Key Points
Question
Does the lung immune prognostic index composite (LIPI) score (the derived neutrophil to lymphocyte ratio and the lactate dehydrogenase level) provide prognostic information for patients with metastatic non–small cell lung cancer treated with immune checkpoint inhibitors, targeted therapy, or cytotoxic chemotherapy?
Findings
An exploratory pooled analysis of the LIPI score was performed of data from 11 immune checkpoint inhibitor and targeted therapy clinical trials for metastatic non–small cell lung cancer submitted to the US Food and Drug Administration. The LIPI score was associated with overall survival and progression-free survival among patients receiving either immune checkpoint inhibitors, targeted therapy, or cytotoxic chemotherapy.
Meaning
Baseline lactate dehydrogenase level and derived neutrophil to lymphocyte ratio may be important prognostic biomarkers irrespective of treatment drug class.
Abstract
Importance
Previous studies have suggested the importance of the baseline derived neutrophil to lymphocyte ratio (dNLR) and lactate dehydrogenase (LDH) level as prognostic markers. The lung immune prognostic index (LIPI) was shown to be associated with progression-free survival (PFS) and overall survival (OS) among patients with metastatic non–small cell lung cancer (mNSCLC) treated with immune checkpoint inhibitors (ICIs) but not cytotoxic chemotherapy (CCT).
Objective
To determine whether the LIPI is associated with long-term outcomes in pooled analyses of clinical studies of ICI and targeted therapy (TT) for patients with mNSCLC.
Design, Setting, and Participants
An exploratory pooled analysis was performed of the LIPI on data from 11 randomized clinical multinational trials evaluating ICIs and TT submitted to the US Food and Drug Administration between January 1, 2013, and December 31, 2017, for 4914 patients with mNSCLC. Lung immune prognostic index scores were calculated based on the dNLR and the LDH level per previous publications to generate good, intermediate, and poor composite scores. Multivariable Cox proportional PFS and OS hazard ratios were generated for the dNLR, the LDH level, age, smoking status, histologic characteristics, and Eastern Cooperative Oncology Group performance score.
Main Outcomes and Measures
Overall survival and PFS and their association with good, intermediate, or poor prognostic LIPI scores.
Results
Eleven mNSCLC randomized trials were analyzed, including 3987 patients with available data. In 5 ICI trials comprising 2440 patients, 1368 patients received ICIs and 1072 patients received CCT. In 6 TT trials comprising 1547 patients, 1110 patients received TT and 437 patients received CCT. A good LIPI score was associated with better OS among patients receiving ICIs (hazard ratio, 0.34; 95% CI, 0.28-0.42), TT (hazard ratio, 0.28; 95% CI, 0.21-0.37), and CCT (hazard ratio, 0.49; 95% CI, 0.40-0.60 in ICI trials; hazard ratio, 0.41; 95% CI, 0.27-0.61 in TT trials) than those with poor LIPI scores. Similar findings were observed in terms of PFS (ICIs: hazard ratio, 0.59; 95% CI, 0.48-0.72; TT: hazard ratio, 0.46; 95% CI, 0.37-0.57; and CCT: hazard ratio, 0.56; 95% CI, 0.45-0.68 in ICI trials; hazard ratio, 0.51; 95% CI, 0.38-0.69 in TT trials).
Conclusions and Relevance
The baseline LDH level and dNLR are important prognostic biomarkers irrespective of treatment modality for patients with mNSCLC. As further prospective clinical trial information is collected, the role of the LIPI score can be better defined.
This exploratory pooled analysis of 11 randomized clinical trials examines whether the lung immune prognostic index is associated with long-term outcomes of treatment with immune checkpoint inhibitors and targeted therapy for patients with metastatic non–small cell lung cancer.
Introduction
Previous work has suggested the importance of the baseline neutrophil to lymphocyte ratio (NLR) and the baseline lactate dehydrogenase (LDH) level in determining outcomes of patients with various cancers, including non–small cell lung cancer (NSCLC).1,2,3,4 The advent of novel immunotherapies has led to the exploration of various biomarkers that may be associated with response, which is particularly important given the multitude of approvals of immune checkpoint inhibitors by the US Food and Drug Administration (FDA). The NLR has been previously associated with disease control and treatment response to checkpoint inhibitors in NSCLC.5,6
To strengthen the prognostic power of the derived NLR (dNLR), a surrogate for the NLR, in NSCLC, Mezquita and colleagues7 recently generated a lung immune prognostic index (LIPI) based on 161 patients who received immune checkpoint inhibitors (ICIs), specifically programmed death 1/programmed death ligand 1 (PD-1/PD-L1) inhibitors. This composite index included a dNLR greater than 3 and an LDH level higher than the upper limit of normal, which characterized 3 risk groups: good, intermediate, and poor. The authors found the LIPI to be associated with survival in an independent ICI-treated cohort of 305 patients but not associated with survival in a cohort of 162 patients treated with cytotoxic chemotherapy (CCT). We performed an exploratory retrospective analysis of the LIPI on pooled clinical trial data from studies evaluating ICIs or targeted therapy (TT) in NSCLC studies submitted to the FDA.
