Abstract
Postchemotherapy cognitive impairment, or PCCI, is a common complaint, particularly among breast cancer patients. However, the exact nature of PCCI appears complex. To model the human condition, ovariectomized C57BL/6J mice were treated intravenous weekly for 4 weeks with saline, 2 mg/kg doxorubicin (DOX), 50 mg/kg cyclophosphamide (CYP), or DOX + CYP. For the subsequent 10 weeks, mice were assessed on several behavioral tests, including those measuring spatial learning and memory. After sacrifice, hippocampal spine density and morphology in the dentate gyrus, CA1, and CA3 regions were measured. Additionally, hippocampal levels of total glutathione, glutathione disulfide, MnSOD, CuZnSOD, and cytokines were measured. Body weight decreased in all groups during treatment, but recovered post-treatment. Most behaviors were unaffected by drug treatment: Open field activity, motor coordination, grip strength, water maze and Barnes maze performance, buried food test performance, and novel object and object location recognition tests. There were some significant effects of CYP and DOX + CYP treatment during the initial test of home cage behavior, but these did not persist into the second and third test times. Density of stubby spines, but not mushroom or thin spines, in the dentate gyrus was significantly decreased in the DOX, CYP, and DOX + CYP treatment groups. There were no significant effects in the CA1 or CA3 regions. CuZnSOD levels were significantly increased in DOX + CYP-treated mice; other hippocampal antioxidant levels were unaffected. Most cytokines showed no treatment-related effects, but IL-1β, IL-6, and IL-12 were slightly reduced in mice treated with DOX + CYP. Although the animal model, route of exposure, and DOX and CYP doses used here were reflective of human exposure, there were only sporadic effects due to chemotherapeutic treatment.
Keywords: doxorubicin, cyclophosphamide, behavior, postchemotherapy cognitive impairment, mouse, female
First described over 30 years ago (Silberfarb, 1983), postchemotherapy cognitive impairment (PCCI) or “chemo-brain” seems to be particularly prevalent and concerning in female breast cancer patients with a reported incidence of 10%–40% (Matsuda et al., 2005). PCCI is characterized by problems with memory, concentration, attention, and executive functioning (Ahles, 2012; Joly et al., 2011) which can be particularly long-lasting (Koppelmans et al., 2012). Overall, the findings are not entirely conclusive due in part to baseline cognitive deficits which may exist prior to chemotherapeutic treatment. In a recent review (McDougall et al., 2014), the results of studies investigating cognitive impairment in breast cancer patients were described as inconsistent. Perhaps more convincing are imaging studies documenting neural alterations in women who received chemotherapy for breast cancer. As reviewed by McDonald and Saykin (2013), structural MRI studies have described decreased gray and white matter, particularly in the frontal and temporal lobes. Results from such imaging studies provide physiological and anatomical evidence for PCCI.
A clear understanding of the mechanisms involved in PCCI would provide important information for potential treatment or therapeutics. There has been limited preclinical research on the neurotoxic effects of chemotherapeutics because the first reports in 2006 (Dietrich et al., 2006; Lee et al., 2006). Although not entirely consistent [as reviewed in Seigers and Fardell (2011)], the majority of studies have indicated significant chemotherapeutic-induced cognitive deficits in laboratory rodents, particularly in hippocampal-dependent assessments. For example, deficits in contextual fear conditioning (Christie et al., 2012; Macleod et al., 2007), novel object/location recognition (Seigers et al., 2015; Yang et al., 2010), and water maze performance (Briones and Woods, 2011) have been described. Such deficits, however, are not always apparent in those assessments (Lee et al., 2006; Long et al., 2011), especially when more human-relevant doses are used (Seigers et al., 2015).
An examination of the animal models used in previous studies of chemotherapeutic-induced behavioral alterations indicates that there are some potentially significant differences between those models and the human patient. For example, some studies intended to model chemotherapeutic-induced cognitive deficits in breast cancer patients have used male rodents (eg, Christie et al., 2012). PCCI in men who received chemotherapeutics is a valid focus (Amidi et al., 2015); however, PCCI in women with breast cancer must be modeled in a female subject.
It was our goal to use a model which would be maximally translational in nature. To this end, 2 common chemotherapeutics were selected for study: Doxorubicin (DOX) and cyclophosphamide (CYP) and their combination. Categorization of breast cancer patients from a MarketScan database and a Cancer Registry-Medicare database indicated that the combination of DOX (trade name: Adriamycin) and CYP (trade name: Cytoxan) was a very common chemotherapeutic regimen during 2003–2007 (Barcenas et al., 2014). Female ovariectomized mice served as subjects. Women with breast cancer who received CYP or other alkylating agents experienced a 40%–80% prevalence rate of amenorrhea (Overbeek et al., 2017) while those who received CYP and DOX treatment experienced a 64% prevalence rate (Gadducci et al., 2007). Further, CYP treatment can reduce serum estrogen levels in laboratory rodents (Jarrell et al., 1987; Plowchalk and Mattison, 1992). Due to the well-known effects of estrogen on cognitive function (Chisholm and Juraska, 2013; Luine, 2014), ovariectomized female rodents are suggested as the most appropriate model for these types of studies (Macleod et al., 2007). In addition, because long-lasting effects of PCCI have been reported (Koppelmans et al., 2012), a comprehensive behavioral assessment with sufficient duration to detect both acute and long-term effects would be valuable. Finally, because DOX and CYP are typically administered intravenously (IV) to humans, the route of administration here was also intravenous. Although others have used similar models (Macleod et al., 2007; Rendeiro et al., 2016; Salas-Ramirez et al., 2015), few have comprehensively surveyed for long-term behavioral effects.
MATERIALS AND METHODS
Animals and Housing
Nonlittermate female C57BL/6J (B6) mice (The Jackson Laboratory, Bar Harbor, Maine; stock No. 000664) (n = 80) were used for this study. All mice were ovariectomized at approximately postnatal day (PND) 56 at the Jackson Laboratory (service No. SRG0046) and arrived at the NCTR on PND 62. Mice were individually housed in standard polycarbonate cages with ad lib access to food (NIH-41 open formula rodent irradiated diet) and water in a vivarium with a 12/12 h light/dark schedule (lights on at 0900) and temperature of 22 ± 1 °C and 45%–55% humidity. The NCTR is AAALAC accredited and all animal procedures were approved in advance by the NCTR Institutional Animal Care and Use Committee.
Treatment
After a 2-week acclimation period, each mouse was assigned to 1 of the 4 treatment groups based on body weight such that each group had approximately the same average weight. Treatment groups were administered 2 mg/kg DOX hydrochloride (LKT Laboratories, Inc., St. Paul, Minnesota) (DOX; n = 20), 50 mg/kg CYP monohydrate (Sigma Aldrich, St. Louis, Missouri) (CYP; n =20, but see below), 2 mg/kg DOX and 50 mg/kg CYP (DOX + CYP; n = 20), or saline vehicle control (SAL; n = 20). Drugs were mixed in sterile physiological saline. One mouse in the CYP group died shortly after the first injection due to events unrelated to treatment; thus, the CYP group contained 19 subjects. New dosing solutions were mixed every 4 weeks. Dose certification analyses were performed on each dose via HPLC-PDA by the Division of Biochemical Toxicology/NCTR. All doses were determined to be within 89%–110% of the targeted concentrations.
Doses were selected based on the typical human chemotherapeutic doses and the study focus. Typical CYP doses for breast cancer treatment range from 1 to 5 mg/kg (see http://www.empr.com/cyclophosphamide/drug/2059/; last accessed July 1, 2017). For a mouse, the CYP dose of 50 mg/kg (150 mg/m2) is equivalent to a human dose of 4.1 mg/kg (151.7 mg/m2). The typical DOX dose for human breast cancer patients is 40–75 mg/m2 (https://www.drugs.com/dosage/doxorubicin.html; last accessed July 1, 2017). The chosen DOX dose of 2 mg/kg (6 mg/m2) is equivalent to a human dose of 0.16 mg/kg (or 5.9 mg/m2), slightly below what is typically given to breast cancer patients. However, in previous studies of mice treated IV with DOX (Desai et al., 2013, 2014), there were significant indications of toxicity at a cumulative dose of 3 mg/kg (here, the cumulative DOX dose is 8 mg/kg); specifically, platelet counts were elevated. Furthermore, body weight was decreased by a cumulative dose of 6 mg/kg and a cumulative dose of 12 mg/kg caused indications of cardiotoxicity (ie, elevated cardiac troponin T plasma levels) as well as decreased organ weights. Because the focus here is on the potential long-lasting effects of DOX, it was essential that the mice survive 10 weeks post-treatment without severe toxicity. Thus, the selected weekly DOX dose was 2 mg/kg.
Beginning on PND 76, each mouse was injected IV weekly via 1 of the 2 lateral tail veins for 4 weeks (ie, on PNDs 76, 83, 90, and 97). Briefly, each was placed in a 39 °C animal warmer for 5 min to vasodilate the tail vein for better visualization prior to placement in a restrainer. The tail was swabbed with 70% ethanol and subsequently injected. Following injection, gentle pressure was applied to the tail vein injection site to ensure that the dosing solution was not lost. With the exception of normal animal husbandry and daily measurement of body weights and food and water intake, the mice were undisturbed during this time. On PNDs 98–170, each was tested on series of behavioral tasks (see the section “Behavioral Testing” and Table 1).
Table 1.
Behavioral Assessments and Test Ages
| Assessment | Ages (PNDs) | Days After Last Injection |
|---|---|---|
| Locomotor activity | 98–99, 127–128, 153–154 | 1–2, 30–31, 56–57 |
| Motor coordination and grip strength | 100, 129, 157 | 3, 32, 60 |
| Home cage behavior | 101–102, 125–126, 155–156 | 4–5, 28–29, 58–59 |
| Water maze performance | 104–108, 132–136 | 7–11, 35–39 |
| Buried food test | 113, 140, 168 | 16, 43, 71 |
| Object location memory | 115, 170 | 18, 73 |
| Novel object recognition | 142 | 45 |
| Barnes maze performance | 118–122, 146–150 | 21–25, 49–53 |
Body Weight, Food, and Water Intake
Body weight and food and water consumption were recorded daily during drug treatment (ie, PNDs 76–97) and weekly thereafter.
