Abstract
Objective
There is an unmet need for tissue-engineered three-dimensional (3D) muscle constructs for laryngeal reconstruction. Functional engineered muscle could be used to repair post-oncologic or traumatic defects, or to medialize the vocal fold in cases of paresis/paralysis. Autologous, organized, engineered muscle that has adequate bulk, integrates into host tissue, and restores function does not currently exist.
Methods
Primary skeletal muscle progenitor cells (MPCs) were isolated from F344 rats. 3D muscle constructs were created by encapsulating MPCs via flow-alignment in a customized collagen formulation and cultured under passive tension. Muscle-specific immunohistochemistry and confocal microscopy were used to evaluate muscle tissue differentiation. After 2 weeks of culture, muscle constructs were implanted into surgically created defects in the rat larynx. Post mortem function testing and histology was performed at 1 and 3 months.
Results
Immunohistochemistry with confocal microscopy demonstrated well differentiated myotubes which were well aligned and distributed throughout the engineered construct in vitro. There was evidence of restoration of normal laryngeal function at 1 month post-op, as indicated by safe swallow (no aspiration events), weight gain, and excellent animal survival. Post-mortem specimens demonstrated functional muscle contraction on ex vivo testing, and histology confirmed integration into host tissue.
Conclusions
This is the first study to demonstrate that functional, 3D tissue-engineered skeletal muscle can be developed from primary MPCs and standardized oligomeric collagen. Collectively, these findings may have tremendous clinical implications for autologous laryngeal muscle repair and reconstruction.
Level of Evidence
NA
Keywords: muscle progenitor cells, type I collagen oligomers, tissue-engineered skeletal muscle, laryngeal reconstruction, tissue regeneration
INTRODUCTION
Compromised laryngeal function, whether due to congenital malformations, trauma, cancer, or surgical defects, affects thousands of individuals worldwide each year1. Unfortunately, therapeutic options to restore lost muscle and dynamic laryngeal functions for these patients are limited. As a result, patients suffer devastating quality of life consequences, including severe voice impairment, an ineffective or unsafe swallow, or airway obstruction, often necessitating gastrostomy and/or tracheostomy tubes. Advanced tissue engineering and regenerative strategies aimed to develop skeletal muscle implants may provide clinicians with new tools and therapeutic strategies for treating these patients. To achieve maximum therapeutic benefit, it has been proposed that engineered muscle should i) be constructed from autologous cell sources, ii) recapitulate the structure and functional properties of native skeletal muscle, which represents aligned muscle fibers interfacing within an appropriate, well-organized extracellular matrix (ECM), iii) integrate rapidly into host tissue with associated neovascularization and innervation, and iv) support scalable and patient-specific design2,3. While significant advances have been made in achieving this goal, persistent shortcomings remain, especially related to identification of standardized and customizable natural biomaterials which effectively recapitulate the guiding interface between muscle and its ECM and accelerate interfacial tissue regeneration.
Evaluation of the hierarchical structure-function of skeletal muscle reveals an intricate tissue design, including muscle fibers with their associated contractile machinery and a rich neurovascular supply which is essential for inducing and sustaining dynamic contraction. Furthermore, muscle ECM plays a critical role in guiding the muscle-nerve-vascular interface as well as supporting muscle’s mechanical function, adaptability, and repair4. Therefore, it is not surprising that the majority of muscle engineering approaches focus on capturing these essential design features. At present, the most widely used cell source for engineering skeletal muscle is the putative muscle progenitor cell (MPC; satellite cell), which can be readily isolated from muscle biopsies and cultured to produce myoblasts5. In turn, these cells are interfaced with a variety of natural and synthetic biomaterials, designed to promote myoblast fusion, differentiation, and maturation in vitro.
Synthetic polymers, including polycaprolactone and poly(lactic-co-glycolic) acid, are often the engineering material of choice largely owing to their mechanical stability, design versatility, and amenability to micro- and nano-fabrication techniques (e.g., electrospinning, patterning)6. Unfortunately, upon implantation in vivo, these materials are sensed as “foreign” to cells, yielding an inflammatory-mediated, foreign-body response7,8. Decellularized tissues (e.g., skeletal muscle), which are processed to maintain the complex composition, structural integrity, and architectural features of tissue ECM, have also been applied. However, these graft materials also induce an inflammatory reaction, and their dense microstructure prevents complete recellularization and muscle recovery9. As an alternative, natural polymers, such as fibrinogen, type I collagen, and Matrigel alone or in combination have been employed10–13. For these applications, cell-matrix constructs are cast within silicone or polydimethylsiloxane molds to form cylindrical or rectangular-shaped constructs. Constructs are then anchored at each end to provide passive tension, which is known to promote unidirectional cell alignment and fusion14. Although it is evident that these natural materials provide essential cell adhesion sites and associated bioinstructive properties, they are known to exhibit high batch-to-batch variability and are less amenable to the control of their physicochemical properties than synthetic polymers2. Although these strategies involving synthetic and natural materials have resulted in significant advancements with respect to muscle engineering, the search continues for a next-generation muscle design that harnesses myogenesis outside the body and rapid functional integration and neurovascular regeneration following implantation within the body.