Methods
A total of 5631 patients were enrolled in 20 randomized trials evaluating either ICIs or TT between January 1, 2013, and December 31, 2017, for metastatic NSCLC (mNSCLC) (all lines of therapy) in data submitted to the FDA, of whom 3987 patients were included in the final analysis (eFigure 1 in the Supplement). We pooled patients into 1 of 4 subgroups: patients receiving ICI, CCT in ICI trials, TT, or CCT in TT trials. The LIPI composite scores were calculated based on the dNLR (absolute neutrophil count/[white blood cell count − absolute neutrophil count]) and the LDH level per Mezquita et al.7 As this work is not considered human participants research, under 45 CFR 46, subpart A, it required no human research participants protections review or institutional board review.
Statistical Analysis
Univariate and multivariable Cox proportional PFS and OS hazard ratios (HRs) were generated for the dNLR, the LDH level, and other major covariates. Median PFS and OS estimates were generated using the Kaplan-Meier method. A global log-rank test was used to compare the whole survival distribution. R software, version 3.4.2, with RStudio, version 1.1.383 (R Foundation for Statistical Computing) was used for statistical analyses. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. A significance level for statistical testing was not prespecified and this study was exploratory in nature, designed to characterize the magnitude of LIPI across the trials selected. The P value reported in this analysis simply conveys an overall measure of whether LIPI would show a significant effect across all studies when assessed via conventional measures.
Results
Patient Demographics and Disease Characteristics
In the 5 ICI trials (n = 2440), 1368 patients received ICIs and 1072 received CCT. In the 6 TT trials (n = 1547), 1110 (587 [52.9%] EGFR mutant and 523 [47.1%] ALK positive) received TT and 437 (140 [32.0%] EGFR mutant and 297 [68.0%] ALK positive) received CCT (eFigure 1 and eTable 1 in the Supplement). Baseline demographics and disease characteristics were relatively balanced between groups (eTable 2 in the Supplement).
Association of LIPI Scores with OS and PFS Among Patients Receiving ICI or CCT
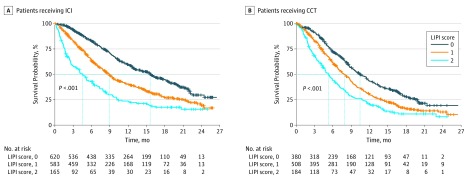
For the ICI, a good LIPI score (LDH level lower than the upper limit of normal and dNLR ≤3) was associated with longer OS compared with a poor LIPI score, with an estimated median survival of 15.6 vs 4.5 months (HR, 0.34; 95% CI, 0.28-0.42) (Figure 1 and Table 1). A similar prognostic association was observed for patients who received CCT: patients with a good LIPI score had longer survival than patients with a poor score, with an estimated median survival of 10.4 vs 5.3 months (HR, 0.49; 95% CI, 0.40-0.60) (Figure 1 and Table 1). Similar associations between good LIPI scores and longer PFS were observed (eFigure 2 in the Supplement). Multivariable analysis showed that, when controlling for the covariates of age, sex, histologic characteristics, smoking, PD-L1 expression, and Eastern Cooperative Oncology Group performance score, the albumin level, LDH level, and dNLR were associated with both longer OS and longer PFS (Table 2). As expected, PD-L1 expression of 1% or more was independently associated with improved outcomes as well as higher albumin levels.
Figure 1. Overall Survival Based on LIPI Score and Treatment: ICI Studies.
CCT indicates cytotoxic chemotherapy; ICI, immune checkpoint inhibitor; and LIPI, lung immune prognostic index.
