Abstract
Objective:
To evaluate the hypothesis that metabolic measures (fasting glucose, insulin and Homeostatic Model of Assessment for Insulin Resistance [HOMA-IR] levels) are inversely associated with performance on cognitive tasks using data from young (4- to 6-year-old), typically developing, healthy children.
Study design:
Data were obtained from children participating in the Healthy Start study, a pre-birth cohort in Colorado. HOMA-IR, glucose and insulin values were centered and scaled using the study sample means and standard deviations (SD). Thus, they are reported in number of SD units from the mean. Fully corrected T-scores for inhibitory control (Flanker task), cognitive flexibility (Dimensional Change Card Sort test), and receptive language (Picture Vocabulary test) were obtained via the NIH Toolbox cognition battery.
Results:
Children included in this analysis (n = 137) were 4.6 years-old, on average. Per 1 SD unit, fasting glucose (B = - 2.02, 95% CI: −3.54, −0.49), insulin (B = −1.67, 95% CI: −2.96, - 0.38), and HOMA-IR values (B = −1.79, 95% CI: −3.09, −0.49) were each significantly and inversely associated with inhibitory control (P < .05 for all, respectively). Fasting glucose levels were also inversely associated with cognitive flexibility (B = −1.96, 95% CI: −3.71, −0.21, p = 0.03).
Conclusion(s):
Our data suggest that metabolic health may impact fluid cognitive function in healthy, young children.
Keywords: insulin resistance, cognition, childhood, executive function
Cognitive functions, such as attention, memory, language, inhibitory control and cognitive flexibility contribute significantly to academic [1, 2] and professional success [3], as well as health-related quality of life [4]. Both perinatal and postnatal exposures can disrupt the cognitive developmental trajectory leading to delay or underdevelopment of cognitive skills [5–7]. The increasing prevalence of metabolic abnormalities [8] and overt type 2 diabetes in children and youth [9] is of particular concern for child cognition, given the established link between poor metabolic health and cognitive decline in adults [10–14].
Although limited data exists for healthy, young children, several studies have found a significant association between indicators of poor metabolic health, specifically fasting glucose levels and insulin resistance, and poorer performance on cognitive tests in youth [15, 16]. These data highlight metabolic health as an understudied factor in the progression or disruption of child cognitive development and brings peripheral metabolism into focus as an early life course issue for developmental morbidity.
Hallmarks of poor metabolic health, such as impaired glucose tolerance (elevated blood glucose levels) and insulin resistance have been observed in typically developing, young children, suggesting that poor metabolic health may begin early in the life course. Although most pronounced in children with obesity [17], indicators of poor metabolic health are also found in 4–6% of children without obesity [8, 18, 19], implicating a broader risk group for negative cognitive outcomes. However, to date, no study has investigated the association between metabolic markers and cognition in young, typically developing children. Therefore, we tested the hypothesis that higher levels of fasting blood glucose, insulin and estimated insulin resistance would be significantly and inversely associated with performance on cognitive function tests in healthy, young children.
METHODS
Data used in this analysis were obtained from participants enrolled in the ongoing Healthy Start study, a pre-birth longitudinal cohort of ethnically diverse children, ages 4 to 6 years old, living in Colorado. Mother-offspring dyads participating in the Healthy Start study have been followed from early pregnancy (<24 weeks of gestation). Information on pregnancy and birth characteristics have been reported elsewhere [20, 21]. Written informed consent was obtained from the mother or legal guardian of the child. The Healthy Start protocol was approved by the Colorado Multiple Institution Review Board.
Study Visit Procedures
Mother-offspring dyads participating in the Healthy Start study were invited to the University of Colorado for an in-person research visit. Prior to arriving for the in-person research visit children fasted for at least 8 hours. During the in-person visit, trained research professionals drew blood on the child participant and made direct measurements of child weight, height, body composition via air displacement plethysmography (PeaPod, Cosmed, Italy) and anthropometry (eg, skin folds, waist circumference, etc.). Child body mass index (BMI) was calculated as weight (kg) divided by height (m2) and BMI-for-age z-scores were derived using the Centers for Disease Control growth charts [22]. The participant’s mother or parent/guardian was also asked to report on self, child, and household demographics.