Behavioral Testing
Table 1 lists age at each behavioral assessment as well as the number of days after the last drug treatment (ie, the 4th injection). Most behaviors were assessed multiple times post-treatment. Anxiety-like behavior was assessed via behavior on an elevated plus maze and each subject was placed on the apparatus for a 5 min session at PNDs 111, 139, and 167. However, the low contrast of a dark mouse on a narrow black Perspex apparatus on the videorecordings prohibited accurate behavioral assessment for this task and it will not be described further.
Locomotor activity
The procedure for locomotor activity assessment has been described in detail (Ferguson et al., 2013, 2015 b). Briefly, each mouse was placed in 1 of the 12 Plexiglas chambers for 30 min on each of 2 consecutive days. Each subject was tested in the same chamber for the 3 test times (ie, PNDs 98–99, 127–128, and 153–154). Urine and feces were removed after each session but no alcohol or detergent was used. Horizontal and vertical (rearing) activities were automatically recorded via a 16×16 array of photobeams (PAS-Open Field, San Diego Instruments, San Diego, California) interfaced with a computer. Endpoints included total horizontal activity (sum of lower beam breaks/session), total vertical activity (sum of upper beam breaks/session), distance traveled/session (cm), and speed traveled/session (cm/s).
Motor coordination and grip strength
Motor coordination and grip strength were measured as described (Ferguson et al., 2015b). A Rotarod apparatus (AccuScan Instruments Inc., Columbus, Ohio) automatically increased revolutions in 20 s intervals from 0 to 4, 4 to 8, 8 to 12, 12 to 17, 17 to 22, and 22 to 25 rpm. Revolutions then gradually increased to 30 rpm during the next 180 s and subsequently slowed to 0 rpm during the last 30 s. Each subject received 3 consecutive trials at each of the 3 test times (ie, PNDs 100, 129, 157). A trial ended when the animal fell, or the final deceleration cycle concluded. Latency to fall (s), distance traveled (cm), and maximum rpm achieved were averaged for the 3 trials/test time prior to analysis.
Fore- and hind-limb grip strength was assessed subsequent to motor coordination testing. The tester allowed the mouse’s forepaws and then hindpaws to grasp bars attached to a strain gauge (Chatillon Model DPP, San Diego Instruments, San Diego, California), and the mouse’s body was then quickly pulled away. Each subject received 2 consecutive trials at each of the 3 test times. Force (kg) measured for the 2 forelimb and the 2 hindlimb trials was averaged for each prior to analysis.
Home cage behavior
Home cage behavior was assessed similar to that previously reported for rats (Ferguson et al., 2015a). Clean cages identical to the normal home cage were placed on a metal housing rack that was modified to ensure the cages remained stationary. A video camera faced the longest side of each cage. Infrared backlighting allowed dark period recording. Cameras were interfaced with computers with HomeCageScan software (CleverSys Inc., Reston, Virginia) which automatically categorized each subject’s behaviors in real-time. Each subject was placed into a test cage 2 h after the beginning of the light-period and behaviors were recorded for 24 h on PNDs 101–102, 125–126, and 155–156. The dark/light schedule in the test room was identical to that of the normal housing room and animals had ad lib access to food and water. Hourly durations of locomotion, immobility, “in place” activity, and rearing were used as primary endpoints for statistical analyses (see Supplementary Online Material Part 12 in Ferguson et al., 2015 b for a detailed description of these behaviors). Following each 24 h test session, subjects were returned to their original cages and housing room.
Water maze performance
Spatial learning and memory were assessed in a white circular water maze (119.4 cm diameter) similar to that previously described (Ferguson et al., 2013). A small white platform (6 cm diameter) was located 1 cm below the water surface and 30.2 cm from the tank wall in the northeast quadrant for the first test time (ie, PNDs 104–108) and in the southwest quadrant for the second test time (ie, PNDs 132–136). The water was made opaque with white nontoxic paint (Crayola, Easton, Pennsylvania) and maintained at 24°C. Clearly visible large cues were located on the test room walls, and the human tester remained in the same location throughout each test trial. An overhead camera interfaced with a computer and software (Water 2100, HVS Image, UK) recorded each subject’s behavior.
Each test time consisted of 4 consecutive sessions of 3 trials/day (ie, acquisition) followed on the fifth day by a single probe trial. During acquisition, each mouse was gently placed in the water maze facing the wall from 1 of the 7 locations excluding the location immediately next to the platform (ie, northeast and southwest starting locations were excluded for the first and second test times, respectively). If the subject failed to find the platform within the maximum allotted 120 s, it was gently placed onto the platform for 20 s. If the subject found the platform, it remained there for 20 s. Between each trial, each subject was placed in a clean housing cage lined with dry towels. Intertrial intervals were 210 s. After each trial, any feces were removed from the maze and the water was gently stirred to dissipate potential odor cues around the platform. The platform was removed during the probe trial on the fifth day and each subject was released from the location opposite the former location of the platform (ie, southwest for the first test time and northeast for the second test time) and behaviors were recorded for 60 s.
Acquisition endpoints included latency to reach the platform (or the maximum trial duration of 120 s if the animal failed to locate the platform), path length (m), swim speed (m/s), percentage time floating (duration of swim speeds less than 0.05 m/s), and percentage time in thigmotaxis behavior (duration spent 11.9 cm or less from the wall). Endpoints were averaged over the 3 daily trials during the 4 acquisition days prior to analysis. During the probe trial, percentage time spent in the formerly correct quadrant of the water maze, number of crossings over the former platform location, and swim speed (m/s) were recorded.
Buried food test
Olfaction and food motivation were assessed on PNDs 113, 140, and 168 via the buried food test as described (Yang and Crawley, 2009). To habituate subjects to the reinforcer, a small piece of breakfast cereal (Kroger Fruit Rings Cereal, Kroger, Cincinnati, Ohio) was placed in each subject’s home cage for 2 consecutive days prior to the test. Each cage was inspected the following morning to ensure complete consumption. On the afternoon prior to testing, all food was removed from each subject’s home cage. On the following day, each subject was allowed 5 min to habituate after being placed in a clean cage with 3 cm of fresh hardwood bedding. The subject was briefly removed and one-fourth of a cereal piece was buried in 1 of the 4 cage corners 1 cm below the bedding level which was then smoothed even. The subject was placed back into the test cage and latency to retrieve and begin eating the cereal piece was recorded by a trained observer blind to treatment conditions (max 10 min).
Object location memory and novel object recognition
Object location memory was assessed similarly to that described (Vogel-Ciernia and Wood, 2014). The testing apparatus consisted of 1 of the 4 black Plexiglas chambers 41 ×35×49 cm. Each chamber was illuminated with an overhead 25 W incandescent bulb in addition to ambient fluorescent room lighting which produced an average illumination of 99.75 lux at the apparatus floor. Four magnets were located beneath the floor in the corners of each chamber (10 cm from each neighboring wall) which allowed the test objects to be firmly secured to the floor. Inverted glass votive candle holders (item No. 424472, Koyal Wholesale, Fullerton, California) with the opening covered with a Plexiglas piece and magnet served as objects. Between trials, the testing apparatus and objects were cleaned with 70% ethanol and allowed to dry.
On the day prior to testing, each subject was placed in an empty apparatus chamber for 5 min for habituation. The following day, each subject was placed in the same chamber which now had 2 identical objects on 1 side of the chamber and allowed to explore for 10 min after which, each was returned to its home cage for 1.5 h. One object was then moved 15 cm to the other side of the chamber and the subject was again placed in the chamber and allowed to explore for 10 min. Object locations and which was designated as stationary or relocated were randomized and balanced across treatment groups. Each subject was tested in a different but identical apparatus chamber on the second object location memory test time (ie, PND 170). Thus, there were 3 exposures to the apparatus chambers at each test time: A 5 min habituation trial followed on the subsequent day by a 10 min familiarization trial with 2 identical objects and then 1.5 h later, the 10 min test trial in which 1 of the objects was relocated. Each test trial (ie, the trial when 1 object was relocated) was video recorded with an overhead camera interfaced with a computer. TopScan software (CleverSys Inc., Reston, Virginia) automatically recorded the duration of sniffing each object via the software’s “sniffing module”. The percentage preference for the relocated object was calculated as: ([duration sniffing the relocated object]/[duration sniffing the relocated object + duration sniffing the stationary object]) ×100. Because the vast majority of activity occurred during the first half of the test trial, only the first 5 min were analyzed.
Novel object recognition was assessed using the same testing apparatus chambers and methods except that in the test trial, objects remained in the same locations but one of the glass votive candle holders was replaced with an interlocked set of square Lego type blocks (Mega Bloks, Target, Minneapolis, Minnesota) which had a magnet and Plexiglas piece glued to the bottom. Duration of sniffing each object was recorded and percentage preference for the novel object was calculated as: ([duration sniffing the novel object]/[duration sniffing the novel object + duration sniffing the familiar object]) ×100. Similar to what was done for object location memory testing, only the first 5 min of the 10 min test trial were analyzed.
