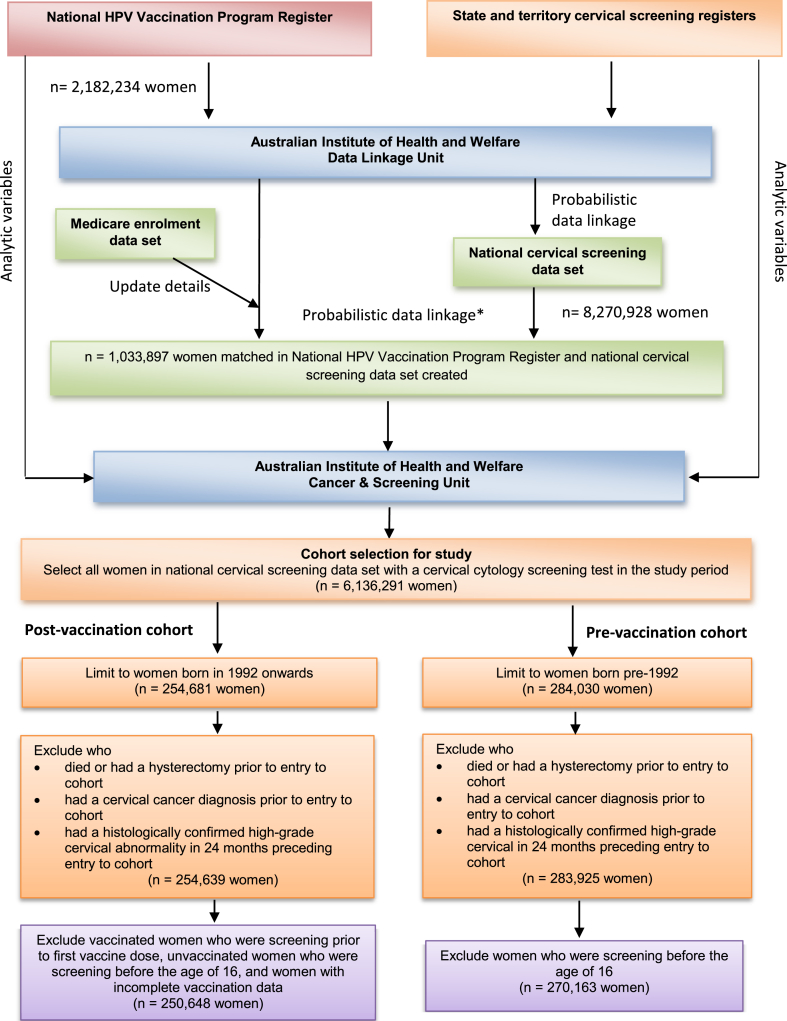
Probabilistic data linkage was performed using established protocols to preserve privacy through separating identifying details (surname, given names, date of birth, postcode, Medicare (Australia's government funded health insurance) number) from analytic variables. Briefly, a step–wise approach was used, whereby records with exact matches on all variables were linked first, followed by subsequent iterations allowing variables to vary with probable pairs assigned a likelihood of a true match, supported by clerical review [18,19]. Prior to linkage, NHVPR records were updated against current Medicare enrolment data. The linked analytic data set contained vaccination status, cervical screening data and demographic data for each woman (date of birth, remoteness area [20] and area–level socioeconomic status [21]) (Fig. 1).
Fig. 1.
Data linkage process and exclusions for analysis. Figure 1 footnote: as per the methods of Fellegi and Sunter [18].
2.2. Study cohort and definitions
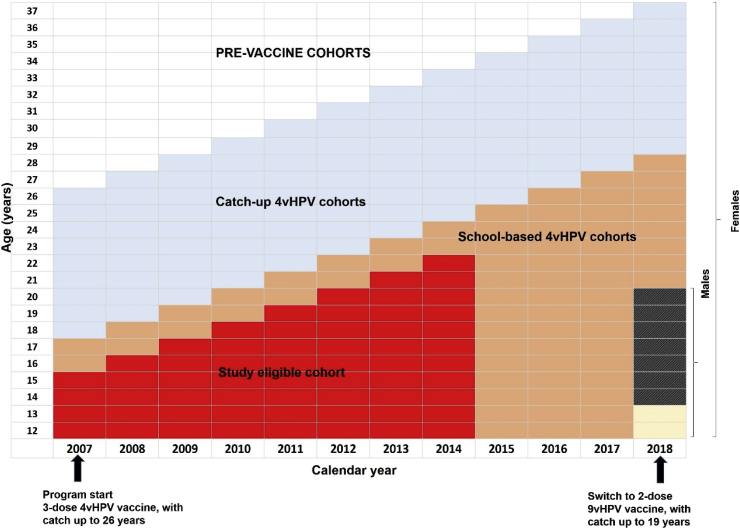
We defined the study cohort as females participating in cervical screening who were age eligible to have been vaccinated two years prior to the Australian median age of sexual debut (age 17 [22]) i.e. females born in or after 1992, as the 1992 birth cohort were eligible for vaccination in 2007 at age 15 (Fig. 2). Any female screening prior to first vaccination (an indication of sexual activity) was excluded. To avoid introducing a systematic bias, we excluded unvaccinated women who commenced screening prior to age 16. The outcome of interest was histologically confirmed CIN2, CIN3, adenocarcinoma–in–situ (AIS), CIN3/AIS or cervical cancer) [23]. HPV typing of cervical lesions or cancers is not routinely undertaken in Australia: therefore, the outcome used was histologically confirmed high–grade lesions due to any HPV type. Two state registries did not code histology data specifically to the level of CIN3+ (only CIN2+); therefore, a sensitivity analysis restricted to this endpoint, with those two states excluded, was undertaken.
Fig. 2.
Study eligible cohort indicated in red in relation to age over time and the roll out of Australia's National HPV Vaccination Program. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
The study period was defined as 1 April 2007 (when HPV vaccination commenced in Australia) until 31 Dec 2014. Women entered the cohort, with events and person-time counted, from the date of their first screening test in the study period until the outcome, two and a half years after their last negative screening test, the end of the study period, hysterectomy, or death, whichever came first. Given that CIN2/AIS+ is diagnosed via screening, person–time at risk is only included for persons who could be diagnosed with the outcome of interest (i.e. screening women).