Cognitive Function Assessment
The National Institutes of Health (NIH) Toolbox cognition battery was used to assess cognitive function in children participating in the Healthy Start study. The NIH Toolbox employs an online interface to deliver a battery of tests assessing cognitive subdomains including executive functions (e.g. set-shifting or cognitive flexibility, working memory, and attention/inhibitory control), language (i.e. receptive), and processing speed for individuals ranging in age from 3 to 85 years old [23]. Due to the young age of child participants, only cognitive flexibility, attention/inhibitory control, and receptive language were measured via the Dimensional Card Sort test, Flanker Inhibitory Control and Attention Test, and Picture Vocabulary test, respectively. These tests were administered to participating children on an iPad during the in-person research visit with a trained professional research assistant providing instruction and supervision. The Spanish-language version of the NIH Toolbox was used with Spanish-only speaking children.
Fully corrected T-scores for age and sex based upon the normative population were generated for each completed test and used for the analysis.
Metabolic Biomarker Analysis
Fasting blood samples were analyzed by the Colorado Clinical and Translational Sciences Institute core laboratory. Blood glucose was measured via the Hexokinase method (Beckman Coulter) and insulin was measured via chemiluminescence immunoassay (Beckman Coulter).
The Homeostatic Model for Assessment of Insulin Resistance (HOMA-IR) was derived from fasting blood glucose and insulin values [24] and used in the present analyses as an estimate for insulin resistance. HOMA-IR, glucose and insulin values were centered and scaled using the study sample means and standard deviations for each measure and used in all statistical models.
Statistical Analyses
Descriptive characteristics of the study sample were derived and are reported as means and frequencies. To test the association between childhood metabolic biomarkers glucose, insulin, and HOMA-IR and performance on tests for cognitive flexibility, inhibitory control, and receptive language we employed multiple univariate linear regression modeling. One model was run for each metabolic biomarker and cognitive outcome pair, resulting in nine total models. Covariates were defined a priori and included ethnicity (Hispanic vs non-Hispanic), APGAR-5 score at birth, birthweight z-score (for age and sex), maternal education (Bachelor’s degree or higher vs. all others) and smoking in pregnancy (yes vs. no). Ethnicity was not included as a covariate in models with receptive language as the outcome. Finally, covariates with a p-value greater than 0.1 were removed from the final models in a backward-stepwise manner.
Given that children with obesity are more likely to be dysmetabolic [17], we additionally tested the association between child BMI z-score and the cognitive outcomes of interest, controlling for the aforementioned a priori covariates. All analyses were performed in SAS version 9.4 (SAS Institute Inc., Carey, NC).
RESULTS
Healthy Start children included in the present analysis had completed the in-person research visit at 4–6 y/o as of April 2018 (N = 479), had data on fasting glucose and insulin levels (n = 337), and complete data on all three cognitive function tests (n = 155). Children were excluded from the study sample if they were born preterm (<37 weeks of gestation) (n = 11). This resulted in a study sample of n = 144. Missing information on covariates minimally reduced the final sample size for complete case analysis to n = 137.
Children included in the study sample were 47% female, 4.6 years-old, on average, and predominantly non-Hispanic white (60%). Children had an average BMI z-score of 0.2, waist-tohip ratio of 1.0, and 21% fat mass. Further, on average, fasting glucose, insulin and HOMA-IR values were all within normal range (Table I).
Table 1.
Analytic sample characteristics (N = 144).
| Child age (months), mean (range) | 56 (43 – 82) |
| Child sex (female), n (%) | 70 (47) |
| Child race, n (%): | |
| BMI-for-age z-score, mean (SD) | 0.2 (1.1) |
| Weight-for-age z-score, mean (SD) | 0.1 (0.9) |
| Waist-to-hip ratio, mean (SD) | 1 (0.04) |
| Fat mass percent, mean (SD) | 21 (6) |
| Mother’s education, n (%): | |
| Metabolic Biomarkers, mean (SD): | |
| NIH Toolbox Scores2, mean (SD) | |
NHW = non-Hispanic white; NHB = non-Hispanic black
Fully corrected T-scores (for age and sex).
