Abstract
Identifying the needs of dementia caregivers is critical for supporting dementia home care. This study identified a typology of expert interventions delivered to dementia caregivers during an innovative telehealth trial that used in-home video recordings to directly observe care challenges. Qualitative content analysis was used to analyze narrative notes describing interventions that were developed based on video data submitted by 33 caregiver–care recipient dyads. Two major themes emerged: education and skills for dementia care and caregiver support. Ten subthemes included education and skills related to: behavioral and psychological symptoms of dementia, disease expectations, safety, activities of daily living, medical care optimization, and medication utilization and caregiver support related to: respite, positive reinforcement, social and financial support, and self-care. Families providing in-home dementia care experience a wide range of care challenges. By using video data, dementia care experts were able to witness and evaluate challenging care situations and provide individualized feedback.
Keywords: Behavioral Symptoms, Alzheimer Disease, Telemedicine
Enhancing in-home care has become a priority to support persons living with dementia (PLWD) and their family caregivers (Borson et al., 2016; U.S. Department of Health and Human Services, 2016). Aiding family caregivers in managing challenging care situations, particularly behavioral and psychological symptoms of dementia, is essential to support families in caring for PLWD at home, reducing premature nursing home placement (Balestreri, Grossberg, & Grossberg, 2000; Kunik et al., 2010). However, deciphering dementia behaviors can be difficult for caregivers due to the complexity of assessment and reduced ability of PLWD to communicate their needs (Kunik et al., 2010; Algase et al., 1996). Guidance from healthcare professionals is necessary to help caregivers face challenges including learning new skills to manage challenging care situations and behavioral and psychological symptoms that are common, progressive and changing in dementia (Fick, Kolanowski, Waller, & Inouye, 2005; Moniz‐Cook et al., 2008; Woods & Clare, 2008).
Technology Supported Dementia Care Guidance
Worldwide, cost-effective dementia care that supports quality of life is considered a public health priority (World Health Organization [WHO], 2012). The population of PLWD is projected to expand from 47 million in 2015 to 132 million by 2050 (Prince, Comas-Herrera, Knapp, Guerchet, & Karagiannidou, 2016). In the U.S. alone this will increase the annual costs of dementia care from $277 billion to $1.1 trillion with similar financial strain reported globally (Alzheimer’s Association [AA], 2018; Prince et al., 2016).
Avoiding premature nursing home care is essential if society is to meet the care needs of a rapidly expanding aging population with dementia. In 2017, there were approximately 15.9 million unpaid in-home dementia caregivers, who provided an estimated 18.4 billion hours of care, saving the United States $232 billion in healthcare costs (AA, 2018). However, in-home caregiving is not without personal and financial costs. Family caregivers of PLWD must cope with the care recipient’s progressive memory loss, self-care impairment, and communication breakdown, which may lead to caregiver depression, insomnia, psychotropic medication use, and increased morbidity and mortality (Monin & Schulz, 2009; Talley & Montgomery, 2013). The stress, strain, and burden of dementia caregiving can contribute to negative physical and mental health outcomes of informal caregivers which further contribute to healthcare costs (AA, 2018). Specifically, informal caregivers who are married, care for someone with dementia, and provide care in the presence of behavioral and psychological symptoms of dementia have significantly higher rates of depression, anxiety and/or insomnia (Vaingankar et al., 2016).
The Need-Driven Dementia Compromised Behavior Model has been used extensively to explain how fixed and modifiable factors contribute to challenging dementia behaviors (Algase et al., 1996; Kovach, Noonan, Schlidt, & Wells, 2005). The more recent conceptual model of factors associated with behavioral and psychological symptoms of dementia expands the focus from just PLWD needs to also include caregiver and environmental factors and posits the need for individualized interventions based on the dyad and the environment (Kales, Gitlin, & Lyketsos, 2015). However, understanding and deciphering challenging behaviors and eliciting individualized approaches is complex and requires professional expertise. Family caregivers interpret behavioral symptoms that present as resistiveness to care with fear, uncertainty, negative emotions, and physical health symptoms (Spigelmyer, Hupcey, Smith, Loeb, & Kitko, 2018; Shirai & Koerner, 2018) and are often unaware of the meaning of nonverbal behavior (Fuchs-Lacelle, Hadjistavropoulos, & Lix, 2008). Instead of understanding and addressing contributing factors to challenging behaviors, they may treat the behavior itself, such as using sedation to reduce agitation, which may result in negative secondary effects (Bradford et al., 2012). Family caregivers and persons in early stage dementia attest that managing behavioral symptoms without sedating medication and with adequate caregiver support is a priority goal for dementia care (Jennings et al., 2017).
Currently caregivers must report challenging behaviors retrospectively to care providers to receive care guidance. Yet, caregivers may be unaware of how symptoms are manifested in PLWD and forget or fail to observe specific details that are important in identifying precipitating factors. Dementia care professionals are therefore limited in providing the appropriate individualized interventions because important contextual and precipitating factors remain unknown. To ensure tailored and individualized guidance, providers must have an accurate understanding of the care challenges caregivers are experiencing at home. Innovative technologies, particularly telehealth, increase the potential for supporting caregivers to keep PLWD in the community (Godwin, Mills, Anderson, & Kunik, 2013; Bossen, Kim, Williams, Steinhoff, & Strieker, 2015). Caregivers of older adults have identified the desire to use technology as a resource to assist in caregiving, specifically identifying the need for technologies that allow personalized professional consultation and guidance for providing care (American Association of Retired Persons [AARP], 2016).
