Abstract
Peripheral membrane proteins associate reversibly with biological membranes that, compared to protein binding partners, are structurally labile and devoid of specific binding pockets. Membranes in different subcellular compartments vary primarily in their chemical composition and physical properties, and recognition of these features is therefore critical for allowing such proteins to engage their proper membrane targets. Intrinsically disordered proteins are well-suited to accomplish this task using highly specific and low-to-moderate-affinity interactions governed by recognition principles that are both similar to and different from those that mediate the membrane interactions of rigid proteins. IDPs have also evolved multiple mechanisms to regulate membrane (and other) interactions and achieve their impressive functional diversity. Moreover, IDP-membrane interactions may have a kinetic advantage in fast processes requiring rapid control of such interactions, such as synaptic transmission or signaling. Herein we review the biophysics, regulation and functional implications of IDP-membrane interactions and include a brief overview of some of the methods that can be used to study such interactions. At each step, we use the example of alpha-synuclein, a protein involved in the pathogenesis of Parkinson’s disease and one of the best characterized membrane-binding IDP, to illustrate some of the principles discussed.
Keywords: IDP, membrane, synuclein, synaptic vesicle, neurodegeneration, Parkinson’s
1. Introduction
In contrast to well-folded proteins, intrinsically disordered proteins or protein regions (IDPs or IDRs) are not limited to one or a few predominant conformations. They typically sample many different conformations separated by low energy barriers, allowing rapid interconversion. This enables such proteins to interact with diverse binding partners and respond to a variety of environmental stimuli. IDP interactions are often transient or reversible, range from low- to high-affinity, and are ubiquitous in biologically significant contexts. IDPs/IDRs have been found to interact with other proteins, nucleic acids and lipid membranes. Here we focus on and discuss the membrane interactions of IDPs/IDRs and their potential implications in health and disease, using the Parkinson’s disease protein alpha-synuclein as an example.
1.1. IDP-membrane interactions in physiology:
Protein-membrane interactions are involved in virtually every aspect of cellular function, with fundamental roles in key tasks such as signaling, membrane trafficking between organelles and transport through cellular membranes. These tasks typically require interactions that are highly specific and regulated. To achieve this, IDPs have evolved to feature remarkable specificity in binding to various types of biological membranes. This specificity is made possible by different membrane chemical properties (charged content, headgroup chemistry) as well as physical properties (membrane curvature, packing density). Membrane specificity is encoded in part in the primary sequence of an IDP but can also be modified or regulated by post-translational modifications. Many IDPs gain structure on binding to protein partners, and this can occur upon binding to biological membranes as well. Oftentimes, membrane-interacting IDRs adopt a helical structure upon binding, undergoing a ‘folding upon binding’ transition. Membrane binding by alpha-synuclein is one of the best studied cases of IDP/membrane interactions and can therefore serve as an example to discuss general concepts.
1.2. Alpha-synuclein:
Alpha-synuclein is a small, 140-residue presynaptic peripheral membrane protein of indeterminate function that is genetically and pathologically linked to Parkinson’s disease. The membrane-binding domain of alpha-synuclein consists of its N-terminal ~100 residues. The primary sequence of this domain features seven imperfect repeats, with a core hexapeptide consensus motif: KTKEGV. Experiments have shown that this domain forms amphipathic helical structure upon binding to membranes and membrane-mimetics with an unusual periodicity of 3 full turns per 11 residues [1,2]. It has been suggested that this non-canonical superhelical twist facilitates formation of a continuously curved helical structure, increasing its specificity in binding to highly curved membranes such as synaptic vesicles [3]. Indeed, alpha-synuclein has been described as a curvature-sensing protein with a higher affinity to highly curved membrane surfaces [4,5]. In addition, this configuration may be strained and may thereby reduce the binding affinity of alpha-synuclein to membranes in a way that may tune its affinity and prevent tight binding to isolated synaptic vesicles [6]. Alpha-synuclein binds specifically to membranes containing negatively charged lipid headgroups, and an increase in the proportion of negatively charged lipids like phosphatidylserine (PS) causes an increased binding affinity [7,8].
2. Biophysics of IDP-membrane interactions
2.1. Folding upon binding:
In contrast to integral membrane proteins, peripheral membrane proteins exhibit reversible binding to biological membranes. Reversible membrane binding domains have been studied extensively for several families of peripheral membrane proteins [9,10]. Whereas many of these domains retain their tertiary structure in their free state, membrane binding IDPs/IDRs can exhibit a radical change in structure when bound to cognate membranes. It is instructive to first briefly review the mechanisms governing the interactions of IDPs with other proteins. Although some IDP-protein interactions do not involve folding [11– 15], many do, and the mechanisms governing coupled folding and binding remain an important area of investigation [16]. On one extreme there is the conformational selection model which in which the correct conformation is chosen from an ensemble of conformations as an IDP binds to its target. On the other extreme is the induced fit model, in which binding occurs first and folding occurs subsequently and is ‘induced’ by the IDP interactions with its partner. Induced fit processes can also include a ‘fly casting model’ which allows an IDP to have a greater capture radius for binding when unfolded, albeit with a lower affinity, followed by folding and consequent reduction in distance between the partners [17]. This mechanism has been proposed to enhance the binding rate significantly over that of a well-folded domain, an effect more pronounced for a proteins present at low concentrations in the cell, such as transcription factors [17]. Experimental approaches to probe the sequential steps involved in such interactions may involve direct detection of sparse intermediates [18–20], or detailed analyses of the reaction kinetics [21]. A decrease in the reaction rate constant as a function of increasing ligand concentration is characteristic of conformational selection, while an increase of the same could arise in either model [22–24]. In the latter case, the two models can be distinguished by employing an excess of either reactant to approach a pseudo-first order reaction condition [21,25]. Although these experimental methods have limitations, some general principles have emerged from experimental studies involving specific IDPs. In some cases, studies support an induced fit or ‘binding before folding’ model, including elements of’fly-casting’ [26]. As an unstructured protein approaches a binding surface, transient encounter complexes are formed, eventually leading to a stable interaction [20]. Binding of the intrinsically disordered p-KID domain of the transcription factor CREB to the KIX domain of the transcriptional coactivator CBP is a classic example involving an encounter complex and follows the induced fit model [27]. Interestingly, when binding to the same KIX domain of CBP, the activation domain of a different transcription factor, c-Myb, exhibits a combination of conformational selection and induced fit [28]. Another study found that either mechanism can take precedence depending on the strength of the binding interactions [29]. Pure conformational selection, while common in structured proteins [18,23,30,31], appears to be relatively rare for IDPs, perhaps because the intrinsic secondary structure propensity determines the favored model [28], and IDPs typically lack highly stable secondary structure in their free state. All told, these and other studies reveal that the mechanism of folding upon binding is dependent on the specific system and does not appear to favor either model exclusively.
While IDP-protein binding reactions have been studied in great detail, IDP-membrane interactions remain less well explored. Studies on alpha-synuclein binding to lipid vesicles have consistently supported the induced fit model of binding. In particular, the N-terminus of alpha-synuclein binds to lipid membranes with a higher affinity than the rest of the protein. This is especially true for the physiologically relevant N-terminally acetylated form of the protein, for which the N-terminal ~10 residues bind more tightly to lipid vesicles than the remainder of the protein [7,32]. Subsequent to lipid binding by at the N-terminus, folding upon binding proceeds from the N-to the C-terminus of the lipid-binding domain. Important evidence for this is provided by mutations that interrupt helix formation (e.g. familial Parkinson’s disease mutant A30P) [33] or disrupt vesicle binding (G51D) [34]. Such mutations reduce binding of residues C-terminal to the mutation site, presumably by interrupting the propagation of the folding-upon-binding reaction. Interestingly, we also observe an increased binding affinity for residues N-terminal to such mutation sites suggesting some degree of anti-cooperativity in alpha-synuclein membrane binding, i.e., there is an energetic cost to folding an extended length of the protein that impacts the overall binding affinity [35], perhaps as a consequence of the altered periodicity of the extended helix [6].
2.2. Membrane targeting of IDPs:
The cell has an elaborate mechanism for transporting integral membrane proteins and lipids to specific subcellular membranes. For most of these proteins, membrane targeting is needed only once during their lifetime, and it is intricately coupled with translation, protein folding and vesicle transport. However, the transient nature of membrane binding by peripheral membrane proteins, including membrane-binding IDPs, poses a challenge for specific membrane recognition [36]. In general, IDPs bind to membranes as a result of a combination of hydrophobic and electrostatic interactions [4]. Lipid composition, packing and membrane curvature all play roles in imparting membrane specificity via these interactions. Many IDPs bind to membranes as amphipathic helices, one surface of which is chiefly hydrophobic while the other surface is populated by more hydrophilic residues. Finer control of binding specificity is impacted by various factors such as size of the side chains of those residues, positioning of the residues with respect to the interface and helical periodicity [37]. However, IDPs or IDRs are also able to bind to membranes while remaining unstructured, using individual side chains to interact with either the charged lipid headgroups or the hydrophobic membrane interiors [38–44].
Certain peripheral membrane proteins have unique properties that enable them to specifically target highly curved membrane surfaces. These include the Bin/Amphiphysin/Rvs (BAR) domain proteins [45,46]; ALPS motifs found in proteins like ArfGAPl [47]. Epsin N-terminal Homology (ENTH) domains [48] and AP 180 N-terminal Homology (ANTH) domains [49]; the C-terminal domain of complexin [44]; and the lipid binding domain of alpha-synuclein [4,50]. Although BAR domains are typically structured even when isolated in solution, the membrane curvature sensing properties of ArfGAP 1 and of the ENTH and ANTH domain proteins arise from an accessory ALPS motif helix which is usually intrinsically disordered in absence of lipids [48,49], and the curvature sensing domains of both complexin and alpha-synuclein are also disordered in the absence of membranes [4,44,50]. Interestingly, in the case of complexin, curvature sensitivity is mediated by two adjacent membrane-binding motifs, one of which remains unstructured in the membrane-bound state, and the other of which folds into an amphipathic helical structure only upon binding to highly curved membranes [44,51]. The case of alpha-synuclein is somewhat unique in that it binds to membranes via its ~100 residue N-terminal lipid-binding domain, which contains 7 imperfect repeats including the hexapeptide consensus sequence KTKEGV. The amphipathic helix inserts partially into the lipid membrane, while the lysines are laterally oriented and positioned at the lipid/water interface, allowing interactions with negatively charged lipid headgroups [2,3,50,52–54] (Figure 1). This is a unique property of Class A amphipathic helices, a structure common in apolipoproteins [53]. The insertion of the hydrophobic face of a helix into the membrane interior is generally considered to favor positively curved membranes which feature an increase in packing defects capable of accommodating helix insertion. Interestingly, the details of the side chain distribution on the different helix faces appears to modulate curvature sensing [37,55,56]. Similarly, insertion of bulky sidechains such as phenylalanine can confer curvature sensitivity in the absence of helical structure [42]. Meanwhile, electrostatic interactions can clearly guide IDPs or other peripheral membrane proteins to charged membrane surfaces.
