Abstract
Background:
High-degree AV block (HDAVB) is a known complication after both transcatheter aortic valve implantation (TAVI) and surgical aortic valve replacement (SAVR). Data about the contemporary practice pattern of permanent pacing, especially the timing of implantation, for HDAVB after TAVI compared to SAVR are limited.
Methods:
Using the National Inpatient Sample database, we identified patients who underwent TAVI or SAVR from 2012 to 2014. We excluded patients with a prior pacemaker and implantable cardioverter defibrillator. The incidence of HDAVB, the rate of permanent pacemaker implantation and its timing were compared between TAVI and SAVR groups.
Results:
We identified 33,690 and 202,110 patients who underwent TAVI and SAVR, respectively. HDAVB occurred in 3,480 patients (10.3%) in the TAVI group and 11,405 patients (5.6%) in the SAVR group (p<0.001). Among the patients who developed HDAVB, patients in the TAVI group were more likely to undergo permanent pacemaker implantation than those in the SAVR group (74.1% versus 64.7%; p<0.001). The median interval from TAVI to pacemaker implantation was 2 days (interquartile range 1–3 days) versus 5 days (interquartile range 3–7 days) from SAVR to pacemaker implantation (p<0.001). Among the patients who developed HDAVB after either TAVI or SAVR, TAVI was independently associated with permanent pacemaker implantation after adjusting for other comorbidities (odds ratio 1.41: 95% confidence interval 1.13–1.77; p=0.003)
Conclusions:
HDAVB occurred more commonly after TAVI compared to SAVR. HDAVB after TAVI compared to SAVR was more likely to lead to permanent pacemaker implantation at earlier timing from the index procedure.
Introduction:
The indication of transcatheter aortic valve implantation (TAVI) has expanded following the promising results of randomized trials in prohibitive, high and intermediate surgical risk patients (1)(2)(3)(4). Although the overall procedural complications of TAVI are decreasing, AV conduction disorders requiring permanent pacemaker remains relatively frequent, occurring in about 6% of patients following balloon-expandable valve (BEV) implantation (interquartile range 5–7%) and about 28% following self-expanding valve (SEV) implantation (interquartile range 24–35%) versus 6.6% with SAVR (5)(6)(7). The majority of permanent pacemakers implantation following TAVI are for high-degree AV block (HDAVB) including complete AV block and Mobitz-type II second-degree AV block (5)(8)(9).
Importantly, spontaneous recovery from HDAVB can be observed after TAVI often within a few days, although it can also happen weeks or even months after the procedure (10)(11). Given the potential recovery of HDAVB, clinicians often face a dilemma about the optimal timing of permanent pacemaker implantation for HDAVB following TAVI. The European guideline recommends a period of clinical observation up to 7 days to assess the “persistence” of HDAVB (11). However, several large registries reported that the large majority of permanent pacemaker following TAVI are implanted within 3 days after the procedure (8)(12).
The rate of permanent pacemaker implantation after TAVI has been well described, however, the overall incidence of HDAVB after TAVI in comparison to SAVR and the rate of permanent pacemaker implantation among those who developed HDAVB remains incompletely described (13)(14)(15). The rate of spontaneous recovery of HDAVB after TAVI and SAVR is of clinical interest as it may impact on the decision to implant a pacemaker and its timing. In this context, we aimed to investigate the incidence of HDAVB and the contemporary practice pattern of permanent pacing, especially the timing of implantation, for HDAVB following TAVI in comparison to SAVR using a large-scale inpatient database.
Methods:
Data source
The National Inpatient Sample (NIS) database is the largest publicly available all-payer inpatient care database in the United States, which is a part of the Healthcare Cost and Utilization Project from the Agency for Healthcare Research and Quality. This NIS dataset contains over a hundred clinical and non-clinical data elements from more than 7 million hospital discharges per year. The NIS dataset from 2012 to 2014 was sampled from a stratified sample of approximately 20 percent of discharges from all United States community hospitals. The University of Kentucky Institutional Review Board deemed this study exempt from a formal review as the NIS database is available to the public as aggregate data without direct personal identifiers (16).
