Abstract
Background
Body mass index (BMI) of living kidney donors has increased substantially. Determining candidacy for live kidney donation among obese individuals is challenging as many donation-related risks among this subgroup remain unquantified, including even basic post-donation mortality.
Methods
We used data from the Scientific Registry of Transplant Recipients linked to data from the Centers for Medicare and Medicaid Services to study long-term mortality risk associated with being obese at the time of kidney donation among 119,769 live kidney donors (LKDs) (1987–2013). Donors were followed for a maximum of 20 years (IQR: 6.0–16.0). Cox proportional hazards estimated the risk of post-donation mortality by obesity status at donation. Multiple imputation accounted for missing obesity data.
Results
Obese (BMI≥30) LKDs were more likely male, African American, and had higher blood pressure. Estimated risk of mortality 20 years after donation was 304.3/10,000 for obese and 208.9/ 10,000 for non-obese LKDs. Adjusting for age, sex, race/ethnicity, blood pressure, baseline estimated glomerular filtration rate (eGFR), relationship to recipient, smoking, and year of donation, obese LKDs had a 30% increased risk of long-term mortality compared to their non-obese counterparts (aHR: 1.32, 95%CI: 1.09–1.60, p=0.006). The impact of obesity on mortality risk did not differ significantly by sex, race/ethnicity, biological relationship, baseline eGFR, or among donors who did and did not develop post-donation kidney failure.
Conclusions
These findings may help to inform selection criteria and discussions with obese persons considering living kidney donation.
Keywords: living kidney donation, obesity, mortality, Scientific Registry of Transplant Recipients, end-stage renal disease
Graphical Abstract
Obese donors had 30% increased risk for post-donation mortality versus non-obese living donors. These findings may help to inform selection criteria and discussions with obese persons considering living kidney donation.
INTRODUCTION
Since 2004 there has been a 13% decline in living kidney donation in the United States (US),(1) and this observed trend differs significantly from recent increases in living donation seen in other parts of the world including the United Kingdom, Japan, Netherlands, Mexico and Australia.(1) The reasons for declining rates of living donation in the US are likely multifactorial,(1) but correspond with an increasingly unhealthy US general population(2–12) and questions surrounding donation-related health risks, particularly among living kidney donors with isolated medical abnormalities at the time of donation, such as obesity (body mass index [BMI] ≥30kg/m2).(13)
The prevalence of obesity in the US has increased from 27.5% in 1999 to 36.5% in 2014,(2) and along with the general population, BMI of living kidney donors has also risen, with more than 25% of all contemporary living kidney donors with obesity at time of donation compared to fewer than 8% in the 1970s.(14) As newer data emerge on donation-related health risks, the transplant community continues to debate the optimal acceptable BMI threshold for living donors. Prior guidelines have suggested that live donor candidates with BMI ≥ 35kg/m2 should be discouraged from donating,(15) while others have suggested that patients with BMI >30kg/m2 should reduce weight before donation.(16) Currently, the 2017 Kidney Disease Improving Global Outcomes (KDIGO) clinical practice guidelines suggest that the decision to approve living donor candidates with BMI >30kg/m2 should be individualized based on patients’ demographic and health profiles, in relation to the transplant program’s acceptable risk threshold.(17)
Within the general population, obesity is strongly associated with an increased risk for cardiovascular disease, diabetes, CKD, end-stage renal disease (3–7, 11, 18)(ESRD) and mortality.(19, 20) Studies among obese living donors, however, have primarily focused on risk of ESRD, demonstrating a 1.16-fold and a 1.86-fold higher ESRD risk among obese potential living donor candidates (adjusted hazard ratio (aHR): 1.16; 95%CI: 1.04–1.29)(21) and actual obese living kidney donors (aHR: 1.86; 95%CI: 1.05–3.30)(22), respectively. The two major studies addressing mortality risk among living donors failed to risk-stratify by BMI (23, 24) and therefore no study to date has specifically quantified the long-term mortality risks faced by obese donors.
Not surprisingly, tremendous variation in BMI thresholds for living donation exist across US transplant centers, highlighting persistent knowledge gaps in our current understanding of living donor risks among obese donors and the need for continued focused research among this at-risk subgroup.(25) To improve our understanding of risk of mortality in obese living kidney donors in order to enhance donor selection practices, we utilized a national registry to examine the association between BMI and post-donation risk of long-term mortality among living kidney donors, adjusting for potential confounders and exploring the presence of effect modification.