The present work represents the first step towards a fabrication strategy for patient-specific human laryngeal muscle, featuring i) alignment of component MPCs and a collagen-fibril ECM immediately upon fabrication and ii) induction of motor endplate expression to accelerate innervation following in-vivo implantation7. Fabrication involved interfacing MPCs with collagen-fibril matrices formed with type I collagen oligomers, an uncommon soluble collagen subdomain which retains natural intermolecular crosslinks15–17. Oligomers are ideally suited for tissue engineering and regeneration strategies since they i) exhibit rapid suprafibrillar self-assembly yielding highly interconnected collagen-fibril matrices resembling those found in vivo; ii) are standardized based upon their fibril-forming capacity; iii) support cell encapsulation and distribution throughout the construct; and iv) allow customized multi-scale design across the broadest range of tissue architectures and physical properties18. Published work shows that oligomer-encapsulated cells remain viable and respond to local matrix mechanophysical cues displaying altered morphology, cytomechanics, phenotype, and tissue morphogenesis15,19. Additional in-vivo studies have shown that the collagen-fibril material persists, inducing neovascularization, innervation, and little to no inflammatory response20,21.
In the current study, preliminary design optimization studies were performed assessing the effect of flow-alignment, MPC density, and collagen-matrix stiffness on MPC fusion and myotube formation in vitro. In turn, optimized muscle constructs prepared with MPCs or motor endplate expressing MPCs (MEEs) were then evaluated for their ability to restore muscle and cartilaginous hemilaryngeal defects in rats. We hypothesized that aligned MEE-oligomer constructs would contribute to rapid tissue integration and innervation in the absence of significant inflammatory reaction. Collectively, the integration of our knowledge and toolkits related to laryngeal reconstruction, standardized polymerizable collagens, and therapeutic muscle cell populations will provide important new insights towards achieving tissue-engineered muscle constructs for functional laryngeal restoration.
MATERIALS AND METHODS
Myoblast Cell Line and Primary MPCs
C2C12 mouse myoblasts (ATCC, Rockville, Maryland) were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Fisher Scientific, Chicago, IL) supplemented with 1% penicillin, streptomycin, amphotericin B (PSF-1; HyClone, Logan, UT), and 10% fetal bovine serum (HyClone; Logan Utah) at 370;C and 5% CO2. Cells were cultured to 70% confluency and used in experiments at passages 5–8. Primary MPCs were generated from skeletal muscle biopsies obtained from 12-week-old male Fischer 344 rats (Envigo, Indianapolis, IN) as previously described7. In brief, fresh muscle tissue was placed in myogenic growth medium (MGM), which consisted of DMEM supplemented with 1% PSF-1, 20% fetal bovine serum, and 0.1% chick embryo extract (Accurate Chemicals, Westbury, NY). Muscle was minced and digested in 0.2% collagenase type I (EMD Millipore, Temecula, CA) at 37°C for 2 hours. Digested tissue was filtered through a 100μm cell strainer and washed 3 times with MGM. Resulting muscle fibers were suspended in MGM, plated onto untreated 100mm petri dishes (Fisher Scientific), and cultured overnight at 37°C within a humidified environment of 5% CO2 in air. The supernatant was removed the next morning and transferred to culture flasks (Corning Life Sciences, Corning, NY). Cells were cultured to 70% confluency and used in experiments at passages 3 to 5.
Fabrication of Engineered Skeletal Muscle Constructs
Type I collagen oligomers were acid-solubilized from the dermis of market-weight pigs and lyophilized for storage as described previously15. The oligomer formulation was standardized on the basis of molecular composition as well as polymerization capacity according to ASTM International standard F3089–1422. Here the polymerization capacity is defined by the matrix shear storage modulus, G’ (in Pa), as a function of oligomer concentration in the polymerization reaction. Each collagen solution was diluted with 0.01 N HCl to achieve the desired concentration. Neutralized solutions were kept on ice prior to induction of polymerization by warming to 37°C.
C2C12 cells or MPCs were suspended in neutralized oligomer collagen solution at either 10E6 or 10E7 cells/mL. The collagen-cell suspension (500μL) was injected into a pre-warmed 4-mm diameter Ultem (McMaster-Carr, Chicago, IL) cylinder mold and then allowed to polymerize at 37°C for 10 minutes. Immediately following polymerization, the construct was transferred to a customized culture chamber and secured with Velcro. Constructs were cultured in DMEM supplemented with 10% fetal bovine serum and 1% PSF-1 for 5 days with medium changes every 2 days. On day 5, medium was changed to differentiation medium, representing DMEM supplemented with 8% horse serum (HyClone) and 1% PSF-1, and constructs were cultured for an additional 7 days to induce myotube formation.