Inclusion and exclusion criteria
We analyzed the NIS database from 2012 to 2014 and identified all hospitalized patients 18 years or older who underwent TAVI or SAVR. This study period was chosen based on the FDA approval of TAVI in the United States (late 2011). TAVI was identified by using International Classification of Diseases, Ninth Edition, Clinical Modification (ICD-9-CM) procedure codes 35.05 (trans-femoral) and 35.06 (trans-apical). SAVR was identified by ICD-9-CM codes 35.21 (bioprosthetic valve) and 35.22 (mechanical valve). We excluded patients who underwent both TAVI and SAVR during the same admission, those with missing survival status, those with a prior pacemaker (ICD-9-CM code V45.01) or implantable cardioverter defibrillator implantation (ICD-9-CM code V45.02). We also excluded patients with missing procedure date for TAVI, SAVR or pacemaker implantation and those who underwent permanent pacemaker implantation before TAVI or SAVR.
Patients characteristics and outcomes of interest
Patients’ baseline demographics and relevant clinical comorbidities were obtained using ICD-9-CM codes and the Elixhauser Comorbidity adjustment method (17). A list of codes used to identify comorbidities is presented in a supplemental table. Patients were divided into TAVI and SAVR groups, with a comparison between their characteristics and clinical outcomes. The primary outcome of interest was the occurrence of high-degree AV block (HDAVB) consisting of complete AV block (code 426.0) or Mobitz-type II second-degree AV block (code 426.12). The secondary outcomes included the rate of permanent pacemaker implantation and the timing of implantation (days after TAVI or SAVR), all-cause in-hospital mortality, and hospital length of stay.
Statistical analyses
Continuous data were expressed as mean± standard deviation or median with interquartile range (IQR) and were compared using Mann-Whitney U test. Categorical variables were expressed in percentage and compared using the Pearson chi-square test. The intervals from TAVI or SAVR to permanent pacemaker implantation were calculated based on procedure date and compared among the year 2012, 2013 and 2014. We also compared the yearly trend on the length of stay and timing of pacemaker implantation over the three-year period. We evaluated an association between TAVI (in comparison to SAVR) and permanent pacemaker implantation in the subgroup of the patients who developed HDAVB after either TAVI or SAVR, using multivariable logistic regression analysis with adjusting for demographics and comorbidities listed in Table 1. Discharge weights provided in the NIS dataset were used to create national estimates. Weighted data were used for all analysis except for the analysis regarding procedure day (timing of pacemaker implantation), for which unweighted data were used. We used the complex sample analysis capabilities of SPSS to account for strata and clustering in analyzing dichotomous variables. Those p values <0.05 were considered statistically significant. All statistical analyses were performed with SPSS version 24.0 (IBM Corp, Armonk, New York, United States).
Table 1: Demographics, risk factors and hospital characteristics in patients who underwent TAVI and SAVR.
| TAVI (n=33,690) | SAVR (n=202,110) | p-value | |
|---|---|---|---|
| Admission year 2012 | 6320 | 65805 | |
| Admission year 2013 | 11420 | 68405 | |