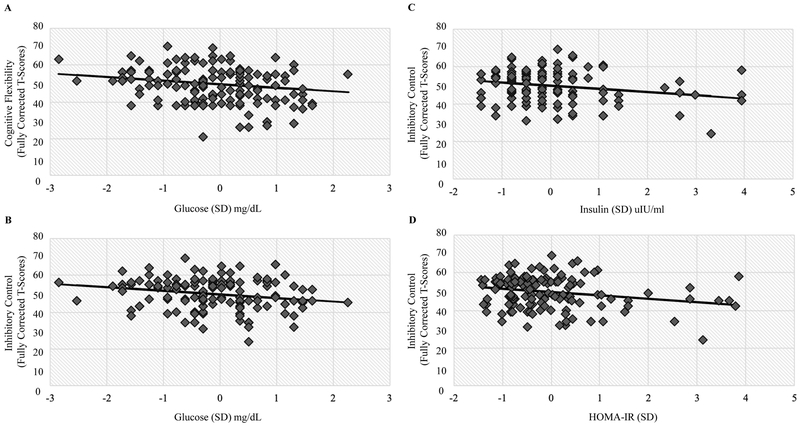
Fasting glucose, insulin, and HOMA-IR values were significantly and inversely associated with inhibitory control (p < 0.01 for all, respectively). On average, a one standard deviation higher value of fasting glucose corresponded with a 2-point lower difference in Flanker test scores (B = −2.02, SE = 0.77). Similarly, a one standard deviation higher value in either fasting insulin or HOMA-IR corresponded with a 1.7- and 1.8-point lower difference in Flanker test scores (B = −1.67, SE = 0.65; B = −1.79, SE = 0.66, respectively). Fasting glucose levels were also inversely associated with cognitive flexibility (B = −1.96, SE = 0.88, p = 0.03). No significant associations were found with receptive language (picture vocabulary test). Child BMI z-score was not associated with any of the measured cognitive outcomes. Table 2 includes further details. The Figure displays example scatter plots of observed neurocognitive test scores by metabolic measures and the fitted regression line.
Table 2.
Effect estimates for the association between fasting blood biomarkers of metabolic health and performance on cognitive function tasks.
| Cognitive Domain | |||
|---|---|---|---|
| Cognitive Flexibility B (95% CI; p) |
Inhibitory Control B (95% CI; p) |
Receptive Language B (95% CI; p) |
|
| Glucose (SD) mg/dL1 | −2.0 (−3.7, −0.2; 0.03) | −2.0 (−3.5, −0.5; 0.01) | −1.0 (−3.2, 1.2; 0.36) |
| Insulin (SD) uIU/ml1 | −0.9 (−2.4, 0.5; 0.21) | −1.7 (−3.0, −0.4; 0.01) | −0.4 (−2.3, 1.5; 0.67) |
| HOMA-IR (SD)1 | −1.0 (−2.5, 0.5; 0.20) | −1.8 (−3.1, −0.5; 0.01) | −0.5 (−2.5, 1.4; 0.60) |
| BMI-for-age z-score1 | −0.6 (−2.4, 1.2; 0.50) | −0.7 (−2.4, 1.0; 0.42) | 0.7 (−1.3, 2.7; 0.49) |
Unstandardized beta estimates are shown for variables included in the final models, only.
Maternal education categories were Bachelor’s degree or higher versus all others (reference).
Gestational smoking categories were smoking during pregnancy, yes versus no (reference).
Figure.
Observed NIH Toolbox scores for cognitive flexibility and inhibitory control by standard deviation of fasting blood glucose (A and B), insulin (C), and HOMA-IR (D) values overlaid by the fitted regression line.