For a subset of experiments, MPCs were induced to express motor endplates (MEE) as described previously7. Briefly, muscle constructs were allowed to differentiate for 5 days at which point acetylcholine chloride (40 nM; Tocris Bioscience, Bristol, England), agrin (10 nM; R&D Systems, Minneapolis, Minnesota), and neuregulin (2 nM; R&D Systems) were added to the medium. Constructs were cultured an additional 7 days with medium changes every 3 days. Motor endplate expression was confirmed by immunostaining with Alexa Fluor 594 conjugated α-bungarotoxin (Molecular Probes, Eugene, Oregon).
Laryngectomy and Implantation of Engineered Muscle
Engineered muscle constructs were evaluated in our established rat partial laryngectomy model8. The animal study protocol was approved by Purdue Animal Care and Use Committee, and institutional guidelines, in accordance with the National Institutes of Health guidelines, were followed for the handling and care of the animals. In brief, 12 Fisher 344 rats were anesthetized with intraperitoneal injection of xylazine and ketamine and then maintained on 1–4% isoflurane. The ventral larynx was exposed via a midline incision. The sternohyoid muscle was incised and reflected to expose the thyroid cartilage. A relatively large section of hemi-thyroid cartilage and associated adductor muscle was removed from the left side. Animals were randomized into the following experimental groups: MPC-oligomer construct (n=4), MEE-oligomer construct (n=4), oligomer construct only (n=2), and defect only control (n=2). The construct was then placed into the defect (similar to a medialization laryngoplasty implant), with extrusion prevented by suturing overlying sternohyoid muscles to one another over the cartilaginous defect. The subcutaneous tissue and skin were then closed with 5–0 Vicryl suture.
Laryngeal Electomyography (EMG)
At 1 month and 3 month endpoints, laryngeal electromyography was performed under anesthesia as previously described14. Electromyogram (Niking Viking Quest electromyography machine, Madison, Wisconsin) was used with a 25-gauge bipolar concentric needle, settings with amplitude of 50 to 100μV, 10- to 100-ms sweep speeds, and a grounding clamp at the exposed lateral sternocleidomastoid muscle. The EMG recording needle was inserted directly into the center of the defect/implant site, the adductor (TA) muscle complex, and the posterior cricoarytenoid (PCA) muscle during laryngospasm and at rest. Immediately following electromyography, rats were humanely euthanized and tissue collected.
Histopathological and Histochemistry Assessment
After euthanasia, rat larynges with implanted engineered muscle were harvested en bloc, fixed in 4% paraformaldehyde overnight, and then transferred to 30% sucrose at 4°C for an additional 24 hours. Cryosections (25μm thickness) were prepared on a Thermo Cryotome FE (Fisher Scientific, Kalamazoo, MI). Sections were stained with hematoxylin & eosin (H&E) for histopathological analysis. Slides were viewed on a Nikon upright microscope (Eclipse E200, Nikon, Melville, NY) and images captured with a Leica camera (DFC480, Leica Buffalo Groove, IL).
For histochemistry analysis, all specimens were washed with phosphate buffered saline (PBS) 3 times, permeabilized with 0.1% Triton X-100 for 20 minutes, and blocked with 1% BSA for 2 hours. Whole engineered muscle constructs were incubated overnight at 4°C with Alexaflour 488 conjugated phalloidin (1:25 Molecular Probes, Eugene, OR) for visualization of F-actin and counterstained with Draq5 (1:1000 Cell Signaling, Danvers, MA 4084L) nuclear stain. For staining tissue explant cryosections, beta III tubulin conjugated primary antibody (1:10 NL647 Molecular Probes) was applied and incubated overnight at 4°C. After rinsing extensively, slides were incubated with Alexa Fluor 594 conjugated 〈-bungarotoxin (1:100) for 2 hours at room temperature. Slides were rinsed and mounted with Fluorogel for imaging on an Olympus Fluoview confocal microscope (IX81, Olympus Waltham, MA).