METHODS
The study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donors, waitlisted candidates, and transplant recipients in the United States, submitted by members of the Organ Procurement and Transplantation Network (OPTN). The Health Resources and Services Administration of the US Department of Health and Human Services provides the oversight to the activities of the OPTN and SRTR contractors. We included all adult kidney-only living donors reported to the OPTN between 10/1/1987 and 6/30/2013 (N=119,769), with a maximum time since donation of 28.2 years.
To determine post-donation mortality, donors were linked to the Social Security Death Master File. We also assessed whether donors developed post-donation ESRD by linkage to Centers for Medicare and Medicaid Services (CMS) data. To perform the linkage, we used a combination of Social Security number (SSN), last name, first name, middle name (or all three), date of birth, and sex for those who donated on or after April 1, 1994, as the OPTN did not begin collecting SSN until that date. For donors prior to 4/½004, linkage to CMS occurred using identifiers other than SSN. We considered donors whose date of donation was prior to April 1, 1994 to be late entries and assumed they had not developed ESRD prior to that date, as CMS ascertainment of ESRD via the 2728 form began in April 1994. Therefore, these donors were left truncated, and their time at risk began on April 1, 1994. All donors were followed until date of death or administrative end of study on December 31, 2015.
Given changes over time to the variables collected in the OPTN living donor registration forms, there was a high prevalence of missing data. These data were assumed to be missing at random, wherein the probability of missing variables was assumed to not depend on the unobserved values, conditional on observed values of other variables. We used multiple imputation by chained equations to impute missing BMI (in the range of 10–70 kg/m2), pre-operative blood pressure, baseline estimated glomerular filtration rate (eGFR), insurance type, donor’s relationship with the recipient, and history of smoking. We imputed missing values based on completely observed variables of outcome, age, sex, year of donation, race/ethnicity, and baseline hazard of both ESRD and mortality. We ran twenty imputations with twenty burn-in periods, and we checked convergence of imputations using trace file plots.
Using the World Health Organization classification of BMI, donors were defined as obese if their BMI at donation was ≥ 30 kg/m2. We calculated baseline eGFR using the CKD-EPI equation, which was the most reliable method available to assess donor pre-operative kidney function and has been demonstrated to be superior to the MDRD equation for eGFR among individuals with creatinine clearance expected to be greater than 60 ml/min/1.73m2 (the typical lower threshold of a living kidney donor), as previously described.(26) We compared donor characteristics by obesity at donation in the non-imputed dataset among all those with observed values.
We performed survival analyses among the imputed datasets, using Rubin’s rules to combine estimates from all imputations and adjusting coefficients and standard errors for the variability between imputations. To illustrate absolute risk of obesity, we estimated cumulative incidence of mortality from baseline hazards in a regression model and displayed the estimates with Loess curve fitting. We used Cox proportional hazards regression to estimate risk of mortality by obesity at donation, adjusting for the following donor characteristics: age, sex, race, blood pressure, baseline eGFR, relationship to recipient, history of smoking at donation, and year of donation. Given that ESRD development is a time-variant factor, we also generated an extended Cox model that allowed ESRD status to vary over time. This was achieved using the mi stsplit command in the imputed data, and robust standard errors were used to account for multiple observations for donors who developed ESRD.
We explored the potential for effect modification by testing for interactions and with stratification, although due to lack of statistical significance, interaction terms were not included in the final model. We assessed the proportional hazards assumption using time-dependent variables. In the case of nonproportionality, a model was generated to include a strata statement for the violating variable. Inferences were consistent with the primary analysis, and as such, the primary analyses are presented.
We applied the final model to the complete case cohort (among the 2/3 of donors without missing pre-donation risk factors). Inferences from both the imputed and complete case data were congruent. Additionally, we explored BMI as both a continuous variable and according to the World Health Organization classification categories, and our inferences were confirmed. All analyses were performed using Stata 12.0 (Stata Corp), and all hypothesis tests were 2-sided with a significance level of α=0.05.