Barnes maze
Spatial learning and memory were assessed using a Barnes maze in a manner similar to that described for rats (Ferguson et al., 2015a; Johnson et al., 2016; Rosenfeld and Ferguson, 2014). The maze consisted of a round white Plexiglas surface 92 cm in diameter with 20 holes (each 5.5 cm diameter) evenly spaced around the perimeter. For each subject, one of the holes led to a small dark escape box and the remainder had false bottoms. Location of the escape hole was pseudorandom and balanced across treatment groups. Three overhead 500 W halogen lamps and the ambient fluorescent room lighting produced an illumination of 3680 lux at the maze surface. For habituation and on the first day only of each of the 2 test times, each subject was placed into the escape box which was then covered for 2 min. Subsequently, the subject was then placed in an opaque tube (16 cm diameter) in the center of the maze. Lifting of the tube initiated the trial. If the subject failed to find the escape box within the maximum allotted 300 s, it was gently guided to it and allowed to remain inside for 15 s before being returned to its home cage. If the subject located the escape box, it remained inside for 15 s before being returned to its home cage. The 4 subsequent days of testing were conducted in the same manner but without the 2 min habituation period inside the escape box. Trials were videorecorded by an overhead camera interfaced with a computer. TopScan software (CleverSys, Reston, Virginia) calculated latency to reach the escape box (s), distance traveled (m), and speed (m/s).
Golgi Staining and Tissue Preparation
Subjects were sacrificed 30 min after the last object location memory test on PND 170. Brains were removed immediately following cervical dislocation. Half brains (n = 4/treatment group) were subjected to Golgi-Cox staining. Samples were immersed in mercuric chloride solution for impregnation for 2 weeks and subsequently immersed in postimpregnation buffer for 2 days. Half brains were then cut at 200 µm in 1× phosphate buffered saline (PBS) using a microtome. Samples were placed into wells and washed with 0.01 M PBS buffer (pH 7.4) with Triton X-100 (0.3%; PBS-T) and subsequently stained with ammonium hydroxide solution and then immersed in a poststaining buffer (superGolgi kit, Bioenno Tech, LLC, Santa Ana California). Sections were then washed in PBS-T and mounted on 1% gelatin-coated slides and allowed to dry. Sections were then dehydrated with ethanol solutions, followed by cleaning in xylenes, and coverslipped with Permount (Fisher Scientific, Pittsburgh, Pennsylvania).
Spine Density and Spine Morphology
Spine analyses were conducted blind to treatment conditions on coded Golgi impregnated brain sections containing the dorsal hippocampus. Spines were examined on dendrites of dentate gyrus granule neurons as well as apical (stratum radiatum) and basal (stratum oriens) dendrites of CA1 and CA3 pyramidal neurons. Neurons that satisfied the following criteria were selected for analysis in each of the treatment groups: (1) presence of untruncated dendrites; (2) consistent and dark Golgi staining along the entire extent of the dendrites; and (3) relative isolation from neighboring neurons to avoid interference with analysis (Titus et al., 2007). Three to 5 dendritic segments, each at least 20 nm in length (Magarinos et al., 2011), were analyzed per neuron, and 6–7 neurons were analyzed per brain. Neurons that met staining criteria were traced using a 60× oil objective, a computerized stage, and Neurolucida software (Ver. 11, MBF Bioscience, Inc., Williston, Vermont).
Antioxidant Analysis
To gauge oxidative stress in the hippocampus, the antioxidant activity of superoxide dismutases (SODs) and total glutathione (GSH) and glutathione disulfide (GSSG) levels were measured. The percentage of GSSG was calculated from the total levels of GSH and GSSG. Superoxide dismutase activity was measured in cell homogenates as previously described (Spitz and Oberley, 1989). MnSOD activity was measured as previously described on hippocampal homogenates prepared on ice in 50 mM potassium phosphate buffer. MnSOD activity is measured based on the competition between MnSOD and an indicator molecule (nitroblue tetrazolium) that reacts with superoxide generated by xanthine/xanthine oxidase in the presence of 5 mM sodium cyanide to inhibit CuZnSOD activity (McCord and Fridovich, 1969). Total glutathione (GSH) levels were determined in a recycling assay that spectrophotometrically measures the reduction of Ellman’s reagent (DTNB) (Sigma-Aldrich Corp., St. Louis, Missouri) to 2-nitro-5-thiobenzoate (TNB) in the presence of glutathione reductase (Sigma-Aldrich Corp.) as described (Anderson, 1985; Griffith and Meister, 1979). Biochemical data were normalized to protein content as previously described (Lowry et al., 1951).
Chemokine and Cytokine Analysis
Hippocampi samples were homogenized in 200 µl of ice-cold PBS with cOmplete Protease Inhibitor Cocktail Tablets (Roche, Switzerland) added. Homogenized samples were centrifuged at 500× g for 10 min at 4°C. Supernatants were collected and centrifuged at 15 000×g for 5 min at 4°C. Supernatants were collected and shipped to Quansys Biosciences (Logan, Utah).
Analysis of cytokine biomarkers was assessed using the Q-Plex Mouse Cytokine Screen (Quansys, Logan, Utah), which utilizes enzyme-linked immunosorbent assays (ELISA)-based chemiluminescence. Cytokines evaluated include Interleukins (IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-10, IL-12p70, IL-17), monocyte chemoattractant protein-1 (MCP-1), interferon gamma (IFNγ), tumor necrosis factor alpha (TNF-α), macrophage inflammatory protein 1 alpha (MIP-1α), granulocyte macrophage-colony stimulating factor, and the regulated on activation, normal T-cell expressed and secreted chemokine (RANTES). The concentration of each cytokine was quantified with the Q-View Software (Quansys). Quansys extrapolated any values that outside the standard curve. Cytokine data are expressed as picograms per milliliters protein.
Data Analysis
Data for measures of body weight, food and water intake, motor coordination, grip strength, water maze probe trial, buried food test, and object location memory were analyzed using repeated measures analyses of variance (ANOVA) with drug treatment, day or test time, and the interaction as factors. Novel object recognition endpoints were analyzed using one-way ANOVAs with drug treatment as the independent variable. For object location memory and novel object recognition, single sample t-tests determined if the percentage preference for the relocated or novel object differed from zero. Locomotor activity, home cage behavior, water maze acquisition, and Barnes maze performance were analyzed using a generalized repeated measures linear model with drug treatment, test time, and session or trial nested within test time as factors. Spine morphology and antioxidant activity were analyzed via one-way ANOVAs with drug treatment as the independent variable. Cytokine levels were analyzed with independent t-tests. All analyses were conducted with α = 0.05 and significant effects were followed up by post hoc comparisons or analysis of simple effects as appropriate. All analyses were conducted using SAS 9.4 (SAS Institute, Inc., Cary, North Carolina).
RESULTS
Body Weight
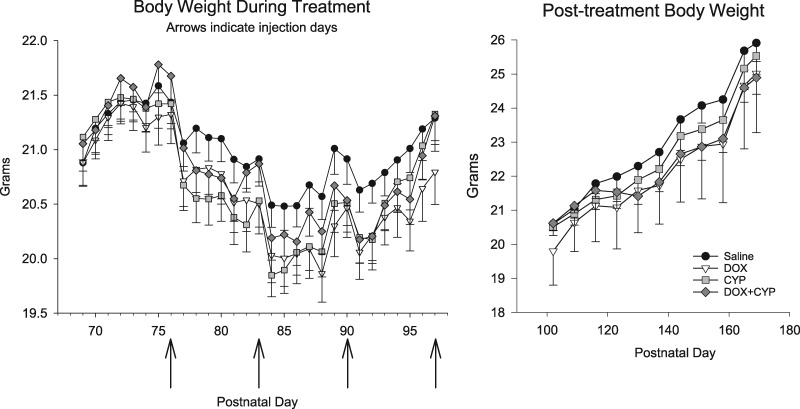
During the drug treatment period, there was a significant PND by drug treatment interaction (F[84, 1960] = 2.14, p = .003) (see Figure 1, left). In general, all groups exhibited minor weight loss following each injection although this was somewhat less so for the SAL group. The greatest difference between groups was less than 0.5 g and analysis of simple effects failed to detect any significant differences; thus, the differences are unlikely to be clinically meaningful. After drug treatment (ie, during behavioral testing), there were no significant effects involving drug treatment (see Figure 1, right). However, there was a significant main effect of PND (F[10, 750] = 334.9, p < .0001), indicating all groups gained weight throughout the study.
Figure 1.
Mean (±SEM) body weights of mice during the SAL, DOX, CYP, or DOX + CYP treatment (left) and after treatment ended (right). Arrows on left abscissa indicate the 4 treatment times. Group sizes were 20 each except for the CYP group which contained 19 subjects.
Food and Water Intake
Analysis of food intake during drug treatment indicated a significant main effect of PND and a marginally significant PND by drug treatment interaction (F[81, 1404] = 1.46, p = .057) (data not shown). All groups consumed less food in the 24 h following each injection and gradually increased consumption until the next treatment time. Following the first injection, CYP-treated mice consumed less food than the SAL group. Prior to the second and fourth injections, the DOX + CYP group consumed more food than SAL-treated mice. Otherwise, no treatment group differed from the SAL group. Food intake during behavioral testing was not affected by prior drug treatment, but there was a main effect of week on average food intake (F[11, 748] = 55.03, p < .001). There was some week-to-week variation in the amount of food consumed, but the greatest observed difference between weeks was only 1.16 g. Analysis of water intake during drug treatment indicated a significant main effect of PND (F[18, 1674] = 16.67, p < .001) (data not shown). Although water intake varied day to day, the greatest difference between days was only 2.28 ml, and there was no clear pattern to the variation. Likewise, water intake was not affected by drug treatment during behavioral testing, but there was a significant main effect of week on average intake (F[24, 488] = 72.69, p < .001). Generally there was little variation in water intake, but during the final week of testing there was a decrease in water intake.