DISCUSSION
We found that higher blood biomarkers of poor metabolic health are related to lower cognitive flexibility and inhibitory control in healthy, young children. These results contribute to the larger body of literature in children with overt type 1 and type 2 diabetes that demonstrates consistent and negative effects of poor metabolic health on cognition. Furthermore, these results are consistent with studies in older children in which elevated blood biomarkers, suggestive of poor metabolic health, were found to be associated with lower cognitive performance on inhibitory control, attention, working memory, and language tests [15, 16, 25]. Our analysis also revealed that child BMI was not associated with cognitive functions, suggesting that insulin and glucose may be the underlying factors contributing to metabolically-related neurodevelopmental outcomes, possibly via their signaling roles in the brain. Importantly, our data contribute evidence from an early life stage to support a potential life course trajectory for the impact of metabolic health on cognitive development in typically developing children.
Executive functions, including cognitive flexibility and inhibitory control, encompass measurably distinct but functionally interrelated skills that govern goal-directed behavior. Cognitive flexibility, also referred to as set-shifting, is defined as an individual’s ability to switch or shift between tasks or mental states. Embedded within cognitive flexibility is one’s ability to inhibit previous mental states or stop an ongoing task in favor of moving on to a new task, which requires inhibitory control. Delay or impairment of cognitive flexibility and inhibitory control processes are found in developmental disorders such as attention-deficit/hyperactivity disorder [26, 27] and autism spectrum disorder [27, 28]. Cognitive flexibility and inhibitory control impairments are also observed in children with psychiatric conditions including bipolar disorder [29]. Executive functions are therefore critical to healthy functioning in children. Cognitive flexibility and inhibitory control are considered “fluid” skills; they do not rely on practiced, or familiar skills. Receptive vocabulary, a crystallized skill, one specifically taught and practiced was not related to metabolic status in this analysis, suggesting that it is the more unique and novel fluid skills that are impacted by glucose, insulin and insulin resistance.
The importance of understanding the effects of metabolic health on cognition in children is 2-fold. First, from birth through adolescence the brain is maturing and refining its neural circuitry thus driving cognitive development of executive functions. However, during this growth period the brain is also vulnerable to insult. For example, animal models have demonstrated detrimental effects of hyperglycemia during critical postnatal developmental windows, specifically in the neonatal period, on brain structure and neurochemistry in brain regions involved in cognitive function [30–32]. Further, several studies have shown white matter alterations that persist over time among young children with type 1 diabetes, as compared with their metabolically healthy counterparts [33, 34]. These studies also showed a significant association between glucose control and extent of white matter abnormalities. Beyond developmentally sensitive periods, adults with metabolic dysfunction (e.g. pre-diabetes, hyperlipidemia, hyperglycemia, etc.) and type 2 diabetes show reductions in tissue volume and altered neural function in brain regions that sub-serve executive function [35, 36]. Together, the animal and adult human data suggest that disruption of neural structure and function may result from metabolic abnormalities and could underlie the cognitive deficits observed in patients with metabolic syndrome and diabetes of any type. Thus, children with altered glucose-insulin metabolism may also be at risk for disrupted cognitive development via underlying brain structural and functional changes.
Finally, from the life course perspective, early and sustained exposure to metabolic dysregulation may have a cumulative, detrimental impact on learning and cognitive development. This is most clearly demonstrated among youth with type 1 and type 2 diabetes who show progressively weaker performance on cognitive tasks as a function of disease duration, specifically processing speed [37, 38]. The inverse relationship between type 1 diabetes duration and cognitive functioning has also been shown in adults with long-standing disease [39, 40]. Further, among a large French cohort of adults without overt diabetes, the cumulative burden of metabolic conditions (e.g. obesity, hyperglycemia) was significantly associated with lower cognitive scores [41]. Thus, either through increased exposure time to elevated glucose, or increased burden from multiple indicators of poor metabolic health, individuals who are exposed in childhood may be at risk for suboptimal cognitive outcomes throughout their lifetime.