Figure 1:
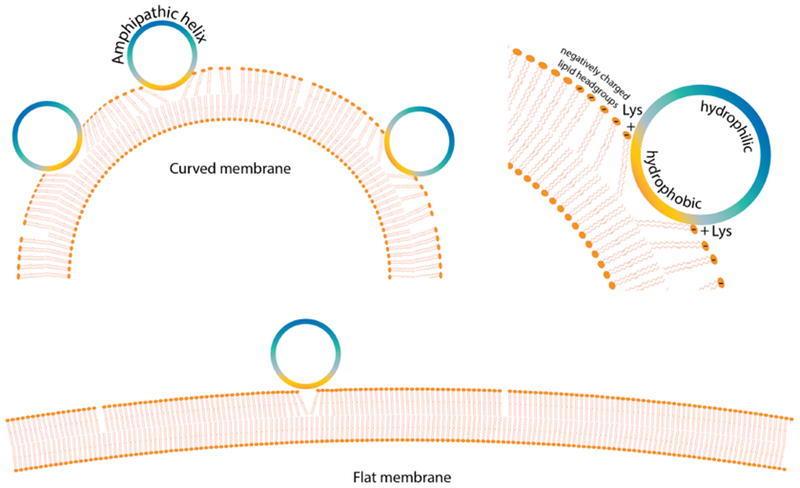
Membrane targeting of IDPs by curvature sensing and electrostatics. Shown here is the example of alpha-synuclein forming an amphipathic helix on preferential binding to highly curved membranes. The outer surface of a highly curved bilayer membrane has a high density of packing defects that allow increased binding and deeper insertion of the hydrophobic face of an amphipathic helix. In the case of alpha-synuclein, such deeper insertion in a negatively charged highly curved membrane is further stabilized by electrostatic interactions between the lysines on the interface and the negatively charged lipid headgroups on the membrane. An imperfectly developed hydrophobic face containing multiple polar threonine residues hinders alpha-synuclein binding on a flat membrane where the packing defects are sparse and shallow, resulting in its curvature sensitivity and preferential binding to negatively charged membranes [55].
2.3. Modulation of membrane curvature:
The same properties that enable an IDP to bind selectively to curved membrane also induce forces that are able to curve a flat membrane, which, in extreme cases, can lead to membrane fission or fusion [57,58]. In order to bend a membrane, the membrane binding energy of a protein domain has to exceed the membrane bending energy [59]. The classical examples of membrane curvature inducing proteins are involved in the processes of endocytosis. Amphiphysin and endophilin, which contain BAR domains [45,60–62], present the membrane with a scaffold for curvature generation. In these cases, the protein must have sufficient rigidity to counteract the elastic force generated by the curved membrane. On the other hand, amphipathic helices that insert into one leaflet of a bilayer membrane can bend the membrane by a spontaneous local curvature mechanism [59]. IDPs/IDRs that do not directly bind the membrane can also induce localized positive membrane curvature through steric forces generated by maximizing IDP conformational entropy, in which case, electrostatic repulsion between IDRs may also contribute to the curvature-inducing force [63]. Change in liposome shape on binding to a protein is commonly used as an in-vitro test for its membrane-deforming ability. The amphipathic ENTH domain of epsin, which is intrinsically disordered, has been shown to form tubules from liposomes [48]. Membrane tubulation and remodeling have been described for alpha-synuclein as well, which is dependent on the lipid composition of liposomes and protein-lipid ratio of the mixture performed in presence of lipid vesicles [64–69].
3. Regulation of membrane binding
Since membrane-bound IDPs play important roles in signaling, membrane trafficking and membrane fusion, their membrane binding is regulatable, most typically by post-translational modifications (PTMs). PTMs offer chemical, structural and functional diversity in the cell’s repertoire of proteins. Localized changes caused by PTMs can cause large-scale conformational rearrangements that can act as signaling switches [70]. Different PTMs exert their influence over different timescales. Some are acquired during translation and stay with the protein for its lifetime, e.g., N-terminal acetylation, whereas others are transient and reversible in nature, e.g., phosphorylation. Phosphorylation at specific sites of an IDP by specific enzymes offers a mode of rapid and reversible control of its function. For example, multisite phosphorylation causes folding of the intrinsically disordered eukaryotic translation initiation inhibitor 4E-BP2 to bury its active site and thus preventing its binding to eIF4E [70]. Synaptic vesicle clustering in neuronal termini is, among other mechanisms, regulated by the phase separating ability of a long IDR in the protein synapsin-1 [71,72]. When phosphorylated at specific sites by calcium and calmodulin-dependent protein kinase II, synapsin-1 phases rapidly disperse, along with the dispersion of the synaptic vesicle clusters [71]. Yet other types of modifications are considered terminal and lead the proteins to degradative pathways (e.g. ubiquitination and ubiquitin-like modifications). Understanding the functional roles of different PTMs and identification of specific enzymes or pathways involved in different PTMs are potentially valuable in order to identify novel drug targets. For example, histone deacetylase (HDAC) inhibitors are clinically approved for certain types of malignancy and a number of small molecules targeting various PTMs on the intrinsically disordered tail of histones are in active clinical trials or preclinical development [73]. Kinases, of course, are also an important class of drug targets in cancer and other diseases. The c-Abl kinase inhibitor nilotinib has shown beneficial effect in Parkinson’s disease patients [74] and disease models [75–78] and its effect could be mediated via the post-translational modification of alpha-synuclein [79]. We again take the example of alpha-synuclein and focus on how different PTMs have been shown to regulate its membrane binding and other functions.
In vivo, alpha-synuclein has been found to possess several PTMs. Some are enriched in the soluble fraction of the protein, while some others are enriched in pathological Lewy body aggregates, providing a first line evidence of potential regulation of protein aggregation propensity by such PTMs. Some of these reported PTMs have been followed up by biochemical and structural studies involving purified proteins, uncovering potential functional roles, as discussed below.
Most eukaryotic proteins are N-terminally acetylated [80] and alpha-synuclein is no exception, as native brain-derived alpha-synuclein, both in the soluble fraction and in the Lewy body associated state, is N-terminally acetylated [81,82]. N-terminal acetylation has been shown to increase the membrane binding affinity of the N-terminal ~10 residues of alpha-synuclein and increase their helical structure upon SDS micelle binding [7]. When binding to more physiological membranes bearing moderate negative charge on their surface, N-terminal acetylated alpha-synuclein shows increased affinity to highly curved membranes bearing curvature similar to that observed for synaptic vesicles [7]. This property likely contributes to its specific synaptic vesicle association in vivo. N-terminal acetylation has been also shown to be critical for the formation of potentially functional oligomers of alpha-synuclein [83], yet to decrease its propensity for pathological aggregation [84]. In addition to N-terminal acetylation, phosphorylation at S87, Y125, S129; ubiquitination at K12, K21 and K23; and various truncations in the C-terminal domain have also reported in native alpha-synuclein [81,85–87]. A novel phosphorylation site at Y39 is particularly interesting because it is located at the linker region between two helices formed in the micelle-bound state of the protein, with the potential to alter the interconversion between the extended and broken helix states (Figure 2) [88,89]. Moreover, Y39 phosphorylation is catalyzed by c-Abl kinase which is upregulated in Parkinson’s disease [79]. In-vitro studies have shown Y39 phosphorylation to decrease binding of helix-2 to SUVs that mimic synaptic vesicles [90]. Hypothetically, this partly helical mode of binding can cause increased interactions between the unbound and therefore unstructured NAC domain and promote aggregation of alpha-synuclein (Figure 2) [90]. The effect of Y39 phosphorylation is similar to that of the G51D Parkinson’s mutation, suggesting that a similarly enhanced membrane-induced aggregation of this mutant may occur in individuals with this mutation [34,90]. There is still an unmet need to investigate the structural and functional significance of many of the other PTMs mentioned here.
Figure 2:
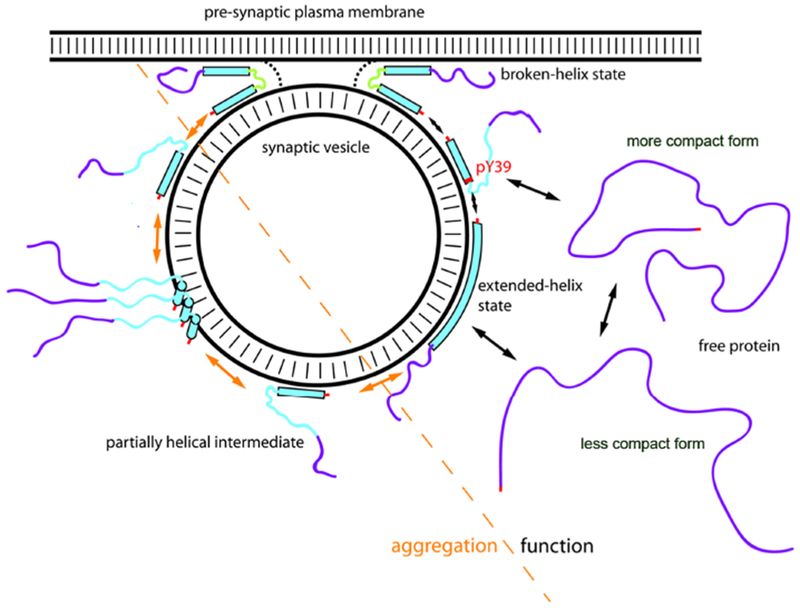
Model for membrane-induced structural changes of alpha-synuclein and their relevance to physiological and disease states. Alpha-helical and intrinsically disordered regions are depicted as filled bars and narrow lines respectively. The membrane-binding domain (residues 1-100) is colored cyan and C-terminal domain (residues 101-140) is colored purple as a visual guide. PTMs including N-terminal acetylation at the N-terminus and phosphorylation at residue tyrosine 39 is indicated are indicated in red. Alpha-synuclein exists in an equilibrium between a disordered monomer in the cytosol that interconverts rapidly between different conformations (although alternative folded oligomeric conformations have been proposed) and multiple membrane-bound conformations on the surface of synaptic vesicles. Upon binding to isolated vesicles, the lipid-binding domain of alpha-synuclein can adopt a highly-extended helical conformation dubbed the extended-helix state, while the C-terminus remains highly disordered, and this interaction is of relatively low affinity in vitro and in vivo. The broken-helix state of the protein, consisting of two shorter helices joined by a non-helical linker, has been observed when alpha-synuclein binds to micelles or to some lipid vesicle compositions, but in this model proposed to allow the protein to bridge between the vesicle and plasma membranes. The broken-helix state binds tightly to micelles in vitro, and membrane-attached synaptic vesicles provide a tight-binding mode for alpha-synuclein in situ as well. In the membrane-bridging mode, the protein is ideally positioned to influence the fusion of the synaptic vesicle with the plasma membrane via a variety of potential mechanisms, including directly modulating the membrane fusion process, or indirect effects mediated by interactions of alpha-synuclein with other proteins that regulate vesicle fusion. The transition to the broken-helix state could be facilitated by phosphorylation of Y39, situated at the beginning of the linker region, which decreases binding of the NAC-containing helix-2 region to isolated vesicles. As a side-effect, phosphorylation of Y39 results in increased population of a partly helical intermediate, similar to that observed in some Parkinson’s disease associated mutants. Such partly helical intermediates are aggregation-prone due to an increased probability of intermolecular interaction of the non-helical and therefore unprotected NAC region and may be the starting point for toxic oligomer and fibril formation in Parkinson’s Disease.