2.3. Dose assignment and sensitivity analyses
We assigned dose status to women using their final dose status (0,1,2,3), counting outcomes for vaccinated women only after her final dose in cases where women had their first screen during the vaccination course, in order to avoid biasing findings by assigning prevalent disease to earlier doses in the vaccine course (‘final dose status last’). In sensitivity analyses, we also added buffer periods, starting outcome counting at 12 and 24 months since last dose. Use of buffer periods allows for prevalent infection or disease to clear prior to outcome measurement. As prevalent disease is more likely to be counted as an outcome against earlier doses in the vaccine course, buffer periods have been commonly used in studies estimating per dose effectiveness against genital warts and CIN in cohorts that include women sexually active prior to vaccination [14]. We counted valid doses according to national dose rules for the three–dose schedule using implied dose numbers assigned by the NHVPR (counting documented doses adherent to recommended minimal dose intervals). In a sensitivity analysis taking into account current recommendations that two dose schedules of HPV vaccine can be used in young adolescents with doses spaced 6–12 months apart, we considered recipients who received two doses spaced 5 months or greater apart as two dose recipients (regardless of age at dose one) and assigned women with closer spacing into the one dose group. We also conducted sensitivity analyses utilising two alternative methods to assign dosage status over time as proposed by Drolet et al. [24] These are ‘time varying dose status’ whereby, a woman's screening outcomes are assigned to her dose status (0,1,2,3) at that time i.e. she contributes person-time to multiple doses. The second alternative method is the ‘final dose status first’ method, whereby a woman's screening outcomes are assigned to her final dose status (0,1,2,3) regardless of whether she had received her final dose number at the time of the outcome i.e. person-time/case counting begins after first dose (but still after screening commences). This approach is designed to minimise bias against one or two dose effectiveness estimates, given that earlier screening episodes are more likely to be impacted by infection prior to vaccination. As an example, if a girl received dose 1 at 15, commenced screening at 16, had dose 2 at 17 and dose 3 at 18 followed by a second screen, in the ‘final dose status last method’ only screening outcomes occurring after the third dose are included so the screening outcome at age 18 would be assigned as occurring in a three dose recipient's person time and the screening outcome at age 16 excluded. With the time varying dose status method, the first screen would be considered as occurring in a one dose recipient's person time and the second screen in a three dose recipient's person time. Using the ‘final dose status method first’, both the screening outcomes at age 16 and 18 would be included as occurring in a three dose recipient's person time.
2.4. Statistical analyses
We used Cox proportional hazard regression, adjusted a priori for area level socioeconomic status and area of residence, with age as the time axis, to estimate hazard ratios (with 95% CIs) of high–grade histopathology for women in our cohort according to their vaccination status. Using age as the time axis allows the baseline hazard to change as a function of age, which is a better method for controlling potential confounding due to age [25]. This method adjusts the analysis effectively for both birth cohort and age at study entry (first screen). In a sensitivity analysis, we also adjusted for age at vaccination among vaccinated women, by comparing hazard ratios between vaccinated dose groups using the three dose group as the reference group. In our primary analysis, unvaccinated women were used as the reference group. We also estimated hazard ratios with one, two or three dose groups as the reference group.
We also evaluated the observed effectiveness of vaccination by number of doses over time using a Kaplan–Meier cumulative failure probability plot. Failure time was calculated as the number of days from the time of their first screen or last vaccination dose (whichever was later) until outcome or censoring.
In an additional analysis, in order to estimate the extent of herd protection, we replicated the study for women aged 15 and under at entry over an identical time span pre vaccination (Jan 2000–Sept 2007). We plotted their failure probability over time so that this historical cohort shows the cumulative probability of CIN2+ in age identical women followed for an identical amount of time in the pre vaccine period. We also repeated our primary analysis using the pre-vaccination cohort as the comparator group.
Analyses were conducted in SAS Enterprise Guide 7·1. Analyses of differences in the distribution of demographic and exposure characteristics of women in the cohort by vaccination status used the Mann–Whitney U test and the Pearson chi–square test.
2.5. Ethics
Ethical approval for the study was given by AIHW Ethics Committee (EO 2014–4–130) and state and territory human research ethics committees.
3. Results
The cohort included 250,648 women (Fig. 1) with a median follow up time of 1.7 years (IQR 0.8–2.5 years). Of these, 48,845 (19·5%) were unvaccinated, 174,995 (69·8%) had received three doses, 18,190 (7·3%) two doses and 8,618 (3·4%) one dose. Using the 2014 estimated resident population of Australia and vaccination status data from the NHVPR, we estimate that of the total Australian female population in the 1992–1995 birth cohorts (n = 617,834), 37.6% had screened by end of 2014 and were included in our cohort. Three dose and two dose recipients had similar screening participation (40.0% and 38.5% respectively), higher than one dose recipients (31.2% participation) or unvaccinated women (31.7%). Characteristics by vaccination status are given in Table 1.
Table 1.
Characteristics of 250,648 women eligible for quadrivalent HPV vaccination at age 12–15 years attending cervical screening 2007–2014, by final vaccination status, Australia.
