Abstract
Deviant auditory steady-state responses (aSSRs) in the gamma range (30–90 Hz) may be translational biomarkers for schizophrenia (SZ). This study tests whether aSSR deviations are (i) specific to SZ across the psychosis dimension, (ii) specific to particular frequency bands, and (iii) present in bipolar I disorder without psychosis (BDNP).
Methods:
Beta (20-), low- (40-), and high-gamma (80-Hz) aSSRs were measured with EEG and compared across 113 SZ, 105 schizoaffective disorder (SAD), 99 bipolar disorder with psychosis (BDP), 68 BDNP, and 137 healthy comparison subjects (HC). Standard aSSR measures (single-trial power [STP] and inter-trial phase coherence [ITC]), as well as evoked responses to stimulus onsets/offsets and pre-stimulus power, were quantified. Multivariate canonical discriminant analysis was used to summarize variables that efficiently and maximally differentiated groups.
Results:
(i) Psychosis groups showed reduced responses on ITC 20Hz, STP/ITC 40 Hz, STP/ITC 80 Hz, indicating dimensional reductions in aSSR across the psychosis spectrum not specific to aSSR frequency. For the 40- and 80-Hz ITCs there was greater reduction in SZ compared to SAD, possibly indexing cortical disruptions linked to psychosis without mood symptoms. (ii) All probands had elevated pre-stimulus power, possibly compromising neural entrainment to the steady-state stimuli. (iii) Onset/Offset and 80 Hz ITC responses were most important for group discrimination and showed dimensional reduction across the schizo-bipolar spectrum.
Conclusions:
Deviant aSSRs were found across the schizo-bipolar spectrum at multiple frequencies with psychosis status and severity linked to greatest reductions at low and high gamma.
Keywords: Auditory Steady-State Response, EEG Biomarkers, Psychosis, Bipolar Disorder, Schizophrenia, Gamma Oscillations
1. Introduction
Cortical oscillations emerge when neural ensembles exhibit temporally coherent coactivity. Beta (14–30 Hz) and gamma (30–90 Hz) oscillations depend on tightly tuned feed- forward and feed-back circuitry composed of reciprocally connected gamma-aminobutryic acid- containing (GABA-ergic) inhibitory interneurons and excitatory pyramidal cells (Womelsdorf et al., 2014; Sohal et al., 2009). It is hypothesized that abnormalities in neural oscillations observed with electroencephalography (EEG) in schizophrenia (SZ) mark underlying disturbances in cortical circuity (Foss-Feig et al., 2017) that are associated with sensory, perceptual, and cognitive deficits (Gonzalez-Burgos et al., 2012).
The auditory steady state response (aSSR) is an oscillatory event arising from neuronal activity entrained to the frequency of a repetitive auditory stimulus (e.g., a train of clicks or an amplitude modulated tone). Most aSSRs below 90Hz (i.e., a stimulus every 11.1 ms) reflect activity in auditory cortices (Hamm et al., 2011), though additional contributions from subcortical/brainstem sources have been demonstrated (Korczak et al., 2012). By varying the frequency of steady-state stimulation, the integrity of neural circuits supporting multiple functions can be assessed (Picton et al., 2003). Further, the aSSR also provides information about early transient (onset) responses like N100 and P200 and other traditional event-related EEG measures (Hamm et al., 2011).
Deviations of 40 Hz aSSRs have been reported frequently in SZ (Thuné et al., 2016). First-degree relatives, first-episode patients, and ultra high-risk individuals may have similar abnormalities (Tada et al, 2016; Rass et al., 2012; Spencer et al., 2008), supporting proposals the aSSR is a translational biomarker for SZ and/or drug response target (O’Donnell et al., 2013). A number of questions remain regarding aSSR’s utility as a biomarker for SZ. In a recent meta-analysis aggregating findings across 20 studies (SZ=606, HC=590), the effect size for aSSR components inter-trial phase coherence (ITC) and single-trial power/evoked power (STP/Evoked) was −.50, a moderate reduction in SZ compared to healthy subjects (Thuné et al., 2016). Some studies have also found aSSR reductions in bipolar disorder in comparison to both healthy and major depressive disorder groups (Rass et al., 2010; Isomura et al, 2016; Oda et al., 2012), questioning the specificity of 40 Hz aSSR deviations to SZ. Additionally, most studies have focused on the 40 Hz aSSR, but there are hints of oscillatory deviations in other frequency ranges (e.g. Hamm et al., 2011, Tsuchimoto et al., 2011), raising questions about neuro- pathological models that assume distinct abnormalities in circuitries specifically responsible for generating the 40 Hz aSSR. Post-mortem SZ studies show molecular and microanatomical abnormalities in multiple interneuron populations, supporting a general cortical neuropathology affecting signal processing in multiple bandwidths (Hashimoto et al., 2008).
The present study used EEG to examine the aSSR at three stimulation frequencies capturing beta (20 Hz), low (40 Hz) and high (80 Hz) gamma oscillations in a large well- characterized group of probands. The study had three major goals: (i) determine whether aSSR deviations are either specific to SZ in comparison to schizoaffective disorder (SAD) and bipolar I disorder with psychosis (BDP) or are dimensional markers of psychosis, (ii) determine if deviations are limited to a specific stimulation frequency, (iii) determine whether individuals with non-psychotic bipolar I disorder (BDNP) have similar aSSR deviations as the psychosis groups, are normal, or show novel characteristics, and iv) determine whether aSSR measures are correlated with clinical symptomatic severity. This comparison will help to clarify whether the aSSR is a specific biomarker for psychosis or a general marker for severe psychopathology.
