Abstract
Chronic hepatitis D (CHD) results from an infection with hepatitis B virus (HBV) and hepatitis D virus (HDV). CHD is the most severe form of human viral hepatitis. Current treatment options consist of interferon alfa, which is effective only in a minority of patients. The study of HDV molecular virology has resulted in new approaches to therapy that have entered clinical trials, the most advanced of which has entered phase-3 studies. These include the entry inhibitor Bulevirtide, the nucleic acid polymer REP 2139-Ca, the farnesyltransferase inhibitor lonafarnib, and pegylated interferon lambda. This review summarizes available data of these emerging therapeutics.
Keywords: Hepatitis d, hepatitis b, clinical trials, therapeutics, cirrhosis
Introduction
Hepatitis D (HDV) was first described by Mario Rizzetto and colleagues in 1977 and today is described as the most severe and rapidly progressive form of chronic viral hepatitis despite being an incomplete virus that requires the presence of hepatitis B virus (HBV) to be a human pathogen.1, 2 Progression to cirrhosis occurs in 10-15% of patients within 2 years and in 70-80% within 5 to 10 years.3, 4 Furthermore, HBV/HDV coinfection results in an increased risk of hepatocellular carcinoma (HCC)5-11 and mortality5, 7, 9, 12 compared to HBV mono-infection.
Although HDV infection has historically been thought of as a rare disease, recent estimations have suggested that the global burden of disease may be close to 62-72 million.13 Despite these concerns, HDV does not currently have a US Food and Drug Administration (FDA) approved therapy. The only treatment that is used outside of clinical trials is pegylated interferon (peginterferon) but this treatment is plagued by significant side effects such as flu-like symptoms, myalgias, and arthralgias while having limited efficacy in HDV.14 Nonetheless, it is currently the only treatment that is endorsed by the major liver societies such as the American Association for the Study of Liver Diseases (AASLD)15 and European Association for the Study of the Liver (EASL)16 due to its proven effect in reducing fibrosis, decreasing risk of hepatic decompensation, and improving mortality.17-19 Numerous other treatments including HBV nucleoside analogs have been studied in clinical trials over the past several decades with and without interferon therapy without improvements in therapeutic response.20-26 However, within the past decade, there has been a resurgence of interest in novel therapies in hopes of defeating this rapidly progressive and devastating disease.
In this review, we will highlight HDV virology and the viral life cycle, past therapeutic approaches, and current recommended therapies and their associated positive and negative aspects. Finally, we will discuss investigational therapies, their mechanisms of action, and the current progress and future of HDV therapeutics.
Virology and life cycle
The HDV virion is small RNA virus measuring ~ 36nm in diameter including an inner nucleocapsid that is made up of a short (~ 1.7 kb) single-stranded, circular RNA of negative polarity and ~ 200 molecules of hepatitis D antigen (HDAg).27-29 This inner nucleocapsid is surrounded by a lipid envelope embedded with all three types of hepatitis B surface antigen (HBsAg) proteins obtained from HBV; without HBsAg, HDV is incapable of being a human pathogen.30 The HDV genome is the smallest among mammalian viruses and shares structural similarity to viroids.27, 28, 31 This genome encodes for one protein that exits in two forms; the small HDAg (SHDAg) and the large HDAg (LHDAg).
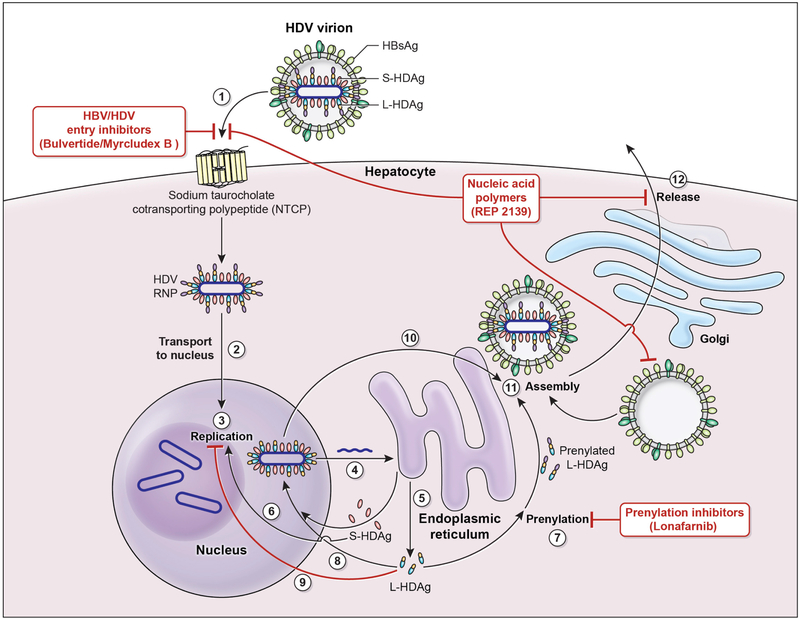
The HDV viral life cycle begins when the HDV virion binds to the human hepatocyte through interaction between the myristoylated N-terminus of the pre-S1 domain of the large HBsAg and the host receptor (Figure 1), also known as the sodium taurocholate cotransporting polypeptide (NTCP) located on the basolateral membrane of hepatocytes.32, 33 After cell entry and uncoating, the HDV genome is translocated to the nucleus via HDAg-mediated interactions where it employs host RNA polymerase II for genome replication. There are no DNA intermediates or archiving events.34 Instead, HDV replication occurs via a double rolling circle mechanism driven by the catalytic activity of RNA polymerase II with the aid of SHDAg to create linear multimeric copies of antigenomic RNA from the incoming negative strand circular template genome.35 These linear multimeric copies then undergo specific cleavage at a unique ribozyme site encoded once in each antigenome. The resulting linear antigenomic monomers are subsequently ligated into antigenomic circles that serve as template for production of linear multimers of opposite polarity genomic RNA. These in turn, self-process into linear genomic monomers via autocatalytic cleavage at another ribozyme site encoded once in each genomic RNA. The genomic monomers are ligated into circles which can either support additional rounds of replication or be packaged into nascent virions.36
Figure 1. HDV viral life cycle and sites of investigative drug targets.
1. Hepatitis D virus (HDV) virion attaches to the hepatocyte via interaction between Hepatitis B surface antigen proteins and the sodium taurocholate cotransporting polypeptide (NTCP).
2. HDV ribonucleoprotein (RNP) is translocated to nucleus mediated by the hepatitis D antigen (HDAg).
3. HDV genome replication occurs via a “rolling circle” mechanism.
4. HDV antigenome is transported out of the nucleus to the endoplasmic reticulum (ER).
5. HDV antigenome is translated in the ER into small HDAg (S-HDAg) and large HDAg (L-HDAg).
6. S-HDAg is transported into the nucleus.
7. S-HDAg promotes HDV replication in the nucleus.
8. L-HDAg undergoes prenylation prior to assembly.
9. L-HDAg inhibits HDV replication in the nucleus.
10. New HDAg molecules are associated with new transcripts of genomic RNA to form new RNPs that are exported to the cytoplasm.