Table 1. Overall Survival in Immune Checkpoint Inhibitor Studies Based on LIPI Score and Treatment.
| Characteristic | Immune Checkpoint Inhibitors | Cytotoxic Chemotherapy | ||||
|---|---|---|---|---|---|---|
| LIPI Score of 0 | LIPI Score of 1 | LIPI Score of 2 | LIPI Score of 0 | LIPI Score of 1 | LIPI Score of 2 | |
| Patients, No. | 620 | 583 | 165 | 380 | 508 | 184 |
| Hazard ratio (95% CI) | 0.34 (0.28-0.42) | 0.59 (0.48-0.73) | 1 [Reference] | 0.49 (0.40-0.60) | 0.70 (0.58-0.85) | 1 [Reference] |
| Overall survival, median, mo (95% CI) | 15.6 (13.5-17.6) | 8.9 (7.9-9.7) | 4.5 (3.0-6.2) | 10.4 (9.2-11.8) | 7.9 (7.0-8.7) | 5.3 (4.3-6.3) |
| Global log-rank P value | <.001 | <.001 | ||||
Abbreviation: LIPI, lung immune prognostic index.
Table 2. Multivariable Analysis for Overall Survival and Progression-Free Survival in Pooled ICI-Treated and TT-Treated Patients.
| Variable | Hazard Ratio (95% CI) | |||
|---|---|---|---|---|
| ICI Group (n = 1368) | TT Group (n = 1110) | |||
| Progression-Free Survival | Overall Survival | Progression-Free Survival | Overall Survival | |
| Age, y | ||||
| <65 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ≥65 | 0.96 (0.82-1.11) | 0.95 (0.80-1.12) | 0.97 (0.80-1.18) | 1.30 (0.97-1.72) |
| P value | .56 | .53 | .78 | .08 |
| Sex | ||||
| Male | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Female | 1.13 (0.97-1.33) | 0.98 (0.82-1.17) | 1.01 (0.82-1.24) | 1.01 (0.75-1.37) |
| P value | .12 | .83 | .96 | .95 |
| Histologic characteristics | ||||
| Nonsquamous | 1 [Reference] | 1 [Reference] | NA | NA |
| Squamous | 1.21 (1.01-1.46) | 1.24 (1.01-1.51) | NA | NA |
| P value | .04 | .04 | NA | NA |
| Smoking | ||||
| Never smoker | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| Anytime smoker | 0.57 (0.44-0.74) | 1.00 (0.74-1.36) | 1.25 (1.02-1.54) | 1.36 (1.01-1.83) |
| P value | <.001 | .98 | .03 | .046 |
| ECOG PS | ||||
| ECOG PS of 0 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| ECOG PS of ≥1 | 1.14 (0.97-1.35) | 1.61 (1.33-1.95) | 1.12 (0.93-1.35) | 1.47 (1.11-1.97) |
| P value | .11 | <.001 | .24 | .008 |
| LDH | ||||
| ≤ULN | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| >ULN | 1.19 (1.02-1.40) | 1.44 (1.21-1.71) | 1.46 (1.20-1.77) | 1.70 (1.27-2.27) |
| P value | .03 | <.001 | <.001 | <.001 |
| dNLR | ||||
| ≤3 | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| >3 | 1.26 (1.06-1.49) | 1.64 (1.37-1.96) | 1.54 (1.26-1.88) | 1.87 (1.41-2.48) |
| P value | .008 | <.001 | <.001 | <.001 |
| Albumin | ||||
| ≥3.5 g/dL | 1 [Reference] | 1 [Reference] | 1 [Reference] | 1 [Reference] |
| <3.5 g/dL | 1.22 (1.00-1.48) | 1.57 (1.29-1.91) | 1.04 (0.84-1.28) | 1.66 (1.24-2.22) |
| P value | .045 | <.001 | .74 | <.001 |
| PD-L1 | ||||
| ≥1% | 1 [Reference] | 1 [Reference] | NA | NA |
| <1% | 1.72 (1.46-2.03) | 1.20 (1.00-1.45) | NA | NA |
| P value | <.001 | .06 | NA | NA |
Abbreviations: dNLR, derived neutrophil to lymphocyte ratio (leukocytes minus neutrophils); ECOG PS, Eastern Cooperative Oncology Group Performance Score; ICI, immune checkpoint inhibitor; LDH, lactate dehydrogenase; NA, not applicable; PD-L1, programmed death ligand 1; TT, targeted therapy; ULN, upper limit of normal.
SI conversion factor: To convert albumin to grams per liter, multiply by 10.0.