In addition to PTMs, specific ligands often play a role in modulating membrane binding of IDPs. Presence of cytosolic proteins influence the partitioning of alpha-synuclein between the membrane and cytosol [91]. In addition, it has been seen that alpha-synuclein disperses from nerve terminals in response to depolarization in a Ca2+-dependent manner [92]. Presence of Ca2+ enhances the binding of alpha-synuclein to isolated synaptosomes, which is in apparent contradiction to the depolarization-induced findings [93]. Dispersal may occur due to the exocytosis and elimination of synaptic vesicles to which alpha-synuclein was bound prior to depolarization. Alpha-synuclein has also been shown to associate with Rab3a, a small GTPase protein cycling between synaptic vesicles and the cytosol, and this interaction may also contribute to its release from membranes in response to depolarization. Alpha-synuclein was found to co-immonoprecipitate with GTP-bound Rab3a on synaptic vesicles and not with GDP-bound Rab3a in the cytosol [94]. In response to depolarization and subsequent cytosolic [Ca2+] increase, Rab3a hydrolyzes GTP and dissociates from the synaptic vesicle membrane, perhaps leading to the release of alpha-synuclein. A GTPase-deficient Rab3a mutant is incapable of this release and causes accumulation of alpha-synuclein in the membrane [94].
Due to the promiscuous nature of their interactions and potential to cause aggregation and toxicity upon overexpression, cells regulate the abundance of IDPs tightly at multiple levels, from transcript clearance, through translation, and up until proteolytic degradation [95]. Overexpression of wild-type alpha-synuclein, as observed in duplication or triplication of the SNCA locus, is known to contribute to early-onset Parkinson’s disease [96,97]. At a molecular level, crowding of IDPs on a membrane has effects on both the protein and the membrane. Molecular crowding promotes partially bound states that can be more aggregation-prone, as demonstrated on alpha-synuclein binding to nanodiscs [98]. On the other hand, molecular crowding causes alteration in membrane fluidity and can lead to membrane curvature changes, tubulation and membrane fission and fusion, as discussed earlier.
Most in-vitro studies of IDP-membrane interactions involve dilute solutions of proteins and lipids. However, proteins in-vivo exist in extremely crowded environments containing other proteins, carbohydrates and nucleic acids, at overall concentrations as high as 400g/l [99]. The excluded volume effect caused by macromolecular crowding has the potential to alter the thermodynamic equilibrium and cause changes in secondary structure, lipid binding affinity as well as cause aggregation/amyloid formation and liquid-liquid phase separation. In vitro, crowding is often mimicked by addition of crowding reagents like PEG, dextran, Ficoll or inert proteins. Some IDPs, e.g. the flagella-regulatory protein FlgM from Salmonella typhimurium, gain structure in response to crowding reagents [100], while other IDPs including alpha-synuclein remain disordered in artificially crowded environments [101] as well as inside the cytosol of living cells [102–104].
4. Functional implications of membrane binding
Because membrane-binding by IDPs is typically observed to be associated with physiological function, studying their membrane-bound conformation is of considerable interest. Membrane-associated IDPs take part in cellular functions such as membrane remodeling, signal transduction and transport across the nuclear membrane. A detailed discussion of all membrane-bound IDPs is beyond the scope of this review, and we instead use the example of alpha-synuclein to illustrate some functional implications of IDP membrane binding.
Although alpha-synuclein is clearly linked with Parkinson’s disease in a pathological context, the physiological function of alpha-synuclein is much less clear. Furthermore, despite its propensity to form pathological aggregates, alpha-synuclein is highly concentrated at the presynaptic termini in healthy neurons. Studies in animal models have linked its normal function to learning and neuronal plasticity [105,106], modulation of neurotransmitter release [107,108] and maintenance of synaptic vesicle pools [109,110]. All of these functional roles involve membranes, and so it is quite likely that the physiologically relevant conformations of alpha-synuclein include its membrane-bound conformations.
4.1. Regulation of synaptic vesicle fusion:
In vitro alpha-synuclein binds to artificial SUVs that mimic synaptic vesicles in terms of lipid composition and size (~40nm diameter), primarily in an extended helix conformation. However, when bound to smaller diameter (~4nm) detergent micelles, the protein adopts a broken helix conformation consisting of an N-terminal helix-1 (residues 1-36), followed by a seven-residue flexible linker (residues 37-43) and a C-terminal helix-2 (residues 44-102). The highly negatively charged C-terminal tail of the protein remains largely unassociated with membranes or detergents. There is no obvious direct physiological correlate to small diameter micelles, but the ability of alpha-synuclein to form two separate membrane-binding helices linked by a flexible linker makes it potentially able to bridge two membranes in close apposition, such as between docked vesicles and the cell membrane at the active zone of a presynapstic nerve terminal (Figure 2) [88]. Accordingly, alpha-synuclein has been shown to remain bound to synaptosome and synaptic vesicle preparations that retain docked vesicles, but to be absence in preparations of isolated synaptic vesicles [111]. Such a binding mode would place alpha-synuclein in a position well-suited to influence the subsequent exocytosis of docked synaptic vesicles, and indeed, multiple reports indicate that alpha-synuclein is capable of influencing this process. In particular, inhibition of vesicle exocytosis has been reported in a number of systems [112–114], and more recently alpha-synuclein has been reported to directly influence the kinetics of the fusion pore opening process that governs the release of vesicle contents into the synaptic cleft [115].
4.2. Regulation of synaptic vesicle clustering:
Synaptic vesicles form clusters at presynaptic termini at regions closely apposed to the active zone, the sites of vesicle release. These clusters are dynamic in nature and disperse on repetitive stimulation of neurons. Hence, these clusters are thought to represent a functional reserve pool of vesicles that maintain a steady supply of vesicles on repetitive stimulation. Alpha-synuclein had previously been implicated in this clustering of vesicles, as observed in some early studies that reported changes in cluster size in synuclein triple-knockout mice lacking all three synuclein-family members (alpha-, beta- and gamma-) [116] and other studies that implicated the binding of alpha-synuclein’s C-terminal domain to VAMP [117], or proposed a double-anchoring of the lipid-binding domain [58] as potential vesicle clustering mechanisms. However, a more recent study on quantitative estimates of vesicle clustering showed increased clustering in synuclein triple-knockout mice, which suggests that synucleins may have an antagonistic effect on vesicle clustering [118], Recently it was suggested that synaptic vesicle clusters are formed by the physical process of liquid-liquid phase separation of the presynaptic protein synapsin and are regulated by synapsin phosphorylation by calcium/calmodulin-dependent protein kinase II [71]. Taken together with the earlier finding that synuclein triple-knockouts exhibit altered baseline phosphorylation of synapsin [118], this suggests the possibility of a hitherto unknown interaction between the synucleins and synapsin that is relevant for regulation of synaptic vesicle pool sizes. On this note, synapsin, which features an N-terminal membrane-binding domain and a long intrinsically disordered domain that phase-separates under physiological conditions, represents a unique example of an IDP whose physiological role involves both phase separation and membrane interaction. This report was also the first observation of a specific interaction between lipid vesicles and phase-separated protein condensates. To date, the physical driving force for this phenomenon and the basis for its specificity to synaptic vesicles is unknown [119].
5. Pathological consequences of membrane interactions
The ability of IDPs to dramatically change membrane physicochemical properties and topology necessitates a fine control of their expression and regulation. An imbalance can result in membrane instability, manifested as changes in membrane curvature, exo/endocytosis and/or membrane trafficking. Moreover, some IDPs including alpha-synuclein are aggregation-prone. The nature and frequency of homotypic intermolecular interactions in the membrane-bound state is thought to promote aggregation of alpha-synuclein. This can arise from both molecular confinement to 2D surface, as well as specific conformational changes that promote aggregation [88,120]. Here we briefly discuss how membrane binding acts as a double-edged sword for the pathological role of alpha-synuclein.
Alpha-synuclein contains a 35-residue (position 61-95) aggregation-prone patch initially described as the NAC (Non-Aβ Component of Alzheimer’s disease amyloid), that is thought to initiate the misfolding and aggregation of alpha-synuclein in Parkinson’s disease [121–123]. At low cytosolic concentrations, the synaptic vesicle-bound conformation of alpha-synuclein can be represented as an extended helix, similar to its binding to isolated lipid SUVs. However, at higher protein: lipid ratios, such as those observed upon duplication or triplication of the SNCA gene locus or upon increased levels of the protein with age, the protein molecules must compete for the available synaptic vesicle membrane-binding surface area, driving more frequent intermolecular interactions. In addition, partially helical conformations that require less membrane surface area may begin to predominate [98]. Such partially helical conformation are highly prone to aggregation, independent of the presence of lipid membranes [120]. Furthermore, confinement of such partly helical conformations to a limited membrane surface area further increases the interaction between the adjacent NAC regions, which are non-helical and unprotected, and is a likely contributor to aggregation (Figure 2) [98]. Some familial PD mutations in alpha-synuclein are thought to increase aggregation propensity due to their altered membrane-bound conformations [33,34].
6. Methods to study membrane interactions of IDPs
IDPs constitute a major fraction of the dark proteome, comprised of protein regions whose conformations are unknown. With the appreciation of the fact that IDPs have important roles in cellular physiology, research into IDP properties and behavior has been progressively increasing [124]. IDPs are involved in promiscuous interactions with multiple partners, including membranes, and act as hubs for signaling pathways [125]. Studying IDP-membrane interactions is not only important as an academic endeavor, such interactions are increasingly being recognized as contributing to important biological processes and in some cases may even comprise novel drug targets [126–129]. In the following section we briefly go over some of the available tools to characterize these types of interactions.
6.1. Direct detection:
Membrane localization of any protein, not just IDPs, can be directly visualized in live or fixed cells using various microscopy-based approaches. Generally speaking, the protein of interest is made visible by tagging with a reporter dye or fluorophore or stained with an antibody in fixed cells, which can then be studied using a plethora of microscopy techniques. The membrane itself can also be labeled with a dye or fluorophore, or directly imaged. For alpha-synuclein bound to lipid vesicles, shape changes and interactions between individual vesicles have been directly visualized by super-resolution light microscopy, transmission electron microscopy (TEM) and cryo-electron microscopy (cryo-EM) methods [58,64,65,69].
6.2. Biochemical methods:
Biochemically, if a protein is co-purified in a membrane fraction and detected by western blot or other suitable method, it can be taken as an evidence that the protein associates with that membrane, either directly or indirectly. Co-sedimentation or co-flotation assays using artificial lipid membranes can be used to study direct membrane interaction in a minimal in-vitro system [130]. Use of artificial membranes allows complete control over the membrane composition and topology (detergent micelles, spherical vesicles of different sizes, supported lipid bilayers or monolayers, nanodiscs with specific diameter etc.).
6.3. Non fluorescence based optical methods:
There are a number of label-free optical methods that can report on IDP-membrane binding. Circular dichroism (CD) spectroscopy is a sensitive method to characterize the secondary structure content of the whole protein molecule. Alpha-helices and beta sheets have characteristic CD spectral signatures and this property is usually employed as a first-line tool to quantify secondary structure changes on binding to lipid membranes [131]. In addition, one can measure apparent membrane-binding affinity by analyzing CD spectra in a titration series [132]. In order to study curvature-dependence of membrane binding, proper characterization of vesicle size is essential. Dynamic light scattering (DLS) allows estimation of vesicle size distribution for binding experiments as well as detection of changes in vesicle size or morphology upon IDP binding, which could arise from shape alterations, vesicle clustering, fusion, fission, and rupture [58,133]. However, DLS data is weighted by particle size and careful interpretation is recommended in the presence of even small quantities of large particles [64]. Finally, surface plasmon resonance (SPR) methods for determining protein-lipid interactions are gaining in popularity with the advent of commercially available chips with functionalized gold surfaces that allow preparation of various membrane-mimetic surfaces on them [134]. The advantages for SPR include rapid label-free detection of protein-lipid interactions in real time with low sample concentration requirement. One can determine binding rate constants and apparent equilibrium constants using SPR, which is essentially the change in plasmon resonance frequency at a thin gold-plated measurement tip due to binding of biomolecules, measured as the change in angle of reflection at which an attenuation of a monochromatic totally internally reflected beam occurs [135]. SPR has been used to study membrane interactions of alpha-synuclein variants [136]. In principle, SPR can be used as a powerful screening tool for membrane interaction modulators of IDPs, much like screening for IDP-protein interaction inhibitors [137,138].