Supporting Family Caregivers for Dementia In-home Care Clinical Trial
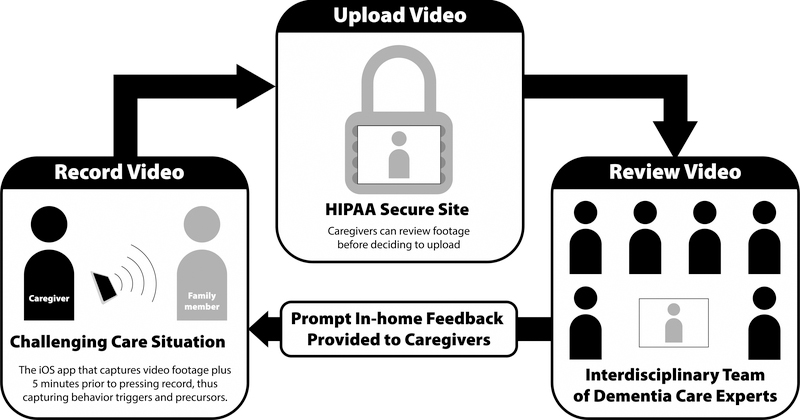
The Supporting Family Caregivers for Dementia Care clinical trial (FamTechCare) evaluates the effects of an innovative telehealth intervention on caregiver well-being and PLWD behaviors. The FamTechCare intervention removes the barrier of retrospective recall by allowing dementia care experts to directly view challenging care situations through in-home video-recording. In addition, rich contextual details (i.e. environmental factors such as noise or clutter) are apparent in video recordings. In the FamTechCare trial, caregivers video-record care situations using an application on an iPad mini. The video-recording application has a buffering component that captures antecedent events by saving retrospective video-data once triggered to record prospectively. The caregiver then uploads the video to a HIPPA-secure website for review by dementia care experts. The interdisciplinary team of experts review the videos and suggest tailored interventions for the specific caregiver-PLWD dyad. The interventions are then provided to the caregiver in a weekly phone call with the research interventionist (Figure 1). In the FamTechCare trial, caregiver-PLWD dyads are randomized to receive either a 12-week video-recording intervention with expert feedback (i.e. experimental group) or a 12-week attention-control intervention based on the traditional retrospective recall without video support (i.e. control group). See Williams et al. (2018) for complete research protocol.
Figure 1.
Study Procedure
Graphic Design by Chris Lorenzen © 2016.
The FamTechCare trial provides a unique and valuable perspective into dementia homecare. Although many telehealth interventions exist to provide support to family caregivers of both persons living with and without dementia (Chi & Demiris, 2015; Waller, Dilworth, Mansfield, & Sanson-Fisher, 2017), the FamTechCare trial is the first to capture in-home video-recordings of challenging care situations to provide tailored feedback for care guidance. Previous telehealth interventions include video or phone conferencing for caregiver support or in-home telemonitoring for safety (Chi & Demiris, 2015; Waller et al., 2017). Although these interventions are important for improving access to caregiver support, they cannot specifically address the barrier of retrospective recall and provide contextually-tailored interventions to guide care and reduce negative caregiver outcomes.
Purpose
The purpose of this qualitative content analysis is to identify a typology of caregiver needs reflected in expert review of video-recordings developed during the FamTechCare trial (R01NR014737). This analysis reports on one aim of the parent-study: to develop a typology of interventions for caregivers based on the unique video-data (i.e. video data from the experimental group). Understanding the caregiver needs related to technological support and expert interventions based on in-home video data is valuable for both clinicians and researchers and can provide new direction for optimizing dementia care.
Methods
Design
Qualitative content analysis was used to develop a typology of in-home dementia expert interventions using study narrative notes (Hsieh & Shannon, 2005; Elo & Kyngäs, 2008). Content analysis was selected as the method to analyze narrative notes in order to enhance understanding and identify shared meaning in the types of interventions provided to in-home dementia caregivers by dementia care experts. All study procedures were IRB approved.
Participants/Sampling
The FamTechCare trial included in-home dementia caregivers. Persons living with dementia were included if they had an actual or probable diagnosis of dementia and excluded if they had a diagnosis of Huntington’s disease, schizophrenia, manic-depressive disorder, deafness, or intellectual disability. Caregivers were included if they provided care to the PLWD on a weekly basis. The PLWD may have had more than one caregiver enroll in the trial, including paraprofessional caregivers who provide regular in-home care. The current analysis included caregiver-PLWD dyads randomly assigned to the experimental (FamTechCare) group, who were enrolled from July 2014 to August 2017. Informed consent was obtained from all participants or their surrogate decision makers and assent was obtained from PLWD who were unable to consent due to cognitive impairment. See protocol for specific details on recruitment, eligibility, the protection of human subjects, and the FamTechCare intervention (Williams et al., 2018).
Data collection
Narrative notes
The interventions provided to caregivers were documented in an electronic video-review form stored in a secured research database (REDCap). The form includes specific fields for a narrative description of the current problems reported by the caregiver and a field for the summary of the suggested interventions made by the interventionist. The interventionist, either a graduate trained nurse, social worker, or clinical psychologist, interacted with the caregiver over the 12-week study period and provided the interventions developed by the expert team who had backgrounds in nursing, geriatric psychiatry, social work, and psychology as well as expertise in dementia care. Other professionals such as geriatric dentistry and occupational therapy were consulted as needed. In the multi-caregiver dyads, separate calls were completed and dyad-specific narrative notes were recorded for each caregiver-PLWD dyad.