Association of LIPI Scores With OS and PFS Among Patients Receiving TT
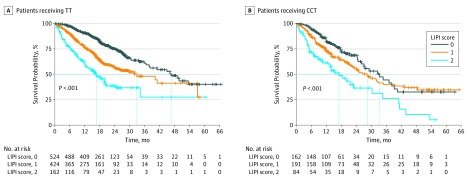
Given the observation that the LIPI score was prognostic for patients receiving ICI and CCT, we investigated whether LIPI was also prognostic in TT trials. Patients with tumors harboring either ALK alterations or EGFR-activating mutations who received TT with a good LIPI score had an estimated median survival of 46.5 months vs 16.6 months for those with a poor score (HR, 0.28; 95% CI, 0.21-0.37) (Figure 2 and Table 3). A similar prognostic association was observed for patients receiving CCT; patients with a good LIPI score had longer survival than patients with a poor score (estimated median survival of 33.4 months vs 17.1 months; HR, 0.41; 95% CI, 0.27-0.61) (Figure 2 and Table 3). Similar associations between LIPI score and PFS were observed (eFigure 3 in the Supplement). Consistently, for patients enrolled in these studies, regardless of receiving TT or CCT, multivariable analysis showed that the dNLR and the LDH level were independently associated with OS and PFS (Table 2).
Figure 2. Overall Survival Based on LIPI Score and Treatment: TT Studies.
CCT indicates cytotoxic chemotherapy; LIPI, lung immune prognostic index; and TT, targeted therapy.
Table 3. Overall Survival in Targeted Therapy Studies Based on LIPI Score and Treatment.
| Characteristic | Targeted Therapy | Cytotoxic Chemotherapy | ||||
|---|---|---|---|---|---|---|
| LIPI Score of 0 | LIPI Score of 1 | LIPI Score of 2 | LIPI Score of 0 | LIPI Score of 1 | LIPI Score of 2 | |
| Patients, No. | 524 | 424 | 162 | 162 | 191 | 84 |
| Hazard ratio (95% CI) | 0.28 (0.21-0.37) | 0.57 (0.43-0.74) | 1 [Reference] | 0.41 (0.27-0.61) | 0.55 (0.38-0.79) | 1 [Reference] |
| Overall survival, median, mo (95% CI) | 46.5 (37.7-NE) | 32.8 (24.3-NE) | 16.6 (12.4-20.0) | 33.4 (26.1-39.2) | 28.6 (21.9-34.9) | 17.1 (10.6-30.4) |
| Global log-rank P value | <.001 | <.001 | ||||
Abbreviations: LIPI, lung immune prognostic index; NE, not estimable.
Finally, we performed exploratory post hoc Kaplan-Meier analysis of PFS and OS for each of the LIPI risk groups (eFigures 4 and 5 in the Supplement). This exploratory supplementary analysis showed that, regardless of the LIPI risk category, the Kaplan-Meier curves appeared similar in terms of PFS, suggesting improvement in the experimental therapies irrespective of LIPI category. In terms of OS, this post hoc exploratory analysis suggests that patients with poor LIPI may not have improved OS when receiving ICI or TT compared with CCT.
Discussion
Our analysis suggests that baseline LIPI score may be a prognostic biomarker irrespective of treatment for patients with mNSCLC. Our supplemental analysis suggested that, for PFS, patients benefited from ICIs and TT compared with CCT irrespective of LIPI score. However, this exploratory analysis suggests that patients with a poor LIPI score who are treated with ICIs or TT did not appear to have improved OS compared with patients with a poor LIPI score treated with CCT. In the TT studies, no OS advantage was demonstrated in the intent-to-treat population, likely owing to crossover. The LIPI score does not appear to be a prognostic biomarker exclusively for ICI therapy. Our observations suggest that the LDH level, which may be a measure of tumor burden, and the dNLR, which may be a measure of the immune system, are important across therapeutic classes and molecular subsets of mNSCLC. In our multivariate analysis, PD-L1 expression and albumin levels were independently associated with PFS and OS as in the original LIPI study (for albumin)7; furthermore, the same group showed in a subsequent report that the prognostic ability of the LIPI score was independent of PD-L1.8 We did not assess changes in LIPI score over time with treatment, which may also be associated with outcome for individual patients. For example, the relative decrease in the NLR from baseline to 6 weeks of treatment was found to be associated with outcome in renal cell carcinoma.9
Limitations
The limitations of our exploratory analysis must be acknowledged. This was a post hoc, retrospective evaluation with potential biases owing to missing trials and missing laboratory values. Another major limitation of our analysis was the lack of data on the number of metastatic sites, which is likely a major prognostic indicator. Nonetheless, if further validated, the LDH level and the dNLR could be considered an inexpensive and practical prognostic marker for potential stratification and enrichment in prospective trials independent of pharmacologic drug class. Future efforts are needed to assess the relative contribution of the LDH level and the dNLR individually, to identify optimal threshold cutoff values, and to longitudinally assess dynamic changes in LIPI score over time in association with treatment and response. Future modeling could incorporate other known prognostic markers such as performance status, smoking status, tumor burden, and other baseline factors and tumor genomic, transcriptomic, proteomic, and metabolomic markers.