| Admission year 2014 | 15950 | 67900 | |
| Age (mean ± standard deviation) | 81 ± 9 | 68 ± 13 | <0.001 |
| Male | 50.6% | 64.5% | <0.001 |
| Race White | 81.4% | 77.9% | <0.001 |
| Hypertension | 79.4% | 73.2% | <0.001 |
| Diabetes | 34.5% | 30.0% | <0.001 |
| Dyslipidemia | 64.0% | 58.9% | <0.001 |
| Current smoking | 3.4% | 10.5% | <0.001 |
| Obesity | 14.8% | 20.3% | <0.001 |
| Coronary artery disease | 29.5% | 42.8% | <0.001 |
| Prior PCI | 18.3% | 8.0% | <0.001 |
| Prior CABG | 20.7% | 4.4% | <0.001 |
| Prior stroke/TIA | 10.0% | 6.2% | <0.001 |
| Anemia | 26.5% | 19.4% | <0.001 |
| Renal failure | 35.4% | 15.9% | <0.001 |
| Chronic pulmonary disease | 33.6% | 20.9% | <0.001 |
| Peripheral vascular disease | 29.9% | 21.0% | <0.001 |
| Atrial fibrillation | 43.2% | 44.9% | 0.02 |
| Hospital location/status | <0.001 | ||
| Rural hospital | 0.7% | 2.3% | |
| Urban teaching hospital | 89.2% | 74.6% | |
| Urban nonteaching hospital | 10.1% | 23.1% | |
| Hospital size | <0.001 | ||
| Small | 4.4% | 6.8% | |
| Medium | 16.9% | 20.4% | |
| Large | 78.7% | 72.8% | |
| Hospital region | 0.04 | ||
| Midwest | 22.7% | 24.9% | |
| Northeast | 26.5% | 22.8% | |
| South | 33.3% | 32.5% | |
| West | 17.5% | 19.8% | |
| Length of stay (days; median [IQR]) | 6 [4–9] | 8 [5–12] | <0.001 |
| Cost ($; median [IQR]) | 195,620 [142,667–274,253] | 161,840 [113,168–246,396] | <0.001 |
CABG: coronary artery bypass grafting, IQR: interquartile range, PCI: percutaneous coronary intervention, SAVR: surgical aortic valve replacement, TAVI: trans-catheter aortic valve implantation, TIA: transient ischemic attack,
Results:
TAVI and SAVR patients
After exclusions, 33,690 inpatients (admissions) who underwent TAVI and 202,110 inpatients who underwent SAVR were included for analysis (Figure 1). The number of TAVI cases increased from the year 2012 to 2014, whereas the number of annual SAVR cases remained stable. Patients’ characteristics of TAVI and SAVR cohorts are shown in Table 1. The majority of TAVI procedures (79.8%) were performed via a trans-femoral approach. Patients who underwent TAVI were older and had a much higher burden of comorbidities burden than those who underwent SAVR.
Figure 1.
Flow chart of patient selection ICD; implantable cardioverter defibrillator, SAVR; surgical aortic valve replacement, TAVI; transcatheter aortic valve implantation
Incidence of HDAVB in TAVI versus SAVR patients
HDAVB occurred in 3,480 patients (10.3%) in the TAVI group and 11,405 patients (5.6%) in the SAVR group (p<0.001). Complete AV block accounted for the majority of HDAVB in both TAVI (98.0%) and SVAR groups (97.3%). In the TAVI cohort, patients who developed HDAVB were older and more likely to have renal failure and atrial fibrillation than those who did not (Table 2). Among the patients who developed HDAVB after either TAVI or SAVR, patients in the TAVI group were more likely to undergo pacemaker implantation than those in the SAVR group (74.1% versus 64.7%; p<0.001). This difference remained significant after excluding those who died (76.0% in TAVI group versus 66.6% in SAVR group; p<0.001). Among the patients with HDAVB, TAVI was independently associated with permanent pacemaker implantation after adjusting for demographics and comorbidities (odds ratio 1.41: 95% confidence interval 1.13–1.77; p=0.003)
Table 2: Demographics and risk factors of patients who developed HDAVB versus no HDAVB after TAVI.