Locomotor Activity
Supplementary Table 1 shows distance traveled and vertical activity for each treatment group at each test time. Analysis of horizontal activity indicated a significant drug treatment by test time interaction (F[6, 150] = 3.51, p = .003) and a significant effect of test time within day (F[3, 213] = 17.12, p < .0001) (data not shown). However, no treatment group differed significantly from the SAL group at any of the 3 test times. That significant interaction effect reflected a more general decrease in activity across test times. For all other endpoints, there were no significant effects involving drug treatment. Analysis of distance traveled indicated a significant effect of test time and day nested within test time (F[3, 213] = 28.22, p < .0001) (see Supplementary Table 1). During the first test, distance traveled was significantly less on the second day which clearly indicated habituation. During the 2 subsequent tests, distance traveled decreased further. A similar pattern was seen for speed with a significant day within test time effect (F[3, 213] = 11.3, p < .0001) which indicated decreasing speed as testing progressed (data not shown). There was a significant effect of day within test time (F[3, 213] = 24.96, p < .0001) detected in the analysis of vertical or rearing activity and this effect indicated that all groups reared less during the second and third test times (see Supplementary Table 1).
Motor Coordination and Grip Strength
There were no significant effects involving drug treatment on latency to fall, distance traveled, or maximum rpm, but there was a significant main effect of test time on each of those endpoints (all F’s > 7.0 and p’s < .0001) (data not shown). In general, all groups improved their performance over test times; that is, longer latencies, increased distance traveled and increased maximum rpm. There were no significant effects of drug treatment, test time, or the interaction on measures of forelimb or hindlimb grip strength (all F’s < 2.31 and p’s > .13; data not shown).
Home Cage Behavior
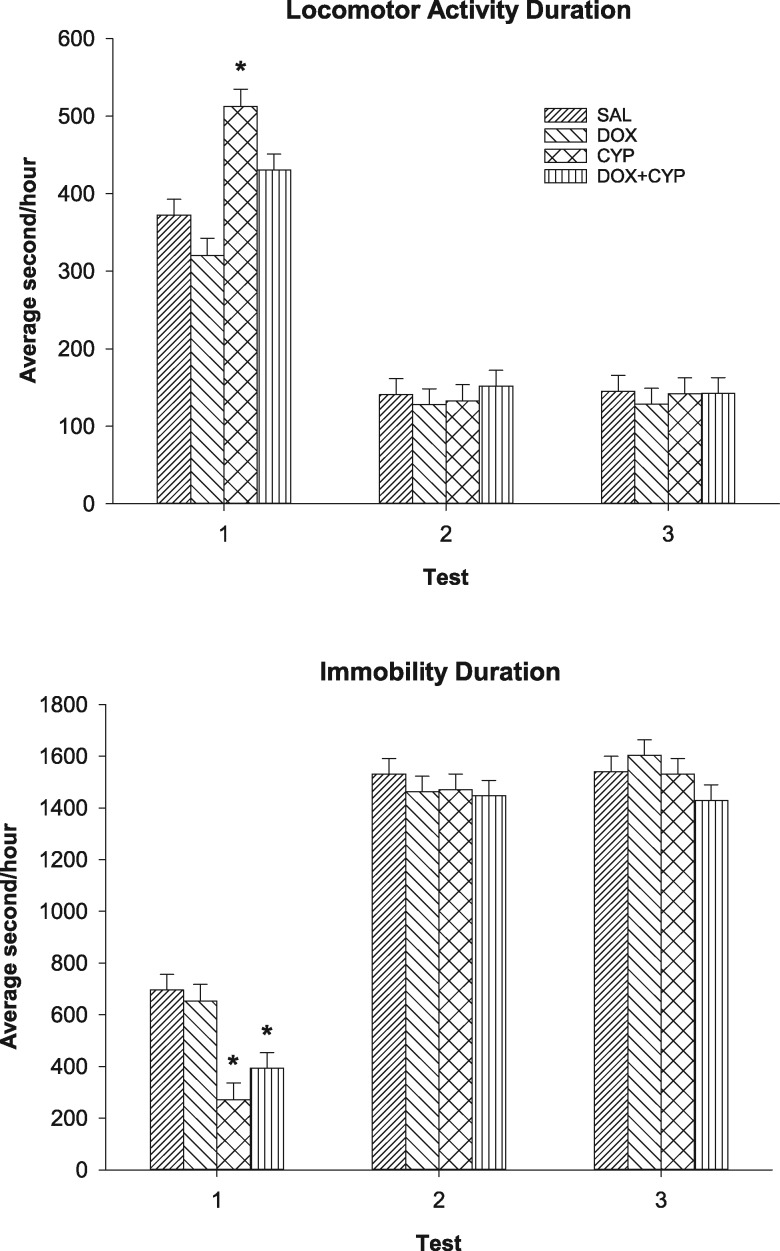
There was a significant drug treatment by test time interaction for locomotion duration (F[6, 145] = 4.62, p < .001) (see Figure 2, top). During the first test time, the CYP group exhibited longer locomotion durations than the SAL group (p < .05). During the second and third test time, no group differed significantly from the SAL group. There was also a significant effect of hour nested within test time (F[69, 5060] = 58.6, p < .0001) for locomotion duration. All groups exhibited longer durations during the first test time for most hours, except between 3 pm and 9 pm (the latter half of the light cycle). For the most part, there were no significant differences between the second and third test times. Likewise, there was a significant drug treatment by test time interaction for immobility duration (F[6, 145] = 3.05, p = .008) (see Figure 2, bottom). During the first test time, the CYP and DOX + CYP groups exhibited decreased immobility durations than the SAL group (p < .05). All groups had higher immobility durations during the second and third test times, but there were no significant effects involving drug treatment. Additionally, there was a significant effect of hour within test time (F[69, 5060] = 23.96, p < .0001) for immobility duration. Generally, all groups were more immobile during the second and third test times. From 1 am to 6 am (the latter portion of the dark phase), all groups were more immobile during the third test time than the second. Analysis of “in place” activity durations indicated a significant drug treatment by test time interaction (F[6, 145] = 2.64, p = .02); however, no drug treated group differed from the SAL group (data not shown). “In place” activity durations were greater during the second and third test times than the first. The effect of hour within test time was significant for “in place” activity durations (F[69, 5060] = 29.39, p < .0001). There were increased durations of “in place” activity during the second and third test times relative to the first, and all groups displayed increased “in place” activity during the first 2 h of the first test. There was a marginally significant main effect of drug treatment for rearing (F[3, 75] = 2.47, p = .068), indicating that CYP and DOX + CYP groups reared somewhat more than the SAL group (data not shown). Further, there was a significant effect of hour within test time (F[69, 5060] = 60.18, p < .0001). All groups reared more during the first test time than the second and third test times for the initial 3 h of testing and the first four hours of the dark phase. Otherwise, there were no significant differences between tests at any given hour.
Figure 2.
Mean (±SEM) home cage behavior during each of the 3 test times. Top: Duration of locomotor activity. During the first test time, the CYP group was more active than the SAL group (*p < .05). There were no statistically significant effects during the second and third test times. Bottom: Duration of immobility. During the first test time, the CYP and DOX + CYP groups were less immobile than the SAL group (*p < .05). There were no statistically significant effects during the second and third test times.
Water Maze Performance
Supplementary Table 2 shows latency, path length, and time in correct quadrant for each treatment group at each test time. During acquisition, there were no significant effects involving drug treatment on latency to reach the platform. A significant day within test time effect (F[6, 447] = 12.86, p < .001) indicated that latencies for all groups were less on days 3 and 4 of the second test time compared with the same days of the first test time. Analysis of path length indicated a significant drug treatment by test time interaction (F[3, 75] = 3.25, p = .027), but no drug treated group was significantly different from the SAL group. There was a significant day within test time effect for path length (F[6, 423] = 18.75, p < .001) which was very similar to that of the latency analysis. Analysis of swim speed did not indicate any drug treatment effects, but there was an effect of day within test time (F[7, 498] = 5.60, p < .001) which indicated that all groups swam slower on the first day of the first test time than subsequent days (data not shown). There was a significant main effect of treatment effect on the percentage of time floating (F[3, 75] = 3.16, p = .03), but no drug-treated group differed significantly from the SAL group (data not shown). Again, there was a significant effect of day within test time (F[7, 498] = 4.67, p < .001) which indicated that all groups floated more on the first day of the first test time than other days. There was a significant main effect of drug treatment effect on the percentage of time in thigmotaxis (F[3, 75] = 3.55, p = .018) indicating that the CYP group exhibited more thigmotaxis than the SAL group (data not shown). However, the analysis of percentage of path length that was thigmotactic did not indicate the same effect (F[3, 75] = 0.68, p = .57). For both measures, there was a significant day within test time effect (F’s = 3.87 and 47.20, respectively) as all groups displayed more thigmotactic behavior on the first day of the first test time than other days.
There were no significant effects of drug treatment during the probe trials. Significant test time effects for percentage of time in the previously correct quadrant (F[1, 75] = 5.41, p = .02) indicated that all groups spent more time in the previously correct quadrant during the probe trial of the second test time relative to the first test time (see Supplementary Table 2). There were no effects of drug treatment, test time, or the interaction on the number of crossings of the platform’s prior location (F’s < 2.05) (data not shown). There was no effects involving drug treatment for swim speed during the probe trial, but there was an effect of test time (F[1, 75] = 21.41, p < .001) with mice swimming faster during second test time (data not shown).
Buried Food Test
Analysis of latency to find the cereal piece indicated a trend toward a drug treatment effect (F[3, 51] = 2.497, p = .07) (data not shown). Although not statistically significant, mice treated with CYP or DOX + CYP took longer to find and consume the food piece. There were no significant differences between the 3 test times on this measure.
Object Location Memory and Novel Object Recognition
Overall performance on the object location memory task was generally poor with no group exhibiting a strong preference for the relocated object. There was no significant effect of drug treatment, but there was a test time effect (F[1, 75] = 5.34, p = .02) which indicated all groups performed slightly better on the second test time (data not shown). Likewise for the novel object recognition task, single sample t-tests indicated that there was no significant preference for the novel object and no effect of drug treatment (data not shown).