| Unvaccinated | 1 dose | 2 doses | 3 doses | |
|---|---|---|---|---|
| Number of observations | 48,845 | 8,618 | 18,190 | 174,995 |
| Mean age in 2007 | 13·2 (±1·3) | 13·1 (±1·5)# | 13·1 (±1·5)#* | 13·2 (±1·4)# |
| Mean age at first screen | 18·9 (±1·5) | 18·4 (±1·6)#* | 18·4 (±1·6)#* | 18·7 (±1·5)# |
| Mean number screens | 1·4 (±0·8) | 1·5 (±0·9)#! | 1·5 (±0·9)#! | 1·5 (±0·8)# |
| Mean age at entry to cohort | 18·9 (±1·5) | 18·4 (±1·6)#* | 18·4 (±1·6)#* | 18·7 (±1·5)# |
| Year of birth | ||||
| 1992 | 16,878 (34·6%) | 2,896 (33·6%) | 5,968 (32·8%)# | 57,139 (32·7%)# |
| 1993 | 13,466 (27·6%) | 2,386 (27·7%) | 4,954 (27·2%) | 47,021 (26·9%)$ |
| 1994 | 9,844 (20·2%) | 1,537 (17·8%)#* | 3,338 (18·4%)#* | 36,178 (20·7%)$ |
| 1995 | 5,523 (11·3%) | 970 (11·3%)! | 2,165 (11·9%)$! | 22,120 (12·6%)# |
| 1996+ | 3,134 (4·7%) | 829 (6·3%)#* | 1,765 (6·7%)#* | 12,537 (5·3%)# |
| Remoteness area | ||||
| Major cities | 34,021 (69·8%) | 5,483 (63·7%)#! | 11,635 (64·0%)#! | 113,616 (65·0%)# |
| Inner regional | 8,920 (18·3%) | 1,932 (22·4%)# | 4,025 (22·1%)# | 38,876 (22·2%)# |
| Outer regional | 4,572 (9·4%) | 925 (10·7%)# | 1,963 (10·8%)#! | 17,988 (10·3%)# |
| Remote | 754 (1·5%) | 145 (1·7%)* | 321 (1·8%)$* | 2,466 (1·4%)$ |
| Very remote | 473 (1·0%) | 126 (1·5%)#! | 228 (1·3%)$! | 1,894 (1·1%)$ |
| Socioeconomic status | ||||
| 1 (lowest) | 10,322 (21·3%) | 2,096 (24·4%)#* | 4,187 (23·1%)#* | 32,423 (18·7%)# |
| 2 | 9,960 (20·6%) | 1,907 (22·2%)$! | 3,982 (22·0%)#! | 36,748 (21·1%)$ |
| 3 | 9,839 (20·3%) | 1,775 (20·7%) | 3,715 (20·5%) | 35,356 (20·3%) |
| 4 | 9,214 (19·0%) | 1,478 (17·2%)$* | 3,368 (18·6%)* | 34,965 (20·1%)# |
| 5 (highest) | 9,076 (18·7%) | 1,319 (15·4%)#* | 2,850 (15·7%)#* | 34,284 (19·7%)# |
| Age at first screen (years) | ||||
| ≤14 | 0 (0·0%) | 74 (0·9%)#* | 158 (0·9%)#* | 541 (0·3%)# |
| 15–17 | 9,032 (18·5%) | 2,455 (28·5%)#* | 4,909 (27·0%)#* | 33,788 (19·3%)# |
| 18+ | 39,813 (81·5%) | 6,089 (70·7%)#* | 13,123 (72·1%)#* | 140,666 (80·4%)# |
| Number of screens | ||||
| 1 only | 35,230 (72·1%) | 5,544 (64·3%)#! | 11,786 (64·8%)# | 114,434 (65·4%)# |
| 2–5 | 13,464 (27·6%) | 3,038 (35·3%)#! | 6,322 (34·8%)# | 59,892 (34·2%)# |
| >5 | 151 (0·3%) | 36 (0·4%) | 82 (0·5%)$ | 669 (0·4%)$ |
| Age commenced vaccination (years) | ||||
| ≤13 | 0 (0·0%) | 2,400 (27·8%)* | 6,079 (33•5%)** | 66,133 (37·8%) |
| 14–15 | 0 (0·0%) | 4,690 (54·4%)* | 10,574 (58•2%)** | 105,223 (60·1%) |
| 16–17 | 0 (0·0%) | 1,465 (17·0%)* | 1,479 (8•1%)** | 3,583 (2·0%) |
| 18+ | 0 (0·0%) | 63 (0·7%)* | 41 (0·2%)* | 56 (0·0%) |
| Year entered cohort | ||||
| 2007 | 5 (0·0%) | 10 (0·1%)# | 23 (0·1%)#! | 111 (0·1%)# |
| 2008 | 252 (0·5%) | 98 (1·1%)#* | 251 (1·4%)#* | 1,307 (0·7%)# |
| 2009 | 1,128 (2·3%) | 433 (5·0%)#* | 823 (4·5%)#* | 4,506 (2·6%)$ |
| 2010 | 2,945 (6·0%) | 834 (9·7%)#* | 1,516 (8·3%)#* | 11,428 (6·5%)# |
| 2011 | 5,948 (12·2%) | 1,300 (15·1%)#! | 2,742 (15·1%)#* | 24,146 (13·8%)# |
| 2012 | 9,234 (18·9%) | 1,772 (20·6%)$ | 3,740 (20·6%)# | 35,753 (20·4%)# |
| 2013 | 13,076 (26·8%) | 1,948 (22·6%)#* | 4,309 (23·7%)#* | 46,145 (26·4%) |
| 2014 | 16,257 (33·3%) | 2,223 (25·8%)#* | 4,786 (26·3%)#* | 51,599 (29·5%)# |
| Mean years between vaccination and screening | 0 | 4·0 (±1·7) | 4·4 (±1·6) | 4·9 (±1·4) |
| Person-time (years) | 85,417 | 18,104 | 37,819 | 334,410 |
| Median person-time (years) (25th–75th percentiles) |
1.6 (0.7–2.5) | 2.1 (0.9–2.7) | 2.0 (0.9–2.6) | 1.7 (0.8–2.5) |
| Cytological abnormalities diagnosed on entry into cohort | ||||
| Unsatisfactory | 1,262 (2·6%) | 223 (2·6%) | 465 (2·6%) | 4,498 (2·6%) |
| Negative | 41,212 (84·4%) | 7,323 (85·0%)* | 15,626 (85·9%)#* | 152,159 (87·0%)# |
| Low–grade | 5,631 (11·5%) | 955 (11·1%)* | 1,897 (10·4%)#! | 16,735 (9·6%)# |
| High–grade | 740 (1·5%) | 116 (1·3%)* | 202 (1·1%)#! | 1,603 (0·9%)# |
| Cancer | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) | 0 (0·0%) |
$ P–value P ≤ 0·05 (reference group ‘unvaccinated’).
# P–value ≤0·0001 (reference group ‘unvaccinated’).
! P–value ≤0·05 (reference group ‘3 doses’).
* P–value ≤0·0001 (reference group ‘3 doses’).
Notes:1. Count is of women; ‘unvaccinated’ refers to women screened who did not receive any dose of HPV vaccine; number of doses refers to a woman's final dose of HPV vaccine.2. Women were assigned to a remoteness area based on a proportional remoteness index. Postcode of usual residence as at entry to cohort was mapped to remoteness index according to the Australian Statistical Geography Standard for 2011 at postcode level [20]. Please note that 285 (0.1%) of women were unable to be assigned to a remoteness area.3. Women were assigned to a socioeconomic status group based on a proportional socioeconomic index. Postcode of usual residence as at entry to cohort was mapped to socioeconomic index according to the Australian Bureau of Statistics Socio–Economic Indexes for Areas (SEIFA) Index of Relative Socio–Economic Disadvantage for 2011 assigned by the Australian Bureau of Statistics at postcode level [21]. Please note that 1,784 (0.7%) of women were unable to be assigned to a socioeconomic status group.4. One woman (<0.01%) did not have information on their cytological abnormality diagnosed on entry into the cohort.