2. Methods and Materials
2.1. Recruitment
Subjects (Total n=522, HC=137, SZ=113, SAD=105, BDP=99, BDNP=68) were recruited at three Psychosis and Affective Research Domains and Intermediate Phenotypes (PARDIP) and Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP2) consortium sites: Boston, Dallas, and Hartford, and followed previously published approaches (for details about study design, recruitment and clinical assessments see Tamminga et al. (2013) and supplemental methods information). Since these subjects were recruited from the ongoing studies from the BSNIP and PARDIP consortiums and have not been classified using the Clementz et al. (2016) biotypes methods, these subjects were analyzed using only their DSM diagnoses. This study was approved by Institutional Review Boards at each consortium site and all participants provided written informed consent before participation. Demographic information and clinical ratings across groups is presented in table 1. For full medication information, see supplemental tables 1–4,
TABLE 1:
Demographics and Clinical scales by group
| HC | SZ | SAD | BDP | BDNP | Statistic | p | |
|---|---|---|---|---|---|---|---|
| N | 137 | 113 | 105 | 99 | 68 | F(4,517) = 1.33 | .26 |
| Mean age | 41.7 | 39 | 38.9 | 40.8 | 39.8 | ||
| Age SD | 11.5 | 12.2 | 11 | 11 | 12.5 | ||
| Sex (M/F) | 71/66 | 62/51 | 49/56 | 43/56 | 2¾5 | x2(4) = 9.27 | .055 |
| Site | |||||||
| Dallas | 41 | 49 | 34 | 43 | 26 | x2(4) = 20.08* | .01 |
| Boston | 44 | 25 | 17 | 22 | 10 | ||
| Hartford | 52 | 39 | 54 | 34 | 32 | ||
| Number of Trials (150 total) | |||||||
| Mean trials | 136 | 128 | 130 | 131 | 133 | F(4,515)=2.48* | .043 |
| SD trials | 16 | 24 | 22 | 13 | 19 | ||
| GAF | F(4, 481) = 182.64*** | <.001 | |||||
| F(3, 363)= 6.55*** | <.001 | ||||||
| N | 130 | 111 | 98 | 98 | 68 | HC> BDNP***, BDP***, SAD***, & SZ*** BDNP> BDP*, SAD*, & SZ*** |
|
| M | 82.53 | 50.35 | 52.84 | 53.16 | 58.35 | ||
| SD | 13.35 | 14.34 | 13.83 | 13.9 | 14.6 | ||
| Birchwood Social Functioning Scale | F(4, 483)= 59.96*** | <.001 | |||||
| F(3, 353)= 9.14*** | <.001 | ||||||
| N | 131 | 102 | 98 | 95 | 62 | HC> BDNP***, BDP***, SAD***, & SZ*** BDNP> SAD*** & SZ*** BDP > SAD* & SZ* |
|
| M | 151.5 | 115 | 115 | 124 | 130 | ||
| SD | 17 | 21.5 | 24 | 23 | 20.5 | ||
| PANSS Positive | F(3,354) = 14.18*** | <.001 | |||||
| N | N/A | 104 | 96 | 93 | 65 | BDNP < SAD*** & SZ*** BDP < SAD** & SZ** |
|
| M | N/A | 18.26 | 18.11 | 15.18 | 12.75 | ||
| SD | N/A | 6.6 | 6.99 | 6.3 | 3.73 | ||
| PANSS Negative | F(3,353) = 5.07* | .002 | |||||
| N | N/A | 104 | 96 | 93 | 65 | BDNP < SAD* & SZ** | |
| M | N/A | 18.78 | 18.34 | 16.62 | 14.86 | ||
| SD | N/A | 7.17 | 7.54 | 6.89 | 6.32 | ||
| PANSS General | F(3,353) = 1.31 | .27 | |||||
| N | N/A | 104 | 96 | 93 | 65 | ||
| M | N/A | 34.53 | 34.18 | 34.67 | 31.74 | ||
| SD | N/A | 10.74 | 9.71 | 10.68 | 9.09 | ||
| PANSS Total | F(3,353) = 5.39* | .001 | |||||
| N | N/A | 103 | 96 | 93 | 65 | BDNP < SAD** & SZ*** | |
| M | N/A | 71.74 | 70.64 | 66.47 | 59.35 | ||
| SD | N/A | 22.33 | 21.54 | 21.94 | 16.33 | ||
| MADRS | F(3,356) = 9.75*** | <001 | |||||
| N | N/A | 104 | 98 | 93 | 65 | BDNP> SZ** BDP> SAD* & SZ*** |
|
| M | N/A | 9.73 | 13.43 | 17.65 | 14.91 | ||
| SD | N/A | 8.74 | 10.5 | 12.48 | 9.63 | ||
| YMRS | F(3,354) = 1.54 | 205 | |||||
| N | N/A | 104 | 96 | 93 | 65 | ||
| M | N/A | 10.12 | 11.74 | 11 | 9.25 | ||
| SD | N/A | 6.84 | 7.5 | 9.37 | 7.29 | ||
Note.
p < .05,
p<.01,
p < .001. Tukey’s test were used for post-hoc analyses.
GAF= Global Assessment of Functioning; Birchwood Social Functioning Scale; PANSS = Positive and Negative Syndrome Scale;
MADRS = Montgomery-Asberg Depression Rating Scale; YMRS = Young Mania Rating Scale. SD = standard deviation.
2.2. Stimuli
Recording conditions, stimulus presentation and recording equipment were standardized across sites. Seated in a sound and electrically shielded booth subjects listened to 166 sinusoidally amplitude modulated broadband noise at 20 (50 trials), 40 (50 trials), and 80 (50 trials) Hz and 16 unmodulated noise (duration 1500 ms; carrier pitch 1000 Hz; randomly ordered). Broadband noise bursts were used since they are known to elicit the most robust aSSRs, especially at higher frequencies (John et al., 1998; Picton et al., 2003; Hamm et al., 2012). Stimuli were presented binaurally through headphones at 75 dB SPL with an inter-trial interval of 1 s. Subjects were instructed to count the number of unmodulated noise bursts to maintain continuous investment in the stimuli.
2.3. EEG Recording
Electroencephalogram (EEG) from 64 sensors was recorded following previously published methods from the B-SNIP consortium (Hamm et al., 2014; supplemental methods).
2.4. EEG Processing
EEG data were pre-processed following previously published methods (Hamm et al., 2014, supplemental methods). Data were segmented into 3000-ms epochs from 750 ms pre- to 750 ms post-stimulus onset and down-sampled to 500 Hz and digitally band pass filtered from .5 Hz to 100 Hz (zero-phase filter; roll-off: 6 and 48 dB/octave, respectively). Epochs containing activity greater than 75 μV at any sensor were not included, see table 1 for number of trials by group.
2.5. EEG Analysis
Event-related potentials (ERPs) were calculated for each trial-type, sensor, and subject and converted to the time-frequency domain yielding complex numbers for points ranging from −500 to 2000 ms in 2ms bins and 1- to 90-Hz following previously published methods (Hamm et al. 2015;supplemental methods). Power values (squared absolute values of complex FFT outputs) were then converted to decibels (10*log10). The baseline period was defined as the average response from −500–0 ms. After averaging over subjects and trial types, 11 sensors with peak auditory response (‘F1’, ‘Fz’, ‘F2’, ‘FC3’, ‘FC1’, ‘FCz’, ‘FC2’, ‘FC4’, ‘C1’, ‘Cz’, ‘C2’) were identified and a grand average time-frequency plot was created by averaging power values over these sensors (figure 1).