11. New HDV RNP associates with Hepatitis B virus (HBV) envelop proteins and assembled into HDV virions.
12. Completed HDV virions are released from the hepatocyte via the trans-Golgi network.
Investigative drug and their targets:
HBV/HDV inhibitors (Bulvertide) - target the NTCP by competitive binding.
Nucleic acid polymers (REP 2139-Ca) – inhibits Hepatitis B surface antigen (HBsAg) and subviral assembly as well as HDV entry.
Prenylation inhibitors (Lonafarnib) – inhibits the process of prenylation of the L-HDAg which is the step leading up to assembly.
A smaller antigenomic sense mRNA is also transcribed off of the genomic template. This mRNA codes for the two forms of HDAg.35 The SHDAg and LHDAg are identical except that the LHDAg features an additional 19-amino acid sequence at the C-terminus resulting from a specific RNA editing event catalyzed by adenosine deaminase acting on RNA 1 (ADAR1) that effects the SHDAg stop codon.37, 38 This results in translation proceeding to the next downstream stop codon and the addition of an extra 19 amino acids that characterize the LHDAg. This extra sequence on the LHDAg contains a CXXX-box motif (C=cysteine, X=one of the last 3 amino acids at the carboxyl terminus of the LHDAg) which then becomes the substrate for host cell farnesyltransferase. The latter covalently attaches a prenyl lipid farnesyl to the cysteine of the CXXX-box. This prenylation event is essential for virion assembly via promoting interaction with HBsAg.39 In addition, the LHDAg is a potent transdominant inhibitor of genome replication while the SHDAg serves to promote genome replication.40-42 Thus, the RNA editing event catalyzed by ADAR1 that changes the production of HDAg from SHDAg to LHDAg serves a key regulatory switch in the virus life cycle, shutting off genome replication and initiating virion assembly.
When the HDV virion is completed, it is ready for release via the trans-golgi network to infect new hepatocytes. After infection, hepatocyte damage caused by HDV infection can be due to a direct cytopathic effect of HDV or via still incompletely understood immune-mediated mechanism.43-45
HDV Therapeutics
Interferon-alpha therapy
Despite the lack of an FDA approved therapy for HDV infection, expert guidelines have recommended the use of peginterferon.16, 46 These therapeutic recommendations stem from various experiences dating back to the early 1990s with the first use interferon alfa therapy. Initial experiences evaluated interferon alfa-2a at low doses (3 million IU TIW) compared with high doses (9 million IU TIW) or with no therapy for one year.47 In this study, a complete response, defined as HDV RNA PCR negativity with ALT normalization, was seen in 21% of those treated with low dose interferon compared to 50% in those treated with high dose and 0% in those who did not receive any therapy. However, no patients demonstrated a sustained virologic response in follow-up up to 48 weeks post-therapy. In this same cohort, a follow-up report of up to 14 years post-therapy with a more sensitive HDV RNA assay revealed that none of the original patients achieved HDV RNA negativity at the end of the original study. More importantly, long-term outcomes from this study demonstrated improved survival in the high dose group in those that achieved a ≥2 log drop in HDV RNA at the end of treatment (EOT) compared to those in the low dose group (p=0.019) and the no treatment group (p=0.003), both of which were unable to achieve a 2 log drop in HDV RNA at EOT.18 Interestingly, there was no difference in survival between the low dose group and controls.
With the efficacy of peginterferon in other viral hepatitis infections, and the initial FDA approval of peginterferon alfa-2b in 2001 for chronic hepatitis C, it was then explored for use in chronic HDV infection. Peginterferon alfa-2b was administered (1.5 ug/kg/wk) for 1 year which resulted in undetectable HDV RNA in serum in 8 of 14 (57%) patients, however after a median post-therapy follow-up of 16 months, the sustained virologic response rate was 43%.48 Prolonged peginterferon monotherapy has been studied for 72 weeks which resulted in low-level or undetectable HDV RNA in 34% of patients at the end of therapy, however with 24 weeks of post-therapy follow-up, only 21% of patients had undetectable HDV RNA.21 Long-term peginterferon alfa-2a with increasing doses up to 360 mcg/wk has been studied for up to 5 years, however this has not resulted in improved response rates; only 30% of patients achieved a complete virologic response, described as HDV RNA negativity and HBsAg seroconversion.49, 50 Thus, in chronic HDV infection, peginterferon appears to be as effective as standard interferon therapy and prolonged therapy does not appear to improve response rates. In fact, HDV RNA levels at 24 weeks of peginterferon therapy may predict response to one year of therapy.51
Interferon-alfa therapy combinations
Interferon alfa-based therapies have been studied in combination with other medications in chronic HDV infection. Interferon alfa, with and without pegylation, in combination with ribavirin has been studied in chronic HDV patients for 1 to 2 years, however, there does not appear be any added value of ribavirin in HDV.20, 21, 52 Alternatively, HBV nucleoside analogues have also been evaluated, without much success, in combination with or without interferon alfa including famicyclovir24, lamivudine53, adefovir26, and tenofovir.54, 55 This is not surprising, since such HBV nucleoside analogs can be quite effective at decreasing serum HBV DNA but have no significant effect on HBsAg—which is all that HDV needs to replicate. One of the largest studies of peginterferon in HDV, the Hep-Net/International Delta Hepatitis Intervention Trial (HIDIT-1) study, randomized 90 patients to adefovir, peginterferon, or the combination. An approximate 2.5 log decline in median HDV RNA was observed at 48 weeks of treatment in both peginterferon arms, with ~25% of these patients achieving HDV RNA negativity at 24 weeks post follow up. No responses were seen in the adefovir arm.26 A follow-on study (HIDIT-2), which evaluated switching the nucleoside analog from adefovir to tenofovir and extending treatment from 48 to 96 weeks, did not show any significant improvement in sustained response rates56. In this study, the primary endpoint of undetectable HDV RNA at the end of therapy was not different between the two groups (peginterferon/tenofovir: 28/59 (48%) vs peginterferon/placebo: 20/61 (33%), p=0.12). Thus, given these various studies, the combination of nucleoside analogues with interferon does not seem to provide additional benefit in chronic HDV infection.
Past Investigational Therapies
Thymus-derived therapies
Thymosins and their synthetic derivatives are believed to induce T-cell differentiation and maturation, increase T cell function and restore immune defects. Given the early promising results in chronic HBV monoinfection57, 58 in the early 1990s, it was explored in two small pilot studies for HDV.59, 60 However, only 1 of 5 (20%) of patients became HDV RNA negative when treated with thymosin-alpha 1 900 ug/m2 twice weekly for 6 months60 and 3 of 11 became HDV RNA negative when treated with thymic humoral factor-gamma 2 (40 ug) when treated for 24 weeks with 2 of 3 demonstrating a virologic relapse. Since these early studies, further thymosin-focused investigation in HDV has not been described.