| HDAVB (n=3,480) | No HDAVB (n=30,210) | p-value | |
|---|---|---|---|
| Age (mean ± standard deviation) | 82 ± 8 | 81 ± 9 | <0.001 |
| Male | 50.9% | 50.6% | 0.89 |
| Race White | 82.6% | 81.2% | 0.35 |
| Hypertension | 79.2% | 79.5% | 0.84 |
| Diabetes | 37.4% | 34.2% | 0.07 |
| Dyslipidemia | 62.8% | 64.1% | 0.49 |
| Current smoking | 2.7% | 3.4% | 0.31 |
| Obesity | 14.5% | 14.8% | 0.83 |
| Coronary artery disease | 30.2% | 70.6% | 0.69 |
| Prior PCI | 17.4% | 18.4% | 0.48 |
| Prior CABG | 18.7% | 20.9% | 0.15 |
| Prior stroke/TIA | 10.6% | 9.9% | 0.54 |
| Anemia | 25.3% | 26.7% | 0.40 |
| Renal failure | 39.4% | 34.9% | 0.02 |
| Chronic pulmonary disease | 32.5% | 33.7% | 0.54 |
| Peripheral vascular disease | 29.2% | 30.0% | 0.61 |
| Atrial fibrillation | 39.1% | 43.7% | 0.02 |
| In-hospital mortality | 5.5% | 4.1% | 0.07 |
CABG: coronary artery bypass grafting, PCI: percutaneous coronary intervention, TAVI: trans-catheter aortic valve implantation, TIA: transient ischemic attack,
Timing of pacemaker implant and yearly trends
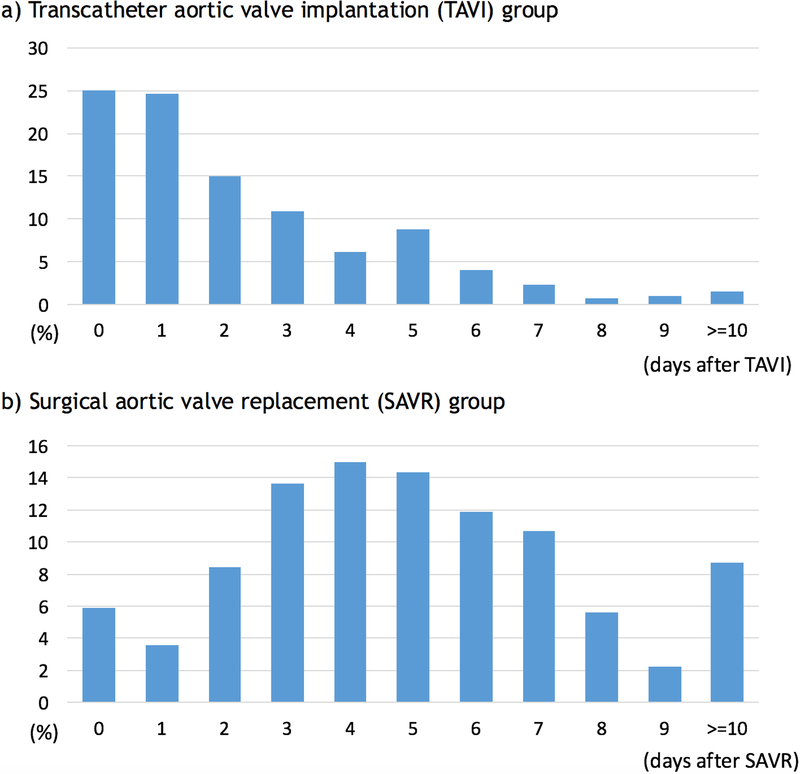
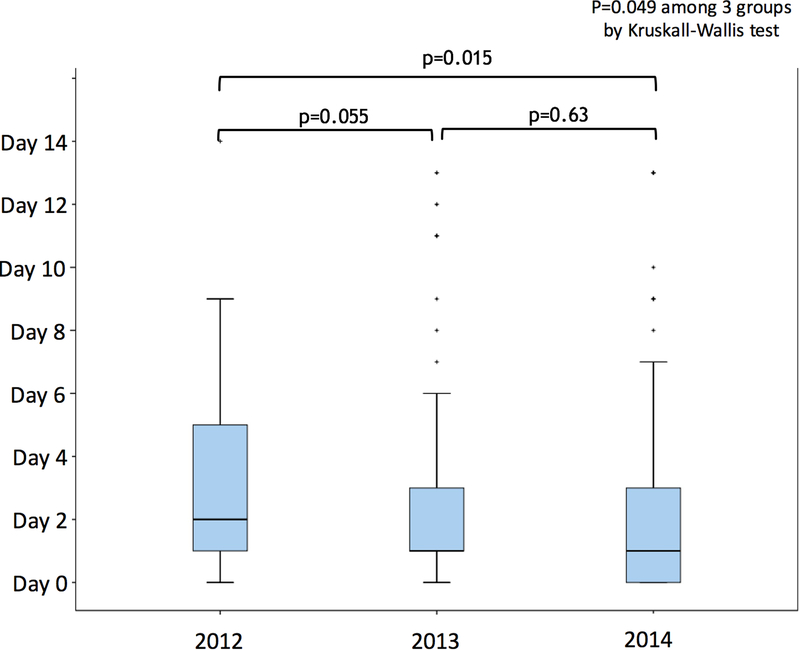
Figure 2 illustrates the timing of pacemaker implantation after TAVI or SAVR. The median interval from TAVI to pacemaker implantation placement was 2 days (interquartile range 1–3 days) versus 5 days (interquartile range 3–7 days) from SAVR to pacemaker implantation (p<0.001). In the TAVI group, 64.5% of permanent pacemakers were placed within 72 hours after the procedure (on day 0, 1, or 2 after TAVI). There was a difference in the interval from TAVI to pacemaker implantation among the admission years of 2012, 2013 and 2014 (p=0.049 by Kruskal-Wallis test) (Figure 3), with pacemakers being implanted earlier after TAVI in 2014 than in 2012. In contrast, there was no significant difference in the interval from SAVR to pacemaker implantation among the years 2012, 2013, and 2014 (p=0.61 by Kruskal-Wallis test).