6.4. Fluorescence based methods:
Fluorescence methods offer sensitive and multidimensional measurements of the membrane interactions of IDPs. A detailed discussion on the multitude of fluorescence-based methods and the questions they address is beyond the scope of this review. Notably, in addition to monitoring IDP properties upon membrane binding, fluorescence methods can also be used to probe how properties of the membrane itself may change upon IDP binding [139–141]. Here we briefly outline various fluorescence-based methods and their utility in addressing specific questions, while guiding the readers to specific literature on the application of such methods.
Membrane-bound fraction of IDPs:
Fluorescence Correlation Spectroscopy (FCS) measures the fluctuation of fluorescence originating from fluorophores in a small confocal volume. Since free and vesicle-bound fluorescently-labeled proteins have different diffusion coefficients in solution, the populations of bound and free protein molecules can be determined from the autocorrelation function of the fluorescence fluctuation in such experiments. As opposed to traditional methods like chromatography or ultracentrifugation, FCS is a rapid and highly sensitive alternative and can be used over a wide range of protein concentrations.
Membrane-bound topology of IDPs:
The fluorescence emission maxima of tryptophan is sensitive to its environment and undergoes a blue shift as the environment becomes more hydrophobic. Exploiting this property, single tryptophan mutants of alpha-synuclein have been used to study its membrane-binding properties in native environment [65,132,142,143]. Labeling single cysteine mutants with environmentally sensitive fluorophores such as acrylodan can also be useful for this purpose. Similar to tryptophan, acrylodan undergoes a blue shift of emission maxima in a hydrophobic environment [144]. Although mostly employed for studies of integral membrane proteins [145–147], this method is equally suitable to probe the topology of IDPs [148].
Membrane-bound dynamics of IDPs:
In their native environment, IDPs undergo chain dynamics at different timescales. Steady state and time-resolved fluorescence anisotropy measurement can probe such local, segmental and global dynamics [149–152] and have been used in the study of alpha-synuclein membrane interactions [153]. For slower timescale motions such as those originating from rotational diffusion of a fluorophore probe within an IDP bound on a membrane surface, proper choice of a fluorophore with a longer fluorescence lifetime is needed for anisotropy measurements [149]. Furthermore, the decay of fluorescence anisotropy on small spherical systems like vesicles is modulated by a combination of rotational and translational diffusion of the fluorophore, both of which can be modeled from experimental observations [154,155]. An orthogonal method, Red Edge Excitation Shift (REES), is based on the heterogeneity of fluorophore energy levels in an ordered polar environment, which leads to a red-shift of emission maxima when excited at a frequency close to the red edge of the excitation envelope [156–158]. REES has been used to directly identify the residues of alpha-synuclein that are juxtaposed (within 15 Å) to the membrane surface [153]
Single molecule methods:
The limitations of population averaging by ensemble biochemical methods can be overcome by single molecule fluorescence based methods. Single molecule Forster Resonance Energy Transfer (smFRET) has been successfully used to probe conformational fluctuations in alpha-synuclein in its lipid-bound state [159–161]. smFRET provides an approximate distance measurement, which can be useful as a complementary approach to ensemble methods such as Electron Paramagnetic Resonance (EPR) spectroscopy.
6.5. Calorimetric methods:
IDP binding to lipid membranes is often associated with change in enthalpy, meaning there is a net release from or uptake of heat into the system. Such transitions can be studied using calorimetric methods. Isothermal titration calorimetry (ITC) is a sensitive method with the advantage of providing a rich set of thermodynamic parameters, namely, binding enthalpy (ΔH), binding entropy (ΔS), association constant (Ka) and binding stoichiometry (n), from a single experiment with label-free reagents [162,163]. However, the concentration requirement of proteins is often a limiting factor [162]. As an example, Bartels et al. used ITC in combination with CD spectroscopy to study lipid vesicle binding of different alpha-synuclein N-terminal truncation mutants and synthetic peptides corresponding to N-terminal sequences of alpha-synuclein [164]. They showed that the cooperative binding and folding of the lipid binding domain is initiated at the N-terminus of alpha-synuclein.
Binding of IDP is also associated with complementary changes at the membrane. In particular, insertion of protein into the lipid bilayer is generally associated with a decrease in lipid packing density and a decrease in the melting temperature (Tm) of the lipid membrane. While other methods based on fluorescence polarization can also be used to determine Tm of artificial lipid membranes [165,166], differential scanning calorimetry is a valuable method to determine such changes as a label-free alternative. In case of alpha-synuclein, the roles of packing defects and of lipid bilayer phases have been investigated using DIC [167–169].
6.6. Nuclear Magnetic Resonance (NMR):
NMR spectroscopy can be used to analyze protein secondary and tertiary structure at single-residue resolution. Secondary chemical shifts are defined as the difference between the observed chemical shift of a nucleus and the predicted random coil chemical shift of the same nucleus. This measure can be indicative of alpha helix or beta sheet propensities even in highly disordered proteins. For a protein molecule partitioned between free and lipid-vesicle bound states, only the free fraction contributes to the detectable NMR spectrum and can be estimated by the peak intensity ratio between matched samples with and without lipids. From this measure, an apparent equilibrium binding constant (KDapp) can be determined [7,90,132]. Although the lipid-bound fraction is ‘invisible’ to traditional NMR methods, this fraction can be selectively saturated using suitable narrow-band off-resonance pulses. This allows the kinetics of the exchange process from bound state to free state to be characterized using DEST (Dark-state Excitation Saturation Transfer) methods [170,171].
6.7. Nanoscale distance measurements:
Distance restraints on a molecular length scale provide valuable information about membrane-bound structures of a protein. Methods using EPR (Electron Paramagnetic Resonance) such as Pulsed Dipolar EPR Spectroscopy (PDS) are able to provide the relatively precise distance information at this length scale. The distance distribution between two paramagnetic (spin) probes covalently attached to the molecule of interest can be measured using PDS, providing distance information up to ~9 nm [172]. In contrast, Paramagnetic relaxation enhancement (PRE) effect on NMR signals can be observed up to ~3 nm, with the advantage that a single spin label can provide multiple distances. PRE can be used to identify spatially proximal residues, and to determine approximate distance restraints [172,173]. However, both PRE and PDS are ensemble methods and are unable to provide time resolution. As alluded to earlier, smFRET is a valuable tool to overcome these limitations [159–161].
6.8. Surface characterization methods:
Protein binding to a lipid surface typically results in alterations of membrane properties, and some thin film characterization methods borrowed from material science have proven useful in characterizing protein effects on biological membranes. Neutron and X-ray reflectivity measurements operate using the same general principles, and provide information about the thickness, density and interfacial roughness of lipid membrane surface [174]. Grazing-incidence X-ray Diffraction (GIXD) provides information about 2D ordering in the plane of the lipid membrane [175]. All of these methods have been applied to study membrane binding of IDPs, including Tau and alpha-synuclein [176–180].
6.9. Simulation:
Since experiments to probe membrane-bound states of IDPs remain highly challenging, molecular dynamics simulation can provide additional insights into these interactions. Due to the computational cost associated with all-atom molecular dynamics simulation, coarse-grain approximation methods are often preferred for such complex systems. MD simulation can be used in conjunction with experimental observations to explore the bound-state conformation. With the increased availability of dedicated supercomputers, all-atom MD simulation is increasingly being applied to study IDP-membrane interaction [180,181]. However, simulation length remains severely limited by computational time, and is therefore still impracticable to simulate transitions that occur on slower timescales, such as slow conformational exchange processes or membrane surface induced protein aggregation [182].
Highlights:
IDP often interact with membranes in physiologically important ways
IDP-membrane interactions are regulated by both protein and membrane features
Alpha-synuclein binds to membranes in different modes and conformations
Alpha-synuclein membrane interactions are important for both normal function and aggregation
7. Acknowledgments
This work was supported in part by NIH grants R37AG019391.
Biographies

Tapojyoti Das is currently a PhD candidate in the Biochemistry and Structural Biology program at Weill Cornell Medicine (2013 - present). He received his Bachelor’s in Medicine and Surgery (MBBS) from University of Calcutta and Master’s in Medical Science and Technology (MMST) from Indian Institute of Technology Kharagpur, India. Before embarking on the PhD program, he was a Young Biotechnologist Fellow at National Institute of Biomedical Genomics, India (2011-2013). His current research interests lie in uncovering the normal structure-function relationship of the Parkinson’s protein alpha-synuclein using structural biology and biophysical tools, chiefly NMR spectroscopy, in parallel with cell-based assays.