Current Investigational Therapies
Peginterferon Lambda-1a
Pegylated interferon lambda-1a is a type-III interferon that has demonstrated antiviral activity against HBV61 and HCV62. Lambda’s antiviral activity was first reported in in vivo models in 200663 and it has been described to utilize similar interferon-stimulated gene (ISG) induction pathways as interferon alfa thereby resulting in broad-spectrum antiviral activities and immunomodulatory properties. Lambda binds to type III interferon receptors which results in dimerization and activation of multiple intracellular signal transduction pathways mediated by the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. While this is similar to interferon alfa, a type-I interferon, type-III interferon lambda receptors are restricted to cells of epithelial origin which includes the liver.64 Thus, initial clinical trials of peginterferon lambda in HBV and HCV demonstrated similar antiviral effects to peginterferon alfa, but demonstrated a substantially improved tolerability profile compared to peginterferon alfa.
In HDV, lambda interferon has demonstrated antiviral activity in in vivo human liver chimeric mouse models.65 In humans, one study evaluating peginterferon lambda monotherapy in 33 chronic HDV patients has recently completed with the end of study results presented in first half of 2019.66 In this randomized, open-label, multicenter study, peginterferon lambda was administered at doses of 120 or 180 ug weekly for 48 weeks. The end of study report has again confirmed that tolerability is improved compared to historical peginterferon alfa data and that both doses of peginterferon lambda have antiviral activity against HDV. Notably, in the high dose group, 9/14 (64%) of patients achieved either a 2 log10 decline in HDV RNA or had levels below the limit of quantification (BLOQ) at the end of therapy which was sustained in 7/14 (50%) of patients 24 weeks after therapy. Additionally, 5/14 (36%) of patients demonstrated a durable virologic response after 24 weeks of therapy (i.e. HDV RNA negative at EOT and 24 weeks post treatment). Given these promising results, peginterferon lambda’s improved tolerability may be an attractive option for treating HDV, either as a monotherapy or in combination with other experimental therapies. Currently, an open-label clinical trial exploring lambda interferon in combination with lonafarnib (LNF) (see below) and ritonavir (RTV) for 24 weeks in 26 patients is being evaluated at the National Institutes of Health Clinical Center ().
HBV/HDV entry inhibitors
Bulevirtide (Myrcludex B), a once daily subcutaneous injection, is a first-in-class HBV and HDV entry inhibitor that targets the human sodium-taurocholate co-transporter peptide (hNTCP) transmembrane protein. (Figure 1) The essential factor for receptor binding is the 47 N-terminal amino acids of the preS1 domain or the L-HBsAg envelope protein67 and competitive binding to hNTCP by Bulevirtide , a myristolytated peptide that includes this N-terminal sequence, has demonstrated entry inhibition by HBV and HDV in in vitro and in vivo models.32, 68, 69 In an early phase 2, randomized, 3-arm, open-label clinical study, 24 HDV patients were treated with Myrcludex B (2mg SQ daily) in combination with peginterferon alfa or Myrcludex B alone or Peginterferon alone for 24 weeks.70 While the primary endpoint in this study, change in serum HBsAg levels, was not achieved, patients that received Myrcludex B experienced significant declines in serum HDV RNA and ALT levels. Notably, the combination of Myrcludex B and peginterferon group experienced mean HDV RNA declines of 2.6 log10 IU/L, the Myrcludex B monotherapy group had a 1.67 log10 IU/L, and the peginterferon group had a 2.2 log10 IU/L decline. HDV RNA became negative in 2 of 8 patients in both the Myrcludex B and peginterferon monotherapy groups and 5 of 7 patients in the combination therapy group. Myrcludex B was reported to be generally well tolerated in this study.
Since this study, a multicenter, open-labeled, randomized, phase 2b clinical trial further exploring the safety and efficacy of Myrcludex B has been performed in 120 HDV patients. Patients were randomized to subcutaneous daily injectable doses of Myrcludex B (2, 5, 10 mg) with oral tenofovir (245 mg/day) for 24 weeks. The primary endpoint of this study was a 2 log10 HDV RNA reduction or negativity in serum. Current end-of-study reports have described median HDV RNA declines in a dose dependent manner, ranging from 1.6 to 2.7 log10 IU/L, with Myrcludex B 10 mg demonstrating the greatest effect.71 Additionally, ALT normalization was seen in up to 50% of patients. HDV RNA relapse occurred in all groups in up to 80% of subjects who responded to Myrcludex B therapy by 12 weeks of follow-up.
Additional studies exploring Bulevirtide in combination with peginterferon alfa is ongoing in Russia (). This multicenter, randomized, open-label phase 2 study is being performed to fUrther investigate the efficacy and safety of Bulevirtide alone and in combination with peginterferon in HDV patients. In this study, 90 patients are anticipated to be randomized into one of six arms: Bulevirtide (subcutaneous injection of 5 mg daily or 5 mg twice daily or 10 mg daily) with peginterferon alfa for 48 weeks, or subcutaneous injection of Bulevirtide 10 mg with Tenofovir for 48 weeks, subcutaneous injection of Bulevirtide 2 mg monotherapy for 48 weeks, or peginterferon alfa monotherapy for 48 weeks. The primary endpoint of this study is the achievement of undetectable HDV RNA by PCR 24 weeks after the end of therapy.
In April of 2019, two additional clinical trials are expected to begin further exploring Bulevirtide. The first is a multicenter, open-label randomized phase 2b clinical trial that will likely enroll 175 patients from Russia, France, Moldova and Romania (). Patients will be randomized to one of four groups: Bulevirtide subcutaneous injection 2mg/day with peginterferon alfa for 48 weeks followed by Bulevirtide 2mg/day subcutaneous injection monotherapy for an additional 48 weeks, subcutaneous injection of Bulevirtide 10 mg/day with peginterferon for 48 weeks followed by subcutaneous injection of Bulevirtide 10 mg/day monotherapy for an additional 48 weeks, subcutaneous injection of Bulevirtide 10 mg/day monotherapy for 96 weeks, or peginterferon alfa for 48 weeks. All groups will then undergo 48 weeks of post-therapy follow-up. The primary endpoint of this study is undetectable HDV RNA in serum 24 weeks after the end of treatment.
The second study is a multicenter, open-label, randomized phase 3 clinical trial anticipated to begin assessing the long-term efficacy and safety of Bulevirtide in patients with HDV (). This three-arm study is estimated to enroll 150 HDV patients with randomization to: observation for 48 weeks followed by therapy with subcutaneous injection of Bulevirtide 10 mg/day for 96 weeks, subcutaneous injection of Bulevirtide 2 mg/day for 144 weeks, or subcutaneous injection of Bulevirtide 10 mg/day for 144 weeks. At the completion of therapy, all groups will undergo 96 weeks of additional follow-up. The primary endpoint of this study is the achievement of undetectable HDV RNA or a ≥ 2 log10 decline from baseline and ALT normalization at 48 weeks of therapy. The rationale for this extended treatment comes from modeling studies that suggest that at least three years continuous treatment with Bulevirtide might be needed to achieve sustained HDV RNA responses.