RESULTS
Flow-aligned cell-oligomer constructs prepared with high cell density and low collagen-fibril density (stiffness) supports enhanced myotube formation in-vitro
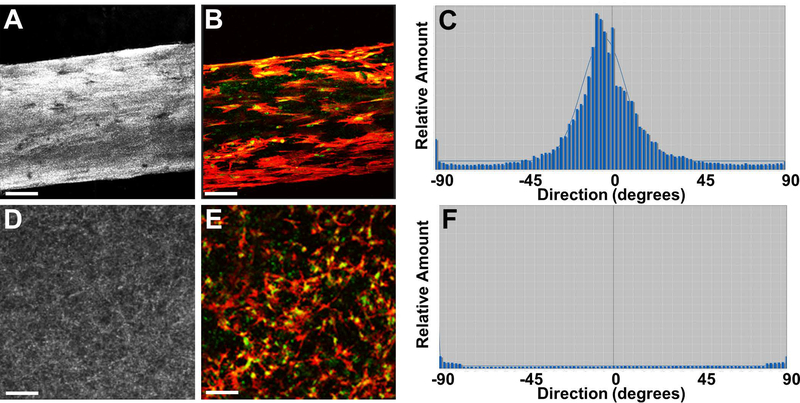
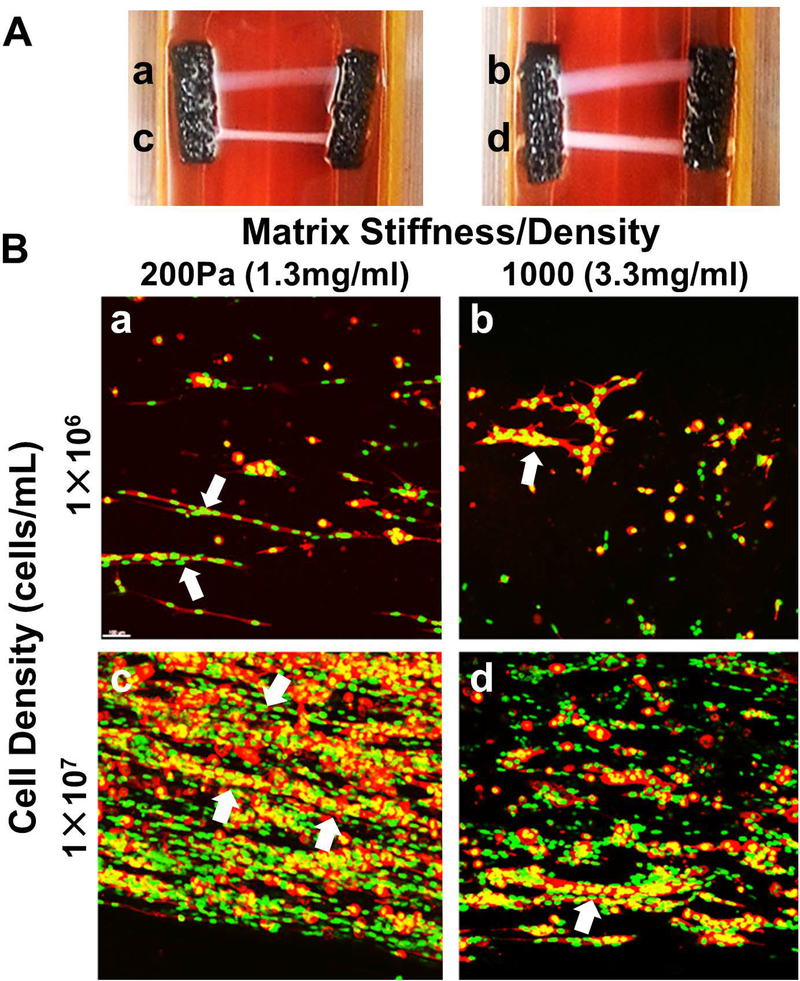
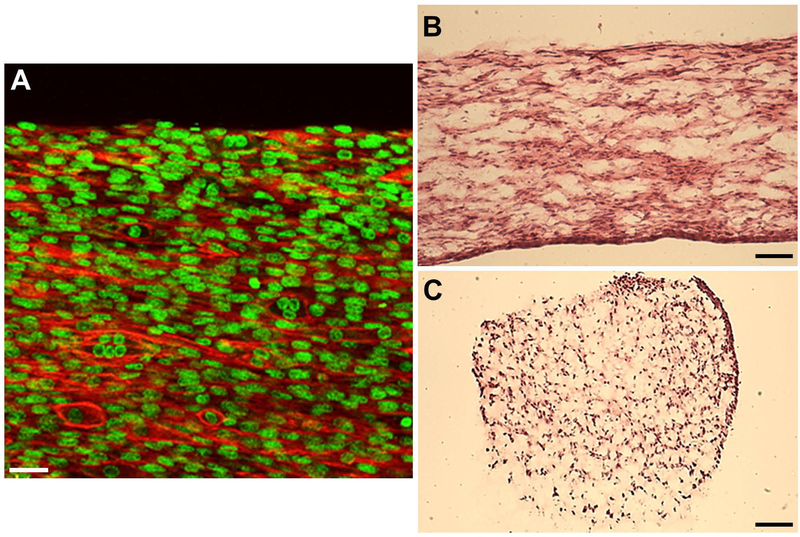
A challenge associated with tissue-engineered muscle is the rapid and reproducible fabrication of muscle constructs with uniform cell alignment and physiologically-relevant ECM associations. Here, polymerization of C2C12-oligomer solutions in flow resulted in constructs with both component collagen-fibrils and myoblasts aligned in the direction of flow (Figure 1A–C). In contrast, when conventional casting techniques were applied, cells were uniformly distributed within a randomly organized collagen-fibril matrix (Figure 1D–F). The flow-alignment method was then applied to determine the optimal cell and collagen-fibril density (stiffness) to achieve myotube formation. As indicated in Figure 2, constructs prepared at high cell density and low collagen-fibril density exhibited the necessary cell-cell interactions and matrix support to facilitate cell fusion and nuclear alignment. Follow-up verification studies revealed that this fabrication strategy was also applicable to primary rat MPCs, yielding highly reproducible engineered muscle with viable cells distributed throughout the construct after 2 weeks of culture (Figure 3).