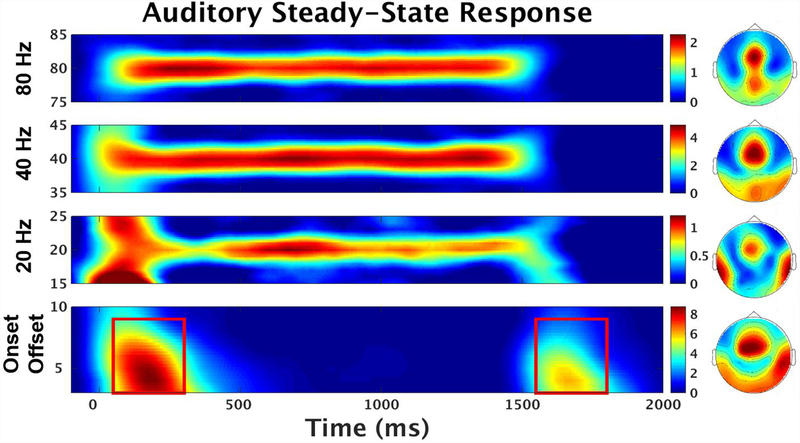
Figure 1: Evoked Power.
Evoked Power (dB) response averaged over all trial-types and all subjects using peak central-frontal sensors. Red Box indicate areas used for onset (3–9 Hz; 50–300 ms) and offset (3–9 Hz; 1550–1800 ms) evoked response analysis. Topographies of each steady-state stimulation frequencies averaged over all trial-types and all subjects from onset of stimulus to offset (0–1500 ms). Bottom topography is the average of both the onset and offset response periods.
2.6. Onset and Offset Evoked Response
Auditory evoked responses to transient stimuli have complex frequency compositions involving stimulus locked alterations in oscillatory amplitudes and phase coherence across multiple frequency bands that is not captured in the temporal domain alone (Hamm et al., 2012). In order to measure initial auditory integration abilities and registration of stimulus change in relation to steady-state initiation and termination, onset and offset evoked responses were examined in the time-frequency domain. The peak responses from the 11-sensor average were between 3–9 Hz and 50–300 ms for the onset and 3–9 Hz and 1550–1800 ms for the offset. See figure 1, supplemental figure 1.
2.7. Single-Trial Analysis
Single-trial voltage data for each subject and sensor were converted to the time-frequency domain using the same method as described above. ”Baseline Power”, a measure of pre-stimulus neuronal activity for each steady-state driving frequency condition, was defined as power values at each frequency averaged from −500 ms to the onset of the stimulus across trials for each trial type. This metric provides a measure of brain activity in preparation for, not in response to, auditory stimulation.
Single-trial power (STP), a measure of stimulation specific neuronal responses, was calculated by averaging power over trials and then subtracting pre-stimulus baseline power (see above; supplemental figure 2). Inter-trial phase coherence (ITC), a measure of the consistency of the phase of oscillatory responses with respect to stimulus onset across trials, was calculated by dividing the complex FFT result by its absolute value (Jammalamadaka and Sengupta, 2001). The resulting values were then averaged across trials within each trial type (supplemental figure 3). Due to the sensitivity of ITC to the number of trials contributed each participant’s chance ITC was subtracted from the observed ITC (Moratti et al. 2007; supplemental methods). The 20 and 40 Hz responses had similar topographies as the evoked aSSR so identical sensors (‘F1’, ‘Fz’, ‘F2’, ‘FC3’, ‘FC1’, ‘FCz’, ‘FC2’, ‘FC4’, ‘C1’, ‘Cz’, ‘C2’) were used; for the 80 Hz response 6 additional sensors from the central-parietal region (‘P1’, ‘Pz’, ‘P2’, ‘PO3’, ‘POz’, ‘PO4’) were included (supplemental figures 2 and 3). STP and ITC were epoched in 300 ms bins to empirically determine if there were changes in entrainment levels over the steady-state periods.
2.8. Principal Component Analysis for Data Reduction
In order to capture maximum explanatory variance across variables and to avoid information redundancy and reduce the number of statistical comparisons, data reduction was accomplished by principal component analysis (PCA, with promax rotation) in SPSS. Three separate PCAs were conducted and the number of components selected was based on an eiginvalue cutoff of 1 (Ethridge et al. 2015, Hamm et al. 2014). The Onset/Offset periods (6 variables) was reduced to a single component (variance accounted: 49%), and the Baseline Power (3 variables) was reduced to a single component (variance accounted: 68%). The Steady- State STP and ITC for each stimulation frequency and time bin (30 variables: 3 frequencies, 2 neural measures [STP ITC], 5 time bins [0–1500 ms in 300 ms bins]) was reduced to five components (20 Hz STP, 20 Hz ITC, 40 Hz Response (STP and ITC), 80 Hz ITC, 80 Hz STP; variance accounted: 71%). The inclusion of 300 ms time bins helped evaluate the consistency of steady-state response across the entire steady-state period. Importantly, if there were different PCA loadings for the time-bin periods it would indicate different levels of entrainment possibly indexing unique properties of the auditory stimuli or cortical disruptions linked to specific time periods. However, for each PCA component, regardless of frequency of stimulation or neural measure that the component indexed, time bins showed a similar pattern: the 0–300 ms time bins had lower loadings [20 Hz STP: .65, 20 Hz ITC: .62, 40 Hz STP/ITC: .78/.75, 80 Hz STP: .58, 80 Hz ITC: .85], but each of the following bins (300–600, 600–900, 900–1200, 1200–1500 ms) had similarly high loadings [20 Hz STP: .75-.83, 20 Hz ITC: .70-.78, 40 Hz STP/ITC: .85-.96, 80 Hz STP: .74-.92, 80 Hz ITC: .87-.91]. Bartlett factor scores were generated for each subject for each PCA component. PCA Pattern Matrices and loadings are presented in supplemental tables 5–7.
2.9. Statistical Analysis
All statistics were performed in SPSS Statistics version 23 (Armonk, NY: IBM Corp.). For each PCA component (7) a 1-way ANOVA was conducted to determine group differences. All ANOVAs were corrected using the Holm-Bonferroni method (Holm, 1979). Significant effects involving group membership were followed with hypothesis driven orthogonal contrasts (Healthy vs. Probands; BDNP vs. [BDP, SAD & SZ]; BDP vs. [SZ & SAD]; SAD vs. SZ). Equal variance was not assumed. Full statistical results are presented in supplemental tables 8 and 9.