There were significant differences across all characteristics examined. Mean age at first screen was significantly lower in women who received one or two doses (18·4) than in either fully vaccinated (18·7) or unvaccinated women (18·9) (p < 0·0001). Women who received one or two doses were also more highly represented in regional and remote areas, and in areas of lower socioeconomic status compared to fully vaccinated or unvaccinated women, with unvaccinated women more likely to reside in major cities and fully vaccinated in areas of higher socioeconomic status. The majority of women in each vaccine dose group initiated vaccination at the age of 14 or 15, with age 12–13 the next most frequent age of initiation. However, one dose women were least likely to have initiated vaccination at 12–13 (27·8% compared to 33·4% 2 dose and 37·8% 3 dose) and most likely to have initiated at either 16–17 (17·0% vs 8·2% vs 2·0%) or at 18 years or older (0·7% vs 0·2% vs 0·03%).
An increasing proportion of women in each vaccine dose group commenced screening and entered the cohort every calendar year as expected. On entry to the cohort, unvaccinated women were the least likely to have a negative Pap test (84·4%) and fully vaccinated women the most (87·0%). Univariate analysis confirmed that area level socioeconomic status (HR least disadvantaged compared to most disadvantaged 0·84 (95%CI 0·73–0·95)), remoteness (HR very remote compared to major cities 1·47 (1·07–2·02)) and for vaccinated women, age at vaccination (HR age 16 + compared to ≤ 13 1·67 (1·34–2.9)), were significant predictors of high–grade cervical disease (Appendix, Table S1). Three cases of cervical cancer (two squamous cell carcinomas and one adenocarcinoma) were diagnosed in the cohort during the study period.
The crude rate of histologically confirmed CIN2/AIS+ was highest amongst unvaccinated women (13·2 per 1000 women) with reducing frequency by vaccine dose status (1 dose 10·3, 2 doses 9·5, 3 doses 8·5) (Table 2). However, the adjusted hazard ratio was significantly lower and comparable between the vaccine dose groups compared to unvaccinated women (1 dose 0·65 (95%CI 0·52–0·81), 2 doses 0·61 (0·52–0·72) and 3 doses 0·59 (0·54–0·65)). Importantly when adjusted for age at vaccination, effectiveness of one or two doses was equivalent to the effect of three doses (one dose HR1.01 (95%CI 0.81–1.26), two doses 1.00 (0.85–1.17)) (Table 3). This was also the case when women vaccinated after 15 were excluded (Table S8). Results were consistent, although less precise due to smaller numbers and with loss of power in the one dose group, for the analysis restricted to CIN3/AIS+ (Table 3). Results presented using one, two or three dose recipients as the comparator groups highlight no evidence of inferior protection for one dose compared to two or three dose recipients (Appendix, Tables S2–4). Two alternative analyses, without the exclusion of unvaccinated women aged under 16 and restricted to women who started screening at age 18 and over, demonstrate equivalent findings (Appendix, Tables S5 and S6).
Table 2.
Rate of histologically confirmed CIN2/AIS+ (due to any HPV type) and hazard ratios by number of quadrivalent human papillomavirus vaccine doses received*, national cohort of screening women born in 1992 or later, 2007–2014, Australia.
| Abnormalities | No. women | Person –time (years) | No. abnormalities | Rate per 1000 women | Rate per 1000 women- years | Hazard ratio** | |
|---|---|---|---|---|---|---|---|
| CIN2+/AIS | Unvaccinated | 48,845 | 85,417 | 645 | 13·2 | 7.6 | 1·0 |
| 1 dose | 8,618 | 18,104 | 89 | 10·3 | 4.9 | 0·65 (0·52–0·81) | |
| 2 doses | 18,190 | 37,819 | 174 | 9·6 | 4.6 | 0·61 (0·52–0·72) | |
| 3 doses | 174,995 | 334,410 | 1,496 | 8·5 | 4.5 | 0·59 (0·54–0·65) |
* Vaccine dose status assigned to outcome using ‘Final status last’ method.
** From Cox proportional hazard regression, with age as the time–scale, adjusted for area of residence and socioeconomic status.
Table 3.
Sensitivity analyses: Rate of histologically confirmed CIN2/AIS+ (caused by any HPV type) per 1000 women and hazard ratio by number of quadrivalent human papillomavirus vaccine doses received, national cohort of screening women born in 1992 or later, 2007–2014, Australia.
| Abnormalities | No. women | No. abnormalities | Rate per 1000 | Adjusted hazard ratioa | |
|---|---|---|---|---|---|
| Analysis variation: adjusted for age at vaccination amongst vaccinated women | |||||
| CIN2/AIS+ | 1 dose | 8,618 | 89 | 10·3 | 1·01 (0·81–1·26) |
| 2 doses | 18,190 | 174 | 9·6 | 1·00 (0·85–1·17) | |
| 3 doses |
174,995 |
1,496 |
8·5 |
1·0 |
|
|
Analysis variation: 2 doses reallocated: < 5 months = 1 dose, ≥ 5 months = 2 doses | |||||
| CIN2/AIS+ | Unvaccinated | 48,845 | 645 | 13·2 | 1·0 |
| 1 dose | 21,853 | 213 | 9·7 | 0·62 (0·53–0·72) | |
| 2 doses | 4,955 | 50 | 10.1 | 0·63 (0·48–0·85) | |
| 3 doses |
174,995 |
1,496 |
8·5 |
0·59 (0·54–0·65) |
|
|
Analysis variation: Application of buffer 12 months after entry to cohort | |||||
| CIN2/AIS+ | Unvaccinated | 32,574 | 287 | 8·8 | 1·0 |
| 1 dose | 6,390 | 36 | 5·6 | 0·54 (0·38–0·76) | |
| 2 doses | 13,399 | 89 | 6·6 | 0·64 (0·51–0·82) | |
| 3 doses |
123,318 |
768 |
6·2 |
0·64 (0·56–0·74) |
|
|
Analysis variation: Application of buffer 24 months after entry to cohort | |||||
| CIN2/AIS+ | Unvaccinated | 19,501 | 165 | 8·5 | 1·0 |
| 1 dose | 4,446 | 26 | 5·8 | 0·59 (0·39–0·89) | |
| 2 doses | 9,090 | 56 | 6·2 | 0·61 (0·45–0·83) | |
| 3 doses |
77,211 |
444 |
5·8 |
0·61 (0·51–0·73) |
|
|
Analysis variation: Dose status assigned using time varying dose status method | |||||
| CIN2/AIS+ | Unvaccinated | 48,847 | 645 | 13·2 | 1·0 |
| 1 dose | 8,695 | 90 | 10·4 | 0·65 (0·52–0·81) | |
| 2 doses | 18,248 | 173 | 9·5 | 0·61 (0·51–0·72) | |
| 3 doses |
174,738 |
1,491 |
8·5 |
0·59 (0·54–0·65) |
|
|
Analysis variation: Dose status assigned using final dose status first method | |||||
| CIN2/AIS+ | Unvaccinated | 48,845 | 645 | 13·2 | 1·0 |
| 1 dose | 8,618 | 89 | 10·3 | 0·65 (0·52–0·81) | |
| 2 doses | 18,190 | 175 | 9·6 | 0·61 (0·51–0·72) | |
| 3 doses |
174,995 |
1,502 |
8·6 |
0·59 (0·54–0·65) |
|
|
Analysis variation: Restricted to CIN3 + outcomes (two States excluded which did not code to CIN3 level)) | |||||
| CIN3/AIS + histopathology | Unvaccinated | 24,202 | 145 | 6·0 | 1·0 |
| 1 dose | 4,035 | 19 | 4·7 | 0·66 (0·41–1·06) | |
| 2 doses | 8,641 | 25 | 2·9 | 0·42 (0·27–0·64) | |
| 3 doses | 80,435 | 227 | 2·8 | 0·43 (0·35–0·53) | |
From Cox proportional hazard regression, with age as the time–scale, adjusted for area of residence and socioeconomic status.