Figure 1.
Representative images and directionality analyses for engineered muscle constructs created using flow-alignment method (A-C) compared to conventional mono-dispersion technique (D-F). Fabricated muscle constructs were cultured 24 hours and analyzed by confocal reflection microscopy for visualization of fibril microstructure (A,D), confocal fluorescence microscopy for visualization of myoblast morphology (B,E; green=actin; blue=nuclei), and ImageJ Directionality algorithm for alignment determination (C,F). Scale bar=50μm
Figure 2.
Tissue-engineered muscle construct culture and optimization. Muscle constructs were created by flow-alignment of C2C12 myoblasts at 10E6 cells/mL (a,b) or 10E7 cells/mL (c,d) within type I collagen oligomer matrices prepared at 200Pa (a,c) and 1000Pa (b,d). A. Constructs were cultured within a custom culture device for 14 days under passive tension. B. Confocal images (100μm thickness) of 14-day constructs stained with phalloidin (F-actin; red) and Draq5 (nucleus, green) show more extensive myotube formation (white arrows) for constructs prepared at high cell densities (10E7 cells/mL) within low stiffness matrices (200Pa). Scale bar=50μm
Figure 3.
Engineered muscle constructs (14 days) prepared via flow-alignment of F344 primary MPC (10E7 cells/mL) and 200Pa oligomer collagen. A. Confocal images (50μm thickness) of 14-day constructs stained with phalloidin (F-actin, red) and Draq5 (nucleus, green) show highly aligned cell morphology and fused myotube formation. Cells were distributed throughout the entire construct volume as indicated by H&E staining of longitudinal (B) and transverse (C) sections. Scale bars=30μm (A), 500μm (B-C)