Figure 2.
Timings of permanent pacemaker implantation for high-degree AV block: a) in transcatheter aortic valve implantation group and b) surgical aortic valve replacement group. Permanent pacemakers were implanted sooner after TAVI than SAVR (the median interval 2 days [interquartile range 1–3 days] versus 5 days [interquartile range 3–7 days]; p<0.001)
Figure 3.
Timings of permanent pacemaker implantation for high-degree AV block after TAVI according to admission year Permanent pacemakers tended to be implanted earlier after TAVI in 2014 than in 2012.
Mortality and length of stay
Among the patients who developed HDAVB, there was no statistically significant difference in in-hospital mortality between TAVI and SAVR cohorts (5.5% versus 4.0%; p=0.07). The median length of stay for TAVI was 6 days [interquartile range 4–9 days]. In the TAVI cohort, there was a significant difference in length of stay among the year 2012, 2013 and 2014 (p<0.001 by Kruskal-Wallis test), and the length of stay in 2014 (median 6 days [IQR 4–9]) was shorter than that in 2012 (median 6 days [IQR 4–10]; p<0.001) or 2013 (median 7 days [IQR 4–10]; p<0.001).
Discussion:
The main findings of our study include 1) HDAVB occurred in 10.3% in the TAVI group and 5.6% in the SAVR group; 2) permanent pacemakers were implanted earlier after TAVI than SAVR (the median interval of 2 days versus 5 days); and 3) among the patients who developed HDAVB, patients in the TAVI group were more likely to undergo permanent pacemaker implantation than those in the SAVR group (74.1% versus 64.7%).
Incidence of HDAVB after TAVI and SAVR
The reported incidences of HDAVB after TAVI varied depending on the type of the valve; 6.8% to 10.8% with BEV and 19.3% to 35.7% with SEV (18)(13)(14)(15). Although we did not have the data on specific types of the valve, our analysis reflects mostly BEV, since the national registry data in the United States up to September 2014 only showed an 11.2% rate of SEV use (12). Considering these factors, the 10.3% incidence of HDAVB after TAVI in our cohort is overall consistent with prior reports. The rate of HDAVB in SAVR at 5.6% is also in line with prior reports (19).
Mechanisms and recovery of HDAVB after TAVI and SAVR
Mechanisms of AV block following SAVR include the removal of the native valve and peri-valvular tissue or sutures placed near the membranous septum. TAVI, on the other hand, involves a direct injury to the conduction system from compression of peri-valvular tissue by the implanted valve cage (20). Although these direct injuries can be irreversible, both SAVR and TAVI often cause transient edema of the surrounding tissue, which leads to transient HDAVB that recovers in a few days to several weeks (10)(11). In a recent study evaluating pacemaker interrogation findings at outpatient follow-up after TAVI, 61% of the HDAVB had resolved, and the majority of recovery occurred within one month after TAVI (18). Similarly, another study reported that only one-third of the patients who underwent pacemaker implantation for complete AV block remained in complete AV block (21).