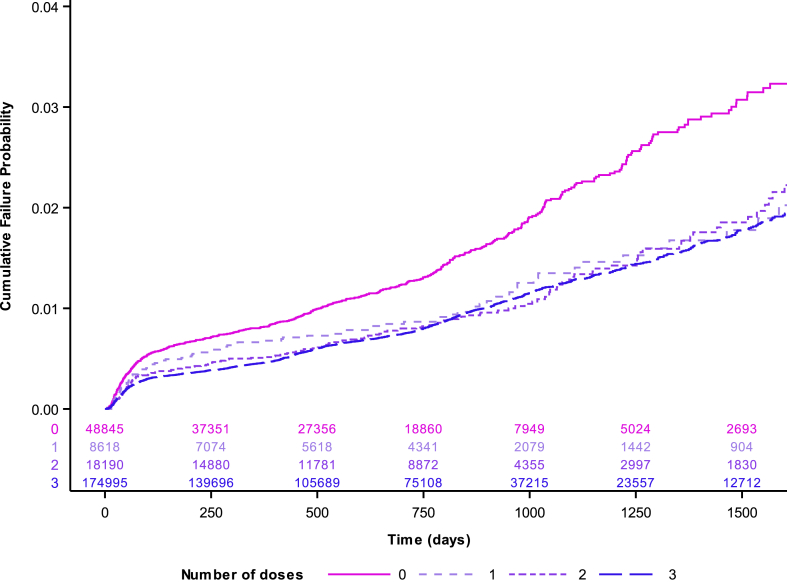
The effectiveness of vaccination by number of doses over time against CIN2/AIS+ is shown in Fig. 3, indicating some initial differences by vaccination status but a clear convergence over time between the cumulative incidence rate in unvaccinated compared to vaccinated women regardless of number of doses, χ [2](3) = 126.76, p < 0.0001. When incidence in a pre-vaccination cohort of 270,613 women was compared (Fig. S1), herd protection of unvaccinated women became apparent (HR 0.73 (0.67–0.79)) and, using the pre-vaccinated group as a comparator, HRs were significant lower for all groups and remained equivalent for one (HR 0.47 (0.38–0.58)), two (HR 0.44 (0.38–0.51)) and three dose groups (HR 0.43 (0.41–0.45)) (Table S7).
Fig. 3.
Cumulative failure probability plot for high grade cervical histopathology (CIN2/AIS+) among 250,648 screening women eligible for quadrivalent HPV vaccine at age 15 or under by final dose status. Figure 3 footnote: Note time 0 is date of first cervical screen.
When only women with two doses spaced over 5 months apart were assigned as two dose recipients, and women with closer spacing considered as one valid dose recipients, crude rates became higher in the two dose group (10·1 per 1000) than one dose group (9.7 per 1000) but adjusted hazard ratios remained similar for all three dosage groups (Table 3). Addition of a 12 or 24-month buffer period lowered crude rates across all groups but the overall finding, of significant and consistent protection across dose groups, remained. Analyses using the ‘Time varying dose’ or ‘First status first’ methods similarly indicated little impact of dose assignment method on the findings (Table 3).
4. Discussion
4.1. Principal findings
In this national data linkage study of screening women first offered vaccine up to seven years previously, we found that one dose of quadrivalent HPV vaccine was as effective as three at preventing histologically confirmed high–grade cervical lesions. This is most robustly demonstrated in our analysis comparing rates of disease between vaccinated groups which also accounted for age at vaccination, and found a hazard ratio for one dose of 1.01 (95%CI 0.81–1.26) and for two doses of 1.00 (0.85–1.17) compared to three. We demonstrated a vaccine effectiveness of approximately 40% when compared to unvaccinated women in the same cohort. This is the effectiveness against all cause high grade lesions (CIN2/AIS+) rather than vaccine type related lesions against which the vaccine has very high efficacy (>95%) in baseline HPV–naïve cohorts. Approximately half of HSIL contain HPV16 or 18, suggesting this effectiveness estimate is consistent with expected overall impact [26] although somewhat lower than previous earlier estimates in younger cohorts in Australia [27] (with wide confidence intervals due to low power). This could be due to a decline over time in 16/18 circulation in adolescents due to both direct and herd protection impacts of vaccination, as previously documented [28,29], and a resulting decrease in the proportion of high grade cervical lesions attributable to 16/18. We demonstrated herd protection of unvaccinated women in our additional analysis comparing a historical cohort (Fig. S1) and note that vaccine effectiveness is higher when using a pre-vaccine period rate as the comparator, at around 55% (Table S7). Herd protection could not explain our finding of equivalent one and two dose effectiveness as three unless herd protection impacted one and two dose recipients to a greater extent than unvaccinated and three dose recipients. This is highly implausible.