RESULTS
We identified 119,769 living kidney donors from October 1, 1987 to June 30, 2013; of these 78,592 had BMI data reported at donation. Obese living donors were more often male (43.1% vs. 39.2%) and African American (16.4% vs. 11.1%). Predonation blood pressures were higher in obese living donors (mean systolic 124.4 mmHg vs. 119.9 mmHg and mean diastolic 75.6 mmHg vs. 72.9 mmHg) than in non-obese donors. Obese donors did not differ significantly from non-obese donors with regard to age, baseline eGFR, type of insurance, their relationship with the recipient, or history of smoking.
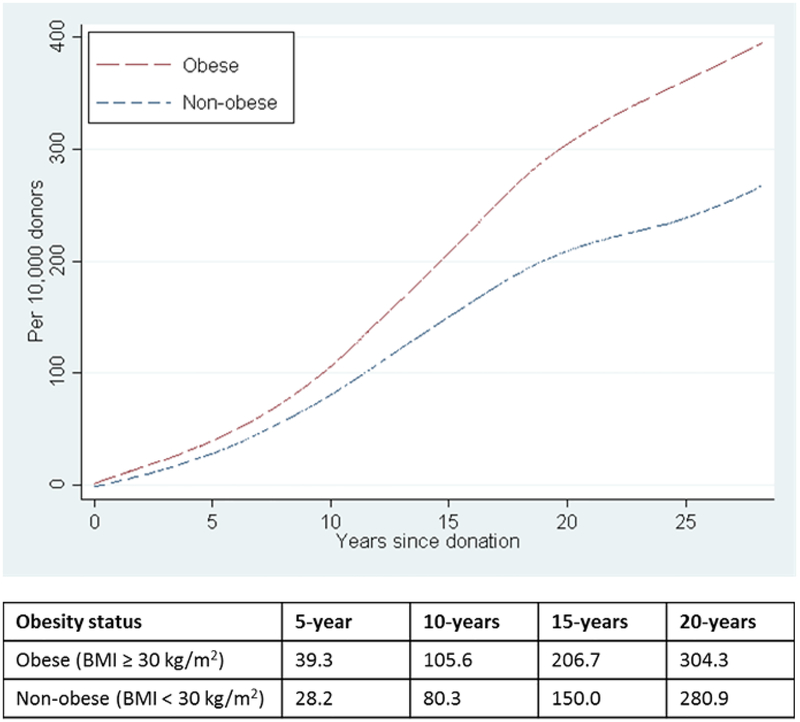
Compared to non-obese donors, the cumulative incidence of long-term mortality per 10,000 living donors was higher among obese living kidney donors, and this increased risk was observed as early as 5-years post-donation (39.3 per 10,000 vs. 28.2 per 10 000, respectively). At 20-years post-donation, obese living kidney donors had a cumulative incidence of mortality of 304.3/10,000 compared to 208.9/10,000 among their non-obese donors (Figure 1).
Figure 1.
Cumulative incidence of post-donation mortality among living kidney donors by obesity status at time of donation.
Table 1 presents results from multivariable models. After adjustment for donor age, ethnicity, gender, baseline eGFR, baseline blood pressure, smoking history, year of donation, and relationship to the recipient, and not accounting for post-donation ESRD development, obesity and smoking (modifiable risk factors) were independently associated with increased risk of post-donation mortality. Compared to non-obese donors, obese living kidney donors had a 1.3-fold increased risk of mortality post-donation (aHR: 1.32, 95%CI: 1.09–1.60, p=0.006) (Table 1). These results were consistent with analyses that limited the cohort only to donors with complete data (aHR: 1.47, 95%CI: 1.09–1.98, p=0.01; Table 2).
Table 1.
Multiple imputation in total cohort (119,769)*
| Characteristic | aHR | 95% CI | p-value |
|---|---|---|---|
| Without adjustment for post-donation ESRD development | |||
| Obese (ref: BMI < 30 kg/m2) | 1.32 | 1.09–1.60 | 0.006 |
| With time varying 4-level variable for post-donation ESRD development | |||
| Obesity/ESRD | |||
| Non-obese/no ESRD | Ref | ||
| Obese/no ESRD | 1.28 | 1.04–1.57 | 0.02 |
| Non-obese/ESRD | 27.11 | 16.72–43.94 | < 0.001 |
| Obese/ESRD | 26.15 | 15.13–45.19 | < 0.001 |
Adjusting for donor age, sex, race/ethnicity, baseline blood pressure, baseline eGFR, relationship to recipient, history of smoking, and year of donation
ESRD indicates end stage renal disease, eGFR indicates estimated glomerular filtration rate, BMI indicates body mass index, aHR indicates adjusted hazard ratio, CI indicates confidence interval
Table 2.