David Eliezer is a professor of Biochemistry and Neuroscience at Weill Cornell Medicine. He obtained BS and MSE degrees in Computer Science at the University of Michigan and completed his PhD in physics at Stanford University, where he studied protein folding. He was a post-doctoral fellow at the Scripps Research Institute, where he applied NMR methods to the characterization of protein-folding intermediates. Upon joining the Weill Cornell faculty, he began applying NMR and other spectroscopic methods to the study of protein misfolding and aggregation as an independent investigator. His laboratory has produced seminal studies of the structural properties of the Parkinson’s and Alzheimer’s proteins alpha-synuclein and tau, and continues to investigate the functional and pathological properties of these proteins as well as others.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
9. References
- [1].Bussell R, Ramlall TF, Eliezer D, Helix periodicity, topology, and dynamics of membrane-associated alpha-synuclein., Protein Sci. 14 (2005) 862–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Jao CC, Der-Sarkissian A, Chen J, Langen R, Structure of membrane-bound alpha-synuclein studied by site-directed spin labeling., Proc. Natl. Acad. Sci. U. S. A. 101 (2004) 8331–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Jao CC, Hegde BG, Chen J, Haworth IS, Langen R, Structure of membrane-bound alpha-synuclein from site-directed spin labeling and computational refinement., Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 19666–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Middleton ER, Rhoades E, Effects of curvature and composition on α-synuclein binding to lipid vesicles, Biophys. J. 99 (2010) 2279–2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Jensen MB, Bhatia VK, Jao CC, Rasmussen JE, Pedersen SL, Jensen KJ, Langen R, Stamou D, Membrane curvature sensing by amphipathic helices: A single liposome study using α-synuclein and annexin B12, J. Biol. Chem. 286 (2011) 42603–42614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bussell R, Eliezer D, A structural and functional role for 11-mer repeats in alpha-synuclein and other exchangeable lipid binding proteins., J. Mol. Biol. 329 (2003) 763–78. [DOI] [PubMed] [Google Scholar]
- [7].Dikiy I, Eliezer D, N-terminal Acetylation stabilizes N-terminal Helicity in Lipid- and Micelle-bound α-Synuclein and increases its affinity for Physiological Membranes, J. Biol. Chem. 289 (2014) 3652–3665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Rhoades E, Ramlall TF, Webb WW, Eliezer D, Quantification of α-Synuclein Binding to Lipid Vesicles Using Fluorescence Correlation Spectroscopy, Biophys. J. 90 (2006) 4692–4700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hurley JH, Membrane binding domains, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 1761 (2006) 805–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Mulgrew-Nesbitt A, Diraviyam K, Wang J, Singh S, Murray P, Li Z, Rogers L, Mirkovic N, Murray D, The role of electrostatics in protein-membrane interactions, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids. 1761 (2006) 812–826. [DOI] [PubMed] [Google Scholar]
- [11].Sigalov AB, Uncoupled binding and folding of immune signaling-related intrinsically disordered proteins, Prog. Biophys. Mol. Biol. 106 (2011) 525–536. [DOI] [PubMed] [Google Scholar]
- [12].Sigalov AB, Structural biology of intrinsically disordered proteins: Revisiting unsolved mysteries, Biochimie. 125 (2016) 112–118. [DOI] [PubMed] [Google Scholar]
- [13].Uversky VN, Intrinsic Disorder-based Protein Interactions and their Modulators, Curr. Pharm. Des. 19 (2013) 4191–4213. [DOI] [PubMed] [Google Scholar]
- [14].Baker JMR, Hudson RP, Kanelis V, Choy W-Y, Thibodeau PH, Thomas PJ, Forman-Kay JD, CFTR regulatory region interacts with NBD1 predominantly via multiple transient helices., Nat. Struct. Mol. Biol. 14 (2007) 738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Bozoky Z, Krzeminski M, Muhandiram R, Birtley JR, Al-Zahrani A, Thomas PJ, Frizzell RA, Ford RC, Forman-Kay JD, Regulatory R region of the CFTR chloride channel is a dynamic integrator of phospho-dependent intra- and intermolecular interactions., Proc. Natl. Acad. Sci. U. S. A. 110 (2013) E4427–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Hammes GG, Chang Y-C, Oas TG, Conformational selection or induced fit: a flux description of reaction mechanism., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 13737–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shoemaker BA, Portman JJ, Wolynes PG, Speeding molecular recognition by using the folding funnel: The fly-casting mechanism, Proc. Natl. Acad. Sci. 97 (2000) 8868–8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Niu W, Chen Z, Gandhi PS, Vogt AD, Pozzi N, Pelc LA, Zapata F, Di Cera E, Crystallographic and kinetic evidence of allostery in a trypsin-like protease, Biochemistry. 50 (2011)6301–6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Clore GM, Interplay between conformational selection and induced fit in multidomain protein-ligand binding probed by paramagnetic relaxation enhancement, Biophys. Chem. 186(2014)3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Tang C, Iwahara J, Clore GM, Visualization of transient encounter complexes in protein-protein association, Nature. 444 (2006) 383–386. [DOI] [PubMed] [Google Scholar]
- [21].Galletto R, Jezewska MJ, Bujalowski W, Kinetics of allosteric conformational transition of a macromolecule prior to ligand binding: analysis of stopped-flow kinetic experiments., Cell Biochem. Biophys. 42 (2005) 121–44. [DOI] [PubMed] [Google Scholar]
- [22].Vogt AD, Di Cera E, Conformational selection is a dominant mechanism of ligand binding, Biochemistry. 52 (2013) 5723–5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vogt AD, Pozzi N, Chen Z, Di Cera E, Essential role of conformational selection in ligand binding, Biophys. Chem. 186 (2014) 13–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Vogt AD, Di Cera E, Conformational selection or induced fit? A critical appraisal of the kinetic mechanism, Biochemistry. 51 (2012) 5894–5902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gianni S, Dogan J, Jemth P, Distinguishing induced fit from conformational selection, Biophys. Chem. 189 (2014) 33–39. [DOI] [PubMed] [Google Scholar]
- [26].Narayanan R, Ganesh OK, Edison AS, Hagen SJ, Kinetics of folding and binding of an intrinsically disordered protein: The inhibitor of yeast aspartic proteinase YPrA, J. Am. Chem. Soc. 130 (2008) 11477–11485. [DOI] [PubMed] [Google Scholar]
- [27].Sugase K, Dyson HJ, Wright PE, Mechanism of coupled folding and binding of an intrinsically disordered protein., Nature. 447 (2007) 1021–5. [DOI] [PubMed] [Google Scholar]
- [28].Arai M, Sugase K, Dyson HJ, Wright PE, Conformational propensities of intrinsically disordered proteins influence the mechanism of binding and folding, Proc. Natl. Acad. Sci. 112 (2015)9614–9619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Onitsuka M, Kamikubo H, Yamazaki Y, Kataoka M, Mechanism of induced folding: Both folding before binding and binding before folding can be realized in staphylococcal nuclease mutants, Proteins Struct. Funct. Genet. 72 (2008) 837–847. [DOI] [PubMed] [Google Scholar]
- [30].Pozzi N, Vogt AD, Gohara DW, Di Cera E, Conformational selection in trypsin-like proteases, Curr. Opin. Struct. Biol. 22 (2012) 421–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Kim YB, Kalinowski SS, Marcinkeviciene J, A pre-steady state analysis of ligand binding to human glucokinase: evidence for a preexisting equilibrium., Biochemistry. 46 (2007) 1423–31. [DOI] [PubMed] [Google Scholar]
- [32].Maltsev AS, Ying J, Bax A, Impact of N-terminal acetylation of α-synuclein on its random coil and lipid binding properties, Biochemistry. 51 (2012) 5004–5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Bodner CR, Maltsev AS, Dobson CM, Bax A, Differential phospholipid binding of α-synuclein variants implicated in Parkinson’s disease revealed by solution NMR spectroscopy, Biochemistry. 49 (2010) 862–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Fares MB, Ait-Bouziad N, Dikiy I, Mbefo MK, Jovičić A, Kiely A, Holton JL, Lee SJ, Gitler AD, Eliezer D, Lashuel HA, The novel Parkinson’s disease linked mutation G51D attenuates in vitro aggregation and membrane binding of α-synuclein, and enhances its secretion and nuclear localization in cells, Hum. Mol. Genet. 23 (2014) 4491–4509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ramezani M, Wilkes MM, Das T, Holowka D, Eliezer D, Baird B, Regulation of Exocytosis and Mitochondrial Relocalization by Alpha-Synuclein in a Mammalian Cell Model, BioRxiv. (2018) 492066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Akopian D, Shen K, Zhang X, Shan S, Signal recognition particle: an essential proteintargeting machine., Annu. Rev. Biochem. 82 (2013) 693–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Antonny B, Mechanisms of membrane curvature sensing., Annu. Rev. Biochem. 80 (2011)101–23. [DOI] [PubMed] [Google Scholar]
- [38].Zhang W, Crocker E, McLaughlin S, Smith SO, Binding of peptides with basic and aromatic residues to bilayer membranes. Phenylalanine in the myristoylated alanine-rich C kinase substrate effector domain penetrates into the hydrophobic core of the bilayer, J. Biol. Chem. 278 (2003) 21459–21466. [DOI] [PubMed] [Google Scholar]
- [39].Gambhir A, Hangyas-Mihályné G, Zaitseva I, Cafiso DS, Wang J, Murray D, Pentyala SN, Smith SO, McLaughlin S, Electrostatic sequestration of PIP2 on phospholipid membranes by basic/aromatic regions of proteins., Biophys. J. 86 (2004) 2188–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Wang J, Gambhir A, Hangyas-Mihalyne G, Murray D, Golebiewska U, McLaughlin S, Lateral sequestration of phosphatidylinositol 4,5-bisphosphate by the basic effector domain of myristoylated alanine-rich C kinase substrate is due to nonspecific electrostatic interactions, J. Biol. Chem. 277 (2002) 34401–34412. [DOI] [PubMed] [Google Scholar]
- [41].Ellena JF, Burnitz MC, Cafiso DS, Location of the myristoylated alanine-rich C-kinase substrate (MARCKS) effector domain in negatively charged phospholipid bicelles, Biophys. J. 85 (2003) 2442–2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Morton LA, Yang H, Saludes JP, Fiorini Z, Beninson L, Chapman ER, Fleshner M, Xue D, Yin H, MARCKS-ED peptide as a curvature and lipid sensor, ACS Chem. Biol. 8 (2013)218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Arbuzova A, Wang L, Wang J, Hangyas-Mihalyne G, Murray D, Honig B, McLaughlin S, Membrane binding of peptides containing both basic and aromatic residues. Experimental studies with peptides corresponding to the scaffolding region of caveolin and the effector region of MARCKS, Biochemistry. 39 (2000) 10330–10339. [DOI] [PubMed] [Google Scholar]