Despite the mounting evidence of a detrimental impact of poor metabolic health on cognitive function, the mechanisms underlying this relationship are incompletely understood, especially among children. However, human studies have highlighted the role of insulin signaling in the brain as important for cognition [42–44]. Insulin signaling in the brain is facilitated by insulin receptors (IR), insulin-like growth factor (IGF) receptors, and insulin receptor substrate proteins, all of which are important for neuron signaling processes [45–47]. In the brain, insulin signaling predominates in structures that are central to cognitive function, especially executive functioning, such as the prefrontal cortex, nucleus accumbens, and hippocampus, among others [48, 49]. Importantly, administration of insulin via intranasal spray or intravenously modulates neuronal activity in these specific brain regions [50–52], demonstrating their insulin sensitivity. Further, lower peripheral insulin sensitivity in adults is associated with reduced neuronal activity in these insulin-sensitive brain regions [44, 53]. Metabolic dysregulation has also been shown to reduce the expression and function of IR and IGF-1 receptors in the brain [54]. These data may be interpreted as abnormal peripheral metabolism impeding neuronal activity in insulin-sensitive regions via disrupted insulin signaling, providing biologic plausibility for our current findings. Additional research in the pediatric-aged population is needed to establish the timing of brain insulin signaling disruption and its relationship to cognitive development.
We demonstrate an inverse association between metabolic markers and cognitive performance on executive function tests in healthy, young children. The Healthy Start cohort, from which the present data was derived, is a well-characterized group of 4 to 6-year-old children allowed us to account for important confounders such as maternal smoking in pregnancy and education level. However, a limitation of the current analysis was that working memory, a third executive function skill [55], was not assessed due to the older age requirement (7 years-old and older) of the working memory test included in the NIH Toolbox cognition battery (List-Sorting Working Memory). Despite this limitation, future analyses will investigate the relationship between peripheral metabolic markers and the full executive function skill set as the Healthy Start cohort is ongoing and executive function will be assessed again when participants are older and more able to complete this demanding task.
Our study provides evidence that peripheral metabolic health is related to cognition in youth and extends the window of potential harm to the early life course. Additional studies are needed to confirm our findings as well as help to establish a possible mechanism for this relationship.
Supplementary Material
ACKNOWLEDGEMENT
We thank the parents and children who participated in the Healthy Start study, without whom this research would not be possible.
The Healthy Start study is funded by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK076648, 5UG3OD023248; Dabelea). The current work was funded, in part, by the National Institute of Mental Health grant T32MH015442 (to A.S.). The authors declare no conflicts of interest.
ABBREVIATIONS
- HOMA-IR
Homeostatic Model of Assessment for Insulin Resistance
- CI
Confidence Interval
- BMI
Body Mass Index
- SD
Standard Deviation
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Borella E, Carretti B, and Pelegrina S. The specific role of inhibition in reading comprehension in good and poor comprehenders. J Learn Disabil. 2010;43: 541–52. [DOI] [PubMed] [Google Scholar]
- 2.Duncan GJ, Dowsett CJ, Claessens A, Magnuson K, Huston AC, Klebanov P, et al. School readiness and later achievement. Dev Psychol. 2007;43:1428–46. [DOI] [PubMed] [Google Scholar]
- 3.Bailey CE, Cognitive accuracy and intelligent executive function in the brain and in business. Ann N Y Acad Sci. 2007;1118:122–41. [DOI] [PubMed] [Google Scholar]
- 4.Brown TE and Landgraf JM. Improvements in executive function correlate with enhanced performance and functioning and health-related quality of life: evidence from 2 large, double-blind, randomized, placebo-controlled trials in ADHD. Postgrad Med. 2010;122:42–51. [DOI] [PubMed] [Google Scholar]