Barnes Maze
Supplementary Table 3 shows latency and distance traveled by group and test time. There were no significant effects involving drug treatment on latency to reach the escape box or distance traveled. For both measures, there were significant effects of day within test time (F[8, 598] = 8.57 and 14.32, respectively, p < .001). All groups exhibited shorter latencies and distance traveled on all days of the second test time relative to the first test time. Likewise, there was no effect of drug treatment on speed, but there was a significant effect of day nested within test time (F[8, 598] = 9.69, p < .001) (data not shown). There were no differences between tests on the speed traveled except on the last day of testing when mice were slower during the second test time than the first.
Changes in Spine Density and Spine Morphology
Dentate gyrus
Overall spine density in the DG was not altered by treatment (F[3, 28] = 2.39, p = .09; Table 2). Analyses of the density of different spine types indicated a significant main effect of drug treatment on the density of stubby spines (F[3, 28] = 5.91, p < .01; Table 2). Relative to the SAL group, all treatment groups exhibited a significant decrease in stubby spine density (DOX, p < .01; CYP, p < .01; DOX + CYP, p < .05). There were no significant effects detected in the analyses of the densities of mushroom spines or thin spines.
Table 2.
Total Spine Density and Spine Density Type in the Dentate Gyrus (mean ± SEM)
| Type | SAL | DOX | CYP | DOX + CYP | p Value |
|---|---|---|---|---|---|
| Total spine density | 20.21±0.42 | 20.78±0.41 | 22.08±0.60 | 20.47±0.67 | .09 |
| Thin | 55.51±1.46 | 58.37±2.07 | 57.09±1.60 | 54.62±1.79 | .45 |
| Stubby | 45.88±3.60 | 32.51±2.15 | 33.97±1.75 | 35.66±2.03 | <.01a |
| Mushroom | 9.95±0.49 | 9.30±0.71 | 8.94±0.53 | 9.72±0.48 | .59 |
DOX, CYP, and DOX + CYP groups were significantly less than the SAL group.
CA1 and CA3 pyramidal dendrites
Spine type and density for the CA1 and CA3 apical and basal cells for each treatment group are shown in Supplementary Table 4. In CA1 apical pyramidal dendrites, there were no significant effects in the analysis of the overall density of spines. In addition, analysis of spine type did not indicate significant effects in thin, stubby, or mushroom spines. In the basal pyramidal dendrites of the CA1 region, overall spine density was also unaffected after treatment. Likewise, there were no significant effects in the analyses of thin, stubby, or mushroom spines.
In the CA3 apical pyramidal dendrites, there were no significant effects in the overall density of spines. In addition, analyses of spine types did not indicate any significant effects in thin and stubby spines but there was a marginally significant effect for mushroom spine density (F[3, 12] = 3.09, p = .06). Similar to that observed in CA3 apical spine analysis, drug treatment did not significantly affect the overall density in CA3 basal spines. Likewise, there were no significant effects in the analyses of thin, stubby, or mushroom spine densities.
Antioxidant Assays
Drug treatment did not significantly affect levels of GSH or GSSG (data not shown). Analysis of MnSOD levels did not indicate a significant drug treatment effect, but CuZnSOD levels were significantly increased in the DOX + CYP group relative to the SAL group (data not shown).
Hippocampal Cytokine/Chemokine Assays
Supplementary Table 5 shows the levels of 13 hippocampal cytokines. Due to assay costs, available tissue amounts, and few significant behavioral effects, only samples (n = 6) from the DOX + CYP and SAL groups were assayed. Levels of IL-1β (t = 4.55, p < .01), IL-6 (t = 2.50, p < .05), and IL-12 (t = 2.79, p < .05) in DOX + CYP-treated mice were significantly decreased compared with SAL-treated mice. Levels of 3 analytes (IL-17, MIP-1α, and RANTES) were too low for further analysis. For the remaining 10 analytes, cytokine/chemokine levels were not significantly different between DOX + CYP- and SAL-treated mice.
DISCUSSION
Chemotherapeutic-induced cognitive alterations are reported to be relatively common and have been best described for women with breast cancer. To better understand such potential deficits, ovariectomized female mice were treated IV with DOX, CYP, or DOX + CYP and a wide range of behaviors was assessed as well as hippocampal spine densities, brain antioxidant activity, and hippocampal cytokine levels. Body weight was acutely decreased by the injections, but food and water intake were only mildly affected. Overall, there were few significant behavioral alterations and only stubby spine density in the dentate gyrus and hippocampal IL-1β, IL-6, and IL-12 levels were affected by drug treatment. These results suggest that, at these doses of 2 common chemotherapeutics, there are few significant effects in the endpoints measured here.
Every attempt was made to decrease the stress associated with the tail vein injections; however, body weight of all groups decreased in the days following treatment. Body weights subsequently recovered and there were no long-term effects. Others have reported no effect of CYP (Janelsins et al., 2016) or DOX + CYP (Philpot et al., 2016) treatment on murine body weight, although some have found significant reductions following treatment with DOX (Desa et al., 2013; Zombeck et al., 2013), CYP, or DOX + CYP (Seigers et al., 2015).
There were few significant behavioral effects of drug treatment. Specifically, there were no effects on open field locomotor activity, motor coordination, grip strength, or buried food test performance. A general increase in activity was observed in mice treated with CYP or DOX + CYP during home cage monitoring at the first test time but not subsequent times. Doxorubicin (Zombeck et al., 2013) and DOX + CYP + 5-fluorouracil (Rendeiro et al., 2016) treatment were reported to have no effects on the open field activity of mice, but DOX (Lira et al., 2016) and DOX + CYP (Salas-Ramirez et al., 2015) treatments were shown to decrease the open field activity of rats. Similar to our findings, others have described unaltered motor coordination in rodents after chemotherapeutic treatments (Bon et al., 2003; Iarkov et al., 2016; Rendeiro et al., 2016; Zombeck et al., 2013). There appears to be only 1 study that examined the effects of DOX or CYP treatment on grip strength. Contrary to the results in mice described here, 10 mg/kg DOX IV in nonovariectomized (intact) female rats reduced both hind- and forelimb grip strength and resulted in a 10% decrease in muscle mass (Maurissen et al., 2003). This is likely due to the substantially higher dose of DOX in that study. We are not aware of previous reports of chemotherapeutic effects on olfaction or buried food test performance in rodents. Although we describe here an initial increased activity in the home cage activity assessment, it has been reported that DOX treatment decreased running on a residential running wheel (Zombeck et al., 2013) and DOX and CYP treatment decreased home cage activity (Seigers et al., 2015). Increased home cage activity was observed during the first test time but a similar increase was not apparent in open field activity which likely reflects the enhanced fidelity afforded by a 24 h observation period in a familiar environment.
Here, there were no significant effects of chemotherapeutic treatment on spatial memory in water maze or Barnes maze performance, but all groups exhibited performance improvements during the second test time. Previous reports of DOX and CYP treatment effects on water maze performance are varied. Mice that were trained in the water maze prior to DOX + CYP treatment showed no deficits during re-training of the task, although females, but not males, displayed recall deficits during a probe trial (Philpot et al., 2016). Likewise, ovariectomized female mice treated with DOX + CYP + 5-fluorouracil exhibited water maze acquisition and recall deficits (Rendeiro et al., 2016). Further complicating the literature, CYP treatment was reported to improve the water maze performance of young, but not old, rats (Lee et al., 2006) although CYP + 5-fluorouracil treatment had no effect in rats (Long et al., 2011). It was reported that CYP + DOX treatment had no effects on Barnes maze performance of male mice, but in a version of a “probe trial” the treated mice spent less time in the previously correct area (Seigers et al., 2015). Although the drug treatments here did not induce spatial memory impairments, the significant behavioral improvements between the first and second test times allow a measure of confidence in these results. That the platform and escape box were relocated between the test times and the subjects displayed improved performance during the second test time is likely due to prior experience with the test apparatus suggesting that they retained some memory for the environments and tasks over the 3-week inter-test time.
Performance on the object location memory and novel object recognition tasks was relatively poor. For the most part, no group displayed preference for the relocated or novel object. In fact, the mice spent the vast majority of the session in the corners of the apparatus. For example, in the novel object recognition task, the duration of novel or familiar object investigation was only 7.2% (M = 21.68 ± 1.20 s) of the analyzed portion of the recognition trial. Similarly, others have reported that male mice treated with DOX or CYP did not perform significantly better than chance on these tasks (Seigers et al., 2015). In rats, the effects of DOX or CYP treatment are more complex with some studies reporting deficits in object location memory (Iarkov et al., 2016; Kitamura et al., 2017; Salas-Ramirez et al., 2015) whereas others reported no effects on the same task (Lyons et al., 2011) or novel object recognition (Salas-Ramirez et al., 2015). Further, some have suggested that ovariectomized mice perform poorly on these tasks even in the absence of drug treatment (Frye and Walf, 2008; Walf et al., 2008).
Here, CYP, DOX, and CYP + DOX treatments decreased the density of stubby spines in the dentate gyrus, which could hamper the management of previously acquired information (Gonzalez-Burgos et al., 2007). Previous studies have shown that chemotherapeutic treatment can alter the number and morphology of dendritic spines (Andres et al., 2014; Groves et al., 2017; Lomeli et al., 2017; Zhou et al., 2016), and CYP treatment has been shown to reduce dendritic spine length, volume, and complexity in the dentate gyrus of athymic nude rats (Acharya et al., 2015). Dendritic spines are important for the transfer of information between neurons and are the sites of neurotransmitter exchange (Andersen, 1990; Von Bohlen und Halbach et al., 2006). Their numbers can be indicative of the amount of information that is transferred between neurons (Leuner and Shors, 2013), and the various types of spines are characteristic of the synapse strength. For example, mushroom and stubby spines allow for more receptor anchoring. Because of their strong excitatory potential, mushroom and stubby spines are more stable, and are able to last for longer periods of time, thus earning the role of memory spines (Harris and Stevens, 1989; Peters and Kaiserman-Abramof, 1970). Given the lack of drug effects on the spatial memory tasks here, it is difficult to interpret the stubby spine density decrease and this effect awaits replication.