- [44].Snead D, Wragg RT, Dittman JS, Eliezer D, Membrane curvature sensing by the C-terminal domain of complexin, Nat. Commun. 5 (2014) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Peter BJ, Kent HM, Mills FG, Vallis Y, Butler PJG, Evans PR, McMahon HT, BAR domains as sensors of membrane curvature: the amphiphysin BAR structure., Science. 303 (2004) 495–9. [DOI] [PubMed] [Google Scholar]
- [46].Frost A, Linger VM, De Camilli P, The BAR Domain Superfamily: Membrane-Molding Macromolecules, Cell. 137 (2009) 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Bigay J, Casella JF, Drin G, Mesmin B, Antonny B, ArfGAPl responds to membrane curvature through the folding of a lipid packing sensor motif, EMBO J. 24 (2005) 2244–2253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Ford MGJ, Mills LG, Peter BJ, Vallis Y, Praefcke GJK, Evans PR, McMahon HT, Curvature of clathrin-coated pits driven by epsin., Nature. 419 (2002) 361–6. [DOI] [PubMed] [Google Scholar]
- [49].Miller SE, Mathiasen S, Bright NA, Pierre F, Kelly BT, Kladt N, Schauss A, Merrifield CJ, Stamou D, Honing S, Owen DJ, CALM Regulates Clathrin-Coated Vesicle Size and Maturation by Directly Sensing and Driving Membrane Curvature, Dev. Cell. 33 (2015)163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Davidson WS, Jonas A, Clayton DF, George JM, Stabilization of alpha-synuclein secondary structure upon binding to synthetic membranes., J. Biol. Chem. 273 (1998) 9443–9. [DOI] [PubMed] [Google Scholar]
- [51].Wragg RT, Snead D, Dong Y, Ramlall TF, Menon I, Bai J, Eliezer D, Dittman JS, Synaptic Vesicles Position Complexin to Block Spontaneous Fusion, Neuron. 77 (2013) 323–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Segrest JP, Jackson RL, Morrisett JD, Gotto AM, A molecular theory of lipid-protein interactions in the plasma lipoproteins, FEBS Lett. 38 (1974) 247–253. [DOI] [PubMed] [Google Scholar]
- [53].Segrest JP, De Loof H, Dohlman JG, Brouillette CG, Anantharamaiah GM, Amphipathic helix motif: classes and properties., Proteins. 8 (1990) 103–17. [DOI] [PubMed] [Google Scholar]
- [54].Eliezer D, Kutluay E, Bussell R, Browne G, Conformational properties of alpha-synuclein in its free and lipid-associated states., J. Mol. Biol. 307 (2001) 1061–1073. [DOI] [PubMed] [Google Scholar]
- [55].Pranke IM, Morello V, Bigay J, Gibson K, Verbavatz JM, Antonny B, Jackson CL, α-Synuclein and ALPS motifs are membrane curvature sensors whose contrasting chemistry mediates selective vesicle binding, J. Cell Biol. 194 (2011) 89–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Giménez-Andrés M, Čopič A, Antonny B, The Many Faces of Amphipathic Helices., Biomolecules. 8 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Snead WT, Hayden CC, Gadok AK, Zhao C, Lafer EM, Rangamani P, Stachowiak JC, Membrane fission by protein crowding, Proc. Natl. Acad. Sci. 114 (2017) E3258–E3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Fusco G, Pape T, Stephens AD, Mahou P, Costa AR, Kaminski CF, Kaminski Schierle GS, Vendruscolo M, Veglia G, Dobson CM, De Simone A, Structural basis of synaptic vesicle assembly promoted by α-synuclein., Nat. Commun. 7 (2016) 12563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zimmerberg J, Kozlov MM, How proteins produce cellular membrane curvature, Nat. Rev. Mol. Cell Biol. 7 (2006) 9–19. [DOI] [PubMed] [Google Scholar]
- [60].Boucrot E, Ferreira APA, Almeida-Souza L, Debard S, Vallis Y, Howard G, Bertot L, Sauvonnet N, McMahon HT, Endophilin marks and controls a clathrin-independent endocytic pathway., Nature. 517 (2015) 460–5. [DOI] [PubMed] [Google Scholar]
- [61].Cui H, Lyman E, Voth GA, Mechanism of membrane curvature sensing by amphipathic helix containing proteins, Biophys. J. 100 (2011) 1271–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Renard H-F, Simunovic M, Lemiere J, Boucrot E, Garcia-Castillo MD, Arumugam S, Chambon V, Lamaze C, Wunder C, Kenworthy AK, Schmidt A. a., McMahon HT, Sykes C, Bassereau P, Johannes L, Endophilin-A2 functions in membrane scission in clathrin-independent endocytosis., Nature. 517 (2015) 493–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Zeno WF, Baul U, Snead WT, DeGroot ACM, Wang L, Lafer EM, Thirumalai D, Stachowiak JC, Synergy between intrinsically disordered domains and structured proteins amplifies membrane curvature sensing, Nat. Commun. 9 (2018) 4152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Bodner CR, Dobson CM, Bax A, Multiple Tight Phospholipid-Binding Modes of α-Synuclein Revealed by Solution NMR Spectroscopy, J. Mol. Biol. 390 (2009) 775–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Jiang Z, de Messieres M, Lee JC, Membrane remodeling by α-synuclein and effects on amyloid formation., J. Am. Chem. Soc. 135 (2013) 15970–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Zhu M, Fink AL, Lipid binding inhibits α-synuclein fibril formation, J. Biol. Chem. 278 (2003)16873–16877. [DOI] [PubMed] [Google Scholar]
- [67].Jo E, McLaurin JA, Yip CM, George-Hyslop P St, Fraser PE, α-Synuclein membrane interactions and lipid specificity, J. Biol. Chem. 275 (2000) 34328–34334.au [DOI] [PubMed] [Google Scholar]
- [68].Madine J, Hughes E, Doig AJ, Middleton DA, The effects of alpha-synuclein on phospholipid vesicle integrity: a study using 31P NMR and electron microscopy., Mol. Membr. Biol. 25 (2008) 518–27. [DOI] [PubMed] [Google Scholar]
- [69].Varkey J, Isas JM, Mizuno N, Jensen MB, Bhatia VK, Jao CC, Petrlova J, Voss JC, Stamou DG, Steven AC, Langen R, Membrane curvature induction and tubulation are common features of synucleins and apolipoproteins., J. Biol. Chem. 285 (2010)32486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Bah A, Vernon RM, Siddiqui Z, Krzeminski M, Muhandiram R, Zhao C, Sonenberg N, Kay LE, Forman-Kay JD, Folding of an intrinsically disordered protein by phosphorylation as a regulatory switch, Nature. 519 (2015) 106–109. [DOI] [PubMed] [Google Scholar]
- [71].Milovanovic D, Wu Y, Bian X, De Camilli P, A liquid phase of synapsin and lipid vesicles., Science. 361 (2018) 604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Milovanovic D, De Camilli P, Synaptic Vesicle Clusters at Synapses: A Distinct Liquid Phase?, Neuron. 93 (2017) 995–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Bennett RL, Licht JD, Targeting Epigenetics in Cancer., Annu. Rev. Pharmacol. Toxicol. 58 (2018) 187–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Pagan F, Hebron M, Valadez EH, Torres-Yaghi Y, Huang X, Mills RR, Wilmarth BM, Howard H, Dunn C, Carlson A, Lawler A, Rogers SL, Falconer RA, Ahn J, Li Z, Moussa C, Nilotinib Effects in Parkinson’s disease and Dementia with Lewy bodies., J. Parkinsons. Dis. 6 (2016) 503–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Tanabe A, Yamamura Y, Kasahara J, Morigaki R, Kaji R, Goto S, A novel tyrosine kinase inhibitor AMN107 (nilotinib) normalizes striatal motor behaviors in a mouse model of Parkinson’s disease., Front. Cell. Neurosci. 8 (2014) 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Imam SZ, Trickier W, Kimura S, Binienda ZK, Paule MG, Slikker W, Li S, Clark RA, Ali SF, Neuroprotective efficacy of a new brain-penetrating C-Abl inhibitor in a murine Parkinson’s disease model., PLoS One. 8 (2013) e65129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Hebron ML, Lonskaya I, Moussa CE-H, Nilotinib reverses loss of dopamine neurons and improves motor behavior via autophagic degradation of α-synuclein in Parkinson’s disease models., Hum. Mol. Genet. 22 (2013) 3315–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Karuppagounder SS, Brahmachari S, Lee Y, Dawson VL, Dawson TM, Ko HS, The c-Abl inhibitor, nilotinib, protects dopaminergic neurons in a preclinical animal model of Parkinson’s disease., Sci. Rep. 4 (2014) 4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Mahul-Mellier A-L, Fauvet B, Gysbers A, Dikiy I, Oueslati A, Georgeon S, Lamontanara AJ, Bisquertt A, Eliezer D, Masliah E, Halliday G, Hantschel O, Lashuel HA, c-Abl phosphorylates α-synuclein and regulates its degradation: implication for α-synuclein clearance and contribution to the pathogenesis of Parkinson’s disease., Hum. Mol. Genet. 23 (2014) 2858–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Arnesen T, Van Damme P, Polevoda B, Helsens K, Evjenth R, Colaert N, Varhaug JE, Vandekerckhove J, Lillehaug JR, Sherman F, Gevaert K, Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 8157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Anderson JP, Walker DE, Goldstein JM, De Laat R, Banducci K, Caccavello RJ, Barbour R, Huang J, Kling K, Lee M, Diep L, Keim PS, Shen X, Chataway T, Schlossmacher MG, Seubert P, Schenk D, Sinha S, Gai WP, Chilcote TJ, Phosphorylation of Ser-129 is the dominant pathological modification of α-synuclein in familial and sporadic lewy body disease, J. Biol. Chem. 281 (2006) 29739–29752. [DOI] [PubMed] [Google Scholar]
- [82].Burré J, Vivona S, Diao J, Sharma M, Brunger AT, Siidhof TC, Properties of native brain α-synuclein, Nature. 498 (2013) 107–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Trexler AJ, Rhoades E, N-Terminal acetylation is critical for forming α-helical oligomer of α-synuclein., Protein Sci. 21 (2012) 601–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Bartels T, Kim NC, Luth ES, Selkoe DJ, N-alpha-acetylation of α-synuclein increases its helical folding propensity, GM1 binding specificity and resistance to aggregation, PLoS One. 9 (2014) 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Paleologou KE, Oueslati A, Shakked G, Rospigliosi CC, Kim H-YH-Y, Lamberto GR, Fernandez CO, Schmid A, Chegini F, Gai WP, Chiappe D, Moniatte M, Schneider BL, Aebischer P, Eliezer D, Zweckstetter M, Masliah E, Lashuel HA, Phosphorylation at S87 is enhanced in synucleinopathies, inhibits alpha-synuclein oligomerization, and influences synuclein-membrane interactions., J. Neurosci. 30 (2010) 3184–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Paleologou KE, Schmid AW, Rospigliosi CC, Kim H-Y, Lamberto GR, Fredenburg RA, Lansbury PT, Fernandez CO, Eliezer D, Zweckstetter M, Lashuel HA, Phosphorylation at Ser-129 but not the phosphomimics S129E/D inhibits the fibrillation of alpha-synuclein., J. Biol. Chem. 283 (2008) 16895–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Hejjaoui M, Butterfield S, Fauvet B, Vercruysse F, Cui J, Dikiy F, Prudent M, Olschewski D, Zhang Y, Eliezer D, Lashuel HA, Elucidating the role of C-terminal post-translational modifications using protein semisynthesis strategies: a-synuclein phosphorylation at tyrosine 125., J. Am. Chem. Soc. 134 (2012) 5196–5210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Dikiy F, Eliezer D, Folding and misfolding of alpha-synuclein on membranes., Biochim. Biophys. Acta. 1818 (2012) 1013–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Snead D, Eliezer D, A-Synuclein Function and Dysfunction on Cellular Membranes, Exp. Neurobiol. 23 (2014) 292–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Dikiy F, Fauvet B, Jovičić A, Mahul-Mellier AL, Desobry C, El-Turk F, Gitler AD, Lashuel HA, Eliezer D, Semisynthetic and in Vitro Phosphorylation of Alpha-Synuclein at Y39 Promotes Functional Partly Helical Membrane-Bound States Resembling Those Induced by PD Mutations, ACS Chem. Biol. 11 (2016) 2428–2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Wislet-Gendebien S, D’Souza C, Kawarai T, St George-Hyslop P, Westaway D, Fraser P, Tandon A, Cytosolic proteins regulate alpha-synuclein dissociation from presynaptic membranes., J. Biol. Chem. 281 (2006) 32148–55. [DOI] [PubMed] [Google Scholar]