Reconstruction of laryngeal defects with MPC-oligomer constructs yields myogenesis, neurovascular regeneration, and tissue integration in absence of an inflammatory-mediated foreign body response
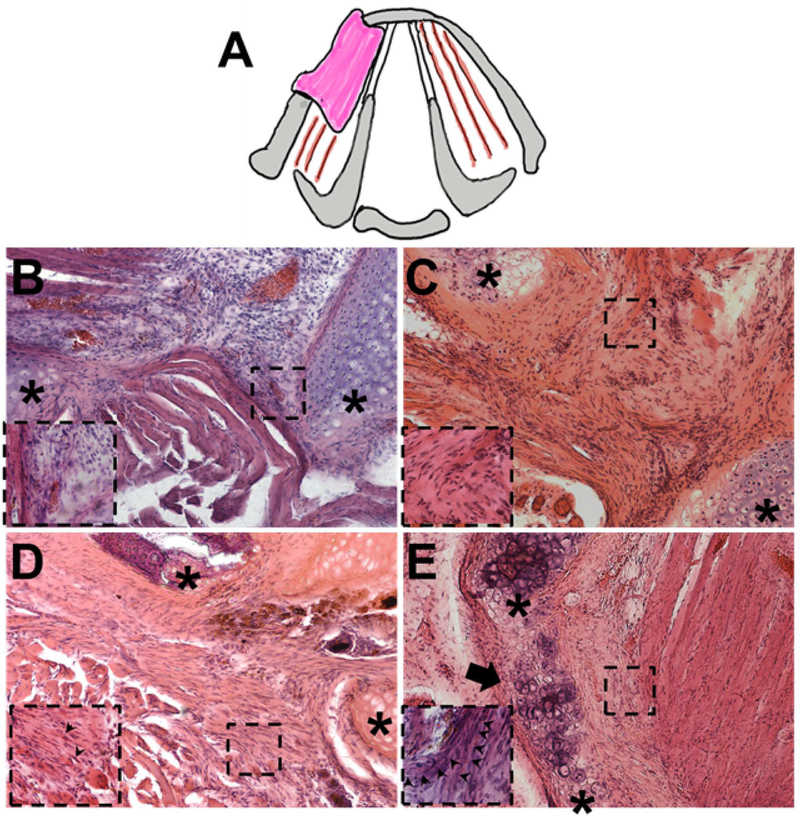
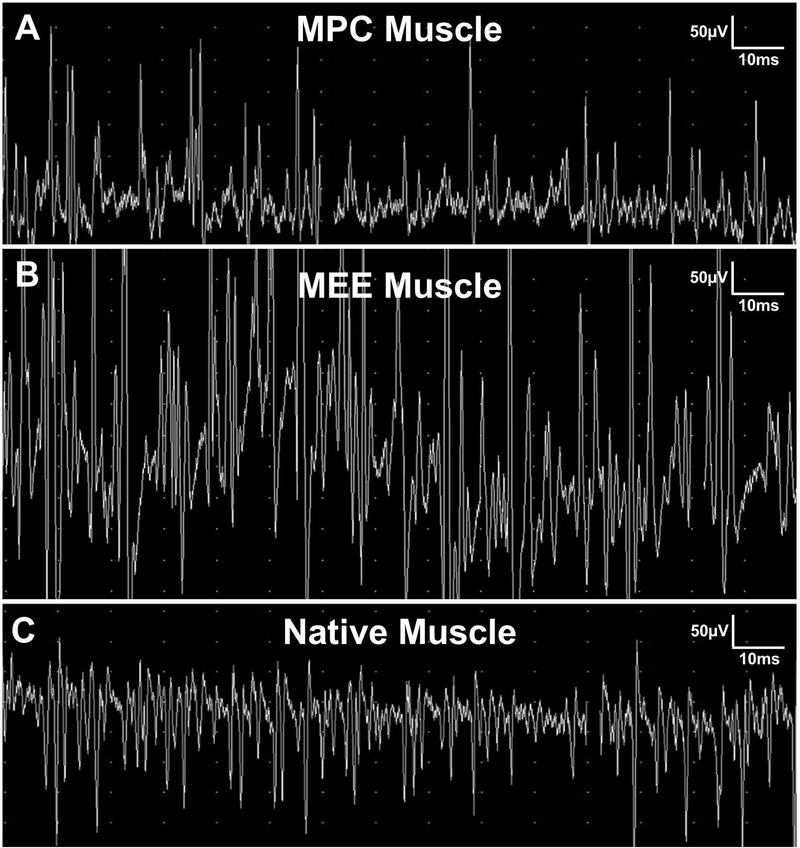
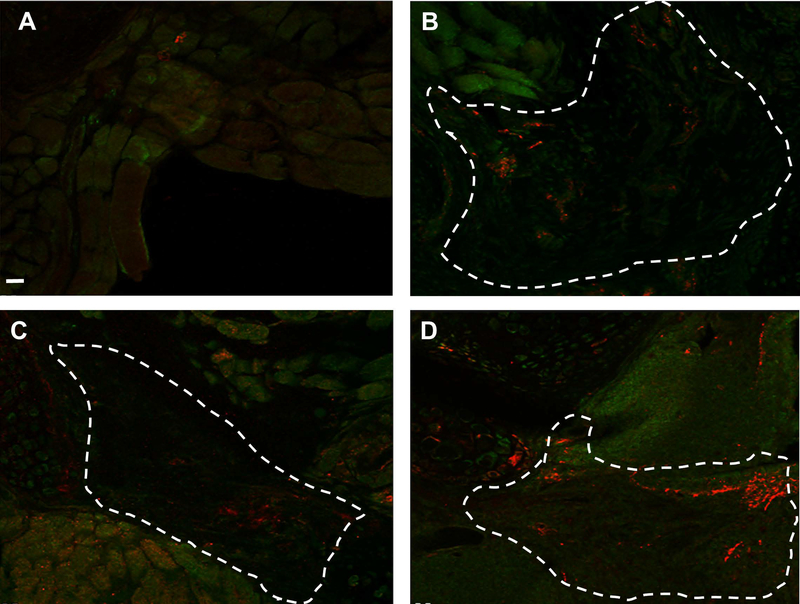
During the post-surgical period, all animals steadily gained weight and showed no signs of laryngeal compromise. As expected, partial laryngectomy with resection of cartilage and muscle and no treatment resulted in a healing response marked by inflammation and fibrous tissue formation within the defect area (Figure 4A). In contrast, oligomer implants, with and without MPCs, showed no significant inflammatory reaction and integrated rapidly with adjacent host tissues (Figure 4B–D). A surprising finding was that chondrogenesis accompanied muscle regeneration over time (Figure 4D). Compared to the oligomer-only group, MPC-oligomer and MEE-oligomer constructs showed more rapid muscle regeneration and maturation as evidenced by obvious striations within the implants as early as 1 month that became more evident at 3 months. By 3 months, the relative extent of striated muscle increased, and was supported by neurogenesis, as evidenced by prominent beta III tubulin staining arising from the graft-host tissue interface (Figure 5). Interestingly, results based on beta III tubulin and 〈-bungarotoxin staining indicated that the greatest level of innervation was achieved with the MEE-oligomer group. Qualitative EMG measurements provided further corroborating evidence of enhanced innervation of the MEE-oligomer constructs, with the MEE-oligomer group demonstrating the greatest level of motor unit activity (recruitment) during laryngospasm (Figure 6).
Figure 4.