Conclusions
Baseline LIPI risk group appears to be an important prognostic biomarker irrespective of pharmacologic class of treatment for patients with NSCLC. As further prospective clinical trials are conducted, the role of the LIPI score can be better defined.
eTable 1. Summary of Selected Clinical Studies
eTable 2. Summary of Key Demographics and Disease Characteristics of Pooled Cohorts
eFigure 1. Flow Chart of Clinical Studies and Patients Selected for Analysis
eFigure 2. Progression-Free Survival Based on LIPI Score and Treatment: ICI Studies
eFigure 3. Progression-Free Survival Based on LIPI Score and Treatment: TT Studies
eFigure 4. Kaplan Meier Progression-Free Survival Estimates by Treatment Arm for Each of the LIPI Categories
eFigure 5. Kaplan Meier Overall Survival Estimates by Treatment Arm for Each of the LIPI Categories
References
- 1.Kiriu T, Yamamoto M, Nagano T, et al. . The time-series behavior of neutrophil-to-lymphocyte ratio is useful as a predictive marker in non-small cell lung cancer. PLoS One. 2018;13(2):e0193018. doi: 10.1371/journal.pone.0193018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Suh KJ, Kim SH, Kim YJ, et al. . Post-treatment neutrophil-to-lymphocyte ratio at week 6 is prognostic in patients with advanced non-small cell lung cancers treated with anti-PD-1 antibody. Cancer Immunol Immunother. 2018;67(3):459-470. doi: 10.1007/s00262-017-2092-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakaya A, Kurata T, Yoshioka H, et al. . Neutrophil-to-lymphocyte ratio as an early marker of outcomes in patients with advanced non-small-cell lung cancer treated with nivolumab. Int J Clin Oncol. 2018;23(4):634-640. doi: 10.1007/s10147-018-1250-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhu L, Li X, Shen Y, et al. . A new prognostic score based on the systemic inflammatory response in patients with inoperable non-small-cell lung cancer. Onco Targets Ther. 2016;9:4879-4886. doi: 10.2147/OTT.S107279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maymani H, Hess K, Groisberg R, et al. . Predicting outcomes in patients with advanced non-small cell lung cancer enrolled in early phase immunotherapy trials. Lung Cancer. 2018;120:137-141. doi: 10.1016/j.lungcan.2018.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zer A, Sung MR, Walia P, et al. . Correlation of neutrophil to lymphocyte ratio and absolute neutrophil count with outcomes with PD-1 axis inhibitors in patients with advanced non-small-cell lung cancer. Clin Lung Cancer. 2018;19(5):426-434.e1. doi: 10.1016/j.cllc.2018.04.008 [DOI] [PubMed] [Google Scholar]
- 7.Mezquita L, Auclin E, Ferrara R, et al. . Association of the lung immune prognostic index with immune checkpoint inhibitor outcomes in patients with advanced non–small cell lung cancer. JAMA Oncol. 2018;4(3):351-357. doi: 10.1001/jamaoncol.2017.4771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mezquita L, Park W, Arbour K, et al. . Correlation of the lung immune prognostic index (LIPI) and PDL1 status with outcomes for immune checkpoint inhibitors in advanced NSCLC patients. J Thorac Oncol. 2018;13(10)(suppl):S488. doi: 10.1016/j.jtho.2018.08.624 [DOI] [Google Scholar]
- 9.Lalani AA, Xie W, Martini DJ, et al. . Change in neutrophil-to-lymphocyte ratio (NLR) in response to immune checkpoint blockade for metastatic renal cell carcinoma. J Immunother Cancer. 2018;6(1):5. doi: 10.1186/s40425-018-0315-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Summary of Selected Clinical Studies
eTable 2. Summary of Key Demographics and Disease Characteristics of Pooled Cohorts
eFigure 1. Flow Chart of Clinical Studies and Patients Selected for Analysis
eFigure 2. Progression-Free Survival Based on LIPI Score and Treatment: ICI Studies
eFigure 3. Progression-Free Survival Based on LIPI Score and Treatment: TT Studies
eFigure 4. Kaplan Meier Progression-Free Survival Estimates by Treatment Arm for Each of the LIPI Categories
eFigure 5. Kaplan Meier Overall Survival Estimates by Treatment Arm for Each of the LIPI Categories