- [92].Fortin DL, Nemani VM, Voglmaier SM, Anthony MD, Ryan TA, Edwards RH, Neural activity controls the synaptic accumulation of alpha-synuclein., J. Neurosci. 25 (2005)10913–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Lautenschläger J, Stephens AD, Fusco G, Strohl F, Curry N, Zacharopoulou M, Michel CH, Laine R, Nespovitaya N, Fantham M, Pinotsi D, Zago W, Fraser P, Tandon A, St George-Hyslop P, Rees E, Phillips JJ, De Simone A, Kaminski CF, Schierle GSK, C-terminal calcium binding of α-synuclein modulates synaptic vesicle interaction., Nat. Commun. 9 (2018) 712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Chen RHC, Wislet-Gendebien S, Samuel F, Visanji NP, Zhang G, Marsilio D, Langman T, Fraser PE, Tandon A, α-Synuclein membrane association is regulated by the Rab3a recycling machinery and presynaptic activity., J. Biol. Chem. 288 (2013) 7438–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Gsponer J, Futschik ME, Teichmann SA, Babu MM, Tight regulation of unstructured proteins: from transcript synthesis to protein degradation., Science. 322 (2008) 1365–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL, Mutation in the alpha-synuclein gene identified in families with Parkinson’s disease., Science. 276 (1997) 2045–7. [DOI] [PubMed] [Google Scholar]
- [97].Singleton AB, Farrer M, Johnson J, Singleton A, Hague S, Kachergus J, Hulihan M, Peuralinna T, Dutra A, Nussbaum R, Lincoln S, Crawley A, Hanson M, Maraganore D, Adler C, Cookson MR, Muenter M, Baptista M, Miller D, Blancato J, Hardy J, Gwinn-Hardy K, alpha-Synuclein locus triplication causes Parkinson’s disease., Science. 302 (2003) 841. [DOI] [PubMed] [Google Scholar]
- [98].Viennet T, Wordehoff MM, Uluca B, Poojari C, Shaykhalishahi H, Willbold D, Strodel B, Heise H, Buell AK, Hoyer W, Etzkorn M, Structural insights from lipid-bilayer nanodiscs link α-Synuclein membrane-binding modes to amyloid fibril formation., Commun. Biol. 1 (2018) 44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Zimmerman SB, Trach SO, Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli., J. Mol. Biol. 222 (1991) 599–620. [DOI] [PubMed] [Google Scholar]
- [100].Dedmon MM, Patel CN, Young GB, Pielak GJ, FlgM gains structure in living cells., Proc. Natl. Acad. Sci. U. S. A. 99 (2002) 12681–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Munishkina LA, Cooper EM, Uversky VN, Fink AL, The effect of macromolecular crowding on protein aggregation and amyloid fibril formation, J. Mol. Recognit. 17 (2004) 456–464. [DOI] [PubMed] [Google Scholar]
- [102].Binolfi A, Theillet F-X, Selenko P, Bacterial in-cell NMR of human α-synuclein: a disordered monomer by nature?, Biochem. Soc. Trans. 40 (2012) 950–4. [DOI] [PubMed] [Google Scholar]
- [103].Theillet FX, Binolfi A, Bekei B, Martorana A, Rose HM, Stuiver M, Verzini S, Lorenz D, Van Rossum M, Goldfarb D, Selenko P, Structural disorder of monomeric α-synuclein persists in mammalian cells, Nature. 530 (2016) 45–50. [DOI] [PubMed] [Google Scholar]
- [104].Waudby CA, Camilloni C, Fitzpatrick AWP, Cabrita LD, Dobson CM, Vendruscolo M, Christodoulou J, In-Cell NMR Characterization of the Secondary Structure Populations of a Disordered Conformation of α-Synuclein within E. coli Cells, PLoS One. 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].George JM, Jin H, Woods WS, Clayton DF, Characterization of a novel protein regulated during the critical period for song learning in the zebra finch, Neuron. 15 (1995) 361–372. [DOI] [PubMed] [Google Scholar]
- [106].Watson JB, Hatami A, David H, Masliah E, Roberts K, Evans CE, Levine MS, Alterations in corticostriatal synaptic plasticity in mice overexpressing human alpha-synuclein., Neuroscience. 159 (2009) 501–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Chandra S, Gallardo G, Fernández-Chacón R, Schlüter OM, Südhof TC, Alpha-synuclein cooperates with CSPalpha in preventing neurodegeneration., Cell. 123 (2005) 383–96. [DOI] [PubMed] [Google Scholar]
- [108].Burre J, Sharma M, Tsetsenis T, Buchman V, Etherton MR, Südhof TC, Alpha-synuclein promotes SNARE-complex assembly in vivo and in vitro., Science. 329 (2010) 1663–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Abeliovich A, Schmitz Y, Farinas I, Choi-Lundberg D, Ho WH, Castillo PE, Shinsky N, Verdugo JM, Armanini M, Ryan A, Hynes M, Phillips H, Sulzer D, Rosenthal A, Mice lacking alpha-synuclein display functional deficits in the nigrostriatal dopamine system., Neuron. 25 (2000) 239–52. [DOI] [PubMed] [Google Scholar]
- [110].Murphy DD, Rueter SM, Trojanowski JQ, Lee VM, Synucleins are developmentally expressed, and alpha-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons., J. Neurosci. 20 (2000) 3214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Burré J, Sharma M, Südhof TC, α-Synuclein assembles into higher-order multimers upon membrane binding to promote SNARE complex formation., Proc. Natl. Acad. Sci. U.S. A. Ill (2014) E4274–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Larsen KE, Schmitz Y, Troyer MD, Mosharov E, Dietrich P, Quazi AZ, Savalle M, Nemani V Chaudhry FA, Edwards RH, Stefanis L, Sulzer D, Alpha-synuclein overexpression in PC12 and chromaffin cells impairs catecholamine release by interfering with a late step in exocytosis., J. Neurosci. 26 (2006) 11915–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Darios F, Ruipérez V, Lopez I, Villanueva J, Gutierrez LM, Davletov B, Alpha-synuclein sequesters arachidonic acid to modulate SNARE-mediated exocytosis., EMBO Rep. 11 (2010) 528–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Nemani VM, Lu W, Berge V, Nakamura K, Onoa B, Lee MK, Chaudhry FA, Nicoll RA, Edwards RH, Increased Expression of α-Synuclein Reduces Neurotransmitter Release by Inhibiting Synaptic Vesicle Reclustering after Endocytosis, Neuron. 65 (2010) 66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Logan T, Bendor J, Toupin C, Thorn K, Edwards RH, α-Synuclein promotes dilation of the exocytotic fusion pore, Nat. Neurosci. 20 (2017) 681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Cabin DE, Shimazu K, Murphy D, Cole NB, Gottschalk W, Mcllwain KL, Orrison B, Chen A, Ellis CE, Paylor R, Lu B, Nussbaum RL, Synaptic vesicle depletion correlates with attenuated synaptic responses to prolonged repetitive stimulation in mice lacking alpha-synuclein., J. Neurosci. 22 (2002) 8797–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Diao J, Burré J, Vivona S, Cipriano DJ, Sharma M, Kyoung M, Südhof TC, Brunger AT, Native α-synuclein induces clustering of synaptic-vesicle mimics via binding to phospholipids and synaptobrevin-2/VAMP2, Elife. 2 (2013) 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Vargas KJ, Schrod N, Davis T, Fernandez-Busnadiego R, Taguchi YV, Laugks U, Lucic V, Chandra SS, Synucleins Have Multiple Effects on Presynaptic Architecture, Cell Rep. 18 (2017) 161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Boczek EE, Alberti S, Phase changes in neurotransmission., Science. 361 (2018) 548–549. [DOI] [PubMed] [Google Scholar]
- [120].Anderson VL, Ramlall TF, Rospigliosi CC, Webb WW, Eliezer D, Identification of a helical intermediate in trifluoroethanol-induced alpha-synuclein aggregation., Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 18850–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ueda K, Fukushima H, Masliah E, Xia Y, Iwai A, Yoshimoto M, Otero DA, Kondo J, Ihara Y, Saitoh T, Molecular cloning of cDNA encoding an unrecognized component of amyloid in Alzheimer disease., Proc. Natl. Acad. Sci. 90 (1993) 11282–11286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Giasson BI, Murray IVJ, Trojanowski JQ, Lee VMY, A Hydrophobic Stretch of 12 Amino Acid Residues in the Middle of α-Synuclein Is Essential for Filament Assembly, J. Biol. Chem. 276 (2001) 2380–2386. [DOI] [PubMed] [Google Scholar]
- [123].Rodriguez JA, Ivanova MI, Sawaya MR, Cascio D, Reyes FE, Shi D, Sangwan S, Guenther EL, Johnson LM, Zhang M, Jiang L, Arbing MA, Nannenga BL, Hattne J, Whitelegge J, Brewster AS, Messerschmidt M, Boutet S, Sauter NK, Gonen T, Eisenberg DS, Structure of the toxic core of α-synuclein from invisible crystals, Nature. 525 (2015) 486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Oldfield CJ, Dunker AK, Intrinsically disordered proteins and intrinsically disordered protein regions., Annu. Rev. Biochem. 83 (2014) 553–84. [DOI] [PubMed] [Google Scholar]
- [125].Dunker AK, Cortese MS, Romero P, Iakoucheva LM, Uversky VN, Flexible nets. The roles of intrinsic disorder in protein interaction networks., FEBS J. 272 (2005) 5129–48. [DOI] [PubMed] [Google Scholar]
- [126].Ambadipudi S, Zweckstetter M, Targeting intrinsically disordered proteins in rational drug discovery, Expert Opin. Drug Discov. 11 (2016) 65–77. [DOI] [PubMed] [Google Scholar]
- [127].Babu MM, van der Lee R, de Groot NS, Gsponer J, Intrinsically disordered proteins: Regulation and disease, Curr. Opin. Struct. Biol. 21 (2011) 432–440. [DOI] [PubMed] [Google Scholar]
- [128].Neira JL, Bintz J, Arruebo M, Rizzuti B, Bonacci T, Vega S, Lanas A, Velázquez-Campoy A, Iovanna JL, Abian O, Identification of a Drug Targeting an Intrinsically Disordered Protein Involved in Pancreatic Adenocarcinoma, Sci. Rep. 7 (2017) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Fonseca-Ornelas L, Eisbach SE, Paulat M, Giller K, Fernández CO, Outeiro TF, Becker S, Zweckstetter M, Small molecule-mediated stabilization of vesicle-associated helical α-synuclein inhibits pathogenic misfolding and aggregation, Nat. Commun. 5 (2014) 5857. [DOI] [PubMed] [Google Scholar]
- [130].Zhao H, Lappalainen P, A simple guide to biochemical approaches for analyzing protein-lipid interactions, Mol. Biol. Cell. 23 (2012) 2823–2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Greenfield NJ, Using circular dichroism spectra to estimate protein secondary structure, Nat. Protoc. 1 (2007) 2876–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Pfefferkorn CM, Lee JC, Tryptophan probes at the alpha-synuclein and membrane interface., J. Phys. Chem. B. 114 (2010) 4615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].vanRooijen BD, Claessens MMAE, Subramaniam V, Membrane Permeabilization by Oligomeric α-Synuclein: In Search of the Mechanism, PLoS One. 5 (2010) e14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Beseničar M, Macek P, Lakey JH, Anderluh G, Surface plasmon resonance in protein-membrane interactions, Chem. Phys. Lipids. 141 (2006) 169–178. [DOI] [PubMed] [Google Scholar]
- [135].Hodnik V, Anderluh G, Surface plasmon resonance for measuring interactions of proteins with lipid membranes., Methods Mol. Biol. 974 (2013) 23–36. [DOI] [PubMed] [Google Scholar]