There were no significant changes due to drug treatment in levels of GSH, GSSG, or MnSOD. However, DOX + CYP treatment caused a significant increase in CuZnSOD levels, which are likely from cytosolic sources. This could be a response to increased levels of superoxide. However, we were unable to measure hippocampal levels of reactive species due to limited tissue amounts. Both CYP and DOX treatment are associated with oxidative stress. For example, CYP and its metabolites have been linked to antioxidant depletion (Crook et al., 1986a,b; Lind et al., 1989), and CYP administration in mice caused oxidative damage (ie, lipid peroxidation) (Bhatia et al., 2006). DOX administration can induce peripheral cytokines which can cross into the CNS. Once in the CNS, local cytokine production occurs which may lead to oxidative imbalance (Aluise et al., 2010; Gutierrez et al., 1993; Osburg et al., 2002). DOX treatment has been reported to increase TNF-α in murine cortex and hippocampus (Tangpong et al., 2006; Usta et al., 2004), which can lead to overproduction of reactive species (Szelenyi, 2001; Tangpong et al., 2008). Similarly, increases in several hippocampal cytokines, including IL-1β, were identified following 5-fluorouracil treatment in aged mice in which altered hippocampal dendritic complexity was also described (Groves et al., 2017). Here, hippocampal levels of IL-1β, IL-6, and IL-12 were decreased approximately 10 weeks after the last DOX + CYP treatment in ovariectomized mice. Although cytokines are multifunctional proteins acting in intricate networks and often performing dual roles, IL-1β, IL-6, and IL-12 are generally considered proinflammatory cytokines (Monastero and Pentyala, 2017; Park et al., 2017; Ramesh et al., 2013). In particular, IL-1β production is regulated by the inflammasome, a multiprotein oligomer component of the innate immune system, and plays a key role in acute and chronic inflammation (Ren and Torres, 2009). IL-12 is a ligand for the JAK-STAT signal transduction pathway inducing a chief proinflammatory signaling cascade (Monastero and Pentyala, 2017). When its receptor is located on the same cell, IL-6 can also promote inflammation via the JAK-STAT pathway (Monastero and Pentyala, 2017; Scheller et al., 2011). Although the results found here would appear to contradict much of the literature, it is important to note the time course following drug treatment and the relatively small decrease in observed cytokine levels. Although a rapid increase in proinflammatory cytokines following chemotherapy might be expected based on clinical literature, hippocampal levels may have diminished or cytokine soluble receptors may have upregulated following the weeks of behavioral testing. Given the likely role of inflammation in PCCI, it would be of interest to explore markers of reactive microglia in this model. A preliminary screen of hippocampal expression of 8 genes associated with reactive microglia (Cd68, Tnf, Il6, Nos2, Fcgr3, Mrc1, Arg1, and Chil3) using quantitative polymerase chain reaction indicated that drug treatment had no effects on the expression of most of these genes, but CD68 and Tnf expressions were decreased in CYP- and DOX + CYP-treated mice (data not shown). However, we were unable to fully explore this given the limits of available tissue.
It is important to note the potential limitations of our study. B6 mice were used as they are one of the most common and well characterized strains available. However, this strain may not have been the most suitable for determination of the cognitive effects of chemotherapeutics. For example, B6 and other strains of mice were more resistant to the pain-related behavioral changes following an acute CYP IP injection (Bon, Lichtensteiger, Wilson and Mogil, 2003), and CYP metabolism has been reported to differ between inbred strains of mice (Pukhalsky et al., 1990). On the other hand, B6 mice were described as more susceptible to the effects of the chemotherapeutic topotecan than FVB mice (Seigers et al., 2016). Although incorporation of multiple strains in any study is costly, the results would be methodologically informative and could reveal potential genetic elements of vulnerability or resistance to PCCI.
We examined neuronal morphology using Golgi staining, which requires a great deal of tissue as adjacent tissue sections are needed to reconstruct individual neurons. Therefore, the analyses here were limited by the available tissue, despite harvesting the brains of all mice in the study. Additional histopathological examinations would add to the knowledge of the effects of DOX and CYP on neuronal stress and survival at the local level. Although functional imaging is costly, future preclinical studies of the effects of chemotherapeutics in vivo in real time would be very valuable.
Given the few significant effects of DOX and CYP treatment here, it is possible that the doses were too low to elicit effects. This is unlikely to be the case for DOX treatment based on pharmacokinetics results (Collins et al., 1986; Gustafson et al., 2002); however, there is some indication that males may be more sensitive to certain DOX-induced toxicities (eg, cardiotoxicity) (Jenkins et al., 2016). Nevertheless, CYP has been shown to be metabolized more rapidly by mice than humans (Voelcker et al., 1984). Additionally, it may be worthwhile to perform baseline behavioral assessments prior to treatment which might detect very subtle cognitive effects of chemotherapy treatment. PCCI is a complex phenomenon occurring in the presence of the disease state itself, the psychological stress accompanying the disease (Hermelink et al., 2017), as well as the effects of the drugs used to treat the disease. Because PCCI affects some, but not all, patients suggests that multiple factors are at play. Further clinical work considering the effects of the disease state, the stress associated with cancer, and the doses and types of chemotherapeutic drugs are warranted. Those results can then guide the preclinical studies to examine mechanisms of and potential treatments for PCCI.
The current study was designed specifically to increase the translational nature of the results through use of an appropriate animal model of PCCI. Specifically, ovariectomized mice resembled and controlled for premature menopause that often occurs in breast cancer patients undergoing chemotherapeutic treatment. Two frequently used cytostatic agents were studied individually and in combination via an IV route at doses reflective of those used in humans to further mimic the clinical situation. Further, behavioral assessments continued for approximately 2 months after the last treatment to detect any long lasting or late emerging effects. Given the results here, we look forward to increased research in this area.
SUPPLEMENTARY DATA
Supplementary data are available at Toxicological Sciences online.
Supplementary Material
ACKNOWLEDGMENTS
We appreciate the careful chemical analyses performed by Dr Matthew Bryant of the Division of Biochemical Toxicology/NCTR. The authors are grateful for the technical expertise provided by the animal care staff of the Priority One Corporation and appreciate the careful study supervision by Mr C. Delbert Law of the Division of Neurotoxicology.
FUNDING
National Center for Toxicological Research (E07581) and the Center for Drug Evaluation of the Food and Drug Administration. Support also came from the Core Facilities of the UAMS Center for Translation Neuroscience IDeA program award P30 GM110702.
REFERENCES
- Acharya M. M., Martirosian V., Chmielewski N. N., Hanna N., Tran K. K., Liao A. C., Christie L. A., Parihar V. K., Limoli C. L. (2015). Stem cell transplantation reverses chemotherapy-induced cognitive dysfunction. Cancer Res. 75, 676–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahles T. A. (2012). Brain vulnerability to chemotherapy toxicities. Psycho-Oncology 21, 1141–1148. 10.1002/pon.3196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aluise C. D., Sultana R., Tangpong J., Vore M., St Clair D., Moscow J. A., Butterfield D. A. (2010). Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: Role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv. Exp. Med. Biol. 678, 147–156. [DOI] [PubMed] [Google Scholar]
- Amidi A., Wu L. M., Pedersen A. D., Mehlsen M., Pedersen C. G., Rossen P., Agerbæk M., Zachariae R. (2015). Cognitive impairment in testicular cancer survivors 2 to 7 years after treatment. Supportive Care Cancer 23, 2973–2979. [DOI] [PubMed] [Google Scholar]
- Andersen P. (1990). Chapter synaptic integration in hippocampal CA1 pyramids. Progr. Brain Res. 83, 215–222. [DOI] [PubMed] [Google Scholar]
- Anderson M. E. (1985). Determination of glutathione and glutathione disulfide in biological samples. Methods Enzymol. 113, 548–555. [DOI] [PubMed] [Google Scholar]
- Andres A. L., Gong X., Di K., Bota D. A. (2014). Low-doses of cisplatin injure hippocampal synapses: A mechanism for ‘chemo’ brain? Exp. Neurol. 255, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barcenas C. H., Niu J., Zhang N., Zhang Y., Buchholz T. A., Elting L. S., Hortobagyi G. N., Smith B. D., Giordano S. H. (2014). Risk of hospitalization according to chemotherapy regimen in early-stage breast cancer. J. Clin. Oncol. 32, 2010–2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatia A. L., Manda K., Patni S., Sharma A. L. (2006). Prophylactic action of linseed (Linum usitatissimum) oil against cyclophosphamide-induced oxidative stress in mouse brain. J. Med. Food 9, 261–264. 10.1089/jmf.2006.9.261 [DOI] [PubMed] [Google Scholar]