2.10. Post Hoc Analyses: Canonical Correlation and Discriminant Analysis
To parsimoniously evaluate the relationships between the PCA neural components and clinical measures (GAF, SFS, PANSS Negative, PANSS Positive, PANSS General, Young Mania Rating Scale, Montgomery-Asberg Depression Rating Scale), canonical correlation analyses (CCA) across all proband groups was performed (Lambert et al., 1988; supplemental methods).
To summarize variables that efficiently and maximally differentiated groups based on aSSR-related neural responses, PCA components that were significant after Holm-Bonferroni correction were submitted to a canonical discriminant analysis (CDA) (Hamm et al. 2014; supplemental methods). For each significant canonical variate, means and standard error of the mean were calculated and plotted (figure 3). A Post-Hoc Tukey’s B test was performed to identify homogenous sub-groupings. The CDA results and PCA correlations entered into the CDA are listed in table 2.
Figure 3:
Canonical Variate. Show a dimensional pattern of severity of psychopathology. The variate correlated strongest with the onset and offset PCA component and the 80 Hz ITC PCA component. HC, Healthy Comparison subjects (n=137); SZ, probands with schizophrenia (n=113); SAD, probands with schizoaffective disorder (n=105); BDP, probands with bipolar disorder I with psychosis (n=99); BDNP, probands with bipolar disorder I without psychosis (n=68). Error bars=SEM. *p < .05, **p<.01, ***p < .001 based on Tukey HSD post-hoc tests.
Table 2:
Canonical Discriminant Analysis
| Eigenvalue | % of Variance | Wilks’ Lambda | Chi-square | Df | Canonical Correlation | Sig. | |
|---|---|---|---|---|---|---|---|
| Canonical Variate 1: | 0.14 | 87.2 | 0.86 | 75 24*** | 24 | 0 34*** | <0.001 |
| Correlations: | Onset and Offset | ITC 80 Hz | STP and ITC 40 Hz | STP 80 Hz | ITC 20 Hz | Baseline Activity | |
| PCA components and Canonical Variate 1 | 0.765 | 0.71 | 0.45 | 0.44 | 0.42 | −0.37 | |
| Group Centroids: | |||||
|---|---|---|---|---|---|
| Canonical Variate 1 | N | Mean | SD | Lower 95% | Upper 95% |
| HC | 137 | 0.56 | 1.11 | 0.37 | 0.75 |
| BDNP | 68 | 0.04 | 0.94 | −0.18 | 0.27 |
| BDP | 99 | −0.07 | 1.06 | −0.28 | 0.14 |
| SAD | 105 | −0.25 | 0.96 | −0.43 | −0.06 |
| SZ | 113 | −0.41 | 0.87 | −0.58 | −0.25 |
Note.
p < .05,
p<.01,
p < .001.
3. Results
3.1. Onset and Offset Evoked Response
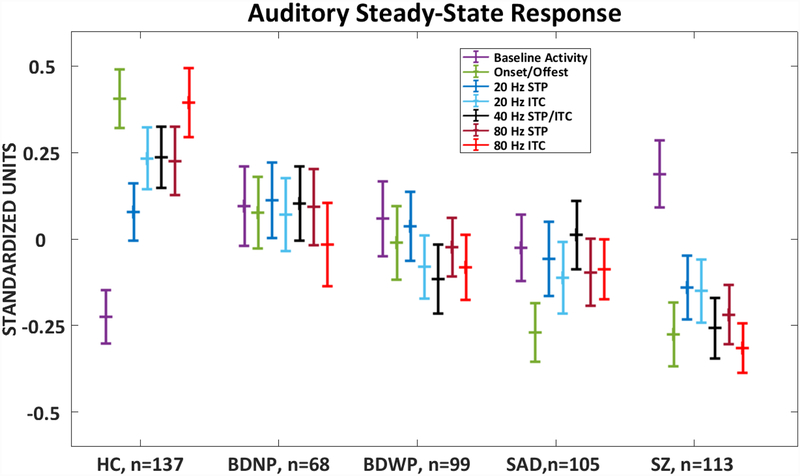
The ANOVA on the Onset- and Offset-evoked response component (Average of 3–9 Hz from 50–300 ms and 1550–1800 ms) revealed a significant main effect for Group (F=10.51, df=4, 517, p<.001; p<.001, Holm-Bonferroni corrected). Contrasts showed that HC> probands (t=5.35, df=229, p< .001), BDNP> psychosis groups (t=2.22, df=107, p=.028), and BDP> SZ/SAD (t=2.22, df=107, p=.035). The SZ and SAD groups did not differ. See figure 2, supplemental tables 8 and 9; PCA loadings are presented in supplemental table 7.
Figure 2:
PCA scores derived from the unadjusted single-trial power from the pre-stimulus period (−500–0ms), the onset (3–9 Hz; 50–300 ms) and offset (3–9 Hz; 1550–1800 ms) evoked response and the Steady-State response at 20, 40 and 80 Hz, resulting in unique 7 components by group. See supplemental tables 6, 7 and 8 for PCA weights. HC, Healthy Comparison subjects (n=137); SZ, probands with schizophrenia (n=113); SAD, probands with schizoaffective disorder (n=105); BDP, probands with bipolar disorder I with psychosis (n=99); BDNP, probands with bipolar disorder I without psychosis (n=68). Error bars=SEM. Full orthogonal contrast results can be found in supplemental table 9.
3.2. Baseline Power
The ANOVA on the Baseline Power component (Overall neuronal activity from −500 → stimulus onset at each frequency of stimulation) revealed a significant main effect for Group (F=3.02, df=4, 517, p=.018; p=.044, Holm-Bonferroni corrected). Contrasts showed that HC< probands (t=−3.24, df=267, p=.001), but the proband groups did not differ (p’s>.12). See figure 2, supplemental tables 8 and 9; PCA loadings are presented in supplemental table 6.
3.3. Steady-State Responses
Five separate ANOVAs were conducted on PCA components that corresponded to 5 separate neural responses: 20 Hz ITC, 20 Hz STP, 40 Hz Response, 80 Hz ITC, 80 Hz STP. See supplemental table 5 for PCA pattern matrix loadings.
3.3.1. 20 Hz Response
The ANOVA on the 20 Hz ITC (measure of phase consistency across trials) component revealed a significant main effect for Group (F=3.13, df=4, 517, p=.015; p=.044, Holm-Bonferroni corrected). Contrasts showed that HC> probands (t=2.92, df=221, p=.004), but the proband groups did not differ (p’s>.12). The ANOVA on the 20 Hz STP (measure of the magnitude of response across trials) component did not show a significant main effect for Group (F=1.1, df=4, 517, p=.36). See figure 2, supplemental tables 8 and 9.