Equivalent impact regardless of number of doses was suggested despite demographic differences between fully vaccinated and partially vaccinated women, such as lower socioeconomic status, increased remoteness of residence, older age at vaccination, and younger age at first screen, all of which will likely limit our ability to detect equivalent vaccine effectiveness in partially vaccinated women because these factors are associated with an increased risk of HPV infection, CIN and cervical cancer. These differences are the likely reason for initially higher rates of CIN in the one and two dose groups due to higher rates of prevalent infection at cohort entry (Fig. 3). Our findings were robust to alterations in definition of two valid doses, the addition of buffering periods, alternative methods of dose assignment and adjustment for age at vaccination. That we did not observe any substantial increase in effectiveness with buffering periods may be due to the young age of vaccination and relatively long duration between vaccination and screening in this cohort. Our secondary analysis restricted to CIN3/AIS+ was underpowered, due to the lower frequency of these lesions and loss of data from two populous states, but was also consistent with the main analysis. An analysis restricted to the youngest birth cohorts routinely eligible for vaccination at age 12–13 (1994 and after) was also consistent but underpowered (data not shown). Our overall results are consistent with the substantial declines in CIN2/3/AIS observed in Australia since vaccination commenced, which is steadily impacting extended age groups as the vaccinated cohorts age [24]. They are also consistent with our previous study, which linked HPV vaccination and cervical screening data from women in Victoria, Australia [13]. That analysis, which included a majority of women vaccinated in their late teens/early twenties, suggested that in women vaccinated prior to screening initiation, partial vaccination had increased effectiveness over time, as prevalent infection and disease within the cohort was cleared [13].
4.2. Strengths and limitations of the study
Compared to our previous analysis, this analysis is national rather than for one state only and has the majority likely vaccinated prior to sexual debut, and improved linkage accuracy due to updating of Medicare details prior to linkage. The major strengths of our study are that it is national data from the country with the first high uptake national HPV vaccination program, and the use of high quality registry data to determine vaccination status, screening participation and outcomes, and vital status.
Limitations include some degree of under–linkage and inaccurate linkage because Australia does not have a unique national identifier: this will result in some misclassification of vaccinated women as unvaccinated, which will decrease our ability to distinguish differences in outcomes between vaccinated and unvaccinated women. Vaccination status may also be slightly under–reported, meaning some unvaccinated women may have been vaccinated and some one and two dose recipients could have received a second or third dose. However, because all individuals in the analysis were eligible for vaccination at school, where all doses were notified in contrast to the known 5–15% under–reporting of doses given in general practice, we do not believe that mis–assignment to dose groups would be sufficiently large to possibly explain the findings of equivalence [17]. The vaccination register routinely sent reminder statements to apparently incompletely vaccinated individuals with good response rates, including updating of the register with doses not previously notified from general practice [30]. We could not adjust for all known CIN risk factors, such as sexual history and smoking. It is probable that some residual confounding between dose groups remains. It may be that, even when the impact of prevalent HPV infection at vaccination is minimised through using a younger cohort, a higher risk of HPV infection and disease throughout life suggested by the characteristics of women who only received one dose, cannot be entirely mitigated. Estimated screening participation was highest amongst fully vaccinated women and participation in screening in these young cohorts was low overall. Follow up analyses as these women age and more enter screening will be important to confirm these findings in a larger proportion of the population.
4.3. Comparison with other studies
These findings support previous post–hoc analyses of the bivalent HPV vaccine trials [8] and non–randomised data from India of the quadrivalent HPV vaccine [10] suggesting sufficient immunogenicity and effectiveness against HPV infection from one dose. Compared to previous observational studies, the current work is better powered and more representative of target populations rather than catch up cohorts, given the length of time since the national vaccination program was started in Australia. A recent study from Denmark and Sweden produced findings consistent with our data in observing similar vaccine effectiveness against CIN2+ in women vaccinated with the quadrivalent HPV vaccine at age <16 years by dose number, although the protection given by one or two doses was not statistically significant with wide confidence intervals due to small numbers [31]. Encouragingly an extended analysis of these data for Denmark, with more follow up time and power, found a similar and lower incidence of CIN3+ in one, two and three dose recipients at the population level than unvaccinated women (Incidence Rate Ratios one dose 0.38 (95%CI 0.14–0.98), two doses 0.38 (95%CI 0.22–0.66), three doses 0.37 (95% CI 0.30–0.45) [32]. However neither of these studies fully accounted for screening participation, with the incidence of CIN2+/3 + calculated for the entire female population regardless of whether they had attended screening or not. The United States also commenced HPV vaccination early but has struggled to achieve high coverage. A recent US study using insurance data, and with the notable strength of being able to adjust for other HPV risk factors such as other sexually transmitted infections, found no difference in genital warts incidence by number of doses in females and males who were vaccinated at age 15–19; incidence rates were too low in the under 15 year old age group to detect any vaccine effect [33]. These recent observational data hopefully foreshadow that we are entering a time, more than a decade on from the first HPV vaccination programs, where many countries will start to report HPV-related outcomes for females routinely vaccinated at 9–14 years of age cohorts as they enter adulthood rather than for catch up cohorts who were not HPV naive at baseline.
4.4. Implications for clinicians, policymakers and future research
In summary, these real–world data are consistent with the hypothesis that one dose of quadrivalent HPV vaccine may be as effective as two or three in preventing high–grade cervical disease. We believe that these data support decision makers to consider how a one dose HPV vaccination schedule, or a planned schedule with a 3–5 year interval between doses, could reduce vaccine demand globally (which currently exceeds supply) whilst awaiting confirmation of equal protection from one dose against HPV infection from the randomised trials currently underway [9]. Although one dose could have waning protection, the time span of this analysis includes girls screening seven years after vaccination. Analyses with more follow–up time will be undertaken, as will analyses including only girls vaccinated at 12–13 years. Recent data supports both the immunogenicity of one dose of quadrivalent vaccine given routinely to 9–14 year olds and the strong response to boosting achievable 3–8 years later using a dose of nonavalent HPV vaccine, supporting that a booster dose would be effective, if trial data or long term follow up data indicated it was required, for cohorts who had been given one dose [34]. There is a strong and successful precedent for trial implementation of reduced dose schedules with HPV vaccines, with both Quebec and Mexico initially implementing a two–dose schedule with a third ‘booster’ dose scheduled years later if needed (not required to date) [35]. We believe that one dose HPV vaccination should be considered a viable strategy as part of the recent WHO call for action to develop strategies, which will include vaccination, screening, early diagnosis and treatment, and palliative care, to achieve the elimination of cervical cancer as a public health problem [36,37]. Requiring only one dose of vaccine greatly reduces program costs and requirements, and may open the way for a global mass vaccination campaign. It may make the difference for many countries, and at the moment for cohorts of girls who may otherwise miss out altogether due to supply constraints, especially those countries who are otherwise unable to afford either screening or vaccination and are therefore suffering what is now a largely preventable burden of cervical cancer.
Funding
The data linkage and analysis of this study were funded by the Australian Department of Health. The funder had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the article for publication. JMLB, AB and MS are investigators in the National Health and Medical Research Council funded Centre for Research Excellence in Cervical Cancer Control (APP1135172).