- Bon K., Lichtensteiger C. A., Wilson S. G., Mogil J. (2003). Characterization of cyclophosphamide cystitis, a model of visceral and referred pain, in the mouse: Species and strain differences. J. Urol. 170, 1008–1012. 10.1097/01.ju.0000079766.49550.94 [DOI] [PubMed] [Google Scholar]
- Briones T. L., Woods J. (2011). Chemotherapy-induced cognitive impairment is associated with decreases in cell proliferation and histone modifications. BMC Neurosci. 12, 124.. 10.1186/1471-2202-12-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chisholm N. C., Juraska J. M. (2013). Factors influencing the cognitive and neural effects of hormone treatment during aging in a rodent model. Brain Res. 1514, 40–49. 10.1016/j.brainres.2013.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie L. A., Acharya M. M., Parihar V. K., Nguyen A., Martirosian V., Limoli C. L. (2012). Impaired cognitive function and hippocampal neurogenesis following cancer chemotherapy. Clin. Cancer Res. 18, 1954–1965. [DOI] [PubMed] [Google Scholar]
- Collins J. M., Zaharko D. S., Dedrick R. L., Chabner B. A. (1986). Potential roles for preclinical pharmacology in phase I clinical trials. Cancer Treat Rep. 70, 73–80. [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., McLean A. E. (1986a). Cytotoxicity, DNA cross-linking, and single strand breaks induced by activated cyclophosphamide and acrolein in human leukemia cells. Cancer Res. 46, 5029–5034. [PubMed] [Google Scholar]
- Crook T. R., Souhami R. L., Whyman G. D., McLean A. E. (1986b). Glutathione depletion as a determinant of sensitivity of human leukemia cells to cyclophosphamide. Cancer Res. 46, 5035–5038. [PubMed] [Google Scholar]
- Desai V. G., Herman E. H., Moland C. L., Branham W. S., Lewis S. M., Davis K. J., George N. I., Lee T., Kerr S., Fuscoe J. C. (2013). Development of doxorubicin-induced chronic cardiotoxicity in the B6C3F1 mouse model. Toxicol. Appl. Pharmacol. 266, 109–121. [DOI] [PubMed] [Google Scholar]
- Desai V. G., C. Kwekel J., Vijay V., Moland C. L., Herman E. H., Lee T., Han T., Lewis S. M., Davis K. J., Muskhelishvili L. et al. , (2014). Early biomarkers of doxorubicin-induced heart injury in a mouse model. Toxicol. Appl. Pharmacol. 281, 221–229. [DOI] [PubMed] [Google Scholar]
- Dietrich J., Han R., Yang Y., Mayer-Proschel M., Noble M. (2006). CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J. Biol. 5, 22.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. A., Delbert Law C., Sahin L., Montenegro S. V. (2015a). Effects of perinatal methylphenidate (MPH) treatment on postweaning behaviors of male and female Sprague-Dawley rats. Neurotoxicol. Teratol. 47, 125–136. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Law C. D., Sarkar S. (2015b). Chronic MPTP treatment produces hyperactivity in male mice which is not alleviated by concurrent trehalose treatment. Behav. Brain Res. 292, 68–78. [DOI] [PubMed] [Google Scholar]
- Ferguson S. A., Sarkar S., Schmued L. C. (2013). Longitudinal behavioral changes in the APP/PS1 transgenic Alzheimer's disease model. Behav. Brain Res. 242, 125–134. 10.1016/j.bbr.2012.12.055 [DOI] [PubMed] [Google Scholar]
- Frye C. A., Walf A. A. (2008). Progesterone to ovariectomized mice enhances cognitive performance in the spontaneous alternation, object recognition, but not placement, water maze, and contextual and cued conditioned fear tasks. Neurobiol. Learn Mem. 90, 171–177. 10.1016/j.nlm.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadducci A., Cosio S., Genazzani A. R. (2007). Ovarian function and childbearing issues in breast cancer survivors. Gynecol. Endocrinol. 23, 625–631. 10.1080/09513590701582406 [DOI] [PubMed] [Google Scholar]
- Gonzalez-Burgos I., Letechipia-Vallejo G., Lopez-Loeza E., Morali G., Cervantes M. (2007). Long-term study of dendritic spines from hippocampal CA1 pyramidal cells, after neuroprotective melatonin treatment following global cerebral ischemia in rats. Neurosci. Lett. 423, 162–166. [DOI] [PubMed] [Google Scholar]
- Griffith O. W., Meister A. (1979). Glutathione: Interorgan translocation, turnover, and metabolism. Proc. Natl. Acad. Sci. USA. 76, 5606–5610. 10.1073/pnas.76.11.5606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groves T. R., Farris R., Anderson J. E., Alexander T. C., Kiffer F., Carter G., Wang J., Boerma M., Allen A. R. (2017). 5-Fluorouracil chemotherapy upregulates cytokines and alters hippocampal dendritic complexity in aged mice. Behav. Brain Res. 316, 215–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson D. L., Rastatter J. C., Colombo T., Long M. E. (2002). Doxorubicin pharmacokinetics: Macromolecule binding, metabolism, and excretion in the context of a physiologic model. J. Pharm. Sci. 91, 1488–1501. [DOI] [PubMed] [Google Scholar]
- Gutierrez E. G., Banks W. A., Kastin A. J. (1993). Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J. Neuroimmunol. 47, 169–176. 10.1016/0165-5728(93)90027-V [DOI] [PubMed] [Google Scholar]
- Harris K. M., Stevens J. K. (1989). Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: Serial electron microscopy with reference to their biophysical characteristics. J. Neurosci. 9, 2982–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermelink K., Bühner M., Sckopke P., Neufeld F., Kaste J., Voigt V., Münzel K., Wuerstlein R., Ditsch N., Hellerhoff K. et al. , (2017). Chemotherapy and post-traumatic stress in the causation of cognitive dysfunction in breast cancer patients. J. Natl. Cancer Inst. 109. [DOI] [PubMed] [Google Scholar]
- Iarkov A., Appunn D., Echeverria V. (2016). Post-treatment with cotinine improved memory and decreased depressive-like behavior after chemotherapy in rats. Cancer Chemother. Pharmacol. 78, 1033–1039. 10.1007/s00280-016-3161-0 [DOI] [PubMed] [Google Scholar]
- Janelsins M. C., Heckler C. E., Thompson B. D., Gross R. A., Opanashuk L. A., Cory-Slechta D. A. (2016). A clinically relevant dose of cyclophosphamide chemotherapy impairs memory performance on the delayed spatial alternation task that is sustained over time as mice age. Neurotoxicology 56, 287–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrell J., Lai E. V., Barr R., McMahon A., Belbeck L., O'Connell G. (1987). Ovarian toxicity of cyclophosphamide alone and in combination with ovarian irradiation in the rat. Cancer Res. 47, 2340–2343. [PubMed] [Google Scholar]
- Jenkins G. R., Lee T., Moland C. L., Vijay V., Herman E. H., Lewis S. M., Davis K. J., Muskhelishvili L., Kerr S., Fuscoe J. C. et al. , (2016). Sex-related differential susceptibility to doxorubicin-induced cardiotoxicity in B6C3F1 mice. Toxicol. Appl. Pharmacol. 310, 159–174. [DOI] [PubMed] [Google Scholar]
- Johnson S. A., Javurek A. B., Painter M. S., Ellersieck M. R., Welsh T. H. Jr, Camacho L., Lewis S. M., Vanlandingham M. M., Ferguson S. A., Rosenfeld C. S. (2016). Effects of developmental exposure to bisphenol A on spatial navigational learning and memory in rats: A CLARITY-BPA study. Horm. Behav. 80, 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joly F., Rigal O., Noal S., Giffard B. (2011). Cognitive dysfunction and cancer: Which consequences in terms of disease management? Psycho-Oncology 20, 1251–1258. [DOI] [PubMed] [Google Scholar]
- Kitamura Y., Kanemoto E., Sugimoto M., Machida A., Nakamura Y., Naito N., Kanzaki H., Miyazaki I., Asanuma M., Sendo T. (2017). Influence of nicotine on doxorubicin and cyclophosphamide combination treatment-induced spatial cognitive impairment and anxiety-like behavior in rats. Naunyn. Schmiedebergs Arch. Pharmacol. 390, 369–378. [DOI] [PubMed] [Google Scholar]
- Koppelmans V., Breteler M. M., Boogerd W., Seynaeve C., Gundy C., Schagen S. B. (2012). Neuropsychological performance in survivors of breast cancer more than 20 years after adjuvant chemotherapy. J. Clin. Oncol. 30, 1080–1086. [DOI] [PubMed] [Google Scholar]
- Lee G. D., Longo D. L., Wang Y., Rifkind J. M., Abdul-Raman L., Mamczarz J. A., Duffy K. B., Spangler E. L., Taub D. D., Mattson M. P. et al. , (2006). Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin. Cancer Res. 12, 198–205. [DOI] [PubMed] [Google Scholar]
- Leuner B., Shors T. J. (2013). Stress, anxiety, and dendritic spines: What are the connections? Neuroscience 251, 108–119. [DOI] [PubMed] [Google Scholar]
- Lind M. J., McGown A. T., Hadfield J. A., Thatcher N., Crowther D., Fox B. W. (1989). The effect of ifosfamide and its metabolites on intracellular glutathione levels in vitro and in vivo. Biochem. Pharmacol. 38, 1835–1840. [DOI] [PubMed] [Google Scholar]
- Lira F. S., Esteves A. M., Pimentel G. D., Rosa J. C., Frank M. K., Mariano M. O., Budni J., Quevedo J., Santos R. V., de Mello M. T. (2016). Sleep pattern and locomotor activity are impaired by doxorubicin in non-tumor-bearing rats. Sleep Sci. 9, 232–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomeli N., Di K., Czerniawski J., Guzowski J. F., Bota D. A. (2017). Cisplatin-induced mitochondrial dysfunction is associated with impaired cognitive function in rats. Free Radic. Biol. Med. 102, 274–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long J. M., Lee G. D., Kelley-Bell B., Spangler E. L., Perez E. J., Longo D. L., de Cabo R., Zou S., Rapp P. R. (2011). Preserved learning and memory following 5-fluorouracil and cyclophosphamide treatment in rats. Pharmacol. Biochem. Behav. 100, 205–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. (1951). Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265–275. [PubMed] [Google Scholar]