Given these early results, Bulevirtide has received orphan drug designation for the treatment of HDV infection from the European Medicines Agency (EMA) and from the U.S. Food & Drug Administration (FDA). Additionally, the EMA has granted Bulevirtide priority medicines (PRIME) scheme eligibility and the FDA has granted it breakthrough therapy designation. Interestingly, the appearance of antibodies to Bulevirtide has been demonstrated in some patients from the phase 2 studies; its significance is unknown and further evaluation is ongoing.70 A recent study evaluating in vitro and in vivo models of the impact of cell proliferation on HDV persistence demonstrated that even with hNTCP blockage by myrcludex B, clonal cell expansion permitted amplification of HDV infection which resulted in HDAg- positive hepatocytes to be observed in dividing cells during all study timepoints.72 Finally, the administration of Bulevirtide in healthy volunteers resulted in total plasma bile acids increases by 19.2 fold along with up to 124-fold increase in taurocholic acid, and an inhibition of uptake transporters OATP1B1 and OATP1B3 cytochrome P450 3A activity.73, 74 However, the clinical importance of these findings have yet to be completely understood.
HBsAg secretion inhibitors
Nucleic acid polymers (NAPs) are phosphorothioated oligonucleotides with demonstrated broad-spectrum activities against various infectious agents including herpes simplex viruses, hepatitis B and C virus, and human immunodeficiency virus.75-79 NAPs are hypothesized to have antiviral effects through several mechanisms including blocking viral entry which is dependent upon NAPs’ phosphorothioation/amphipathicity that can interact with hydrophobic surfaces of proteins glycoproteins78, 80, inhibition of HBsAg release79, 81, reduction of intracellular HBsAg via inhibition of subviral particle assembly82, and possibly interactions with the SHDAg and LHDAg leading to inhibition of the HDV replication cycle (Figure 1).83
In human clinical studies, REP 2139-Ca is the lead NAP that has been investigated in HDV infected patients and is given as a once a week intravenous infusion. In a phase 2, proof-of-concept study () treating 12 patients with REP 2139-Ca monotherapy for 15 weeks weekly IV followed by combination therapy of half dose REP 2139-Ca given weekly IV with peginterferon-alfa for 15 weeks, and then peginterferon-alfa monotherapy for 33 weeks, REP 2139-Ca demonstrated antiviral effects against both HBV and HDV.84 REP 2139-Ca appears to be the only one of the investigative therapies to reduce HBsAg rapidly resulting in a 3.5 log10 IU/ml decline in HBsAg from baseline.84 A similar reduction was also seen in a prior safety and tolerability trial.85 In patients who experienced a rapid decline in HBsAg with REP 2139-Ca monotherapy, peginterferon alfa-2a appeared to yield a profound increase in anti-HBs concentration. Overall, 6 of the 12 patients achieved anti-HBs titers above 10 mIU/mL by the end of therapy. Moreover, 9 of 12 patients became HDV RNA negative in serum by the end of treatment with a mean HDV RNA decline of 5.34 log10 IU/L. Substantial HDV RNA reduction was present in patients who had smaller declines in HBsAg which further suggests that NAPs have more than one antiviral mechanism. Moreover, this effect appears persistent as the 5 patients who achieved functional control of HBV maintained this control through 18 months. In addition, the 7 of the 9 patients who became HDV RNA negative maintained their negativity.86 A follow-up study () exploring the durability of these responses through 3 years of follow-up is currently ongoing.
REP2139-Ca is generally well tolerated.84, 85 The most frequently reported side effects with REP 2139-Ca in the initial safety and tolerability study included mild fatigue, dyspepsia, anorexia, dysphagia, dysgeusia, and hair loss. Many of these symptoms were attributed to heavy metal exposure at the trial site and the effect of increased mineral elimination by phosphorotioated oligonucleotides. Similar findings were not described in the more recent trial excluding patients with heavy metal exposure.84, 87 Commonly seen side effects from REP 2139-Ca in the phase 2 study included pyrexia, chills, thrombocytopenia, and leukopenia.84 Asymptomatic, self-resolving, substantial AST and ALT flares were commonly seen in HBV mono-infected patients after reductions of HBsAg raises the possibility of propagating decompensation in patients with advanced liver disease.85 Smaller flares were also seen in HBV/HDV co-infection.84 This is concerning since interferon therapy, which is being studied in combination with REP 2139-Ca, can cause similar flares which prevents its use in decompensated cirrhosis.85, 88-90 One patient in the phase 2 study required discontinuation of the drug due to elevation in ALT and bilirubin after introduction of peginterferon alfa-2a.84 Thus far, cirrhotic patients have not been included in studies investigating REP 2139-Ca. However, these flares may potentially be therapeutic as it was described to result in HBV viral load reduction and may be evidence of reactivation of immune response in the liver.85
Although promising, the interpretation of the results from these trials are limited by the small size of the trials. While studies have been done with intravenous dosing of REP 2139-CA, a subcutaneous formulation, REP2139-Mg, is currently being tested in HBV and a study in HDV is set to begin enrollment in Q3 2019.91 Good patient tolerability of the subcutaneous formulation will be needed for drug sustainability. Finally, additional evidence of the interplay between interferon therapy and NAPs are needed to determine if there is in fact improved rates of functional control of both HBV and HDV with combination therapy.
Virus assembly inhibitors
As previously mentioned in the virology section, prenylation is the process of adding a farnesyl group to the cysteine of the CXXX-box of the LHDAg and is essential for HDV virion assembly.39 Lonafarnib is an orally available farnesyltransferase inhibitor (FTI) that has been extensively studied in cancer92 and progeria93 which disrupts the process of prenylation and in HDV prevents proper interaction of LHDAg with HBsAg (Figure 1).94 In 2014, a proof-of-concept, randomized, placebo-controlled study demonstrated that oral lonafarnib resulted in a dose dependent, significant reduction in serum HDV RNA levels compared to placebo.95 The most common side effects of lonafarnib were noted to be GI-related including nausea, diarrhea, anorexia, and weight loss which was also dose dependent.