3.3.2. 40 Hz Response
The ANOVA on the 40 Hz Response component revealed a significant main effect for Group (F=4.4, df=4, 517, p=.002; p=.008, Holm-Bonferroni corrected). Contrasts showed that HC> probands (t=2.95, df=225, p=.004) and SAD>SZ (t=2.03, df=211, p=.044), however, the contrasts between BDNP vs psychosis subgroups and BDP vs SZ/SAD did not differ (p’s>.07). See figure 2, supplemental tables 8 and 9.
3.3.3. 80 Hz Response
The ANOVA on the 80 Hz ITC component revealed a significant main effect for Group (F=9.04, df=4, 517, p<.001; p<.001, Holm-Bonferonni corrected). Contrasts showed that HC> probands (t=4.7, df=200, p<.001) and SAD>SZ (t=2.01, df=206, p=.046), however, the contrasts between BDNP vs psychosis subgroups and BDP vs SZ/SAD did not differ (p’s>.27). The ANOVA on the 80 Hz STP component revealed a significant main effect for Group (F=3.57, df=4, 517, p=.007; p=.028, Holm-Bonferroni corrected). Contrasts showed that HC> probands (t=2.61, df=202, p=.01), but the proband groups did not differ (p’s>.096). See figure 2, supplemental tables 8 and 9.
3.4. Canonical Correlation Analysis
The six PCA components that showed significant group differences (onset/offset, baseline, 20 Hz ITC, 40 Hz Response (STP and ITC), 80 Hz ITC, and 80 Hz STP) and seven clinical measures (Global Assessment of Functioning scale, Birchwood Social Functioning Scale, PANSS Negative, PANSS Positive, PANSS General, Young Mania Rating Scale, Montgomery-Asberg Depression Rating Scale) were used in the CCA, however, none of the variates reached significance (p’s> .265). See supplemental tables 10 and 11 for individual correlations and CCA loadings.
3.5. Canonical Discriminant Analysis
The six PCA components that showed significant group differences (onset/offset, baseline, 20 Hz ITC, 40 Hz Response (STP and ITC), 80 Hz ITC, and 80 Hz STP) were used in the CDA to efficiently summarize group differentiations (HC, BDNP, BDP, SAD, SZ). Only the first variate (Λ =.34, Wilks’ Lambda= .86, Chi=75.24, df=24, p<.001) was statistically significant. Correlations between each of the components and canonical variate are provided in table 2. The canonical variate (plotted in figure 3) is mostly associated with higher neural activity to stimulus onset and offset (r=.765), and phase coherence in response to the 80 Hz steady-state stimuli (r=.71). This variate showed a pattern of HC (Mean: .56, SEM: .095) > BDNP (Mean: .04, SEM: .11) > BDP (Mean: −.07, SEM: .11) > SAD (Mean: −.25, SEM: .096) >SZ (Mean: −.41, SEM: .087). A follow up Tukey B post-hoc test identified three homogenous sub-groups: Healthy, affective disorders [BDNP, BDP & SAD], and psychosis [BDP, SAD, & SZ].
4. Discussion
We examined the aSSR and related neural activations in a large sample of individuals across the schizophrenia-bipolar spectrum. The sample size of this individual aSSR study of psychosis is 44% that of a previous meta-analysis of gamma aSSR psychosis studies (Thuné et al., 2016) and has a more diverse syndromal representation. The results yield information concerning the utility of this family of neural activations to serve as psychosis and/or severe psychopathology biomarkers. Detailed discussion of the study outcomes and their importance for understanding neuro-pathological correlates of psychosis and bipolar disorder is provided below.
There were five main study outcomes: (i) psychosis groups (SZ, SAD, and BDP) had dimensionally deviant auditory neural responses to the steady-state stimuli, with differences following the pattern of most severe deviations occurring in SZ and the least severe in BDP; (ii) deviant auditory responses in psychosis and BDNP were not limited to a specific stimulation frequency, to magnitude (STP), or phase coherence (ITC). In response to 40 Hz and 80 Hz stimuli, however, ITC values were lower among SZ compared to SAD; (iii) Reductions of onset and offset responses were found in all probands in comparison to HC, between BDNP and psychosis subgroups, BDP and SAD/SZ, but not between SAD and SZ; (iv) The combined proband groups showed statistically similar elevations of neural activity prior to stimuli onset (as measured by neural activity during the baseline period) compared to healthy subjects; (v) there were no substantial associations between clinical and social functioning measures and the auditory neural components in response to the steady-state stimuli. In a recent article Zhou et al. (2018) examined the aSSR across the psychosis spectrum (SZ, SAD, and BDP). The current study replicates the main findings of a reduction to the aSSR across the psychosis spectrum that is not limited to 40 Hz. By utilizing a multivariate approach across the full schizo-bipolar spectrum the current study provides a number of useful insights into the utility of the aSSR as a biomarker for serious mental illness.
4.1. Response to Stimuli Onset and Offset
Due to the complex frequency compositions of early transient responses the onset and offset evoked response was examined in the time-frequency domain. Previous studies have found reduced early transient neural responses in SZ to the aSSR in lower frequencies (Hamm et al. 2012, Hamm et al., 2011). This is consistent with a general reduction in early ERP (traditional N100/P200) responses to auditory stimuli; however, prior studies have been limited by only including SZ (Hamm et al., 2011; Rosburg et al., 2008), SZ and BDP (Hamm et al., 2014; Ethridge et al., 2012), or by not distinguishing between BDP and BDNP (Wang et al., 2014). The current study shows a general pattern of psychopathology severity (HC>BDNP>BDP>SZ/SAD). These reductions may be related to inhibitory-excitatory imbalances in cortical circuits (Javitt et al., 2015; Blatow et al., 2003) especially those involving dysfunction of inhibitory somatostatin- containing (SST) interneurons (Hamm et al., 2016). This study provides evidence that earlier evoked response reductions in the low-frequency range are a marker for severity of serious mental illness (figure 2).
4.2. Baseline Power
High levels of intrinsic neural activity had been shown previously in SZ (Rolls et al., 2008; Clementz et al., 2008; Clementz et al., 2004) and are present in some mouse models of the disease (Hamm et al., 2017). Examining the role of ongoing and pre-stimulus background brain activity at the driving frequencies provides important insight into steady-state deviations. In all proband groups baseline power (−500 to stimulus onset) was increased in comparison to HC (figure 2). The results in the current study show that disturbances are present across the bipolar/schizophrenia spectrum, regardless of psychotic or affective status and could partially explain the overlap in deviations to the steady-state found across the probands in investigation not adjusting for background brain activity. This issue deserves study in other neurophysiological dimensions relevant to serious mental illness and provides an important target for translational future translational studies.