- Luine V. N. (2014). Estradiol and cognitive function: Past, present and future. Horm. Behav. 66, 602–618. 10.1016/j.yhbeh.2014.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons L., Elbeltagy M., Bennett G., Wigmore P. (2011). The effects of cyclophosphamide on hippocampal cell proliferation and spatial working memory in rat. PLoS One 6, e21445.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macleod J. E., DeLeo J. A., Hickey W. F., Ahles T. A., Saykin A. J., Bucci D. J. (2007). Cancer chemotherapy impairs contextual but not cue-specific fear memory. Behav. Brain Res. 181, 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos A. M., Li C. J., Gal Toth J., Bath K. G., Jing D., Lee F. S., McEwen B. S. (2011). Effect of brain-derived neurotrophic factor haploinsufficiency on stress-induced remodeling of hippocampal neurons. Hippocampus 21, 253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda T., Takayama T., Tashiro M., Nakamura Y., Ohashi Y., Shimozuma K. (2005). Mild cognitive impairment after adjuvant chemotherapy in breast cancer patients–evaluation of appropriate research design and methodology to measure symptoms. Breast Cancer 12, 279–287. [DOI] [PubMed] [Google Scholar]
- Maurissen J. P., Marable B. R., Andrus A. K., Stebbins K. E. (2003). Factors affecting grip strength testing. Neurotoxicol. Teratol. 25, 543–553. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055. [PubMed] [Google Scholar]
- McDonald B. C., Saykin A. J. (2013). Alterations in brain structure related to breast cancer and its treatment: Chemotherapy and other considerations. Brain Imaging Behav. 7, 374–387. 10.1007/s11682-013-9256-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougall G. J. Jr, Oliver J. S., Scogin F. (2014). Memory and cancer: A review of the literature. Arch. Psychiatr. Nurs. 28, 180–186. 10.1016/j.apnu.2013.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monastero R. N., Pentyala S. (2017). Cytokines as biomarkers and their respective clinical cutoff levels. Int. J. Inflamm. 2017, 4309485 (Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osburg B., Peiser C., Domling D., Schomburg L., Ko Y. T., Voigt K., Bickel U. (2002). Effect of endotoxin on expression of TNF receptors and transport of TNF-alpha at the blood-brain barrier of the rat. Am. J. Physiol. Endocrinol. Metab. 283, E899–E908. [DOI] [PubMed] [Google Scholar]
- Overbeek A., van den Berg M. H., van Leeuwen F. E., Kaspers G. J., Lambalk C. B., van Dulmen-den Broeder E. (2017). Chemotherapy-related late adverse effects on ovarian function in female survivors of childhood and young adult cancer: A systematic review. Cancer Treat. Rev. 53, 10–24. [DOI] [PubMed] [Google Scholar]
- Park J. H., Peyrin-Biroulet L., Eisenhut M., Shin J. I. (2017). IBD immunopathogenesis: A comprehensive review of inflammatory molecules. Autoimmun. Rev. 16, 416–426 (Review). [DOI] [PubMed] [Google Scholar]
- Peters A., Kaiserman-Abramof I. R. (1970). The small pyramidal neuron of the rat cerebral cortex. The perikaryon, dendrites and spines. Am. J. Anat. 127, 321–355. 10.1002/aja.1001270402 [DOI] [PubMed] [Google Scholar]
- Philpot R. M., Ficken M., Wecker L. (2016). Doxorubicin and cyclophosphamide lead to long-lasting impairment of spatial memory in female, but not male mice. Behav. Brain Res. 307, 165–175. 10.1016/j.bbr.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Plowchalk D. R., Mattison D. R. (1992). Reproductive toxicity of cyclophosphamide in the C57BL/6N mouse: 1. Effects on ovarian structure and function. Reprod. Toxicol. 6, 411–421. 10.1016/0890-6238(92)90004-D [DOI] [PubMed] [Google Scholar]
- Pukhalsky A. L., Toptygina A. P., Viktorov V. V. (1990). Pharmacokinetics of alkylating metabolites of cyclophosphamide in different strains of mice. Int. J. Immunopharmacol. 12, 217–223. [DOI] [PubMed] [Google Scholar]
- Ramesh G., MacLean A. G., Philipp M. T. (2013). Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm 2013, 480739 (Research Support, N.I.H., Extramural Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren K., Torres R. (2009). Role of interleukin-1beta during pain and inflammation. Brain Res. Rev. 60, 57–64 (Research Support, N.I.H., Extramural Review). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rendeiro C., Sheriff A., Bhattacharya T. K., Gogola J. V., Baxter J. H., Chen H., Helferich W. G., Roy E. J., Rhodes J. S. (2016). Long-lasting impairments in adult neurogenesis, spatial learning and memory from a standard chemotherapy regimen used to treat breast cancer. Behav. Brain Res. 315, 10–22. [DOI] [PubMed] [Google Scholar]
- Rosenfeld C. S., Ferguson S. A. (2014). Barnes maze testing strategies with small and large rodent models. J. Vis. Exp., doi:10.3791/51194(84), e51194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salas-Ramirez K. Y., Bagnall C., Frias L., Abdali S. A., Ahles T. A., Hubbard K. (2015). Doxorubicin and cyclophosphamide induce cognitive dysfunction and activate the ERK and AKT signaling pathways. Behav. Brain Res. 292, 133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J., Chalaris A., Schmidt-Arras D., Rose-John S. (2011). The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim. Biophys. Acta 1813, 878–888 (Research Support, Non-U.S. Gov't Review). [DOI] [PubMed] [Google Scholar]
- Seigers R., Fardell J. E. (2011). Neurobiological basis of chemotherapy-induced cognitive impairment: A review of rodent research. Neurosci. Biobehav. Rev. 35, 729–741. 10.1016/j.neubiorev.2010.09.006 [DOI] [PubMed] [Google Scholar]
- Seigers R., Loos M., Van Tellingen O., Boogerd W., Smit A. B., Schagen S. B. (2015). Cognitive impact of cytotoxic agents in mice. Psychopharmacology (Berl) 232, 17–37. [DOI] [PubMed] [Google Scholar]
- Seigers R., Loos M., Van Tellingen O., Boogerd W., Smit A. B., Schagen S. B. (2016). Neurobiological changes by cytotoxic agents in mice. Behav. Brain Res. 299, 19–26. [DOI] [PubMed] [Google Scholar]
- Silberfarb P. M. (1983). Chemotherapy and cognitive defects in cancer patients. Annu. Rev. Med. 34, 35–46. 10.1146/annurev.me.34.020183.000343 [DOI] [PubMed] [Google Scholar]
- Spitz D. R., Oberley L. W. (1989). An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal. Biochem. 179, 8–18. 10.1016/0003-2697(89)90192-9 [DOI] [PubMed] [Google Scholar]
- Szelenyi J. (2001). Cytokines and the central nervous system. Brain Res. Bull. 54, 329–338. 10.1016/S0361-9230(01)00428-2 [DOI] [PubMed] [Google Scholar]
- Tangpong J., Cole M. P., Sultana R., Joshi G., Estus S., Vore M., St Clair W., Ratanachaiyavong S., St Clair D. K., Butterfield D. A. (2006). Adriamycin-induced, TNF-alpha-mediated central nervous system toxicity. Neurobiol. Dis. 23, 127–139. [DOI] [PubMed] [Google Scholar]
- Tangpong J., Sompol P., Vore M., St Clair W., Butterfield D. A., St Clair D. K. (2008). Tumor necrosis factor alpha-mediated nitric oxide production enhances manganese superoxide dismutase nitration and mitochondrial dysfunction in primary neurons: An insight into the role of glial cells. Neuroscience 151, 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titus A. D., Shankaranarayana Rao B. S., Harsha H. N., Ramkumar K., Srikumar B. N., Singh S. B., Chattarji S., Raju T. R. (2007). Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience 145, 265–278. [DOI] [PubMed] [Google Scholar]
- Usta Y., Ismailoglu U. B., Bakkaloglu A., Orhan D., Besbas N., Sahin-Erdemli I., Ozen S. (2004). Effects of pentoxifylline in adriamycin-induced renal disease in rats. Pediatr. Nephrol. 19, 840–843. [DOI] [PubMed] [Google Scholar]
- Voelcker G., Wagner T., Wientzek C., Hohorst H. J. (1984). Pharmacokinetics of “activated” cyclophosphamide and therapeutic efficacies. Cancer 54(6 Suppl), 1179–1186. [DOI] [PubMed] [Google Scholar]
- Vogel-Ciernia A., Wood M. A. (2014). Examining object location and object recognition memory in mice. Curr. Protoc. Neurosci. 69, 8.31.1–8.31.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Bohlen und Halbach O., Zacher C., Gass P., Unsicker K. (2006). Age‐related alterations in hippocampal spines and deficiencies in spatial memory in mice. J. Neurosci. Res. 83, 525–531. [DOI] [PubMed] [Google Scholar]
- Walf A. A., Koonce C. J., Frye C. A. (2008). Estradiol or diarylpropionitrile administration to wild type, but not estrogen receptor beta knockout, mice enhances performance in the object recognition and object placement tasks. Neurobiol. Learn. Mem. 89, 513–521. 10.1016/j.nlm.2008.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Crawley J. N. (2009). Simple behavioral assessment of mouse olfaction. Curr. Protoc. Neurosci. Chapter, Unit 48(suppl):8.24.1–8.24.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang M., Kim J. S., Song M. S., Kim S. H., Kang S. S., Bae C. S., Kim J. C., Wang H., Shin T., Moon C. (2010). Cyclophosphamide impairs hippocampus-dependent learning and memory in adult mice: Possible involvement of hippocampal neurogenesis in chemotherapy-induced memory deficits. Neurobiol. Learn. Mem. 93, 487–494. [DOI] [PubMed] [Google Scholar]
- Zhou W., Kavelaars A., Heijnen C. J., Borchelt D. R. (2016). Metformin prevents cisplatin-induced cognitive impairment and brain damage in mice. PLoS One 11, e0151890.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck J. A., Fey E. G., Lyng G. D., Sonis S. T. (2013). A clinically translatable mouse model for chemotherapy-related fatigue. Comp. Med. 63, 491–497. [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.