This study was followed by the LOWR (LOnfarnib With and without Ritonavir) HDV-1, 2, 3, and 4 studies. Ritonavir, an inhibitor of CYP3A4 which metabolizes lonafarnib, was added to allow for the use of lower doses of lonafarnib compared to the proof-of-concept study thereby minimizing GI-related side effects in a manner akin to the drug boosting tactic used with highly active antiretroviral therapy (HAART) in HIV. 42, 143 LOWR HDV-1 was a 7-arm, parallel, open-label study of 15 patients that were treated for up to 12 weeks that proved that the combination of lonafarnib with ritonavir improved patient tolerability and achieved higher serum lonafarnib concentrations, and that the addition of peginterferon alfa-2a was possible for future trials.96
This was followed by LOWR HDV-2, a dose-optimization, open-label study of various combinations of lonafarnib with ritonavir with or without peginterferon alfa-2a for 12, 24, or 48 weeks in 55 patients.97 At 24 weeks, a dose-dependent response was seen between all oral lonafarnib 25 mg twice daily vs. 50 mg twice daily, each with ritonavir 100 mg twice daily. Addition of peginterferon alfa-2a to either of these regimens demonstrated additive to synergistic effects. The most impressive results thus far with lonafarnib occurred in this study in the low dose lonafarnib groups (oral 25 or 50 mg twice daily) with low dose ritonavir (oral 100 mg twice daily) and peginterferon alfa-2a triple combination therapy. 8 of 9 patients achieved serum HDV RNA levels BLOQ or ≥ 2 log10 IU/L decline in serum HDV RNA by week 24. Patients in the 50 mg group experienced an impressive 3.81 log10 IU/L decline in HDV RNA at 24 weeks. Finally, LOWR HDV-3 and 4 were two additional dose-finding and titration studies that have been recently conducted.98, 99 LOWR HDV-3 demonstrated that once a day lonafarnib of 50 mg with ritonavir had superior results compared higher doses of lonafarnib (75mg or 100mg) with ritonavir.98 Meanwhile, LOWR HDV-4 described that dose escalation of up to lonafarnib 100 mg twice daily with ritonavir was feasible.99
It is reassuring that resistance has thus far not been reported with lonafarnib.95, 96 Interestingly, in LOWR-1 and LOWR-2, a subset of patients who did not achieve HDV RNA negativity on treatment experienced post-treatment, “therapeutic”, ALT flares with resultant HDV RNA negativity and ALT normalization.96, 97, 100 In those patients who had a liver biopsy before starting treatment, follow up biopsy performed after lonafarnib associated flare and ALT normalization revealed regression of fibrosis.101 Similar to NAPs, these findings need to be studied further in patients who are not at risk for decompensation. The main side effects in the LOWR HDV studies with the low doses of lonafarnib (25 mg or 50 mg orally twice daily) to be taken into the phase 3 registration study are mild to moderate GI-related, which can be symptomatically managed with antidiarrheals, proton pump inhibitors, or antiemetics.
Due to these results, lonafarnib has received orphan drug designation for the treatment of HDV infection from the EMA and from the U.S. FDA. In addition, lonafarnib in combination with ritonavir has been granted Breakthrough Therapy designation by the FDA and PRIME designation by the EMA for HDV infection. This has resulted in the first phase 3 for HDV, which is a randomized, placebo-controlled, trial named D-LIVR (Delta Liver Improvement and Virologic Response) () studying lonafarnib with ritonavir with or without peginterferon alfa-2a in 400 patients, and which is expected to be fully enrolled by the end of 2019.
Finally, the above data have supported the initiation of the first combination study of two investigational agents for HDV. As previously mentioned, a smaller phase 2 open label study evaluating the combination of peginterferon lambda, lonafarnib and RTV is currently ongoing at the National Institutes of Health Clinical Center ().
Conclusion
Despite being discovered over 40 years ago and being known as the most severe form of chronic viral hepatitis, the availability of adequate treatment options continues to an ongoing issue in chronic HDV infection. Although peginterferon alpha can be used with limited efficacy, it is plagued by significant side effects that limits its routine use. Multiple promising investigative therapies are now in clinical trials targeting the ISG-induction pathways (peginterferon lambda), viral entry (Bulevirtide, REP2139-Ca), subviral particle assembly/secretion (REP 2139-Ca/REP 2139-Mg), and virus assembly (lonafarnib). In the near term, therapies for patients afflicted with this devastating disease will be through participation in clinical trials and the likely success story will require some form of combination therapy.
KEY POINTS.
Hepatitis D infection represents the most serious form of viral hepatitis in humans.
Pegylated interferon alfa therapy is currently the recommended therapy but has attenuated efficacy at the cost of substantial side effects.
With increased understanding of HDV, several promising drugs (pegylated interferon lambda, lonafarnib, Bulevirtide, REP2139-Ca) have been developed to target various stages of the HDV life cycle.
Clinical trials of combination therapy with investigative drugs and pegylated interferon are currently underway.
SYNOPSIS.
Chronic hepatitis D (CHD) results from an infection with hepatitis B virus (HBV) and hepatitis D virus (HDV). CHD is the most severe form of human viral hepatitis. Current treatment options consist of interferon alfa, which is effective only in a minority of patients. The study of HDV molecular virology has resulted in new approaches to therapy that have entered clinical trials, the most advanced of which has entered phase-3 studies. These include the entry inhibitor Bulevirtide, the nucleic acid polymer REP 2139-Ca, the farnesyltransferase inhibitor lonafarnib, and pegylated interferon lambda. This review summarizes available data of these emerging therapeutics
Acknowledgments
Financial Support: This research was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health.
Footnotes
DISCLOSURE STATEMENT
Drs. Koh and Da have nothing to disclose. Dr. Glenn is a board member and has equity interest in Eiger Biopharmaceutics, Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Christopher Koh, Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Ben L. Da, Digestive Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Jeffrey S. Glenn, Departments of Medicine and Microbiology & Immunology, Division of Gastroenterology and Hepatology, Stanford University School of Medicine, Stanford, CA, USA.