Procedure for Typology Development and Analysis
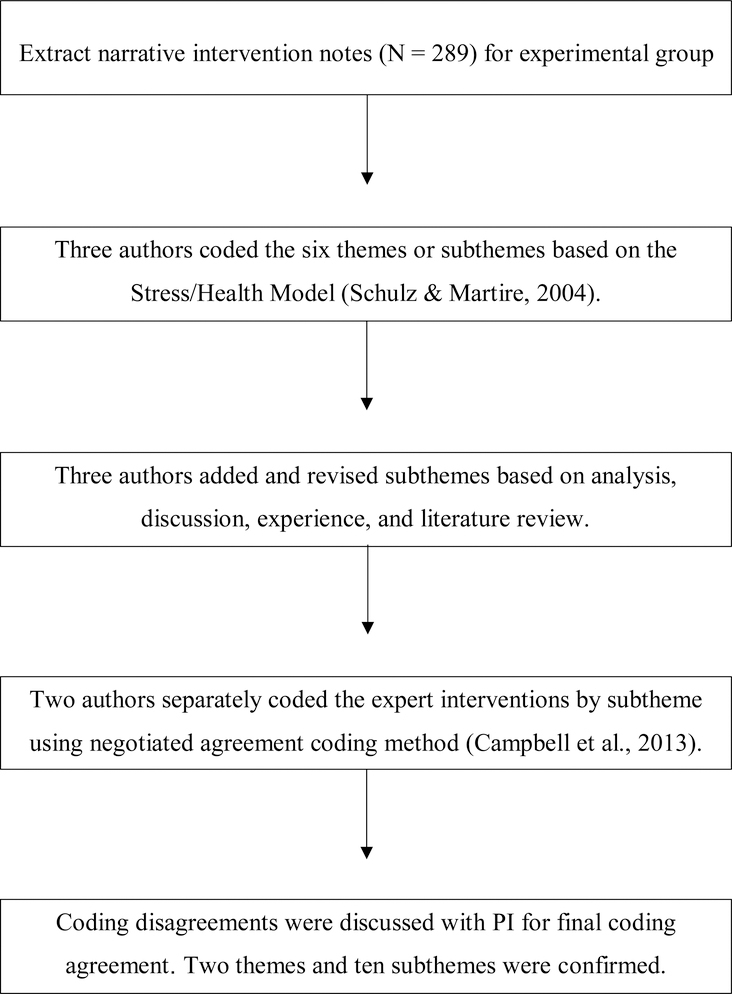
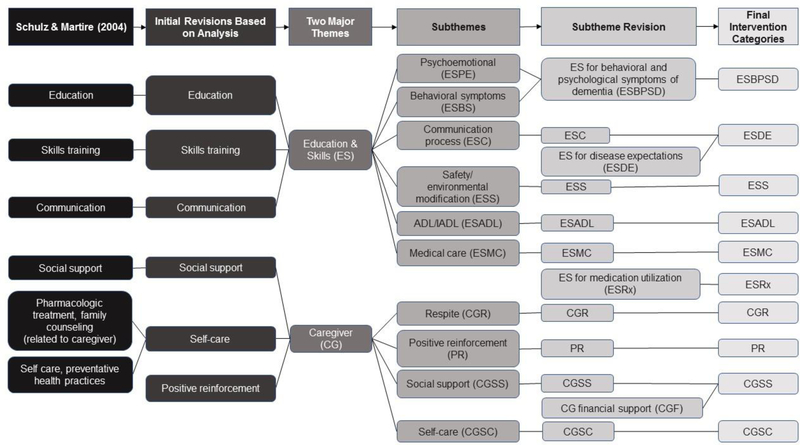
To guide initial typology development, the six intervention categories identified in the Stress/Health Model for Caregiving were applied by hand to the narrative notes by three researchers (Schulz & Martire, 2004). The “Stress/Health Process” intervention categories include pharmacologic treatment/family counseling, social support, education, skills training, self-care/preventative health practices, and communication which impact secondary stressors, appraisal of demands and adaptive capacities, and emotional/behavioral response. The initial deductive approach of applying the Stress/Health Model for Caregiving was chosen to make sense of the data through established caregiving intervention themes (Elo & Kyngäs, 2008). The application of this model did not fully represent our data, however it represented the two major themes related to education and skills for dementia care and caregiver support. To develop subthemes, an inductive and iterative process commenced with the three researchers hand-coding each narrative note and meeting weekly to discuss subthemes. Researchers independently developed generic categories of general descriptions while coding and then came together and refined categories into detailed subthemes. As the codebook for the expert interventions evolved, subthemes were added, revised, or deleted to fully explain different subthemes of the interventions. For example, when there was significant redundancy or overlap, subthemes were combined into one subtheme and the operational definition was revised. The typology development process is shown in Figure 2. Figure 3 documents the evolution of the codebook.
Figure 2.
Flow Chart of Typology Development Process
Figure 3.
Codebook Creation Process
Once the typology was created, each narrative note was reanalyzed to quantify each typology subtheme. Two trained graduate-research assistants hand-coded all the narrative notes using a negotiated agreement coding method (Campbell, Quincy, Osserman, & Pedersen, 2013). All coding disagreements were highlighted and discussed with the study PI to achieve final coding agreement. In the quantification coding, there was no limit to the number of intervention subthemes coded for each note. However, each individual code was only counted once per week. Thus, the coding of interventions does not reflect the depth or dose of the expert interventions, but instead reflects an overview of the types of interventions provided to the caregivers based on in-home video data. In multi-caregiver dyads each caregiver’s interventions were counted separately because each caregiver received personalized interventions that varied based on their unique interaction with the PWD. Narrative notes were organized in Microsoft Excel to apply and quantify the subthemes. Certain weeks were coded as no contact if the telehealth team was unable to reach the caregiver.
Results
Narrative notes based on videos submitted by 33 caregiver-PLWD dyads were included (n = 33 caregivers; n = 27 PLWD). Among the 33 caregivers, participants were mostly female (n = 24; 72.7%), spouses (n = 21; 63.7%) or daughters (n = 4, 12.1%) of the PLWD, and non-Hispanic white (n = 27; 81.8%). Over half (n = 19; 57.6%) had less than a bachelor’s degree. The mean age was 63.8 years (SD = 11.8; range = 32 – 81 years). Caregivers reported an average of 4.0 years (SD = 2.9) as a caregiver.
The PLWD participants were primarily male (n = 16; 59.2%), non-Hispanic white (n = 25; 92.6%), with an average age of 74.9 years (SD = 9.3). Alzheimer’s disease versus other types of dementia (n = 14; 51.9%) was the most common with diagnosis and PLWD were diagnosed for an average of 4.5 years (SD = 3.2). Using the Functional Assessment Staging (FAST) most participants were staged as moderately severe dementia (n=13; 48.2%) (Reisberg, 2007; Sclan & Reisberg, 1992). Additional demographic is provided in Table 1.
Table 1.