4.3. Steady-State Response
Across a range of aSSR stimulation frequencies and measures there was not any single feature of the specific steady-state responses associated with any syndrome. In an exploratory analysis, there was no group by steady-state PCA components interaction (F(13.5, 1741)=1.06, p=.39; Greenhouse Geisser corrected). SZ tended to have lower amplitude responses to 40- and 80-Hz stimulation than the other groups, but these differences were qualitative rather than quantitative with considerable between-groups overlap. The dimensional nature of abnormalities observed is summarized by the discriminant analysis outcome displayed in figure 3, combined with the statistical outcome of those results that indicated the healthy, affective disorder, and psychosis cases seemed to yield an ordered severity continuum.
These results might inspire questions about understanding of aSSR deviations among clinically defined psychiatric groups (figure 2), but they might simultaneously yield an important development in our understanding of this interesting family of translational biomarkers. These data seem to be most consistent with the thesis that EEG deviations, including the ERPs to stimulus change as well as the actual steady-state components, especially in response to gamma range stimuli, index severity across affective and nonaffective syndromes. This pattern of results is consistent with molecular and micro-anatomical abnormalities in SZ-like psychosis in multiple interneuron families (Hashimoto et al., 2008), and suggests pathology is not limited to parvalbumin containing interneurons related specifically to the 40 Hz aSSR (Sivarao, 2015; Sohal et al., 2009; Kim et al., 2015) or to cortical somatostatin-containing interneurons which naturally control sensory responses in the beta (20Hz) band (Hamm et al., 2017; Veit et al., 2017), and that these mechanisms might transcend any psychosis syndrome.
Further, each of the steady-state frequencies may probe different spatial scales of cortical communication. Beta (20 Hz) has been implicated in long-range cortical to cortical communication (Kopell et al., 2000), auditory gamma (40 Hz) is primary generated in Heschel’s gryus with contributions from the thalamus and midbrain (Herdman et al., 2002), and high gamma (80 Hz) may have significant subcortical/brainstem sources (Korczak et al., 2012). The specific deviations at low and high gamma could be due feedfoward inhibition dysfunction from thalamus to layer IV parvalbumin interneurons (Womelsdorf et al., 2014). However, future systematic translational and animal model studies linking cell- and circuit-level motifs to macro- level biomarkers like aSSR could provide insight into the neurophysiological deviations present across serious mental illness. Alternatively, a more fundamental alteration affecting the stability of cortical ensembles and/or attractors (Hamm et al., 2017) could affect neuronal computation across all bandwidths and time courses, offering an intriguing, more parsimonious basis for the diverse EEG abnormalities seen in serious mental illness.
4.4. Relation to Clinical and Social Functioning
Some previous studies have found an association between clinical and social functioning and the auditory steady-state response (Zhou et al., 2018; Hamm et al., 2012; Spencer et al. 2009), the multivariate approach used in this study did not find associations that met conventional statistical significance thresholds. Nevertheless, the variates did show patterns consistent with previous findings (see supplemental table 11). The first canonical correlation indicated a relationship between mostly 20 Hz ITC, and overall functioning as indexed by the GAF. The second canonical variate indicated a relationship between mostly 40 Hz ITC/STP and PANSS negative symptom ratings and the MADRS. These associations warrant additional study to further understanding of relationships between the auditory steady-state response and clinical correlates of psychosis.
4.5. Strengths and Limitations
The strengths of our study include a large carefully collected sample covering a broad range of serious mental illness syndromes, and a rich characterization of clinical and cognitive measures. Among limitations, almost all patients were receiving psychiatric medications, which in some studies have been shown to interact with the aSSR (Rass et al., 2010), therefore the effect of treatment cannot be ruled out. However several medication considerations should be taken into account. Importantly, while the majority of SZ, SAD and BDP were treated with antipsychotics (SZ=86%, SAD=79%, BDP=78%), a nontrivial portion of BDNP cases were also receiving antipsychotics (38%). CPZ dosages were similar across groups and did not correlate with any PCA component (p’s> .29). Additionally, while there was a significant overall difference in frequency of mood stabilizer use, BDP and BDNP showed similar rates (BDP=65%, BDNP=59%; lithium: BDP=27%, BDNP=18%) and lithium dosage level were similar across groups and did not correlate with any PCA component (p’s> .23). There were no differences in anti-depressant use (supplemental tables 1–4). No study to date has examined the aSSR in psychosis samples before and after receiving antipsychotic, mood stabilizer, and antidepressant medications, which could be an important area for follow up research. Since this study used a cross-sectional design, it is unable to assess how stable the aSSR is across time in psychiatric populations, although the aSSR has been shown to have high test-retest reliability in healthy persons (Legget et al., 2017). A majority of the cases reported on here were chronic, stable mid-course outpatients. While there is some evidence of aSSR abnormalities in pre-onset and first episode psychosis, and first-degree relatives of SZ (Tada et al., 2016; Rass et al., 2012; Spencer et al., 2008), this has not been assessed in BD. Such an investigation could provide a biomarker of partially distinct etiologies or risk profiles across serious mental illness.
4.6. Conclusions
The aSSR provides useful information about the auditory system and neural circuitry abnormalities/disruptions that are shared across the dimensions of psychosis, and appear to be shared dimensionally across the bipolar-schizophrenia spectrum. In order to investigate its utility as a biomarker the current study used a multivariate approach to examine intrinsic neural activity, transient evoked responses, and the steady-state response across beta, low, and high gamma frequencies. The aSSR and intrinsic activity deviations are shared across the bipolar- schizophrenia spectrum that suggest an overlap in shared cortical disruptions, with the most severe reductions occurring in the most SZ-like cases. Transient evoked responses suggest a more severe reduction occurring in both affective and psychosis syndromes. Future studies could usefully examine temporal stability of the aSSR across the bipolar-schizophrenia spectrum and examine its relationship to prodromal/high risk populations.
Supplementary Material
Acknowledgments:
The authors thank Francis Kittle, Brad Witte, and Gaurav Poudyal for their contributions to data management and organization. They thank the numerous researchers and clinicians who assisted in recruitment and data collection. Most importantly, they express gratitude to the patients who contributed their time and effort to participate in this study.