- 5.Galera C, Côté SM, Bouvard MP, Pingault JB, Melchior M, Michel G, et al. Early risk factors for hyperactivity-impulsivity and inattention trajectories from age 17 months to 8 years. Arch Gen Psychiatry. 2011;68:1267–75. [DOI] [PubMed] [Google Scholar]
- 6.Cheng ER, Poehlmann-Tynan J, Mullahy J, Witt WP. Cumulative social risk exposure, infant birth weight, and cognitive delay in infancy. Acad Pediatr. 2014;14:581–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hillemeier MM, Morgan PL, Farkas G, Maczuga SA. Perinatal and socioeconomic risk factors for variable and persistent cognitive delay at 24 and 48 months of age in a national sample. Matern Child Health J. 2011;15:1001–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among u.s. Adolescents, 1999–2000. Diabetes Care. 2004;27:2438–43. [DOI] [PubMed] [Google Scholar]
- 9.Mayer-Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L, et al. Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002–2012. N Engl J Med. 2017;376:1419–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bokura H, Nagai A, Oguro H, Kobayashi S, Yamaguchi S. The association of metabolic syndrome with executive dysfunction independent of subclinical ischemic brain lesions in Japanese adults. Dement Geriatr Cogn Disord. 2010;30:479–85. [DOI] [PubMed] [Google Scholar]
- 11.Goh DA, Dong Y, Lee WY, Koay WI, Tay SZ, Soon D, et al. A pilot study to examine the correlation between cognition and blood biomarkers in a Singapore Chinese male cohort with type 2 diabetes mellitus. PLoS One. 2014;9:e96874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goh VH and Hart WG, The association of metabolic syndrome and aging with cognition in Asian men. Aging Male. 2014;17:216–22. [DOI] [PubMed] [Google Scholar]
- 13.Hawkins MA, Gunstad J, Calvo D, Spitznagel MB. Higher fasting glucose is associated with poorer cognition among healthy young adults. Health Psychol. 2016;35:199–202. [DOI] [PubMed] [Google Scholar]
- 14.Sims Wright R, Levy SA, Katzel LI, Rosenberger WF, Manukyan Z, Whitfield KE, et al. Fasting glucose and glucose tolerance as potential predictors of neurocognitive function among nondiabetic older adults. J Clin Exp Neuropsychol. 2015;37:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Akin O, Eker I, Arslan M, Yavuz ST, Akman S, Taşçılar ME, et al. Relation of insulin resistance to neurocognitive function and electroencephalography in obese children. J Pediatr Endocrinol Metab. 2017;30:1027–32. [DOI] [PubMed] [Google Scholar]
- 16.Mangone A, Yates KF, Sweat V, Joseph A, Convit A. Cognitive functions among predominantly minority urban adolescents with metabolic syndrome. Appl Neuropsychol Child. 2018;7:157–63. [DOI] [PubMed] [Google Scholar]
- 17.Ahrens W, Moreno LA, Mårild S, Molnár D, Siani A, De Henauw S, et al. Metabolic syndrome in young children: definitions and results of the IDEFICS study. Int J Obes (Lond). 2014;38:S4–14. [DOI] [PubMed] [Google Scholar]
- 18.Nishimura R, Sano H, Onda Y, Tsujino D, Ando K, Ebara F, et al. Population-based cross-sectional study on insulin resistance and insulin-secretory capacity in Japanese school children. J Diabetes Investig. 2017:8:672–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peplies J, Jiménez-Pavón D, Savva SC, Buck C, Günther K, Fraterman A, et al. Percentiles of fasting serum insulin, glucose, HbA1c and HOMA-IR in pre-pubertal normal weight European children from the IDEFICS cohort. Int J Obes (Lond). 2014;38:S39–47. [DOI] [PubMed] [Google Scholar]
- 20.Starling AP, Brinton JT, Glueck DH, Shapiro AL, Harrod CS, Lynch AM, et al. Associations of maternal BMI and gestational weight gain with neonatal adiposity in the Healthy Start study. Am J Clin Nutr. 2015;101:302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shapiro AL, Kaar JL, Crume TL, Starling AP, Siega-Riz AM, Ringham BM, et al. Maternal diet quality in pregnancy and neonatal adiposity: the Healthy Start Study. Int J Obes (Lond). 2016;40:1056–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A SAS Program for the 2000 CDC Growth Charts (ages 0 to <20 years) [Internet]. 2016. Available from: https://www.cdc.gov/nccdphp/dnpao/growthcharts/resources/sas.htm.