Demographics of Caregiver and Persons Living with Dementia
| Caregivers (N = 33) | PLWD (N = 27) | |
|---|---|---|
| n(%) | n(%) | |
| Gender | ||
| Female | 24(72.7) | 11(40.7) |
| Male | 9(27.3) | 16(59.3) |
| Race/Ethnicity | ||
| Non-Hispanic White | 27(81.8) | 25(92.6) |
| Non-Hispanic Black | 6(18.2) | 2(7.4) |
| Education | ||
| Less than bachelor’s degree | 19(57.6) | 17(63.0) |
| Bachelor’s degree | 9(27.3) | 4(14.8) |
| Master’s degree or higher | 5(15.1) | 6(22.2) |
| Relationship to PLWD | ||
| Spouse | 21(63.7) | |
| Daughter | 4(12.1) | |
| Paid caregiver | 4(12.1) | |
| Son | 2(6.1) | |
| Daughter in law | 1(3.0) | |
| Sister | 1(3.0) | |
| Type of dementiaa | ||
| Alzheimer’s disease | 14(51.9) | |
| Lewy body | 5(18.5) | |
| Parkinson’s disease | 3(11.1) | |
| Fronto-temporal | 3(11.1) | |
| Vascular | 2(7.4) | |
| Unknown | 2(7.4) | |
| Aphasic | 1(3.7) | |
| Stage of Dementia (FAST) | ||
| Mild | 9(33.3) | |
| Moderate | 5(18.5) | |
| Moderately Severe | 13(48.2) | |
Note. FAST = functional assessment staging; PLWD = persons living with dementia.
Some subjects have more than 1 type of dementia, therefore percentages is over 100% due to rounding.
The description of major themes and subthemes is shown in Table 2. The final codes belonged to one of two related major themes: (1) education and skills for caring for a PLWD, and (2) caregiver support. Within these two major themes, ten subthemes emerged. The typology was developed from 289 narrative notes, representing the 289 phone calls to the 33 experimental group caregivers. On average, each caregiver received 8.8 phone calls (SD = 2.6) over the 12-week trial. The mean duration of these intervention calls was 16.6 minutes (SD = 12.1 minutes). A total of 372 intervention topics representing the ten subthemes were provided to the experimental group caregivers. Below is a description of each subtheme which includes an example of a video and the expert interventions based on video review. Each video may have produced multiple suggested interventions, but only the specific intervention related to the subtheme is included in the description.
Table 2.
Expert Interventions Themes
| Expert Interventions (N = 372) |
||
|---|---|---|
| n(%) | Rank | |
| Major theme: Education & Skills… | ||
| Subtheme | ||
| For managing behavioral and psychological symptoms of dementia | 70 (18.8) | 1 |
| For greater understanding of dementia disease expectations | 49 (13.2) | 2 |
| For improving safety and creating a safe environment | 43 (11.6) | 3 |
| For improving performance of activities of daily living and instrumental activities of daily living | 39 (10.5) | 4 |
| For enhancing use of medical care | 38 (10.2) | 5a |
| For more effective medication utilization | 20 (5.4) | 9 |
| Major theme: Caregiver… | ||
| Subtheme | ||
| Respite | 38 (10.2) | 5a |
| Social and financial support | 24 (6.4) | 8 |
| Self-care | 17 (4.6) | 10 |
| Positive reinforcement | 34 (9.1) | 7 |
Note.
Tie Rank.
Education and Skills
This major theme referred to educational interventions and skills training that related to one of the six identified subthemes that guides the caregiver in caring for the PLWD.
Education and Skills for Managing Behavioral and Psychological Symptoms of Dementia
Behavioral and psychological symptoms of dementia (BPSD) were defined as agitation, aggression, apathy, anxiety, delusions, depression, disinhibition, hallucinations, irritability, motor disturbances, or nighttime wakefulness exhibited by the PLWD. Interventions to manage BPSD are based on assessing and understanding the PLWD, caregiver, and environmental factors (Kales et al., 2015) to create approaches around altering specific behaviors (Kales, Gitlin, & Lyketsos, 2014). Discussion about the use of meaningful activities was also common in this subtheme.
Video of caregiver babysitting grandchildren at home while PLWD present, PLWD appears agitated: “Discussed keying into his [PLWD] anxiety and separating him from them [grandchildren and PLWD] at these times. When you [caregiver] notice agitation, provide quiet distraction and remove him from the area.”
Education and Skills Regarding Disease Expectations
Although all typology subthemes have some component of disease expectations, this subtheme specifically focuses on education and skills related to memory loss. Caregivers were often unaware of dementia’s progressive trajectory and expressed uncertainty over the PLWD increasing forgetfulness, difficulty to follow directions, and increasing functional decline. The expert team frequently advised caregivers that it was necessary to prompt the PLWD, instead of the PLWD remembering themselves, and that care activities should be broken into simple steps. This was evident in the video-recordings with caregivers asking the PLWD complex questions or quizzing them on family and past life events. Frequently caregivers would ask the PLWD “why” questions on the videos, such as, “Why did you just do that?” Often the PLWD would appear perplexed and occasionally agitated if the caregiver persisted with this type of questioning.
Video of caregiver and PLWD eating dinner, caregiver appears frustrated when PLWD cannot follow conversation and answer questions: “Discussed how husband has decreased ability to remember. Keep things simple. Repetitive questions/topics are expected. He really can’t remember.”
Education and Skills for Improved Safety
This subtheme refers to improving PLWD safety. In-home safety issues were often noted by the expert team based on the video recordings. These were often related to the physical environment or the caregiver-PLWD routines.
Video continues running after caregiver and PLWD finished dinner, caregiver leaves room to complete housework and video captures PLWD going outside and smoking without caregiver’s knowledge: “Consider smoking safety, recommended she [caregiver] supervise him, concerned he could start fire or wander off if unsupervised. Check into getting ID bracelet.”
Education and Skills for Improved Performance of Activities of Daily Living (ADLs) and Instrumental Activities of Daily Living (IADLs)
Caregivers frequently described caregiving burden related to ADL and IADL care for the PLWD. Video recordings included a variety challenges related to mealtimes, medication administration, oral hygiene, daily care, and dressing.
Video of PLWD putting shoes and button-up sweater on, PLWD tries to put sweater on inside-out, doing well with buttons but going slow: “Create new normal, plan for getting dressed to take longer in morning. Know which pieces are difficult for PLWD. As disease progresses consider easier clothing without buttons and zippers.”