Complete case with time-varying covariate for ESRD development (N=46,133)
| Characteristic | aHR | 95% CI | p-value |
|---|---|---|---|
| Obese (ref: BMI < 30 kg/m2) | 1.47 | 1.09–1.98 | 0.01 |
| Age, per 1-year increase | 1.04 | 1.02–1.05 | < 0.001 |
| Female | 0.56 | 0.42–0.75 | < 0.001 |
| African American (ref=non-African American) | 1.56 | 1.02–2.36 | 0.04 |
| Systolic blood pressure, per 1-unit increase | 1.02 | 1.01–1.03 | 0.003 |
| Diastolic blood pressure, per 1-unit increase | 1.00 | 0.98–1.01 | 0.67 |
| eGFR, per 1 mL/min/1.73m2 increase | 1.00 | 0.99–1.01 | 0.80 |
| Biologically related to recipient | 0.94 | 0.71–1.25 | 0.68 |
| History of smoking at donation | 2.76 | 2.08–3.66 | < 0.001 |
| Year of donation | 0.88 | 0.83–0.94 | < 0.001 |
| Developed ESRD | 28.43 | 3.71–217.71 | 0.001 |
ESRD indicates end stage renal disease, eGFR indicates estimated glomerular filtration rate, BMI indicates body mass index, aHR indicates adjusted hazard ratio, CI indicates confidence interval
Post-donation ESRD was then included as a time-varying exposure. Even among obese donors who did not develop post-donation ESRD, there remained a 1.28-fold increased risk for mortality (aHR: 1.28, 95%CI: 1.04–1.57, p=0.02; Table 1). Overall long-term mortality risk was greatest among donors who developed post-donation ESRD regardless of obesity status (non-obese aHR: 27.11, 95%CI: 16.72–43.94, p<0.001; obese aHR: 26.15, 95%CI: 15.13–45.19, p<0.001); as demonstrated in our effect modification analyses, this finding was not modified by BMI (aHR: 0.77, 95%CI: 0.39–1.54, p=0.46). Moreover, there were no statistically significant interactions between obesity and age, eGFR, systolic or diastolic blood pressure, race/ethnicity, gender, relationship to the recipient, smoking history, or year of donation (Supplemental Table).
DISCUSSION
In this national study of 119,769 living kidney donors, we calculated a post-donation long-term death rate at 20 years of 209/10,000 among non-obese donors and 304/10,000 for obese living donors. While the absolute risk for post-donation mortality remains low, the magnitude of the mortality risk difference between these two donor groups is significant. After adjusting for age, ethnicity, gender, baseline eGFR, baseline blood pressure, smoking history, year of donation, and relationship to the recipient, donors with obesity had 30% increased risk for post-donation mortality compared to non-obese living donors. This excess mortality risk was maintained even in models that accounted for the development of post-donation ESRD. In other words, obesity is very clearly independently associated with long-term mortality following living donation.
This association between obesity and excess mortality risk is not entirely surprising. Studies from the general US population have also shown a negative association with obesity and mortality. Importantly though, these non-donor cohorts contained individuals with diabetes and hypertension, conditions which are correlated with increased mortality.(11, 27) Living kidney donors are by definition, generally healthy, but even in this cohort of actual living donors we found that obesity was an independent risk factor for long-term mortality. Differences in mortality rate by obesity status were apparent as early as five years after donation and more pronounced by 10 years of follow up. We found this effect was not modified by the development of ESRD, age, eGFR, systolic blood pressure, diastolic blood pressure, African American ethnicity, gender, relationship to the recipient, smoking history, or year of donation, again highlighting that obesity is an independent risk factor for post-donation long-term mortality. These findings are useful for current living donor selection practices and can be used to inform BMI thresholds for absolute contraindications for donation.