- 23.Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, et al. Cognition assessment using the NIH Toolbox. Neurology. 2013;80:S54–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bonora E, Targher G, Alberiche M, Bonadonna RC, Saggiani F, Zenere MB, et al. Homeostasis model assessment closely mirrors the glucose clamp technique in the assessment of insulin sensitivity: studies in subjects with various degrees of glucose tolerance and insulin sensitivity. Diabetes Care. 2000;23:57–63. [DOI] [PubMed] [Google Scholar]
- 25.Rubens M, Ramamoorthy V, Saxena A, George F, Shehadeh N, Attonito J, et al. Relationship Between Metabolic Syndrome and Cognitive Abilities in U.S. Adolescents. Metab Syndr Relat Disord. 2016;14:397–403. [DOI] [PubMed] [Google Scholar]
- 26.Kofler MJ, Irwin LN, Soto EF, Groves NB, Harmon SL, Sarver DE, Executive Functioning Heterogeneity in Pediatric ADHD. J Abnorm Child Psychol. 2018. April. doi: 10.1007/s10802-018-0438-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Karalunas SL, Hawkey E, Gustafsson H, Miller M, Langhorst M, Cordova M, et al. Overlapping and Distinct Cognitive Impairments in Attention-Deficit/Hyperactivity and Autism Spectrum Disorder without Intellectual Disability. J Abnorm Child Psychol. 2018;46:1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carter Leno V, Chandler S, White P, Pickles A, Baird G, Hobson C, et al. Testing the specificity of executive functioning impairments in adolescents with ADHD, ODD/CD and ASD. Eur Child Adolesc Psychiatry. 2018;27:899–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dickstein DP, Nelson EE, McClure EB, Grimley ME, Knopf L, Brotman MA, et al. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–55. [DOI] [PubMed] [Google Scholar]
- 30.Satrom KM, Ennis K, Sweis BM, Matveeva TM, Chen J, Hanson L, et al. Neonatal hyperglycemia induces CXCL10/CXCR3 signaling and microglial activation and impairs long-term synaptogenesis in the hippocampus and alters behavior in rats. J Neuroinflammation. 2018;15:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rao R, Nashawaty M, Fatima S, Ennis K, Tkac I. Neonatal hyperglycemia alters the neurochemical profile, dendritic arborization and gene expression in the developing rat hippocampus. NMR Biomed. 2018;31:e3910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rosa AP, Mescka CP, Catarino FM, de Castro AL, Teixeira RB, Campos C, et al. Neonatal hyperglycemia induces cell death in the rat brain. Metab Brain Dis. 2018;33:333–42. [DOI] [PubMed] [Google Scholar]
- 33.Barnea-Goraly N, Raman M, Mazaika P, Marzelli M, Hershey T, Weinzimer SA, et al. Alterations in white matter structure in young children with type 1 diabetes. Diabetes Care. 2014;37:332–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fox LA, Hershey T, Mauras N, Arbelaez AM, Tamborlane WV, Buckingham B, et al. Persistence of abnormalities in white matter in children with type 1 diabetes. Diabetologia. 2018;61:1538–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alfaro FJ, Lioutas VA, Pimentel DA, Chung CC, Bedoya F, Yoo WK, et al. Cognitive decline in metabolic syndrome is linked to microstructural white matter abnormalities. J Neurol. 2016;263:2505–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Bussel FC, Backes WH, van Veenendaal TM, Hofman PA, van Boxtel MP, Schram MT, et al. Functional Brain Networks Are Altered in Type 2 Diabetes and Prediabetes: Signs for Compensation of Cognitive Decrements? The Maastricht Study. Diabetes. 2016;65:2404–13. [DOI] [PubMed] [Google Scholar]
- 37.Kirchhoff BA, Jundt DK, Doty T, Hershey T. A longitudinal investigation of cognitive function in children and adolescents with type 1 diabetes mellitus. Pediatr Diabetes. 2017;18:443–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brady CC, Vannest JJ, Dolan LM, Kadis DS, Lee GR, Holland SK, et al. Obese adolescents with type 2 diabetes perform worse than controls on cognitive and behavioral assessments. Pediatr Diabetes. 2017;18:297–303. [DOI] [PubMed] [Google Scholar]