Education and Skills for Improved Medical Care Utilization (ESMC)
While previous subthemes focus on behavioral interventions and education, this subtheme included situations in which the expert team advised the caregiver to seek professional medical care for the PLWD. Caregivers struggled with navigating the healthcare system and were unaware of special consultations that may be helpful (e.g., occupational or speech therapy). Video data also identified acute medical problems that the caregivers considered expected, but that indicated changes in condition warranting medical evaluation.
Video of PLWD and caregiver talking, PLWD has near syncopal episode, falls to side, and is able to catch self on wall: “Consider taking him [PLWD] to see his health care provider due to dizziness.”
Education and Skills Related to Medication Utilization (ESRx)
The telehealth team noticed that caregivers often lacked knowledge about prescribed medications and how to optimize their use. Many caregivers were unable to explain medication purposes, when the medications were needed, and what dose to administer if needed. Since many of the prescriptions were psychotropic and as-needed (PRN), the expert team educated caregivers on when the medications were appropriate to be given, what to watch for, and when to contact medical providers.
Video of PLWD tremors [Parkinson’s related]: “Keep real-time log of symptom occurrence. Contact healthcare provider after the two-week trial of discontinuing Galantamine is over. Note if tremors increase with anxiety. Note if Lorazepam use impacts tremors.”
Caregiver Support
The second major theme was caregiver support, which was defined as expert interventions related to any intervention to improve caregiver wellbeing. Although caregiver frustration or fatigue was sometimes observed in the videos, caregiver specific support needs were also determined based on the weekly phone conversation.
Caregiver Respite
Caregivers often stated that they wanted to use formal respite services, but also expressed fears and guilt in initiating these services. The telehealth team frequently encouraged caregivers to utilize respite, identified available community resources, discussed how to make the transition, and presented respite in general planning for the future.
Video of caregiver and PLWD eating dinner, caregiver verbalizing stress related to all of the things she wants to accomplish: “Encouraged caregiver to consider respite help. Discussed respite help through [local agencies].”
Positive reinforcement
Interventions in this subtheme are based on providing caregivers positive reinforcement about their caregiving to build rapport, trust, and reinforce positive behavior. For example, when a caregiver engaged in meaningful activities with the PLWD the experts affirmed and reinforced the value of that caregiving behavior.
Video of caregiver redirecting PLWD after he asks her about the boat they no longer own: “Good job being patient, waiting for response, and redirecting him [PLWD].”
Caregiver Social and Financial Support
Social support included suggestions to caregivers to attend support groups or seek the support of peers and family. Additionally, although less common, this subtheme included seeking financial support that the PLWD may qualify, including governmental programs.
Video of PLWD eating dinner with spouse, daughter, and grandchild, PLWD having difficulty eating with few verbal responses; during the weekly phone call the caregiver expresses guilt about considering long-term care due to PLWD continuing decline: “Encouraged using children for social support while considering difficult decisions like long term care placement.”
Caregiver Self-Care
This general self-care subtheme is for other caregiver support interventions that are not included in social support or respite. Examples include discussions of taking time for self, participating in activities outside of caregiving, and brainstorming supportive activities like journaling or exercise.
Video of caregiver helping PLWD during dinner, pleasantly discussing day where caregiver completed housework and tasks related to caregiving: “Encouraged caregiver to set goals to do things for self, for example attending the card game day she [caregiver] likes during the last week of April.”
Frequencies of Subthemes
A total of 372 expert interventions were provided to the 33 caregivers who received the intervention based on the video-data over the 12-week study period (Table 2). The top three subthemes for interventions were: 1) education and skills for managing behavioral and psychological symptoms of dementia (n = 70, 18.8%); 2) education and skills for greater understanding of disease expectations (n = 49, 13.2%); 3) education and skills for improving safety and creating a safe environment (n = 43, 11.6%).
Discussion
This study identified a typology of dementia family caregiver support interventions specific to a telehealth intervention. Data triangulation, integrating data derived from videos submitted by caregivers and interventions developed by experts enabled us to discover issues that caregivers may not self-report. Overall, the typology reflects traditional intervention frameworks with a few key differences (Jennings et al., 2017; Schulz & Martire, 2004; Toot, Hoe, Ledgerd, Burnell, Devine, & Orrell, 2013). The primary focus of the typology is on needs for support with education and skills: to manage behavioral symptoms, provide activities of daily living, achieve a realistic expectation for disease progression, and support caregivers themselves. The typology notably aligns with the recent recommendations for Families Caring for an Aging America (National Academies of Sciences, Engineering, and Medicine, 2016). Based on video data that effectively linked the experts to observations in the home, the FamTechCare typology identified additional caregiver needs for support related to safety, medication utilization, ongoing support in achieving realistic disease-related expectations as dementia progresses, and provision of positive reinforcement. This typology is unique in that it portrays how interdisciplinary providers can use in-home video data for contextual observations to provide individualized interventions to families in the home setting.
As the population with dementia expands and caregiving becomes recognized as a growing public health crisis, it is important to use all available tools to assist caregivers and minimize negative effects of caregiving (Talley & Crews, 2007). The FamTechCare intervention responds to each caregiver’s self-identified care challenges to meet their needs using available technologies that caregivers desire (AARP, 2016). Research testing outcomes of technology use for PLWD and their caregivers is needed, and concerns about technological naivety, privacy, and trust issues must be addressed to reach the full potential for technological support. FamTechCare effectively links caregivers to dementia experts in the home by providing direct observation of care situations, communication, and environmental concerns and provides caregivers with individualized contextually-based interventions.
The need to assist caregivers to achieve realistic perceptions and expectations for PLWD related to progressive cognitive decline may reflect the fact that caregivers may be overwhelmed at the time of diagnosis when they receive information regarding the dementia disease process (Pozzebon, Douglas, & Ames, 2016; Robinson, Clare, & Evans, 2005). In addition, we found that caregivers needed updated information related to expectations as new and different care challenges occurred. Although other frameworks for caregiver support do not specifically identify disease expectations as a focus, we assigned a category because caregivers consistently exhibited a knowledge deficit of hallmark symptoms of dementia. In addition, caregivers may need help in applying expectations to actual ADL and IADL activities. These findings support the work of others endeavoring to provide support to family caregivers with traditional interventions (Gitlin & Hodgson, 2015; Schulz & Martire, 2004; Talley & Montgomery, 2013).