Pacemaker implants after TAVI
There is a variation in the rate of permanent pacemaker implantation in patients who developed peri-procedural HDAVB, ranging from 83.3% to 100% (13)(14)(15). Our study found that three-quarters of patients who developed HDAVB after TAVI underwent permanent pacemaker implantation, suggesting 25% chance of early spontaneous recovery. Therefore, clinicians often face a dilemma to determine the optimal timing of permanent pacemaker implantation for HDAVB following TAVI. The European guideline recommends a period of clinical observation up to 7 days to assess the “persistence” of HDAVB with an exception for cases with a low rate of escape rhythm in which this observation period can be shortened (11). Such recommendation for a long waiting period is reasonable considering the relatively common short- and long-term complications related to permanent pacemaker implants (22). In current clinical practice, however, the large majority of permanent pacemaker following TAVI are implanted within 3 days after the procedure (8)(12). In our cohort, we found a trend with permanent pacemakers implanted earlier in 2014 (median 1 day) than in 2012, along with a shorter overall length of hospital stay in 2014 than previous years. Length of stay continues to be a highly monitored and important quality metric to hospital systems nationwide and will likely continue to decrease in the years ahead. This will encourage an even more rapid pacemaker implantation if there are any signs of HDAVB post-procedure. Such early implantations will certainly facilitate recovery from the procedure, which is especially valuable to TAVI patients who are much older than SAVR patients. Furthermore, TAVI patients do not need prolonged in-hospital care after their less invasive procedure in contrast to SAVR patients who require post-thoracotomy wound care, pain management, and rehabilitation. The higher rate of pacemaker implantation for HDAVB in TAVI cohort compared to SAVR cohort observed in our study is likely related to different risk profiles such as advanced age in the TAVI cohort. An independent association between TAVI and pacemaker implantation among those with HDAVB suggests a potential impact of earlier implantation in the TAVI cohort on the higher pacemaker rate in comparison to SAVR. Considering the lack of the established optimal waiting period prior to pacemaker implantation after TAVI, the decision on the timing of pacemaker implantation should entail a detailed discussion with patients. Further studies focusing on predictors of early spontaneous recovery of HDAVB after TAVI would be helpful in such decision. When a pacemaker is implanted, programming to minimize ventricular pacing, such as search AV algorithms, should be implemented, given the potential of atrioventricular conduction recovery and the possible detrimental effect from chronic ventricular pacing.
Pacemaker implants after SAVR
The incidence of HDAVB and pacemaker implantation after SAVR are overall consistent with prior studies. One study for instance including 3,534 SAVR patients showed that 6.6% of the patients underwent permanent pacemaker implantation and 97% of them were placed for HDAVB.(7) In this study, 43.6 % of HDAVB occurred intraoperatively and the remaining HDAVB occurred during hospitalization with an average time of 1.96 days from SAVR (7). The interval from SAVR to permanent pacemaker ranged from 4.4 to 11 days on prior reports (7)(23).
Study limitations
There are several limitations to our study inherent to the nature of the study using a large administrative database. Since the NIS database does not contain the date of each diagnosis, we could not ascertain the timing of its occurrence, and progression or regression of HDAVB. The nature of the database derived from administrative coding did not allow us to ascertain indications or clinical rationale for pacemaker implantation. As HDAVB is the indication for pacemaker implantation after TAVI in the large majority of the patients (5)(8)(9), we focused on permanent pacemaker implantation for HDAVB and did not evaluate pacemaker implantation for sinus node dysfunction. Importantly, we assumed that coded diagnosis of HDAVB is a new-onset following TAVI or SAVR. This is very reasonable since we excluded patients with prior pacemaker implantation and persistent HDAVB usually requires pacemaker implantation and chronic HDAVB without pacemaker is very rare. For the same reason, we assumed that HDAVB recovered in patients with a coded diagnosis of HDAVB but who did not undergo permanent pacemaker implantation during hospitalization. Lastly, we did not intend to identify independent predictors for HDAVB or permanent pacemaker implantation because we could not differentiate the chronicity (pre-existing versus post-procedure) of well-known predictors for HDAVB such as right bundle branch block.
Conclusion:
HDAVB occurred in 10.3% in the TAVI group and 5.6% in the SAVR group. Permanent pacemakers were implanted earlier after TAVI than SAVR (the median interval of 2 days versus 5 days). In patients who developed HDAVB after either TAVI or SAVR, patients who underwent TAVI were more likely to undergo pacemaker implantation than those who underwent SAVR and TAVI was independently associated with pacemaker implantation. The optimal waiting periods prior to pacemaker implantation for HDAVB after TAVI remains to be established and further studies focusing on predictors of recovery of HDAVB are awaited.