- [136].Ghosh D, Sahay S, Ranjan P, Salot S, Mohite GM, Singh PK, Dwivedi S, Carvalho E, Banerjee R, Kumar A, Maji SK, The newly discovered Parkinson’s disease associated Finnish mutation (A53E) attenuates α-synuclein aggregation and membrane binding., Biochemistry. 53 (2014) 6419–21. [DOI] [PubMed] [Google Scholar]
- [137].VErkizan H, Kong Y, Merchant M, Schlottmann S, Barber-Rotenberg JS, Yuan L, Abaan OD, Chou T, Dakshanamurthy S, Brown ML, Uren A, Toretsky JA, A small molecule blocking oncogenic protein EWS-FLI1 interaction with RNA helicase A inhibits growth of Ewing’s sarcoma., Nat. Med. 15 (2009) 750–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Patching SG, Surface plasmon resonance spectroscopy for characterisation of membrane protein-ligand interactions and its potential for drug discovery., Biochim. Biophys. Acta. 1838(2014)43–55. [DOI] [PubMed] [Google Scholar]
- [139].Iyer A, Petersen NO, Claessens MMAE, Subramaniam V, Amyloids of alpha-synuclein affect the structure and dynamics of supported lipid bilayers, Biophys. J. 106 (2014)2585–2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Iyer A, Schilderink N, Claessens MMAE, Subramaniam V, Membrane-Bound Alpha Synuclein Clusters Induce Impaired Lipid Diffusion and Increased Lipid Packing, Biophys. J.111 (2016)2440–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kamp F, Beyer K, Binding of α-synuclein affects the lipid packing in bilayers of small vesicles, J. Biol. Chem. 281 (2006) 9251–9259. [DOI] [PubMed] [Google Scholar]
- [142].Shvadchak VV, Subramaniam V, A four-amino acid linker between repeats in the α-synuclein sequence is important for fibril formation, Biochemistry. 53 (2014) 279–281. [DOI] [PubMed] [Google Scholar]
- [143].Munishkina LA, Fink AL, Fluorescence as a method to reveal structures and membrane-interactions of amyloidogenic proteins, Biochim. Biophys. Acta - Biomembr. 1768 (2007) 1862–1885. [DOI] [PubMed] [Google Scholar]
- [144].Prendergast FG, Meyer M, Carlson GL, Iida S, Potter JD, Synthesis, spectral properties, and use of 6-acryloyl-2-dimethylaminonaphthalene (Acrylodan). A thiol-selective, polarity-sensitive fluorescent probe., J. Biol. Chem. 258 (1983) 7541–4. [PubMed] [Google Scholar]
- [145].Palmer M, Saweljew P, Vulicevic I, Valeva A, Kehoe M, Bhakdi S, Membrane-penetrating domain of streptolysin O identified by cysteine scanning mutagenesis., J. Biol. Chem. 271 (1996) 26664–7. [DOI] [PubMed] [Google Scholar]
- [146].Valeva A, Weisser A, Walker B, Kehoe M, Bayley H, Bhakdi S, Palmer M, Molecular architecture of a toxin pore: a 15-residue sequence lines the transmembrane channel of staphylococcal alpha-toxin., EMBO J. 15 (1996) 1857–64. [PMC free article] [PubMed] [Google Scholar]
- [147].Anderluh G, Barlic A, Podlesek Z, Macek P, Pungercar J, Gubensek F, Zecchini ML, Serra MD, Menestrina G, Cysteine-scanning mutagenesis of an eukaryotic pore-forming toxin from sea anemone: topology in lipid membranes., Eur. J. Biochem. 263 (1999) 128–36. [DOI] [PubMed] [Google Scholar]
- [148].Li X-H, Culver JA, Rhoades E, Tau Binds to Multiple Tubulin Dimers with Helical Structure., J. Am. Chem. Soc. 137 (2015) 9218–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Majumdar A, Mukhopadhyay S, Fluorescence Depolarization Kinetics to Study the Conformational Preference, Structural Plasticity, Binding, and Assembly of Intrinsically Disordered Proteins, 1st ed., Elsevier Inc., 2018. [DOI] [PubMed] [Google Scholar]
- [150].Jain N, Narang D, Bhasne K, Dalai V, Arya S, Bhattacharya M, Mukhopadhyay S, Direct Observation of the Intrinsic Backbone Torsional Mobility of Disordered Proteins, Biophys. J.111 (2016) 768–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [151].Arya S, Singh AK, Bhasne K, Dogra P, Datta A, Das P, Mukhopadhyay S, Femtosecond Hydration Map of Intrinsically Disordered α-Synuclein., Biophys. J. 114 (2018)2540–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [152].Swasthi HM, Mukhopadhyay S, Electrostatic lipid–protein interactions sequester the curb amyloid fold on the lipopolysaccharide membrane surface, J. Biol. Chem. 292 (2017) 19861–19872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Jain N, Bhasne K, Hemaswasthi M, Mukhopadhyay S, Structural and dynamical insights into the membrane-bound α-synuclein, PLoS One. 8 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Krishna MMG, Das R, Periasamy N, Nityananda R, Translational diffusion of fluorescent probes on a sphere: Monte Carlo simulations, theory, and fluorescence anisotropy experiment, J. Chem. Phys. 112 (2000) 8502–8514. [Google Scholar]
- [155].Krishna MMG, Srivastava A, Periasamy N, Rotational dynamics of surface probes in lipid vesicles., Biophys. Chem. 90 (2001) 123–33. [DOI] [PubMed] [Google Scholar]
- [156].Demchenko AP, The red-edge effects: 30 years of exploration, Luminescence. 17 (2002) 19–42. [DOI] [PubMed] [Google Scholar]
- [157].Chattopadhyay A, Mukherjee S, Red Edge Excitation Shift of a Deeply Embedded Membrane Probe: Implications in Water Penetration in the Bilayer, J. Phys. Chem. B. 103 (2002)8180–8185. [Google Scholar]
- [158].Chattopadhyay A, Haidar S, Dynamic insight into protein structure utilizing red edge excitation shift, Acc. Chem. Res. 47 (2014) 12–19. [DOI] [PubMed] [Google Scholar]
- [159].Ferreon ACM, Gambin Y, Lemke EA, Deniz AA, Interplay of alpha-synuclein binding and conformational switching probed by single-molecule fluorescence., Proc. Natl. Acad. Sci. U. S. A. 106 (2009) 5645–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Trexler AJ, Rhoades E, α-Synuclein binds large unilamellar vesicles as an extended helix, Biochemistry. 48 (2009) 2304–2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Trexler AJ, Rhoades E, Single molecule characterization of α-synuclein in aggregation-prone states, Biophys. J. 99 (2010) 3048–3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Rajarathnam K, Rosgen J, Isothermal titration calorimetry of membrane proteins - progress and challenges., Biochim. Biophys. Acta. 1838 (2014) 69–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Swamy MJ, Sankhala RS, Probing the thermodynamics of protein-lipid interactions by isothermal titration calorimetry., Methods Mol. Biol. 974 (2013) 37–53. [DOI] [PubMed] [Google Scholar]
- [164].Bartels T, Ahlstrom LS, Leftin A, Kamp F, Haass C, Brown MF, Beyer K, The N-terminus of the intrinsically disordered protein α-synuclein triggers membrane binding and helix folding, Biophys. J. 99 (2010) 2116–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Papahadjopoulos D, Jacobson K, Nir S, Isac T, Phase transitions in phospholipid vesicles. Fluorescence polarization and permeability measurements concerning the effect of temperature and cholesterol., Biochim. Biophys. Acta. 311 (1973) 330–48. [DOI] [PubMed] [Google Scholar]
- [166].Chen LA, Dale RE, Roth S, Brand L, Nanosecond time-dependent fluorescence depolarization of diphenylhexatriene in dimyristoyllecithin vesicles and the determination of “microviscosity”., J. Biol. Chem. 252 (1977) 2163–9. [PubMed] [Google Scholar]
- [167].Pirc K, Ulrih NP, α-Synuclein interactions with phospholipid model membranes: Key roles for electrostatic interactions and lipid-bilayer structure., Biochim. Biophys. Acta. 1848 (2015)2002–12. [DOI] [PubMed] [Google Scholar]
- [168].Nuscher B, Kamp F, Mehnert T, Odoy S, Haass C, Kahle PJ, Beyer K, α-synuclein has a high affinity for packing defects in a bilayer membrane: A thermodynamics study, J. Biol. Chem. 279 (2004) 21966–21975. [DOI] [PubMed] [Google Scholar]
- [169].Galvagnion C, Brown JWP, Ouberai MM, Flagmeier P, Vendruscolo M, Buell AK, Sparr E, Dobson CM, Chemical properties of lipids strongly affect the kinetics of the membrane-induced aggregation of α-synuclein, Proc. Natl. Acad. Sci. 113 (2016) 7065–7070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [170].Fawzi NL, Ying J, Ghirlando R, Torchia DA, Clore GM, Atomic-resolution dynamics on the surface of amyloid-β protofibrils probed by solution NMR, Nature. 480 (2011)268–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Fawzi NL, Ying J, Torchia DA, Clore GM, Probing exchange kinetics and atomic resolution dynamics in high-molecular-weight complexes using dark-state exchange saturation transfer NMR spectroscopy, Nat. Protoc. 7 (2012) 1523–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Eliezer D, Distance information for disordered proteins from NMR and ESR measurements using paramagnetic spin labels., Methods Mol. Biol. 895 (2012) 127–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Dedmon MM, Lindorff-Larsen K, Christodoulou J, Vendruscolo M, Dobson CM, Mapping long-range interactions in α-synuclein using spin-label NMR and ensemble molecular dynamics simulations, J. Am. Chem. Soc. 127 (2005) 476–477. [DOI] [PubMed] [Google Scholar]
- [174].Hossain KR, Holt SA, Le Brun AP, Al Khamici H, Valenzuela SM, X-ray and Neutron Reflectivity Study Shows That CLIC1 Undergoes Cholesterol-Dependent Structural Reorganization in Lipid Monolayers., Langmuir. 33 (2017) 12497–12509. [DOI] [PubMed] [Google Scholar]
- [175].Als-Nielsen J, Jacquemain D, Kjaer K, Leveiller F, Lahav M, Leiserowitz L, Principles and applications of grazing incidence X-ray and neutron scattering from ordered molecular monolayers at the air-water interface, Phys. Rep. 246 (1994) 251–313. [Google Scholar]
- [176].Jones EM, Dubey M, Camp PJ, Vernon BC, Biernat J, Mandelkow E, Majewski J, Chi EY, Interaction of Tau protein with model lipid membranes induces tau structural compaction and membrane disruption, Biochemistry. 51 (2012) 2539–2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Hahl H, Moller I, Kiesel I, Campioni S, Riek R, Verdes D, Seeger S, α-Synuclein insertion into supported lipid bilayers as seen by in situ X-ray reflectivity, ACS Chem. Neurosci. 6 (2015) 374–379. [DOI] [PubMed] [Google Scholar]
- [178].Hsieh M-H, Huang P-T, Liou H-H, Liang P-H, Chen P-M, Holt SA, Yu IF, James M, Shiau Y-S, Lee M-T, Lin T-L, Lou K-L, The Penetration Depth for Hanatoxin Partitioning into the Membrane Hydrocarbon Core Measured with Neutron Reflectivity., Langmuir. 34 (2018) 9036–9046. [DOI] [PubMed] [Google Scholar]
- [179].Richter AG, Kuzmenko I, Using in situ X-ray reflectivity to study protein adsorption on hydrophilic and hydrophobic surfaces: Benefits and limitations, Langmuir. 29 (2013) 5167–5180. [DOI] [PubMed] [Google Scholar]
- [180].Pfefferkorn CM, Heinrich F, Sodt AJ, Maltsev AS, Pastor RW, Lee JC, Depth of α-synuclein in a bilayer determined by fluorescence, neutron reflectometry, and computation., Biophys. J. 102 (2012) 613–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Wragg RT, Parisotto DA, Li Z, Terakawa MS, Snead D, Basu I, Weinstein H, Eliezer D, Dittman JS, Evolutionary Divergence of the C-terminal Domain of Complexin Accounts for Functional Disparities between Vertebrate and Invertebrate Complexins., Front. Mol. Neurosci. 10 (2017) 146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [182].Press-Sandler O, Miller Y, Molecular mechanisms of membrane-associated amyloid aggregation: Computational perspective and challenges, Biochim. Biophys. Acta - Biomembr. 1860 (2018) 1889–1905. [DOI] [PubMed] [Google Scholar]