Funding:
National Institute of Health, National Institute of Mental Health (NIMH): MH096942, MH078113, MH096900, MH103366, MH096913, MH077851, MH096957, MH077945, MH103368.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures and Conflict of Interest:
DA Parker, JP Hamm, JE McDowell, SK Keedy, ES Gershon, EI Ivleva, GD Pearlson, MS Keshavan, and JA Sweeney report no financial interests or potential conflicts of interest. BA Clementz has served as a consultant for Astellas. Dr. Tamminga is a Deputy Editor of the Journal. She has served as an ad Hoc Consultant to Astellas, and Eli Lilly Pharmaceuticles, a consultant for Kaye Scholer LLC and TAISHO Pharmaceutical Co LTD and is on the Advisory Board for Intra-cellular Therapies (ITI, Inc.); She has served as an unpaid volunteer at The Brain & Behavior Foundation (Council Member), Institute of Medicine (Council Member), Lieber Institute (Scientific Advisory Board), and NAMI (Council Member).
References:
- Blatow M, Rozov A, Katona I, Hormuzdi SG, Meyer AH, Whittington MA, Caputi A, Monyer H, 2003. A novel network of multipolar bursting interneurons generates theta frequency oscillations in neocortex. Neuron 38, 805–817. 10.1016/S0896-6273(03)00300-3 [DOI] [PubMed] [Google Scholar]
- Clementz B. a., Keil A, Kissler J, 2004. Aberrant brain dynamics in schizophrenia: delayed buildup and prolonged decay of the visual steady-state response. Cogn Brain Res 18, 121–129. 10.1016/j.cogbrainres.2003.09.007 [DOI] [PubMed] [Google Scholar]
- Clementz B. a, Wang J, Keil A, 2008. Normal electrocortical facilitation but abnormal target identification during visual sustained attention in schizophrenia. J Neurosci 28, 13411–8. 10.1523/JNEUROSCI.4095-08.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementz BA, Sweeney JA, Hamm JP, Ivleva EI, Ethridge LE, Pearlson GD, Keshavan MS, Tamminga CA, 2016. Identification of distinct psychosis biotypes using brain-based biomarkers. Am J Psychiatry 173, 373–384. 10.1176/appi.ajp.2015.14091200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ethridge LE, Hamm JP, Pearlson GD, Tamminga CA, Sweeney JA, Keshavan MS, Clementz BA, 2015. Event-related potential and time-frequency endophenotypes for schizophrenia and psychotic bipolar disorder. Biol Psychiatry 77, 127–136. 10.1016/j.biopsych.2014.03.032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foss-Feig JH, Adkinson BD, Ji JL, Yang G, Srihari VH, McPartland JC, Krystal JH, Murray JD, Anticevic A, 2017. Searching for Cross-Diagnostic Convergence: Neural Mechanisms Governing Excitation and Inhibition Balance in Schizophrenia and Autism Spectrum Disorders. Biol Psychiatry 81, 848–861. 10.1016/j.biopsych.2017.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Burgos G, Lewis DA, 2012. NMDA receptor hypofunction, parvalbumin-positive neurons, and cortical gamma oscillations in schizophrenia. Schizophr Bull 38, 950–957. 10.1093/schbul/sbs010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Bobilev AM, Hayrynen LK, Hudgens-Haney ME, Oliver WT, Parker DA, McDowell JE, Buckley PA, Clementz BA, 2015. Stimulus train duration but not attention moderates Gamma-band entrainment abnormalities in schizophrenia. Schizophr Res 165, 97–102. 10.1016/j.schres.2015.02.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Ethridge LE, Boutros NN, Keshavan MS, Sweeney J. a, Pearlson GD, Tamminga C. a, Clementz B. a, 2014. Diagnostic specificity and familiality of early versus late evoked potentials to auditory paired stimuli across the schizophrenia-bipolar psychosis spectrum. Psychophysiology 51, 348–57. 10.1111/psyp.12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Clementz BA, 2012. Augmented gamma band auditory steady-state responses: Support for NMDA hypofunction in schizophrenia. Schizophr Res 138, 1–7. 10.1016/j.schres.2012.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Gilmore CS, Picchetti N. a M., Sponheim SR, Clementz B. a, 2011. Abnormalities of neuronal oscillations and temporal integration to low- and high-frequency auditory stimulation in schizophrenia. Biol Psychiatry 69, 989–96. 10.1016/j.biopsych.2010.11.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Peterka DS, Gogos JA, Yuste R, 2017. Altered Cortical Ensembles in Mouse Models of Schizophrenia. Neuron 94, 153–167.e8. 10.1016/j.neuron.2017.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm JP, Yuste R, 2016. Somatostatin Interneurons Control a Key Component of Mismatch Negativity in Mouse Visual Cortex. Cell Rep 16, 597–604. 10.1016/j.celrep.2016.06.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Bazmi HH, Mirnics K, Wu Q, Sampson AR, 2008. Conserved Regional Patterns of GABA-Related Transcript Expression in the Neocortex of Subjects With Schizophrenia. Am J Psychiatry 165(4), 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herdman AT, Lins O, Van Roon P, Stapells DR, Scherg M, Picton TW, 2002. Intracerebral sources of human auditory steady-state responses. Brain Topogr 15, 69–86. 10.1023/A:1021470822922 [DOI] [PubMed] [Google Scholar]
- Holm S, 1979. A Simple Sequentially Rejective Multiple Test Procedure. Scand J Stat 65–70. [Google Scholar]
- Isomura S, Onitsuka T, Tsuchimoto R, Nakamura I, Hirano S, Oda Y, Oribe N, Hirano Y, Ueno T, Kanba S, 2016. Differentiation between major depressive disorder and bipolar disorder by auditory steady-state responses. J Affect Disord 190, 800–806. 10.1016/j.jad.2015.11.034 [DOI] [PubMed] [Google Scholar]
- Jammalamadaka SR, SenGupta A, 2001. 3. Some Sampling Distributions, in: Topics in Circular Statistics. 10.1016/B978-0-12-146150-8.50013-X [DOI] [Google Scholar]
- Javitt DC, Sweet RA, 2015. Auditory dysfunction in schizophrenia: integrating clinical and basic features. Nat Rev Neurosci 16, 535–550. 10.1038/nrn4002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John MS, Lins OG, Boucher BL, Picton TW, 1998. Multiple auditory steady-state responses (MASTER): stimulus and recording parameters. Audiology 37 (2), 59–82. [DOI] [PubMed] [Google Scholar]