A. Schematic showing partial laryngectomy model, which involved removal of a section of hemi-thyroid cartilage and associated underlying adductor muscle from the left side. Cross section of rat larynx showing surgical procedure and implant placement. B-E. Representative H&E stained sections of untreated control defect (B, 2 months) and defects treated with oligomer-only implant (C, 2 months), engineered muscle implant (D, 1 month), and engineered muscle implant (E, 3 months). Untreated defect (A) showed gap filled with native sternohyoid muscle and healing via inflammation. Oligomer-only group showed no significant inflammatory response and construct populated with mesenchymal cells. Engineered muscle implants showed progressive increase in volume of striated muscle (arrows) and cartilage regeneration over time with similar responses observed for both MPC- and MEE-oligomer implants). Asterix (*) indicate edges of cartilage defect. Inserts represent high magnification of boxed areas. Scale bar=200μm
Figure 5.
Representative electromyography (EMG) tracings captured at 50μV amplitude and 10ms sweep speeds during active laryngospasm to detect firing within engineered muscle implant 3 months following implantation (A,B) or native adductor muscle complex (C). A. Defect treated with engineered muscle prepared with muscle progenitor cells (MPC Muscle) generated abundant, variable-sized motor unit potentials. B. Engineered muscle prepared with motor endplate expressing MPC (MEE Muscle) generated potentials that were significantly larger in amplitude and frequency compared to MPC Muscle and native adductor muscle. C. Native adductor muscle complex demonstrated bursts of motor unit potentials during laryngospasm.
Figure 6.
Anti-beta III tubulin (red) and 〈-bungarotoxin staining A. defect only control B. collagen only control C. MSC implant at 3 month post-op D. MEE implant at 3 month post op. The MEE-oligomer construct (D) has increased innervation when compared to the MSC-oligomer construct (C). Both the MEE- and MSC-oligomer constructs have increased innervation when compared to the oligomer-only and untreated controls. Dotted line outlines approximate construct boundaries. Scale bar=100μm
DISCUSSION
We and others have shown that significant muscular injuries result in a reparative inflammatory-mediated healing response yielding fibrotic scar and dysfunctional muscle. Here, the myogenic potential of MPCs was interfaced with a standardized self-assembling type I collagen-fibril matrix to create a 3D tissue-engineered muscle for laryngeal reconstruction. Flow-alignment and an optimized MPC-collagen-fibril density ratio were used to achieve cell-matrix physical and biochemical associations that mimicked those found between muscle cells and the endomysium in vivo, resulting in accelerated in-vitro myotube formation. Additional benefit of recapitulating the muscle-ECM interface was evident from the time-dependent recovery of muscle volume and function along with regeneration of supporting cartilaginous structures following implantation in vivo.
Results of these studies suggest that aligned MEE-oligomer constructs contributed to rapid tissue integration and regeneration in the absence of any significant inflammatory reaction, yielding engineered muscle with enhanced innervation on histology, and functional elicitation of motor unit potentials. Major advantages of the model include the use of autologous cells strategically aligned, robust muscle neovascularization/innervation/survival, and a scalable model which could be translated into patient-specific designs. While the current study allowed a variety of constructs to be tested in vivo, the major limitation of such a rodent model is the small anatomic size and physiologic differences (lack of cough) preventing complete transmural hemilaryngectomies from being safely performed. Thus, animal studies with engineered muscle and cartilage will be needed in the future to better assess the full potential of the engineered muscle to recapitulate laryngeal structure and function.
CONCLUSION
Collectively, this work represents initial design optimization of a 3D tissue-engineered muscle implant prepared from therapeutic MPCs and type I collagen oligomers. This strategy overcomes a number of significant challenges related to skeletal muscle engineering including i) the use of a standardized and customizable self-assembling collagen formulation, ii) promotion of interfacial tissue regeneration by minimizing inflammation and iii) acceleration of functional muscle recovery through rapid reinnervation in MEE constructs. While our current study suggests that MEE constructs receive robust innervation after implantation, further studies are needed to determine if these findings will translate into improved muscle contractility and function. Thus, these experiments represent initial steps towards the successful design and application of engineered autologous muscle implants for laryngeal repair and reconstruction.
ACKNOWLEDGEMENTS
These projects were supported in part by award number R01DC014070-01A1 from the National Institute on Deafness and Other Communication Disorders (NIDCD) within the National Institutes of Health (NIH). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the NIDCD or NIH. The authors would like to thank Alexis Zobel (2015 Summer Research Fellow) for her assistance with flow alignment method, Melissa Bible and the rest of the Weldon School of Biomedical Engineering animal technical staff for their animal study support, and finally Will Hoggatt and Richard Brookes for the design and fabrication of the Ultem mold and custom culture device.
This project was supported in part by award number R01DC014070-01A1 from the National Institute on Deafness and Other Communication Disorders (NIDCD) within the National Institutes of Health (NIH).
This study was performed in accordance with the PHS Policy on Humane Care and Use of Laboratory Animals, the NIH Guide for the Care and Use of Laboratory Animals, and the Animal Welfare Act (7 U.S.C. et seq.); the animal use protocol was approved by the Purdue Animal Care and Use Committee (PACUC).