Supplementary Material
Table 3: Demographics and risk factors of the patients with HDAVB who underwent permanent pacemaker implantation versus those did not.
| Pacemaker (n=2,580) | No pacemaker (n=900) | p-value | |
|---|---|---|---|
| Age (mean ±standard deviation) | 82 ± 8 | 82 ± 8 | 0.64 |
| Male | 52.1% | 47.2% | 0.23 |
| Race White | 82.8% | 82.2% | 0.86 |
| Hypertension | 80.0% | 76.7% | 0.27 |
| Diabetes | 38.8% | 33.3% | 0.19 |
| Dyslipidemia | 65.5% | 55.0% | 0.01 |
| Current smoking | 2.7% | 2.8% | 0.96 |
| Obesity | 16.3% | 9.4% | 0.01 |
| Coronary artery disease | 29.7% | 31.7% | 0.58 |
| Prior PCI | 18.0% | 15.6% | 0.37 |
| Prior CABG | 19.8% | 15.6% | 0.13 |
| Prior stroke/TIA | 11.6% | 7.8% | 0.11 |
| Anemia | 25.6% | 24.4% | 0.71 |
| Renal failure | 40.1% | 37.2% | 0.45 |
| Chronic pulmonary disease | 32.8% | 31.7% | 0.78 |
| Peripheral vascular disease | 28.9% | 30.0% | 0.74 |
| Atrial fibrillation | 38.0% | 42.2% | 0.22 |
| In-hospital mortality | 3.1% | 12.2% | <0.001 |
CABG: coronary artery bypass grafting, PCI: percutaneous coronary intervention, TAVI: trans-catheter aortic valve implantation, TIA: transient ischemic attack,
Acknowledgments:
Dr. Abdel-Latif is supported by the University of Kentucky Clinical and Translational Science Pilot Award (UL1TR000117), the UK COBRE Early Career Program (P20 GM103527) and the NIH Grant R56 HL124266.
This work was supported by the Penny Warren Research Award from the University of Kentucky (Lexington, Kentucky, United States).
Footnotes
Disclosure of any relationship with industry: none
References:
- 1.Leon MB, Smith CR, Mack M, et al. Transcatheter Aortic-Valve Implantation for Aortic Stenosis in Patients Who Cannot Undergo Surgery. N. Engl. J. Med 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 2.Smith CR, Leon MB, Mack MJ, et al. Transcatheter versus Surgical Aortic-Valve Replacement in High-Risk Patients. N. Engl. J. Med 2011;364:2187–2198. [DOI] [PubMed] [Google Scholar]
- 3.Adams DH, Popma JJ, Reardon MJ, et al. Transcatheter Aortic-Valve Replacement with a Self-Expanding Prosthesis. N. Engl. J. Med 2014;370:1790–1798. [DOI] [PubMed] [Google Scholar]
- 4.Leon MB, Smith CR, Mack MJ, et al. Transcatheter or Surgical Aortic-Valve Replacement in Intermediate-Risk Patients. N. Engl. J. Med 2016;374:1609–1620. [DOI] [PubMed] [Google Scholar]
- 5.Siontis GCM, Jüni P, Pilgrim T, et al. Predictors of permanent pacemaker implantation in patients with severe aortic stenosis undergoing TAVR: a meta-analysis. J. Am. Coll. Cardiol 2014;64:129–40. [DOI] [PubMed] [Google Scholar]
- 6.Auffret V, Puri R, Urena M, et al. Conduction Disturbances After Transcatheter Aortic Valve Replacement. Circulation 2017;136:1049–1069. [DOI] [PubMed] [Google Scholar]
- 7.Schurr UP, Berli J, Berdajs D, et al. Incidence and risk factors for pacemaker implantation following aortic valve replacement. Interact. Cardiovasc. Thorac. Surg 2010;11:556–560. [DOI] [PubMed] [Google Scholar]
- 8.Nazif TM, Dizon JM, Hahn RT, et al. Predictors and Clinical Outcomes of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv 2015;8:60–69. [DOI] [PubMed] [Google Scholar]
- 9.Mauri V, Reimann A, Stern D, et al. Predictors of Permanent Pacemaker Implantation After Transcatheter Aortic Valve Replacement With the SAPIEN 3. JACC Cardiovasc. Interv 2016;9:2200–2209. [DOI] [PubMed] [Google Scholar]