Declarations of interest
Julia Brotherton was an investigator on investigator-initiated research grants that provided funding for laboratory testing for a study of cervical cancers (Seqirus) and recurrent respiratory papillomatosis (Merck) more than three years ago, but has never received personal financial benefits. All other authors declare no conflicts of interest.
Acknowledgements
We thank each state and territory Health Department for their permission to use their registry data and the Australian Department of Health for permission to use NHVPR data. We thank the Cancer Institute NSW for providing data from the NSW Pap Test Register, the Cancer Screening Unit, Department of Health Queensland, for provision of data from the Queensland Pap Smear Register and the Cervix Screening Program, SA Health, for provision of data from the SA Cervix Screening Register.
The NHVPR was owned by the Australian Department of Health and operated by VCS Foundation. We also thank the staff of the AIHW Data Linkage Unit for conducting the data linkage for this study, the state and territory cancer registries for use of their data through the Australian Cancer Database, and Karen Winch for preparation of the data from the NHVPR.
Data for the National Death Index are provided to the AIHW by the State and Territory Registries of Births, Deaths and Marriages and the National Coronial Information System (managed by the Victorian Department of Justice) and include cause of death coded by the Australian Bureau of Statistics. We also thank Philip Castle and Marc Brisson for their helpful advice regarding aspects of the analysis and Dorothy Machalek for the production of Fig. 2.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.pvr.2019.100177.
Contributor Information
Julia ML. Brotherton, Email: jbrother@vcs.org.au.
Alison Budd, Email: Alison.budd@aihw.gov.au.
Christopher Rompotis, Email: christopher.rompotis@aihw.gov.au.
Natasha Bartlett, Email: Natasha.Bartlett@aihw.gov.au.
Michael J. Malloy, Email: mmalloy@vcs.org.au.
Rachael L. Andersen, Email: Rachael.Andersen@dhhs.vic.gov.au.
Kim AR. Coulter, Email: Kim.Coulter@nt.gov.au.
Peter W. Couvee, Email: Peter.Couvee@act.gov.au.
Nerida Steel, Email: Nerida.Steel@health.wa.gov.au.
Gail H. Ward, Email: gail.ward@ths.tas.gov.au.
Marion Saville, Email: msaville@vcs.org.au.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Bouvard V., Baan R., Straif K. A review of human carcinogens– Part B: biological agents. Lancet Oncol. 2009;10(4):321–322. doi: 10.1016/s1470-2045(09)70096-8. [DOI] [PubMed] [Google Scholar]
- 2.Arbyn M., Xu L., Simoens C., Martin–Hirsch P.P.L. Prophylactic vaccination against human papillomaviruses to prevent cervical cancer and its precursors. Cochrane Database Syst. Rev. 2018;(5) doi: 10.1002/14651858.CD009069.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stanley M. Preventing cervical cancer and genital warts – how much protection is enough for HPV vaccines? J. Infect. 2016;72(Suppl) doi: 10.1016/j.jinf.2016.04.018. S23–8. [DOI] [PubMed] [Google Scholar]
- 4.Schiller J., Lowy D. Explanations for the high potency of HPV prophylactic vaccines. Vaccine. 2018;36(32 Pt A):4768–4773. doi: 10.1016/j.vaccine.2017.12.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.LaMontagne D.S., Bloem P.J.N., Brotherton J.M.L., Gallagher K.E., Ndiaye C., Badiane O. Progress in HPV vaccination in low and lower–middle income countries. Int. J. Gynecol. Obstet. 2017;138(Suppl. 1):7–14. doi: 10.1002/ijgo.12186. [DOI] [PubMed] [Google Scholar]
- 6.Bruni L., Diaz M., Barrionuevo–Rosas L. Global estimates of human papillomavirus vaccination coverage by region and income level: a pooled analysis. Lancet Glob. Health. 2016;4(7):e453–e463. doi: 10.1016/S2214-109X(16)30099-7. [DOI] [PubMed] [Google Scholar]
- 7.D'Addario M., Redmond S., Scott P. Two–dose schedules for human papillomavirus vaccine: systematic review and meta–analysis. Vaccine. 2017;35(22):2892–2901. doi: 10.1016/j.vaccine.2017.03.096. [DOI] [PubMed] [Google Scholar]
- 8.Kreimer A.R., Struyf F., Del Rosario, Raymundo M.R. Efficacy of fewer than three doses of an HPV– 16/18 AS04 adjuvanted vaccine: combined analysis of data from the Costa Rica vaccine trial and the PATRICIA Trial. Lancet Oncol. 2015;16(7):775e86. doi: 10.1016/S1470-2045(15)00047-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kreimer A.R., Herrero R., Sampson J.N. Evidence for single–dose protection by the bivalent HPV vaccine–Review of the Costa Rica HPV vaccine trial and future research studies. Vaccine. 2018;36(32):4774–4782. doi: 10.1016/j.vaccine.2017.12.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sankaranarayanan R., Joshi S., Muwonge R. Can a single dose of human papillomavirus (HPV) vaccine prevent cervical cancer? Early findings from an Indian study. Vaccine. 2018;36(32):4783–4791. doi: 10.1016/j.vaccine.2018.02.087. [DOI] [PubMed] [Google Scholar]
- 11.Drolet M., Benard E., Boily M.C. Population–level impact and herd effects following human papillomavirus vaccination programmes: a systematic review and meta–analysis. Lancet Infect. Dis. 2015 May;15(5):565e80. doi: 10.1016/S1473%5f3099(14)71073%5f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Markowitz L.E., Drolet M., Perez N., Jit M., Brisson M. Human papillomavirus vaccine effectiveness by number of doses: systematic review of data from national immunization programs. Vaccine. 2018;36(32):4806–4815. doi: 10.1016/j.vaccine.2018.01.057. [DOI] [PubMed] [Google Scholar]
- 13.Brotherton J.M.L., Malloy M., Budd A., Saville M., Drennan K., Gertig D.M. Effectiveness of less than three doses of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia when administered using a standard dose spacing schedule: observational cohort of young women in Australia. Papillomavirus Res. 2015;1:59–73. [Google Scholar]
- 14.Victorian Cervical Cytology Registry . 2017. Annual Statistical Report 2015. Victorian Cytology Service.http://www.vccr.org/data-research/statistical-reports/annual-statistical-reports Available at. [Google Scholar]