Education and skills related to safety was identified as the third most suggested expert intervention. The insidious onset and progression of dementia may leave caregivers unaware of developing safety issues, which related to a lack of understanding of disease expectations may contribute to significant safety concerns. Providers who directly observed the in-home situation often noted needed safety and environmental modifications that were not the caregivers intended focus for the video. Further research should focus on safety interventions in dementia care, especially considering statistics reporting high fall rates in PLWD that frequently necessitate hospitalization (Rudolph et al., 2010; Meuleners & Hobday, 2017).
Interventions related to education and skills surrounding medical care utilization were frequent enough to warrant designation as a subtheme (10.2%). Interventions in this area alerted the caregiver of the need to consult the PLWD’ healthcare provider. We found that some caregivers needed assistance in determining when care adjustments were needed. The same was true for use of and adjustment of medications (5.4%). Caregivers were typically unaware of medication management and optimization. Because participation in the study was not intended to replace medical care, caregivers were referred to their health care provider for medical and medication needs.
Our typology is congruent with advancing theories for dementia caregiver support that advocate individualized interventions for specific dyads and expand the focus of support to caregivers themselves (AARP, 2016; Jennings et al., 2016; Kales et al., 2015; Spigelmyer et al., 2018). Our findings agree with current research, in which caregivers report that reduced attention to physical and mental self-care and limitations on their social contribute to their caregiving distress, and are partially relieved by respite services (Roberts & Struckmeyer, 2018; Spigelmyer et al., 2018; Wang, Liu, Robinson, Shawler, & Zhou, 2018). Positive reinforcement for caregiver performance has also been emphasized in the research literature. It has been reported that positive reinforcement improves caregiver confidence and promotes coping to relieve caregiving stress (Stockwell‐Smith, Moyle, & Kellett, 2018). Given the current evidence on caregiver support, future interventions targeting specific needs of individual caregivers are essential.
Dementia care experts also integrated caregiver support interventions (i.e. respite, self-care, and social support) frequently (21.2%). The need to focus on the well-being and needs of the caregiver is reflected by statistics about the stress and burden of dementia caregiving, including evidence of increased morbidity for caregivers such as clinical depression in approximately 50% of this population (AA, 2018). Positive reinforcement was also a new intervention category identified in the FamTechCare typology. Being observed and receiving a positive evaluation by dementia care experts was well received by caregivers, particularly validating their skills and strengths in meeting care challenges. We found positive reinforcement essential in this study to build rapport and trust so that caregivers took the risk of submitting videos displaying their care skills that could have been criticized. This strength-reinforcing approach was helpful in establishing a trusting relationship and for crediting caregivers with their hard work and their positive approaches to providing care.
A number of limitations of this study should be addressed in ongoing research. Our sample included only those caregivers and their care recipient partners who volunteered to participate in the trial and who were agreeable to submitting videos of in-home care challenges. Caregivers selected the videos to record and upload and may have failed to share videos of extremely uncomfortable situations that may have limited needed caregiver interventions. The sample may not represent dementia caregivers overall. The sample had limited diversity so that cultural and ethnic factors that influence the caregiving experience were not evaluated (Knight & Sayegh, 2010). Additionally, this study focused on videos of challenging care situations, future research should collect and analyze videos of positive care interactions in order to gain insight into practical strategies for dementia care.
In addition, multiple health expert interventionists worked with the caregivers in this study, and their individual differences and detail in documentation of research notes may have varied from day-to-day and between interventionists. Although the interventionists varied in their disciplinary backgrounds, they were all reporting interventions as suggested by the expert team based on video-review. Thus, difference in disciplines should be minimized. Interventions addressing issues such as behavioral symptoms frequently included multifaceted interventions and our coding and quantification only counted bundled suggestions only once, thus potentially discounting the dose of interventions provided. This process may have resulted in an underestimation of the interventions provided. However, the alternative would have most likely provided an overestimation because the research team did not directly apply these interventions, rather suggested them to the caregivers. We also had variations in the number and quality of videos submitted by families, and there were weeks when weekly phone contact did not occur.
The typology of expert interventions based on in-home video data include: (1) education and skills for managing behavioral and psychological symptoms, improving ADLs and IADLs, achieving realistic disease expectations, improving safety, and managing medical care and medication utilization; and (2) caregiver support including social support, self-care, respite, and positive reinforcement. This typology extends that of traditional approaches to support caregivers, by identifying needs for interventions that address safety and environmental modifications, provide positive reinforcement, and continue teaching about disease expectations as dementia progresses.
Ultimately, future research can inform practice by identifying typical interventions needed based on characteristics of the dementia care dyad (such as caregiver relationship and the person with dementia’s disease stage). In addition, evaluation of the congruence between dementia caregiver perceived needs and what experts identify is warranted. This study demonstrated the value of technology in providing rich contextual data to develop an enhanced typology of interventions to support dementia caregivers.
Acknowledgements
The authors thank other research team members who were involved in the conduct of the FamTechCare study including Eric Vidoni, Diane Blyler, Denise Seabold, JoEllen Wurth, Ann Arthur, and Michelle Niedens. The University of Kansas Alzheimer’s Disease Center (P30AG035982) provided essential infrastructure and recruitment support.
Funding statement
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute of Nursing Research of the National Institutes of Health [grant number R01NR014737]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Clinical Trials registration NCT02483520.
Footnotes
Conflicting Interests
The Authors declare that there is no conflict of interest.