References
- 1.Rizzetto M, Canese MG, Arico S, et al. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977;18:997–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor JM. Structure and replication of hepatitis delta virus RNA. Curr Top Microbiol Immunol 2006;307:1–23. [DOI] [PubMed] [Google Scholar]
- 3.Rizzetto M, Verme G, Recchia S, et al. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the delta antigen. An active and progressive disease unresponsive to immunosuppressive treatment. Ann Intern Med 1983;98:437–41. [DOI] [PubMed] [Google Scholar]
- 4.Yurdaydin C, Idilman R, Bozkaya H, et al. Natural history and treatment of chronic delta hepatitis. J Viral Hepat 2010;17:749–56. [DOI] [PubMed] [Google Scholar]
- 5.Kushner T, Serper M, Kaplan DE. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J Hepatol 2015;63:586–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toukan AU, Abu-el-Rub OA, Abu-Laban SA, et al. The epidemiology and clinical outcome of hepatitis D virus (delta) infection in Jordan. Hepatology 1987;7:1340–5. [DOI] [PubMed] [Google Scholar]
- 7.Romeo R, Del Ninno E, Rumi M, et al. A 28-year study of the course of hepatitis Delta infection: a risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009;136:1629–38. [DOI] [PubMed] [Google Scholar]
- 8.Tamura I, Kurimura O, Koda T, et al. Risk of liver cirrhosis and hepatocellular carcinoma in subjects with hepatitis B and delta virus infection: a study from Kure, Japan. J Gastroenterol Hepatol 1993;8:433–6. [DOI] [PubMed] [Google Scholar]
- 9.Fattovich G, Giustina G, Christensen E, et al. Influence of hepatitis delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 2000;46:420–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ji J, Sundquist K, Sundquist J. A population-based study of hepatitis D virus as potential risk factor for hepatocellular carcinoma. J Natl Cancer Inst 2012;104:790–2. [DOI] [PubMed] [Google Scholar]
- 11.Abbas Z, Abbas M, Abbas S, et al. Hepatitis D and hepatocellular carcinoma. World J Hepatol 2015;7:777–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Beguelin C, Moradpour D, Sahli R, et al. Hepatitis delta-associated mortality in HIV/HBV-coinfected patients. J Hepatol 2017;66:297–303. [DOI] [PubMed] [Google Scholar]
- 13.Chen HY, Shen DT, Ji DZ, et al. Prevalence and burden of hepatitis D virus infection in the global population: a systematic review and meta-analysis. Gut 2018. [DOI] [PubMed] [Google Scholar]
- 14.Sleijfer S, Bannink M, Van Gool AR, et al. Side effects of interferon-alpha therapy. Pharm World Sci 2005;27:423–31. [DOI] [PubMed] [Google Scholar]
- 15.Terrault NA, Lok ASF, McMahon BJ, et al. Update on prevention, diagnosis, and treatment of chronic hepatitis B: AASLD 2018 hepatitis B guidance. Hepatology 2018;67:1560–1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J Hepatol 2017;67:370–398. [DOI] [PubMed] [Google Scholar]
- 17.Wranke A, Serrano BC, Heidrich B, et al. Antiviral treatment and liver-related complications in hepatitis delta. Hepatology 2017;65:414–425. [DOI] [PubMed] [Google Scholar]
- 18.Farci P, Roskams T, Chessa L, et al. Long-term benefit of interferon alpha therapy of chronic hepatitis D: regression of advanced hepatic fibrosis. Gastroenterology 2004;126:1740–9. [DOI] [PubMed] [Google Scholar]
- 19.Yurdaydin C, Keskin O, Kalkan C, et al. Interferon Treatment Duration in Patients With Chronic Delta Hepatitis and its Effect on the Natural Course of the Disease. J Infect Dis 2018;217:1184–1192. [DOI] [PubMed] [Google Scholar]
- 20.Gunsar F, Akarca US, Ersoz G, et al. Two-year interferon therapy with or without ribavirin in chronic delta hepatitis. Antivir Ther 2005;10:721–6. [PubMed] [Google Scholar]
- 21.Niro GA, Ciancio A, Gaeta GB, et al. Pegylated interferon alpha-2b as monotherapy or in combination with ribavirin in chronic hepatitis delta. Hepatology 2006;44:713–20. [DOI] [PubMed] [Google Scholar]
- 22.Yurdaydin C, Bozkaya H, Onder FO, et al. Treatment of chronic delta hepatitis with lamivudine vs lamivudine + interferon vs interferon. J Viral Hepat 2008;15:314–21. [DOI] [PubMed] [Google Scholar]
- 23.Lau DT, Doo E, Park Y, et al. Lamivudine for chronic delta hepatitis. Hepatology 1999;30:546–9. [DOI] [PubMed] [Google Scholar]
- 24.Yurdaydin C, Bozkaya H, Gurel S, et al. Famciclovir treatment of chronic delta hepatitis. J Hepatol 2002;37:266–71. [DOI] [PubMed] [Google Scholar]
- 25.Wedemeyer H, Yurdaydin C, Ernst S, et al. Prolonged therapy of hepatitis delta for 96 weeks with pegylated-interferon-2a plus tenofovir or placebo does not prevent HDV RNA relapase after treatment: the HIDIT-2 study. Journal of Hepatology 2014;60:S2–S3. [Google Scholar]
- 26.Wedemeyer H, Yurdaydin C, Dalekos GN, et al. Peginterferon plus adefovir versus either drug alone for hepatitis delta. N Engl J Med 2011;364:322–31. [DOI] [PubMed] [Google Scholar]
- 27.Sureau C The role of the HBV envelope proteins in the HDV replication cycle. Curr Top Microbiol Immunol 2006;307:113–31. [DOI] [PubMed] [Google Scholar]
- 28.Bonino F, Heermann KH, Rizzetto M, et al. Hepatitis delta virus: protein composition of delta antigen and its hepatitis B virus-derived envelope. J Virol 1986;58:945–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rizzetto M, Canese MG, Gerin JL, et al. Transmission of the hepatitis B virus-associated delta antigen to chimpanzees. J Infect Dis 1980;141:590–602. [DOI] [PubMed] [Google Scholar]
- 30.Polo JM, Jeng KS, Lim B, et al. Transgenic mice support replication of hepatitis delta virus RNA in multiple tissues, particularly in skeletal muscle. J Virol 1995;69:4880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sureau C, Negro F. The hepatitis delta virus: Replication and pathogenesis. J Hepatol 2016;64:S102–S116. [DOI] [PubMed] [Google Scholar]
- 32.Barrera A, Guerra B, Notvall L, et al. Mapping of the hepatitis B virus pre-S1 domain involved in receptor recognition. J Virol 2005;79:9786–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yan H, Zhong G, Xu G, et al. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. Elife 2012;3. [DOI] [PubMed] [Google Scholar]
- 34.Hughes SA, Wedemeyer H, Harrison PM. Hepatitis delta virus. Lancet 2011;378:73–85. [DOI] [PubMed] [Google Scholar]
- 35.Chang J, Nie X, Chang HE, et al. Transcription of hepatitis delta virus RNA by RNA polymerase II. J Virol 2008;82:1118–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lai MM. The molecular biology of hepatitis delta virus. Annu Rev Biochem 1995;64:259–86. [DOI] [PubMed] [Google Scholar]
- 37.Glenn JS. Prenylation of HDAg and antiviral drug development. Curr Top Microbiol Immunol 2006;307:133–49. [DOI] [PubMed] [Google Scholar]
- 38.Purcell RH, Satterfield WC, Bergmann KF, et al. Experimental hepatitis delta virus infection in the chimpanzee. Prog Clin Biol Res 1987;234:27–36. [PubMed] [Google Scholar]