- 15.Australian Institute of Health and Welfare (AIHW) AIHW; Canberra: 2016. Cervical Screening in Australia Report 2013–14. Cancer Series No. 97. Cat. No. CAN 95. [Google Scholar]
- 16.Brotherton J.M.L., Murray S.L., Hall M.A. Human papillomavirus vaccine coverage among female Australian adolescents: success of the school–based approach. Med. J. Aust. 2013;199:614–617. doi: 10.5694/mja13.10272. [DOI] [PubMed] [Google Scholar]
- 17.Brotherton J.M.L., Liu B., Donovan B., Kaldor J.M., Saville M. Human papillomavirus (HPV) vaccination coverage in young Australian women is higher than previously estimated: independent estimates from a nationally representative mobile phone survey. Vaccine. 2014;32:592–597. doi: 10.1016/j.vaccine.2013.11.075. [DOI] [PubMed] [Google Scholar]
- 18.Fellegi I.P., Sunter A.B. A theory for record linkage. J. Am. Stat. Assoc. 1969;64:1183–1210. [Google Scholar]
- 19.Australian Institute of Health and Welfare . AIHW; Canberra: 2018. Analysis of Cancer Outcomes and Screening Behaviour for National Cancer Screening Programs in Australia. Cancer Series No. 111. Cat. No. CAN 115. [Google Scholar]
- 20.Australian Bureau of Statistics . ABS; 2011. 1216.0 – Australian Standard Geographical Classification (ASGC), 2011 Canberra (AUST) [Google Scholar]
- 21.Australian Bureau of Statistics . ABS; Canberra (AUST): 2013. 2033.0.5.5.001 – Census of Population and Housing: Socio–Economic Indexes for Areas (SEIFA), Australia, 2011. [Google Scholar]
- 22.Rissel C., Heywood W., de Visser R.O. First vaginal intercourse and oral sex among a representative sample of Australian adults: the Second Australian Study of Health and Relationships. Sex. Health. 2014 Nov;11(5):406–415. doi: 10.1071/SH14113. [DOI] [PubMed] [Google Scholar]
- 23.Australian Institute of Health and Welfare (AIHW) AIHW; Canberra: 2018. Cervical Screening in Australia 2018. Cat. No. CAN 111. [Google Scholar]
- 24.Drolet M., Lemieux-Mellouki P., Mondor M., Fournier A., Markowitz L., Brotherton J., Kreimer A., Brisson M. Presented at International Papillomavirus Conference, Sydney. October 2018. Reduced-dose HPV vaccination effectiveness in post-vaccination surveillance studies: using mathematical modeling to quantify the impact of biases. IPVC8-0507. [Google Scholar]
- 25.Korn E.L., Graubard B.I., Midthune D. Time–to–event analysis of longitudinal follow–up of a survey: choice of the time–scale. Am. J. Epidemiol. 1997;145:72–80. doi: 10.1093/oxfordjournals.aje.a009034. [DOI] [PubMed] [Google Scholar]
- 26.Smith J.S., Lindsay L., Hoots B., Keys J., Franceschi S., Winer R. Human papillomavirus type distribution in invasive cervical cancer and high–grade cervical lesions: a meta–analysis update. Int. J. Cancer. 2007 Aug 1;121(3):621–632. doi: 10.1002/ijc.22527. [DOI] [PubMed] [Google Scholar]
- 27.Gertig D.M., Brotherton J.M.L., Budd A.C., Drennan K., Chappell G., Saville A.M. Impact of a population –based HPV vaccination program on cervical abnormalities: a data linkage study. BMC Med. 2013;11:227. doi: 10.1186/1741-7015-11-227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garland S.M., Cornall A.M., Brotherton J.M.L., Wark J.D., Malloy M.J. Tabrizi SN on behalf of the VACCINE study group. Final analysis of a study assessing genital human papillomavirus genoprevalence in young Australian women, following eight years of a national vaccination program. Vaccine. 2018;36(23):3221–3230. doi: 10.1016/j.vaccine.2018.04.080. [DOI] [PubMed] [Google Scholar]
- 29.Tabrizi S., Brotherton J.M.L., Kaldor J.M. Assessment of herd immunity and cross–protection following a human papillomavirus vaccination programme: a repeat cross–sectional study. Lancet Infect. Dis. 2014;14(10):958–966. doi: 10.1016/S1473-3099(14)70841-2. [DOI] [PubMed] [Google Scholar]
- 30.Brotherton J.M.L., Batchelor M., Winch K. Utility of reports and routine correspondence from the national HPV vaccination program register. Med. J. Aust. 2013;199(7):463. doi: 10.5694/mja13.10737. [DOI] [PubMed] [Google Scholar]
- 31.Dehlendorff C., Sparén P., Baldur-Felskov B., Herweijer E., Arnheim-Dahlström L., Ploner A., Uhnoo I., Kjaer S.K. Effectiveness of varying number of doses and timing between doses of quadrivalent HPV vaccine against severe cervical lesions. Vaccine. 2018;36:6373–6378. doi: 10.1016/j.vaccine.2018.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Verdoodt F., Dehlendorff C., Kjaer S.K. Dose-related effectiveness of quadrivalent human papillomavirus vaccine against cervical intraepithelial neoplasia: a Danish nationwide cohort study. Clin. Infect. Dis. 2019 Mar 20 doi: 10.1093/cid/ciz239. pii: ciz239. [DOI] [PubMed] [Google Scholar]
- 33.Zeybek B., Lin Y.L., Kuo Y.F., Rodriguez A.M. The impact of varying numbers of quadrivalent human papillomavirus vaccine doses on anogenital warts in the United States: a database study. J. Low. Genit. Tract Dis. 2018;22(3):189–194. doi: 10.1097/LGT.0000000000000401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gilca V., Sauvageau C., Panicker G., De Serres G., Schiller J., Ouakki M., Unger E. Long intervals between two doses of HPV vaccines and magnitude of the immune response: a post-hoc analysis of two clinical trials. Hum. Vaccines Immunother. 2019 doi: 10.1080/21645515.2019.1605278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gilca V., Salmerón–Castro J., Sauvageau C. Early use of the HPV 2–dose vaccination schedule: leveraging evidence to support policy for accelerated impact. Vaccine. 2018;36(32):4800–4805. doi: 10.1016/j.vaccine.2018.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.World Health Organization WHO Director–General Calls for All Countries to Take Action to Help End the Suffering Caused by Cervical Cancer. http://www.who.int/reproductivehealth/call-to-action-elimination-cervical-cancer/en/References Available at.
- 37.World Health Organization WHO Leads the Way towards the Elimination of Cervical Cancer as a Public Health Concern. https://www.who.int/reproductivehealth/cervical-cancer-public-health-concern/en/ Available at.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.