- 10.Urena M, Rodés-Cabau J. Managing heart block after transcatheter aortic valve implantation: from monitoring to device selection and pacemaker indications. EuroIntervention 2015;11 Suppl W:W101–5. [DOI] [PubMed] [Google Scholar]
- 11.Brignole M, Auricchio A, Baron-Esquivias G, et al. 2013 ESC Guidelines on cardiac pacing and cardiac resynchronization therapy. Eur. Heart J 2013;34:2281–2329. [DOI] [PubMed] [Google Scholar]
- 12.Fadahunsi OO, Olowoyeye A, Ukaigwe A, et al. Incidence, Predictors, and Outcomes of Permanent Pacemaker Implantation Following Transcatheter Aortic Valve Replacement. JACC Cardiovasc. Interv 2016;9:2189–2199. [DOI] [PubMed] [Google Scholar]
- 13.Calvi V, Puzzangara E, Pruiti GP, et al. Early conduction disorders following percutaneous aortic valve replacement. Pacing Clin. Electrophysiol 2009;32 Suppl 1:S126–30. [DOI] [PubMed] [Google Scholar]
- 14.Toggweiler S, Stortecky S, Holy E, et al. The Electrocardiogram After Transcatheter Aortic Valve Replacement Determines the Risk for Post-Procedural High-Degree AV Block and the Need for Telemetry Monitoring. JACC. Cardiovasc. Interv 2016;9:1269–76. [DOI] [PubMed] [Google Scholar]
- 15.Guetta V, Goldenberg G, Segev A, et al. Predictors and course of high-degree atrioventricular block after transcatheter aortic valve implantation using the CoreValve Revalving System. Am. J. Cardiol 2011;108:1600–5. [DOI] [PubMed] [Google Scholar]
- 16.Anon. Overview of the National (Nationwide) Inpatient Sample (NIS). Available at: https://www.hcup-us.ahrq.gov/nisoverview.jsp. Accessed July 13, 2017.
- 17.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med. Care 1998;36:8–27. [DOI] [PubMed] [Google Scholar]
- 18.Raelson CA, Gabriels J, Ruan J, et al. Recovery of atrioventricular conduction in patients with heart block after transcatheter aortic valve replacement. J. Cardiovasc. Electrophysiol 2017;28:1196–1202. [DOI] [PubMed] [Google Scholar]
- 19.Hwang YM, Kim J, Lee JH, et al. Conduction disturbance after isolated surgical aortic valve replacement in degenerative aortic stenosis. J. Thorac. Cardiovasc. Surg 2017;154:1556–1565.e1. [DOI] [PubMed] [Google Scholar]
- 20.Young Lee M, Chilakamarri Yeshwant S, Chava S, Lawrence Lustgarten D. Mechanisms of Heart Block after Transcatheter Aortic Valve Replacement - Cardiac Anatomy, Clinical Predictors and Mechanical Factors that Contribute to Permanent Pacemaker Implantation. Arrhythmia Electrophysiol. Rev 2015;4:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simms AD, Hogarth AJ, Hudson EA, et al. Ongoing requirement for pacing post-transcatheter aortic valve implantation and surgical aortic valve replacement. Interact. Cardiovasc. Thorac. Surg 2013;17:328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cantillon Daniel J., Exner Derek V., Badie Nima, Davis Kevin, Gu Ning Yan N Y and D R. Complications and Health Care Costs Associated With Transvenous Cardiac Pacemakers in a Nationwide Assessment. JACC Clin. Electrophysiol 2017;3:1296–1305. [DOI] [PubMed] [Google Scholar]
- 23.Dawkins S, Hobson AR, Kalra PR, Tang ATM, Monro JL, Dawkins KD. Permanent pacemaker implantation after isolated aortic valve replacement: incidence, indications, and predictors. Ann. Thorac. Surg 2008;85:108–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.