As with any study, there are important limitations. We used multiple imputation in order to include the one third of the cohort who donated prior to when BMI was routinely captured for donors. We confirmed our inferences from multiple imputation in the complete-case analyses among donors who were not missing any baseline risk factors, underscoring the robustness of our findings. The relatively short time frame of the study (median follow-up of 10.7 years) may not have allowed for complete understanding of the long-term risk of mortality. However, it is likely that the risk observed here is an underestimate. Additionally, we are limited by the completeness and the nature of pre-donation and follow up data collected by the OPTN. These data do not contain pre-donation lipid measurements, and as such, we could not identify presence of pre-donation metabolic syndrome, which is known to be associated with increased risk of mortality.(28) We are also unable to assess for post-donation development of hypertension, diabetes or cardiovascular disease, all comorbidities associated with obesity in the general population. However, in single center series, development of these comorbidities has not negatively impacted living donor survival.(29) There is a strong possibility of residual confounding resulting from not including factors not collected reliably by the OPTN (e.g. socioeconomic status, medication use). Finally, the incremental risk of mortality directly attributable to living donation and loss of nephron mass was not assessed in this study. Obesity in the absence of kidney donation confers increased risk of premature mortality, but the additional risk of development of mortality conferred by living donation in the setting of obesity remains unknown.
CONCLUSION
In summary, while the absolute risk of post-donation mortality remains low, the risk of long-term mortality is 30% higher among obese living kidney donors compared to non-obese donors. These data can be used to inform donor selection, pre-donation management, and informed consent discussions with obese persons who are considering living donation. Further research is needed to better understand the relationships between changes in weight pre- and post- donation and long-term risk for post-donation mortality.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by National Institutes of Health grant numbers K23-DK103918 (PI: Locke), R01-DK113980 (PI: Locke), K01DK-101677 (Massie), the American Society of Transplantation Clinical Scientist Faculty Development Grant (PI: Locke), and K24-DK101828 (PI: Segev). The data reported here have been supplied by the Minneapolis Medical Research Foundation (MMRF) as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy of or interpretation by the National Institutes of Health, SRTR, OPTN, or the American Society of Transplantation.
Funding: This work was supported by National Institutes of Health grant numbers K23-DK103918 (PI: Locke), R01-DK113980 (PI: Locke), K01DK-101677 (Massie), the American Society of Transplantation Clinical Scientist Faculty Development Grant (PI: Locke), and K24-DK101828 (PI: Segev).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
None
REFERENCES
- 1.Rodrigue JR, Schold JD, Mandelbrot DA. The decline in living kidney donation in the United States: random variation or cause for concern? Transplantation. 2013;96(9):767–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of Obesity Among Adults and Youth: United States, 2011–2014. NCHS data brief. 2015(219):1–8. [PubMed] [Google Scholar]
- 3.Chertow GM, Hsu CY, Johansen KL. The enlarging body of evidence: obesity and chronic kidney disease. J Am Soc Nephrol. 2006;17(6):1501–2. [DOI] [PubMed] [Google Scholar]
- 4.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113(6):898–918. [DOI] [PubMed] [Google Scholar]
- 5.Abdullah A, Amin FA, Hanum F, Stoelwinder J, Tanamas S, Wolf R, et al. Estimating the risk of type-2 diabetes using obese-years in a contemporary population of the Framingham Study. Glob Health Action. 2016;9:30421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gelber RP, Kurth T, Kausz AT, Manson JE, Buring JE, Levey AS, et al. Association between body mass index and CKD in apparently healthy men. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;46(5):871–80. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Chen X, Song Y, Caballero B, Cheskin LJ. Association between obesity and kidney disease: a systematic review and meta-analysis. Kidney international. 2008;73(1):19–33. [DOI] [PubMed] [Google Scholar]
- 8.Ejerblad E, Fored CM, Lindblad P, Fryzek J, McLaughlin JK, Nyren O. Obesity and risk for chronic renal failure. J Am Soc Nephrol. 2006;17(6):1695–702. [DOI] [PubMed] [Google Scholar]
- 9.Fox CS, Larson MG, Leip EP, Culleton B, Wilson PW, Levy D. Predictors of new-onset kidney disease in a community-based population. Jama. 2004;291(7):844–50. [DOI] [PubMed] [Google Scholar]