References
- American Association of Retired Persons (2016). Caregivers & Technology: What They Want and Need. Retrieved from https://www.aarp.org/content/dam/aarp/home-and-family/personal-technology/2016/04/Caregivers-and-Technology-AARP.pdf
- Algase DL, Beck C, Kolanowski A, Whall A, Berent S, Richards K, & Beattie E (1996). Need-driven dementia-compromised behavior: An alternative view of disruptive behavior. American Journal of Alzheimer’s Disease, 11(6), 10–19. 10.1177/153331759601100603 [DOI] [Google Scholar]
- Alzheimer’s Association (2018). 2018 Alzheimer’s Disease Facts and Figures. Alzheimer’s & Dementia, 14, 367–429. 10.1016/j.jalz.2018.02.001 [DOI] [PubMed] [Google Scholar]
- Balestreri L, Grossberg A, & Grossberg GT (2000). Behavioral and psychological symptoms of dementia as a risk factor for nursing home placement. International Psychogeriatrics, 12(S1), 59–62. 10.1017/S1041610200006773 [DOI] [Google Scholar]
- Borson S, Boustani MA, Buckwalter KC, Burgio LD, Chodosh J, Fortinsky RH, . . . Lynn J (2016). Report on milestones for care and support under the US National Plan to Address Alzheimer’s Disease. Alzheimer’s & Dementia, 12(3), 334–369. 10.1016/j.jalz.2016.01.005 [DOI] [PubMed] [Google Scholar]
- Bossen AL, Kim H, Williams KN, Steinhoff AE, & Strieker M (2015). Emerging roles for telemedicine and smart technologies in dementia care. Smart Homecare Technology and Telehealth, 3, 49–57. 10.2147/SHTT.S59500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford A, Shrestha S, Snow AL, Stanley MA, Wilson N, Hersch G, & Kunik ME (2012). Managing pain to prevent aggression in people with dementia: A nonpharmacologic intervention. American Journal of Alzheimer’s Disease & Other Dementias, 27(1), 41–47. 10.1177/1533317512439795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell JL, Quincy C, Osserman J, & Pedersen OK (2013). Coding in-depth semistructured interviews: Problems of unitization and intercoder reliability and agreement. Sociological Methods & Research, 42(3), 294–320. 10.1177/0049124113500475 [DOI] [Google Scholar]
- Chi NC & Demiris G (2015). A systematic review of telehealth tools and interventions to support family caregivers. Journal of Telemedicine and Telecare, 21(1), 37–44. 10.1177/1357633X14562734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elo S, & Kyngäs H (2008). The qualitative content analysis process. Journal of Advanced Nursing, 62(1), 107–115. 10.1111/j.1365-2648.2007.04569.x [DOI] [PubMed] [Google Scholar]
- Fick DM, Kolanowski AM, Waller JL, & Inouye SK (2005). Delirium superimposed on dementia in a community-dwelling managed care population: a 3-year retrospective study of occurrence, costs, and utilization. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences, 60(6), 748–753. 10.1093/gerona/60.6.748 [DOI] [PubMed] [Google Scholar]
- Fuchs-Lacelle S, Hadjistavropoulos T, & Lix L (2008). Pain assessment as intervention: a study of older adults with severe dementia. The Clinical Journal of Pain, 24(8), 697–707. 10.1097/AJP.0b013e318172625a [DOI] [PubMed] [Google Scholar]
- Gitlin LN, & Hodgson N (2015). Caregivers as therapeutic agents in dementia care: the context of caregiving and the evidence base for interventions. In Family Caregiving in the New Normal (pp. 305–353). 10.1016/B978-0-12-417046-9.00017-9 [DOI] [Google Scholar]
- Godwin KM, Mills WL, Anderson JA, & Kunik ME (2013). Technology-driven interventions for caregivers of persons with dementia a systematic review. American Journal of Alzheimer’s Disease and Other Dementias, 28(3), 216–222. 10.1177/1533317513481091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh H-F, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15(9), 1277–1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Jennings LA, Palimaru A, Corona MG, Cagigas XE, Ramirez KD, Zhao T, ... & Reuben DB (2017). Patient and caregiver goals for dementia care. Quality of Life Research, 26(3), 685–693. 10.1007/s11136-016-1471-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, & Lyketsos CG (2014). Management of neuropsychiatric symptoms of dementia in clinical settings: Recommendations from a multidisciplinary expert panel. Journal of the American Geriatrics Society, 62(4), 762–769. 10.1111/jgs.12730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kales HC, Gitlin LN, & Lyketsos CG (2015). Assessment and management of behavioral and psychological symptoms of dementia. The BMJ, 350, h369 10.1136/bmj.h369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight BG, & Sayegh P (2010). Cultural values and caregiving: The updated sociocultural stress and coping model. The Journals of Gerontology: Series B, 65(1), 5–13. 10.1093/geronb/gbp096 [DOI] [PubMed] [Google Scholar]
- Kovach CR, Noonan PE, Schlidt AM, & Wells T (2005). A model of consequences of need‐driven, dementia‐compromised behavior. Journal of Nursing Scholarship, 37(2), 134–140. 10.1111/j.1547-5069.2005.00025_1.x [DOI] [PubMed] [Google Scholar]
- Kunik ME, Snow AL, Davila JA, McNeese T, Steele AB, Balasubramanyam V, . . . Walder A (2010). Consequences of aggressive behavior in patients with dementia. The Journal of Neuropsychiatry and Clinical Neurosciences, 22(1), 40–47. 10.1176/jnp.2010.22.1.40 [DOI] [PubMed] [Google Scholar]
- Meuleners LB, & Hobday MB (2017). A population‐based study examining injury in older adults with and without dementia. Journal of the American Geriatrics Society, 65(3), 520–525. 10.1111/jgs.14523 [DOI] [PubMed] [Google Scholar]
- Monin JK, & Schulz R (2009). Interpersonal effects of suffering in older adult caregiving relationships. Psychology and Aging, 24(3), 681–695. 10.1037/a0016355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moniz‐Cook E, Elston C, Gardiner E, Agar S, Silver M, Win T, & Wang M (2008). Can training community mental health nurses to support family carers reduce behavioral problems in dementia? An exploratory pragmatic randomized controlled trial. International Journal of Geriatric Psychiatry, 23(2), 185–191. 10.1002/gps.1860 [DOI] [PubMed] [Google Scholar]