Footnotes
Financial Disclosure/conflict of interest: None
REFERENCES
- 1.Howlader N, Noone AM, Krapcho M, Miller D, Bishop, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds). SEER Cancer Statistics Review, 1975–2013, National Cancer Institute; Bethesda, MD, http://seer.cancer.gov/csr/1975_2013/, based on November 2015 SEER data submission, posted to the SEER web site, April 2016. [Google Scholar]
- 2.Bian W, Bursac N. Tissue engineering of functional skeletal muscle: challenges and recent advances. IEEE Eng Med Biol Mag 2008; 27:109–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mertens JP, Sugg KB, Lee JD, Larkin LM. Engineering muscle constructs for the creation of functional engineered musculoskeletal tissue. Regen Med 2014; 9:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve 2011; 44:318–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng CS, Davis BN, Madden L, Bursac N, Truskey GA. Physiology and metabolism of tissue-engineered skeletal muscle. Exp Biol Med (Maywood) 2014; 239:1203–1214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ostrovidov S, Hosseini V, Ahadian Set al. Skeletal muscle tissue engineering: methods to form skeletal myotubes and their applications. Tissue Eng Part B Rev 2014; 20:403–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Halum SL, Bijangi-Vishehsaraei K, Zhang H, Sowinski J, Bottino MC. Stem cell-derived tissue-engineered constructs for hemilaryngeal reconstruction. Ann Otol Rhinol Laryngol 2014; 123:124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morehead JM, Holt GR. Soft-tissue response to synthetic biomaterials. Otolaryngol Clin North Am 1994; 27:195–201. [PubMed] [Google Scholar]
- 9.Porzionato A, Sfriso MM, Pontini A et al. Decellularized Human Skeletal Muscle as Biologic Scaffold for Reconstructive Surgery. Int J Mol Sci 2015; 16:14808–14831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hinds S, Bian W, Dennis RG, Bursac N. The role of extracellular matrix composition in structure and function of bioengineered skeletal muscle. Biomaterials 2011; 32:3575–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhim C, Lowell DA, Reedy MC et al. Morphology and ultrastructure of differentiating three-dimensional mammalian skeletal muscle in a collagen gel. Muscle Nerve 2007; 36:71–80. [DOI] [PubMed] [Google Scholar]
- 12.Rossi CA, Pozzobon M, Ditadi A et al. Clonal characterization of rat muscle satellite cells: proliferation, metabolism and differentiation define an intrinsic heterogeneity. PLoS One 2010; 5:e8523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol (1985) 2005; 98:706–713. [DOI] [PubMed] [Google Scholar]
- 14.Powell CA, Smiley BL, Mills J, Vandenburgh HH. Mechanical stimulation improves tissue-engineered human skeletal muscle. Am J Physiol Cell Physiol 2002; 283:C1557–1565. [DOI] [PubMed] [Google Scholar]
- 15.Kreger ST, Bell BJ, Bailey Jet al. Polymerization and matrix physical properties as important design considerations for soluble collagen formulations. Biopolymers 2010; 93:690–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey JL, Critser PJ, Whittington C, Kuske JL, Yoder MC, Voytik-Harbin SL. Collagen oligomers modulate physical and biological properties of three-dimensional self-assembled matrices. Biopolymers 2011; 95:77–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blum KM, Novak T, Watkins L, Neu CP, Wallace JM, Bart ZR, Voytik-Harbin SL. Acellular and cellular high-density, collagen-fibril constructs with suprafibrillar organization. Biomater Sci 2016; 4:711–723. [DOI] [PubMed] [Google Scholar]
- 18.Voytik-Harbin SL, Han B. Collagen-cell interactions and modeling in microenvironments In: Neu CP, Genin G, eds. CRC Handbook of Imaging in Biological Mechanics. Boca Raton, Florida: Taylor & Francis Group; 2015:261–273. [Google Scholar]
- 19.Whittington CF, Yoder MC, Voytik-Harbin SL. Collagen-polymer guidance of vessel network formation and stabilization by endothelial colony forming cells in vitro. Macromol Biosci 2013; 13:1135–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Critser PJ, Kreger ST, Voytik-Harbin SL, Yoder MC. Collagen matrix physical properties modulate endothelial colony forming cell-derived vessels in vivo. Microvasc Res 2010; 80:23–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yrineo AA, Adelsperger AR, Durkes AC et al. Murine ultrasound-guided transabdominal para-aortic injections of self-assembling type I collagen oligomers. J Control Release 2017; 249:53–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.ASTM Standard F3089: Characterization and Standardization of Polymerizable Collagen-Based Products and Associated Collagen-Cell Interactions; ASTM International: West Conshohocken, PA, 2014; DOI: 10.1520/F3089-14 ; 10.1520/F3089-14www.astm.org; www.astm.org . [Google Scholar]