- 10.Kramer H, Luke A, Bidani A, Cao G, Cooper R, McGee D. Obesity and prevalent and incident CKD: the Hypertension Detection and Follow-Up Program. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2005;46(4):587–94. [DOI] [PubMed] [Google Scholar]
- 11.Hsu CY, McCulloch CE, Iribarren C, Darbinian J, Go AS. Body mass index and risk for end-stage renal disease. Ann Intern Med. 2006;144(1):21–8. [DOI] [PubMed] [Google Scholar]
- 12.Reed RD, Sawinski D, Shelton BA, MacLennan PA, Hanaway M, Kumar V, et al. Population Health, Ethnicity and Rate of Living Donor Kidney Transplantation. Transplantation. Accepted for Publication April 30, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Locke JE, Qu H, Shewchuk R, Mannon RB, Gaston R, Segev DL, et al. Identification of strategies to facilitate organ donation among African Americans using the nominal group technique. Clin J Am Soc Nephrol. 2015;10(2):286–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taler SJ ME, Leichtman AB, Gilllespie BW, Kew CE, Stegall MD, Merion RM, Matas AJ, Ibrahim HN, for the RELIVE Study Group. Demographic, Metabolic, and Blood Pressure Characteristics of Living Kidney Donors Spanning Five Decades. American Journal of Transplantation. 2012;13(2):390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Delmonico F, Council of the Transplantation S. A Report of the Amsterdam Forum On the Care of the Live Kidney Donor: Data and Medical Guidelines. Transplantation. 2005;79(6 Suppl):S53–66. [PubMed] [Google Scholar]
- 16.Abramowicz D, Cochat P, Claas FH, Heemann U, Pascual J, Dudley C, et al. European Renal Best Practice Guideline on kidney donor and recipient evaluation and perioperative care. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2015;30(11):1790–7. [DOI] [PubMed] [Google Scholar]
- 17.Lentine KL, Kasiske BL, Levey AS, Adams PL, Alberu J, Bakr MA, et al. KDIGO Clinical Practice Guideline on the Evaluation and Care of Living Kidney Donors. Transplantation. 2017;101(8S Suppl 1):S1–S109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Massie AB, Muzaale AD, Luo X, Chow EKH, Locke JE, Nguyen AQ, et al. Quantifying Postdonation Risk of ESRD in Living Kidney Donors. J Am Soc Nephrol. 2017;28(9):2749–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borrell LN, Samuel L. Body mass index categories and mortality risk in US adults: the effect of overweight and obesity on advancing death. American journal of public health. 2014;104(3):512–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flegal KM, Kit BK, Orpana H, Graubard BI. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. Jama. 2013;309(1):71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grams ME, Sang Y, Levey AS, Matsushita K, Ballew S, Chang AR, et al. Kidney-Failure Risk Projection for the Living Kidney-Donor Candidate. N Engl J Med. 2016;374(5):411–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Locke JE, Reed RD, Massie A, MacLennan PA, Sawinski D, Kumar V, et al. Obesity increases the risk of end-stage renal disease among living kidney donors. Kidney international. 2017;91(3):699–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ibrahim HN, Foley R, Tan L, Rogers T, Bailey RF, Guo H, et al. Long-term consequences of kidney donation. N Engl J Med. 2009;360(5):459–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segev DL, Muzaale AD, Caffo BS, Mehta SH, Singer AL, Taranto SE, et al. Perioperative mortality and long-term survival following live kidney donation. Jama. 2010;303(10):959–66. [DOI] [PubMed] [Google Scholar]
- 25.Naik AS, Cibrik DM, Sakhuja A, Samaniego M, Lu Y, Shahinian V, et al. Temporal trends, center-level variation, and the impact of prevalent state obesity rates on acceptance of obese living kidney donors. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2018;18(3):642–9. [DOI] [PubMed] [Google Scholar]
- 26.Stevens LA, Schmid CH, Zhang YL, Coresh J, Manzi J, Landis R, et al. Development and validation of GFR-estimating equations using diabetes, transplant and weight. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2010;25(2):449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hsu CY, Iribarren C, McCulloch CE, Darbinian J, Go AS. Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med. 2009;169(4):342–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Panwar B, Hanks LJ, Tanner RM, Muntner P, Kramer H, McClellan WM, et al. Obesity, metabolic health, and the risk of end-stage renal disease. Kidney international. 2015;87(6):1216–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibrahim HN, Berglund DM, Jackson S, Vock DM, Foley RN, Matas AJ. Renal Consequences of Diabetes After Kidney Donation. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2017;17(12):3141–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.