- Kim T, Thankachan S, McKenna JT, McNally JM, Yang C, Choi JH, Chen L, Kocsis B, Deisseroth K, Strecker RE, Basheer R, Brown RE, M. R, 2015. Cortically projecting basal forebrain parvalbumin neurons regulate cortical gamma band oscillations. Proc Natl Acad Sci 112, E2848–E2848. 10.1073/pnas.1507465112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopell N, Ermentrout GB, Whittington MA, Traub RD, 2000. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci U S A 97, 1867–1872. 10.1073/pnas.97.4.1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korczak P, Smart J, Delgado R, Strobel TM, Bradford C, 2012. Auditory Steady-State Responses. J Am Acad Audiol 23, 146–170. 10.3766/jaaa.23.3.3 [DOI] [PubMed] [Google Scholar]
- Lambert Z, Wildt A, Durand RM, 1988. Redundancy analysis: An alternative to canonical correlation and multivariate multiple regression in exploring interset associations. Psychol Bull. 10.1037/0033-2909.104.2.282 [DOI] [Google Scholar]
- Moratti S, Clementz B. a, Gao Y, Ortiz T, Keil A, 2007. Neural mechanisms of evoked oscillations: stability and interaction with transient events. Hum Brain Mapp 28, 1318–33. 10.1002/hbm.20342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donnell BF, Vohs JL, Krishnan GP, Rass O, Hetrick WP, M. S, 2013. The auditory steady-state response (ASSR): a translational biomarker for schizophrenia. Suppl Clin Neurophysiol 62, 101–12. 10.1530/ERC-14-0411.Persistent [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda Y, Onitsuka T, Tsuchimoto R, Hirano S, Oribe N, Ueno T, Hirano Y, Nakamura I, Miura T, Kanba S, 2012. Gamma band neural synchronization deficits for auditory steady state responses in bipolar disorder patients. PLoS One 7, 1–8. 10.1371/journal.pone.0039955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, John MS, Dimitrijevic A, Purcell D, 2003. Human auditory steady-state responses: Respuestas auditivas de estado estable en humanos. Int J Audiol 42, 177–219. 10.3109/14992020309101316 [DOI] [PubMed] [Google Scholar]
- Rass O, Forsyth JK, Krishnan GP, Hetrick WP, Klaunig MJ, Breier A, O’Donnell BF, Brenner CA, 2012. Auditory steady state response in the schizophrenia, first-degree relatives, and schizotypal personality disorder. Schizophr Res 136, 143–149. 10.1016/j.schres.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rass O, Krishnan G, Brenner CA, Hetrick WP, Merrill CC, Shekhar A, O’Donnell BF, 2010. Auditory steady state response in bipolar disorder: Relation to clinical state, cognitive performance, medication status, and substance disorders. Bipolar Disord 12, 793–803. 10.1111/j.1399-5618.2010.00871.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolls ET, Loh M, Deco G, Winterer G, 2008. Computational models of schizophrenia and dopamine modulation in the prefrontal cortex. Nat Rev Neurosci 9, 696–709. 10.1038/nrn2462 [DOI] [PubMed] [Google Scholar]
- Rosburg T, Boutros NN, Ford JM, 2008. Reduced auditory evoked potential component N100 in schizophrenia - A critical review. Psychiatry Res 161, 259–274. 10.1016/j.psychres.2008.03.017 [DOI] [PubMed] [Google Scholar]
- Sivarao DV, 2015. The 40-Hz auditory steady-state response: A selective biomarker for cortical NMDA function. Ann N Y Acad Sci 1344, 27–36. 10.1111/nyas.12739 [DOI] [PubMed] [Google Scholar]
- Sivarao DV, Chen P, Senapati A, Yang Y, Fernandes A, Benitex Y, Whiterock V, Li YW, Ahlijanian MK, 2016. 40 Hz Auditory Steady-State Response Is a Pharmacodynamic Biomarker for Cortical NMDA Receptors. Neuropsychopharmacology 10.1038/npp.2016.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohal VS, Zhang F, Yizhar O, Deisseroth K, 2009. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 459, 1–5. 10.1038/nature07991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Salisbury DF, Shenton ME, McCarley RW, 2008. Gamma-Band Auditory Steady-State Responses Are Impaired in First Episode Psychosis. Biol Psychiatry 64, 369–375. 10.1016/j.biopsych.2008.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz M. a, Nestor PG, Shenton ME, McCarley RW, 2009. Left auditory cortex gamma synchronization and auditory hallucination symptoms in schizophrenia. BMC Neurosci 10, 85 10.1186/1471-2202-10-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tada M, Nagai T, Kirihara K, Koike S, Suga M, Araki T, Kobayashi T, Kasai K, 2016. Differential Alterations of Auditory Gamma Oscillatory Responses between Pre-Onset High-Risk Individuals and First-Episode Schizophrenia. Cereb Cortex 26, 1027–1035. 10.1093/cercor/bhu278 [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Ivleva EI, Keshavan MS, Pearlson GD, Clementz BA, Witte B, Morris DW, Bishop J, Thaker GK, Sweeney JA, 2013. Clinical Phenotypes of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). Am J Psychiatry 170, 1263–1274. 10.1176/appi.ajp.2013.12101339 [DOI] [PubMed] [Google Scholar]
- Thuné H, Recasens M, Uhlhaas PJ, 2016. The 40-Hz Auditory Steady-State Response in Patients With Schizophrenia. JAMA Psychiatry 73, 1145–1153. 10.1001/jamapsychiatry.2016.2619 [DOI] [PubMed] [Google Scholar]
- Tsuchimoto R, Kanba S, Hirano S, Oribe N, Ueno T, Hirano Y, Nakamura I, Oda Y, Miura T, Onitsuka T, 2011. Reduced high and low frequency gamma synchronization in patients with chronic schizophrenia. Schizophr Res 133, 99–105. 10.1016/j.schres.2011.07.020 [DOI] [PubMed] [Google Scholar]
- Veit J, Hakim R, Jadi MP, Sejnowski TJ, Adesnik H, 2017. Cortical gamma band synchronization through somatostatin interneurons. Nat Neurosci 20, 951–959. 10.1038/nn.4562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jia Y, Feng Y, Zhong S, Xie Y, Wang W, Guan Y, Zhu D, Huang L, 2014. Overlapping auditory M100 and M200 abnormalities in schizophrenia and bipolar disorder: A MEG study. Schizophr Res 160, 201–207. 10.1016/j.schres.2014.10.042 [DOI] [PubMed] [Google Scholar]
- Womelsdorf T, Valiante T. a, Sahin NT, Miller KJ, Tiesinga P, 2014. Dynamic circuit motifs underlying rhythmic gain control, gating and integration. Nat Neurosci 17, 1031–9. 10.1038/nn.3764 [DOI] [PubMed] [Google Scholar]
- Zhou T, Mueller NE, Spencer KM, Mallya SG, Eve K, Norris LA, Levy DL, Cohen BM, Öngür D, Hall M, 2018. Auditory steady state response de fi cits are associated with symptom severity and poor functioning in patients with psychotic disorder. Schizophr Res 10.1016/j.schres.2018.05.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.