- 39.Awad A, Lundqvist R, Rolandsson O, Sundström A, Eliasson M. Lower cognitive performance among long-term type 1 diabetes survivors: A case-control study. J Diabetes Complications. 2017;31:1328–31. [DOI] [PubMed] [Google Scholar]
- 40.Broadley MM, White MJ, Andrew B. A Systematic Review and Meta-analysis of Executive Function Performance in Type 1 Diabetes Mellitus. Psychosom Med. 2017;79:684–96. [DOI] [PubMed] [Google Scholar]
- 41.Kesse-Guyot E, Julia C, Andreeva V, Fezeu L, Hercberg S, Galan P. Evidence of a cumulative effect of cardiometabolic disorders at midlife and subsequent cognitive function. Age Ageing. 2015;44:648–54. [DOI] [PubMed] [Google Scholar]
- 42.Kern W, Peters A, Fruehwald-Schultes B, Deininger E, Born J, Fehm HL. Improving influence of insulin on cognitive functions in humans. Neuroendocrinology. 2001;74:270–80. [DOI] [PubMed] [Google Scholar]
- 43.Novak V, Milberg W, Hao Y, Munshi M, Novak P, Galica A, et al. Enhancement of vasoreactivity and cognition by intranasal insulin in type 2 diabetes. Diabetes Care. 2014;37:751–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang H, Hao Y, Manor B, Novak P, Milberg W, Zhang J, et al. Intranasal insulin enhanced resting-state functional connectivity of hippocampal regions in type 2 diabetes. Diabetes. 2015;64:1025–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abbasi Z, Behnam-Rassouli F, Ghahramani Seno MM, Fereidoni M. A transient insulin system dysfunction in newborn rat brain followed by neonatal intracerebroventricular administration of streptozotocin could be accompanied by a labile cognitive impairment. Neurosci Res. 2018. July;132:17–25. [DOI] [PubMed] [Google Scholar]
- 46.Derakhshan F and Toth C. Insulin and the brain. Curr Diabetes Rev. 2013;9:102–16. [PubMed] [Google Scholar]
- 47.Fernandez AM and Torres-Aleman I. The many faces of insulin-like peptide signalling in the brain. Nat Rev Neurosci. 2012;13:225–39. [DOI] [PubMed] [Google Scholar]
- 48.Kleinridders A Deciphering Brain Insulin Receptor and Insulin-Like Growth Factor 1 Receptor Signalling. J Neuroendocrinol. 2016;28:doi: 10.1111/jne.12433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kleinridders A, Ferris HA, Cai W, Kahn CR. Insulin action in brain regulates systemic metabolism and brain function. Diabetes. 2014;63:2232–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kullmann S, Heni M, Veit R, Scheffler K, Machann J, Häring HU, et al. Selective insulin resistance in homeostatic and cognitive control brain areas in overweight and obese adults. Diabetes Care. 2015;38:1044–50. [DOI] [PubMed] [Google Scholar]
- 51.Kullmann S, Veit R, Peter A, Pohmann R, Scheffler K, Häring HU, et al. Dose-Dependent Effects of Intranasal Insulin on Resting-State Brain Activity. J Clin Endocrinol Metab. 2018;103:253–62. [DOI] [PubMed] [Google Scholar]
- 52.Maimaiti S, Anderson KL, DeMoll C, Brewer LD, Rauh BA, Gant JC, et al. Intranasal Insulin Improves Age-Related Cognitive Deficits and Reverses Electrophysiological Correlates of Brain Aging. J Gerontol A Biol Sci Med Sci. 2016;71:30–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ryan JP, Karim HT, Aizenstein HJ, Helbling NL, Toledo FG. Insulin sensitivity predicts brain network connectivity following a meal. Neuroimage. 2018. May;171:268–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Agrawal R and Gomez-Pinilla F. ‘Metabolic syndrome’ in the brain: deficiency in omega-3 fatty acid exacerbates dysfunctions in insulin receptor signalling and cognition. J Physiol. 2012;590:2485–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: a latent variable analysis. Cogn Psychol. 2000;41:49–100. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.