- 39.Guilhot S, Huang SN, Xia YP, et al. Expression of the hepatitis delta virus large and small antigens in transgenic mice. J Virol 1994;68:1052–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sato S, Cornillez-Ty C, Lazinski DW. By inhibiting replication, the large hepatitis delta antigen can indirectly regulate amber/W editing and its own expression. J Virol 2004;78:8120–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Glenn JS, White JM. trans-dominant inhibition of human hepatitis delta virus genome replication. J Virol 1991;65:2357–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chao M, Hsieh SY, Taylor J. Role of two forms of hepatitis delta virus antigen: evidence for a mechanism of self-limiting genome replication. J Virol 1990;64:5066–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cole SM, Gowans EJ, Macnaughton TB, et al. Direct evidence for cytotoxicity associated with expression of hepatitis delta virus antigen. Hepatology 1991;13:845–51. [PubMed] [Google Scholar]
- 44.Niro GA, Smedile A. Current concept in the pathophysiology of hepatitis delta infection. Curr Infect Dis Rep 2012;14:9–14. [DOI] [PubMed] [Google Scholar]
- 45.Fiedler M, Roggendorf M. Immunology of HDV infection. Curr Top Microbiol Immunol 2006;307:187–209. [DOI] [PubMed] [Google Scholar]
- 46.Terrault NA, Bzowej NH, Chang KM, et al. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016;63:261–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farci P, Mandas A, Coiana A, et al. Treatment of chronic hepatitis D with interferon alfa-2a. N Engl J Med 1994;330:88–94. [DOI] [PubMed] [Google Scholar]
- 48.Castelnau C, Le Gal F, Ripault MP, et al. Efficacy of peginterferon alpha-2b in chronic hepatitis delta: relevance of quantitative RT-PCR for follow-up. Hepatology 2006;44:728–35. [DOI] [PubMed] [Google Scholar]
- 49.Heller T, Rotman Y, Koh C, et al. Long-term therapy of chronic delta hepatitis with peginterferon alfa. Aliment Pharmacol Ther 2014;40:93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Guedj J, Rotman Y, Cotler SJ, et al. Understanding early serum hepatitis D virus and hepatitis B surface antigen kinetics during pegylated interferon-alpha therapy via mathematical modeling. Hepatology 2014;60:1902–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Keskin O, Wedemeyer H, Tuzun A, et al. Association Between Level of Hepatitis D Virus RNA at Week 24 of Pegylated Interferon Therapy and Outcome. Clin Gastroenterol Hepatol 2015;13:2342–49 e1-2. [DOI] [PubMed] [Google Scholar]
- 52.Kaymakoglu S, Karaca C, Demir K, et al. Alpha interferon and ribavirin combination therapy of chronic hepatitis D. Antimicrob Agents Chemother 2005;49:1135–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wolters LM, van Nunen AB, Honkoop P, et al. Lamivudine-high dose interferon combination therapy for chronic hepatitis B patients co-infected with the hepatitis D virus. J Viral Hepat 2000;7:428–34. [DOI] [PubMed] [Google Scholar]
- 54.Wedemeyer H, Yurdaydin C, Ernst S, et al. 96 weeks of pegylated-interferon-alpha-2a plus tenofovir or placebo for the treatment of hepatitis delta: the HIDIT-2 study. Hepatology 2013;58:222A–223A. [Google Scholar]
- 55.Wranke A, Heidrich B, Ernst S, et al. Anti-HDV IgM as a marker of disease activity in hepatitis delta. PLoS One 2014;9:e101002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wedemeyer H, Yurdaydin C, Hardtke S, et al. Peginterferon alfa-2a plus tenofovir disoproxil fumarate for hepatitis D (HIDIT-II): a randomised, placebo controlled, phase 2 trial. Lancet Infect Dis 2019;19:275–286. [DOI] [PubMed] [Google Scholar]
- 57.Mutchnick MG, Appelman HD, Chung HT, et al. Thymosin treatment of chronic hepatitis B: a placebo-controlled pilot trial. Hepatology 1991;14:409–15. [PubMed] [Google Scholar]
- 58.Andreone P, Cursaro C, Gramenzi A, et al. A randomized controlled trial of thymosin-alpha1 versus interferon alfa treatment in patients with hepatitis B e antigen antibody-- and hepatitis B virus DNA--positive chronic hepatitis B. Hepatology 1996;24:774–7. [DOI] [PubMed] [Google Scholar]
- 59.Rosina F, Conoscitore P, Smedile A, et al. Treatment of chronic hepatitis D with thymus-derived polypeptide thymic humoral factor-gamma 2: a pilot study. Dig Liver Dis 2002;34:285–9. [DOI] [PubMed] [Google Scholar]
- 60.Zavaglia C, Bottelli R, Smedile A, et al. A pilot study of thymosin-alpha 1 therapy for chronic hepatitis D. J Clin Gastroenterol 1996;23:162–3. [DOI] [PubMed] [Google Scholar]
- 61.Chan HLY, Ahn SH, Chang TT, et al. Peginterferon lambda for the treatment of HBeAg-positive chronic hepatitis B: A randomized phase 2b study (LIRA-B). J Hepatol 2016;64:1011–1019. [DOI] [PubMed] [Google Scholar]
- 62.Foster GR, Chayama K, Chuang WL, et al. A randomized, controlled study of peginterferon lambda-1a/ribavirin +/− daclatasvir for hepatitis C virus genotype 2 or 3. Springerplus; 2016;5:1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ank N, West H, Bartholdy C, et al. Lambda interferon (IFN-lambda), a type III IFN, is induced by viruses and IFNs and displays potent antiviral activity against select virus infections in vivo. J Virol 2006;80:4501–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lasfar A, Abushahba W, Balan M, et al. Interferon lambda: a new sword in cancer immunotherapy. Clin Dev Immunol 2011;2011:349575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Giersch K, Homs M, Volz T, et al. Both interferon alpha and lambda can reduce all intrahepatic HDV infection markers in HBV/HDV infected humanized mice. Sci Rep 2017;7:3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Etzion O, Hamid SS, Lurie Y, et al. End of Study Results from LIMT HDV Study: 36% Durable Virologic Response at 24 Weeks Post-Treatment with Pegylated Interferon Lambda Monotherapy in Patients with Chronic Hepatitis Delta Virus Infection. J Hepatol 2019;70. [Google Scholar]
- 67.Engelke M, Mills K, Seitz S, et al. Characterization of a hepatitis B and hepatitis delta virus receptor binding site. Hepatology 2006;43:750–60. [DOI] [PubMed] [Google Scholar]
- 68.Lutgehetmann M, Mancke LV, Volz T, et al. Humanized chimeric uPA mouse model for the study of hepatitis B and D virus interactions and preclinical drug evaluation. Hepatology 2012;55:685–94. [DOI] [PubMed] [Google Scholar]
- 69.Gripon P, Cannie I, Urban S. Efficient inhibition of hepatitis B virus infection by acylated peptides derived from the large viral surface protein. J Virol 2005;79:1613–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bogomolov P, Alexandrov A, Voronkova N, et al. Treatment of chronic hepatitis D with the entry inhibitor myrcludex B: First results of a phase Ib/IIa study. J Hepatol 2016;65:490–8. [DOI] [PubMed] [Google Scholar]
- 71.Wedemeyer H, Bogomolov P, Blank A, et al. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J Hepatol 2018;68:S3. [Google Scholar]