- National Academies of Sciences, Engineering, and Medicine. (2016). Families caring for an aging America. National Academies Press; Retrieved from http://www.nationalacademies.org/hmd/Reports/2016/families-caring-for-an-aging-america.aspx [PubMed] [Google Scholar]
- Prince M, Comas-Herrera A, Knapp M, Guerchet M, & Karagiannidou M (2016). World Alzheimer report 2016: improving healthcare for people living with dementia: coverage, quality and costs now and in the future. Retrieved from http://eprints.lse.ac.uk/67858/
- Pozzebon M, Douglas J & Ames D (2016). Spouses’ experience of living with a partner diagnosed with dementia: A synthesis of the qualitative research. International Psychogeriatrics, 28(4), 537–556. 10.1017/S1041610215002239 [DOI] [PubMed] [Google Scholar]
- Reisberg B (2007). Global measures: Utility in defining and measuring treatment response in dementia. International Psychogeriatrics, 19(3), 421–456. 10.1017/S1041610207005261 [DOI] [PubMed] [Google Scholar]
- Roberts E, & Struckmeyer KM (2018). The Impact of Respite Programming on Caregiver Resilience in Dementia Care: A Qualitative Examination of Family Caregiver Perspectives. INQUIRY: The Journal of Health Care Organization, Provision, and Financing, 55, 0046958017751507 10.1177/0046958017751507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson L, Clare L, & Evans K (2005). Making sense of dementia and adjusting to loss: Psychological reactions to a diagnosis of dementia in couples. Aging & Mental Health, 9(4), 337–347. 10.1080/13607860500114555 [DOI] [PubMed] [Google Scholar]
- Rudolph JL, Zanin NM, Jones RN, Marcantonio ER, Fong TG, Yang FM, . . . Inouye SK (2010). Hospitalization in community‐dwelling persons with Alzheimer’s Disease: Frequency and causes. Journal of the American Geriatrics Society, 58(8), 1542–1548. 10.1111/j.1532-5415.2010.02924.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz R, & Martire LM (2004). Family caregiving of persons with dementia: Prevalence, health effects, and support strategies. The American Journal of Geriatric Psychiatry, 12(3), 240–249. 10.1097/00019442-200405000-00002 [DOI] [PubMed] [Google Scholar]
- Sclan SG, & Reisberg B (1992). Functional assessment staging (FAST) in Alzheimer’s disease: Reliability, validity, and ordinality. International Psychogeriatrics, 4(3), 55–69. 10.1017/S1041610292001157 [DOI] [PubMed] [Google Scholar]
- Shirai Y, & Koerner S (2018). Examining the Influence of Care-Recipient Resistance on Family Caregiver Emotional and Physical Well-Being: Average Frequency Versus Daily Fluctuation. Journal of Applied Gerontology, 37(2), 203–227. 10.1177/0733464816631594 [DOI] [PubMed] [Google Scholar]
- Spigelmyer PC, Hupcey JE, Smith CA, Loeb SJ, & Kitko L (2018). Resistiveness to Care as Experienced by Family Caregivers Providing Care for Someone With Dementia. Journal of Nursing Scholarship, 50(1), 36–46. 10.1111/jnu.12345 [DOI] [PubMed] [Google Scholar]
- Stockwell‐Smith G, Moyle W, & Kellett U (2018). The impact of early psychosocial intervention on self‐efficacy of care recipient/carer dyads living with early‐stage dementia—A mixed‐methods study. Journal of advanced nursing, 74(9), 2167–2180. 10.1111/jan.13710 [DOI] [PubMed] [Google Scholar]
- Talley RC, & Crews JE (2007). Framing the public health of caregiving. American Journal of Public Health, 97(2), 224–228. 10.2105/AJPH.2004.059337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley RC, Montgomery RJV (2013). Caregiving: A Developmental, Life-Long Perspective In: Talley R, Montgomery R (eds) Caregiving Across the Lifespan. Caregiving: Research • Practice • Policy. Springer, New York, NY: 10.1007/978-1-4614-5553-0_1 [DOI] [Google Scholar]
- Toot S, Hoe J, Ledgerd R, Burnell K, Devine M, & Orrell M (2013). Causes of crises and appropriate interventions: The views of people with dementia, carers and healthcare professionals. Aging & Mental Health, 17(3), 328–335. 10.1080/13607863.2012.732037 [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services (2016). National plan to address Alzheimer’s disease. Retrieved from https://aspe.hhs.gov/national-plan-address-alzheimers-disease-2015-update
- Vaingankar JA, Chong SA, Abdin E, Picco L, Shafie S, Seow E, ... & Subramaniam M (2016). Psychiatric morbidity and its correlates among informal caregivers of older adults. Comprehensive psychiatry, 68, 178–185. 10.1016/j.comppsych.2016.04.017 [DOI] [PubMed] [Google Scholar]
- Waller A, Dilworth S, Mansfield E, & Sanson-Fisher R (2017). Computer and telephone delivered interventions to support caregivers of people with dementia: A systematic review of research output and quality. BMC Geriatrics, 17(1), 265 10.1186/s12877-017-0654-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XR, Liu SX, Robinson KM, Shawler C, & Zhou L (2018). The impact of dementia caregiving on self‐care management of caregivers and facilitators: a qualitative study. Psychogeriatrics. 10.1111/psyg.12354 [DOI] [PubMed] [Google Scholar]
- Williams K, Blyler D, Vidoni E, Shaw C, Wurth J, Seabold D, … Van Schiver A (2018). A randomized trial using telehealth technology to link caregivers with dementia care experts for in-home caregiving support: FamTechCare protocol. Research in Nursing and Health, 41(3), 219–227. 10.1002/nur.21869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods RT, & Clare L (2008). Handbook of the Clinical Psychology of Ageing: John Wiley & Sons; 10.1002/9780470773185 [DOI] [Google Scholar]
- World Health Organization. (2012). Dementia: a public health priority. World Health Organization; Retrieved from http://apps.who.int/iris/bitstream/handle/10665/75263/9789241564458_eng.pdf?sequence=1 [Google Scholar]