- 72.Giersch K, Bhadra OD, Volz T, et al. Hepatitis delta virus persists during liver regeneration and is amplified through cell division both in vitro and in vivo. Gut 2019;68:150–157. [DOI] [PubMed] [Google Scholar]
- 73.Blank A, Eidam A, Haag M, et al. The NTCP-inhibitor Myrcludex B: Effects on Bile Acid Disposition and Tenofovir Pharmacokinetics. Clin Pharmacol Ther 2018;103:341–348. [DOI] [PubMed] [Google Scholar]
- 74.Blank A, Meier K, Urban S, et al. Drug-drug interaction potential of the HBV and HDV entry inhibitor myrcludex B assessed in vitro. Antivir Ther 2018;23:267–275. [DOI] [PubMed] [Google Scholar]
- 75.Bernstein DI, Goyette N, Cardin R, et al. Amphipathic DNA polymers exhibit antiherpetic activity in vitro and in vivo. Antimicrob Agents Chemother 2008;52:2727–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Guzman EM, Cheshenko N, Shende V, et al. Amphipathic DNA polymers are candidate vaginal microbicides and block herpes simplex virus binding, entry and viral gene expression. Antivir Ther 2007;12:1147–56. [PubMed] [Google Scholar]
- 77.Matsumura T, Hu Z, Kato T, et al. Amphipathic DNA polymers inhibit hepatitis C virus infection by blocking viral entry. Gastroenterology 2009;137:673–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Vaillant A, Juteau JM, Lu H, et al. Phosphorothioate oligonucleotides inhibit human immunodeficiency virus type 1 fusion by blocking gp41 core formation. Antimicrob Agents Chemother 2006;50:1393–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Noordeen F, Vaillant A, Jilbert AR. Nucleic acid polymers inhibit duck hepatitis B virus infection in vitro. Antimicrob Agents Chemother 2013;57:5291–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beilstein F, Blanchet M, Vaillant A, et al. Nucleic Acid Polymers Are Active against Hepatitis Delta Virus Infection In Vitro. J Virol 2018;92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Noordeen F, Scougall CA, Grosse A, et al. Therapeutic Antiviral Effect of the Nucleic Acid Polymer REP 2055 against Persistent Duck Hepatitis B Virus Infection. PLoS One 2015;10:e0140909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Blanchet M, Sinnathamby V, Vaillant A, et al. Inhibition of HBsAg secretion by nucleic acid polymers in HepG2.2.15cells. Antiviral Res 2019;164:97–105. [DOI] [PubMed] [Google Scholar]
- 83.Shamur M P- NR, Mayer R, Vaillant A. Interaction of nucleic acid polymers with the large and small forms of hepatitis delta antigen protein. Hepatology 2017;66. [Google Scholar]
- 84.Bazinet M, Pantea V, Cebotarescu V, et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): a non-randomised, open-label, phase 2 trial. Lancet Gastroenterol Hepatol 2017;2:877–889. [DOI] [PubMed] [Google Scholar]
- 85.Al-Mahtab M, Bazinet M, Vaillant A. Safety and Efficacy of Nucleic Acid Polymers in Monotherapy and Combined with Immunotherapy in Treatment-Naive Bangladeshi Patients with HBeAg+ Chronic Hepatitis B Infection. PLoS One 2016;11:e0156667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bazinet M, Pantea V, Cebotarescu V, et al. Establishment of persistent functional remission of HBV and HDV infection following REP 2139 and pegylated interferon alpha 2a therapy in patients with chronic HBV/HDV co-infection: 18 month follow-up results from the REP 301-LTF study. Journal of Hepatology 2018;68:S509. [Google Scholar]
- 87.Mata JE, Bishop MR, Tarantolo SR, et al. Evidence of enhanced iron excretion during systemic phosphorothioate oligodeoxynucleotide treatment. J Toxicol Clin Toxicol 2000;38:383–7. [DOI] [PubMed] [Google Scholar]
- 88.Alfaiate D, Negro F. Nucleic acid polymers: much-needed hope for hepatitis D? Lancet Gastroenterol Hepatol 2017;2:841–842. [DOI] [PubMed] [Google Scholar]
- 89.Janssen HL, Brouwer JT, Nevens F, et al. Fatal hepatic decompensation associated with interferon alfa. European concerted action on viral hepatitis (Eurohep). BMJ 1993;306:107–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iacobellis A, Andriulli A. Antiviral therapy in compensated and decompensated cirrhotic patients with chronic HCV infection. Expert Opin Pharmacother 2009;10:1929–38. [DOI] [PubMed] [Google Scholar]
- 91.Bazinet MPV, Placinta G. Update on safety and efficacy in the REP 401 protocol: REP 2139-Mg or REP 2165-Mg used in combination with tenofovir disoproxil fumarate and pegylated Interferon alpha-2a in treatment naive Caucasian patients with chronic HBeAg negative HBV infection, In International Liver Congress, Amsterdam, Netherlands, April 19–23, 2017. [Google Scholar]
- 92.Wong NS, Morse MA. Lonafarnib for cancer and progeria. Expert Opin Investig Drugs 2012;21:1043–55. [DOI] [PubMed] [Google Scholar]
- 93.Gordon LB, Shappell H, Massaro J, et al. Association of Lonafarnib Treatment vs No Treatment With Mortality Rate in Patients With Hutchinson-Gilford Progeria Syndrome. JAMA 2018;319:1687–1695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bordier BB, Marion PL, Ohashi K, et al. A prenylation inhibitor prevents production of infectious hepatitis delta virus particles. J Virol 2002;76:10465–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Koh C, Canini L, Dahari H, et al. Oral prenylation inhibition with lonafarnib in chronic hepatitis D infection: a proof-of-concept randomised, double-blind, placebo-controlled phase 2A trial. Lancet Infect Dis 2015;15:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yurdaydin C, Keskin O, Kalkan C, et al. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology 2018;67:1224–1236. [DOI] [PubMed] [Google Scholar]
- 97.Yurdaydin C, Idilman R, Keskin O, et al. A phase 2 dose-optimization study of lonafarnib with ritonavir for the treatment of chronic delta hepatitis-end of treatment results from the LOWR HDV-2 study. J Hepatol 2017;66:S33–34. [Google Scholar]
- 98.Koh C, Surana P, Han T, et al. A phase 2 study exploring once daily dosing of ritonavir boosted lonafarnib for the treatment of chronic delta hepatitis – end of study results from the LOWR HDV-3 study. Journal of Hepatology 2017;66:S101–S102. [Google Scholar]
- 99.Wedemeyer H, Port K, Deterding K, et al. A Phase 2 Study Of Titrating-Dose Lonafarnib Plus Ritonavir In Patients With Chronic Hepatitis D: Interim Results From The Lonafarnib With Ritonavir In HDV-4 (LOWR HDV-4) Study. Hepatology 2016;64:121A. [Google Scholar]
- 100.Yurdaydin C IR, Kalkan C, Karakya F, Kartal AC, Keskin O, Karatayli E, Karatayli SC, Bozdayi AM, Koh C, Heller T, Glenn J. The Prenylation Inhibitor Lonafarnib Can Induce Post-Treatment Alt Flares With Viral Cle. Hepatology 2016;64:927A. [Google Scholar]
- 101.Yurdaydin C, Idilman R, Kalkan C, et al. The prenylation inhibitor lonafarnib can induce post-treatment viral clearance in patients with chronic delta hepatitis resulting in ALT normalization and regression of fibrosis. J Hepatol 2017;66:S259. [Google Scholar]